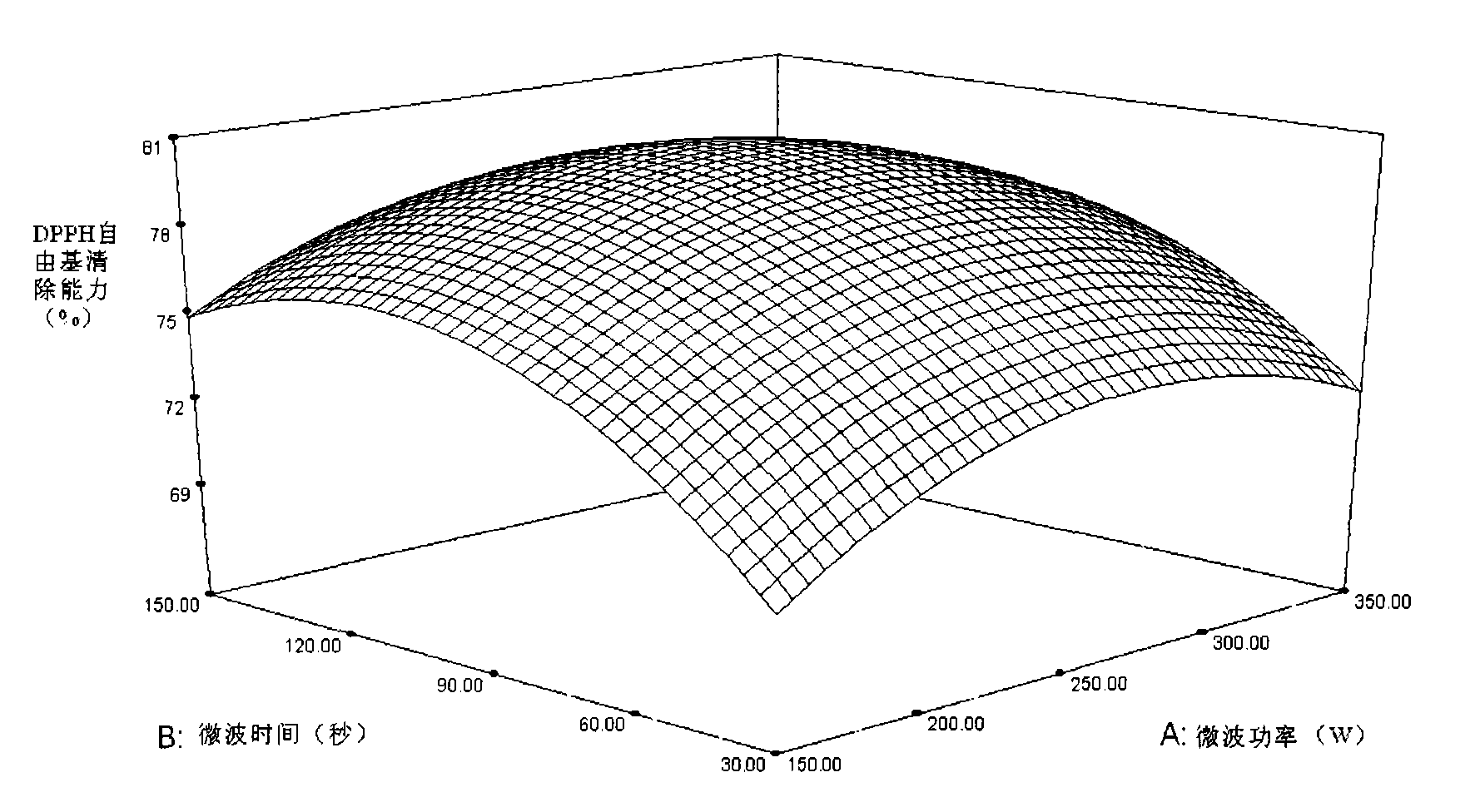
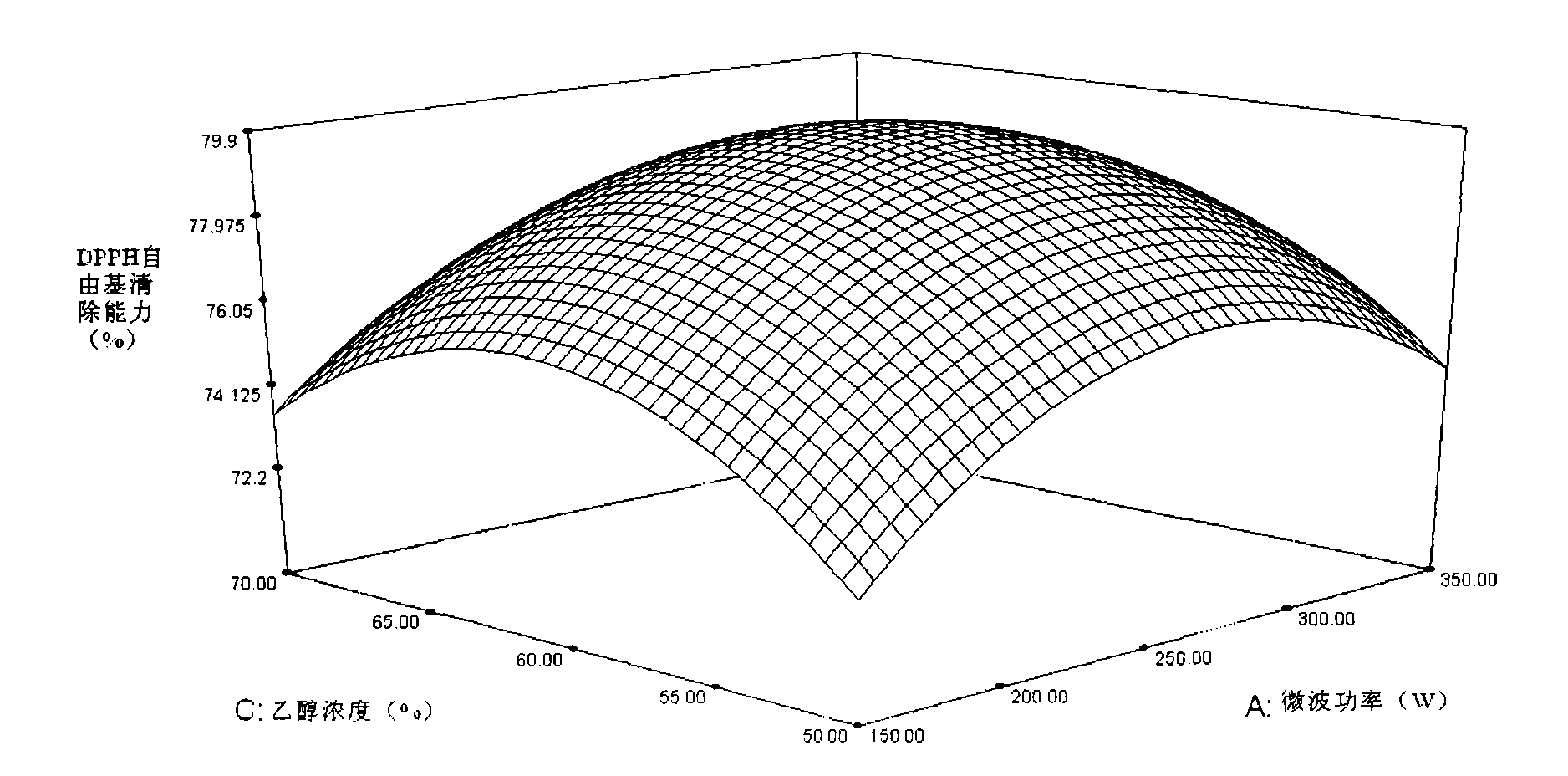
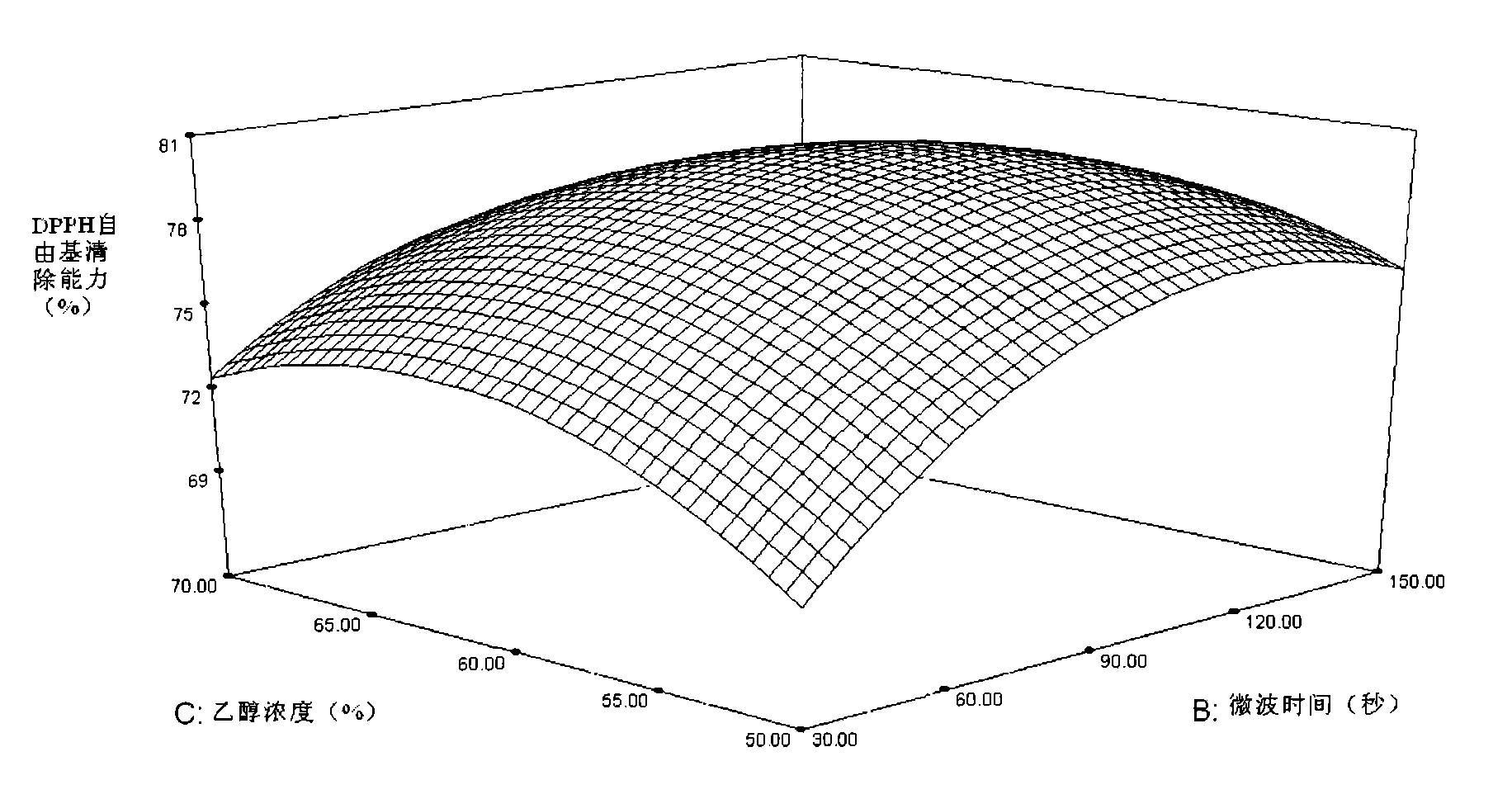
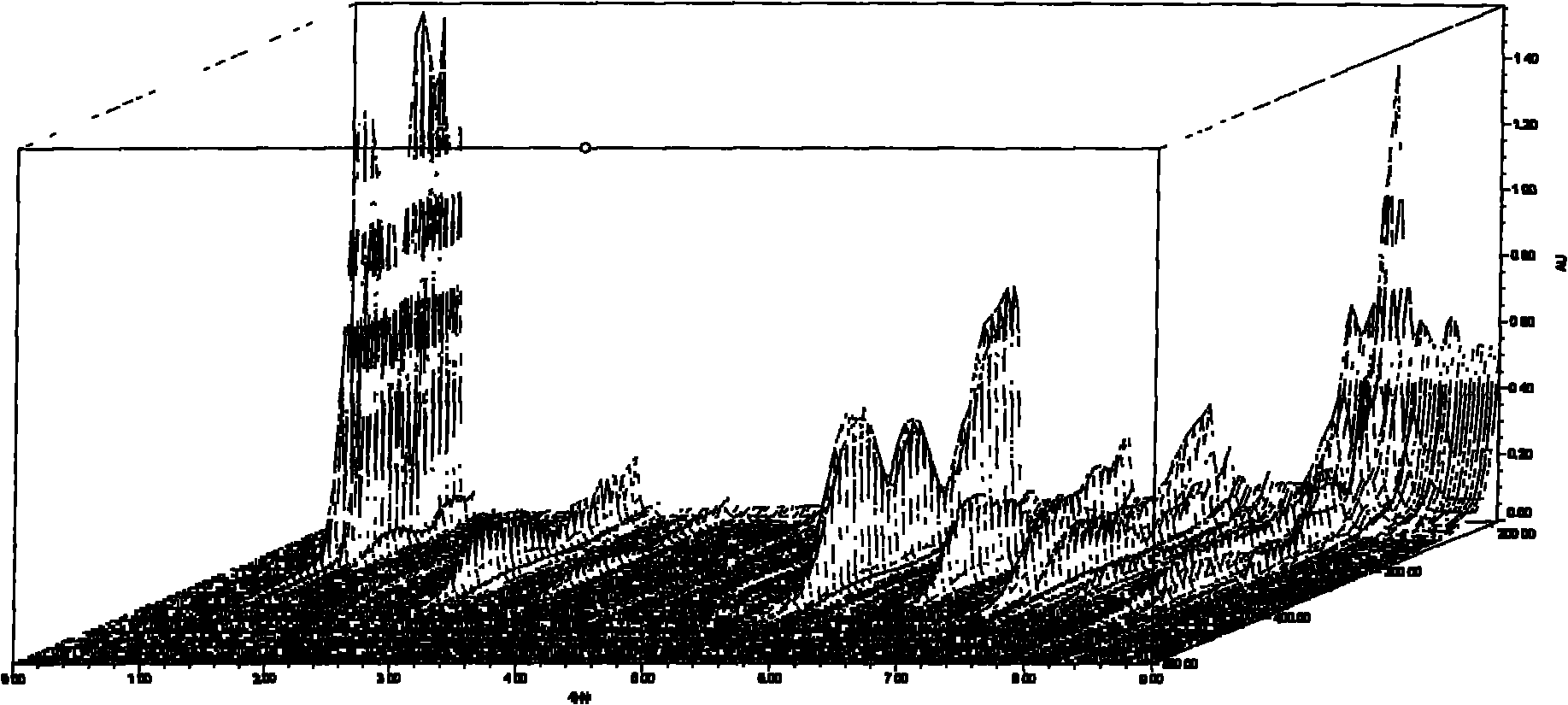
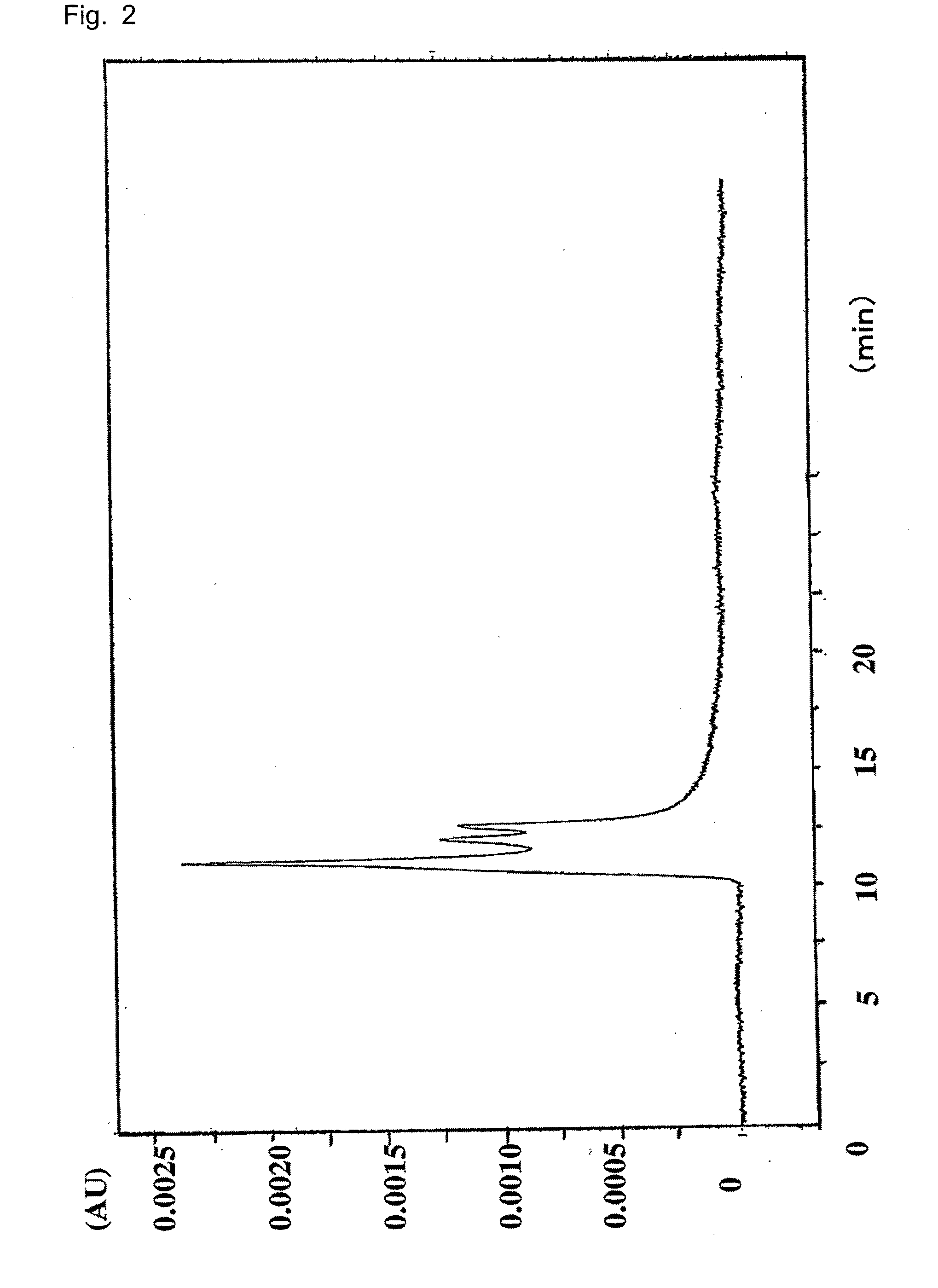
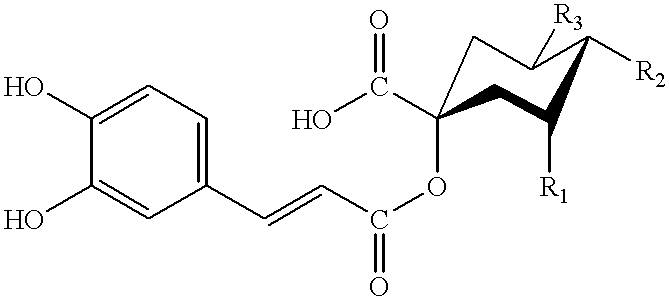
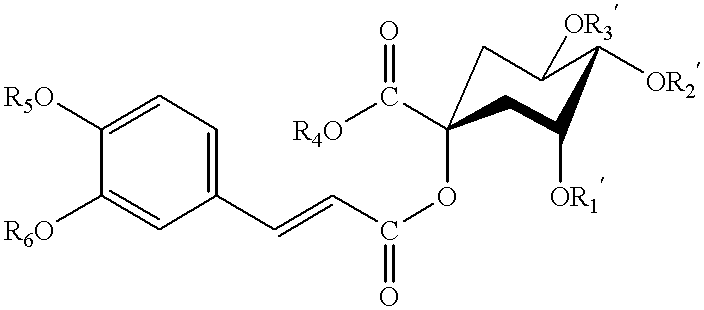
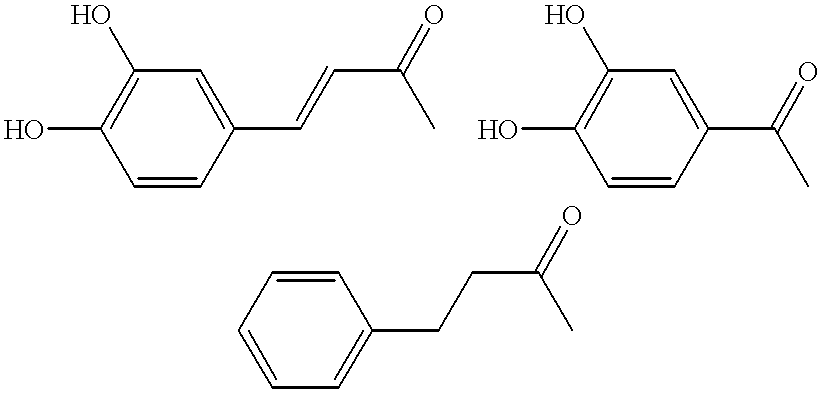
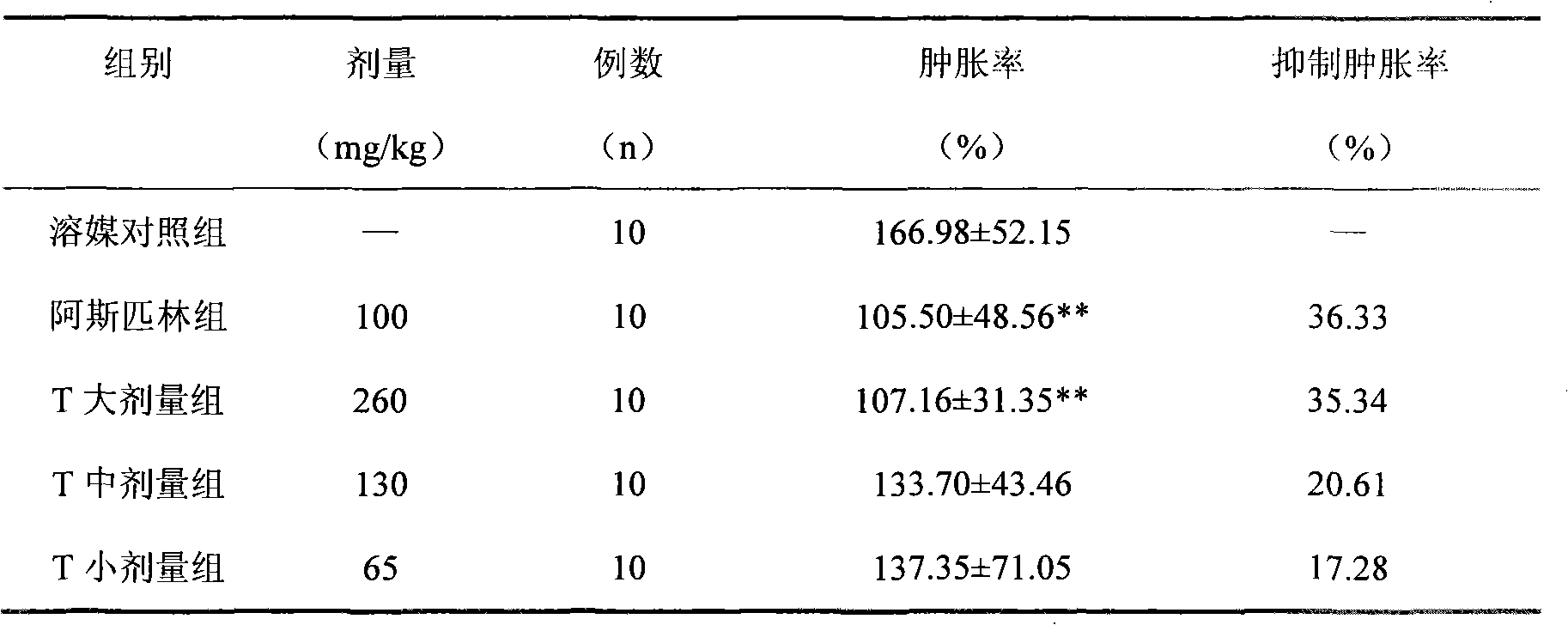
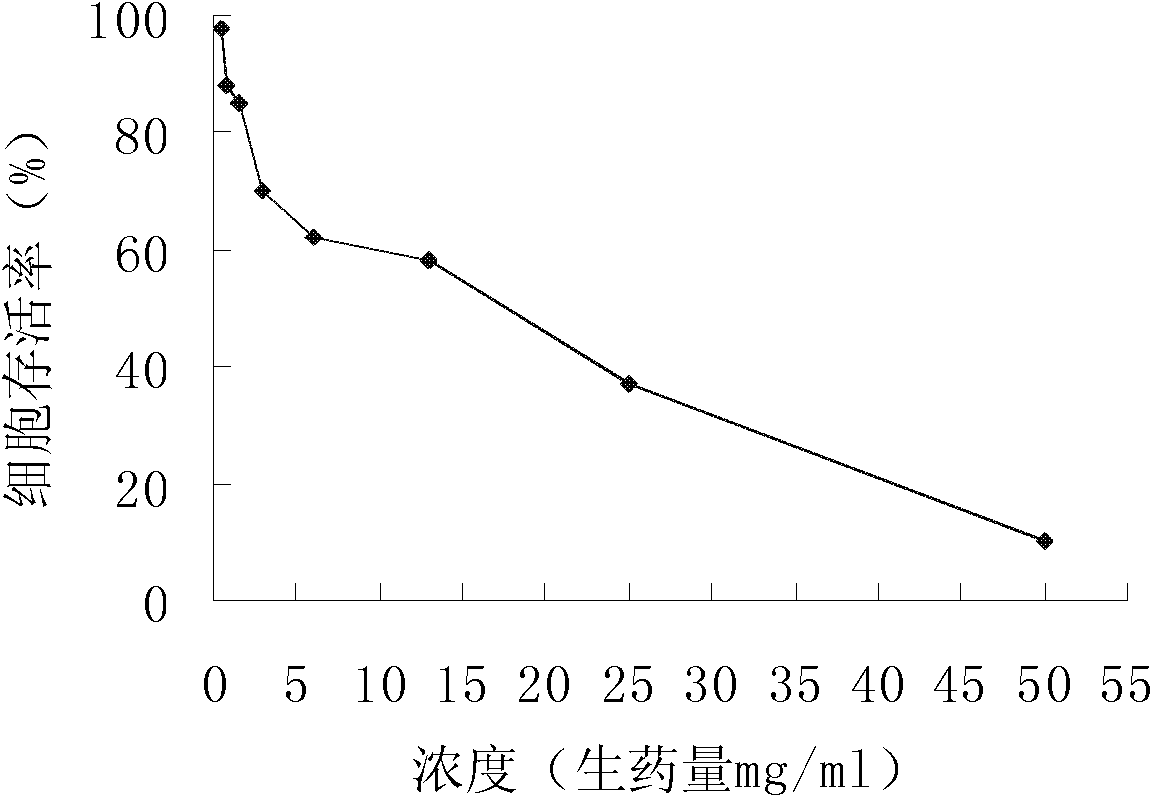
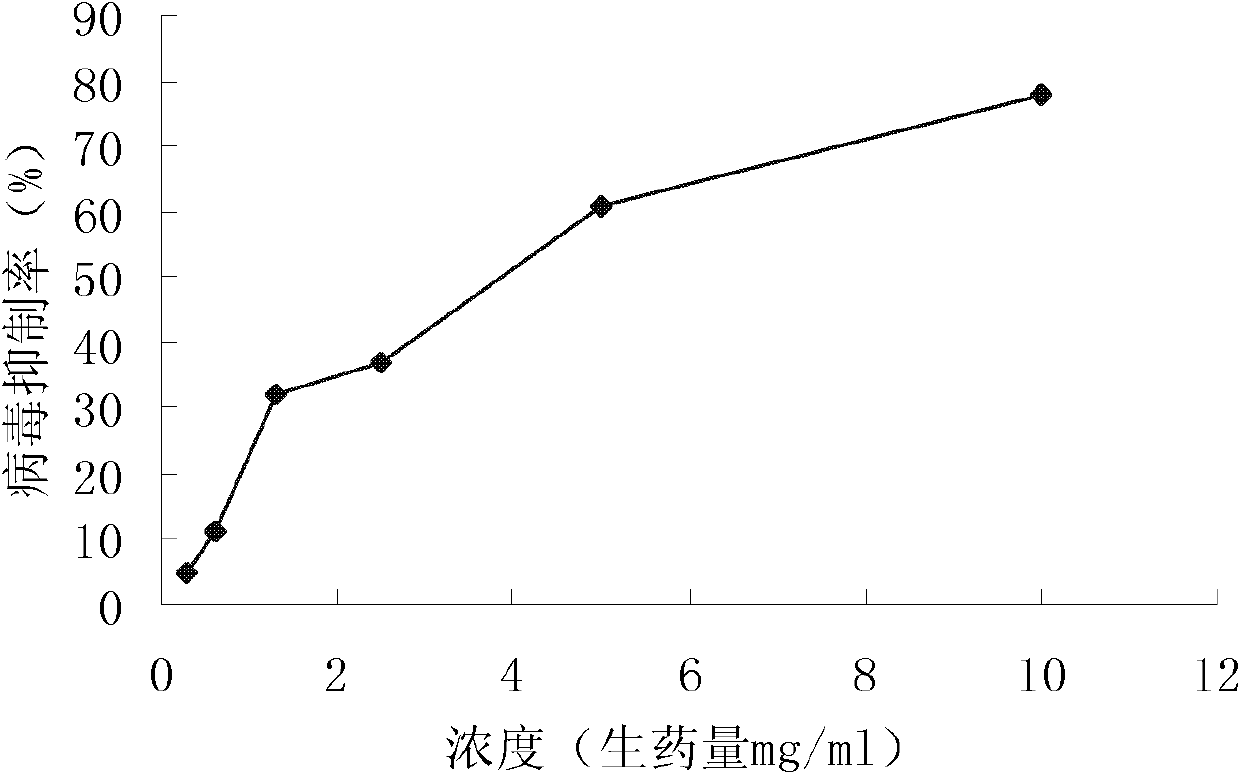
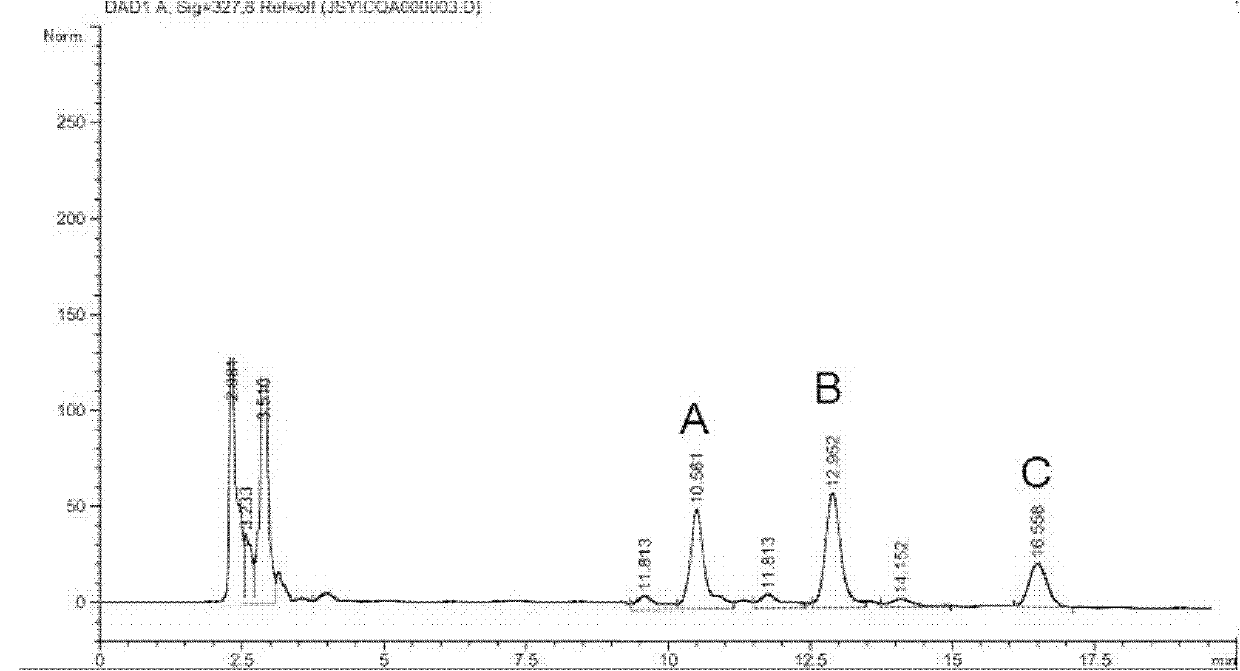
Patents
Literature
110 results about "Dicaffeoylquinic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fleabane extract and dicaffeoylquinic acid medicine composition and its application
The present invention relates to a medicine composition formed from breviscapine which is effective component extracted from Chinese medicinal material erigeron breviscapus and cynarin and its new application for curing acute and chronic hepatitides. The breviscapine and cynarin are mixed according to a certain proportion, and proper auxiliary material can be added, and can be made into various preparations for curing and preventing the diseases of fibrosis of liver, cirrhosis of liver and fatty liver, etc.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for preparing antioxidative active extractive of sweet potato leaves
ActiveCN101773593AEasy to process and useImprove cleanlinessAntinoxious agentsPlant ingredientsDicaffeoylquinic acidCaffeoylquinic acid
The invention relates to a method for preparing an antioxidative active extractive of sweet potato leaves, and belonging to the field of agriculture products processing. The method comprises the following steps: drying and crushing fresh sweet potato leaves; performing microwave assistant extraction after the decoloring and degreasing treatment is carried out; decompressing and concentrating extract in vacuum; chromatographing through a column, and absorbing by maroporous resin; collecting eluent; decompressing and concentrating the eluent in vacuum to obtain extract of the sweet potato leaves; and atomizing and drying the extract to obtain powdered extractive of the sweet potato leaves. The product is powdered, has the unique smell of the sweet potato leaves, contains 65 to 75 percent of total phenol, and contains chlorogenic acid and 3,5-O-dicaffeoylquinic acid. The method adopts mass waste sweet potato leaves to prepare the extractive with strong antioxidative activity, turns waste into wealth, is a green industry, has simple process, no need of larger equipment investment and high extracting rate of antioxidative active substances, and is applicable to industrial production.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Stevia rebaudian valid target as well as its activity and application
InactiveCN101156883ASignificant hypoglycemiaSignificant lipid-lowering effectMetabolism disorderPharmaceutical delivery mechanismApigeninAcute hyperglycaemia
The invention discloses an effective part of stevia and the activity and the application thereof. The effective part mainly comprises stevioside category and flavone category which are obtained by extracting and separating from dried stevia leaves, wherein the sum of the percentage content of the stevioside components in the stevioside part is 5 to 100 percent (w / w), and the stevioside components mainly contains the stevioside, stevibioside, rebaudioside A, B, C, D, E, and F, dulcoside A and the derivative thereof, etc.; the sum of the percentage content of the flavone components in the flavone part is 5 to 100 percent (w / w), and the flavone components mainly contains luteolin, quercetin, luteolin-7-O-Beta-D-glucoside, apigenin-7-O-Beta-D-glucoside, quercitrin, quercetin-3-O-Beta-D-arabinoside, quercetin-3-O-(4-O-anti form-caffeoyl acyl-Alpha-L-rhamnose-(1 to 6)-Beta-D-galactoside) and the derivative thereof, etc., and 4, 5-dicaffeoylquinic acid and the derivative thereof, etc. The effective part has significant sugar-reducing and fat-reducing effects, can be used singly or combined with any other Chinese medicines and Western medicines or foods in any proportion, is used for preparing medicines or functional foods, and is used for treating hyperglycemia and hyperlipoidemia.
Owner:石任兵
Quality control method for erigeron breviscapus (Vant.) hand-mazz.
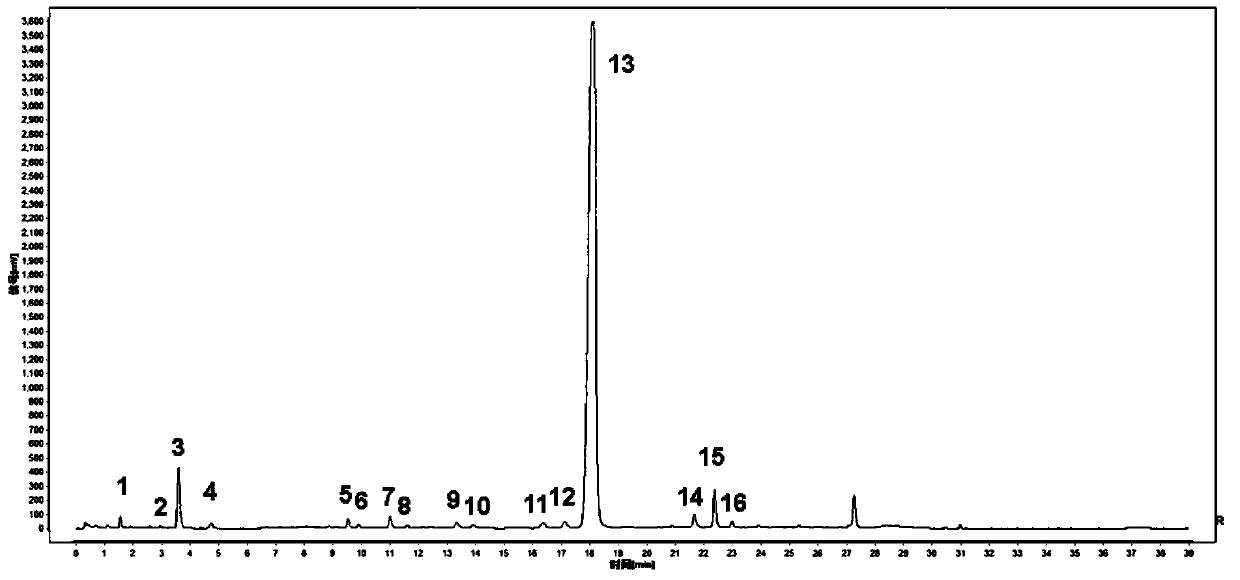
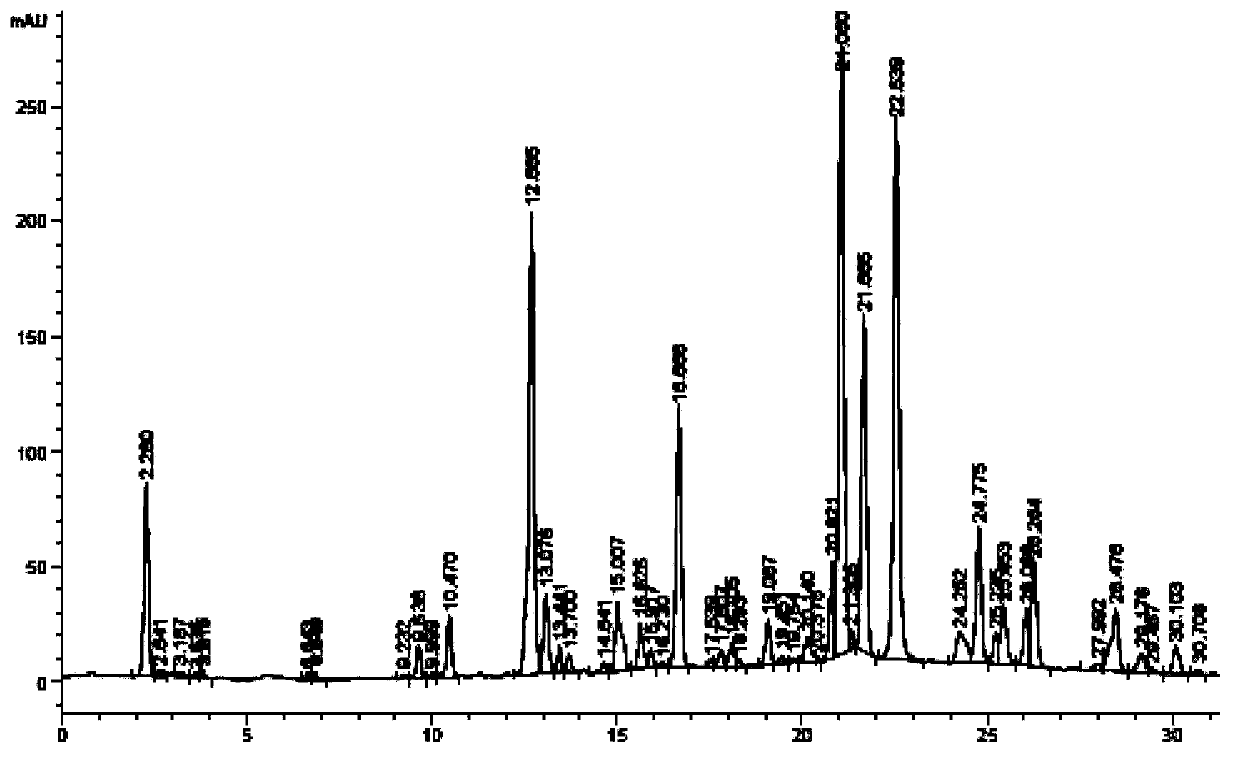
InactiveCN102038728APerfect quality control systemIntrinsic qualityComponent separationAntipyreticUplc pdaDicaffeoylquinic acid
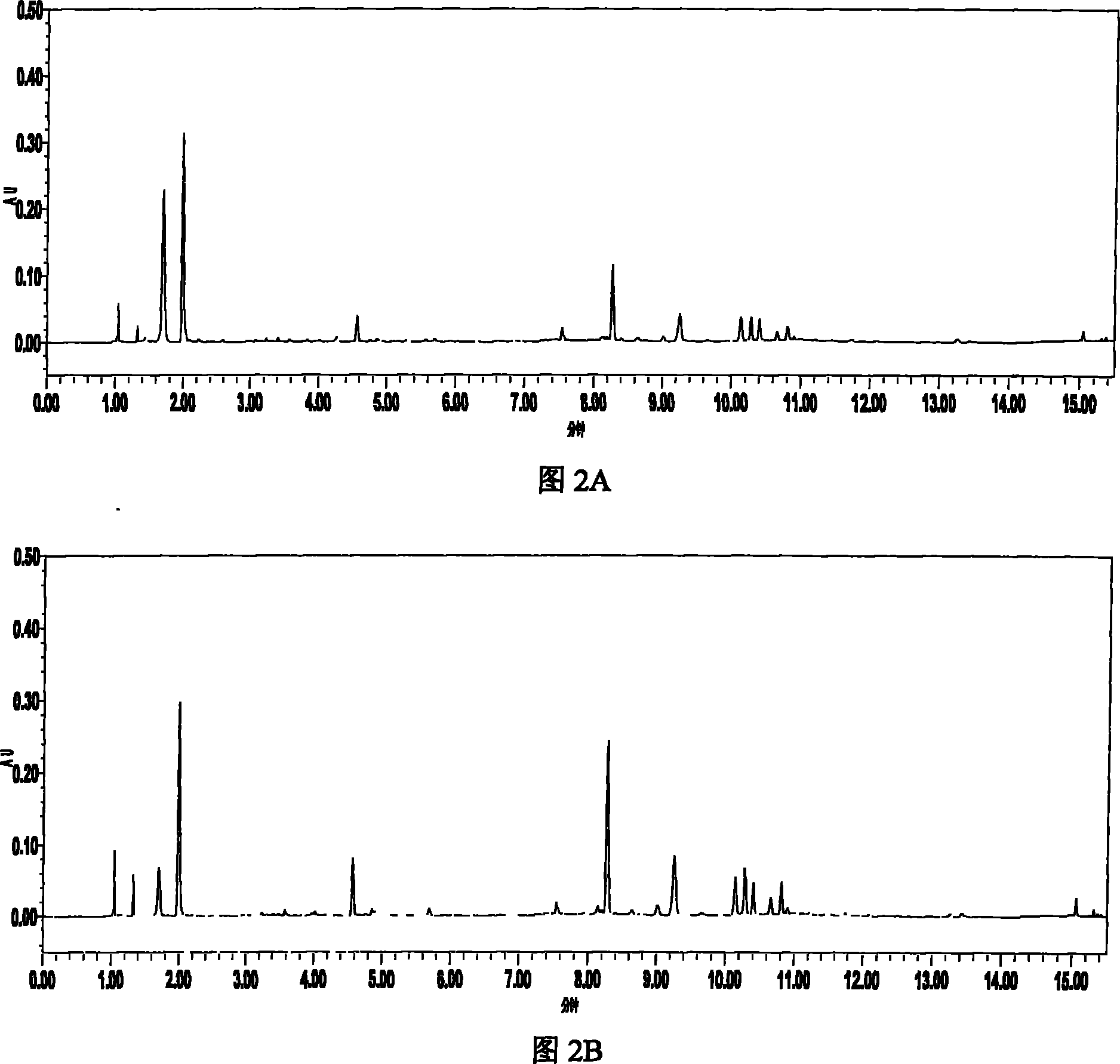
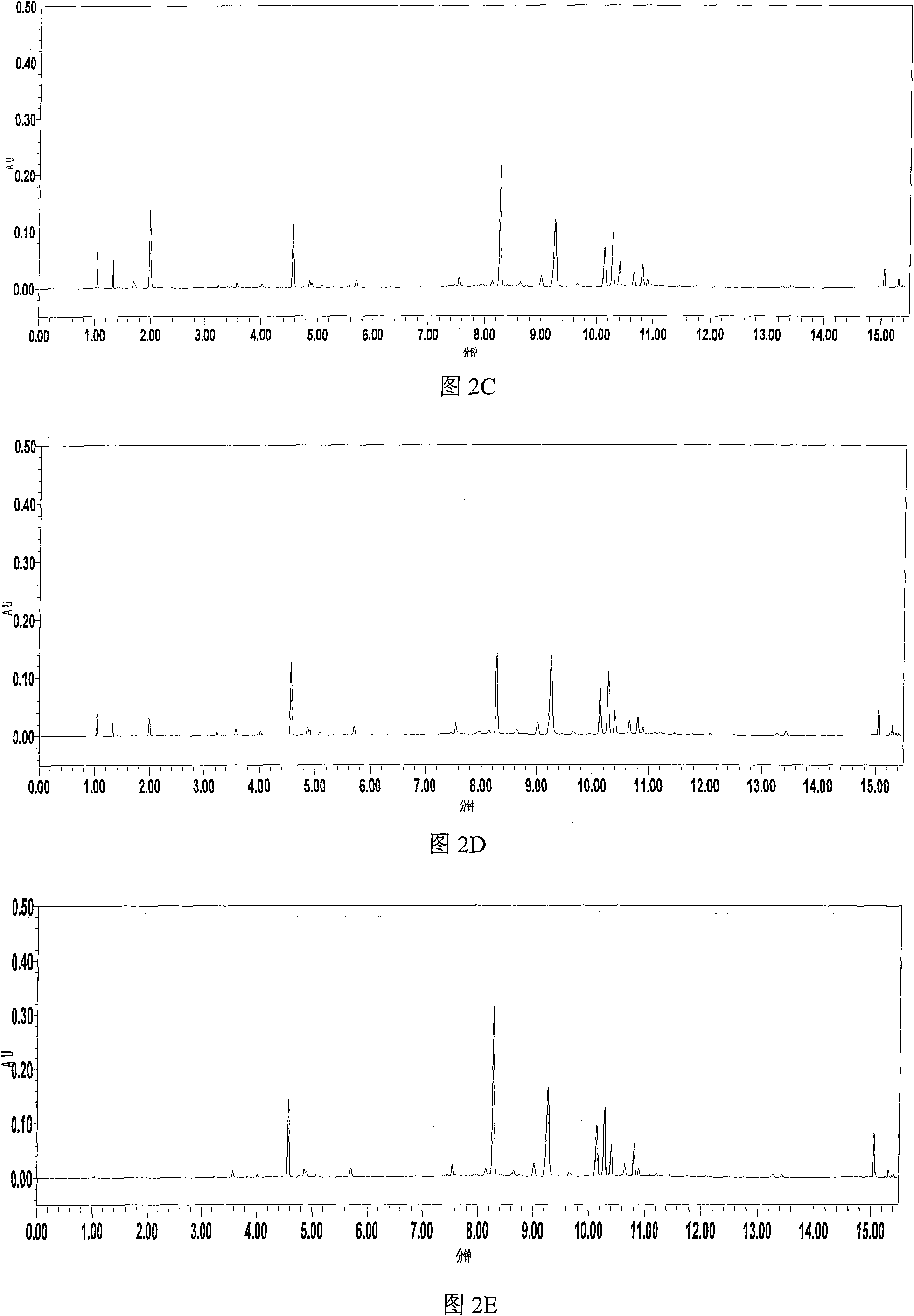
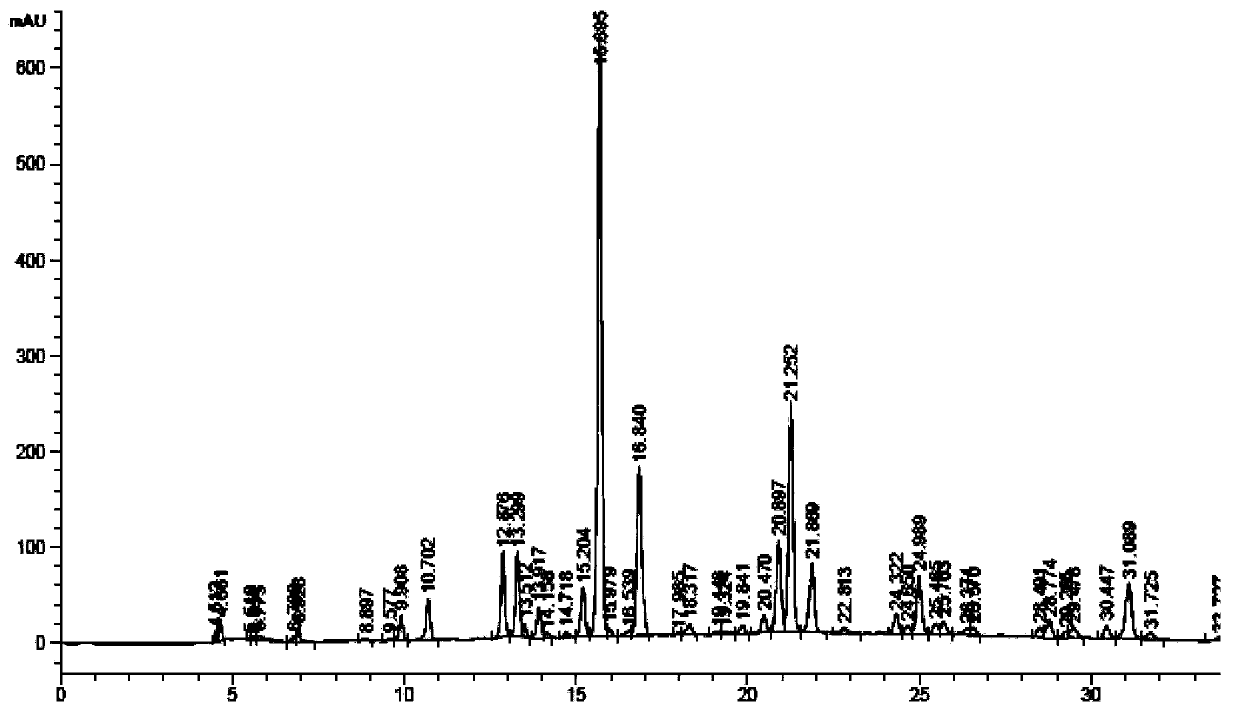
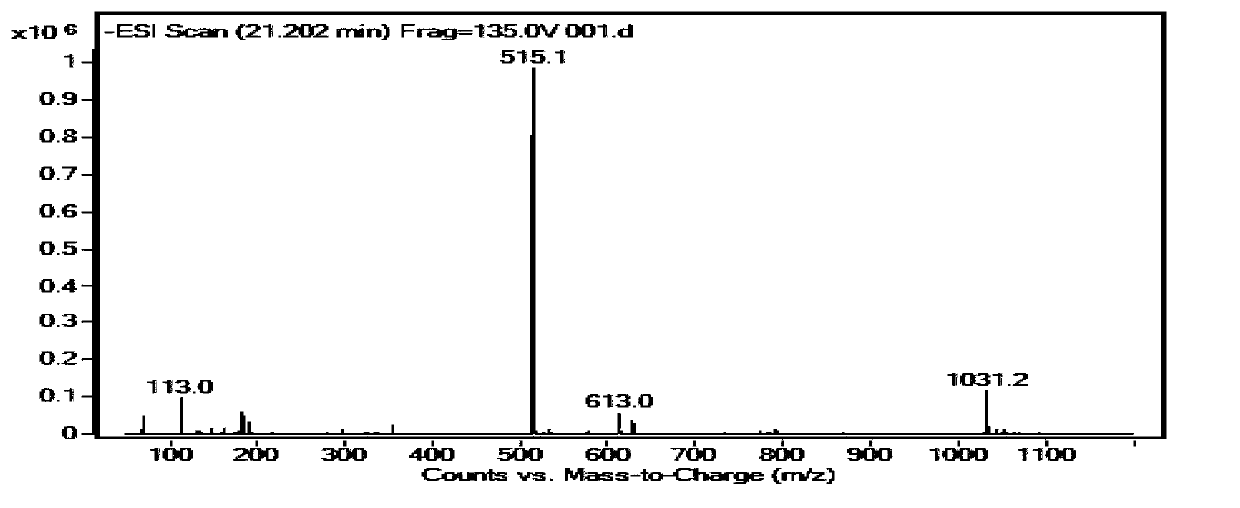
The invention relates to the field of the quality control on traditional Chinese medicine, specifically to a quality control method for erigeron breviscapus(Vant.) hand-mazz. The method comprises a fingerprint detection method and a multiple-indicative content detection method for erigeron breviscapus (Vant.) hand-mazz, wherein the fingerprint detection method employs UPLC detection method, through which 24 characteristic peaks are found and 8 characteristic peaks are ascribed. By using content detection method for erigeron breviscapus(Vant.) hand-mazz., the UPLC-PDA method, contents of following constituents are determined: erigeroside, chlorogenic acid, breviscpini glycoside, Scutellarin, 3,4-0-dicaffeoylquinic acid, 3,5-0-dicaffeoylquinic acid and 4,5-0-dicaffeoylquinic acid. Compared with the prior art, the fingerprint detection method and the multiple-indicative content detection method for erigeron breviscapus (Vant.) hand-mazz are improved in the invention, so that the quality of erigeron breviscapus (Vant.) hand-mazz. can be controlled in a convenient, rapid and accurate manner, thereby ensuring the quality of erigeron breviscapus (Vant.) hand-mazz. stable, uniform and controllable.
Owner:GUIYANG MEDICAL UNIVERSITY
Packaged coffee drink
ActiveUS20090035421A1Prevent precipitationReady-for-oven doughsFruit and vegetables preservationHigh concentrationAdditive ingredient
To provide a packaged coffee beverage, which contains chlorogenic acids at high concentration, has good flavor and taste, and is suppressed in the occurrence of sediment during long-term storage. A packaged coffee beverage subjected to heat sterilization treatment, the beverage comprising (A) monocaffeoylquinic acid, (B) feruloylquinic acid and (C) dicaffeoylquinic acid, wherein (a) a total content of the ingredients (A), (B) and (C) contained in dissolved states in the beverage is from 0.14 to 4% by weight based on the beverage, and the beverage comprises (b) 80% by weight or more of water, (c) magnesium and sodium at a Mg / Na weight ratio of from 0.04 to 1, (d) a coffee extract obtained from roasted coffee beans having an L value of form 16 to 25, and (e) from 0.0024 to 0.0122% by weight of brown color in terms of Food Yellow No. 4.
Owner:KAO CORP
Dicaffeoylquinic acid for treating hepatitis B and the diseases associated with retrovirus, and the new caffeoylquinic acid derivatives
InactiveUS6331565B1Increased proliferationAvoid problemsBiocideOrganic chemistryDiseaseDicaffeoylquinic acid
This invention relates to the new use of dicaffeoylquinic acid derivatives for treating Hepatitis B and diseases associated with retrovirus (such as HIV), the new caffeoylquinic acid derivatives and the composition containing the same.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Preparation method for high-purity di-coffee mesitoyl quinine acid compounds
InactiveCN101343225AAvoid lostInhibitory activityDigestive systemAntinoxious agentsChromatographic separationAlcohol
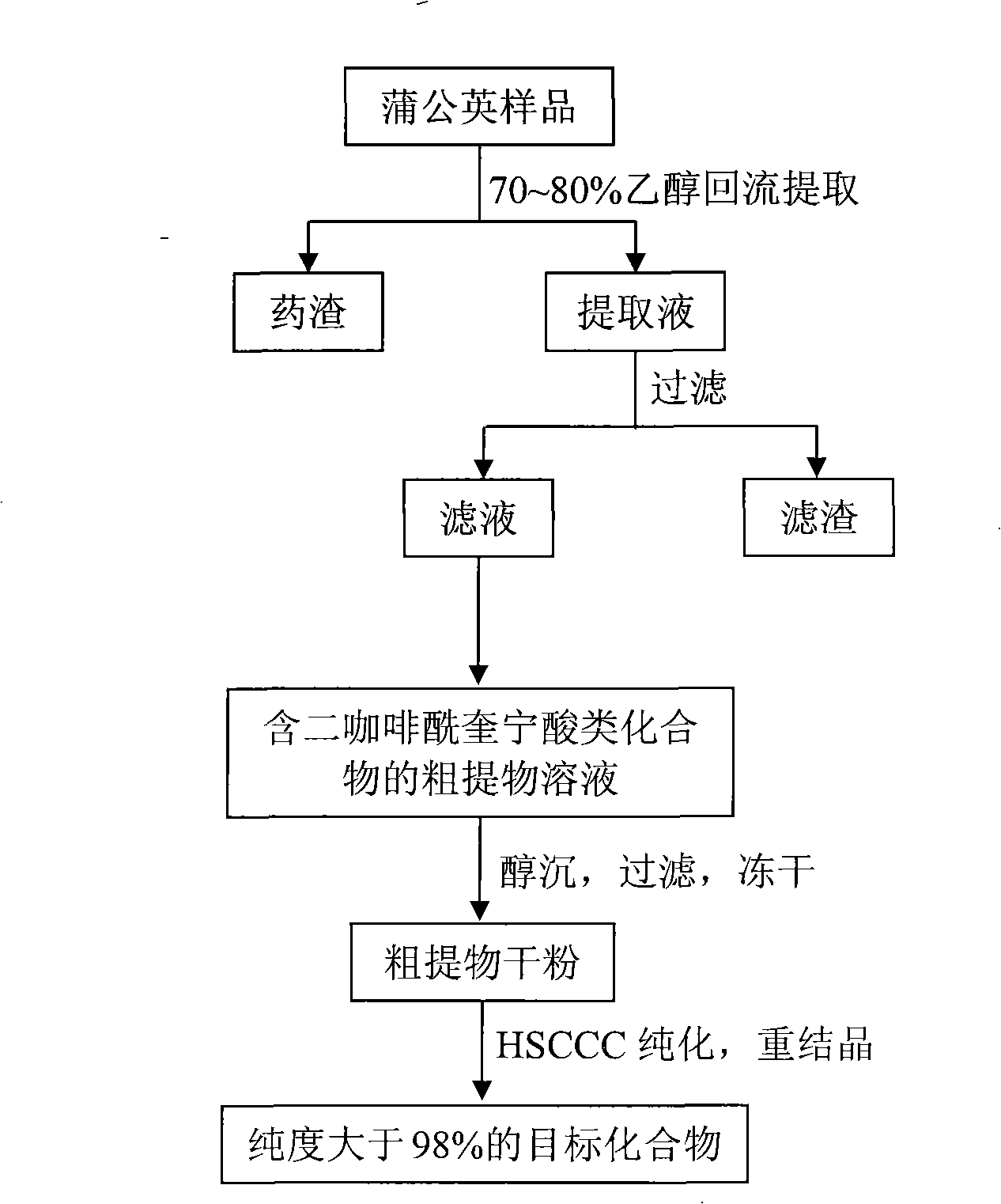
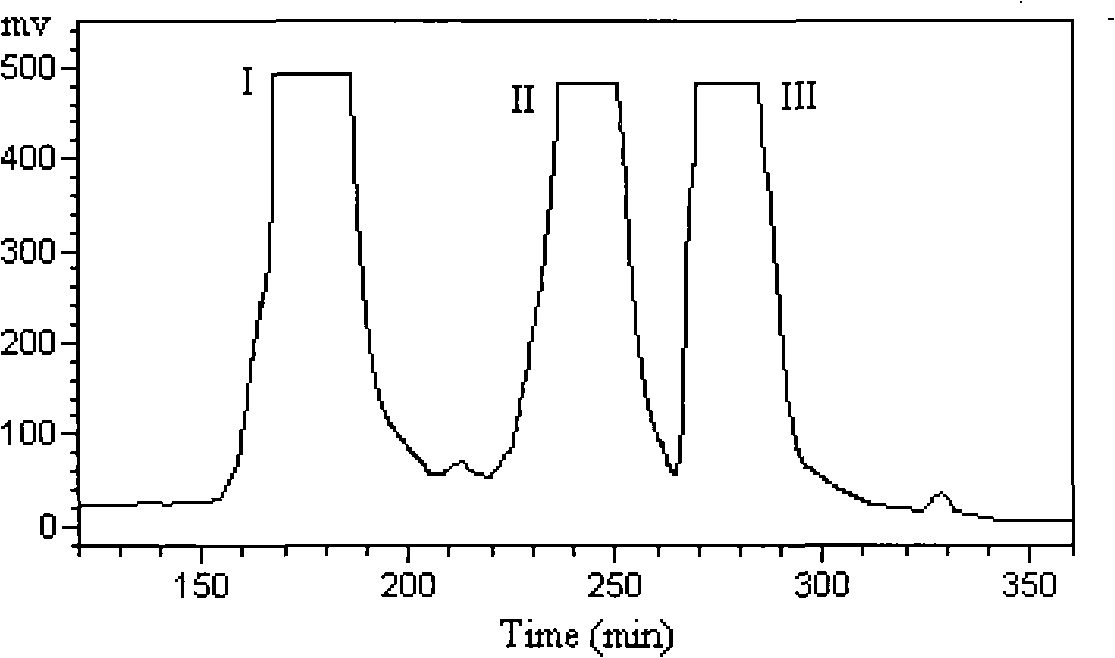
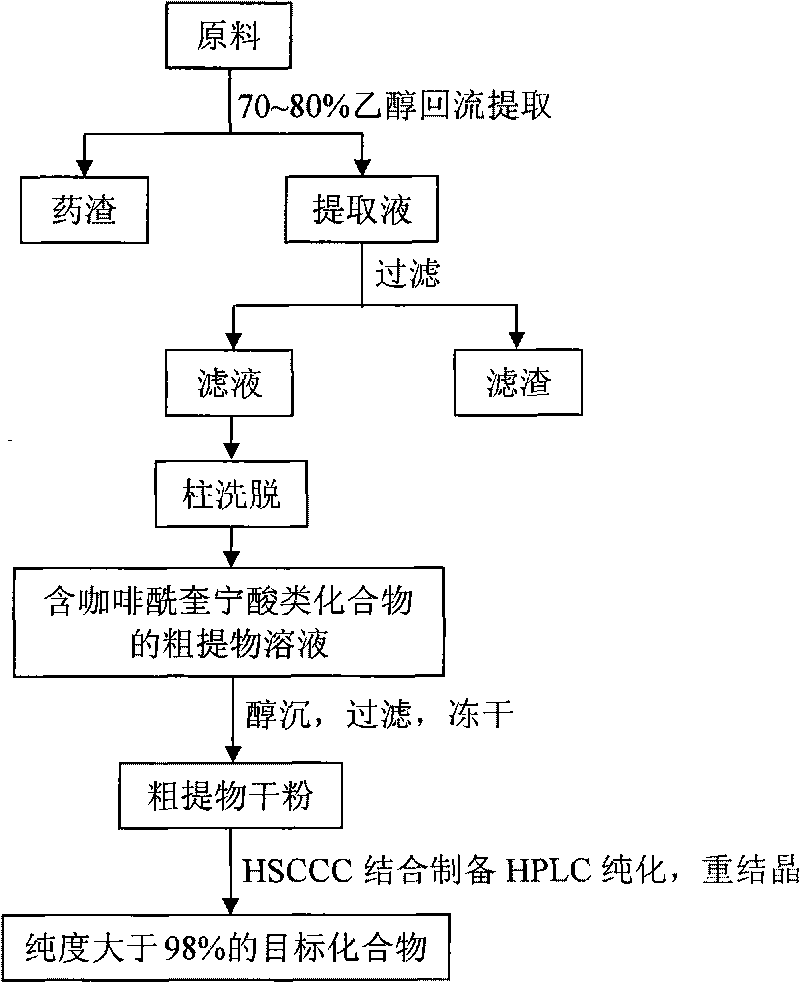
The invention discloses a method for preparing a high purity dicaffeoylquinic acid compound by high-efficient separation, mainly comprising: using an alcohol-water solution to extract taraxacum fresh plant or dry plant, carrying out the column chromatographic separation after the concentration, preparing a crude extract rich in dicaffeoylquinic acid compound, then isolating and purifying the crude extract by high-speed countercurrent chromatography, and finally adopting a recrystallization process for refining so as to obtain 3,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid with high purity. The method is applied to the preparation of high purity monomers by taking various natural products or natural product extracts containing one or a plurality of ingredients in the dicaffeoylquinic acid compound and acquired through various approaches as raw materials, and has advantages of simple steps, simple operation, high efficiency, low cost, easy repetition, and large output of separation and preparation. The purity of dicaffeoylquinic acid compound prepared by the method can reach 98 percent.
Owner:施树云 +1
Separation and purification methods of highly purified antiviral active components in artichoke
InactiveCN101691330ALarge separation capacityImprove performanceIon-exchange process apparatusOrganic compound preparationChromatographic separationPurification methods
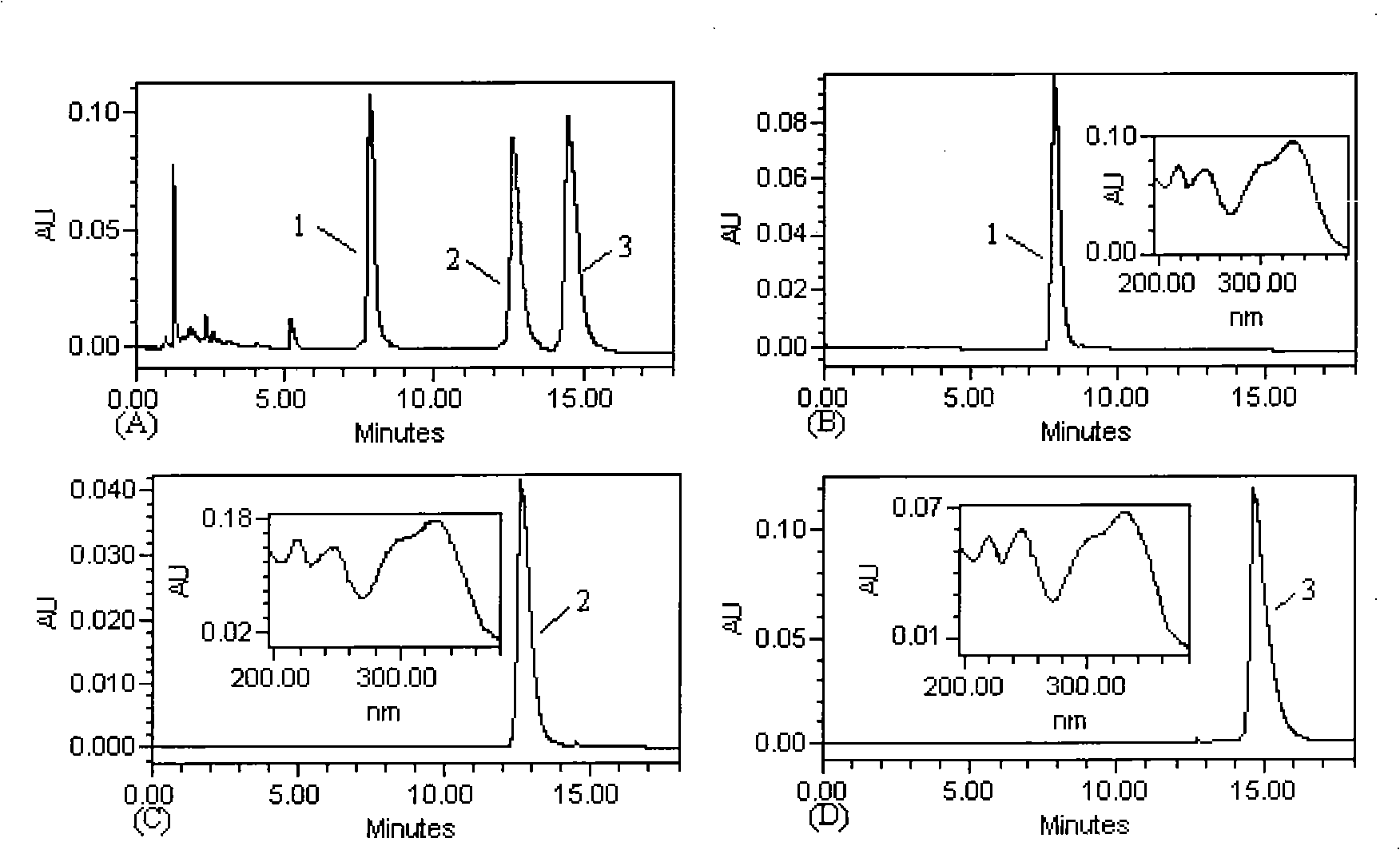
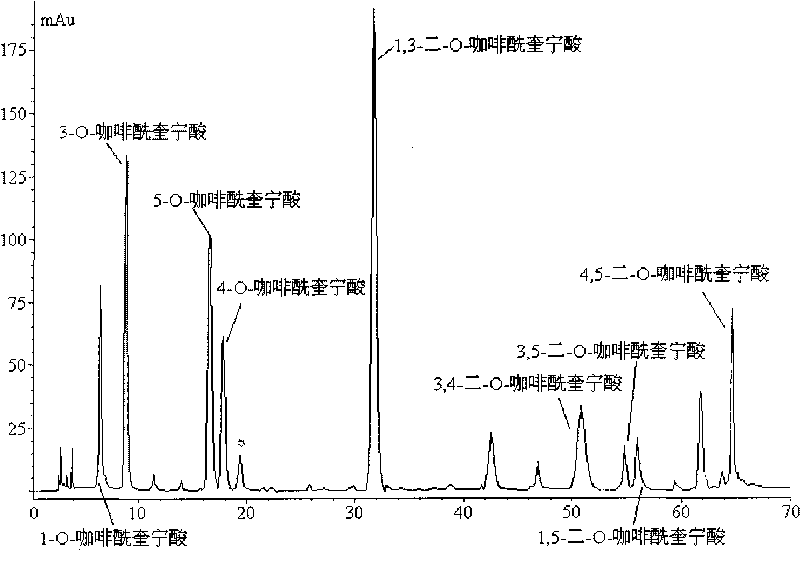
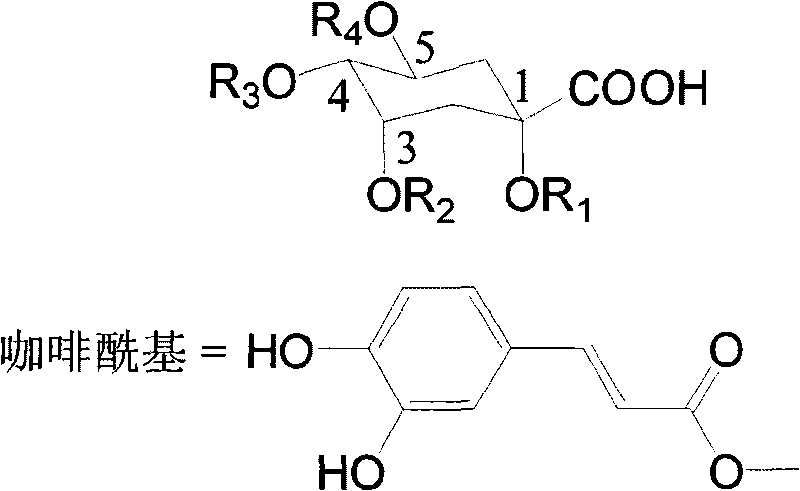
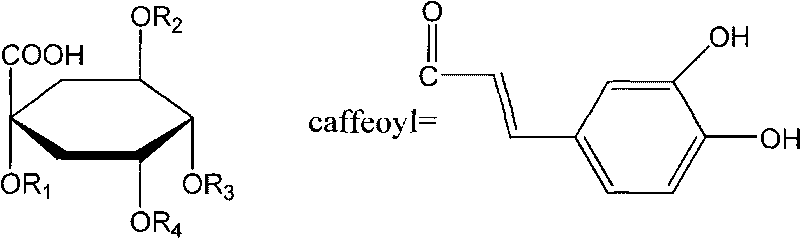
The invention discloses a method for efficiently separating and preparing highly purified antiviral active components (single caffeoylquinic acid compound and dicaffeoylquinic acid compound) in artichoke, mainly comprising the following steps: utilizing alcohol aqueous solution to extract fresh or dry artichoke, concentrating, carrying out column chromatographic separation, preparing crude extract rich in the antiviral active components, carrying out separation and purification on the crude extract by combing high speed counter-current chromatography with high preparative performance liquid chromatography, and finally adopting a recrystallization method for refining to obtain highly purified 1-O-caffeoylquinic acid, 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 1,3-di-O-caffeoylquinic acid, 1,5-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, 3,4-di-O-caffeoyl quinine acid and 4,5-di-O-caffeoylquinic acid. The method is suitable for preparing highly purified monomer by utilizing various natural products or the extractives of the natural products containing one or more components containing the above caffeoylquinic acid compounds as raw materials, wherein the natural products or the extractives are obtained from various ways; and the method has simple steps, simple operation, high efficiency, low cost and large separating preparation quantity, is easy to repeat; and the purification of the caffeoylquinic acid compounds prepared by the method can be up to 98%.
Owner:CENT SOUTH UNIV
Separation purifying method for DCQA (dicaffeoylquinic acid) component in jerusalem artichoke
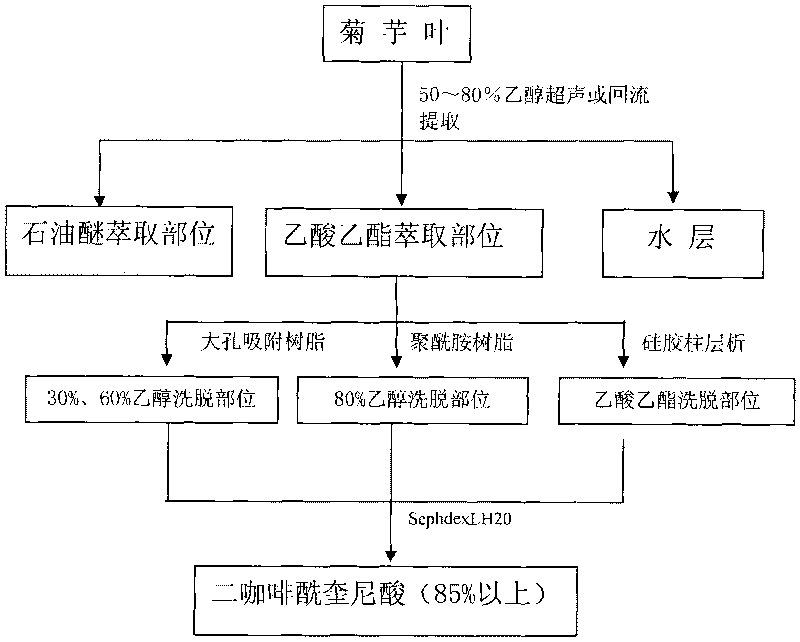
InactiveCN101747195AIncrease contentHigh yieldCarboxylic acid esters separation/purificationPlant ingredientsChromatographic separationDicaffeoylquinic acid
The invention discloses a separation purifying method for a DCQA (dicaffeoylquinic acid) component in jerusalem artichoke, belonging to the field of effective composition developing and utilizing. The main processing steps for extracting and separating a chemical compound in jerusalem artichoke are as follows: dried medicinal materials of jerusalem artichoke are crushed, ultrasonic or backflow extraction is carried out by ethanol (50 to 80%) in the quantity of 10 to 20 times, petroleum ether is degreased, extraction is carried out by ethyl acetate, and extraction liquid is decompressed and concentrated into an extractum. The extractum is preliminarily purified by resin or silica gel column chromatography and is further purified by SephdexLH20 so as to obtain the DCQA component with the content of higher than 85%. With simple technique and easy operation, the separation purifying method adopts diversified column chromatography to separate and purify the chemical compound of DCQA in jerusalem artichoke; the content of DCQA in a finished product is high, the source of raw materials is rich, the resin or SephdexLH20 can be recycled, and the industrialization is easy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for separating and purifying chlorogenic acid and 3,5-dicaffeoylquinic acid from honeysuckle flower
ActiveCN103641718ASimple compositionNot pollutedCarboxylic acid esters separation/purificationFluid phaseDicaffeoylquinic acid
The invention relates to a method for separating and purifying chlorogenic acid and 3,5-dicaffeoylquinic acid from a honeysuckle flower. According to the method, the honeysuckle flower is taken as a raw material and the method comprises the following steps: (1) preparing a honeysuckle flower crude extract; (2) extracting; (3) separating and purifying through macroporous adsorption resin; (4) separating and purifying through a semi-preparation type high efficiency liquid chromatography, namely separating and purifying by adopting the semi-preparation type high efficiency liquid chromatography to obtain two high-purity components by taking methanol-water as a mobile phase, wherein the two high-purity components are respectively identified as the chlorogenic acid and the 3,5-dicaffeoylquinic acid. The process disclosed by the invention is environment-friendly in process without severe harm on the environment and is low in integrated cost.
Owner:GUANGZHOU WANGLAOJI MAJOR HEALTH IND
Effect section of frangrant ainsliaea herb, preparation method and application thereof
ActiveCN101293003AEnhanced inhibitory effectEnhance anti-inflammatoryAntibacterial agentsAntipyreticDicaffeoylquinic acidAdditive ingredient
The invention relates to effective part of frangrant ainsliaea herb, a preparation method and application thereof, the invention (1) adopts 10 percent to 80 percent of ethanol to carry out percolation or heating reflux extraction; (2) ethyl acetate is used for extraction or macroporous absorption resin is used for purification. The method adopts water, alcohol, ethyl acetate and other commonly used solvents and has the advantages of simple operation, short process, low cost, high content of active ingredients and so on. The effective part of the frangrant ainsliaea herb mainly contains chlorogenic acid, 3, 5-dicaffeoylquinic acid and other phenolic acid ingredients, has antibacterial and anti-inflammatory effects, has significant inhibition effects on hepatitis B virus, aids virus, human papilloma virus, etc. and has a certain efficacy on cardiovascular diseases; and the effective part of the frangrant ainsliaea herb can be separately used or be matched with other drugs for the development of the new drugs for anti-inflammatory, anti-hepatitis B virus, anti-HIV, anti-human papilloma virus and the treatment of cardiovascular diseases.
Owner:江西佑美制药有限公司 +1
Sweet potato leaf extract and preparation method and use thereof
ActiveCN102210737ASignificant effect of protecting liver and reducing enzymesLow costDigestive systemAntiviralsBiotechnologyDicaffeoylquinic acid
The invention provides a sweet potato leaf extract and a preparation method and use thereof and belongs to the technical field of Chinese medicine preparations and preparation methods. The sweet potato leaf extract comprises the following active components in percentage: 3.2 to 7.2 percent of 4,5-dicaffeoylquinic acid, 8.3 to 38.1 percent of 3,5-dicaffeoylquinic acid, and 4.6 to 11.2 percent of 3,4-dicaffeoylquinic acid. The preparation method comprises: leaching sweet potato leaves in water, mixing extract, and filtering; and standing at a low temperature, filtering under vacuum, recovering,concentrating, loading concentrate on macroporous resin column, eluting with water and ethanol in turn, concentrating ethanol eluent, drying and obtaining the sweet potato leaf extract. The sweet potato leaf extract can treat hand-foot-and-mouth disease (EV71), respiratory syncytial virus(RSV) disease, hepatitis B and hepatitis and can protect liver and reduce enzymes. The invention has the advantages that: the sweet potato leaf extract can be used as substitutes for the conventional expensive medicines; and the preparation process is simple.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
New application of dicaffeoylquinic acid
InactiveCN101642450AOrganic active ingredientsNervous disorderAdditive ingredientDicaffeoylquinic acid
The invention provides an application of dicaffeoylquinic acid for the preparation of drugs of cerebral neuroprotection and the drugs can be used for curing ischemic strokes. The invention also provides a drug composite of cerebral neuroprotection. Drug effect tests and researches show that dicaffeoylquinic acid component is a new kind of effective ingredients which can protect cerebral nerve better, can both promote the survival of cerebral cortical neurons in vitro and the antioxidation and has a certain function of passing blood-brain barrier.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Method for preparing dicaffeoylquinic acid methyl compound and composition thereof
InactiveCN101774921AThe synthesis method is simpleMild reaction conditionsOrganic active ingredientsOrganic compound preparationDicaffeoylquinic acidAdditive ingredient
The invention relates to a method for preparing a dicaffeoylquinic acid methyl compound and an anti-influenza and antihepatitic medicine composition thereof. The method for preparing a dicaffeoylquinic acid methyl compound has the following steps of: extracting from woodbine, separating and modifying to obtain the dicaffeoylquinic acid methyl compound; and preparing the anti-influenza and antihepatitic medicine composition thereof. A great amount of anti-influenza and antihepatitic medicine composition containing dicaffeoylquinic acid methyl compound used as effective ingredients can be produced from plants with low dicaffeoylquinic acid methyl compound content by the invention.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Dicaffeoylquinic acid-containing drink
InactiveUS20150328179A1Suppressing astringencyBiocideOrganic active ingredientsArginineDicaffeoylquinic acid
A dicaffeoylquinic acid-containing beverage of the present invention includes the following components (A) and (B): (A) 0.02 to 0.18 mass % of dicaffeoylquinic acids; and (B) 0.1 to 1.0 mass % of L-arginine, in which a mass ratio between the component (A) and the component (B), [(B) / (A)], is from 2 to 18.
Owner:KAO CORP
Cynara scolymus extracts, the use thereof and formulations containing them
InactiveUS20090285911A1Reduce actionRaise the ratioBiocideMetabolism disorderThio-Dicaffeoylquinic acid
The present invention relates to the preparation of a Cynara scolymus extract obtainable by fractioning on a resin. The process of the invention allows to obtain an extract, starting from the aerial parts of the plant Cynara scolymus, containing three classes of active principles, namely dicaffeoylquinic acids, luteolin and cynaropicrin glycosides, in a constant ratio. Cynaropicrin is stabilized by addition of precise amounts of sulfated amino acids or suitable thio-derivatives. These extracts have hypolipemizing, anti-dyspeptic and vascular anti-inflammatory activities. The extracts are mainly formulated in Enothera biennis oil or in oils rich in ω-3 and ω-6 acids which enhance the vascular activity.
Owner:INDENA SPA
Method for preparing phenolic acid compound through fatsia japonica
InactiveCN105385747ALarge biomassIncrease planting areaMicroorganism based processesCarboxylic acid esters separation/purificationDicaffeoylquinic acidAspergillus niger
The invention relates to a method for preparing a phenolic acid compound through fatsia japonica. According to the method, the phenolic acid compound is obtained with fatsia japonica as a raw material through extraction and separation or obtained through biological conversion of aspergillus niger and eurotium after extraction and separation. According to the method, the phenolic acid compound in fatsia japonica is researched and quantitatively analyzed for the first time, and the total amount of the phenolic acid compound in fatsia japonica is 0.279-1.38%. Besides, the yield of a dicaffeoylquinic acid compound is increased through the biological conversion method, and a new raw material and a new method are provided for obtaining the phenolic acid compound.
Owner:NANJING NORMAL UNIVERSITY
Phlegm-heat clearing injection fingerprint spectrum establishment method and fingerprint spectrum thereof
ActiveCN110187041AAvoid one-sidednessHigh precisionComponent separationDicaffeoylquinic acidInjection solution
The invention relates to the technical field of medicine detection, and in particular relates to a phlegm-heat clearing injection fingerprint spectrum establishment method and a fingerprint spectrum thereof; the phlegm-heat clearing injection is prepared from scutellaria baicalensis, bear gall powder, cornu gorais, honeysuckle and fructus forsythiae, and a phlegm-heat clearing injection fingerprint spectrum is established by detecting the phlegm-heat clearing injection components by adopting an ultra-high performance liquid chromatography method; the method specifically comprises the followingsteps of S1, preparing a reference substance solution: taking a proper amount of caffeic acid, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3, 4-dicaffeoylquinic acid and 3, 5-dicaffeoylquinic acid, 4, 5-dicaffeoylquinic acid, forsythiaside D, baicalin, scutellarin, chrysin-7-O-glucuronide, oroxylin-7-O-glucuronide and a wogonin reference substance, and adding methanol to prepare a solution which is 20-50 [mu]g in 1ml. according to the phlegm-heat clearing injection fingerprint spectrum establishment method provided by the invention, the method is simple and convenient to operate, stable in result, high in reproducibility and high in precision.
Owner:SHANGHAI KAIBAO PHARMA
Production method for chlorogenic acid and dicaffeoylquinic acid through induction and culture of hairy roots of stevia rebaudiana
ActiveCN103695462AHigh and stable chlorogenic acidHigh and stable dicaffeoylquinic acid contentOrganic chemistryGenetic engineeringDicaffeoylquinic acidRhizobium rhizogenes
The invention belongs to the field of genetic engineering, specifically to a production method for chlorogenic acid and dicaffeoylquinic acid by establishing a hairy root culture system through genetic transformation of stevia rebaudiana with agrobacterium rhizogenes. The method comprises the following steps: preparation of an explant of stevia rebaudiana, activation and culture of agrobacterium rhizogenes, suspension culture and production of chlorogenic acid and dicaffeoylquinic acid. PCR detection results show that hairy roots of stevia rebaudiana are generated through transformation; HPLC detection results show that the hairy roots of stevia rebaudiana can produce chlorogenic acid and dicaffeoylquinic acid. Since the hairy roots used in the invention have the characteristic of rapid growth on a hormone-free medium, the method provided by the invention has the advantages of simple operation, low cost, no restriction by natural conditions like climate and land, etc. A stable and sustainable novel drug source and food function factors are provided for production of chlorogenic acid and dicaffeoylquinic acid through culture of the hairy roots, and a reliable source and a reliable substance base are provided for large scale production of chlorogenic acid and dicaffeoylquinic acid by using a bioreactor in the future.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Thin-layer chromatography discriminating method of schizomussaenda dehiscens
ActiveCN107677759AEasy to operateEasy to develop colorComponent separationDicaffeoylquinic acidThin layer chromatogram
The invention discloses a thin-layer chromatography discriminating method of schizomussaenda dehiscens. According to the invention, schizomussaenda dehiscens is taken as a main component, a mixed solution of chlorogenic acid, 3,5-O-dicaffeoylquinic acid, and 4,5-O-dicaffeoylquinic acid is taken as a reference substance solution, and a thin-layer chromatography (Chinese pharmacopoeia 2015 edition general rule 0502) is referred for testing. The method has the advantages of convenient operation, simple equipment, easy color development, and fast expansion rate, and provides basis for discriminating the schizomussaenda dehiscens.
Owner:广西壮族自治区食品药品检验所
Thin-layer chromatography identification method forwild chrysanthemum medicinal material and formula granule and application
ActiveCN112067739AImprove efficiencyLow costComponent separationPreparing sample for investigationBiotechnologyMedicinal herbs
The invention relates to a thin-layer chromatography identification method for a wild chrysanthemum medicinal material and formula granules. The thin-layer chromatograms of the wild chrysanthemum medicinal material and formula granules are compared with the chromatograms of a wild chrysanthemum reference medicinal material as well as chlorogenic acid, linarin, 4,5-dicaffeoylquinic acid, 3, 5-O-dicaffeoylquinic acid, galuteolin and luteolin reference substance; so that effective components in the wild chrysanthemum medicinal material and formula granules can be rapidly and effectively identified. three isomers namely, chlorogenic acid, 4,5-dicaffeoylquinic acid and 3, 5-O-dicaffeoylquinic acid and two natural flavonoid compounds, namely,the galuteolin and luteolin in the wild chrysanthemumflower medicinal material and formula granules are identified by thin-layer chromatography for the first time; and the wild chrysanthemum flower medicinal material and the formula granules can be enriched, separated and purified at corresponding Rf positions of a silica gel plate by using the method. The chlorogenic acid, the isomeride component of chlorogenic acid, and the flavonoid compounds canbe prepared, so that high commercial value can be realized. The method is environmentally friendly and convenient to operate, good in repeatability and specificity and good in application prospect.
Owner:劲牌持正堂药业有限公司
Packaged coffee drink
ActiveUS8088428B2Prevent precipitationReady-for-oven doughsFruit and vegetables preservationHigh concentrationDicaffeoylquinic acid
To provide a packaged coffee beverage, which contains chlorogenic acids at high concentration, has good flavor and taste, and is suppressed in the occurrence of sediment during long-term storage. A packaged coffee beverage subjected to heat sterilization treatment, the beverage comprising (A) monocaffeoylquinic acid, (B) feruloylquinic acid and (C) dicaffeoylquinic acid, wherein (a) a total content of the ingredients (A), (B) and (C) contained in dissolved states in the beverage is from 0.14 to 4% by weight based on the beverage, and the beverage comprises (b) 80% by weight or more of water, (c) magnesium and sodium at a Mg / Na weight ratio of from 0.04 to 1, (d) a coffee extract obtained from roasted coffee beans having an L value of form 16 to 25, and (e) from 0.0024 to 0.0122% by weight of brown color in terms of Food Yellow No. 4.
Owner:KAO CORP
Construction method and quality detection method of UPLC feature map of Hangzhou chrysanthemum medicinal material
ActiveCN109374786AEasy to harvestConvenient sourceComponent separationChemical compositionDicaffeoylquinic acid
The invention relates to a construction method and a quality detection method of a UPLC feature map of a Hangzhou chrysanthemum medicinal material. The construction method comprises the following steps: sample solution preparation; taking the Hangzhou chrysanthemum medicinal material, crushing, adding a solvent for extraction and filtering, wherein a filtrate is taken as a sample solution; reference solution preparation: taking chlorogenic acid, 3,5-O-dicaffeoylquinic acid, 4,5-O-dicaffeoylquinic acid, galuteolin and linarin and adding a solvent for dissolution, wherein an obtained solution isa reference solution; ultra performance liquid chromatography detection: absorbing the sample solution and the reference solution, injecting into a liquid chromatograph and detecting to obtain the UPLC feature map of the Hangzhou chrysanthemum medicinal material. The UPLC feature map is rich in characteristic peak information, can fully reflect the chemical composition of the Hangzhou chrysanthemum medicinal material and can also effectively characterize the qualities of a Hangzhou chrysanthemum decoction piece and standard decoction and identify the product authenticity.
Owner:GUANGDONG YIFANG PHARMA
Method for simultaneously determining content of five chemical components in chrysanthemum medicinal materials
InactiveCN110133153AFully reflect the quality characteristicsHigh sensitivityComponent separationApigeninDicaffeoylquinic acid
The invention relates to the technical field of effective component determination, and in particular relates to a method for simultaneously determining content of five chemical components in chrysanthemum medicinal materials. By adoption of the method provided by the invention, the content of chlorogenic acid, luteolin-7-O-beta-D-glucoside, luteolin-7-O-beta-D-glucuronide, 3, 5-dicaffeoylquinic acid and apigenin-7-O-beta-D-glucoside can be measured simultaneously. According to the method provided by the invention, the separation degree is high, the separation of all the components can achievethe baseline separation, the linear range is large, the sensitivity is high, and the repeatability is high; the sample addition recovery rate of the five chemical components ranges from 95% to 105% ina sample addition recovery test, which all meet the specification. Therefore, the method provided by the invention provides an effective detection means for the chrysanthemum medicinal materials withthe five chemical components as quality detection indexes, and the quality characteristics of the chrysanthemum medicinal materials can be comprehensively reflected.
Owner:BEIJING CHENGYI INVESTMENT CO LTD
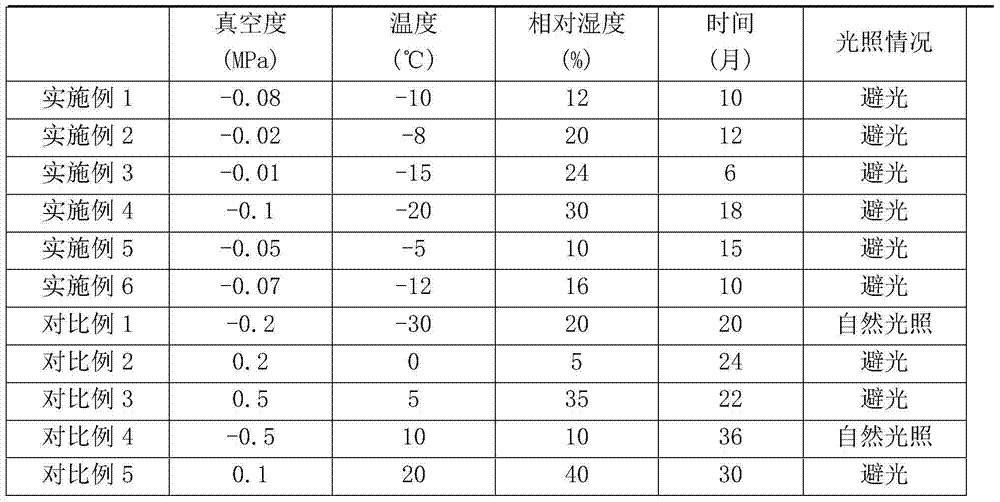
Method for storing largeflower-like honeysuckle flower bud medicinal material
ActiveCN103877141AQuality improvementColor unchangedComponent separationPlant ingredientsDicaffeoylquinic acidCaffeic acid
The invention provides a method for storing honeysuckle flowers. The method comprises the following steps: removing water from buds or blossoms of newly collected largeflower-like honeysuckle by virtue of steam, and drying to obtain fresh honeysuckle; putting the fresh honeysuckle in vacuum package with vacuum degree of -0.01-0.1MPa, and storing in shade for 0-18 months in an environment with relative humidity of 10-30 percent and temperature of (-20)-5 DEG C. The honeysuckle stored by adopting method, and the quality of the medicinal material is more stable, wherein the color value of the medicinal material is slightly changed, and the contents of characteristic components, such as chlorogenic acid, caffeic acid, 3,5-dicaffeoylquinic acid, galuteolin, macranthoidin B, dipsacoside B and the like are slightly changed as well.
Owner:CHONGQING ACAD OF CHINESE MATERIA MEDICA
Method for bioconversion of chlorogenic acids in plants by adopting aspergillus aculeatus
ActiveCN103421853AShort conversion timeEfficient conversionMicroorganism based processesFermentationDicaffeoylquinic acidCaffeic acid
The invention relates to a method for directionally converting and purifying chlorogenic acids by using aspergillus aculeatus CCTCC No.M2011264 and an enzyme liquid of the aspergillus aculeatus. The method comprises the following steps: the aspergillus aculeatus is inoculated to a liquid or solid enzyme generation culture medium, the fungus or enzyme obtained from fermentation is used for converting plant extracting solution or powder containing the chlorogenic acids. According to the invention, 1, 5-dicaffeoylquinic acid in the system of the method is reserved in the solution rather than being converted, and the solution can be purified as partial compositions are consumed during the conversion process (for example, 99% above of 4, 5-dicaffeoylquinic acid with property extremely similar to the that of the 1, 5-dicaffeoylquinic acid is consumed), so that the follow-up separation and purification steps are eliminated, and the method can be taken as a novel method for purifying the 1, 5-dicaffeoylquinic acid; besides, through controlling the conversion condition, the limitation that only caffeic acid, ferulic acid and quinic acid can be obtained by adopting the conventional conversion method is overcome.
Owner:巴州正达绿源生物科技有限公司
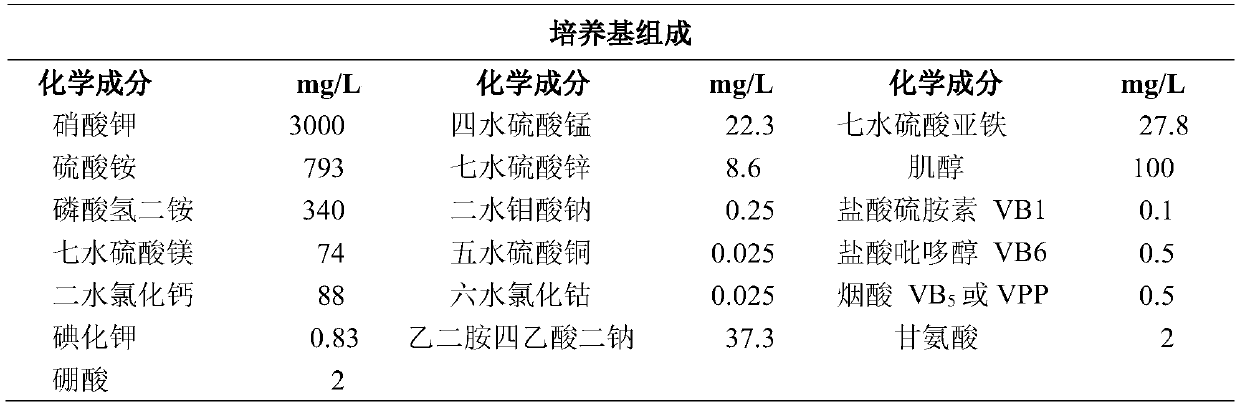
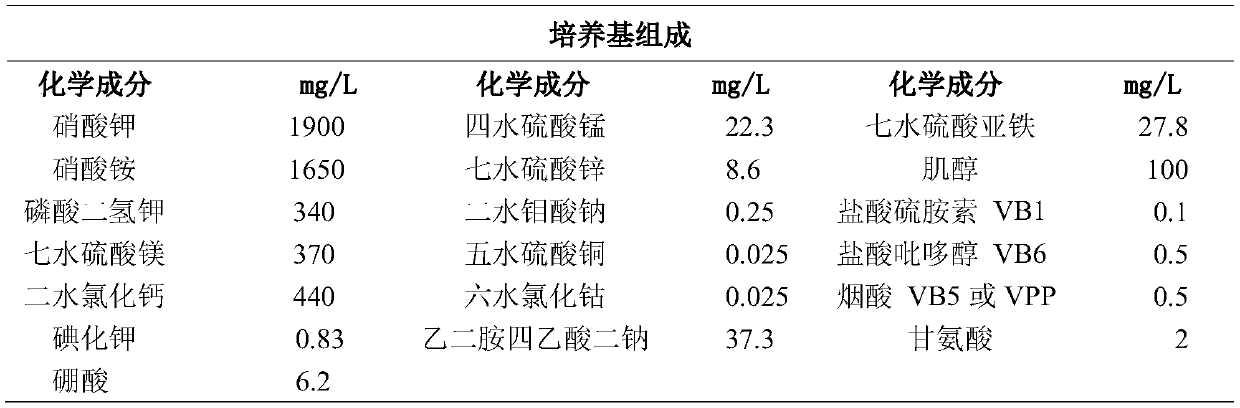
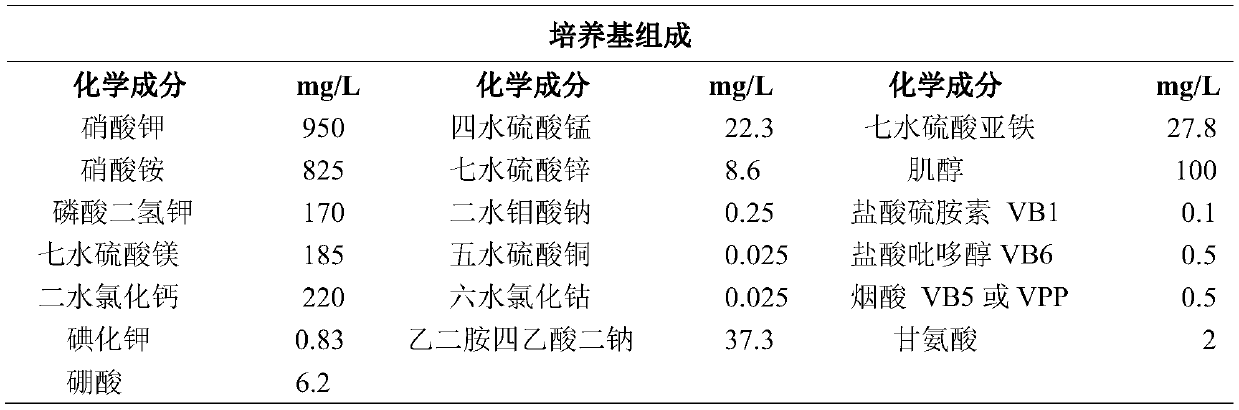
Method for improving flavonoid phenylpropanoid compounds of saussurea involucrate cell culture
PendingCN110923190AHigh in flavonoidsIncrease contentFermentationPlant tissue cultureDicaffeoylquinic acidCaffeoylquinic acid
The invention belongs to the field of plant biotechnology engineering, and particularly relates to a method for improving flavonoid phenylpropanoid compounds of a saussurea involucrate cell culture. In a culture medium used in the method, the phosphorus element concentration is 0.5-3 mmol / L, the NO3-ion concentration is 20-35 mmol / L, the NH4+ ion concentration is 0-30 mmol / L, and the Ca 2+ ion concentration is 0.5-3 mmol / L; the Mg 2+ ion concentration is 0.2-1.5 mmol / L, and the concentration of boron ions is 0.02-0.1 mmol / L; inducers or precursors or inhibitors of bypass metabolism may be added to the culture medium. Finally, the total flavone content of the saussurea involucrate cell culture and the content of rutin, chlorogenic acid, Syringin and 1, 5-dicaffeoylquinic acid are obviouslyimproved.
Owner:DALIAN PRACTICAL BIOTECH
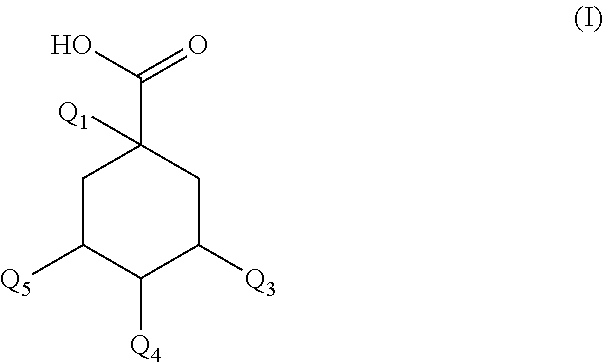
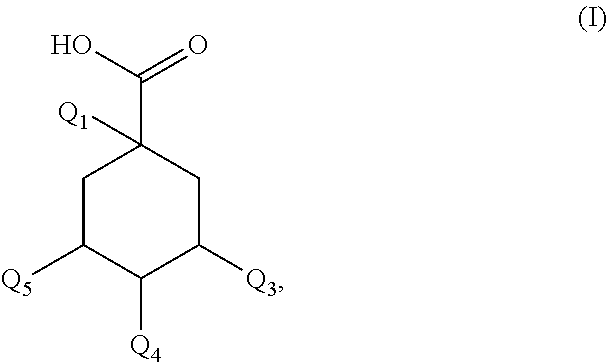
Use of a new composition for preventing or slowing the appearance of signs of inflammation
PendingUS20210154112A1Additional componentImprove microcirculationCosmetic preparationsToilet preparationsDicaffeoylquinic acidAcyl group
A composition for prevents or slows the appearance of blemishes associated with inflammation of the skin and / or scalp, or eliminates such, the composition including, per 100% by mass: a) 60.0%-75.0% by mass of an organic solvent chosen from 1,2-propanediol, 1,3-propanediol, 1,4-butanediol, 1,3-butanediol, 1,2-butanediol, 2-methyl-2,4-pentanediol, 1,6-hexanediol, 1,8-octanediol, or a blend of the above compounds b) 0.1%-2.0% by mass of a composition including a mass quantity x1, expressed as mass equivalent of 1-O-(2-caffeyol) maloyl-3,5-O-dicaffeoylquinic acid, greater than or equal to 200 mg / g of a compound of general formula (I):in which Q1-Q5 independently of each other represent the hydroxyl radical or a salt thereof or a radical selected among the caffeoyl, maloyl, caffeoyl maloyl and maloyl caffeoyl radicals. At least one of Q1-Q5 represents neither the —OH radical nor a salt thereof; and c) 20.0%-35.0% by mass water.
Owner:SOC DEXPLOITATION DE PROD POUR LES IND CHEM SEPPIC
Method for rapidly separating and purifying polyphenol compounds in sorbus pohuashanensis berries
ActiveCN109503373AOvercome operabilityOvercome the cycleSugar derivativesOrganic compound preparationDicaffeoylquinic acidPolyamide
The invention discloses a method for rapidly separating and purifying polyphenol compounds in sorbus pohuashanensis berries. The method comprises the steps as follows: mashing sorbus pohuashanensis berries, adding an ethanol solution for ultrasonic extraction, and performing concentration under reduced pressure to obtain crude extract of sorbus pohuashanensis berries; performing gradient elution by a polyamide chromatographic column with ethanol as an eluant, and performing concentration under reduced pressure to obtain eluates with different gradient fractions; separating and purifying the eluates with different gradient fractions with HSCCC (high-speed counter-current chromatography) to obtain neochlorogenic acid, chlorogenic acid, quercetin-3-O-(6''-alpha-L)-rhamnosyl-4'''-alpha-L-rhamnosyl)-beta-D-glucoside, 3,5-O-dicaffeoylquinic acid and rutin with purity higher than 95% respectively. The method adopts a simple process, is high in separation speed and low in comprehensive cost and has very good promotional and use values, and the product purity is high.
Owner:LIAONING UNIVERSITY
Method for preparing a plurality of caffeoylquinic acid monomers from honeysuckle
InactiveCN102942483AIncrease profitReasonable ideaCarboxylic acid esters separation/purificationOrganic solventSeparation technology
The invention relates to a method for preparing a plurality of caffeoylquinic acid monomers from honeysuckle, and belongs to preparation methods for chemical substances or drugs. In the prior art, caffeoylquinic acid substances prepared by the existing method do not have high purity; the existing chlorogenic acid extraction and preparation technology has a disadvantage of high organic solvent consumption so as to cause environmental pollution, or adopts a complex separation technology, such that production enlarging is difficult; and extraction efficiency is not high, raw material utilization rate is low, and other problems exist. With the present invention, the problems in the prior art are solved, and high purity 3-caffeoylquinic acid, high purity 4-caffeoylquinic acid, high purity 2-caffeoylquinic acid, high purity 4,5-dicaffeoylquinic acid, high purity 3,4-dicaffeoylquinic acid and other monomer components are obtained. In addition, the project technology comprises a raw material pretreatment step, an extraction step, a macromolecule precipitation step, a macroporous resin separation step, a reversed phase column separation step, a crystallization step and the like, such that production cost is saved, production efficiency is improved, the process is simple and easily enlarged into industrialization production, and the raw material utilization rate can be greatly improved.
Owner:HUBEI CHUTIANSHU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com