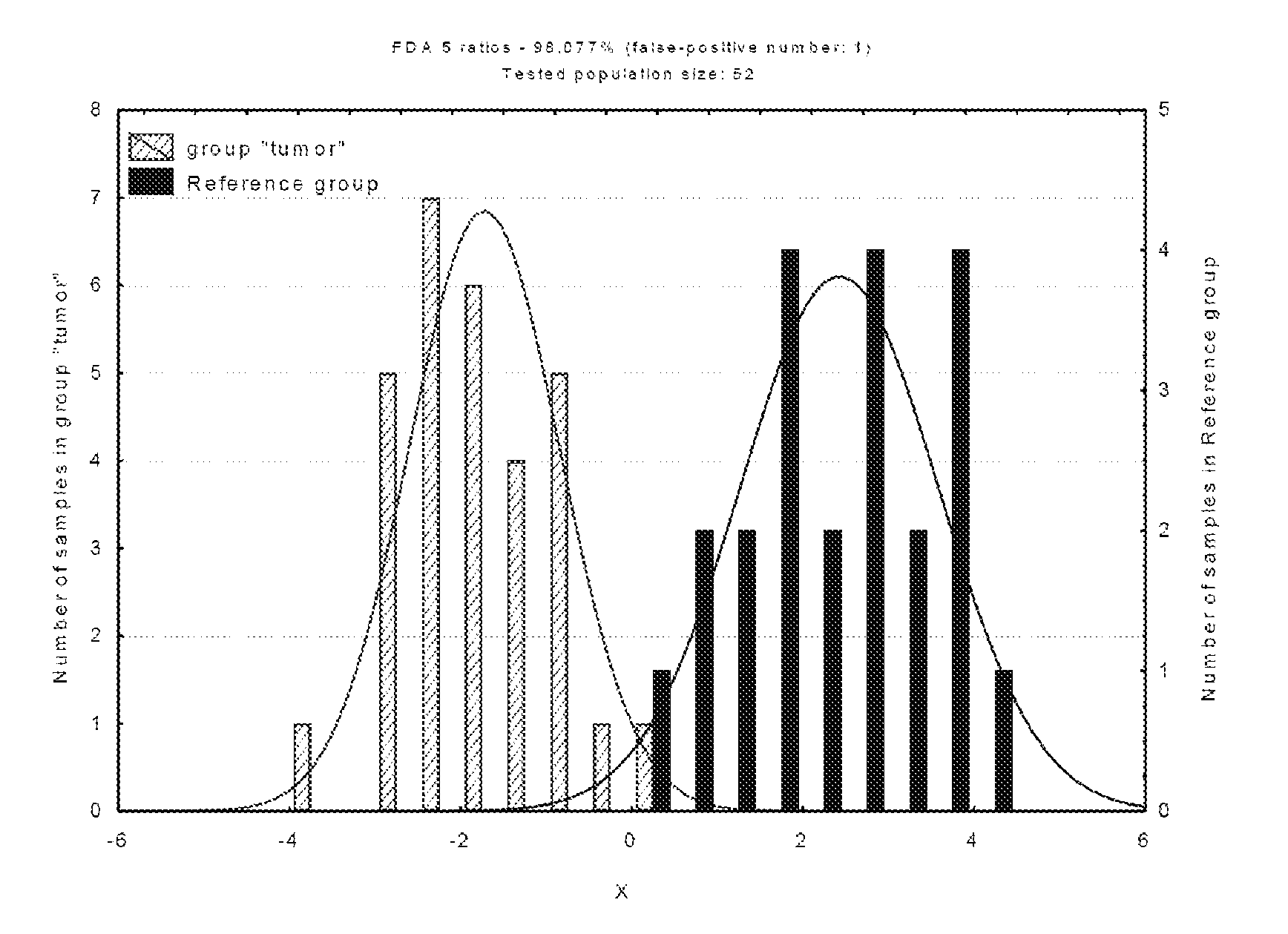
Patents
Literature
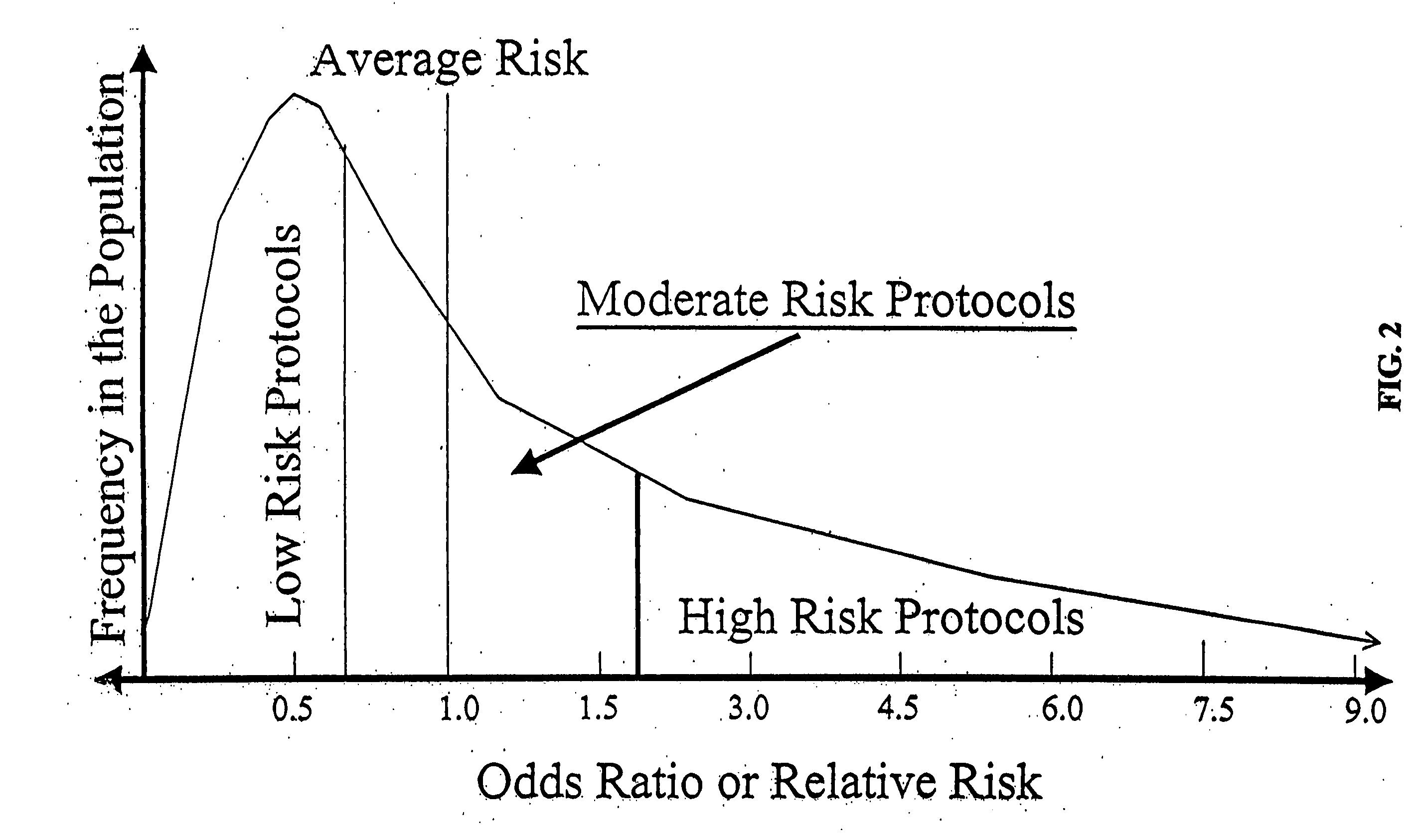
243 results about "Cancer risk" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method and apparatus for noninvasive measurement of carotenoids and related chemical substances in biological tissue
InactiveUS6205354B1Rapid and noninvasive and quantitative measurementRiskRadiation pyrometrySurgeryResonance Raman spectroscopyAntioxidant
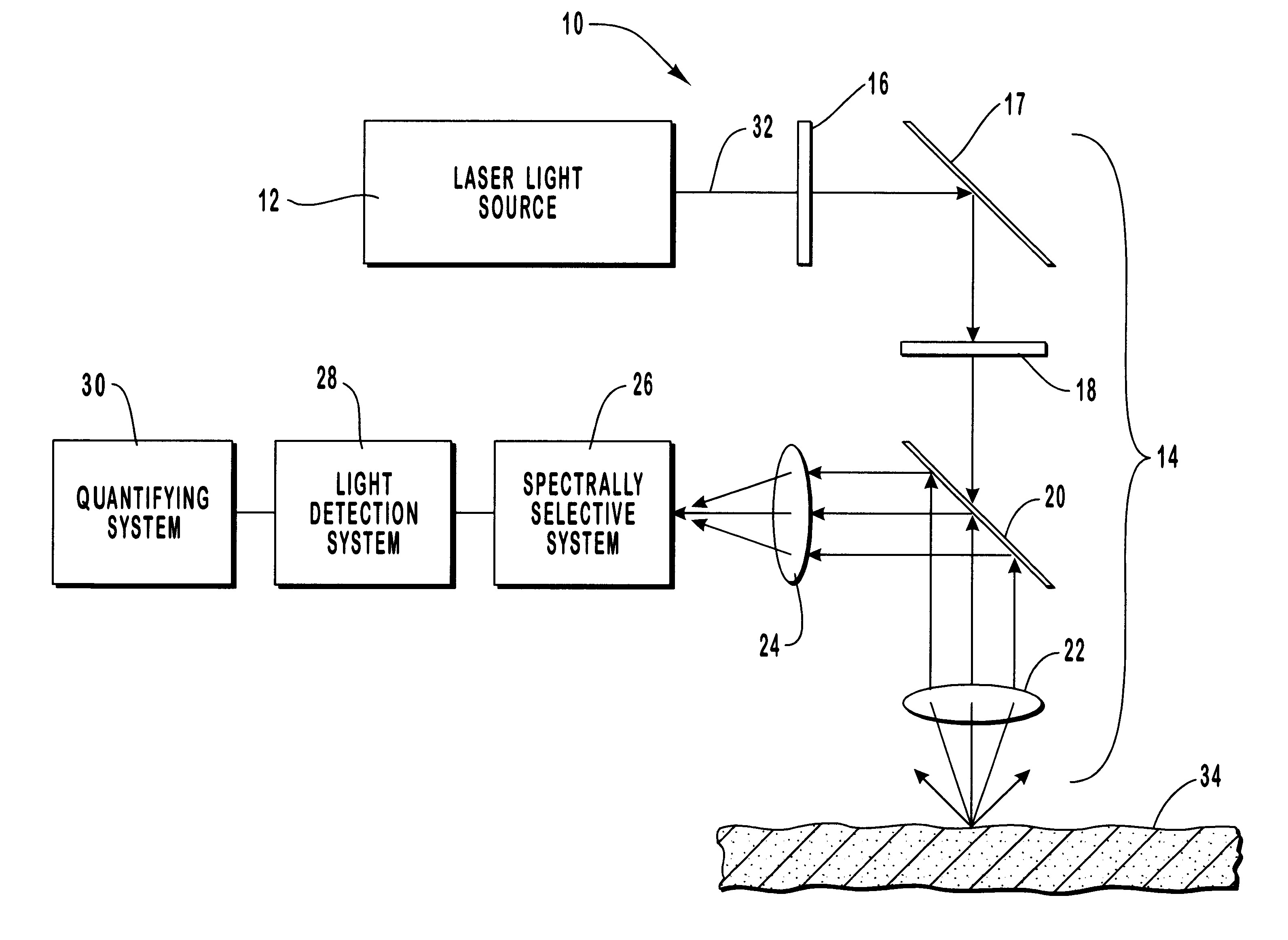
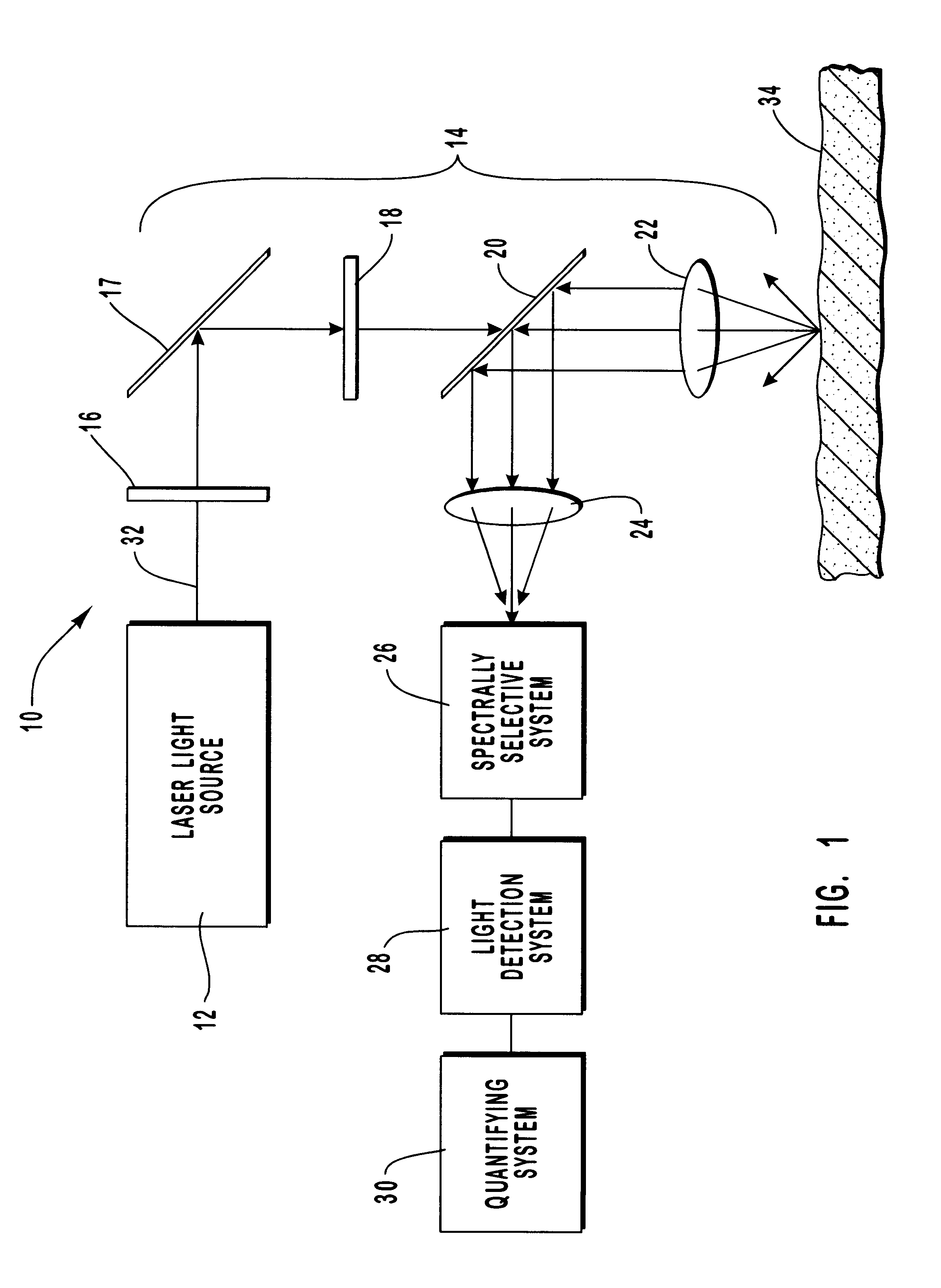
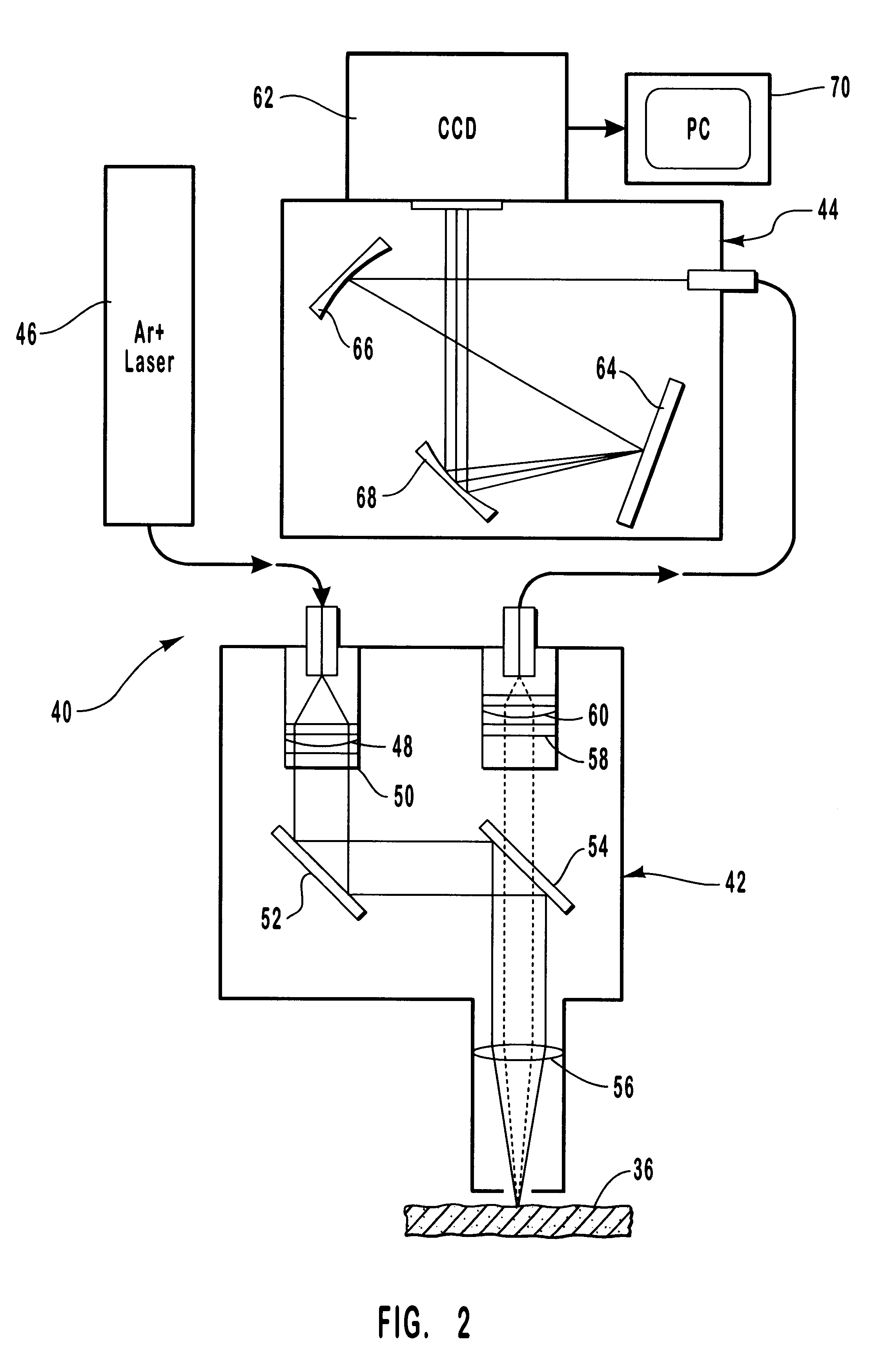
A method and apparatus are provided for the determination of levels of carotenoids and similar chemical compounds in biological tissue such as living skin. The method and apparatus provide a noninvasive, rapid, accurate, and safe determination of carotenoid levels which in turn can provide diagnostic information regarding cancer risk, or can be a marker for conditions where carotenoids or other antioxidant compounds may provide diagnostic information. Such early diagnostic information allows for the possibility of preventative intervention. The method and apparatus utilize the technique of resonance Raman spectroscopy to measure the levels of carotenoids and similar substances in tissue. In this technique, laser light is directed upon the area of tissue which is of interest. A small fraction of the scattered light is scattered inelastically, producing the carotenoid Raman signal which is at a different frequency than the incident laser light, and the Raman signal is collected, filtered, and measured. The resulting Raman signal can be analyzed such that the background fluorescence signal is subtracted and the results displayed and compared with known calibration standards.
Owner:UNIV OF UTAH RES FOUND
Compositions and methods for cancer immunotherapy
The invention relates to immunotherapeutic compounds and to methods for stimulating an immune response in a subject individual at risk for developing cancer, diagnosed with a cancer, in treatment for cancer, or in post-therapy recovery from cancer or the compounds of the invention can be administered as a prophylactic to a subject individual to prevent or delay the development of cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
Method and Apparatus for Categorizing Breast Density and Assessing Cancer Risk Utilizing Acoustic Parameters
ActiveUS20080275344A1Ultrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsMedicineWhole breast
A method for categorizing whole-breast density is disclosed. The method includes the steps of exposing breast tissue to an acoustic signal; measuring a distribution of an acoustic parameter by analyzing the acoustic signal; and obtaining a measure of whole-breast density from said measuring step. An apparatus is also disclosed.
Owner:DELPHINUS MEDICAL TECH
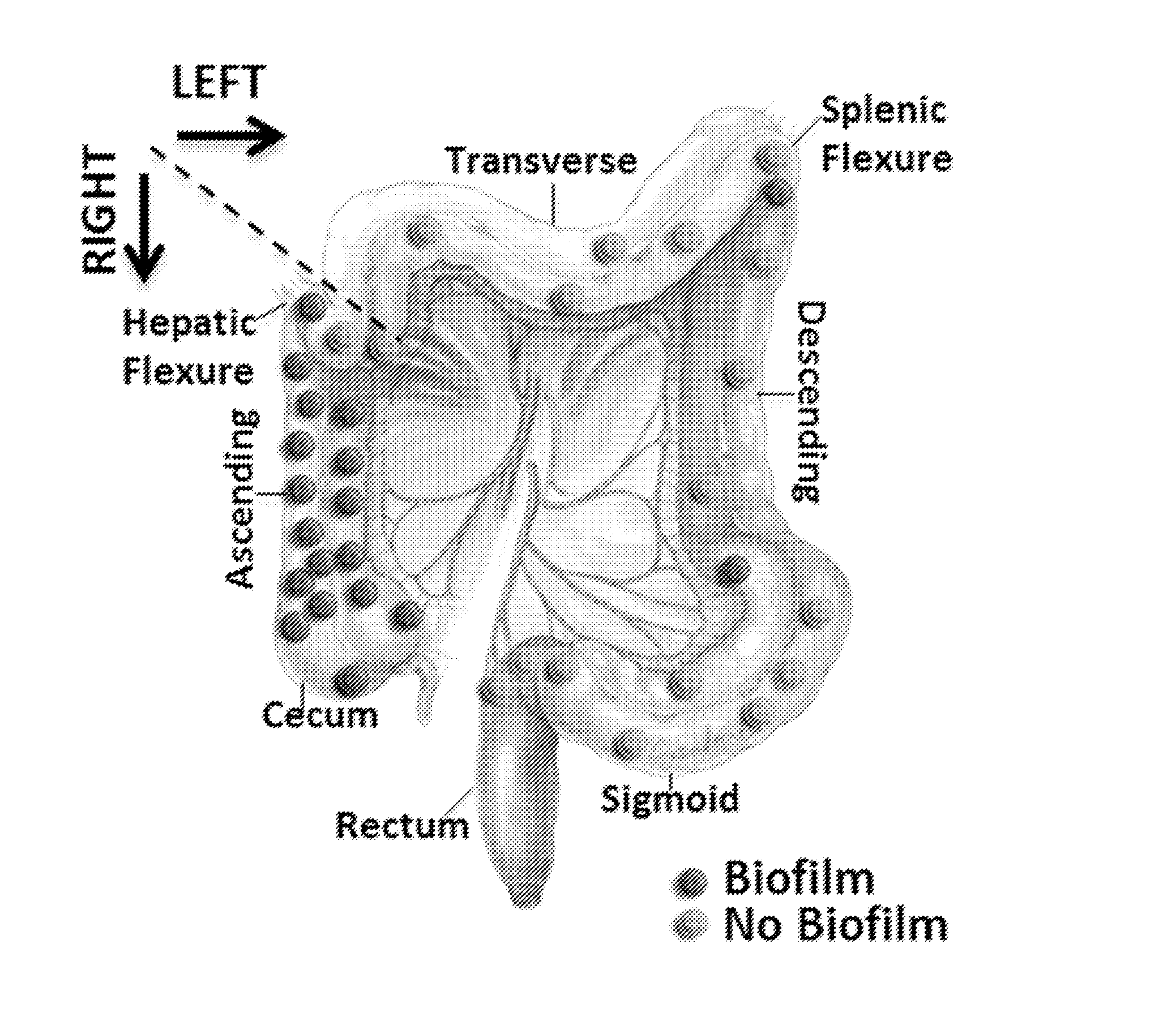
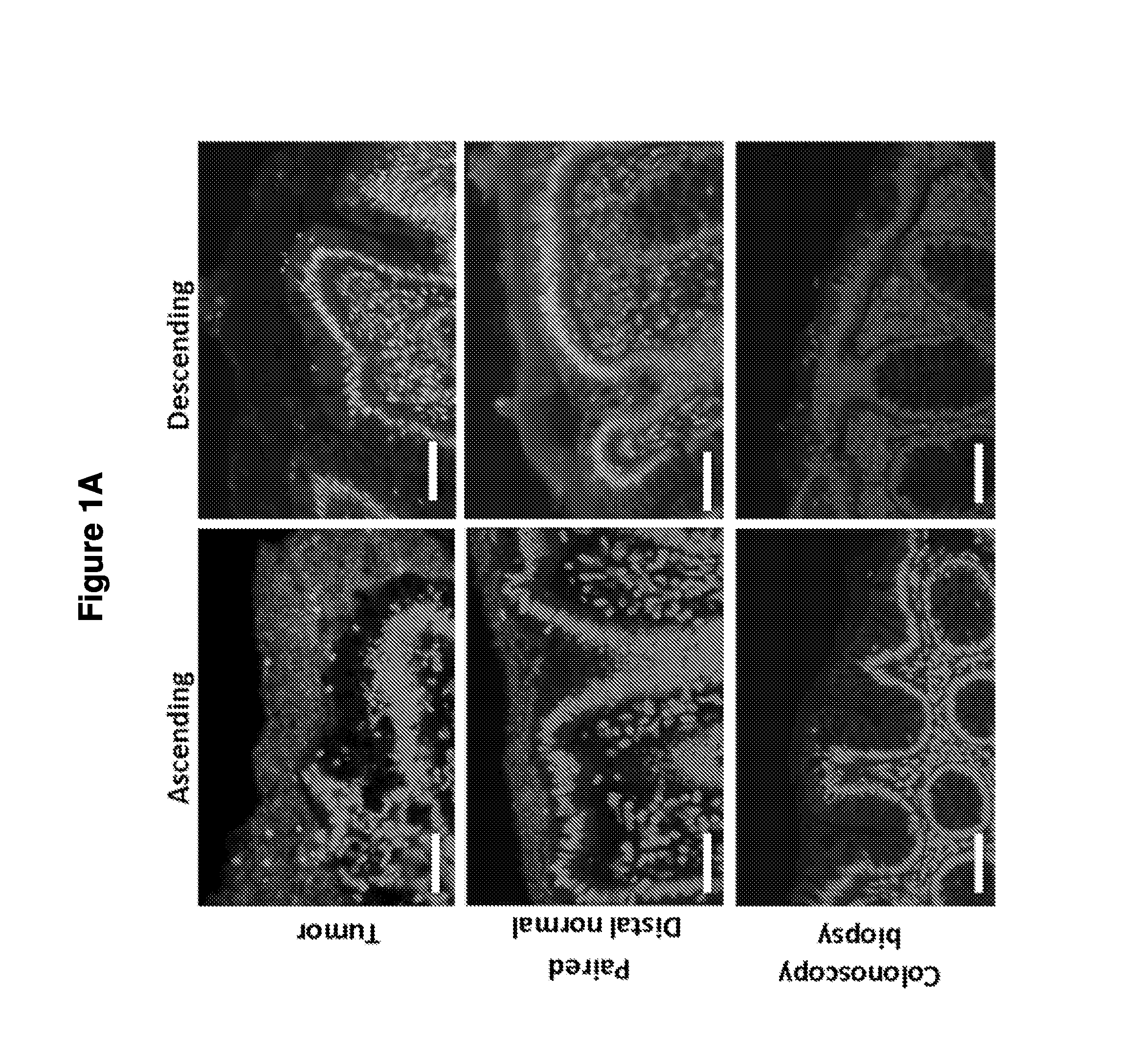
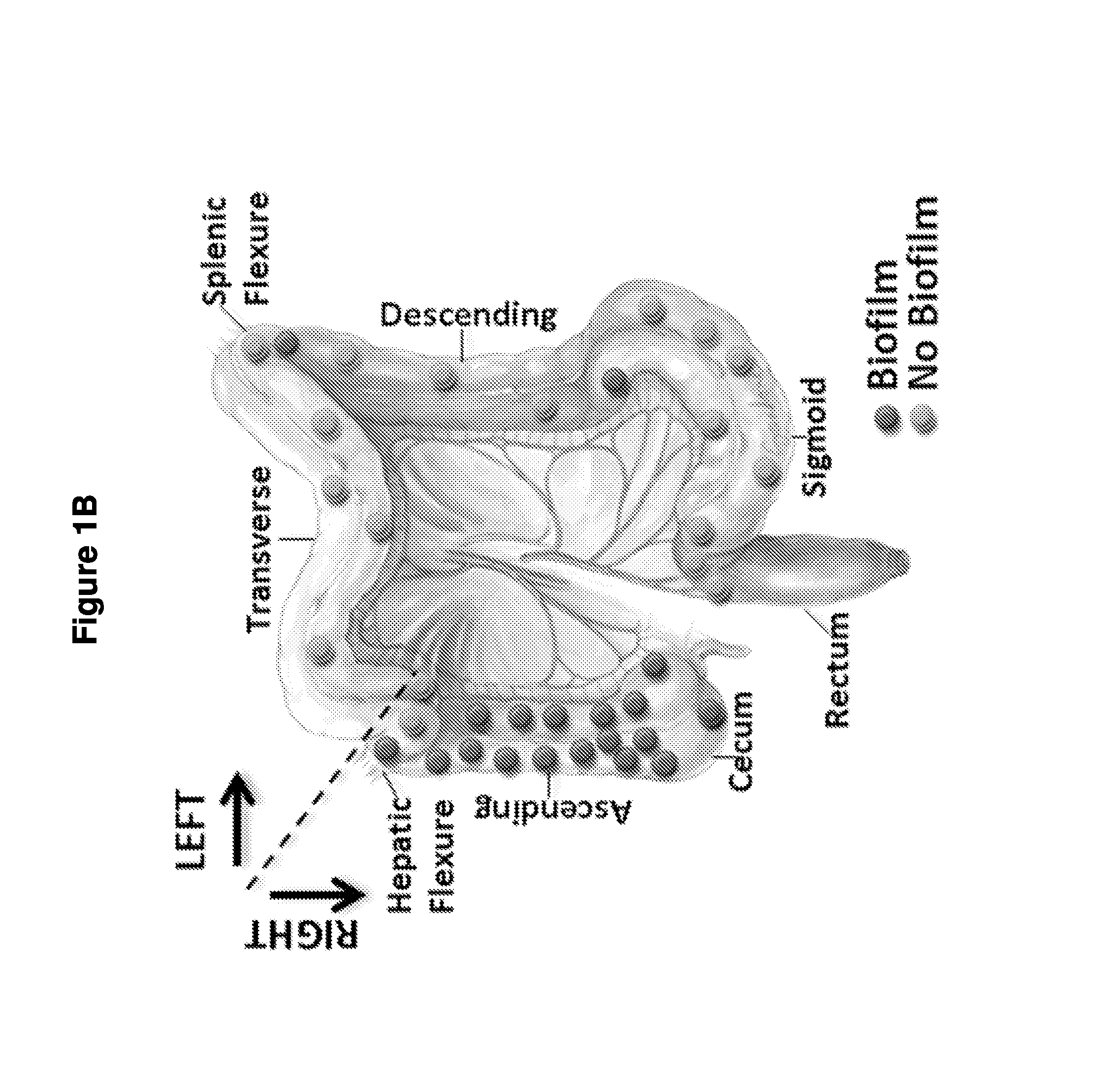
Biofilm formation to define risk for colon cancer
ActiveUS20160223553A1Preventing and diminishing developmentIncreased proliferationMicrobiological testing/measurementUnknown materialsFecesIncreased risk
Methods of identifying subjects at increased risk of cancer, based upon detection of biofilms and / or biofilm-associated microbes within a subject, are disclosed. Therapies designed to prevent formation and / or reduce the size of biofilms in a subject identified to be at increased risk of cancer based upon detection of biofilms and / or biofilm-associated microbes are disclosed. In particular embodiments, the invention provides for identification of a subject at elevated risk of developing or having colorectal cancer and / or a colorectal adenoma, based upon detection of a biofilm and / or biofilm-associated bacteria within the gastrointestinal tract of the subject (optionally, within a biopsy specimen and / or stool sample of such subject). Therapies involving administration of an antibiotic agent and / or a probiotic agent to a subject, to prevent or reduce biofilm formation within the gastrointestinal tract of the subject, optionally provided in combination with additional cancer therapy, are also disclosed.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
System and method for 3-d biopsy
ActiveUS20080039723A1Improve efficiencyMaximizing similarityUltrasonic/sonic/infrasonic diagnosticsSurgeryCancers locationBiopsy procedure
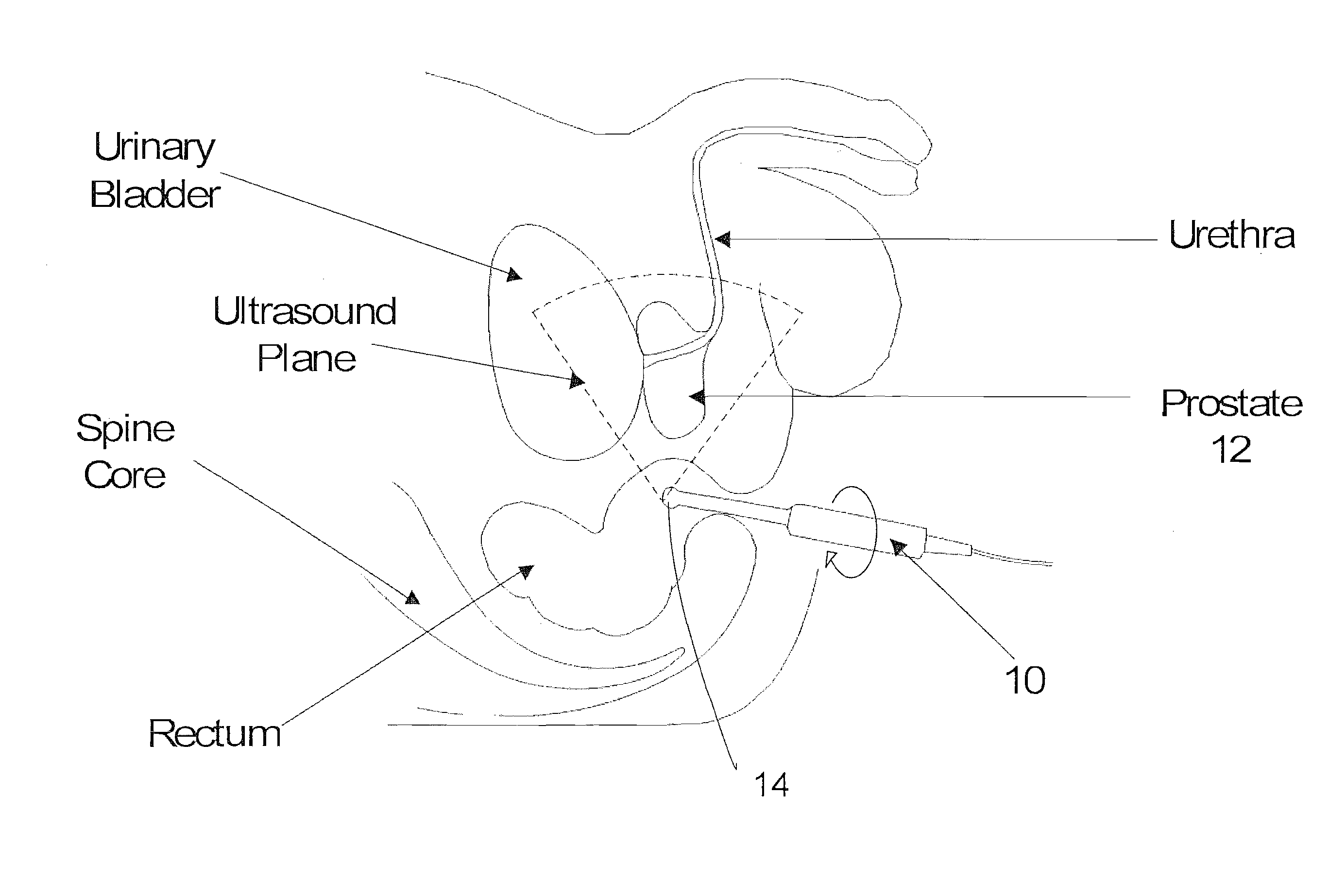
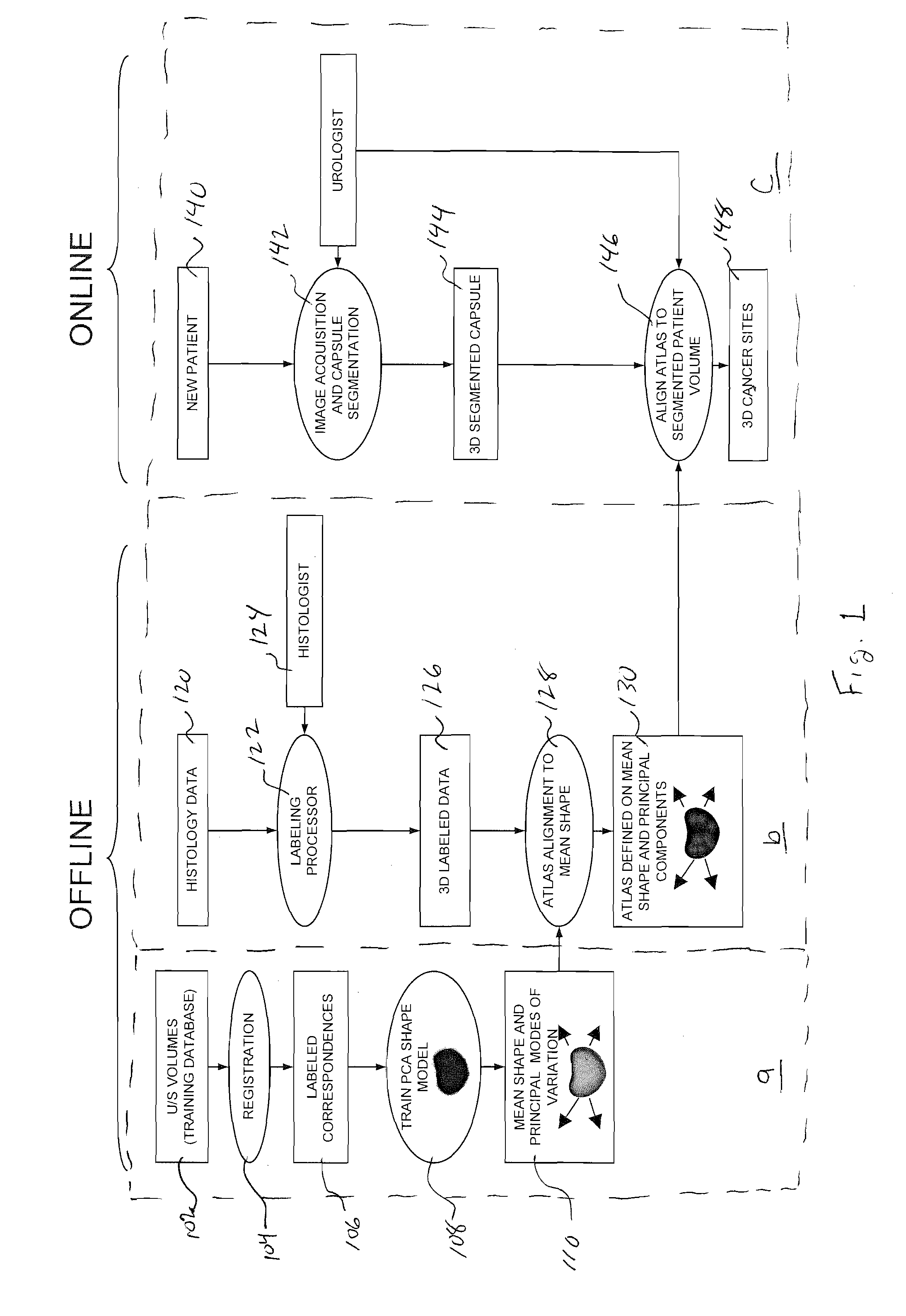
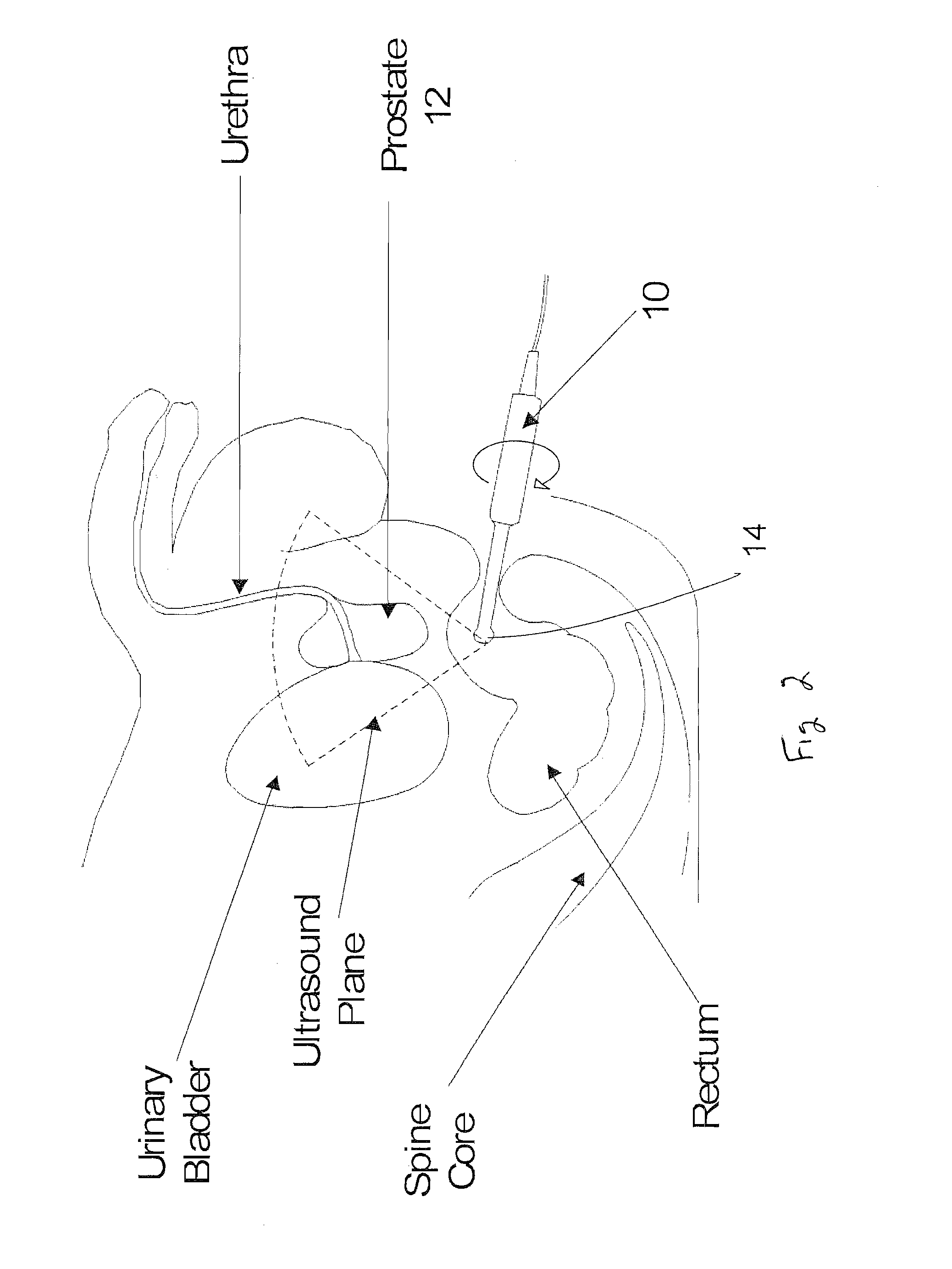
A system and method (i.e, utility) are disclosed for positioning a needle in three-dimensions based on patient related statistics for extracting tissue during biopsy procedures. Aspects of the utility can be applied independently or serve as an aid to the urologist when regions of interest are hard to discern in an ultrasound image. Regions of interest that correspond to high cancer risk regions (e.g., statistically) are automatically superimposed on an ultrasound image of a patient in real time. Additionally a statistical map based on one or more demographic parameters of a patient and containing cancer probability locations are also automatically mapped on the ultrasound image in real time displaying potential cancer locations. Aspects of the system are also capable of displaying optimal needle placement positions based on statistical priors and will be able to accurately navigate the needle to that position for biopsy extraction and / or treatment.
Owner:EIGEN HEALTH SERVICES LLC
STK15 (STK6) gene polymorphism and methods of determining cancer risk
The present invention provides methods for determining cancer susceptibility in a human subject by identifying in a nucleic acid sample from the subject, a nucleotide occurrence of a single nucleotide polymorphism (SNP) of the STK15 gene, including the STK15 Ile31 polymorphism. The invention provides isolated polynucleotides, polypeptides, specific binding pair members, and cells useful for identifying agents that affect tumor susceptibility. Furthermore, the invention provides methods for detecting low penetrance tumor susceptibility genes.
Owner:RGT UNIV OF CALIFORNIA
Genetic markers for predicting disease and treatment outcome
InactiveUS20060115827A1Reduce and delay recurrenceProlong survival timeBiocideMicrobiological testing/measurementNucleic Acid ProbesIncreased risk
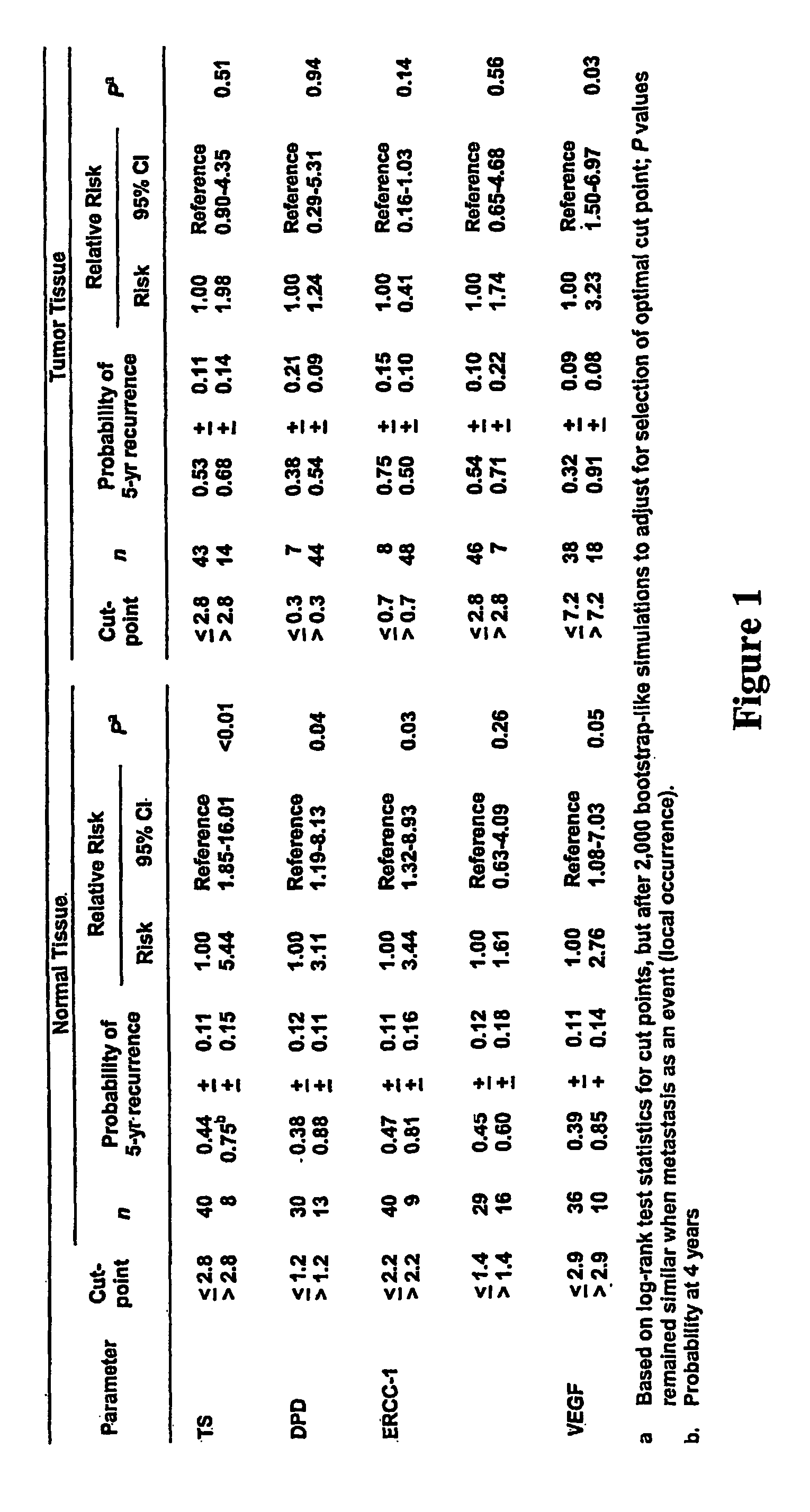
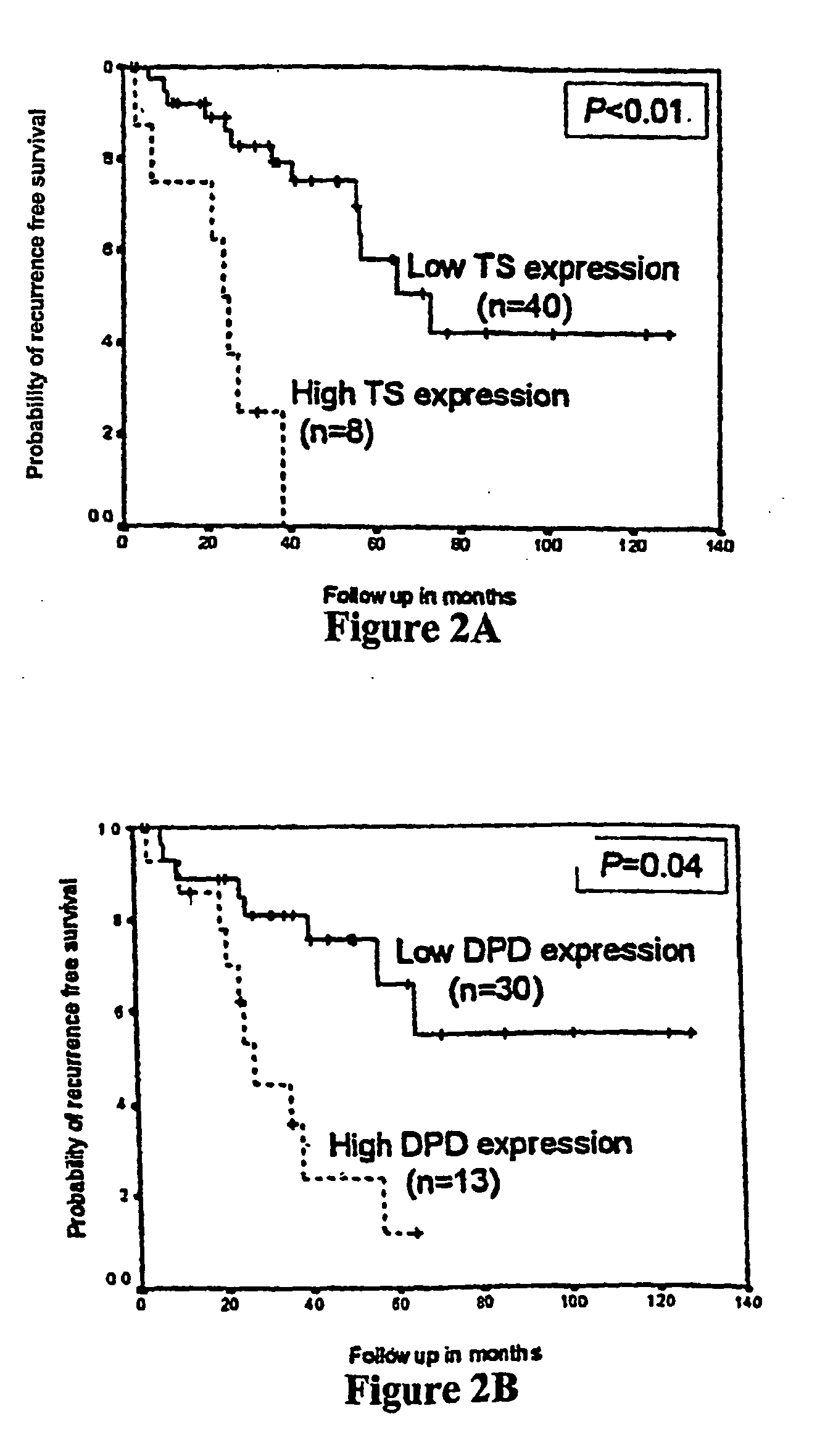
The invention provides compositions and methods for determining the increased risk for recurrence of certain cancers and the likelihood of successful treatment with one or both of chemotherapy and radiation therapy. The methods comprise determining the type of genomic polymorphism present in a predetermined region of the gene of interest isolated from the subject or patient. Also provided are nucleic acid probes and kits for determining a patient's cancer risk and treatment response.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method of preventing or reducing the risk or incidence of cancer
InactiveUS20070237780A1Prevent and reduce riskDestroy, reduce or remove unwanted cells in the targeted tissueOrganic active ingredientsPeptide/protein ingredientsPeptideCancer risk
Owner:NYMOX CORP
Methods and uses thereof of prosaposin
InactiveUS20100144603A1Extended half-lifeFacilitating recombinant protein expressionAntibacterial agentsBiocideAbnormal tissue growthLymphatic Spread
The invention relates to methods for treating of tumor metastasis, methods for preventing, inhibiting, and predicting tumor recurrence and tumor metastasis, and methods of preventing cancer development for one at risk of developing cancer, for one diagnosed with a benign cancer, and / or for one diagnosed with malignant cancer. The invention also relates to methods of treating angiogenesis-dependent diseases and disorders. In addition, the invention provides: (1) a method for screening for tumor / cancer derived angiogenesis and metastasis factors; (2) a method for screening for compounds that inhibit angiogenesis and metastasis; (3) a method for screening for compounds that promote anti-angiogenesis and anti-metastasis activities; (4) a method for cancer prognosis evaluation; (5) a method for predicting the tissue specificity of a metastatic cancer; (6) a method for determining likelihood of metastasis; and (7) a method for cancer prognosis evaluation by the surveillance of metastasis development.
Owner:CHILDRENS MEDICAL CENT CORP
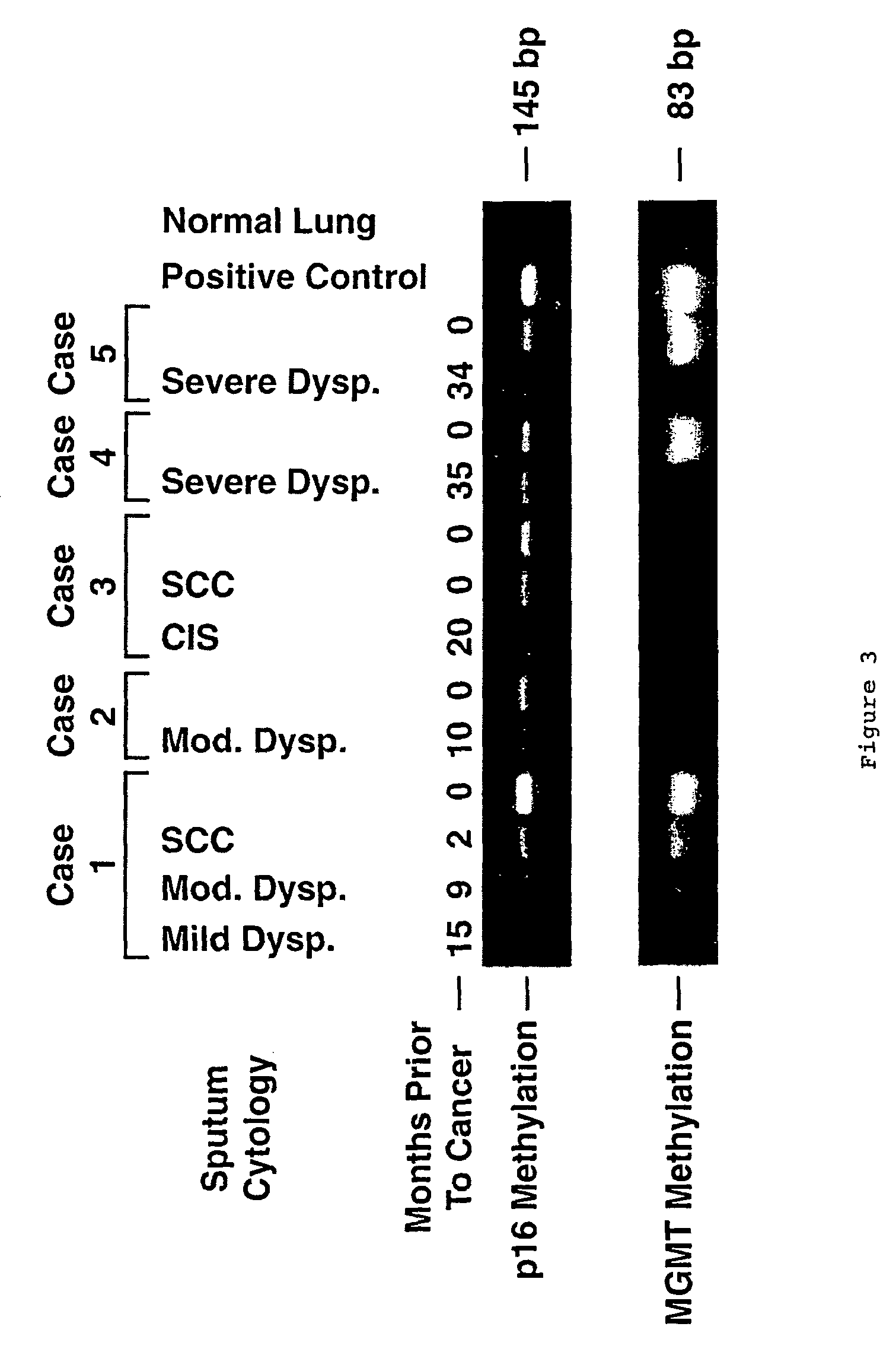
Nested methylation-specific polymerase chain reaction cancer detection method
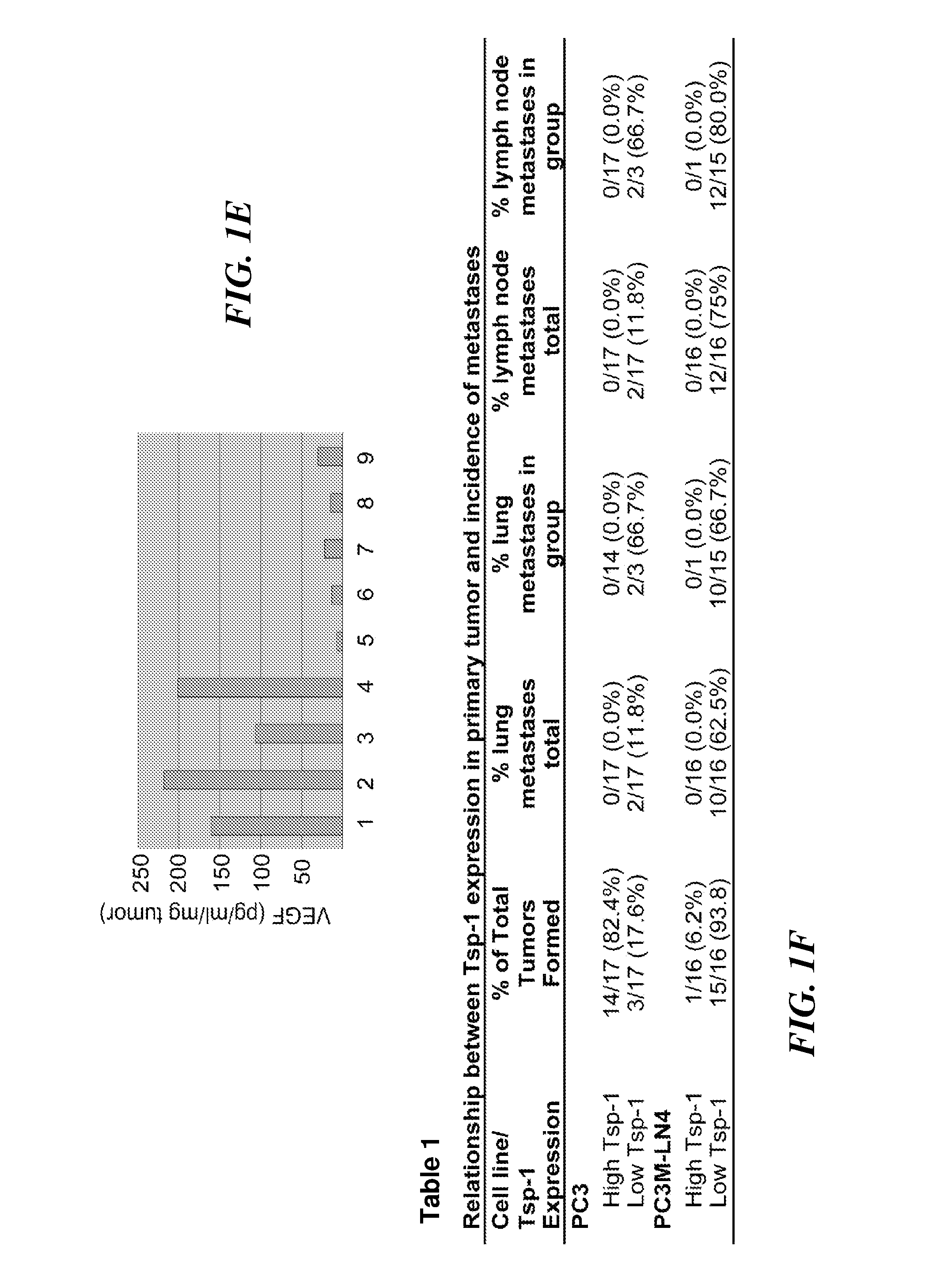
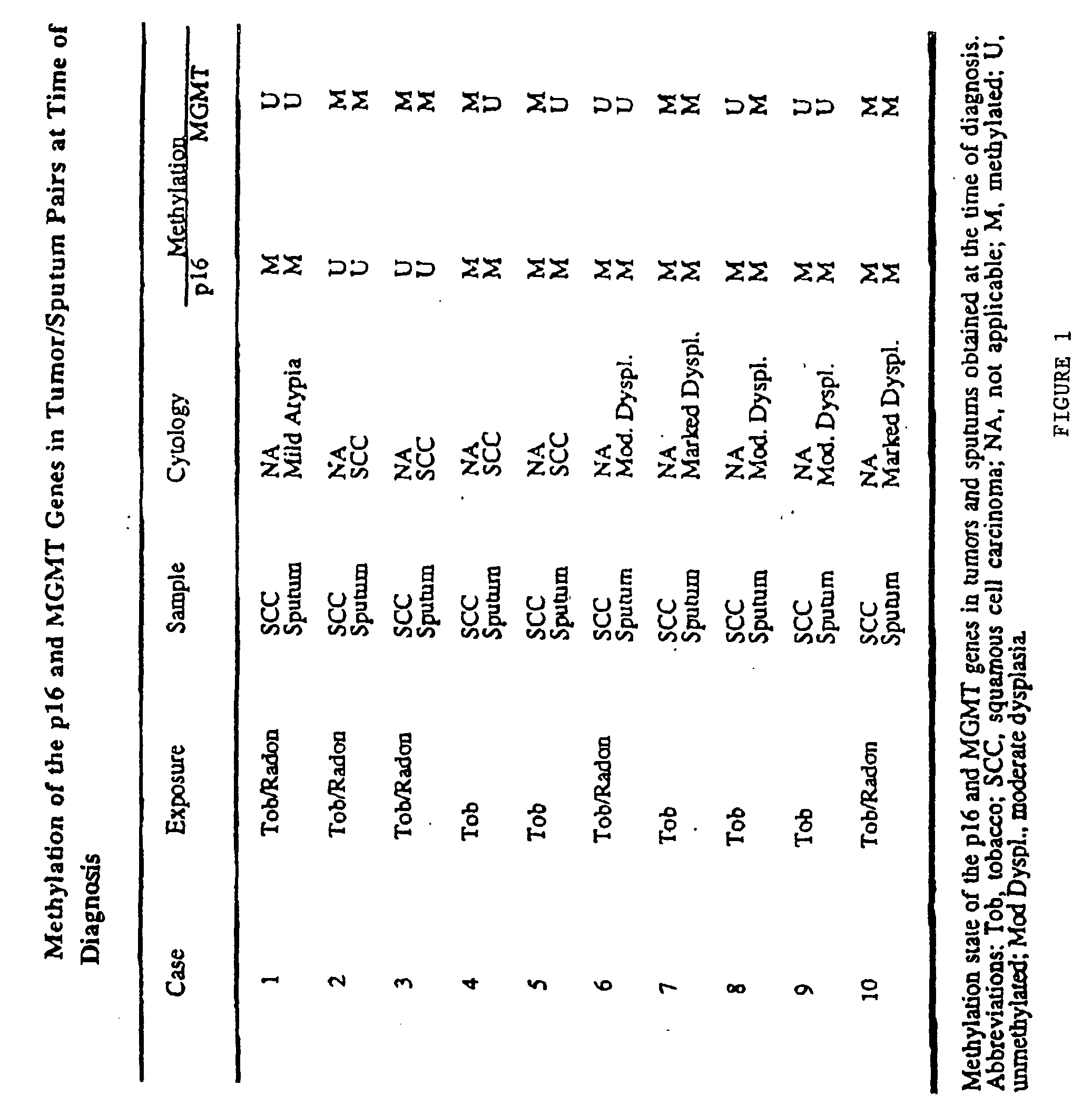
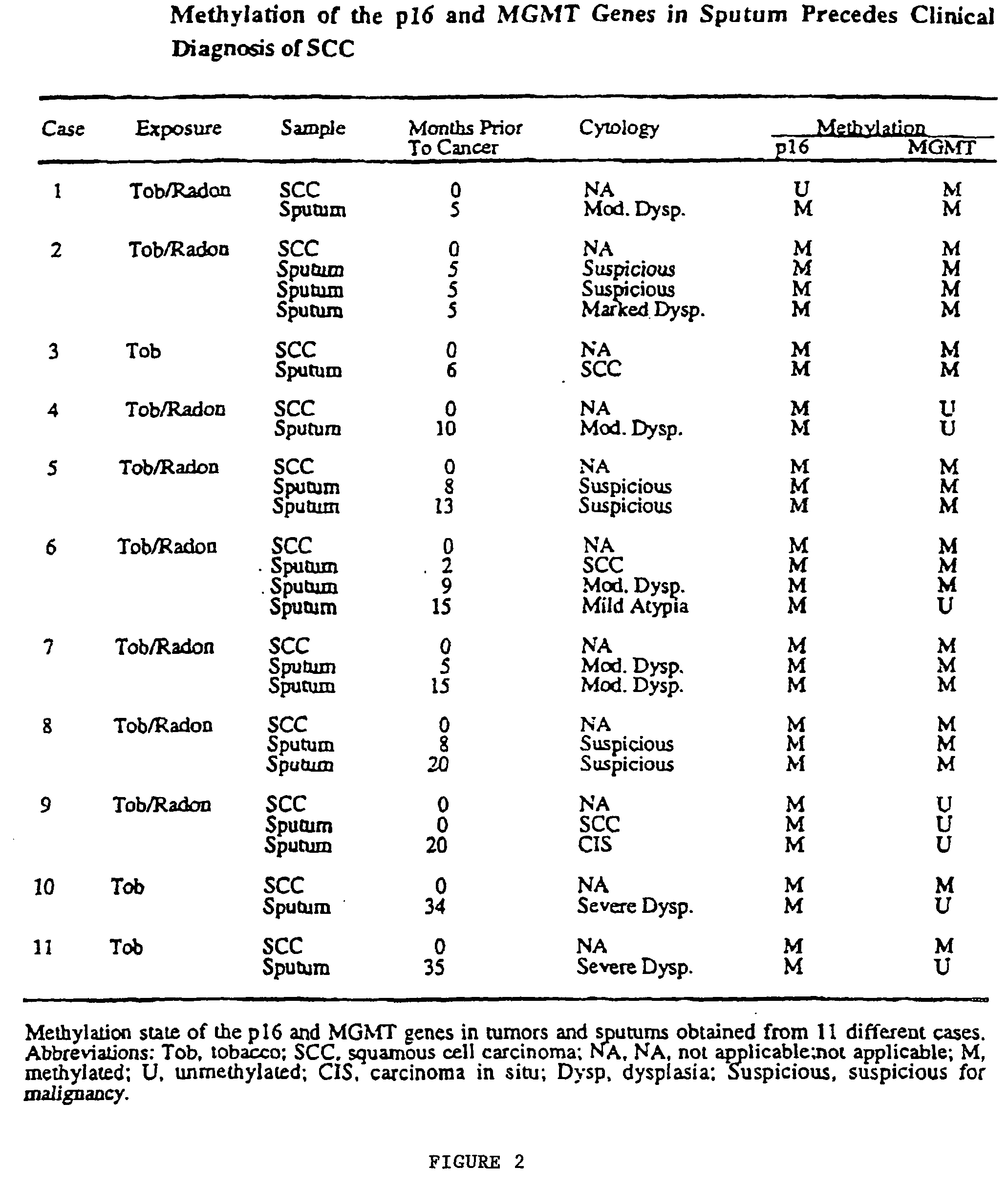
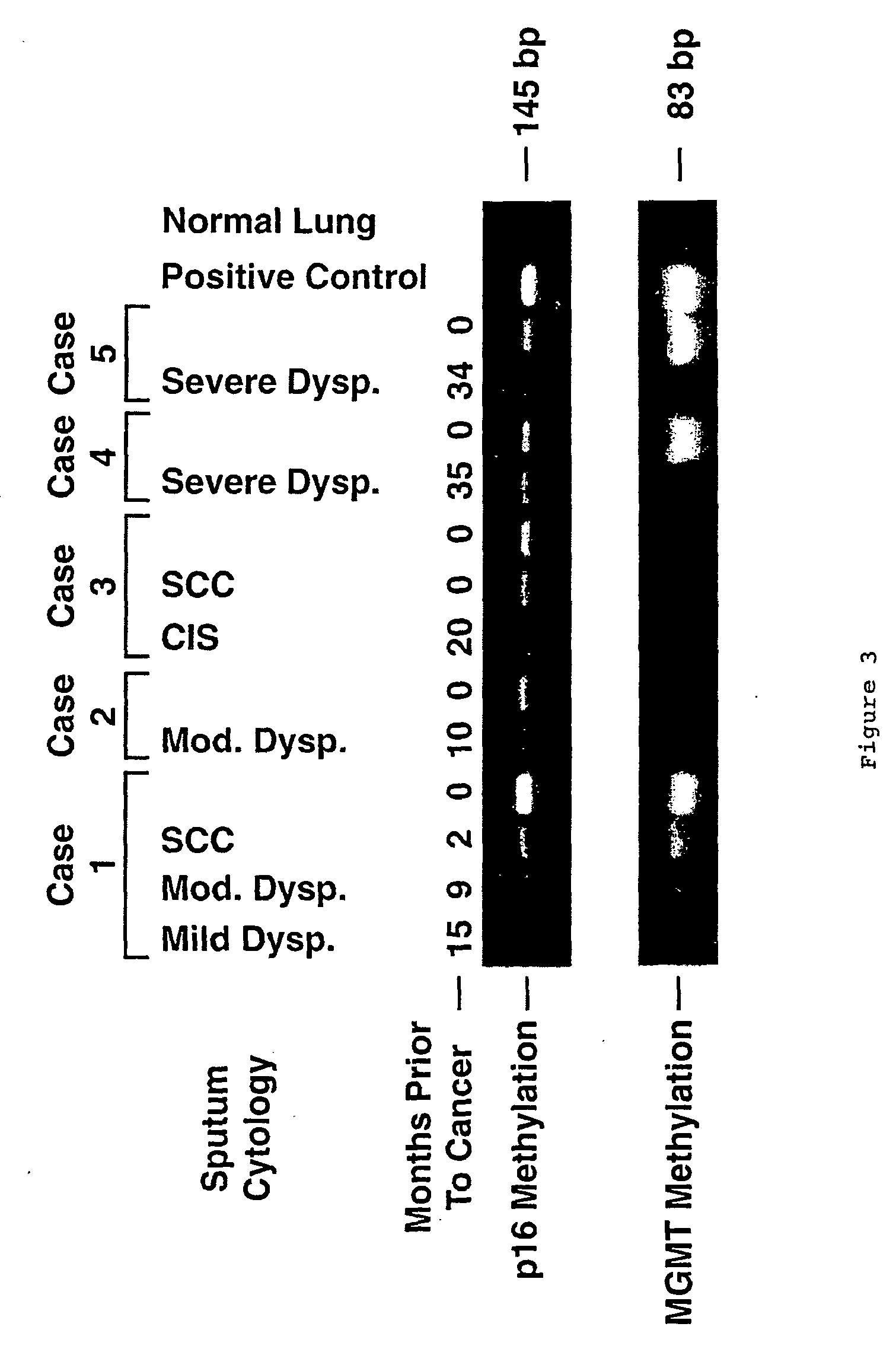
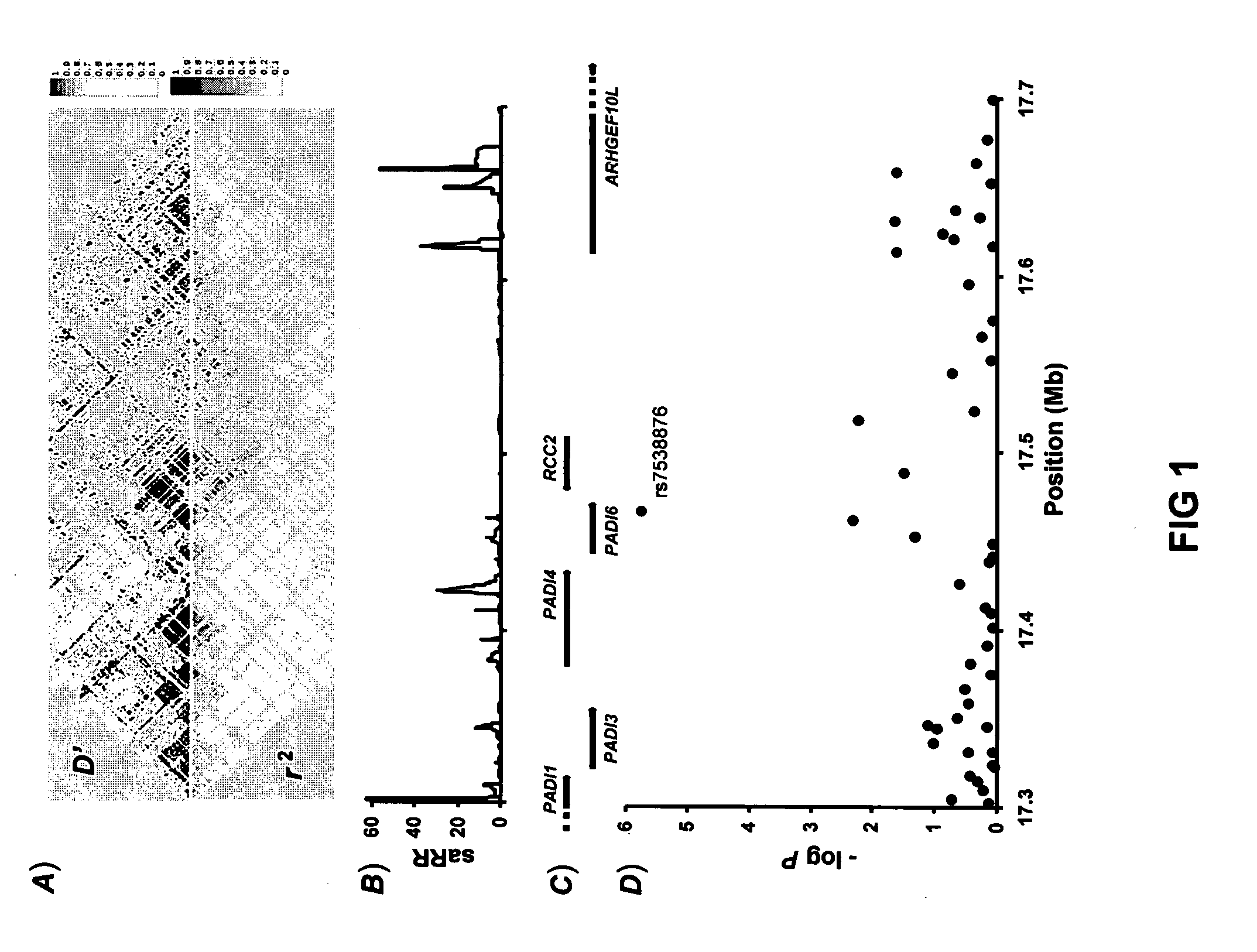
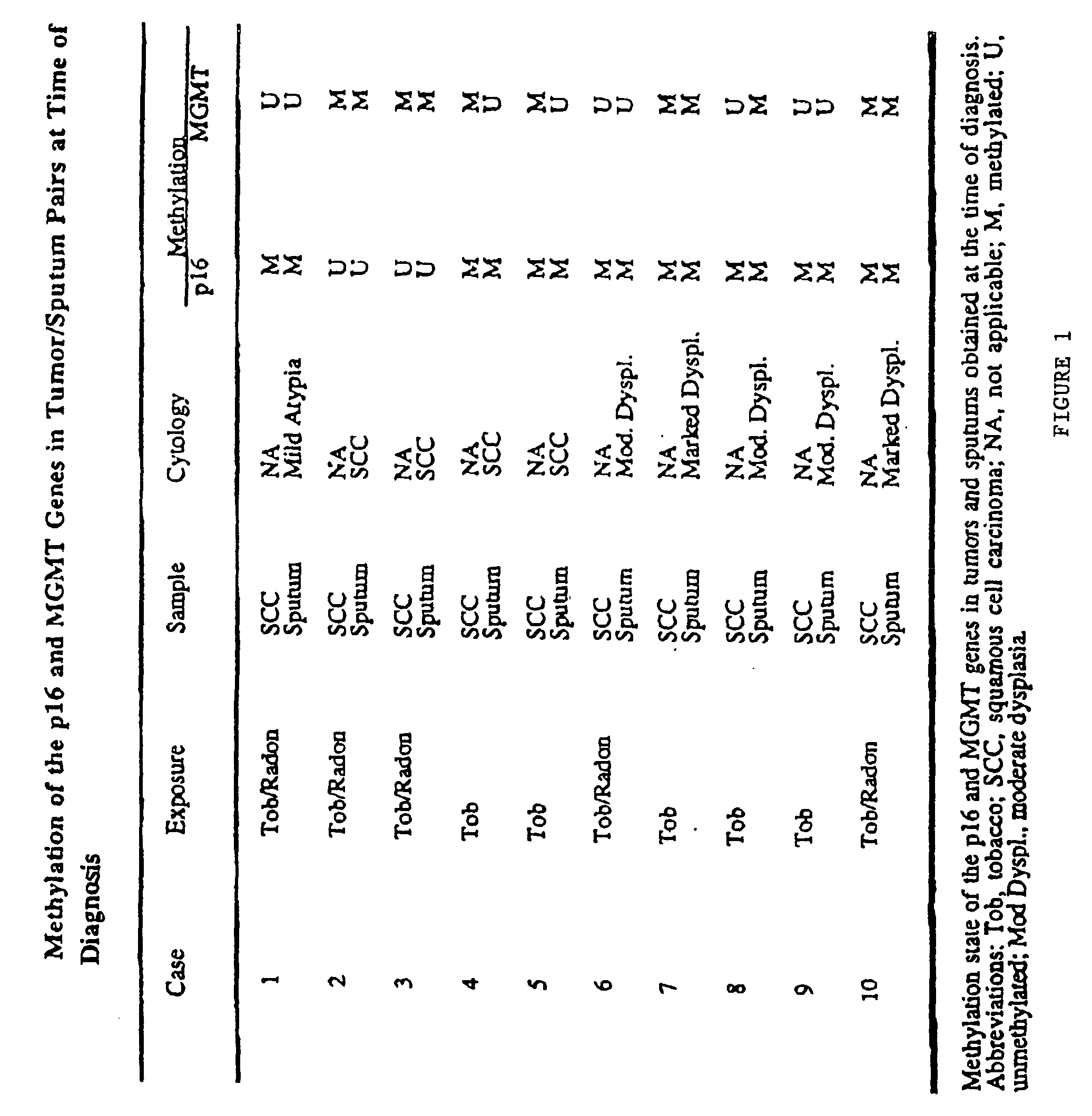
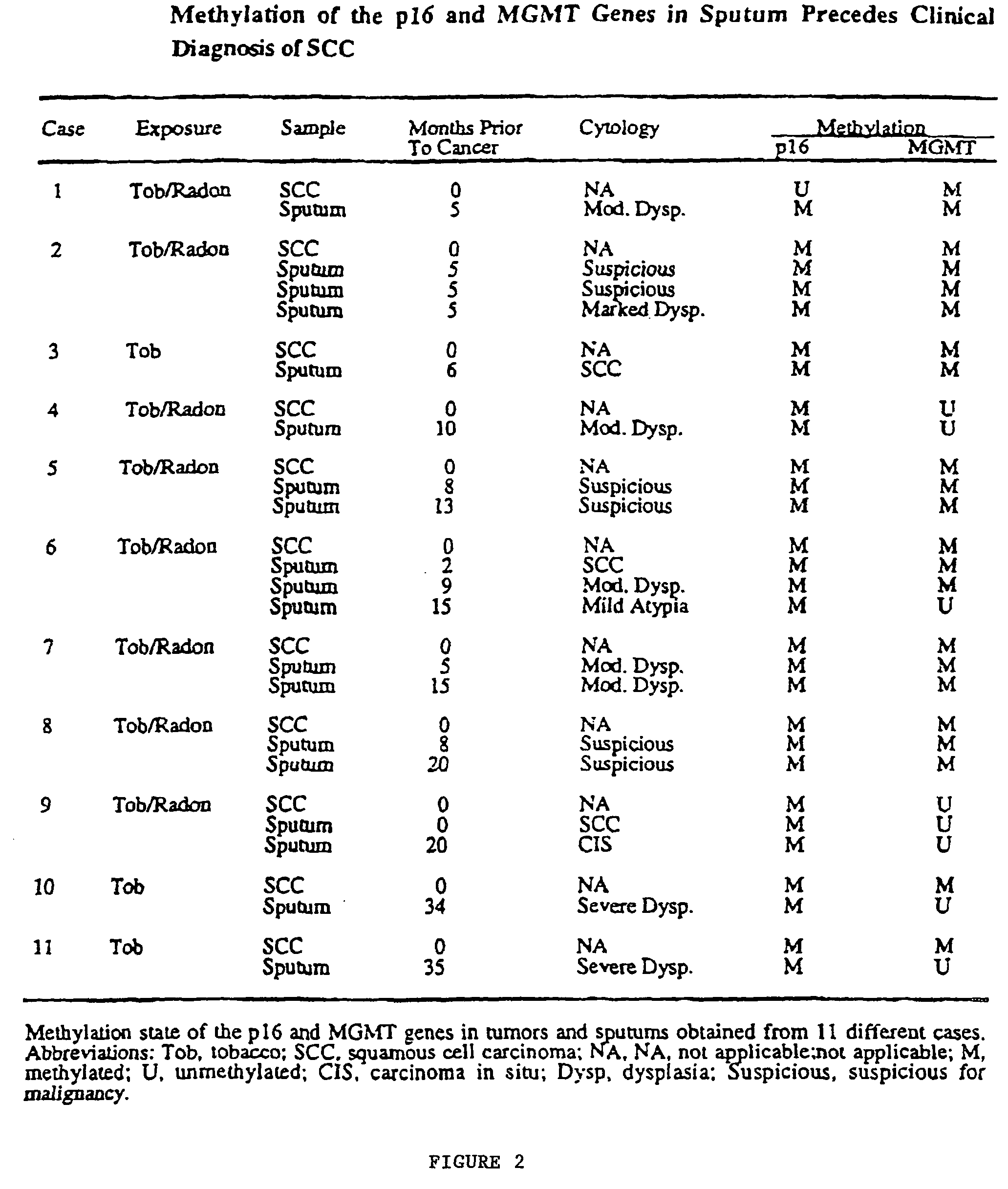
A molecular marker-based method for monitoring and detecting cancer in humans. Aberrant methylation of gene promoters is a marker for cancer risk in humans. A two-stage, or "nested" polymerase chain reaction method is disclosed for detecting methylated DNA sequences at sufficiently high levels of sensitivity to permit cancer screening in biological fluid samples, such as sputum, obtained non-invasively. The method is for detecting the aberrant methylation of the p16 gene, O 6-methylguanine-DNA methyltransferase gene, Death-associated protein kinase gene, RAS-associated family 1 gene, or other gene promoters. The method offers a potentially powerful approach to population-based screening for the detection of lung and other cancers.
Owner:LOVELACE RESPIRATORY RES INST
Diagnoses and therapeutics for cancer
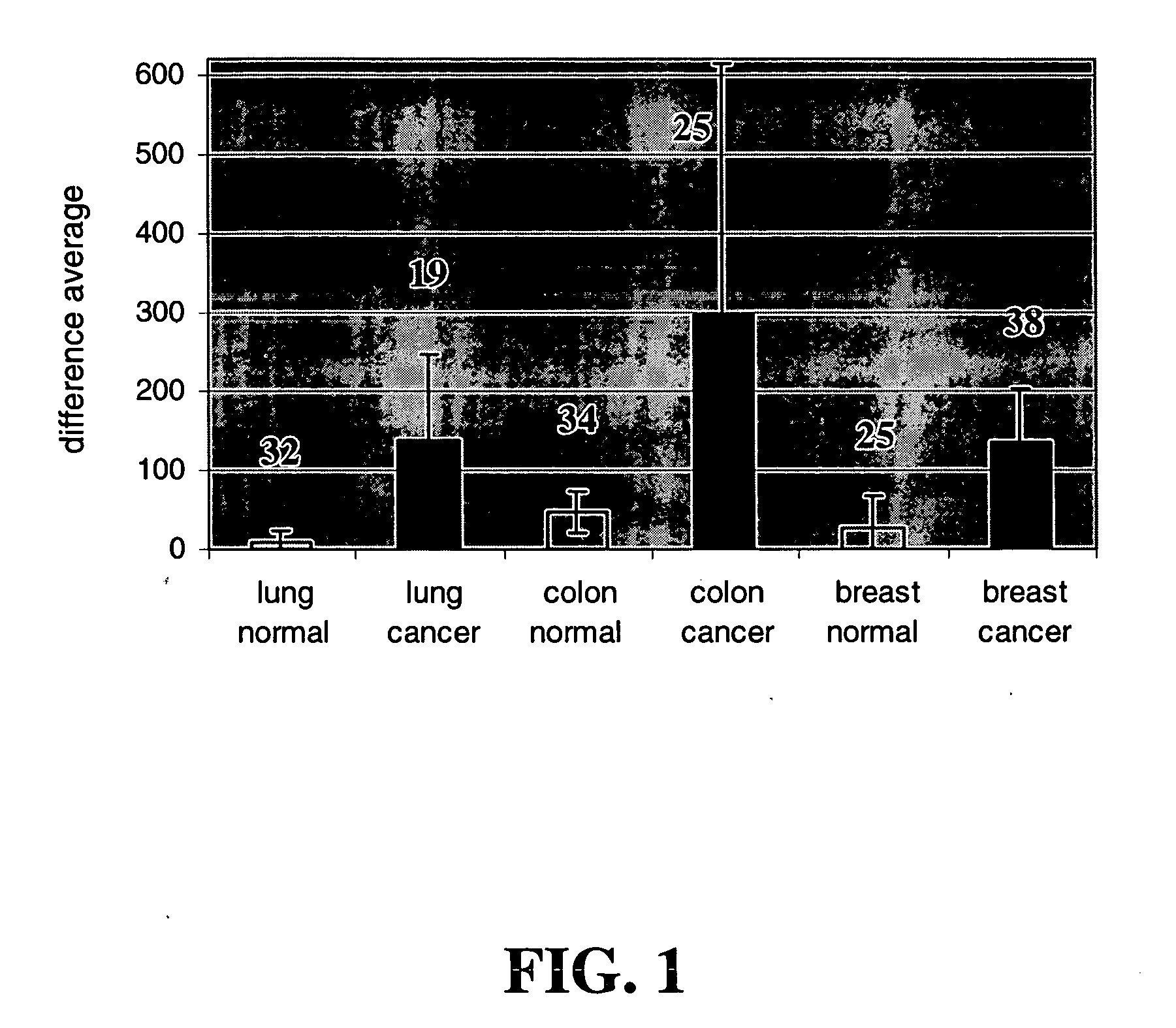
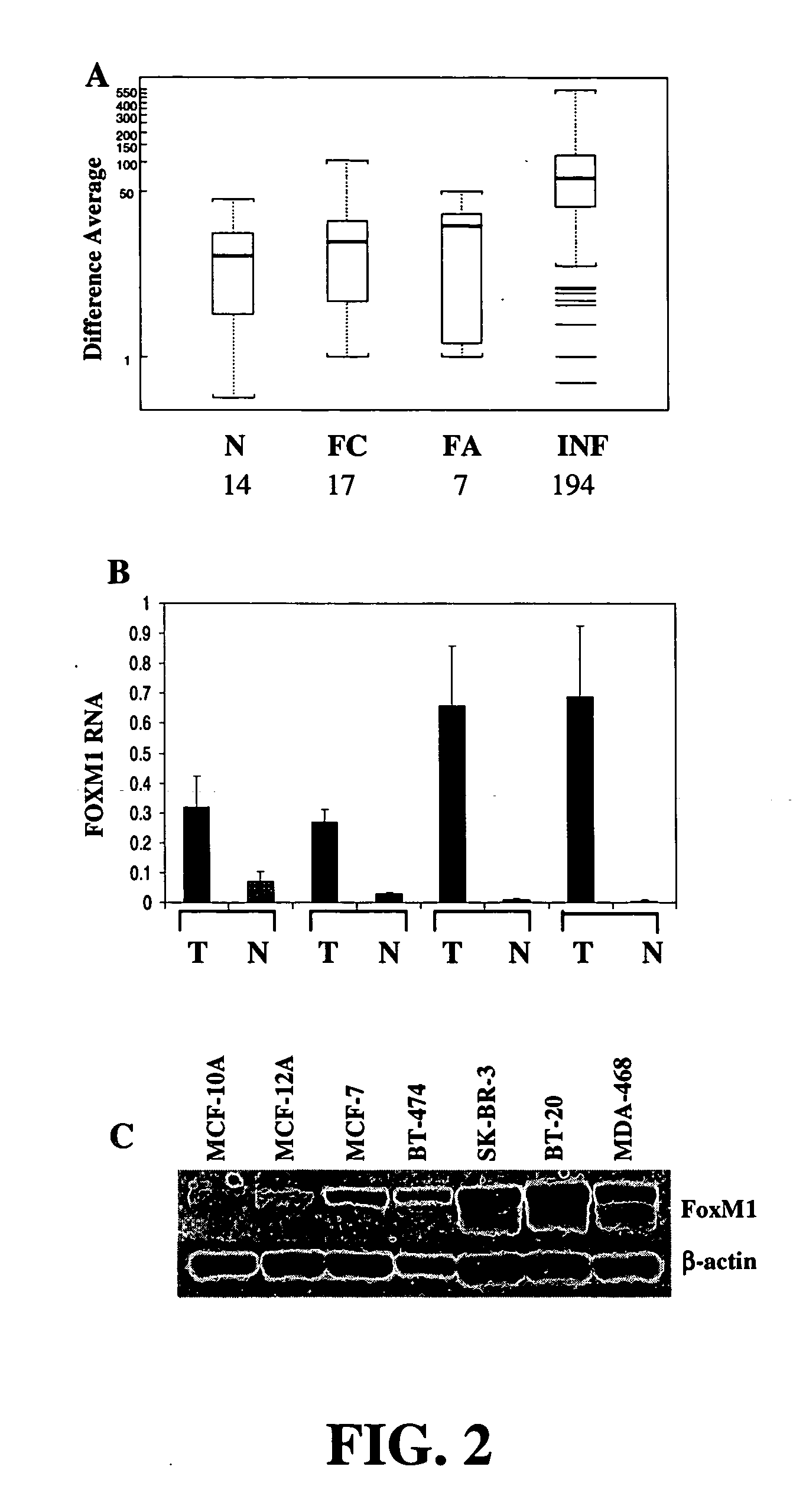
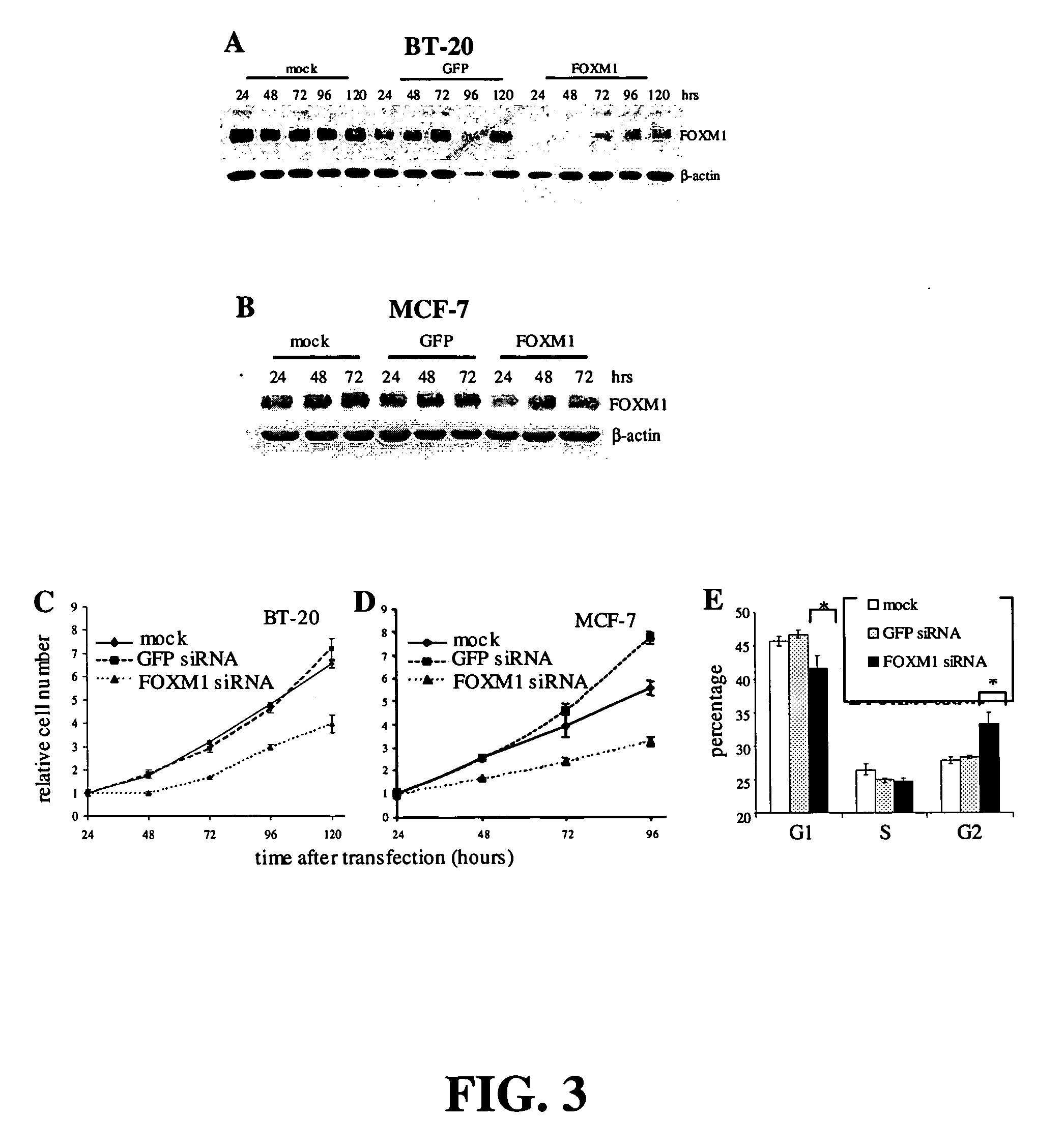
InactiveUS20060014686A1Decreasing cancer cell proliferationDecrease lungTumor rejection antigen precursorsPeptide/protein ingredientsCell growthBiology
Methods and compositions for treating and diagnosing cancer and screening for agents for such treatment and diagnosis are provided. The methods involve screening for agents that modulate the activity or expression of FOXM1, which has been discovered herein to play a role in cell growth and cell cycle regulation. Methods for treating cancer, methods for modulating the activity or expression of FOXM1, methods for diagnosing a subject that has or is at risk of developing cancer, and pharmaceutical compositions are also provided.
Owner:WYETH LLC
Method for processing cured meat
The invention discloses a method for processing cured meat, which mainly comprises the following steps: a, selecting materials, refrigerating and slitting; b, smearing spice, kneading and standing; c, cleaning; d, airing and hanging; and e, smoke curing. The method for processing the cured meat has the following characteristics: firstly, the selected spice is prepared by mixing green tea, wild pepper, anise, tsaoko, geranium, ganoderma lucidum, patchouli and cumin, the selection of the spice is scientific and the using amount is reasonable, wherein the green tea component not only can inhibit the generation of nitrite but also can inhibit the absorption of human large intestines on fat so that the cured meat reduces the burden of the human intestines and stomach caused by the fat at the same time of having unique fragrance; and secondly, the method strictly controls various process indexes during processing, and adopts active carbon to absorb benzopyrene in fume mist at a smoke curing stage so that the nitrite residue in a cured meat product is little, the benzopyrene content is low, and the cancer risk of the human body caused by the salt-cured product is further reduced.
Owner:巫溪县李大姐肉食品有限公司
Process to modulate disease risk with doses of a nutraceutical
A dietary supplement is created, comprised of material from the following nutrients, vitamins, herbs, minerals, and food and plant substances and food and plant derivatives: lycopene, vitamin E, selenium, green tea, coenzyme Q10, garlic, folic acid, vitamin C, curcumin, seaweed, Cordyceps sinsensis mushroom, Lentinus edodes (shiitake) mushroom, and Ganoderma lucidum (reishi) mushroom. The composition is administered orally for individuals who wish to reduce their risk of disease, particularly cancer-risk.
Owner:GENETIC SERVICES MANAGEMENT
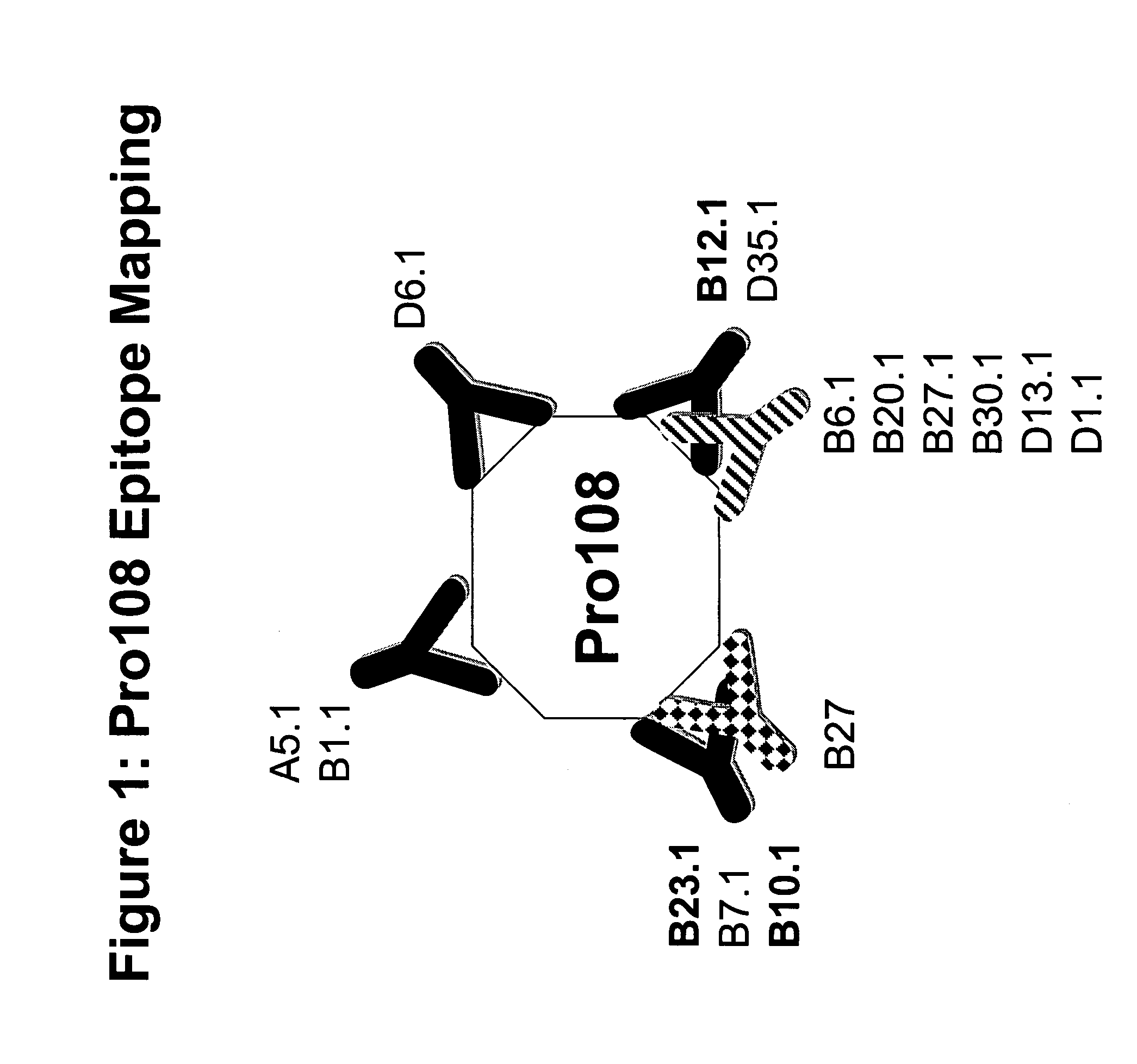
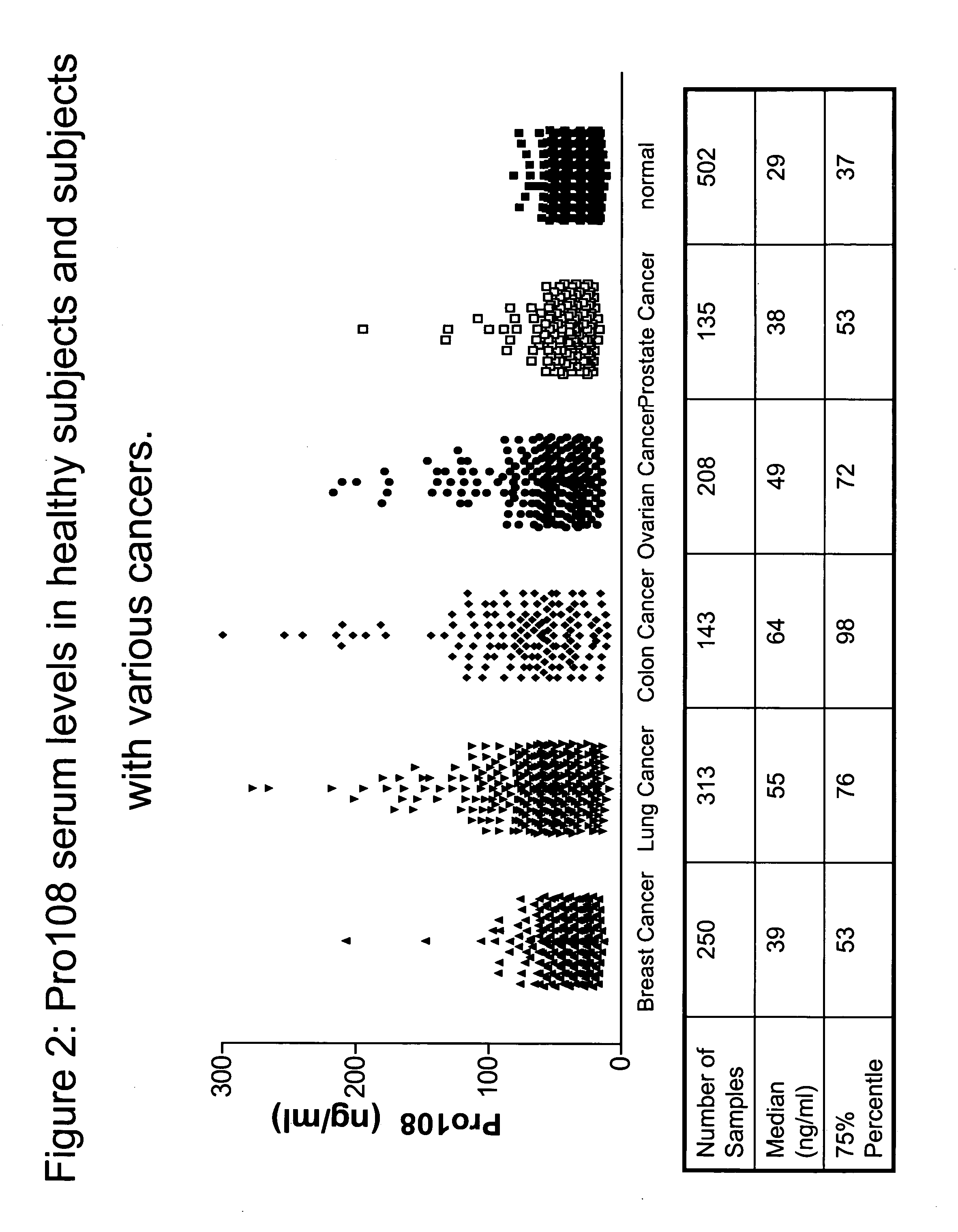
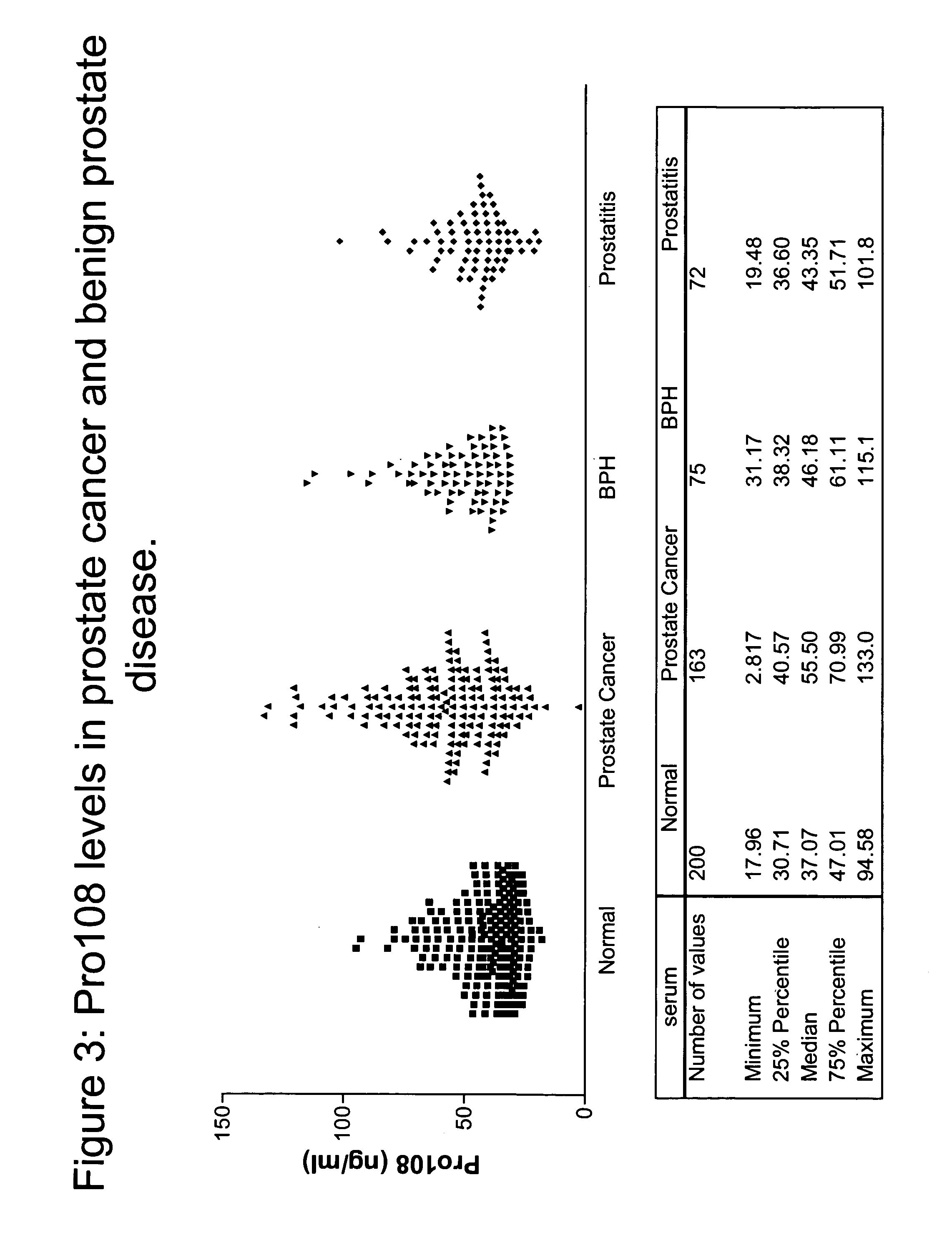
Pro108 antibody compositions and methods of use and use of Pro108 to assess cancer risk
InactiveUS7294704B2Reduce riskHigh profileAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellMammal
Owner:DIAZYME LAB INC
Polymorphisms for predicting disease and treatment outcome
The invention provides compositions and methods for determining the increased risks for recurrence of certain cancers and the likelihood of successful treatment with one or both of chemotherapy and radiation therapy. The methods comprising determining the type of genomic polymorphism present in a predetermined region of the gene of interest isolated from the subject or patient. Also provided are nucleic acid probes and kits for determining a patient's cancer risk and treatment response.
Owner:UNIV OF SOUTHERN CALIFORNIA
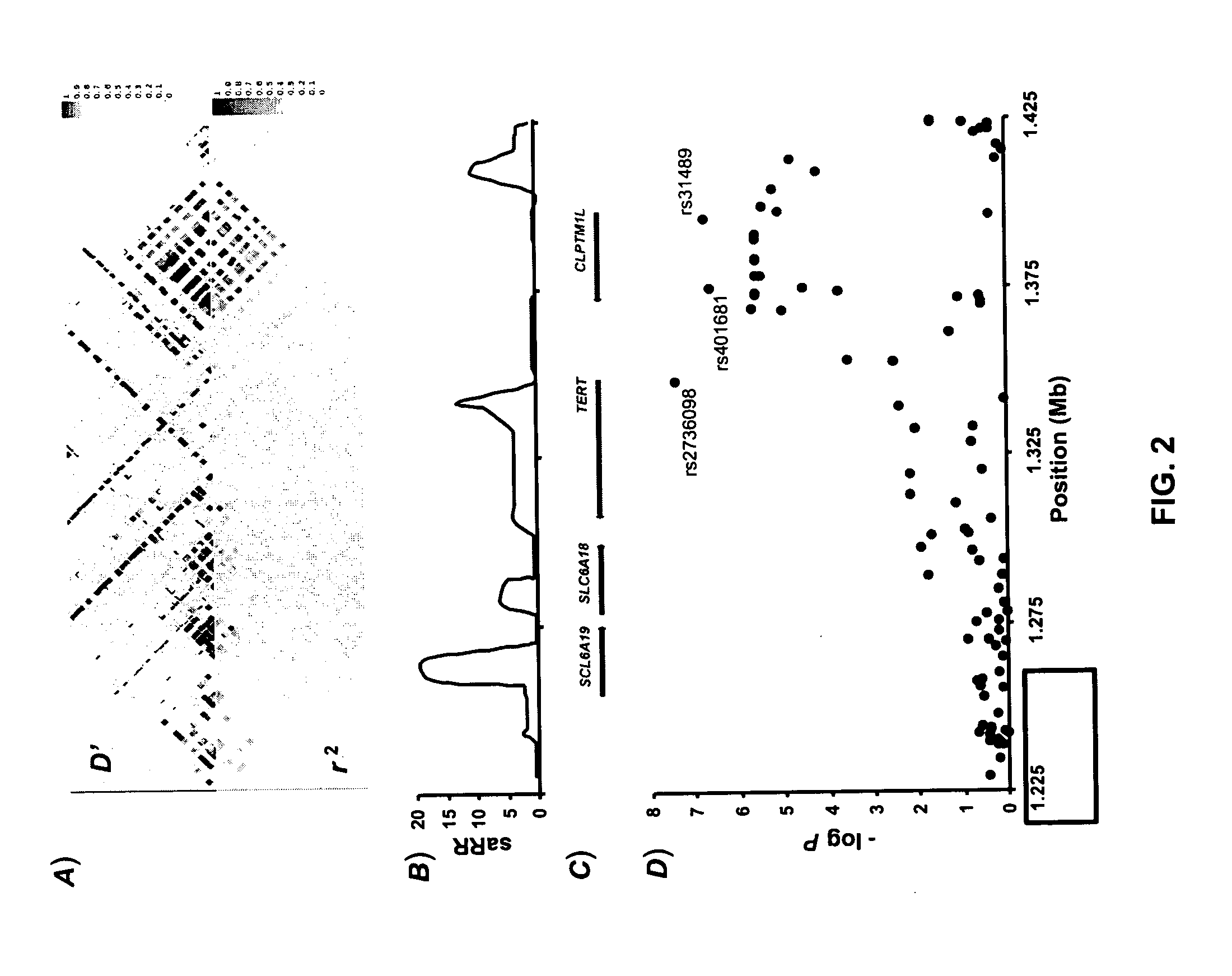
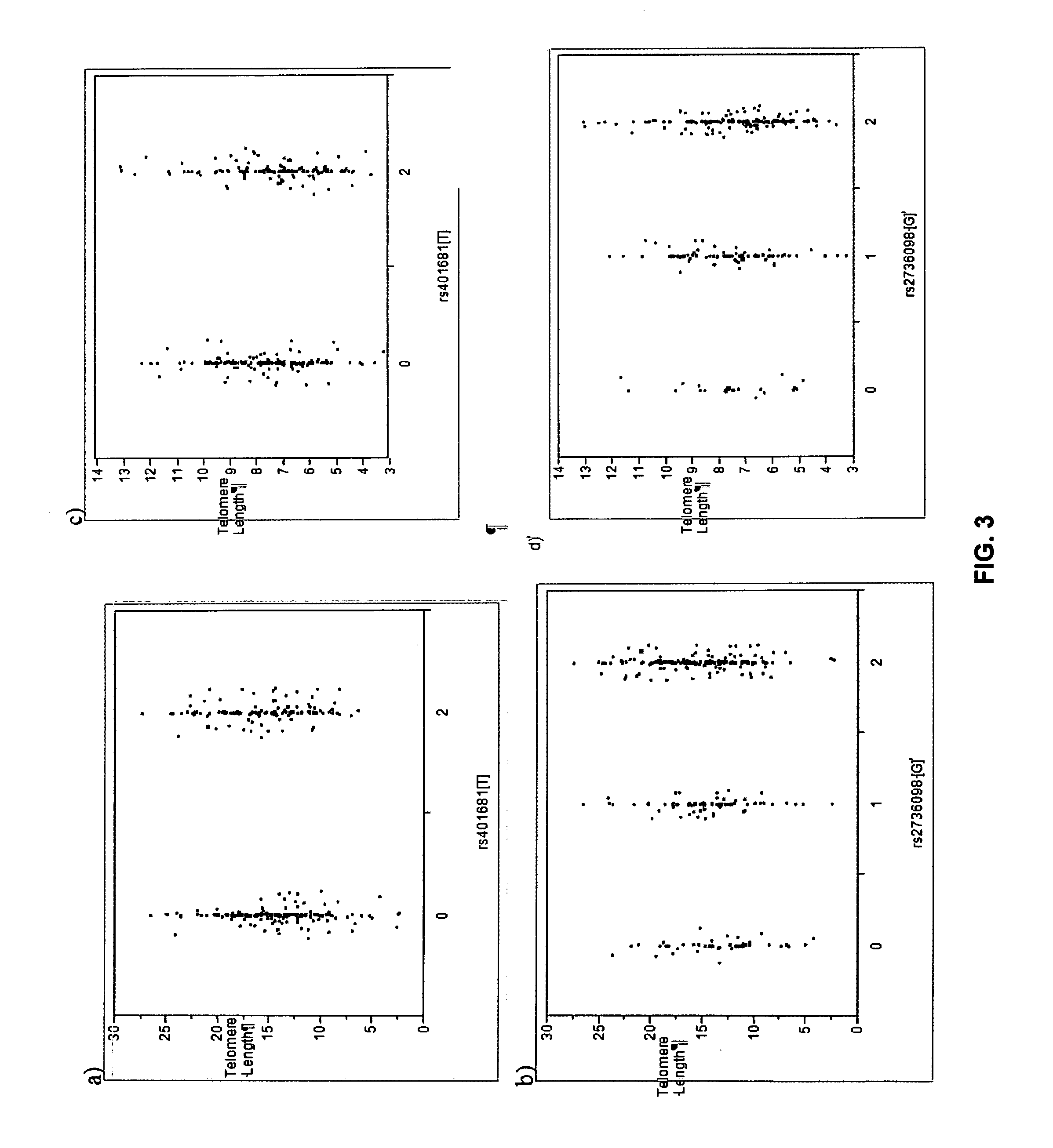
Genetic Variants Predictive of Cancer Risk
InactiveUS20110212855A1Increased susceptibilityHigh riskMicrobiological testing/measurementLibrary screeningDiseaseGenetic variants
The invention discloses genetic variants that have been determined to be susceptibility variants of cancer. Methods of disease management, including determining increased susceptibility to cancer, methods of predicting response to therapy and methods of predicting prognosis of cancer using such variants are described. The invention further relates to kits useful in the methods of the invention.
Owner:DECODE GENETICS EHF
Compositions and methods for prognosis of cancers
InactiveUS7402389B2Early stageLower Level RequirementsMicrobiological testing/measurementProstate cancerOncology
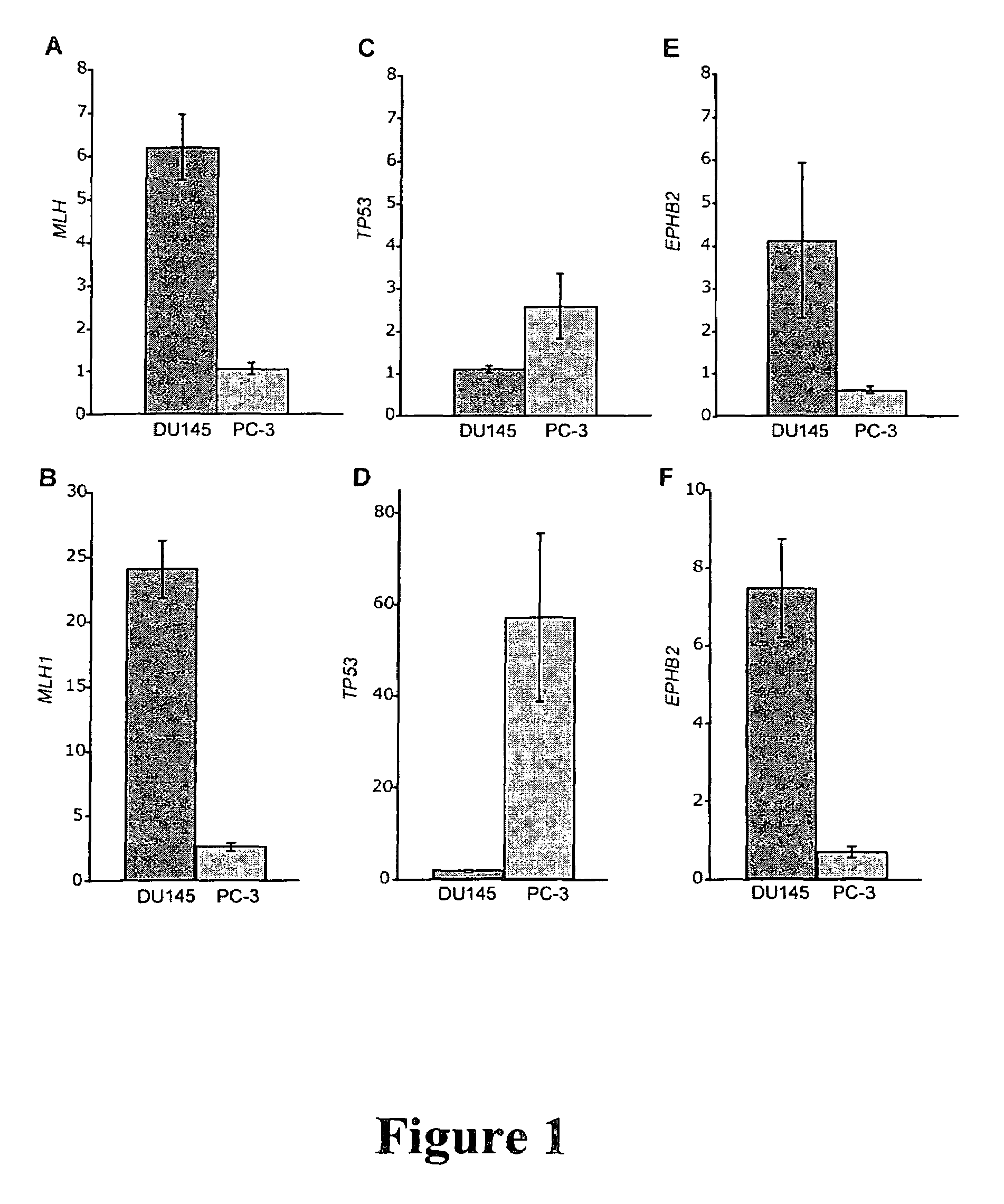
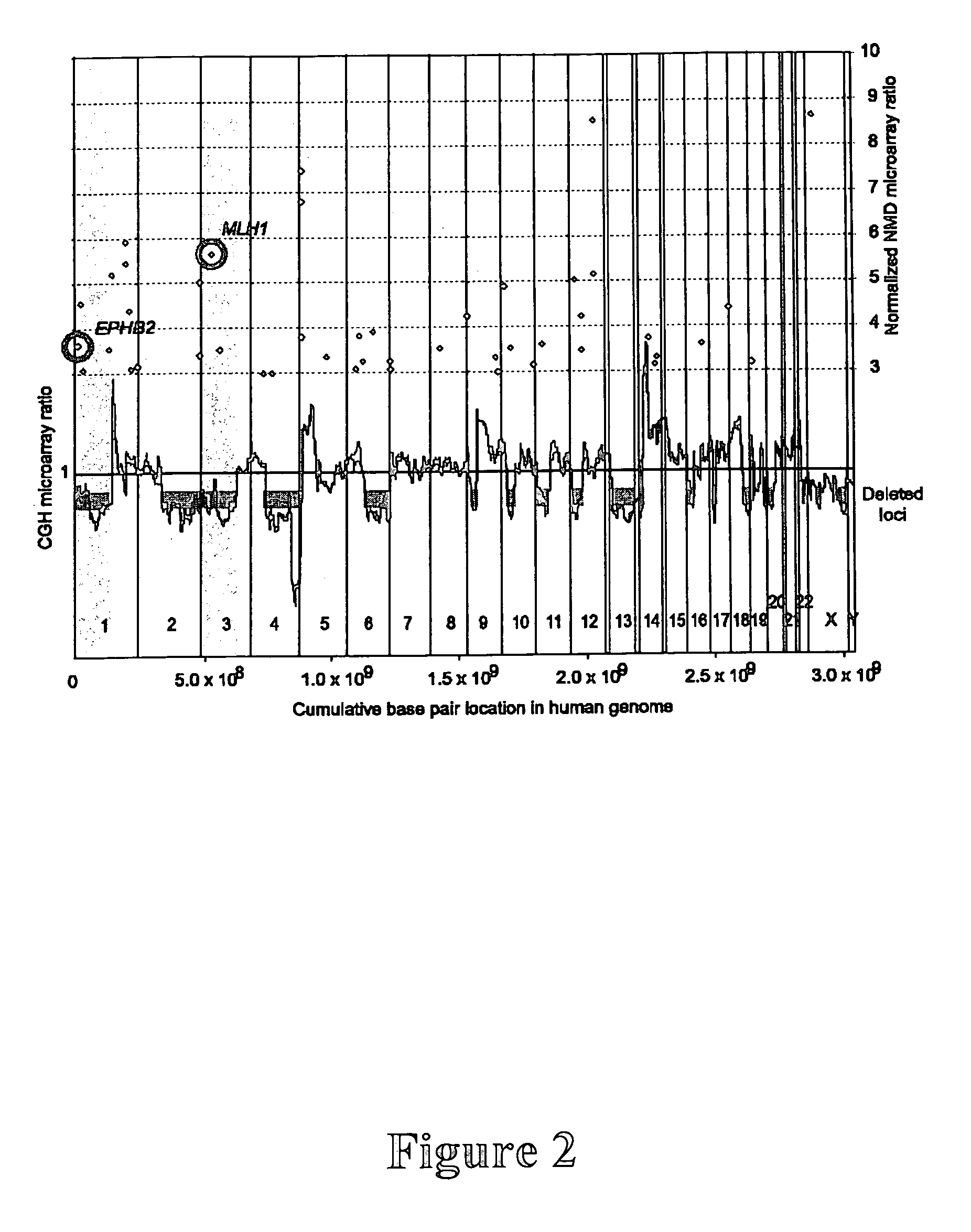
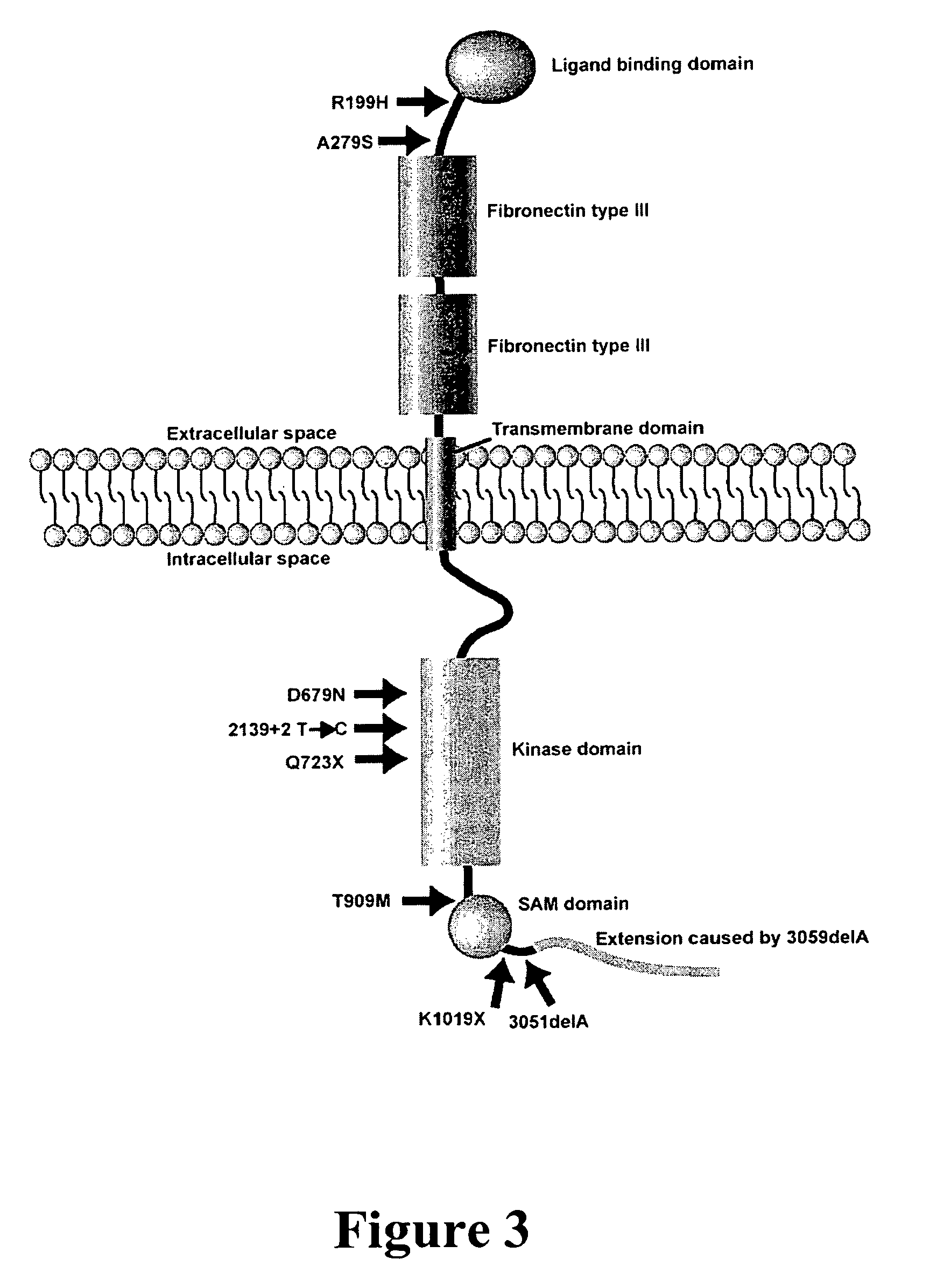
The present invention provides compositions and methods of using the EPHB2 gene or its related signaling pathways to detect, prognosticate, assess the risk of, prevent, or treat cancers. Cancers amenable to the present invention include, but are not limited to, prostate cancer, breast cancer, and neuroblastoma. In one aspect, the present invention provides compositions which comprise an agent capable of eradicating or alleviating an abnormality in the EPHB2 gene or its related signaling pathways. This abnormality may cause or contribute to the development or progression of cancers. In another aspect, the present invention provides methods comprising detecting an abnormality in the EPHB2 gene or its related signaling pathways. The presence or absence of such an abnormality is indicative of the risk or disease status of cancer in a person of interest.
Owner:TRANSLATIONAL GENOMICS RES
Methods of Predicting Cancer Risk Using Gene Expression in Premalignant Tissue
ActiveUS20100291573A1Use can be riskyIncreased riskMicrobiological testing/measurementBiological testingBraf genesExpression gene
The present disclosure provides methods for assessing a patient's cancer risk and / or recurrence risk, which methods comprise assaying, in a biological sample obtained from the gastrointestinal (GI) tract of the patient, an expression level of a risk gene. The present disclosure also provides methods involving a cancer risk / recurrence risk sequence, i.e. the V600E mutation of the BRAF gene, which is useful for assessing cancer risk and / or recurrence risk in a patient.
Owner:GENOMIC HEALTH INC
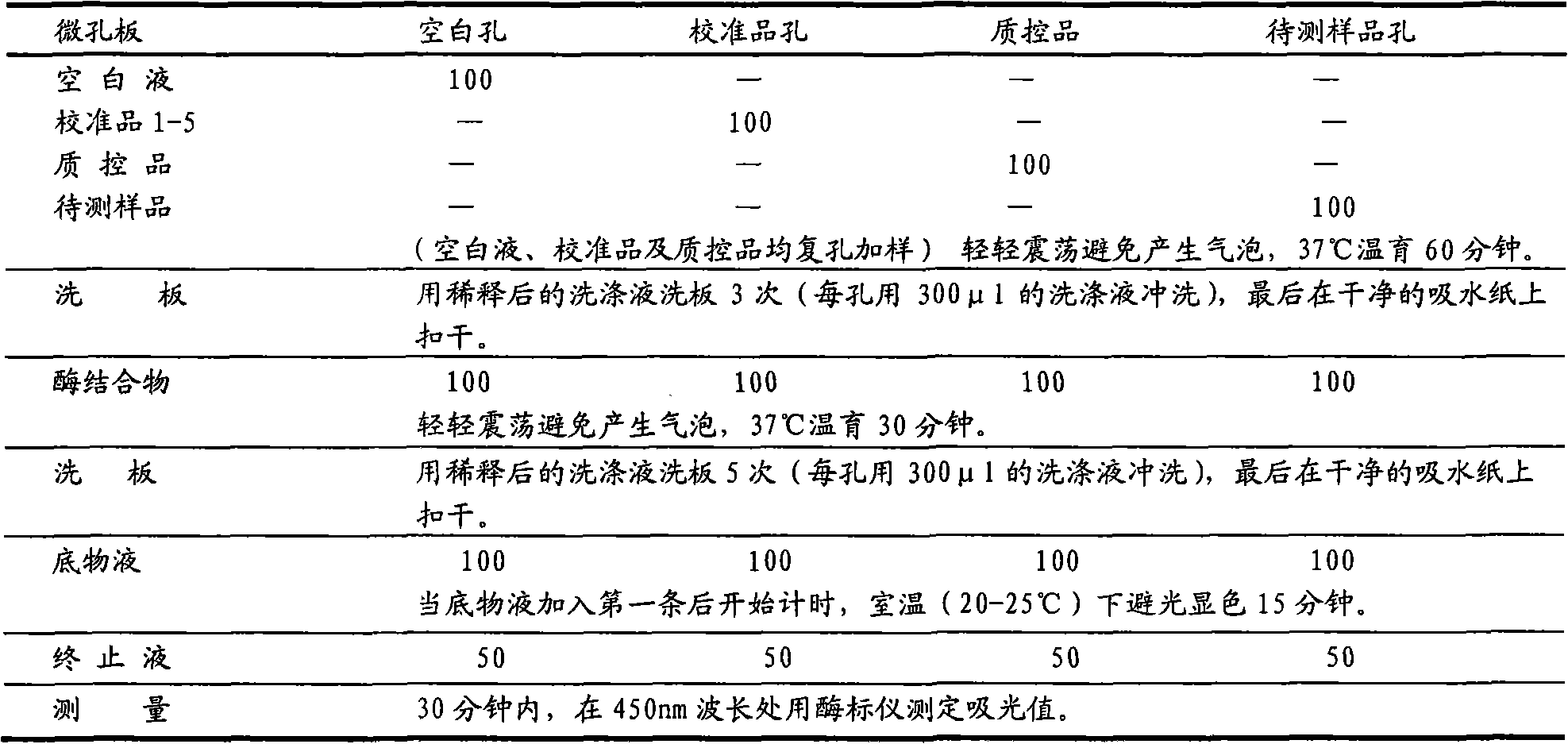
Enzyme linked immunosorbent assay kit for combined diagnosis of gastrosis or evaluation of gastric cancer risks
InactiveCN102087279AIncreased sensitivityImprove featuresComponent separationTissue cultureAntigenPepsinogen I
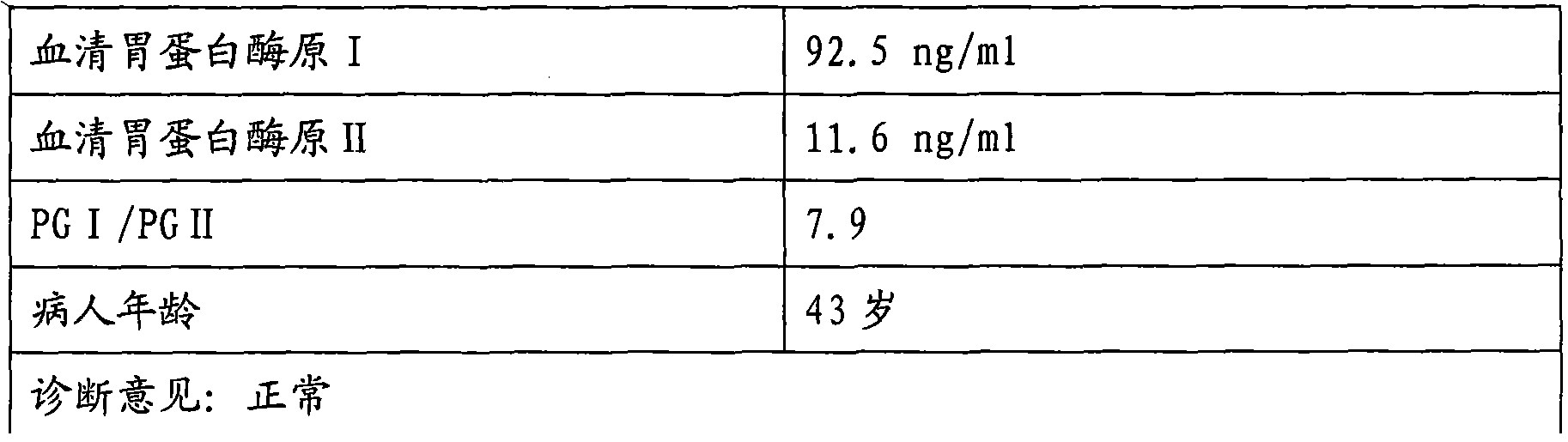
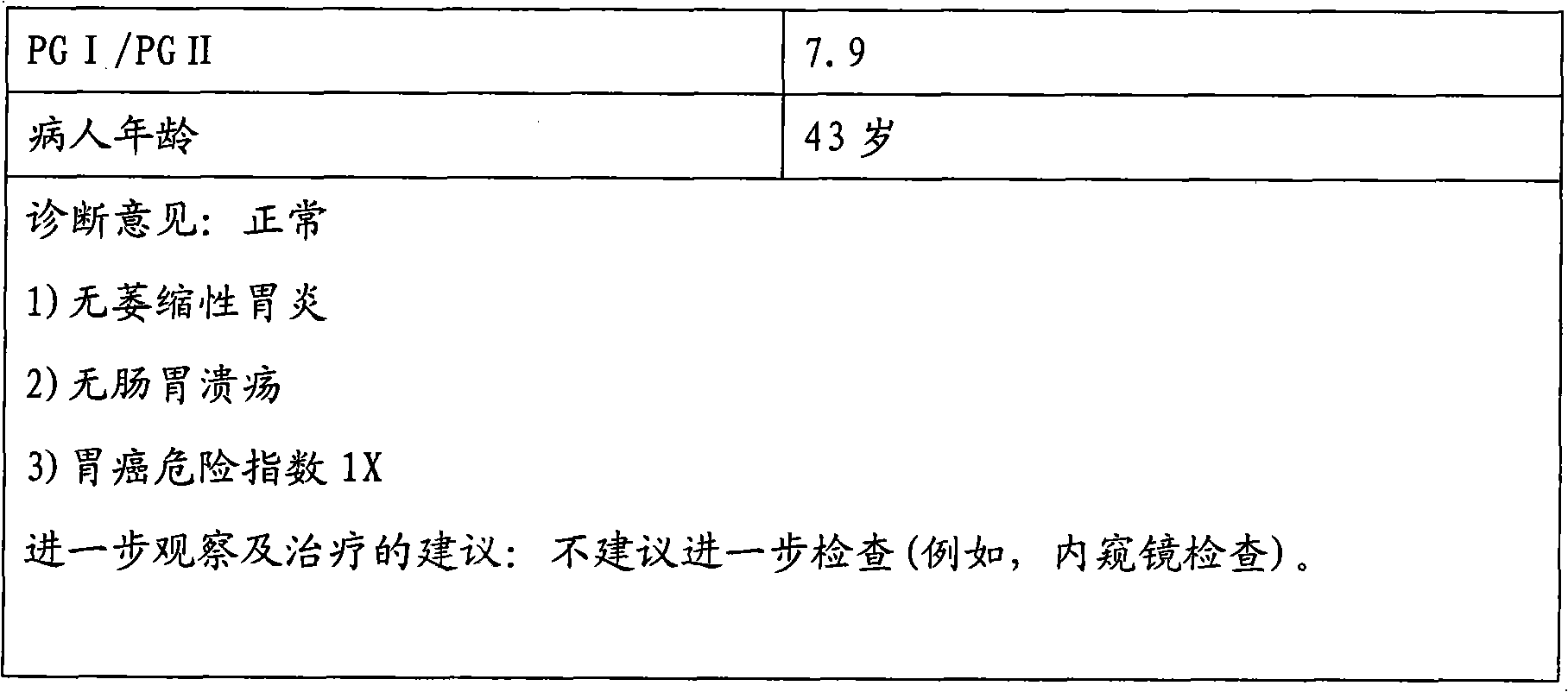
The invention discloses an enzyme linked immunosorbent assay kit for combined diagnosis of the gastrosis or evaluation of gastric cancer risks and a preparation method thereof. The kit comprises a micropore plate coated with an antibody against a pepsin antigen I or an antibody against a pepsin antigen II, an enzyme labeled antibody, a color-developing agent, a stop solution and a concentrated cleaning solution, wherein the pepsin antigen I or the pepsin antigen II is a natural protein obtained from extraction of human gastric mucosa tissue. The kit disclosed by the invention adopts a mouse immunized with pepsinogen I and pepsinogen II which are separated from human gastric mucosa to prepare immunogen of a monoclonal antibody, the used standard sample also adopts the pepsin antigen I or the pepsin antigen II separated from the human gastric mucosa, thereby the defects caused by adopting different structures of animal pepsinogen and human pepsinogen are filled. The kit can be used for accurately diagnosing the gastrosis or early gastric cancer and has the advantages of high sensitivity, strong specificity, good accuracy and the like.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Method of determining risk for cancer
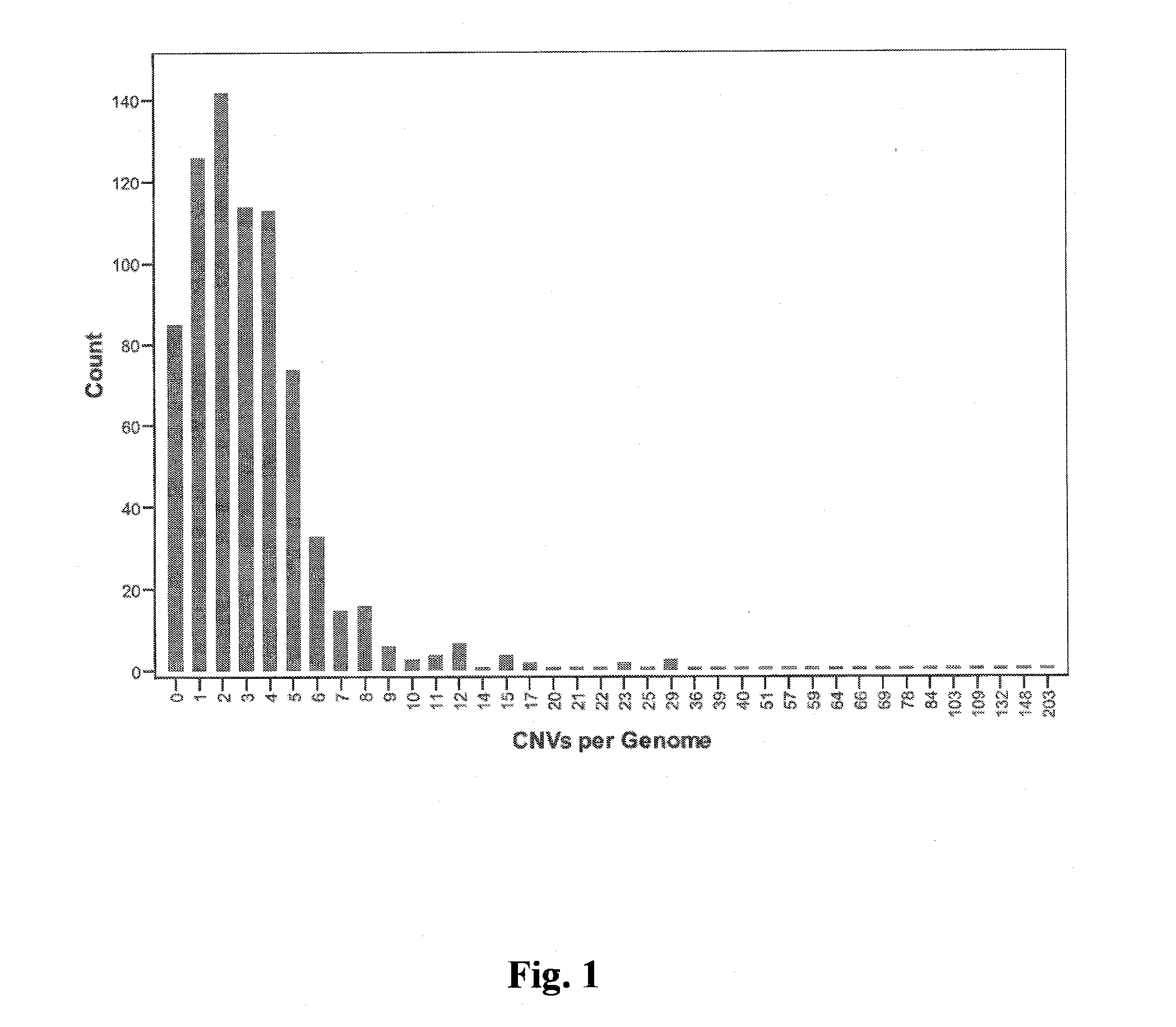
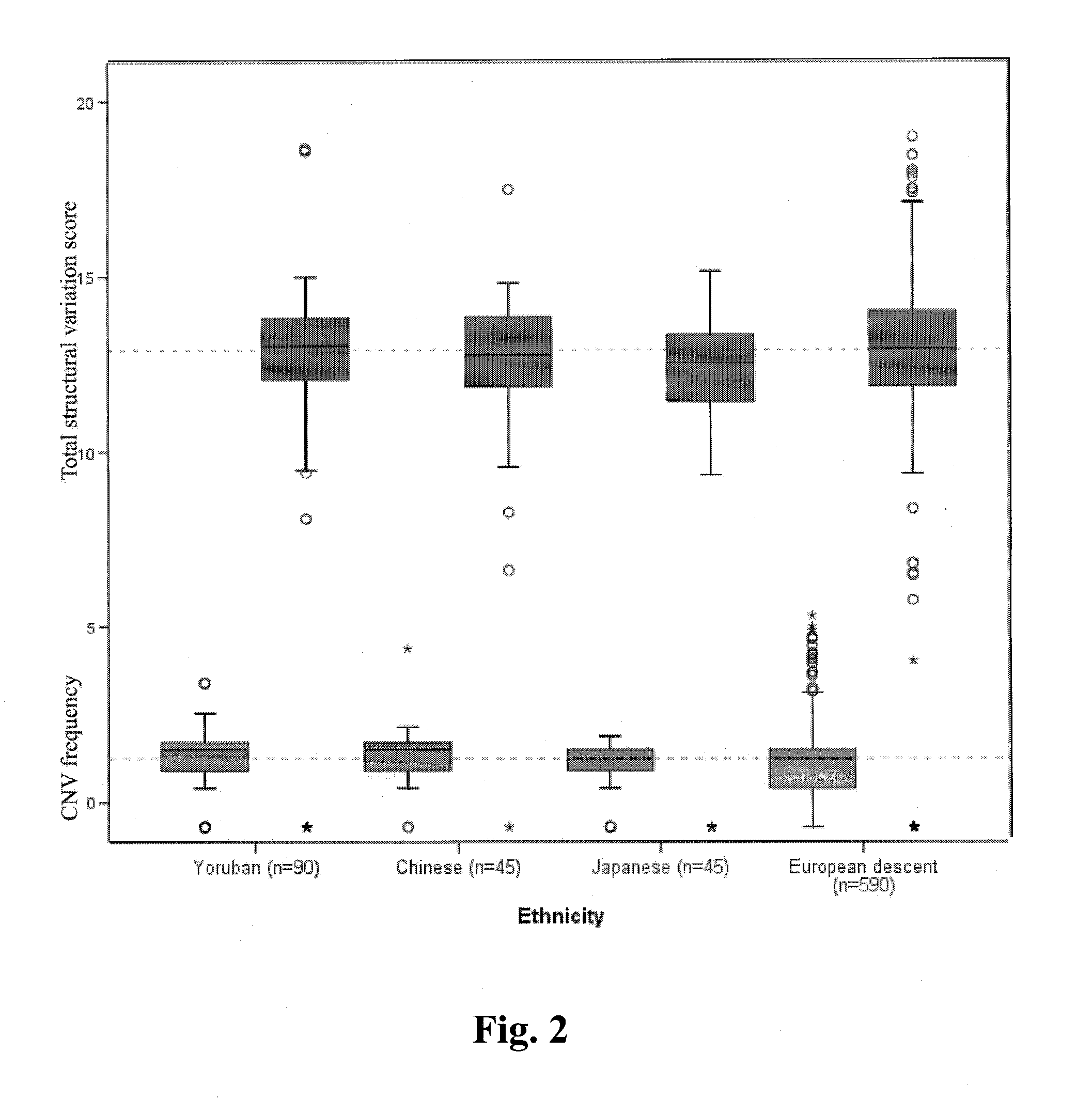
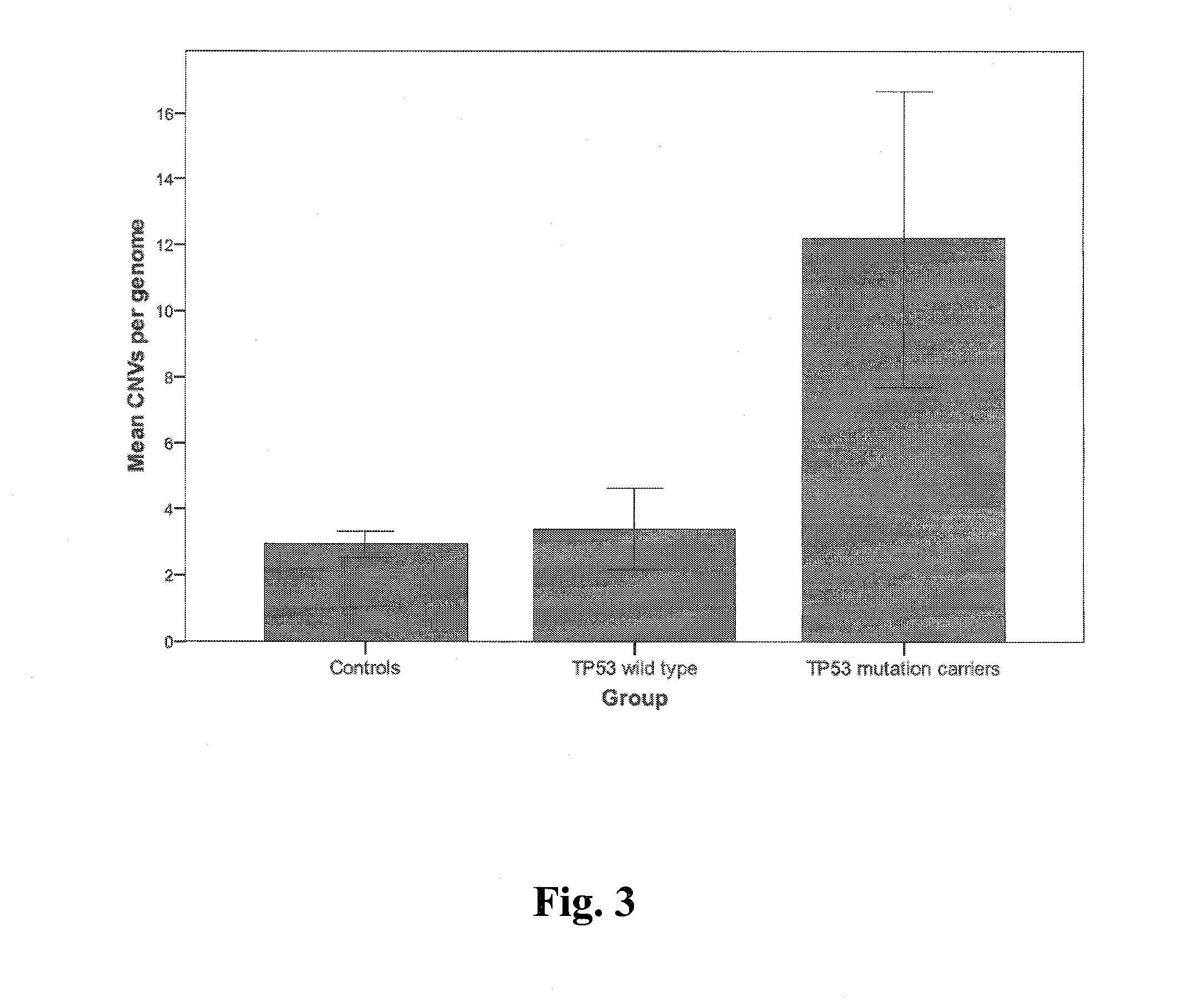
A method of determining risk of cancer in a mammal is provided. The method includes analyzing the genomic DNA of the mammal and determining genomic CNV frequency or genomic structural variation. An increase in either CNV frequency or genomic structural variation in comparison to a baseline mean value is indicative of cancer.
Owner:HOSPITAL FOR SICK CHILDREN
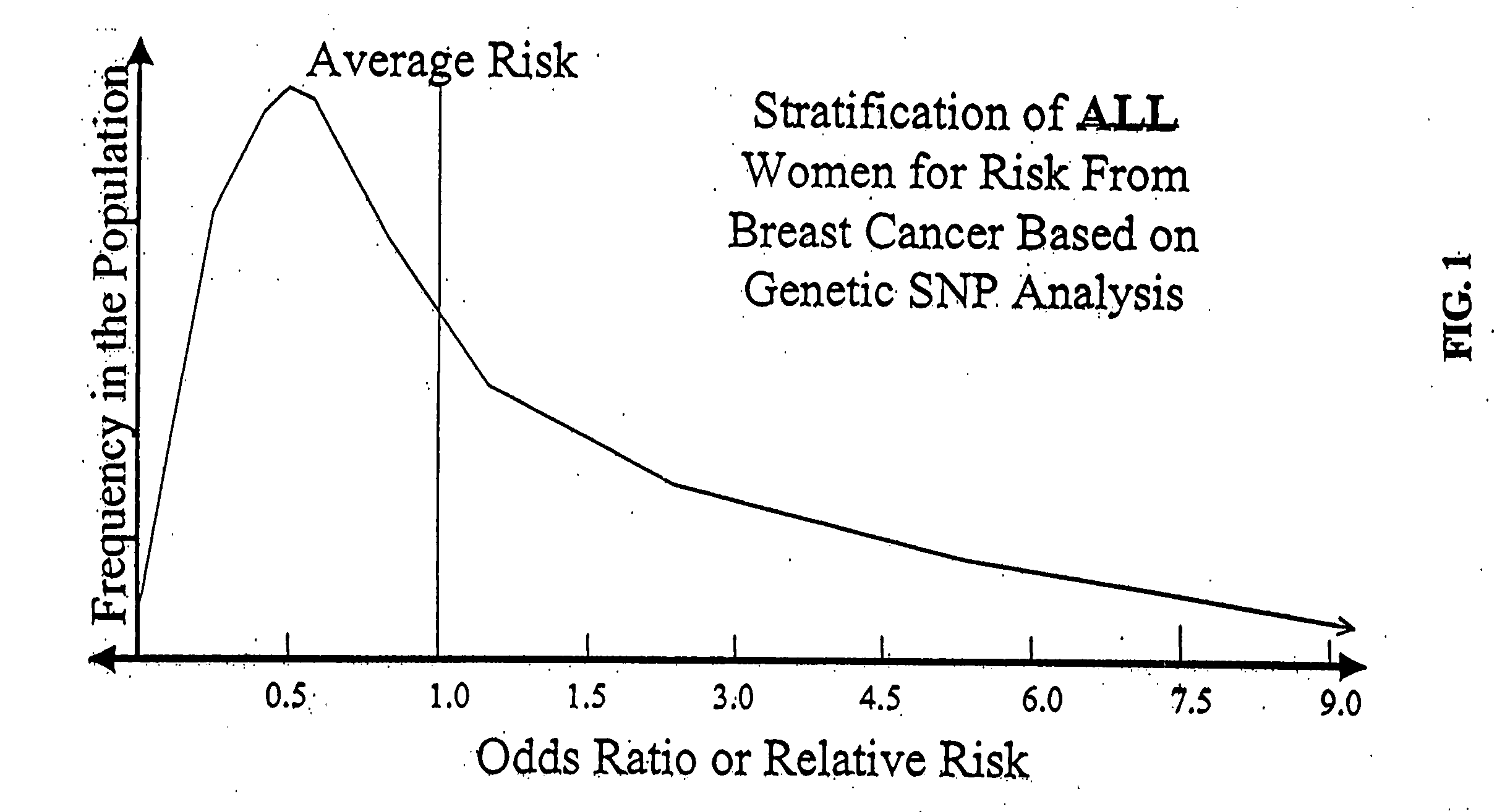
Genetic models for stratification of cancer risk
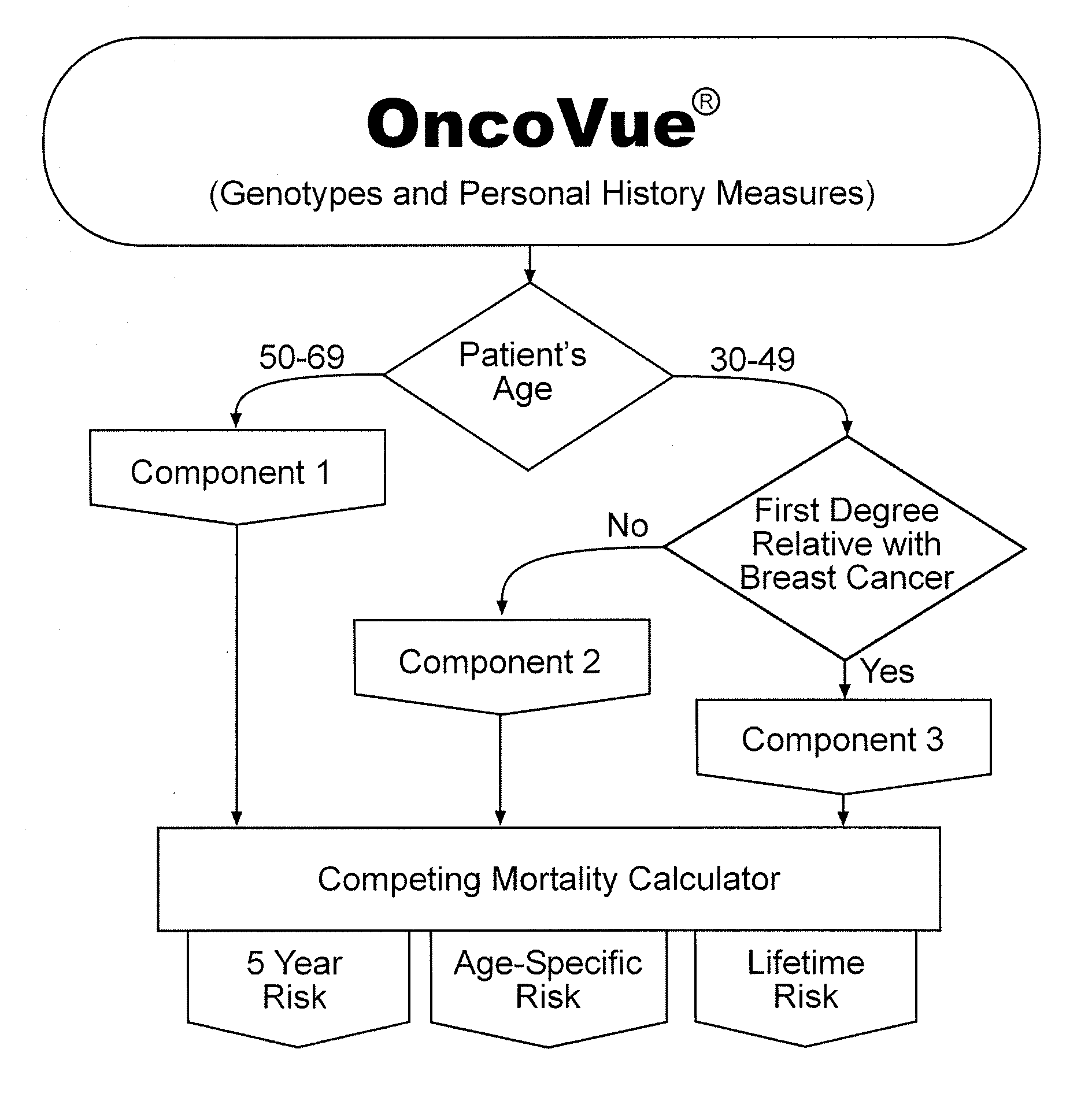
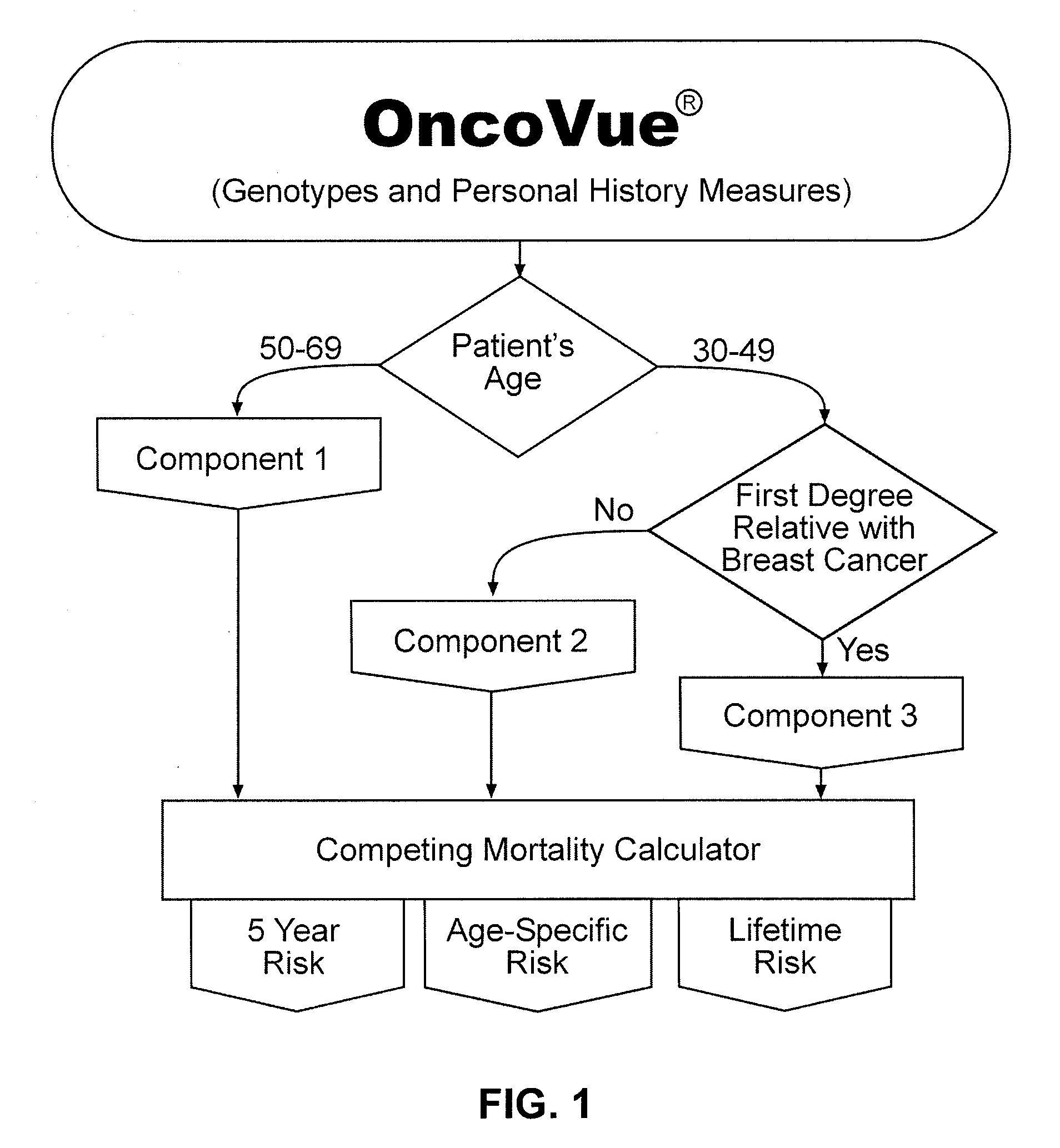
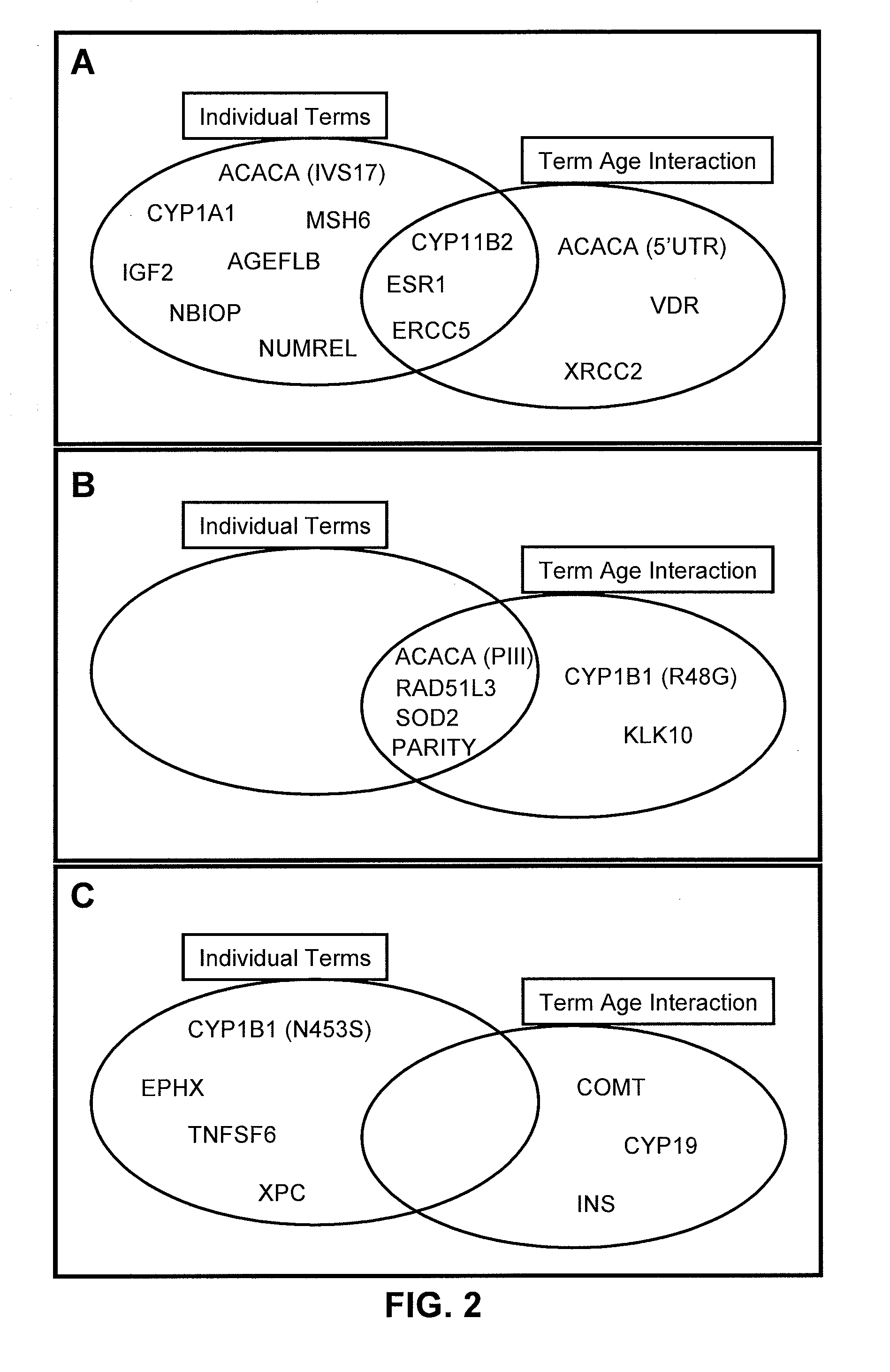
InactiveUS20090029375A1Microbiological testing/measurementProteomicsHeightened Cancer RiskProphylactic treatment
The present invention provides new methods for the assessment of cancer risk in the general population. These methods utilize particular alleles of in multiple selected genes to identify individuals with increased or decreased risk of breast cancer. In addition, personal history measures such as age and family history are used to further refine the analysis. Using such methods, it is possible to reallocate healthcare costs in cancer screening to patient subpopulations at increased cancer risk. It also permits identification of candidates for cancer prophylactic treatment.
Owner:INTERGENETICS +1
Method and kit for predicting cancer
The present invention relates to a method and a kit for diagnosing and / or predicting the occurrence of cancer or the risk of contracting a cancer by measuring the concentration of a cancer screening antigen(CSA) in blood, which changes before the occurrence of the cancer in a patient. The method of diagnosing or predicting the occurrence of cancer or the risk of contracting a cancer comprising the steps of: determining a concentration of galectin-3 in a blood sample by reacting the blood sample with a monoclonal antibody of the galectin-3; comparing the determined concentration of the galectin-3 with concentration of the galectin-3 in a blood sample of a normal human; and predicting the risk of contracting a cancer if the determined concentration is greater than the concentration of the galectin-3 in blood of the normal human.
Owner:HAE JONG WOO
Genetic Variants Predictive of Cancer Risk in Humans
InactiveUS20120122698A1Reduce sensitivityMicrobiological testing/measurementLibrary member identificationComputerized systemBasal cell carcinoma
The present invention discloses genetic variants that have been found to be predictive of risk of particular forms of cancer, in particular basal cell carcinoma and cutaneous melanoma. The invention provides methods of predicting risk of developing such cancers, and other methods pertaining to risk management of cancer utilizing such risk variants. The invention furthermore provides kits and computer systems for use in such methods.
Owner:DECODE GENETICS EHF
Nested methylation-specific polymerase chain reaction cancer detection method
A molecular marker-based method for monitoring and detecting cancer in humans. Aberrant methylation of gene promoters is a marker for cancer risk in humans. A two-stage, or “nested” polymerase chain reaction method is disclosed for detecting methylated DNA sequences at sufficiently high levels of sensitivity to permit cancer screening in biological fluid samples, such as sputum, obtained non-invasively. The method is for detecting the aberrant methylation of the p16 gene, O 6-methylguanine-DNA methyltransferase gene, Death-associated protein kinase gene, RAS-associated family 1 gene, or other gene promoters. The method offers a potentially powerful approach to population-based screening for the detection of lung and other cancers.
Owner:LOVELACE RESPIRATORY RES INST
Macrophage-Enhanced MRI (MEMRI)
Methods for assessing stage of cancer in a subject are provided, comprising administering a macrophage imaging agent to the subject, making a magnetic resonance image of regions of the subject's body at cancer risk, and using the image to assess macrophage density and displacement associated with any primary cancer or metastatic cancer in the subject, such density and displacement being indicative of neoplasia. The macrophage imaging agent may be an ultrasmall superparamagnetic iron oxide particle and in particular embodiments, the macrophage imaging agent has a blood half-life sufficient to permit microphage trapping throughout the regions at cancer risk. Additional embodiments provide methods for assessing efficacy of an anticancer treatment in a subject, methods for determining frequency of follow-up MEMRI evaluation in a subject, methods for determining metastatic potential of cancer foci in a subject, and methods for determining prognosis of cancer in a subject. Methods for directing site of biopsy in a subject by performing a whole body MEMRI evaluation of the subject to identify macrophage density at a tumor site of interest and assessing the macrophage density to identify the site of biopsy in the subject, macrophage density being an indicator of tumor growth are also provided, in addition to methods for providing individualized cancer treatment to a subject in need thereof using whole body MEMRI evaluation.
Owner:AMAG PHARMA
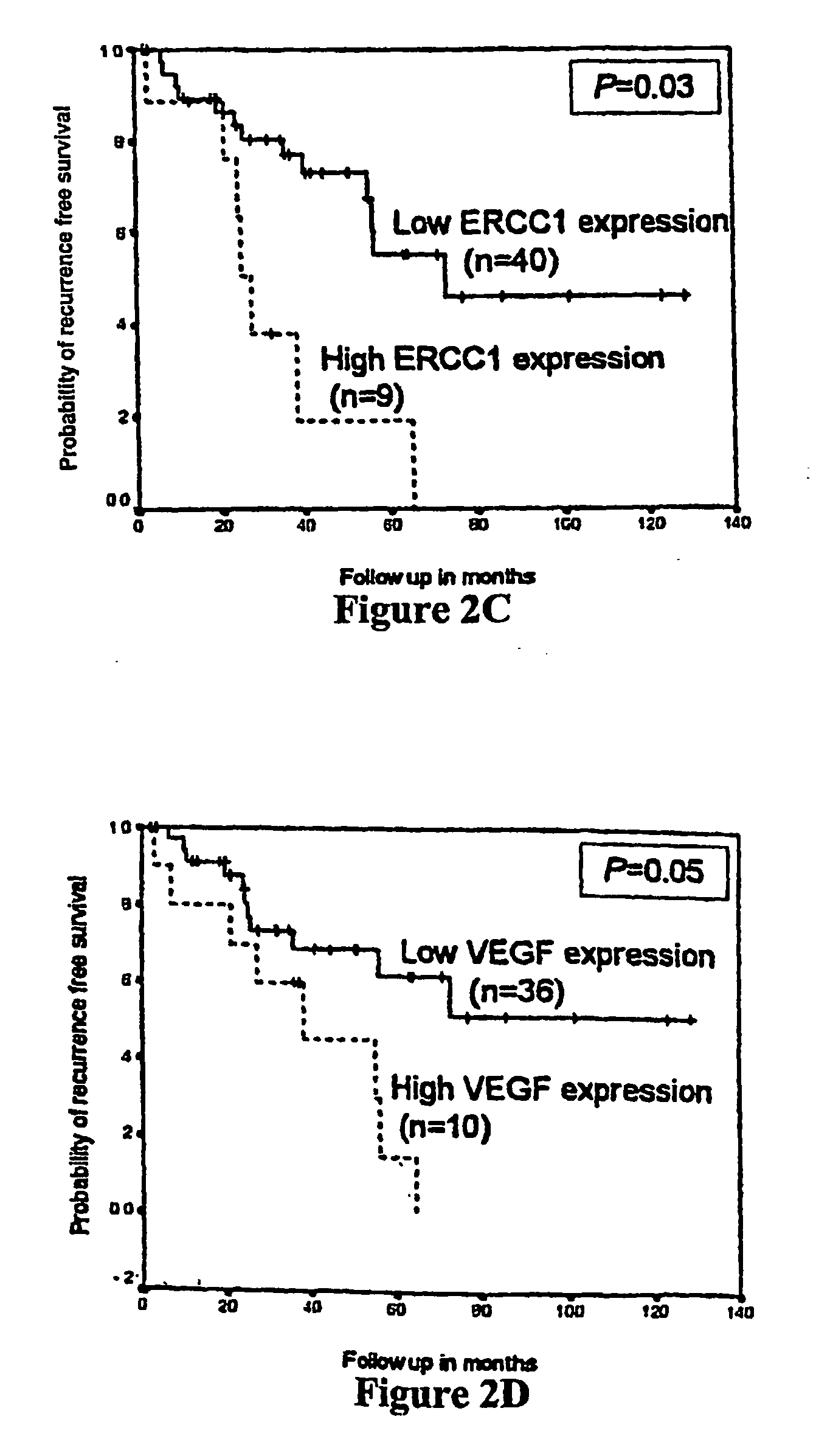
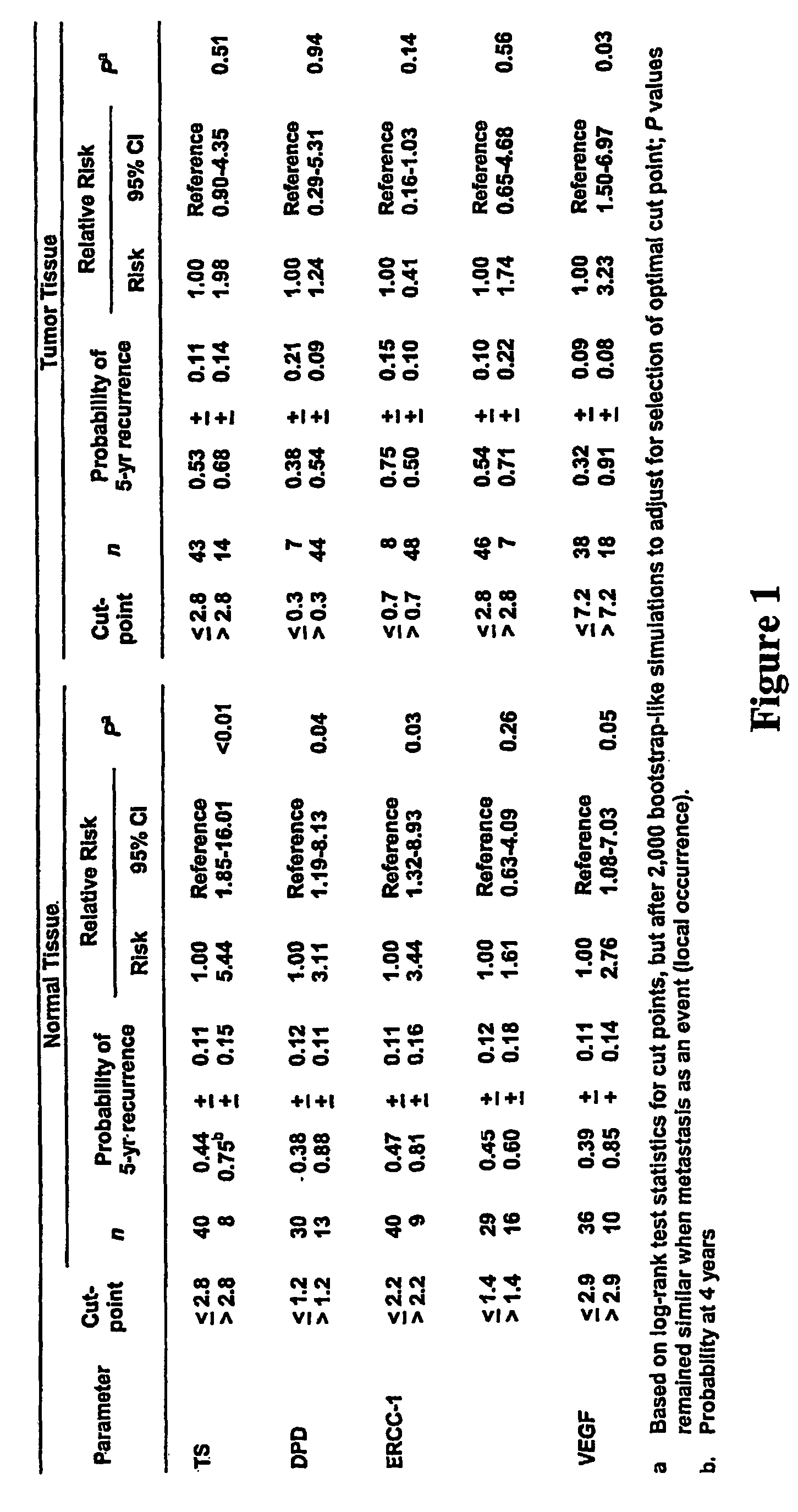
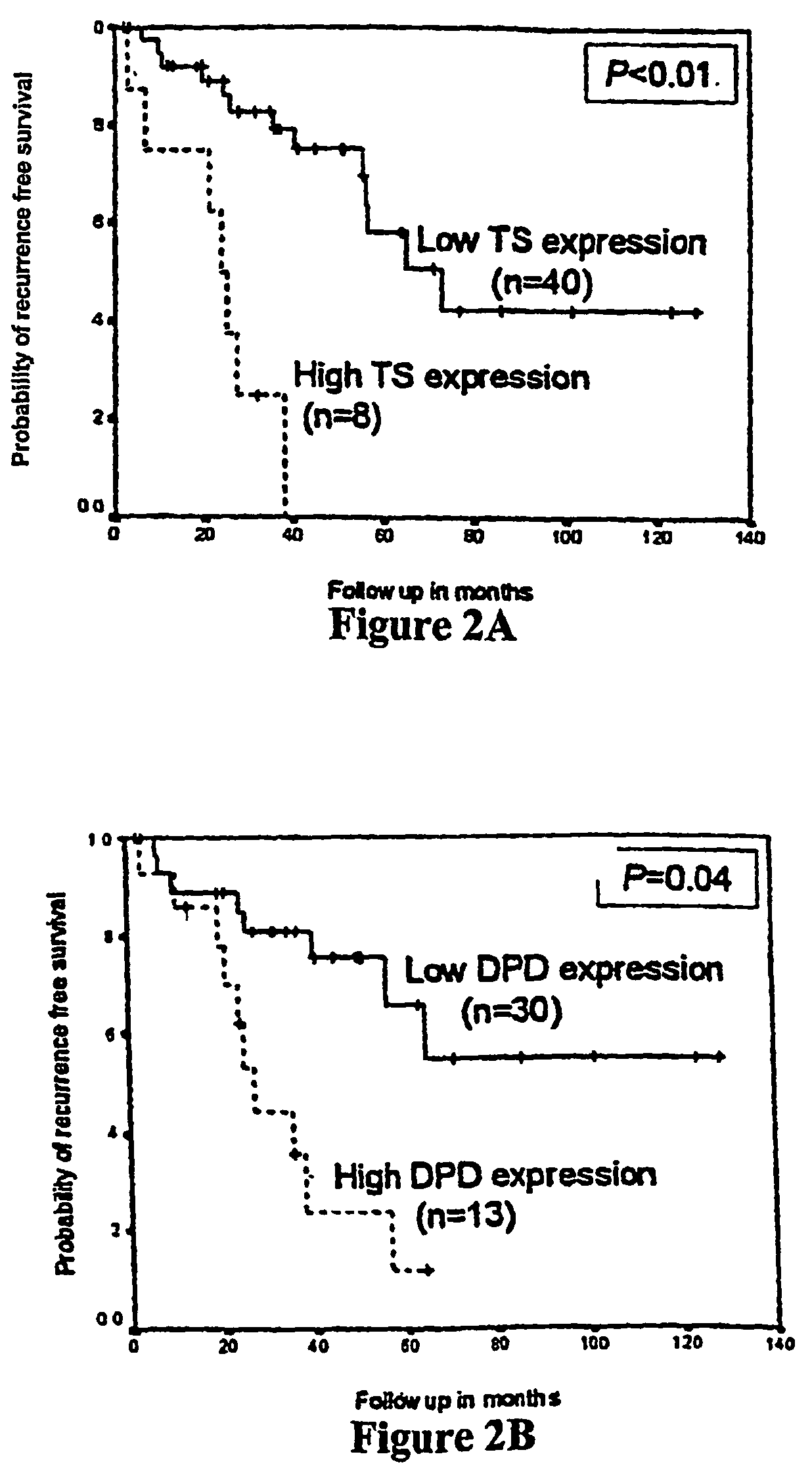
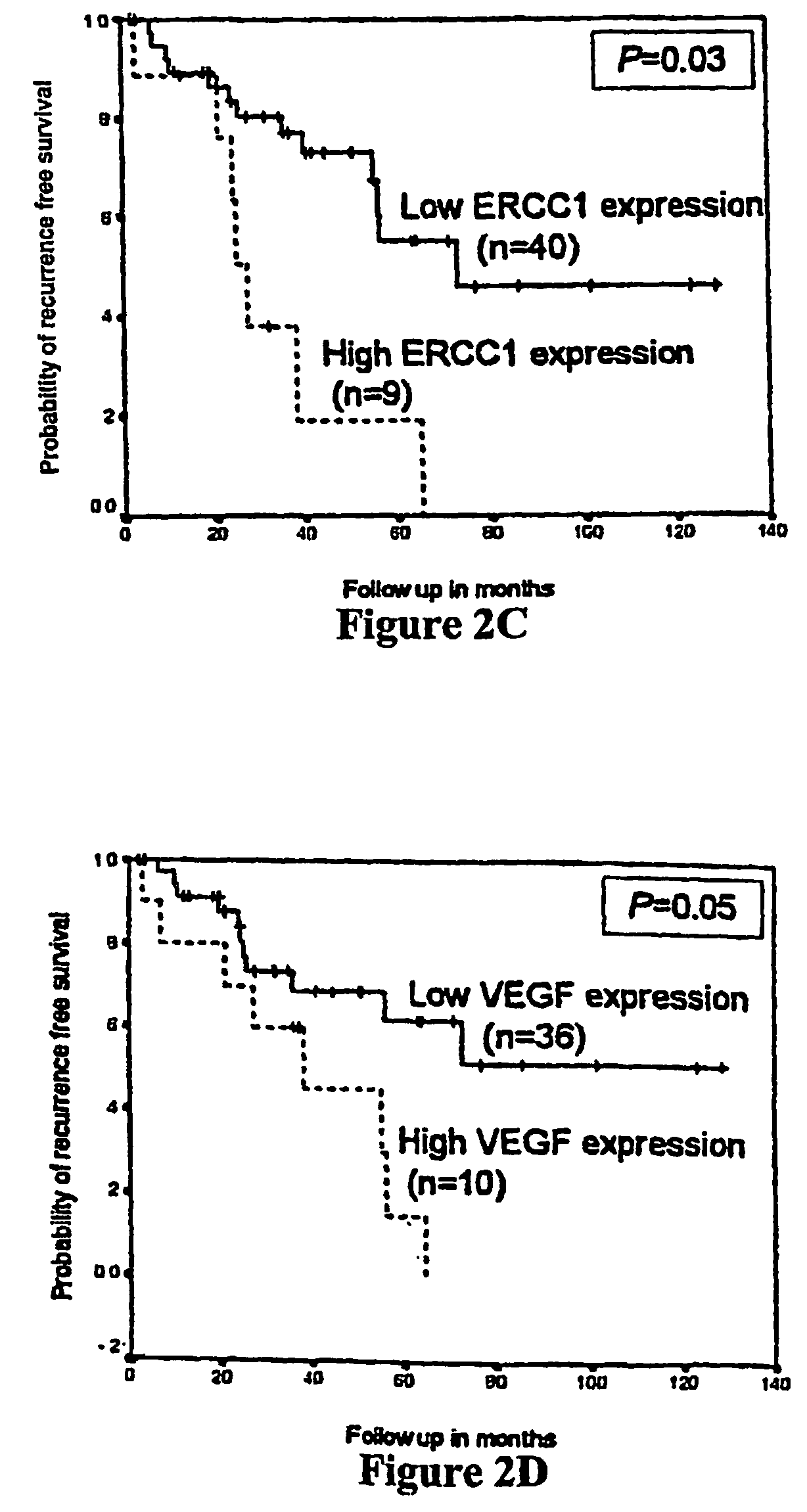
Polymorphisms in the ERCC1 gene for predicting treatment outcome
The invention provides compositions and methods for determining the increased risks for recurrence of certain cancers and the likelihood of successful treatment with one or both of chemotherapy and radiation therapy. The methods comprising determining the type of genomic polymorphism present in a predetermined region of the gene of interest isolated from the subject or patient. Also provided are nucleic acid probes and kits for determining a patient's cancer risk and treatment response.
Owner:UNIV OF SOUTHERN CALIFORNIA
Genetic analysis for stratification of cancer risk
InactiveUS20050136438A1Data processing applicationsHealth-index calculationProphylactic treatmentOncology
The present invention provides new methods for the assessment of cancer risk in the general population. These methods utilize particular alleles of two or more genes, in combination, to identify individuals with increased or decreased risk of cancer. Exemplified is risk assessment for breast cancer in women. In addition, personal history measures such as age and race are used to further refine the analysis. Using such methods, it is possible to reallocate healthcare costs in cancer screening to patient subpopulations at increased cancer risk. It also permits identification of candidates for cancer prophylactic treatment.
Owner:INTERGENETICS +1
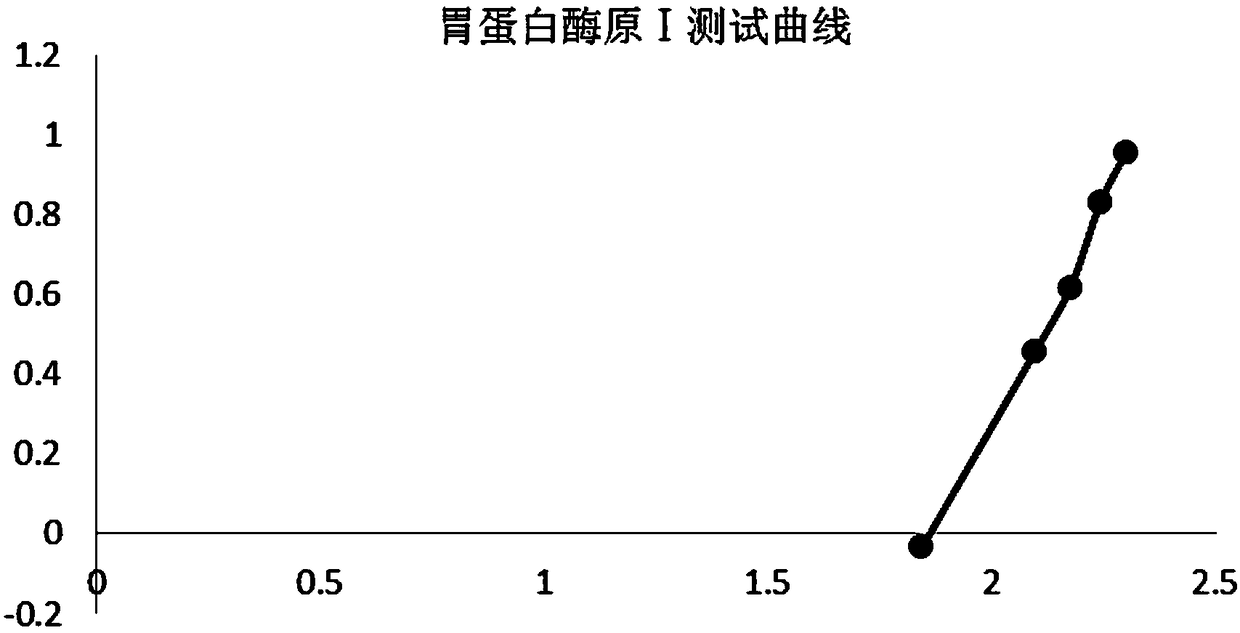
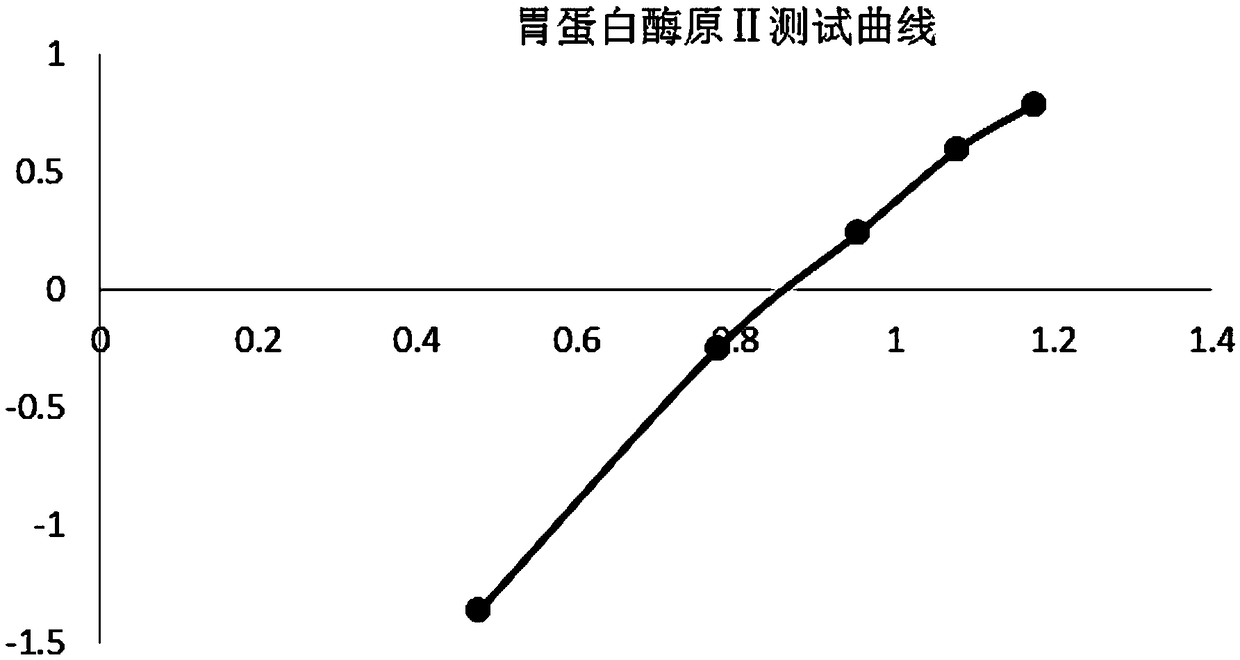
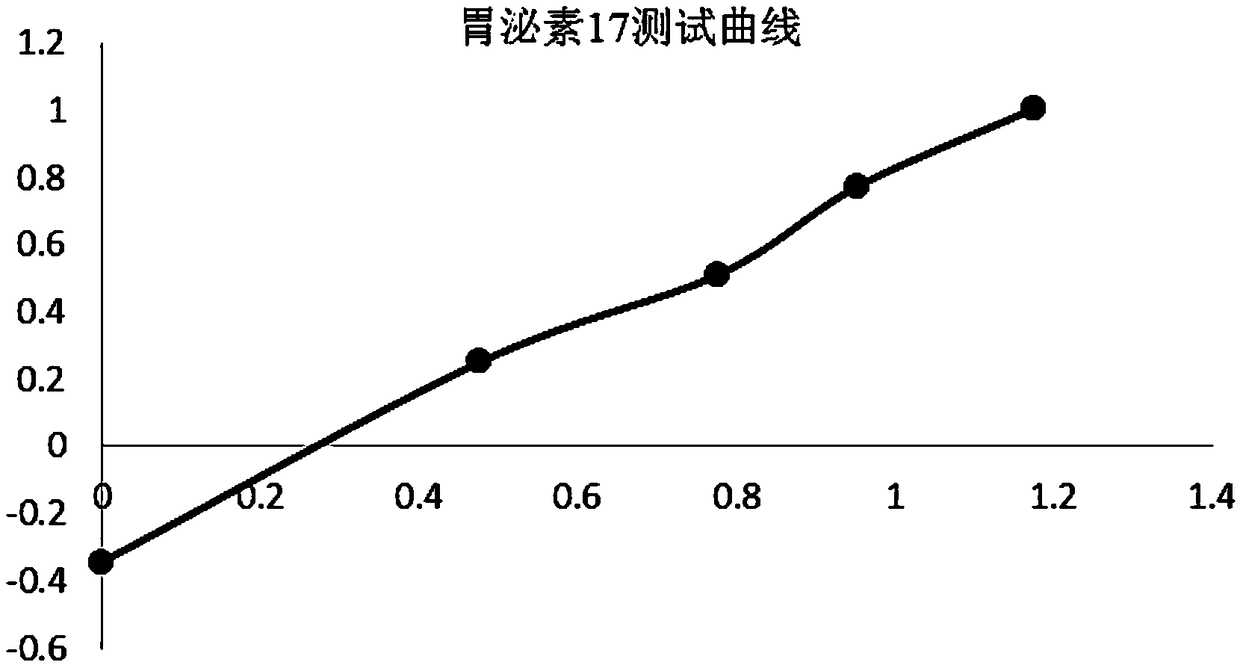
United quantitative detection test paper of gastric cancer risk markers and preparation method
The invention relates to united quantitative detection test paper of gastric cancer risk markers and a preparation method. The gastric cancer risk markers include pepsinogen I, pepsinogen II and gastrin 17. A test paper strip comprises a sample pad, a gastric cancer risk marker antibody magnetic rare earth fluorescent microsphere labelling pad, a coating membrane and a water absorption pad, wherein three gastric cancer risk marker quantitative detection lines and a quality control region C line are arranged on the coating membrane. After tested substances in samples are concentrated through amagnetic field, a low-background high-sensitivity fluorescence immunochromatographic method is utilized for detecting the tested substances, and the problem of low sensitivity of a POCT type product is solved. The problem that when blood samples are detected through an immunity lateral chromatography reagent, in order to reduce the interference of other substances, usually the samples are tested after being diluted, so that the sensitivity is slightly low can be solved.
Owner:广州华澳生物科技有限公司
Method of evaluating cancer risk in human
Disclosed is a method of providing a risk evaluation and diagnosis of human cancer, by examining the presence, in the volatile fraction of a human saliva sample, of a combination of particulate biochemical volatile organic compounds, which is indicative of an increased risk of developing cancer.
Owner:INSTITUT CLINIDENT
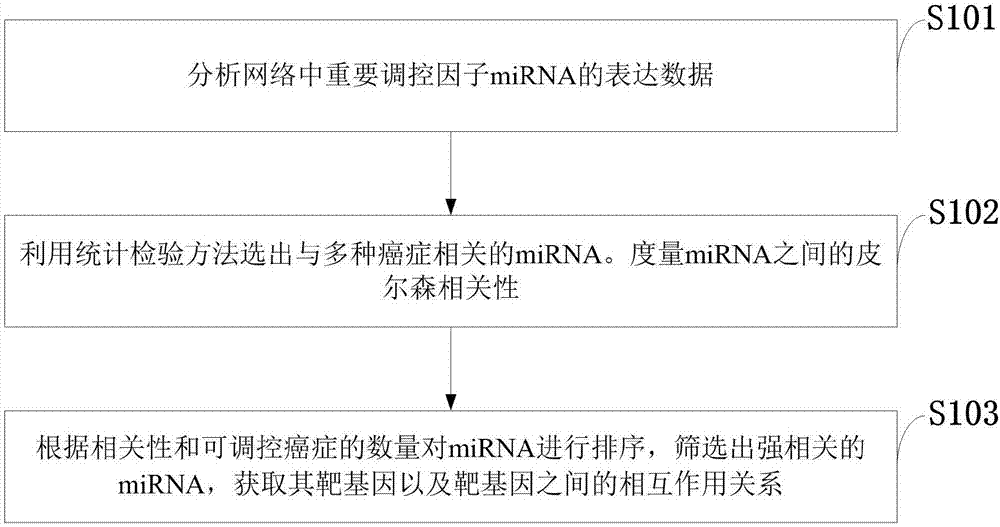
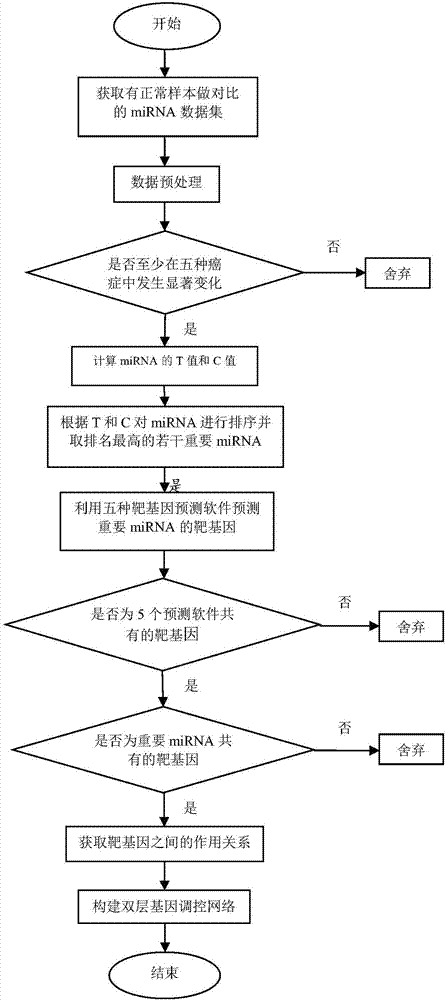
Method for constructing double-layer gene regulation and control network
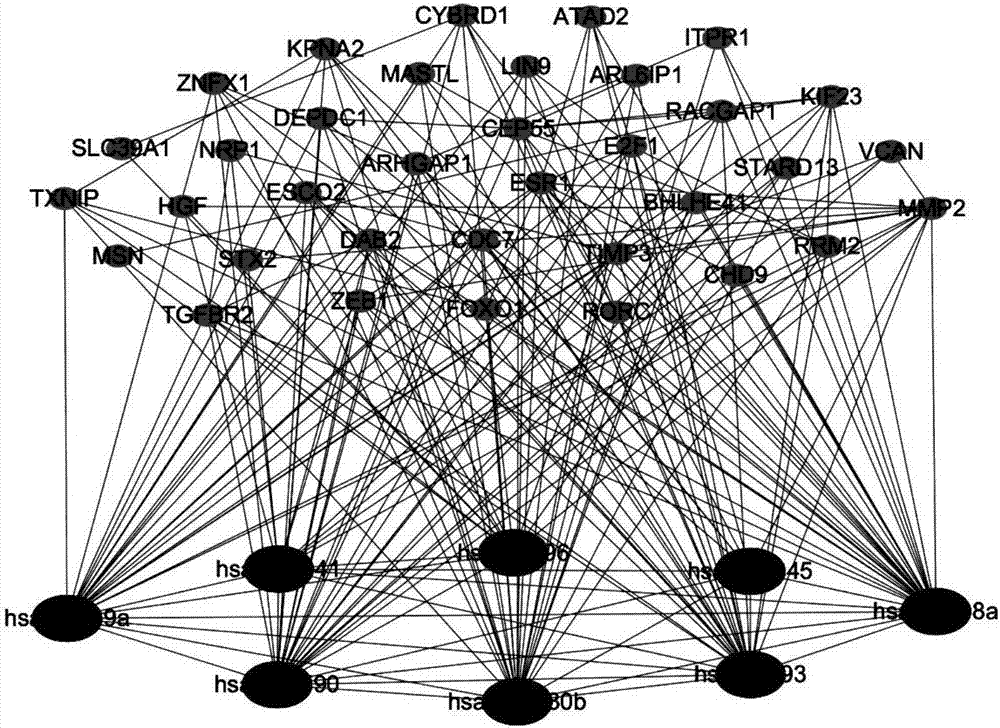
ActiveCN107358062AUnderstanding PathogenesisUnderstand the processProteomicsGenomicsKey factorsTargeted therapy
The invention belongs to the technical field of data processing and discloses a method for constructing a double-layer gene regulation and control network. The method comprises following steps: analyzing expression data of an important regulation and control factor miRNA in a network; selecting miRNA relevant to multiple types of cancer by means of the statistics examination method; measuring Pearson correlation among the miRNA; ordering the miRNA according to the correlation and the number of controllable cancer, screening out strongly correlated miRNA and obtaining target genes thereof and interacting relations among the target genes. The present invention discloses the mechanism of gene and miRNA involved in the regulation of biological processes such as cancer and helps to understand the relationship between genes and cancer and other complex diseases and provides reference for the development of biopharmaceuticals and targeted therapy for pan-cancer. Identifying cancer-associated miRNAs and genes can be used to elucidate the mechanisms of cooperation that are involved in the regulation of key factors that influence a variety of cancer processes, cancer risk prediction, and the development of bio-targeted drugs.
Owner:XIDIAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com