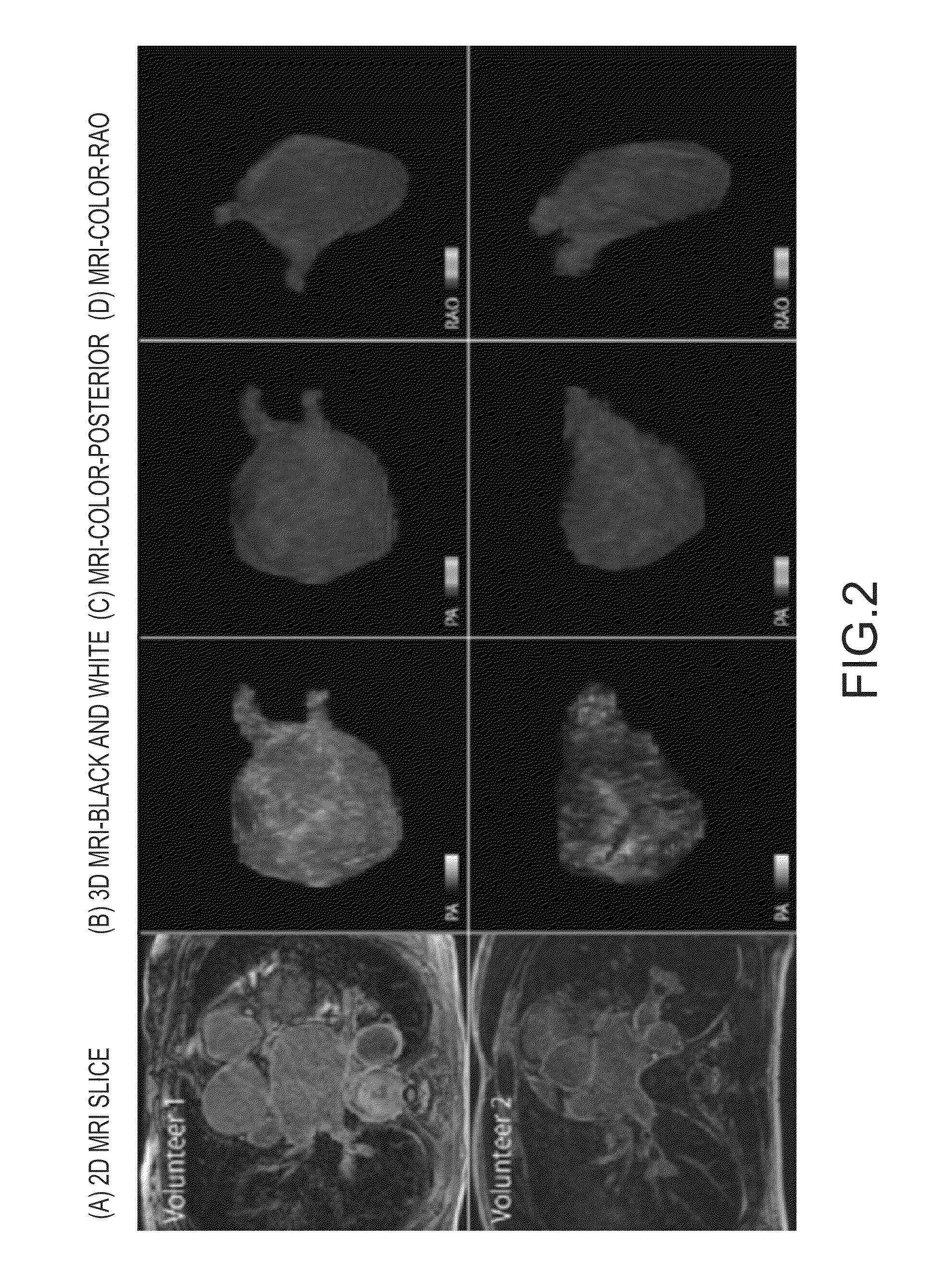
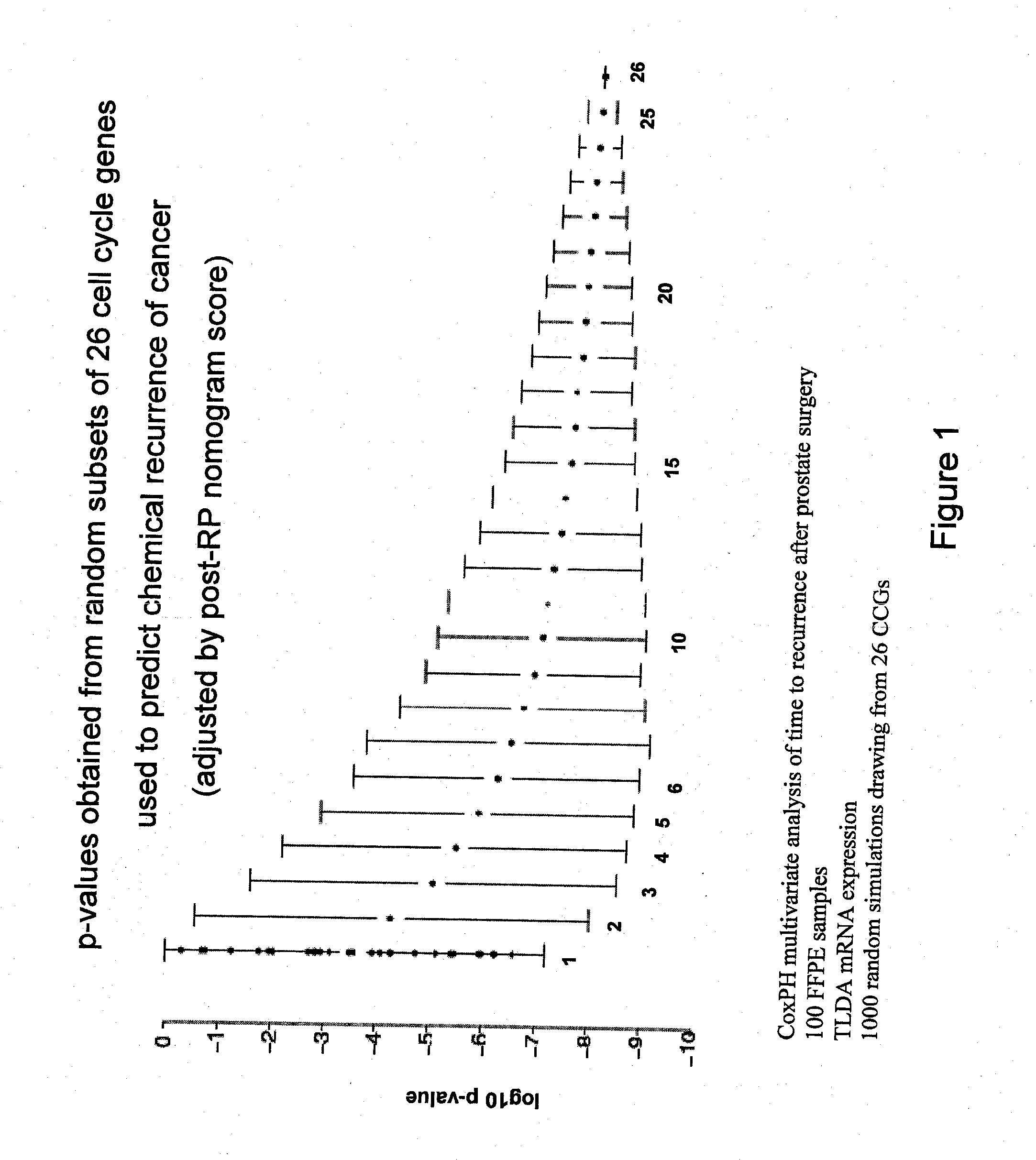
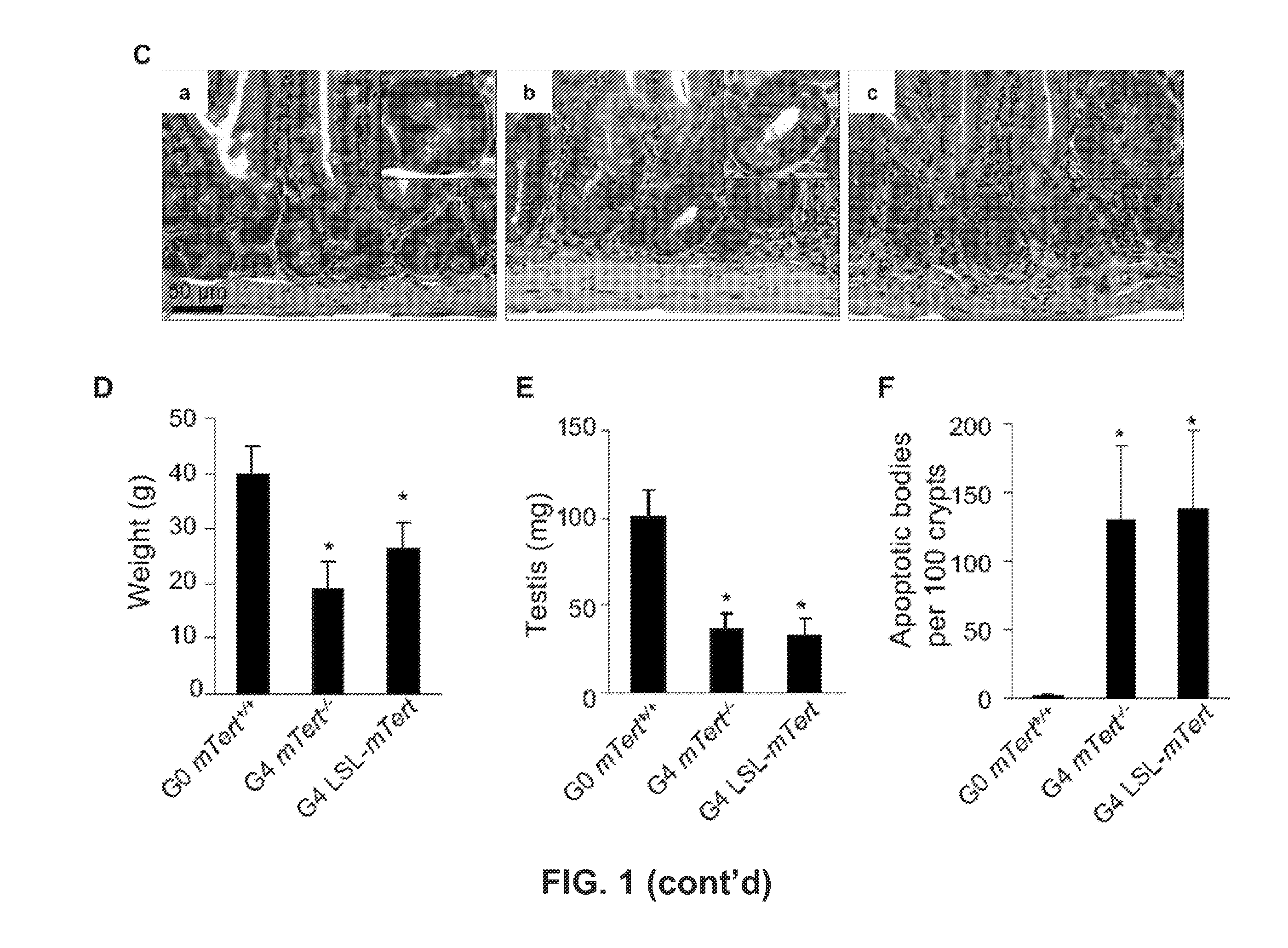
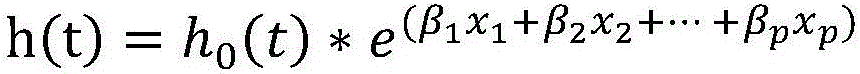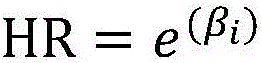Patents
Literature
195 results about "Recurrence risk" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
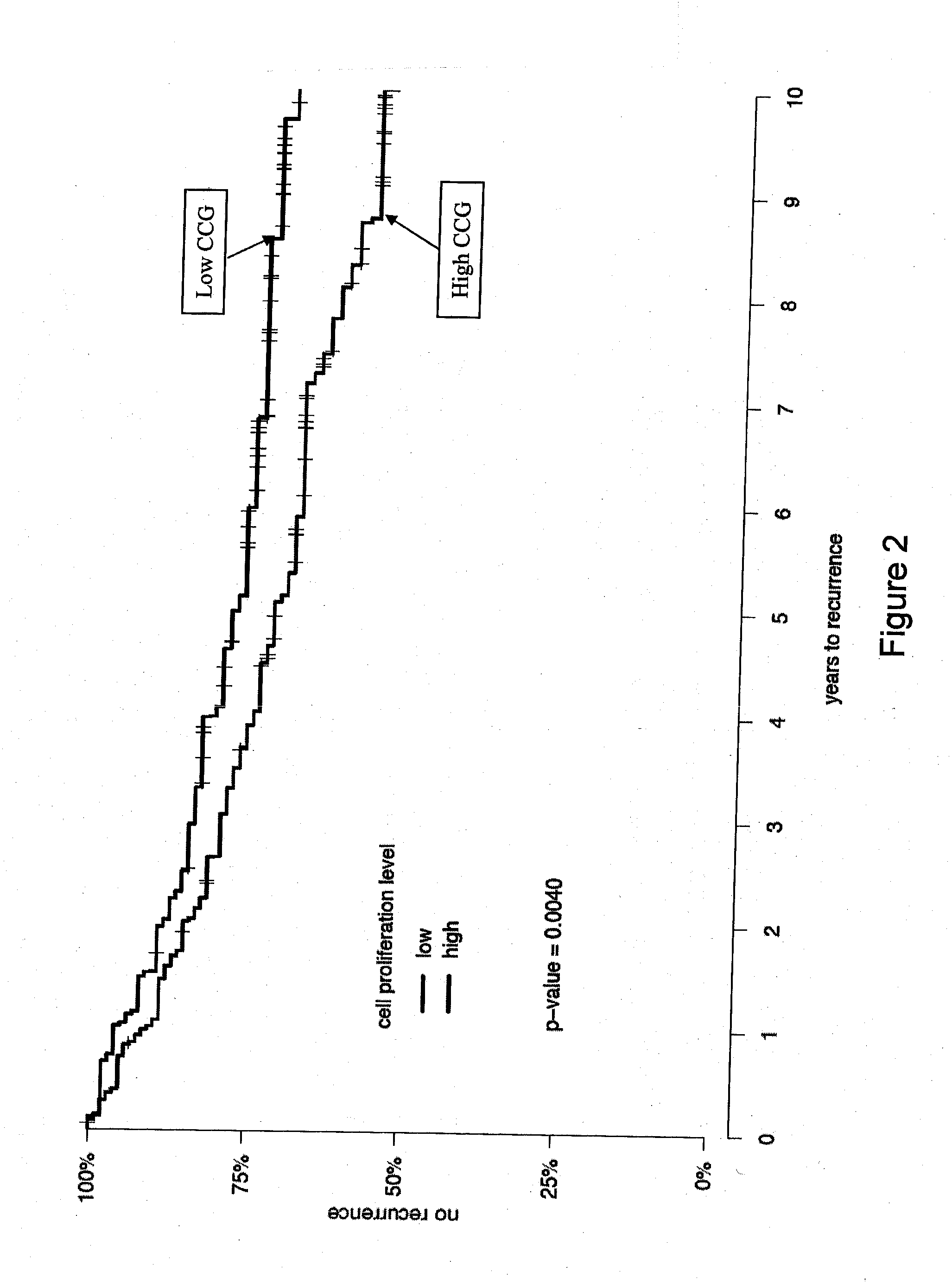
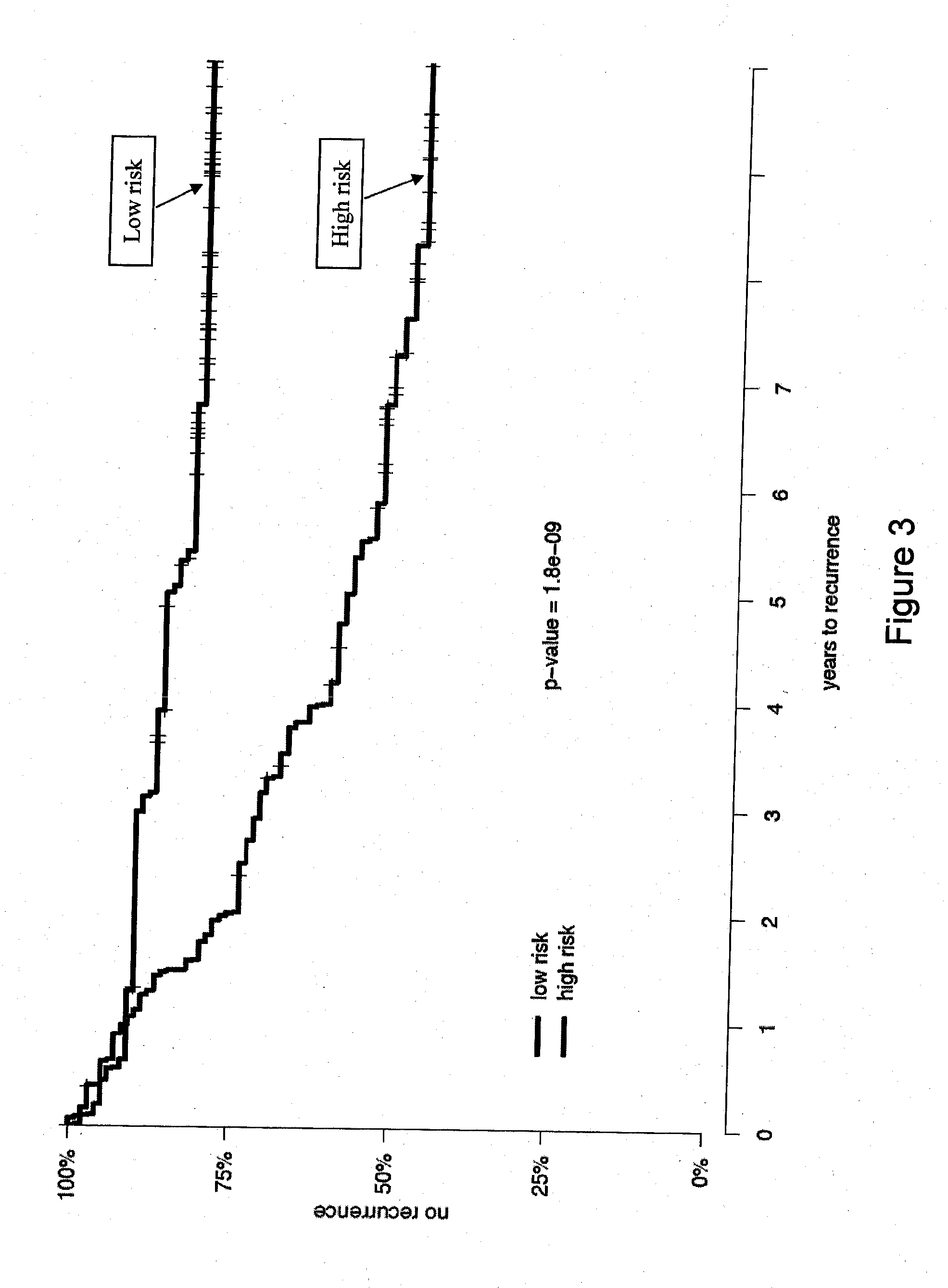
Recurrence risk: The chance that a disease will strike again. In medical genetics, the chance that an inherited disease that is present in a family will recur in that family, affecting another person or. persons.
Expression profile algorithm and test for cancer prognosis
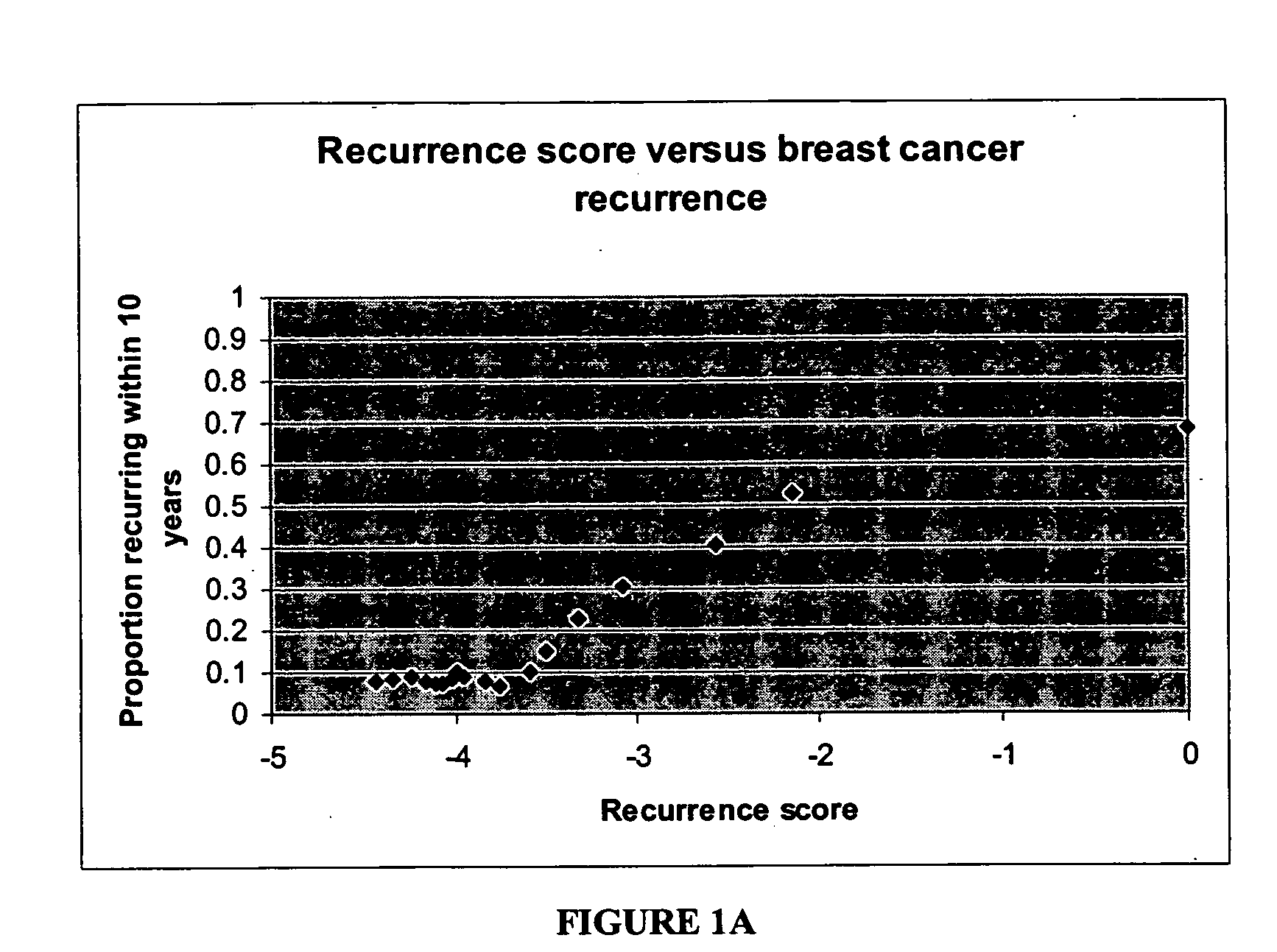
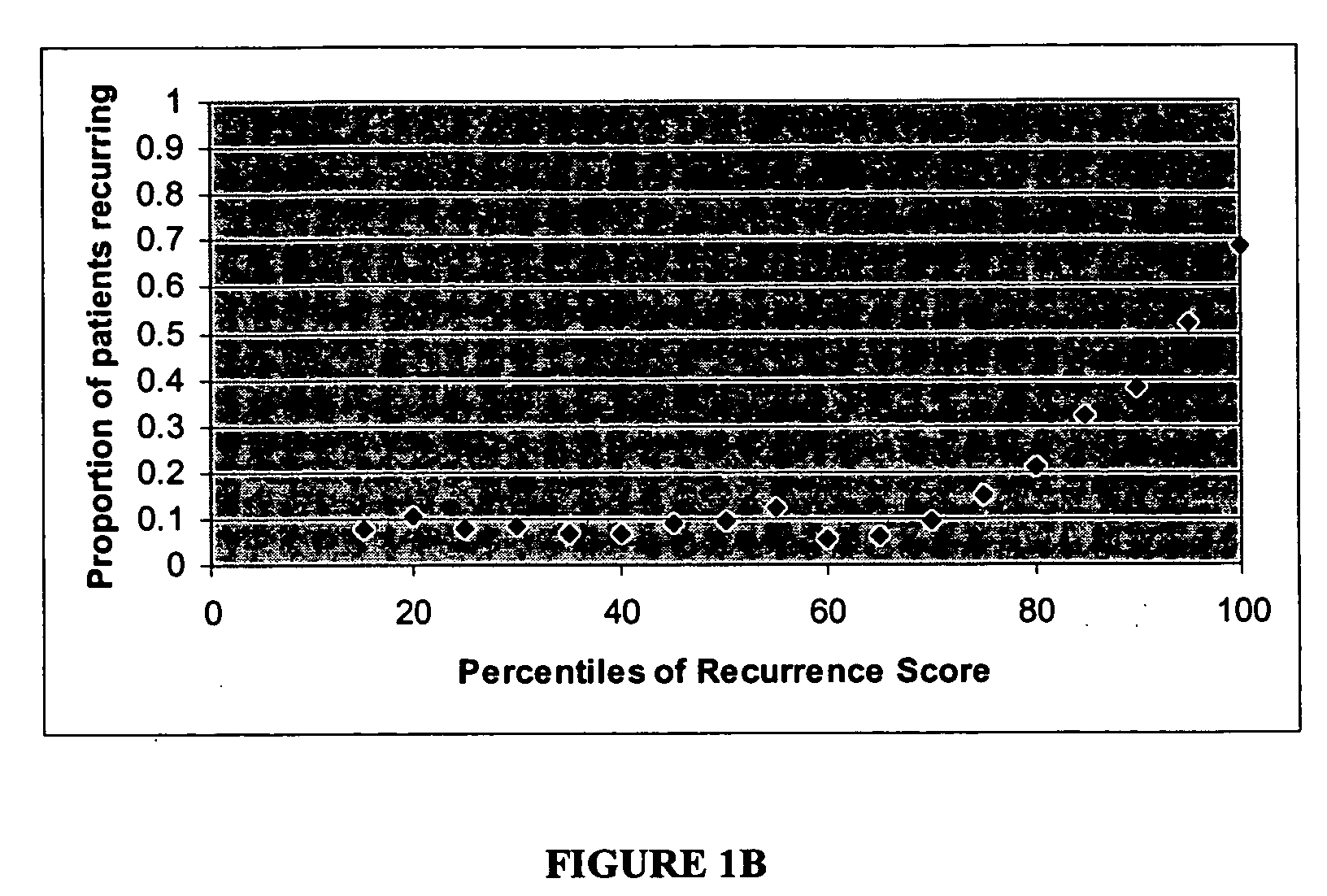
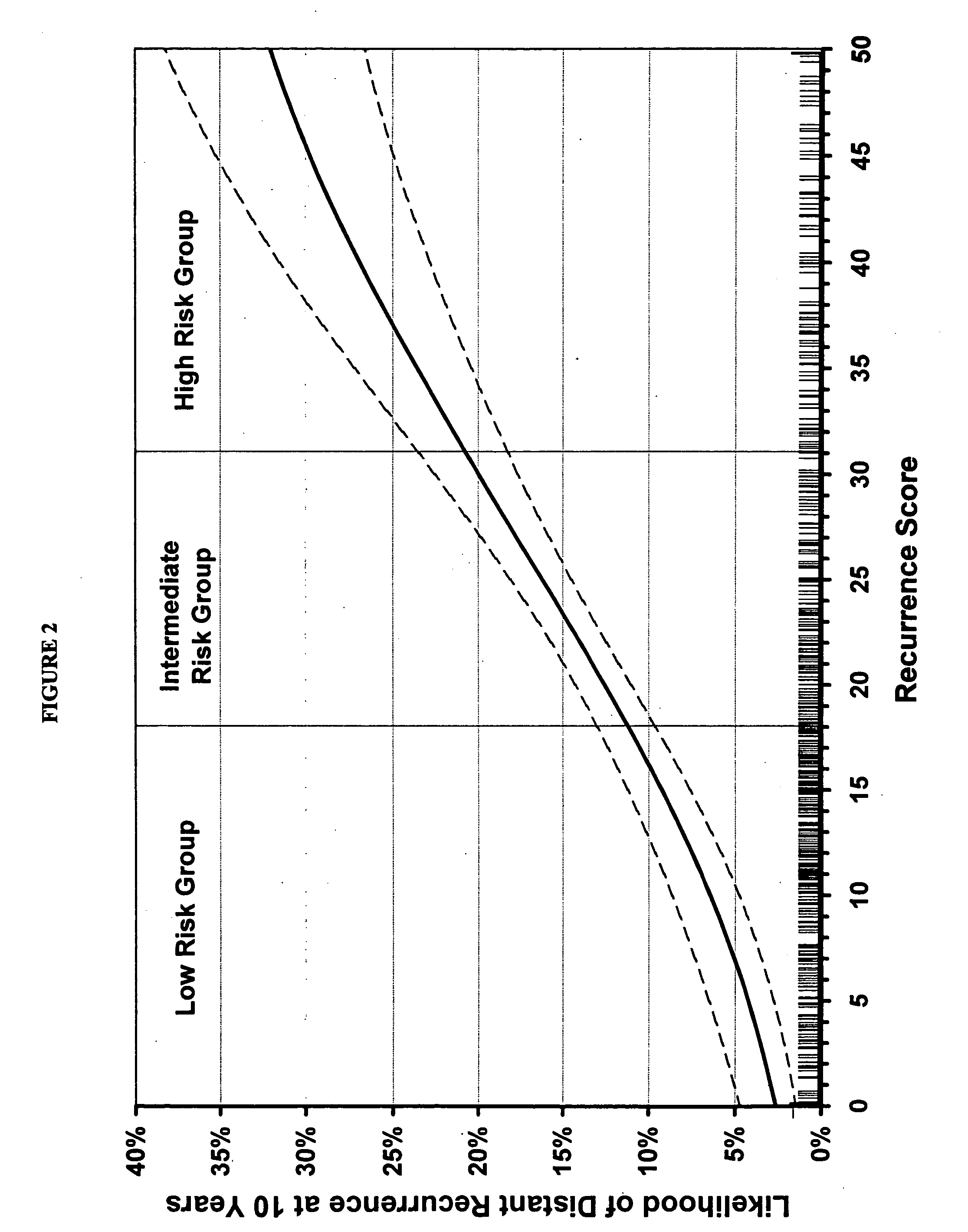
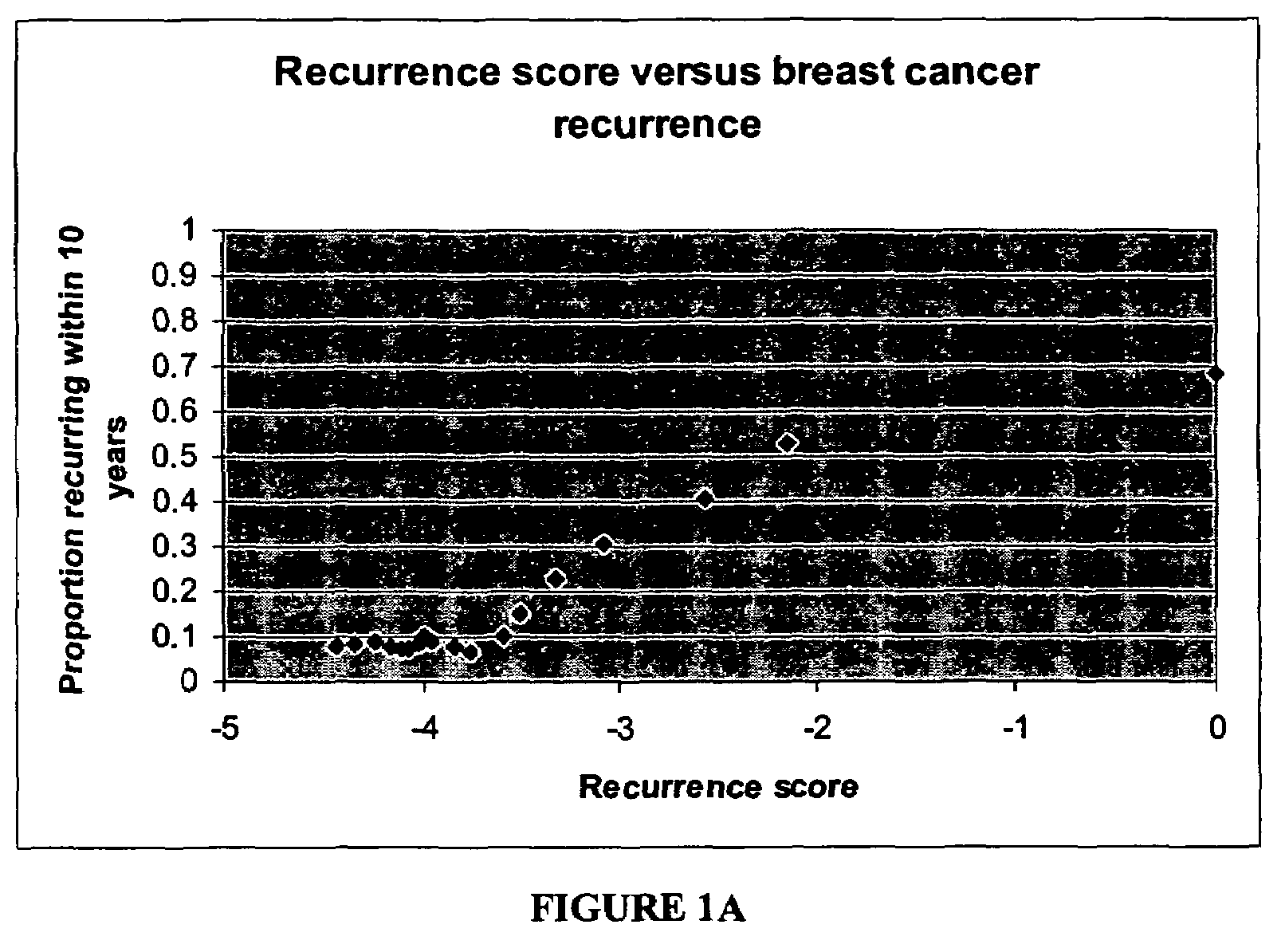
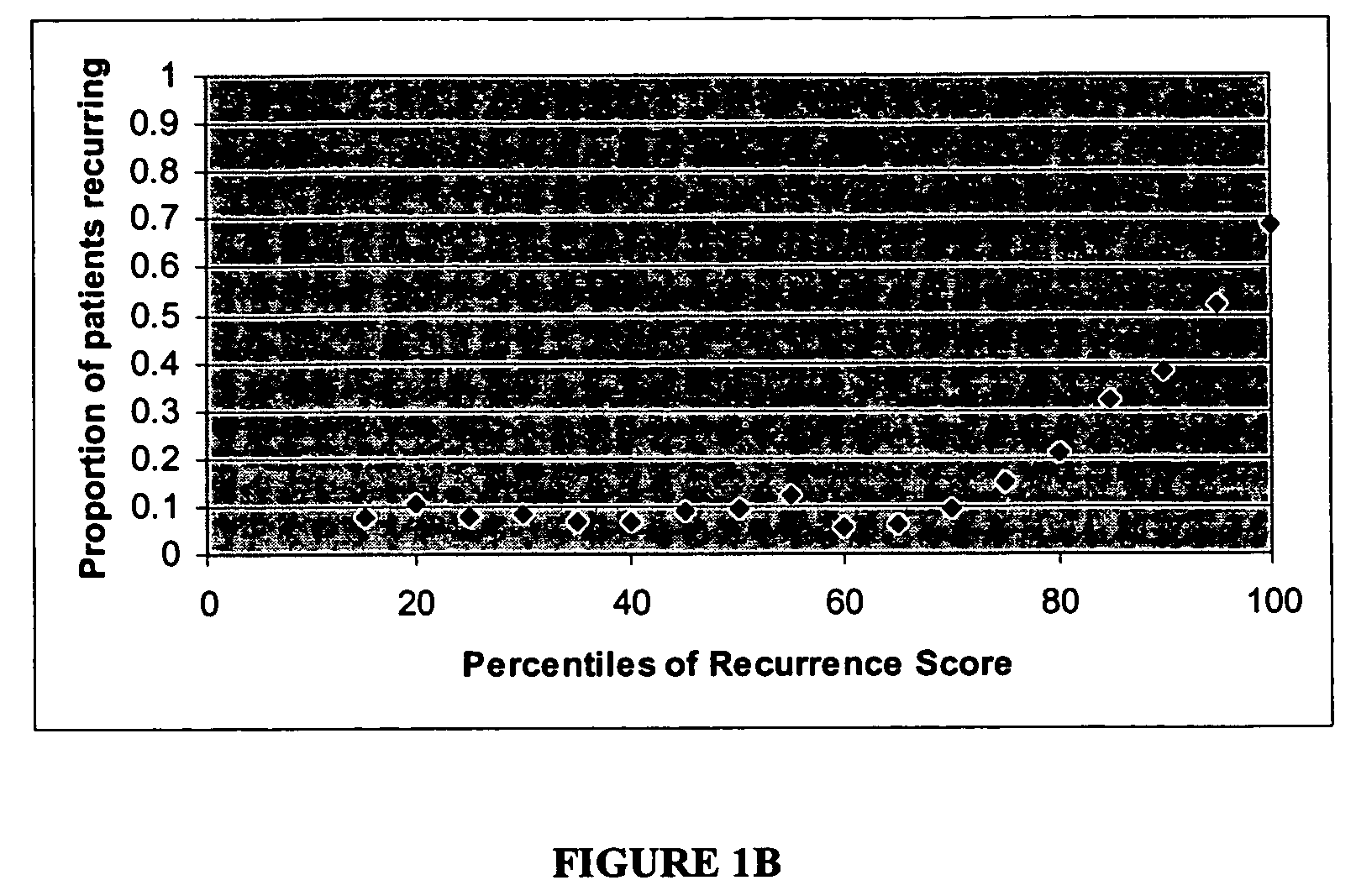
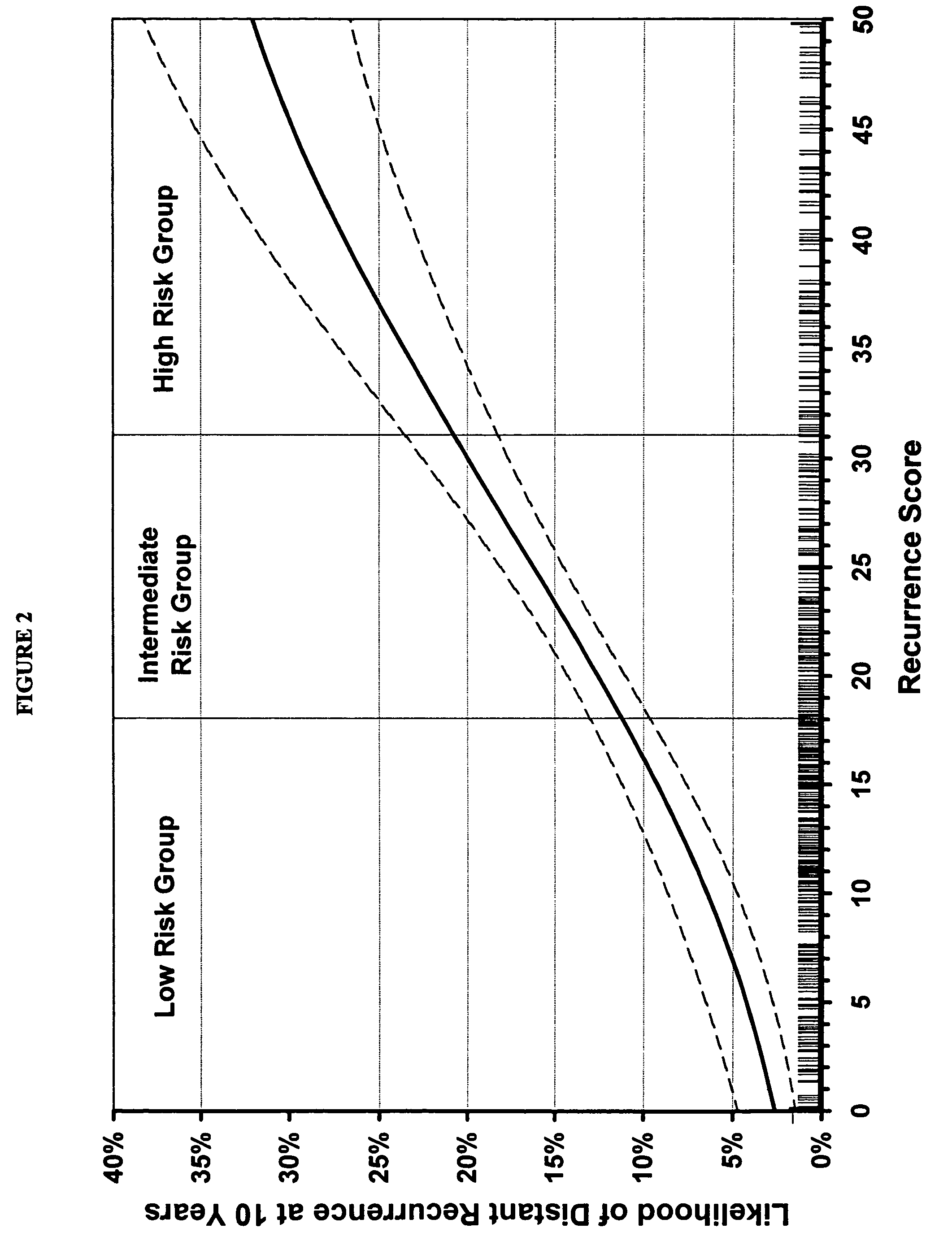
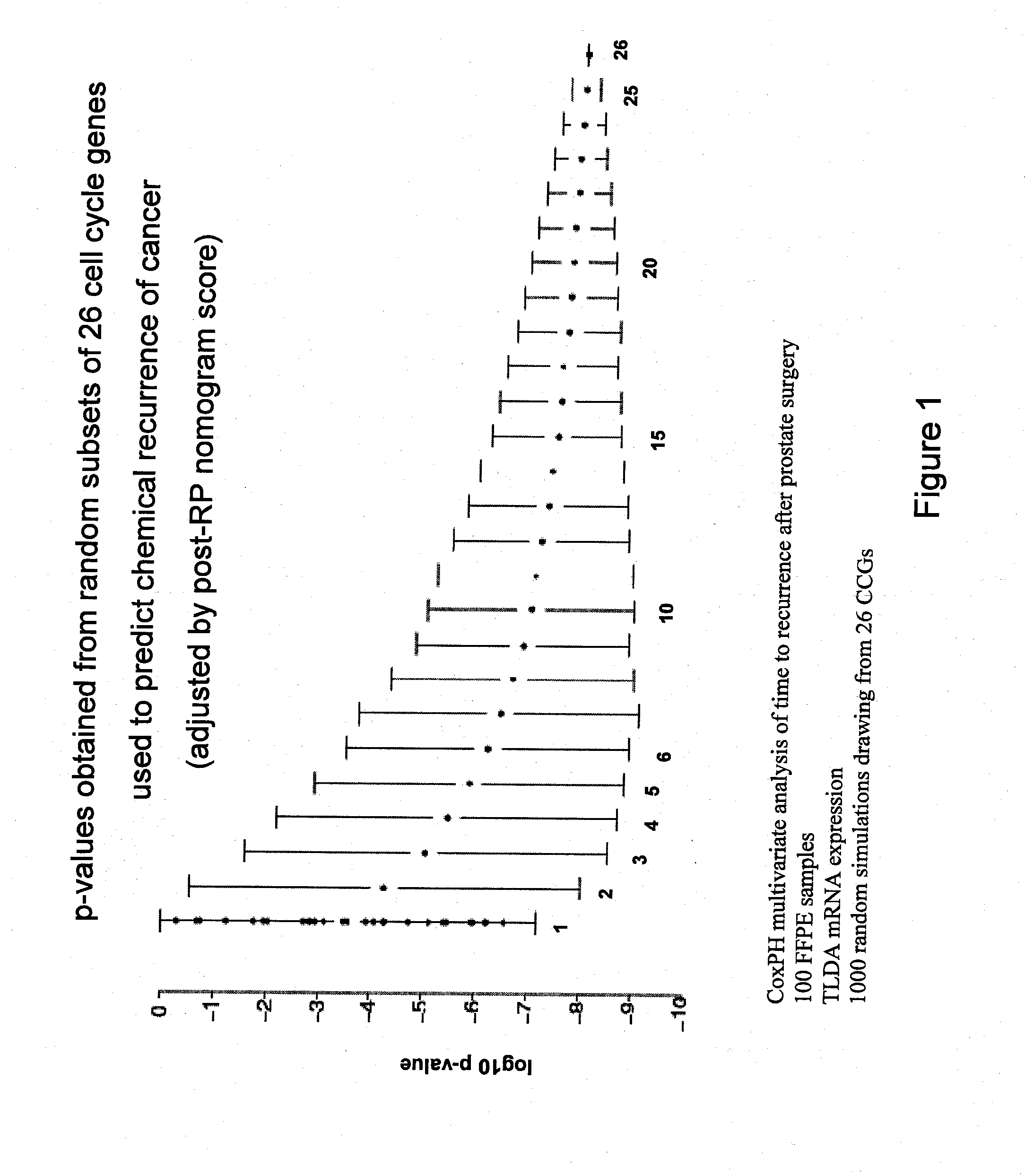
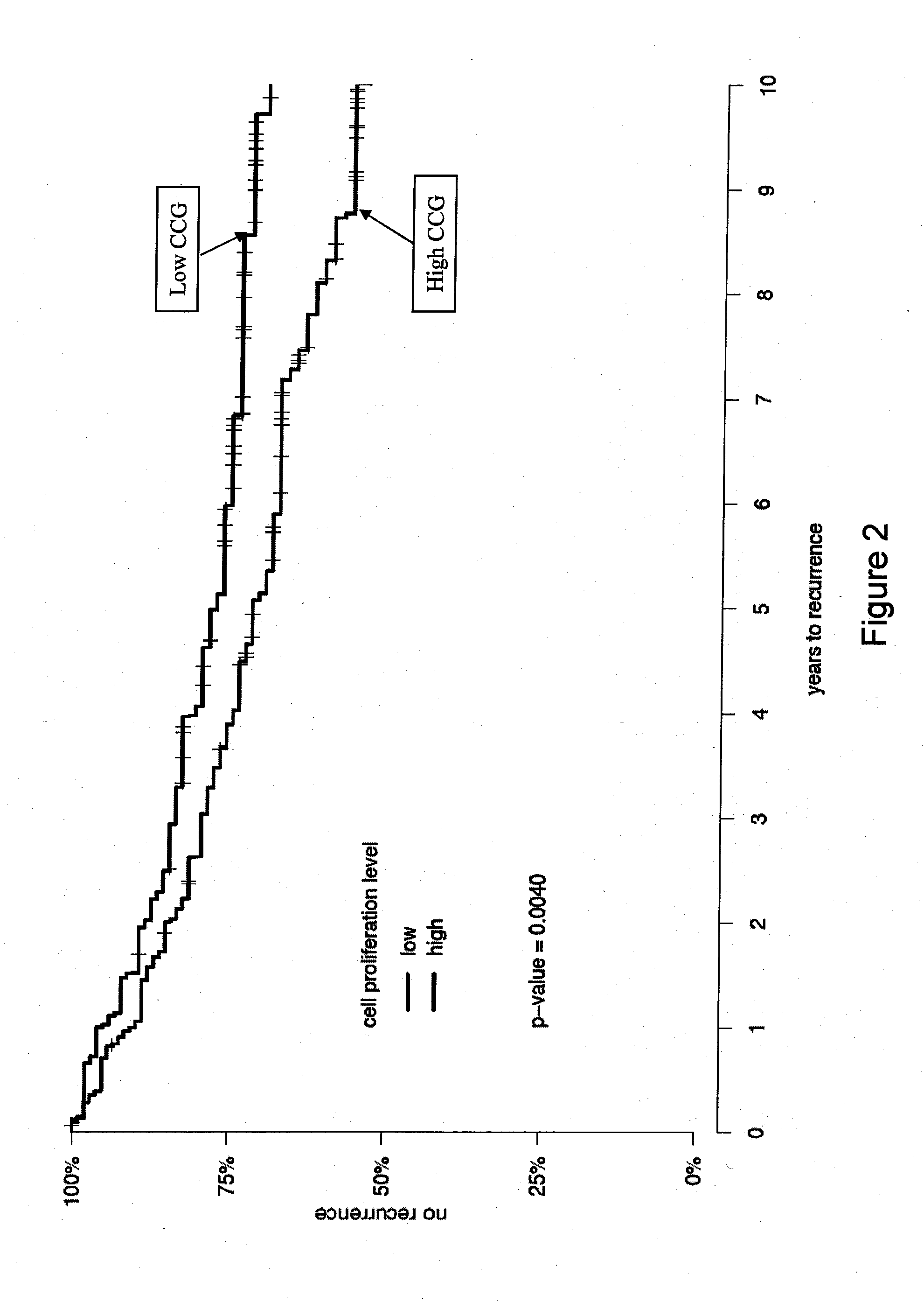
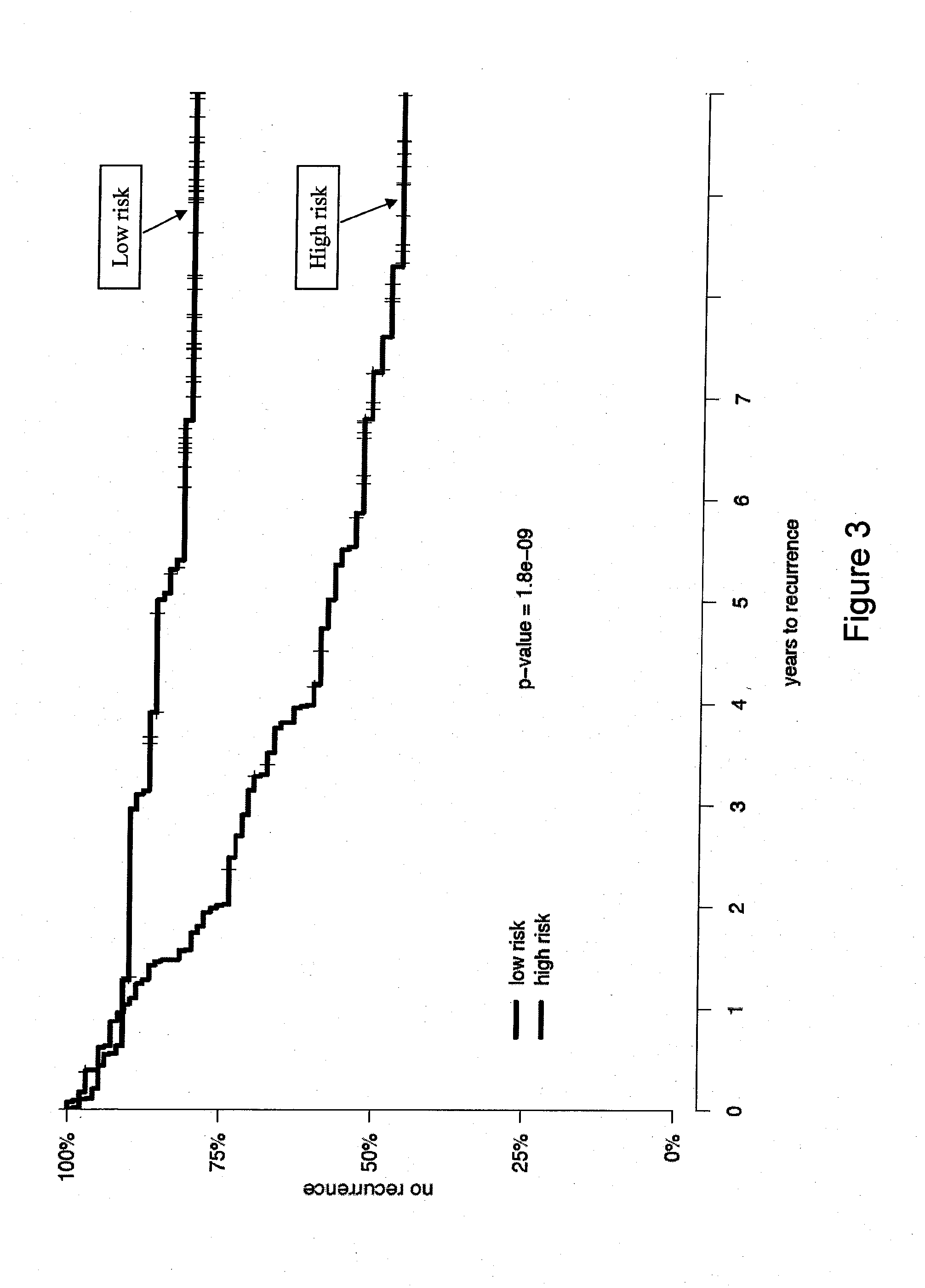
The present invention provides a noninvasive, quantitative test for prognosis determination in cancer patients. The test relies on measurements of the tumor levels of certain messenger RNAs (mRNAs). These mRNA levels are inserted into a polynomial formula (algorithm) that yields a numerical recurrence score, which indicates recurrence risk.
Owner:GENOMIC HEALTH INC
Expression profile algorithm and test for cancer prognosis
The present invention provides a noninvasive, quantitative test for prognosis determination in cancer patients. The test relies on measurements of the tumor levels of certain messenger RNAs (mRNAs). These mRNA levels are inserted into a polynomial formula (algorithm) that yields a numerical recurrence score, which indicates recurrence risk.
Owner:GENOMIC HEALTH INC
Computational pathology systems and methods for early-stage cancer prognosis
ActiveUS20170270666A1Reduce computing costInformed choiceImage enhancementImage analysisComputational pathologyLow risk group
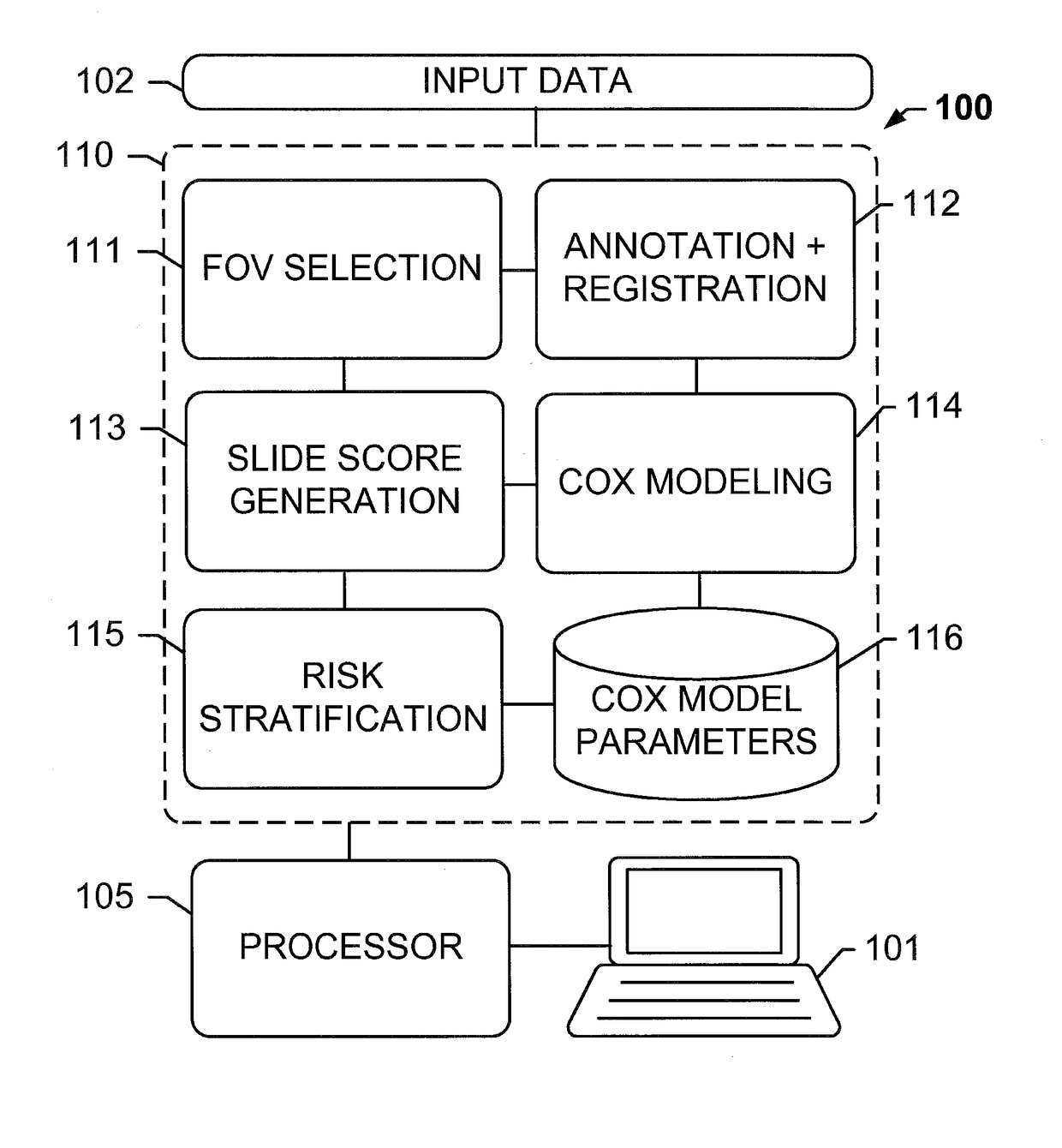
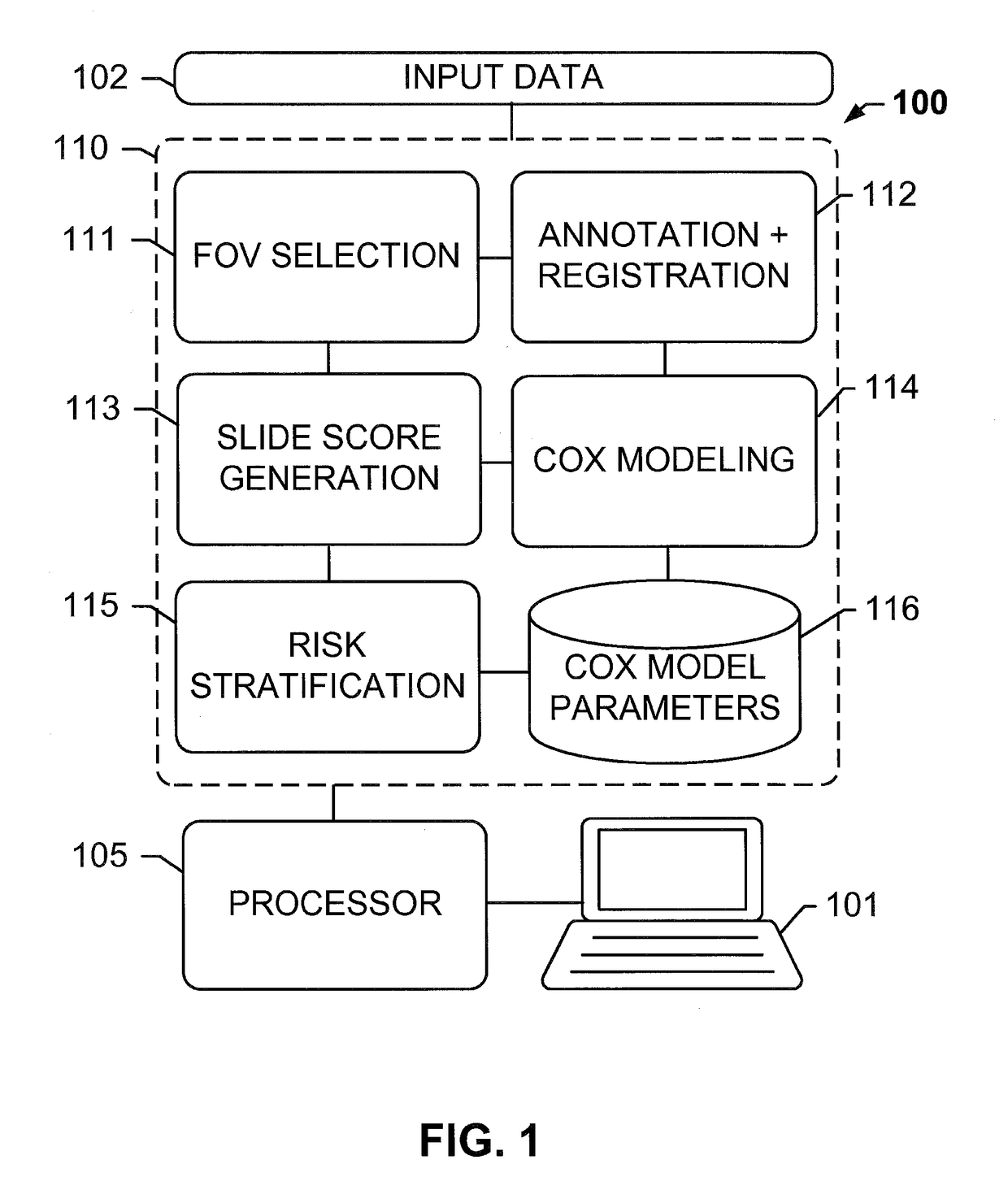
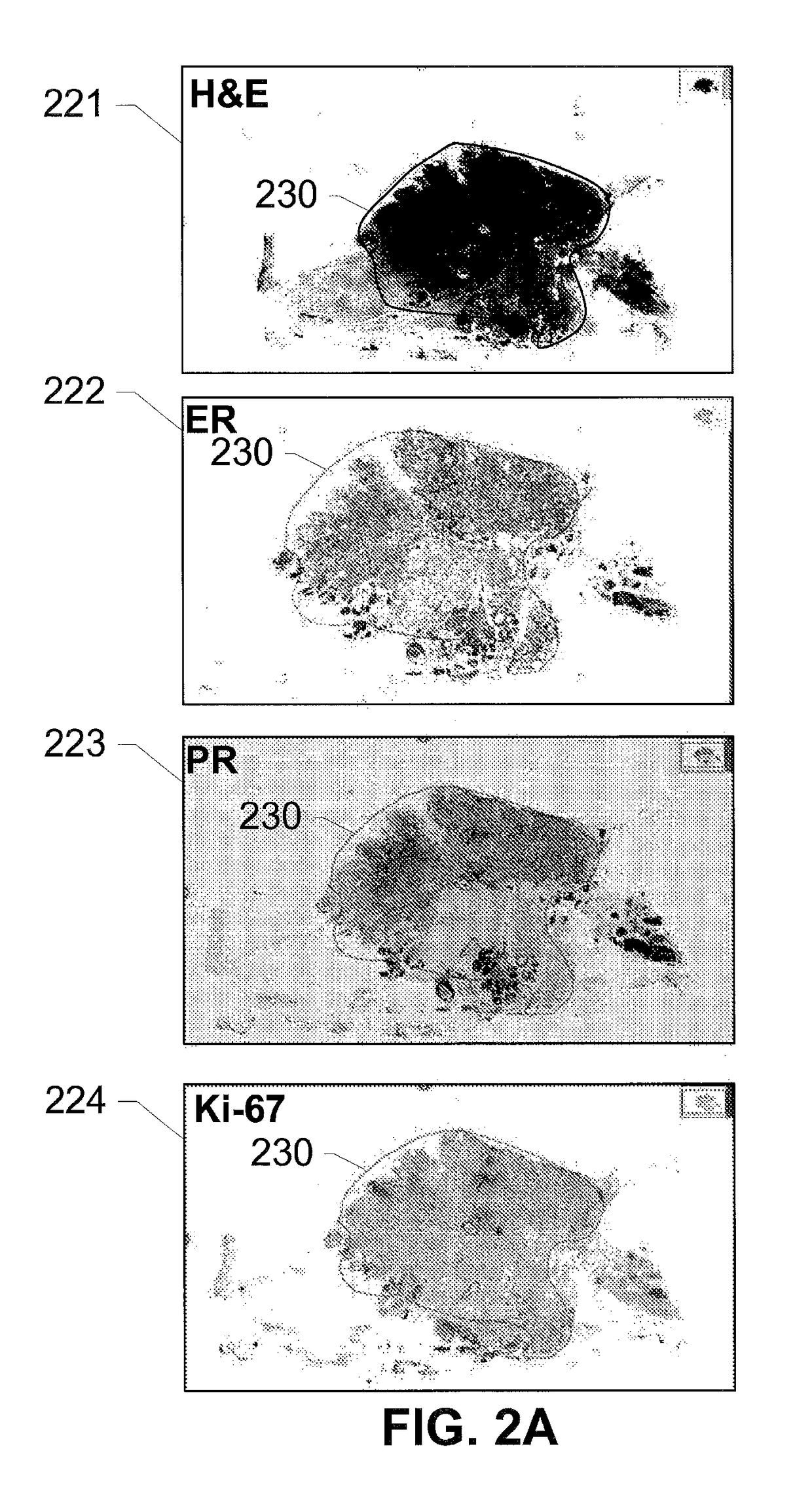
The subject disclosure presents systems and computer-implemented methods for providing reliable risk stratification for early-stage cancer patients by predicting a recurrence risk of the patient and to categorize the patient into a high or low risk group. A series of slides depicting serial sections of cancerous tissue are automatically analyzed by a digital pathology system, a score for the sections is calculated, and a Cox proportional hazards regression model is used to stratify the patient into a low or high risk group. The Cox proportional hazards regression model may be used to determine a whole-slide scoring algorithm based on training data comprising survival data for a plurality of patients and their respective tissue sections. The coefficients may differ based on different types of image analysis operations applied to either whole-tumor regions or specified regions within a slide.
Owner:VENTANA MEDICAL SYST INC
Cancer biomarkers
InactiveUS20120041274A1Improve predictive performanceIncreased likelihood of recurrenceBioreactor/fermenter combinationsBiological substance pretreatmentsTumor BiomarkersBiologic marker
Biomarkers and methods using the biomarkers for the prediction of the recurrence risk of cancer in a patient are provided.
Owner:MYRIAD GENETICS
Stomach cancer prognostic marker screening and classifying method based on gene expression profile
InactiveCN106407689AReduce dimensionalityPredict prognostic riskHealth-index calculationBiostatisticsRisk modelClassification methods
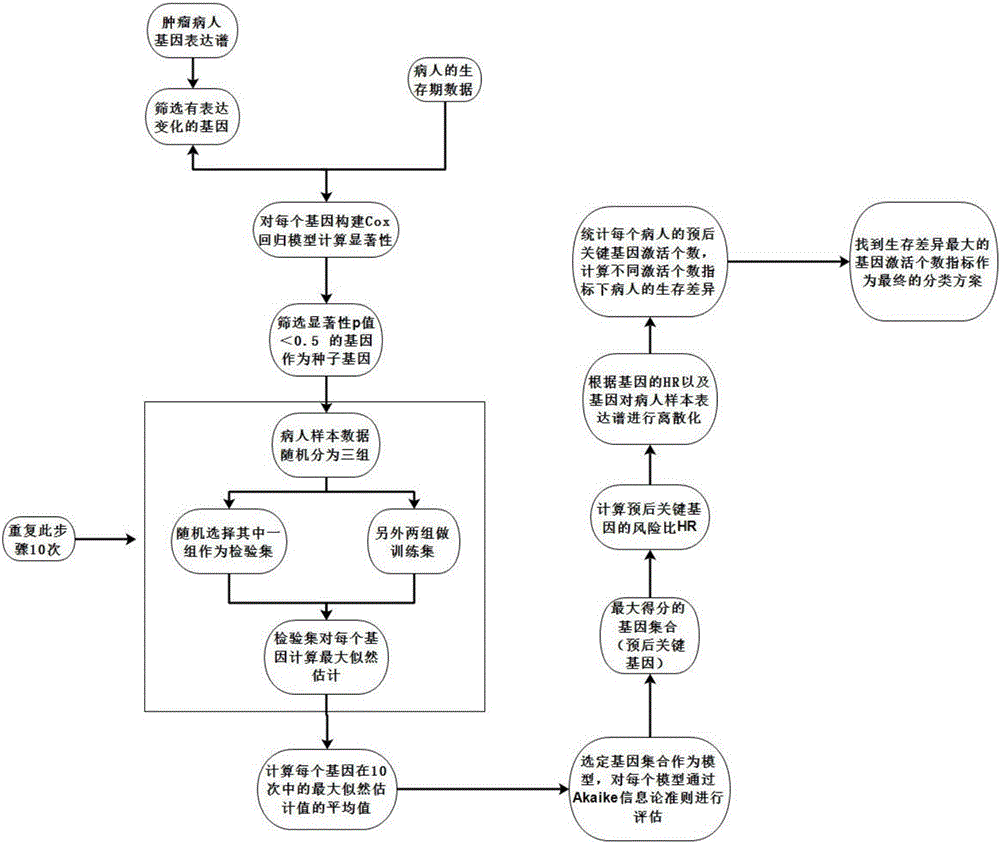
The invention discloses a stomach cancer prognostic marker screening and classifying method based on a gene expression profile. The stomach cancer prognostic marker screening and classifying method comprises that a gene the expression of which is changed is screened out from data of the gene expression profile Cox proportional hazard model analysis is established aiming at the screened gene, a gene which has statistical significance is selected as a seed gene, a maximum likelihood model is constructed by means of clinical follow-up information data of the patient to screen out prognosis key genes, then the risk coefficient of each prognosis key gene in the patient is calculated, classification and statistic verification are carried out in dependence on the number of prognosis key genes of the patient, and an optimal classification mode is selected. According to the invention, dimensionality of high-dimension miscellaneous gene expression profile data can be effectively reduced, several key genes which can be applied to clinical detection easily are screened out from tens of thousands of genes, survival and the recurrence risk of the patient are predicted by means of the expression case of the several key genes.
Owner:牟合(上海)生物科技有限公司
Methods of Predicting Cancer Risk Using Gene Expression in Premalignant Tissue
ActiveUS20100291573A1Use can be riskyIncreased riskMicrobiological testing/measurementBiological testingBraf genesExpression gene
The present disclosure provides methods for assessing a patient's cancer risk and / or recurrence risk, which methods comprise assaying, in a biological sample obtained from the gastrointestinal (GI) tract of the patient, an expression level of a risk gene. The present disclosure also provides methods involving a cancer risk / recurrence risk sequence, i.e. the V600E mutation of the BRAF gene, which is useful for assessing cancer risk and / or recurrence risk in a patient.
Owner:GENOMIC HEALTH INC
Preparation method for probes related to breast cancer molecular markers and application of same
ActiveCN102399772AImprove the detection rateAccurate typingMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceMortality rate
The invention relates to a preparation method for probes related to breast cancer molecular markers and to preparation of a breast cancer fluorescence in situ hybridization detection kit by using the probes. The breast cancer fluorescence in situ hybridization detection kit can be prepared from HER2, TOP2A and AGTR1 gene probes prepared by using the method provided in the invention, human chromosome 17 counting probe, hybridization buffer, unlabelled competitive DNA and DAPI counter strain; application of the kit enables the detection rate of breast cancers to be substantially improved and type sorting of breast cancers to be more accurate and provides guidance to formulation of an individual therapeutic schedule and selection of proper therapeutic drugs, thereby lowering down mortality, reducing recurrence risk and achieving the goal of optimizing the effect of diagnosis and treatment.
Owner:DAAN GENE CO LTD
Therapeutic success prediction for atrial fibrillation
Certain embodiments of the invention provide methods of assessing a patient's risk for atrial fibrillation (AF) recurrence after receiving treatment with an AF treatment modality, that include determining, from left atrium (LA) tissue image data of a patient, a level of a parameter that is positively proportional to an amount of unhealthy tissue in a wall of the LA of the patient; and outputting, to an output device, an indicator of a comparison between (i) the determined level and (ii) a first threshold level of the parameter, the first threshold level derived from LA tissue image data of at least one other patient, who experienced an AF recurrence after treatment with the AF treatment modality. In certain embodiments, levels of the parameter equal to or greater than the first threshold level are indicative of a significant risk of AF recurrence after treatment with the AF treatment modality.
Owner:UNIV OF UTAH RES FOUND
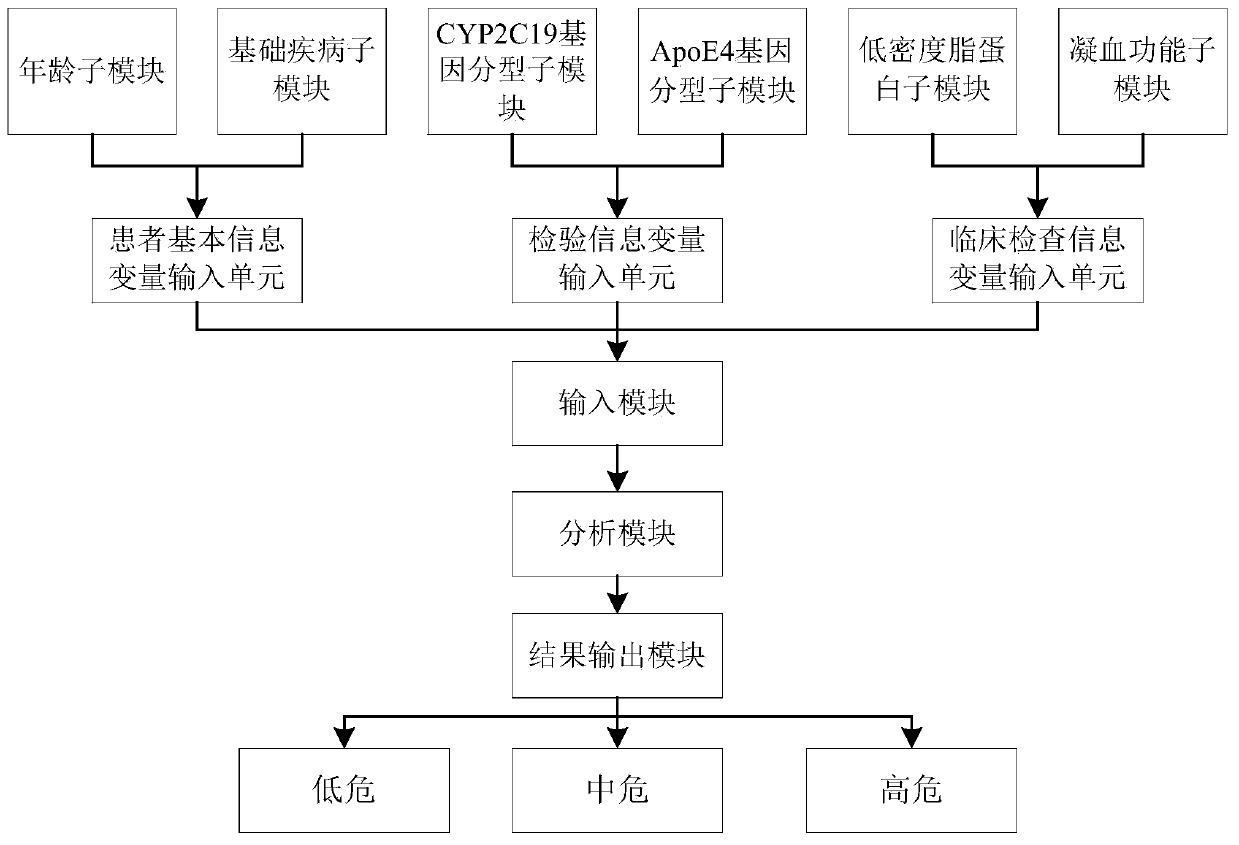
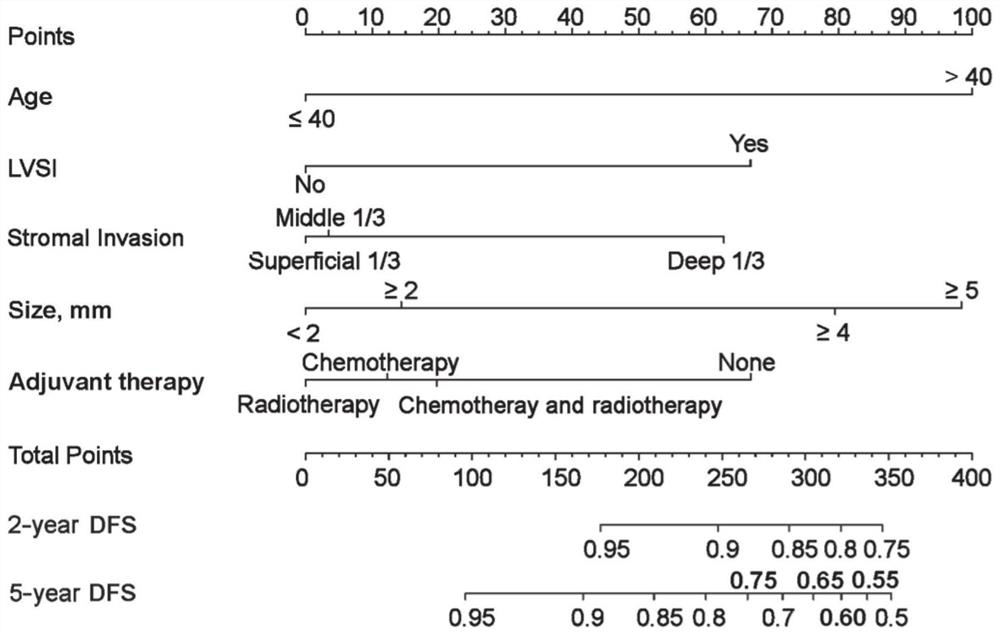
Clinical cerebral infarction patient recurrence risk early warning scoring visual model system and evaluation method thereof
InactiveCN110634573AEasy to operateEasy to handleMedical simulationHealth-index calculationLow risk groupLower risk
The invention discloses a clinical cerebral infarction patient recurrence risk early warning scoring visual model system. The system comprises an input module, an analysis module and a result output module. The invention also discloses an evaluation method of the clinical cerebral infarction patient recurrence risk early warning scoring visual model system. A clinical cerebral infarction patient recurrence risk evaluation nomogram is concise, popular and easy to understand and convenient for clinicians and patients to operate, and a current cerebral infarction recurrence risk of the patients is predicted. And meanwhile, high-risk, medium-risk and low-risk groups can be clearly distinguished according to patient risk scores calculated by a risk scoring formula in the model so that the clinicians are assisted to formulate an efficient treatment scheme. In the invention, a doctor and the patient are guided to evaluate early warning scores of the clinical cerebral infarction patient recurrence risk in combination with a statistical method, and patient data acquisition is verified through a corresponding mathematical statistical method so that the doctor and the patient can quickly grasp the clinical cerebral infarction patient recurrence risk and provide early warning information.
Owner:南昌大学第一附属医院
Gene signatures for cancer prognosis
Biomarkers and methods using the biomarkers for the prediction of the recurrence risk of cancer in a patient are provided.
Owner:MYRIAD GENETICS
Reagent kit for quantitatively assessing long-term recurrence risks of breast cancer
InactiveCN102586410AEasy to operateTest results are stableMicrobiological testing/measurementFluorescence/phosphorescenceGenomicsBreast cancer metastasis
The invention relates to the functional genomic and gene expression detection technology and the analysis technology, and discloses a reagent kit for quantitatively assessing long-term recurrence risks of breast cancer. Particularly, a type of genes capable of being used for breast cancer metastasis and prognostic molecular classification is screened within a human functional genome expression profile range, the detection technology is created, and the reagent kit is prepared and applied to breast cancer metastasis and prognostic assessment for a patient. The reagent kit for quantitatively assessing long-term recurrence risks of breast cancer comprises 21 pairs of primers, 21 specific taqman fluorescent probes, 10XRT-PCR (reverse transcription-polymerase chain reaction) buffer solution, 2.5mM of dNTP (diethyl-nitrophenyl thiophosphate) mixed liquor, reverse transcriptase, DNA (deoxyribose nucleic acid) polymerase, 10XPCR buffer solution and RNA (ribonucleic acid) enzyme inhibitor. The reverse transcription PCR technology is combined with the taqman fluorescent quantitative PCR technology, reverse transcription primers, real-time PCR primers and the taqman fluorescent probes are self-designed and optimized, reverse transcription PCR reagent and taqman fluorescent quantitative PCR reagent are integrated to prepare the detection reagent kit, operation is simple and fast, detection results are more stable, and detection cost is lower.
Owner:苏州科贝生物技术有限公司
Chimeric antigen receptor, gene of chimeric antigen receptor, recombinant expression vector of chimeric antigen receptor, CD22-CD19 double-targeting T cell and application of cell
ActiveCN109678965AGood antitumor activityAvoid immune escapeAntibody mimetics/scaffoldsMammal material medical ingredientsAntigen receptorsHinge region
The invention belongs to the field of tumor biological products, and particularly relates to an chimeric antigen receptor, a gene of the chimeric antigen receptor, the recombinant expression vector ofthe chimeric antigen receptor, a CD22-CD19 double-targeting T cell and application of the T cell. The chimeric antigen receptor is CD22ScFv-L-CD19ScFv-CD8-CD137-CD3zeta and comprises CD22ScFv, connecting peptide L, CD19ScFv, a hinge region and a transmembrane region of CD8, an intracellular signal structural domain of CD137 and an intracellular signal structural domain of the CD3zeta which are connected in series. According to the chimeric antigen receptor, the gene of the chimeric antigen receptor, the recombinant expression vector of the chimeric antigen receptor, the CD22-CD19 double-targeting T cell and the application of the T cell, when B-cell line hematological malignant tumors are treated, the T cell modified by the chimeric antigen receptor not only can specifically recognize a tumor cell which is only expressed by a single target spot of a CD19 antigen or a CD22 antigen, but also can recognize tumor cells co-expressed by the two target spots of the CD19 antigen and the CD22antigen, compared with the single target spot CAR T, the double target spots CAR T have stronger anti-tumor activity, so that the immune escape of the tumor cells expressed by the low-abundance antigen is avoided, and therefore the recurrence risk is reduced.
Owner:GENERAL HOSPITAL OF PLA
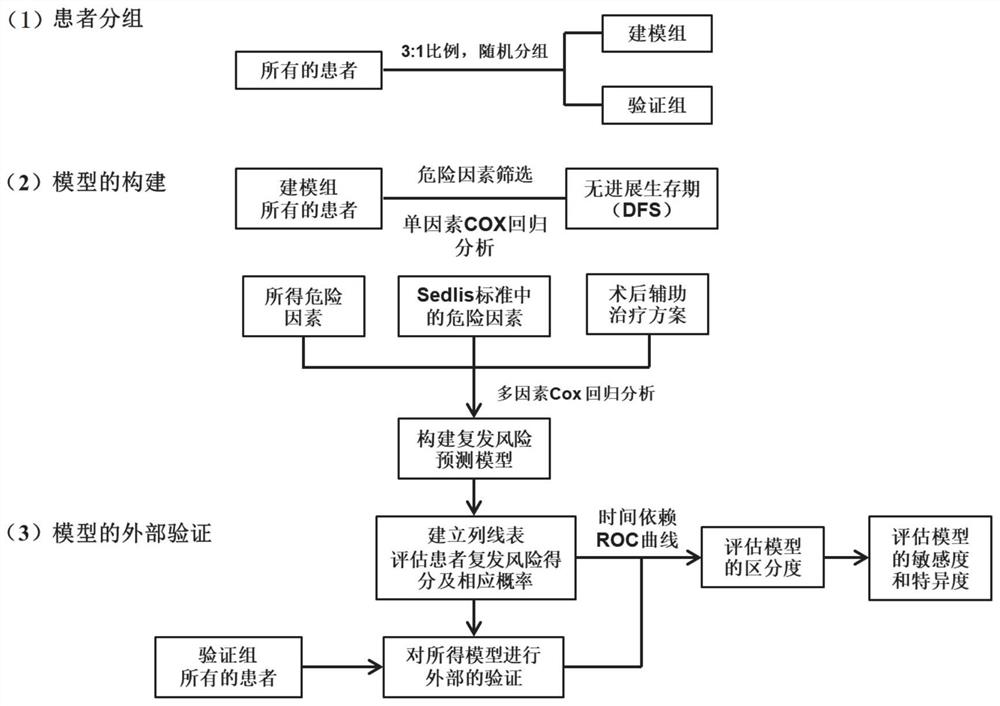
Cervical cancer postoperative recurrence risk prediction method and system
PendingCN111640509AAchieve the predicted effectAchieve forecastMedical simulationMachine learningDiseaseCervical ca
The invention provides a cervical cancer postoperative recurrence risk prediction method and system, and belongs to the technical field of medicine. The invention provides a prediction model based onmedium-risk pathological factors. The effect of predicting the postoperative disease recurrence risk of a early cervical cancer patient is achieved and the foothold is postoperative disease recurrencerisk assessment of early cervical cancer patients without high-risk pathological factors; therefore, the disease recurrence risk prediction of cervical cancer patients at any time point after the operation can be realized; and meanwhile, two model verification methods of data of a verification group and a machine learning algorithm are used, external verification is performed on the obtained model, and the prediction accuracy of the constructed model is further ensured, so that the method has a good practical application value.
Owner:SHANDONG UNIV QILU HOSPITAL
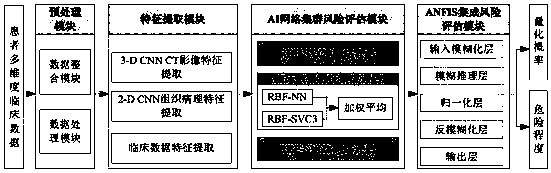
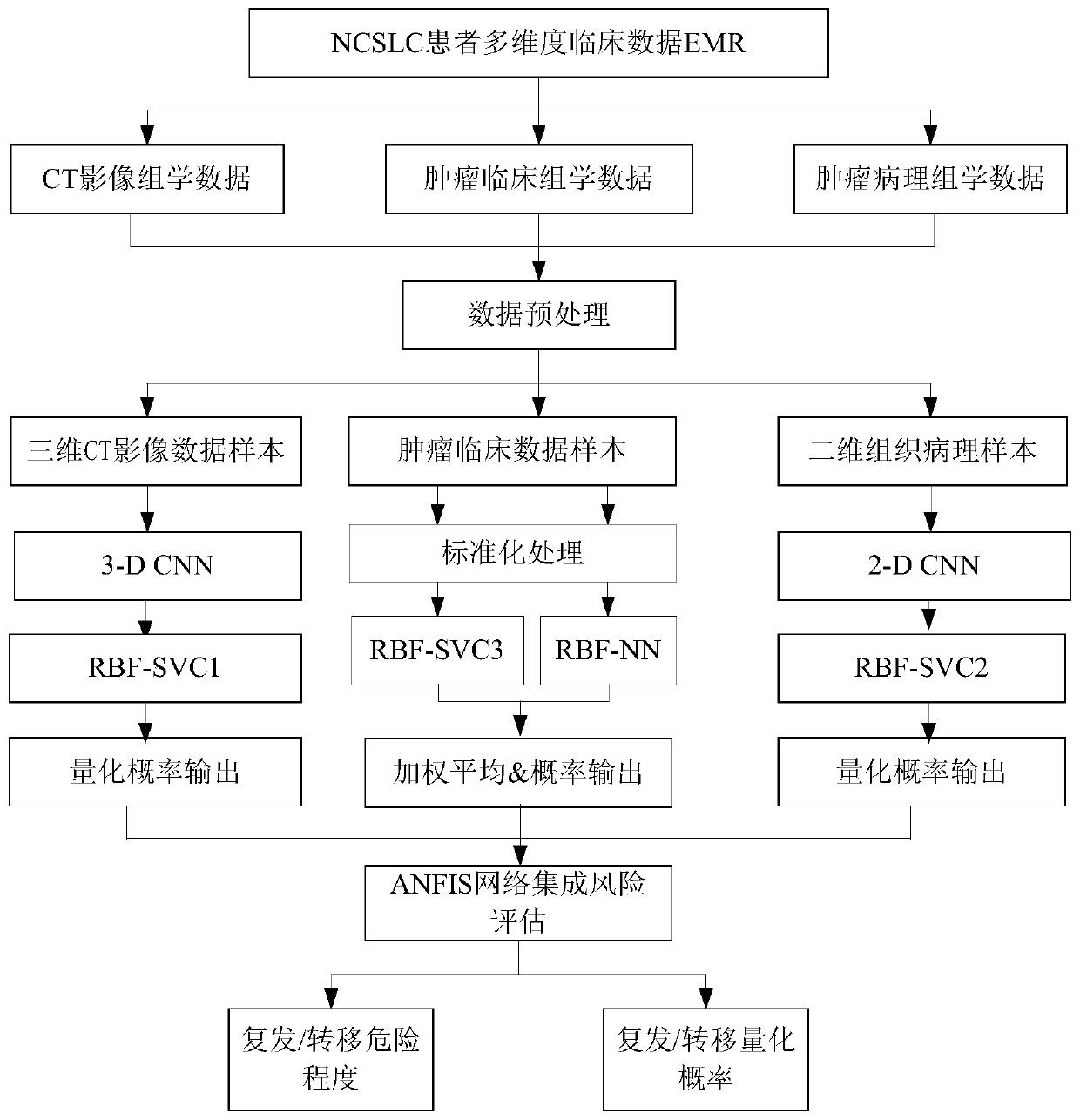
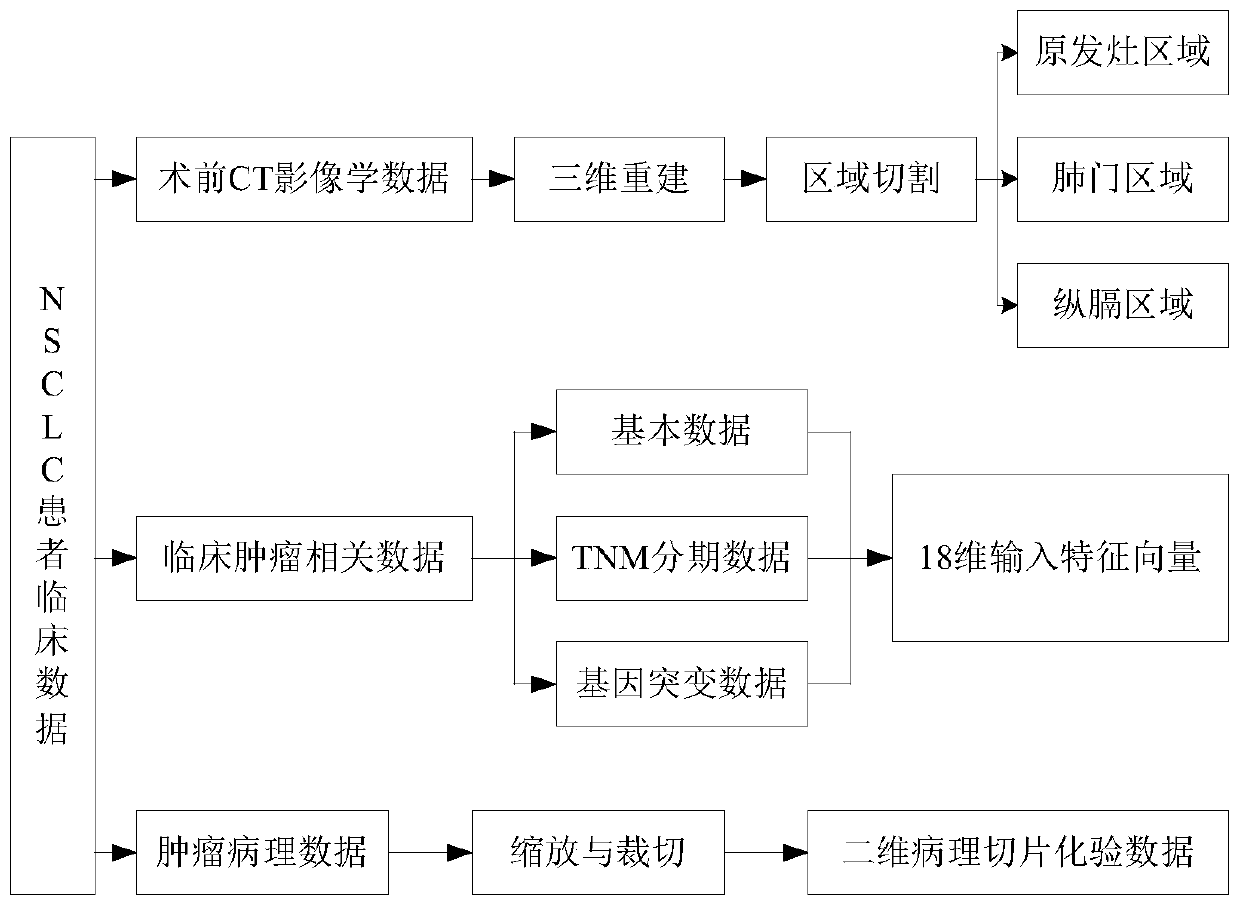
NSCLC patient postoperative short-period recurrence metastasis risk evaluating system
InactiveCN110111892AStrong clinical practicabilityImprove forecast accuracyMedical data miningHealth-index calculationLymphatic SpreadWilms' tumor
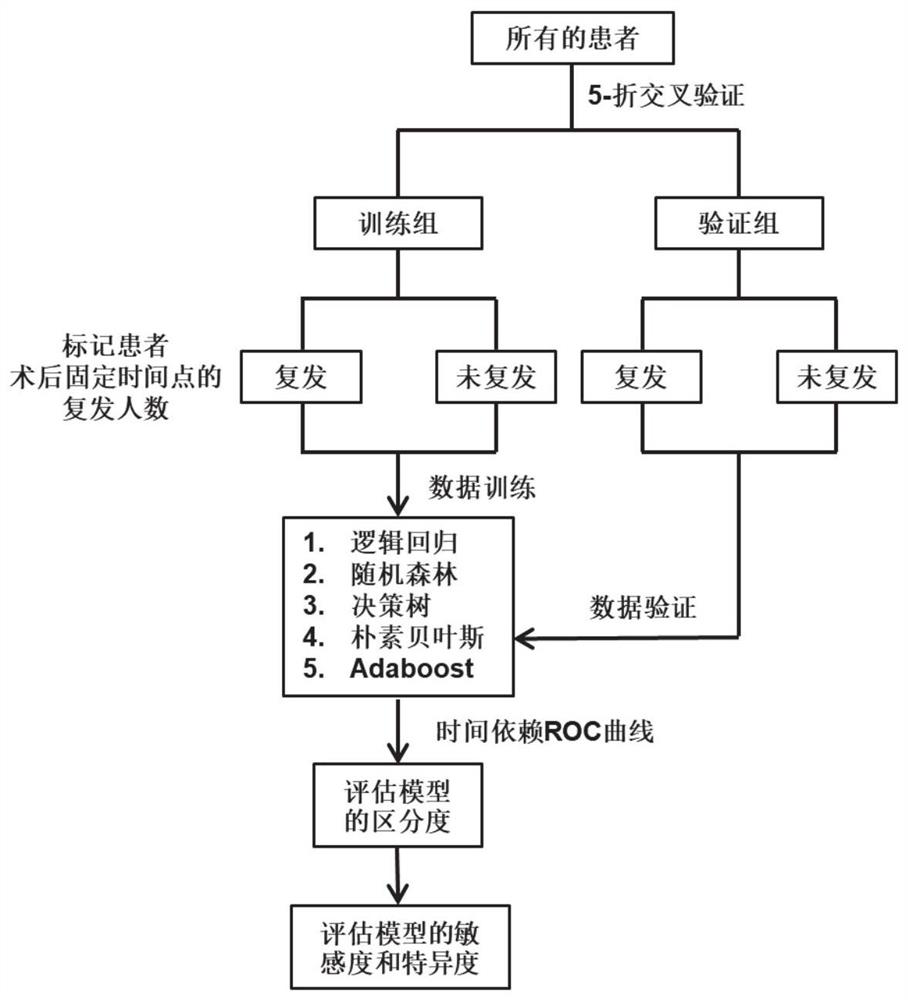
The invention provides an NSCLC patient postoperative short-period recurrence metastasis risk evaluating system which comprises a preprocessing module, a characteristic extracting module, an AI network cluster risk evaluating module and an ANFIS network integrated risk evaluating module. The preprocessing module is used for collecting and preprocessing multidimensional patient clinical data. The multidimensional patient clinical data comprise patient preoperative CT radiomics data, tumor histopathology data and tumor clinical. The characteristic extracting module is used for extracting the characteristic of the data sample after preprocessing. The AI network cluster risk evaluating module is used for performing recurrence risk probability evaluation on the extracted sample characteristic.The ANFIS network integrated risk evaluating module is used for using a recurrence risk probability evaluation result as an input characteristic vector, establishes an ANFIS network, uses a recurrence / metastasis risk quantification probability and dangerous degree as the output, and performs final integrated risk evaluation. The system can supply a reference for selecting a radial curing postoperative comprehensive treatment plan by a lung cancer patient and has high predicting accuracy.
Owner:HANGZHOU DIANZI UNIV
Combination antibody kit for risk assessment of postoperative recurrence of primary hepatocellular carcinoma
InactiveCN102298053AThe experimental method is matureEasy to detectBiological testingPatient survivalCvd risk
The invention discloses a multi-antibody detection kit used in postoperative recurrence risk assessment after a resection operation is performed on a primary hepatocellular carcinoma patient. The kit comprises 8 immunohistochemical antibodies used for paraffin sections. The invention also discloses a method for determining postoperative recurrence risk of a liver cancer patient by using the kit. The prediction effect provided by the invention is superior to an existing clinical index. The kit and the method provided by the invention have characteristics of objective, convenient, direct, and repeatable. The kit can be used in the predictions of recurrence and death after clinical liver cancer resection operations. The kit assists in screening liver cancer patients with high postoperative recurrence risk. Therefore, appropriate auxiliary treatments can be directed, recurrence can be effectively reduced, and patient survival time can be prolonged.
Owner:SUN YAT SEN UNIV CANCER CENT
Kit for jointly detecting and evaluating liver cancer transfer and recurrence risks after orthotopic liver transplantation
ActiveCN106370867AInhibit progressImproved prognosisMicrobiological testing/measurementDisease diagnosisSplit liver transplantationCirculating cancer cell
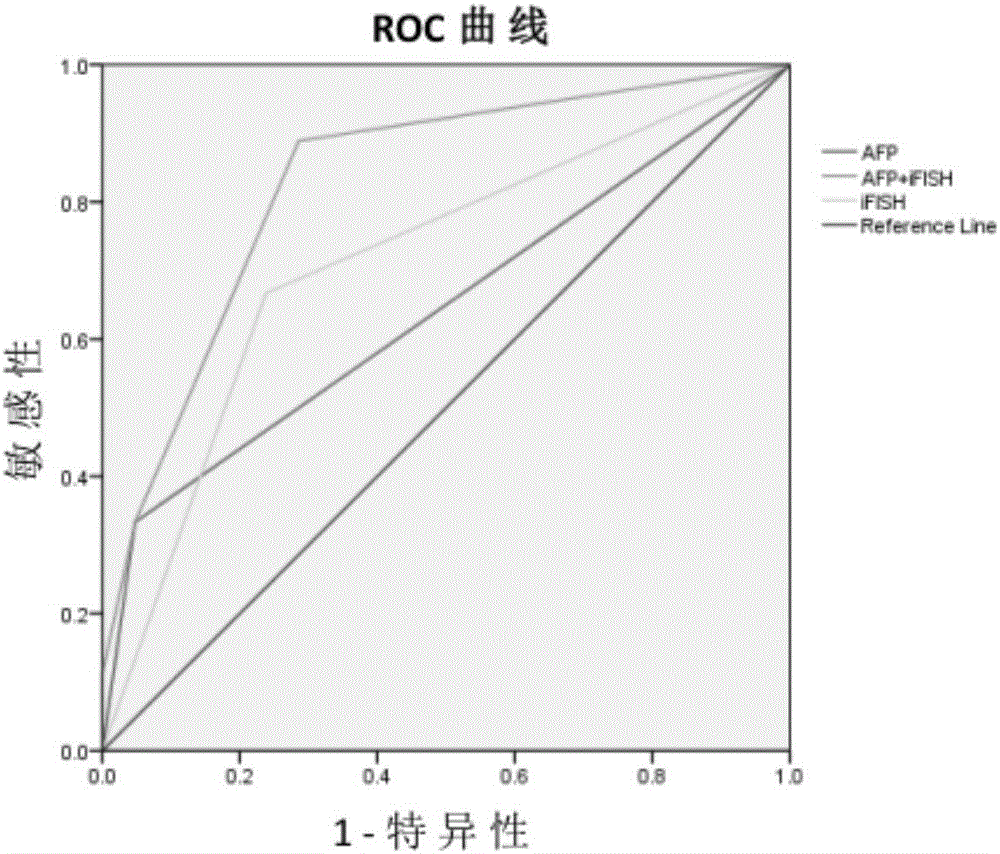
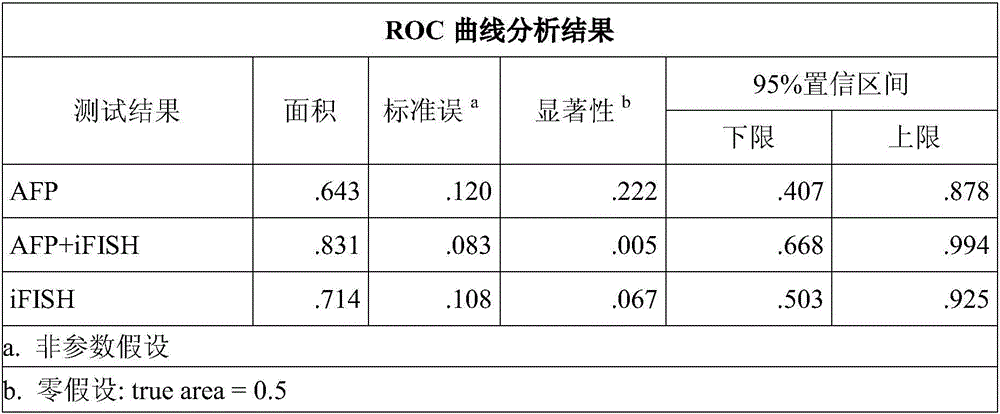
The invention relates to the field of medical biological detection, in particular to a detection reagent or a kit for evaluating liver cancer transfer and recurrence risks after orthotopic liver transplantation through combination of AFP (alpha fetoprotein) detection and iFISH-CTC (circulating tumor cells) detection, and a detection method of the detection reagent or the kit. The liver cancer transfer and recurrence risks after the orthotopic liver transplantation are evaluated through detection of the content of AFP and the number of CTC, so that a new measure is provided for detection of liver cancer transfer and recurrence after the orthotopic liver transplantation so as to start clinical intervention as early as possible, worsening of patients' conditions is prevented, and prognosis of patients is improved.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
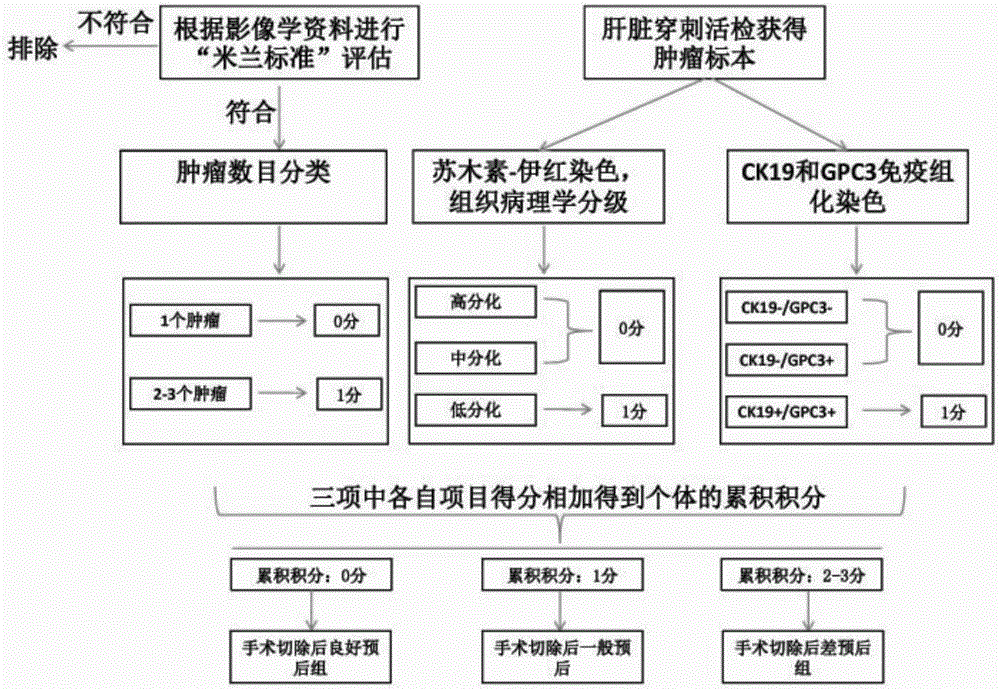
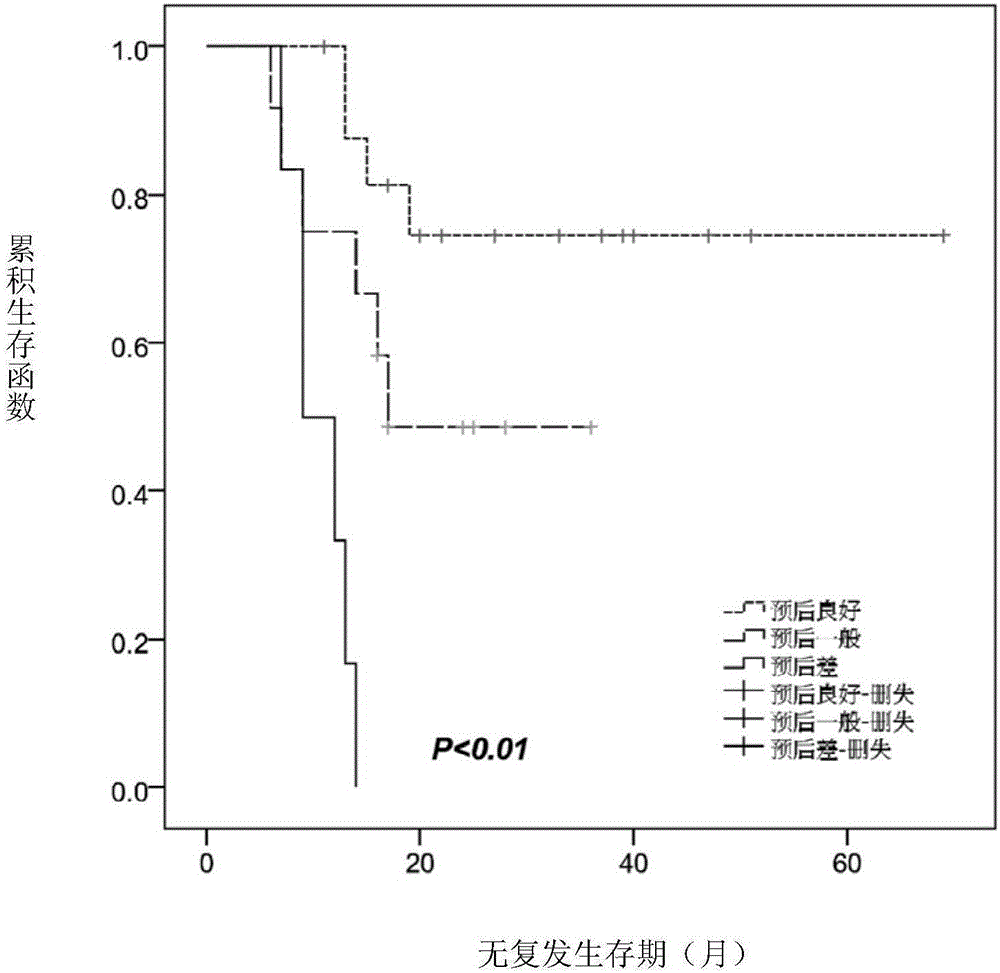
Method and system for performing orthotopic liver transplantation prognosis condition grouping on hepatocellular carcinoma patient with single tumor
InactiveCN107180154AGood treatment effectGood for healthSpecial data processing applicationsMaximum diameterRegression analysis
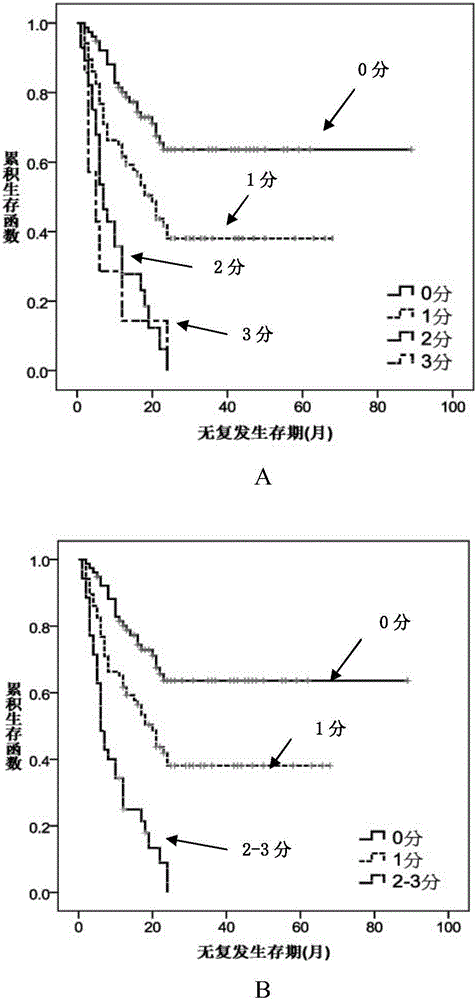
The invention relates to a method for performing prognosis condition grouping on a hepatocellular carcinoma patient who has single tumor and is about to be subjected to or is already subjected to orthotopic liver transplantation. The method comprises the steps of (1) obtaining a sample from general samples after transhepatic biopsy before transplantation or liver transplantation, and detecting CK19 and GPC3 expression conditions of tumor cells in the hepatocellular carcinoma tissue sample of the patient; (2) detecting and recording a maximum diameter of the tumor of the patient; (3) detecting and recording a serum alpha-fetoprotein level of the patient; and (4) based on a pathological section, observing whether microvascular invasion exists or not. Recurrent risks are scored according to beta coefficients of four factors in COX regression analysis; scores in items are subjected to addition; a recurrent risk group with a total score less than or equal to 4 is determined as a low recurrent risk group; a recurrent risk group with a total score equal to 5-7 is determined as a medium recurrent risk group; and a current risk group with a total score more than or equal to 8 is determined as a high recurrent risk group. Through the method, an effective auxiliary information value can be provided for a doctor and the patient; and tumor recurrence or transfer is closely monitored, so that health conditions can be discovered within the shortest time, the doctor and the patient are effectively prompted, and people are reminded to timely examine body conditions.
Owner:冯德昭 +1
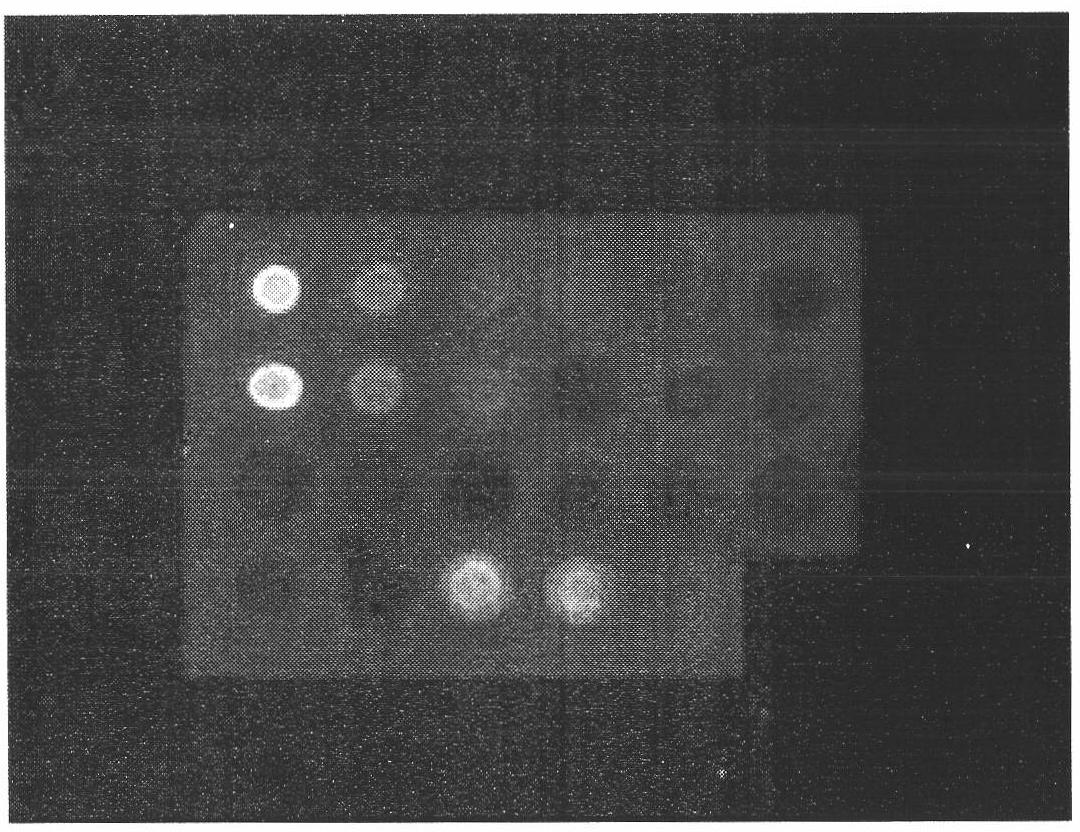
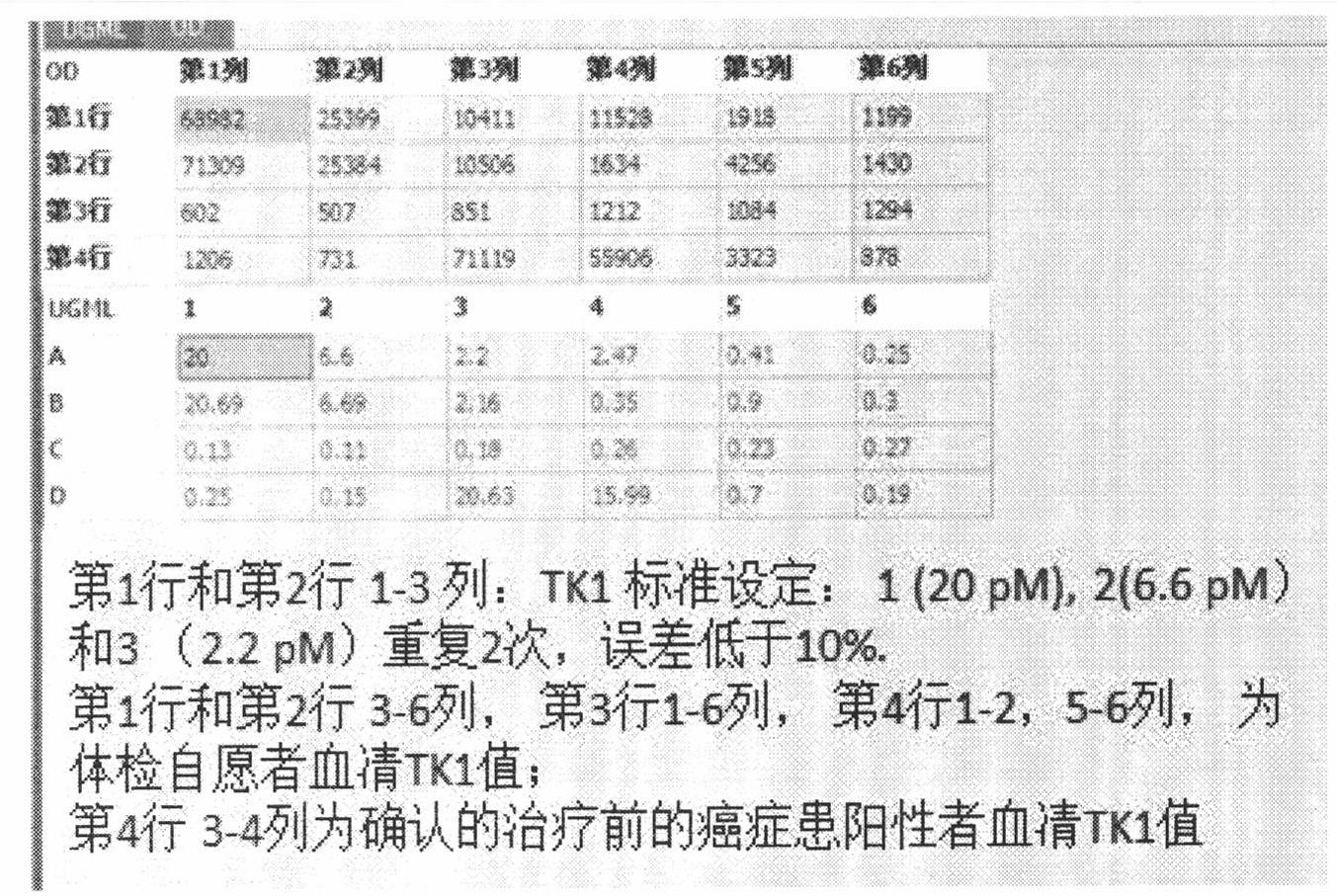
Preparation of multi-epitope TK1 antibody and application of multi-epitope TK1 antibody to evaluation on recurrence risk and prognosis of tumor patient at early stage
ActiveCN102432683ATrue reflection of proliferationReflect proliferationEgg immunoglobulinsTransferasesAntigenCvd risk
The invention provides a high-specificity and high-sensitivity coordination combination anti-human TK1 antibody prepared from an antigenic determinant consisting of 23 peptide at an N end, 20 peptide at a C end and 28 peptide at the C end of human cervical cancer cells, and application thereof to tumor diagnosis. The antigenic determinant comprises the following amino acid sequences: 1) the 23 peptide (3-25) at the N end: CINLPTVLPGSPSKTRGQIQVIL: 2) the 20 peptide (206-225) at the C end: CPVPGKPGEAVAARKLFAPQ; and 3) the 28 peptide at the C end: AGPDNKENCPVPGKPGEAVAARKLFAPQ. The invention simultaneously provides a method applying the antigen for preparing the antibody. An antibody kit provided by the invention has the characteristics of high sensitivity, high specificity, low cost and the like, tumor rehabilitation populations are periodically monitored by an enhanced chemiluminescence dot blot detection method, immunohistochemistry detection and the detection kit, and the recurrence and transfer risk and the prognosis of the tumor rehabilitation people are evaluated.
Owner:SHENZHEN HUARUI TONGKANG BIOTECHNOLOGICAL
Signatures and determinants associated with prostate cancer progression and methods of use thereof
ActiveUS20140314765A1Increased susceptibilityMicrobiological testing/measurementLibrary screeningMedicineProstate cancer
Owner:DANA FARBER CANCER INST INC
Grouping method and system for prognosis of radical operation of hepatocellular carcinoma for hepatocellular carcinoma patients and kit
ActiveCN106248945AGood treatment effectGood curative effectMicrobiological testing/measurementBiological material analysisAbnormal tissue growthLymphatic Spread
The invention provides a method for evaluating postoperative recurrence risk of hepatocellular carcinoma patients prepared to be treated by radical operation of hepatocellular carcinoma on the basis of combined detection of cytokeratin 19 (CK19) and Glypican-3 (GPC3), tumor cells differentiation status and number of tumors as the indexes. According to the method, patients can be reasonably screened before operation so as to reduce metastasis and recurrence after radical operation of tumor. In addition, metastasis and recurrence risks after radical operation of hepatocellular carcinoma patients can be evaluated according to the method so as to further guide clinicians to carry out close monitoring and early treatment for cases with high risks of metastasis and recurrence.
Owner:冯骥良
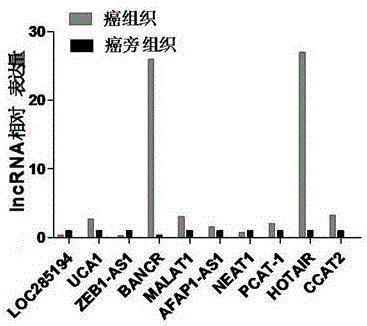
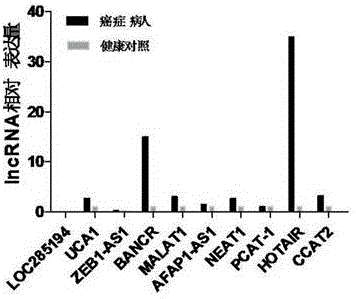
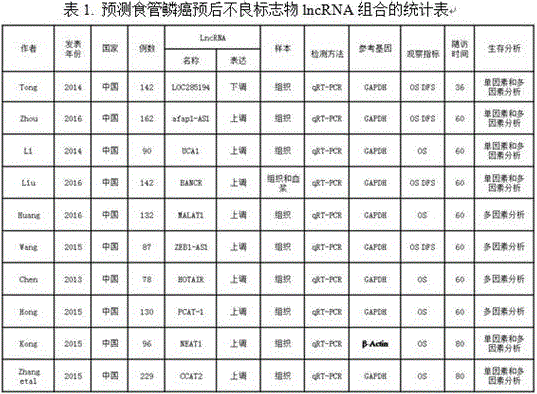
LncRNA composition for detecting prognosis of early esophageal cancer and kit comprising LncRNA composition
InactiveCN107523647APredict risk of poor prognosisImprove survival rateMicrobiological testing/measurementDNA/RNA fragmentationReference sampleCvd risk
The invention discloses an LncRNA composition for detecting prognosis of an early esophageal cancer and a kit comprising the LncRNA composition. By fluorescent quantitative PCR, a sample (which can be tissues / plasma serum and the like) of a patient with an esophageal cancer and difference change of the expression amount of a group of LncRNA in a corresponding para-carcinoma tissue / reference sample are identified, and the risk of esophageal cancerrelapse or transfer is accurately evaluated early. The kit can be used for accurately detecting early esophageal cancer transfer potential, then the transfer or relapse risk of a postoperative esophageal cancer patient can be accurately predicted and evaluated, the patient with high risk is intensively monitored and intervened effectively, relapse or transfer of the esophageal cancer is reduced, and prognosis of the patient is improved further. The poor prognosis related lncRNA composition is adopted, and the shortcomings that sensitivity and specificity are low due to the fact that a kit only uses single lncRNA as a tumor marker, and therefore, rate of fault diagnosis and rate of missed diagnosis on the esophageal cancer are greatly increased are overcome.
Owner:NANYANG NORMAL UNIV
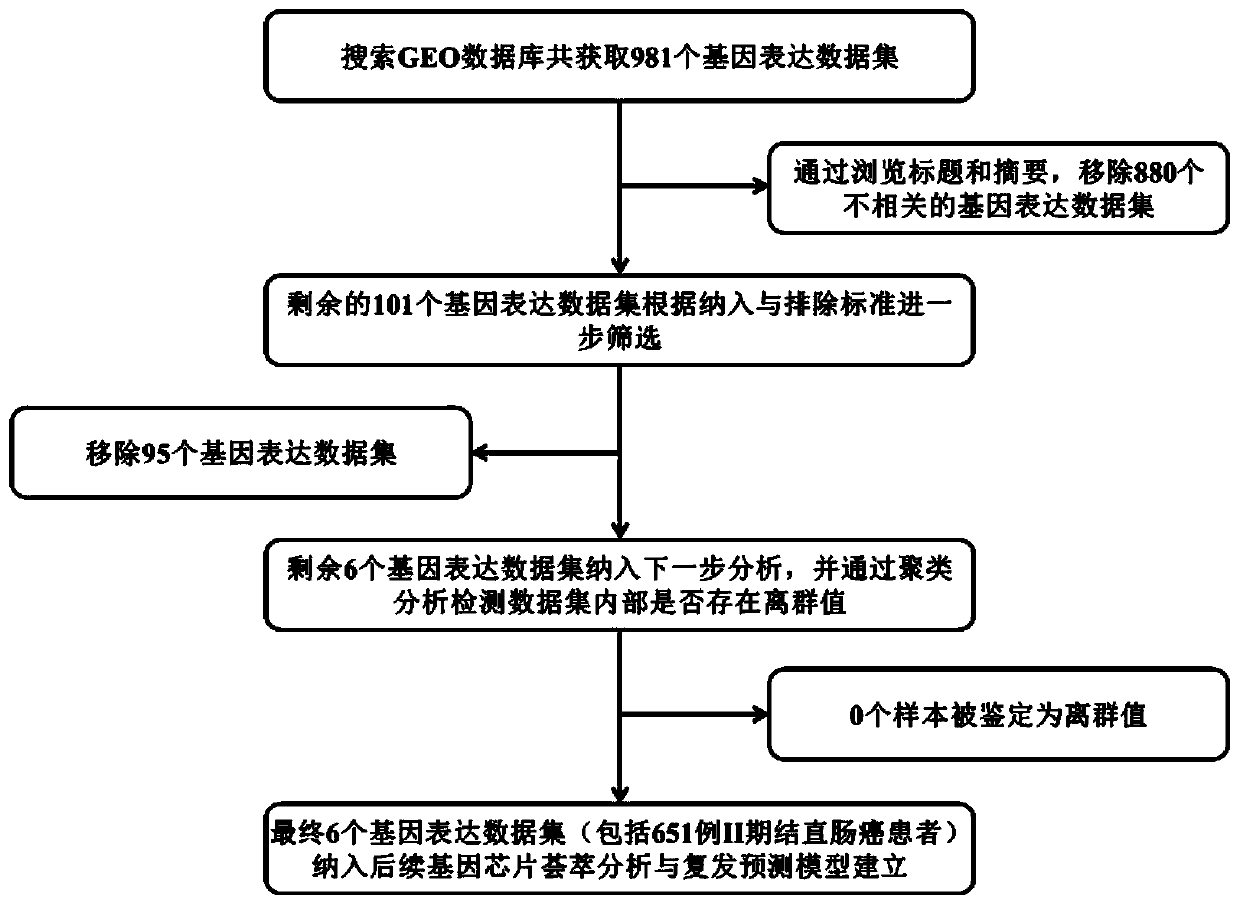
Prognostic marker gene and random survival forest model for predicting recurrence of II-stage colorectal cancer
ActiveCN110791565AVariable dimensionality reductionMicrobiological testing/measurementSystems biologyBiologyRecurrence risk
The invention discloses a prognostic marker gene for predicting recurrence of II-stage colorectal cancer and application. The invention provides a model for predicting the recurrence risk of a patientaccording to gene expression information of tumors of II-stage colorectal cancer patients. The AUC value of the model for predicting the five-year recurrence risk of the II-stage colorectal cancer patients is 0.993, and patients with high recurrence risk and patients with low recurrence risk can be remarkably separated in a test set. For the establishment and selection of the model, a random survival forest model is used, variable screening is carried out according to the minimum depth value of the maximum sub-tree where variables are located, and an important variables are selected to reestablish the model, so that the variable dimension of the model is greatly reduced. After the patients are divided into a high recurrence risk group and a low recurrence risk group by using the random survival forest model in a test set, no-recurrence lifetimes of the patients of the two groups have significant difference.
Owner:ZHEJIANG UNIV
Gene chip for predicting liver cancer metastasis and recurrence risk and manufacturing method and using method thereof
InactiveCN101812507AImprove survivalAccurate predictionMicrobiological testing/measurementLymphatic SpreadCvd risk
The technical scheme of the invention provides a gene chip for predicting liver cancer metastasis and recurrence risk, which comprises a substrate and a probe arranged on the substrate, wherein the probe consists of 149 genes. The gene chip for predicting the liver cancer metastasis and the recurrence risk has the advantages that: due to the adoption of genome comparison research, a large sample independently proves that a prediction model can accurately predict and evaluate the metastasis potential of liver cancer patients. Therefore, postoperative survival and metastasis, and recurrence of the patient can be accurately predicted (even early liver cancer patients can be accurately predicted). The gene chip contributes to early identifying or predicting high-risk patients so as to perform intensive monitoring and effective intervention on the high-risk patients to further prolong the life of the tumor patients.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Breast cancer prognosis evaluation model and establishing method thereof
InactiveCN110656173ALess rapid disease progressionNo disease progressionMicrobiological testing/measurementBiostatisticsSide effectIndividualized treatment
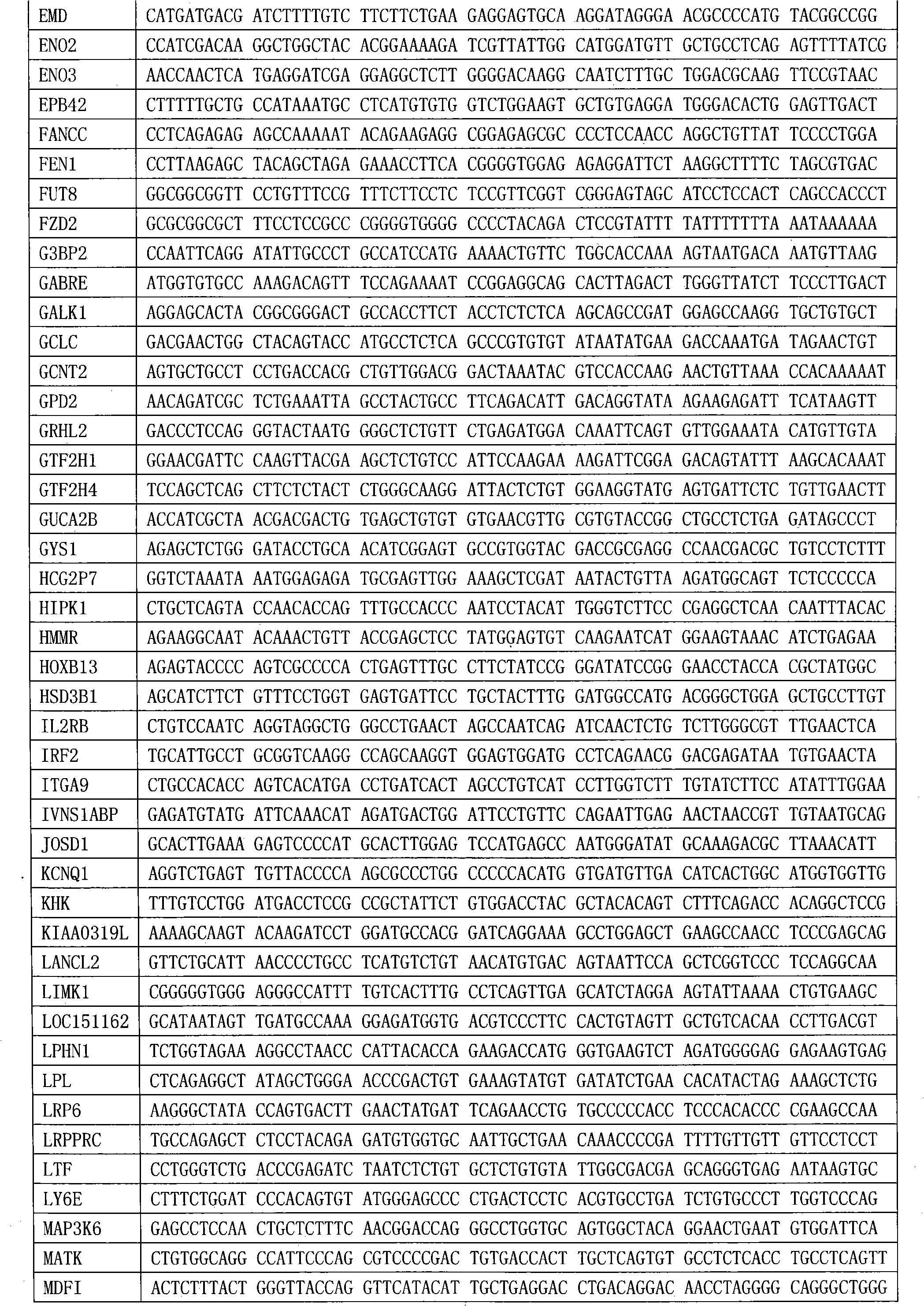
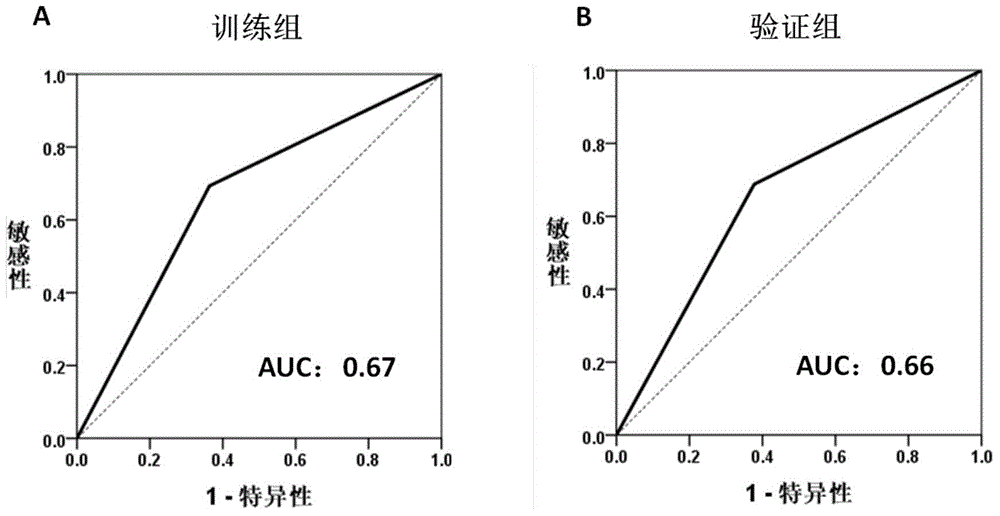
The invention provides a breast cancer prognosis evaluation model and an establishing method thereof. Differentially expressed genes are screened by selecting Chinese population as the research objectand through an RNA-seq method, the genes closely related to recurrence are screened out, and thus the prediction model suitable for the breast cancer recurrence risk of the Chinese population is obtained; and the model comprises the 190 genes which are differentially expressed in recurrence groups and non-recurrence groups and shown in the tablet 1. The model can provide more accurate individualized treatment effect prediction and ten-year recurrence risk prediction for early-stage breast cancer patients of the Chinese population, thus disease progression does not quickly occur in the high-risk population due to the lack of postoperative adjuvant chemotherapy, and the bodies of the low-risk population do not bear unnecessary chemotherapy toxic and side effects due to overtreatment either.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
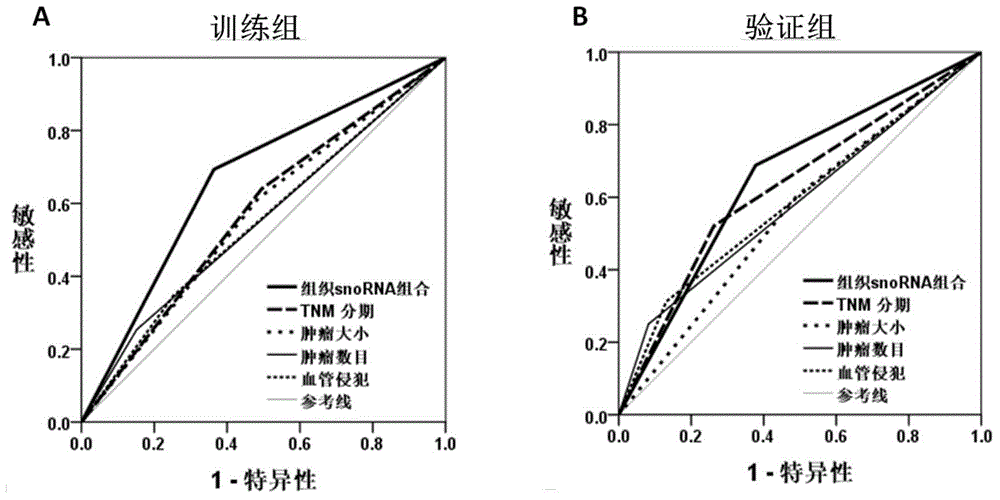
Marker composed of tissue snoRNA and kit used for predicting liver cancer recurrence risk
InactiveCN106282321AAvoid warningEasy to getMicrobiological testing/measurementDNA/RNA fragmentationCvd riskWilms' tumor
Disclosed are a marker composed of tissue snoRNA and a kit used for predicting the liver cancer recurrence risk. The invention discloses a marker used for predicting the postoperative recurrence risk of a patient who suffers from liver cancer. The maker is composed of one or several of the following snoRNA nucleic acid molecules: U15a, U19, ACA21, ACA31, U42b, U52 and / or ACA61. The invention further discloses a multi-gene kit for evaluating the postoperative recurrence risk of the patient who suffers from liver cancer. The kit comprises an agent for detecting the level of the following snoRNA nucleic acid molecules including U15a, U19, ACA21, ACA31, U42b, U52 and / or ACA61 in the tumor tissue of a patient who suffers from liver cancer. The kit can reflect the postoperative recurrence risk of a patient who suffers from liver cancer, allows excessive treatment to a low-risk patient to be avoided, and warns a high-risk patient. The kit is convenient for a clinician to adopt a personalized recurrence prevention scheme in time.
Owner:SUN YAT SEN UNIV
Gene group evaluating breast cancer molecule typing and detection kit thereof
ActiveCN104293910AImprove rationalityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationTypingNormal cell
The invention discloses a gene group evaluating breast cancer molecule typing and a detection kit thereof. The gene group evaluating breast cancer molecule typing is a gene group composed of 216 genes. The invention also discloses the detection kit for the gene group evaluating breast cancer molecule typing. The detection kit comprises a primer capable of detecting the genes in the gene group for evaluating breast cancer molecule typing and cancer recurrence risk. An Illumina second-generation sequencing technology DASL-Seq is employed for detecting expression spectrum of cancer tissue, and on the above basis, breast cancer cases of Chinese women are divided into five molecule typing: 1) luminal type A, 2) luminal type B, 3) HER2 enrichment type, 4) basal cell type and 5) normal-cell like type, and propagation and immunization index are combined for calculating recurrence risk and pridicting recurrence possibility in 10 years, and thus individual treatment on breast cancer is guided.
Owner:SHANGHAI PRECISION DIAGNOSTICS CO LTD
Biomarker and detection method for detecting risk of cancer recurrence
ActiveCN108424970AReduced disease-free survivalReduced overall survivalMicrobiological testing/measurementDNA/RNA fragmentationProstate cancerCvd risk
The invention relates to a biomarker and detection method for detecting the risk of cancer recurrence, wherein the biomarker for detecting the risk of cancer recurrence comprises at least one of the following genes: SLCO2A1, CGNL1, SUPV3L1, TATDN2, MGAT4B, VAV2, SLC25A33, MCCC1, ASNS, CASKIN1, DNMT3B, AURKA, OIP5, CTHRC1 and GOLGA7B. The biomarker for detecting the risk of cancer recurrence, provided by the invention, has an advantage of effectively predicting the recurrence risk of cancers such as prostate cancer and the like.
Owner:SHENZHEN YIKANG BIOTECH CO LTD
Methods for assessing the risk of disease occurrence or recurrence using expression level and sequence variant information
PendingCN107636171AImproved Preoperative Risk PredictionHealth-index calculationMicrobiological testing/measurementRecurrence riskBioinformatics
Provided herein are methods, systems and kits for stratification of risk of disease occurrence of a sample obtained from a subject by combining two or more feature spaces to improve individualizationof subject management.
Owner:VERACYTE INC
Liver Cancer Methods and Compositions
InactiveUS20100304372A1Improve automationThe process is simple and accurateMicrobiological testing/measurementMalignancyWilms' tumor
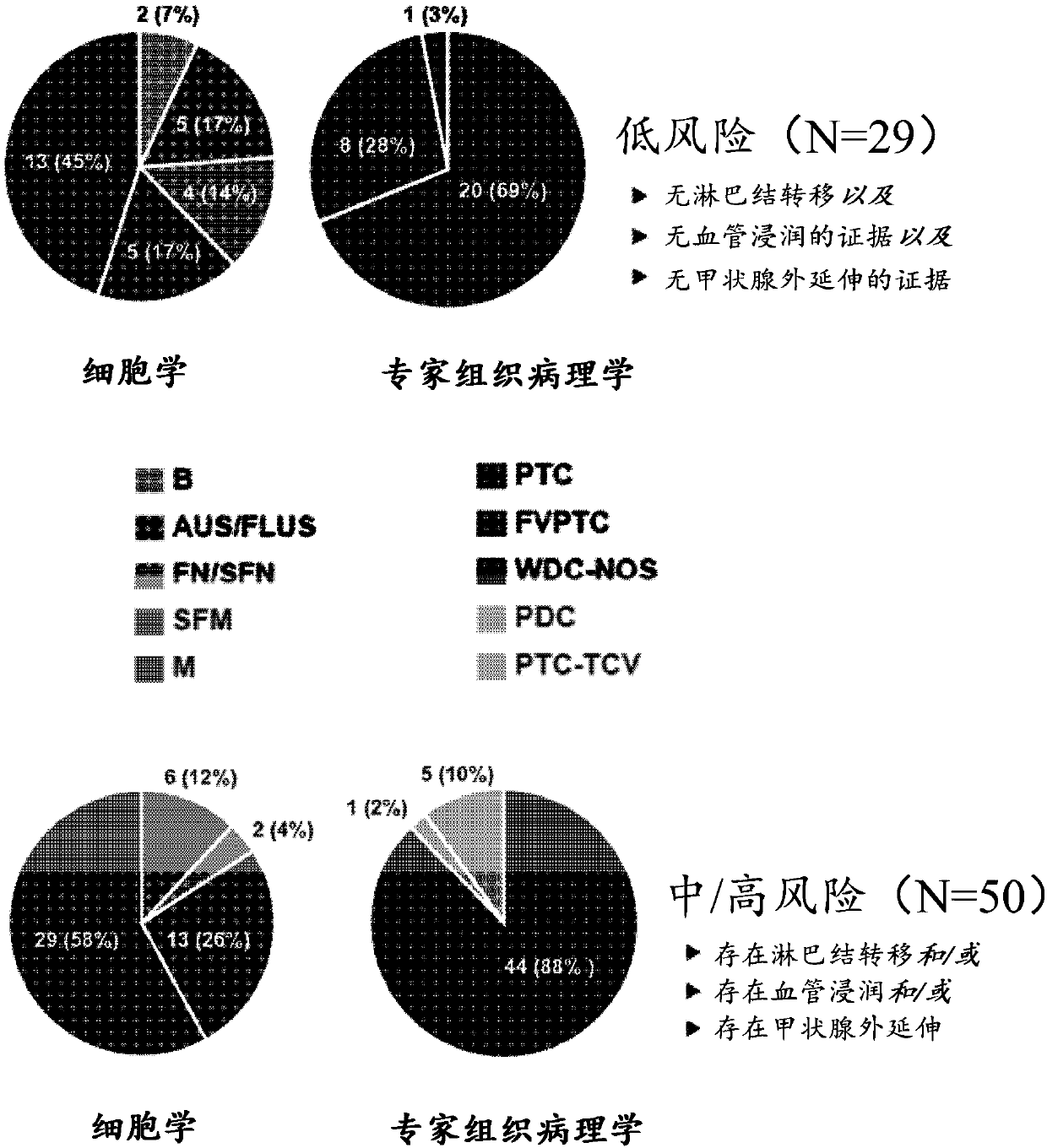
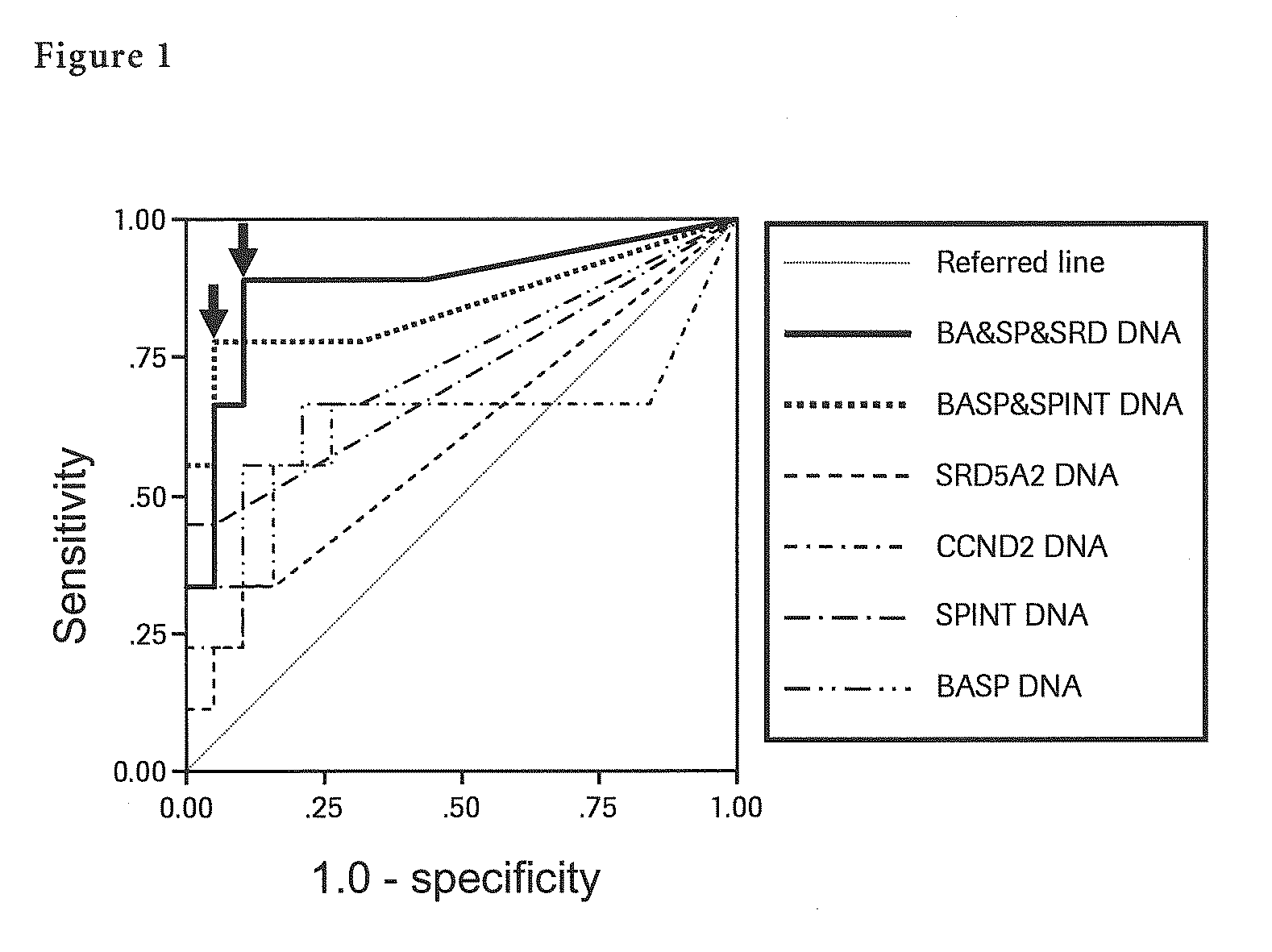
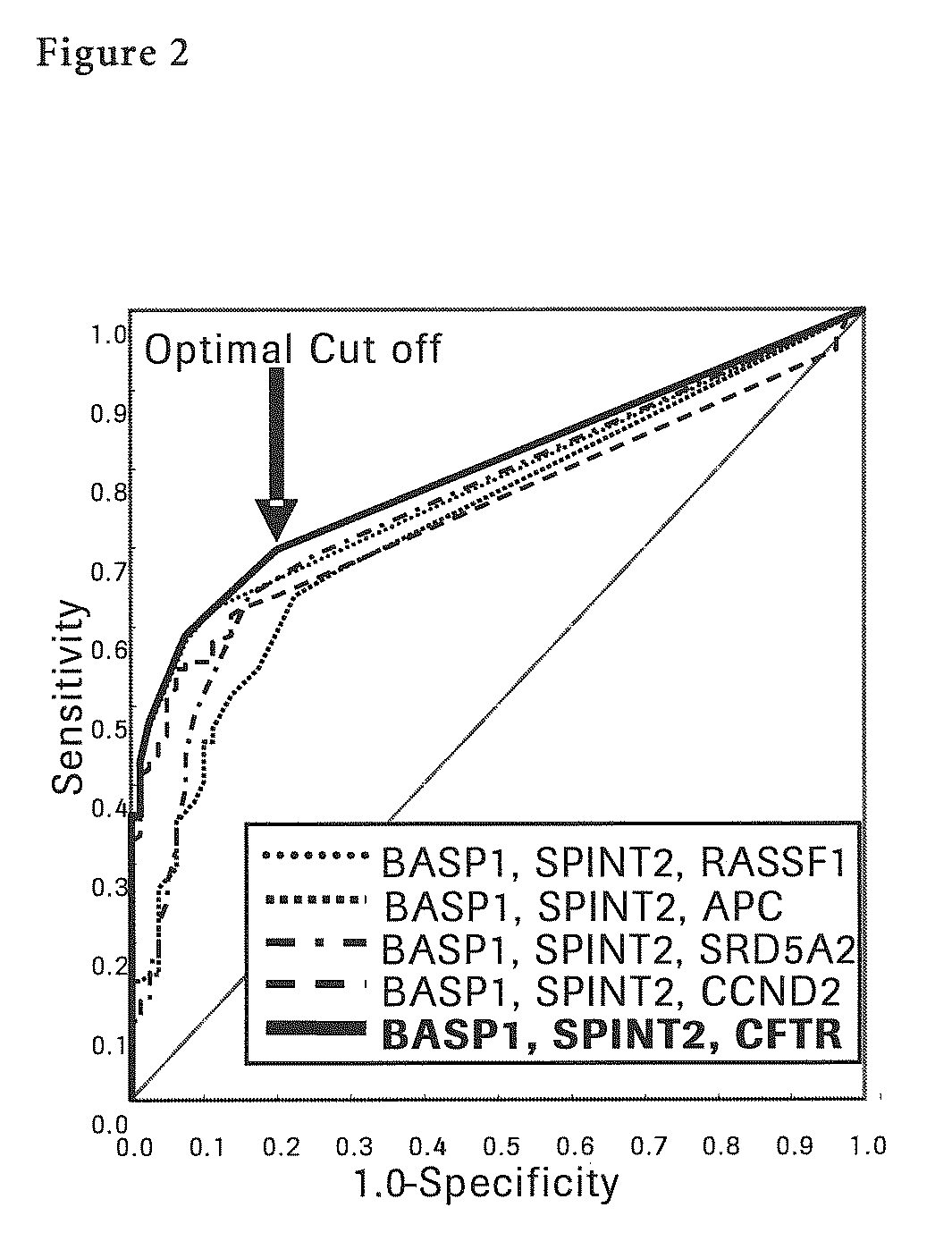
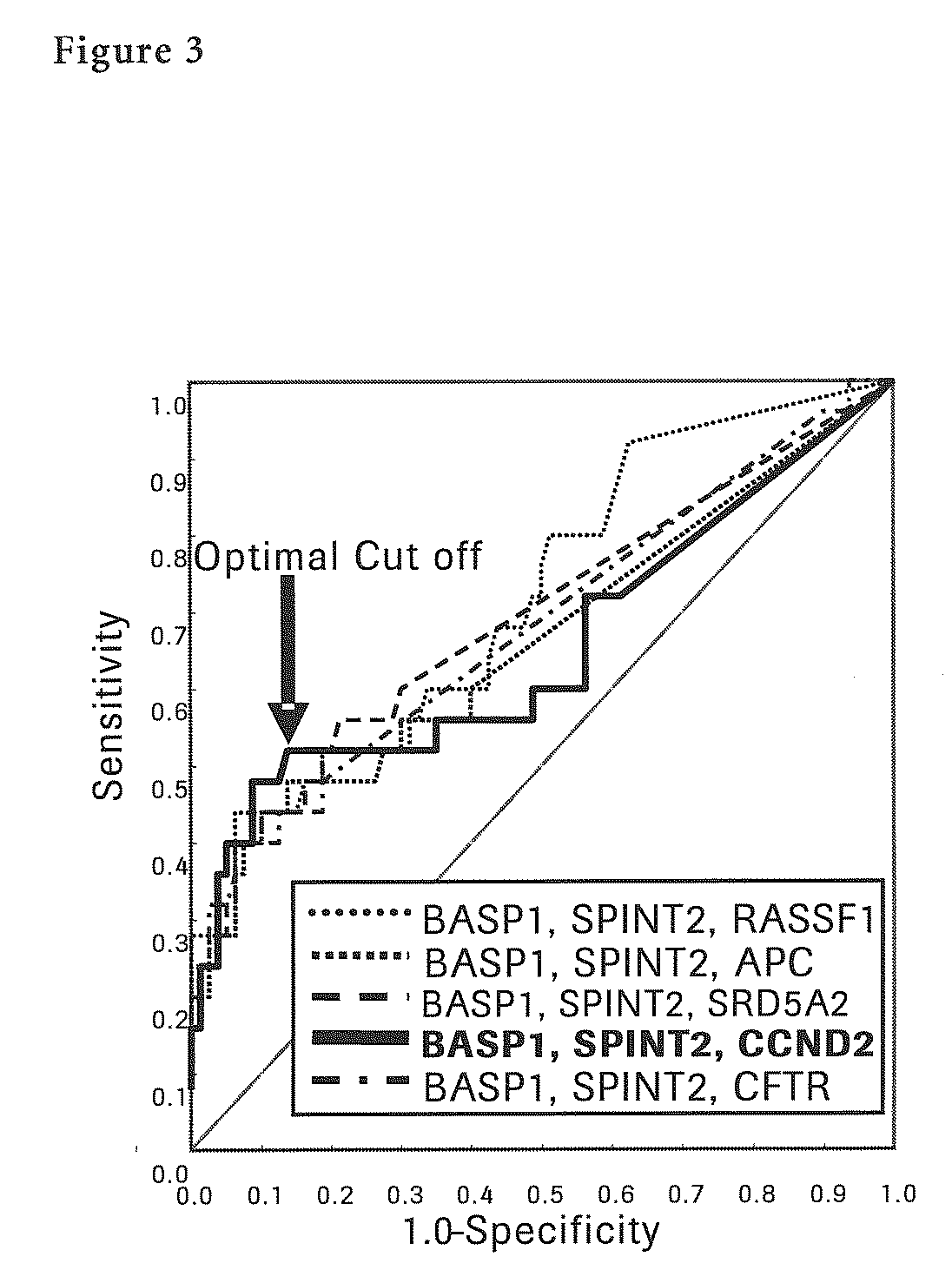
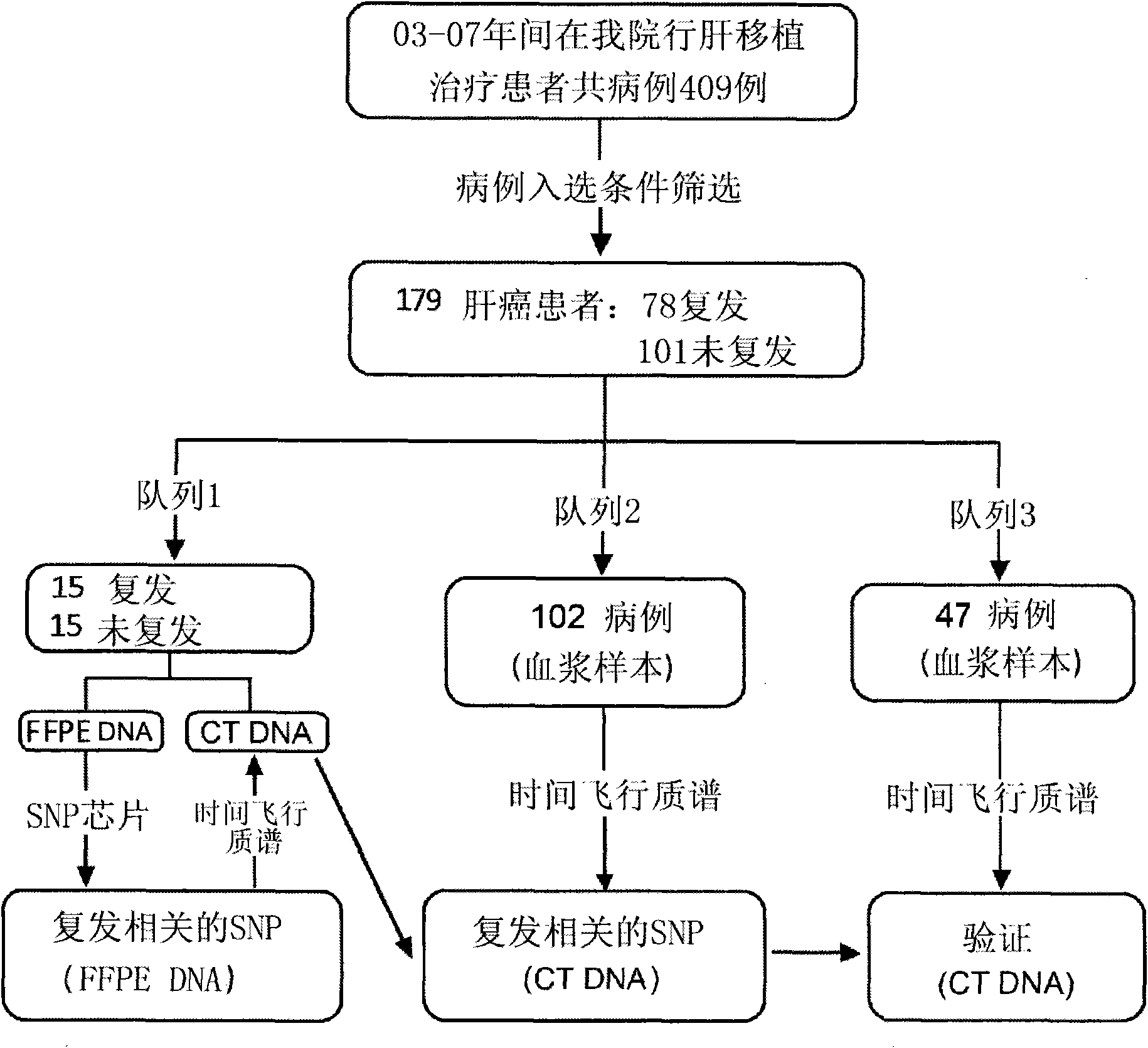
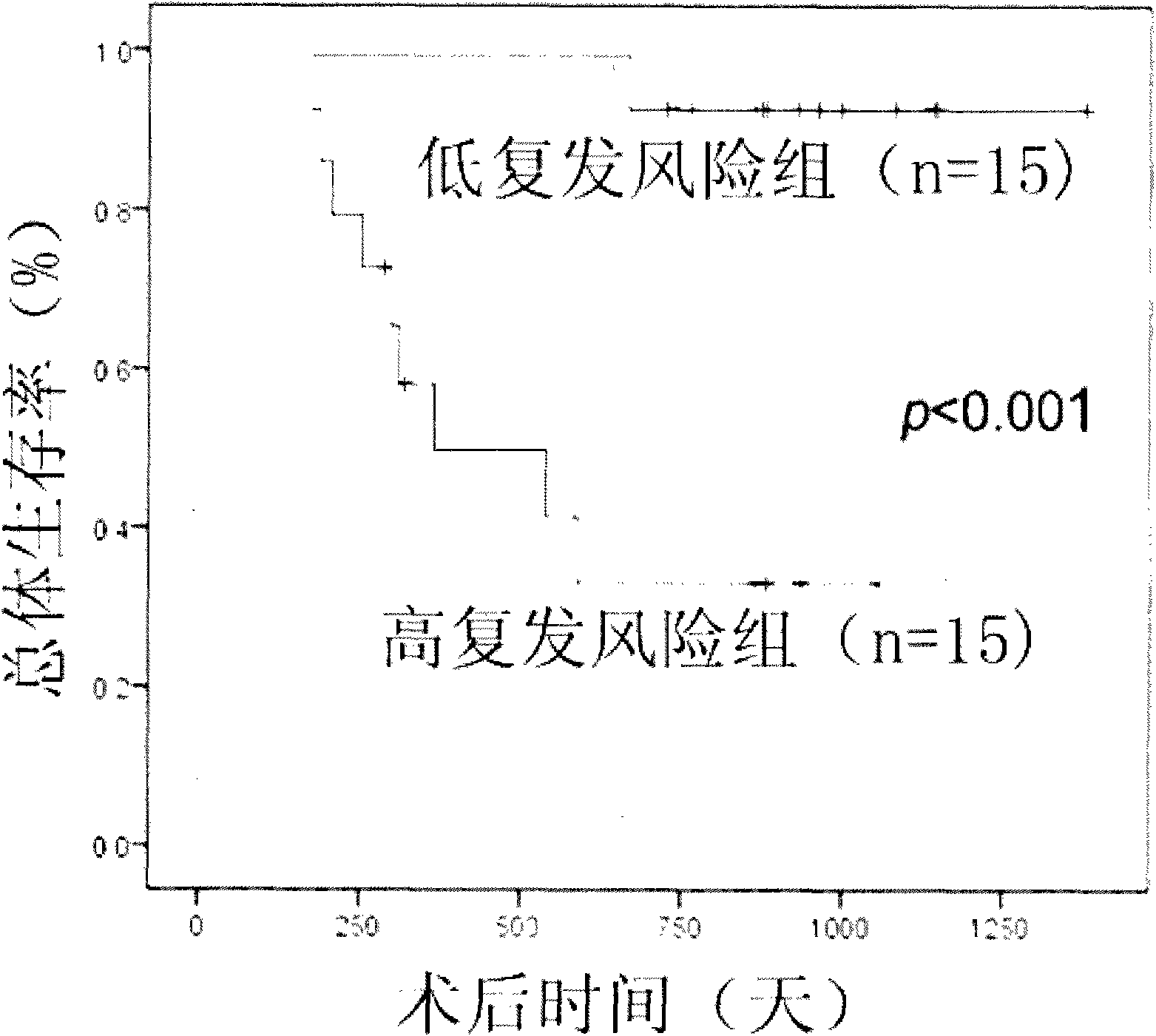
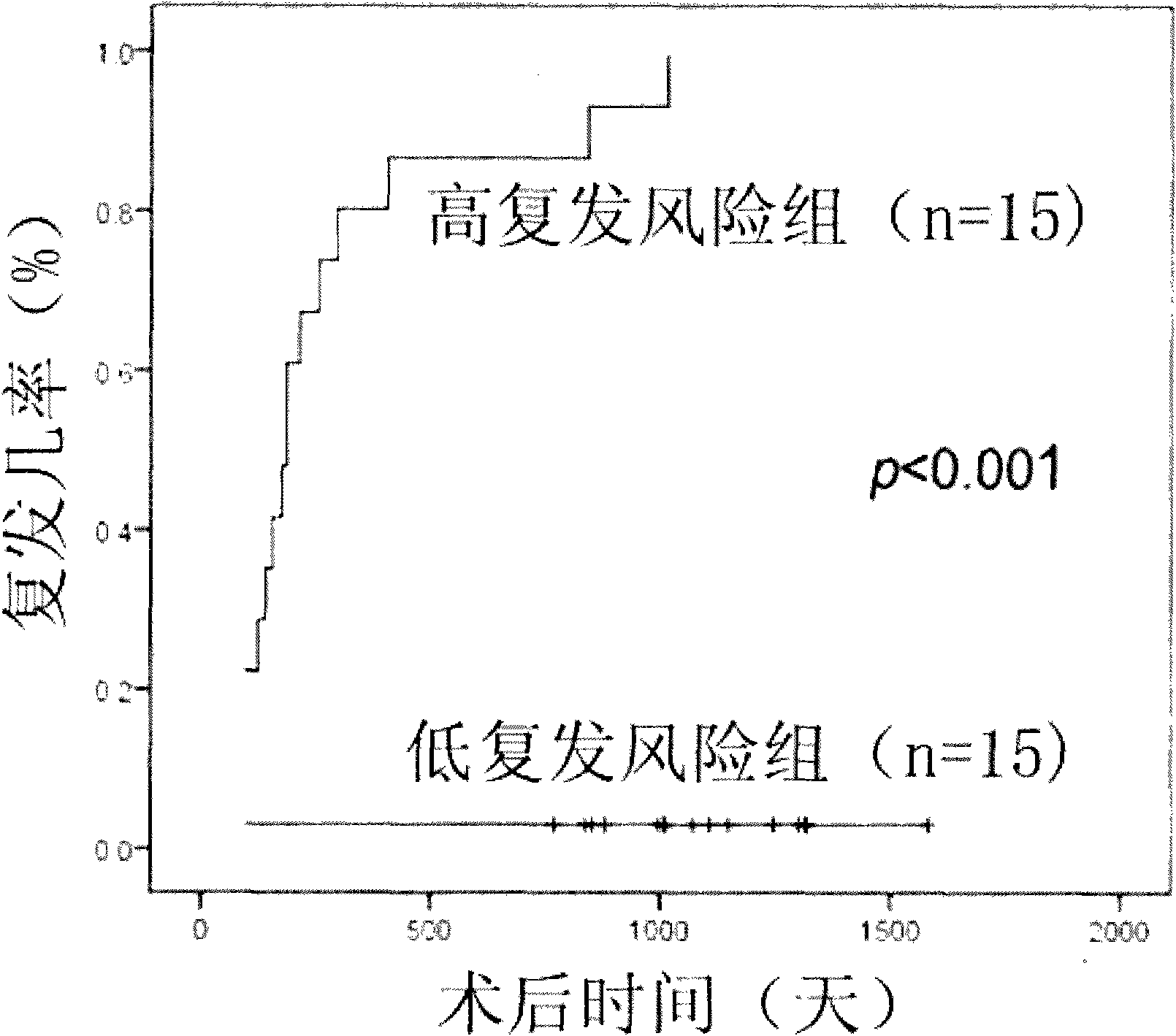
The present invention provides a novel method for detection of liver cancer. This method detects high-sensitively, high-specifically, simply and accurately liver cancer, especially that in early stage by identifying and / or quantifying methylation on particular genes and / or their DNA fragments in clinical specimens, and by combining said methylated DNA values with existing tumor marker values and / or DNA amounts in blood. This invention also detects a precancerous lesion, detects a risk of recurrence after treatment of liver cancer, detects malignancy of liver cancer and monitors progression of liver cancer with time by the same method. As for particular genes, BASP1 gene, SPINT2 gene, APC gene, CCND2 gene, CFTR gene, RASSF1 gene and SRD5A2 gene are mentioned.
Owner:ROCHE MOLECULAR SYST INC
Recurrence predicting reagent kit for liver cancer liver transplantation postoperation
InactiveCN101880715AEffective predictionTraumaMicrobiological testing/measurementCvd riskGastroenterology
The invention provides a recurrence predicting reagent kit for liver cancer liver transplantation postoperation, which is characterized in that the reagent kit uses two SNPrs rs894151 and rs12438080 in a plasma circulation DNA as detection sites, uses upstream sequences and downstream sequences of the rs894151 and the rs12438080 as amplimers, and uses fluorescent probes as genotyping probes. The invention can evaluate the recurrence risk of the liver transplantation operation carried out by liver cancer patients before the operation, and in addition, the invention uses peripheral blood as detection samples, and has the characteristics of high speed, accuracy, convenience, simplicity, easy popularization and application and the like. The invention can be used for sieving the clinical liver cancer liver transplantation patients, can guide the auxiliary treatment after the liver cancer liver transplantation operation, and can reduce the postoperation recurrence rate of high-risk patients, so the prognosis of the liver cancer liver transplantation is improved, and the limited donor livers can be effectively utilized. The reagent kit has important clinical significance to the prognosis judgment on patients exceeding the Milan standard.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com