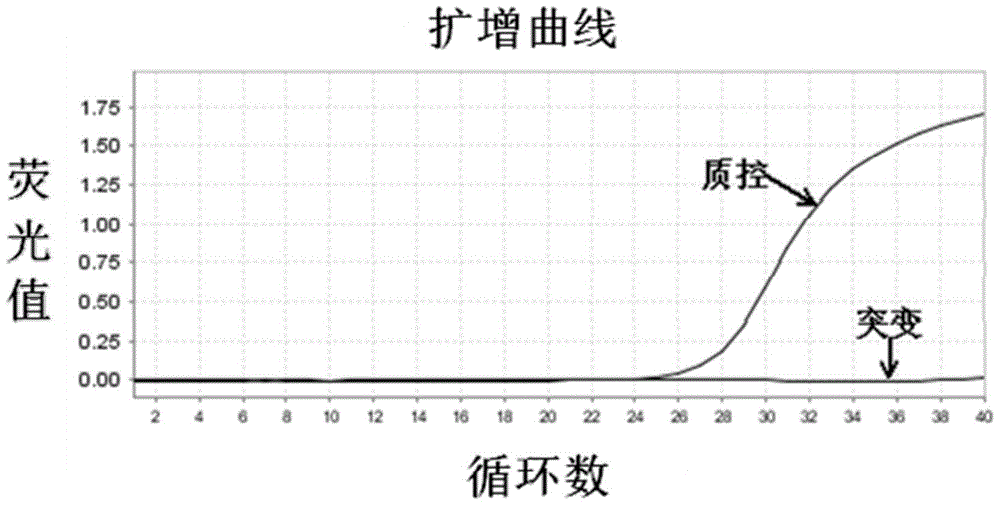
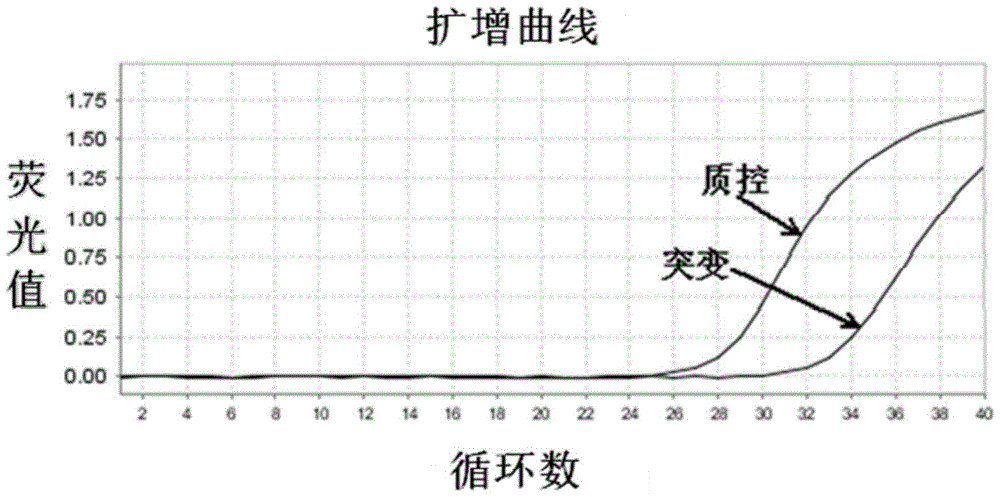
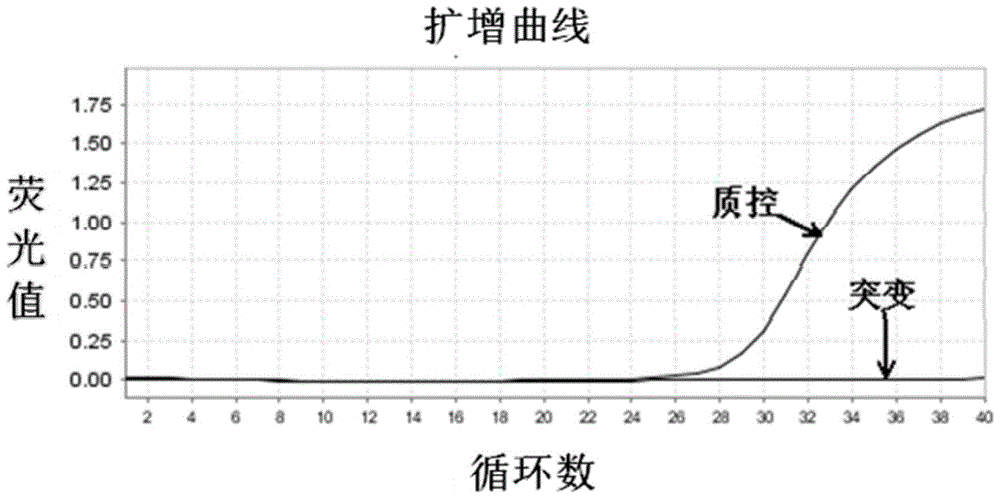
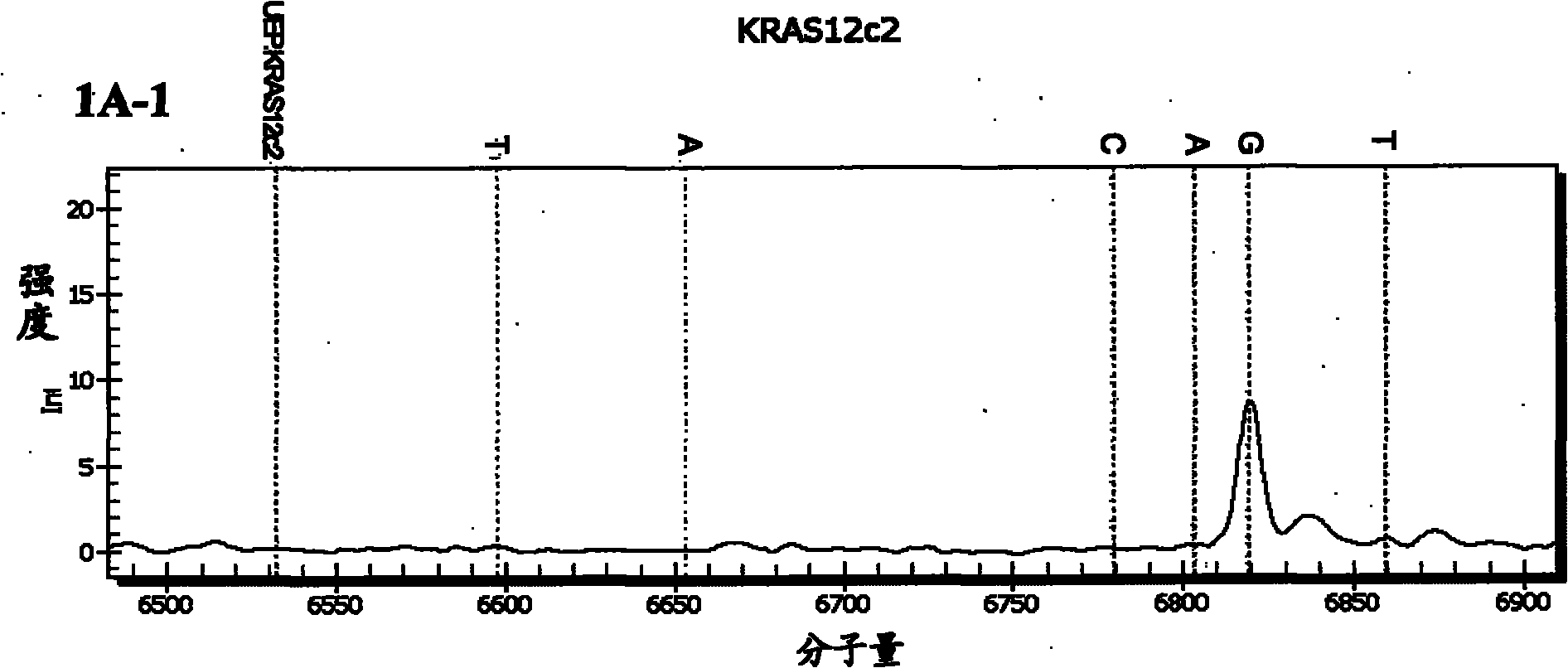
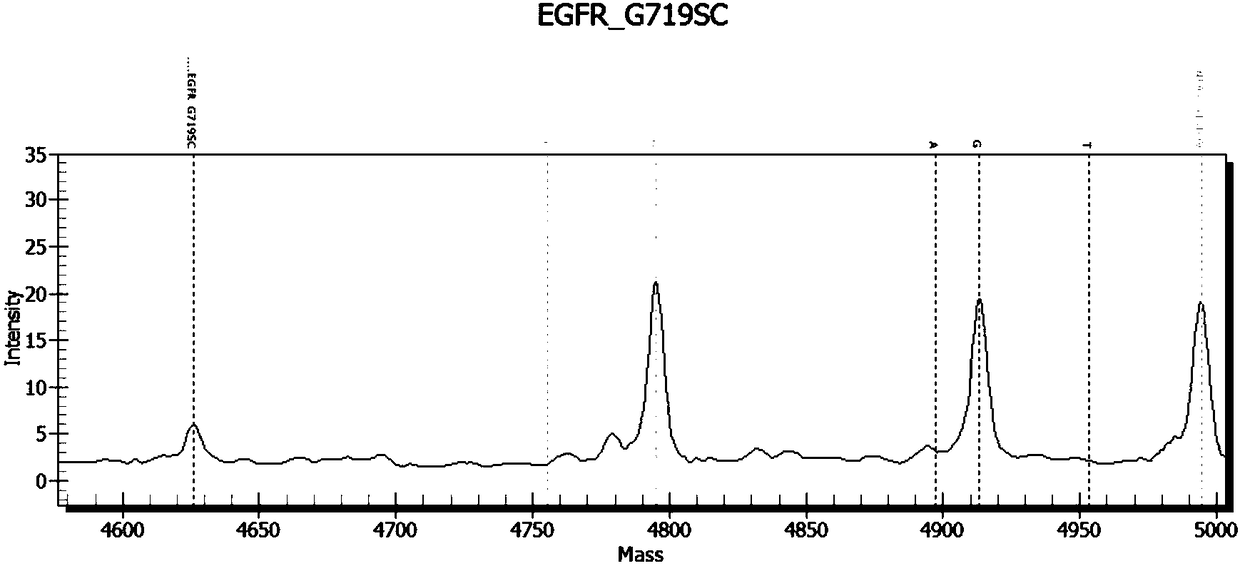
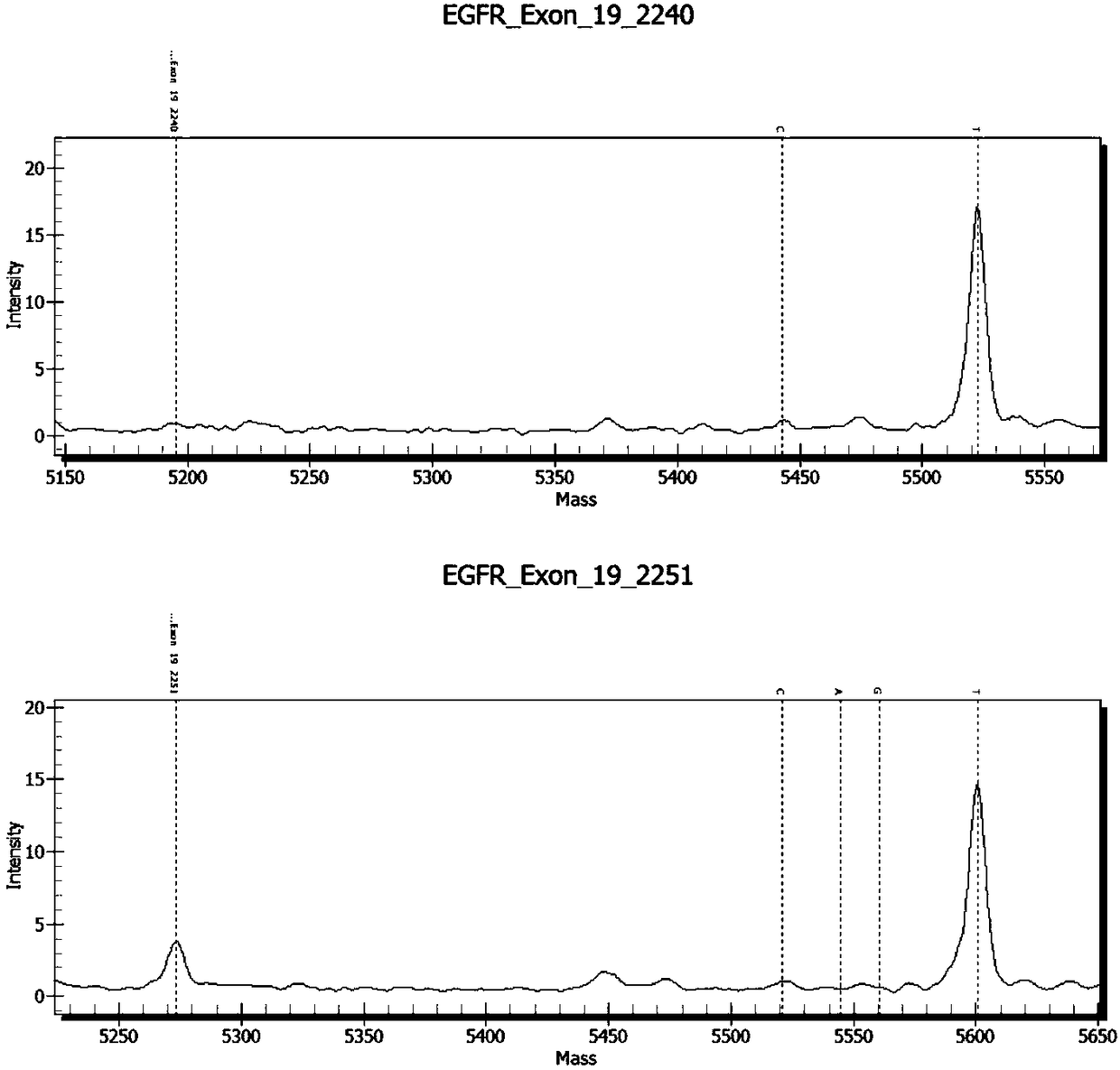
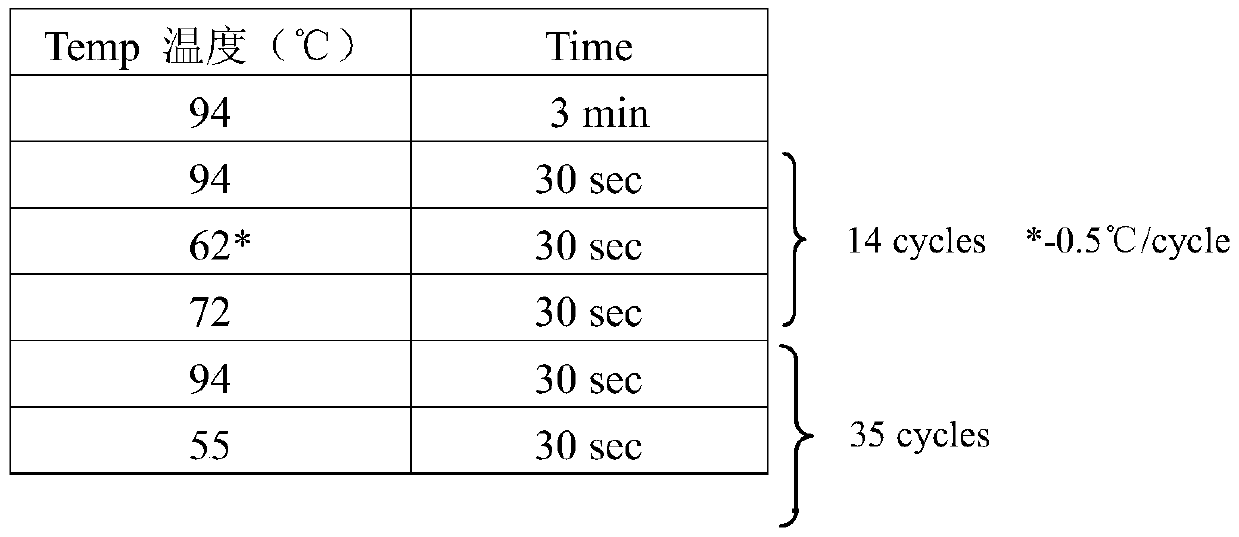
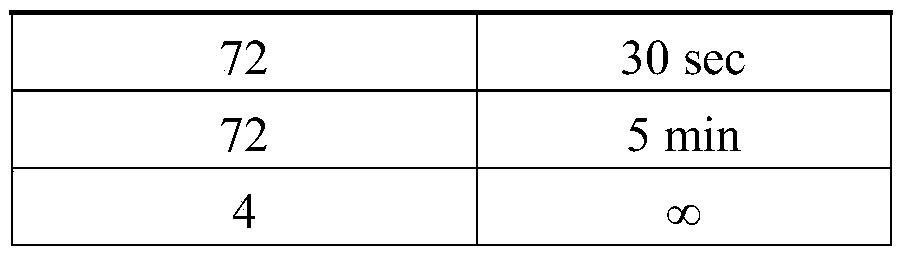
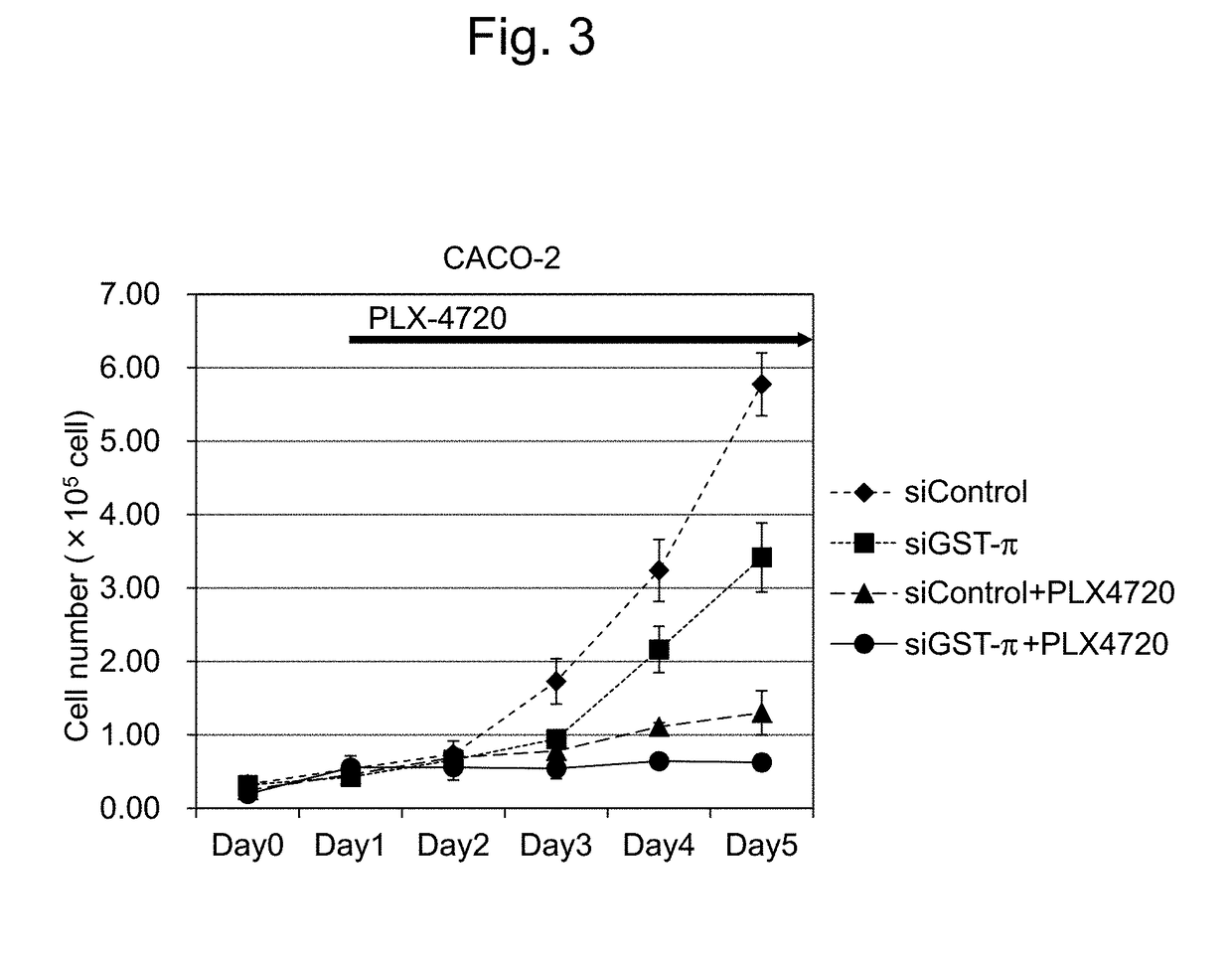
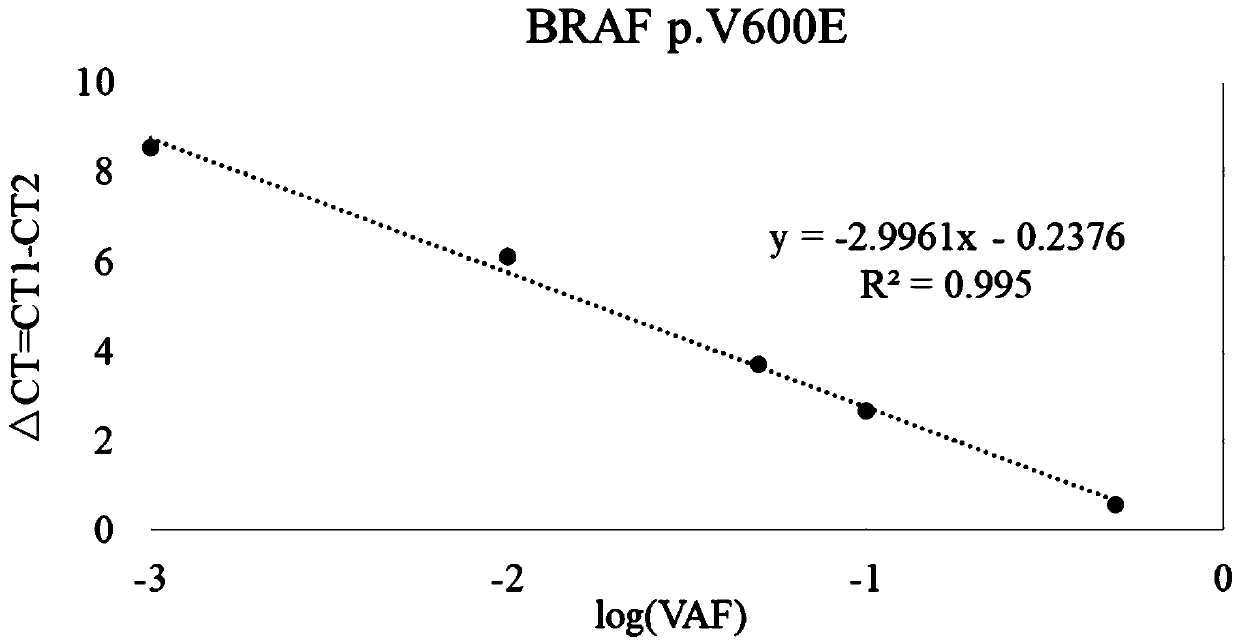
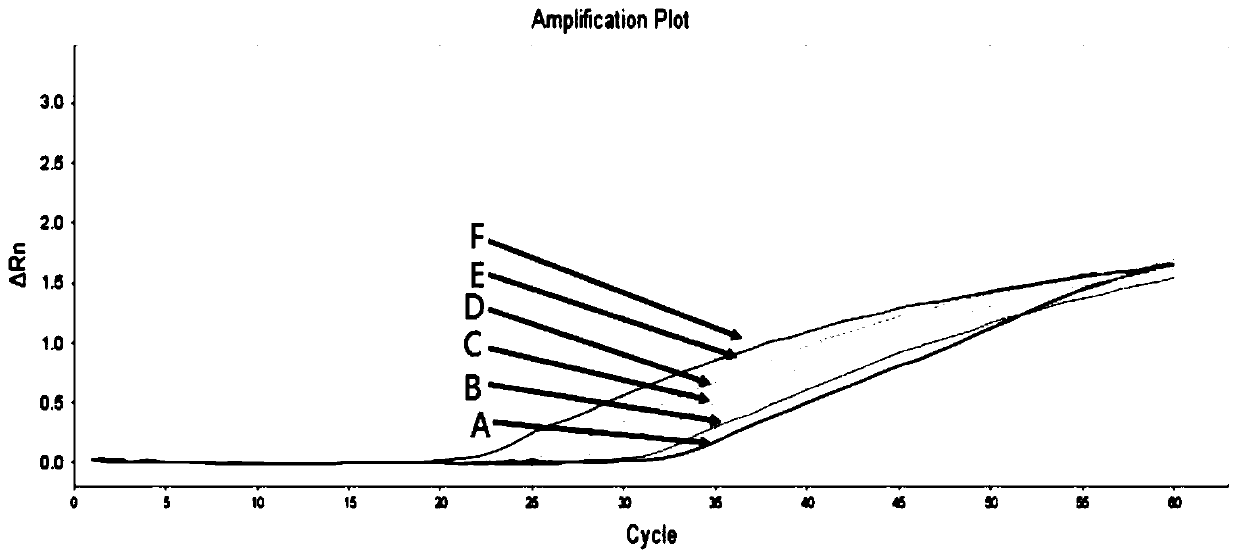
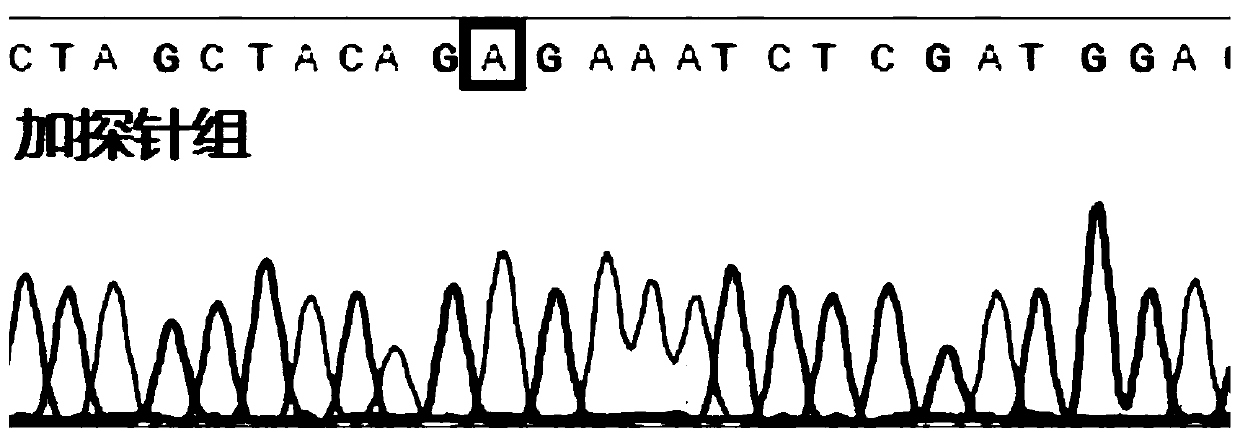
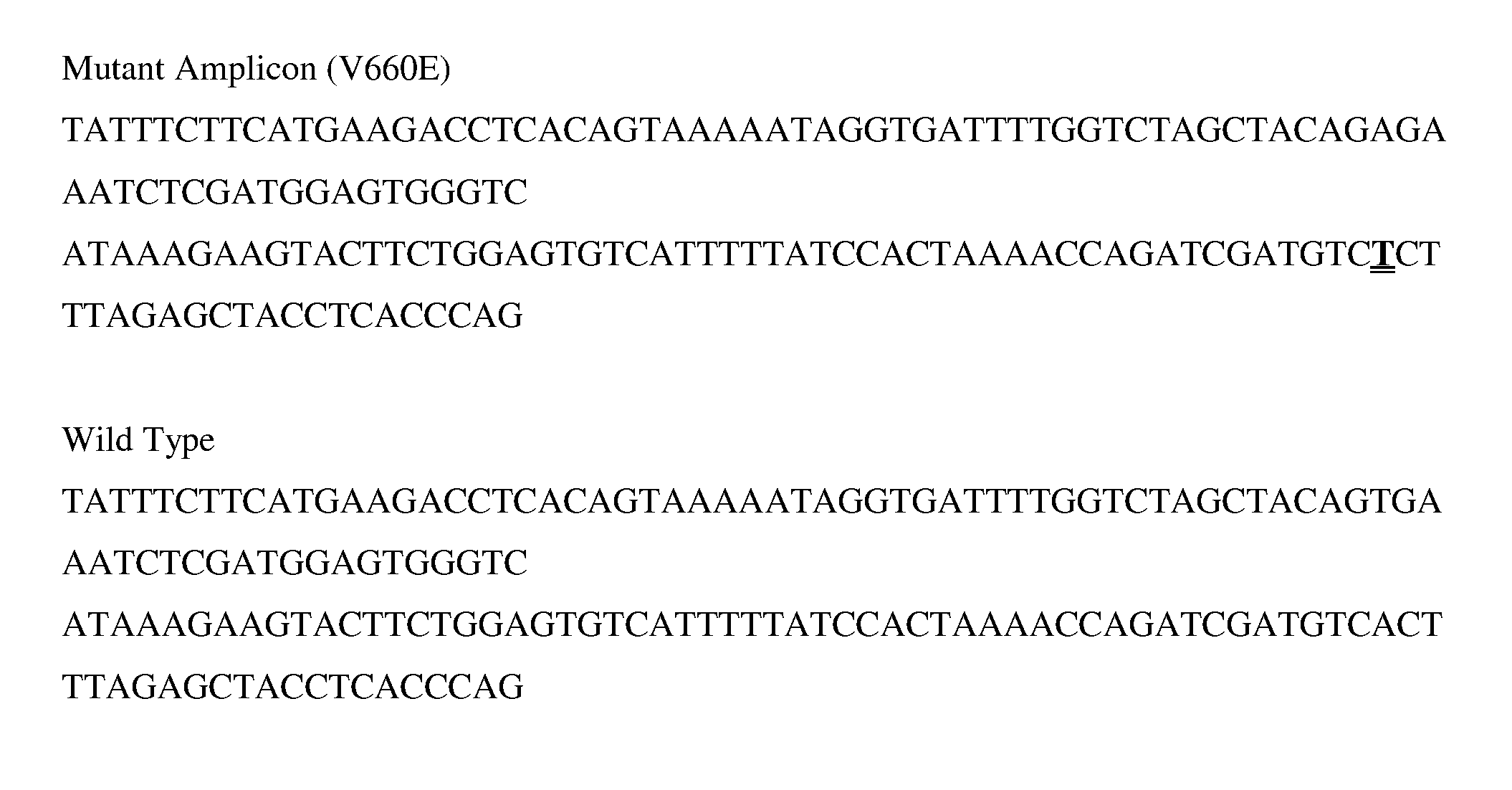Patents
Literature
104 results about "Braf genes" patented technology
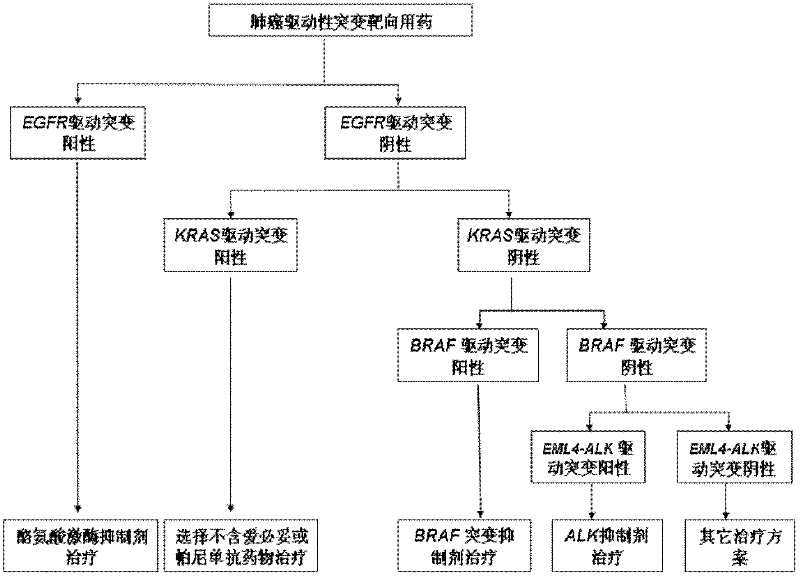
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Primers, probes, detection system and kit for one time detection of lung cancer multiple genes
ActiveCN104818320AWill not interfere with each otherLow costMicrobiological testing/measurementDNA/RNA fragmentationTumor targetBRAF Gene Mutation
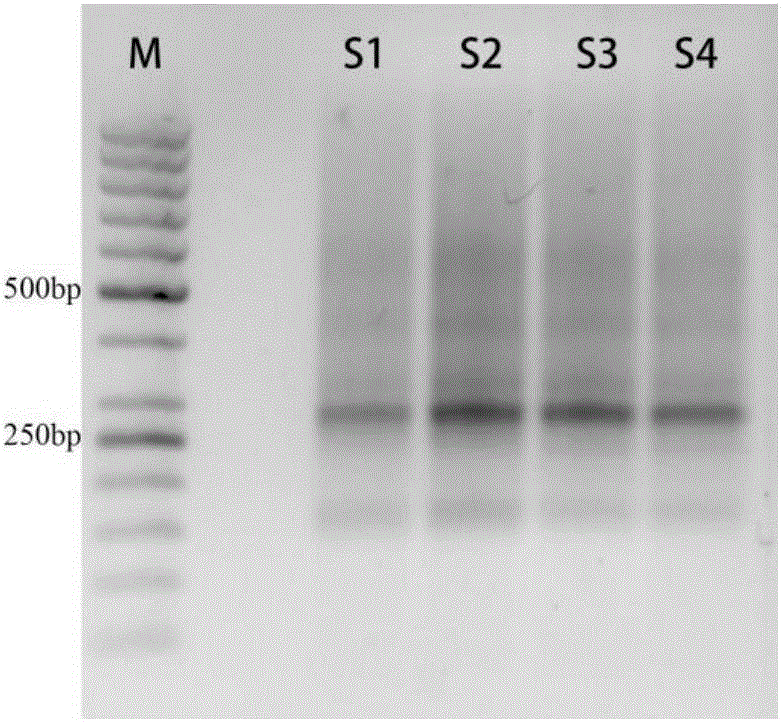
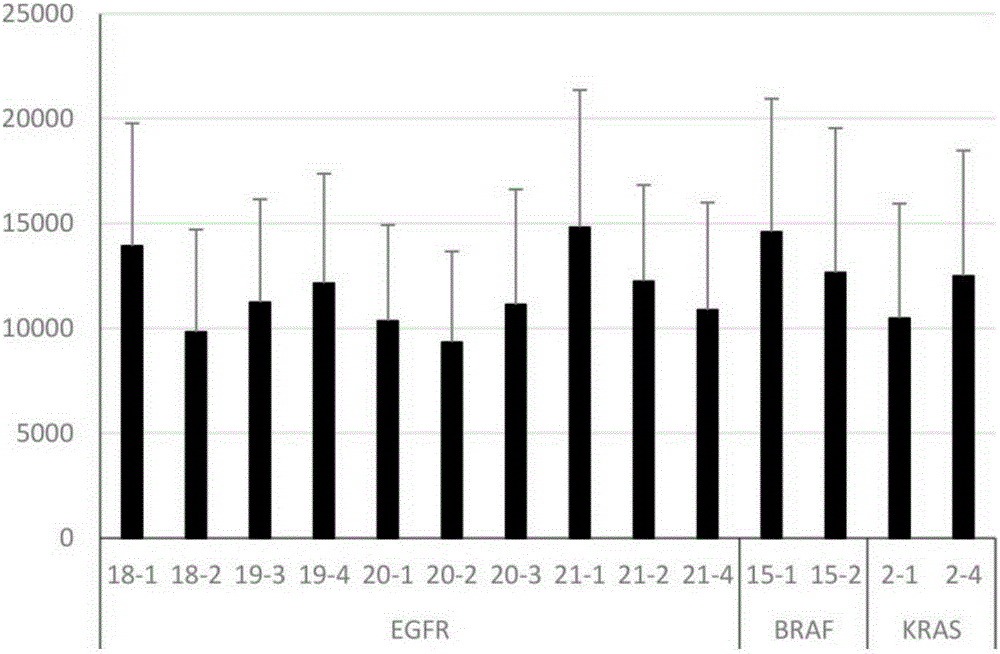
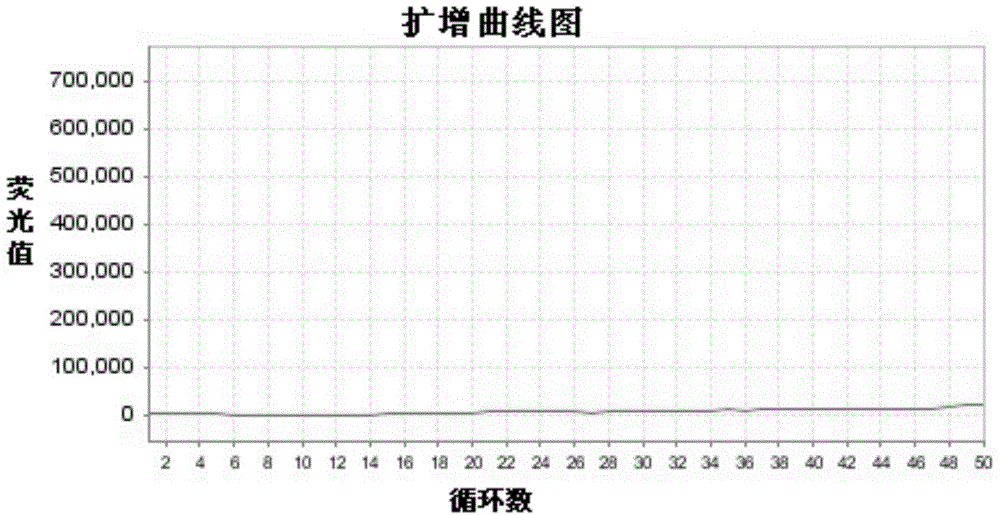
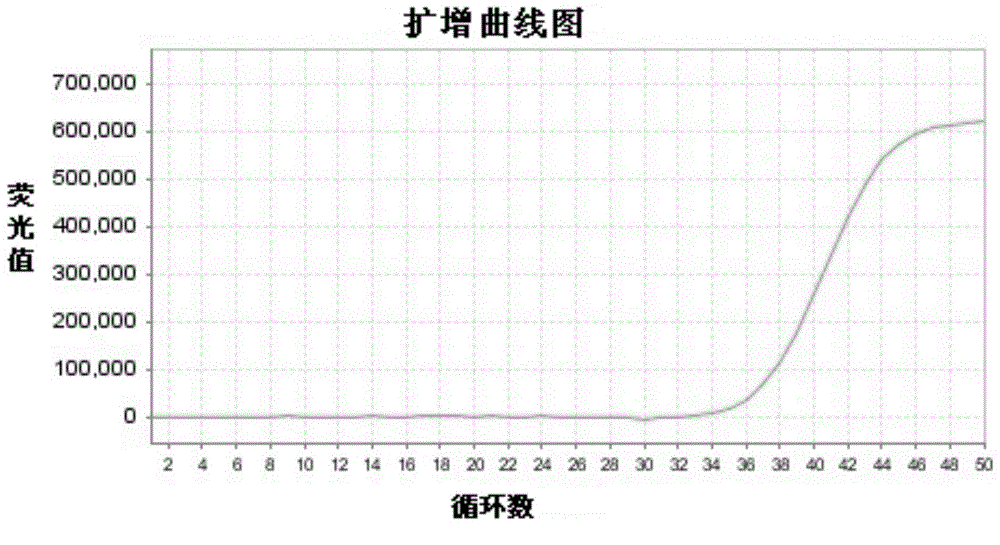
The present invention discloses primers, probes, a detection system and a kit for one time detection of lung cancer multiple genes, wherein the primers, the probes, and the distribution way for detecting 25 EGFR gene mutations, 7 KRAS gene mutations, 6 BRAF gene mutations, 9 NRAS gene mutations, 5 HER2 gene mutations, 2 PIK3CA gene mutations, 5 fusion genes of ALK5, 13 fusion genes of ROS1, and 6 fusion genes of RET are provided. According to the present invention, the detection kit adopts the 12 linking PCR reaction strip design, each 12 linking PCR strip detects multiple genes of a sample, and the corresponding fusion detection reagents and the internal control reagents are filled in the pipes 1-4 of the 12 linking PCR strip; and with the primers, the probes, the detection system and the kit, the one-time detection of the 24 fusions and the 54 mutations of the lung cancer can be achieved, such that the detection time is substantially shortened, the sensitivity is high, the specificity is strong, the operation is simple and rapid, and the reference for selection of tumor targeting drug therapy on lung cancer patients can be provided for clinician.
Owner:AMOY DIAGNOSTICS CO LTD
Methods of Predicting Cancer Risk Using Gene Expression in Premalignant Tissue
ActiveUS20100291573A1Use can be riskyIncreased riskMicrobiological testing/measurementBiological testingBraf genesExpression gene
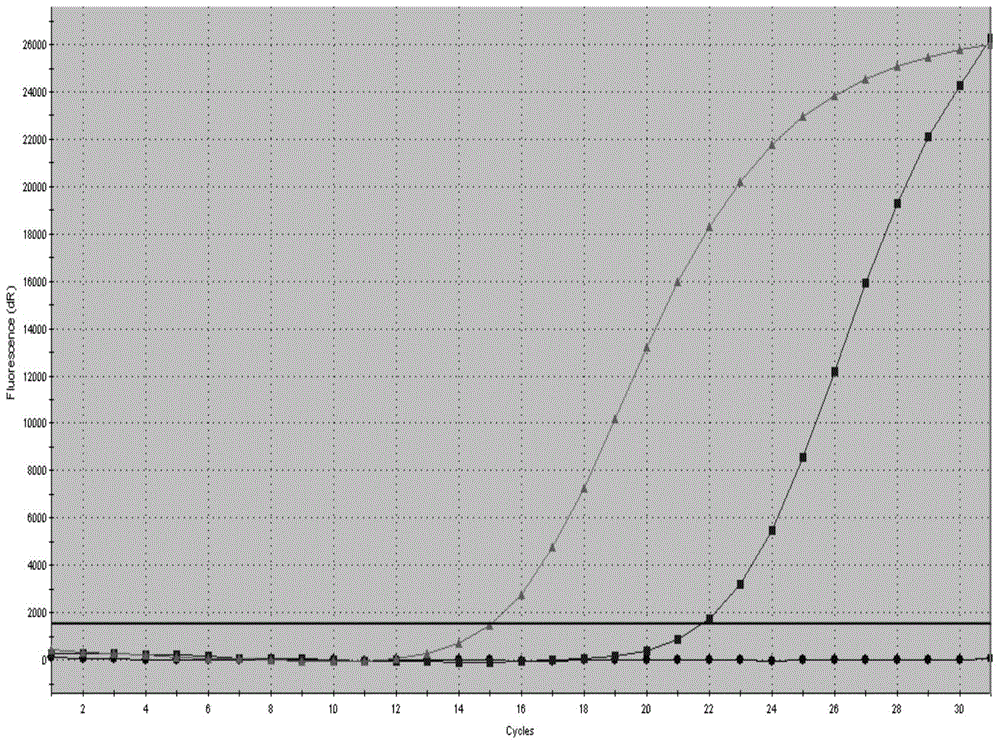
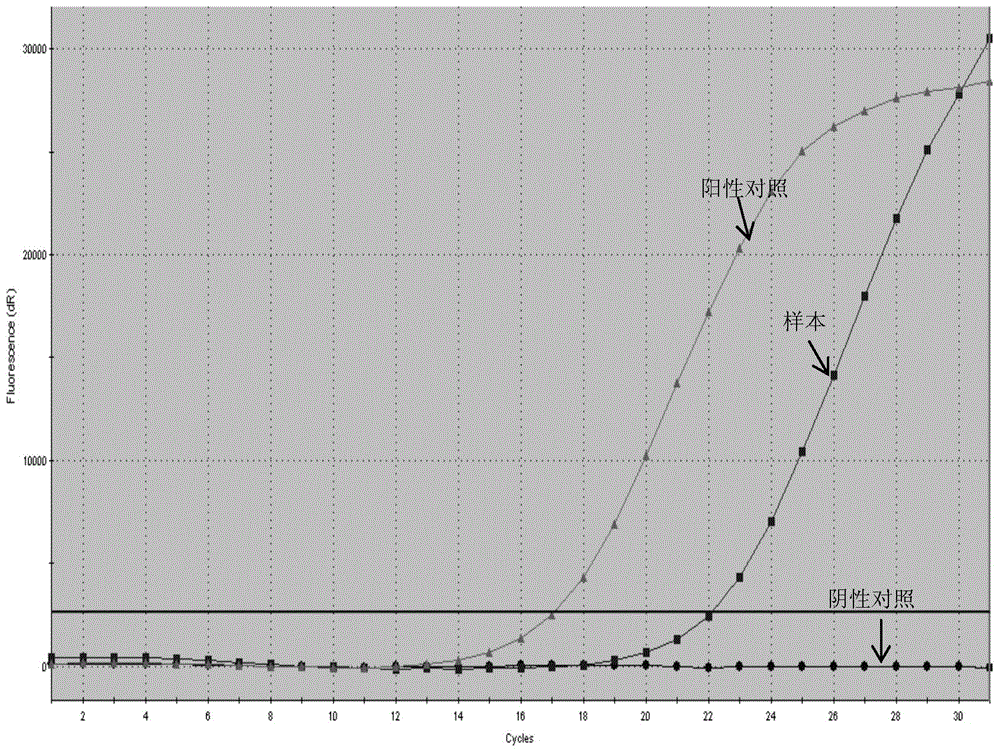
The present disclosure provides methods for assessing a patient's cancer risk and / or recurrence risk, which methods comprise assaying, in a biological sample obtained from the gastrointestinal (GI) tract of the patient, an expression level of a risk gene. The present disclosure also provides methods involving a cancer risk / recurrence risk sequence, i.e. the V600E mutation of the BRAF gene, which is useful for assessing cancer risk and / or recurrence risk in a patient.
Owner:GENOMIC HEALTH INC
Method and kit for detecting mutation of BRAF gene of human colorectal cancer
InactiveCN102586401ASignificant progressEasy to detectMicrobiological testing/measurementFluorescence/phosphorescenceNucleotideV600E
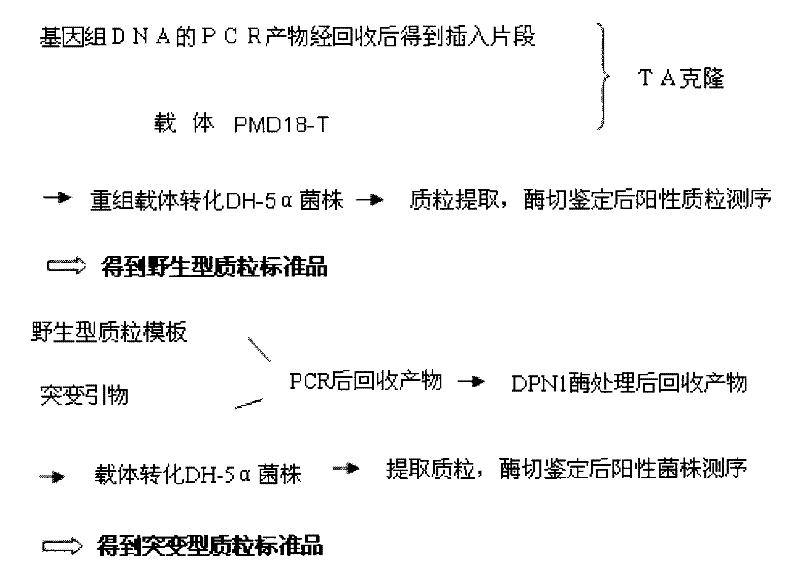
The invention relates to a method and a kit for detecting gene mutation, in particular to a method and a kit for detecting the mutation of BRAF gene. The invention is characterized in that the kit comprises a specific probe used for carrying out genotyping on No. 15 exon codon V600E of the BRAF gene, wherein the specific probe of the No. 15 exon codon V600E comprises a nucleotide sequence of V600E codon. by the technology combining the conventional polymerase chain reaction (PCR) amplification with a Cold-PCR enrichment amplification product and a high resolution melting curve analysis technology, the kit provided by the invention can be used for completing the judgment on sample genotyping.
Owner:苏州科贝生物技术有限公司
Primer, probe, reagent kit and method for detecting mutation of BRAF gene V600E
InactiveCN102816851AHigh detection specificityEasy to readMicrobiological testing/measurementDNA/RNA fragmentationBraf genesPcr ctpp
The invention provides a primer, a probe, a reagent kit and a method for detecting mutation of BRAF gene V600E on the basis of real-time fluorescent quantitative PCR (polymerase chain reaction) technical platform. The primer, the probe, the reagent kit and the method are quick and low in cost and have clinic detection popularization value.
Owner:SUZHOU KUANGYUAN MOLECULAR BIOTECH
Detecting probe and liquid phase chip for BRAF gene mutation and detecting method thereof
ActiveCN101487051AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBRAF Gene MutationBraf genes
The invention discloses a nucleic acid probe, a liquid chip and a detection method that are used for the mutation detection of a BRAF gene exon 15. The liquid chip that is used for the mutation detection of the BRAF gene exon 15 comprises microspheres and a primer; wherein, the microspheres are coated with wild and mutant probes which are decorated with amino groups and aim at the BRAF gene exon 15; and the primer is used for augmenting a target sequence that is enriched in mutant sites needing to be detected by the BRAF gene exon 15 and has a biotin marker in the tail end. The detection method that is provided by the invention has quickness, accuracy, simple and convenient operation, and greatly improves the detection efficiency.
Owner:广州益善医学检验所有限公司
Detection kit for human BRAF gene V600E mutation
InactiveCN105039514AAccurate detection of genetic mutationsReduce pollutionMicrobiological testing/measurementBraf genesLocked nucleic acid
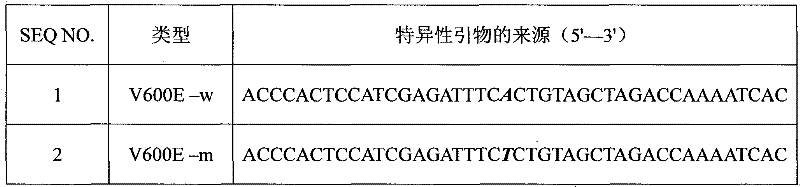
The invention provides a detection kit and a detection method for human BRAF gene V600E mutation. The detection kit comprises a first reagent pack and a second reagent pack, wherein each of the first reagent pack and the second reagent pack comprises PCR buffer solution, dNTP, MgCl2, a specific probe, a locked nucleic acid (LNA) primer, an interior label system, HotStart Taq enzyme and UNG enzyme; the first reagent pack comprises a primer pair SEQ ID NO:1 and SEQ ID NO:2, and a probe SEQ ID NO:3; the second reagent pack comprises a primer pair SEQ ID NO:1 and SEQ ID NO:4, and a probe SEQ ID NO:5; and the SEQ ID NO:4 is LNA primer. The detection kit and the detection method can be used for detecting human BRAF gene V600E mutation, and has the advantages of strong specificity, high sensitivity, simplicity and rapidness in operation, high flux, safety, objective result determination and the like.
Owner:WUHAN YZY MEDICAL SCI & TECH
Detection method of nucleotide mutation points of KRAS gene and/or BRAF gene
ActiveCN101838683AHigh sensitivityImprove accuracyMicrobiological testing/measurementMaterial analysis by electric/magnetic meansBraf genesMass Spectrometry-Mass Spectrometry
The invention provides a method for detecting the nucleotide mutation points of a KRAS gene and / or a BRAF gene, which comprises the following steps that: (1) the mutation points to be detected of the KRAS gene and / or the BRAF gene are determined; (2) according to the mutation points, an amplification primer and the extension primer of each mutation point are designed; (3) PCR amplification; (4) SAP enzyme treatment; (5) extension reaction; (6) resin is adopted to purify an extension reaction product; and (7) mass spectrometry detection, the mutant of the target sites of the KRAS gene and / or the BRAF gene to be detected is determined. The method solves the problems of low sensitivity, limited accuracy, low flux and high cost of a traditional detection method. In addition, the invention also provides a specific primer and a primer combination of the method and the purposes thereof for the detection method.
Owner:BGI GENOMICS CO LTD
Fluorescent PCR detecting kit for V600E mutation of BRAF gene
InactiveCN105296666AStrong specificityEasy to operateMicrobiological testing/measurementBraf genesNucleotide
The invention provides a fluorescent PCR detecting kit for V600E mutation of a BRAF gene. The detecting kit comprises a V600E mutation detection reaction solution, wherein the V600E mutation detection reaction solution comprises a 10*PCR buffering solution, deoxyribonucleoside triphosphate, an upstream primer, a downstream primer and a probe, wherein the upstream primmer and the downstream primer are used for amplifying target polynucleotide, and the probe is used for detecting the target polynucleotide. The fluorescent PCR detecting kit for V600E mutation of the BRAF gene is based on a real-time fluorescent quantitative PCR technology and an ARMS-PCR technology, has favorable specificity and is high in operation speed, simple and convenient in method and high in detection precision; and meanwhile an interior label additionally arranged in a reaction system can be used for effectively avoiding that the detected result is false negative.
Owner:HUNAN SHENGWEI GENE TECH
BRAF gene mutation detection kit and application thereof
InactiveCN106148497AHigh sensitivityThe detection process is fastMicrobiological testing/measurementBraf genesBRAF Gene Mutation
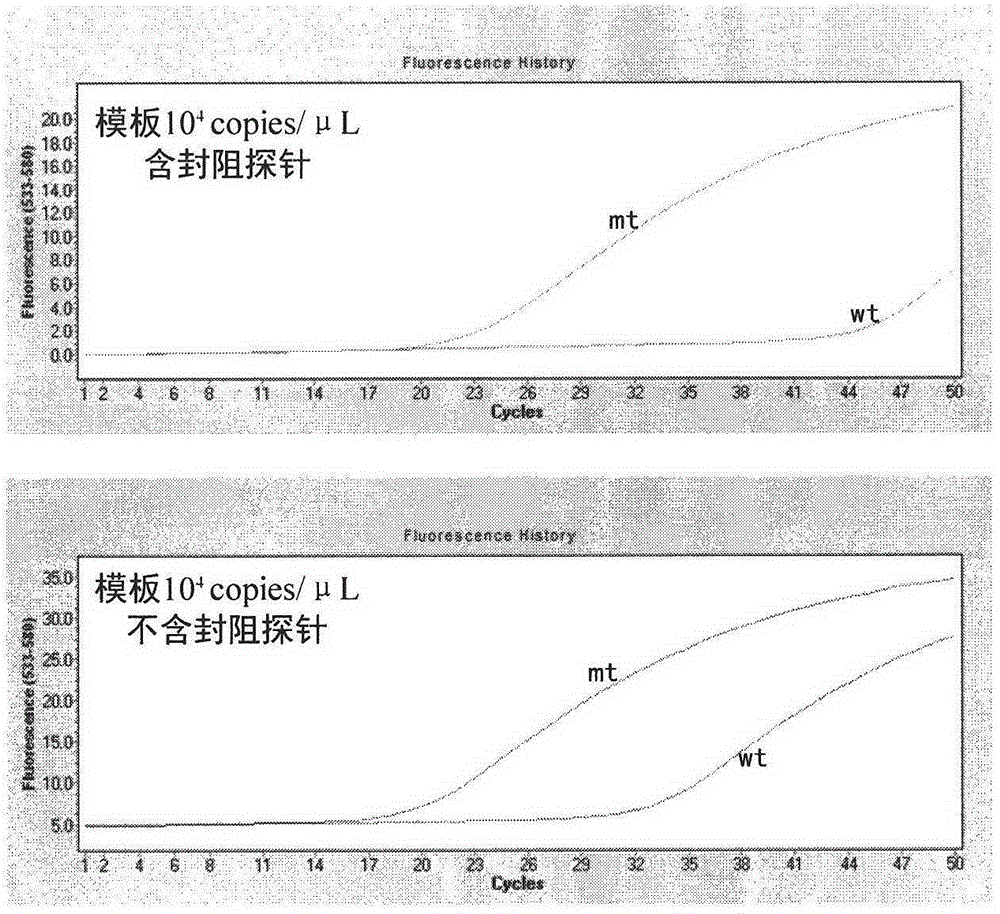
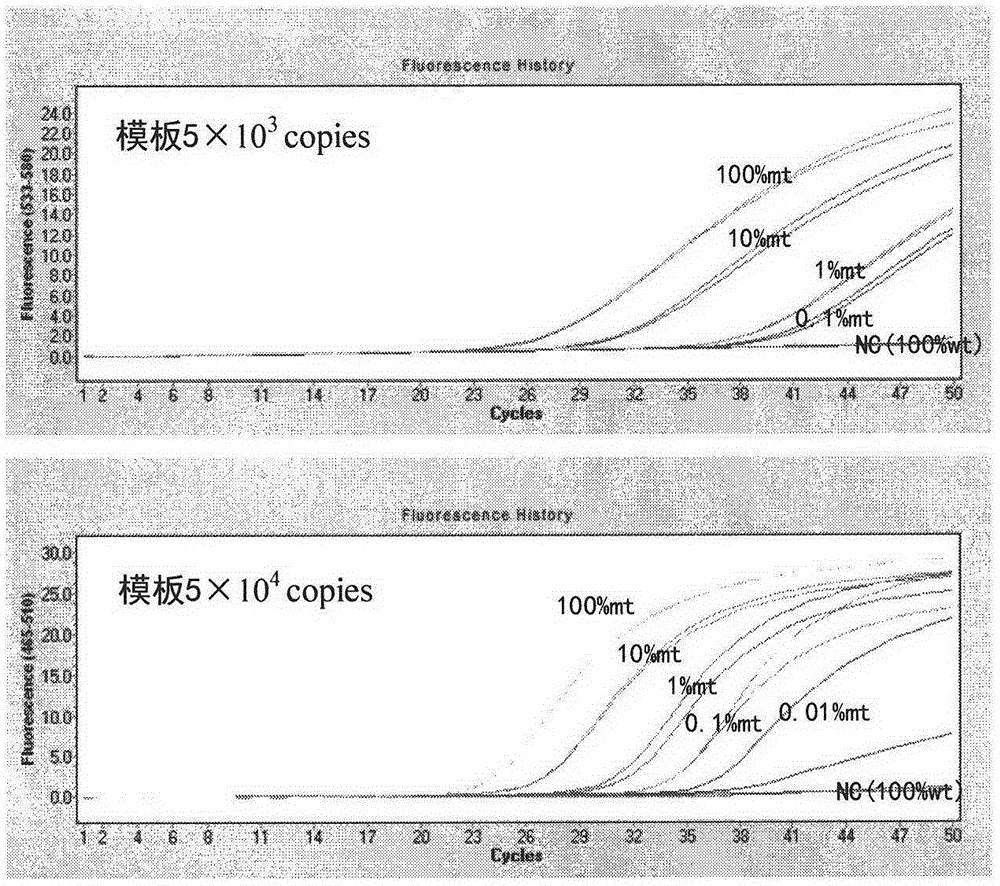
The invention discloses a detection kit and detection method for BRAF gene mutations, and belongs to the technical field of B2D10 currently first developed high-tech industrialization important field guide / biology / novel medical precise diagnosis and treatment equipment. The detection kit is characterized by comprising two pairs of specifically amplified primers for BRAF gene mutation loci, an efficient blocking probe of a wild sequence and a BRAF gene specific TaqMan fluorescent probe. The kit can detect specimens of which the number of the mutant copies is as low as 5 to 10, and the mutation content is as low as 0.1%. The detection kit can detect five gene mutations of a BRAF gene simultaneously, the sensitivity is high, operation is easy, detection is low in price, the clinical application range is wide, samples can adopt fresh pathological tissue or paraffin-embedded tissue or a pleural fluid or serum or plasma, the detection speed is high, and only 90 minutes are needed for completing the detection process.
Owner:上海济远生物科技有限公司
Nucleotide sequence for detecting BRAF gene V600E mutation and application thereof
PendingCN109576372AThe result is accurateImprove stabilityMicrobiological testing/measurementDNA/RNA fragmentationBraf genesMolecular diagnostic techniques
The invention discloses a nucleotide sequence for detecting BRAF gene V600E mutation and application thereof and belongs to the technical field of molecular diagnosis. A primer and a probe which are used for detecting BRAF can be specifically amplified and are used for detecting the BRAF V600E gene mutation. The invention further discloses a detection kit for detecting the BRAF V600E gene mutationbased on a micro-droplet type digital PCR (Polymerase Chain Reaction) system and the mutation of a BRAF gene can be rapidly, sensitively and accurately detected. The nucleotide sequence has the advantages of convenient operation, strong specificity, high sensitivity, low cost, high flux and the like, can be used for rapidly detecting the clinical BRAF V600E gene mutation and provides reference for diagnosis and treatment of the BRAF V600E mutation.
Owner:HANGZHOU D A GENETIC ENG
Multi-PCR primer for detecting EGFR/KRAS/BRAF genetic mutation locus based on next-generation sequencing technology and method and application
The invention provides a multi-PCR primer for detecting EGFR / KRAS / BRAF genetic mutation locus based on a next-generation sequencing technology. The multi-PCR primer comprises two or more pairs of 14 pairs of primers shown as SEQ ID NO: 1-SEQ ID NO: 28; preferably, the multi-PCR primer comprises a primer pair including any two or three genes of amplification EGFR, KRAS and BRAF genes at a minimum, wherein each gene amplifies one or more exon regions, and one or more primer pairs are adopted for each exon region. The multi-PCR primer set can be used for efficient detection of diversity of EGFR / KRAS / BRAF genetic mutation locus and can cover 18th-21th EGFR exon, the second KRAS exon and the 15th BRAF exon.
Owner:GENEMIND BIOSCIENCES CO LTD
Primer pair as well as probe and kit for detecting human BRAF gene mutation
InactiveCN104017887AHigh sensitivityThe detection process is fastMicrobiological testing/measurementDNA/RNA fragmentationMelanomaBRAF Gene Mutation
The invention discloses a primer pair and a probe for detecting a human BRAF gene mutation. The sequences of the primer pair and probe are shown as SEQ ID NO: 1 to SEQ ID NO: 7. The invention also discloses a kit for detecting human BRAF gene mutation and used for detecting T-A base mutation occurring on 1799-postion nucleotide of the BRAF gene exon 15 by a fluorescent PCR. The kit disclosed by the invention has the advantages of simple and quick operation, high specificity and low misdetection rate and is applicable to the auxiliary clinical diagnosis and typing of thyroid cancer, or provides a clinical reference for selecting tyrosine kinase inhibitors of melanoma and colorectal cancer and BRAF gene mutation targeted drugs.
Owner:HELIXGEN GUANGZHOU
Primer and probe composition for detecting V600E of BRAF (V-Raf Murine Sarcoma Viral Oncogene Homolog B1) gene and detecting method of primer and probe composition
PendingCN108277263AEasy to retouchAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationCell freeAgricultural science
The invention provides a primer and probe composition for detecting V600E of a BRAF (V-Raf Murine Sarcoma Viral Oncogene Homolog B1) gene and a detecting method of the primer and probe composition. The primer and probe composition comprises a mutant probe which aims at a V600E site of the BRAF gene and is as shown in SEQ ID NO.1, a wild type probe which aims at the V600E site of the BRAF gene andis as shown in SEQ ID NO.2 and specific primers which aim at the V600E site of the BRAF gene and are as shown in SEQ ID NO.3 to SEQ ID NO.4. Primer designing and probe modification are carried out ona specific zone aiming at the V600E site, a primer and a probe are matched with each other, ddPCR (Differential Display Polymerase Chain Reaction) is combined with a high-specificity primer and probecomposition, extraction of cfDNA (Cell-free DNA), recycle number of PCR and annealing temperature are optimized, all steps are in a synergistic effect, the accuracy, the stability and the sensitivityfor detection are finally increased, and wide application prospect and a market value are obtained.
Owner:江西海普洛斯医学检验实验室有限公司
Kit for detecting BRAF gene V600E trace mutation through pyrosequencing technique and application of kit
InactiveCN105400900AAccurate quantification of mutation frequencyReduce material requirementsMicrobiological testing/measurementDNA/RNA fragmentationLife qualityBraf genes
The invention mainly relates to the field of molecular diagnosis and provides a kit for accurately detecting BRAF gene V600E trace mutation. The kit mainly comprises two specific amplification primers, one blocking primer, one sequencing primer and affinity magnetic beads marked bystreptomycin. By the application of the kit, trace mutation of the BRAF gene V600E which is lowered to 0.1% in concentration can be accurately detected. A detection method is high in sensitivity, the mutation generated when the concentration is lowered to 0.1% can be detected, the result is visual, interpretation is simpler, more accurate and more rapid, the false negative rate of the detection result is greatly lowered, clinical invalid choice of targeted drugs of a patient is avoided, valuable treatment time is saved for the patient, and the living quality of the patient is improved.
Owner:HANGZHOU DIAN BIOTECH CO LTD
Method and kit thereof for detecting BRAF gene mutation
InactiveCN104928355AAccurate detectionEffectively combine applicationsMicrobiological testing/measurementDNA/RNA fragmentationBRAF Gene MutationBraf genes
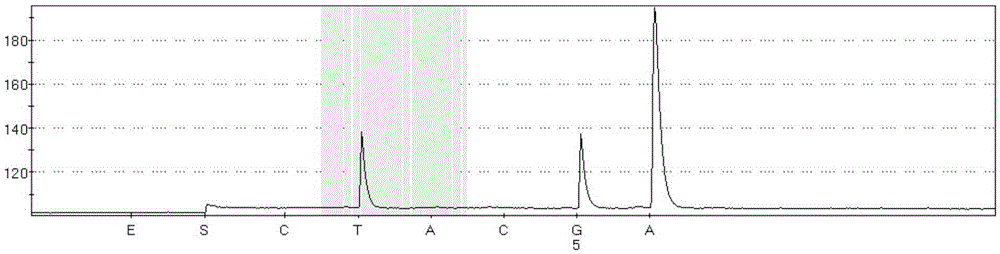
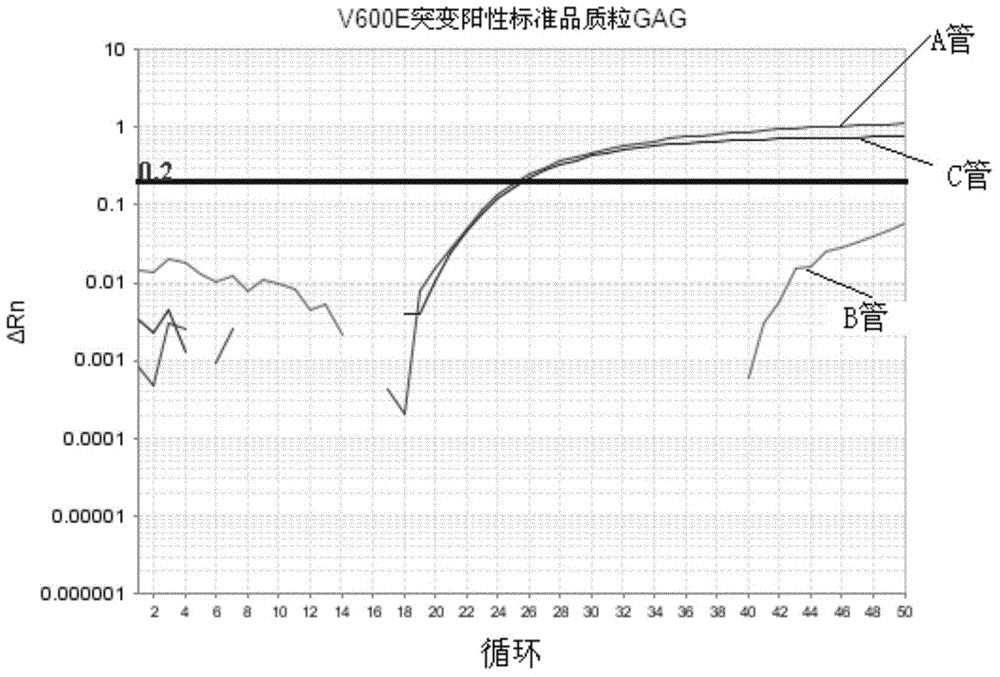
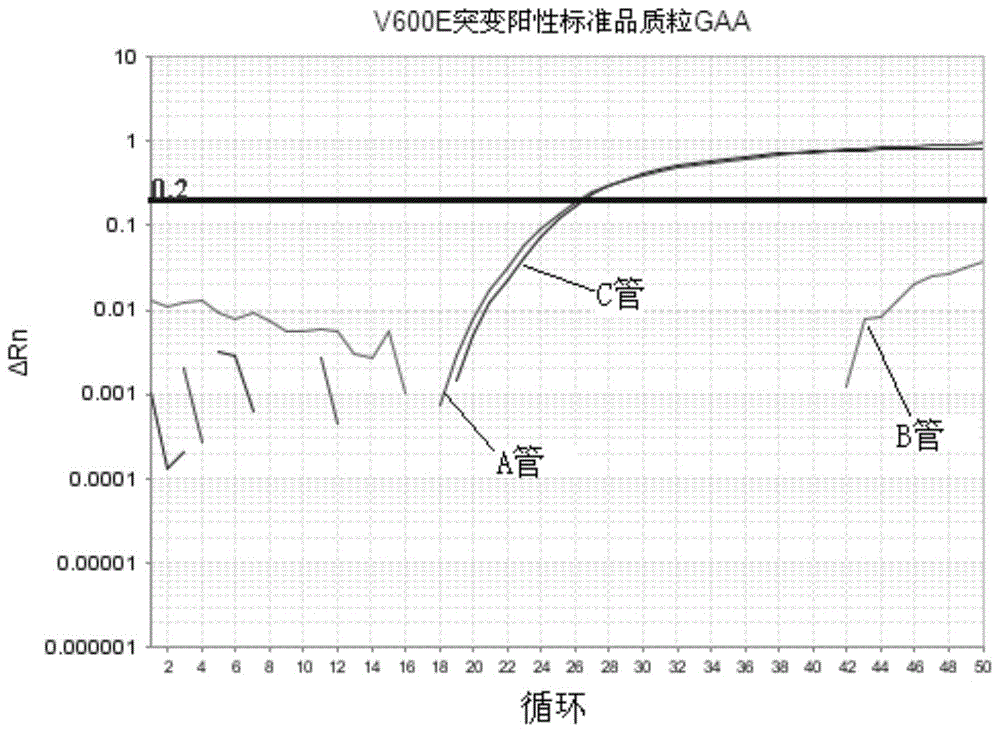
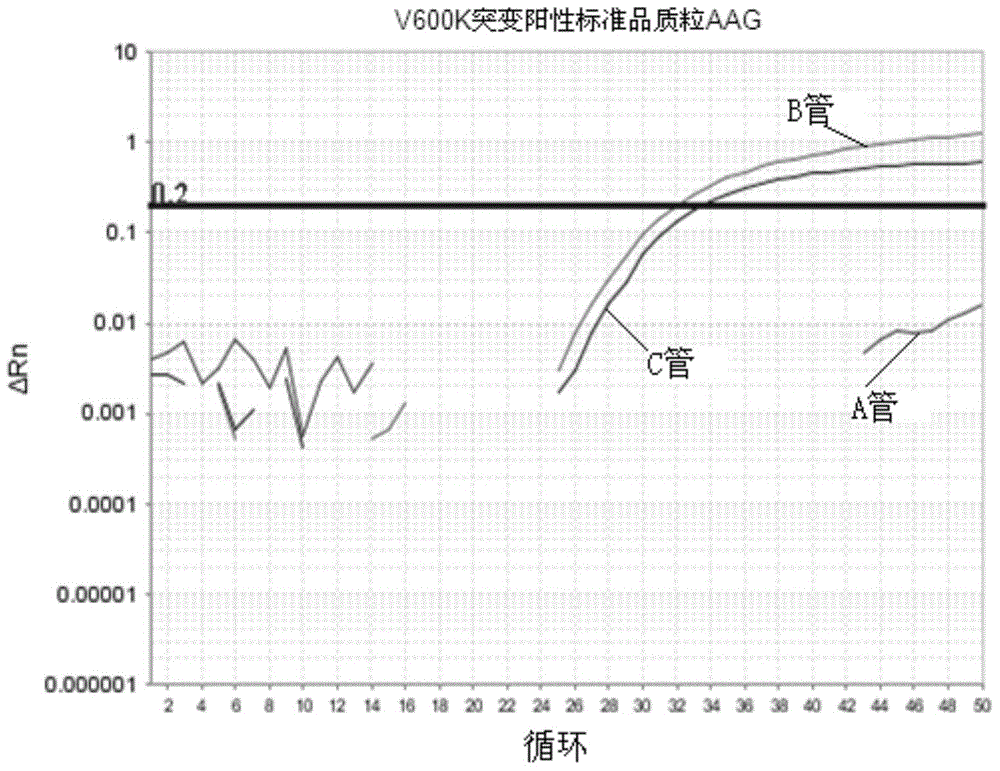
The invention discloses a method and kit thereof for detecting BARF gene mutation. The method comprises the following steps: (1) carrying out reactions in tube A, B, and C at the same time, carrying out BARF gene V600E and V600K mutation realtime quantitative PCR detection on a plasmid standard with a known mutation amount so as to obtain standard curves; (2) extracting and purifying the DNA of a sample, measuring the concentration, carrying out realtime quantitative PCR detection on the sample according to the tube A, B, and C reaction systems in the step (1), judging whether the gene mutation exists or not and the mutation amount according to the standard curves and the amplification fluorescence signals. The kit comprises a primer pair for detecting the No.15 exon mutation V600 E and V600K of BRAF gene, a probe, and a PNA repressing sequence. The provided method and kit can more precisely detect the No.15 exon mutation V600 E and V600K of BRAF gene, and the detection sensitivity is greatly improved.
Owner:李跃 +2
Specific primer and liquid phase chip for BRAF genetic mutation detection
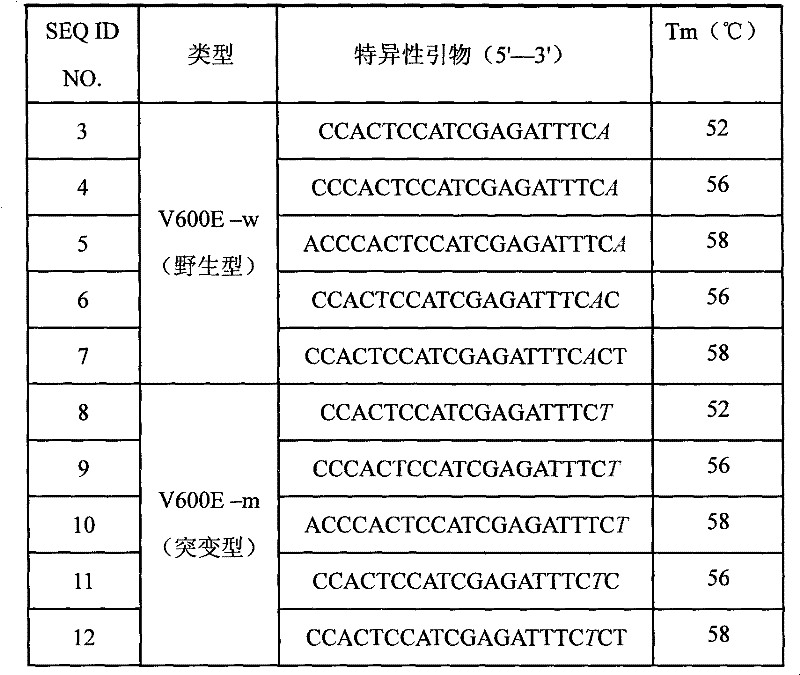
ActiveCN102234684AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementDNA/RNA fragmentationBraf genesSignal-to-noise ratio (imaging)
The invention discloses a liquid phase chip for BRAF genetic mutation detection. The liquid phase chip mainly comprises (A) respectively designed ASPE primer pairs in the wild type and in the mutant type directed to a V600E mutation site of the BRAF: each ASPE primer is composed of a tag sequence at a 5'terminus and a specific primer which is at a 3'terminus and directed to a target gene mutation site, one base of last three bases at the 3'terminus of the specific primer is the mutation site, and the Tm value of the specific primer is from 52 to 58 DEG C; (B) two types of microspheres which have color-different codes and are coated with specific anti-tag sequences which can correspondingly have complementary pairing with the selected tag sequence in the (A); and an amplimer for amplifying the target sequence of the BRAF gene having the V600E mutation site. The liquid phase chip for BRAF genetic mutation detection prepared in the invention has an excellent signal to noise ratio, and a cross reaction does not substantially exist between a designed probe and the anti-tag sequence.
Owner:SUREXAM BIO TECH
Primers, probes, detection system and kit for one time detection of intestinal cancer multiple gene mutation
ActiveCN104818317AWill not interfere with each otherLow costMicrobiological testing/measurementDNA/RNA fragmentationTumor targetTumor targeting
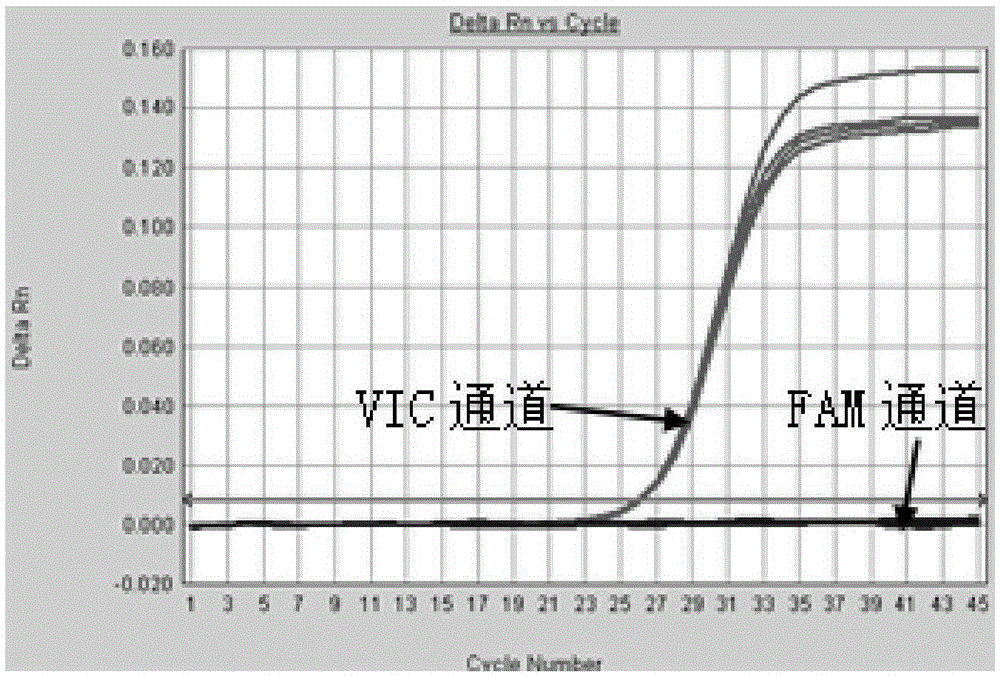
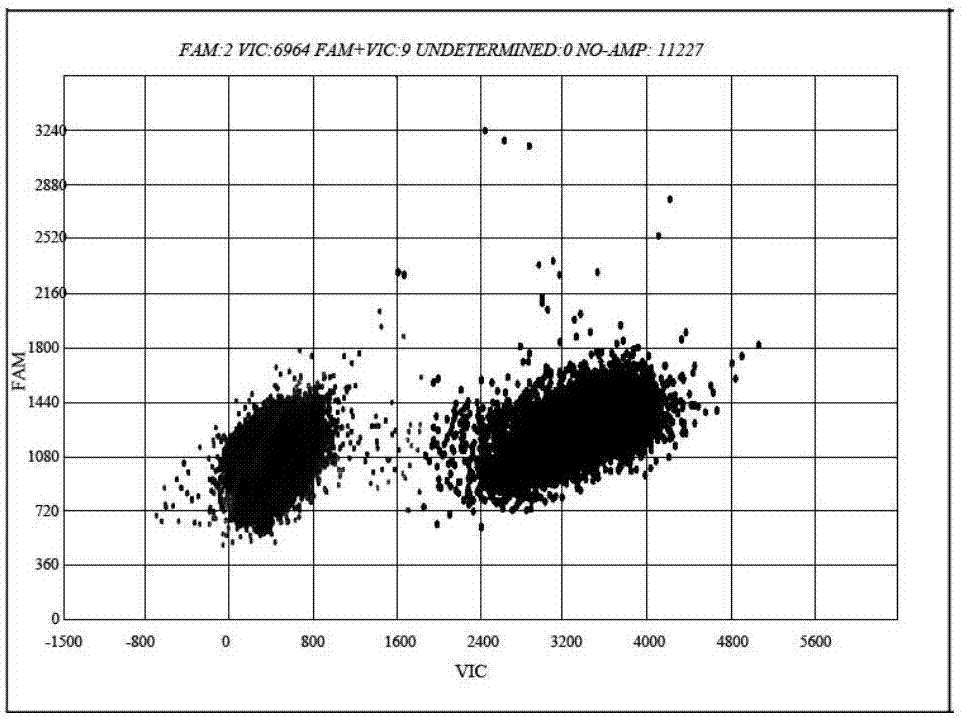
The present invention discloses primers, probes, a detection system and a kit for one time detection of intestinal cancer multiple gene mutation, wherein the primers, the probes, and the distribution way for detecting gene mutations of intestinal cancer genes such as KRAS, NRAS, PIK3CA, and BRAF are provided. According to the present invention, the detection kit adopts the 12 linking PCR reaction strip design, each 12 linking PCR strip detects multiple genes of a sample, the corresponding detection reagents for the 37 mutations of KRAS / NRAS / PIK3CA / BRAF and the internal control reagents are filled in the pipes 1-11 of the 12 linking PCR strip, the mutation is indicated by the FAM signal, and the internal control is indicated by the HEX (or VIC) signal; the pipe 12 is adopted as the DNA extraction quality external control detection pipe and is indicated by the FAM; and with the primers, the probes, the detection system and the kit, the one-time detection of the 37 mutations of the KRAS / NRAS / PIK3CA / BRAF gene can be achieved, such that the detection time is substantially shortened, the sensitivity is high, the specificity is strong, the operation is simple and rapid, and the reference for selection of tumor targeting drug therapy on intestinal cancer patients can be provided for clinician.
Owner:AMOY DIAGNOSTICS CO LTD
Methods of predicting cancer risk using gene expression in premalignant tissue
Owner:GENOMIC HEALTH INC
Kit for quantificationally detecting BRAF (Block Repeat Active Flag) mutation
InactiveCN102161990AAccurate determination of contentEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceBRAF Gene MutationEGFR Tyrosine Kinase Inhibitors
The invention relates to a method and kit for detecting BRAF (Block Repeat Active Flag) mutation relevant to the curative effect of a molecular-targeting anti-cancer medicament, in particular relating to a fluorescence quantificational PCR (Polymerase Chain Reaction) detecting method and kit for detecting mutation in BRAF gene mutation hot spot regions and application thereof. According to the invention, mutation at special positions of BRAF genes is detected, the curative effect of the molecular-targeting anti-cancer medicament such as an EGFR (Epidermal Growth Factor Receptor) tyrosine kinase inhibitor, and the like can be forecasted, and furthermore, the individual medicament use schemes of patients with tumors are directed.
Owner:BEIJING ACCB BIOTECH
Primer and kit for detecting BRAF gene V600E mutation sites, and PCR method of kit
ActiveCN104846106ADifficulty of SimplificationReduce errorsMicrobiological testing/measurementDNA/RNA fragmentationForward primerBraf genes
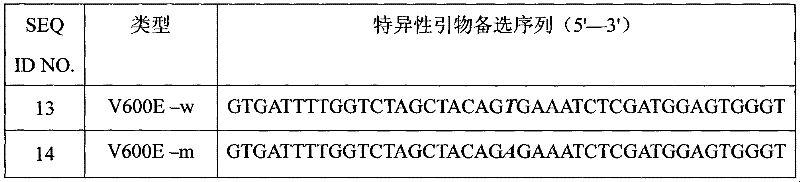
The invention discloses a primer for detecting BRAF gene V600E mutation sites, and a PCR method of the kit. The primer comprises a wild type specific forward primer, a mutant type specific forward primer, and a reverse primer shared by the wild type specific forward primer and the mutant type specific forward primer, wherein the wild type specific forward primer has a sequence shown as SEQ No.17; the mutant type specific forward primer has a sequence shown as SEQ No.14; and the shared reverse primer has a sequence shown as SEQ No.16. The kit has the advantages of simple detection, rapidness, accuracy, low price and the like and provides a powerful tool for scientific research and clinical detection of BRAF gene V600E mutation sites and gene mutation analysis.
Owner:沈阳优吉诺生物科技有限公司
Primer for detecting variation of benign and malignant related genes of thyroid nodules, kit and detection method
ActiveCN110241215AImprove accuracyHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBiologyEIF1AX gene
The invention discloses a primer for detecting variation of benign and malignant related genes of thyroid nodules, a kit and a detection method. According to the primer, the kit and the detection method, variation of 15 loci of the six genes and variation of the three fusion genes can be detected simultaneously, and the BRAF gene, the KRAS gene, the HRAS gene, the NRAS gene, the TERT gene, the EIF1AX gene, RET / PTC1 fusion, RET / PTC3 fusion and PAX8 / PPARgamma fusion are involved. Hotspot mutation and fusion variation of the genes are closely related to the benign and malignant thyroid nodules. Therefore, the primer, the kit and the detection method are used for detecting a sample of a patient and can assist doctors in benign and malignant identification of the thyroid nodules which cannot be clearly diagnosed in the cytology, the accuracy of identifying the benign and malignant thyroid nodules is improved, excessive medical treatment for the patient with the thyroid nodules is reduced, and the primer, the kit and the detection method have important practical significance and high economic benefit for saving medical resources of China.
Owner:润安医学科技(苏州)有限公司
Detection kit for lung cancer driving gene mutation
ActiveCN102443626ARealize multiple comprehensive detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBraf genesA-DNA
The present invention discloses a detection kit for lung cancer driving gene mutation. The kit comprises: primers for detecting mutations of lung cancer driving genes including EGFR, KRAS, BRAF and EML4-ALK, and probes for detecting the mutations of the lung cancer driving genes including EGFR, KRAS, BRAF and EML4-ALK. The method of the present invention comprises: (1) providing 4 groups of the 71 primers and the probes; (2) extracting a DNA template of a sample requiring detection; (3) preparing a fluorescent PCR reaction system for detecting the driven mutations of EGFR, KRAS, BRAF and EML4-ALK; (4) adopting the hybridization of the dual-ring probe and the specific primer to detect the fluorescence intensity of FAM and HEX, or ROX of the reaction system, and determining the result according to the fluorescence intensity of the FAM and the HEX, or the ROX. With the method of the present invention, the driven mutations of the EGFR gene, the KRAS gene, the BRAF gene and the EML4-ALK gene of the lung cancer can be detected, and the method has characteristics of high sensitivity, strong specificity, rapid detection speed, convenient detection and the like.
Owner:AMOY DIAGNOSTICS CO LTD
Kit for detecting 34 mutation sites of lung cancer based on MALDI-TOF-MS
InactiveCN108410982AReduce demandHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBraf genesIndividualized treatment
The invention discloses a kit for detecting 34 mutation sites of lung cancer based on MALDI-TOF-MS. The kit comprises multiple PCR amplification reaction primers which are shown as SEQ ID NO:1-16, andsingle-base extension primers which are shown as SEQ ID NO:17-48. The kit provided by the invention can be used for simultaneously detecting 23 sites of an EGFR gene, 7 sites of a KRAS gene, 1 site of a BRAF gene and 3 sites of a PIK3CA gene. According to the kit provided by the invention, the 34 sites of the four genes can undergo target segment amplification in two holes and single-base extension in eight holes; and with the application of the kit provided by the invention, 34 site mutations of the four genes, which may exist in an abnormal sample, can be simultaneously detected and analyzed. The kit provided by the invention has important guiding significance for mastering related factors of a patient on drug susceptibility, conducting individualized treatment on the patient with the lung cancer and targeting to drug adaptability.
Owner:GUANGZHOU DARUI BIOTECH
Application of BRAF gene detector to preparation of ACTH type pituitary adenoma molecular pathological diagnosis and parting product
The invention discloses application of a BRAF gene detector to preparation of an ACTH type pituitary adenoma molecular pathological diagnosis and parting product. It is proved through tests that BRAF gene mutation is peculiar to ACTH type pituitary adenoma and not found in other types of pituitary adenoma, so that the BRAF gene mutation can serve as a specific molecular pathological diagnosis marker of the ACTH type pituitary adenoma. According to the result that a BRAF gene is mutant or not, the ACTH pituitary adenoma can be further divided into two subtypes, namely the BRAF mutant type and the wild type, the mean volume of the BRAF mutant type ACTH pituitary adenoma is small, the attack degree is low, and the midnight serum cortisol concentration is less than or equal to 22 ug / dl; the mean volume of the wild type ACTH pituitary adenoma is large, the attack degree is high, and the midnight serum cortisol concentration is greater than 22 ug / dl. Therefore, the different subtypes of the ACTH pituitary adenoma can be judged by detecting whether the BRAF gene is mutant or not, and different clinical treatment strategies are adopted in time accordingly.
Owner:SHANGHAI JIAO TONG UNIV +1
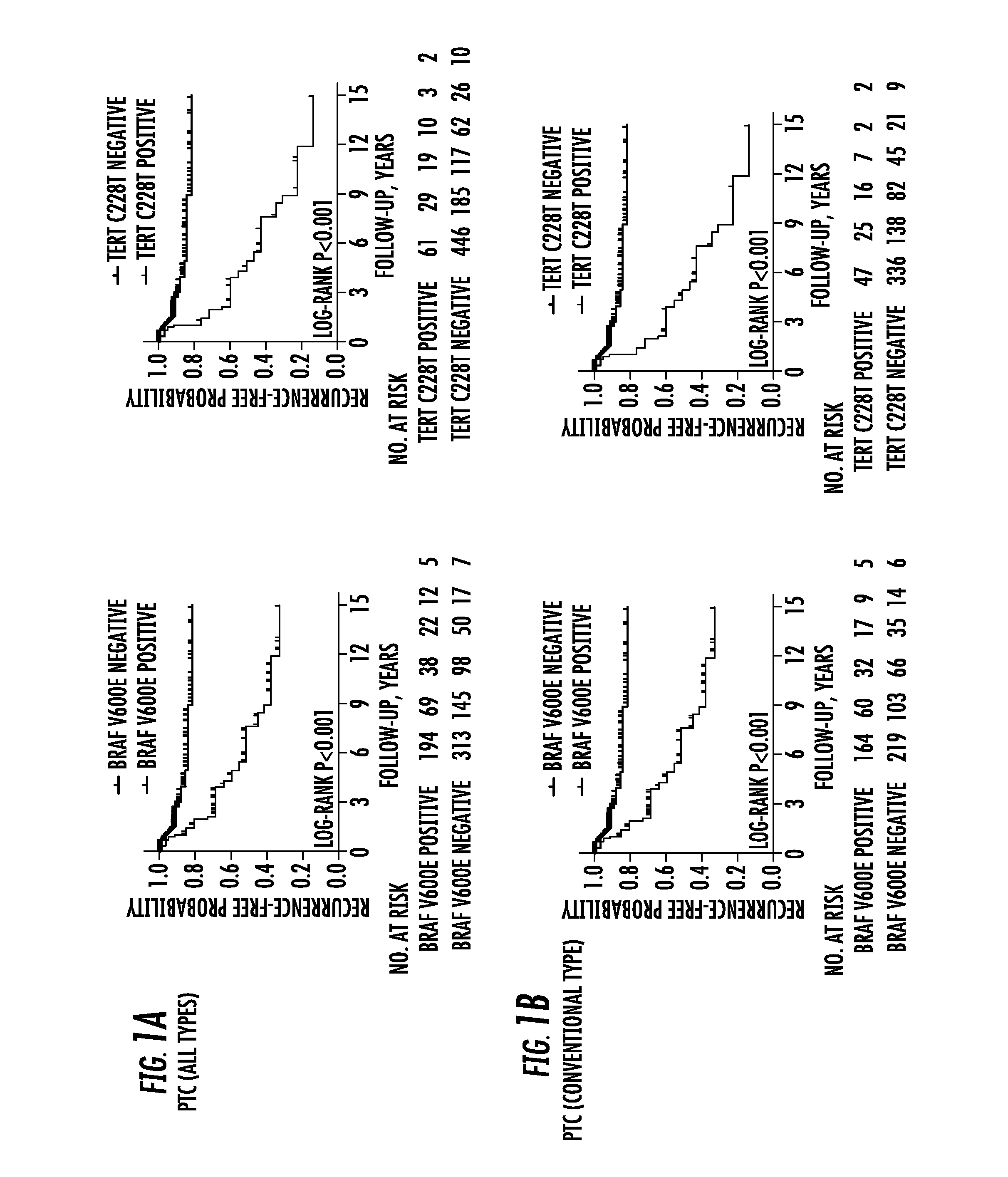
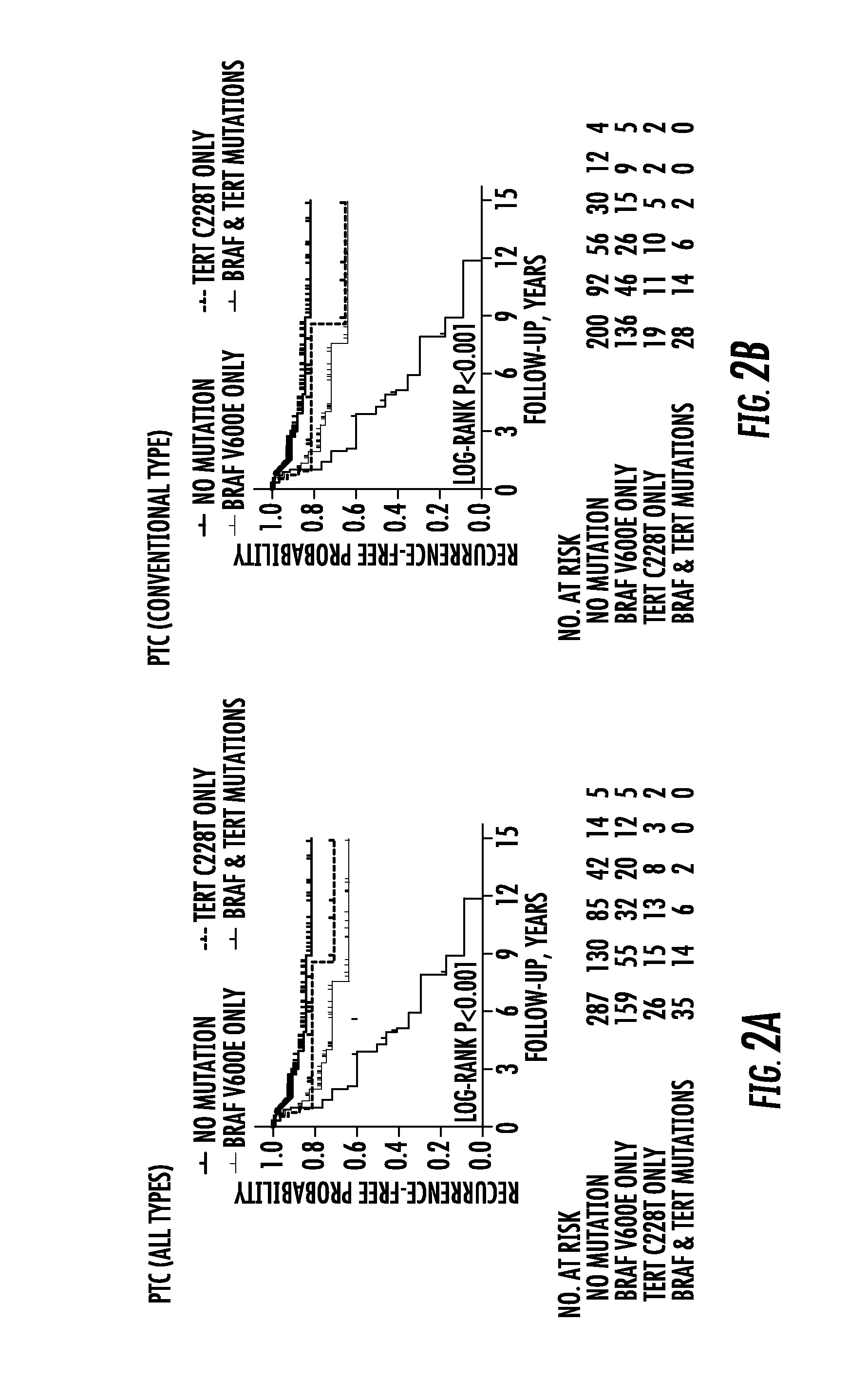
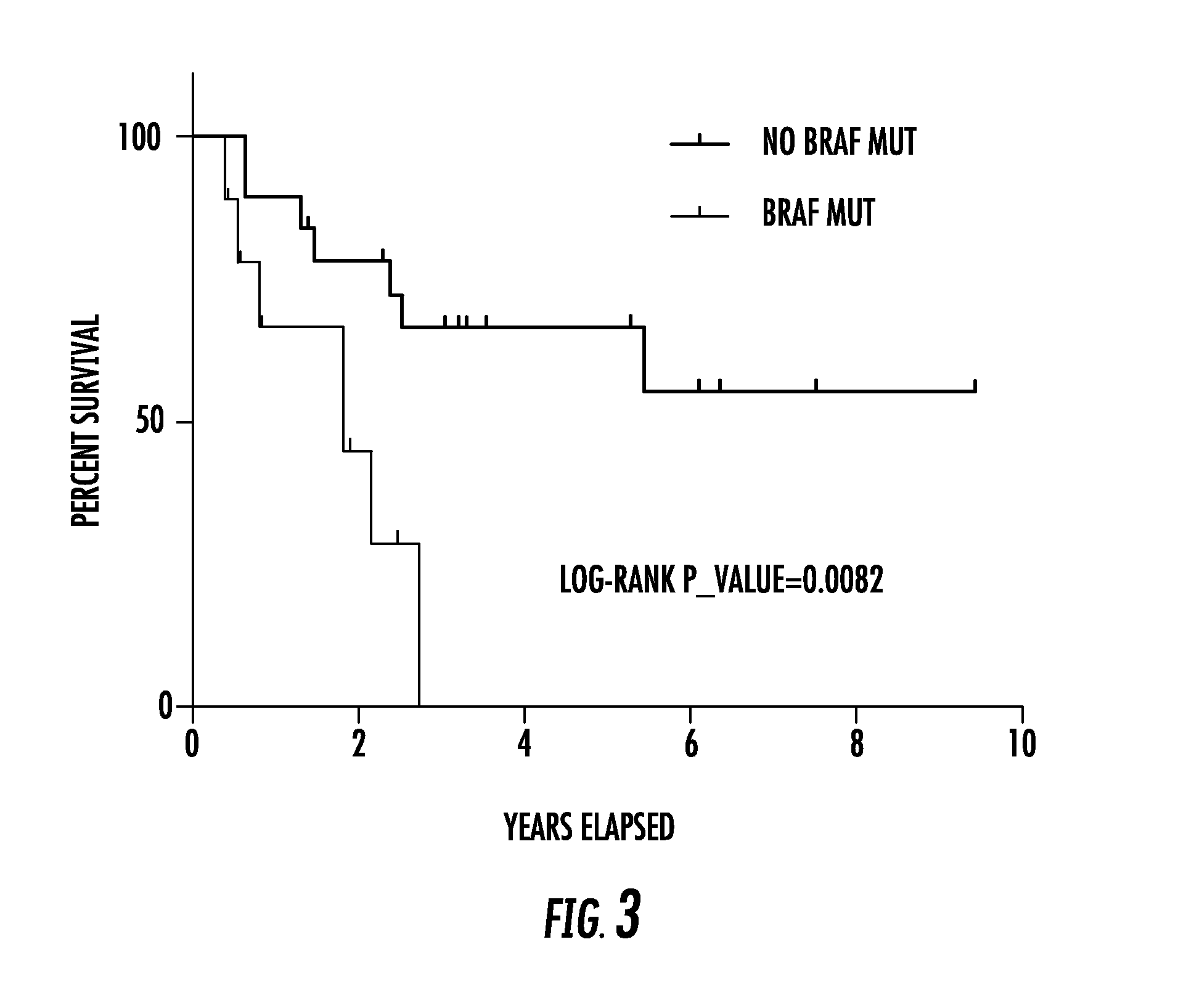
Tert and braf mutations in human cancer
ActiveUS20170022572A1Good risk stratificationEasy to manageMicrobiological testing/measurementReverse transcriptaseTert promoter
The present invention relates to the field of cancer. More specifically, the present invention provides methods and compositions related to certain mutations in cancer. In one embodiment, a method for treating a subject having aggressive thyroid cancer comprises the steps of (a) obtaining a biological sample from the subject; (b) performing an assay on the sample obtained from the subject to identify a mutation at 1 295 228 C>T (C228T), corresponding to −124 C>T from the translation start site in the promoter of the telomerase reverse transcriptase (TERT) gene, and a T1799A mutation in the BRAF gene that results in a V600E amino acid change; (c) identifying the subject as having or likely to develop aggressive thyroid cancer if the C228T and V600E mutations are identified; and (d) treating the subject with one or more treatment modalities appropriate for a subject having or likely to develop aggressive thyroid cancer. Similar approaches are applied to other human cancers harboring both BRAF V600E mutation and TERT promoter mutations.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Primer probe composition, kit and method for detecting KRAS and BRAF gene mutations through 3D (Three-dimensional) digital PCR (Polymerase Chain Reaction)
ActiveCN107488727AImprove detection efficiencyThe result is intuitive and clearMicrobiological testing/measurementDNA/RNA fragmentationIntestinal CancerBRAF Gene Mutation
The invention discloses a primer probe composition, a kit and a method for detecting KRAS and BRAF gene mutations through 3D (Three-dimensional) digital PCR (Polymerase Chain Reaction). The primer probe composition comprises primers and probes for detecting the six amino acid mutations on a No.2 exon of the KRAS gene, and primers and probes for detecting a V600E amino acid mutation on a No.15 exon of the BRAF gene, wherein the six amino acid mutations are respectively G12A, G12C, G12D, G12V, G12S and G13D. The primer probe composition comprises the seven common mutation sites related to intestinal cancer, and the mutation sites are respectively detected through the primers and the probes of carried different fluorescence signals according to the different mutation sites, so that the multiple mutations can be detected, the detection efficiency is high, and a result is more intuitive and clearer since the primer probe composition is combined with a 3D-PCR detection method for detecting.
Owner:GENE CRAB BIOTECH CO
Cell death inducing agent for cells having braf gene mutation, growth suppressing agent for same cells and pharmaceutical composition for therapy of diseases caused by growth defect of same cells
ActiveUS20180135058A1Improve efficacyInduce cell deathCompound screeningOrganic active ingredientsGrowth retardantDisease
Owner:NITTO DENKO CORP
Primer group, kit and method for detecting BRAF gene mutation
ActiveCN110938693AMeet the problem of low sensitivity of sequencingHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBRAF Gene MutationBraf genes
The invention discloses a primer group, a kit and a method for detecting BRAF gene mutation, belonging to the technical field of gene detection. The primer group provided by the invention comprises aBRAF upstream primer and a BRAF downstream primer, wherein the BRAF upstream primer has a nucleotide sequence as shown in a sequence table SEQ ID NO: 1; and the BRAF downstream primer has a nucleotidesequence as shown in a sequence table SEQ ID NO: 2. In addition, the kit provided by the invention comprises a PCR amplification reaction reagent, a positive quality control product, a negative quality control product, a primer mixed solution, a primer probe mixed solution and a standard curve equation. The method for detecting BRAF gene mutation provided by the invention has the characteristicsof high efficiency, simplicity, convenience, intuition, high sensitivity and the like, and can be used for quantitatively analyzing the mutation frequency of a BRAF gene.
Owner:CARRIER GENE TECH SUZHOU CO LTD
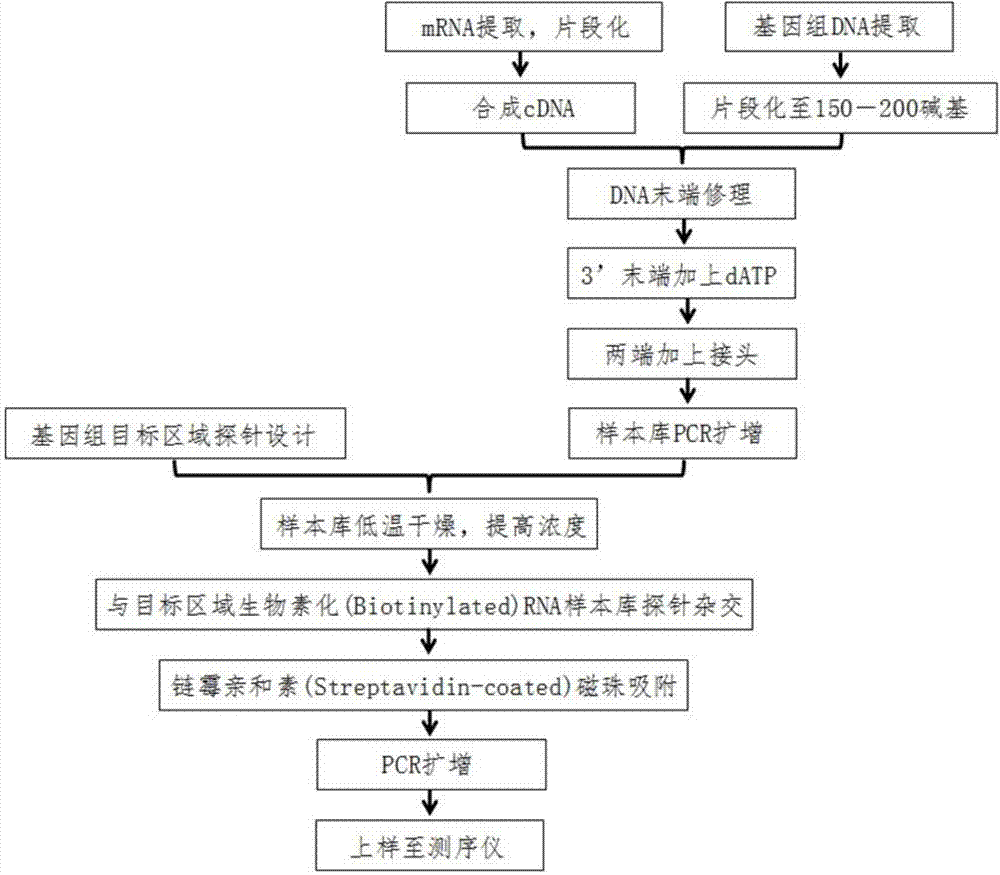
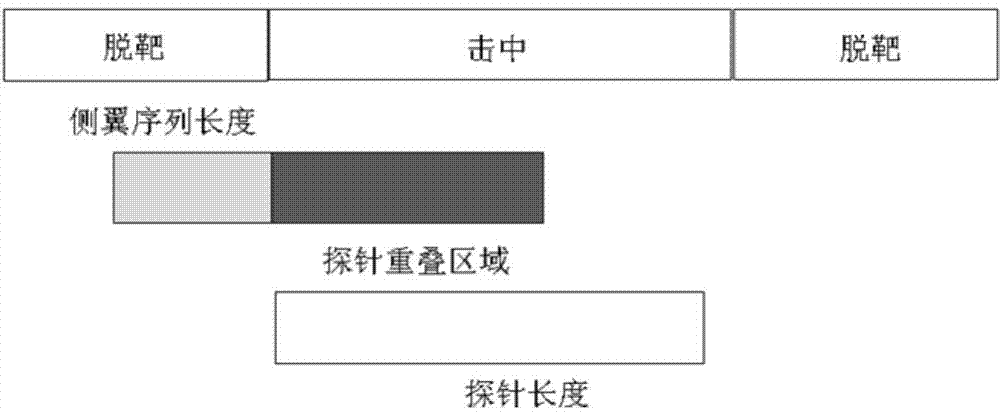
DNA (Deoxyribonucleic Acid) probe library hybridized with BRAF (v-Raf murine sarcoma viral oncogene homolog B1) gene, and method for enriching BRAF gene segments by adopting same
ActiveCN103667268AIncreased sensitivityGuaranteed accuracyNucleotide librariesMicrobiological testing/measurementViral OncogeneGenes mutation
The invention provides a DNA (Deoxyribonucleic Acid) probe library hybridized with BRAF (v-Raf murine sarcoma viral oncogene homolog B1) gene. The DNA probe library comprises one or more DNA probes which can be hybridized with the BRAF gene, each DNA probe comprises the following sequences: SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3, SEQ ID NO.4, SEQ ID NO.5, OR SEQ ID NO.6. The invention also provides a method for enriching BRAF gene segments by adopting the same. Based on this, the invention further provides a method for detecting the gene mutation of the BRAF gene. The BRAF gene segments can be enriched by thousands of times through adopting the method, the BRAF gene segments can be used for next-generation sequencing technology for detecting gene structure mutation including single base mutation, mRNA deficiency or increase, mRNA structure transversion and mRNA splicing change.
Owner:GENESEEQ TECH INC
Kit for quantitative detection of braf mutation
InactiveUS20130095491A1Accurately and quantitatively ratioEasy to operateMicrobiological testing/measurementChemiluminescene/bioluminescenceBRAF Gene MutationBraf genes
The present invention relates to a method and assay kit for BRAF gene mutations which relates to the effect of molecule-targeting anti-tumor drug. Particularly, the present invention relates to a fluorescent quantitative PCR method and kit for detecting mutations at hotspots of BRAF gene, together with the use thereof. The present invention detects the mutations at specific sites of BRAF gene, and can predict the therapeutic efficacy of anti-EGFR tyrosine kinase inhibitors, an anti-tumor drug. Therefore, the present invention can provide a guidance to individualized treatments for cancer patients.
Owner:BEIJING ACCB BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com