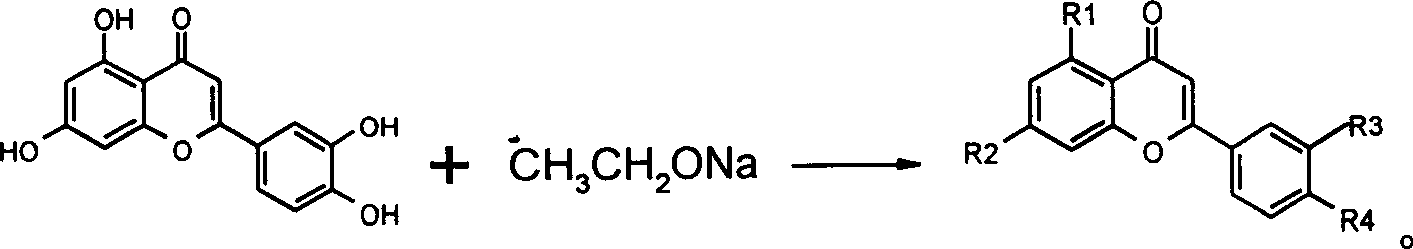Patents
Literature
518 results about "Luteolin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
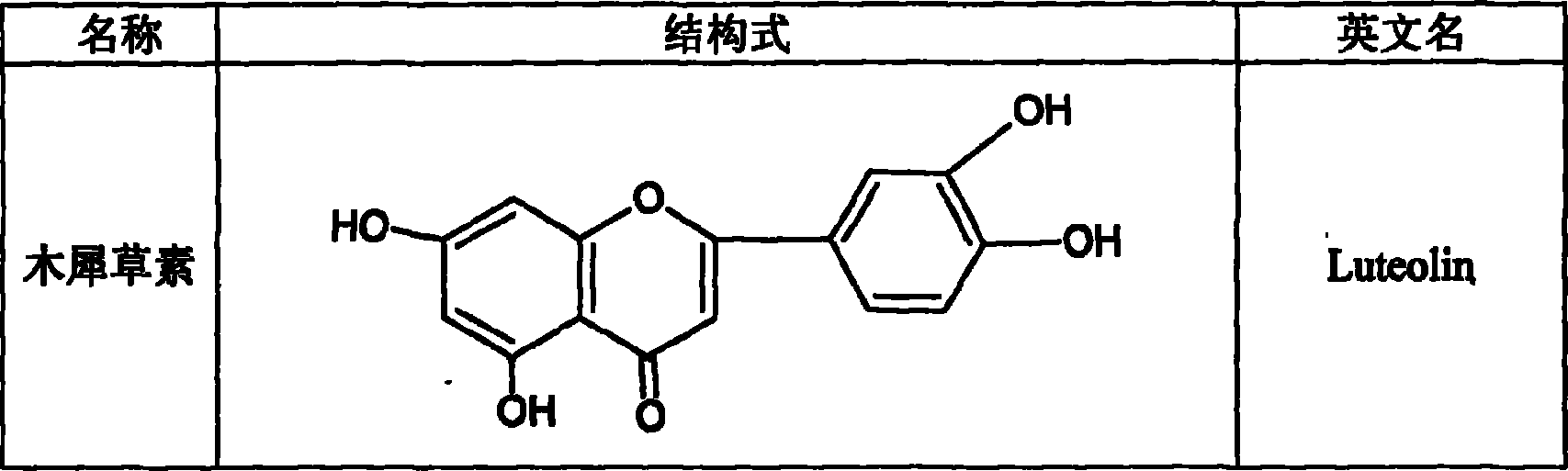
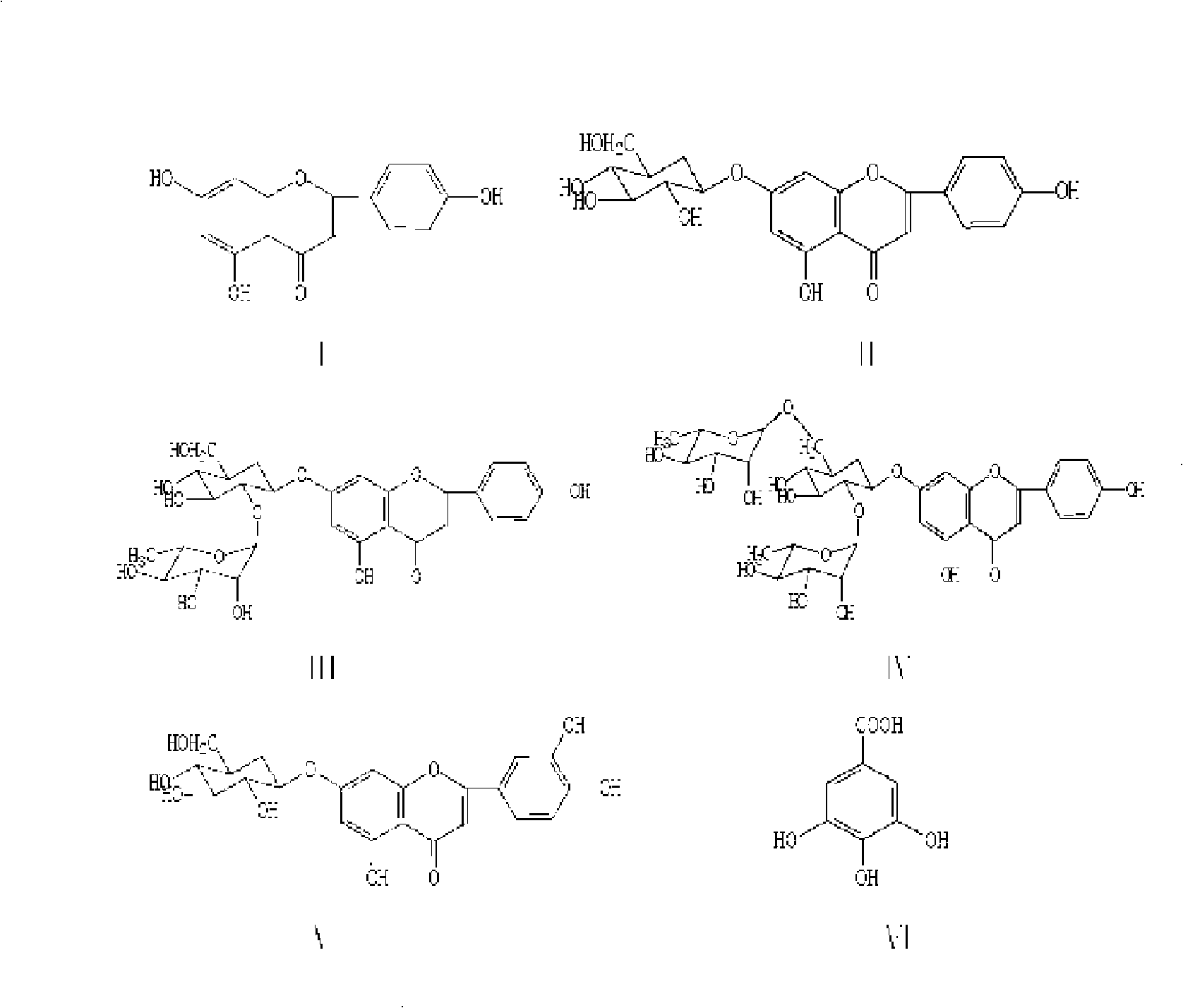
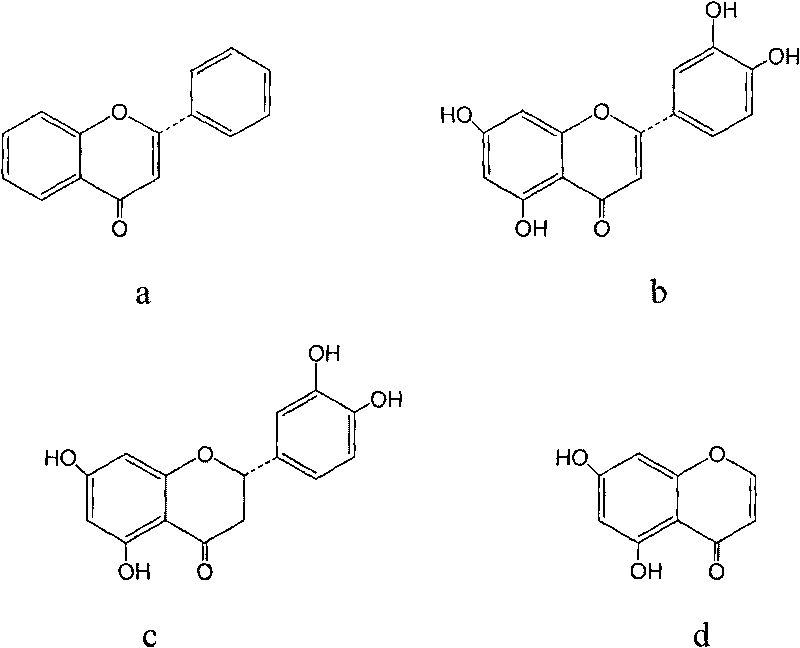
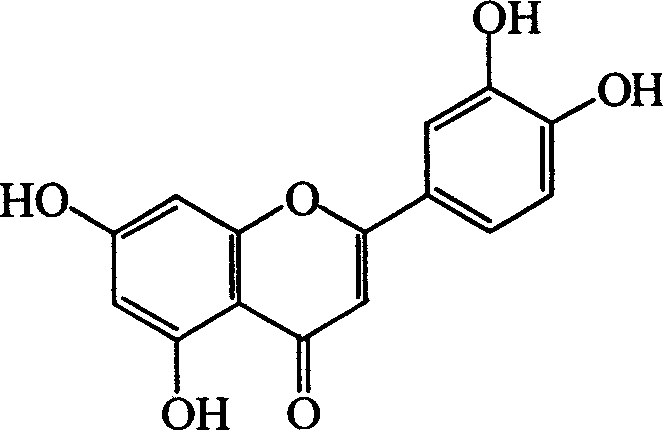
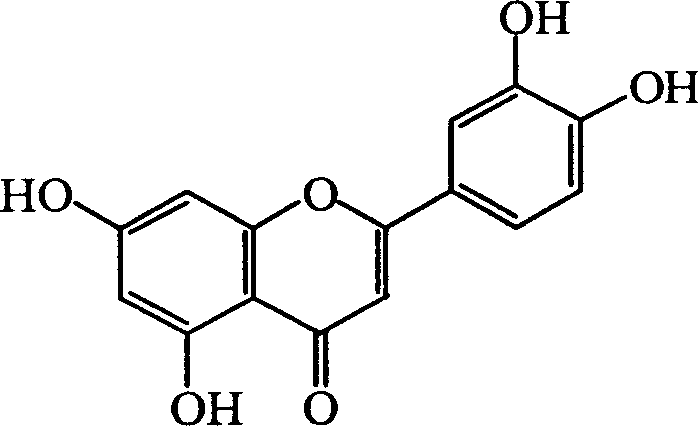
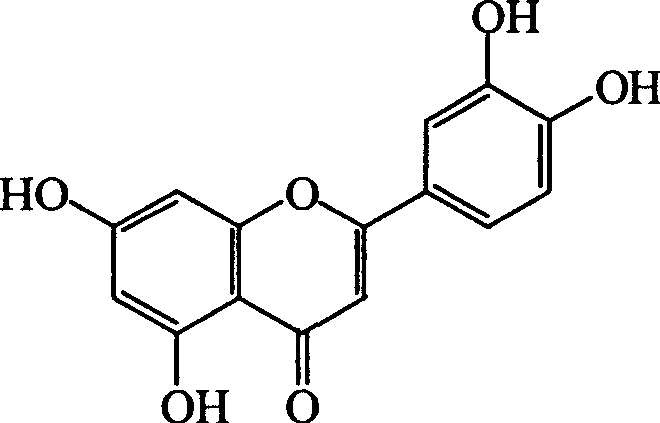
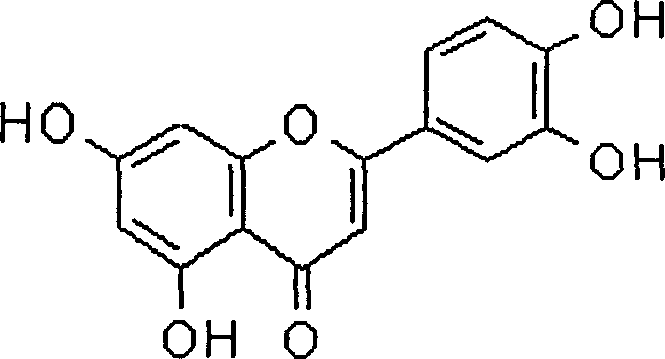
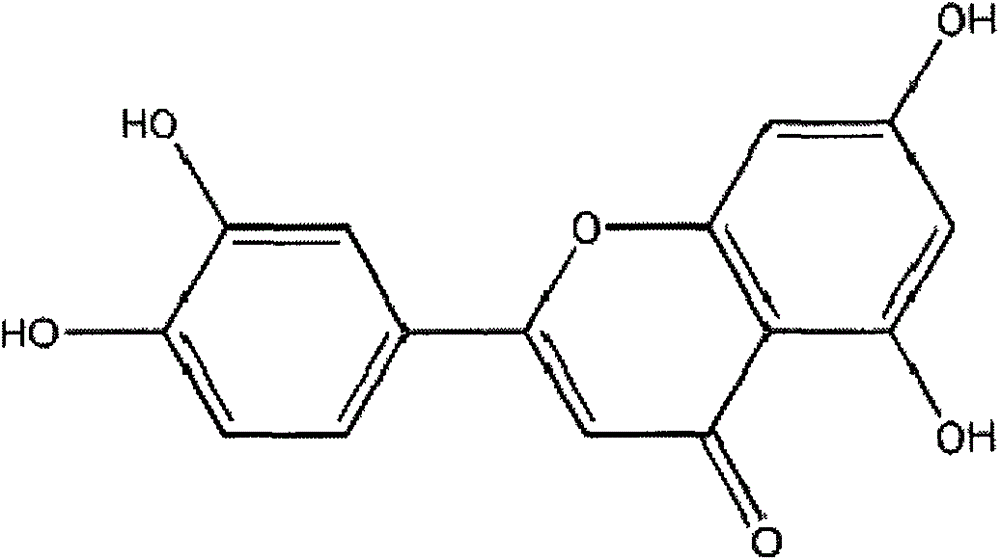
Luteolin is a flavone, a type of flavonoid, with a yellow crystalline appearance. Luteolin is the principal yellow dye compound that is obtained from the plant Reseda luteola, which has been used as a source of the dye since at least the first millenium B.C. Luteolin was first isolated in pure form, and named, in 1829 by the French chemist Michel Eugène Chevreul. Luteolin's empirical formula was determined by the Austrian chemists Heinrich Hlasiwetz and Leopold Pfaundler in 1864. In 1896, the English chemist Arthur George Perkin proposed the correct structure for luteolin. Perkin's proposed structure for luteolin was confirmed in 1900 when the Polish-Swiss chemist Stanislaw Kostanecki (1860–1910) and his students A. Różycki and J. Tambor synthesized luteolin.
Methods and compositions for modulating hair growth or regrowth
InactiveUS20070036742A1Inhibit synthesisIncreased vascularizationBiocideCosmetic preparationsAstaxanthinRed Clover
The present invention relates to compositions and methods for modulating hair growth or regrowth. The compositions of the present invention comprise extracts of one or more of the following: Boswellia serrata, Undaria pinnatifida, green tea (e.g., Camellia sinensis), shiso, Pureraria mirifica, luteolin (e.g. Perilla ocymoides leaf extract), astilbin, vitamin E, amentoflavone, tetrahydropiperine, licochalcone, astaxanthin, red clover, Brassica juncea, unfermented green rooibos, enzyme CoQ10, salvia, ximenynic acid, hops oleoresin, apple, soy, saw palmetto, or ellagic extract, or any derivative thereof. In particular, the compositions and methods of the present invention can be used to stimulate or increase hair growth and / or prevent or slow the loss of hair by having one or more of the following functions: (a) inhibiting synthesis of DHT; (b) inhibiting proteasomal activity; (c) inhibiting IL-1 activity; (d) increasing vascularization; (e) increasing expression of vascular endothelial growth factor; (f) increasing expression of keratinocyte growth factor; (g) inhibiting inflammation; or (h) acting as an antibacterial.
Owner:ACCESS BUSINESS GRP INT LLC
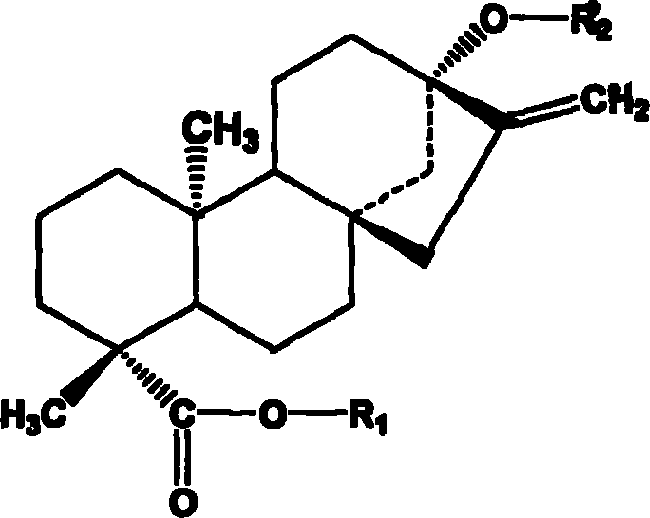
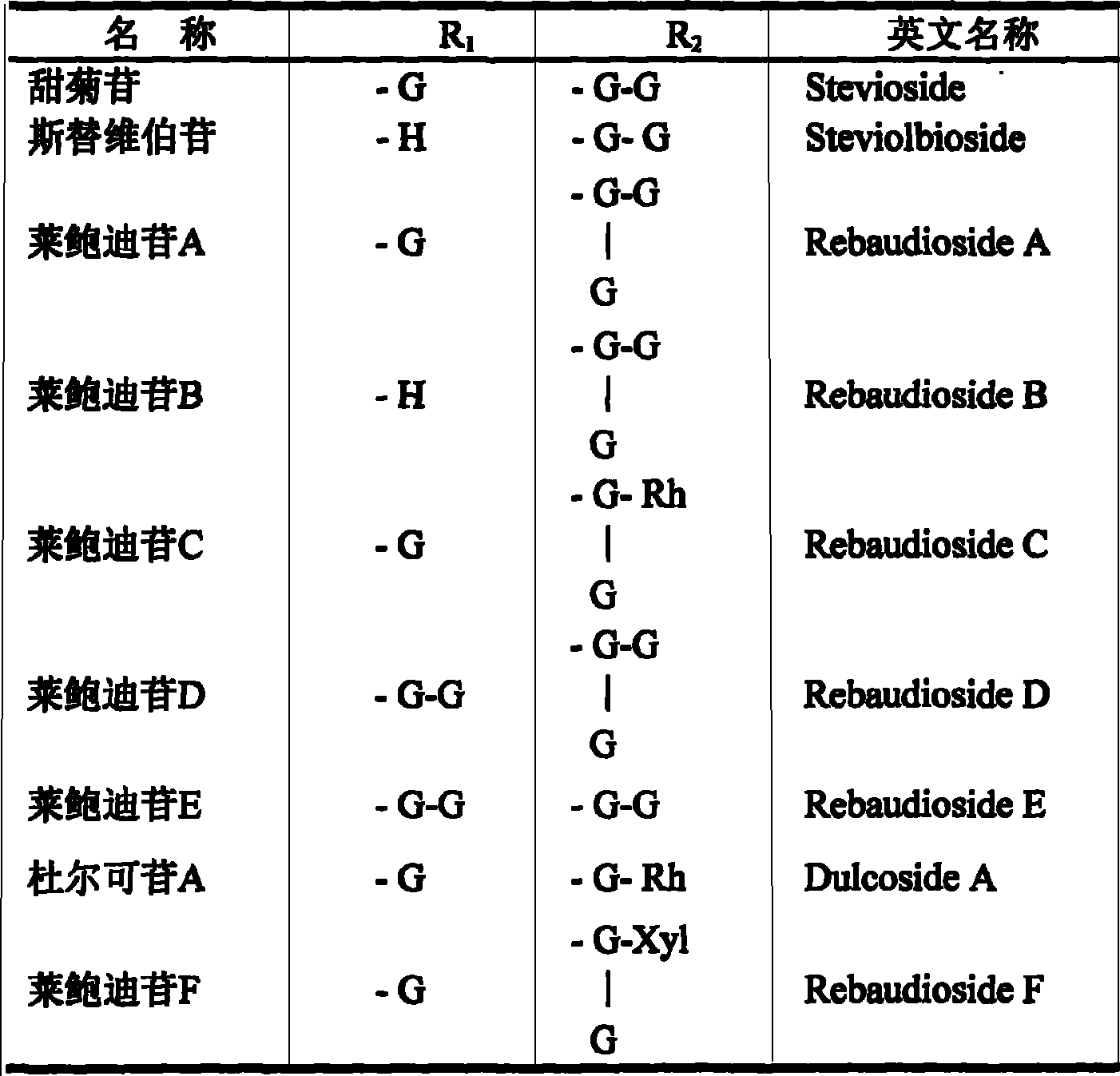
Method for preparing stevia whole stevioside and stevia whole flavone at the same time
ActiveCN101062077AImprove mildew resistanceConducive to food preservationSugar derivativesMetabolism disorderLuteolinGlucoside
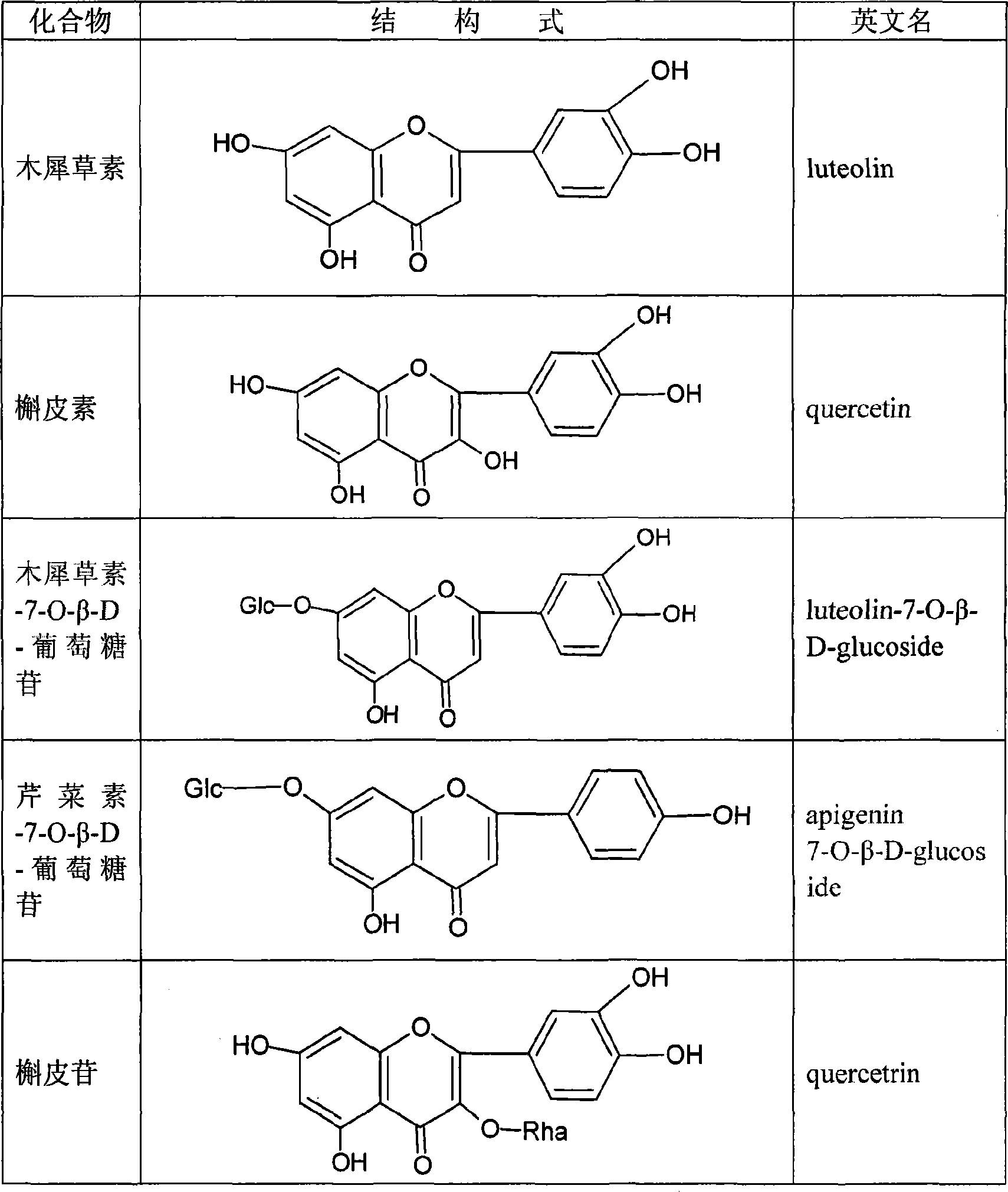
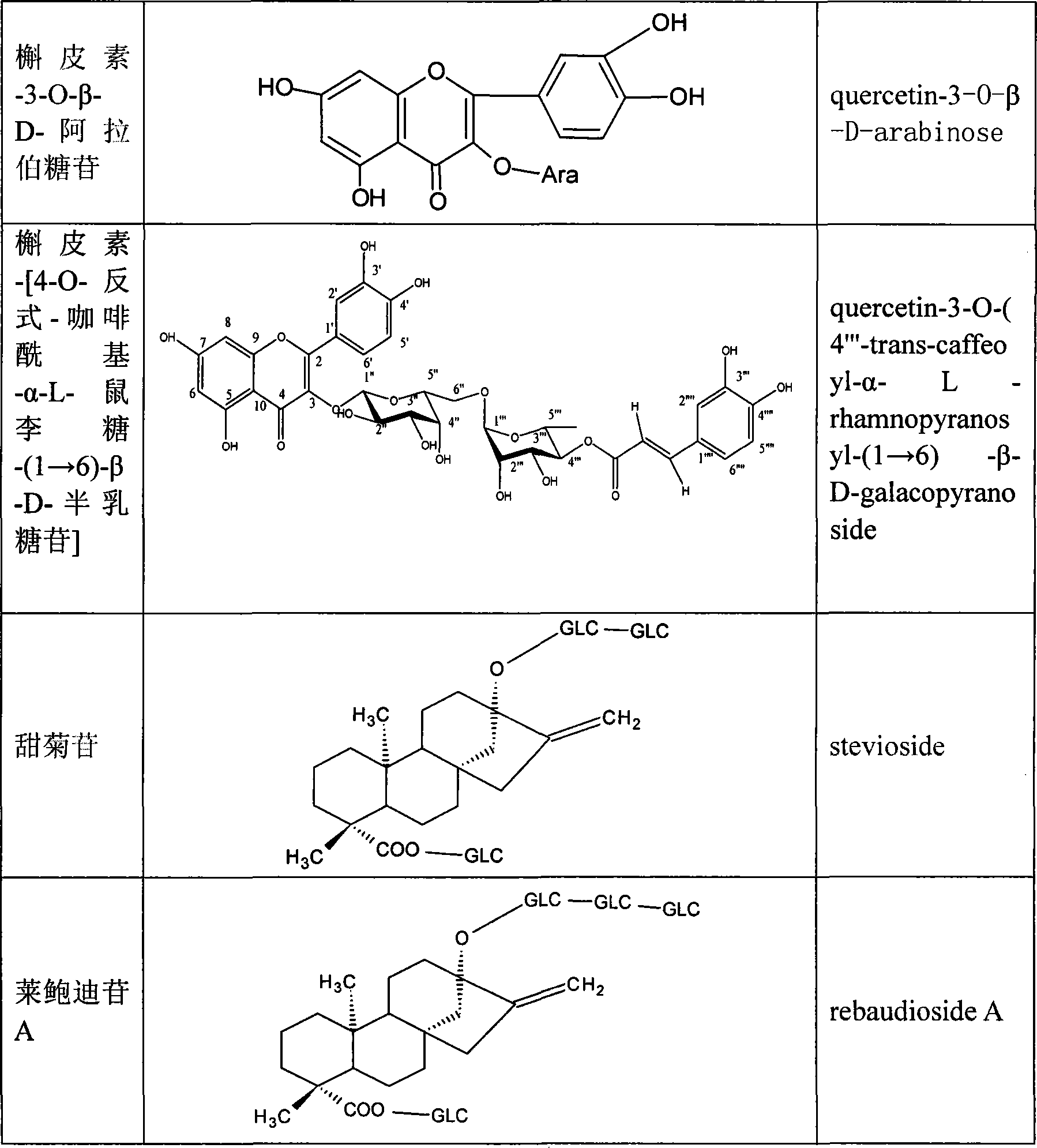
The invention discloses a preparing method of total sweet chrysanthemum glycosides and total chromocor in sweet leaf chrysanthemum, which is characterized by the following: comprising sweet chrysanthemum glycosides, stevi primary glycosides, labroid glycosides A, B, C, D, E, F, duacl glycosides A and so on in total sweet chrysanthemum glycosides; comprising cyanidenon, meletin, cyanidenon-7-0-beta-D glycosides, celery element-7-0-beta-D-glycosides, quercetin, meletin-3-0-beta-D-arabinoside, meletin-3-0-[4-0-trans-coffe acyl-alpha-L-isodulcitol-(1-6)-beta-D-arabinoside] and so on; choosing one or several methods from solvent extraction, solvent extraction process, macroreticular absorption resin method, column chromatography, supercritical fluid chromatography, liquid-liquid counter-current partition chromatography and so on; extracting the total chromocor; setting content of sweet chrysanthemum glycosides element in total sweet chrysanthemum glycosides at 5-100%; counting 5-100% of all sweet chrysanthemum glycosides content with sweet chrysanthemum glycosides and labroid glycosides; counting 5-100% of chromocor element in sweet leaf chrysanthemum total chromocor; counting 5-100% of all total chromocor content with cyanidenon-7-0-beta-D glycosides, quercetin and meletin-3-0-[4-0-trans-coffe acyl-alpha-L-isodulcitol-(1-6)-beta-D-arabinoside].
Owner:石任兵 +1
Extract of stevia whole stevioside and stevia whole flavone and the preparing method thereof
The invention discloses a preparing method of sweet chrysanthemum glycosides and chromocor extract from sweet leaf chrysanthemum, which is characterized by the following: comprising sweet chrysanthemum glycosides, labroid glycosides A, cyanidenon, meletin, cyanidenon-7-0-beta-D glycosides, celery element-7-0-beta-D-glycosides, quercetin, meletin-3-0-beta-D-arabinoside, meletin-3-0-[4-0-trans-coffe acyl-alpha-L-isodulcitol-(1-6)-beta-D-arabinoside], derivant and so on; choosing one or several methods from solvent extraction, solvent extraction process, macroreticular absorption resin method, column chromatography, supercritical fluid chromatography, liquid-liquid counter-current partition chromatography and so on; producing extract; counting 5-100%(w / w) of each sweet chrysanthemum glycosides element and chromocor element content in sweet chrysanthemum glycosides and chromocor extract; setting the chromocor element sum in sweet chrysanthemum glycosides, labroid glycosides A, cyanidenon-7-0-beta-D glycosides, quercetin, meletin-3-0-[4-0-trans-coffe acyl-alpha-L-isodulcitol-(1-6)-beta-D-arabinoside] at 5-100%(w / w).
Owner:石任兵 +1
Dracocephalum moldavica extract and dracocephalum moldavica dropping pills, and method of preparing the same
InactiveCN101219161AEasy to operateEasy to usePill deliveryCardiovascular disorderClinical efficacyDracocephalum moldavica
The invention relates to a moldavica dragonhead extract, a moldavica dragonhead dropping pill and a production method thereof, wherein, the moldavica dragonhead extract contains higher total flavonoids and luteolin and the production method of the moldavica dragonhead extract is simply operated. Pharmacodynamics test result indicates that the moldavica dragonhead dropping pill acquired from the invention has excellent curative effect to rat ischemia myocardial injury; each dose group of the moldavica dragonhead dropping pill has varying degrees of protection; Composite salviae dropping pill has similar effect with the middle-dose group of moldavica dragonhead dropping pill; all dose groups of the moldavica dragonhead dropping pill have better curative effect than Yixing Badiranjibuya Keli at ischemia myocardial; dosages of middle-dose group of moldavica dragonhead dropping pill and low-dose group of moldavica dragonhead dropping pill are only 50 percent and 25 percent of the dosage of Yixing Badiranjibuya Keli respectively, thereby greatly improving the compliance of sufferers. The moldavica dragonhead dropping pill acquired from the invention is a novel preparation of convenient use and good compliance, thus bringing into play better clinical curative effect of the moldavica dragonhead, an age-old Uighur medicinal material. The production method of the moldavica dragonhead dropping pill can be carried out easily.
Owner:XINJIANG INST OF MATERIA MEDICA
Compositions and methods for treatment of diabetes
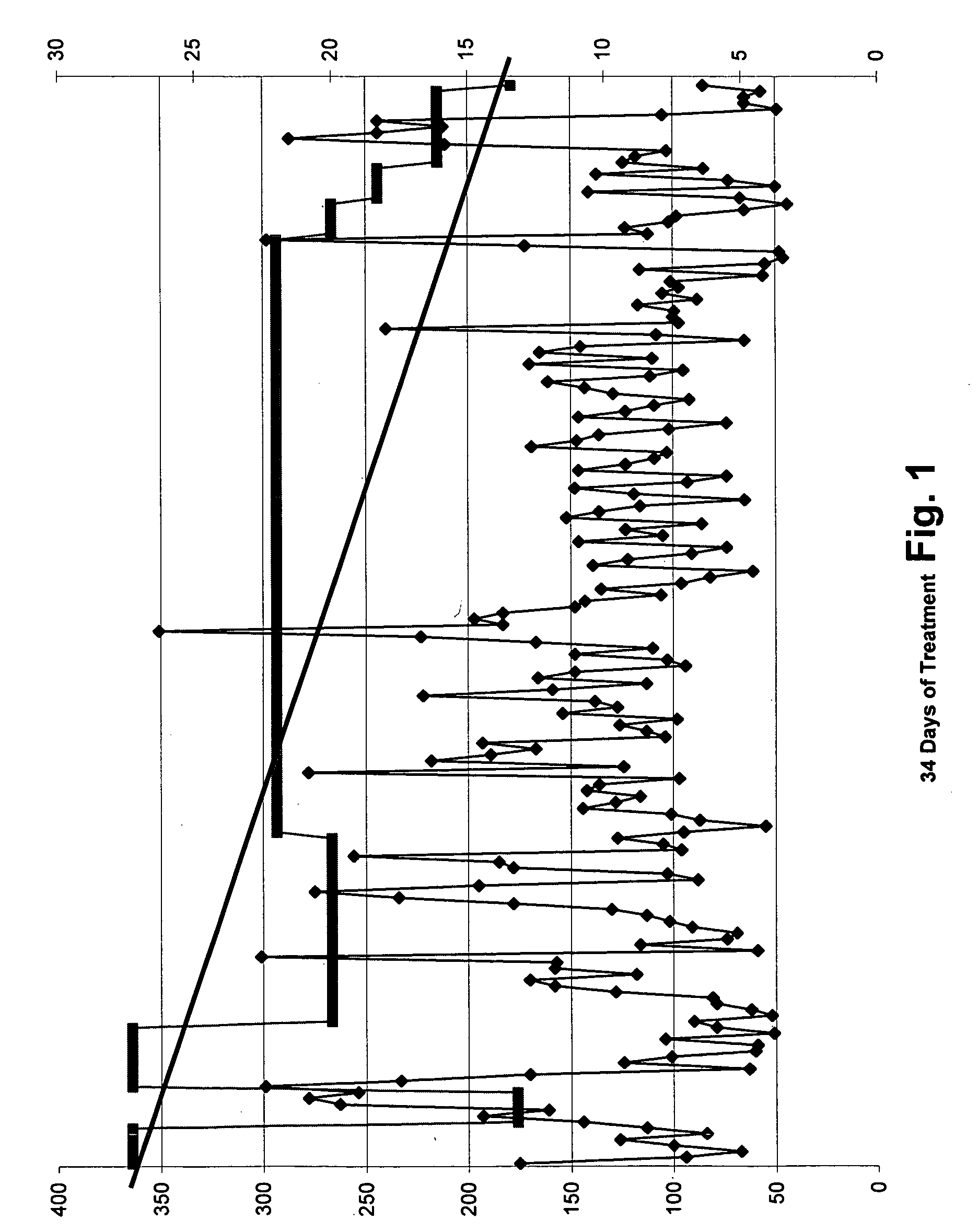
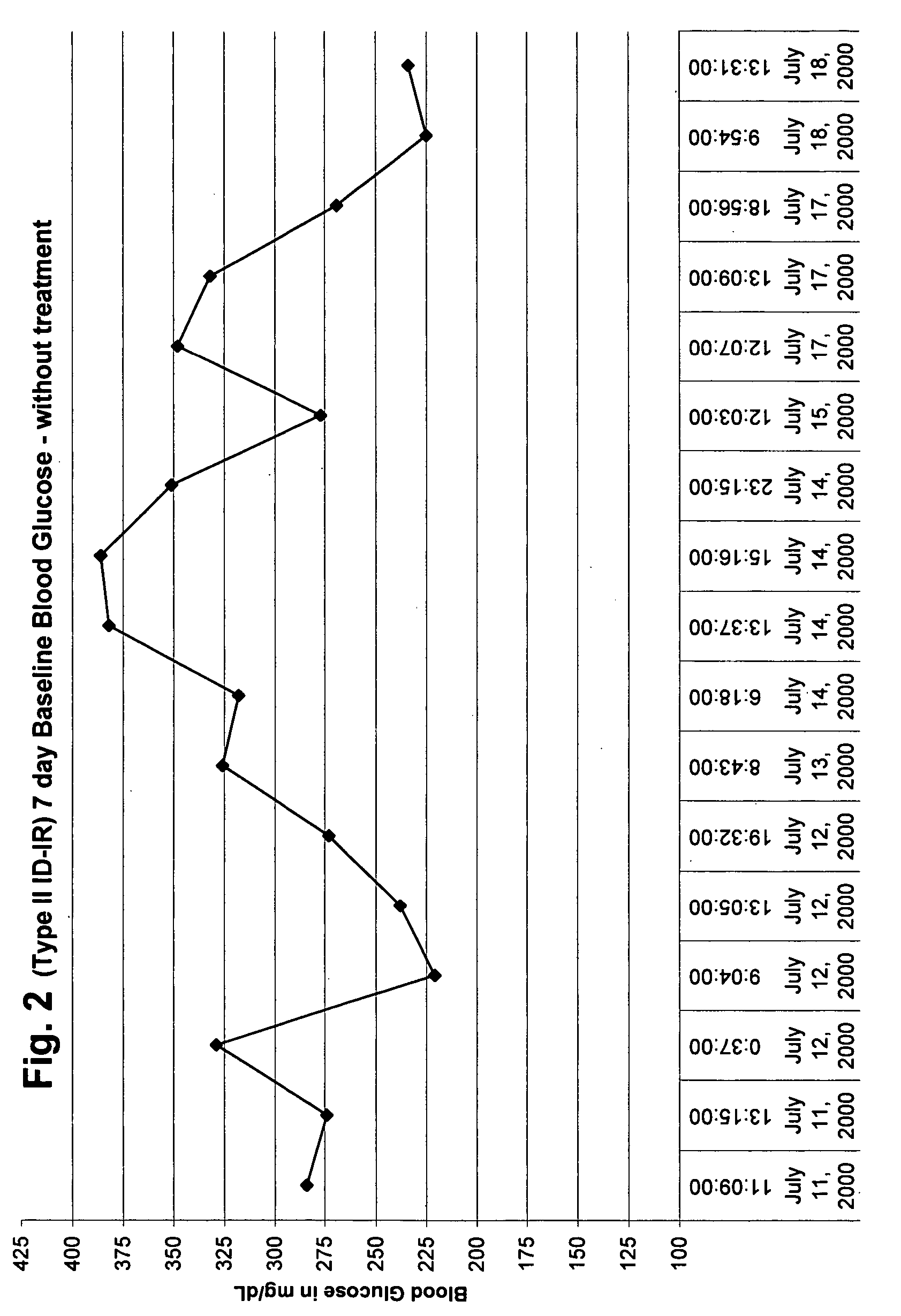
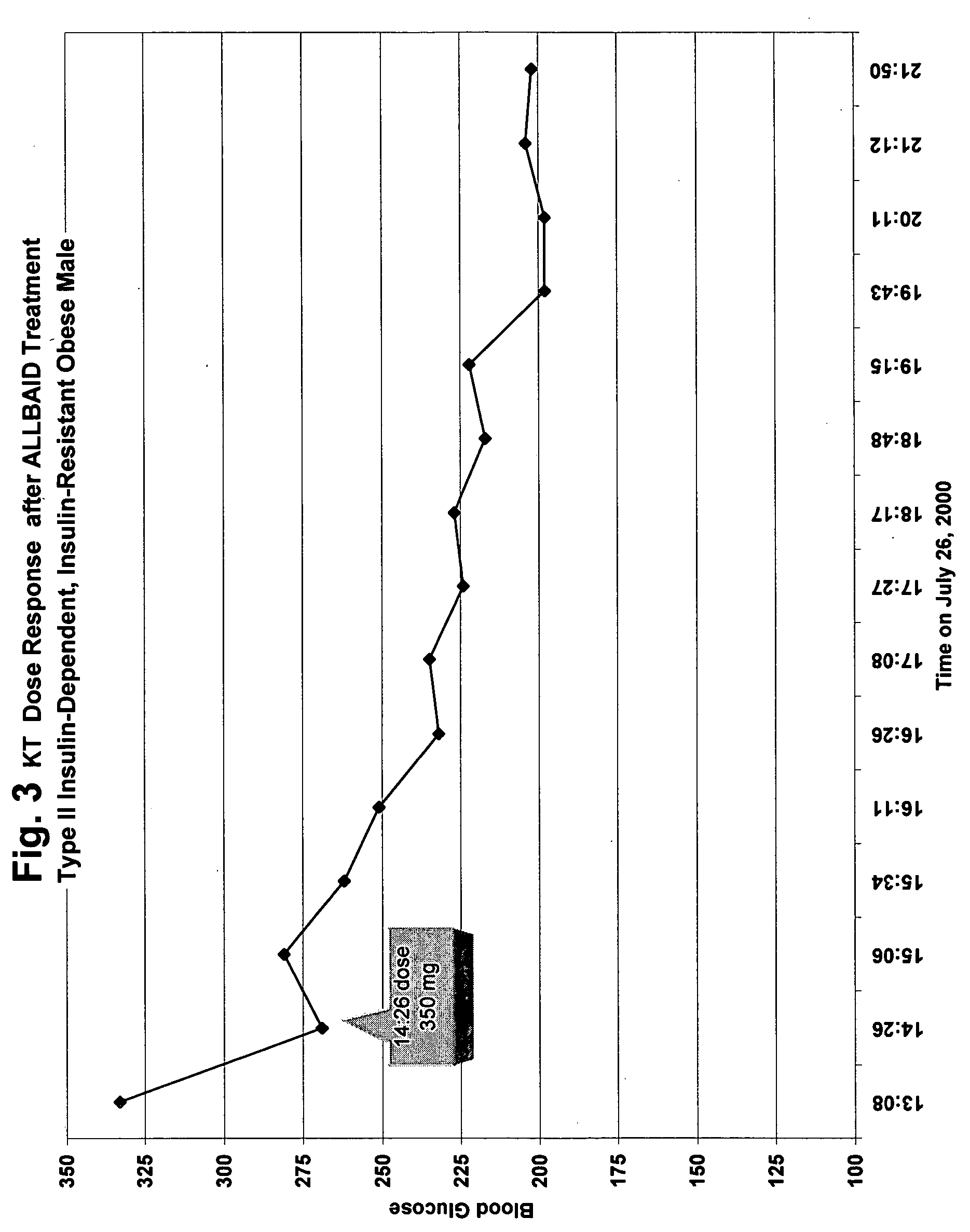
Flavonoids, especially luteolin, are shown to be effective against insulin dependent (Type I) and insulin independent (Type II) diabetes mellitus. It is demonstrated that luteolin works in mammals by binding and blocking the Kv1.3 potassium channel of T-cell and Beta cells. Antidiabetic and anti-autoimmune compounds can be selected by measuring their ability to bind to and block the Kv1.3 channel.
Owner:ZIEGLER RANDY
Chinese medicine extract and medicine use thereof
The invention belongs to the traditional Chinese medicinal material field, and relates to a Turpinia arguta leaf extract, which contains a flavonoid chemical constituent and acceptable salt in the medicine thereof. The content of total flavonoids is more than 15 percent; the content of apigenin aglycon and a flavone glycoside constituent which uses apigenin as aglycon is no less than 1.5 percent measured in the apigenin; a flavonoid chemical constituent contains one or more of the apigenin, apigenin-7-O-Bata-neohesperidoside, apigenin-7-O-2<1>-Bata-rhamanopyranosyl rutinoside, apigenin-7-O-Bata-glucoside, luteolin-7-O-Bata-glucoside. The invention furhter provides a method for preparing the extract thereof, a method for controlling the quality of the extract, and the application of the extract for preparing the medicines such as antibiosis, oxidation resistance, anti-mutation, antitumor, hepatic protection, anti-hepatitis b virus, anti-thrombosis, anti-arteriosclerosis, etc.
Owner:胡军
Composition for controlling the respiratory effects of inhaled pollutants & allergens
InactiveUS20070082071A1Eliminate the effects ofReduced responseBiocidePharmaceutical delivery mechanismPeppermintsRespiratory effect
An anti-inflammatory composition for application to the nasal mucosa includes a combination of essential oils. The composition is intended to help prevent and / or alleviate the effects of exposure caused by the inhalation of pollutants and allergens. Preferred embodiments of the composition include jojoba oil, rosemary oil, any of a variety of citrus oils, coconut oil, sesame oil, soy oil, thyme oil, oregano oil, chamomile oil, peppermint oil, cardiospermum halicacabum, galphimia glauca, luffa operculata, bee's milk, bee's wax, and aloe vera in various combinations and sub-combinations. The composition may further include lauric acid, d-limonene and luteolin.
Owner:GLOBAL LIFE TECH
Stevia rebaudian valid target as well as its activity and application
InactiveCN101156883ASignificant hypoglycemiaSignificant lipid-lowering effectMetabolism disorderPharmaceutical delivery mechanismApigeninAcute hyperglycaemia
The invention discloses an effective part of stevia and the activity and the application thereof. The effective part mainly comprises stevioside category and flavone category which are obtained by extracting and separating from dried stevia leaves, wherein the sum of the percentage content of the stevioside components in the stevioside part is 5 to 100 percent (w / w), and the stevioside components mainly contains the stevioside, stevibioside, rebaudioside A, B, C, D, E, and F, dulcoside A and the derivative thereof, etc.; the sum of the percentage content of the flavone components in the flavone part is 5 to 100 percent (w / w), and the flavone components mainly contains luteolin, quercetin, luteolin-7-O-Beta-D-glucoside, apigenin-7-O-Beta-D-glucoside, quercitrin, quercetin-3-O-Beta-D-arabinoside, quercetin-3-O-(4-O-anti form-caffeoyl acyl-Alpha-L-rhamnose-(1 to 6)-Beta-D-galactoside) and the derivative thereof, etc., and 4, 5-dicaffeoylquinic acid and the derivative thereof, etc. The effective part has significant sugar-reducing and fat-reducing effects, can be used singly or combined with any other Chinese medicines and Western medicines or foods in any proportion, is used for preparing medicines or functional foods, and is used for treating hyperglycemia and hyperlipoidemia.
Owner:石任兵
Method for separating and purifying luteolin
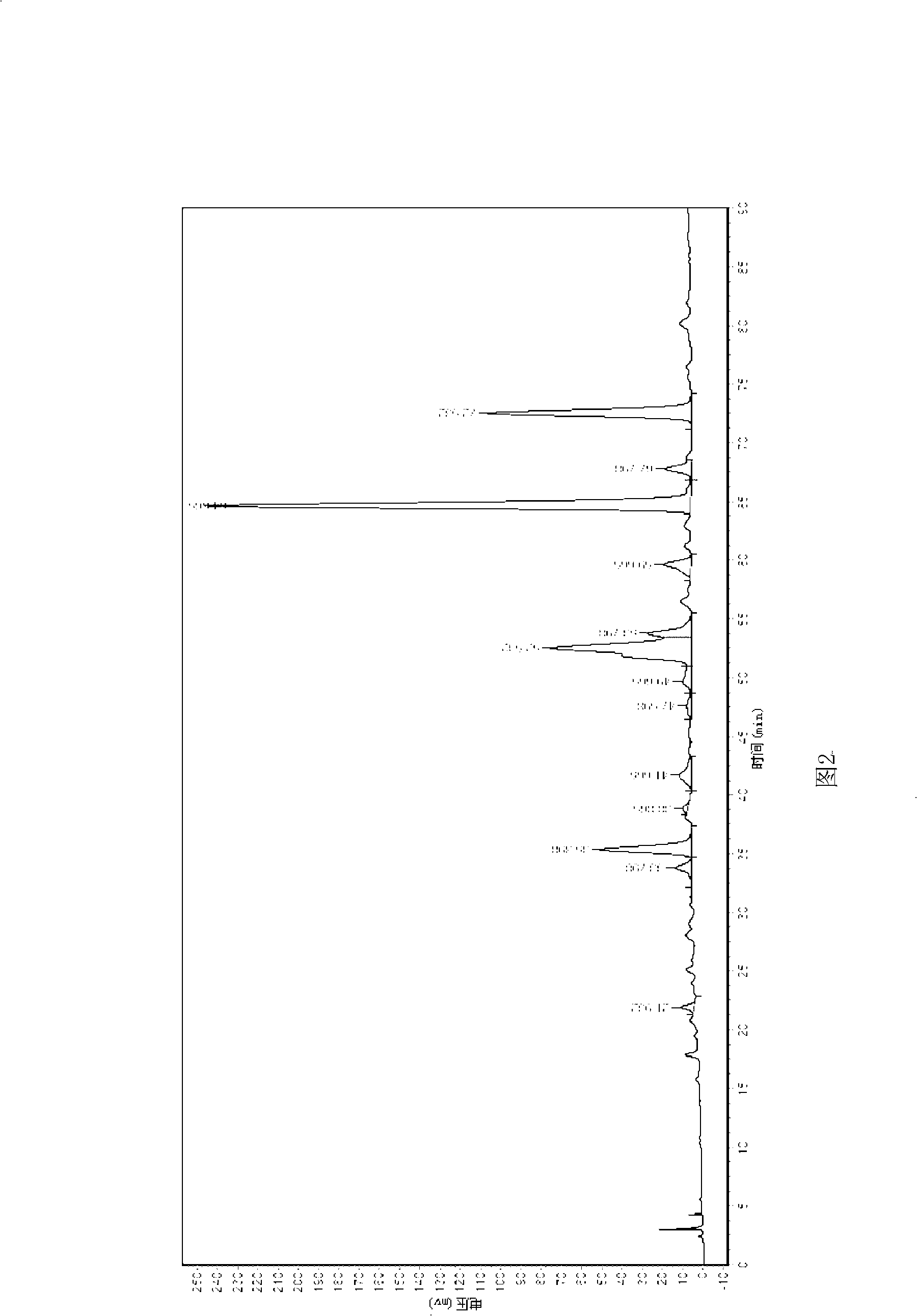
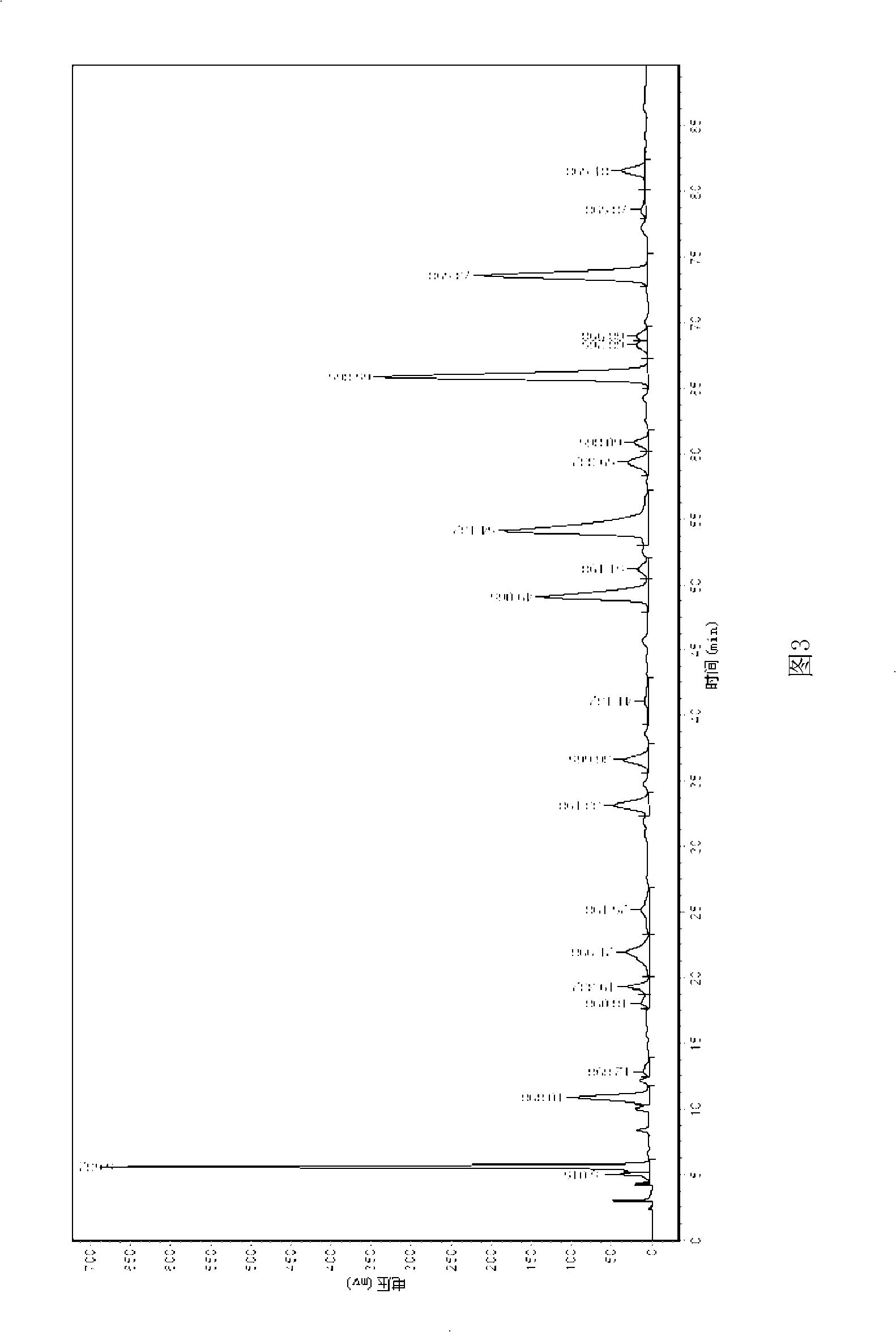
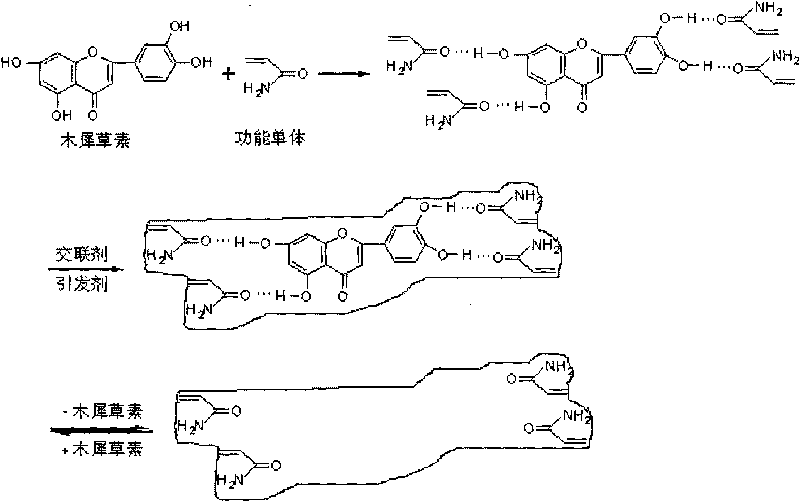
InactiveCN101712669AHigh selectivityEasy to separateNervous disorderOrganic chemistryCross-linkFunctional monomer
The invention aims to provide a material for preparing a molecular imprinted polymer by taking luteolin as a template and adopting a molecular imprinting technique method, and a method for separating and purifying the luteolin in a natural product, and belongs to the field of separation and purification of effective components from the natural product. The method comprises the following steps: (1) crude extraction, namely sequentially washing, drying, crushing and sieving peanut shells, extracting the peanut shell powder with 70 percent ethanol time by time under the assistance of ultrasonic waves, and concentrating and drying the extract; (2) preparation of the polymer, namely weighing the pure luteolin, a functional monomer, a cross-linking agent and an initiating agent in proportion, dissolving the materials in a solvent, performing ultrasonic processing, degassing and polymerization on the solution to obtain the block polymer, and sequentially grinding, sieving and eluting the block polymer to obtain the molecular imprinted polymer of the luteolin; and (3) purification, namely loading the prepared molecular imprinted polymer into a solid-phase extraction column, loading the crude extract, washing off the luteolin left on the column after removing impurities by eluting, concentrating and drying the luteolin to obtain the pure product of the luteolin.
Owner:NORTH CHINA UNIVERSITY OF SCIENCE AND TECHNOLOGY
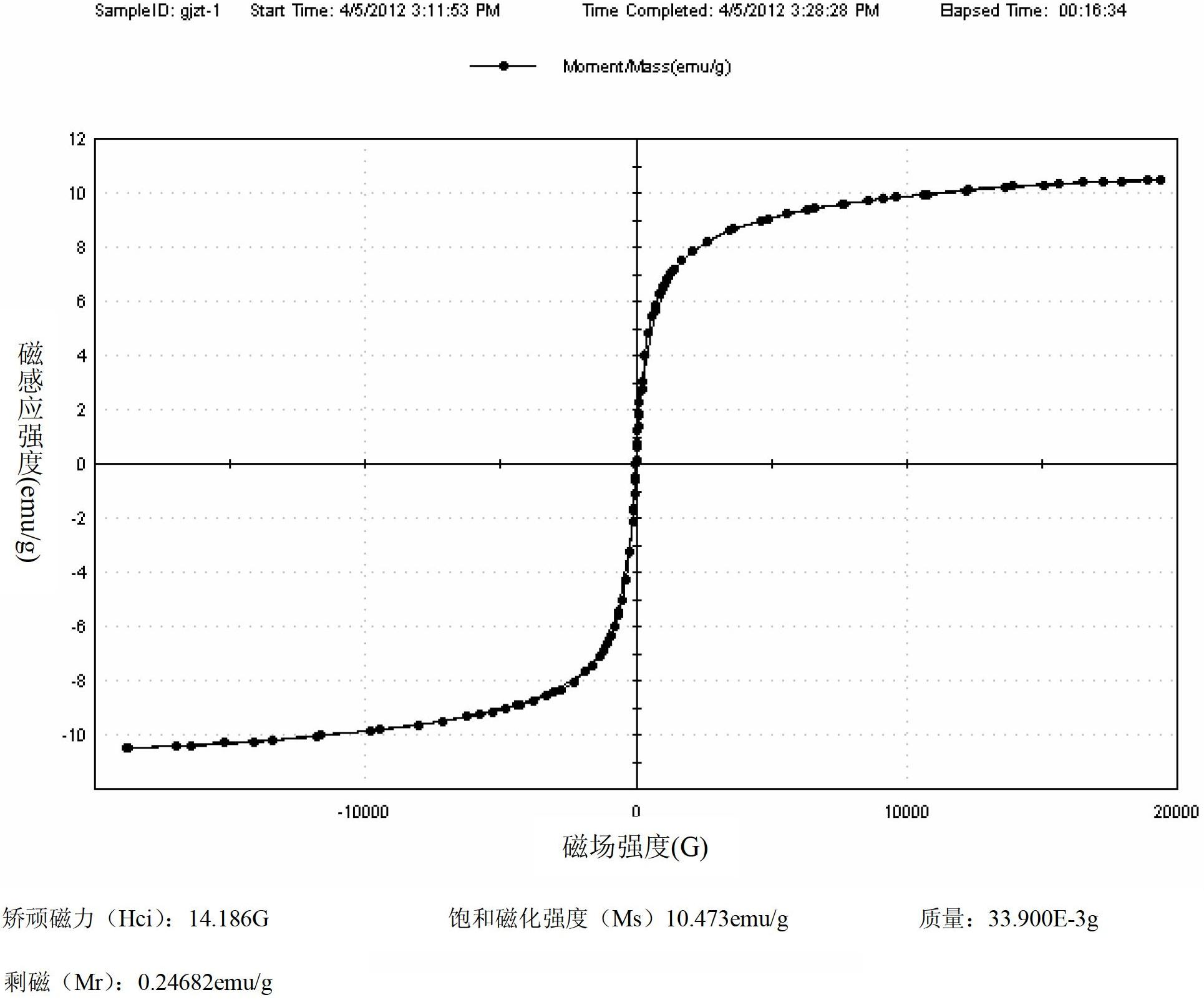
Flavone magnetic molecularly imprinted polymer, preparation of flavone magnetic molecularly imprinted polymer, and application of flavone magnetic molecularly imprinted polymer to bamboo-leaf flavone separation
InactiveCN102659982AWith dynamic memory functionOther chemical processesAlkali metal oxides/hydroxidesCross-linkFunctional monomer
Flavone magnetic molecularly imprinted polymer, preparation of the flavone magnetic molecularly imprinted polymer, and application of the flavone magnetic molecularly imprinted polymer to bamboo-leafflavone separation belong to the technical field of drug separation. Preparation of flavone magnetic molecularly imprinted polymer microspheres include subjecting nano Fe3O4 subjected to surface modification by oleic oil, template molecule luteolin, functional monomer 4-ethylpyridine and cross linking agent ethylene glycol dimethacrylate to suspension polymerization according to a template molecule, functional monomer and crosslinking agent ratio of 1:5-8:80. The flavone magnetic molecularly imprinted polymer microspheres are applicable to selective enrichment and purification of bamboo-leaf flavones in bamboo-leaf extract, and the preparation is simple, quick, high in recovery rate and the like and is widely applicable to the field of separation of natural flavone compounds.
Owner:INT CENT FOR BAMBOO & RATTAN
Method for extracting and preparing flavonoids pigment from chrysanthemum
The invention relates to a method for ultrasonically extracting and preparing flavonoids pigment from chrysanthemum, belonging to the field of food processing and especially used for deep processing chrysanthemum. The process comprises the following steps of: blanching, drying and hermetically storing chrysanthemum at a low temperature, filtering by using a 100-mesh sieve, soaking in 60% ethanol as an extraction solvent in a liquid-material ratio of 40 / 1 (v / w) for 2 h, ultrasonically extracting a dry base in a water bath at 70 DEG C for 10 min to acquire the yield of 13.34 percent; and evaporating in the water bath at 70 DEG C by using the 60% of ethanol extraction liquid in a rotating way to release an ethanol taste; and freezing and drying in vacuum, wherein analytical compositions reach 52.01% of the dry base and comprise flavonoids, linarin, flavonoids (luteolin, acacetin and apigenin), flavonols (quercetin), phenolic acid (chlorogenic acid and caffeic acid), metal elements, and the like. The invention provides a new method for deep processing the chrysanthemum and has strong maneuverability, good safety and good repetitiveness.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for extracting luteolin from peanut roots, stems, leaves and shells
The invention relates to a method for extracting luteolin from peanut roots, stems, leaves and shells, comprising the following steps: washing materials comprising peanut roots, stems, leaves and shells, removing impurities in the peanut roots, stems, leaves and shells, and grinding the peanut roots, stems, leaves and shells subject to impurity removal; extracting by using 75% ethanol, filtering, decoloring, and concentrating at reduced pressure; precipitating by using hot water, filtering, heating filtrate, and concentrating at reduced pressure; absorbing by using KLFC-150 macroporous resin, and eluting by using 75% ethanol; concentrating elution solution at reduced pressureto obtain crude luteolin; dissolving the crude luteolin in ethanol, adding water to dilute the crude luteolin, recovering the ethanol, concentrating, crystallizing, and recrystallizing to obtain the high-purity luteolin product. The operation process is mainly characterized in that the peanut roots, stems, leaves and shells are used as extraction raw materials and are low-cost sustainable resources, the 75% ethanol is used as extraction solvent and eluent, and the product contains no toxic solvent and other residue, is safe and environmentally friendly and is low in production cost and high in yield.
Owner:彭国平
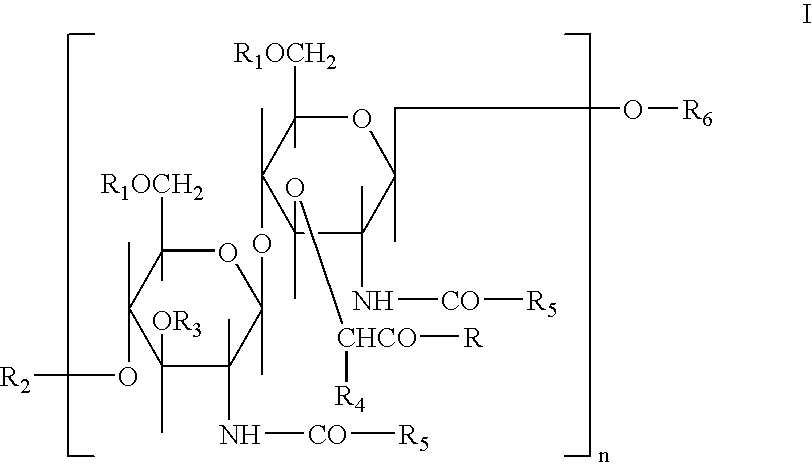
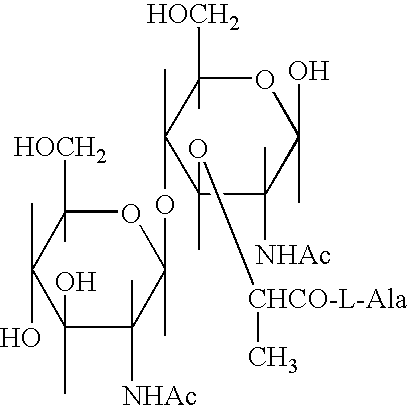
Glycopeptides for the treatment of als and other metabolic and autoimmune disorders
InactiveUS20070185012A1Promote absorptionImprove bioavailabilityMetabolism disorderAntipyreticIsoglutamineGlycopeptide
New compositions and methods for treating patients suffering from Amyotrophic Lateral Sclerosis (ALS) and other metabolic and autoimmune disorders, which include glycopeptides such as N-acetyl-D-glucosaminyl(β1-4)-N-Acetyl-muramyl-L-alanyl-D-isoglutamine (GMDP) and peptide analog-L-alanyl-D-glutamic acid (GMDP-A) of at least 98% purity administered either alone, or in combination with a flavone such as luteolin and / or an isoflavone such as genistein, optionally in combination with a flavonol glycoside such as isoquercitrin or rutin. The high purity glycopeptides have a decreased amount immunogenic impurities and demonstrate a synergistic effect when combined with luteolin and / or genistein in presence of isoquercitrin.
Owner:RAJADHYAKSHA V J +1
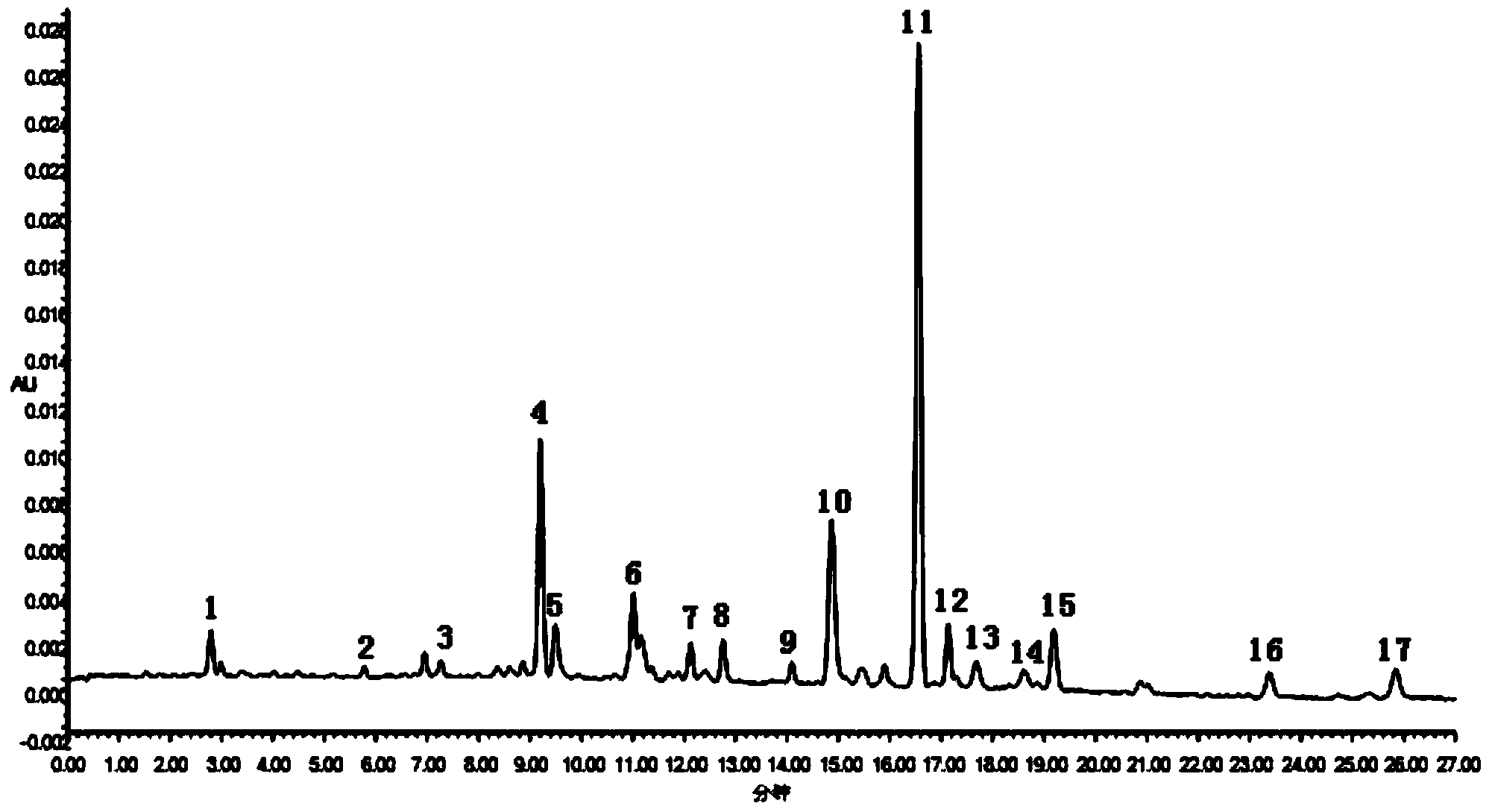
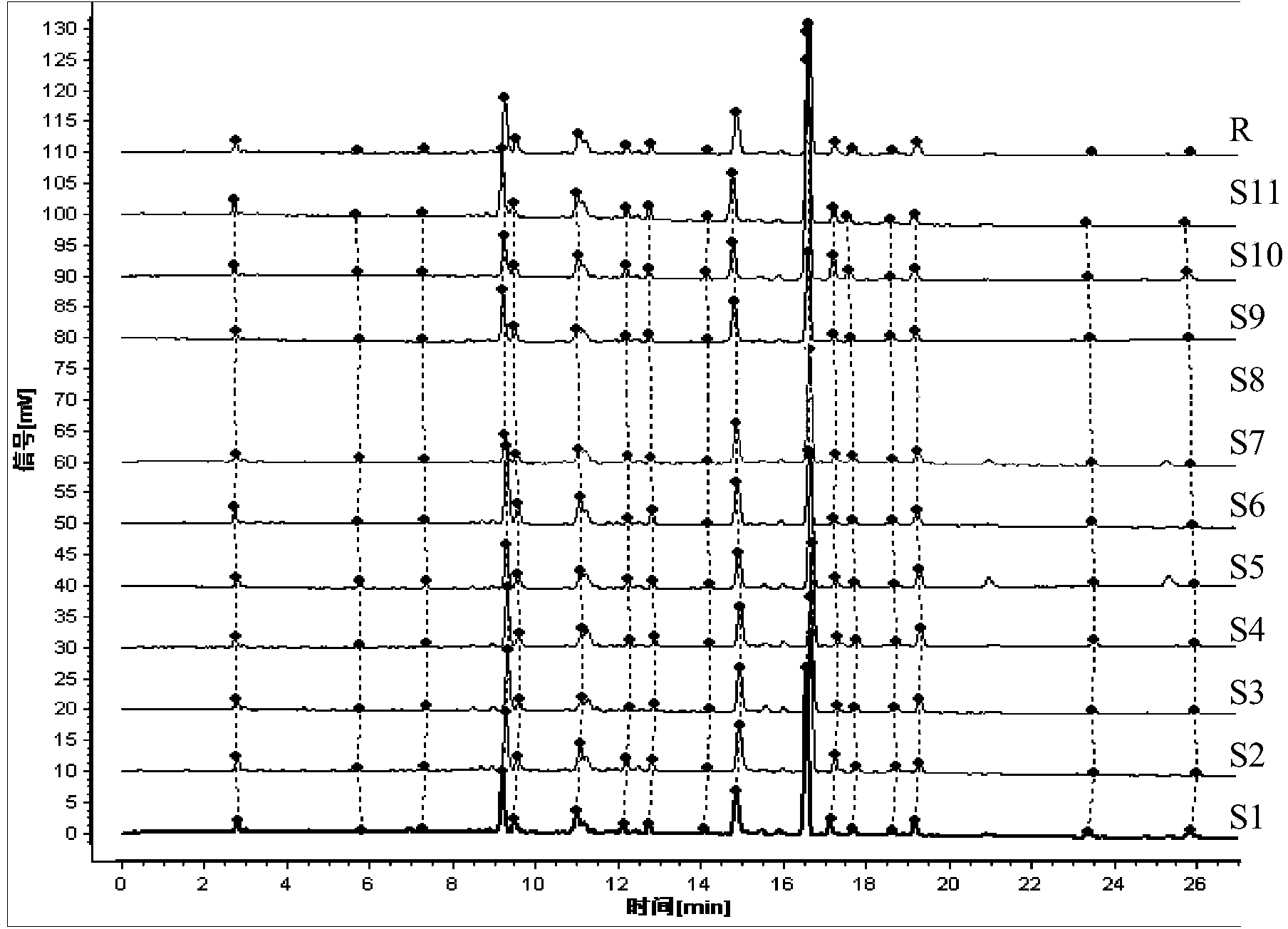
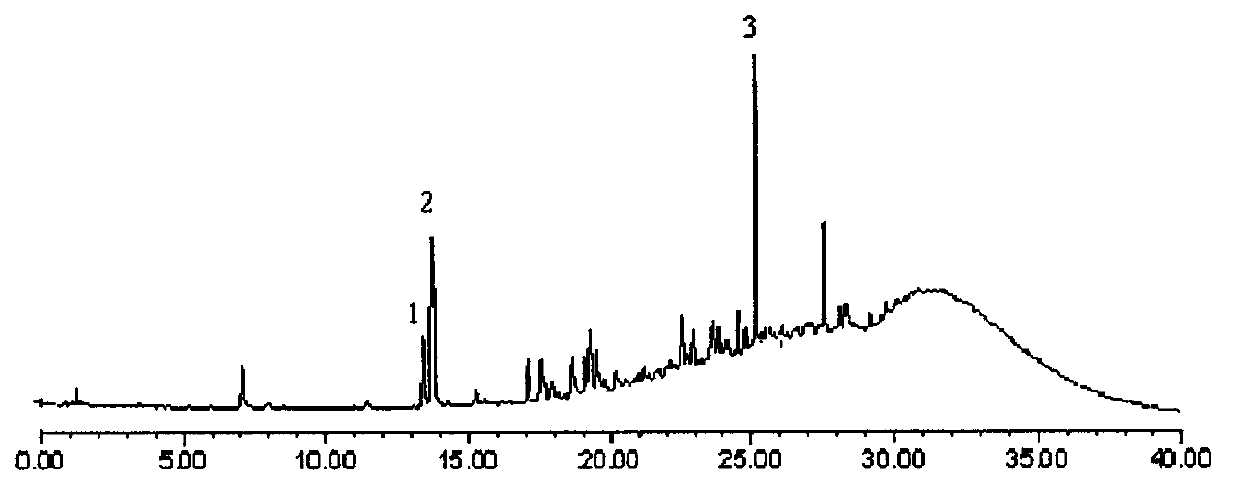
Construction method of wild chrysanthemum flower UPLC (Ultra Performance Liquid Chromatography) fingerprint spectrum
InactiveCN103926355AImprove analytical performanceImprove accuracyComponent separationChlorogenic acidMicrofiltration membrane
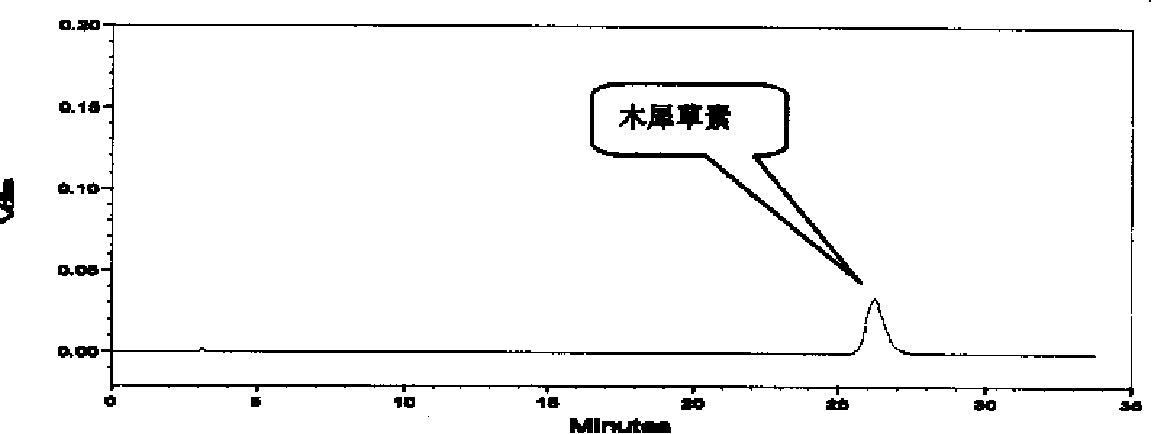
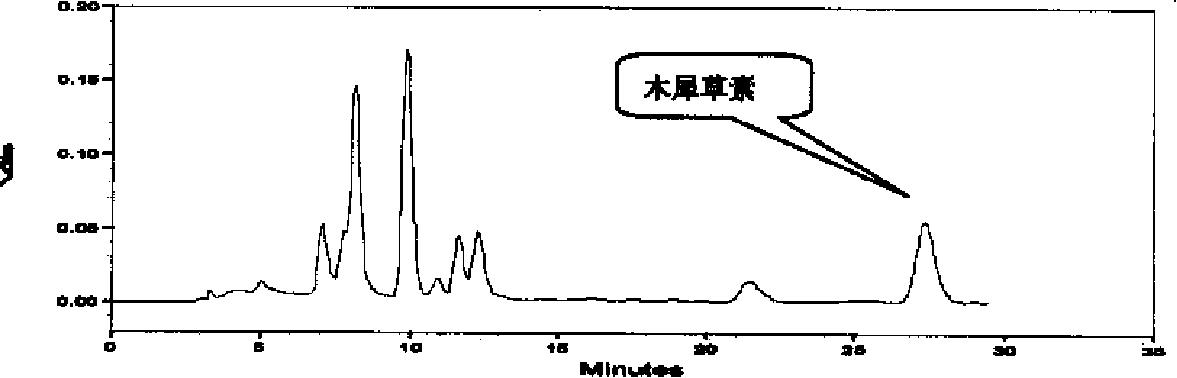
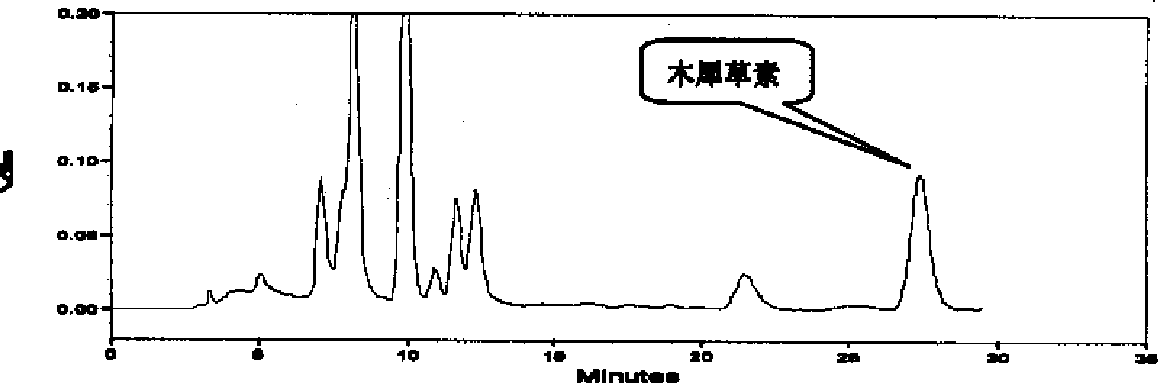
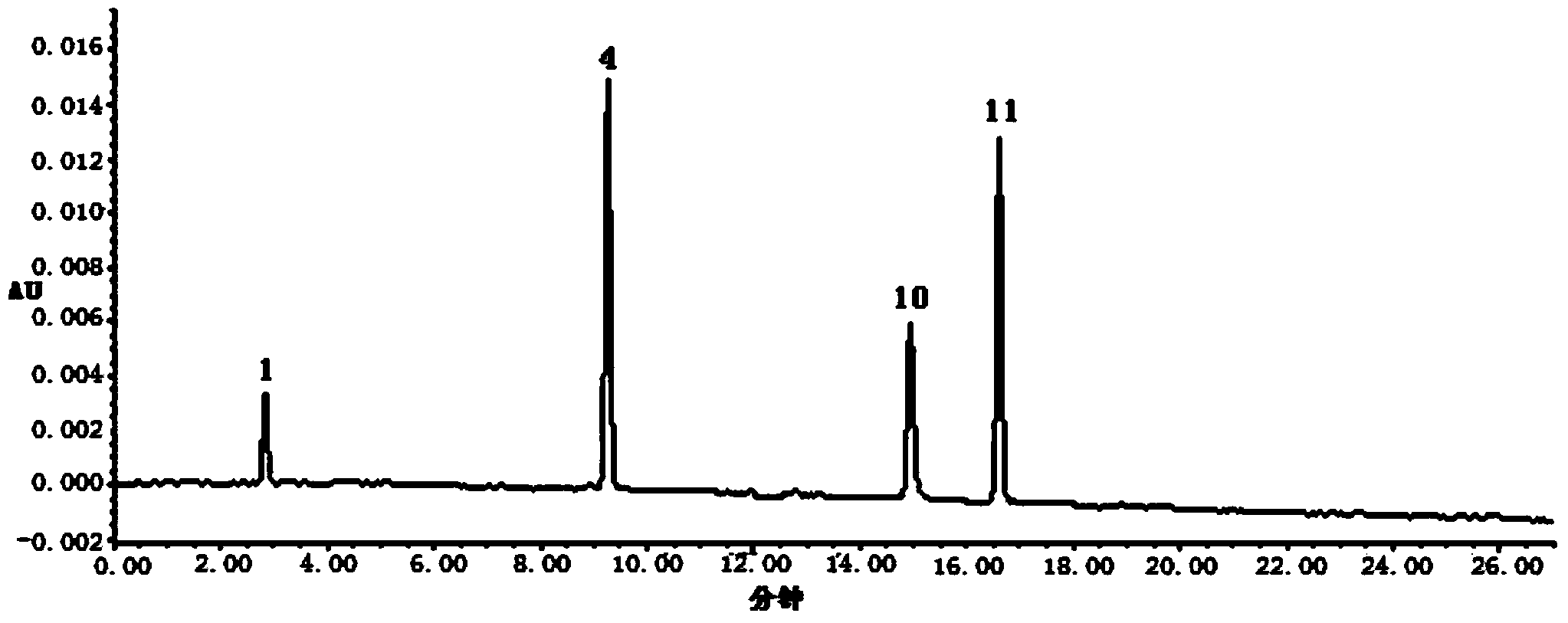
The invention discloses a construction method of a wild chrysanthemum flower UPLC fingerprint spectrum. The method comprises the following steps: crushing a wild chrysanthemum flower medicinal material, precisely weighing, adding methanol, performing ultrasonic treatment after weighing, filtering by use of a microfiltration membrane, and taking subsequent filtrate to obtain a test sample solution; respectively taking chlorogenic acid, galuteolin, luteolin and linarin, precisely weighing, and dissolving by use of the methanol to obtain a reference solution; and respectively measuring the test sample solution and the reference solution by use of an ultrahigh-efficiency liquid chromatogram system to generate chromatogram peaks of the test sample solution and the reference solution so as to generate the wild chrysanthemum flower UPLC fingerprint spectrum. The construction method of the wild chrysanthemum flower UPLC fingerprint spectrum related to the invention is simple, easy to operate and greatly improved in spectrum analytic capability, and has the advantages of improving the accuracy of results and greatly improving the stability and sensitivity of the detection of the fingerprint spectrum.
Owner:GUANGZHOU XINGQUN PHARMA
Method for extracting luteolin and beta-sitosterol from peanut shells
The invention relates to a method for extracting luteolin and beta-sitosterol from peanut shells. The method comprises the following steps: taking the peanut shells as materials, cleaning the peanut shells to remove impurities, crushing the cleaned peanut shells, performing extraction on the crushed peanut shells by an ethanol solution, carrying out filtration, decolorization and concentration under reduced pressure to obtain a luteolin and beta-sitosterol mixture, then mixing the luteolin and beta-sitosterol mixture with an alkali liquor, after that, carrying out extraction separation to obtain beta-sitosterol, performing extraction after pH adjustment to obtain a luteolin crude product, and performing alcohol dissolution, crystallization and recrystallization on the crude product to obtain a luteolin product with relatively high purity. The method is simple to operate, demands less on equipment, lowers the manufacturing cost of luteolin and reduces the risk of environmental pollution.
Owner:HUNAN AGRICULTURAL UNIV
Method for preparing compound of luteolin
The preparation method of luteolin compound includes the following steps: adding raw material rutin or its derivative in water, adding NaOH under the condition of heating and stirring to completely dissolve raw material, cooling to room temperature, regulating pH to 2-6, filtering, wasing and drying to obtain raw material refined product; then adding solid alkali, prepared raw material refined product and Na2S2O4 into water according to mole ratio of raw material refined product; solid alkali: Na2S2O4: water=1:15-20:8-70:1110-3330, using 100-500 W microwave to heat and reflow of 0.25-2 hr, cooling to room temperature, regulating reaction liquor pH to 2-7, filtering, washing, drying, and using organic solvent to make recrystallization so as to obtain the invented product.
Owner:ZHEJIANG UNIV
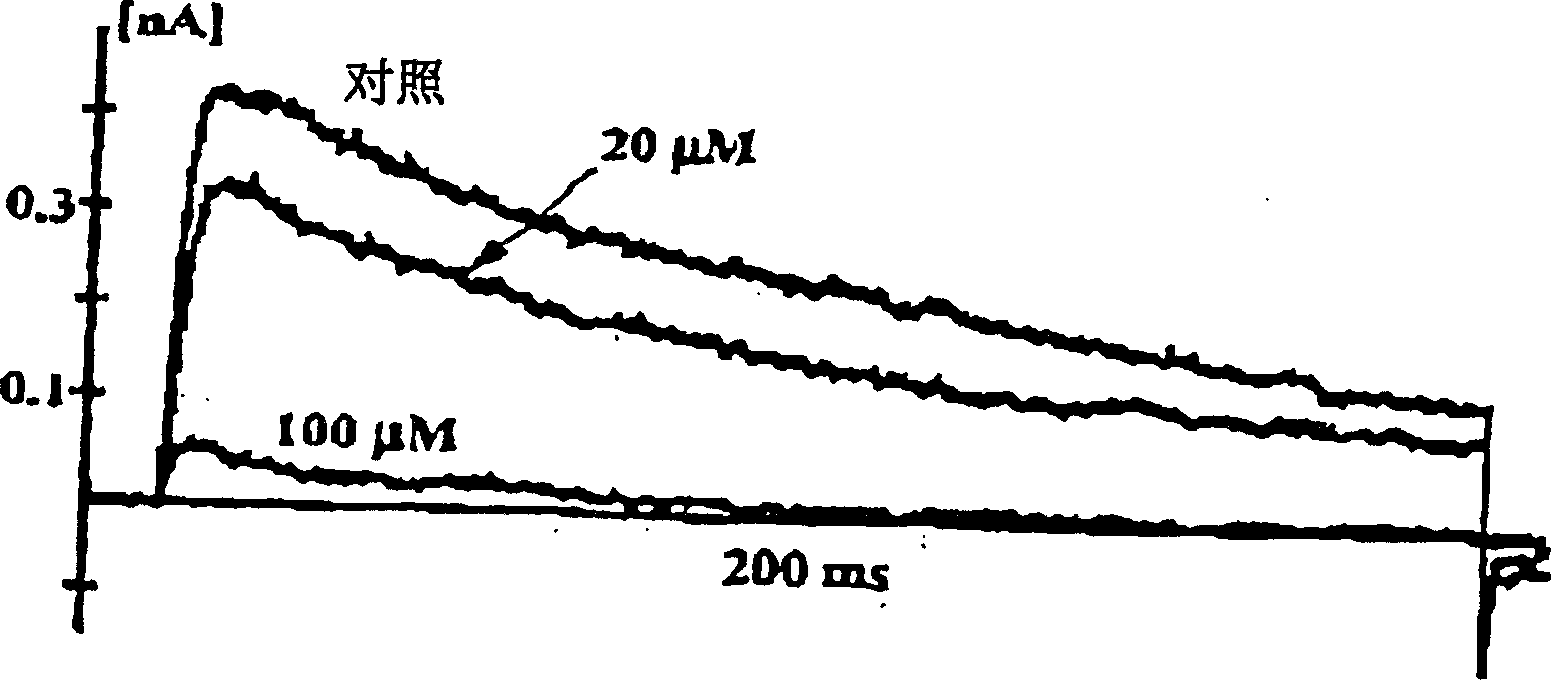
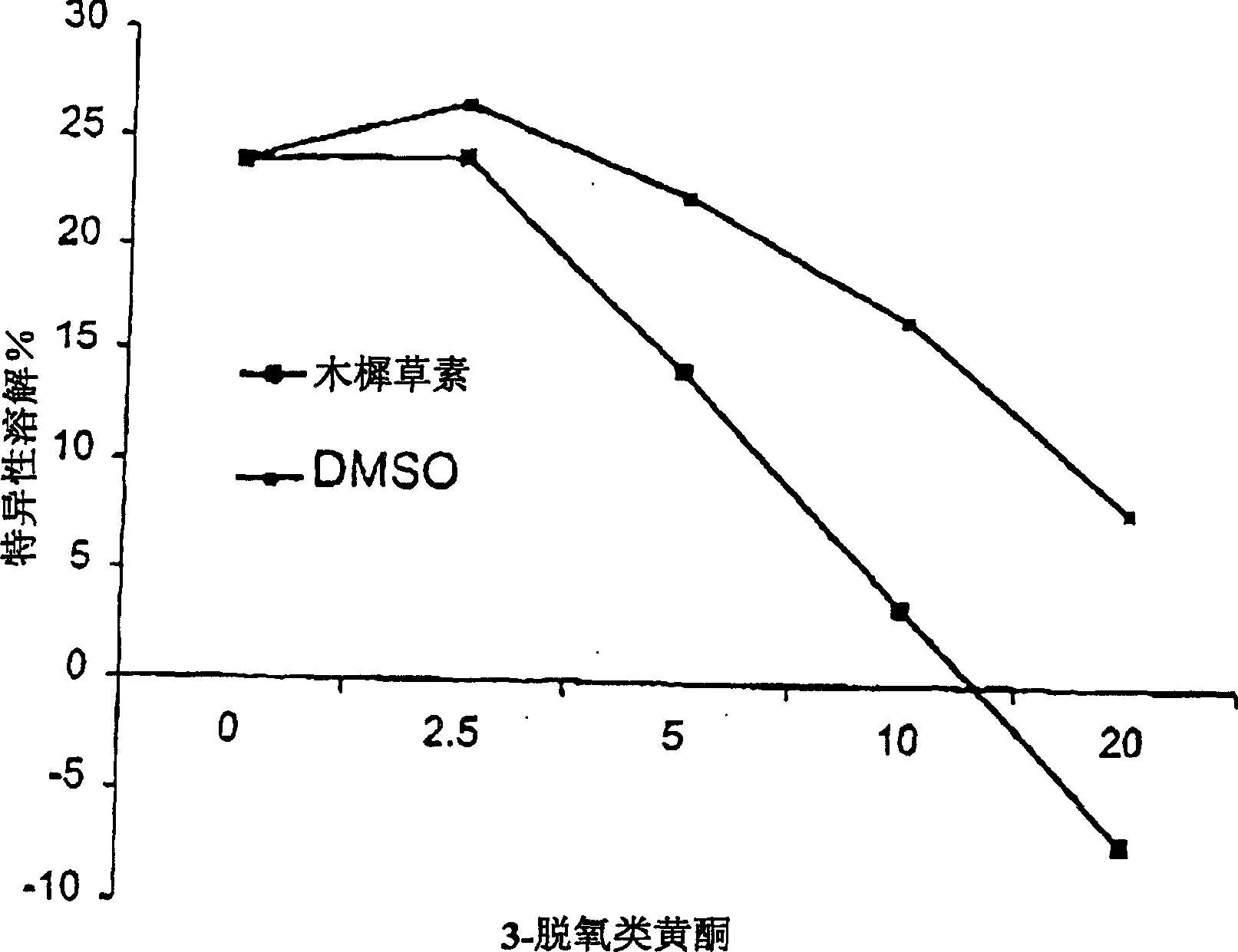
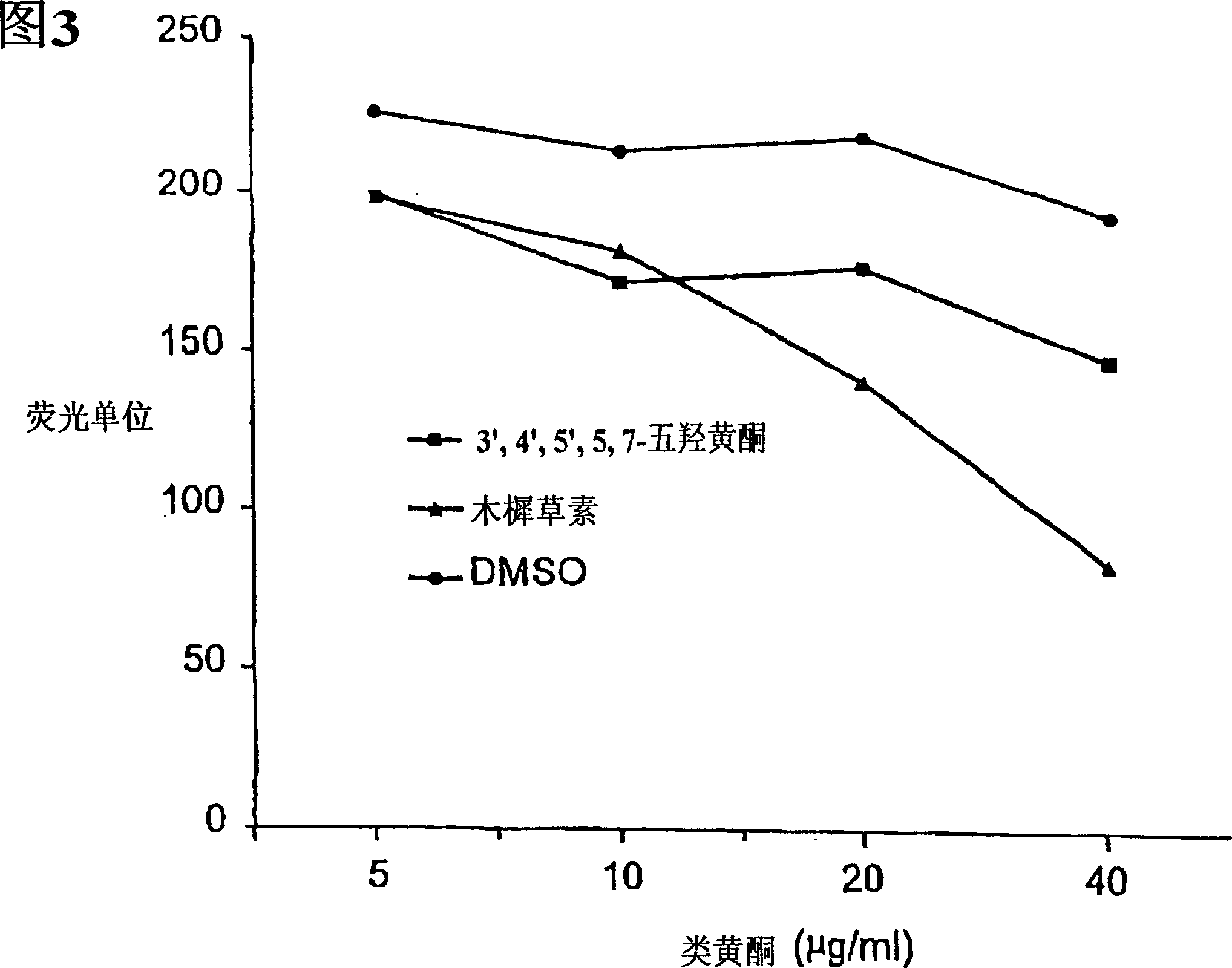
Inhibition by 3-deoxyflavonoids of t-lymphocyte activation and therapies related thereto
InactiveCN1668287AHalogenated hydrocarbon active ingredientsMetabolism disorderAutoimmune diseaseRutin
3-Deoxyflavonoid compounds and methods for inhibiting T-cell activity and treating diseases and disorders (e.g., autoimmune disorders, inflammatory disorders, diabetes, ALS, MS, rheumatoid arthritis, etc.). In some cases the efficacy and / or duration of action of luteolin and / or other 3-dioxyflavinoid compounds may be increased by administering such compounds along with Rutin, a Rutin congener and / or a Rutin derivative. Also, in some cases, first pass metabolism of luteolin or other 3-deoxyflavinoids may be avoided by administering such compounds by parenteral routes (e.g., sublingual, buccal, intranasal, injection, etc.).
Owner:SYNORX
Anti-hepatitis B virus composition taken from fresh dandelion and application of anti-hepatitis B virus composition in preparation of anti-hepatitis B virus drug
InactiveCN103099838AHas anti-HBV activityHigh development valueOrganic active ingredientsDigestive systemCaffeic acidIsochlorogenic acid
The invention relates to an anti-hepatitis B virus composition taken from fresh dandelion and an application of the anti-hepatitis B virus composition in preparation of an anti-hepatitis B virus drug and belongs to the field of traditional Chinese medicines. The anti-hepatitis B virus composition provided by the invention comprises a fresh dandelion polysaccharide component, a phenolic acid component and a flavone component and is characterized in that the weight ratio of the fresh dandelion polysaccharide component to the phenolic acid component to the flavone component is (2-40): (3-70): (2-50). According to an optimized scheme, the phenolic acid component takes caffeic acid, chlorogenic acid and isochlorogenic acid A as typical ingredients; the flavone component takes galuteolin, quercetin and luteolin as typical ingredients; and the weight ratio of the caffeic acid to the chlorogenic acid to the isochlorogenic acid A to the galuteolin to the quercetin to the luteolin is (0.25-10): (2-40): (0.25-20): (0.5-20): (0-10): (1.5-20). According to the invention, the fresh dandelion is taken as raw material, effective ingredients are separated, purified and enriched by macroporous resin, the effective ingredients are mixed to obtain the fresh dandelion anti-hepatitis B virus composition, a process is simple and convenient, related diseases caused by hepatitis B viruses can be effectively treated, cost is low, and mass production can be achieved.
Owner:苏州艾费堂医药科技有限公司
Preparation and use of Linaria vulgaris flavone and total flavone thereof
InactiveCN101475619AIncrease contentHigh purityOrganic active ingredientsSugar derivativesRobininAdditive ingredient
The invention belongs to the technical field of medicine, and relates to a method for preparing flavonoids and total flavonoids in Linaria vulgaris and application thereof. The flavonoids and the total flavonoids come from flavonoid compounds in the Linaria vulgaris (including flavonoid compounds such as pectolinarin, aglycon, acetyl pectolinarin, acetyl linarin, acetyl linarin, hesperidin, robinin, hispidulin, luteolin, vanilla lignin, chrysin and the like). Dried whole herbs of the Linaria vulgaris are subjected to reflux extraction by water or alcohol, then a macroporous resin is used to send to a column, alcohols with different concentrations are used for elution, eluents are collected, then different elution flow ingredients pass through a silica gel chromatographic column, a polyamide chromatographic column, a gel chromatographic column and the like, to obtain various compositions, and the content of an obtained total flavonoid substance is more than 60 percent. The flavonoid compounds can be used for preparing medicines for treating angiocardiopathy, cough, and asthma. An extraction method of the invention is simple, practical and cheap, and is suitable for industrialized production.
Owner:SHENYANG PHARMA UNIVERSITY
Traditional Chinese medicine composition contg. luteolin and capsule of sweeping forsythia and its prepn. method and use
InactiveCN1947747ASynergisticIndicators are stableAntibacterial agentsOrganic active ingredientsViral diseaseForsythia
A composite Chinese medicine for preventing and treating bacterial and viral diseases, inflammation and tumor, relieving pain and cough, decreasing blood fat, improving immunity, etc is proportionally prepared from luteolin and forsythia fruit or its extract. Its preparing process is also disclosed.
Owner:海安江理工技术转移中心有限公司
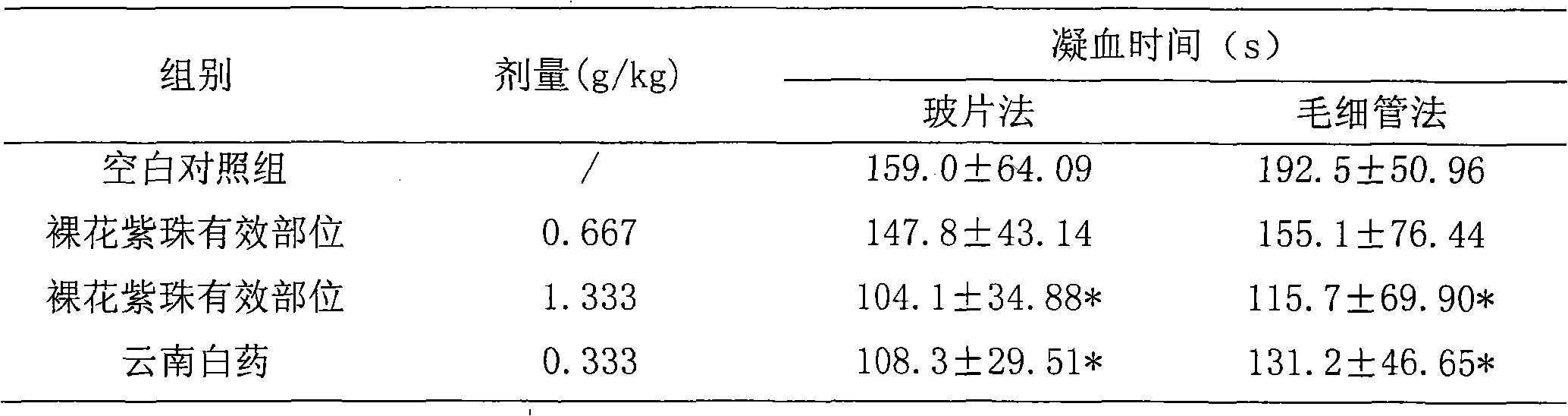
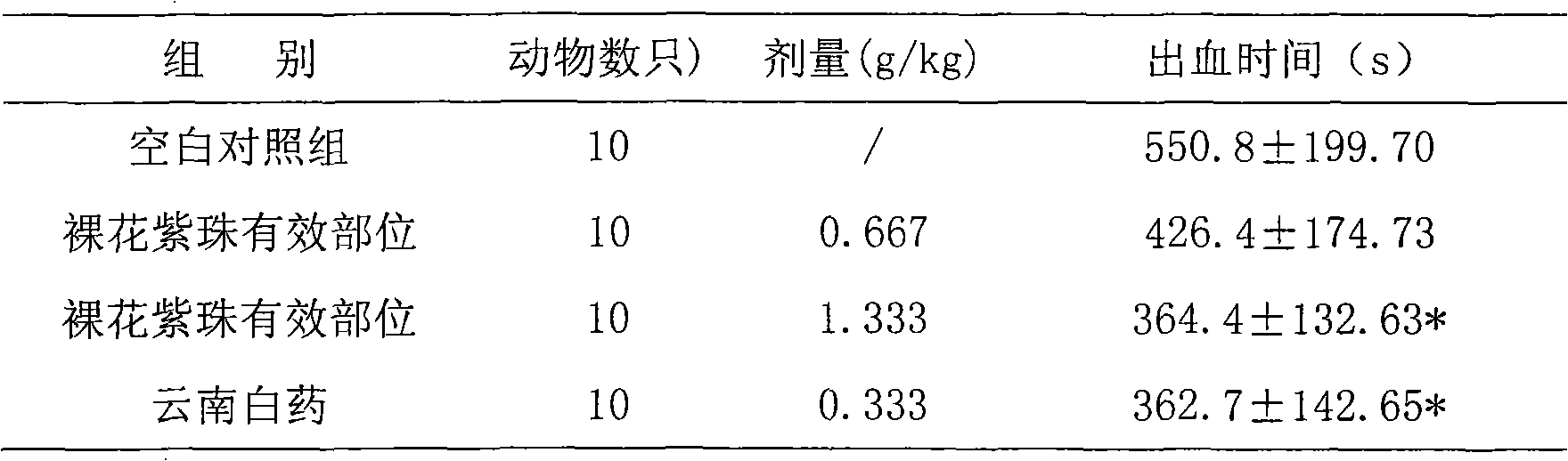
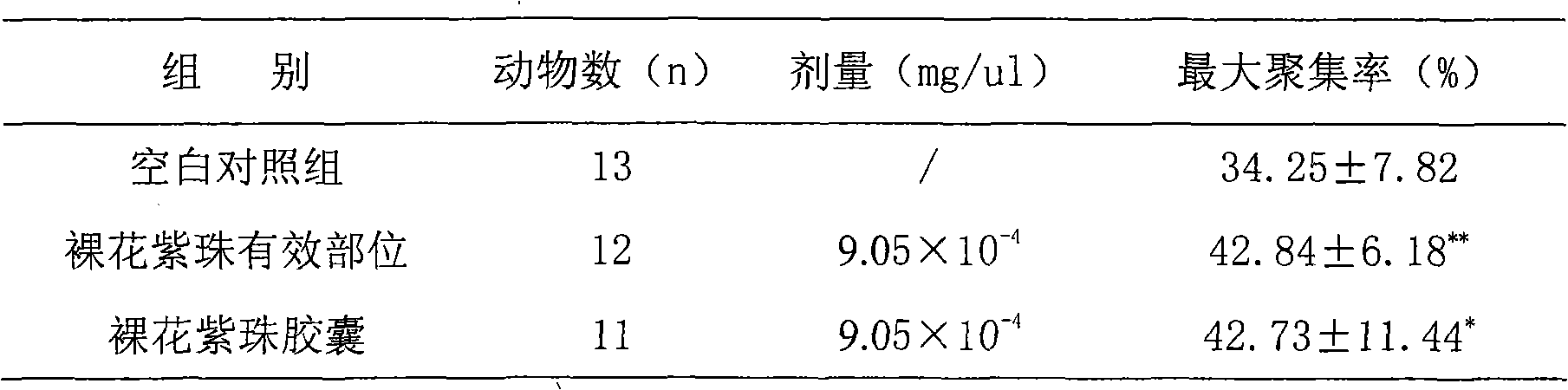
Callicarpa nudiflora effective part with hemostatic effect and pharmaceutical preparation thereof
The invention discloses a callicarpa nudiflora effective part extract with a hemostatic effect and a preparation method of a pharmaceutical preparation of the callicarpa nudiflora effective part extract. The effective part extract and the pharmaceutical preparation contain total flavonoids of the callicarpa nudiflora and are extracted and separated from the callicarpa nudiflora which is a traditional Chinese medicine plant, the content of the total flavonoids is not less than 50%, and the effective part extract and the pharmaceutical preparation mainly contain galuteolin, luteolin-3'-O-beta-D-glucopyranoside, luteolin-4'-O-beta-D-glucopyranoside, calliterpenone, luteolin, apigenin and cosmosiin. The effective part extract and the pharmaceutical preparation have significant hemostatic effects.
Owner:江西省药物研究所
Blood pressure reduction celery tea and manufacturing method thereof
The invention provides blood pressure reduction celery tea and a manufacturing method thereof. The blood pressure reduction celery tea has high apigenin content, is purely natural, can prevent and adjust high blood pressure, and has good health care effects. The blood pressure reduction celery tea is characterized in that 500g of dried celery leaves contain 8 to 20g of apigenin. The manufacturing method of the blood pressure reduction celery tea comprises the following steps of carrying out enzyme deactivation of celery leaves, adding apigenin or celery extract and yeast into the celery leaves obtained by the previous step, carrying out microzyme fermentation, heating the fermented celery leaves, kneading the heated celery leaves so that the heated celery leaves have tea-leaf shapes, feeding the kneaded celery leaves into an oven, and carry out drying. The manufacturing method of the blood pressure reduction celery tea fully utilizes celery medicinal values and completely retains active ingredients in celery. The blood pressure reduction celery tea has high apigenin content, high vegetable protein content, high vitamin content, high trace element content and high nonnutritive factor content, and has the effects of reducing blood pressure and blood fat, improving immunity and delaying aging. Through taking the blood pressure reduction celery tea, people can get enough apigenin and flavonoids containing apiin, persicarin, friedelin, luteolin flavone and the like. Therefore, the blood pressure reduction celery tea has excellent health-care and medicinal functions.
Owner:李家成
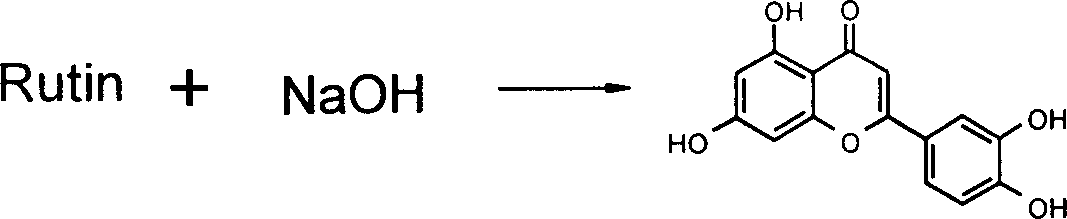
Luteolin metal salt and its preparation method and use
A luteolin-metal salt for preventing and treating pulmonary fibrosis is prepared through adding rutin to the aqueous solution of sodium hydroxide, thermal reflux, cooling, regulating pH=1-4, filtering to obtain yellow solid, dissolving it in water, regulating pH=11-14, filtering, regulating pH=1-4, filtering, baking to obtain coarse product, refining in the mixture of water and methanol by heating, adding it to the solution of Na in absolute alcohol, reacting, and vacuum concentrating until it become solid. Its advantages are high purity and output rate, and low cost.
Owner:HANGZHOU FST PHARMA
Preparation method of boric-acid functionalized porous adsorbent
InactiveCN106964322AImprove permeabilityHigh porosityOther chemical processesSolid sorbent liquid separationCross-linkGlycidyl methacrylate
The invention relates to a preparation method of a boric-acid functionalized porous adsorbent, and belongs to the technical field of preparation of medicinal materials. The preparation method includes preparing boric-acid functionalized nano particles BA-MSNs, taking glycidyl methacrylate as a monomer, taking divinyl benzene as a cross-linking agent, taking azodiisobutyronitrile as an initiator, adding a surfactant Hypermer 2296, stirring to form Pickering high-internal-phase emulsion, and performing thermal-initiation polymerization to obtain boric-acid functionalized porous resin; introducing a B-N coordination compound according to a post-modification method to prepare the boric-acid functionalized porous adsorbent, and applying the boric-acid functionalized porous adsorbent to adsorption separation of luteolin under a neutral condition. The prepared adsorbent has a communicated porous structure, high permeability, high adsorption capacity and high recycle rate, and is environment friendly; adsorption can be achieved under the neutral condition, and secondary pollution and luteolin oxidation are reduced.
Owner:JIANGSU UNIV
Pharmaceutical composition having alpha-glucosidase inhibition activity, and applications thereof
ActiveCN104984346AGood hypoglycemic effectHypoglycemic effect achievedOrganic active ingredientsMetabolism disorderSide effectHypoglycemia
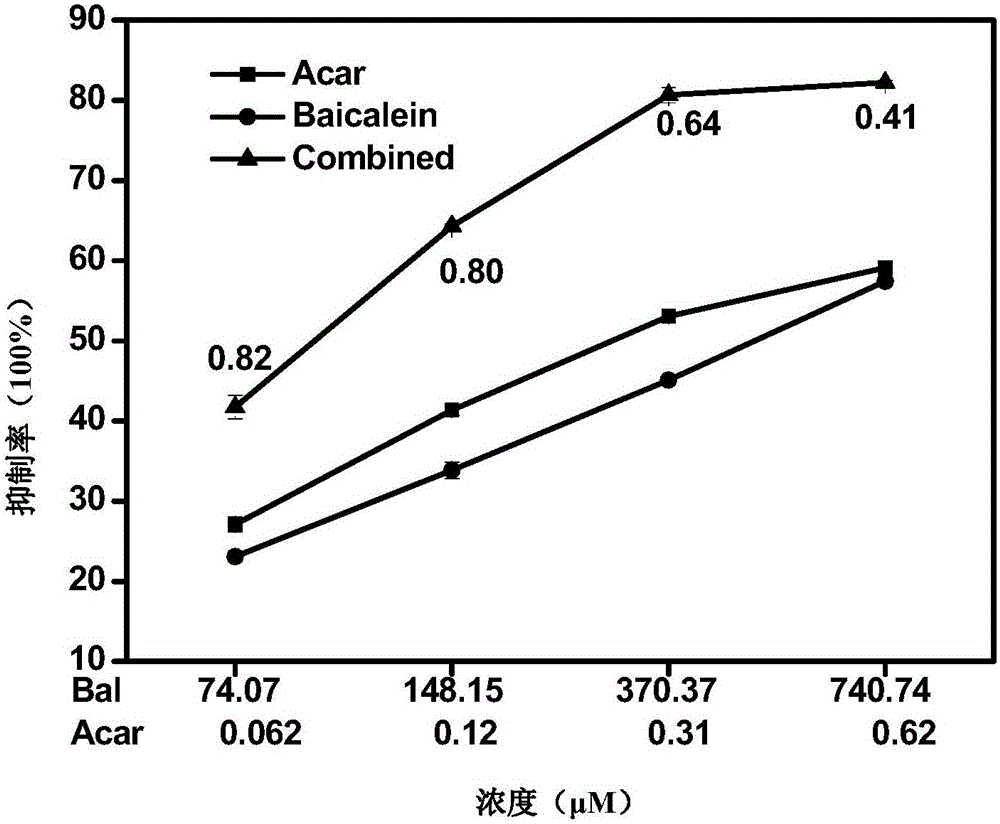
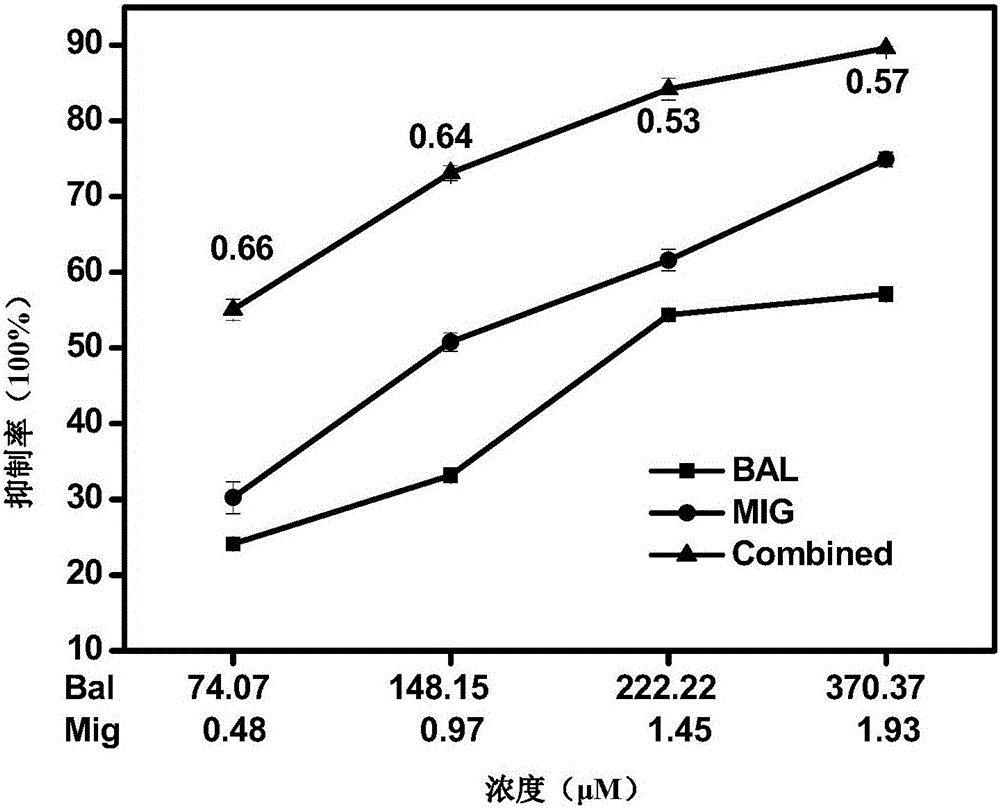
The invention relates to a pharmaceutical composition having alpha-glucosidase inhibition activity, wherein the pharmaceutical composition comprises a flavone compound and an alpha-glucosidase inhibitor, the flavone compound is at least one selected from a monomer such as baicalein, quercetin, luteolin, baicalein-7-O-glucoside and catechin, an organic salt of the monomer, and an inorganic salt of the monomer, and the alpha-glucosidase inhibitor is at least one selected from a monomer such as acarbose, voglibose and miglitol, an organic salt of the monomer, and an inorganic salt of the monomer. According to the present invention, the pharmaceutical composition can effectively reduce postprandial blood glucose, can inhibit the activity of alpha-glucosidase adopting starch, maltose and sucrose as substrates, and less uses the alpha-glucosidase inhibitor, such that the efficacy can be improved, the side effect of the alpha-glucosidase inhibitor can be effectively reduced, and hypoglycemia and other problems easily caused by drug combination are effectively solved.
Owner:上海皋鱼医药科技有限公司
Method for extracting and purifying effective parts of peanut shells
The invention provides a method for extracting and purifying effective parts of peanut shells, and belongs to the technical field of natural medicine extraction. The purity of luteolin at the extracted effective parts of the peanut shells reaches 60%. The method specifically comprises the following steps: (1) carrying out extracting with an ethanol aqueous solution under micropressure; (2) concentrating; (3) performing recrystallization; (4) performing column chromatography. The greatest feature of the method is that the operation is simple and economical, requirements on instrument and equipment are low, the production period is short, and the method is suitable for industrial production; only ethanol and water which are used as reagents are adopted, so that the method is safe and reliable, and the obtained effective parts can be directly used for study of peanut shell efficacy or pharmacology.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Bamboo-leaf-flavonoids molecular imprinted solid-phase extraction column, preparation and application thereof
InactiveCN102671639AEasy to separateEfficient enrichmentIon-exchange process apparatusOrganic chemistryFunctional monomerSolid phase extraction
The invention relates to a bamboo-leaf-flavonoids molecular imprinted solid-phase extraction column, preparation and application thereof, and belongs to the technical field of medicine separation. Template molecule luteolin, functional monomer 4-ethylpyridine and ethylene glycol dimethacrylate which is a cross-linking agent are polymerized into bamboo-leaf-flavonoids molecular imprinted polymer particles in a suspension manner, the molar ratio of template molecules to the functional monomer to the cross-linking agent is 1:(5-8):80. The prepared bamboo-leaf-flavonoids molecular imprinted polymer particles are filled in a polypropylene or glass solid-phase extraction column hollow tube, so that the bamboo-leaf-flavonoids molecular imprinted solid-phase extraction column is prepared. The bamboo-leaf-flavonoids molecular imprinted solid-phase extraction column is used for selectively gathering and purifying bamboo-leaf-flavonoids extracting solution, has the advantages if simplicity, rapidness, high recycling rate and the like, and is wide in application prospect in the field of separation of natural flavonoids compounds.
Owner:INT CENT FOR BAMBOO & RATTAN
Clothing antibacterial softener preparation method
The invention relates to a clothing antibacterial softener preparation method, which comprises: weighing 5-10% by mass of dioctadecyl dimethyl ammonium chloride, 0.3-0.5% by mass of polydimethylsiloxane diquaternary ammonium salt, 7-15% by mass of luteolin, 2-10% by mass of ethylene glycol, and a proper amount of water, mixing, heating to a temperature of 55-60 DEG C, dissolving, heating to a temperature of 75 DEG C, adding 5-15% by mass of a thickening agent polyethylene glycol 6000, adding 0.5-1.0% by mass of eugenol, and cooling to obtain the product. According to the present invention, the luteolin is adopted as the main component so as to inhibit a variety of bacteria and viruses, such as staphylococcus aureus, escherichia coli, herpes simplex virus and poliovirus; the antibacterial function is strengthened under the premise of no influence on softening property and the like; and the clothing antibacterial softener with characteristics of good compatibility with the softener, stable performance, good antibacterial effect, capability of inhibition of odor due to bacterial breeding during a wearing process of clothing, no odor, no irritating, low toxicity, safety and no harm on environment is provided.
Owner:WUJIANG CITY LI DA LUSTRE FINISHED PROD
Preparation, quality control method and application of composition of active ingredients of bitter herb
ActiveCN102362883AHigh yieldLow cost of water extractionComponent separationCardiovascular disorderMedicinal herbsPolyamide
The invention discloses a preparation method, a quality control method and an application of composition of active ingredients ob bitter herb. The composition of the active ingredients of the bitter herb disclosed by the invention mainly comprises cichoric acid and luteolin-7-O-beta-D-glucuronide, and content of the cichoric acid and the luteolin-7-O-beta-D-glucuronide is not less than 80%. An extraction method of the composition comprises that pretreatment is carried out by adopting an enzymatic method, then a supercritical fluid extraction method is adopted, and the obtained extract is respectively subjected to separating, purifying, concentrating and drying by virtue of an AB-8 resin column and a polyamide resin column so as to obtain the composition. Besides, the invention also relates to an application of the composition in preparation of modern bitter herb injection and treatment on coronary heart disease, angor pectoris, myocardial infarction and cerebral infarction and an application of the composition in content determination of the composition of the active ingredients of the bitter herb and quality control of bitter herb medicinal material.
Owner:沈阳双鼎制药有限公司
Preparation method of boron affiliated dual recognition molecularly imprinted material
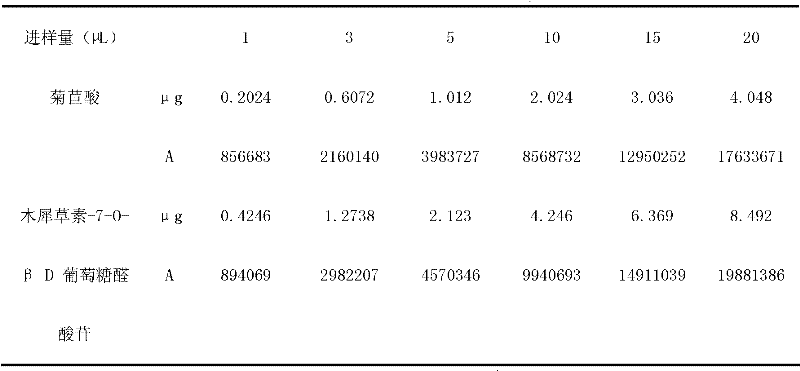
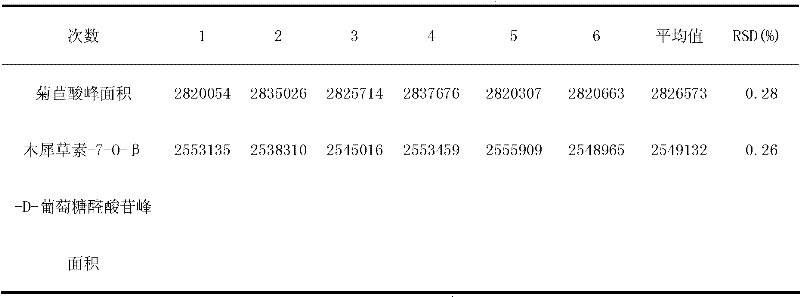
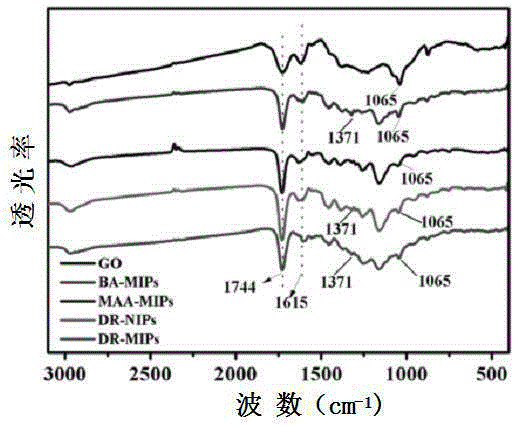
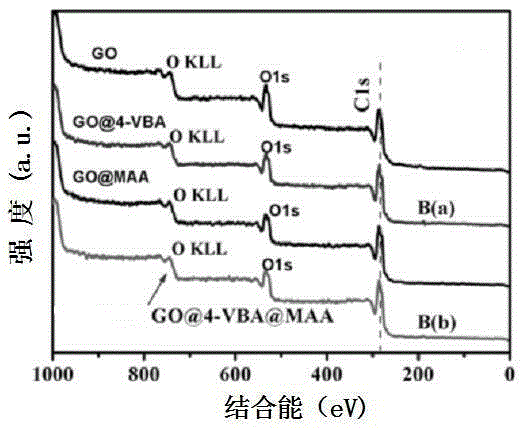
InactiveCN105233801AHigh mechanical strengthGood acid response propertiesOther chemical processesAtom-transfer radical-polymerizationHost material
Belonging to the technical field of preparation of environment functional materials, the invention relates to a preparation method of a boron affiliated dual recognition molecularly imprinted material. The method includes: firstly modifying natural flake graphite powder to form graphene oxide, then taking graphene oxide (GO) as the matrix material to prepare an imprinted polymer (DR-MIPs) with dual recognition molecular imprinting function by atom transfer radical polymerization; and conducting a series of treatment to obtain an adsorbent, and applying the adsorbent to selective recognition and separation of luteolin in an aqueous solution. The boron affiliated dual recognition molecularly imprinted material prepared by the method provided by the invention has good thermal stability, large surface area, high adsorption capacity, the function of reversible adsorption / release of acid-base effect along with acidity and alkalinity, and significant LTL molecular recognition performance.
Owner:JIANGSU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com