Patents
Literature
48 results about "Expression Signature" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A gene signature or gene expression signature is a single or combined group of genes in a cell with a uniquely characteristic pattern of gene expression that occurs as a result of an altered or unaltered biological process or pathogenic medical condition.
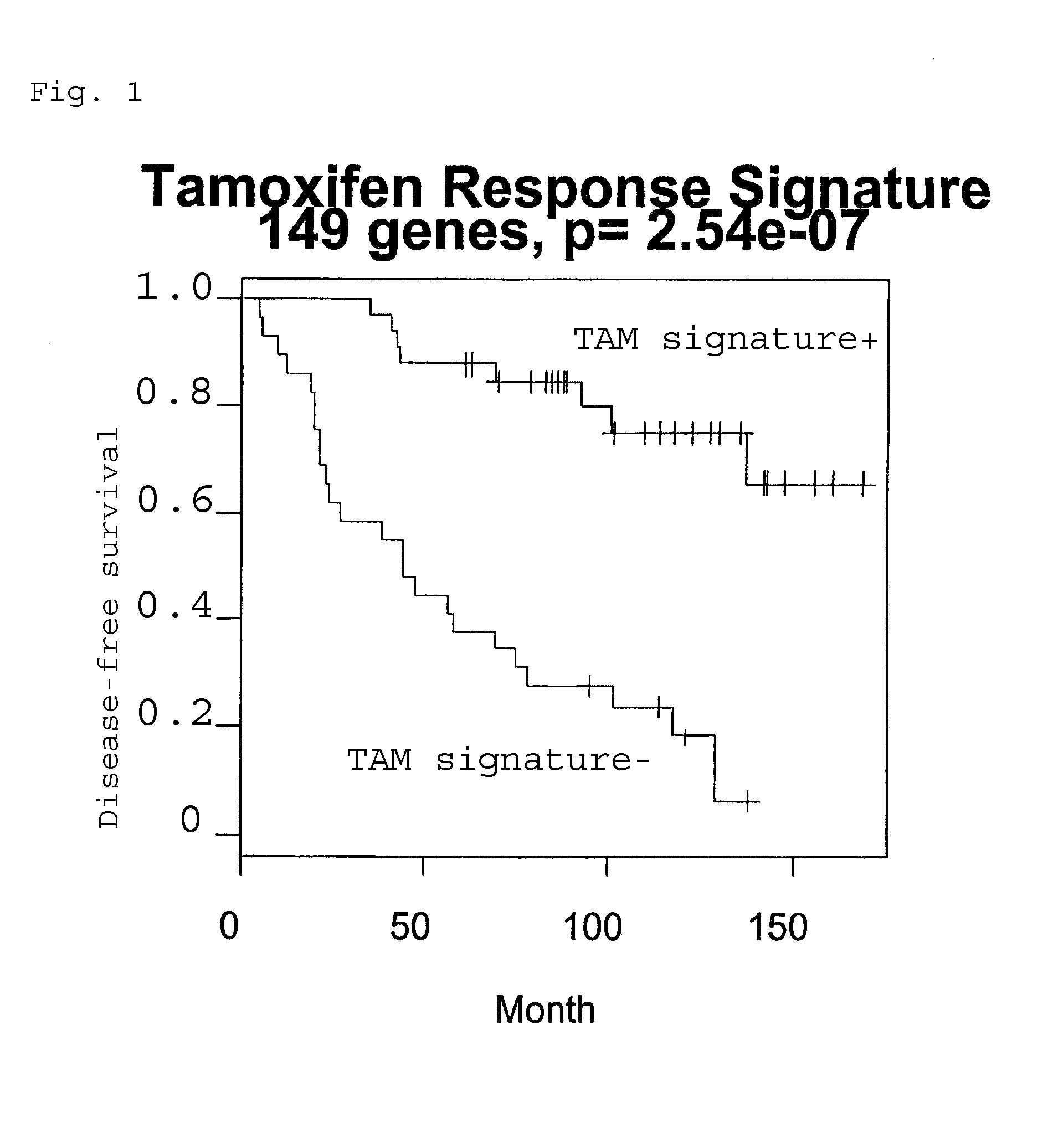
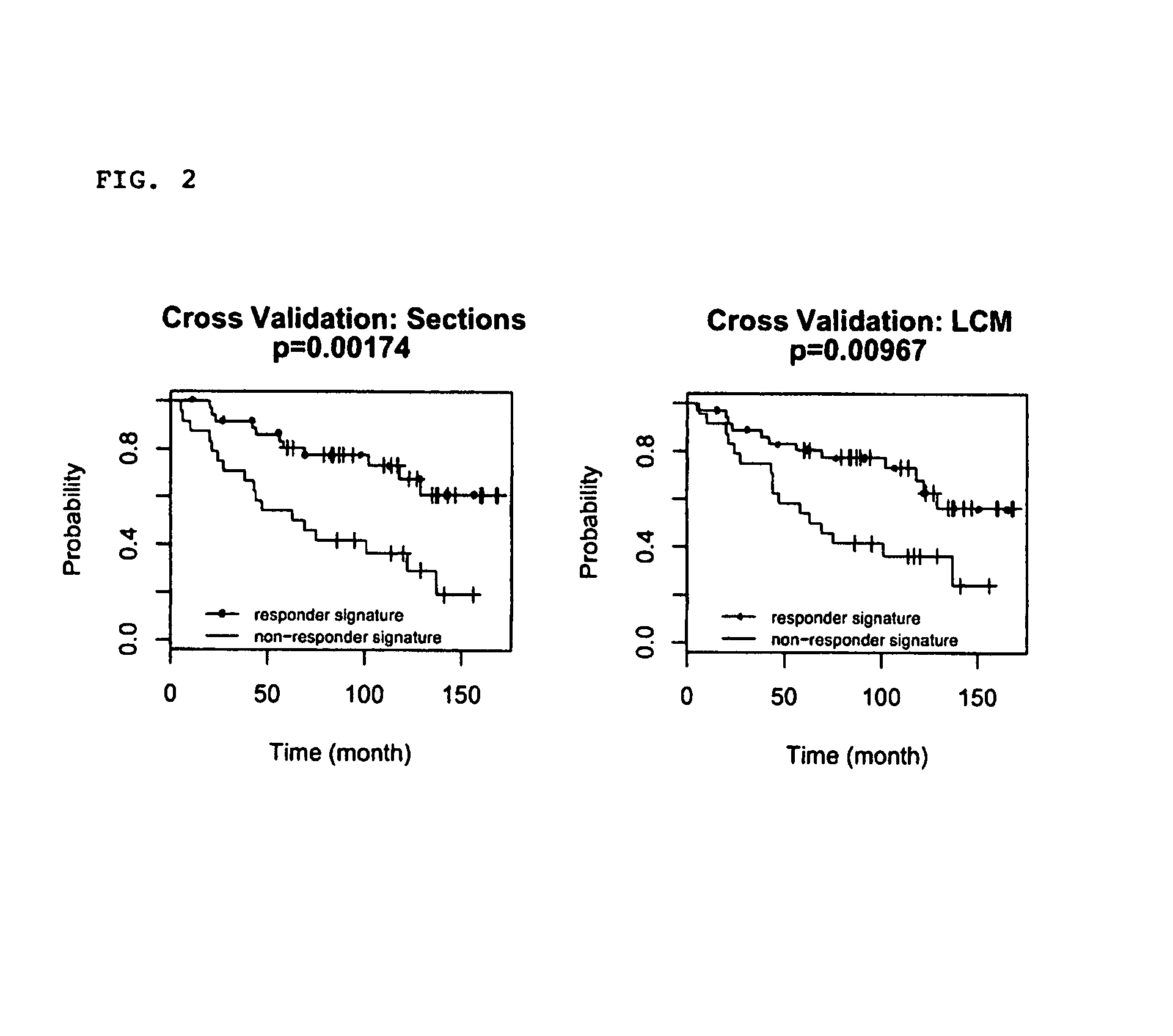
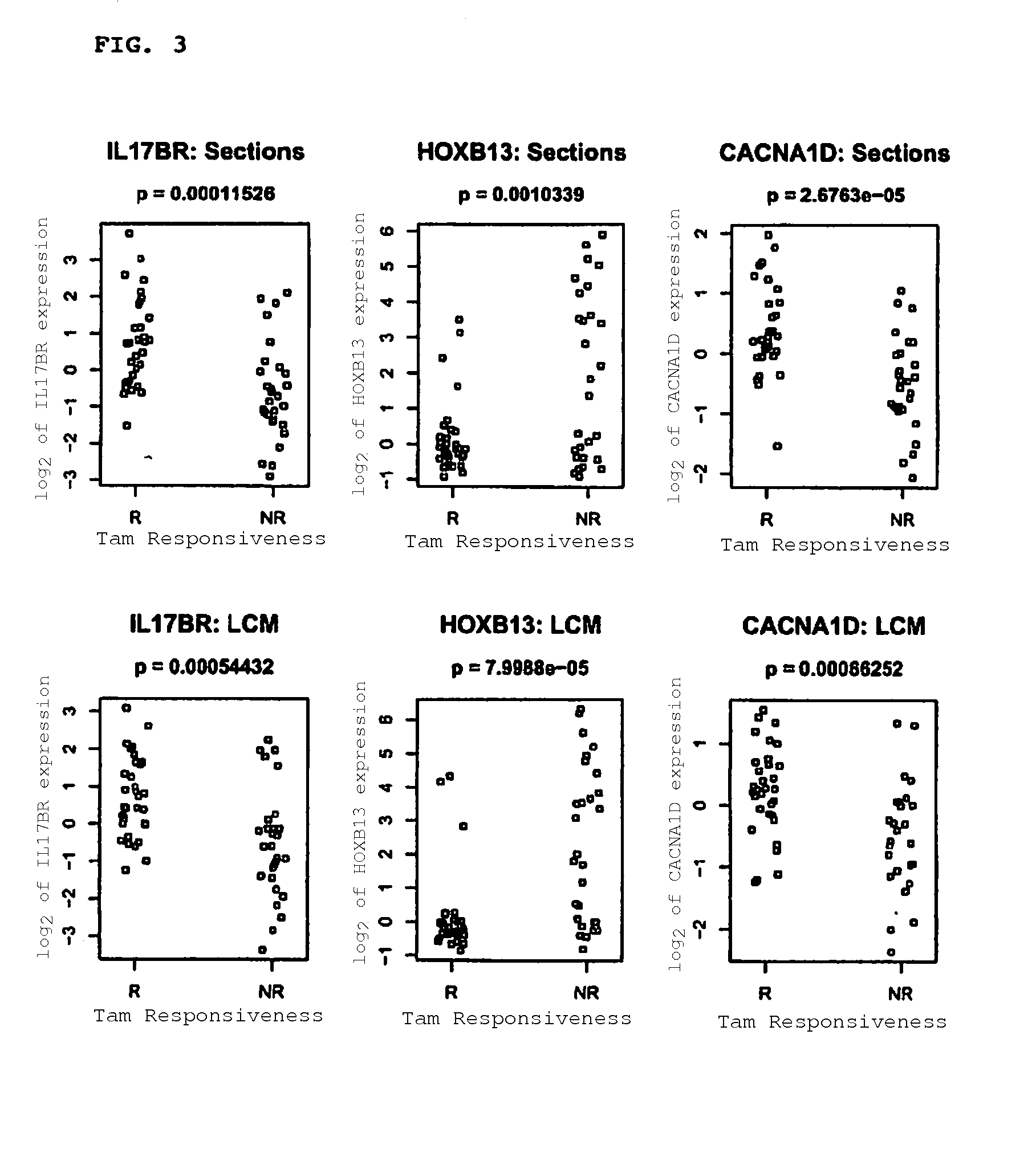
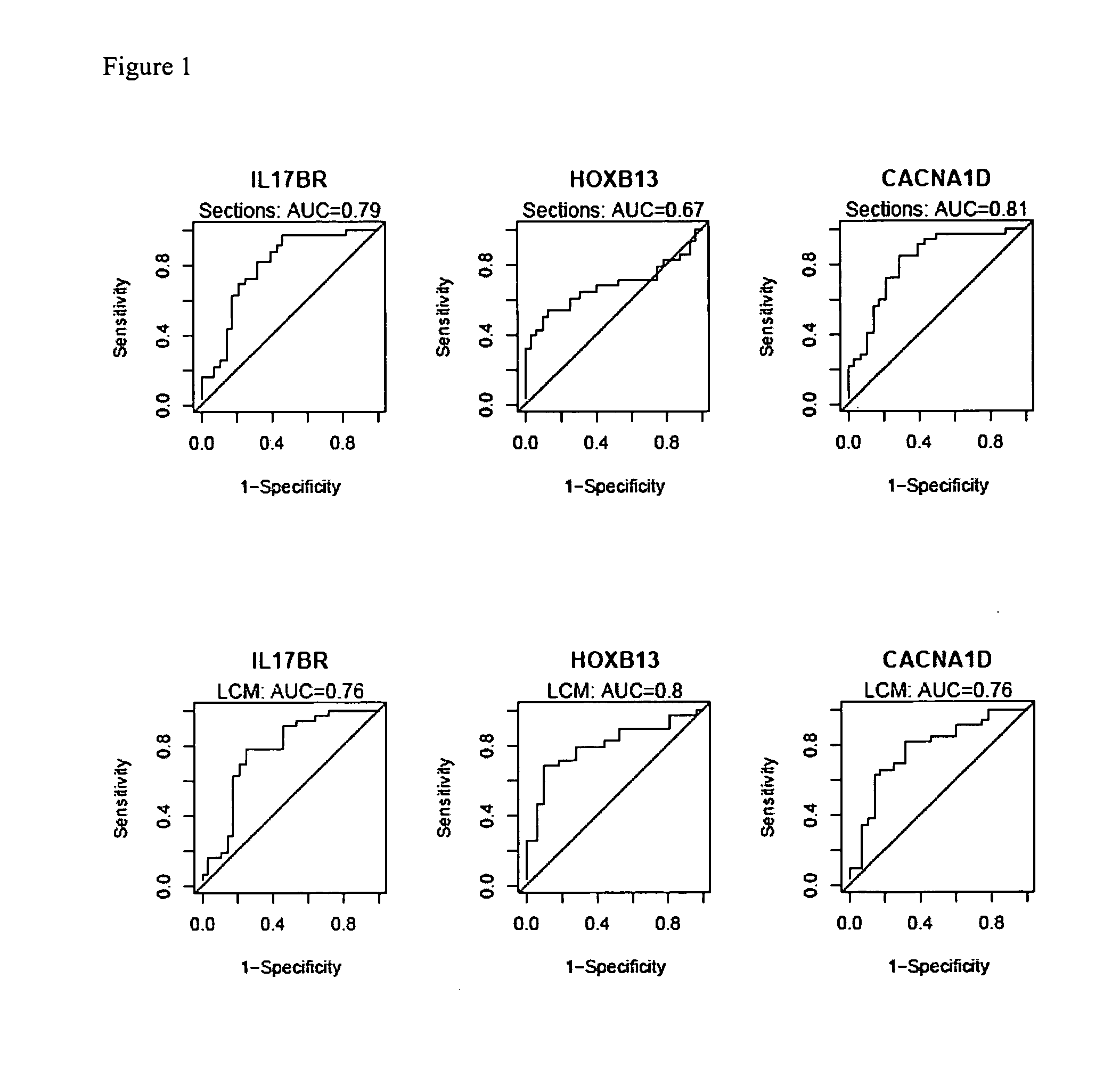
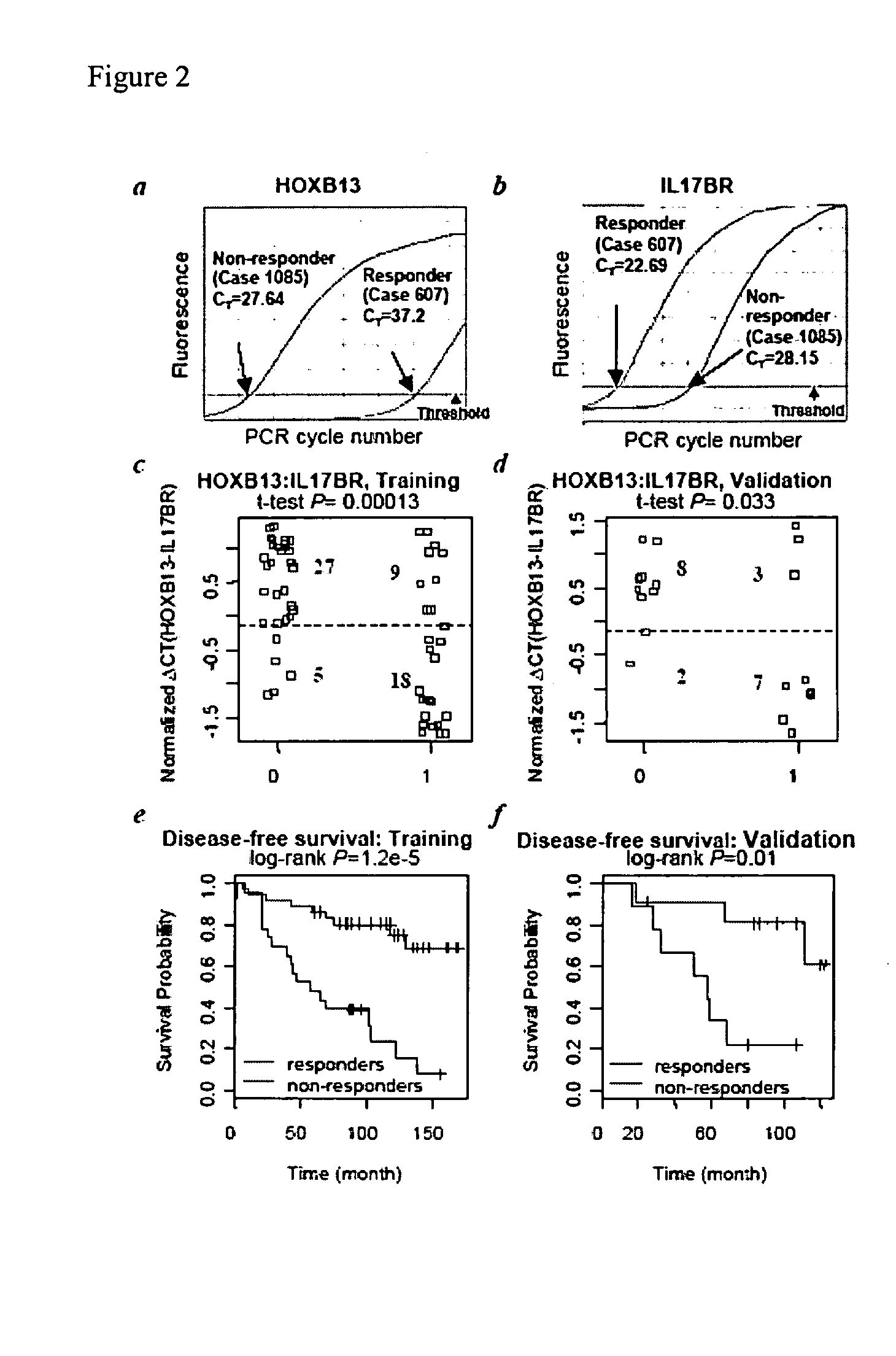
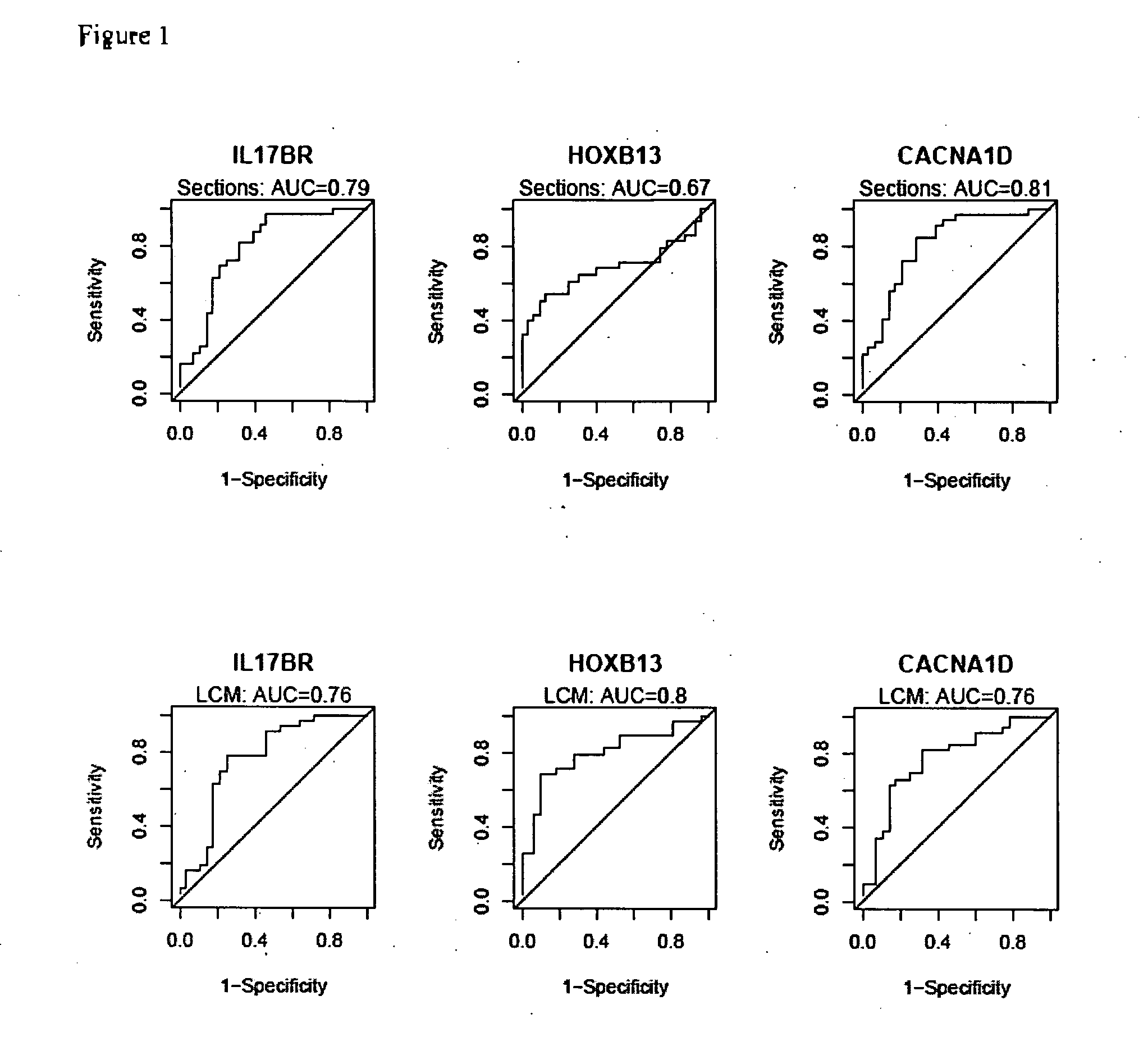
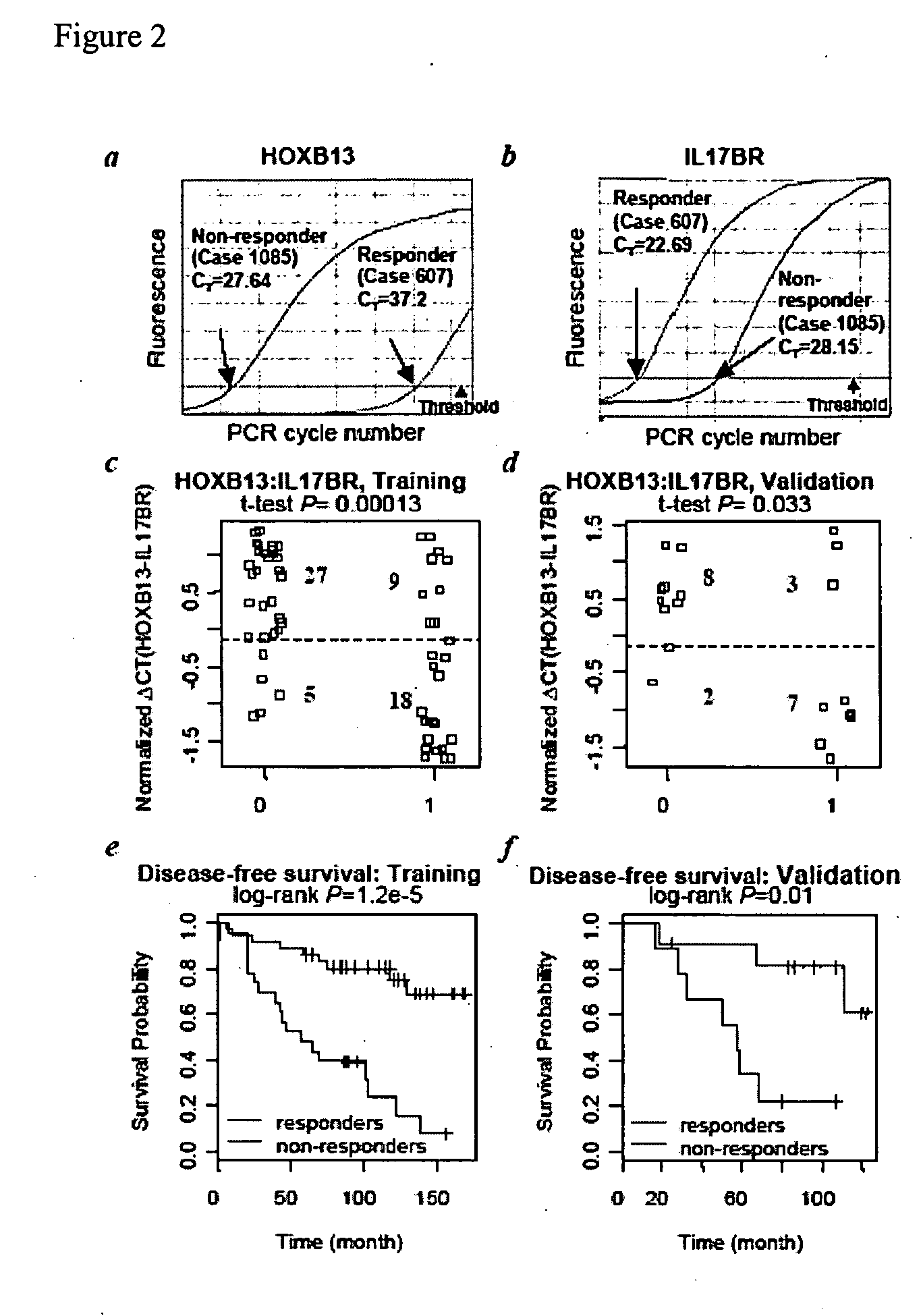
Predicting outcome with tamoxifen in breast cancer
ActiveUS7504214B2Improve predictive performanceBioreactor/fermenter combinationsBiological substance pretreatmentsTamoxifen treatmentOncology
Owner:BIOTHERANOSTICS +1
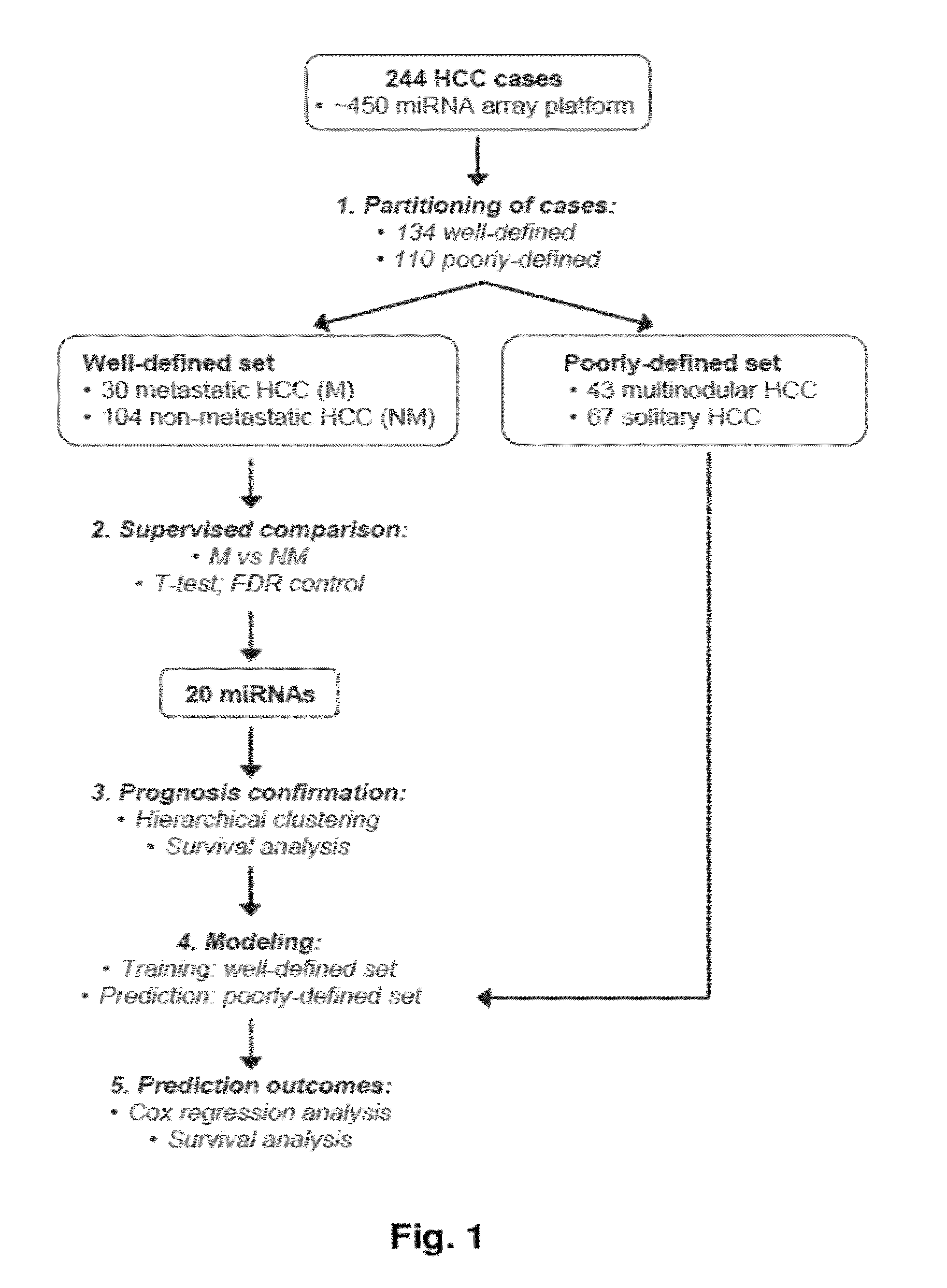
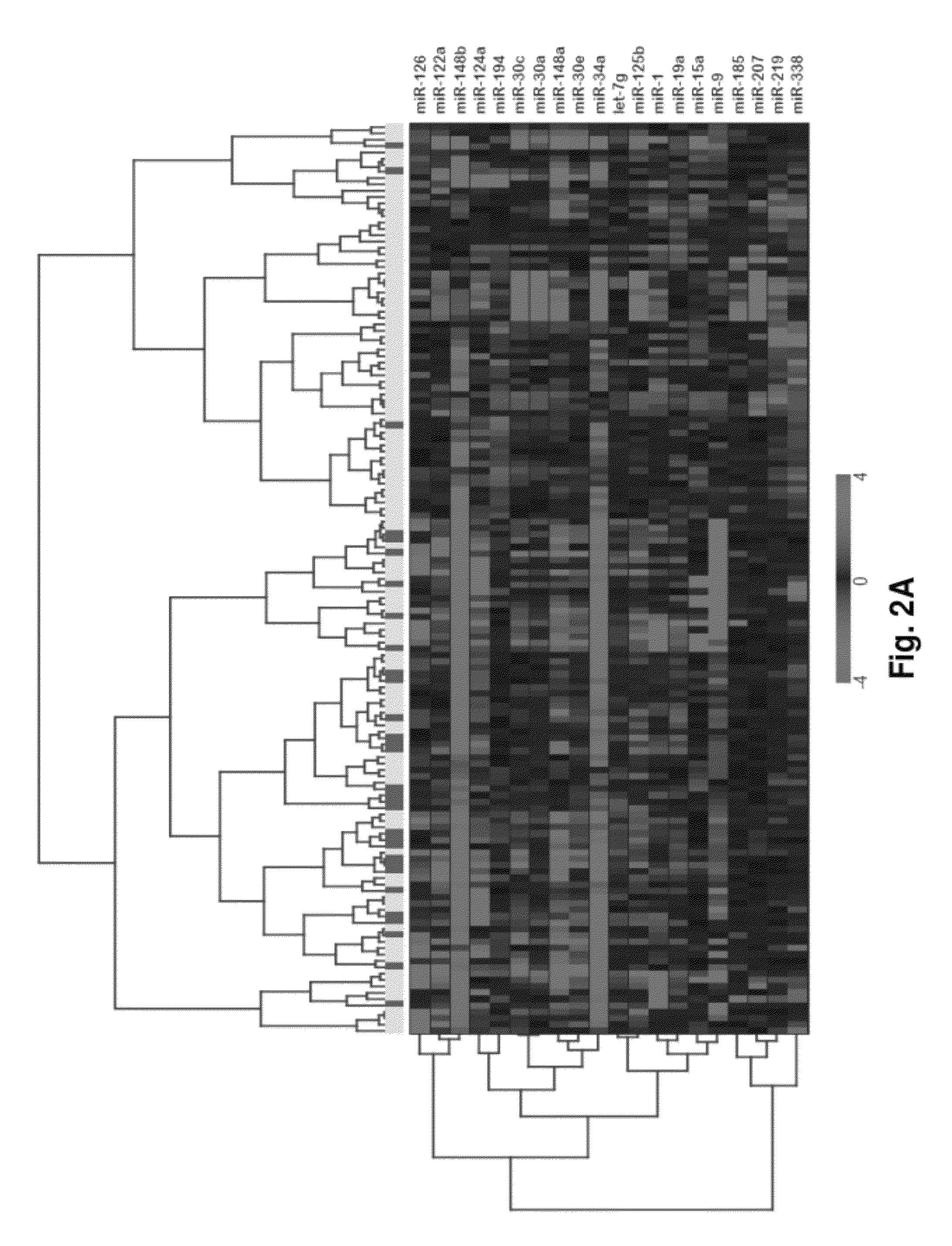
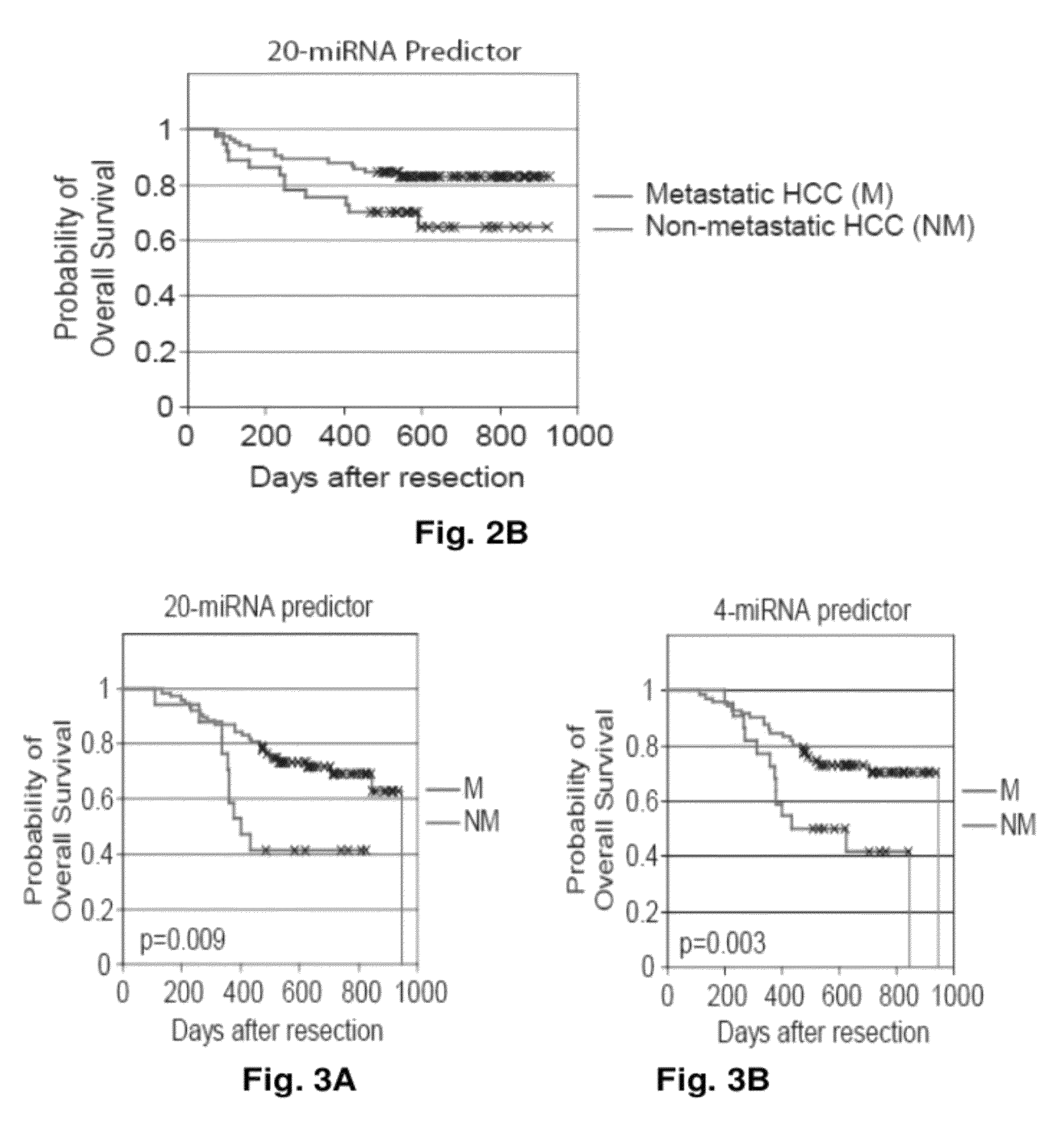
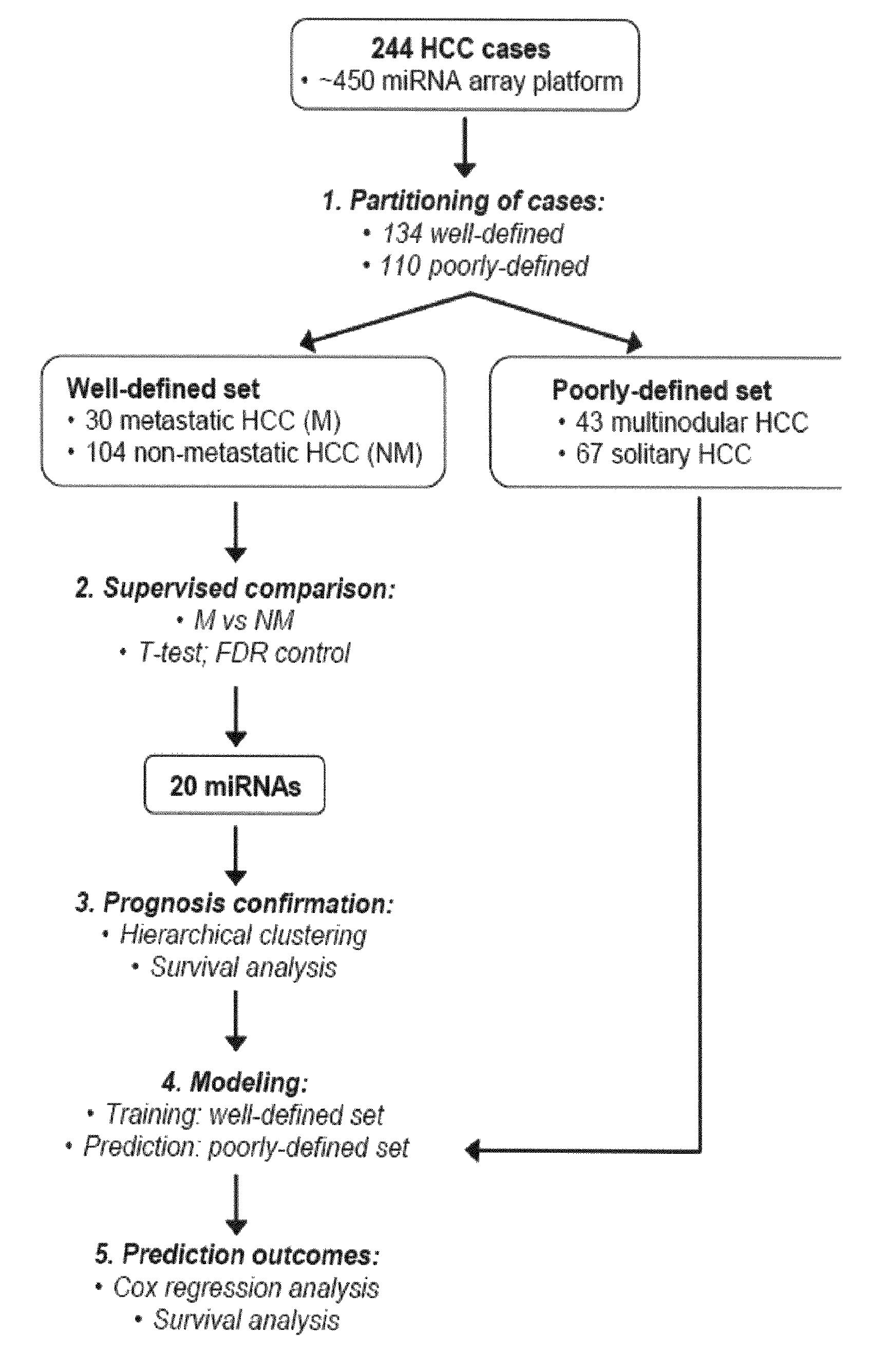
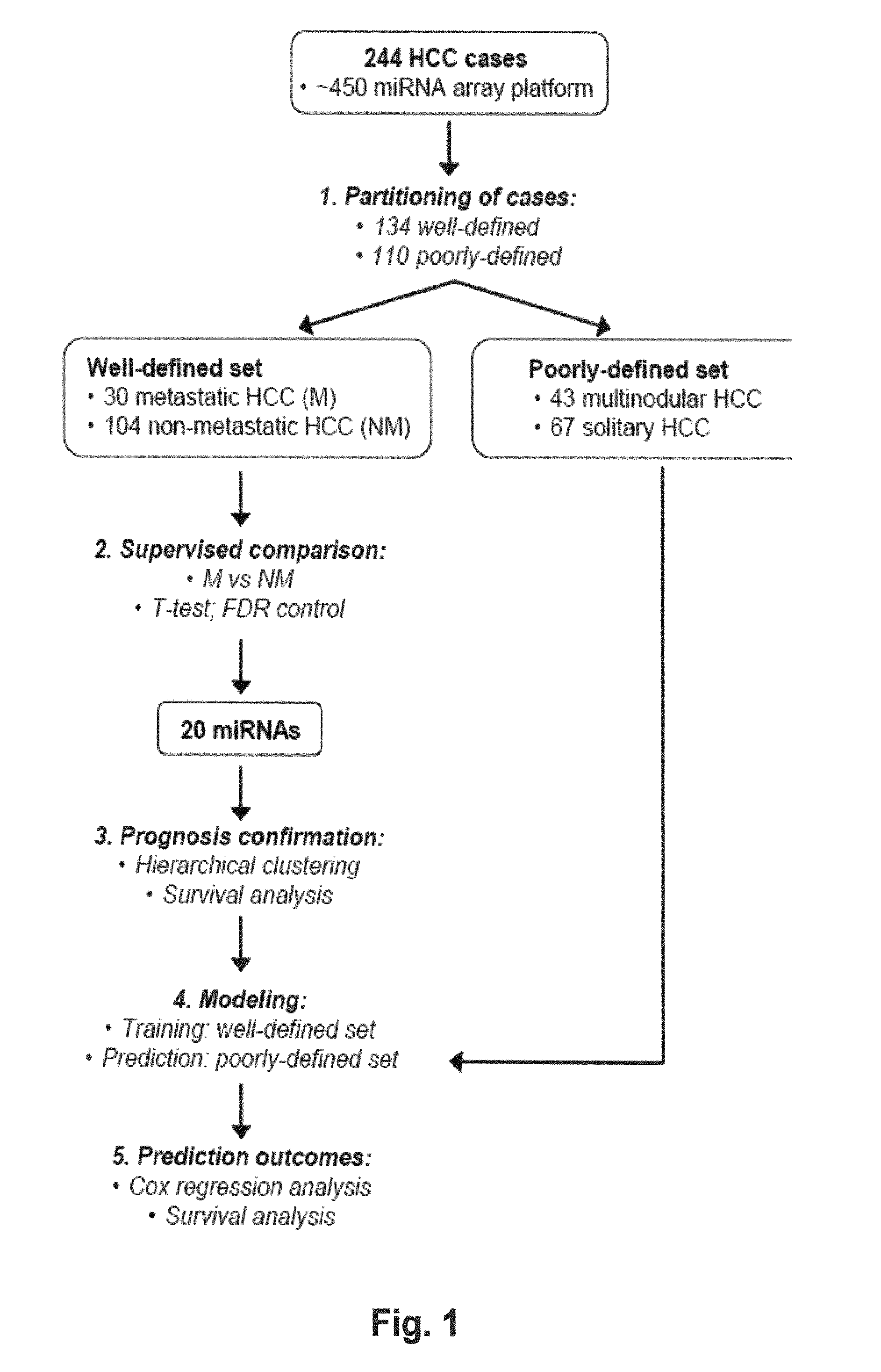
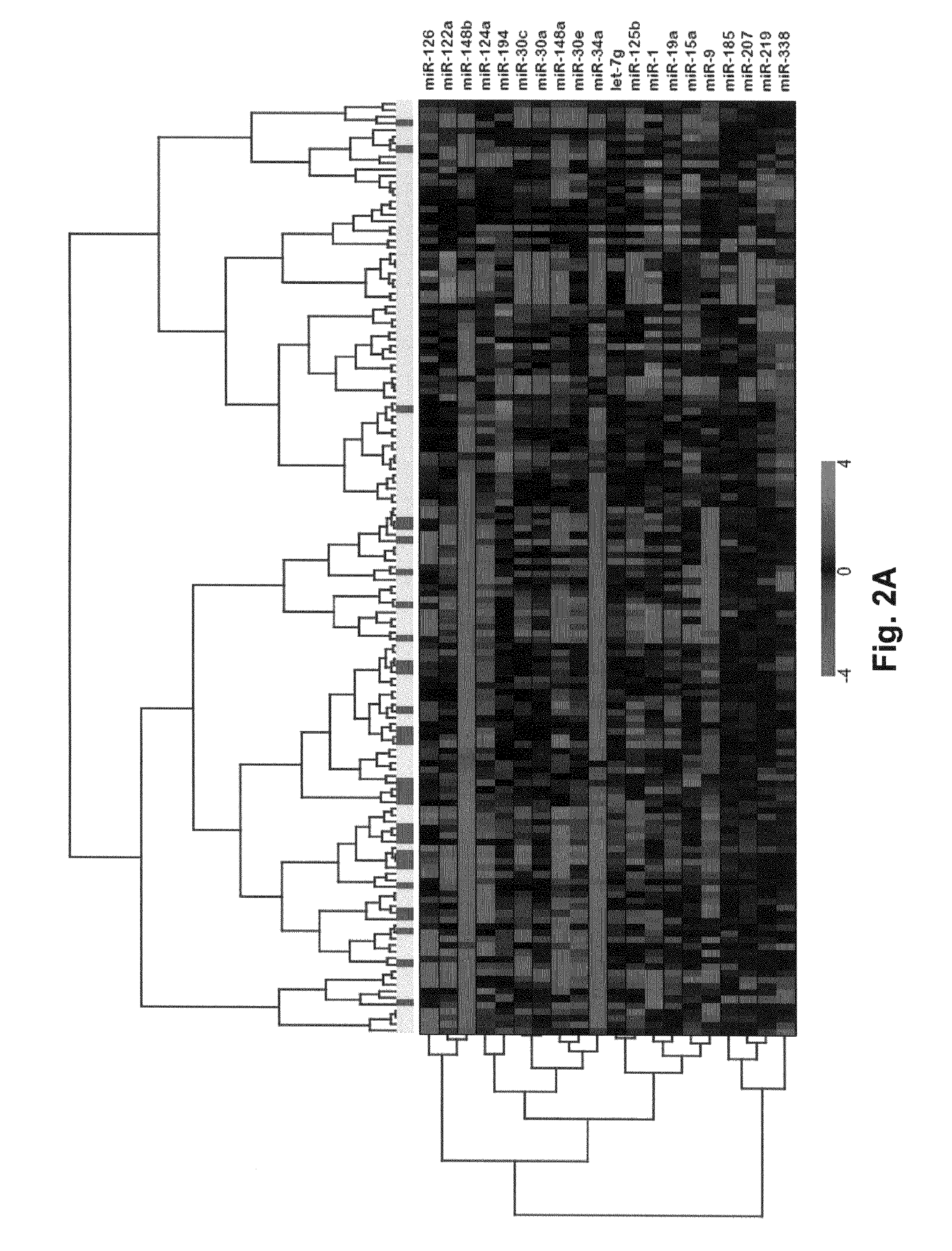
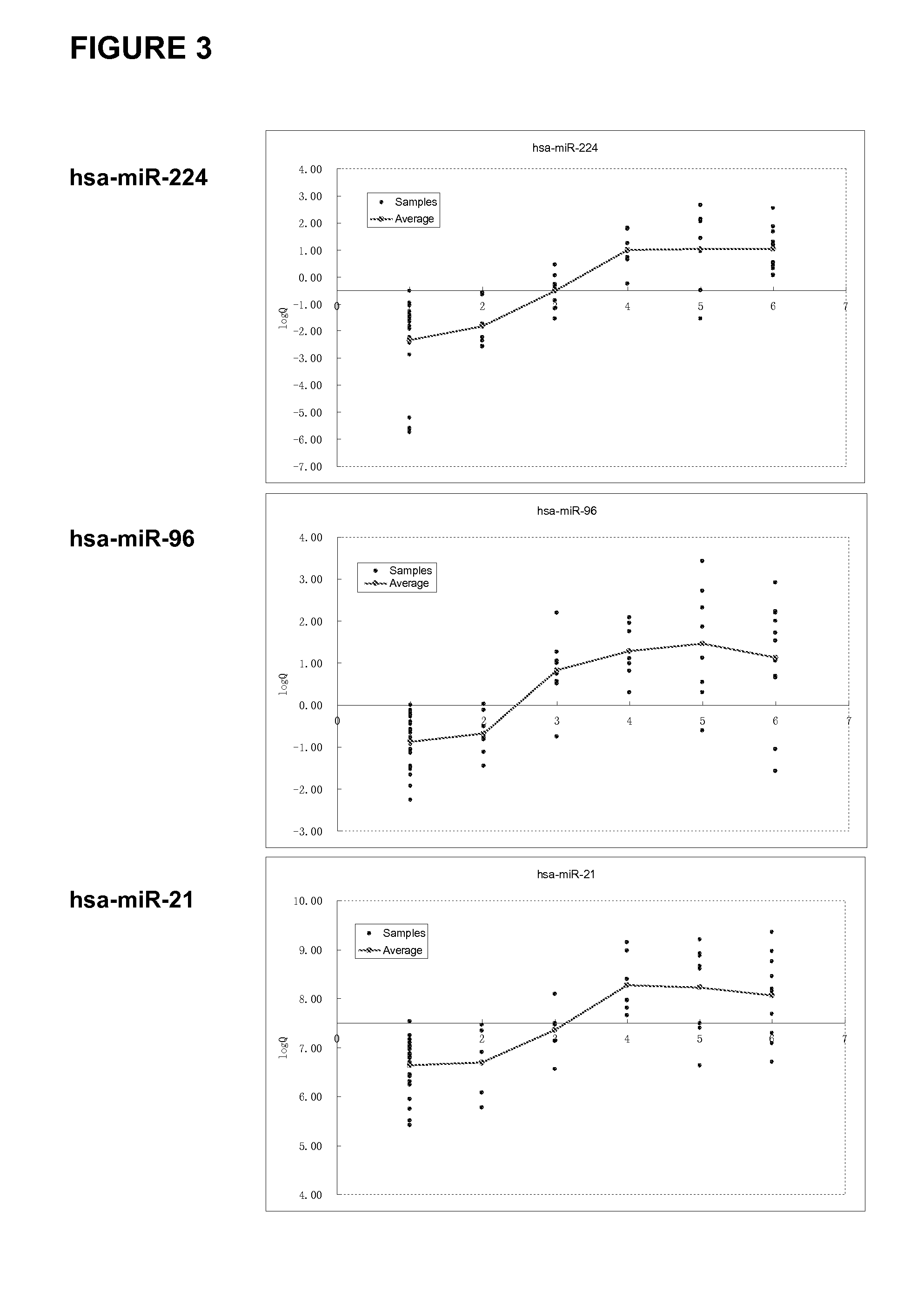
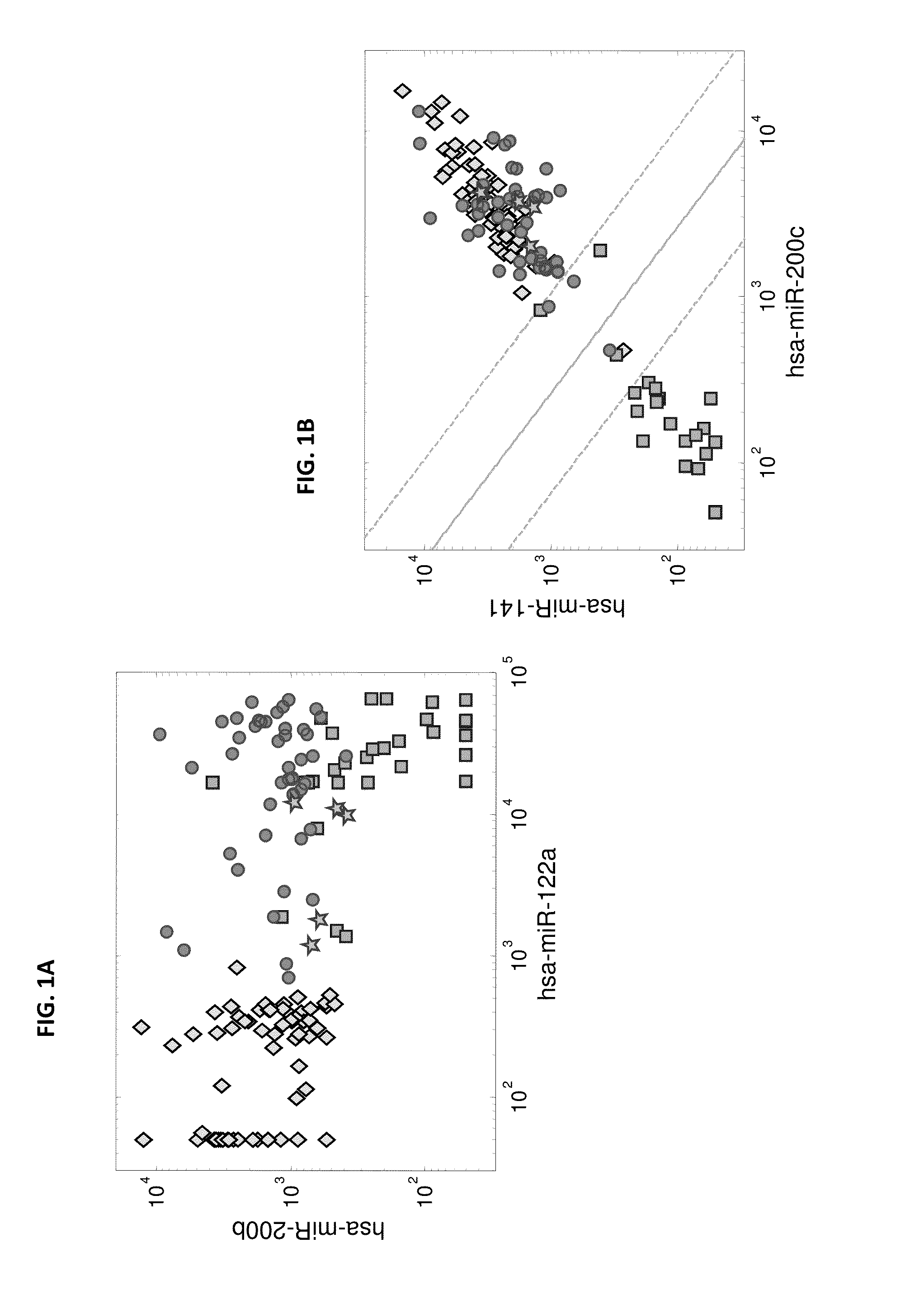
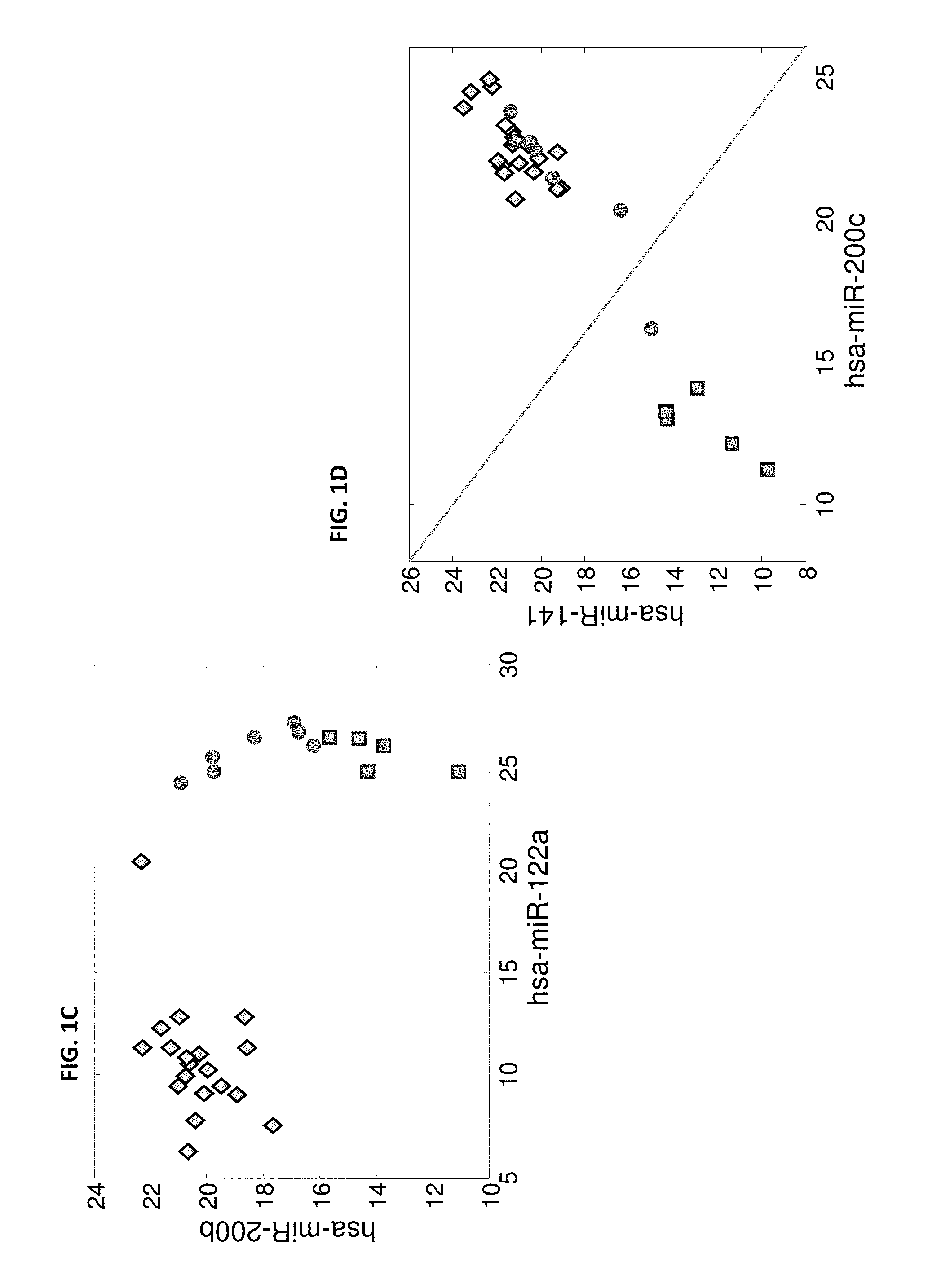
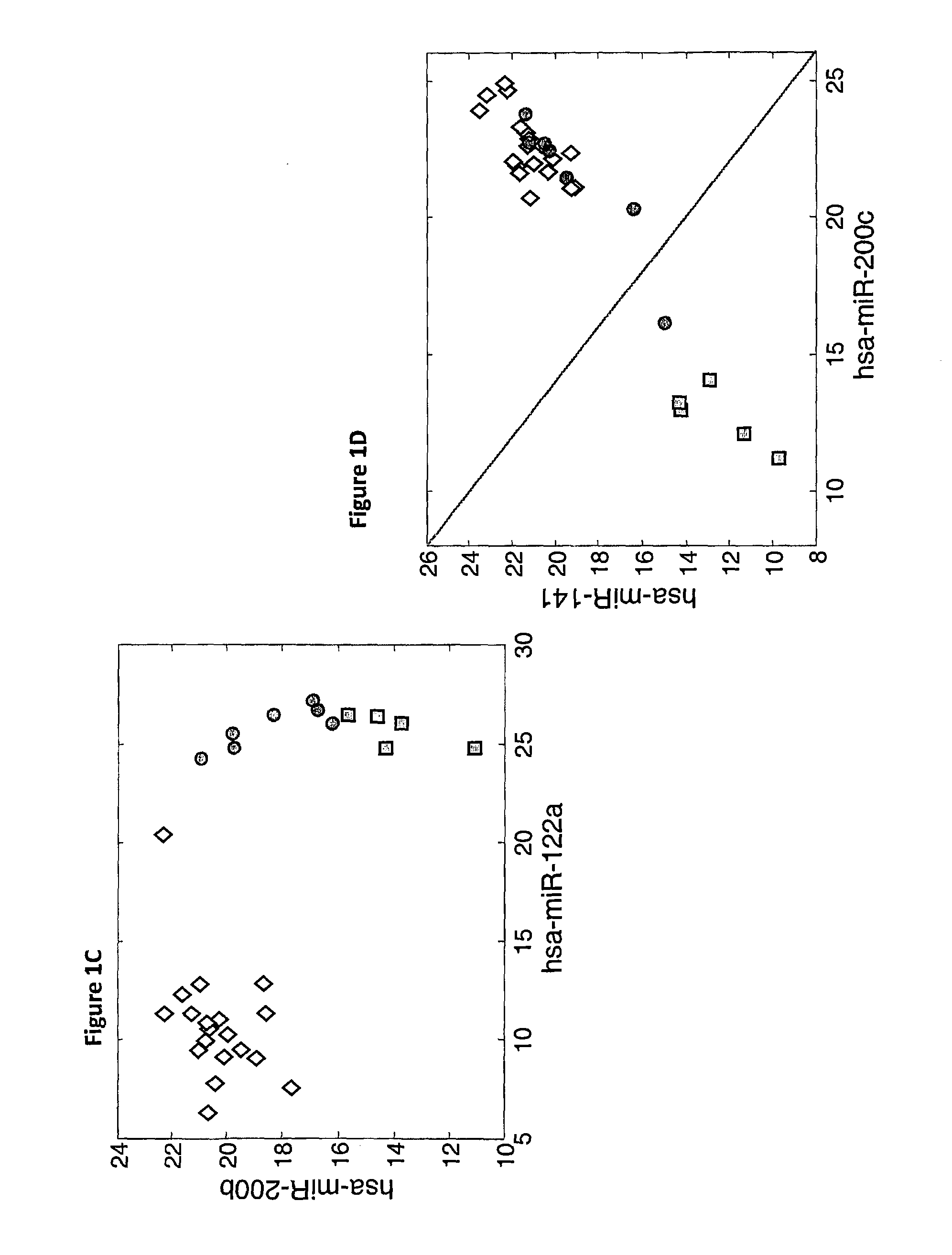
MicroRNA expression signature for predicting survival and metastases in hepatocellular carcinoma
InactiveUS8252538B2Independent and significant predictor of patient prognosis and relapseEnable prognosisSugar derivativesNucleotide librariesLymphatic SpreadHepatocellular carcinoma
Provided herein are methods and compositions for the diagnosis, prognosis and treatment of Hepatocellular carcinoma (HCC). Also provided are methods of identifying anti-HCC agents.
Owner:THE OHIO STATES UNIV +2
Markers for cancer prognosis and therapy and methods of use
InactiveUS20130042333A1Adverse side effectLow costBiocideMicrobiological testing/measurementAdjuvantTreatment field
The invention relates generally to the field of cancer prognosis and treatment. More particularly, the present invention relates to methods and compositions that utilize a particular panel of gene products (“biomarkers”) and their differential expression patterns (“expression signatures”), wherein the expression patterns correlate with responsiveness, or lack thereof, to chemotherapy treatment. The invention is based on the identification of a specific set of biomarkers that are differentially expressed in chemotherapy-treated tumors and which are useful in predicting the likelihood of a therapeutic response, including residual disease persistence and subsequent tumor recurrence in cancer patients receiving chemotherapy. The gene panel is also useful in designing specific adjuvant modalities with improved therapeutic efficiency. Also disclosed are methods for characterizing tumors according to expression of the biomarkers described herein.
Owner:GEN TECH
Compositions and methods for micro-rna expression profiling of cancer stem cells
InactiveUS20120053224A1Organic active ingredientsMicrobiological testing/measurementControl cellRna expression
The present invention relates compositions and methods for microRNA expression profiling of cancer stem cells. In particular, the invention relates to a method for identifying and / or diagnosing one or more cancer stem cells, the method comprising identifying from a plurality of nucleic acid molecules, each encoding a microRNA sequence, one or more nucleic acid molecules are differentially expressed in the cancer stem cells and in one or more control cells, wherein the one or more differentially expressed nucleic acid molecules together represent a nucleic acid expression signature that is indicative for the presence of cancer stem cells. The invention further relates to a corresponding diagnostic kit of molecular markers, namely the nucleic acid expression signature. Finally, the invention is directed to a method using such nucleic acid expression signatures for preventing the proliferation and / or self-renewal of such cancer stem cells as well as to a corresponding pharmaceutical composition.
Owner:UNIV REGENSBURG
MicroRNA Expression Signature for Predicting Survival and Metastases in Hepatocellular Carcinoma
InactiveUS20100120898A1Enable prognosisIndependent and significant predictor of patient prognosis and relapseOrganic active ingredientsSugar derivativesCancer researchExpression Signature
Provided herein are methods and compositions for the diagnosis, prognosis and treatment of Hepatocellular carcinoma (HCC). Also provided are methods of identifying anti-HCC agents.
Owner:THE OHIO STATES UNIV +2
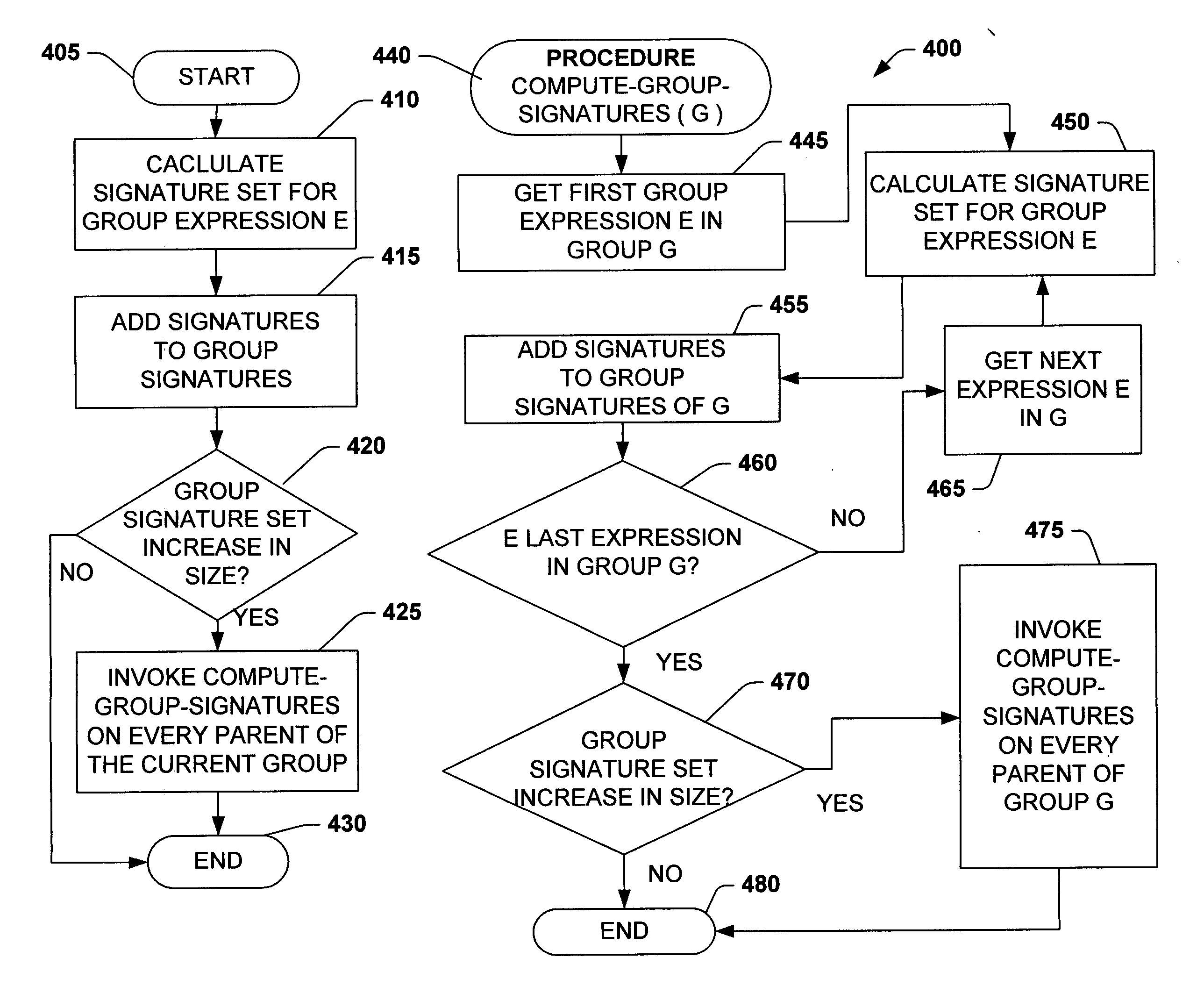
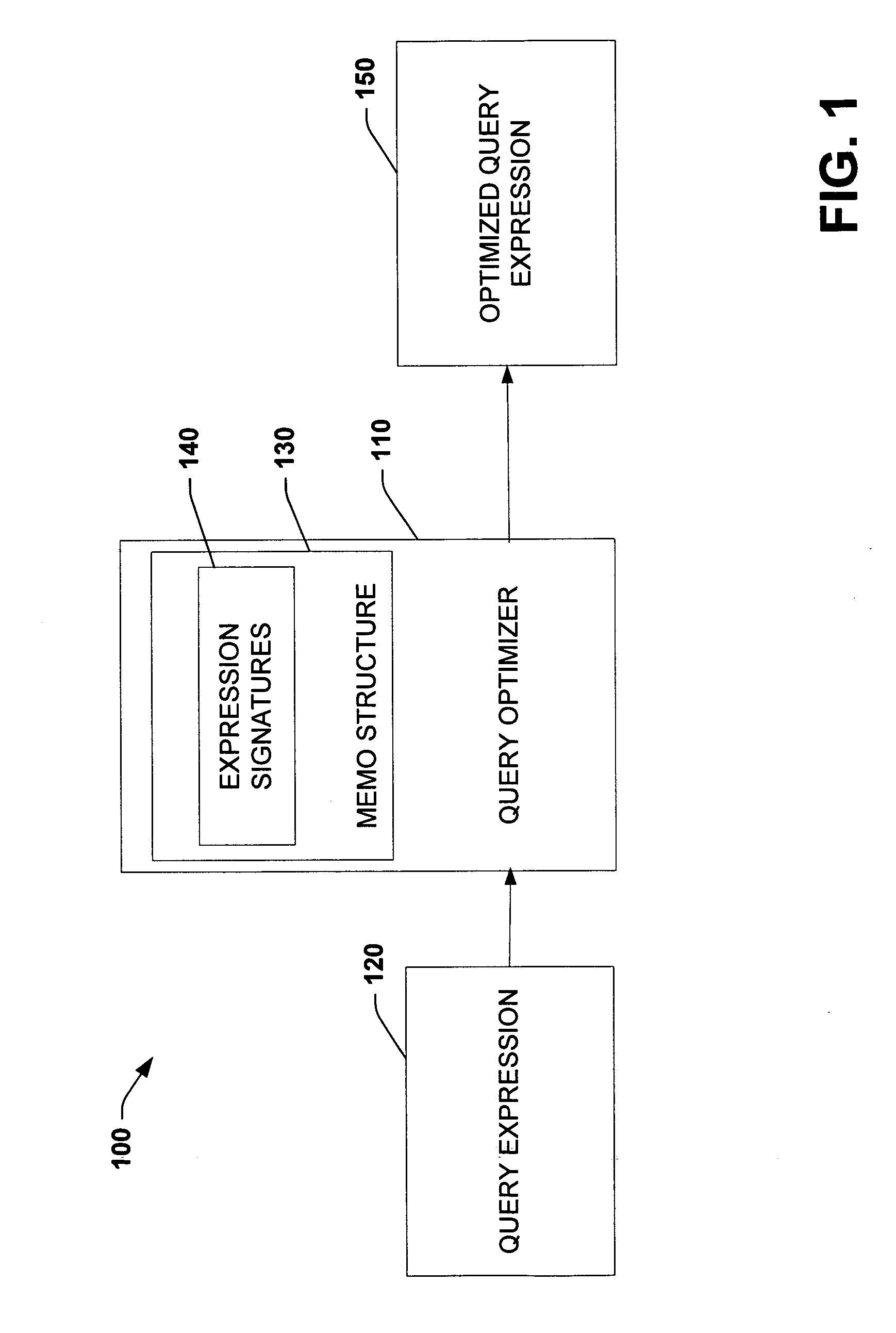
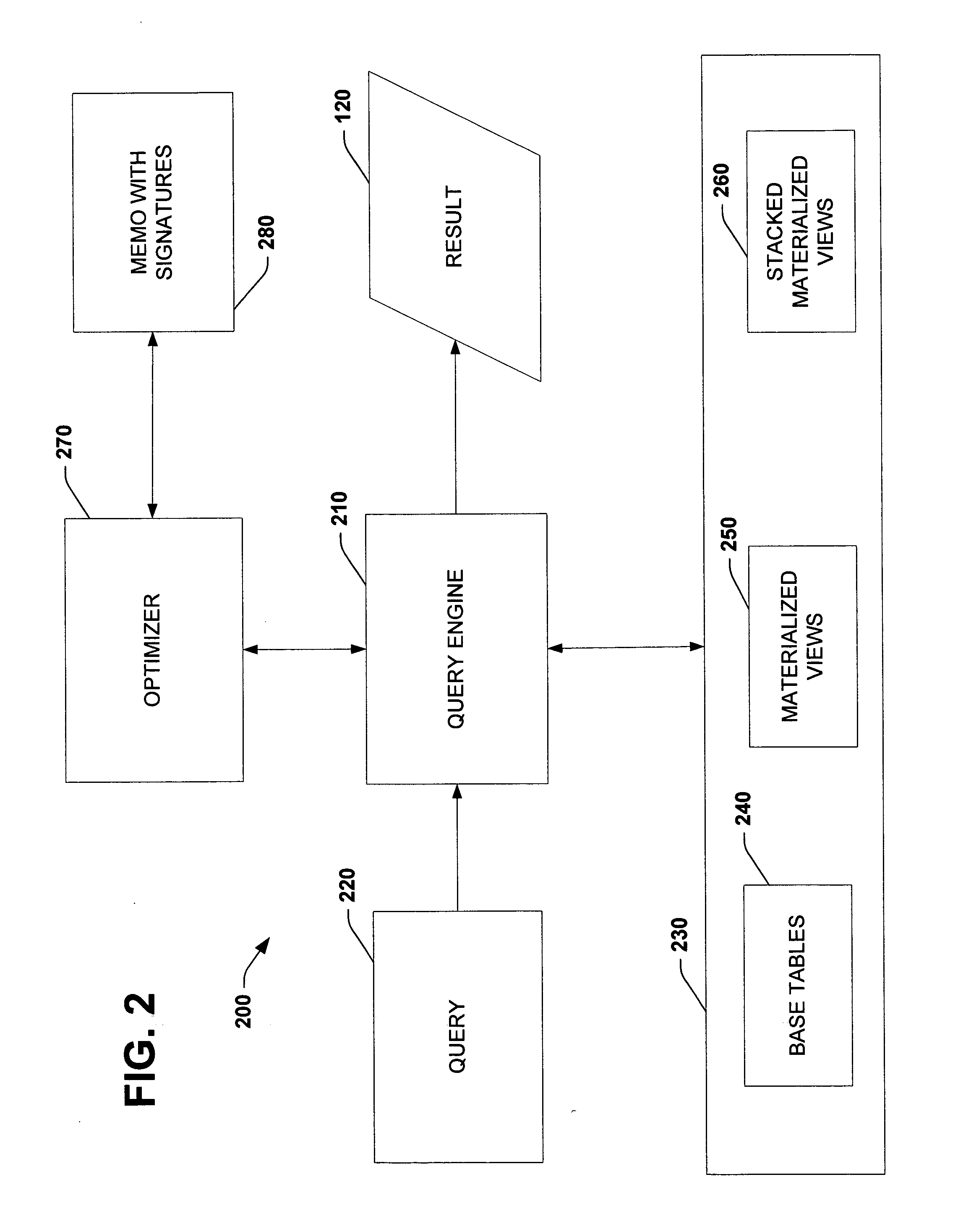
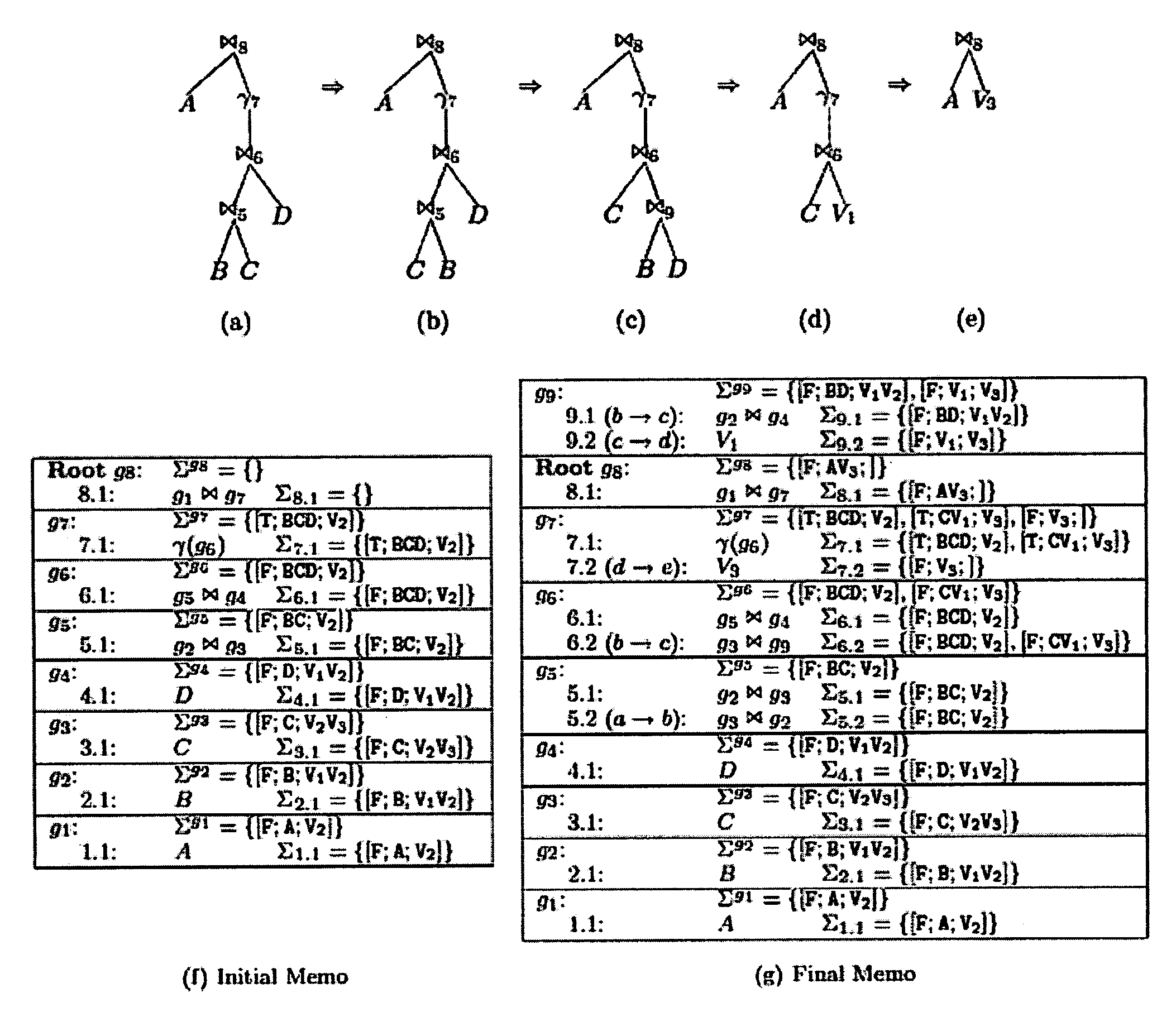
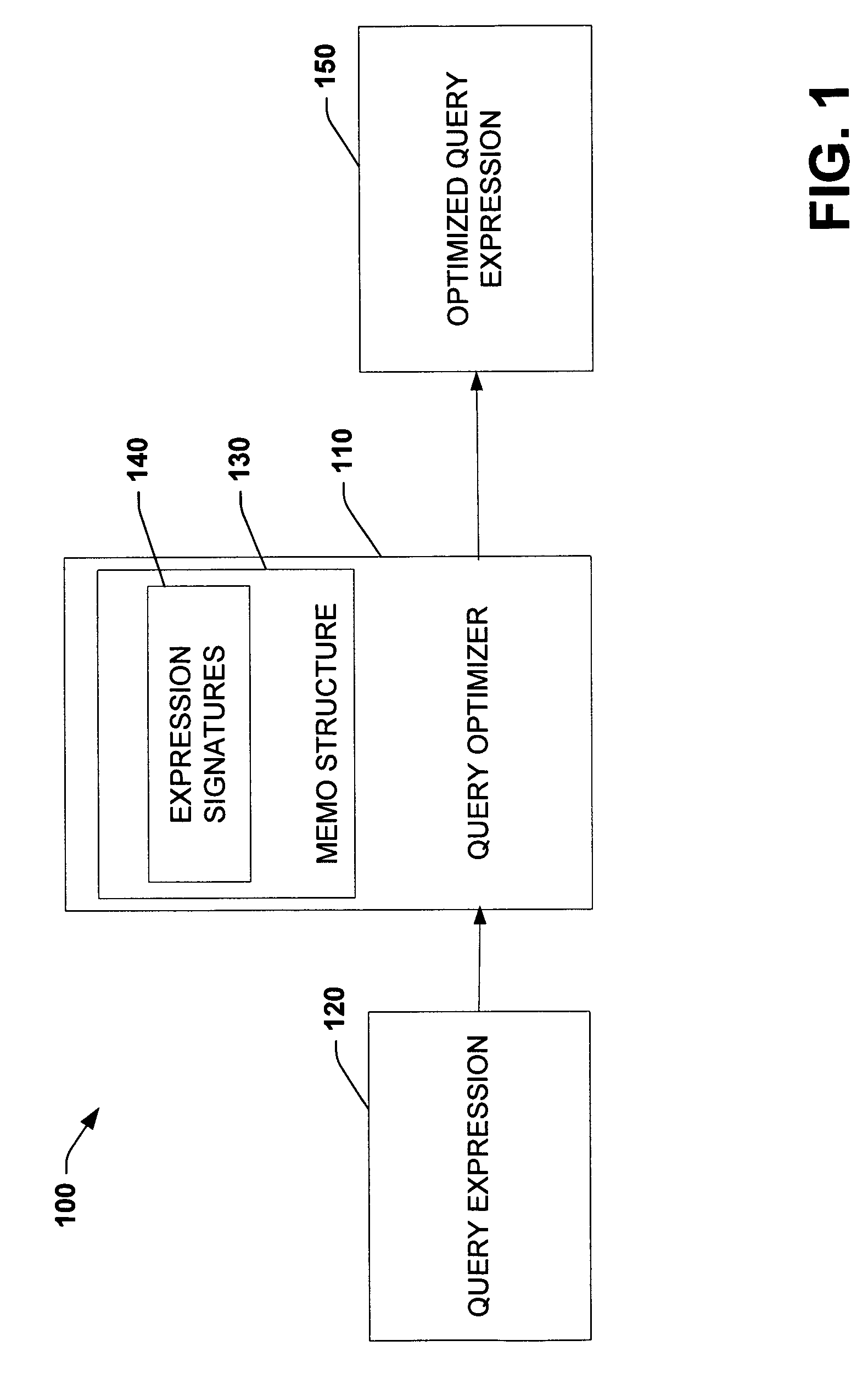
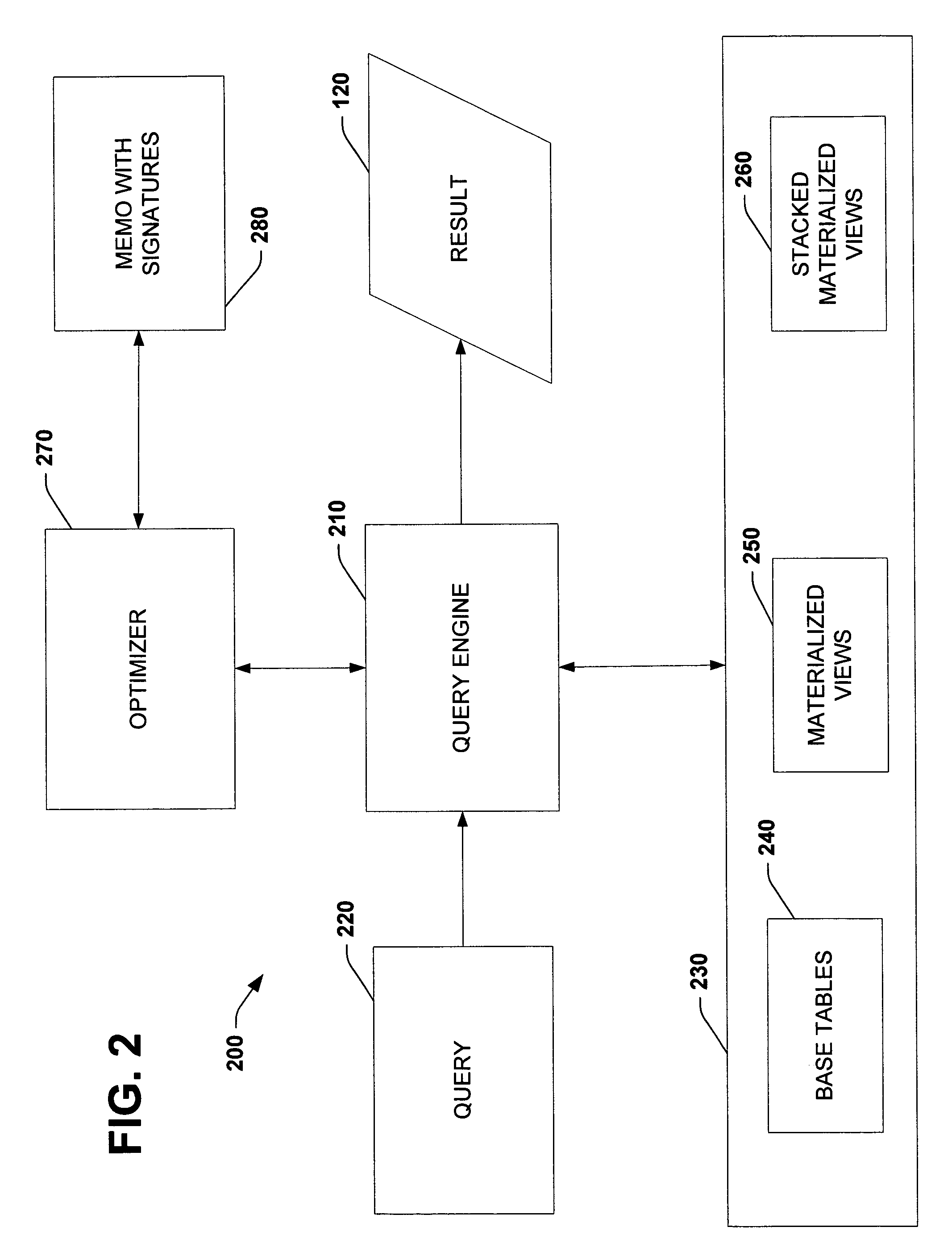
Using query expression signatures in view matching
InactiveUS20060230017A1Easy to handleImprove efficiencyData processing applicationsDigital data information retrievalTheoretical computer scienceExpression Signature
A system for optimizing queries against a database is disclosed. The system comprises a memo structure that encodes a plurality of query expressions. The system also includes a signature mechanism that enables an assignment of the query expressions into equivalence classes. Methods of using such a system are additionally provided.
Owner:MICROSOFT TECH LICENSING LLC
Colon cancer gene expression signatures and methods of use
InactiveUS10196691B2Accurate diagnosisQuality improvementMicrobiological testing/measurementBiological testingCancer genesOncology
A gene expression signature of colon cancer, microarrays including them and methods of using the colon gene expression signature are provided. The gene expression signature is especially useful for determining the prognosis of a patient diagnosed with colon cancer, such as stage II colon cancer. The gene signature described herein is also useful for determining effectiveness of surgical resection with or without adjuvant chemotherapy, and determining possibility of cancer recurrence in patients with colon cancer.
Owner:ALMAC DIAGNOSTICS SERVICES LIMITED
Compositions and methods for micro-rna expression profiling of colorectal cancer
ActiveUS20110281756A1Reliable discriminationSugar derivativesMicrobiological testing/measurementControl cellRna expression
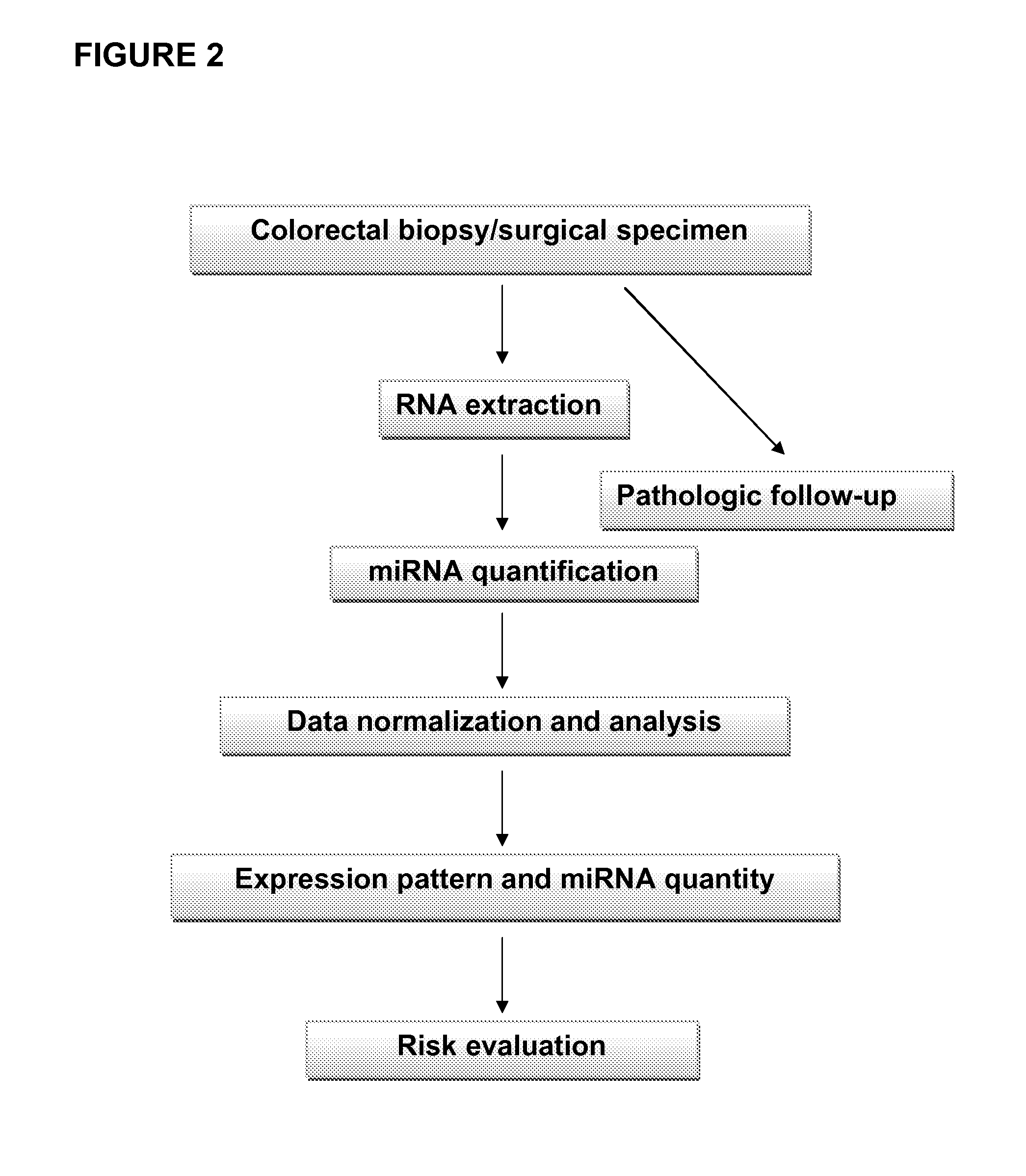
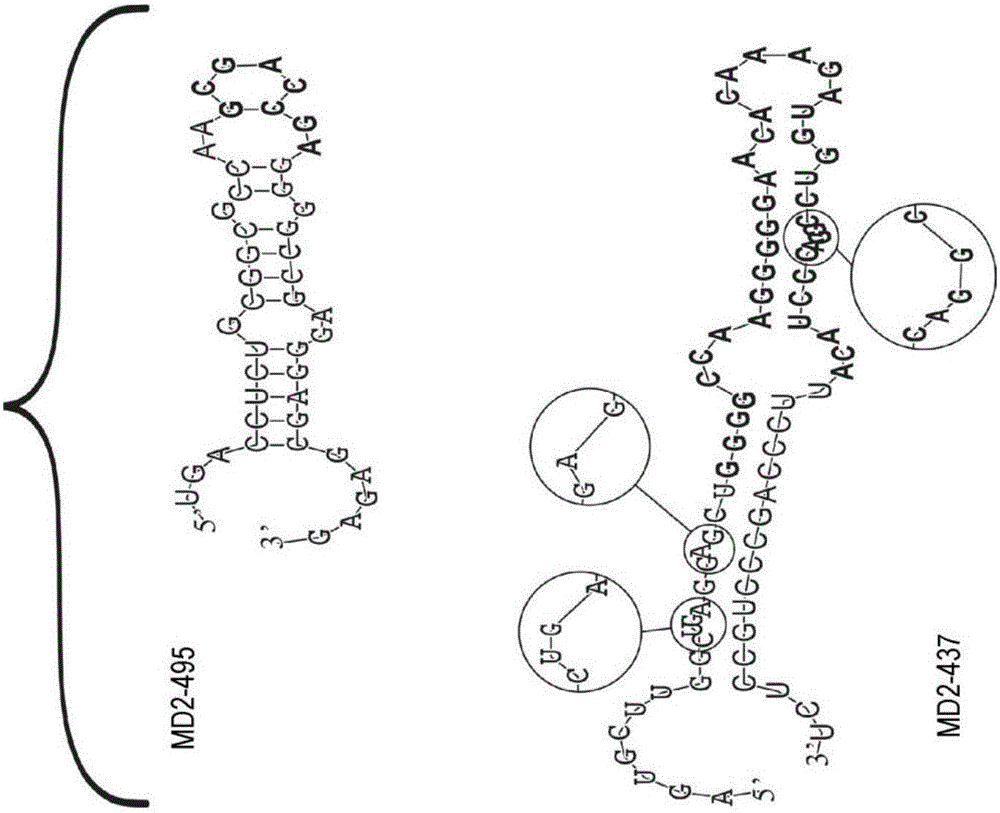
The present invention relates compositions and methods for microRNA (miRNA) expression profiling of colorectal cancer. In particular, the invention relates to a diagnostic kit of molecular markers for identifying one or more mammalian target cells exhibiting or having a predisposition to develop colorectal cancer, the kit comprising a plurality of nucleic acid molecules, each nucleic acid molecule encoding a miRNA sequence, wherein one or more of the plurality of nucleic acid molecules are differentially expressed in the target cells and in one or more control cells, and wherein the one or more differentially expressed nucleic acid molecules together represent a nucleic acid expression signature that is indicative for the presence of or the predisposition to develop colorectal cancer. The invention further relates to corresponding methods using such nucleic acid expression signatures for identifying one or more mammalian target cells exhibiting or having a predisposition to develop colorectal cancer as well as for preventing or treating such a condition. Finally, the invention is directed to a pharmaceutical composition for the prevention and / or treatment of colorectal cancer.
Owner:FUDAN UNIV
Alzheimer's disease signature markers and methods of use
InactiveUS20140304845A1Sugar derivativesMicrobiological testing/measurementBiomarker (petroleum)Biology
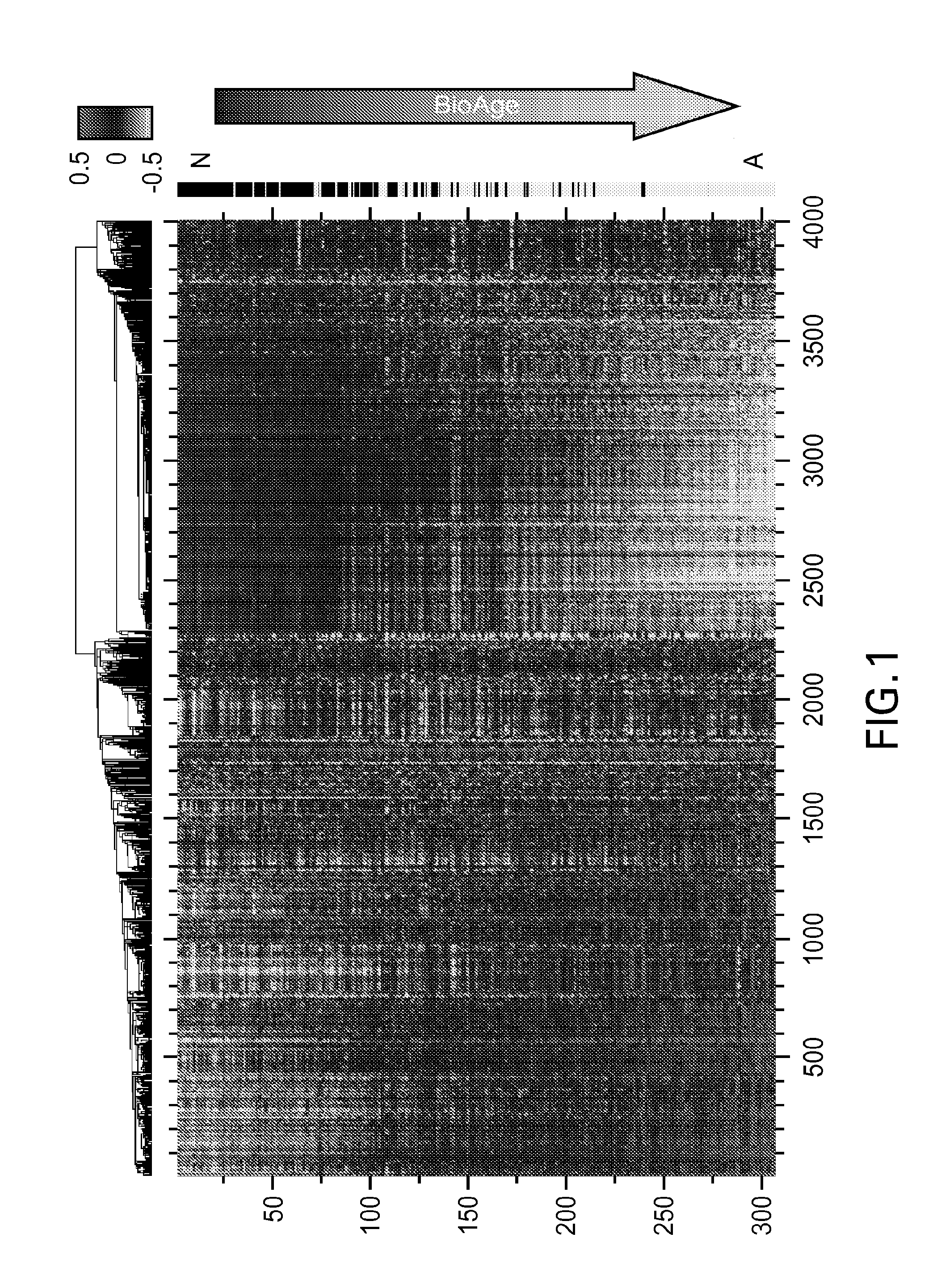
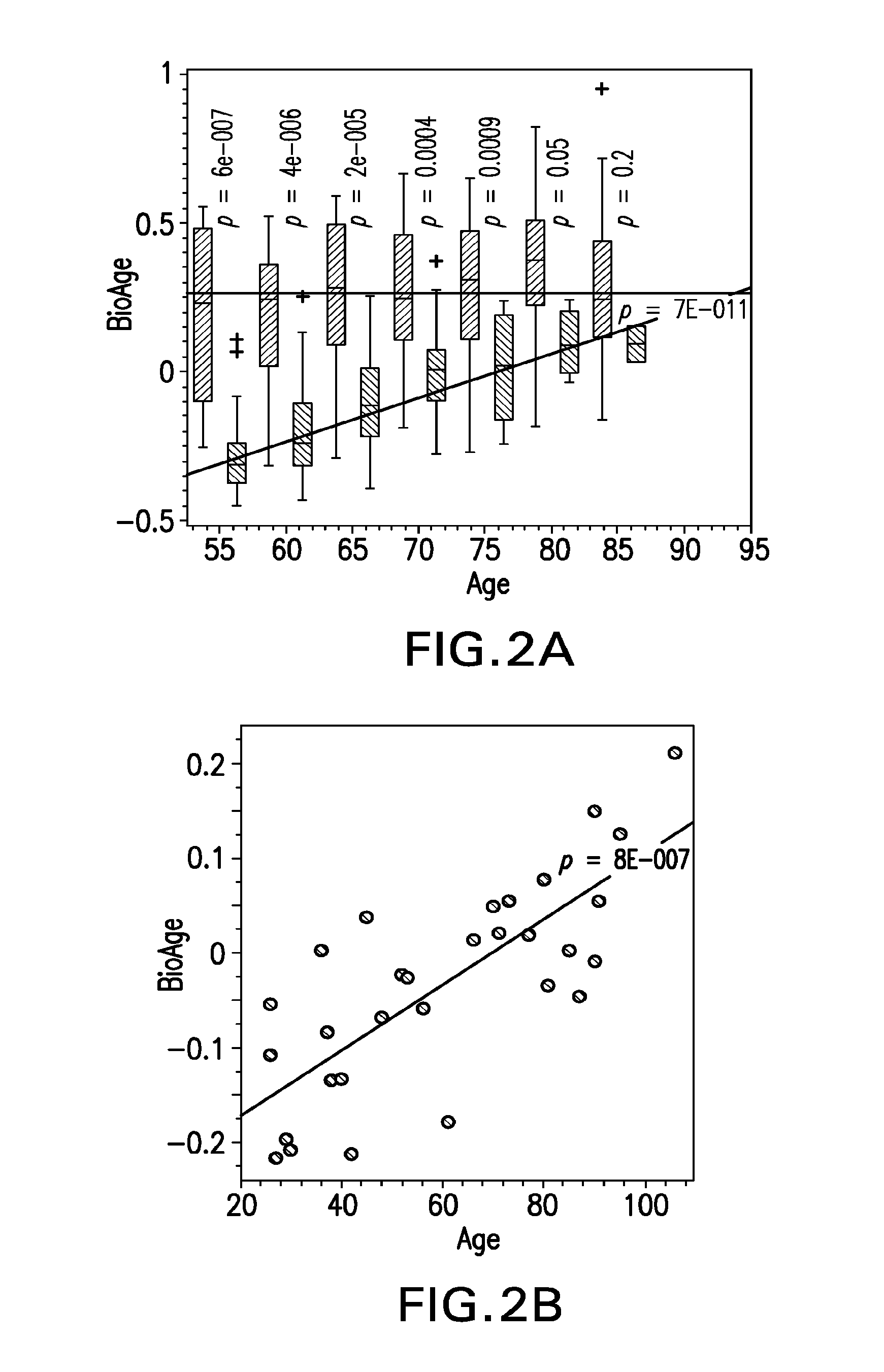
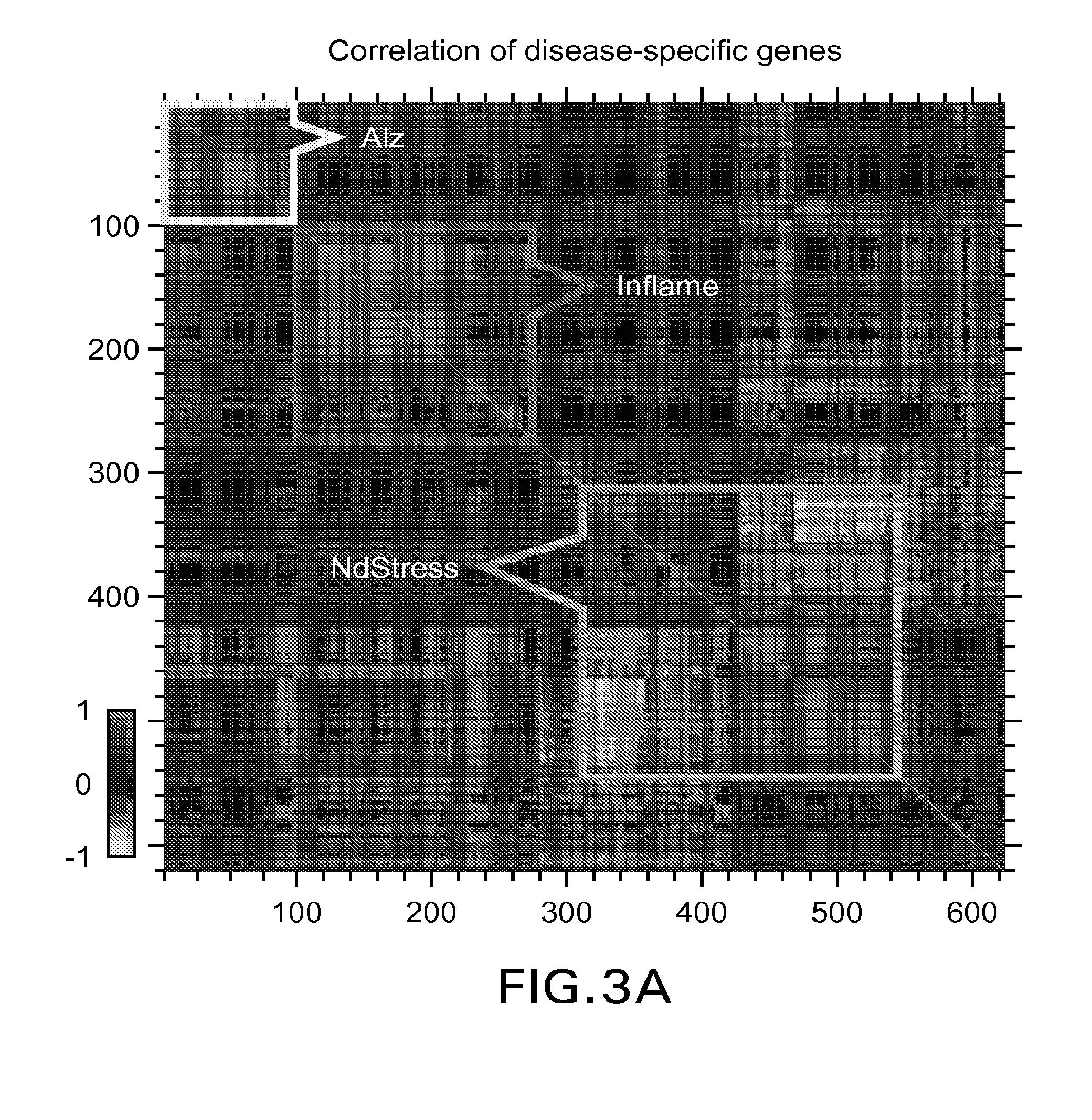
Methods, biomarkers, and expression signatures are disclosed for assessing the disease progression of Alzheimer's disease (AD). In one embodiment, BioAge (biological age), NdStress (neurodegenerative stress), Alz (Alzheimer), and Inflame (inflammation) are used as biomarkers of AD progression. In another aspect, the invention comprises a gene signature for evaluating disease progression. In still another embodiment, methods for evaluating disease progression are provided. In yet another embodiment, the invention can be used to identify animal models for use in the development and evaluation of therapeutics for the treatment of AD.
Owner:MERCK SHARP & DOHME CORP
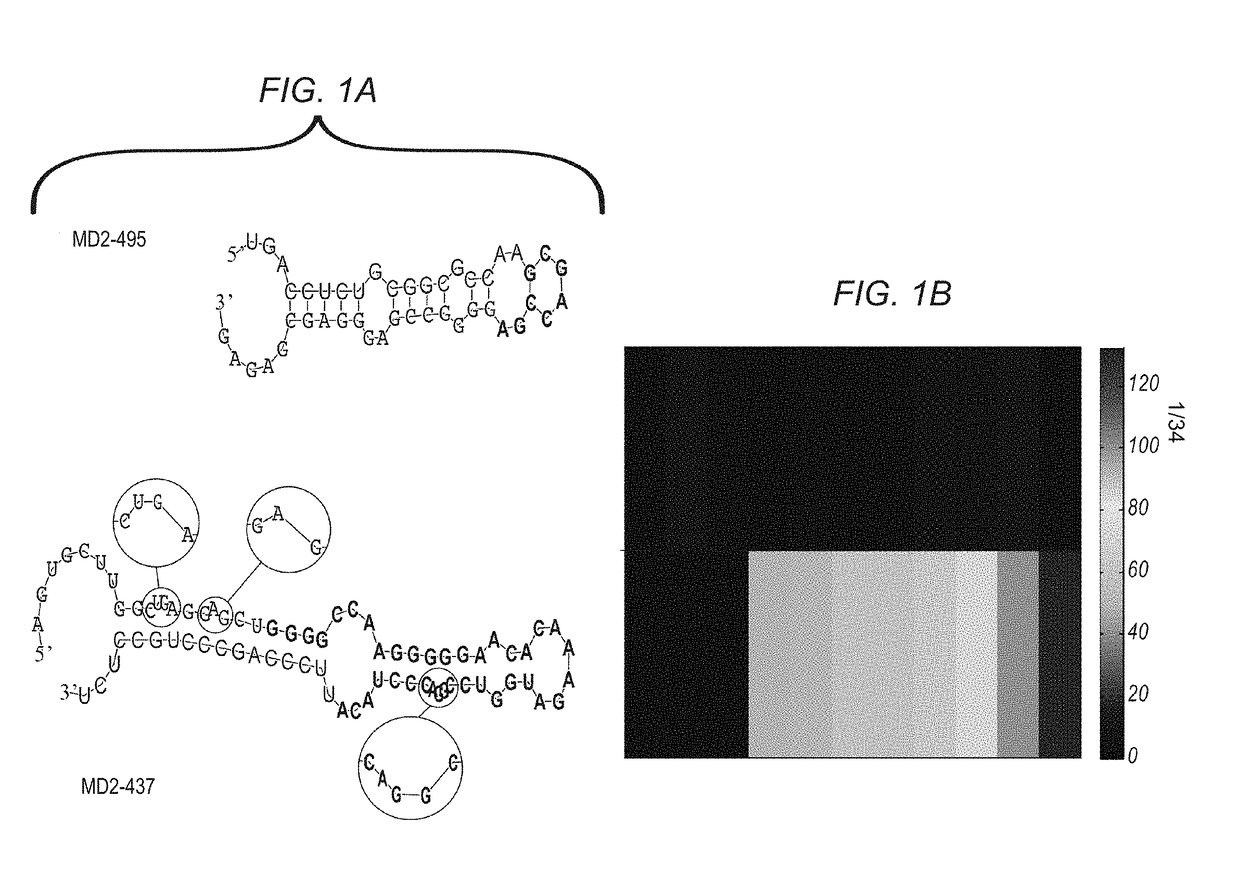
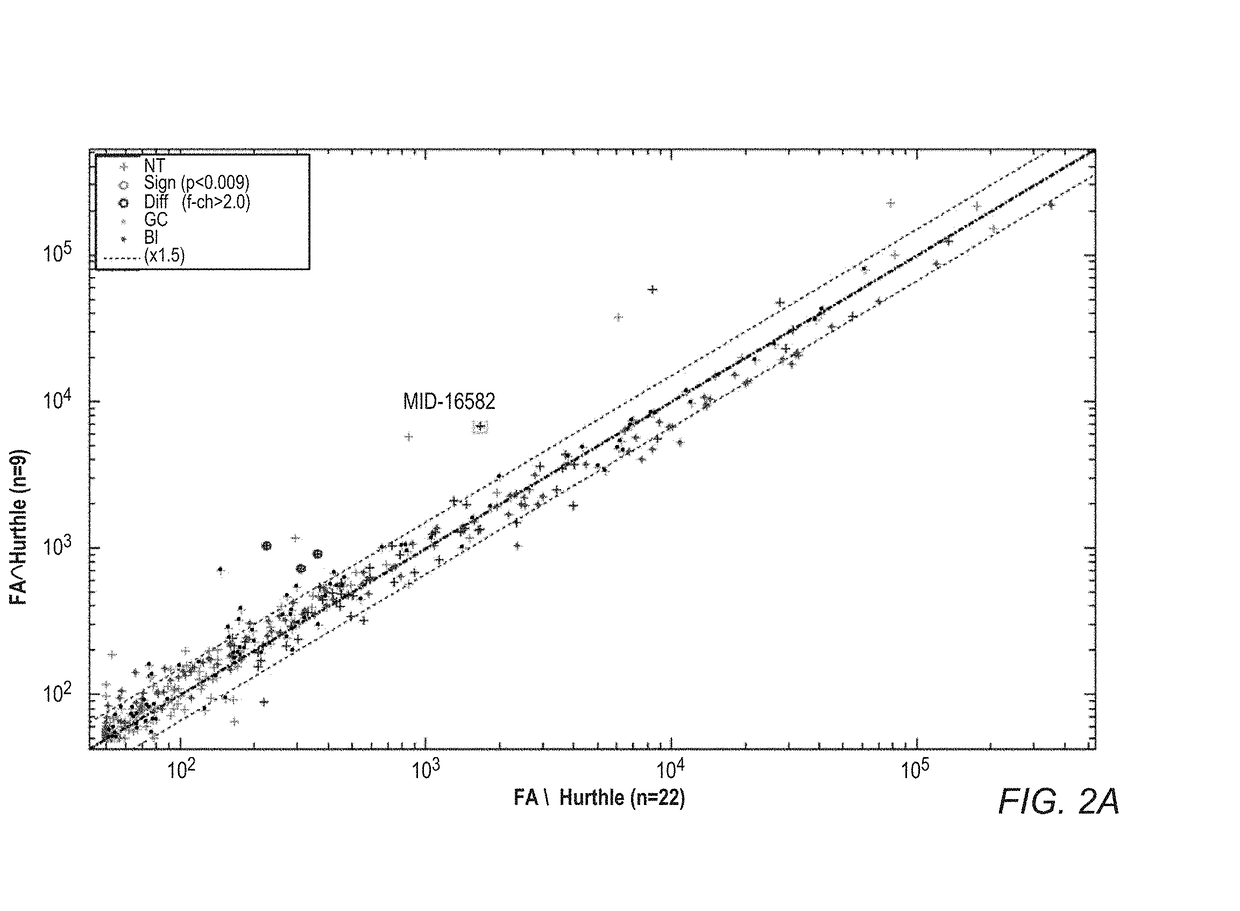
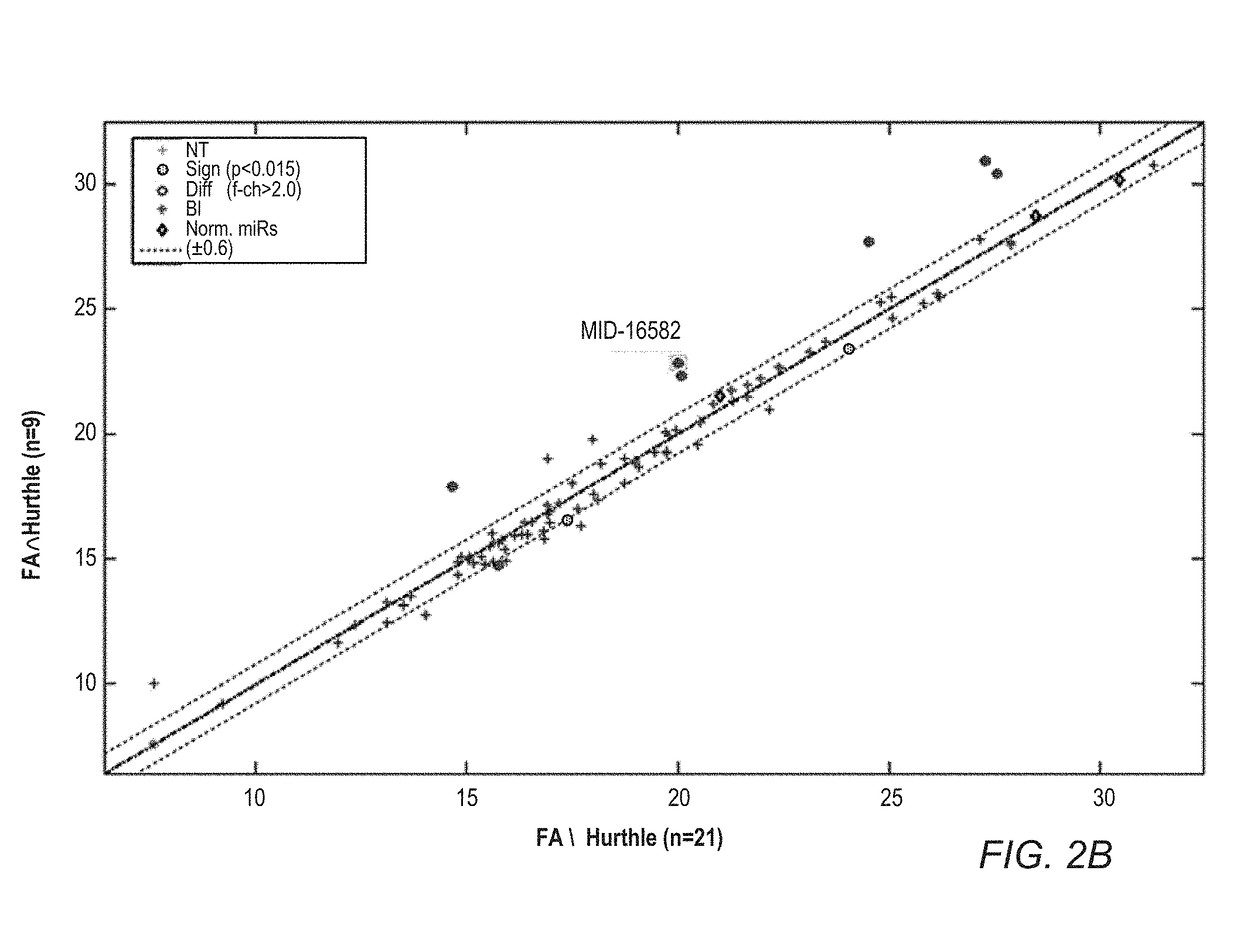
Mirna expression signature in the classification of thyroid tumors
The present invention provides a method for classification of thyroid tumors through the analysis of the expression patterns of specific microRNAs in fine needle aspiration samples. Thyroid tumor classification according to a microRNA expression signature allows optimization of diagnosis and treatment, as well as determination of signature-specific therapy.
Owner:ROSETTA GENOMICS
Using query expression signatures in view matching
InactiveUS7599925B2Easy to handleImprove efficiencyData processing applicationsDigital data information retrievalTheoretical computer scienceQuery expansion
A system for optimizing queries against a database is described. The system comprises a memo structure that encodes a plurality of query expressions. The system also includes a signature mechanism that enables an assignment of the query expressions into equivalence classes. Methods of using such a system are additionally provided.
Owner:MICROSOFT TECH LICENSING LLC
Predicting breast cancer treatment outcome
ActiveUS20050239083A1Improve survival outcomeGene expressionBioreactor/fermenter combinationsBiological substance pretreatmentsOncologyBreast tissue sample
Methods and compositions are provided for the identification of expression signatures in ER+ breast cancer cases, where the signatures correlate with responsiveness, or lack thereof, to treatment with tamoxifen or another antiestrogen agent against breast cancer The signature profiles are identified based upon sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for predicting the efficacy of treating a subject with breast cancer with tamoxifen or another antiestrogen agent against breast cancer. Additional methods and compositions are provided for predicting responsiveness to tamoxifen or another antiestrogen agent against breast cancer in cases of breast cancer by use of three biomarkers. Two biomarkers display increased expression correlated with tamoxifen response while the third biomarker displays decreased expression correlated with tamoxifen response.
Owner:THE GENERAL HOSPITAL CORP +1
MiRNA expression signature in the classification of thyroid tumors
The present invention provides a method for classification of thyroid tumors through the analysis of the expression patterns of specific microRNAs in fine needle aspiration samples. Thyroid tumor classification according to a microRNA expression signature allows optimization of diagnosis and treatment, as well as determination of signature-specific therapy.
Owner:ROSETTA GENOMICS
Identification of signature genes associated with hepatocellular carcinoma
InactiveUS20110257035A1Lower levelImprove survivalPeptide librariesNucleotide librariesRegimenTumor Biomarkers
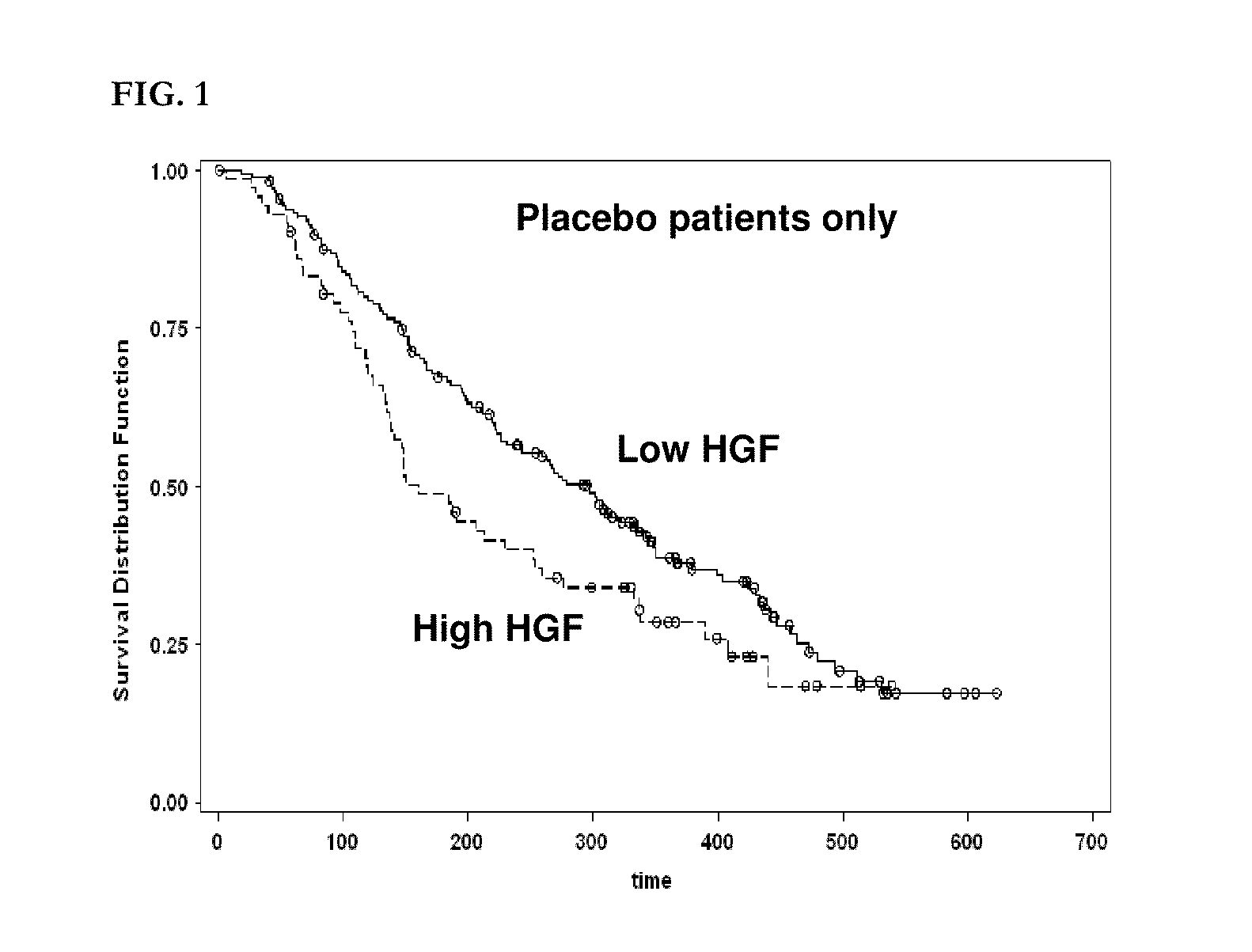
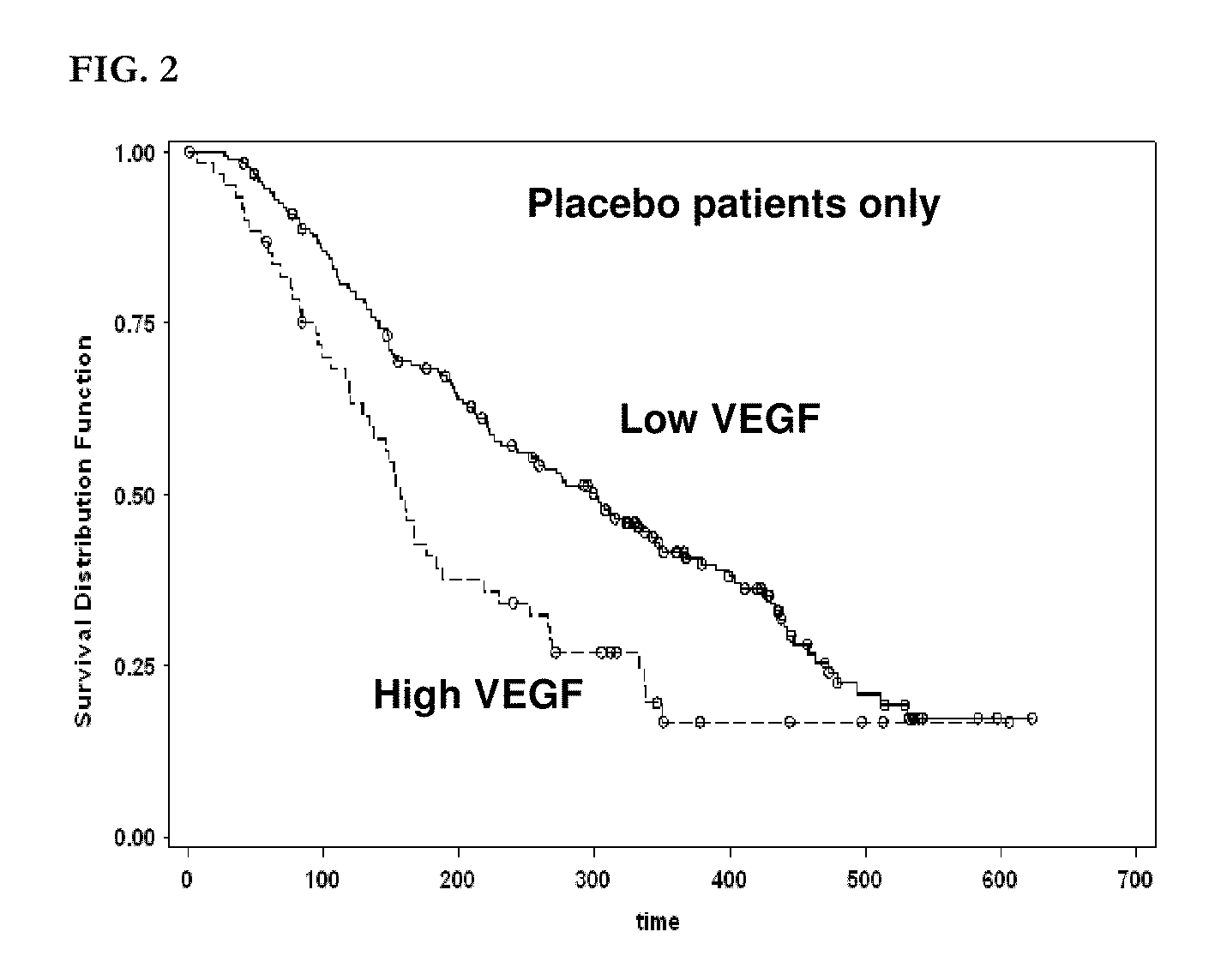
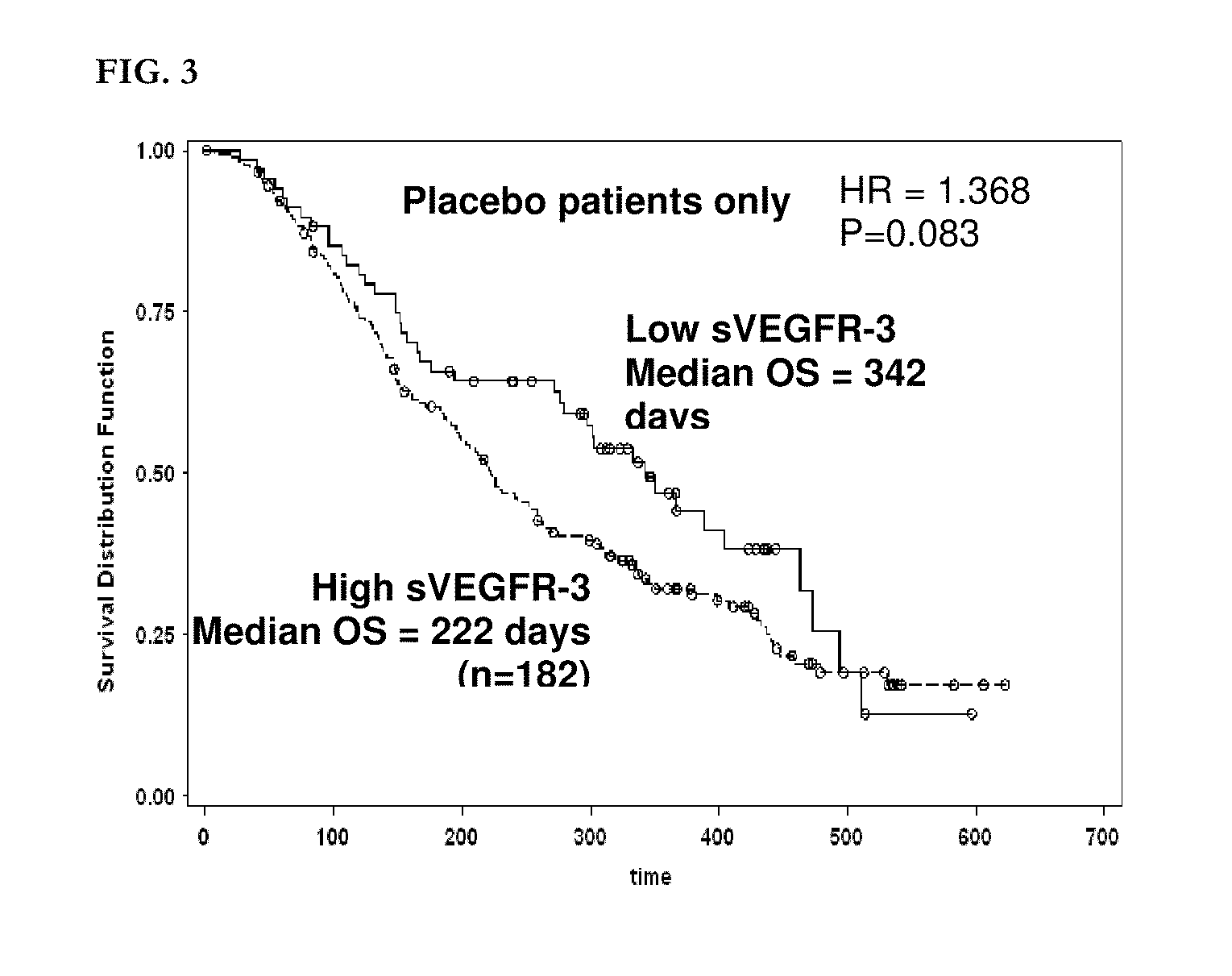
The present invention relates to, for example, (1) a novel method for identification of clinically useful serum and / or tumor biomarkers and expression signatures that can be used for detection, prognostication and guidance for the treatment of patients with hepatocellular carcinoma (HCC); and (2) discovery of an expression signature that can be used to monitor and / or study the efficacy of a chemotherapeutic regimen, such as, for example, sorafenib (solely or in combination with other agents). The present invention also provides a method for predicting clinical outcomes, such as, for example, overall survival (OS), time to progression (TTP) and / or likelihood of benefitting from a chemotherapeutic treatment (i.e., sorafenib) in HCC patients based on the analysis of such biomarkers.
Owner:BAYER HEALTHCARE LLC
Diagnosis and treatment of breast cancer
InactiveUS20060154267A1Improve survival outcomeGene expressionMechanical/radiation/invasive therapiesData processing applicationsCurative effectOncology
Methods and compositions are provided for the identification of expression signatures in ER+ breast cancer cases, where the signatures correlate with responsiveness, or lack thereof, to treatment with tamoxifen or another antiestrogen agent against breast cancer The signature profiles are identified based upon sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for predicting the efficacy of treating a subject with breast cancer with tamoxifen or another antiestrogen agent against breast cancer. Additional methods and compositions are provided for predicting responsiveness to tamoxifen or another antiestrogen agent against breast cancer in cases of breast cancer by use of multiple biomarkers. Two biomarkers display increased expression correlated with tamoxifen response while two other biomarkers display decreased expression correlated with tamoxifen response.
Owner:AVIARADX +1
Micrornas expression signature for determination of tumors origin
Owner:ROSETTA GENOMICS +1
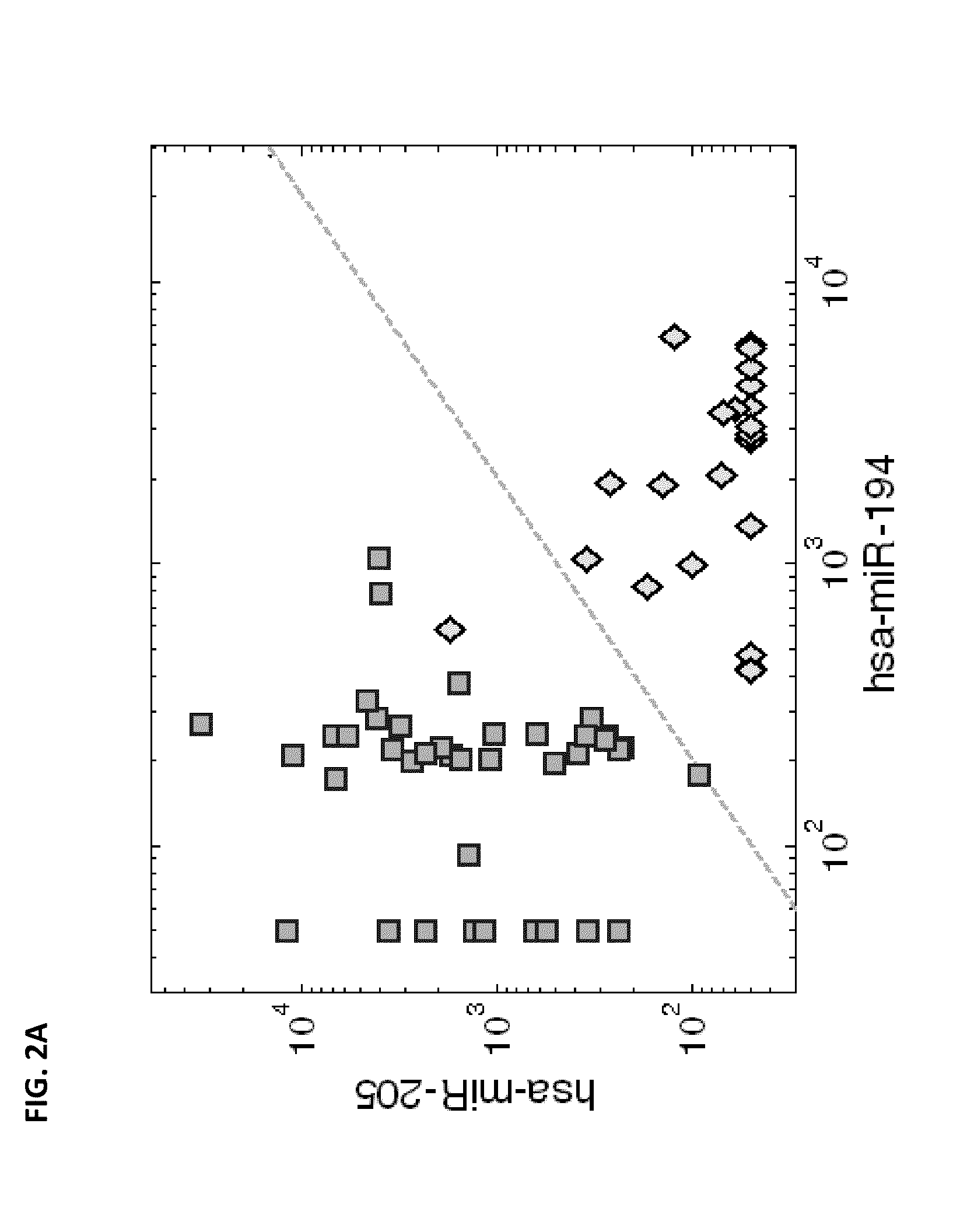
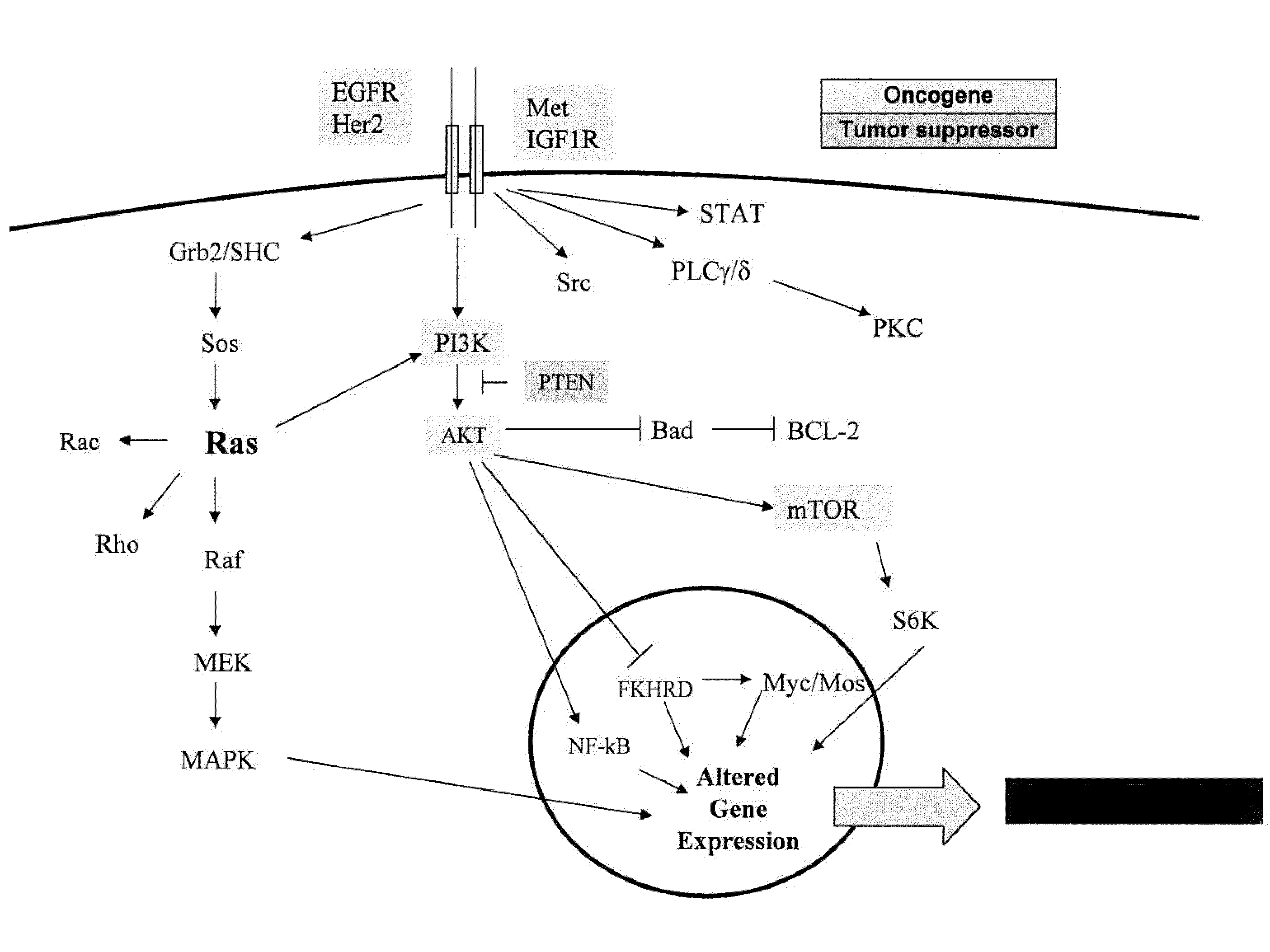
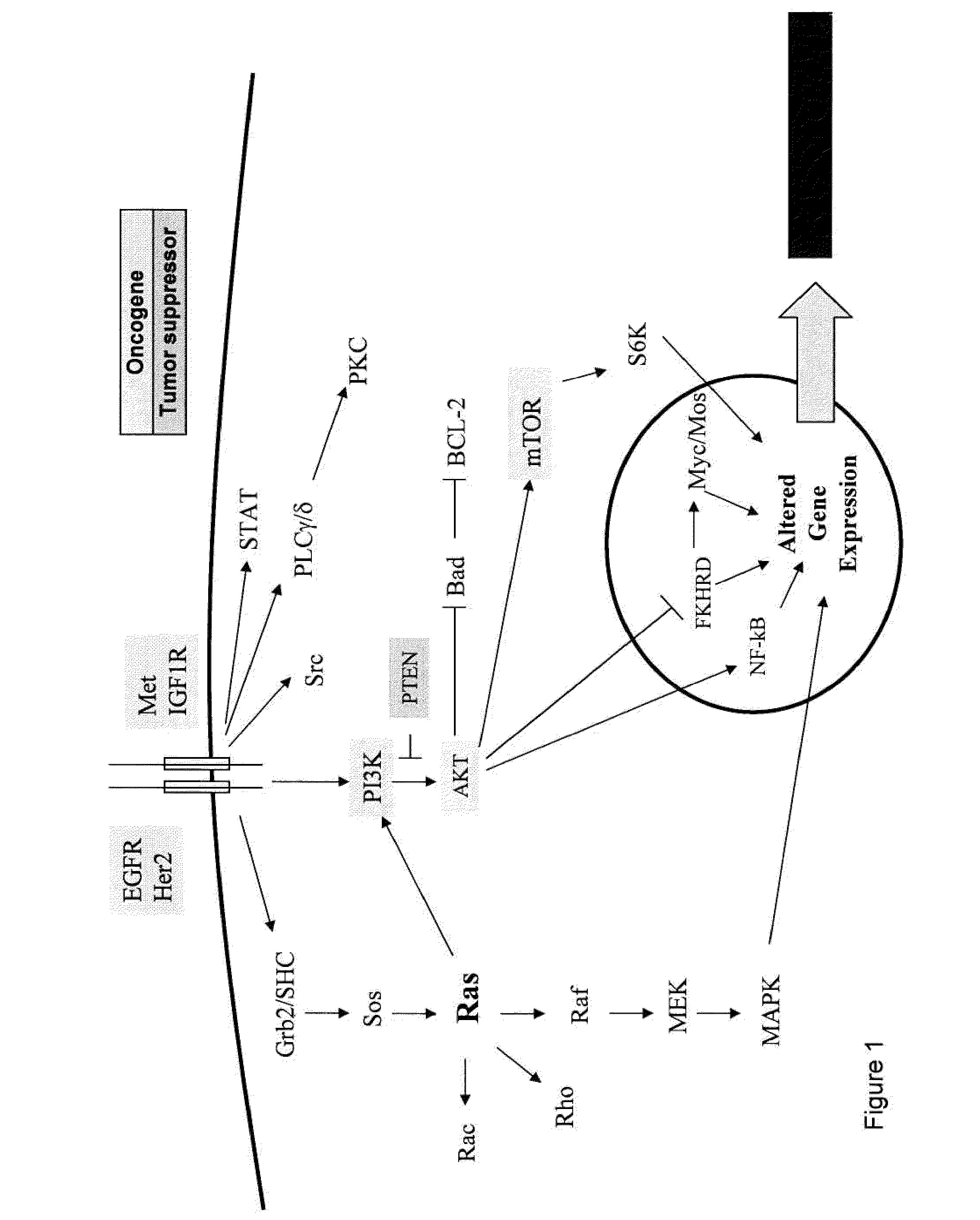
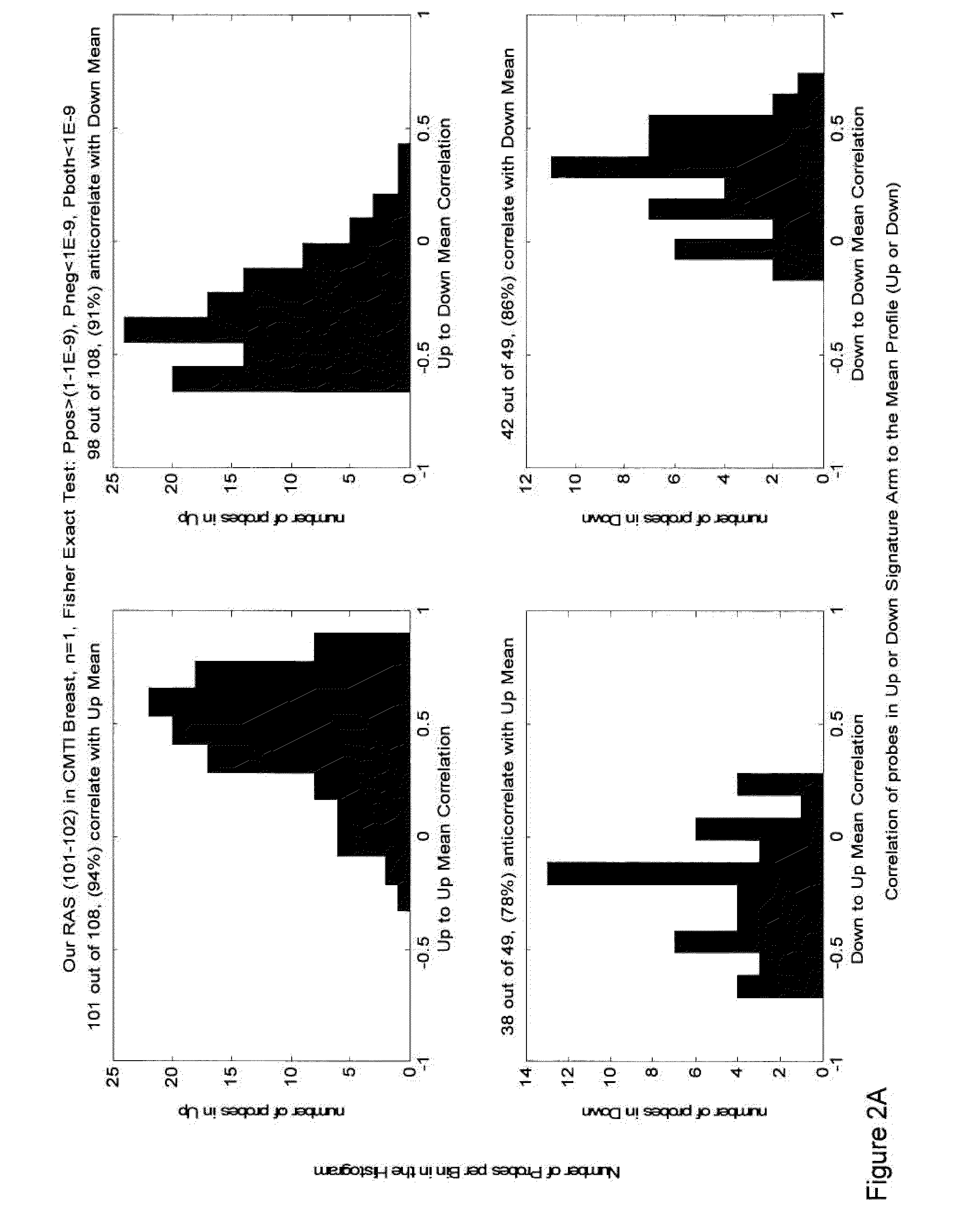
Methods and gene expression signature for assessing ras pathway activity
Methods, biomarkers, and expression signatures are disclosed for assessing the regulation status of RAS pathway signaling in a cell sample or subject. More specifically, several aspects of the invention provide a set of genes which can be used as biomarkers and gene signatures for evaluating RAS pathway deregulation status in a sample; classifying a cell sample as having a deregulated or regulated RAS signaling pathway; determining whether an agent modulates the RAS signaling pathway in sample; predicting response of a subject to an agent that modulates the RAS signaling pathway; assigning treatment to a subject; and evaluating the pharmacodynamic effects of cancer therapies designed to regulate RAS pathway signaling.
Owner:MERCK SHARP & DOHME CORP
Predicting breast cancer treatment outcome
InactiveCN1969047ABioreactor/fermenter combinationsBiological substance pretreatmentsPharmaceutical drugOncology
Methods and compositions are provided for the identification of expression signatures in ER+ breast cancer cases, where the signatures correlate with responsiveness, or lack thereof, to treatment with tamoxifen or another antiestrogen agent against breast cancer. The signature profiles are identified based upon sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for predicting the efficacy of treating a subject with breast cancer with tamoxifen or another antiestrogen agent against breast cancer. Additional methods and compositions are provided for predicting responsiveness to tamoxifen or another antiestrogen agent against breast cancer in cases of breast cancer by use of multiple biomarkers. Two biomarkers display increased expression correlated with tamoxifen response while two other biomarkers display decreased expression correlated with tamoxifen response.
Owner:阿克丘勒斯生物科学股份有限公司
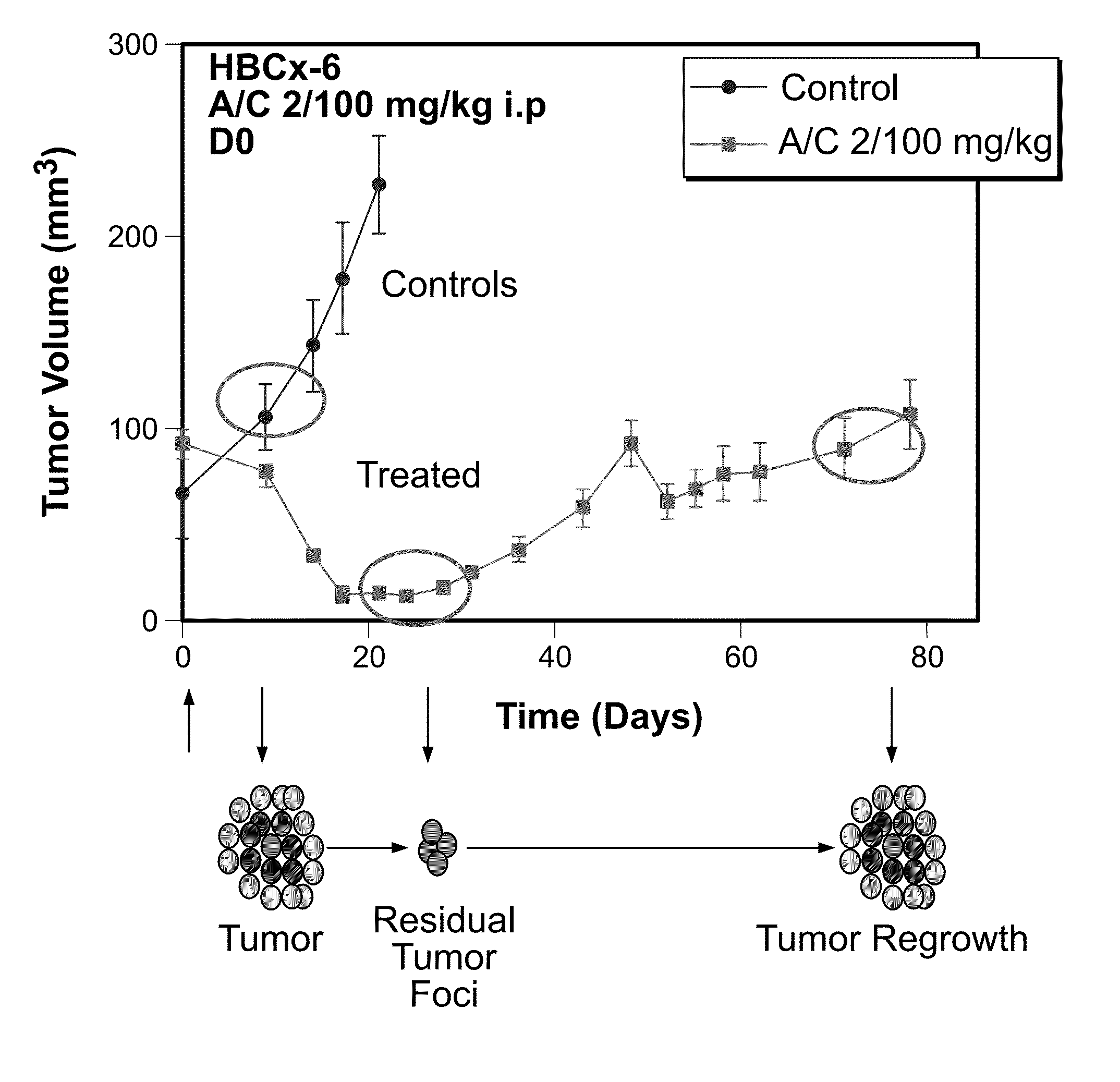
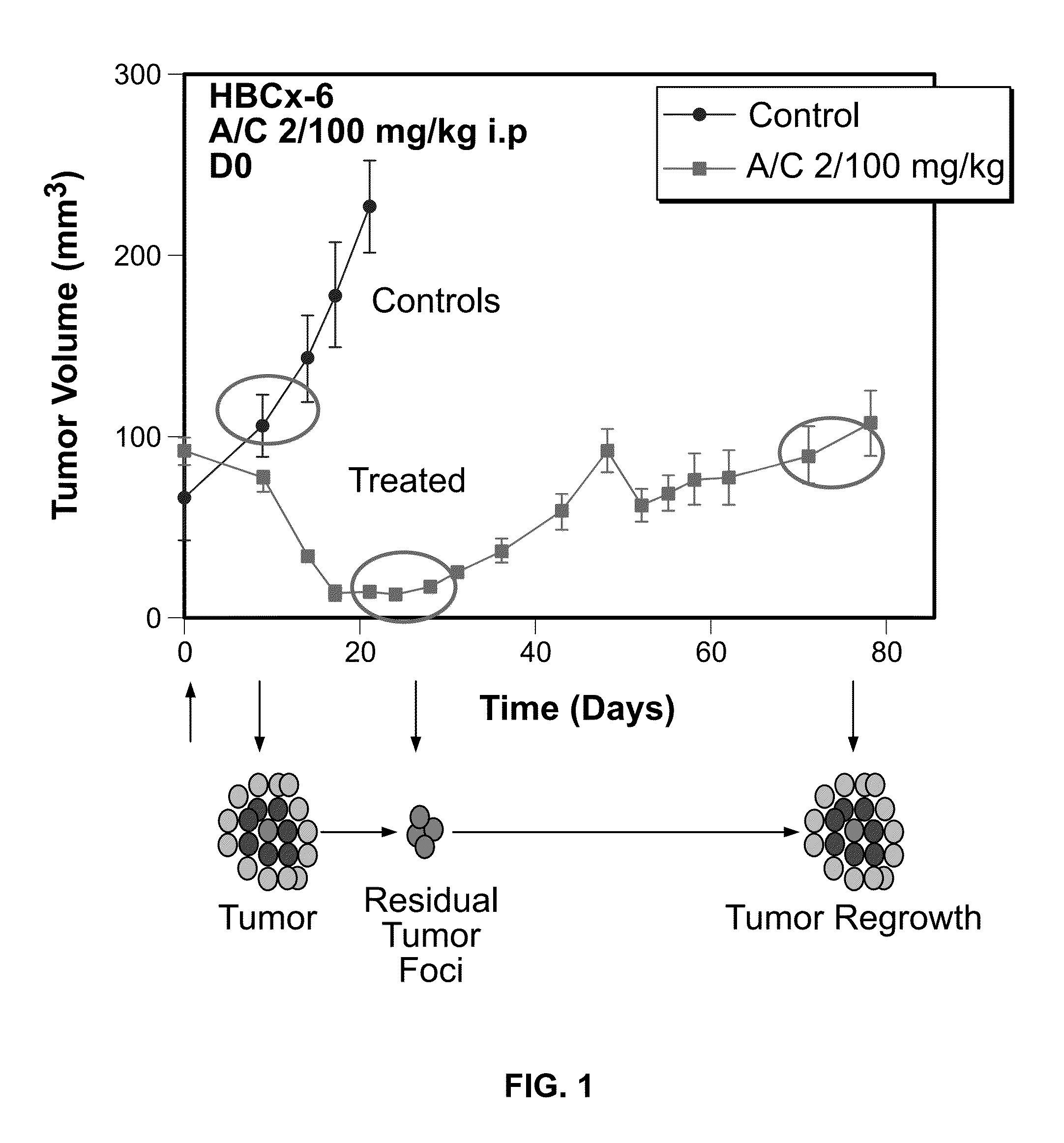
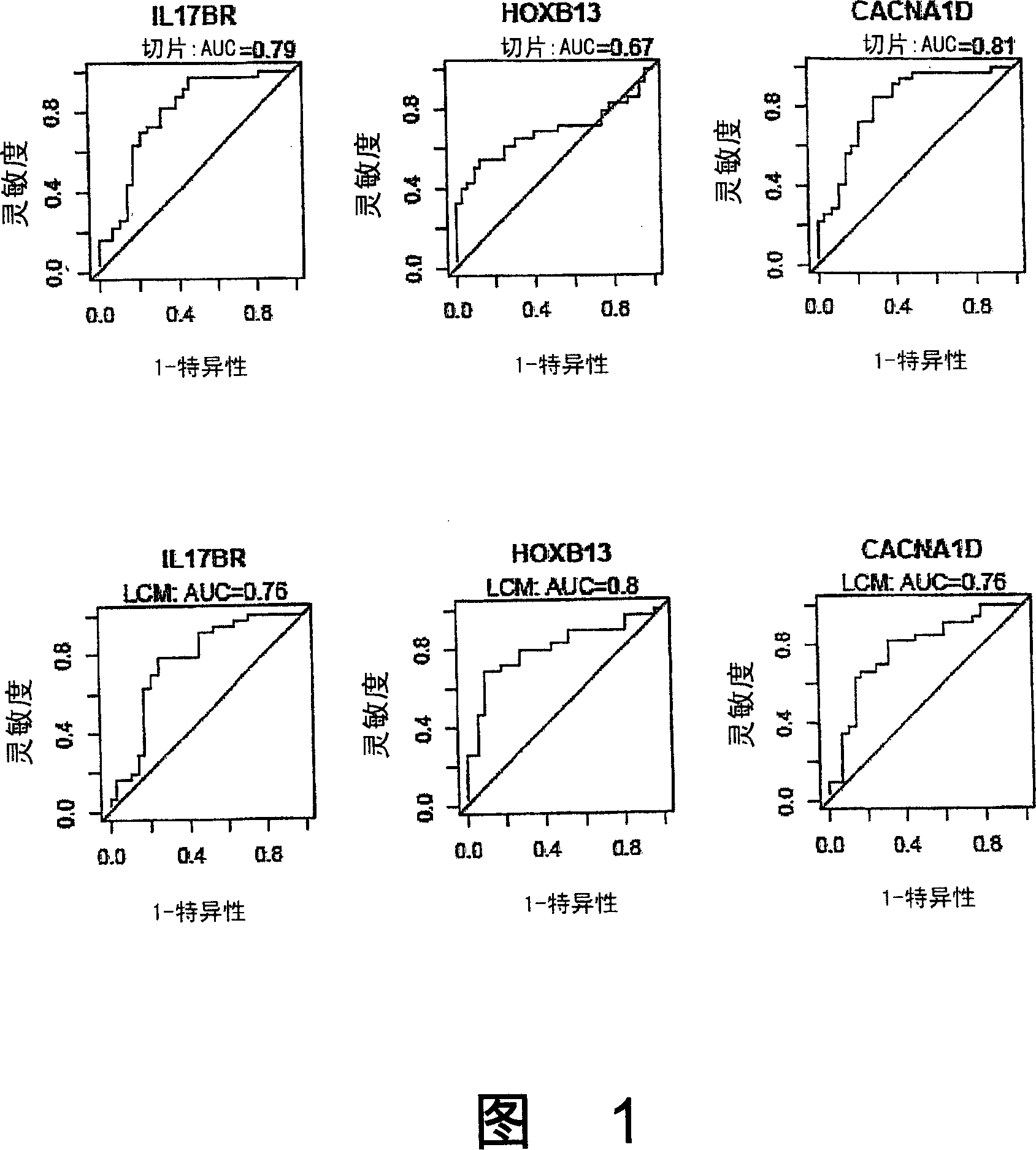
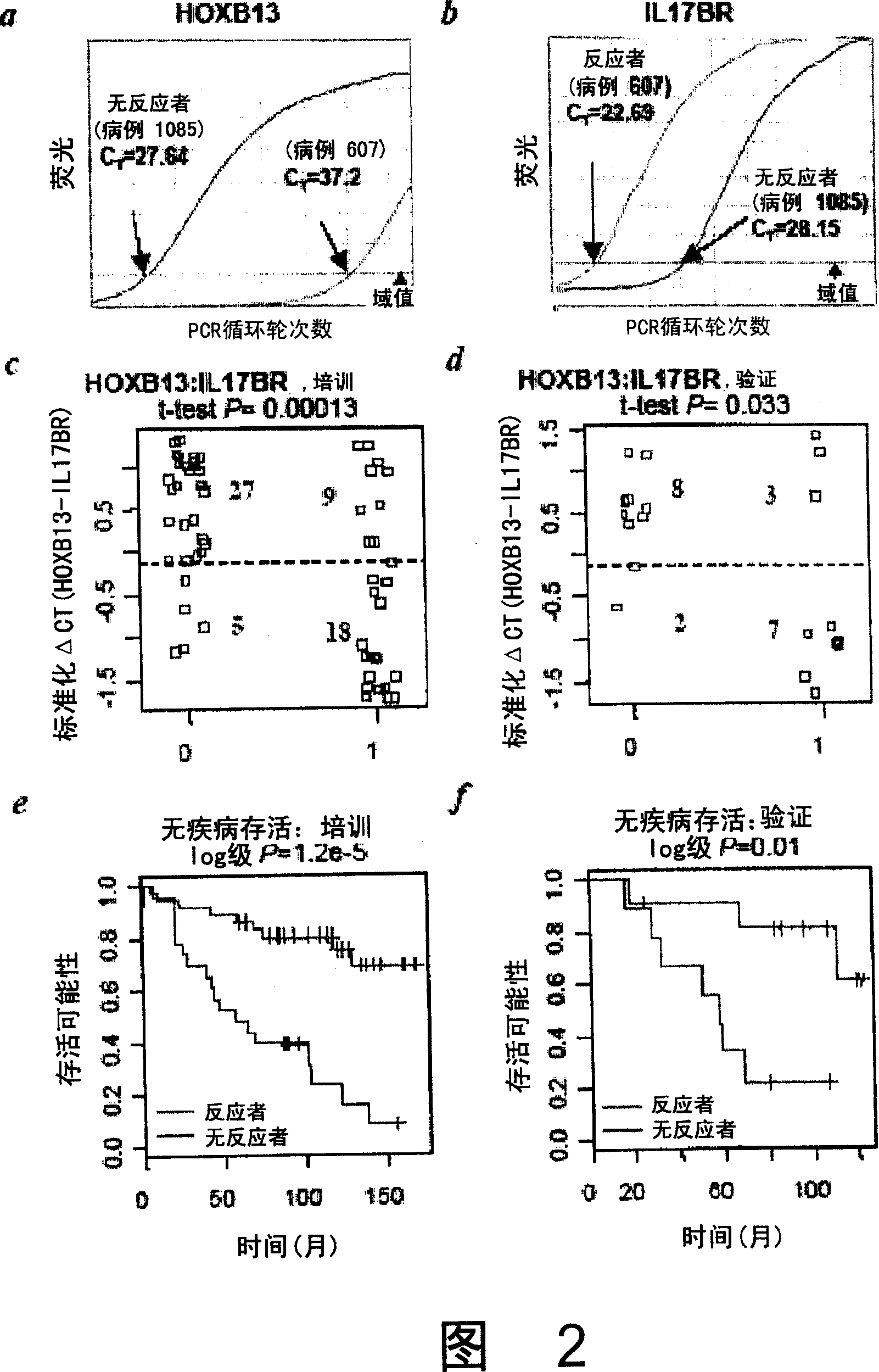
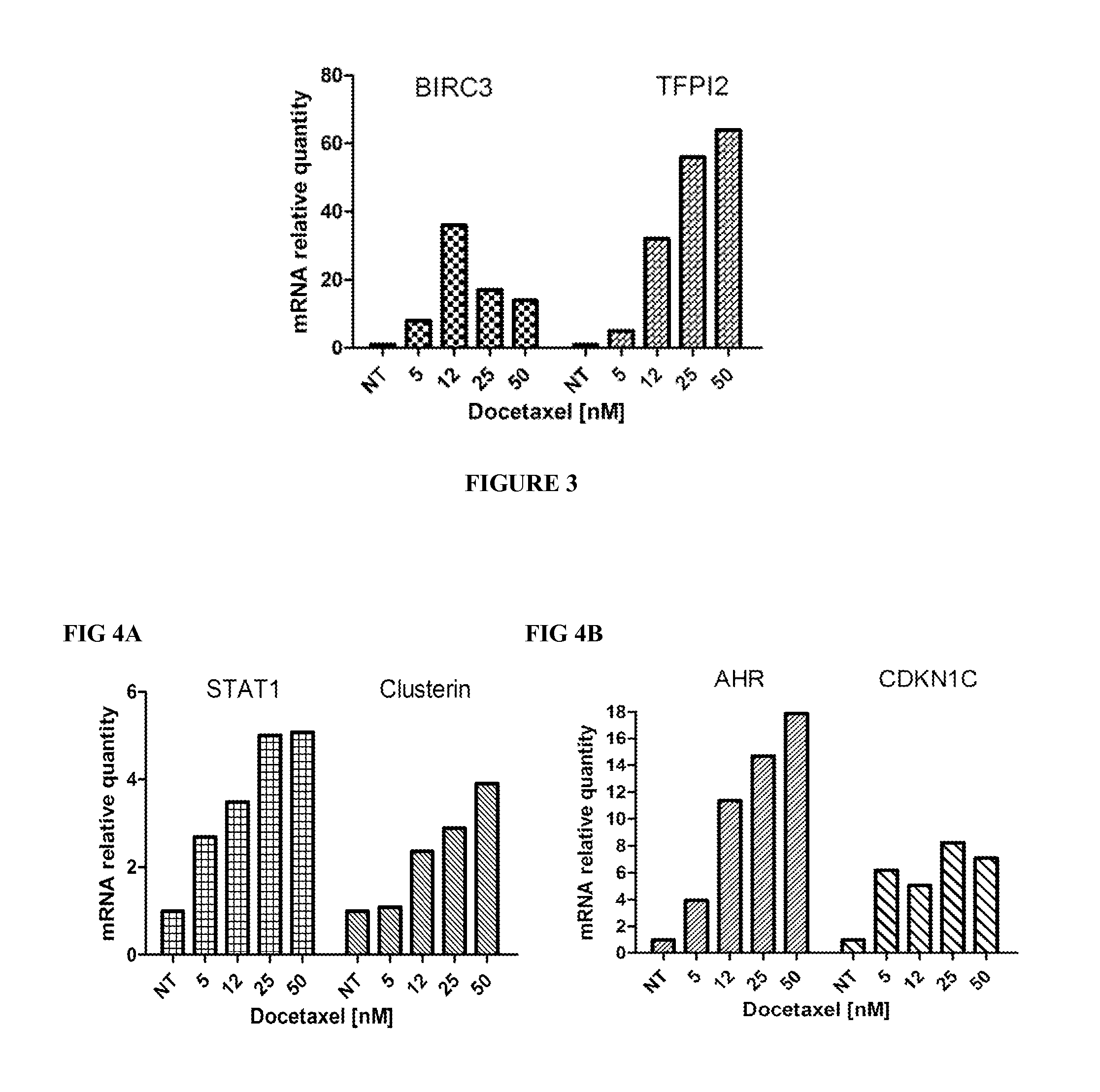
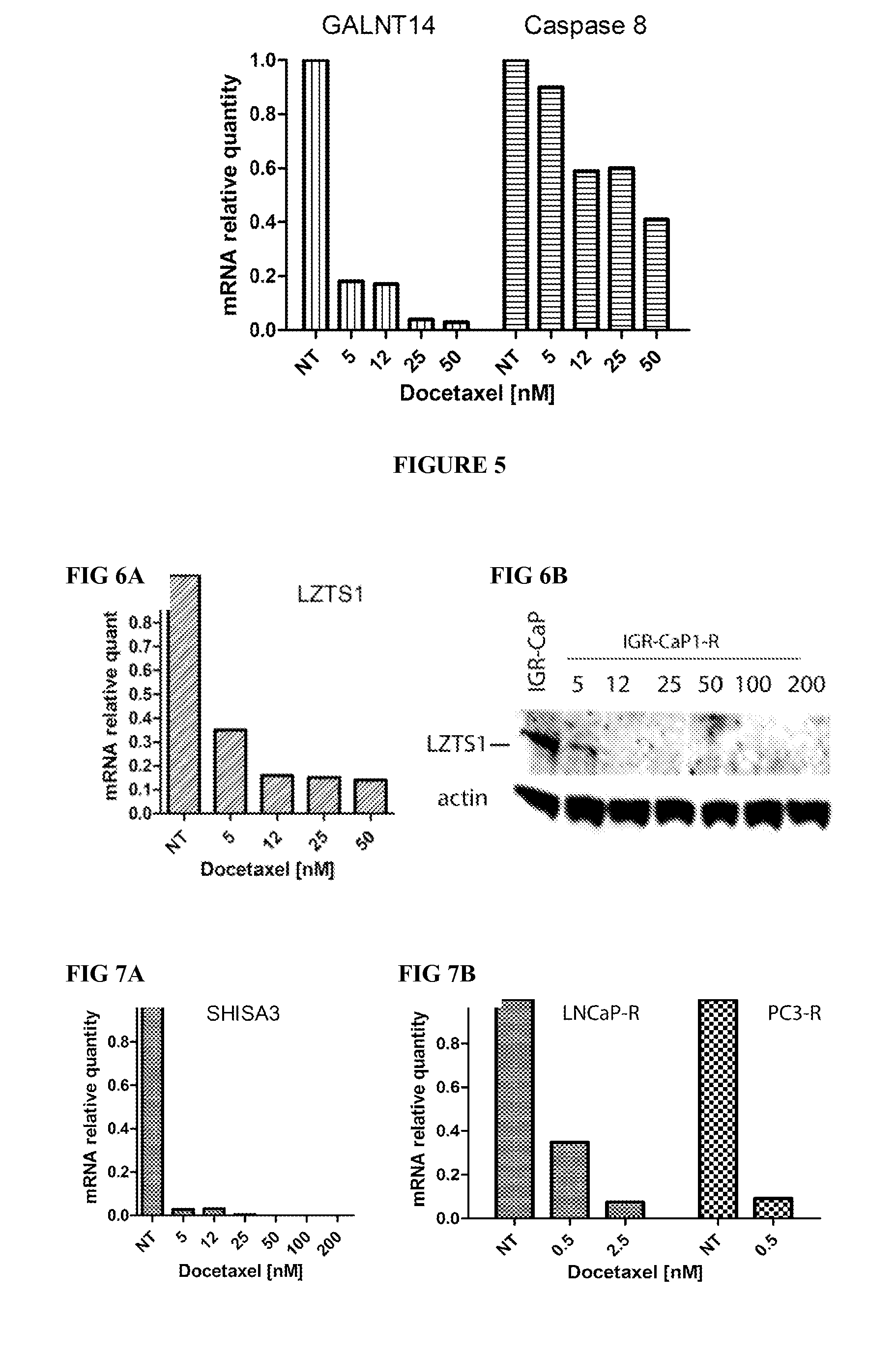
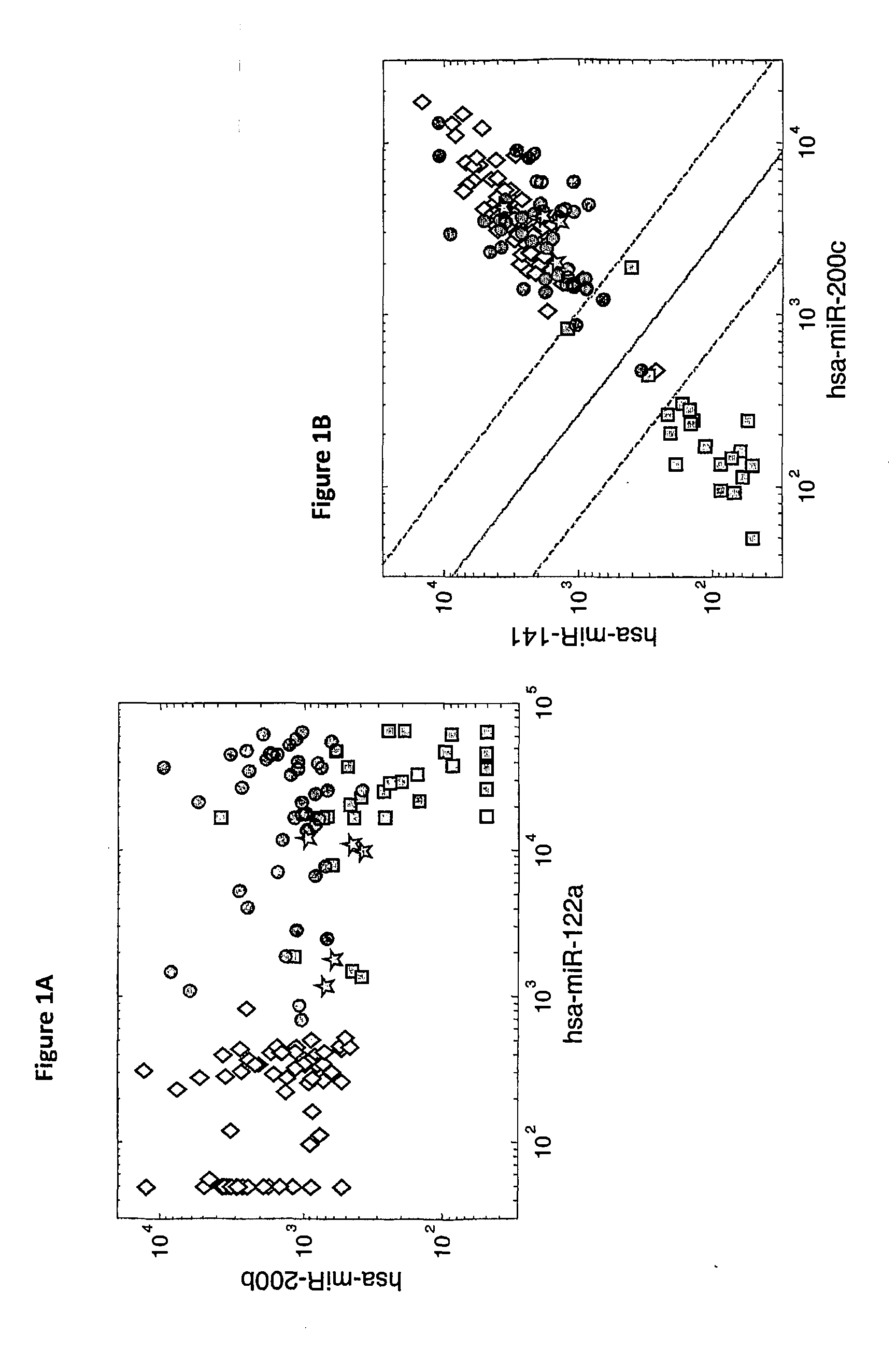
Methods for predicting or monitoring whether a patient affected by a cancer is responsive to a treatment with a molecule of the taxoid family
InactiveUS20110177970A1Improved treatment of cancerReduce development of resistanceNucleotide librariesMicrobiological testing/measurementPatient affectedMedicine
The present invention concerns in vitro methods for predicting or monitoring whether a patient affected by a cancer is responsive to a treatment with a molecule of the taxoid family based on a resistance expression signature, kits for performing the methods, and methods for screening or identifying a compound suitable for improving the treatment of a cancer with a molecule of the taxoid family or for reducing the resistance development during the treatment of a cancer with the molecule of the taxoid family.
Owner:INSTITUT GUSTAVE ROUSSY
Micrornas expression signature for determination of tumors origin
The present invention provides a process for classification of specific cancers and tumors origin through the analysis of the expression patterns of specific microRNAs and nucleic acid molecules relating thereto. Classification according to a microRNA expression framework allows optimization of treatment, and determination of specific therapy.
Owner:ROSETTA GENOMICS +1
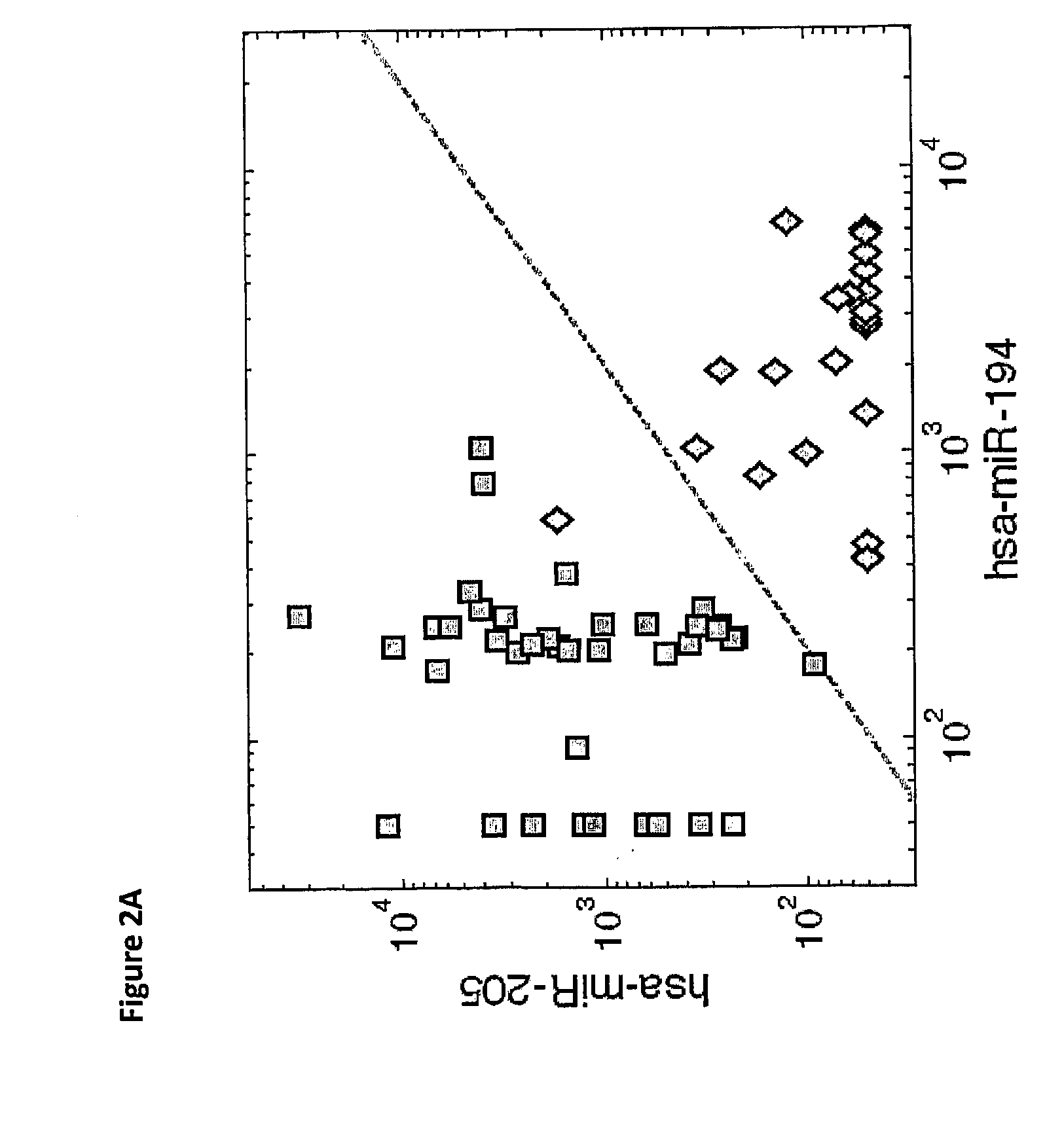
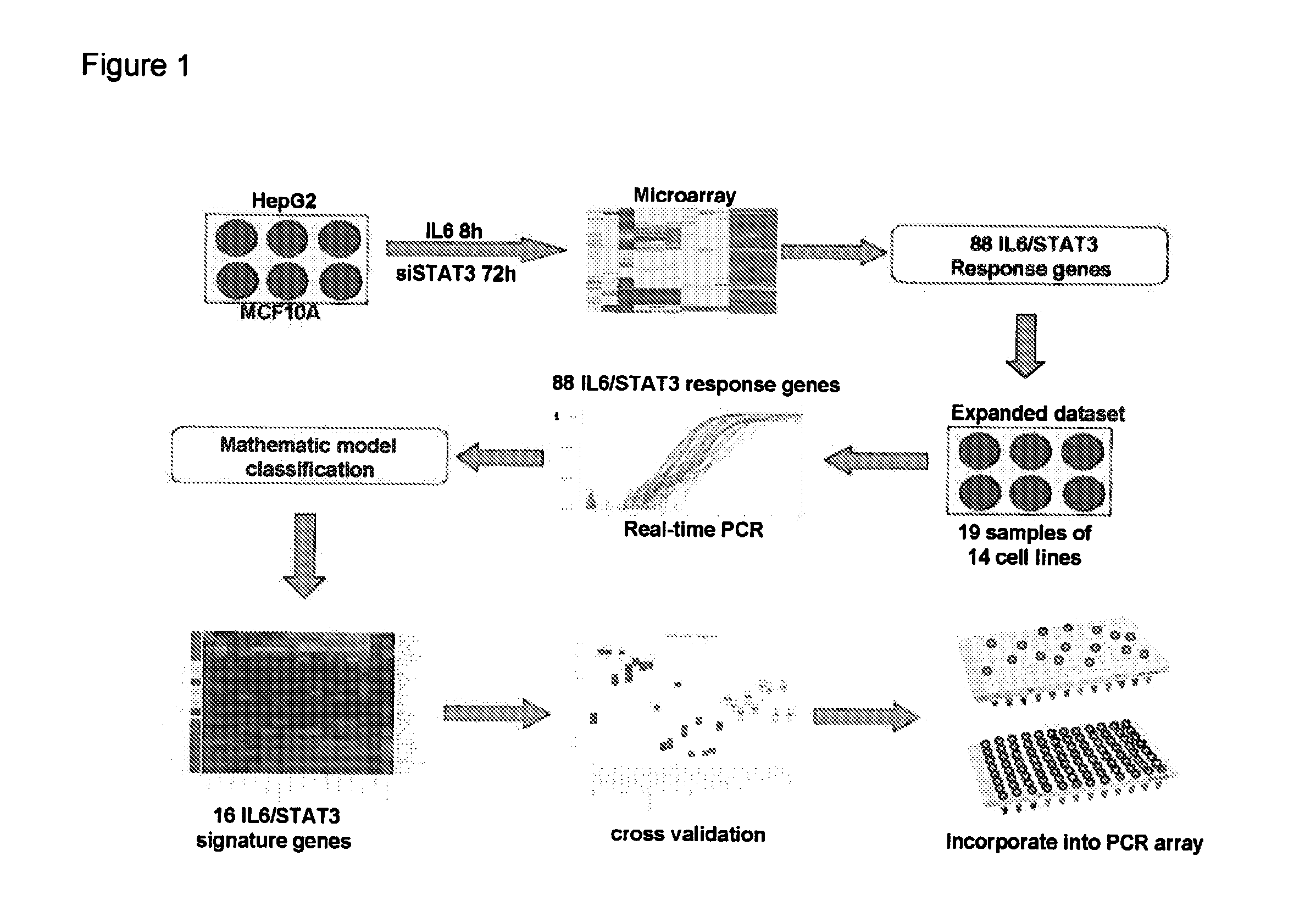
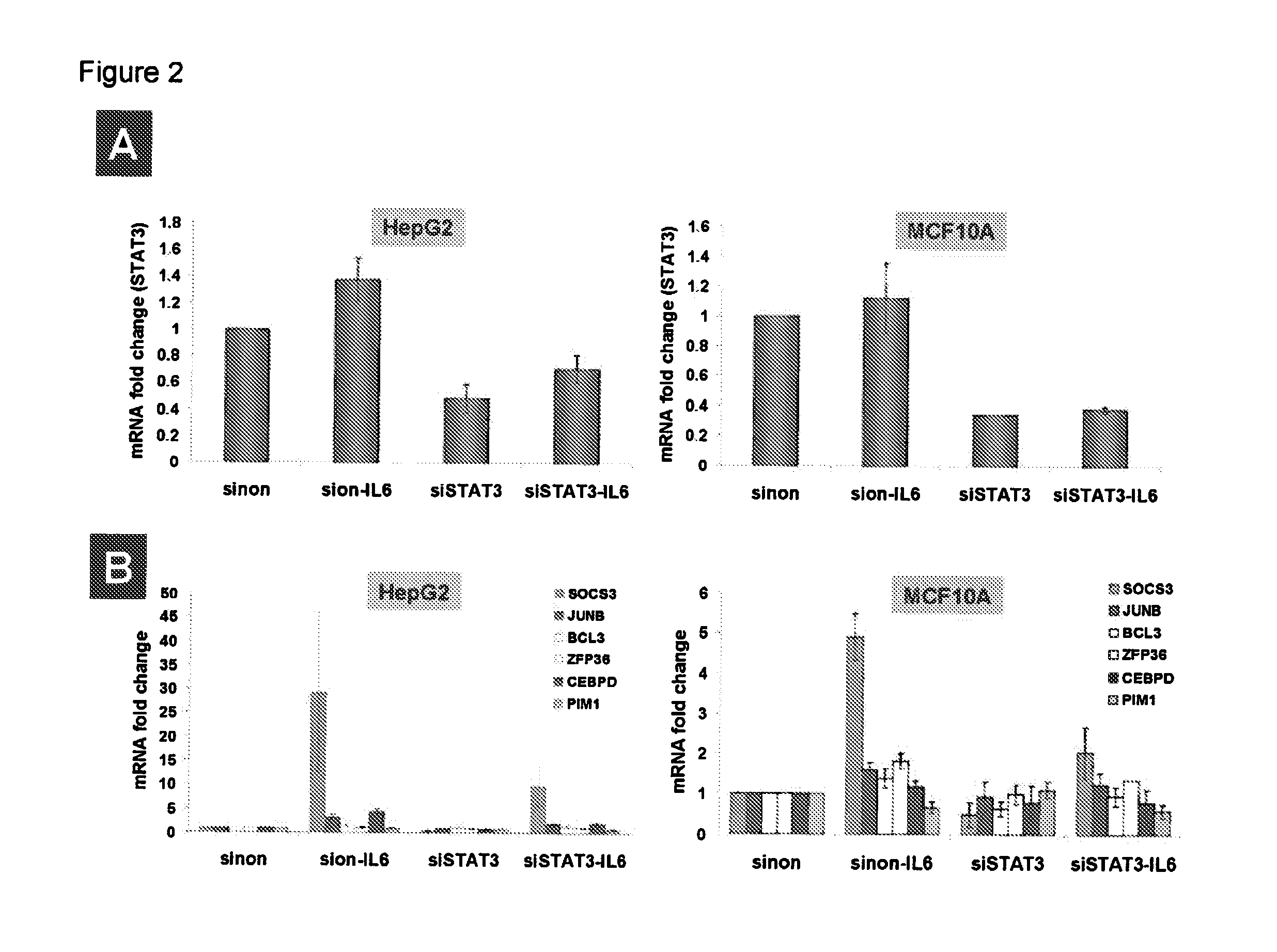
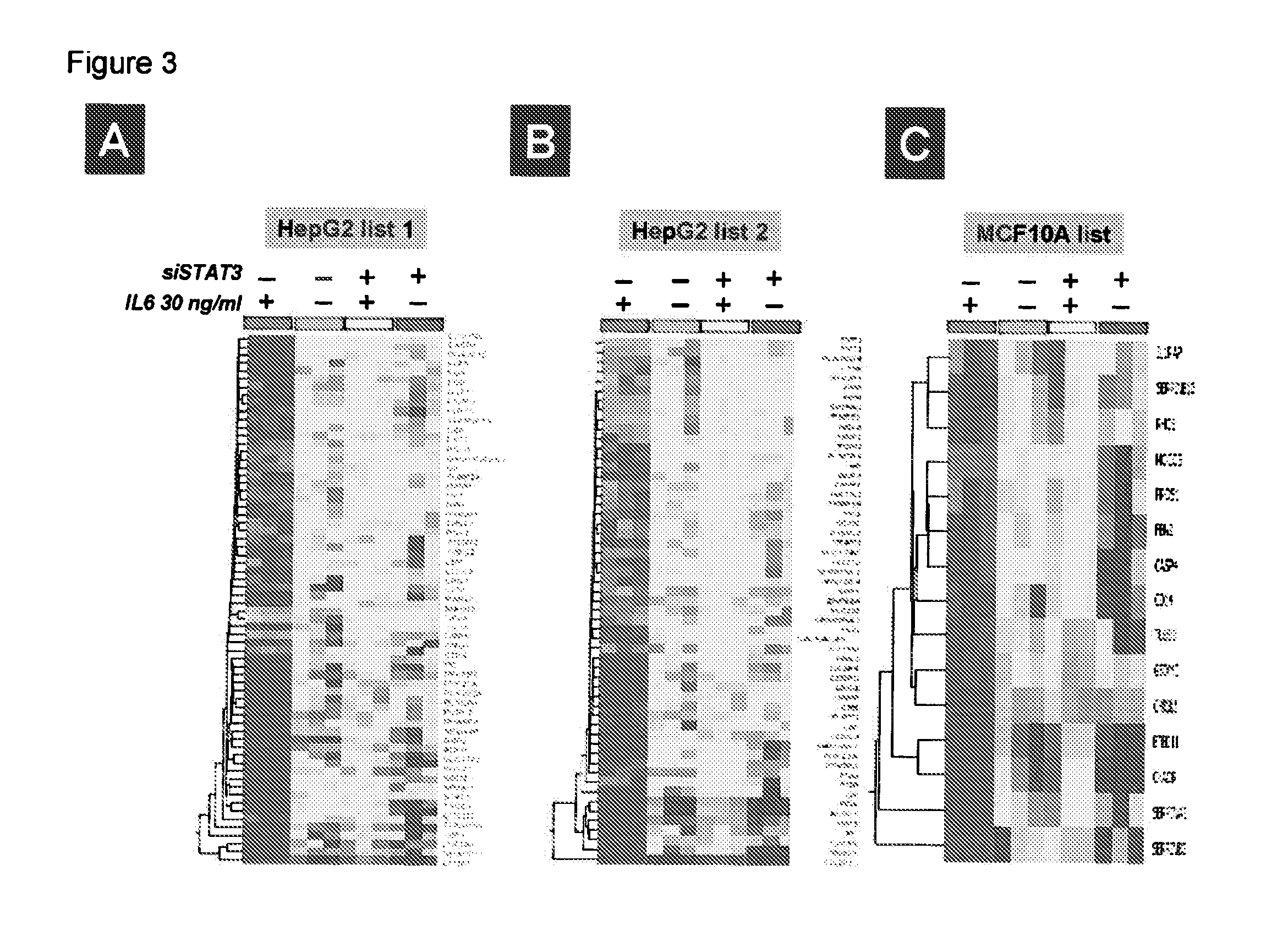
Gene expression signature for il-6/stat3 signaling pathway and use thereof
ActiveUS20150232926A1Reliable measurementMicrobiological testing/measurementDNA/RNA fragmentationRandom forestStat3 Signaling Pathway
The present invention relates to a set of biomarkers, microarrays that provide for detection thereof, an expression signature comprising 16 genes or a subset thereof, and the use thereof in determining the regulation status of IL-6 / STAT3 signaling pathway in a cell sample or subject, as well as compositions for the detection thereof. The regulation status of IL-6 / STAT3 signaling pathway in a cell sample or subject may be assayed based on the level of expression of one or more of these genes. The methods and compositions provided herein may be used to evaluate IL-6 / STAT3 pathway regulation status in a sample; classify a cell sample as having a deregulated or regulated IL-6 / STAT3 signaling pathway; determine whether an agent modulates the IL-6 / STAT3 signaling pathway; predict the response of a subject to an agent that modulates the IL-6 / STAT3 signaling pathway; assign treatment to a subject; and / or evaluate the pharmacodynamic effects of therapies designed to regulate IL-6 / STAT3 pathway signaling. Expression of the biomarkers is preferably determined by RT-PCR using SYBR Green methods, and the expression data analyzed and compared to a control sample by use of the random forest method.
Owner:QIAGEN SCIENCES LLC +1
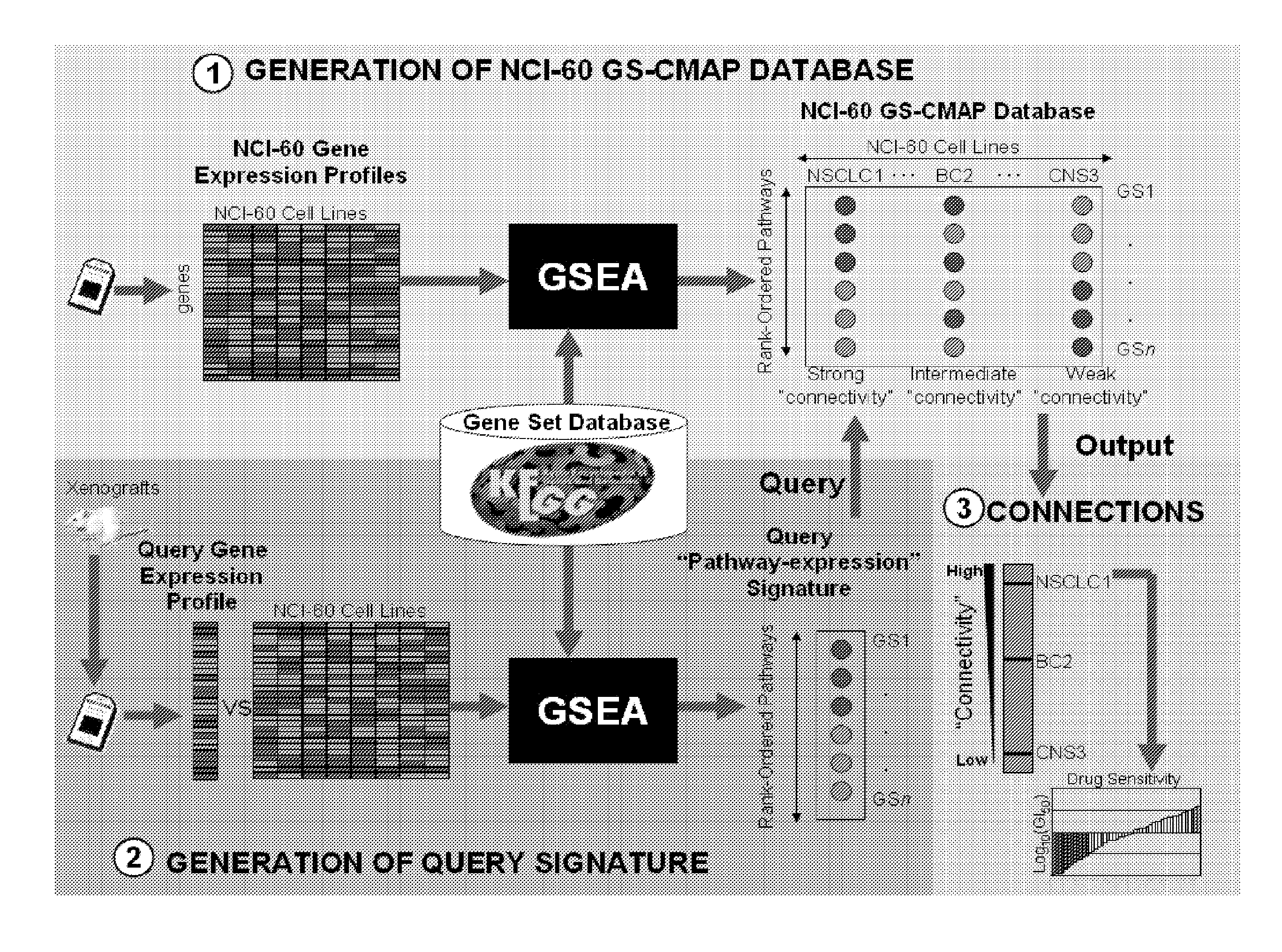
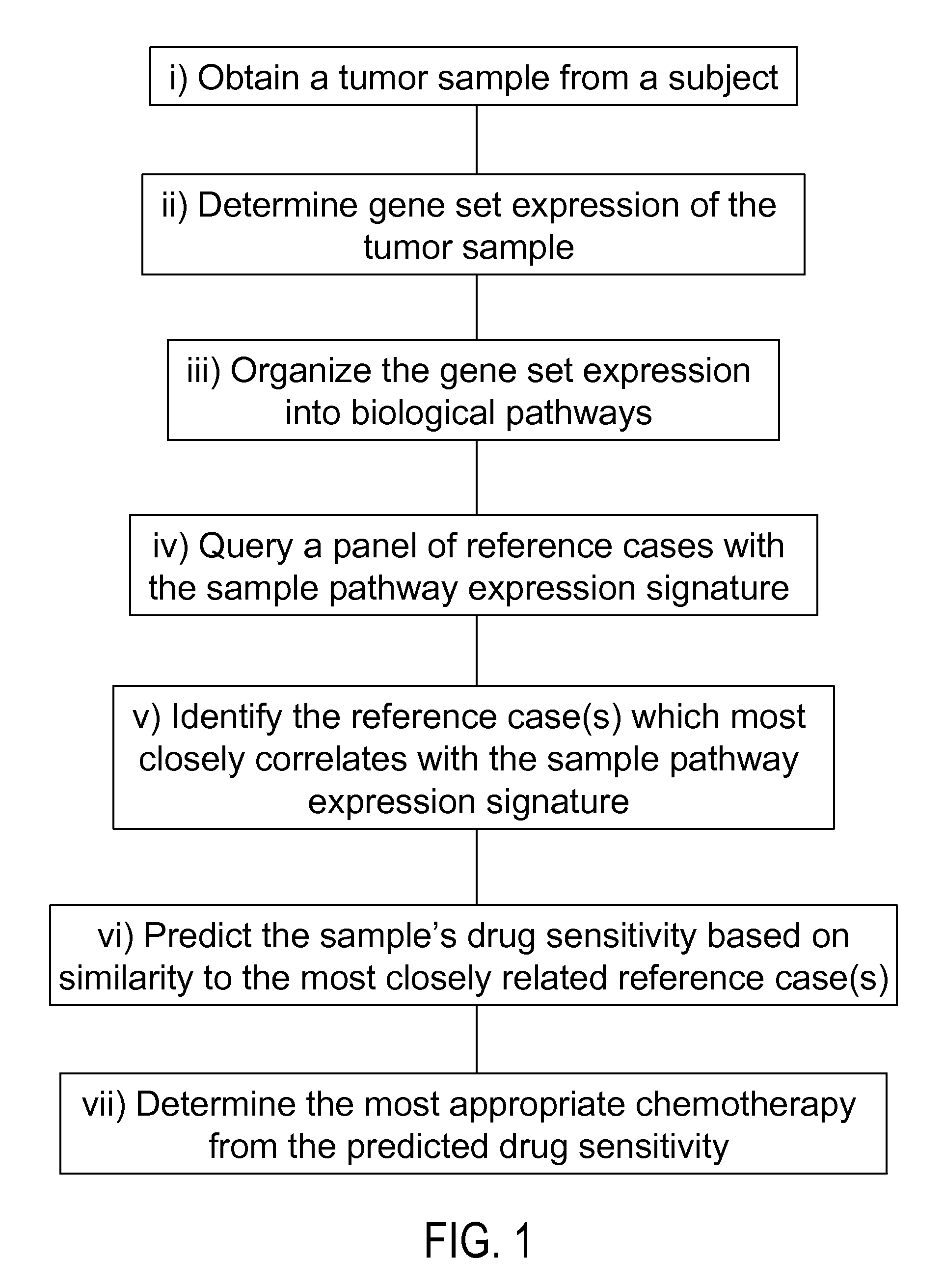
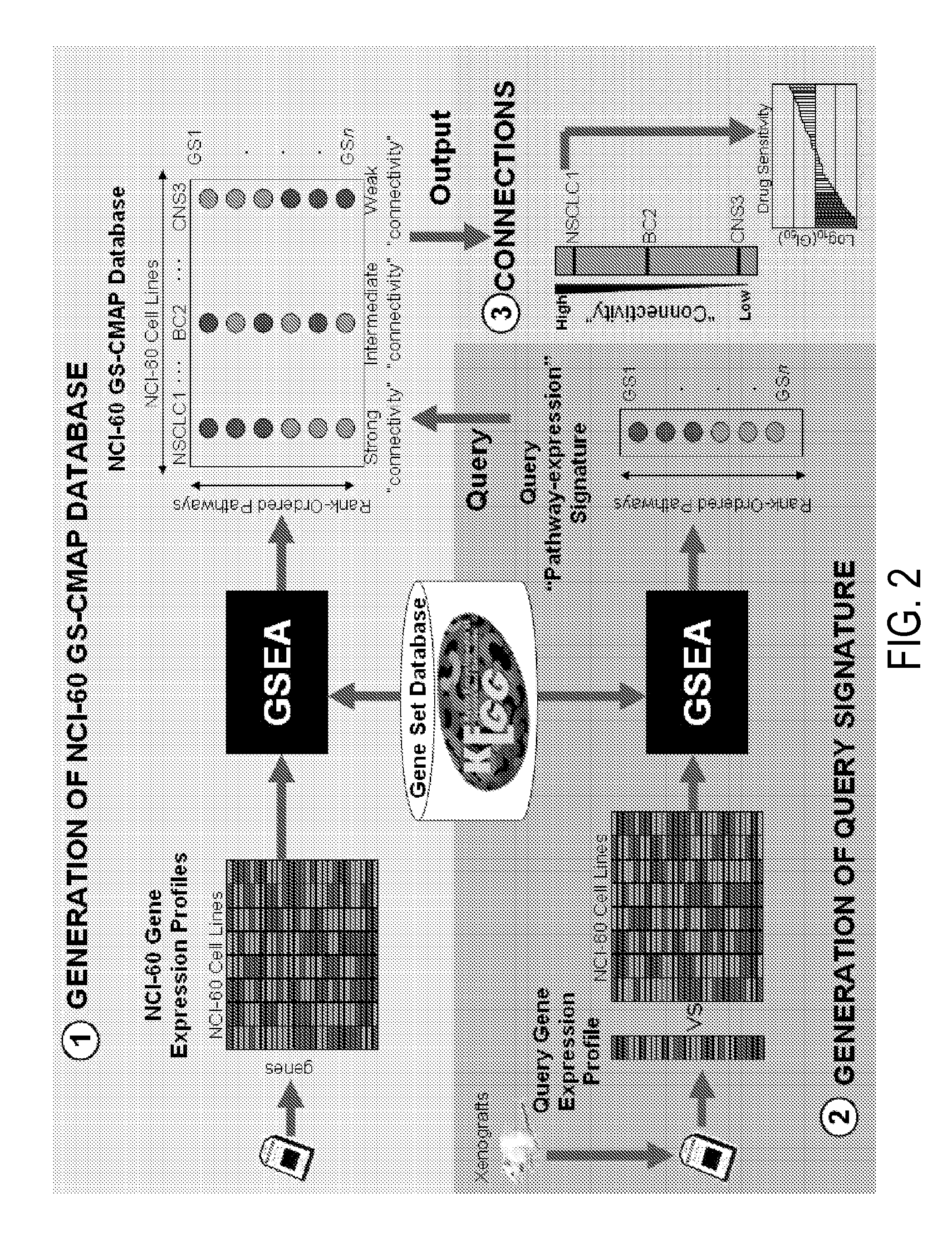
Methods to correct gene set expression profiles to drug sensitivity
The present invention comprises a treatment approach based on gene set-expression signatures that systematically connects a sample to a profile from a reference database to extrapolate the most effective therapeutic agent. Further disclosed are methods to optimize combination treatments.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
MIRNA expression signature in classification of thyroid tumors
InactiveCN106460053AMicrobiological testing/measurementAntineoplastic agentsRna expressionThyroid tumors
The present invention provides a method for classification of thyroid tumors through the analysis of the expression patterns of specific microRNAs in fine needle aspiration samples. Thyroid tumor classification according to a microRNA expression signature allows optimization of diagnosis and treatment, as well as determination of signature-specific therapy.
Owner:ROSETTA GENOMICS
Compositions and methods for micro-RNA expression profiling of colorectal cancer
The present invention relates compositions and methods for microRNA (miRNA) expression profiling of colorectal cancer. In particular, the invention relates to a diagnostic kit of molecular markers for identifying one or more mammalian target cells exhibiting or having a predisposition to develop colorectal cancer, the kit comprising a plurality of nucleic acid molecules, each nucleic acid molecule encoding a miRNA sequence, wherein one or more of the plurality of nucleic acid molecules are differentially expressed in the target cells and in one or more control cells, and wherein the one or more differentially expressed nucleic acid molecules together represent a nucleic acid expression signature that is indicative for the presence of or the predisposition to develop colorectal cancer. The invention further relates to corresponding methods using such nucleic acid expression signatures for identifying one or more mammalian target cells exhibiting or having a predisposition to develop colorectal cancer as well as for preventing or treating such a condition. Finally, the invention is directed to a pharmaceutical composition for the prevention and / or treatment of colorectal cancer.
Owner:FUDAN UNIV
Prediction of Clinical Outcome in Hematological Malignancies Using a Self-Renewal Expression Signature
InactiveUS20140148351A1Inhibit hematological malignancyReduce expressionMicrobiological testing/measurementLibrary screeningScreening methodTissue sample
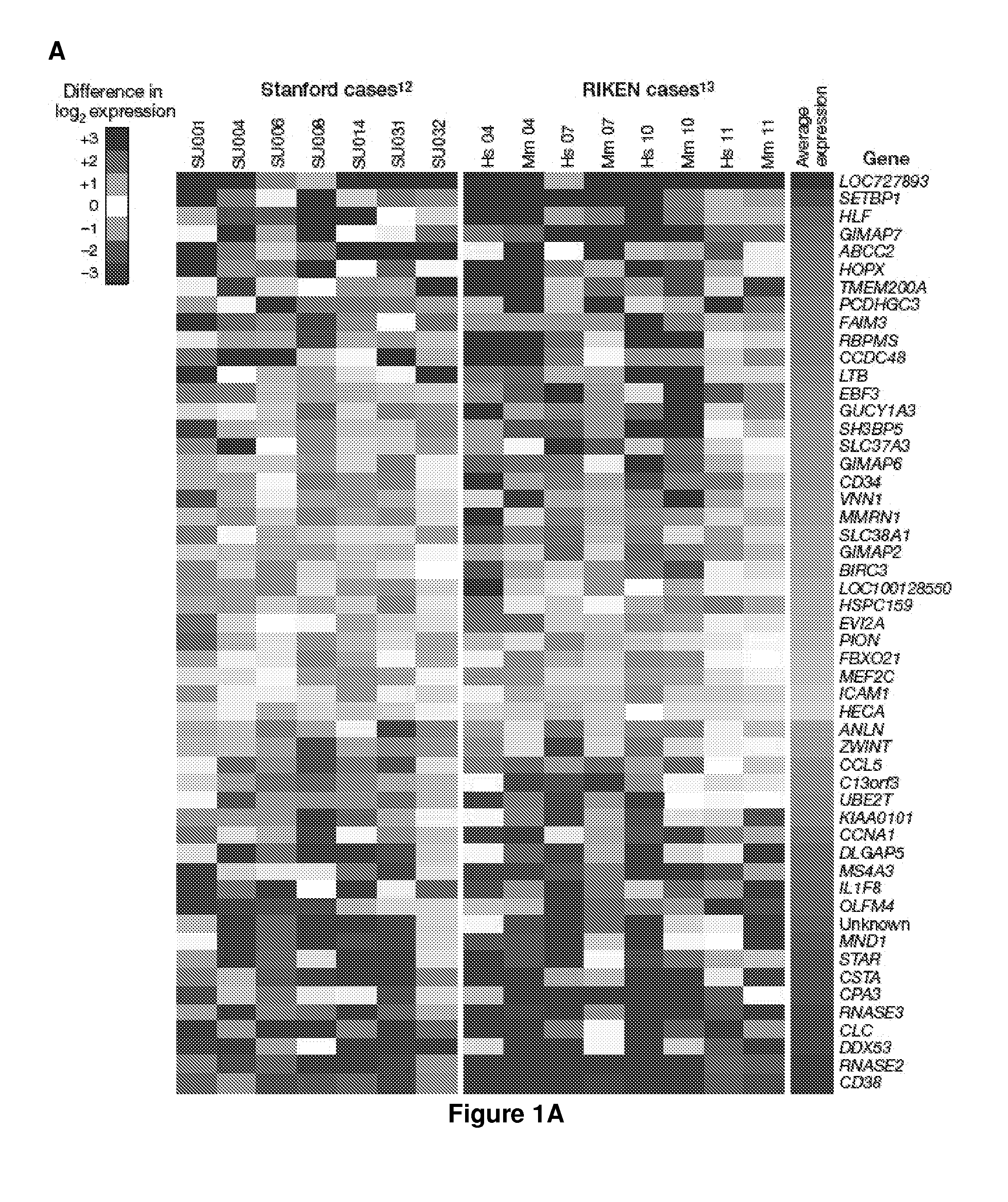
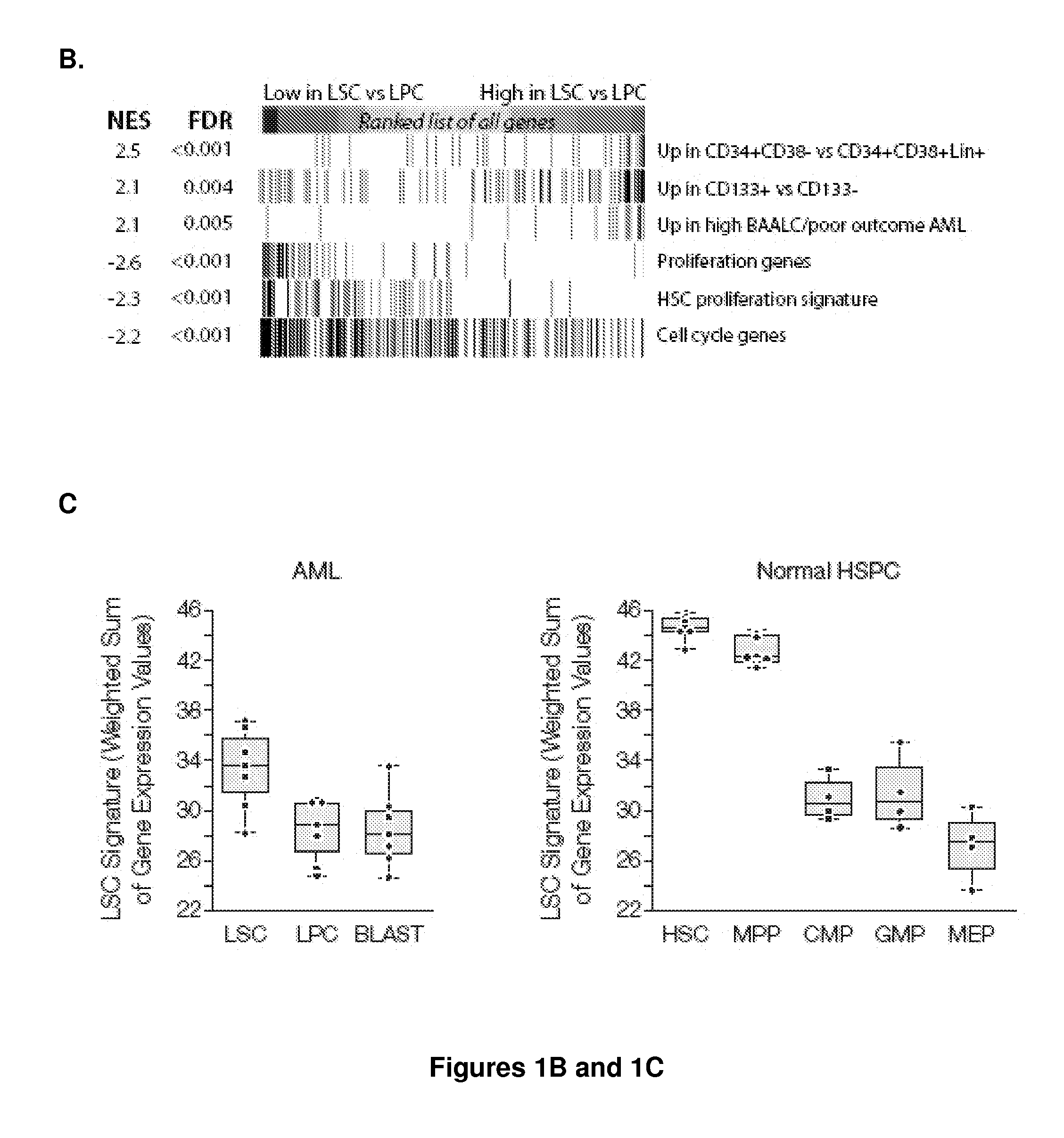
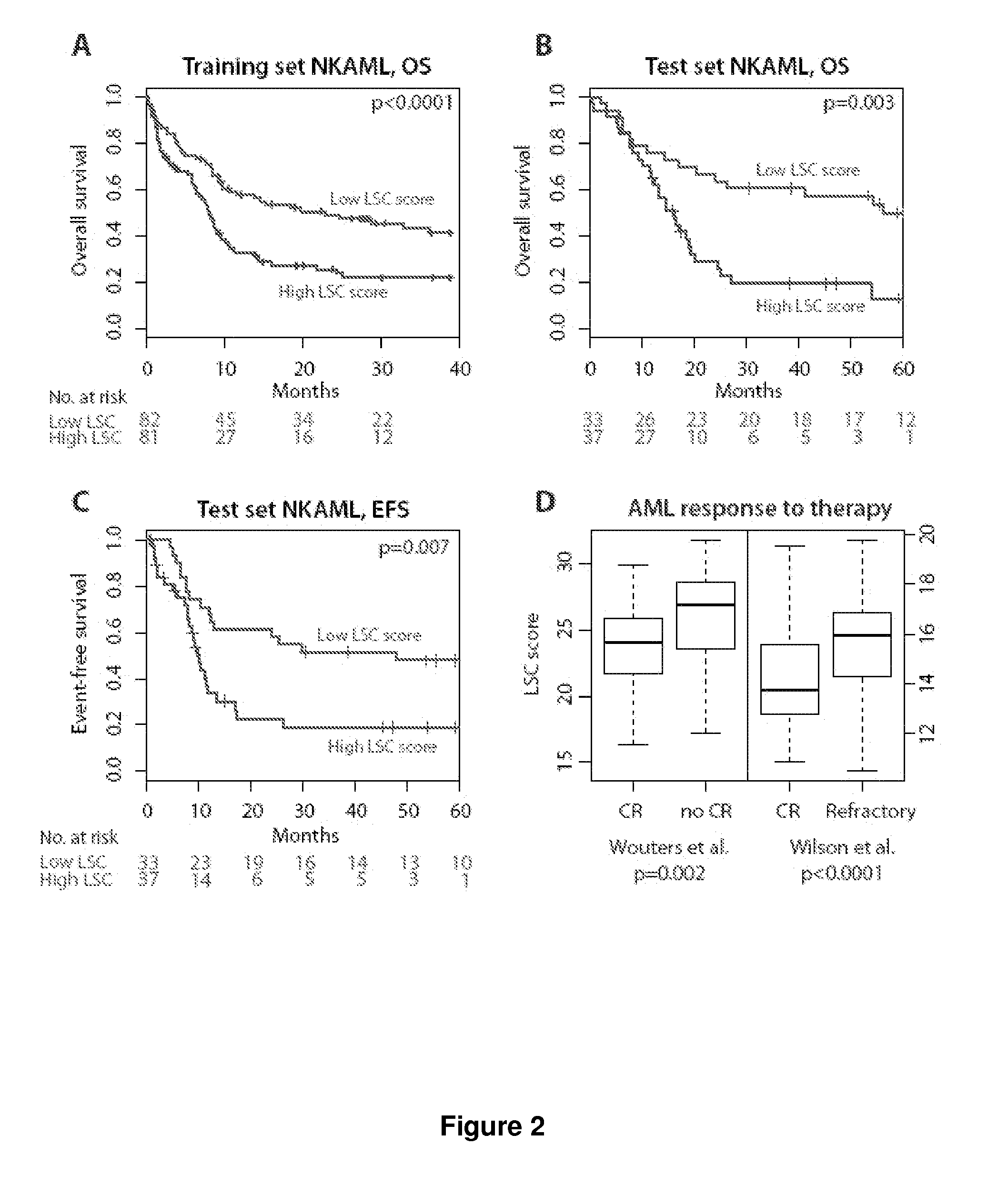
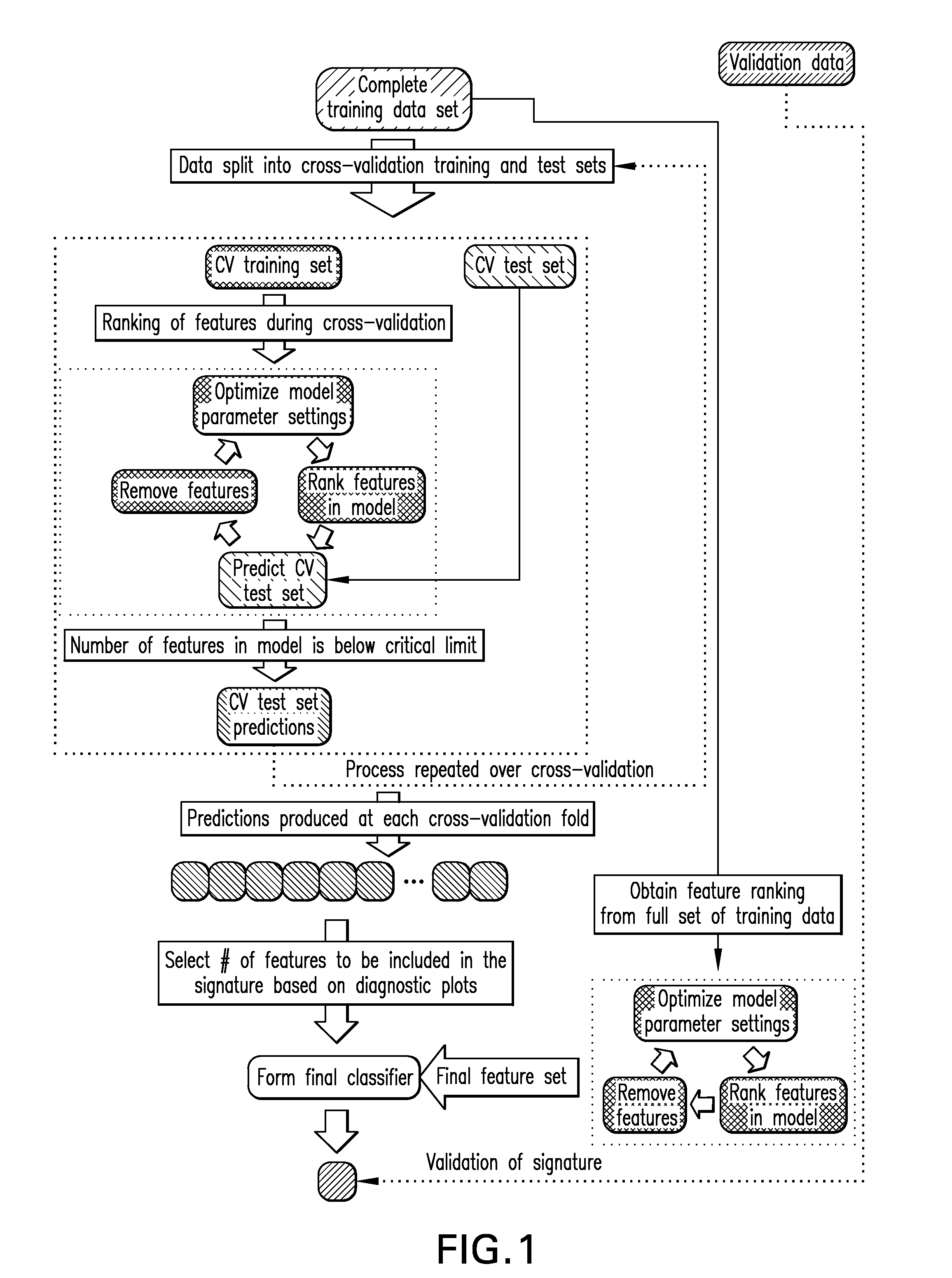
Methods, compositions, and kits are provided for providing a diagnosis, a prognosis, or a prediction of responsiveness to a therapy for a patient with a hematological malignancy. In practicing the subject methods, the expression level of at least one leukemia stem cell (LSC) genes in a tissue sample is assayed to obtain an LSC expression representation. The LSC expression representation is then employed to determine if an individual has a hematological malignancy, to provide a prognosis to a patient with a hematological malignancy, and / or to provide a prediction of the responsiveness of a patient with a hematological malignancy to a therapy. Also provided are screening methods for identifying novel therapies for patients with a hematological malignancy, and compositions and kits for use in these screening methods.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Colon Cancer Gene Expression Signatures and Methods of Use
InactiveUS20140094379A1Predict prognosisAccurate diagnosisSugar derivativesNucleotide librariesCancer genesOncology
A gene expression signature of colon cancer, microarrays including them and methods of using the colon gene expression signature are provided. The gene expression signature is especially useful for determining the prognosis of a patient diagnosed with colon cancer, such as stage II colon cancer. The gene signature described herein is also useful for determining effectiveness of surgical resection with or without adjuvant chemotherapy, and determining possibility of cancer recurrence in patients with colon cancer.
Owner:ALMAC DIAGNOSTICS SERVICES LIMITED
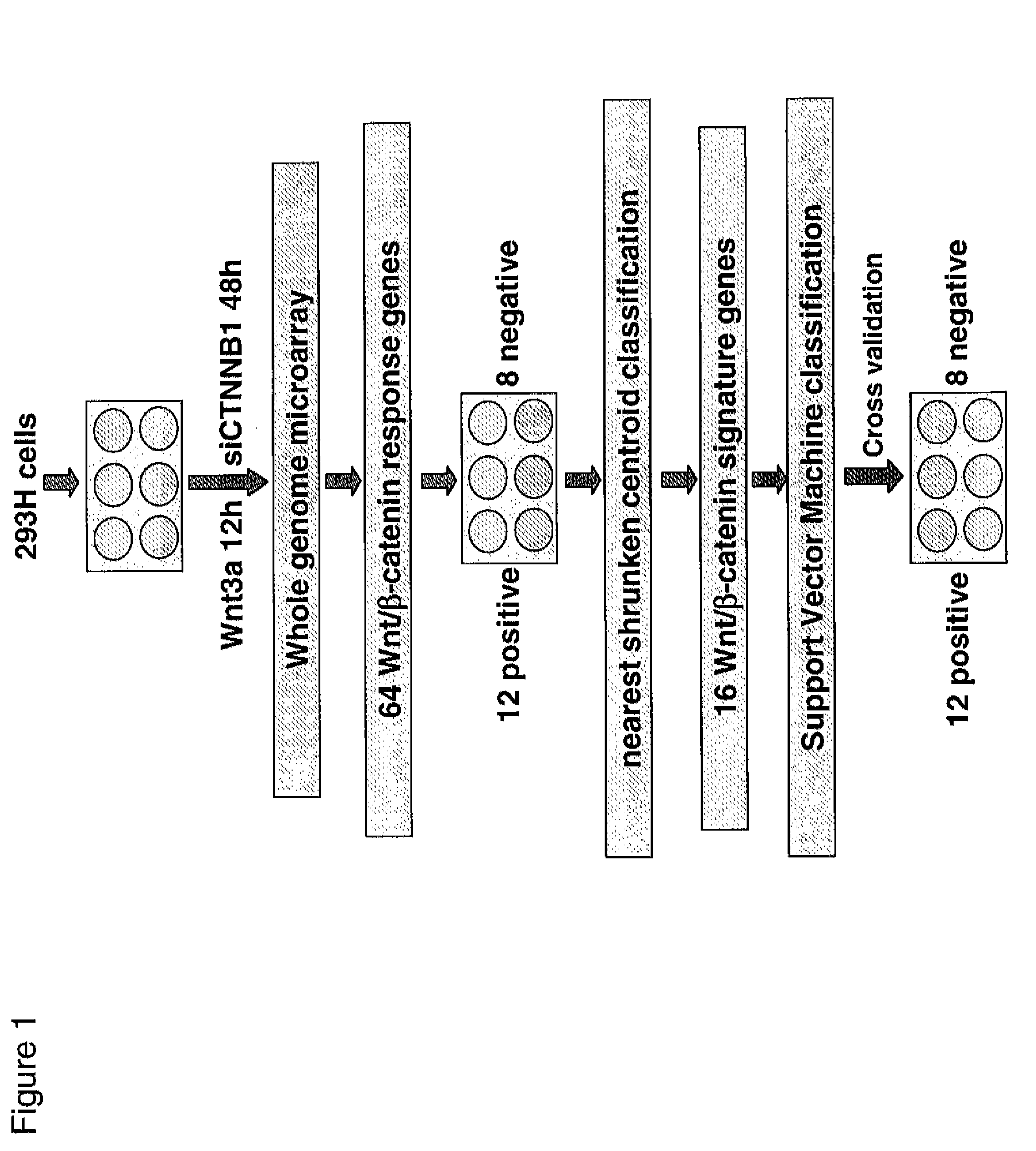
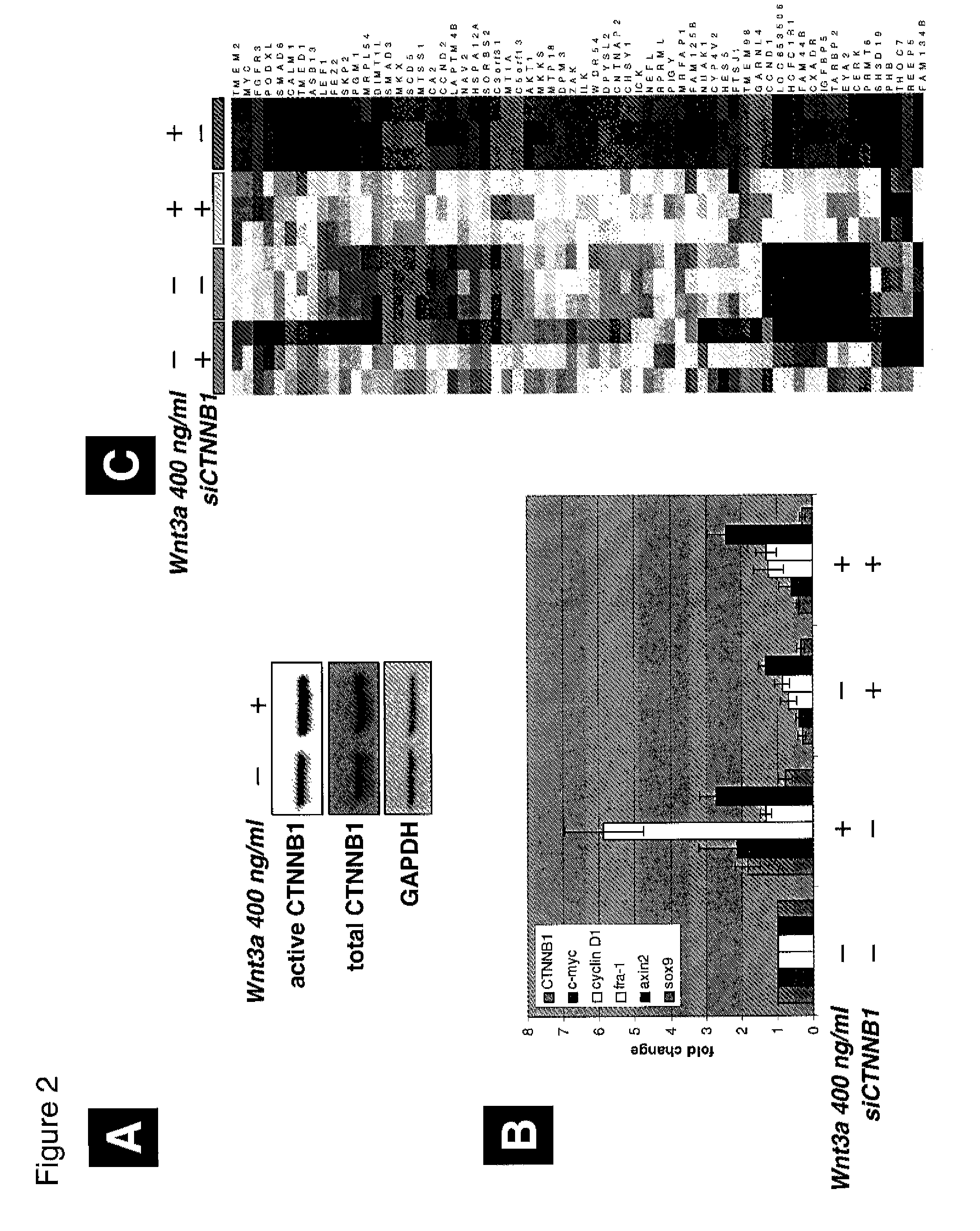
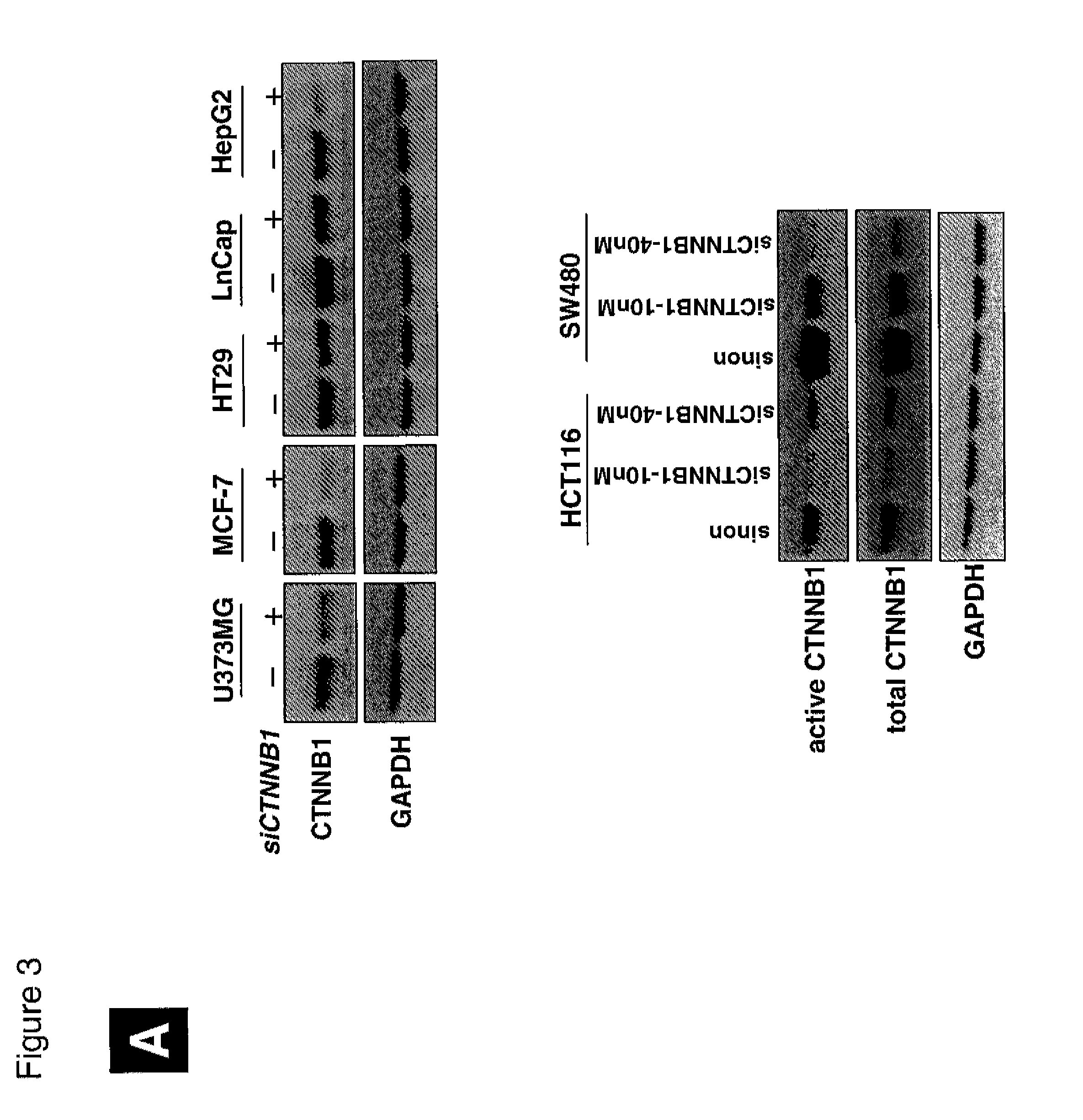
Gene expression signature for wnt/b-catenin signaling pathway and use thereof
InactiveUS20120252689A1Nucleotide librariesMicrobiological testing/measurementBeta-cateninΒ catenin signaling
The present invention relates to a novel set of 16 biomarkers, microarrays that provide for the detection thereof, an expression signature comprising 16 genes or a subset thereof, and the use thereof in determining the regulation status of Wnt / β-catenin signaling pathway. The regulation status of Wnt / β-catenin signaling pathway may be assayed based on the level of expression of one or more of these genes. The expression of these biomarkers may be used to evaluate Wnt / β-catenin pathway deregulation status; classify a cell sample as having a deregulated or regulated Wnt / β-catenin signaling pathway; determine whether an agent modulates the Wnt / β-catenin signaling pathway; predict response of a subject to an agent that modulates the Wnt / β-catenin signaling pathway; assign treatment to a subject; or evaluate the pharmacodynamic effects of therapies designed to regulate Wnt / β-catenin pathway signaling.
Owner:SABIOSCIENCES CORP
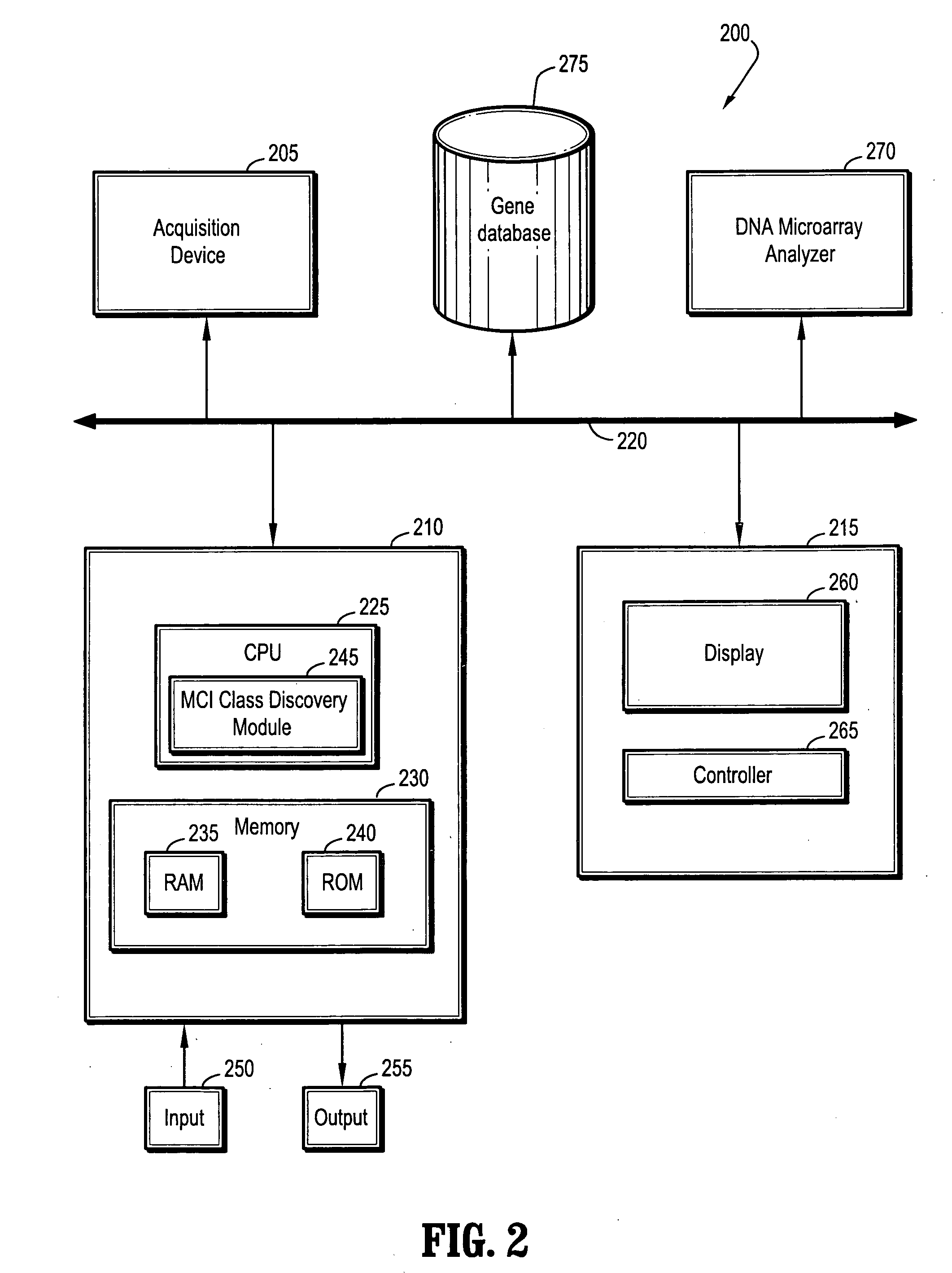
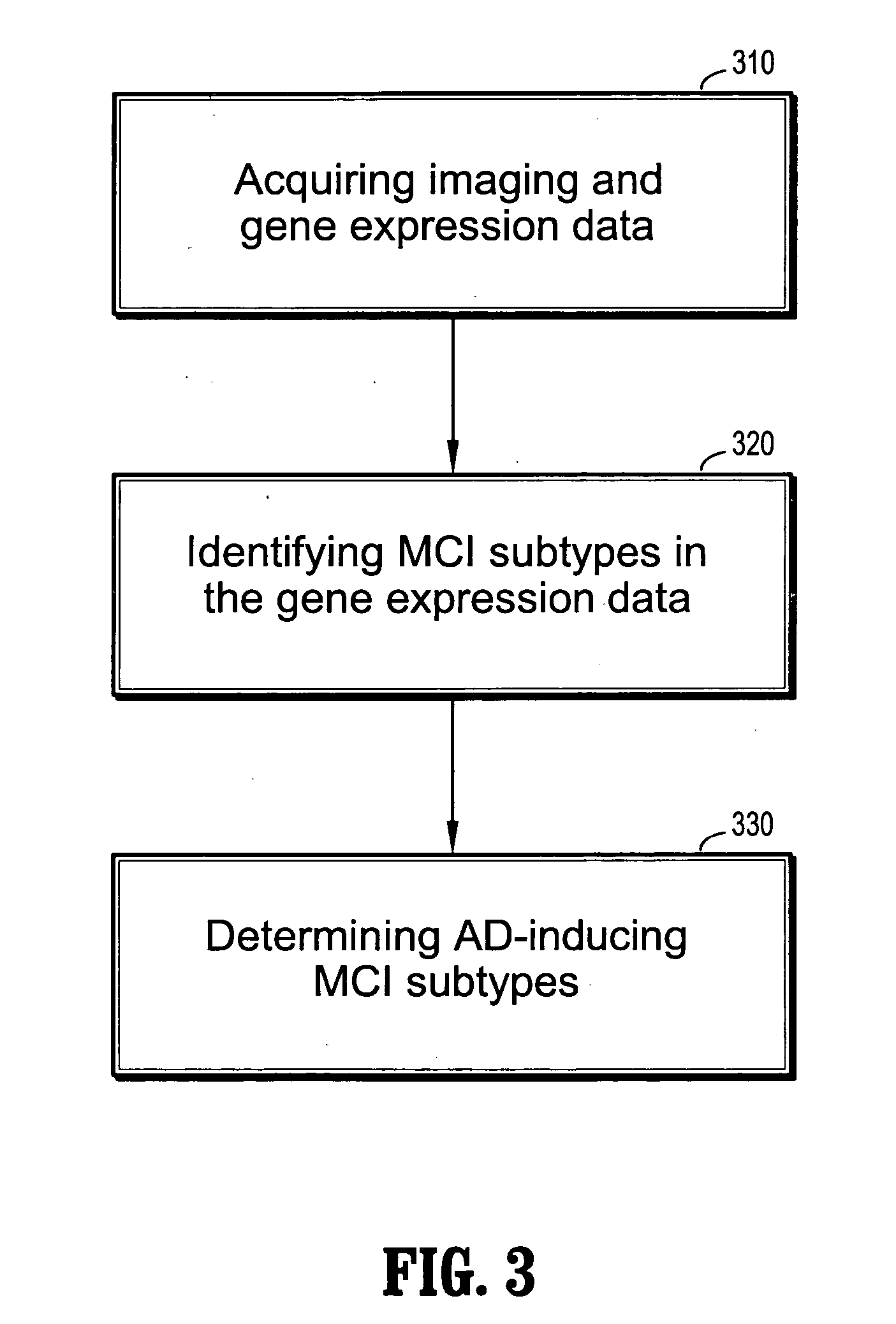
System and method for mild cognitive impairment class discovery using gene expression data
ActiveUS20060094044A1Microbiological testing/measurementSurgeryMinimal cognitive impairmentBioinformatics
A system and method for mild cognitive impairment (MCI) class discovery using gene expression data are provided. The method comprises: acquiring gene expression data of a patient having MCI; and identifying a putative MCI subtype based on an expression signature in the gene expression data, wherein the putative MCI subtype is identified by using a boosting tree.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Mirna expression signature in the classification of thyroid tumors
The present invention provides a method for classification of thyroid tumors through the analysis of the expression patterns of specific microRNAs in fine needle aspiration samples. Thyroid tumor classification according to a microRNA expression signature allows optimization of diagnosis and treatment, as well as determination of signature-specific therapy.
Owner:ROSETTA GENOMICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com