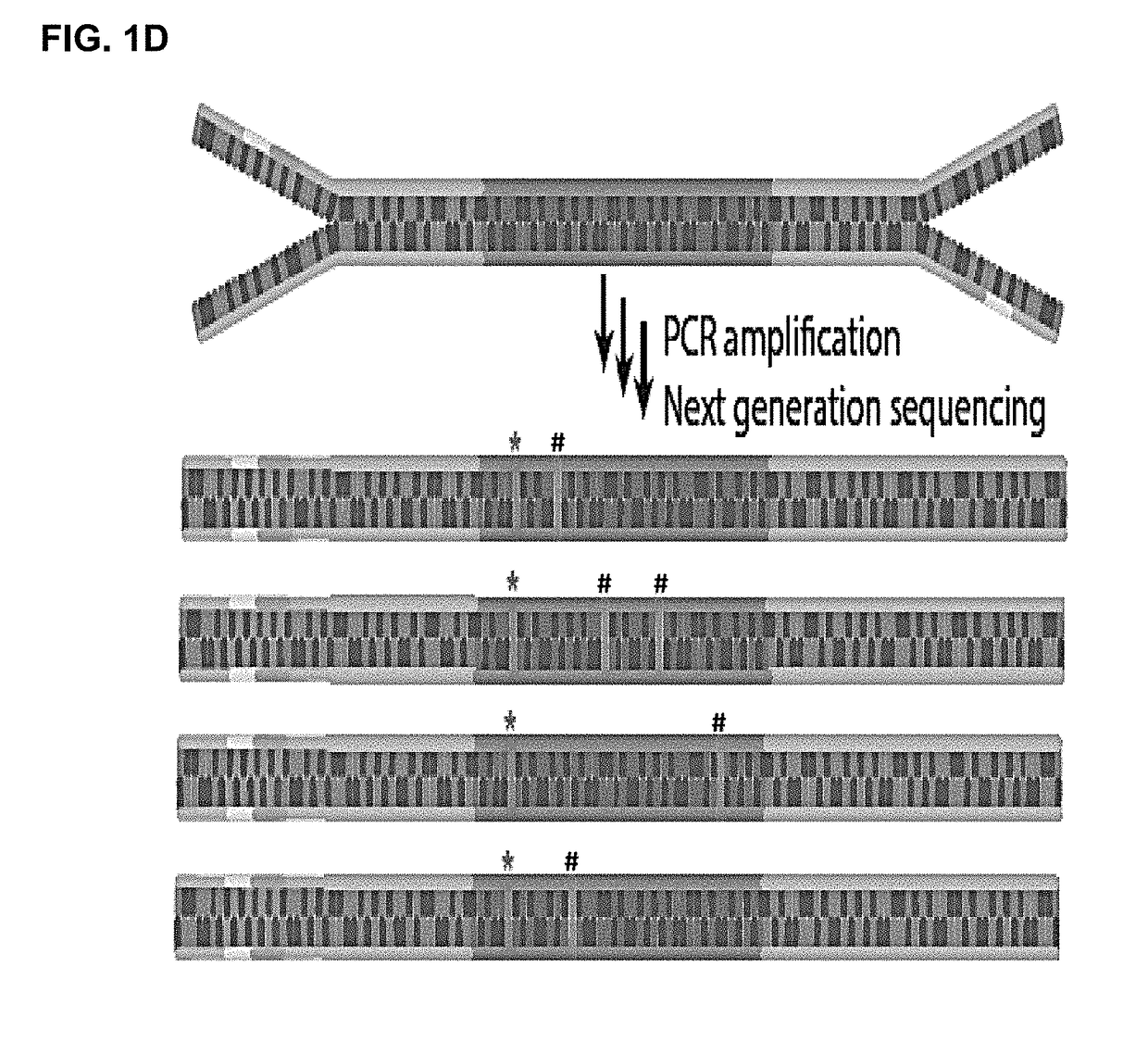
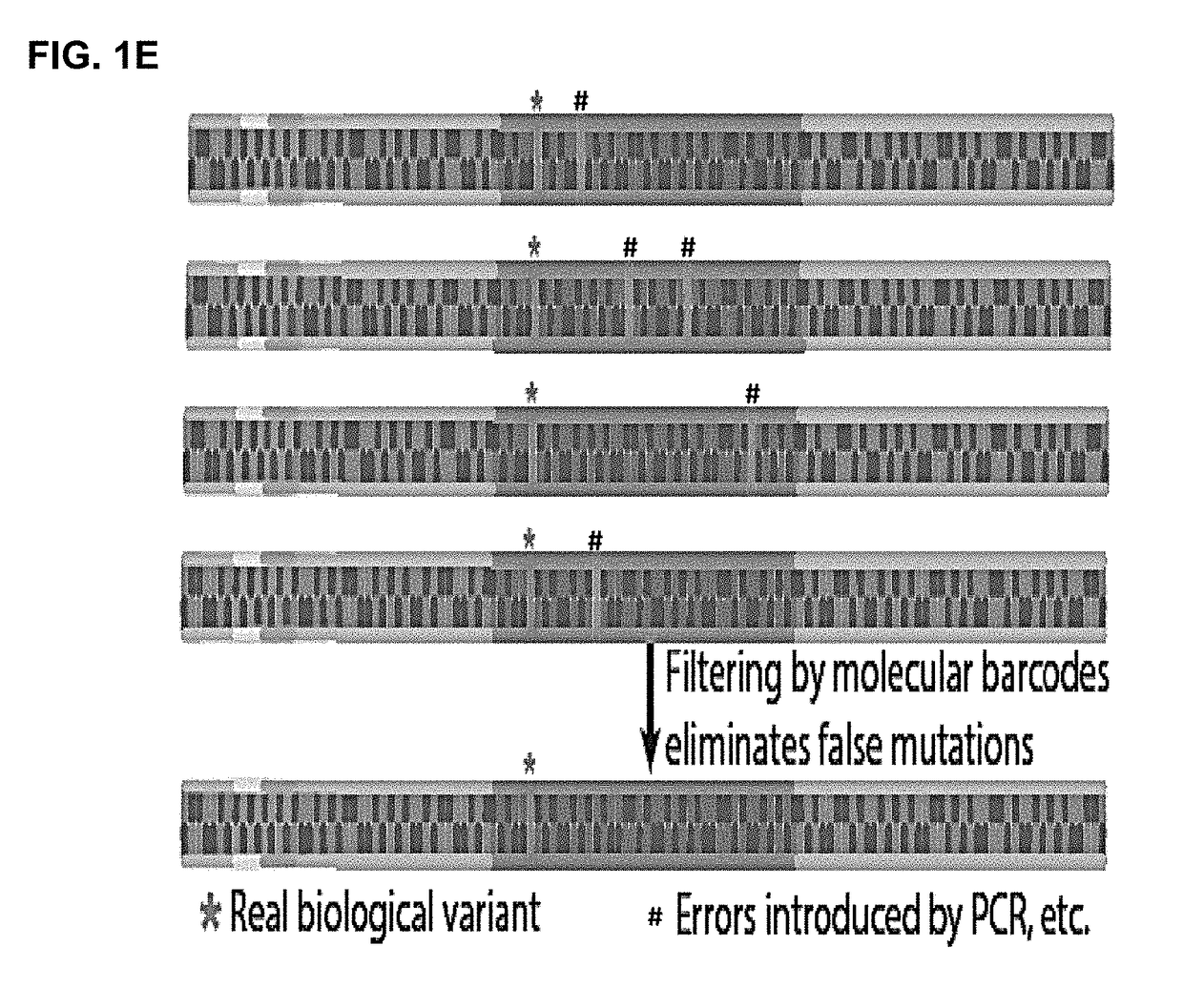
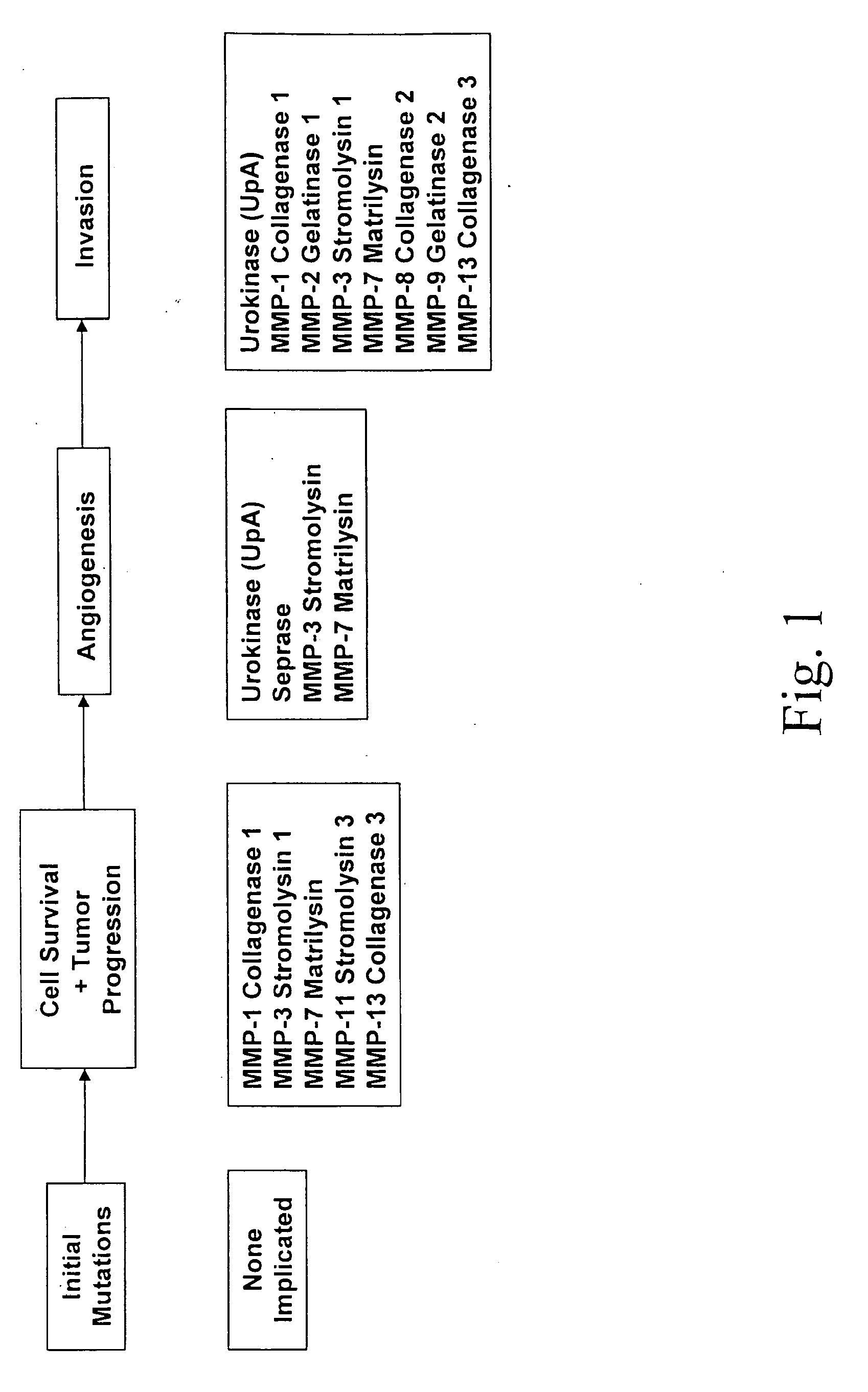
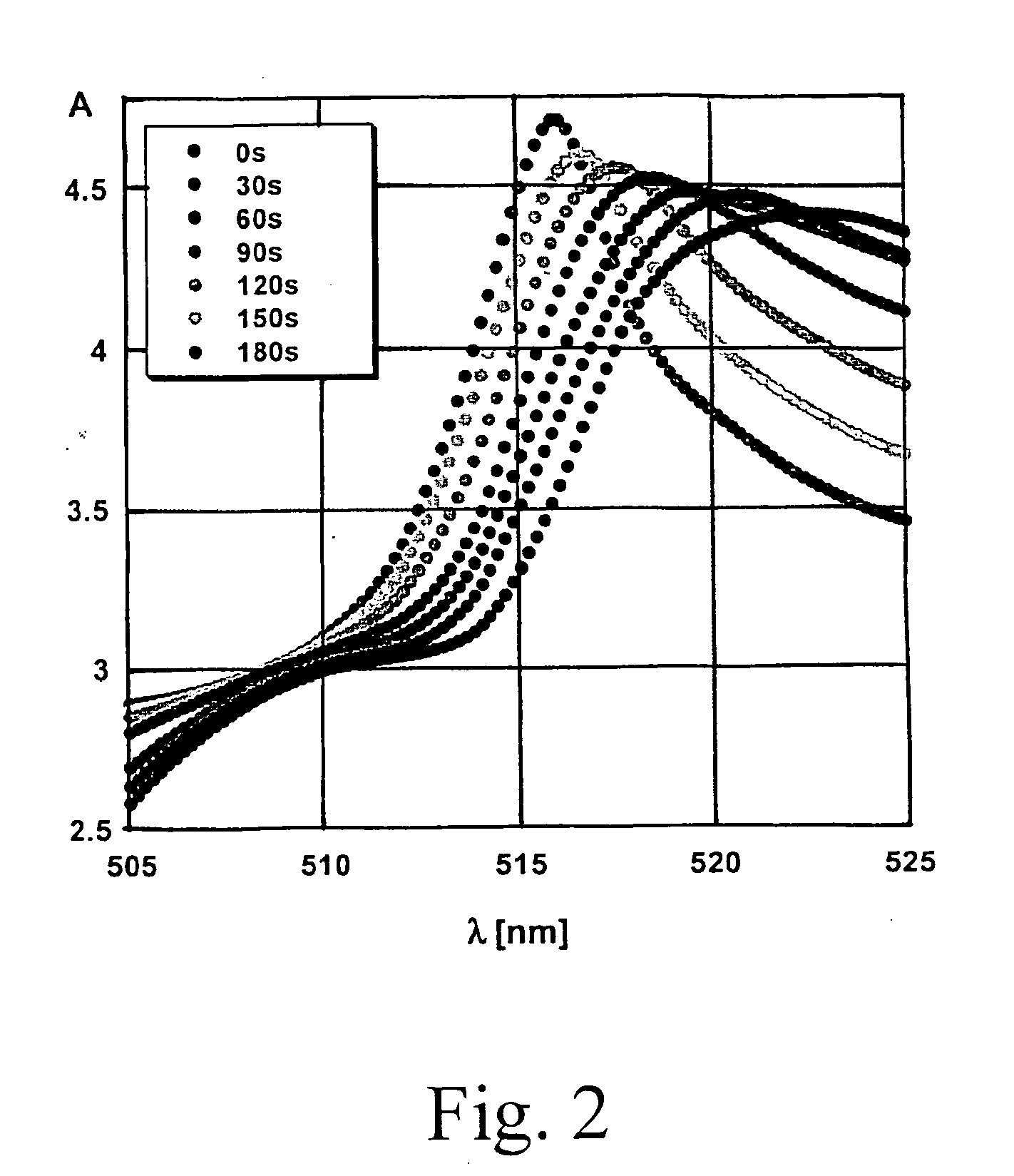
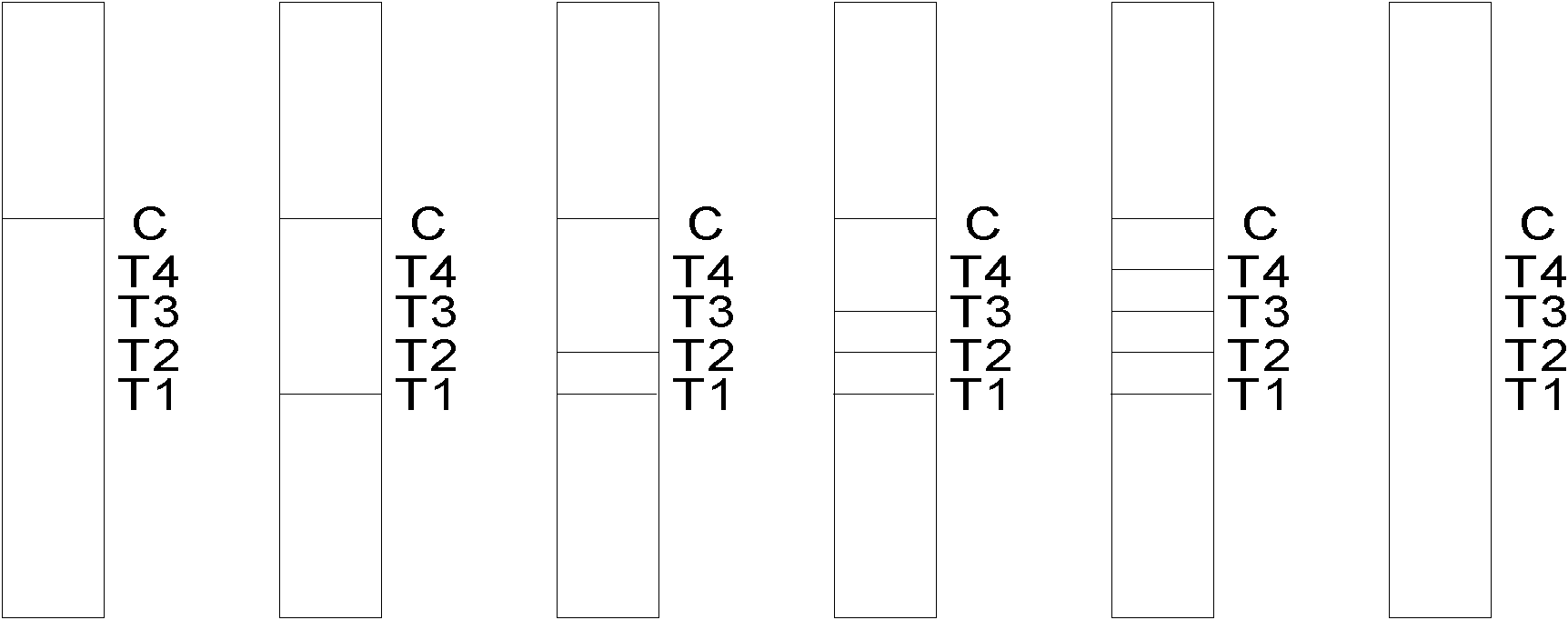Patents
Literature
221 results about "Cancer prognosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
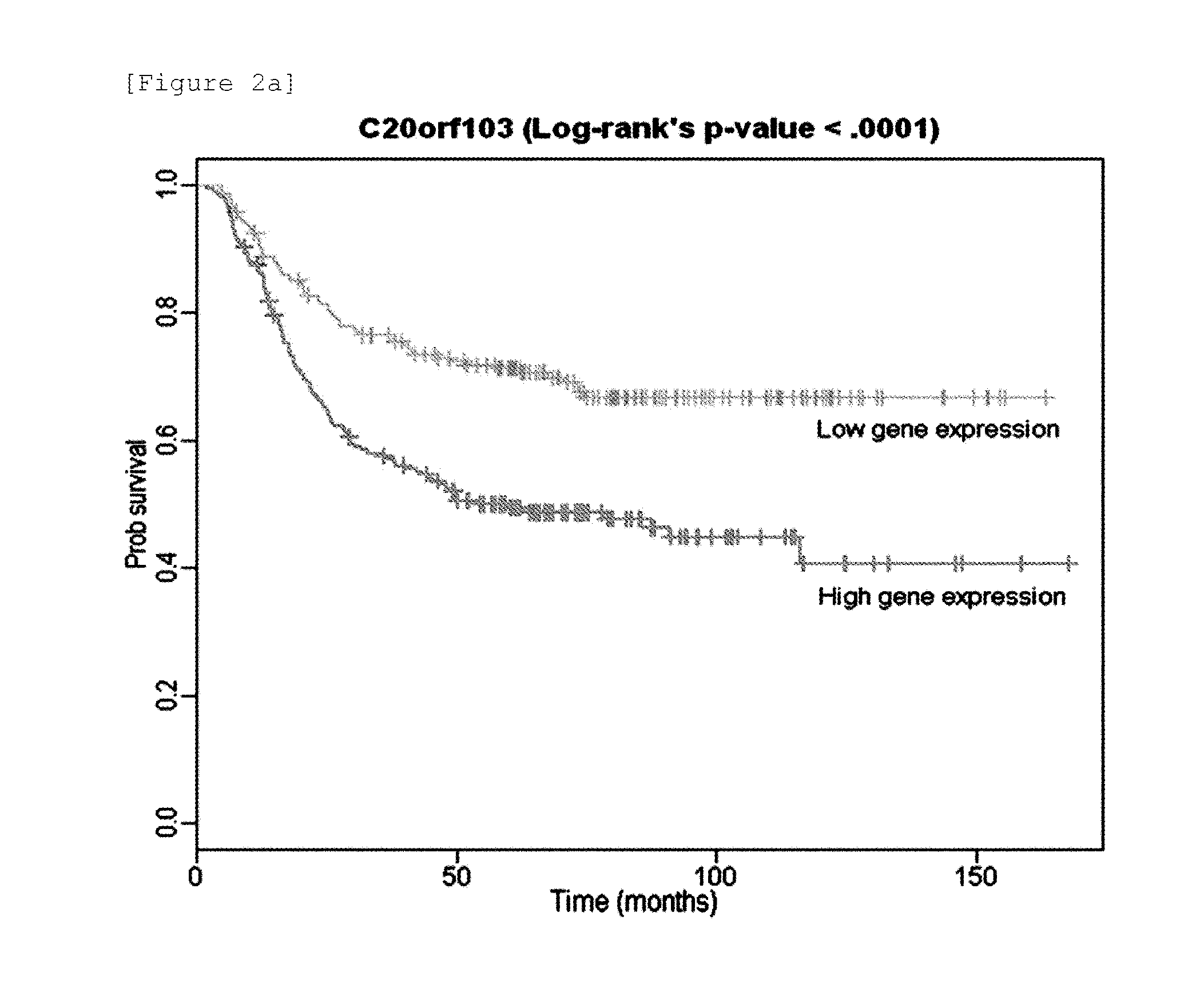
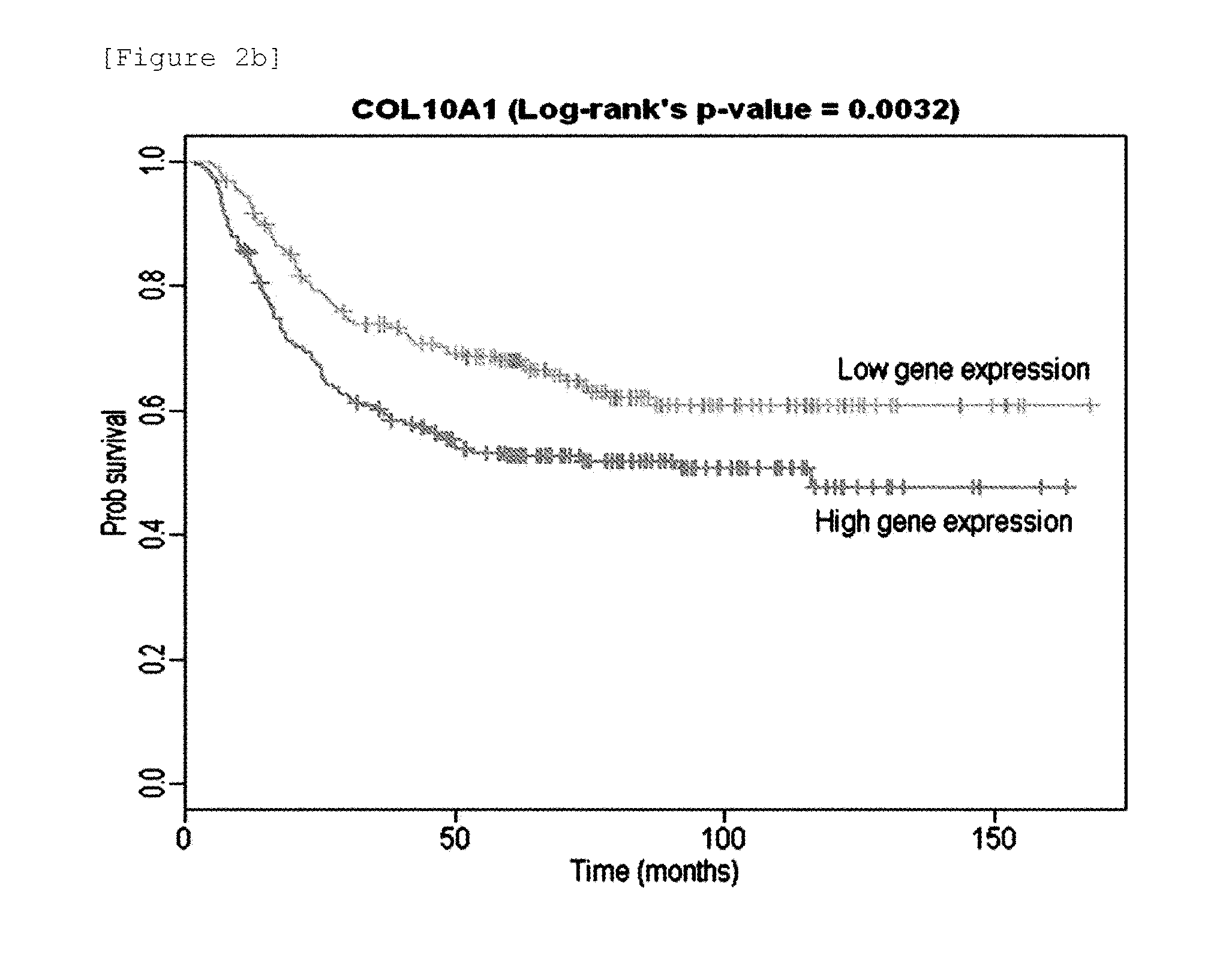
Expression profile algorithm and test for cancer prognosis
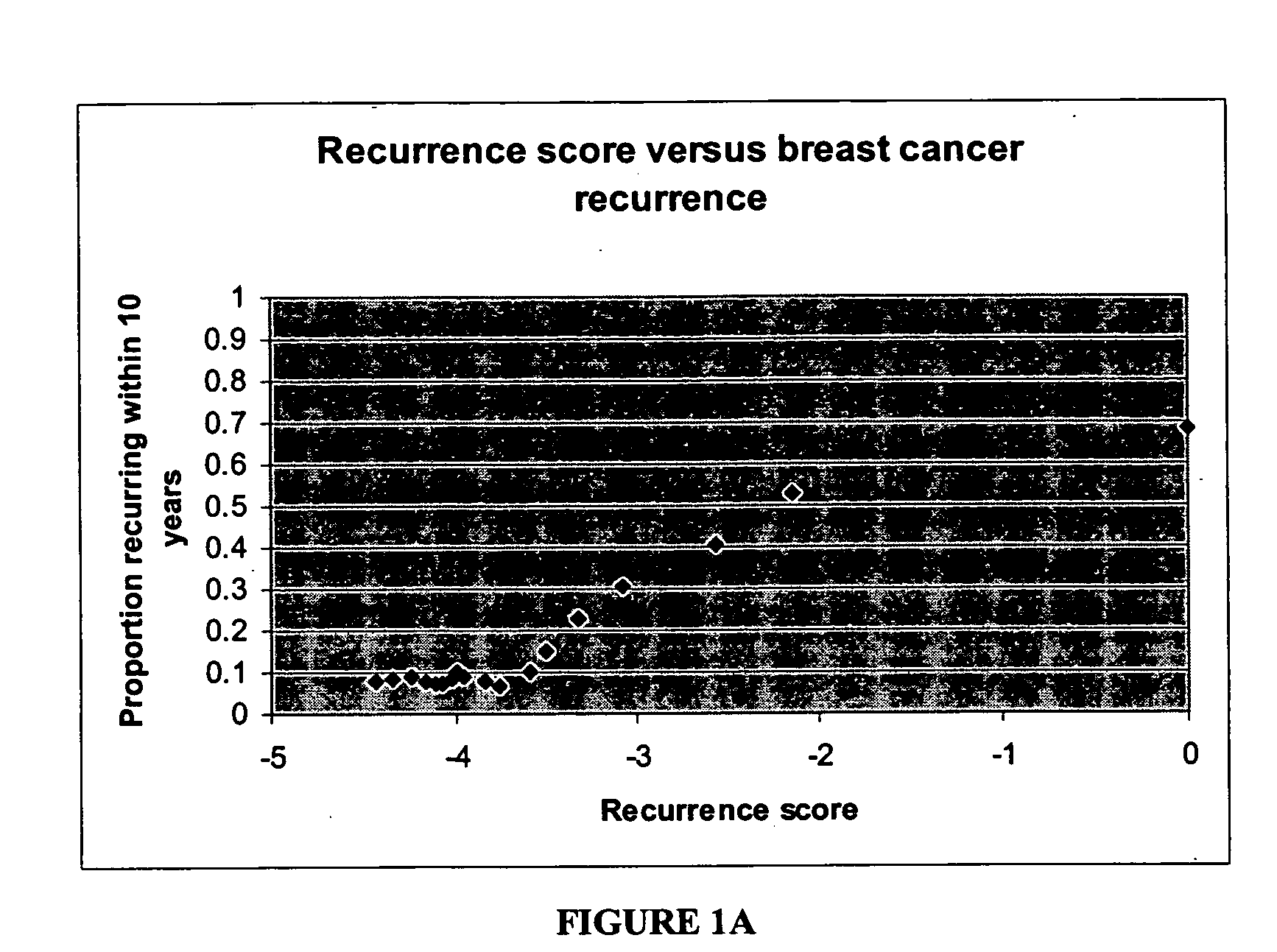
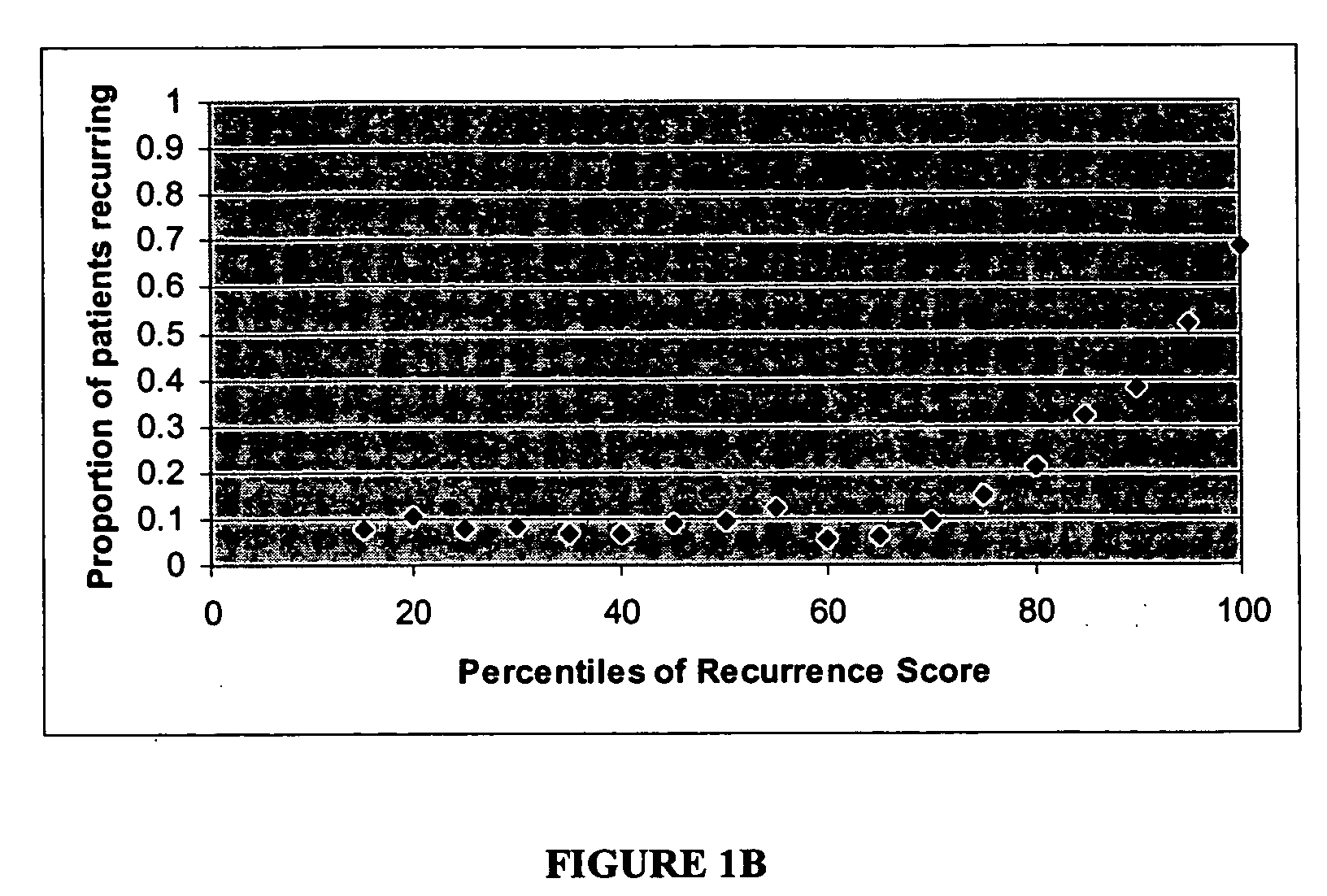
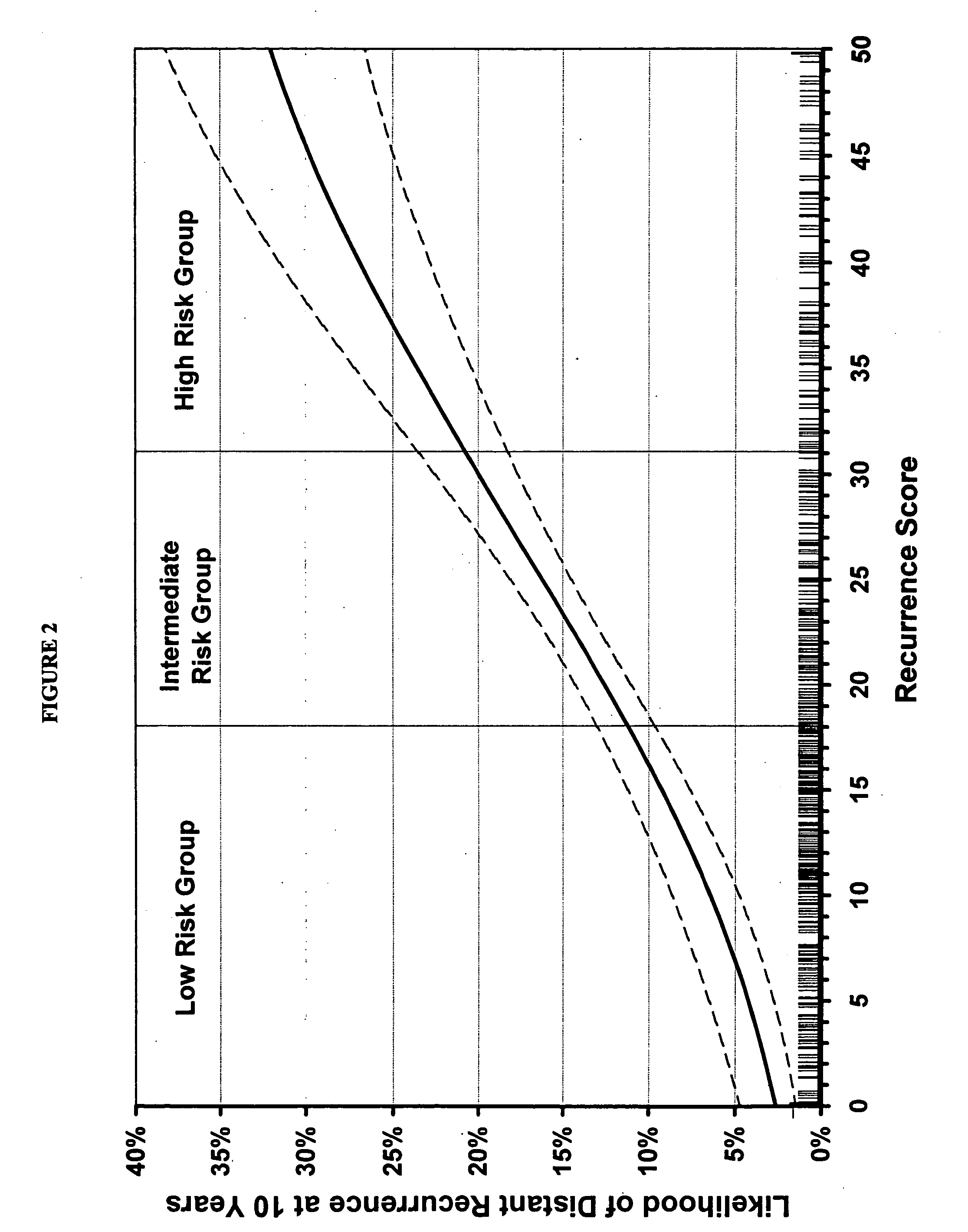
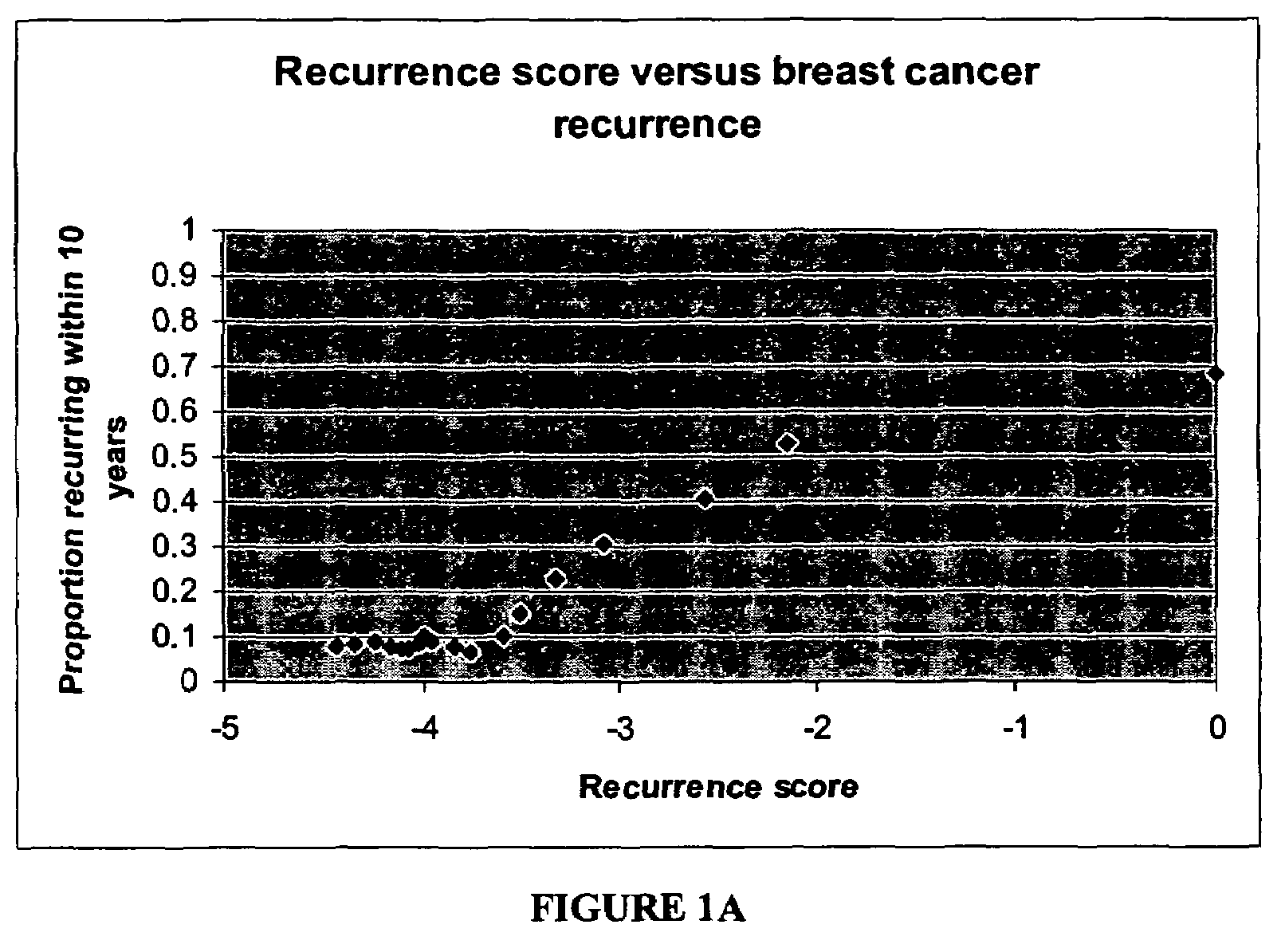
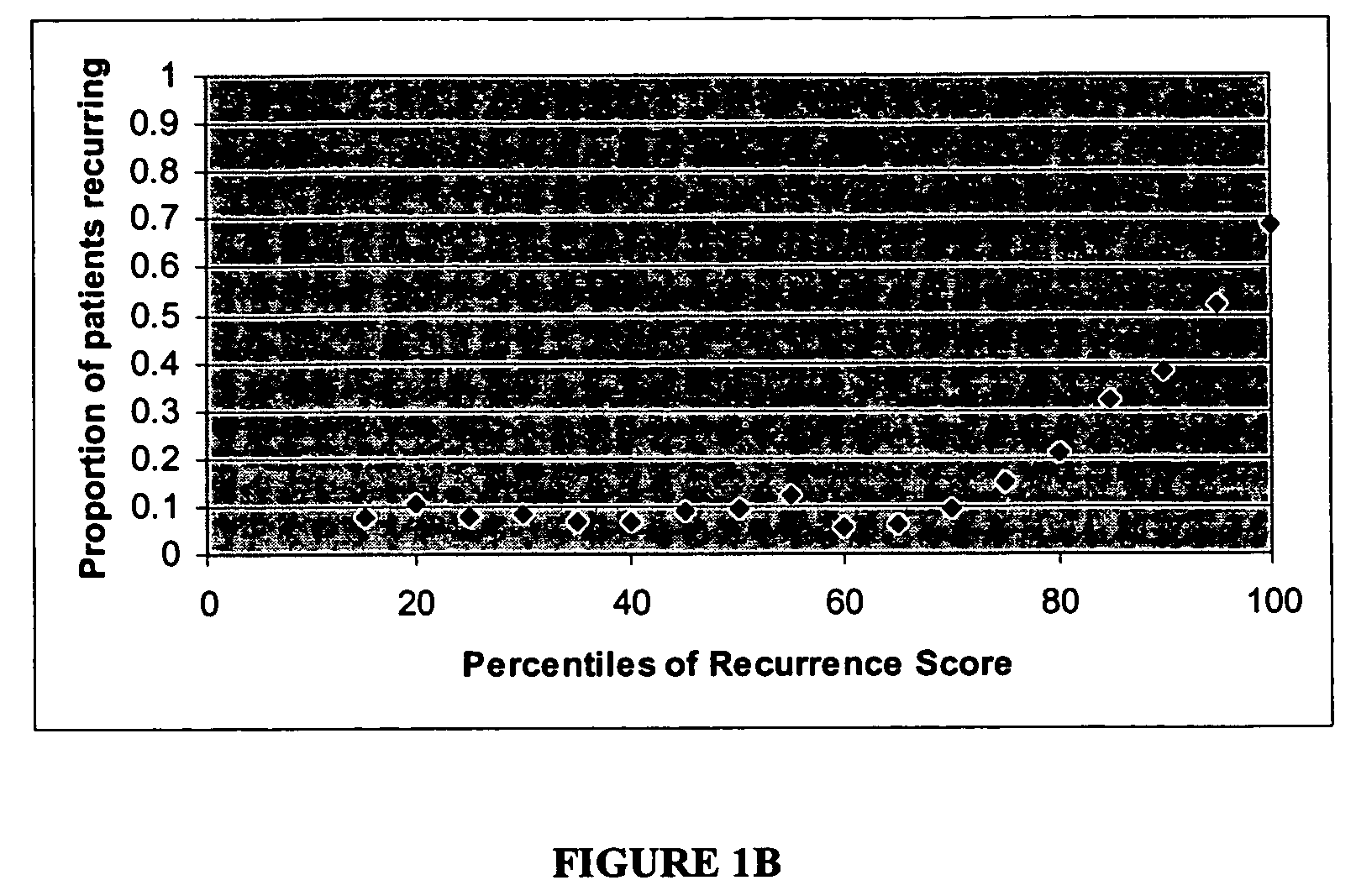
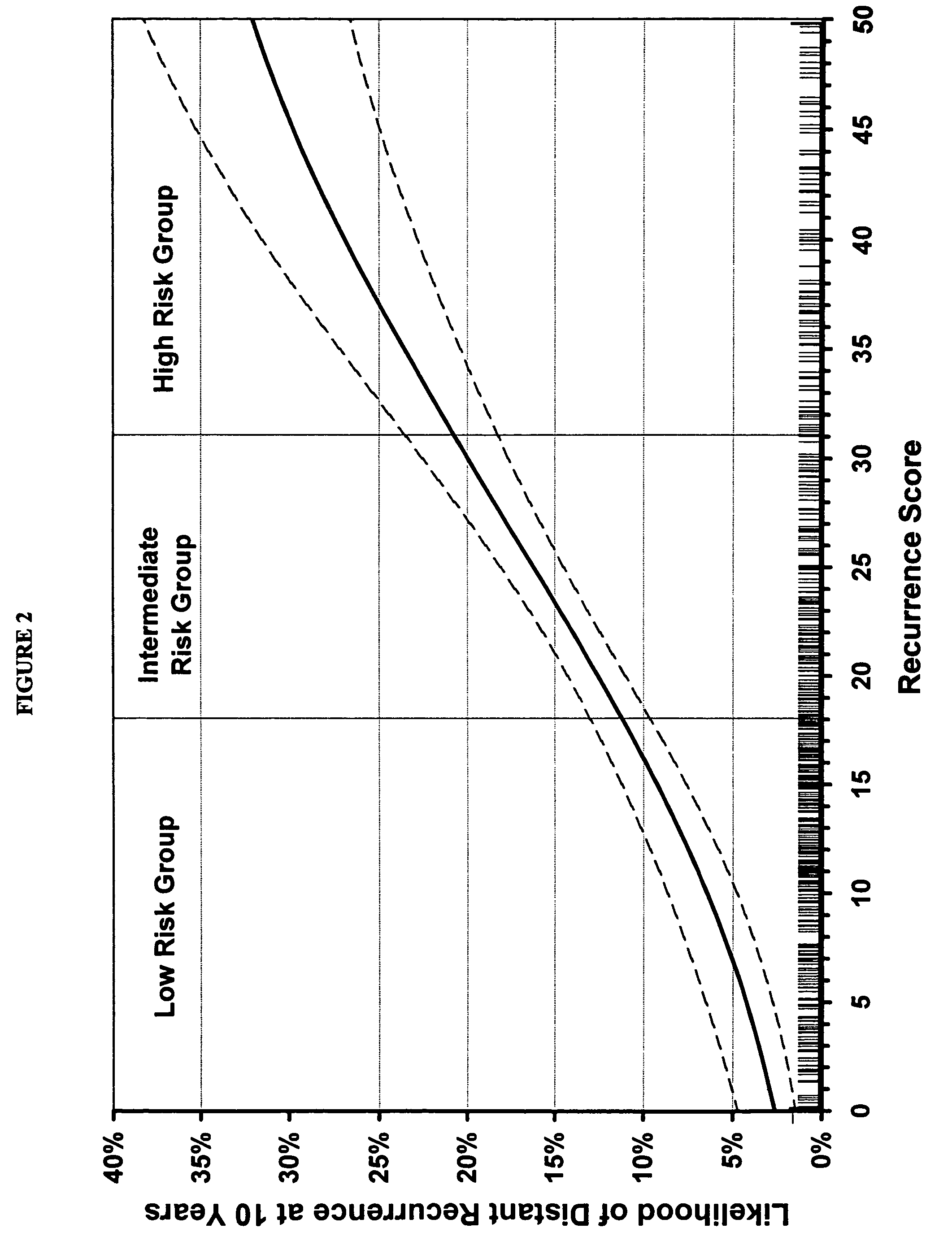
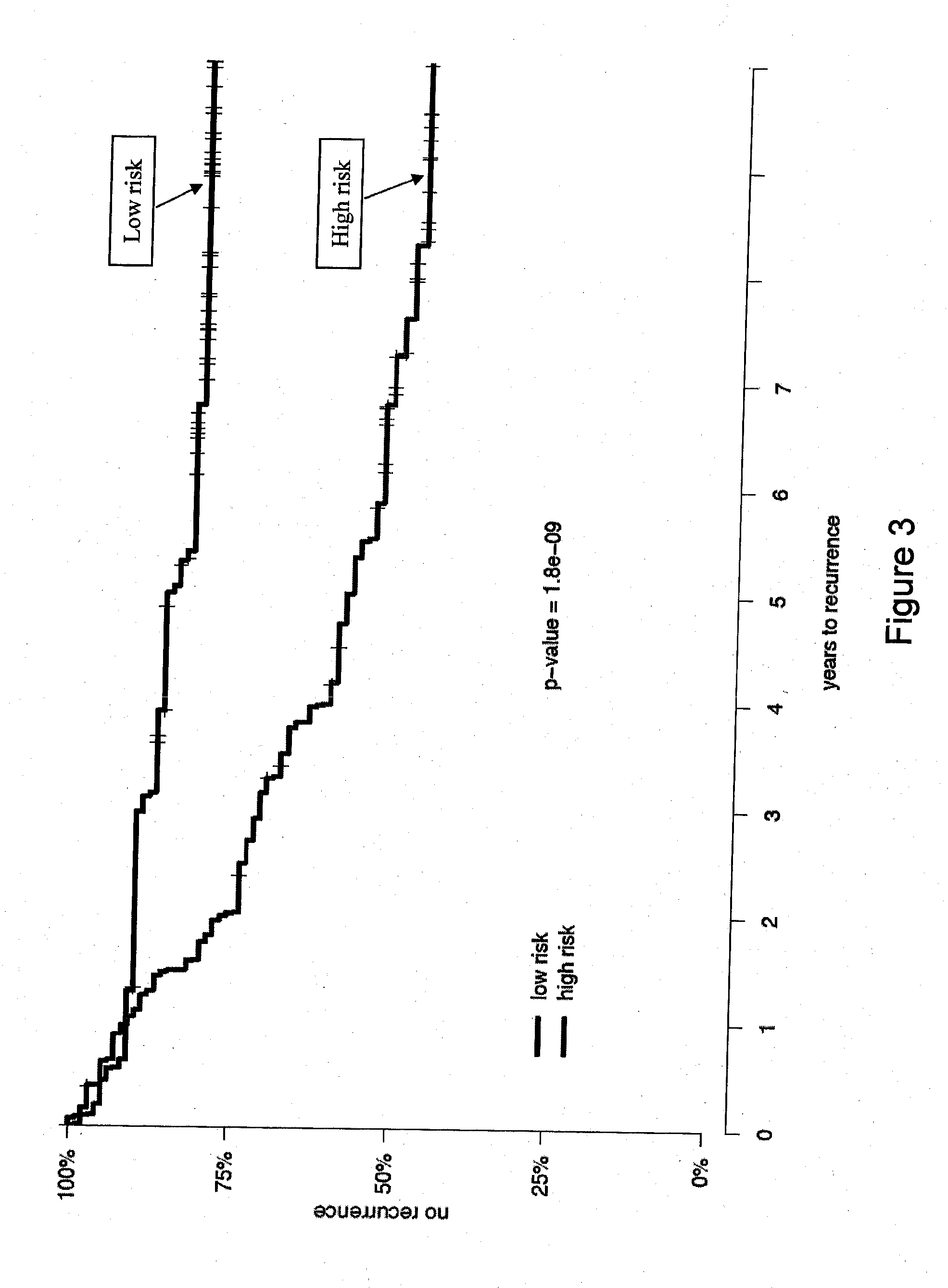
The present invention provides a noninvasive, quantitative test for prognosis determination in cancer patients. The test relies on measurements of the tumor levels of certain messenger RNAs (mRNAs). These mRNA levels are inserted into a polynomial formula (algorithm) that yields a numerical recurrence score, which indicates recurrence risk.
Owner:GENOMIC HEALTH INC
Markers for cancer prognosis and therapy and methods of use
InactiveUS20130042333A1Adverse side effectLow costBiocideMicrobiological testing/measurementAdjuvantTreatment field
The invention relates generally to the field of cancer prognosis and treatment. More particularly, the present invention relates to methods and compositions that utilize a particular panel of gene products (“biomarkers”) and their differential expression patterns (“expression signatures”), wherein the expression patterns correlate with responsiveness, or lack thereof, to chemotherapy treatment. The invention is based on the identification of a specific set of biomarkers that are differentially expressed in chemotherapy-treated tumors and which are useful in predicting the likelihood of a therapeutic response, including residual disease persistence and subsequent tumor recurrence in cancer patients receiving chemotherapy. The gene panel is also useful in designing specific adjuvant modalities with improved therapeutic efficiency. Also disclosed are methods for characterizing tumors according to expression of the biomarkers described herein.
Owner:GEN TECH
Methods and compositions for evaluating breast cancer prognosis
InactiveUS20060063190A1Accurate assessmentImproved prognosisMicrobiological testing/measurementProteomicsLymphatic SpreadNucleic acid hybridisation
Methods and compositions for evaluating the prognosis of a breast cancer patient, particularly an early-stage breast cancer patient, are provided. The methods of the invention comprise detecting expression of at least one, more particularly at least two, biomarker(s) in a body sample, wherein overexpression of the biomarker or a combination of biomarkers is indicative of breast cancer prognosis. In some embodiments, the body sample is a breast tissue sample, particularly a primary breast tumor sample. The biomarkers of the invention are proteins and / or genes whose overexpression is indicative of either a good or bad cancer prognosis. Biomarkers of interest include proteins and genes involved in cell cycle regulation, DNA replication, transcription, signal transduction, cell proliferation, invasion, proteolysis, or metastasis. In some aspects of the invention, overexpression of a biomarker of interest is detected at the protein level using biomarker-specific antibodies or at the nucleic acid level using nucleic acid hybridization techniques.
Owner:TRIPATH IMAGING INC
Antibody-based arrays for detecting multiple signal transducers in rare circulating cells
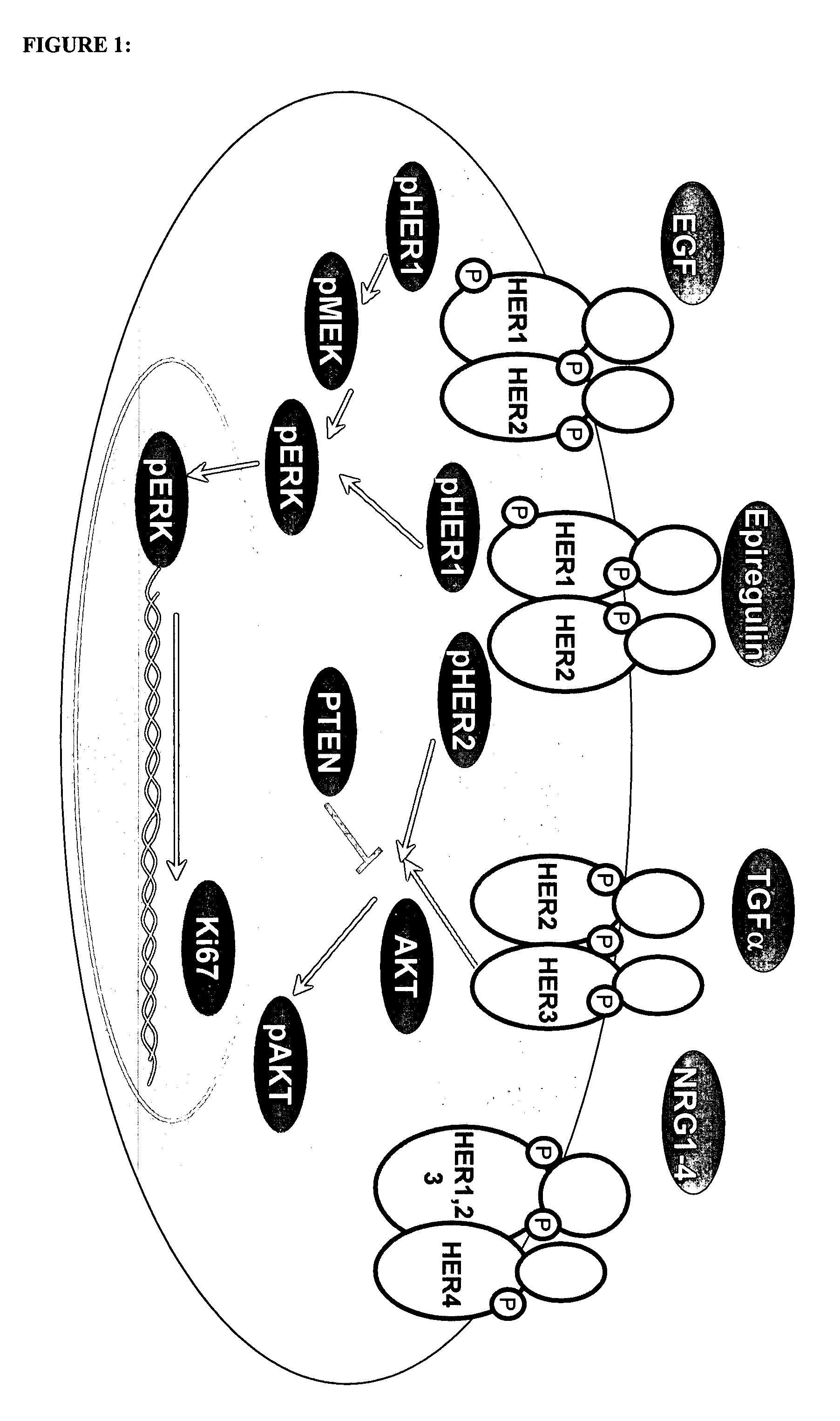
The present invention provides antibody-based arrays for detecting the activation state and / or total amount of a plurality of signal transduction molecules in rare circulating cells and methods of use thereof for facilitating cancer prognosis and diagnosis and the design of personalized, targeted therapies.
Owner:SOC DES PROD NESTLE SA
Expression profile algorithm and test for cancer prognosis
The present invention provides a noninvasive, quantitative test for prognosis determination in cancer patients. The test relies on measurements of the tumor levels of certain messenger RNAs (mRNAs). These mRNA levels are inserted into a polynomial formula (algorithm) that yields a numerical recurrence score, which indicates recurrence risk.
Owner:GENOMIC HEALTH INC
Method for Cancer Prognosis Using Cellular Folate Vitamin Receptor Quantification
Owner:PURDUE RES FOUND INC
MRI and optical assays for proteases
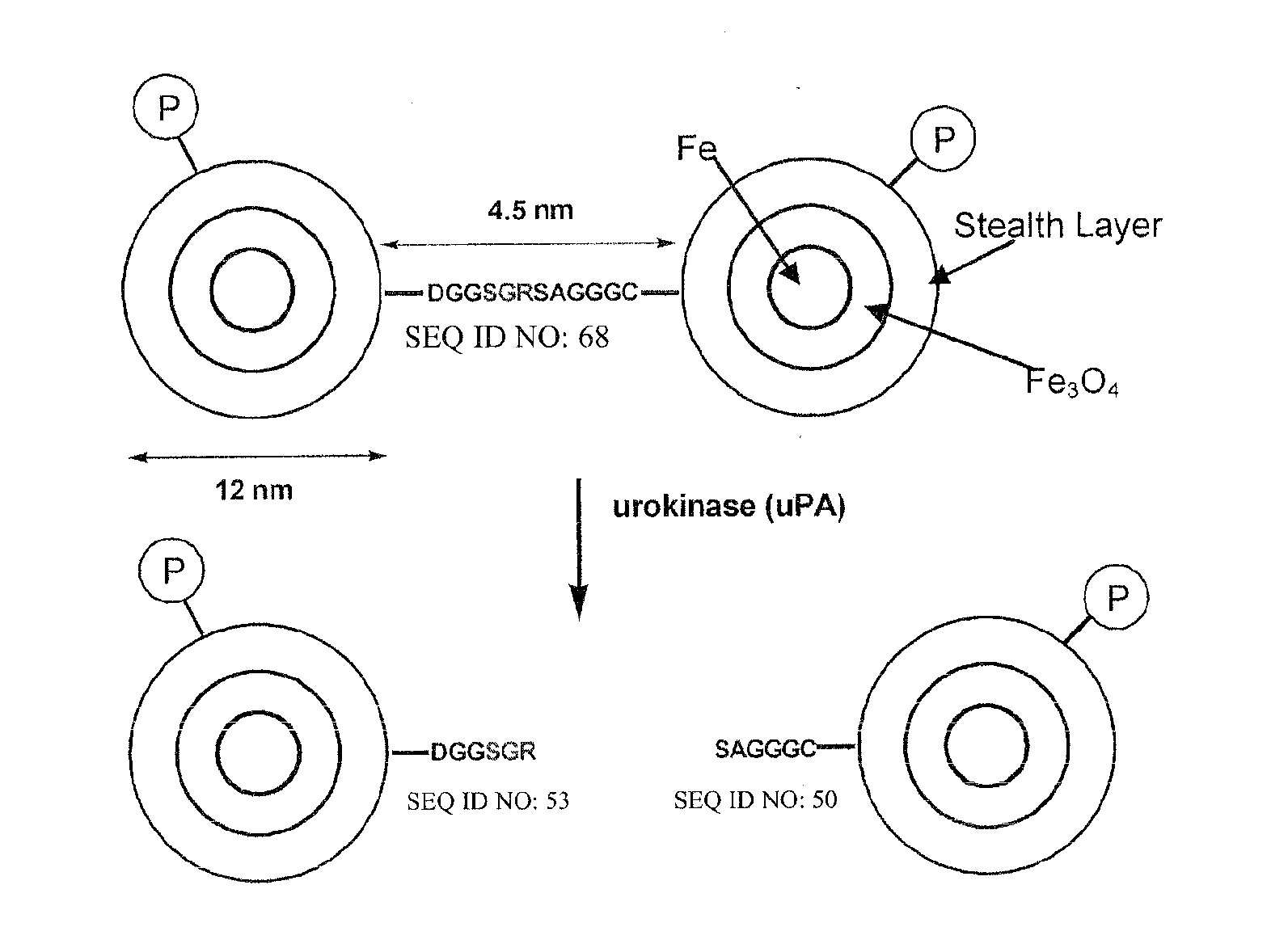
The present invention provides multifunctional nanoplatforms for assessing the activity of a protease in vivo or in vitro, along with methods of imaging and detecting the presence of cancerous or precancerous tissues, and the therapeutic treatment thereof, including monitoring of treatment. The diagnostic nanoplatforms comprise nanoparticles and are linked to each other or other particles via an oligopeptide linkage that comprises a consensus sequence specific for the target protease. Cleavage of the sequence by the target protease can be detected using various sensors, and the diagnostic results can be correlated with cancer prognosis. Individual unlinked nanoplatforms are also adaptable for therapeutic hyperthermia treatment of the cancerous tissue.
Owner:NANOSCALE CORPORATION +1
Methods and uses thereof of prosaposin
InactiveUS20100144603A1Extended half-lifeFacilitating recombinant protein expressionAntibacterial agentsBiocideAbnormal tissue growthLymphatic Spread
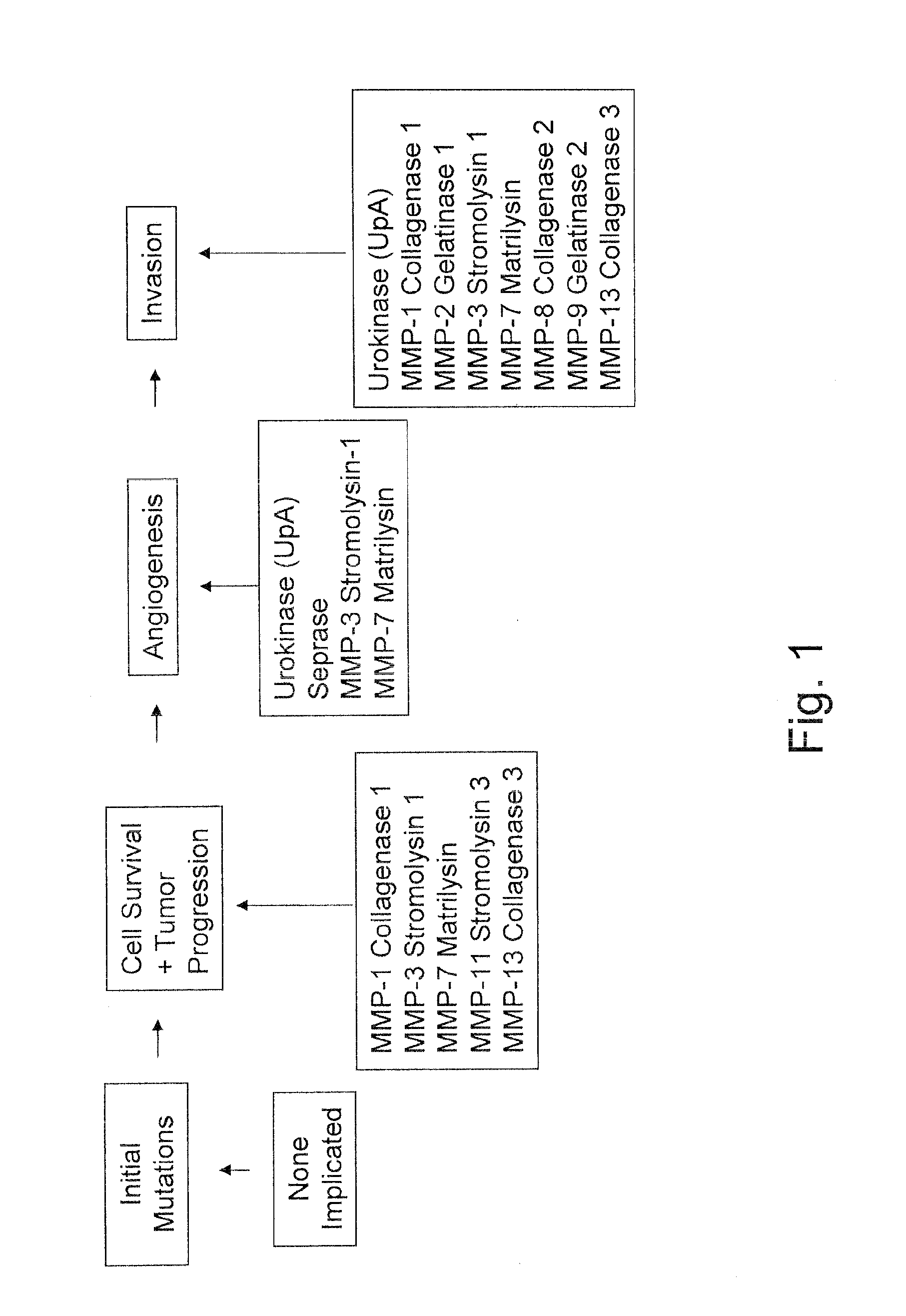
The invention relates to methods for treating of tumor metastasis, methods for preventing, inhibiting, and predicting tumor recurrence and tumor metastasis, and methods of preventing cancer development for one at risk of developing cancer, for one diagnosed with a benign cancer, and / or for one diagnosed with malignant cancer. The invention also relates to methods of treating angiogenesis-dependent diseases and disorders. In addition, the invention provides: (1) a method for screening for tumor / cancer derived angiogenesis and metastasis factors; (2) a method for screening for compounds that inhibit angiogenesis and metastasis; (3) a method for screening for compounds that promote anti-angiogenesis and anti-metastasis activities; (4) a method for cancer prognosis evaluation; (5) a method for predicting the tissue specificity of a metastatic cancer; (6) a method for determining likelihood of metastasis; and (7) a method for cancer prognosis evaluation by the surveillance of metastasis development.
Owner:CHILDRENS MEDICAL CENT CORP
Identification and use of circulating nucleic acid tumor markers
ActiveCN105518151AMicrobiological testing/measurementSequence analysisCancers diagnosisCancer therapy
Methods for creating a selector of mutated genomic regions and for using the selector set to analyze genetic alterations in a cell-free nucleic acid sample are provided. The methods can be used to measure tumor-derived nucleic acids in a blood sample from a subject and thus to monitor the progression of disease in the subject. The methods can also be used for cancer screening, cancer diagnosis, cancer prognosis, and cancer therapy designation.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Identification and use of circulating nucleic acids
ActiveUS20180251848A1Nucleotide librariesMicrobiological testing/measurementPolynucleotideCell-Free Nucleic Acids
Disclosed herein are polynucleotide adaptors and methods of use thereof for identifying and analyzing nucleic acids, including cell-free nucleic acids from a patient sample. Also disclosed herein are methods of using the adaptors to detect, diagnose, or determine prognosis of cancers.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Protease assay
The present invention provides a diagnostic reagent or assay for assessing the activity of a protease in vivo or in vitro and methods of detecting the presence of a cancerous or precancerous cell. The assays are comprised of two particles linked via an oligopeptide linkage that comprises a consensus sequence specific for the target protease. Cleavage of the sequence by the target protease can be detected visually or using various sensors, and the diagnostic results can be correlated with cancer prognosis.
Owner:KANSAS STATE UNIV RES FOUND
Methods and computer program products for analysis and optimization of marker candidates for cancer prognosis
ActiveUS20060078926A1Accurate predictionMinimal numberMicrobiological testing/measurementProteomicsPatient careDisease cause
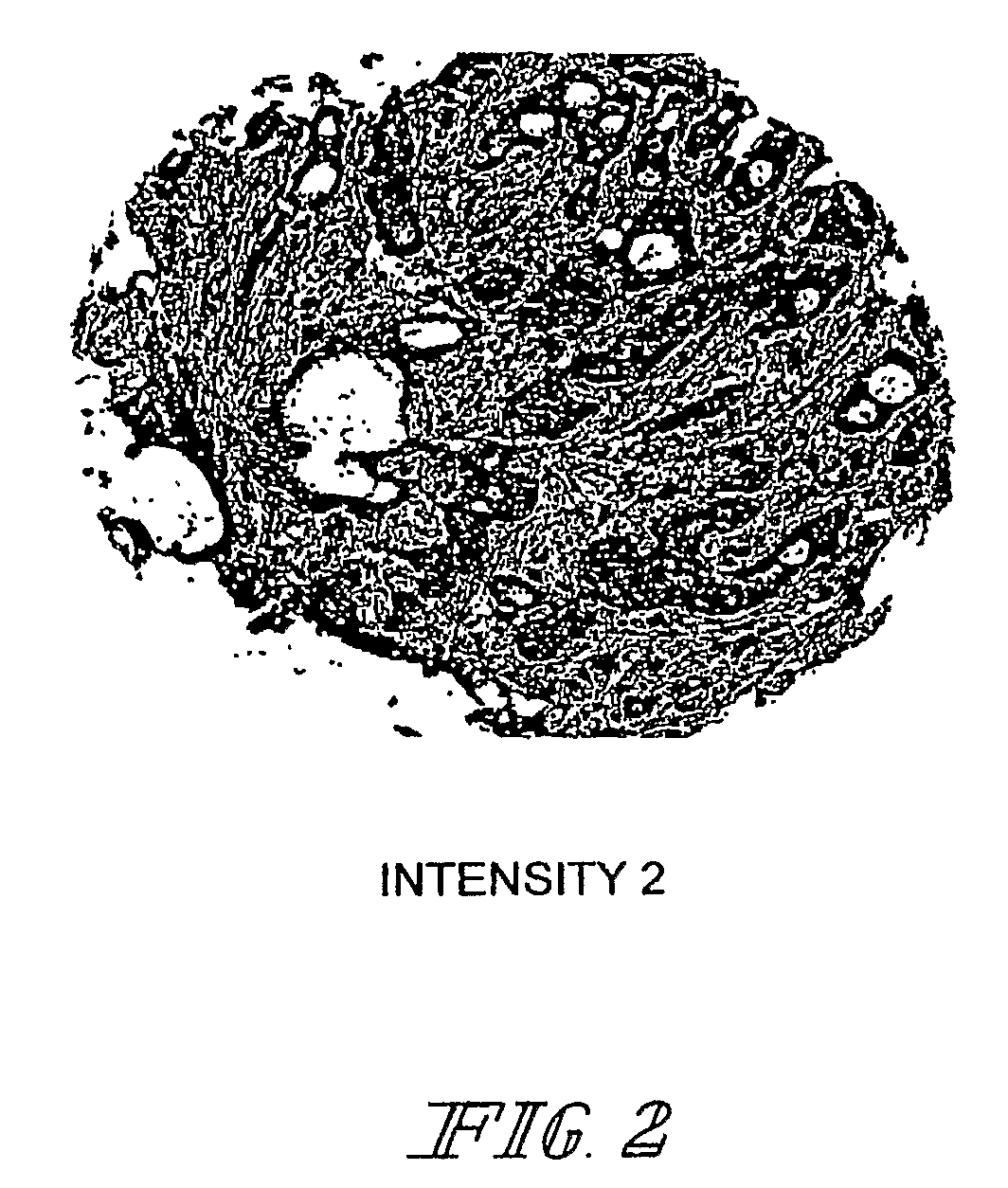
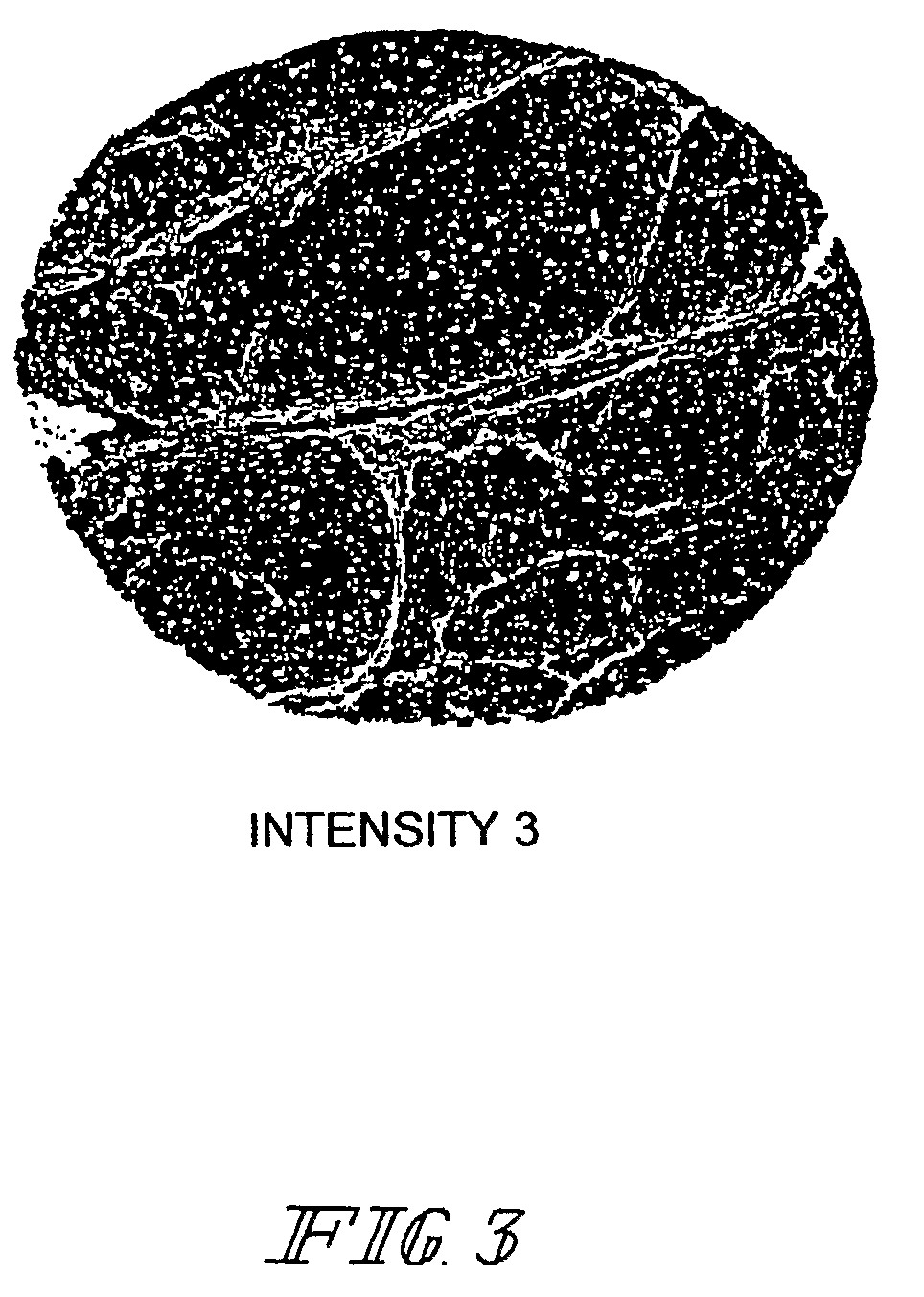
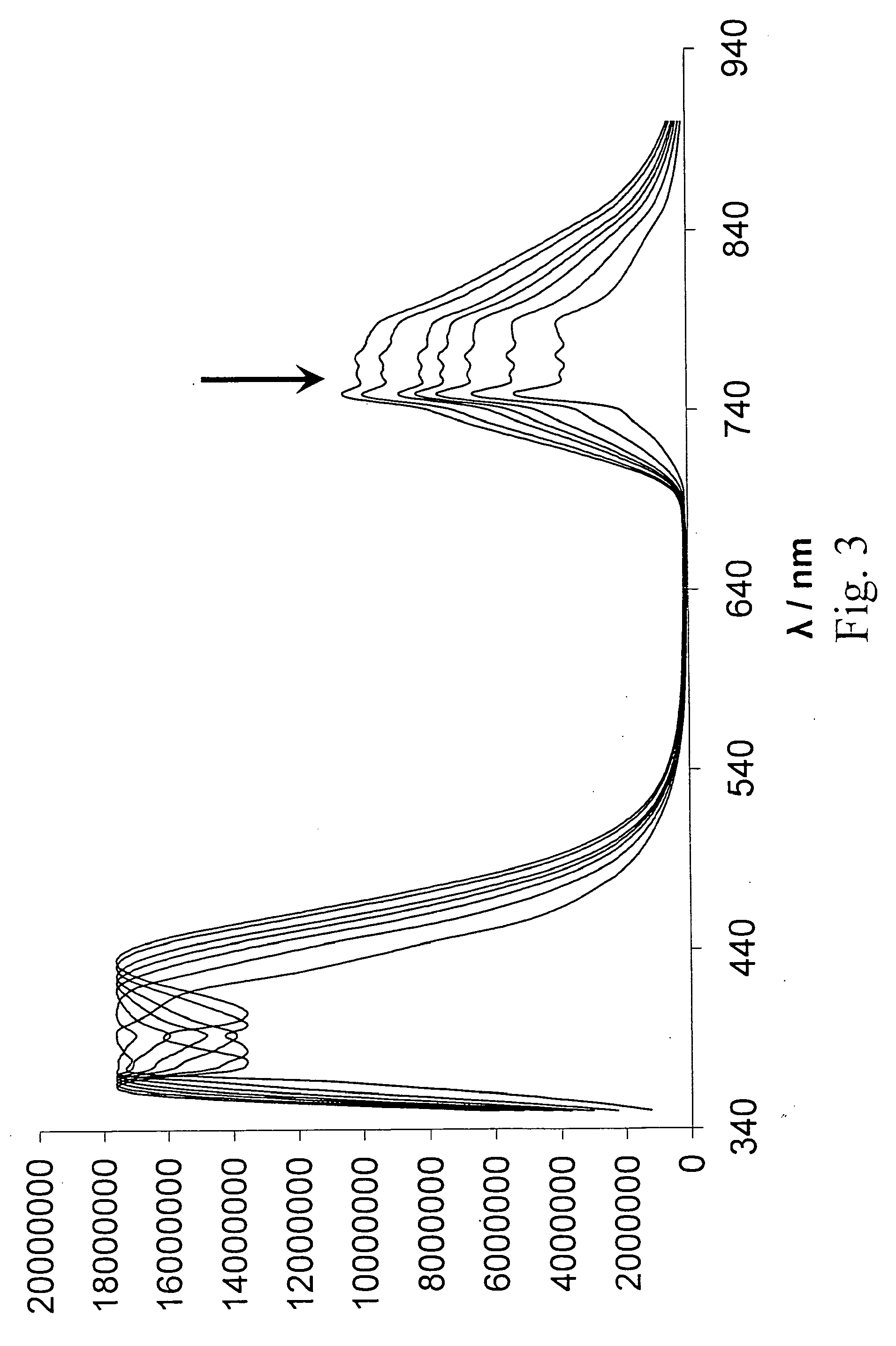
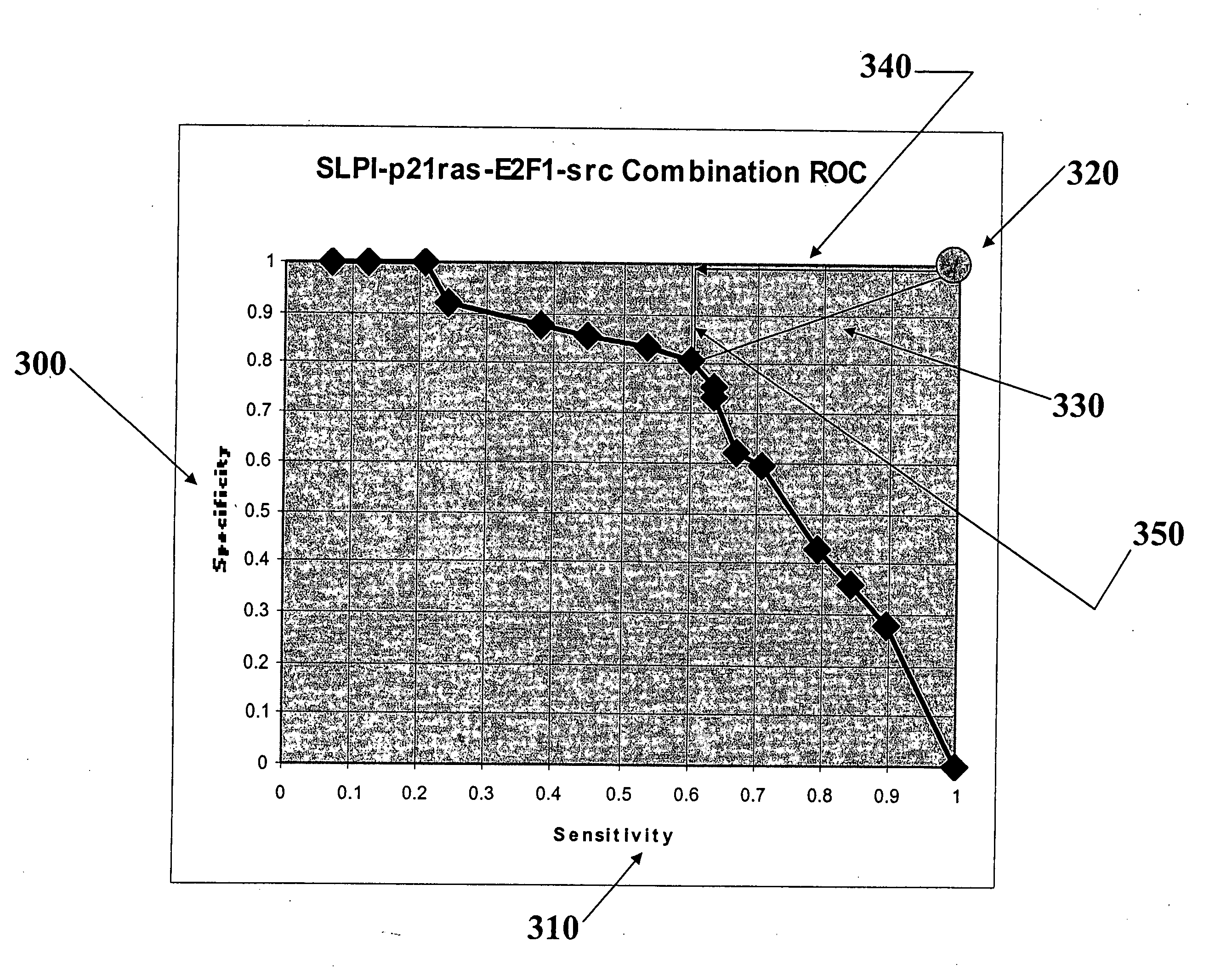
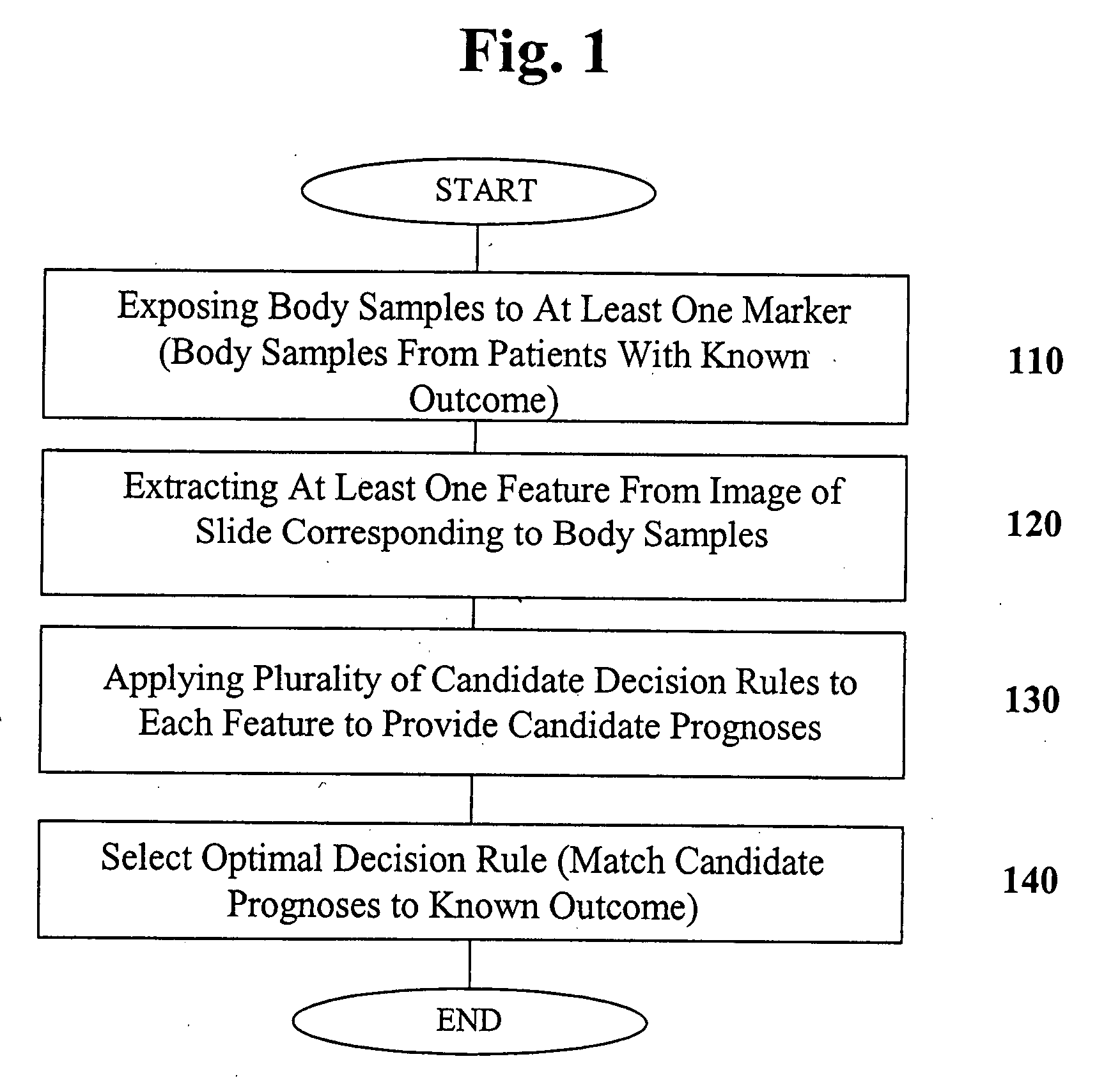
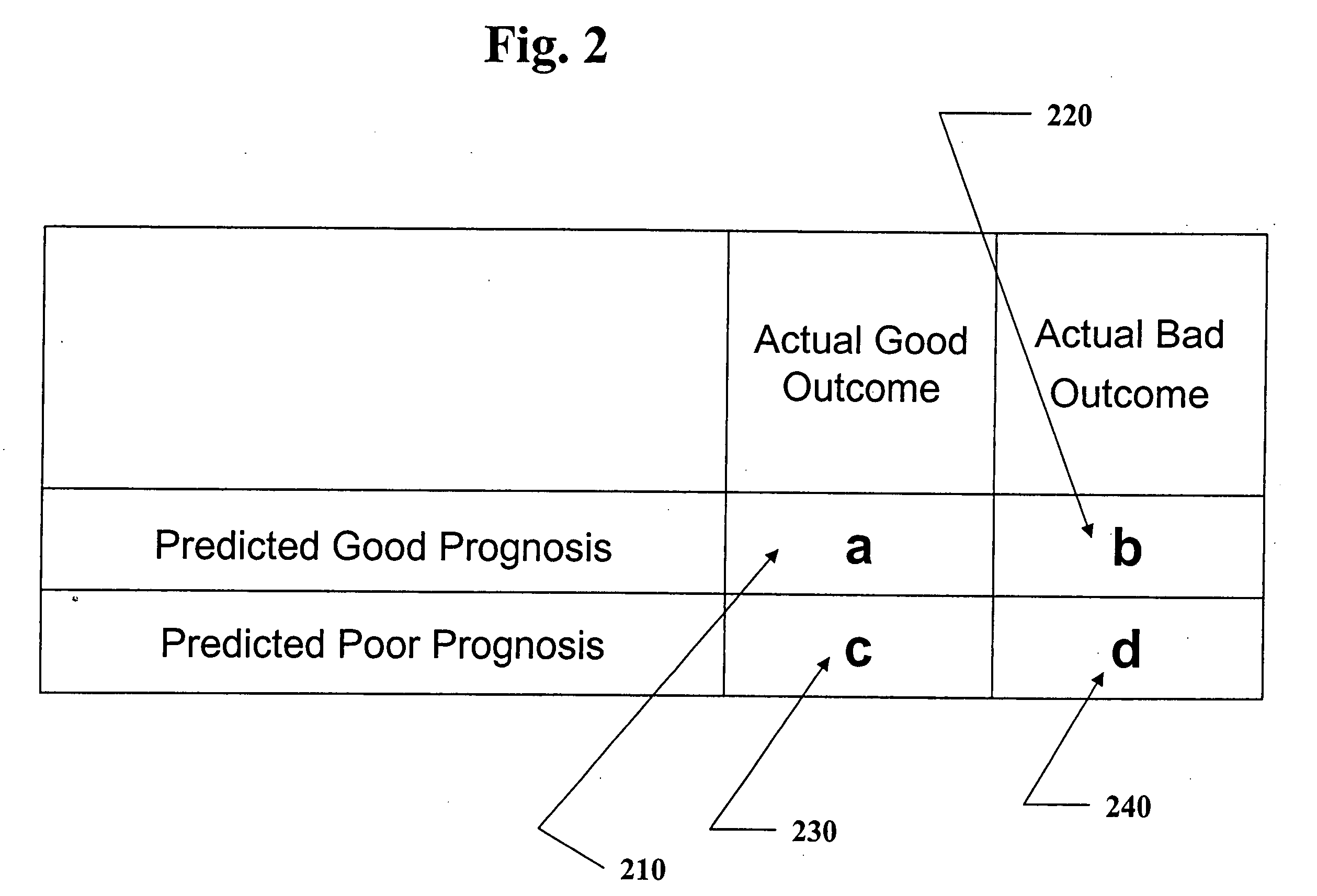
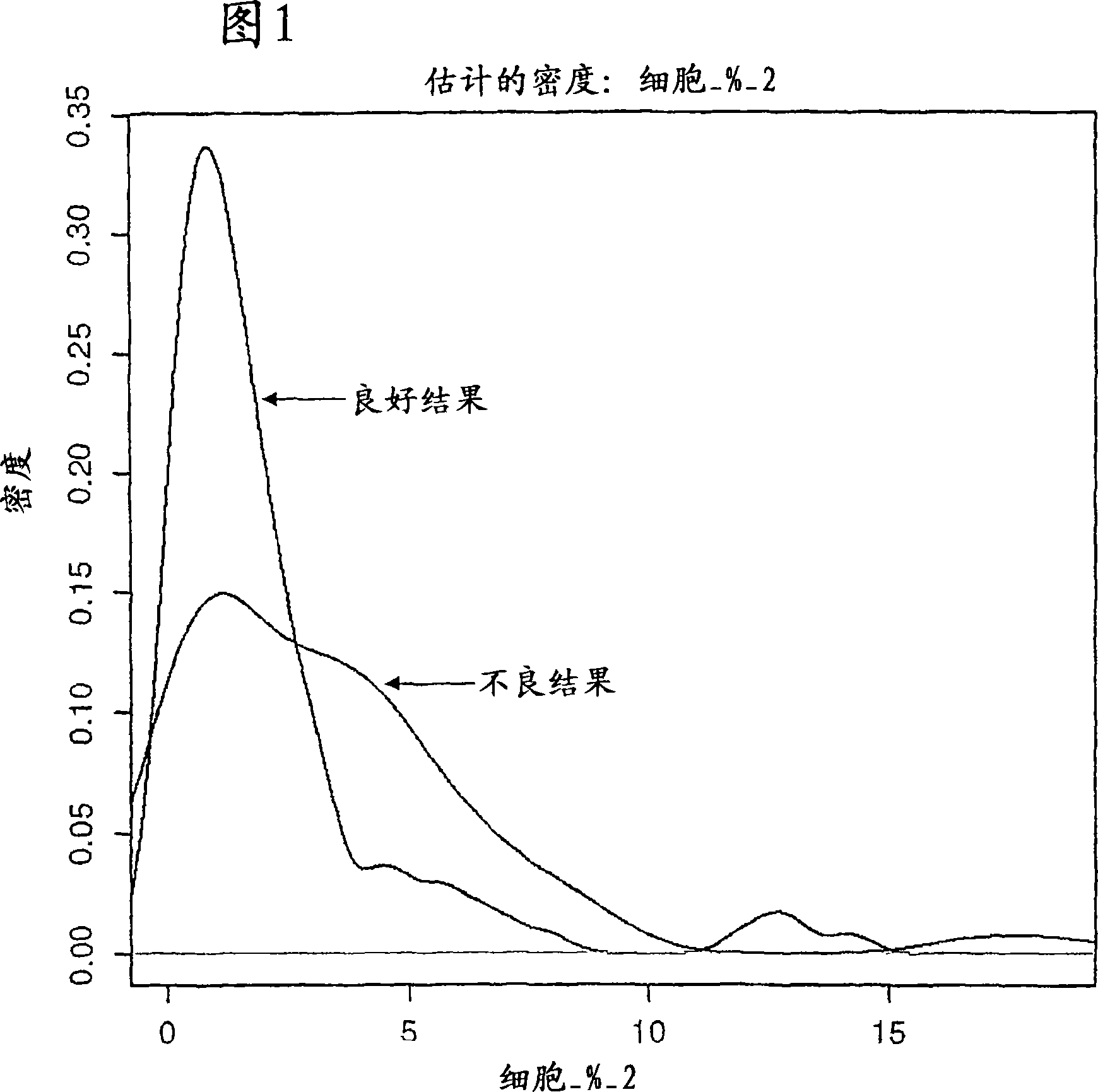
Methods and computer program products for evaluating and optimizing one or more markers for use in establishing a prognosis for a patient suffering from a disease are provided. More particularly, the methods include steps for systematically evaluating a number of features that may be extracted from an image of a body sample, such as a histological slide, that has been exposed to one or more biomarkers so as to establish a prognostic decision rule based on one or more of the extracted features such that the decision rule yields a prognosis that is optimally predictive of actual patient outcome. Thus, the methods and computer program products provided yield optimally predictive prognoses to assist clinicians in developing strategies for effective patient care management.
Owner:TRIPATH IMAGING INC
Protease assay
The present invention provides a diagnostic reagent or assay for assessing the activity of a protease in vivo or in vitro and methods of detecting the presence of a cancerous or precancerous cell. The assays are comprised of two particles linked via an oligopeptide linkage that comprises a consensus sequence specific for the target protease. Cleavage of the sequence by the target protease can be detected visually or using various sensors, and the diagnostic results can be correlated with cancer prognosis.
Owner:KANSAS STATE UNIV RES FOUND
Cancer therapy prognosis and target
InactiveUS20090123925A1Improve the level ofImprove responseMicrobiological testing/measurementProtein nucleotide librariesAnticarcinogenCancer therapy
The present invention relates to a gene and / or protein expression based method of predicting response to platinum based chemotherapy for lung cancer patients, as well as a method of predicting prognosis of survival based on protein expression and type of lung cancer. The invention also provides novel targets for screening candidate anti-cancer agents.
Owner:THE UNIV COURT OF THE UNIV OF ABERDEEN REGENT WALK +1
Gene signatures for cancer prognosis
Biomarkers and methods using the biomarkers for the prediction of the recurrence risk of cancer in a patient are provided.
Owner:MYRIAD GENETICS
Tumor marker colloidal gold immunochromatographic assay quantitative detection test paper and method for preparing same
InactiveCN101900733ARealize quantitative detectionEasy to operateMaterial analysisHuman tumorAntibody conjugate
The invention discloses tumor marker colloidal gold immunochromatographic assay quantitative detection test paper and a method for preparing the same. The test paper comprises a sample pad, a gold-labeled antibody conjugate film, a nitrocellulose membrane, a water absorbing cushion and a reaction support, wherein the nitrocellulose membrane of which the end close to the sample pad is used as a starting end and the end close to the water absorbing cushion is used as a tail end is enveloped by five antibody strips in turn, namely T1, T2, T3 and T4 detection strips of mouse anti-human tumor marker monoclonal antibody I and a C quality control strip of goat anti-mouse IgG respectively; and the gold-labeled antibody conjugate film is a film coated with a colloidal gold-labeled mouse anti-human tumor marker monoclonal antibody II. The test paper and the method realize rapid and accurate quantitative detection of the tumor marker and thus meet the requirement of rapid detection of cancer surveying and screening, family cancer prognosis monitoring and the like.
Owner:CENT SOUTH UNIV
Gene-based algorithmic cancer prognosis
InactiveUS20080275652A1Guaranteed monitoring effectMicrobiological testing/measurementLibrary screeningAbnormal tissue growthTumor Sample
Owner:UNIV LIBRE DE BRUXELIES
Reagents and methods for cancer prognosis and pathological staging
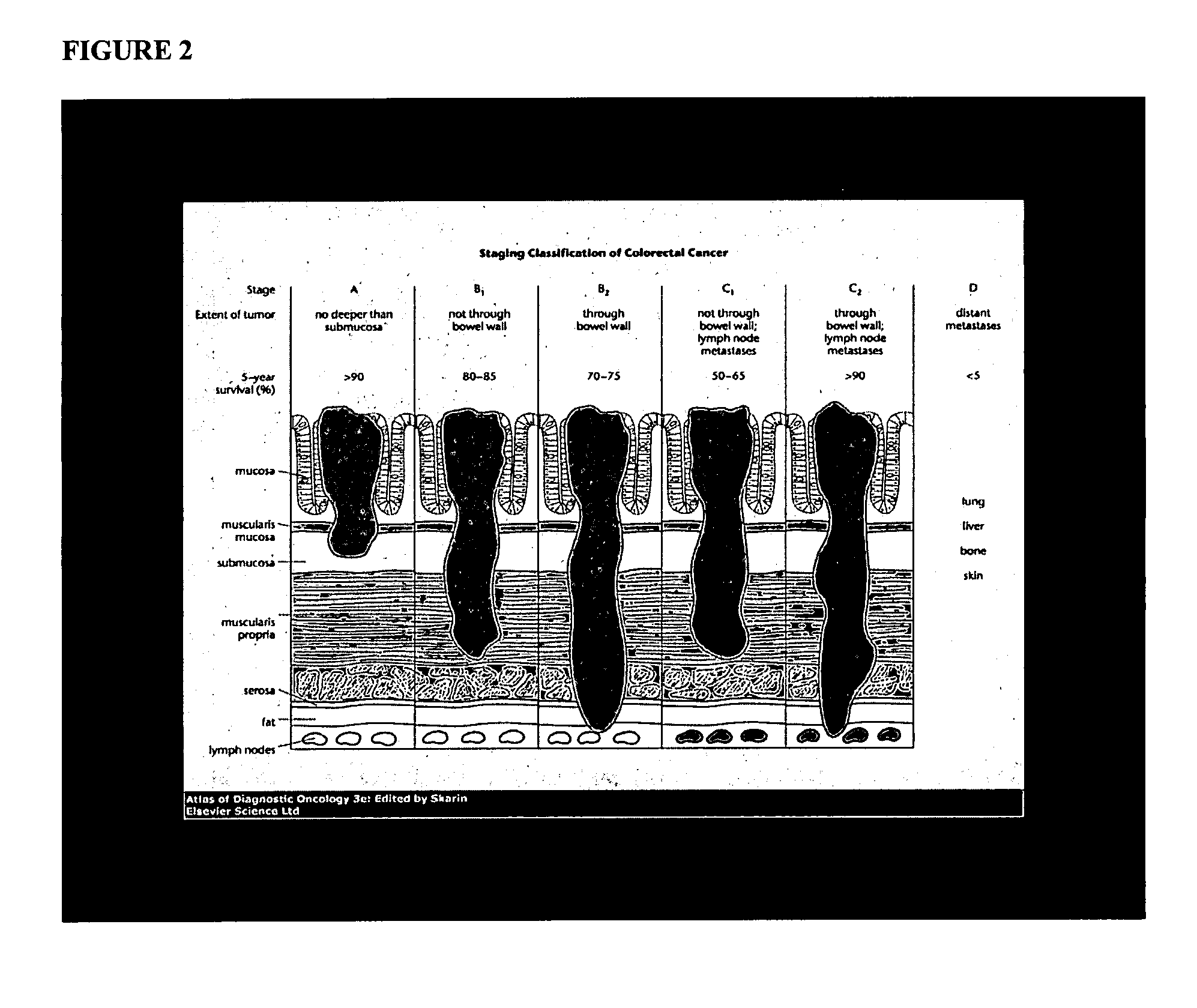
InactiveUS20070207489A1Microbiological testing/measurementMaterial analysisTumor progressionTumor cells
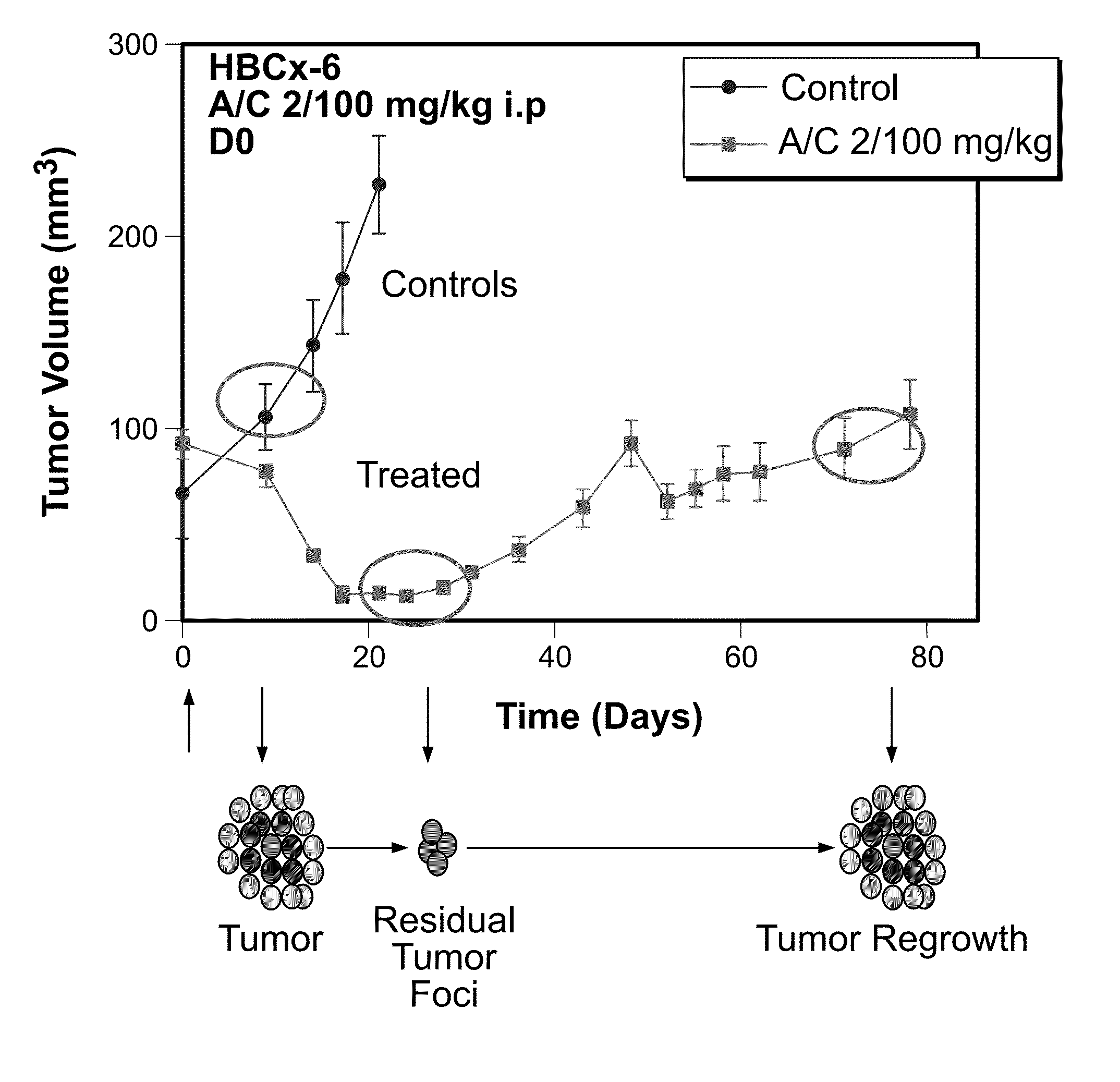
This invention provides reagents and methods for assessing tumor progression using tissue or tumor cell-containing samples from an individual. The invention also provides reagents and methods for assessing response to chemotherapy.
Owner:VENTANA MEDICAL SYST INC
Marker for predicting gastric cancer prognosis and method for predicting gastric cancer prognosis using the same
ActiveUS20130337449A1Improve survival rateAccurate predictionMicrobiological testing/measurementDisease diagnosisStage II Gastric CancerProper treatment
The present invention relates to a marker for predicting a gastric cancer prognosis, a composition and a kit for predicting gastric cancer prognosis comprising an agent for measuring the expression level thereof, and a method for predicting gastric cancer prognosis using the marker. According to the present invention, gastric cancer prognosis may be predicted promptly and accurately, and an appropriate treatment plan can be determined based on the predicted prognosis, which has an advantage of contributing to significant reduction of death caused by gastric cancer. Particularly, according to the present invention, the survival rate can be remarkably increased by using the treatment method for a stage III gastric cancer patient to a patient who has been predicted to have a negative prognosis among stage Ib / II gastric cancer patients.
Owner:SAMSUNG LIFE PUBLIC WELFARE FOUND
Methods and compositions for evaluating breast cancer prognosis
The invention provides a method and a composition used for evaluating patients with breast cancer, particularly the prognosis of the patients with the breast cancer at an early stage. The method of the invention comprises the detection of at least one of body samples, particularly the expression of at least two biological markers, wherein, the over-expression of the biological markers or the combination of the biological markers indicates the prognosis of the breast cancer. The body samples in a plurality of implementation proposals are the mammary tissue samples, particularly the primary breast tumor samples. The biological markers of the invention are proteins and / or genes, and the over-expression indicates the good or poor cancer prognosis. The concerned biological markers include the proteins and genes which participate in cell cycle regulation, DNA replication, transcription, signal transduction, cell proliferation, cell invasion, protein hydrolysis or transfer. The biological marker-specific antibody is used on the protein level or the nucleic acid hybridization technology is used on the nucleic acid level for detecting the over-expression of the concerned biological markers in certain aspects of the invention.
Owner:TRIPATH IMAGING INC
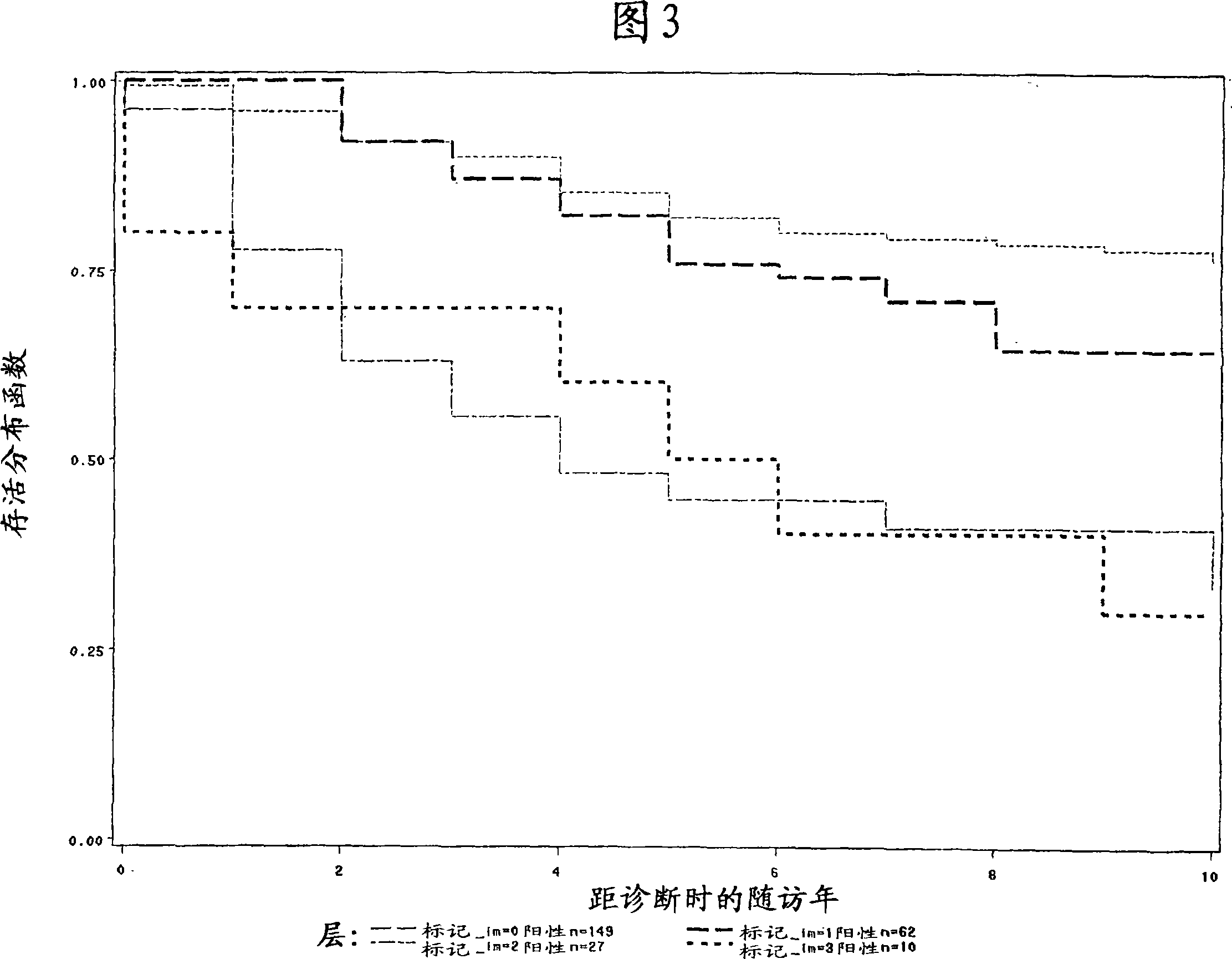
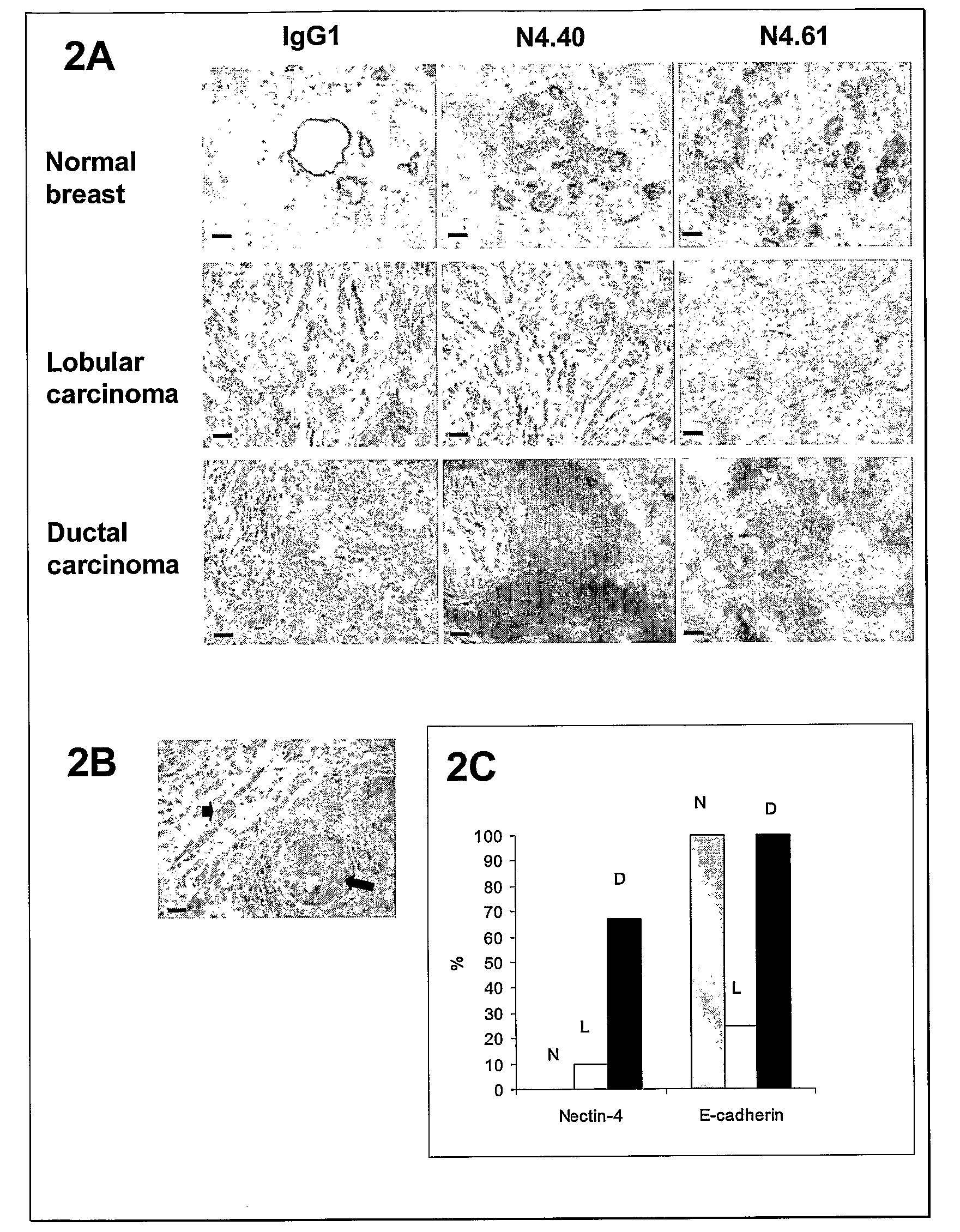
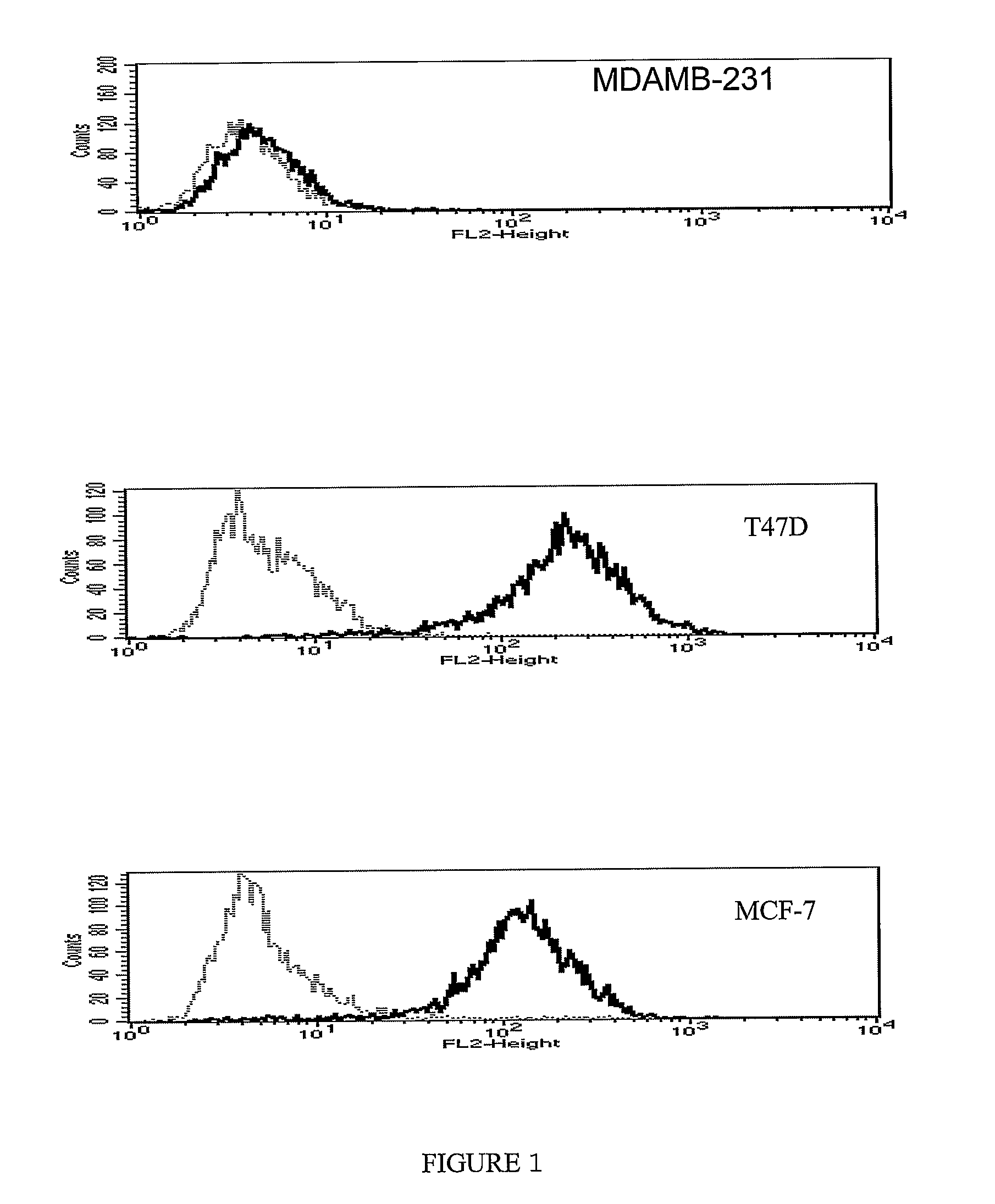
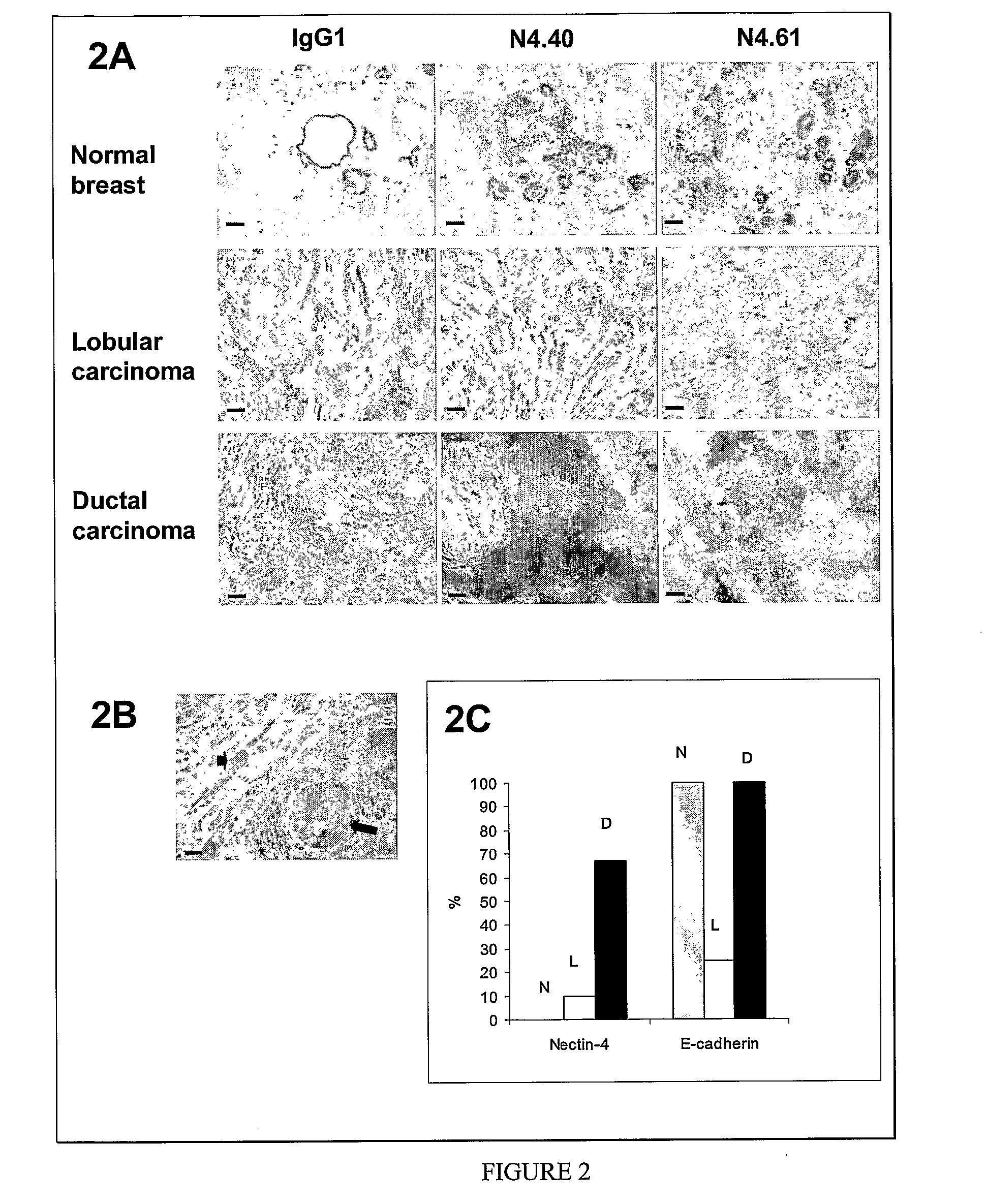
Nectin 4 (N4) as a Marker for Cancer Prognosis
InactiveUS20080268476A1Rapidly characterizing the prevalence and prognostic significanceQuick analysisConnective tissue peptidesMicrobiological testing/measurementNectinOncology
The present invention relate for a method for prognosis cancer, in particular metastatic breast cancer comprising doing a dosage of Nectin 4, in a soluble form or in transmembrane form, in a sample, the presence of Nectin 4 being indicative of a cancer.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Antibody-based arrays for detecting multiple signal transducers in rate circulating cells
The present invention provides antibody-based arrays for detecting the activation state and / or total amount of a plurality of signal transduction molecules in rare circulating cells and methods of use thereof for facilitating cancer prognosis and diagnosis and the design of personalized, targeted therapies.
Owner:SOC DES PROD NESTLE SA
Multiple PCR primer group and kit for detecting gene related to colorectal cancer administration
ActiveCN107400714AImprove efficiencyGuaranteed FeaturesMicrobiological testing/measurementDNA/RNA fragmentationCancer Early DiagnosisChemotherapeutic drugs
The invention discloses a multiple PCR primer group for detecting a gene related to colorectal cancer administration. The primer group comprises an upstream primer group and a downstream primer group, wherein the 5' end sequence of the upstream is complementary to partial sequence of a to-be-detected primer, and the 3' end of the upstream primer is a specific sequence combined to a target zone; the 5' end sequence of the downstream primer is complementary with partial sequence of the to-be-detected primer, and the 3' end of the downstream primer is a specific sequence combined to the target zone, and the sequence of the middle position of the downstream primer is a molecule tag sequence. The invention further discloses a kit containing the multiple PCR primer group and a method for detecting the gene related to colorectal cancer administration, which can be used for simultaneously detecting exon regions of 8 genes related to target medicines for treating colorectal cancer and mutation of 23 polymorphic sites related to chemotherapeutic drugs, can be directly used for assisting clinical colorectal cancer administration, and can be used in cancer early diagnosis, auxiliary diagnosis and screening or cancer prognosis monitoring.
Owner:GUANGZHOU FOREVERGEN BIOTECH CO LTD +1
Novel diagnostic and therapeutic targets associated with or regulated by n-cadherin expression and/or epithelial to mesenchymal transition (EMT) in prostate cancer and other malignancies
InactiveUS20130137584A1Microbiological testing/measurementLibrary member identificationProstate cancerOncology
Owner:RGT UNIV OF CALIFORNIA
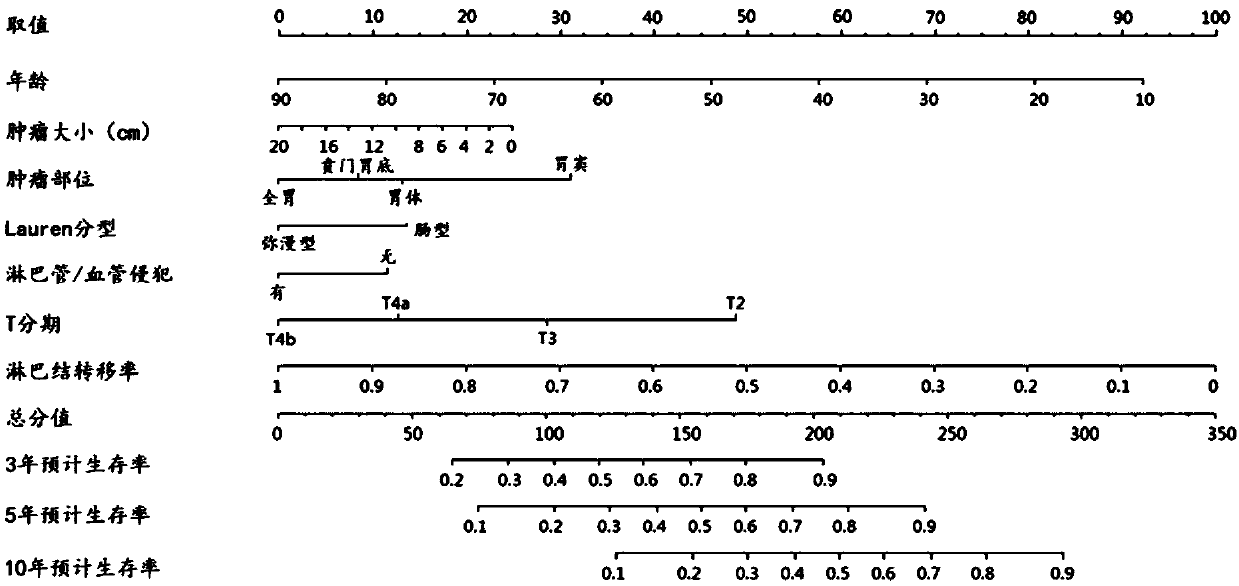
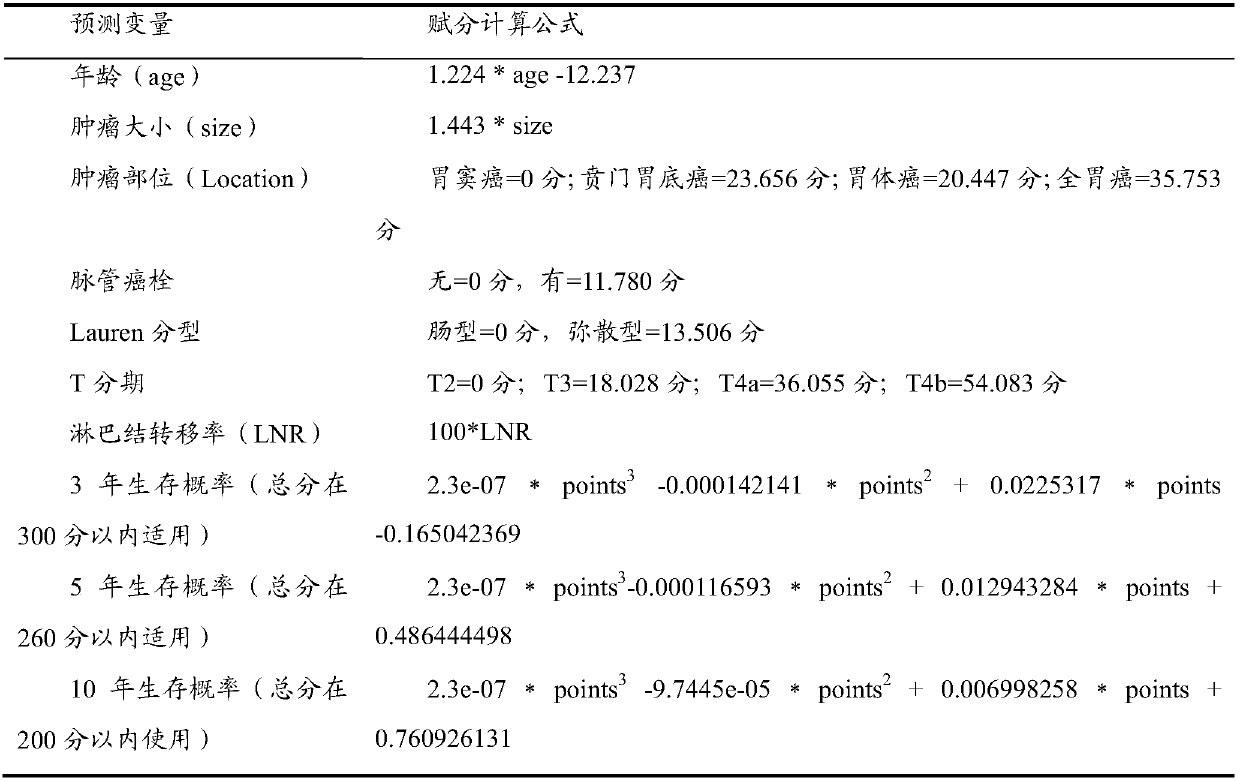
System for accurately predicting prognosis of patient suffering from stomach cancer
InactiveCN107563134AAvoid wastingCorrect understandingSpecial data processing applicationsTNM staging systemNomogram
The present invention provides a system for accurately predicting the prognosis of gastric cancer patients. The system is based on the Nomogram prognostic prediction model constructed by the present invention. Compared with the traditional TNM staging system, the system has high accuracy, individualized prediction, and conforms to Prognostic features of gastric cancer with Chinese characteristics.
Owner:SUN YAT SEN UNIV
Smart microarray cancer detection system
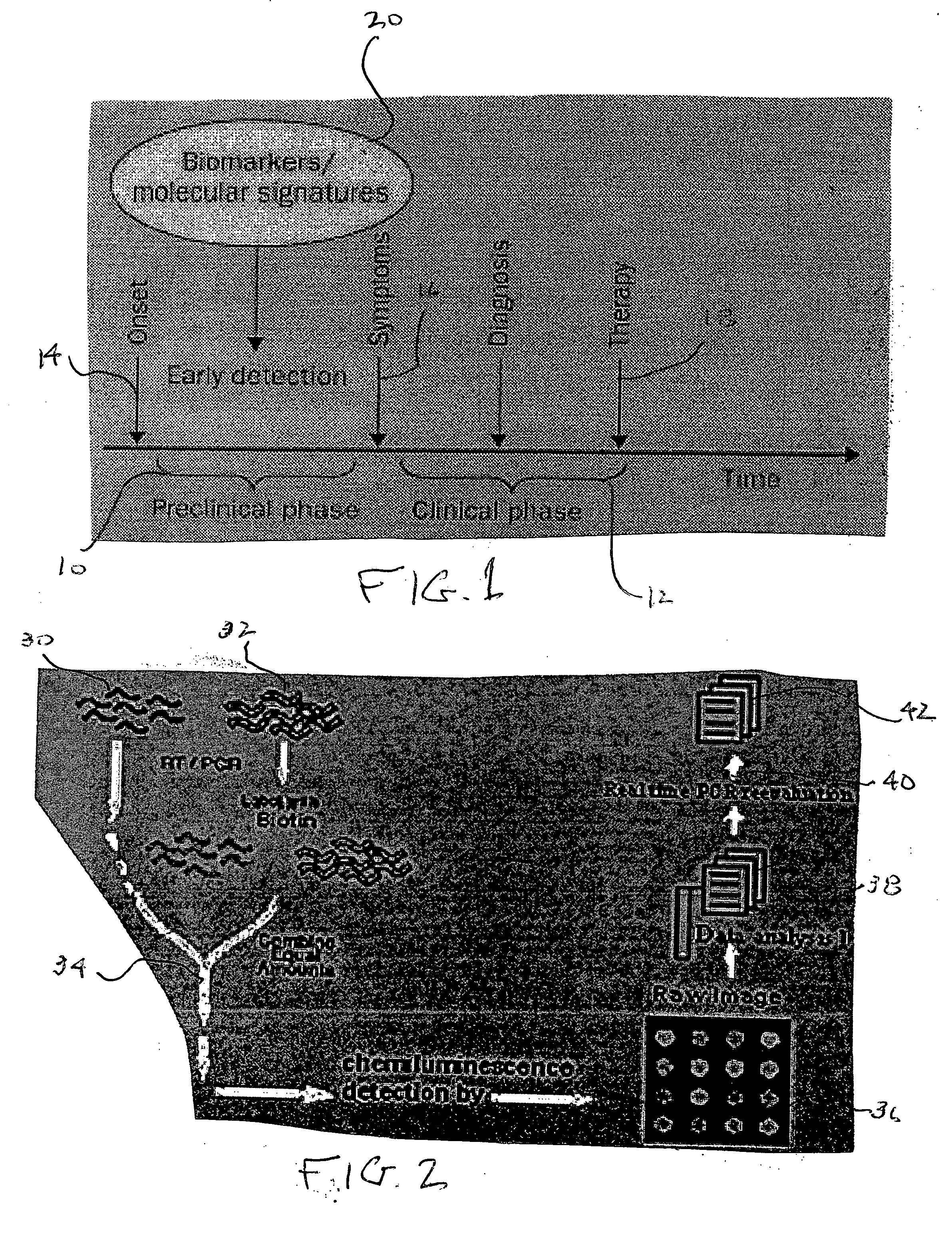
InactiveUS20060041387A1Sensitive highLow costMicrobiological testing/measurementBiological testingMedicineCancer detection
A smart microarray detection system for exploring and detecting cancer specific genes. A microarray is constructed using cancer specific genes that can detect, identify, quantify, and discriminate cancers in biological specimens. Data obtained from the microarray is analyzed with questionable signals reanalyzed with real time PCR. This information is then evaluated using software to determine a cancer prognosis.
Owner:SUN XIUMEI
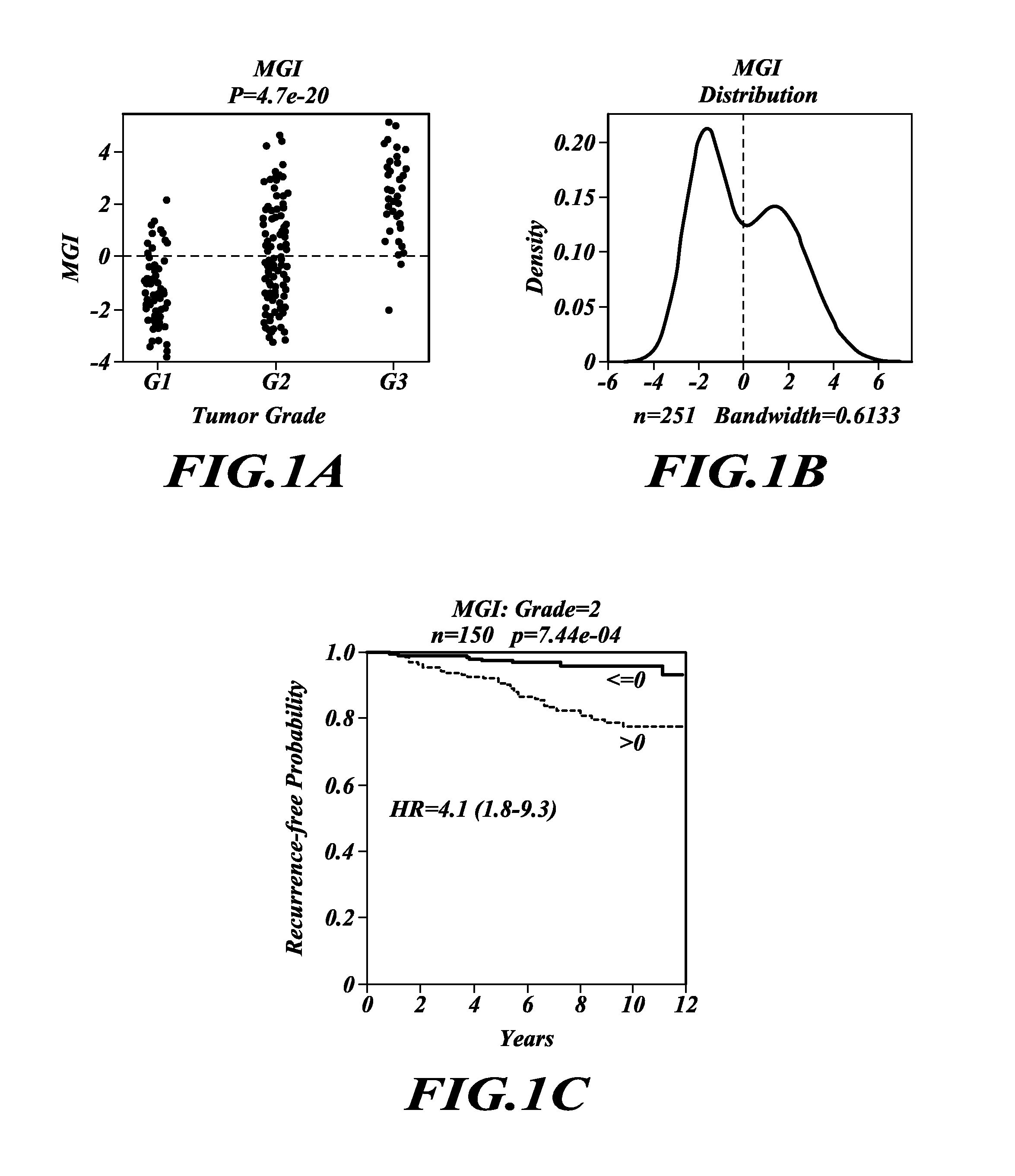
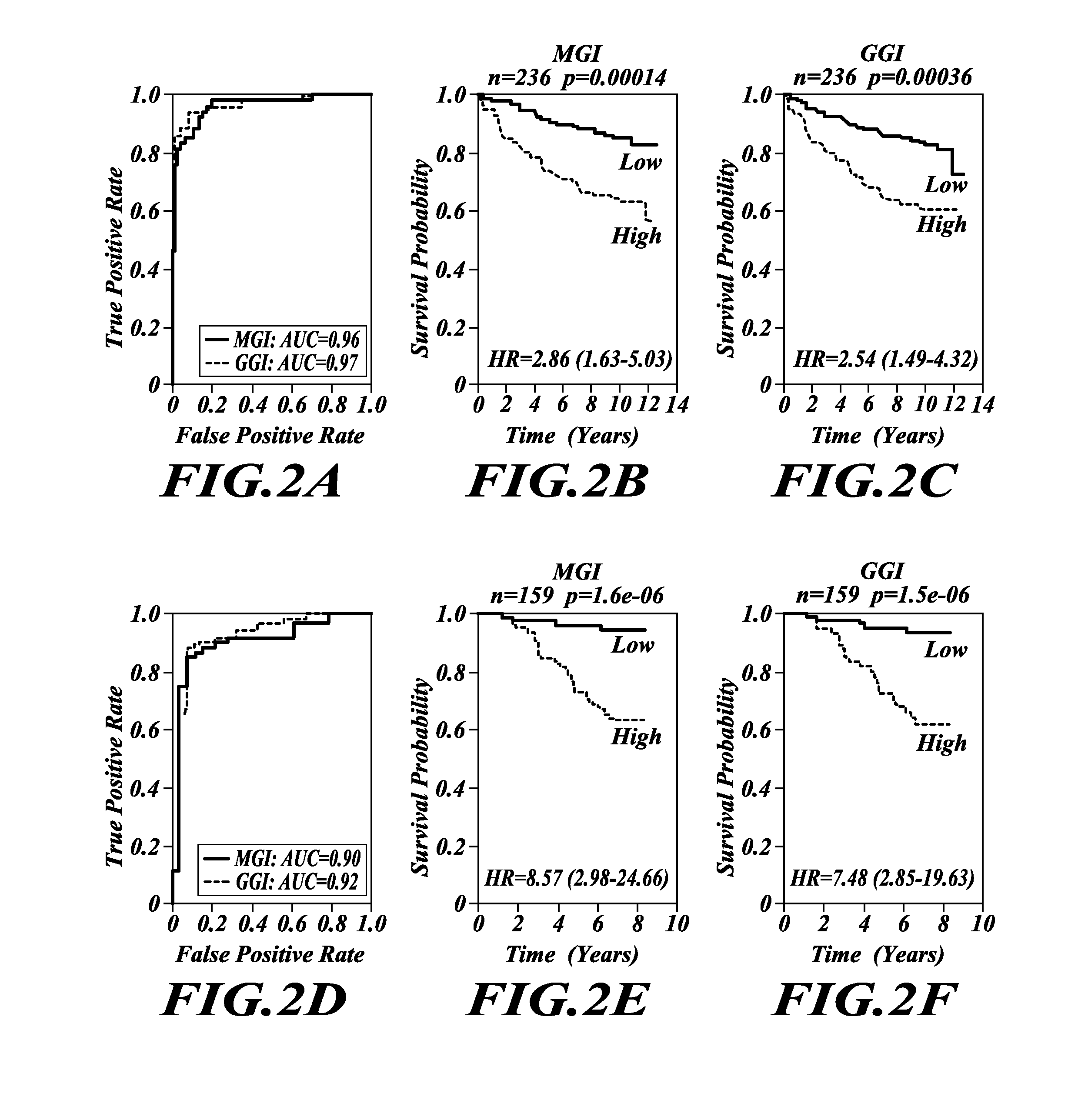
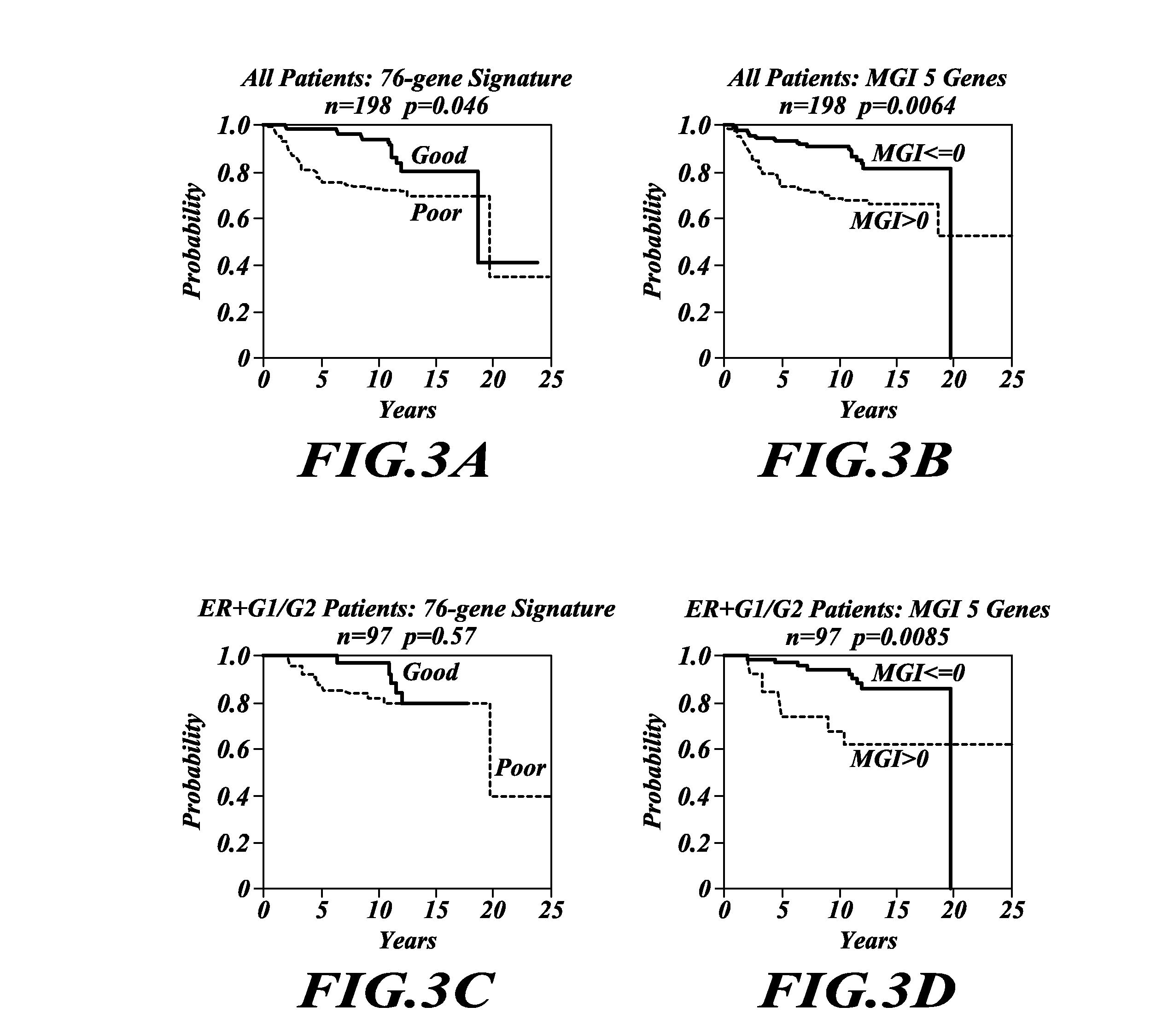
Tumor grading and cancer prognosis
ActiveUS20110136680A1Promote stratificationImproves cancer diagnosisMicrobiological testing/measurementLibrary screeningWho gradeCancer metastasis
The disclosure includes the identification and use of gene expression profiles, or patterns, with clinical relevance to cancer. In particular, the disclosure includes the identities of genes that are expressed in correlation with tumor grade. The levels of gene expression are disclosed as a molecular index for determining tumor grade in a patient and predicting clinical outcome, and so prognosis, for the patient. The molecular grading of cancer may optionally be used in combination with a second molecular index for diagnosing cancer and its prognosis. The disclosure further includes methods for predicting cancer recurrence, and / or predicting occurrence of metastatic cancer. For diagnosis or prognosis, the disclosure further includes methods for determining or selecting the treatment of cancer based upon the likelihood of life expectancy, cancer recurrence, and / or cancer metastasis.
Owner:THE GENERAL HOSPITAL CORP +1
Antibody-based arrays for detecting multiple signal transducers in rare circulating cells
Owner:SOC DES PROD NESTLE SA
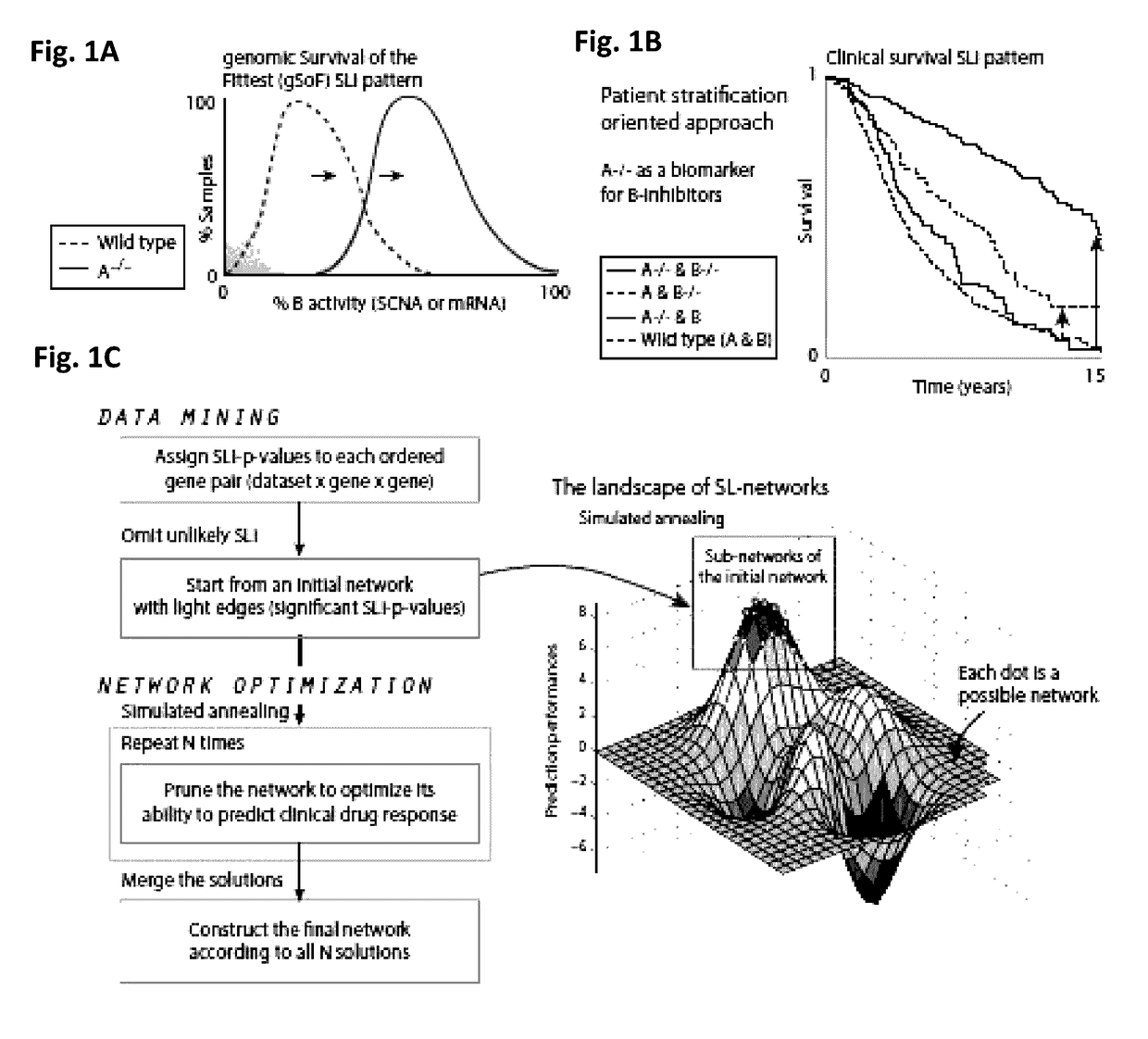
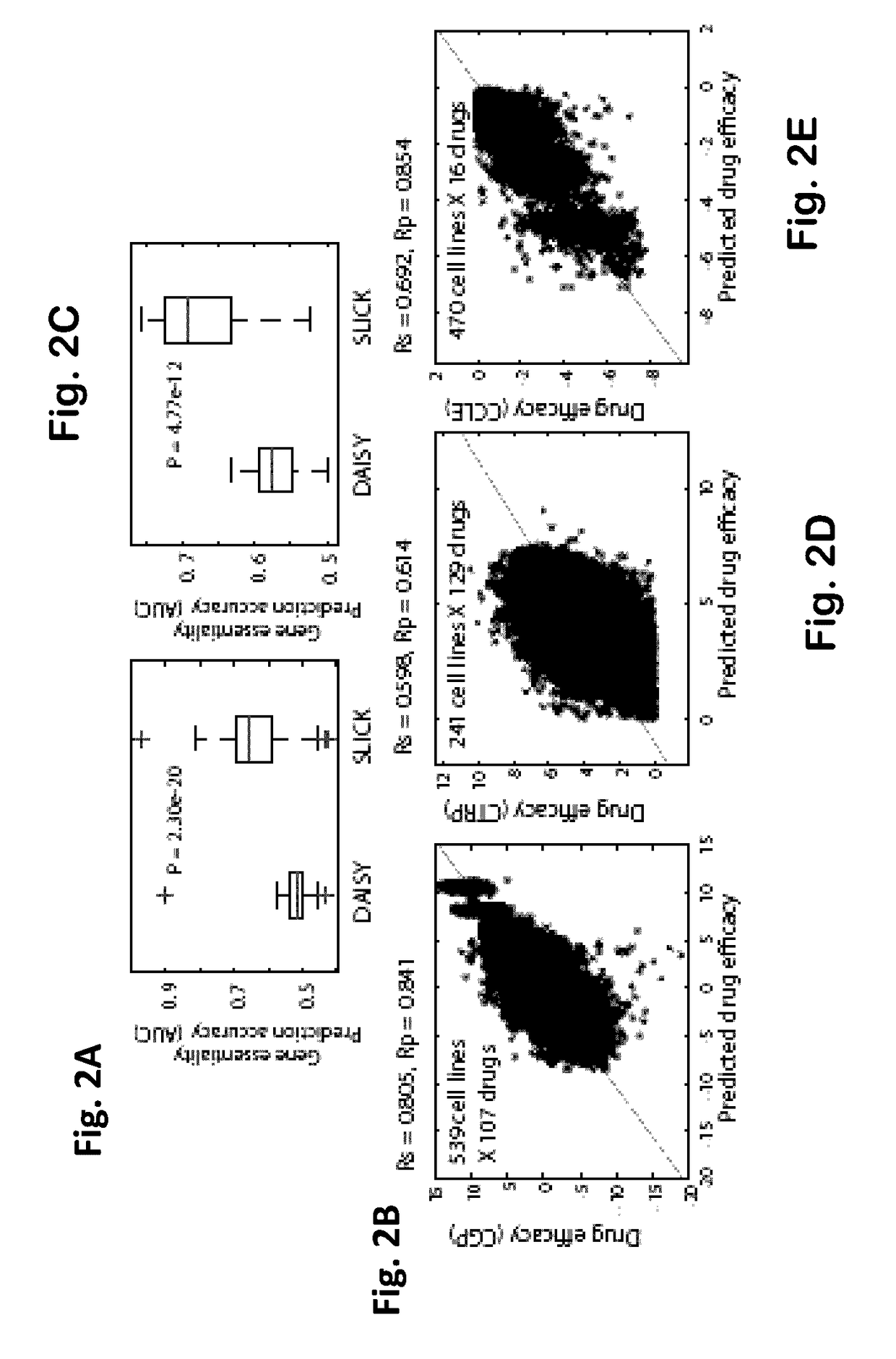
Clinically relevant synthetic lethality based method and system for cancer prognosis and therapy
InactiveUS20170154163A1Improve abilitiesAvoid problemsMedical simulationOrganic active ingredientsSynthetic lethalityCancer data
Systems and methods for identifying clinically relevant Synthetic Lethal interactions SLi by analyzing large and diverse cohorts of clinically relevant cancer data, utilizing a data driven approach, termed SLICK, are provided. Further provided are system and methods of utilizing SLICK to uncover therapeutic possibilities in cancer.
Owner:RAMOT AT TEL AVIV UNIV LTD
Molecular markers for cancer prognosis
InactiveUS20120053842A9Reduce overtreatmentMicrobiological testing/measurementBiostatisticsDiseaseSOX4
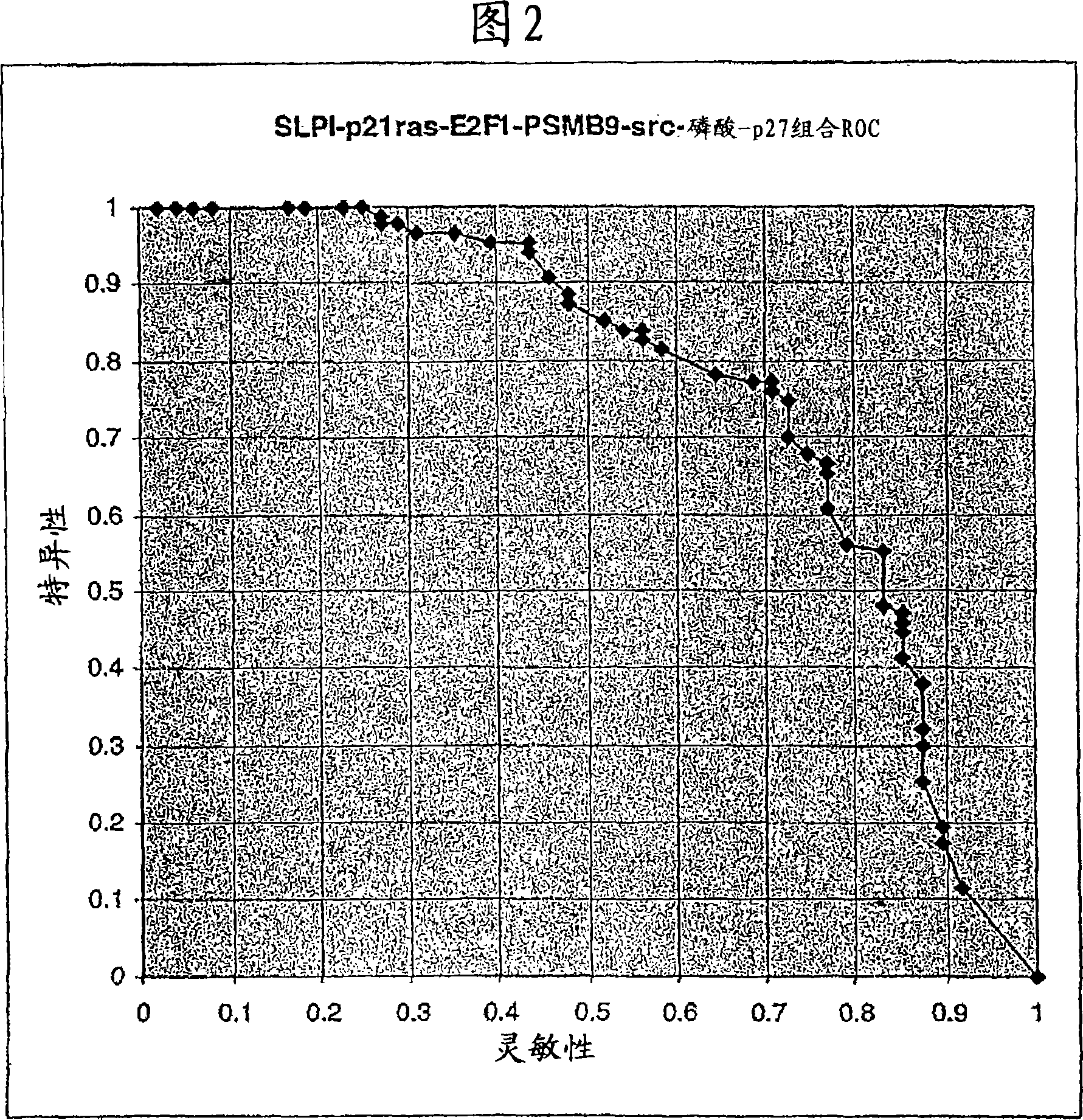
The present invention relates to methods for prediction of an outcome of neoplastic disease or cancer. More specifically, the present invention relates to a method for the prediction of breast cancer by determining in a biological sample from said patient an expression level of a plurality of genes selected from the group consisting of ACTG1, CA12, CALM2, CCND1, CHPT1, CLEC2B, CTSB, CXCL13, DCN, DHRS2, EIF4B, ERBB2, ESR1, FBXO28, GABRP, GAPDH, H2AFZ, IGFBP3, IGHG1, IGKC, KCTD3, KIAA0101, KRT17, MLPH, MMP1, NAT1, NEK2, NR2F2, OAZ1, PCNA, PDLIM5, PGR, PPIA, PRC1, RACGAP1, RPL37A, SOX4, TOP2A, UBE2C and VEGF.
Owner:SIVIDON DIAGNOSTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com