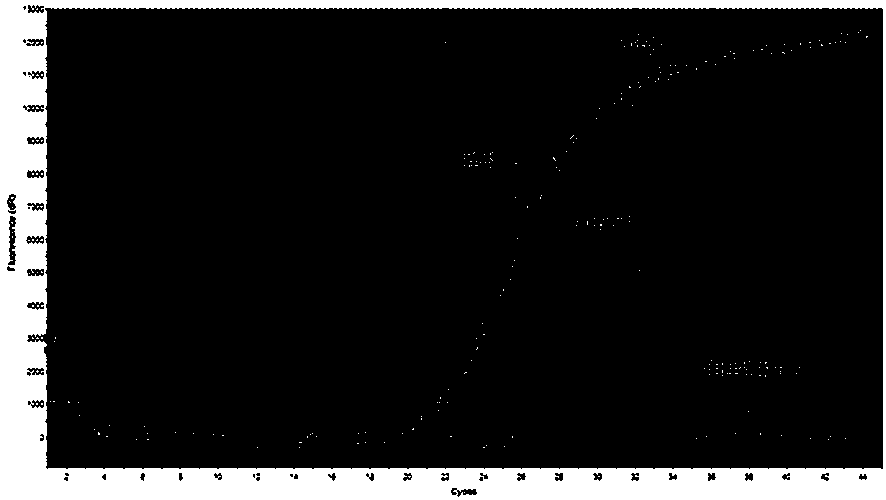
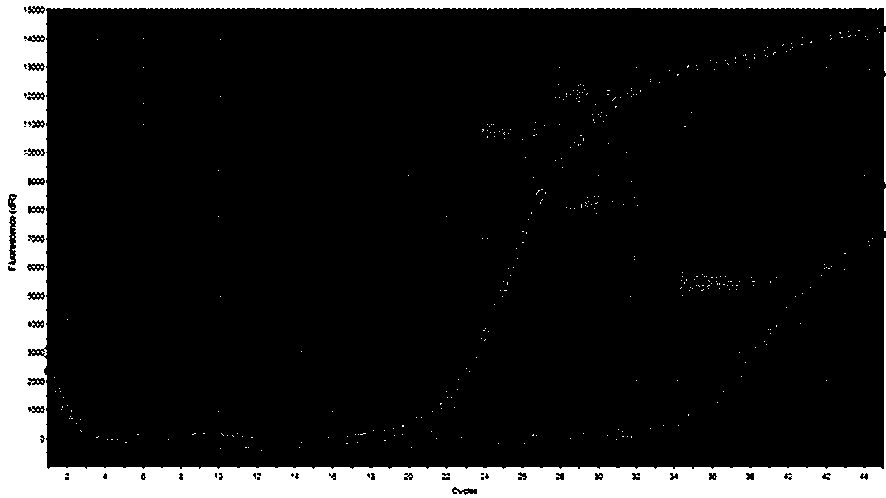
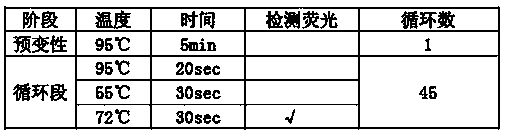
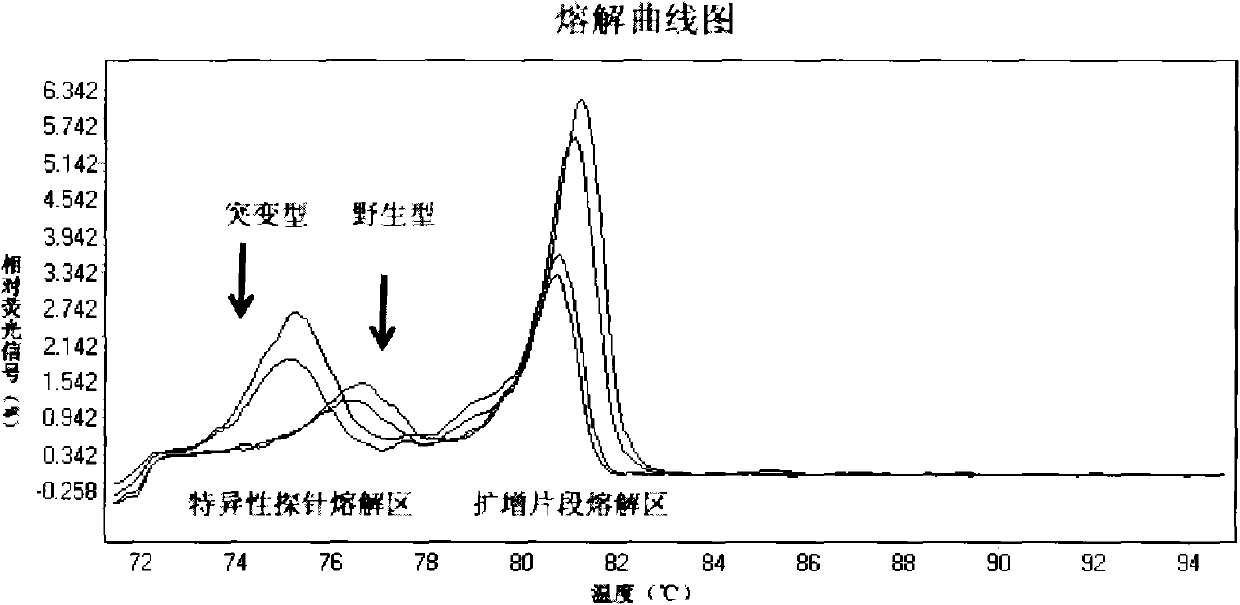
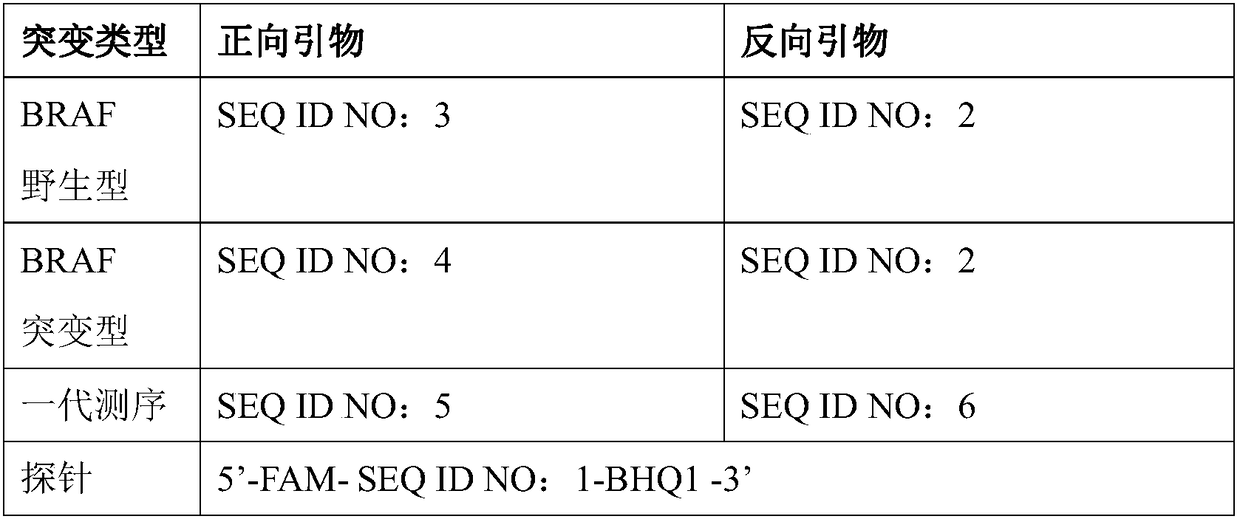
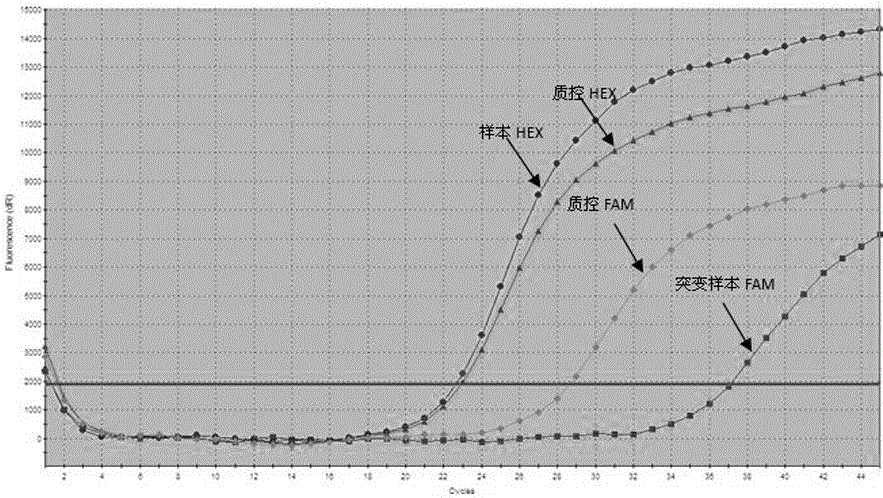
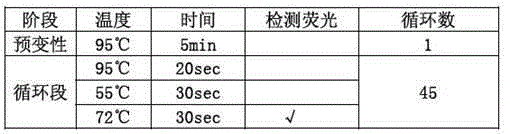
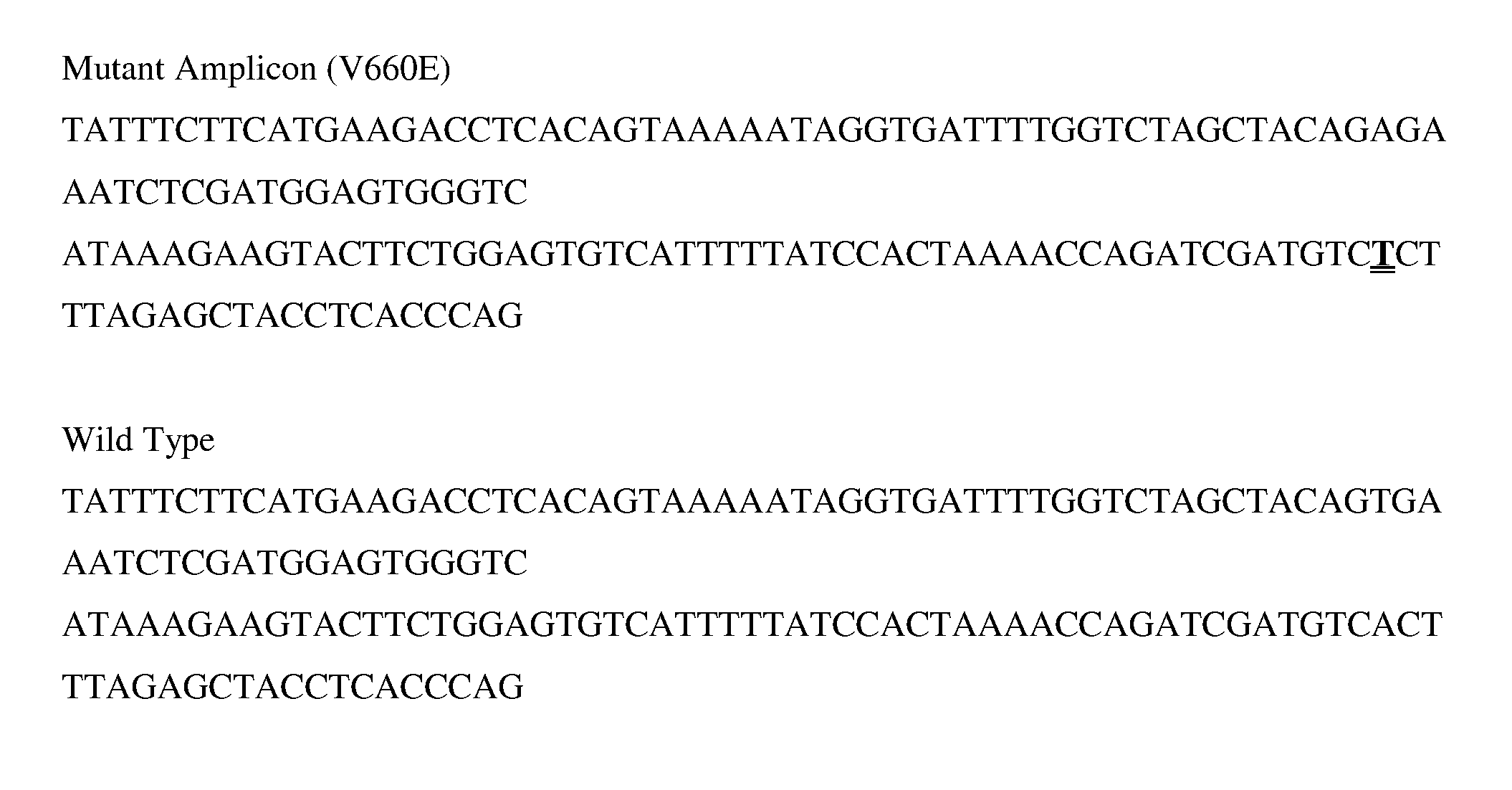Patents
Literature
44 results about "V600E" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
V600E is a mutation of the BRAF gene in which valine (V) is substituted by glutamic acid (E) at amino acid 600. It is a driver mutation in a proportion of certain diagnoses, including melanoma, hairy cell leukemia, papillary thyroid carcinoma, colorectal cancer, non-small-cell lung cancer, Langerhans cell histiocytosis, and ameloblastoma.
Methods of Predicting Cancer Risk Using Gene Expression in Premalignant Tissue
ActiveUS20100291573A1Use can be riskyIncreased riskMicrobiological testing/measurementBiological testingBraf genesExpression gene
The present disclosure provides methods for assessing a patient's cancer risk and / or recurrence risk, which methods comprise assaying, in a biological sample obtained from the gastrointestinal (GI) tract of the patient, an expression level of a risk gene. The present disclosure also provides methods involving a cancer risk / recurrence risk sequence, i.e. the V600E mutation of the BRAF gene, which is useful for assessing cancer risk and / or recurrence risk in a patient.
Owner:GENOMIC HEALTH INC
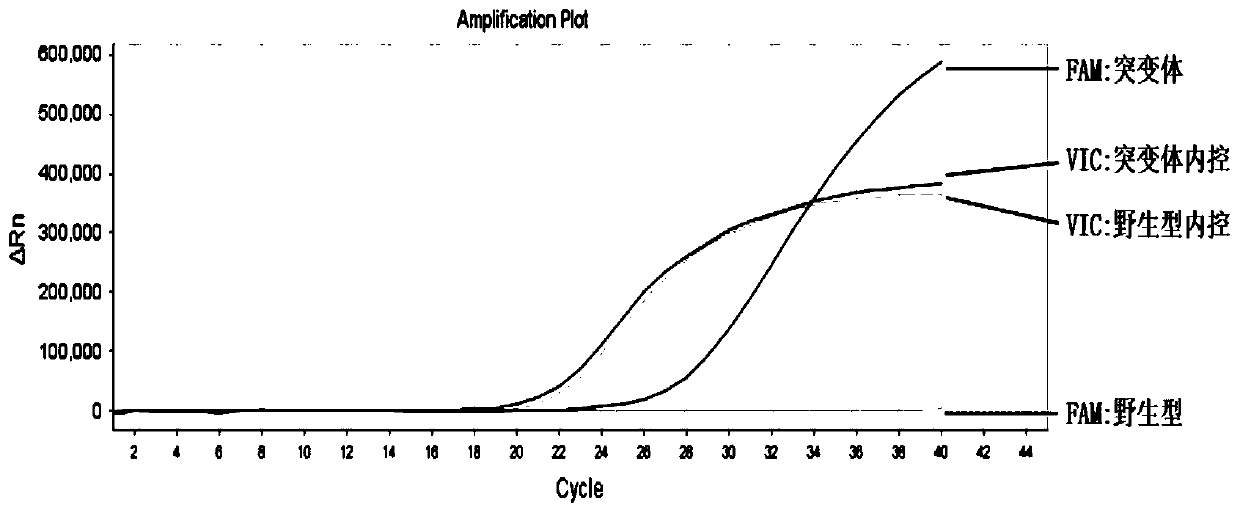
B-raf gene mutation detection kit
ActiveCN104099425AGuaranteed accuracyMonitor for false negativesMicrobiological testing/measurementForward primerB-RAF Gene Mutation
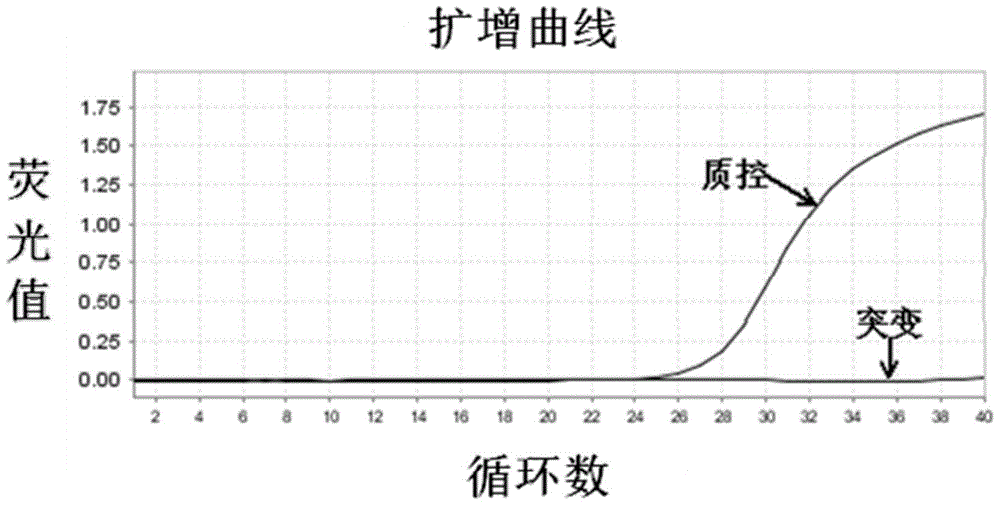
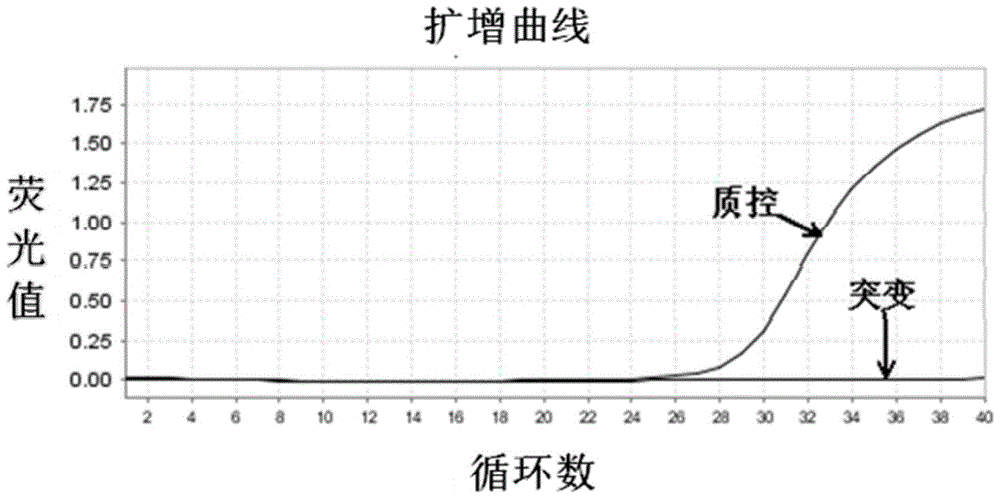
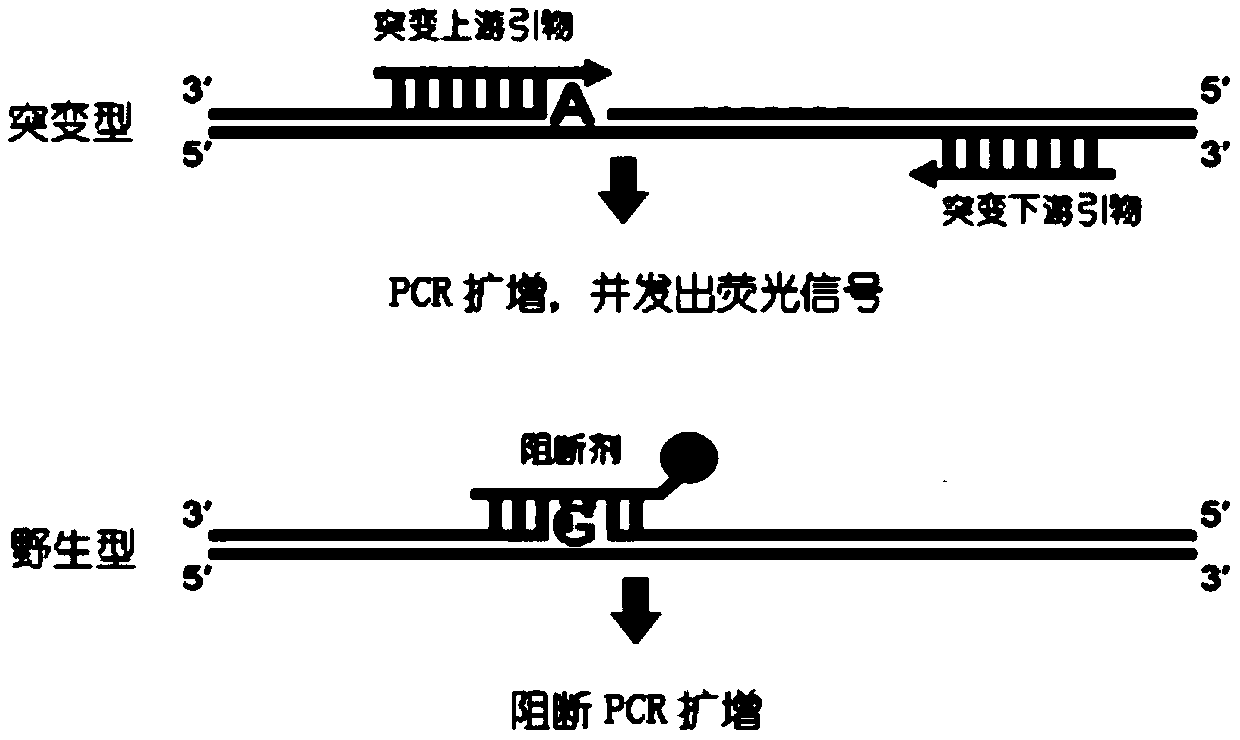
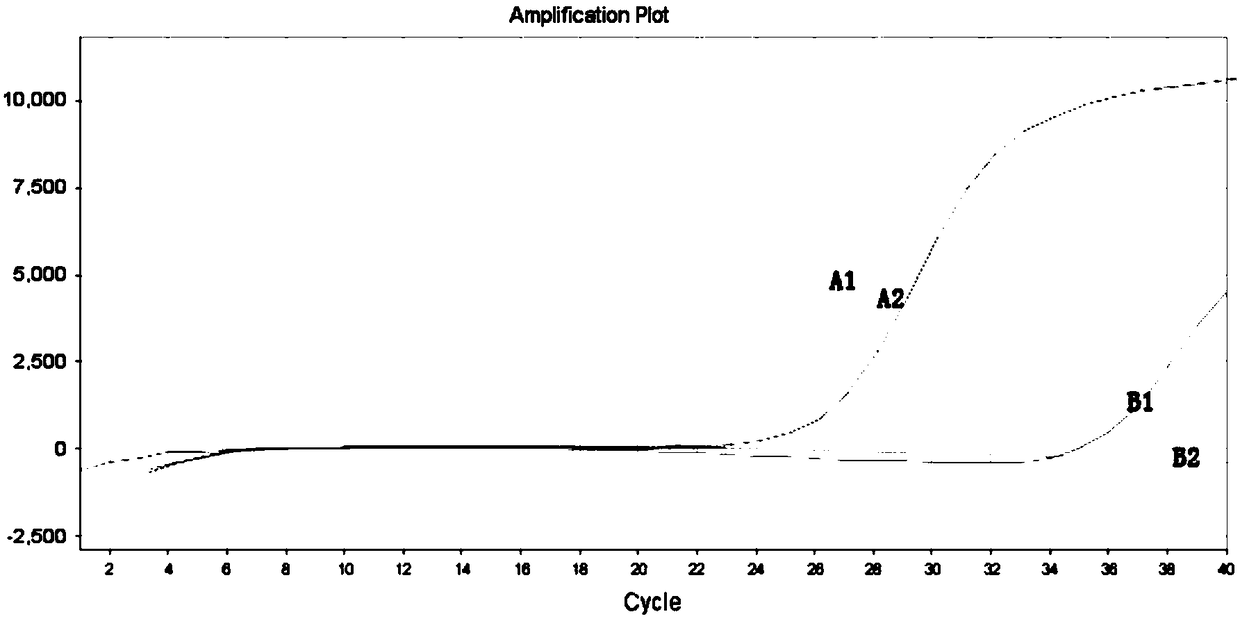
The invention relates to a B-raf gene mutation detection kit. The B-raf gene mutation detection kit comprises quality-control primer probe internal-standard mixed liquor and detection primer probe internal-standard mixed liquor, wherein the quality-control primer probe internal-standard mixed liquor comprises a quality-control primer pair, a B-raf gene specific probe, an internal-standard primer pair, an internal-standard specific probe and an internal-standard template, and the detection primer probe internal-standard mixed liquor comprises a B-raf gene V600E mutation detection specific primer pair, a B-raf gene specific probe, an amplification blocking nucleic acid sequence, an internal-standard primer pair, an internal-standard specific probe and an internal-standard template. The B-raf gene mutation detection kit has the advantages that an amplification refractory mutation system is combined with a wild type amplification blocking nucleic acid sequence with a phosphorylated terminal, deoxyinosine is introduced into detection of a B-raf gene V600E mutation detection specific ARMS (amplification refractory mutation system) forward primer to enable quality-control PCR (polymerase chain reaction) and detection PCR to be performed for detection of samples parallelly, and each reaction system can have an internal-standard system capable of effectively avoiding false negative and intra-assay variation; the B-raf gene mutation detection kit is low in cost, high in sensitivity and more capable of controlling intra-assay and inter-assay variation.
Owner:国九堂山东阿胶有限公司
Method and kit for detecting mutation of BRAF gene of human colorectal cancer
InactiveCN102586401ASignificant progressEasy to detectMicrobiological testing/measurementFluorescence/phosphorescenceNucleotideV600E
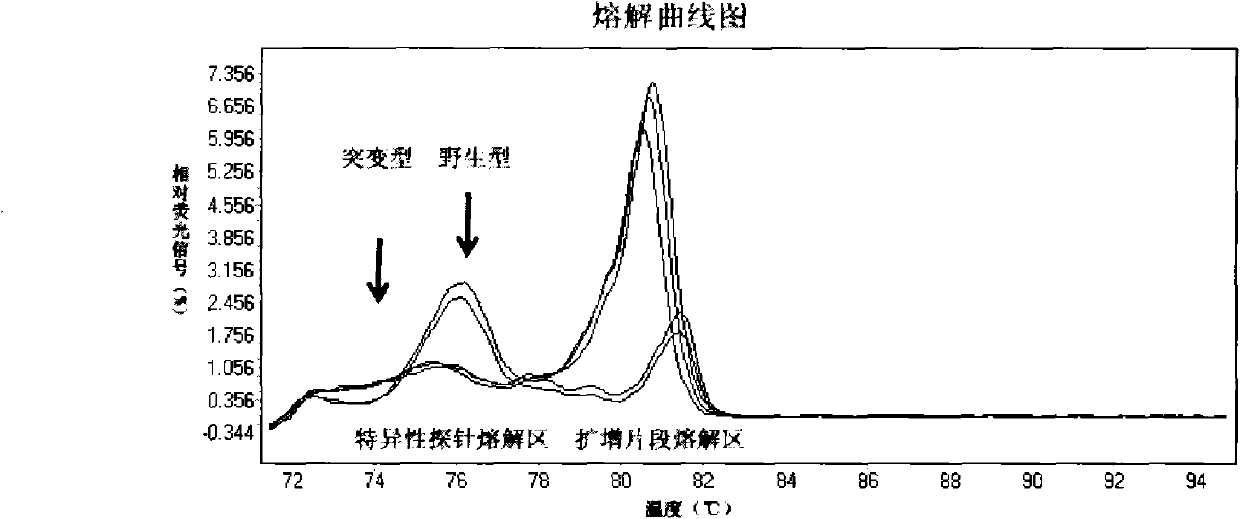
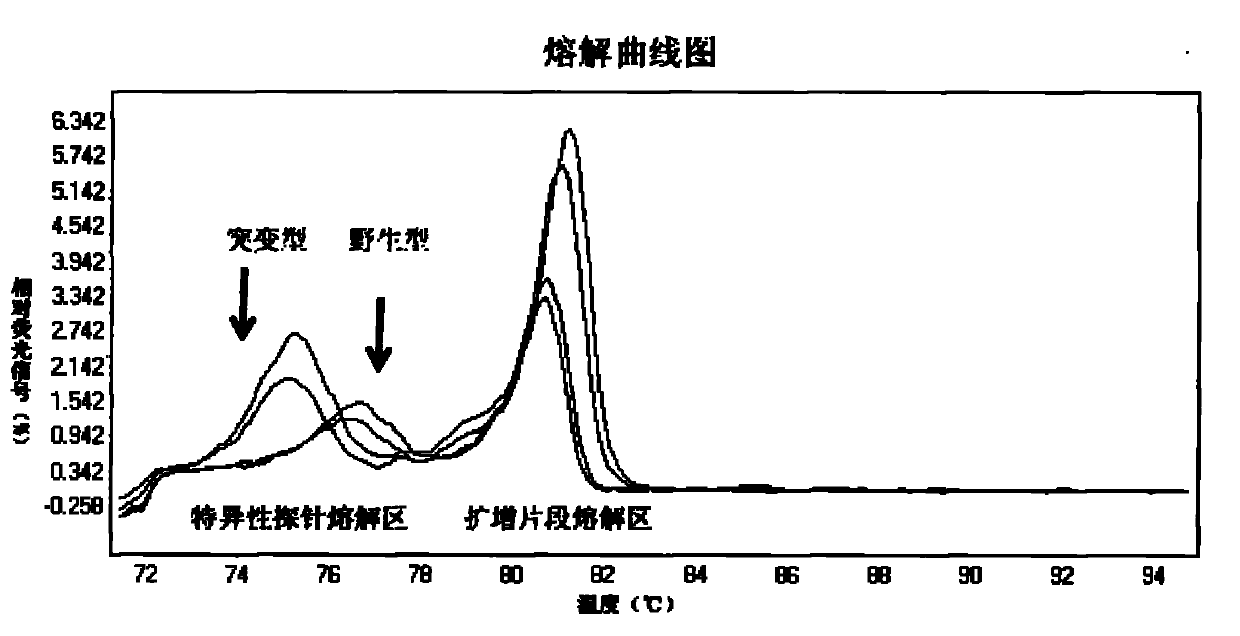
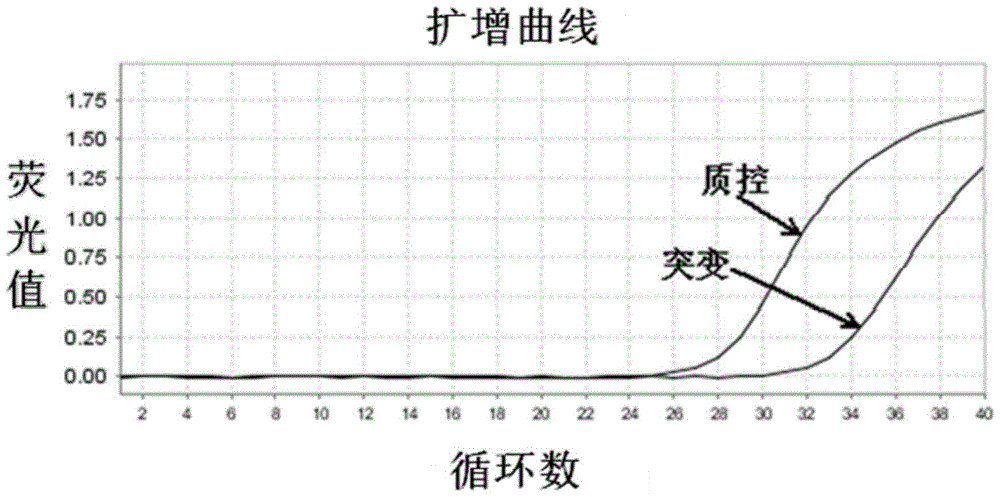
The invention relates to a method and a kit for detecting gene mutation, in particular to a method and a kit for detecting the mutation of BRAF gene. The invention is characterized in that the kit comprises a specific probe used for carrying out genotyping on No. 15 exon codon V600E of the BRAF gene, wherein the specific probe of the No. 15 exon codon V600E comprises a nucleotide sequence of V600E codon. by the technology combining the conventional polymerase chain reaction (PCR) amplification with a Cold-PCR enrichment amplification product and a high resolution melting curve analysis technology, the kit provided by the invention can be used for completing the judgment on sample genotyping.
Owner:苏州科贝生物技术有限公司
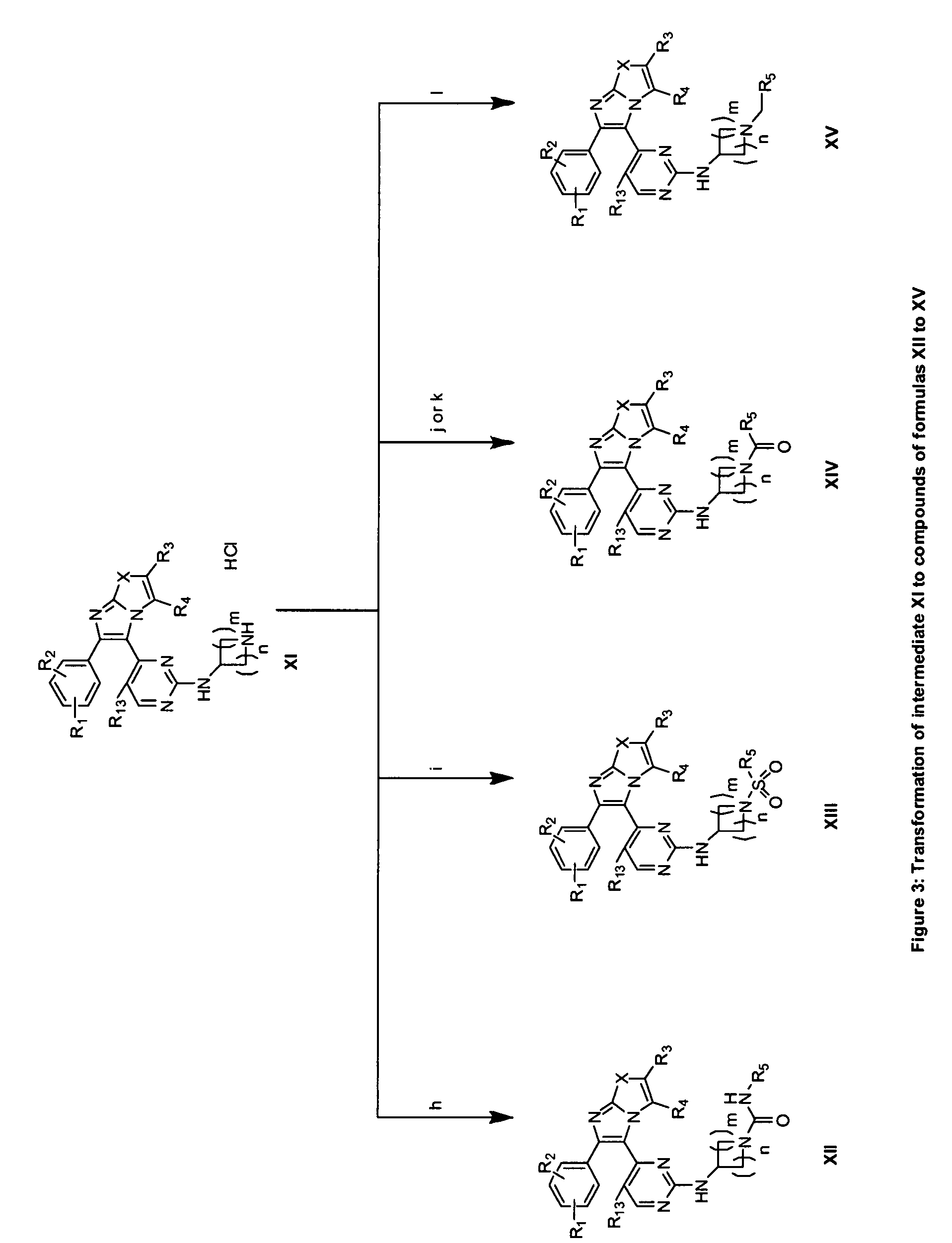
RAF inhibitors and their uses
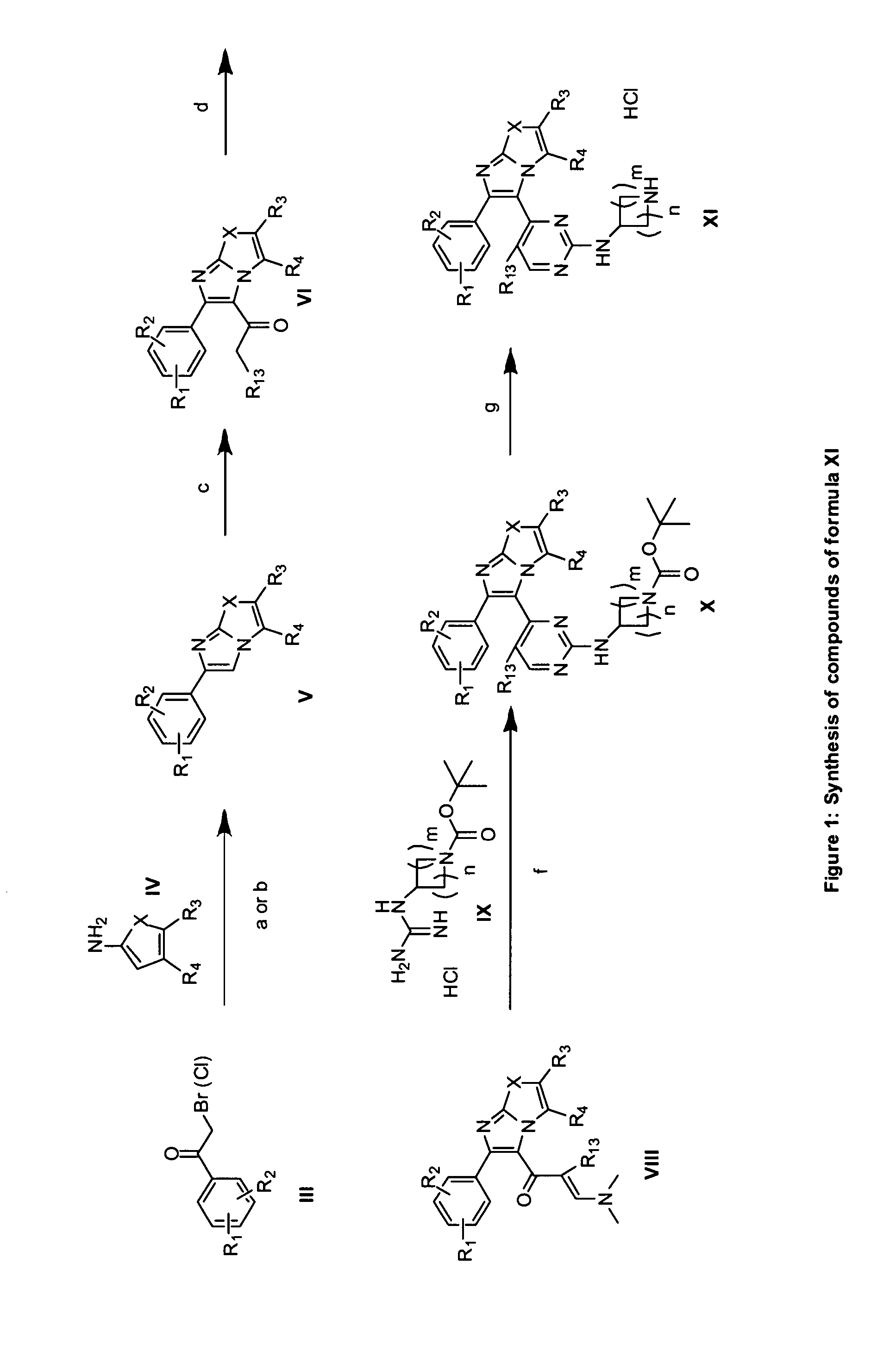
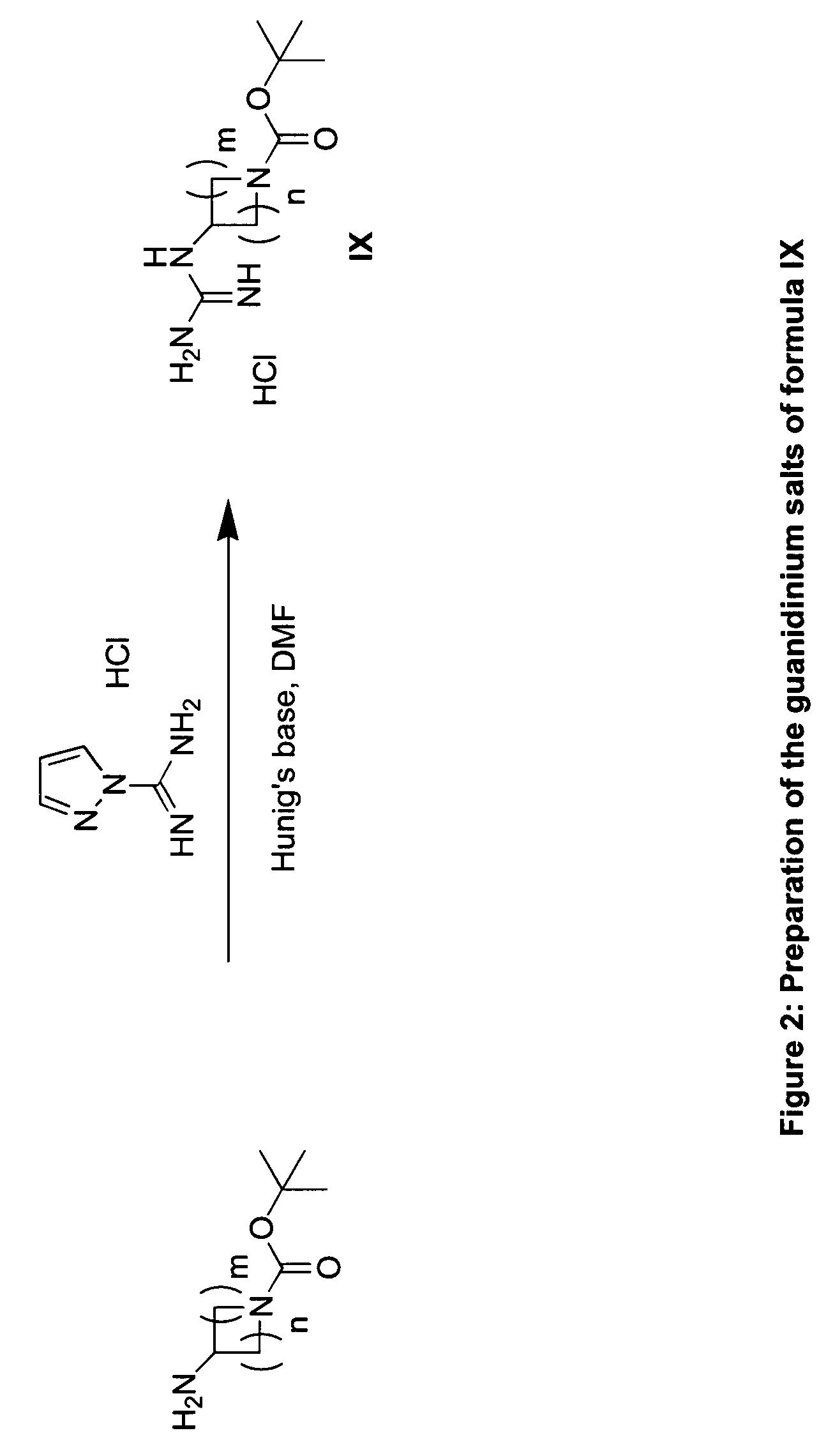
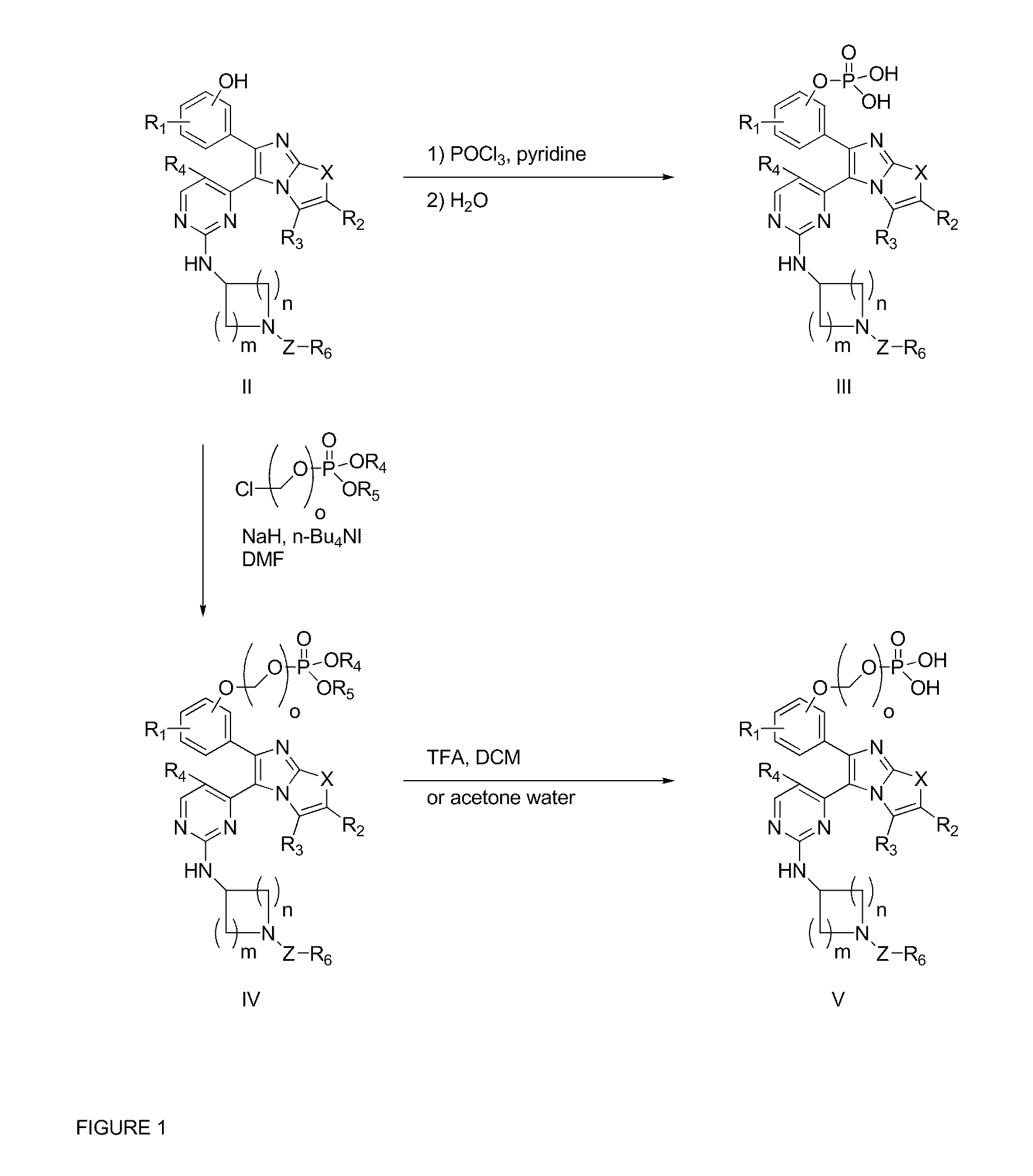
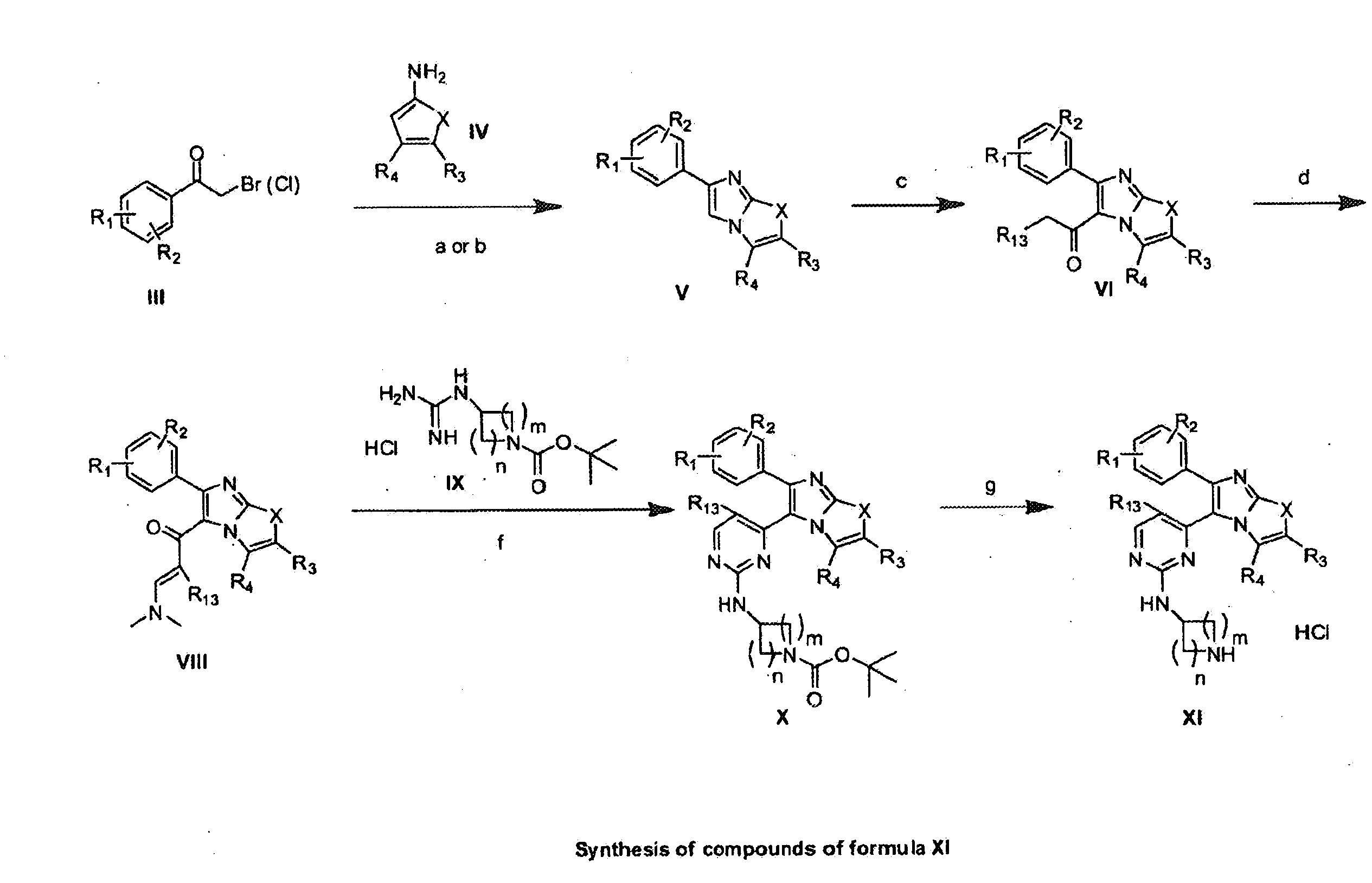
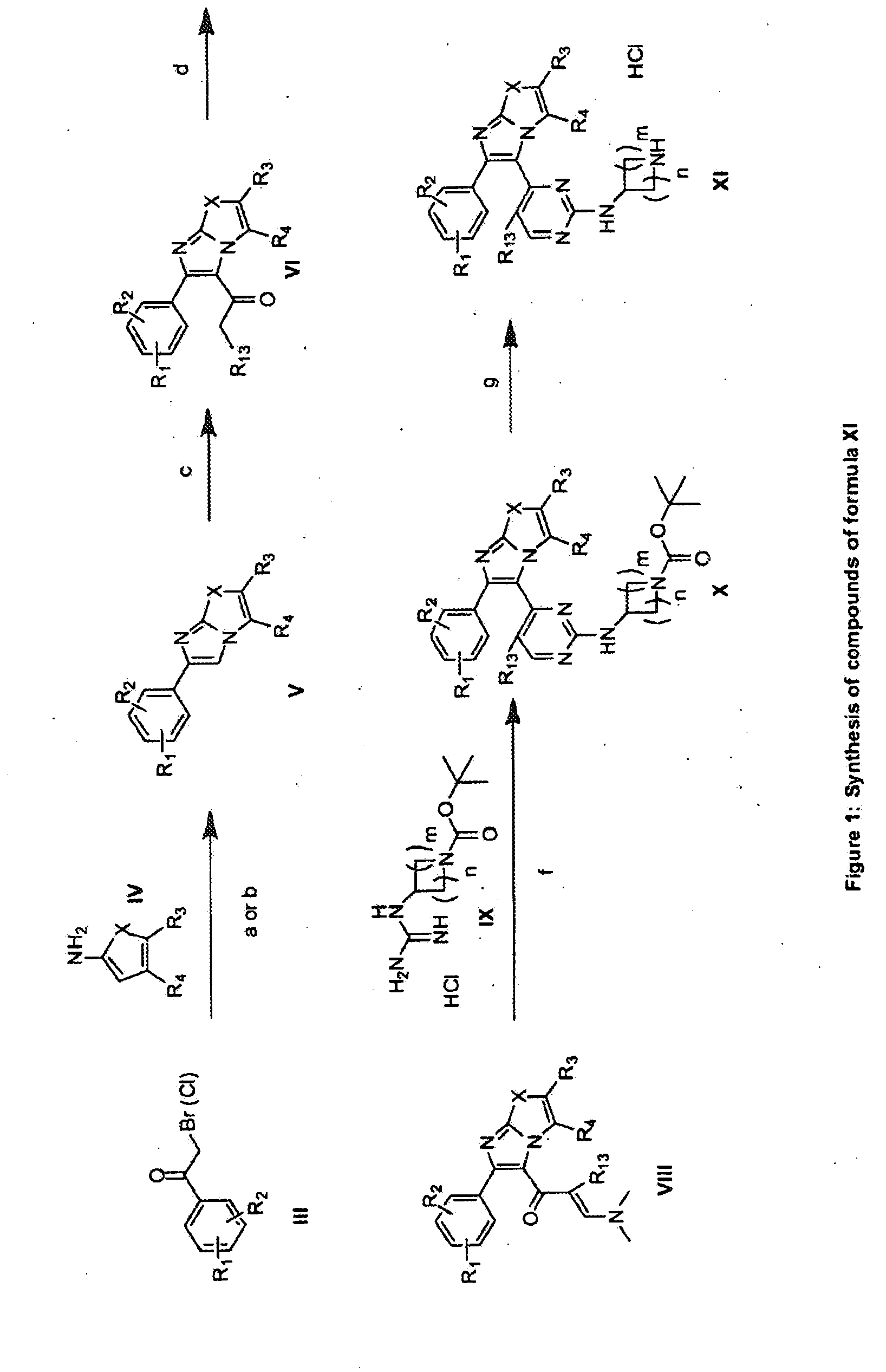
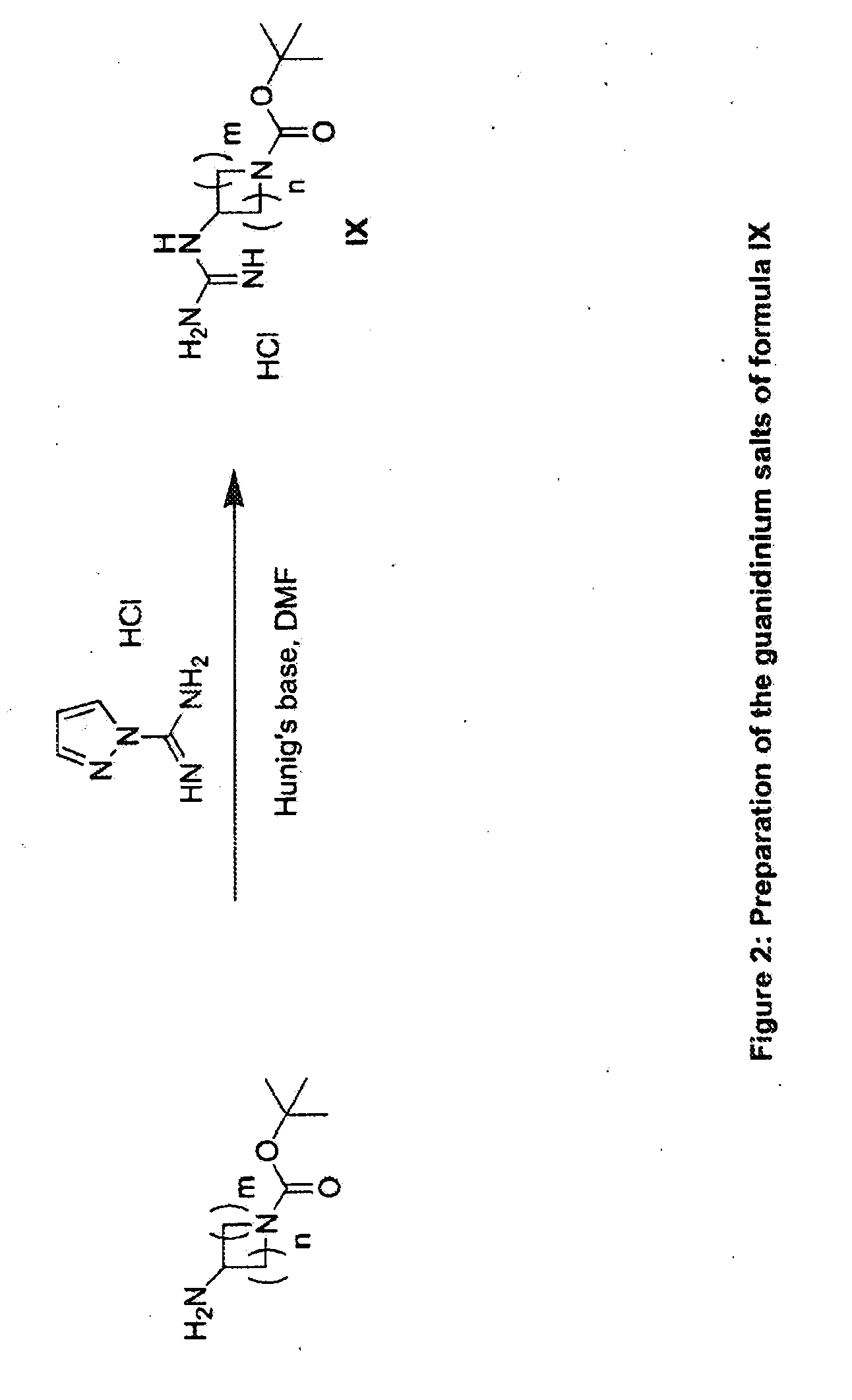
The present invention provides imidazooxazole and imidazothiazole compounds and their synthesis. The compounds of the present invention are capable of inhibiting the activity of RAF kinase, such as B-RAFV600E. The compounds are useful for the treatment of cell proliferative disorders such as cancer.
Owner:ARQULE INC
Primer, probe, reagent kit and method for detecting mutation of BRAF gene V600E
InactiveCN102816851AHigh detection specificityEasy to readMicrobiological testing/measurementDNA/RNA fragmentationBraf genesPcr ctpp
The invention provides a primer, a probe, a reagent kit and a method for detecting mutation of BRAF gene V600E on the basis of real-time fluorescent quantitative PCR (polymerase chain reaction) technical platform. The primer, the probe, the reagent kit and the method are quick and low in cost and have clinic detection popularization value.
Owner:SUZHOU KUANGYUAN MOLECULAR BIOTECH
Detection kit for human BRAF gene V600E mutation
InactiveCN105039514AAccurate detection of genetic mutationsReduce pollutionMicrobiological testing/measurementBraf genesLocked nucleic acid
The invention provides a detection kit and a detection method for human BRAF gene V600E mutation. The detection kit comprises a first reagent pack and a second reagent pack, wherein each of the first reagent pack and the second reagent pack comprises PCR buffer solution, dNTP, MgCl2, a specific probe, a locked nucleic acid (LNA) primer, an interior label system, HotStart Taq enzyme and UNG enzyme; the first reagent pack comprises a primer pair SEQ ID NO:1 and SEQ ID NO:2, and a probe SEQ ID NO:3; the second reagent pack comprises a primer pair SEQ ID NO:1 and SEQ ID NO:4, and a probe SEQ ID NO:5; and the SEQ ID NO:4 is LNA primer. The detection kit and the detection method can be used for detecting human BRAF gene V600E mutation, and has the advantages of strong specificity, high sensitivity, simplicity and rapidness in operation, high flux, safety, objective result determination and the like.
Owner:WUHAN YZY MEDICAL SCI & TECH
RAF Inhibitors and Their Uses
The present invention provides imidazooxazole and imidazothiazole compounds and their syntheses. The compounds of the present invention are capable of inhibiting the activity of RAF kinase, such as B-RAFV600E. The compounds are useful for the treatment of cell proliferative disorders such as cancer.
Owner:ARQULE INC
Nucleotide sequence for detecting BRAF gene V600E mutation and application thereof
PendingCN109576372AThe result is accurateImprove stabilityMicrobiological testing/measurementDNA/RNA fragmentationBraf genesMolecular diagnostic techniques
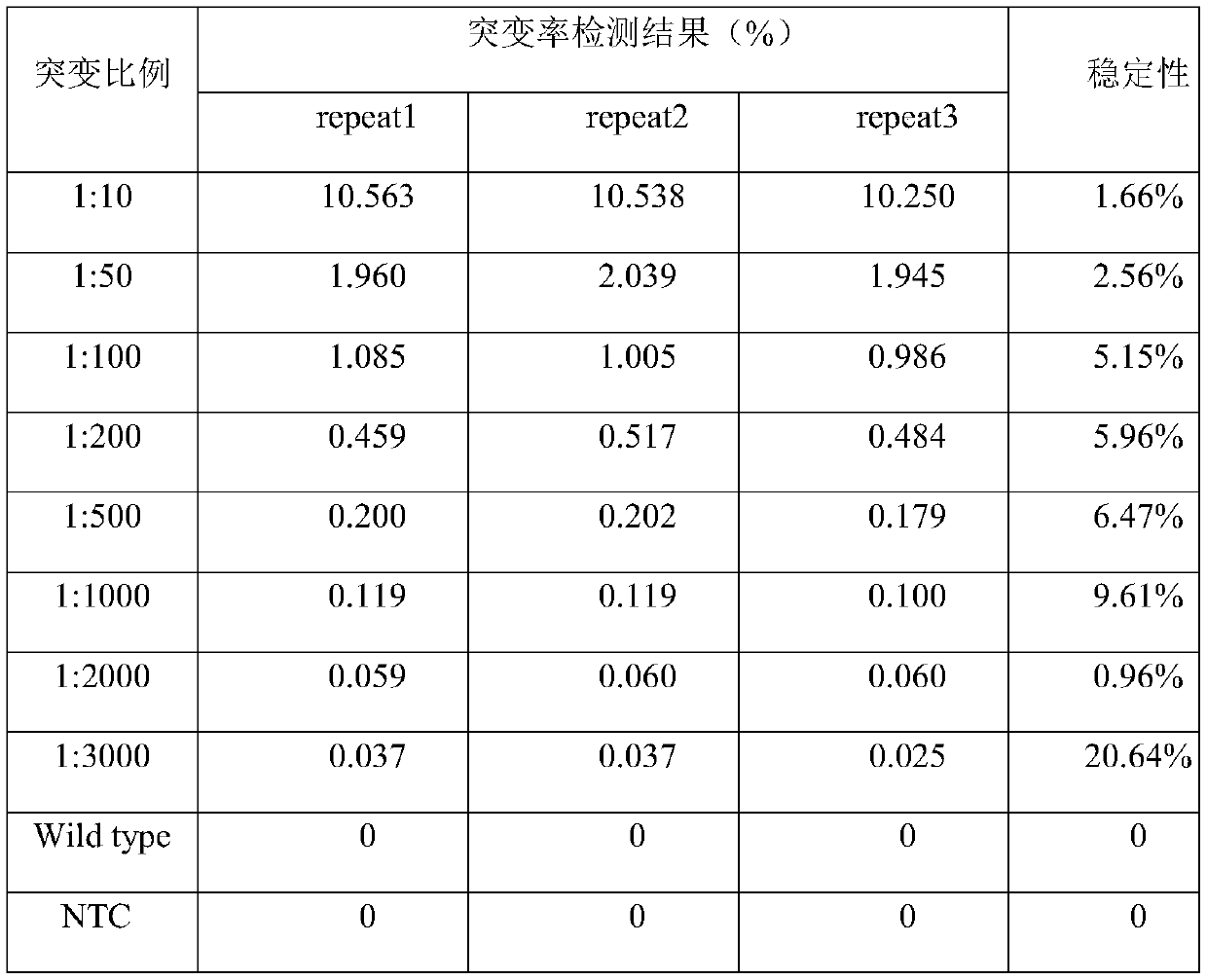
The invention discloses a nucleotide sequence for detecting BRAF gene V600E mutation and application thereof and belongs to the technical field of molecular diagnosis. A primer and a probe which are used for detecting BRAF can be specifically amplified and are used for detecting the BRAF V600E gene mutation. The invention further discloses a detection kit for detecting the BRAF V600E gene mutationbased on a micro-droplet type digital PCR (Polymerase Chain Reaction) system and the mutation of a BRAF gene can be rapidly, sensitively and accurately detected. The nucleotide sequence has the advantages of convenient operation, strong specificity, high sensitivity, low cost, high flux and the like, can be used for rapidly detecting the clinical BRAF V600E gene mutation and provides reference for diagnosis and treatment of the BRAF V600E mutation.
Owner:HANGZHOU D A GENETIC ENG
Kit for detecting BRAF gene V600E trace mutation through pyrosequencing technique and application of kit
InactiveCN105400900AAccurate quantification of mutation frequencyReduce material requirementsMicrobiological testing/measurementDNA/RNA fragmentationLife qualityBraf genes
The invention mainly relates to the field of molecular diagnosis and provides a kit for accurately detecting BRAF gene V600E trace mutation. The kit mainly comprises two specific amplification primers, one blocking primer, one sequencing primer and affinity magnetic beads marked bystreptomycin. By the application of the kit, trace mutation of the BRAF gene V600E which is lowered to 0.1% in concentration can be accurately detected. A detection method is high in sensitivity, the mutation generated when the concentration is lowered to 0.1% can be detected, the result is visual, interpretation is simpler, more accurate and more rapid, the false negative rate of the detection result is greatly lowered, clinical invalid choice of targeted drugs of a patient is avoided, valuable treatment time is saved for the patient, and the living quality of the patient is improved.
Owner:HANGZHOU DIAN BIOTECH CO LTD
Methods of predicting cancer risk using gene expression in premalignant tissue
Owner:GENOMIC HEALTH INC
RAF Inhibitors and Uses Thereof
The present invention provides imidazooxazole and imidazothiazole compounds and their synthesis. The compounds of the present invention are capable of inhibiting the activity of RAF kinase, such as B-RAFV600E. The compounds are useful for the treatment of cell proliferative disorders such as cancer.
Owner:ARQULE INC
Primer and kit for detecting BRAF gene V600E mutation sites, and PCR method of kit
ActiveCN104846106ADifficulty of SimplificationReduce errorsMicrobiological testing/measurementDNA/RNA fragmentationForward primerBraf genes
The invention discloses a primer for detecting BRAF gene V600E mutation sites, and a PCR method of the kit. The primer comprises a wild type specific forward primer, a mutant type specific forward primer, and a reverse primer shared by the wild type specific forward primer and the mutant type specific forward primer, wherein the wild type specific forward primer has a sequence shown as SEQ No.17; the mutant type specific forward primer has a sequence shown as SEQ No.14; and the shared reverse primer has a sequence shown as SEQ No.16. The kit has the advantages of simple detection, rapidness, accuracy, low price and the like and provides a powerful tool for scientific research and clinical detection of BRAF gene V600E mutation sites and gene mutation analysis.
Owner:沈阳优吉诺生物科技有限公司
Methods for treatment of melanoma
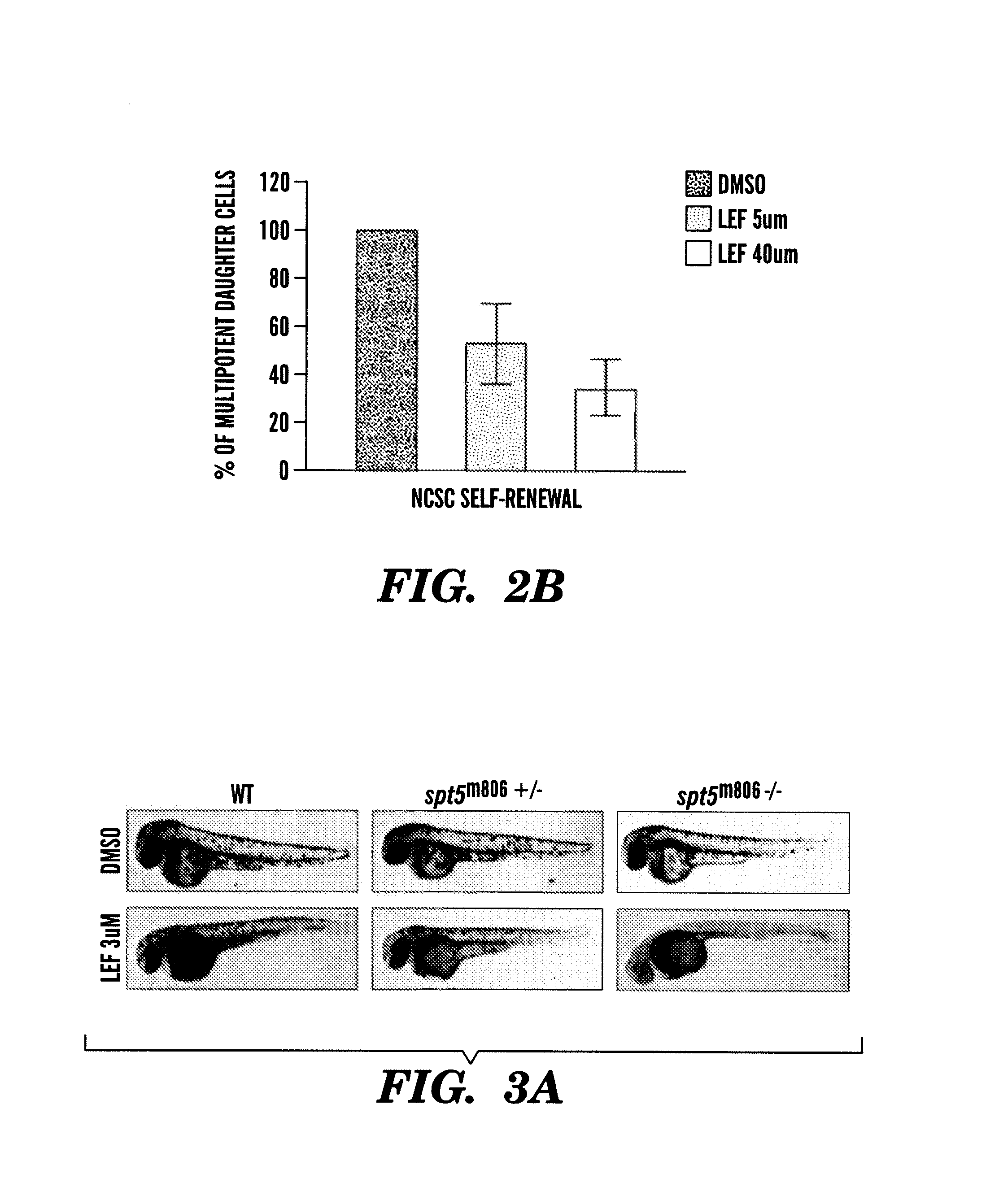
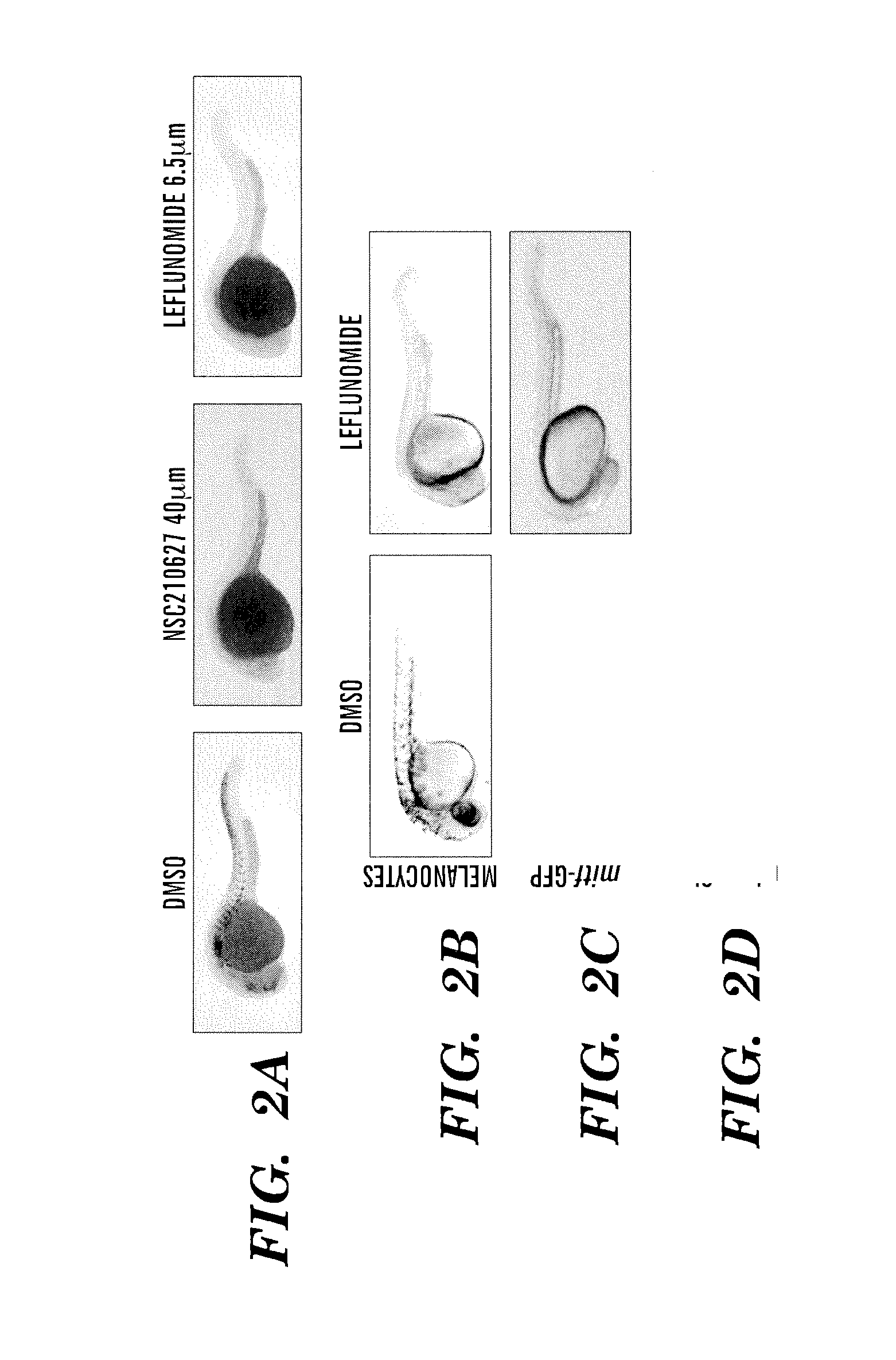
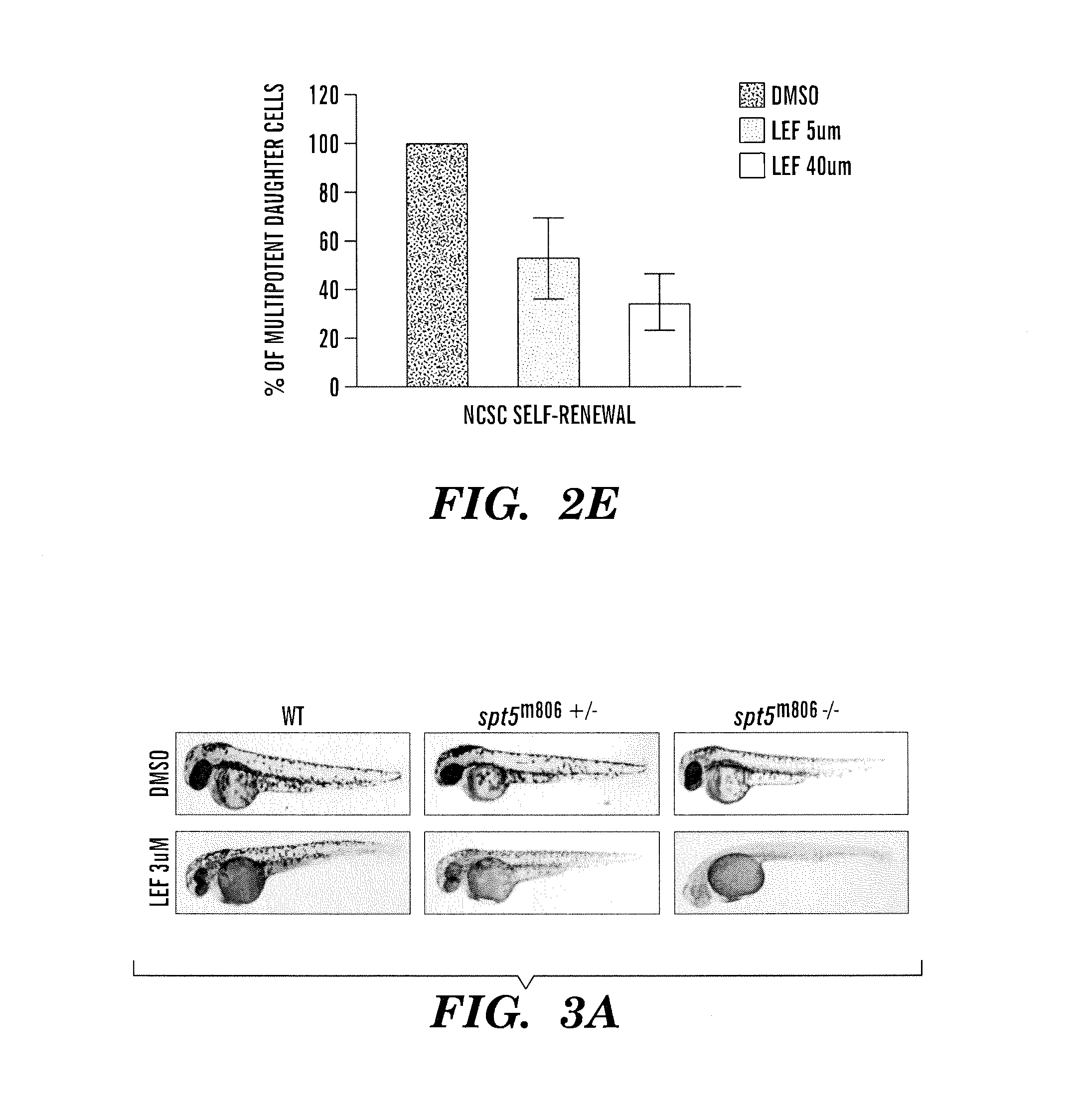
InactiveUS20140031383A1Utility in treatmentUseful in treatmentBiocideMicrobiological testing/measurementProgenitorNeural crest
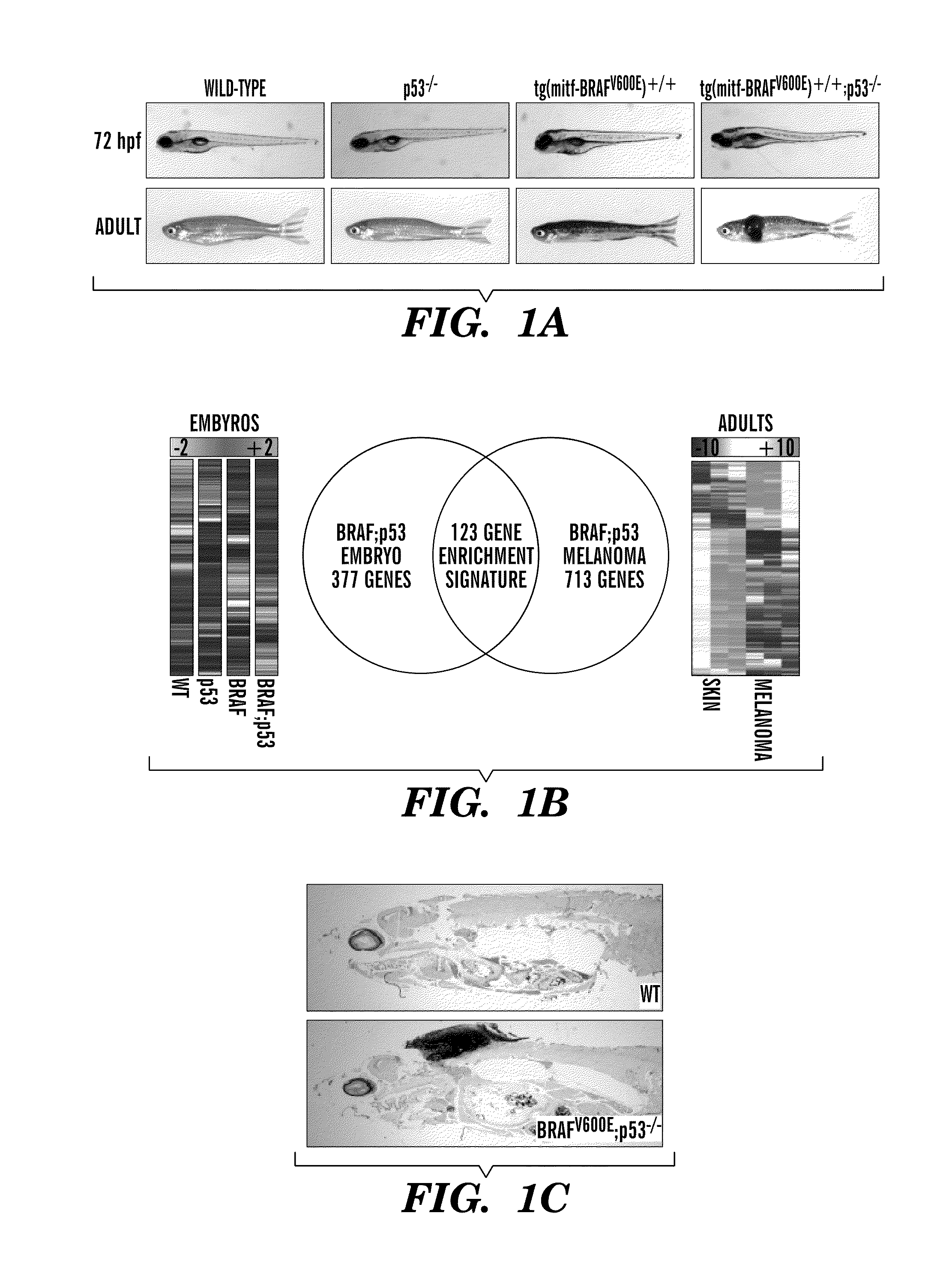
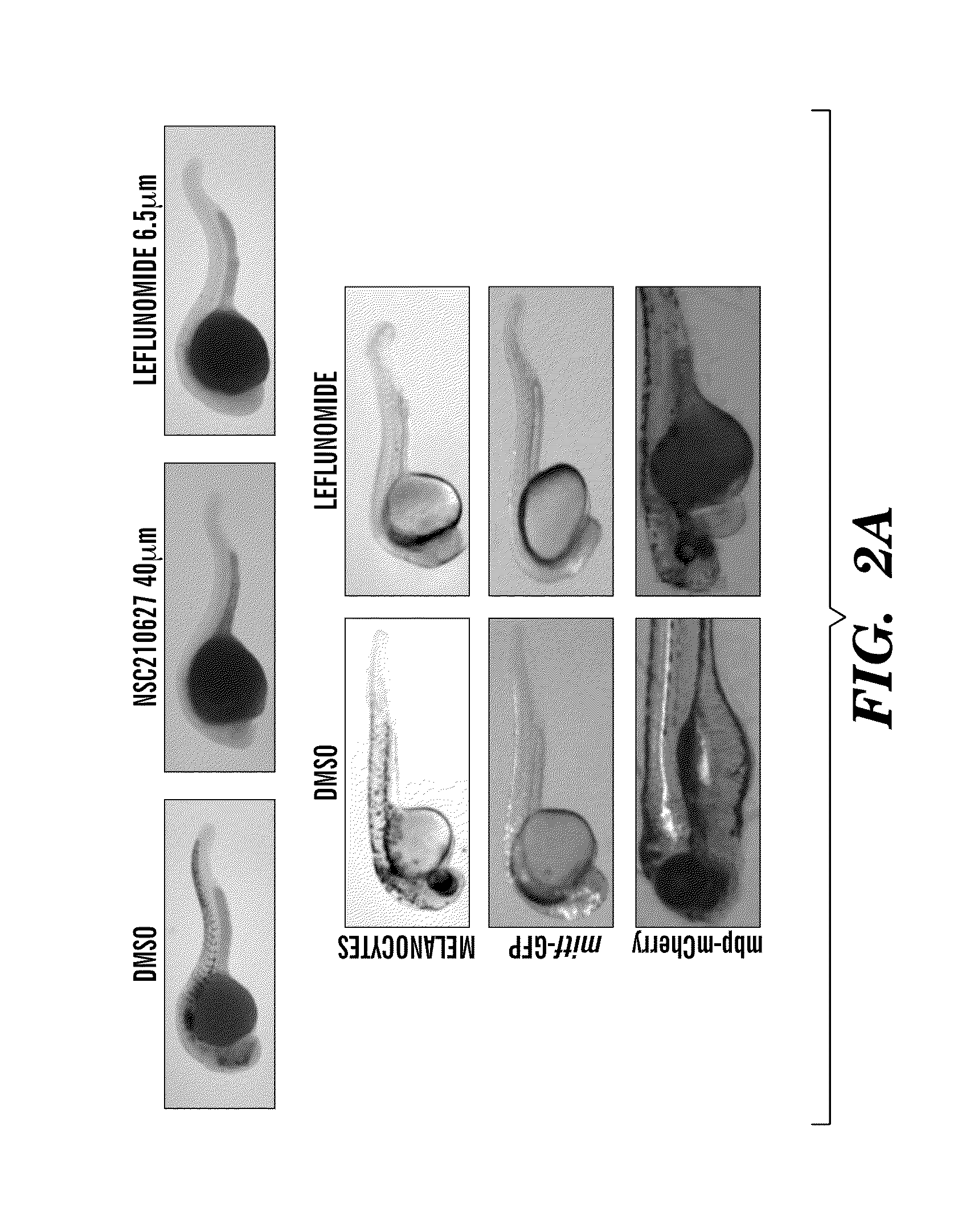
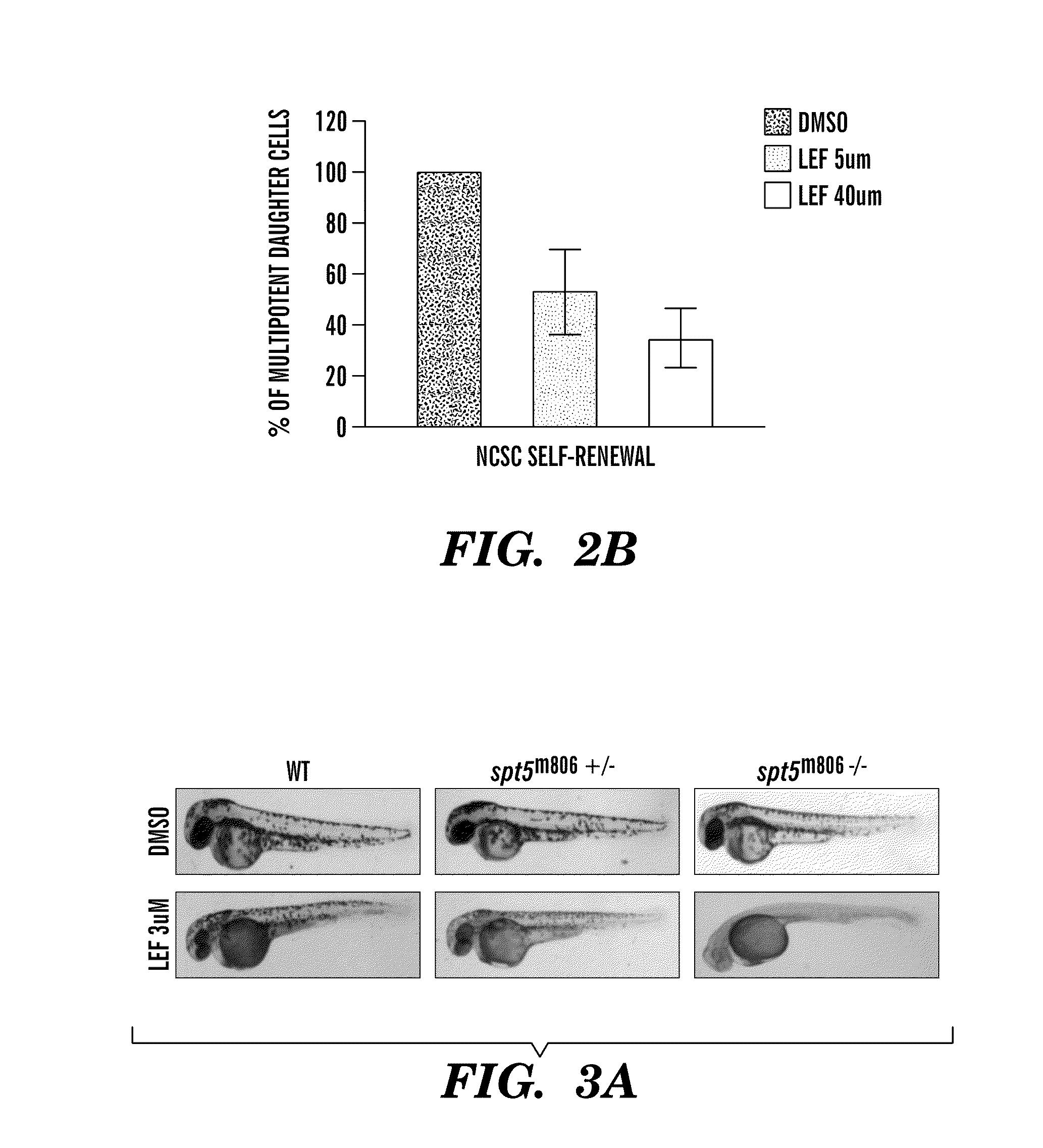
The present invention is directed to methods for treatment of melanoma using an inhibitor of dihydroorotate dehydrogenase (DHODH) and to combination therapies that involve administering to a subject an inhibitor of oncogenic BRAF (e.g. BRAF(V600E)), as well as an inhibitor of dihydroorotate dehydrogenase (DHODH). Assays for identifying compounds useful for the treatment of melanoma are also provided. The methods herein are directed to screening for compounds or agents that inhibit neural crest progenitor formation in a zebra fish model of melanoma.
Owner:DANA FARBER CANCER INST INC +1
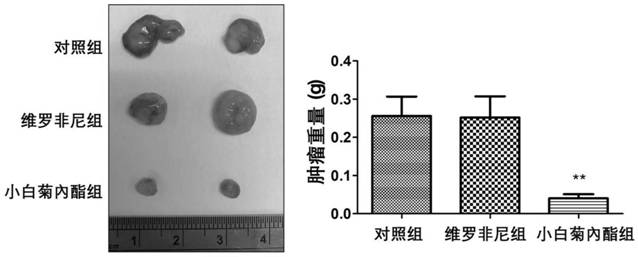
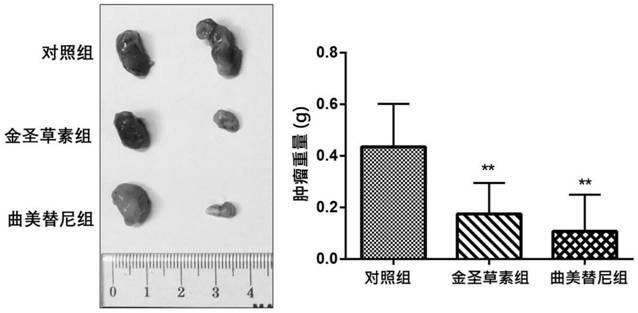
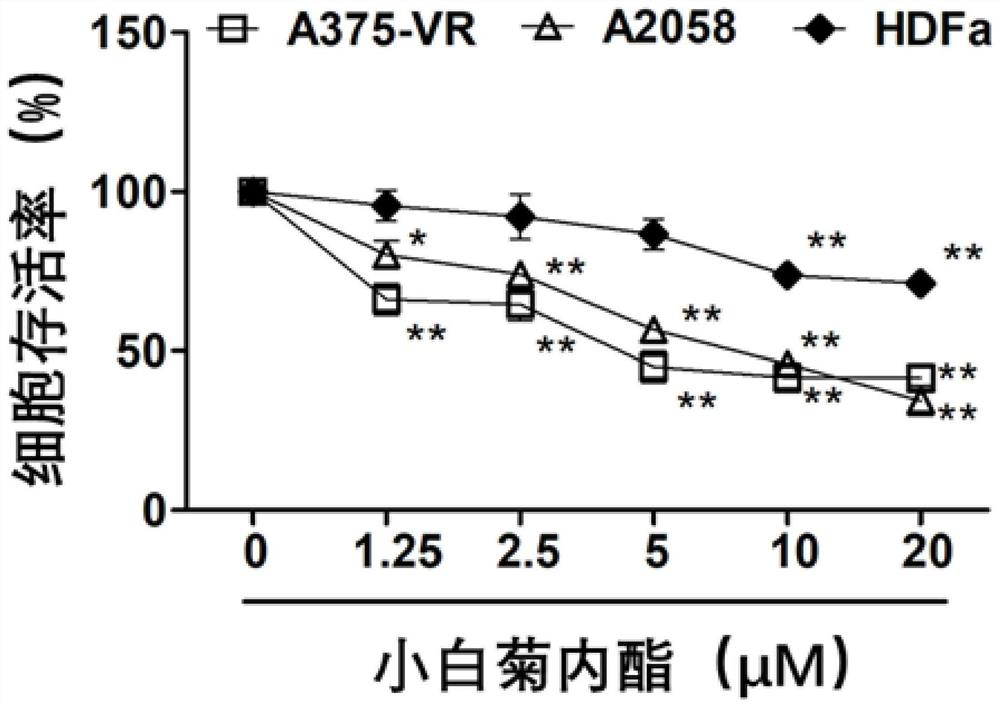
Novel application of parthenolide, luteolin, chrysoeriol and ginsenoside Rg3
InactiveCN111686104APrevent proliferationGrowth inhibitionOrganic active ingredientsAntineoplastic agentsChrysoeriolPharmaceutical drug
The invention provides novel application of parthenolide, luteolin, chrysoeriol and ginsenoside Rg3, and specifically relates to preparation of the compounds or derivatives of the compounds in preparation of drugs for preventing and / or treating drug-resistant BRAF (V600E) mutant-type tumors. It is found that proliferation of tumor cells resistant to BRAF (V600E)-targeted drugs can be inhibited through parthenolide, luteolin, chrysoeriol and ginsenoside Rg3, the tumor cells include inherent and acquired tolerant tumor cells, and growth of tumors is inhibited. It is shown through results that parthenolide, luteolin, chrysoeriol and ginsenoside Rg3 have the potential to be developed as the safe and effective drugs for treating the drug-resistant BRAF (V600E) mutant-type tumors.
Owner:香港浸会大学深圳研究院
Methods for treatment of melanoma
ActiveUS20150306080A1Utility in treatmentUseful in treatmentBiocidePeptide/protein ingredientsProgenitorNeural crest
Embodiments of the present invention are directed to methods for treatment of melanoma using an inhibitor of dihydroorotate dehydrogenase (DHODH) and to combination therapies that involve administering to a subject an inhibitor of oncogenic BRAF (e.g. BRAF(V600E)), as well as an inhibitor of dihydroorotate dehydrogenase (DHODH). Assays for identifying compounds useful for the treatment of melanoma are also provided. The methods comprise screening for compounds or agents that inhibit neural crest progenitor formation in a zebra fish model of melanoma.
Owner:DANA FARBER CANCER INST INC +1
Optimization method of BRAF gene V600E mutation digital PCR detection system and detection product
PendingCN109295226APlay a decisive roleThe result is accurateMicrobiological testing/measurementBraf genesEnzyme digestion
The invention relates to an optimization method of a BRAF gene V600E mutation digital PCR detection system, wherein the detection system includes upstream primers, downstream primers, wild-type probes, mutant-type probes, a wild-type template and a mutant-type template; the wild-type template is normal human gDNA after enzyme digestion, and the mutant-type template is a mutant plasmid inserted with a BRAF gene V600E mutant fragment after enzyme digestion; a standard substance is prepared from the mutant-type template and the wild-type template according to a certain proportion of copy number;reaction is performed with a medium mutation frequency standard substance as a template by digital PCR, a data statistical graph is prepared according to the reaction data, and a wild-type fluorescentregion and a mutant-type fluorescent region are selected. Reaction is performed with the wild-type template as the template by digital PCR, and the background threshold value is determined. A detection product optimized by the optimization method of the BRAF gene V600E mutation digital PCR detection system has higher accuracy degree.
Owner:上海赛安生物医药科技股份有限公司
Kit for detecting mutation of V600E locus of BRAF (v-taf mourine sarcoma viral oncogene homolog B1) gene
PendingCN111304329AAchieving High Sensitivity DetectionVarious sources of samplesMicrobiological testing/measurementDNA/RNA fragmentationViral OncogeneNucleotide
Owner:宁波胤瑞生物医学仪器有限责任公司
PCR (Polymerase Chain Reaction) detection method for mutation of human BRAF (v-raf Murine Sacoma Viral Oncogene Homolog) gene V600E, primer, probe and kit
InactiveCN107400716AAchieving Specific DetectionEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationViral OncogeneBraf genes
Owner:SHANGHAI BIOCHIP
B-raf gene V600E mutation detection method
InactiveCN104611406AHigh sensitivityHigh precisionMicrobiological testing/measurementForward primerNucleotide
The present invention relates to a B-raf gene V600E mutation detection method, and belongs to the field of life science and technology, wherein the method can be used for detecting whether a particular nucleotide variation exists in the B-raf gene in the DNA sample isolated from the patient body in vivo. The method comprises a polymerase chain reaction, wherein the reaction system contains a specific forward primer, a specific downstream primer, and a set of probes treated according to a specific method, a single-stranded nucleotide hybridization and melting process is performed under specific conditions, and a fluorescence quantitative PCR instrument is utilized to collect the fluorescence signal during the melting so as to determine the presence or absence of the variation.
Owner:JIANGSU MOLE BIOSCI
Methods for treatment of melanoma
InactiveUS20150328204A1Reduce the overall heightBiocidePeptide/protein ingredientsProgenitorMedicine
Embodiments of the present invention are directed to methods for treatment of melanoma using an inhibitor of dihydroorotate dehydrogenase (DHODH) and to combination therapies that involve administering to a subject an inhibitor of oncogenic BRAF (e.g. BRAF(V600E)), as well as an inhibitor of dihydroorotate dehydrogenase (DHODH). Assays for identifying compounds useful for the treatment of melanoma are also provided. The methods comprise screening for compounds or agents that inhibit neural crest progenitor formation in a zebra fish model of melanoma.
Owner:DANA FARBER CANCER INST INC +1
Primer for detecting BRAF gene V600E mutation of human colorectal cancer, detection method and kit thereof
The invention provides a primer for detecting BRAF gene V600E mutation of human colorectal cancer, a detection method and a kit thereof. The detection primer provided by the invention can bind to a specific sequence of the BRAF gene, and amplifies a mutant sequence. The detection method adopts a detection fluorescent signal group to bind to an amplified fragment to emit a fluorescent signal, and uses a blocking agent specifically binding to the wild type sequence corresponding to the mutation site to inhibit wild type non-specific amplification. The method adopts a competitive allele specificPCR amplification technology to establish a real-time fluorescent PCR kit, which can accurately detect the BRAF gene V600E mutation. The method of the invention has simple operation, easy result reading, and high sensitivity, can detect a sample containing 0.001% BRAF gene V600E mutation, and realizes ultrasensitive detection of the BRAF gene V600E mutation.
Owner:广州健天医药科技有限公司
Primer pair and probe combined product, composition, and kit used for detecting BRAF mutation, and applications thereof
InactiveCN108374009AStrong specificityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationForward primerBraf genes
The invention belongs to the field of molecular biology, and more specifically relates to a primer pair and probe combined product, a composition, and a kit used for detecting BRAF mutation, and applications thereof. The combined product comprises a LNA fluorescence probe and primers used for detecting BRAF gene V600E mutation; wherein the forward primer sequence of the primer pair comprises at least one locked nucleic acid, and / or, the LNA fluorescence probe sequence comprises at least one locked nucleic acid. The primer pair and the probe composition is rapid and convenient in detection, ishigh in specificity, sensitivity, reliability, detection efficiency, and accuracy, low in detection cost, short in detection time, and low in false positive rate. The kit can be used for detecting BRAF mutation, is capable of providing guidance for clinical diagnosis and treatment of BRAF mutation cancer, and is promising in application prospect.
Owner:求臻医学科技(浙江)有限公司
Detection kit and detection method for human BRAF gene V600E mutation
InactiveCN110964818ARelieve painHigh detection specificityMicrobiological testing/measurementFluoProbesBraf genes
The invention provides a detection kit for human BRAF gene V600E mutation. The detection kit includes primers and a probe for detecting BRAF gene V600E mutation, an inhibitor, internal control primersand a probe, external control primers and a probe, and is characterized in that the detection primers are SEQ ID NO.1 and SEQ ID NO.2, the detection probe is SEQ ID NO.3; the inhibitor is SEQ ID NO.4; the internal control primers are SEQ ID NO. 5 and SEQ ID NO. 6, and the internal control fluorescent probe is SEQ ID NO. 7; and the external control primers are SEQ ID NO. 8 and SEQ ID NO. 9, and the external control fluorescent probe is SEQ ID NO. 10. According to the invention, the peripheral blood of patients is used to efficiently and noninvasively detect the mutation of BRAF gene in ctDNA.The detection kit has advantages of high detection sensitivity, short cycle, low cost, simple interpretation, and has great clinical promotion and application value.
Owner:重庆浦洛通基因医学研究院有限公司
Methods for treatment of melanoma
ActiveUS10016402B2Reduce the overall heightHeterocyclic compound active ingredientsAssayNeural crest
Owner:DANA FARBER CANCER INST INC +1
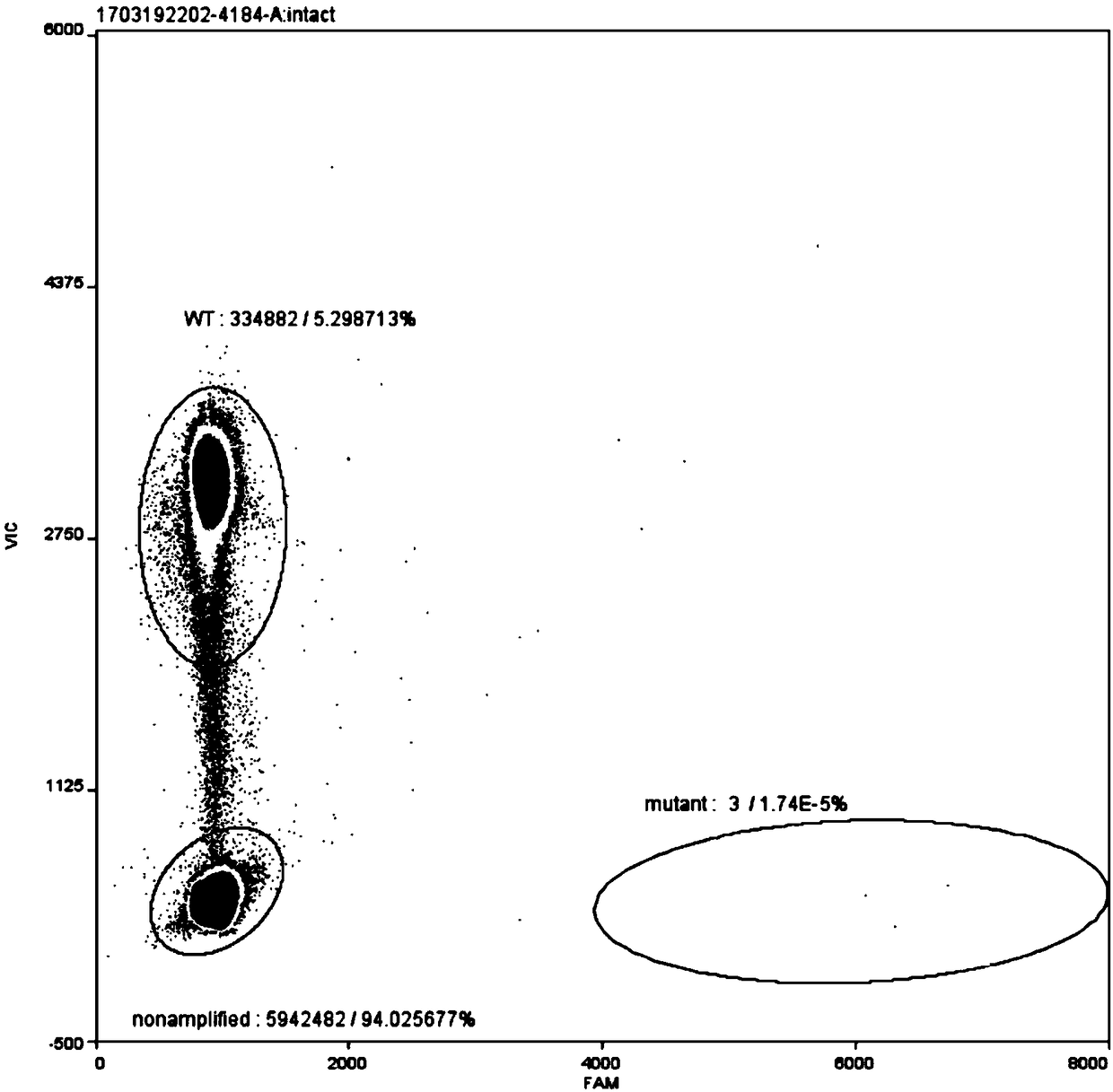
Method for improving diagnosis efficiency of BRAF gene V600E mutation
The invention relates to a method for improving the diagnosis efficiency of BRAF gene V600E mutation. The inventor optimizes a detection reagent for detecting the BRAF gene V600E-site mutation, and onthis basis, a kit for detecting the BRAF gene V600E-site mutation, application and a detection method are provided.
Owner:GENOSABER BIOTECH CO LTD +1
Detecting system and kit for detecting BRAF gene mutation
PendingCN108823311ATo achieve the purpose of specific bindingReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationBRAF Gene MutationNucleotide
The invention discloses a detecting system and a kit for detecting BRAF gene mutation. The kit comprises digital PCR pre-mixed liquor and primer probe mixed liquor, wherein the primer probe mixed liquor comprises an upstream primer for detecting a site V600E of a 600rd codon, an downstream primer and a peptide nucleic acid probe; a nucleotide sequence of the upstream primer is as shown in SEQ ID No.1; a nucleotide sequence of the downstream primer is as shown in SEQ ID No.2; the nucleotide sequence of the peptide nucleic acid probe and the site V600E of the 600rd codon of the BRAF mutant geneare completely complementary, and the nucleotide sequence is as shown in SEQ ID No.3; the end 5 ' of the peptide nucleic acid probe is connected with a strong chelating group which consists of four amino acids. The detecting system and the kit for detecting BRAF gene mutation are high in sensitivity and specificity, are smaller in quantity demanded of samples, and can quickly, conveniently and accurately perform PCR quantitative detection.
Owner:上海赛安生物医药科技股份有限公司
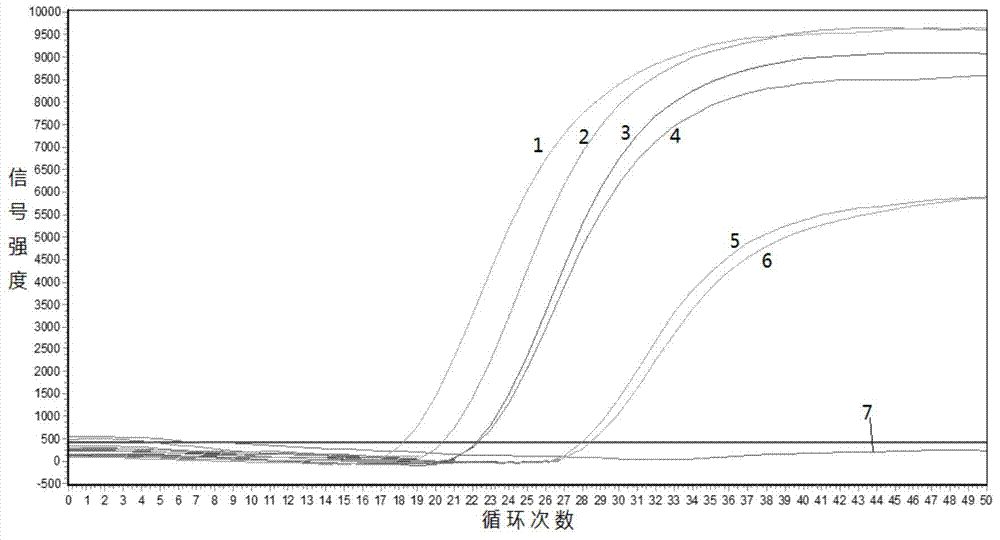
Fluorescence quantitative detection primers and probe for BRAF gene V600E mutation
InactiveCN107090512AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBraf genesAgricultural science
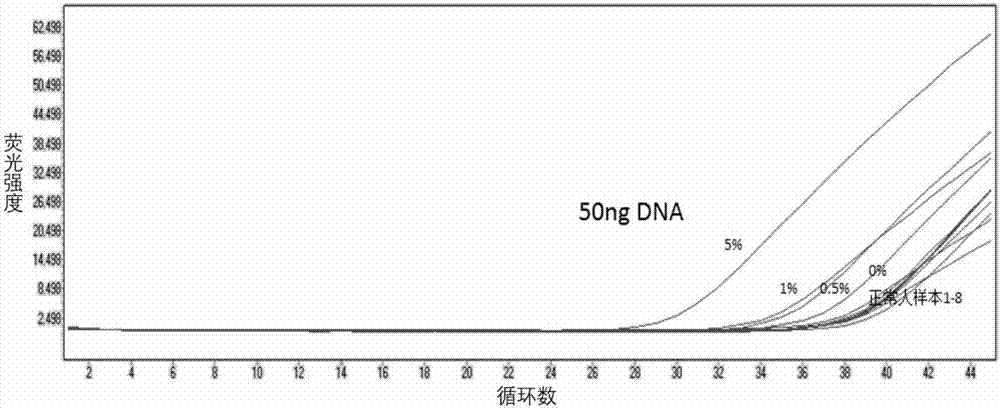
The invention discloses fluorescence quantitative detection primers and a probe for BRAF gene V600E mutation. The fluorescence quantitative detection primers for BRAF gene V600E mutation are primer 1 and primer 2, the primer 1 is a single stranded DNA shown in SEQ ID NO. 1 in a sequence listing, and the primer 2 is a single stranded DNA shown in SEQ ID NO. 2 in the sequence listing; the probe is the single stranded DNA probe shown in SEQ ID NO. 3 in the sequence listing. For tissue sample DNA and FFPE paraffin sections, the fluorescence quantitative detection primers and the probe for BRAF gene V600E mutation can perform detection with a minimum 50 ng DNA, a minimum mutation rate of 0.5% and a final fluorescence curve Ct value being less than or equal to 36, indicating that the primer pair and the probe provided by the invention have good sensitivity. The fluorescence quantitative detection primers and the probe for BRAF gene V600E mutation are significant for screening BRAF gene V600E mutant-associated cancers.
Owner:MYGENOSTICS (CHONGQING) GENE TECH CO LTD
Method for detection of BRAF and PI 3K mutations
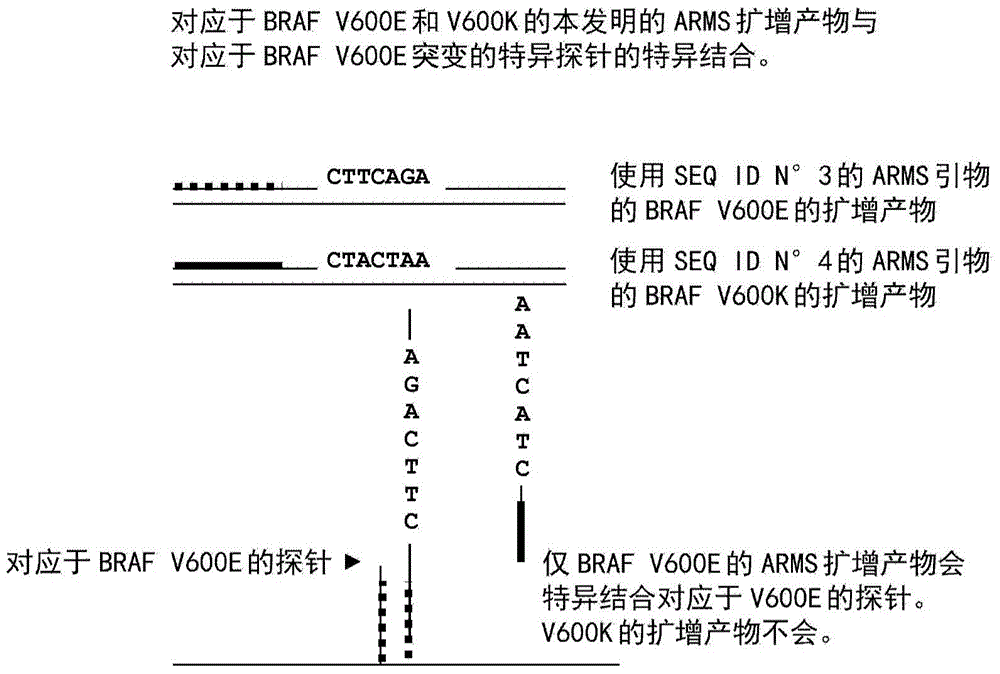
The present invention is based on a detection method of the BRAF mutations V600E and V600K, and of the PI3K mutations E542K, E545D, E545K and H1047R, in a sample susceptible of containing one or more of such mutations, based on amplification of the sample with the primers of the present invention. Further, the present invention relates to (i) a kit which comprises, amongst its components, amplification reagents including one or more of the primers of the present invention; (ii) the primers themselves; and (iii) use of the method, kit and primers of above, for the diagnosis / prognosis of a pathological condition in a patient, particularly, of cancer.
Owner:GENOMICA SAU
Biomarker for thyroid nodule benign and malignant discrimination, multi-gene joint detection kit and application
PendingCN114807350AHigh sensitivitySimple and fast operationMicrobiological testing/measurementDNA/RNA fragmentationBase JBiomarker (medicine)
The invention belongs to the field of biological medicine, and discloses a biomarker for judging benign and malignant thyroid nodules, a multi-gene joint detection kit and application. The biomarker group comprises at least one of eight DNA single base mutations and four gene fusions; the eight kinds of DNA single base mutations are selected from a BRAF gene V600E, a TERT gene C228T / C250T, a KRAS gene G12C / G12V / Q61R, an NRAS gene Q61R and an HRAS gene Q61R, and the four kinds of gene fusion are selected from CCDC6-RET (Exon1-Exon12), NCOA4-RET (Exon8-Exon12), PAX8-PPARG (Exon10-Exon2) and ETV6-NTRK3 (Exon4-Exon14). The method and the kit are high in detection sensitivity, high in specificity and wide in application scene.
Owner:上海睿璟生物科技有限公司
A kit for detecting b-raf gene mutation
ActiveCN104099425BGuaranteed accuracyAvoid duplication of experimentsMicrobiological testing/measurementForward primerB-RAF Gene Mutation
The invention relates to a B-raf gene mutation detection kit. The B-raf gene mutation detection kit comprises quality-control primer probe internal-standard mixed liquor and detection primer probe internal-standard mixed liquor, wherein the quality-control primer probe internal-standard mixed liquor comprises a quality-control primer pair, a B-raf gene specific probe, an internal-standard primer pair, an internal-standard specific probe and an internal-standard template, and the detection primer probe internal-standard mixed liquor comprises a B-raf gene V600E mutation detection specific primer pair, a B-raf gene specific probe, an amplification blocking nucleic acid sequence, an internal-standard primer pair, an internal-standard specific probe and an internal-standard template. The B-raf gene mutation detection kit has the advantages that an amplification refractory mutation system is combined with a wild type amplification blocking nucleic acid sequence with a phosphorylated terminal, deoxyinosine is introduced into detection of a B-raf gene V600E mutation detection specific ARMS (amplification refractory mutation system) forward primer to enable quality-control PCR (polymerase chain reaction) and detection PCR to be performed for detection of samples parallelly, and each reaction system can have an internal-standard system capable of effectively avoiding false negative and intra-assay variation; the B-raf gene mutation detection kit is low in cost, high in sensitivity and more capable of controlling intra-assay and inter-assay variation.
Owner:山东国九堂制药集团股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com