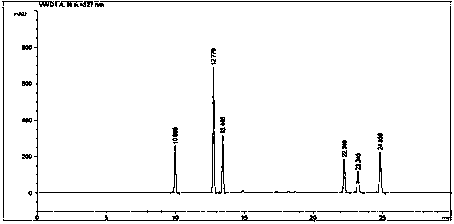
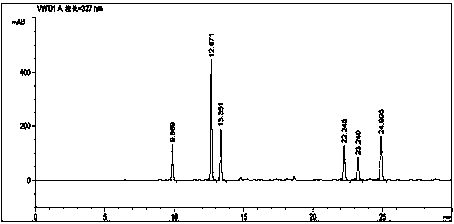
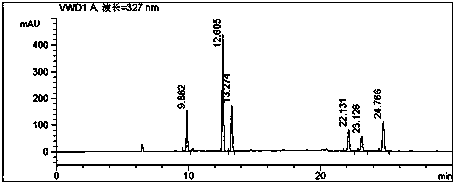
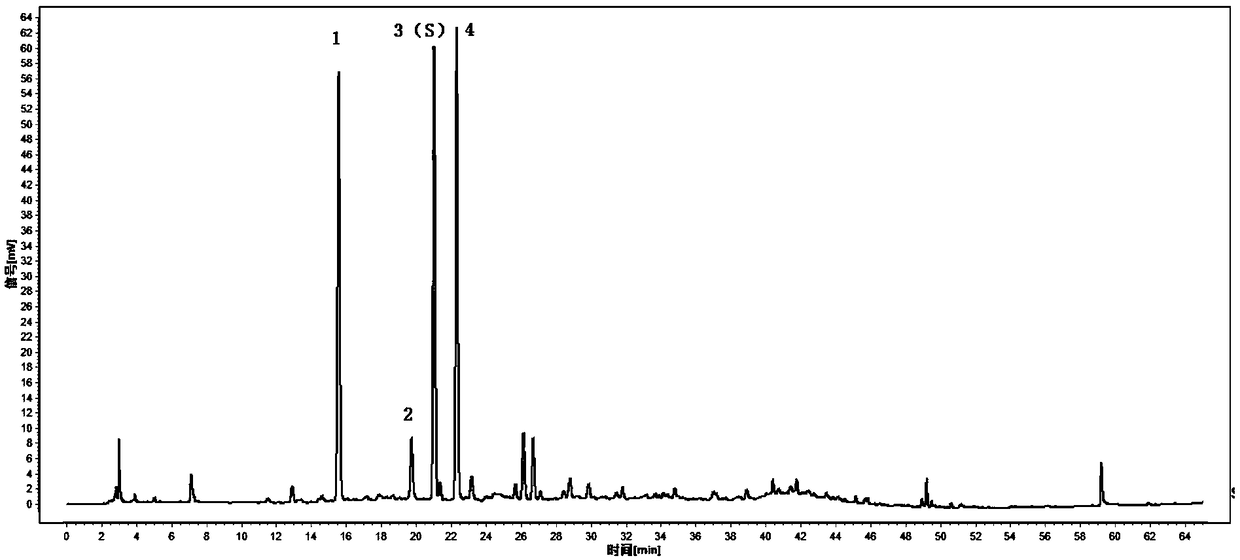
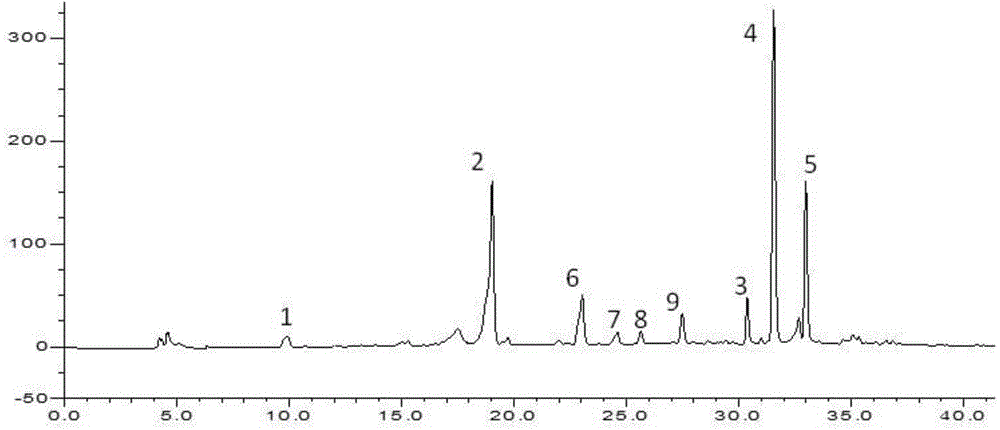
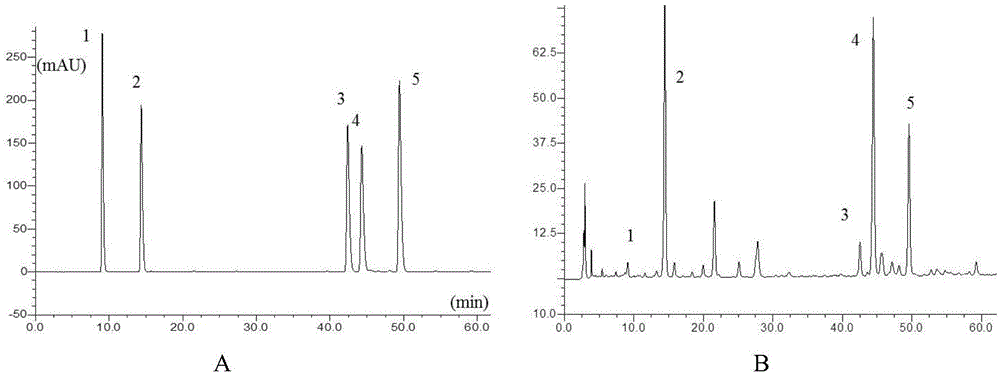
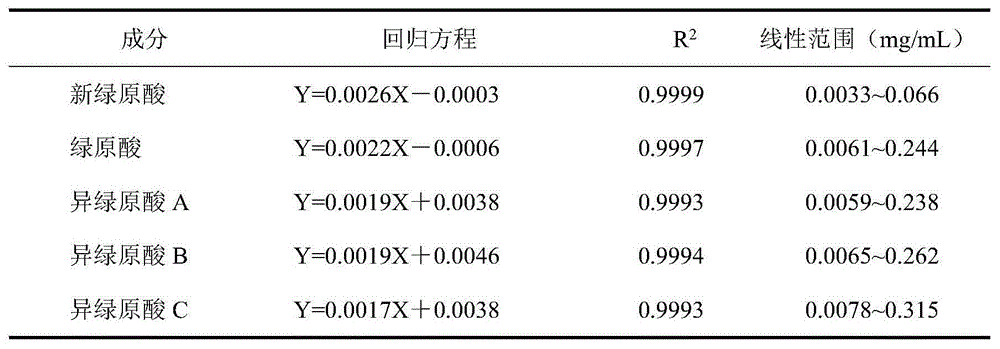
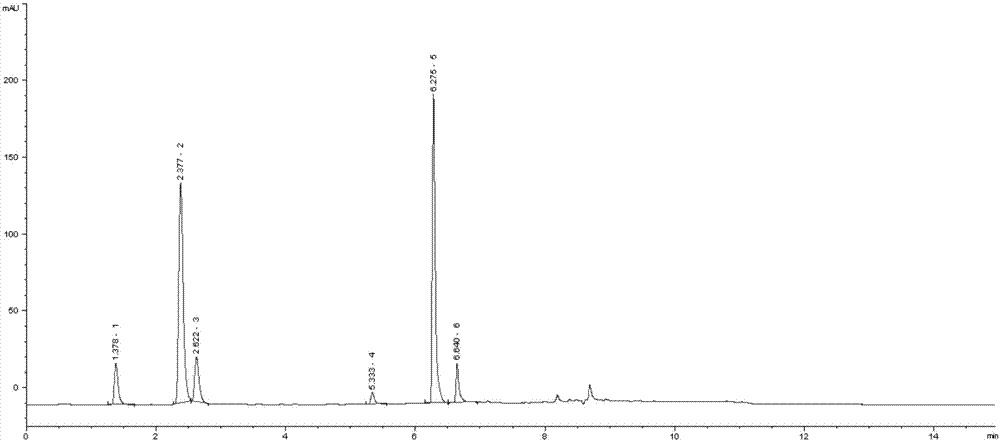
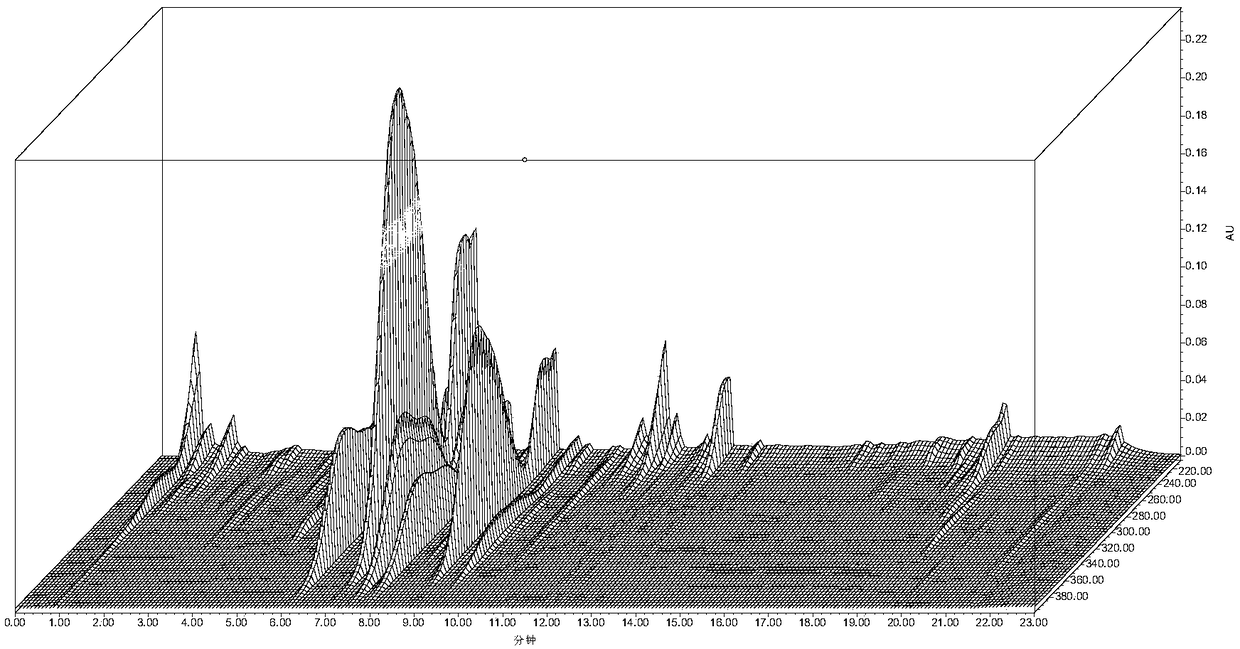
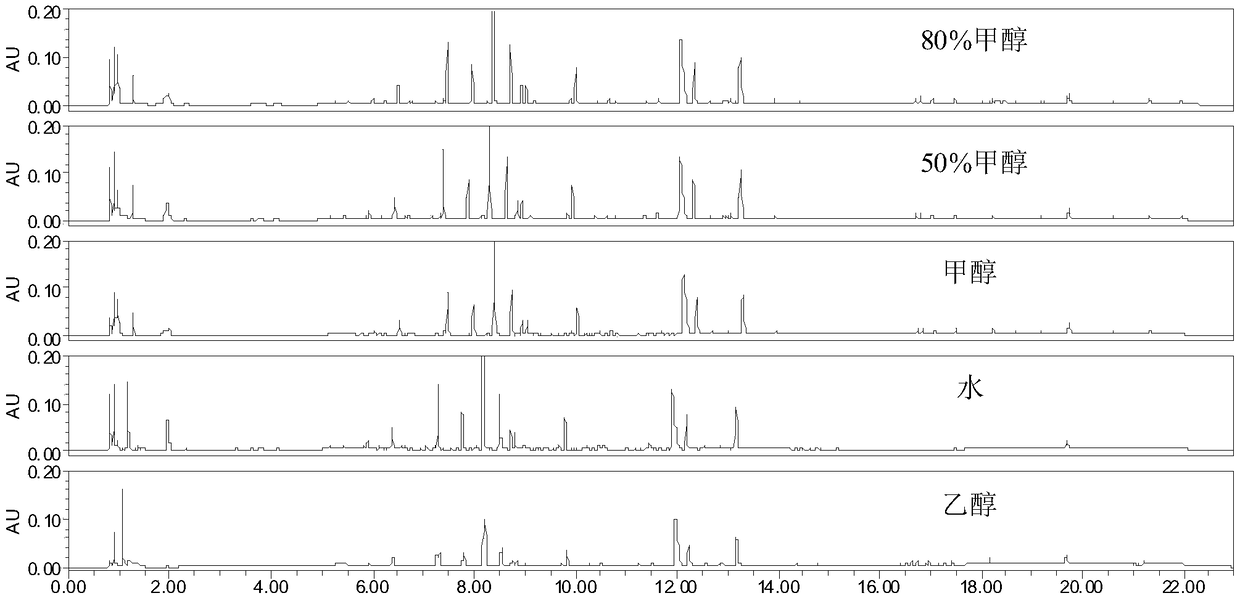
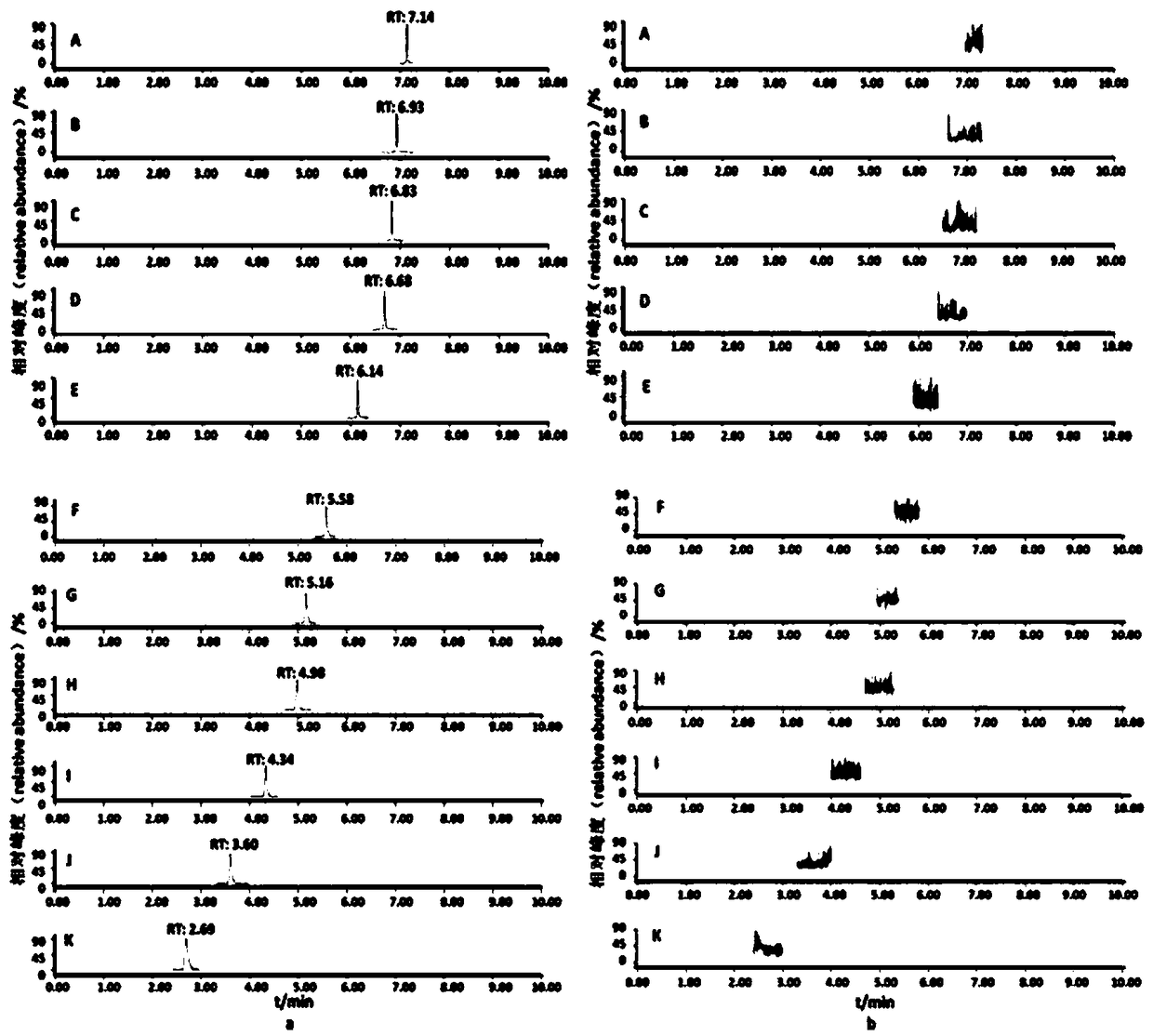
Patents
Literature
93 results about "Neochlorogenic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Neochlorogenic acid or 5-caffeoylquinic acid is a natural polyphenolic compound found in some types of dried fruits and a variety of other plant sources such as peaches. It is also found in Sunflower seed meal, Globe artichoke heads, Chicory [Red], Half-highbush blueberry, Highbush blueberry, Lovage, Burdock root, and Highbush blueberry. It is an isomer of chlorogenic acid; both of these are members of the caffeoylquinic acid class of molecules.
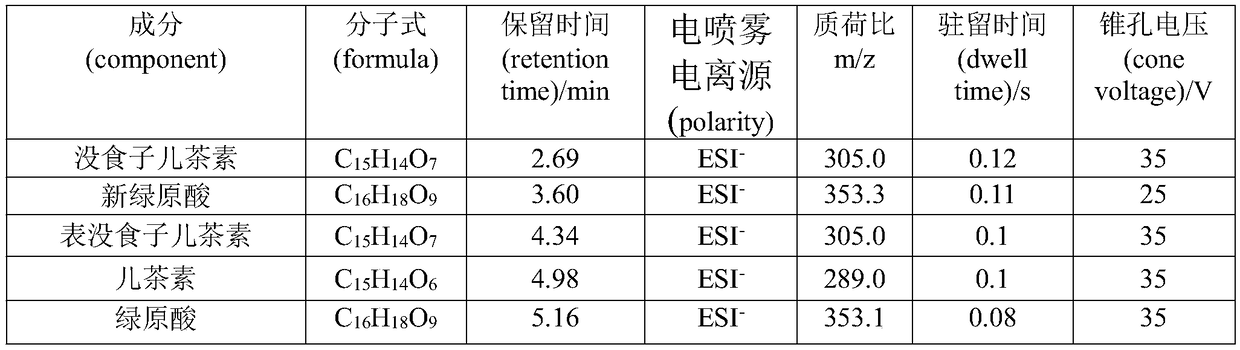
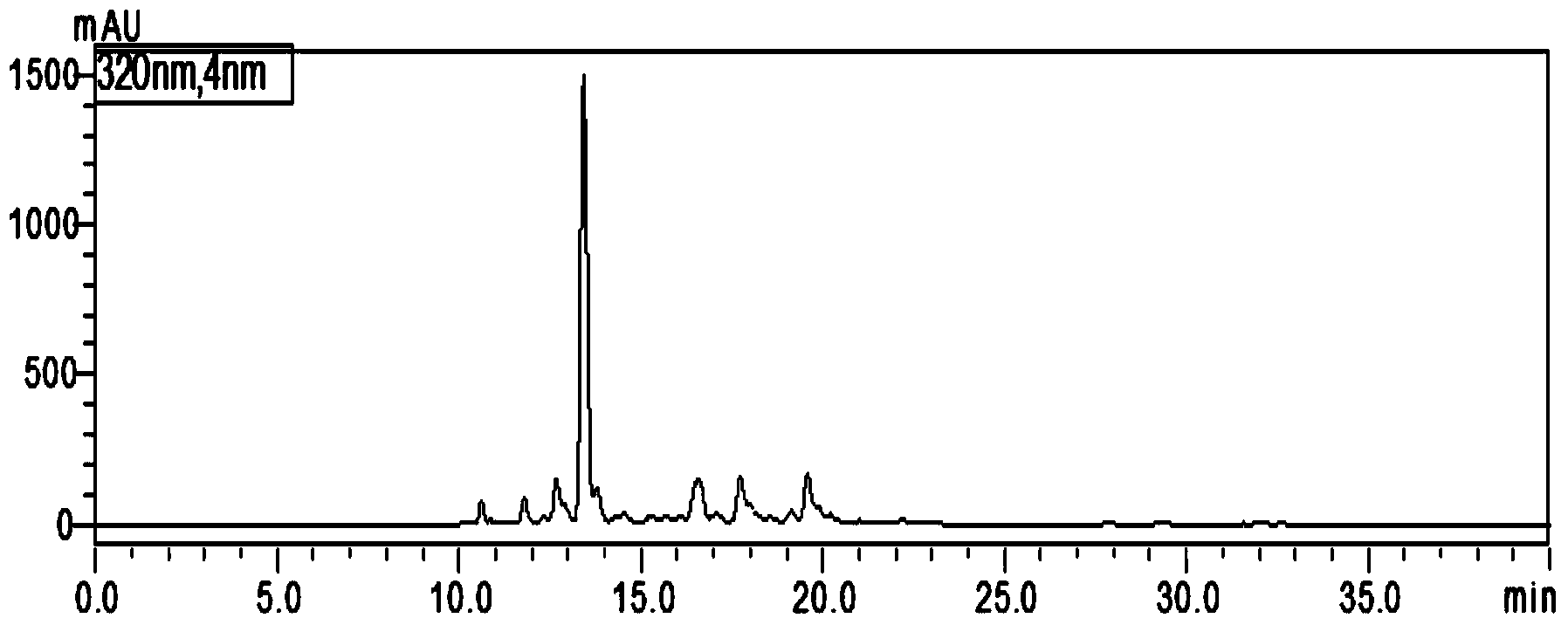
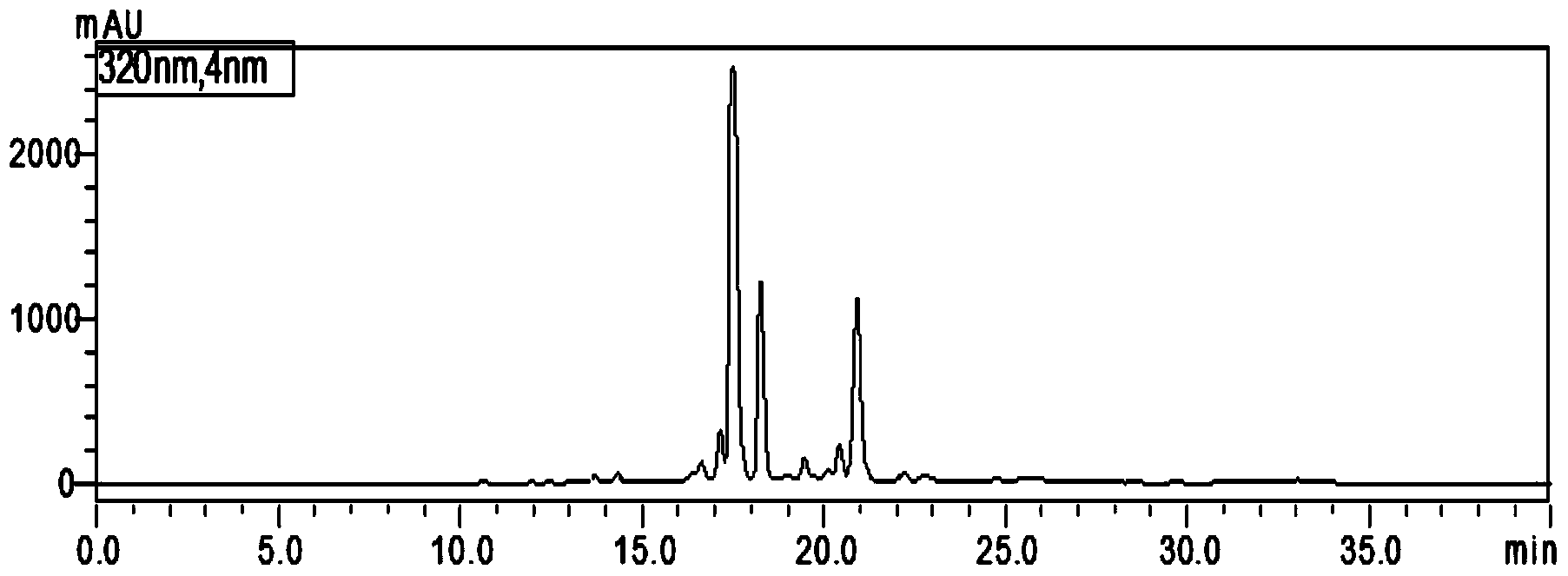
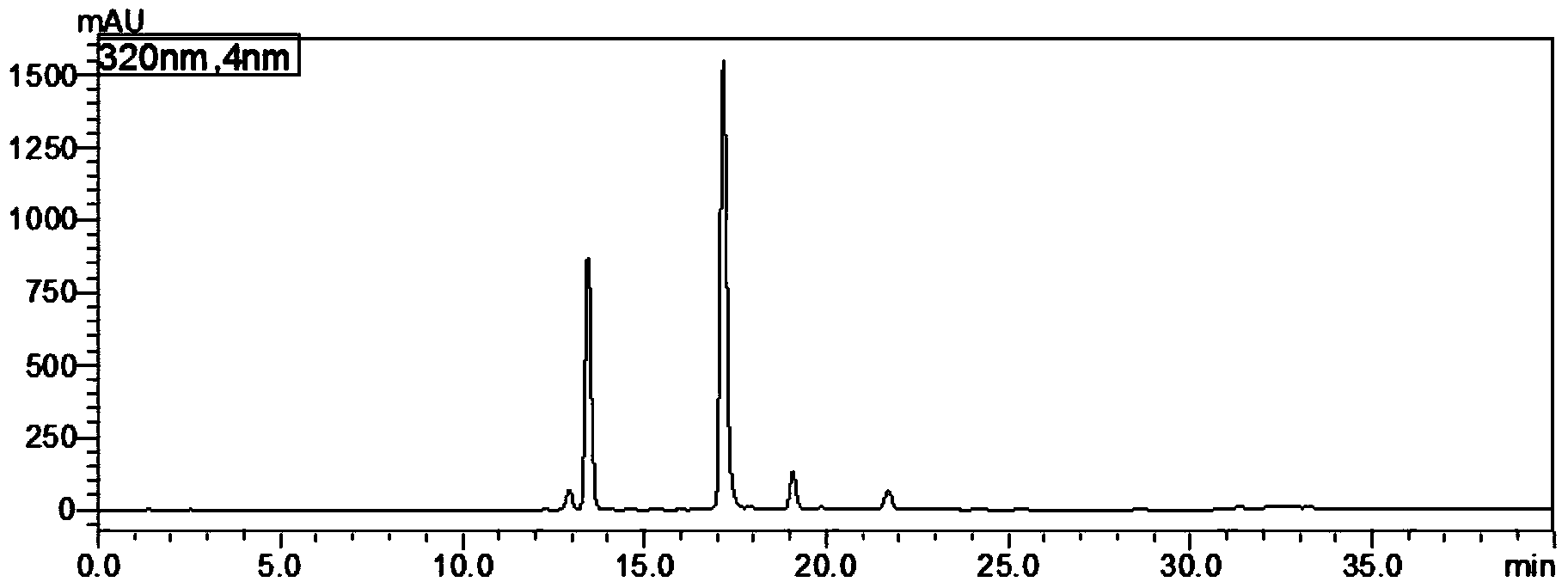
Content measurement method for Chinese patent medicine prepared from sweet wormwood, honeysuckle and gardenia jasminoides fruit
ActiveCN102233021AGood reproducibilityImprove quality controlComponent separationAntipyreticPhosphoric acidIsochlorogenic acid
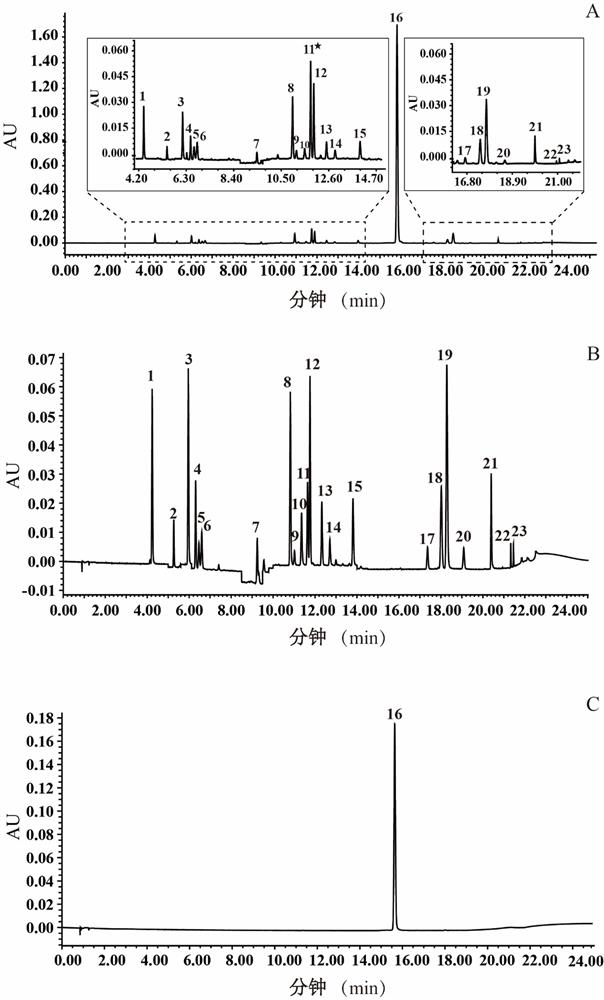
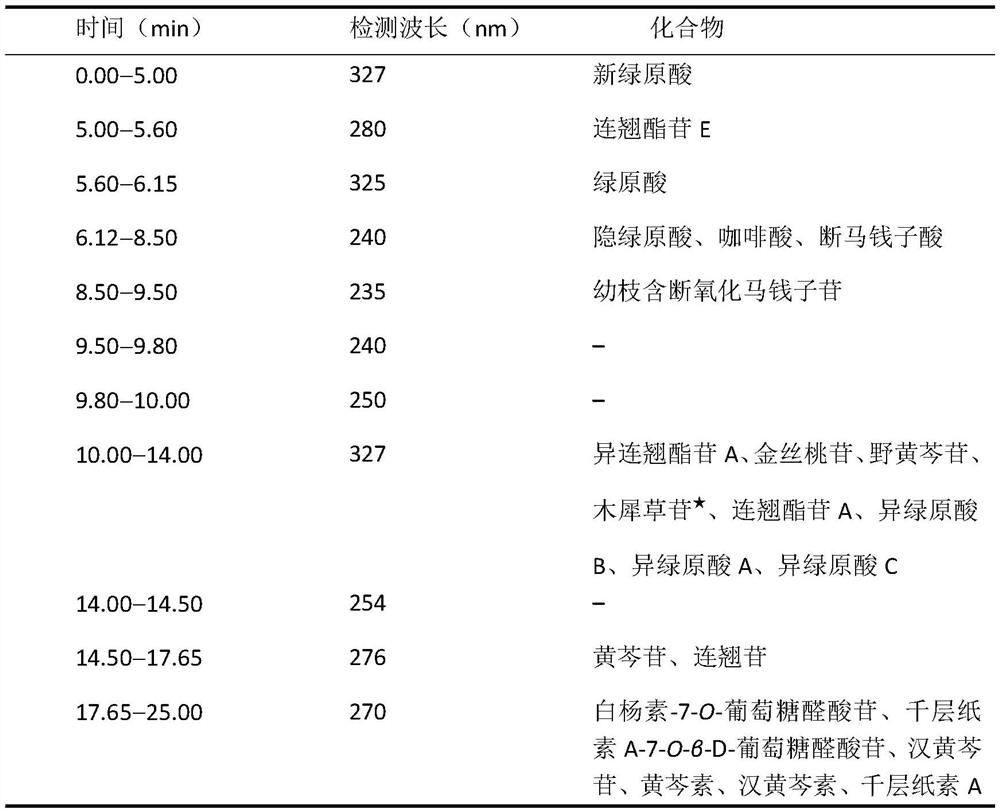
The invention belongs to the field of Chinese medicine analysis and particularly relates to a content measurement method for Chinese patent medicine prepared from sweet wormwood, honeysuckle and gardenia jasminoides fruit. The method measures the content of nine ingredients including neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, geniposide, secoxyloganin, isochlorogenic acid B, isochlorogenic acid A and isochlorogenic acid C in the Chinese patent medicine by reversed-phase high performance liquid chromatography, wherein the chromatographic conditions include: a chromatographic column is C18 column; methanol or acetonitrile is used as a flowing phase A; 0.05-to-1.0 percent phosphoric acid or 0.05-to-1.0 percent acetic acid is used a flowing phase B; the totalpercentage of flowing phases A and B is 100 percent; and gradient elute is performed. The content measurement method has high repeatability; and the method completely reflects the main ingredients and the change of the main ingredient content of the medicine from a qualitative prospect respectively and thus improves the level of the control over the quality of the medicine.
Owner:JIANGSU KANION PHARMA CO LTD
Method for content determination of multiple components in traditional Chinese medicinal preparation Shuanghuanglian for injection
ActiveCN103308615AAvoid driftingSolve the precipitation problemComponent separationIsochlorogenic acidContent determination
The invention relates to a method for content determination of multiple components in traditional Chinese medicinal preparation Shuanghuanglian for injection, aiming at solving the problems of an existing method for content determination of Shuanghuanglian for injection which is complex in sample processing, time, labor and detection equipment-consuming to detect Shuanghuanglian, and long in detection period. The detection method provided by the invention can be used for synchronously determining the contents of more than 10 components such as forsythin, forsythiaside A, caffeic acid, neochlorogenic acid, chlorogenic acid, cryptochlorogenin acid, isochlorogenic acid A, isochlorogenic acid C, baicalin, scutellarin and oroxylin-7-O-glucuronic acid in the Shuanghuanglian for injection, and meanwhile monitoring a fingerprint spectrum. The method can be used for completing previous detection in one time just by one detection system, and is more accurate, stable, comprehensive and rapid in control over the quality of the Shuanghuanglian for injection.
Owner:哈药集团中药有限公司
Production method of wild honeysuckle flower tea
InactiveCN102326648AFull flower shapeComplete flower shapeTea substituesChlorogenic acidDecomposition
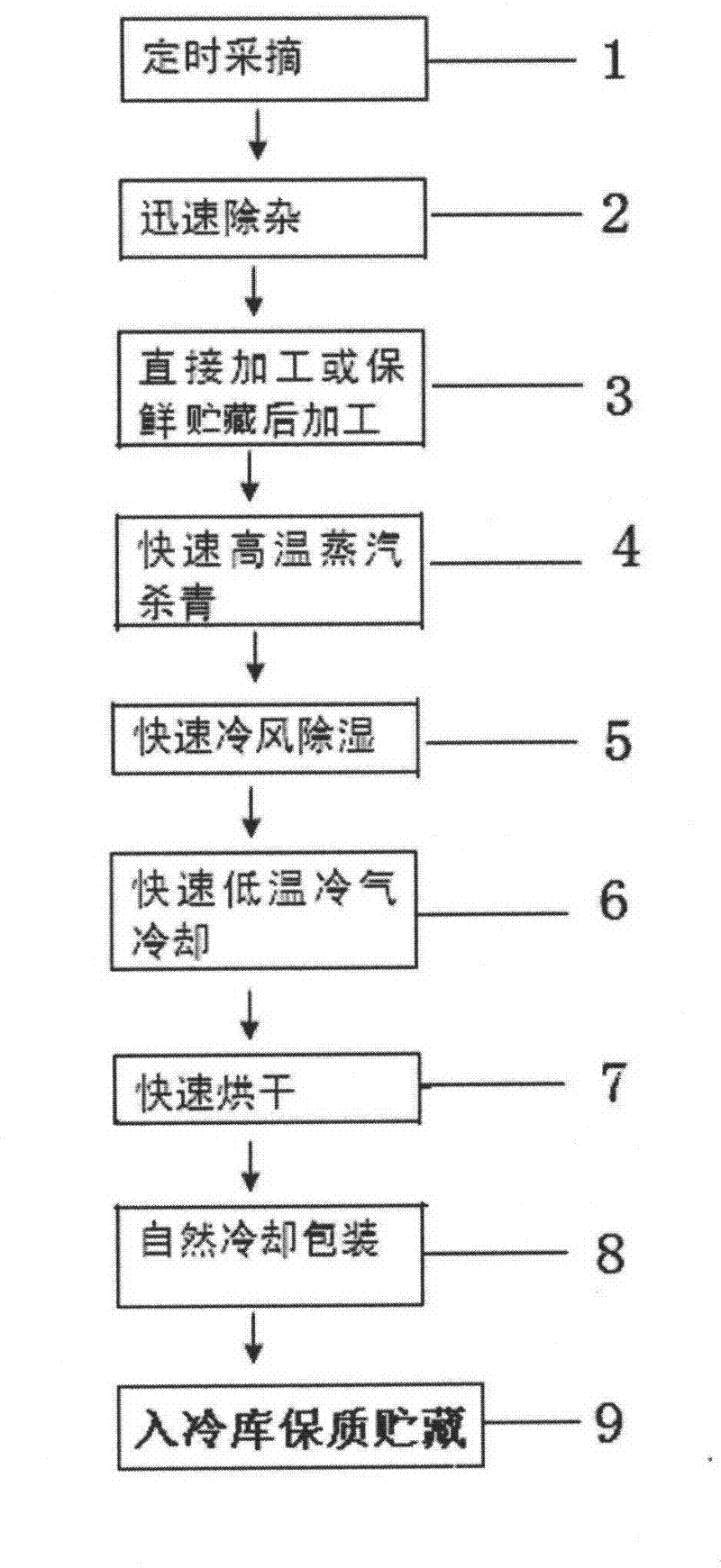
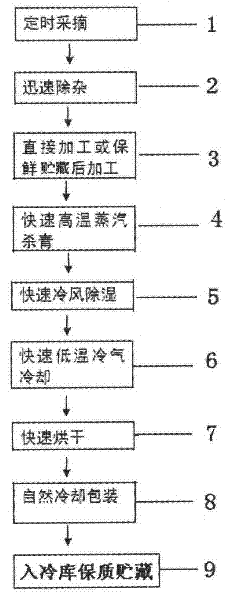
The invention discloses a production method of wild honeysuckle flower tea. By adopting the production method, the original color and shape of wild honeysuckle flower can be kept unchanged and stable chlorogenic acid content can be ensured. The production method specifically comprises the following steps: picking at regular time; rapidly removing impurities; directly processing; carrying out rapid enzyme deactivation by high-temperature steam; rapidly dehumidifying by cold air; rapidly cooling by low-temperature cold air; rapidly drying by high-temperature hot air; naturally cooling and packaging; and warehousing for storage so as to guarantee quality. The production method has the beneficial effects of rapidly destroying activity of polyphenol oxidase in the wild honeysuckle flower, effectively avoiding decomposition of the beneficial substance namely chlorogenic acid contained in the wild honeysuckle flower, preventing the wild honeysuckle flower from browning, avoiding the procedures of turnover as well as pickup and placement, and ensuring that the appearance of the wild honeysuckle flower is not damaged and the flower shape is complete. The wild honeysuckle flower tea processed by the method has the advantages of green color, new shape and less loss of the chlorogenic acid; and the brewed wild honeysuckle flower tea has the advantages of light green and bright and clean color, delicious taste as well as complete and lifelike flower shape. Therefore, the drinking value and appreciation grade of the wild honeysuckle flower tea are obviously improved by adopting the production method.
Owner:XIUSHAN SHENGDA AGRI DEV
Honeysuckle contrasting extract and preparation method thereof
ActiveCN103823034AHigh content of target ingredientsAccurate Qualitative and Quantitative DeterminationTesting medicinal preparationsBiotechnologyIsochlorogenic acid
The invention relates to the technical field of traditional Chinese medicine extracts and traditional Chinese medicine quality control, in particular to a honeysuckle contrasting extract. The honeysuckle contrasting extract is prepared from the following components of neochlorogenic acid, chlorogenic acid, cryptochlorogenin acid, isochlorogenic acid A, isochlorogenic acid B and isochlorogenic acid C at the mass ratio: (0.20-0.40) : 1.00 : (0.30-0.50) : (0.15-0.45) : (0.10-0.40) : (0.15-0.45), and the total mass fraction exceeds 70%. The preparation method comprises the following steps: performing water extracting and alcohol precipitating to obtain crude extracts; dissolving the crude extracts, regulating pH to be acidic, and centrifuging; separating the supernatant by macroporous adsorption resin, silica gel column and gel column to reach the target content. The honeysuckle contrasting extract has high content of target components, can be applied as a mixed reference substance, is used for quality control of traditional Chinese medicine that contains honeysuckle in the prescription and for accurately qualitative and quantitative measurement, and is efficient in detection, low in cost, accurate in results and lower in preparation cost.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Method for detection, identification and content determination of folia eriobotryae or drug containing folia eriobotryae raw material
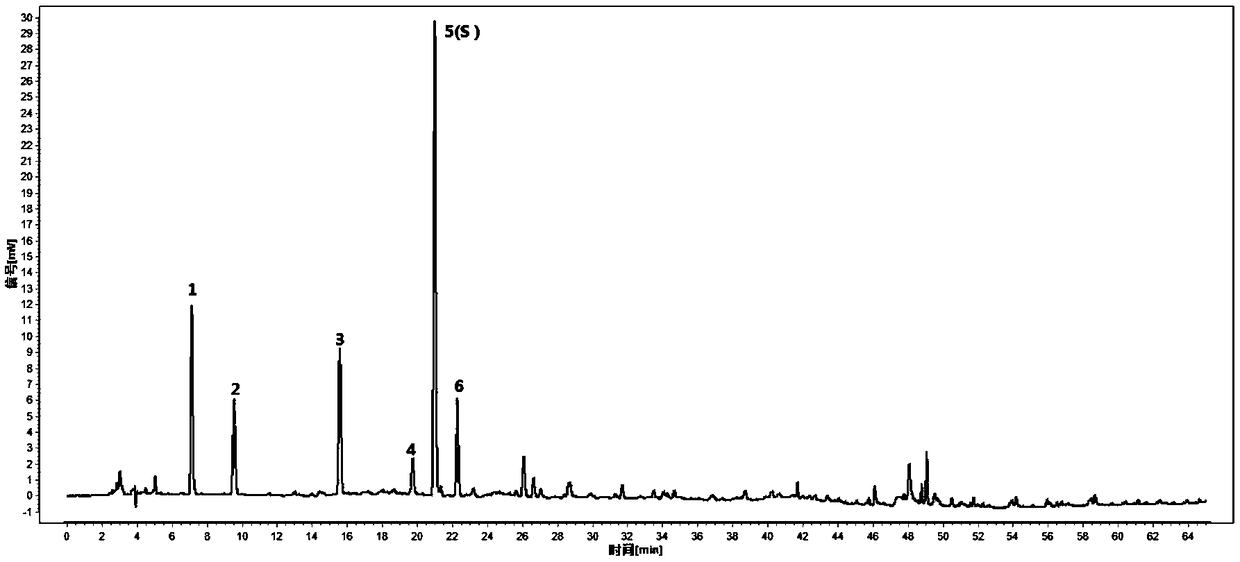
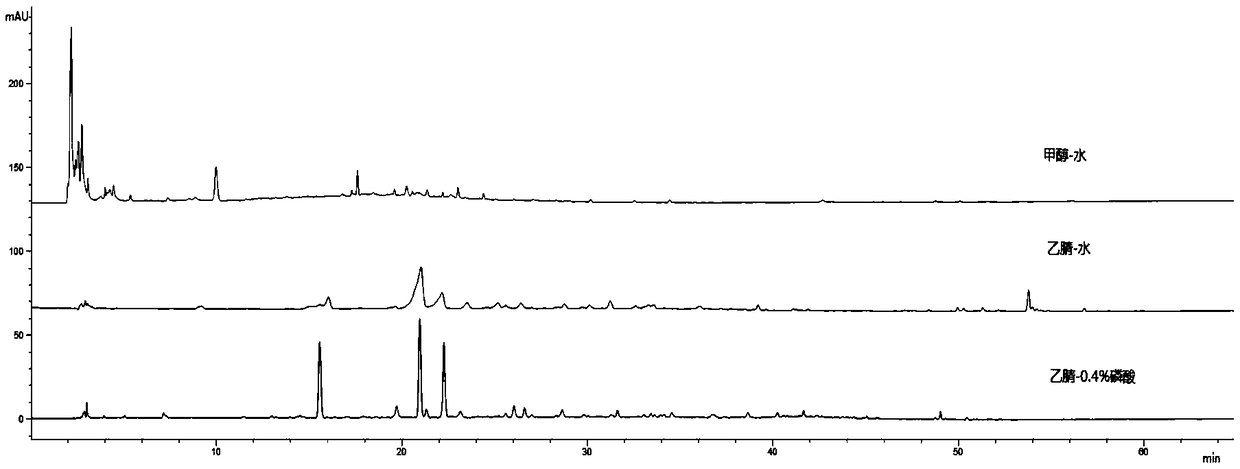
The invention provides a method for the detection of folia eriobotryae or drugs containing folia eriobotryae raw material. The detection method adopting HPLC (high performance liquid chromatography) fingerprint for detection includes the steps of: (1) preparing a test solution; (2) preparing a reference solution by taking chlorogenic acid, neochlorogenic acid and cryptochlorogenic acid; (3) conducting precise drawing of the reference solution and the test solution into a liquid chromatograph and then performing elution with 0.4% of phosphoric acid-acetonitrile as a mobile phase. The quality detection method of the folia eriobotryae or the drugs containing the folia eriobotryae raw material has the advantages of being capable of performing content determination and feature mapping and saving inspection costs, and being capable of distinguishing between folia eriobotryae (medicinal materials, decoction pieces or formula granules) and honeyed folia eriobotryae (medicine, decoction piecesor formula granules) and conducting quality control of the whole process of folia eriobotryae or honeyed folia eriobotryae production.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Method for simultaneously determining contents of 11 kinds of effective components in Reduning injection
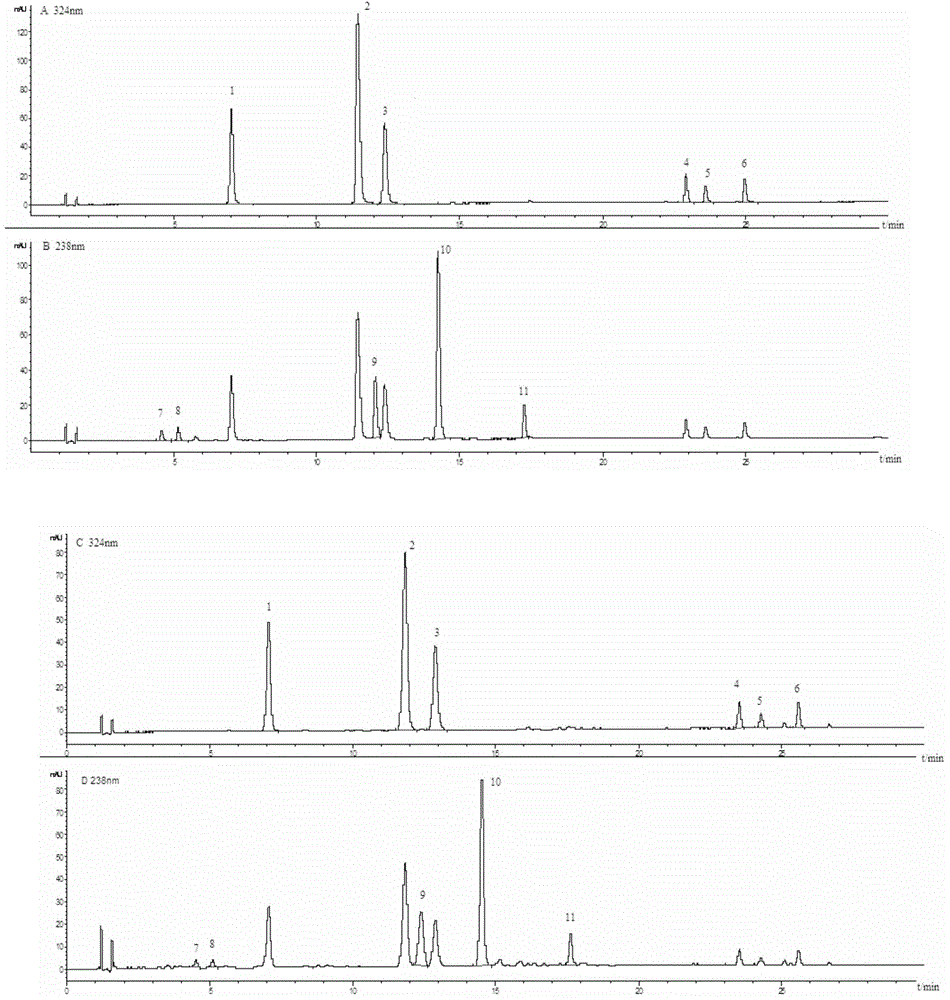
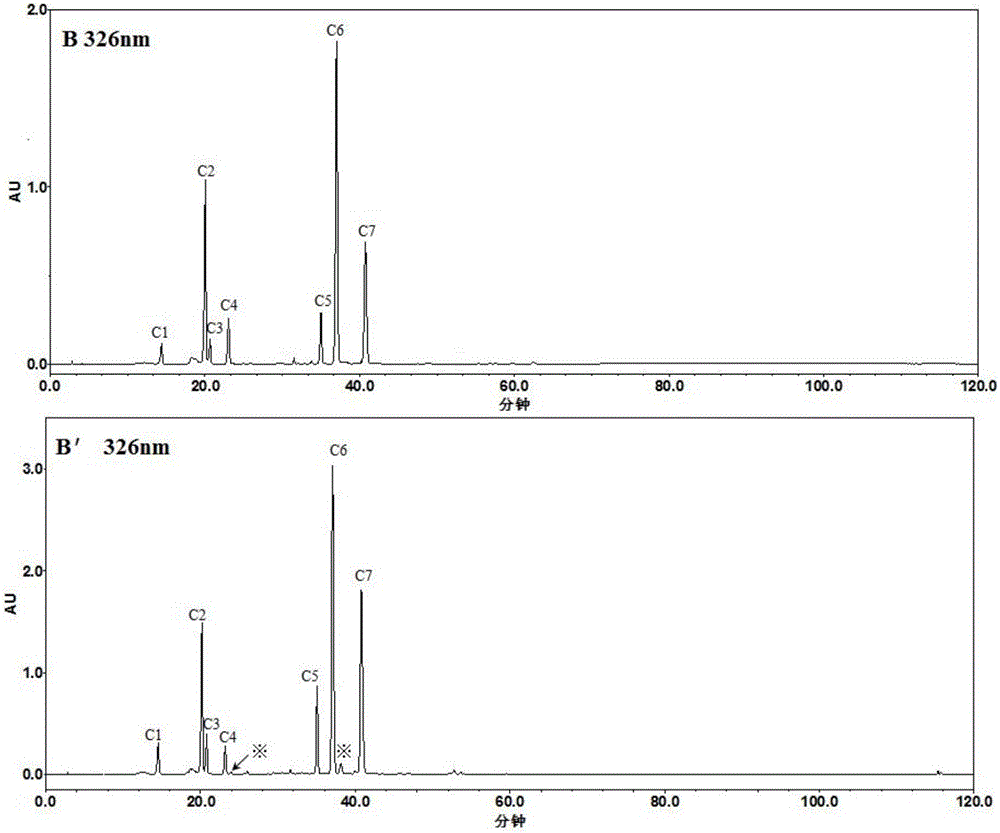
The invention discloses a method for simultaneously determining the contents of 11 kinds of effective components in a Reduning injection. The 11 kinds of effective components comprise a neochlorogenic acid, a chlorogenic acid, a cryptochlorogenic acid, an isochlorogenic acid A, an isochlorogenic acid B, an isochlorogenic acid C, shanzhiside, a geniposidic acid, genipin1-gentiobioside, geniposide and broken oxidation nux vomica glycosides. The method adopts an Agilent ZORBAX SB-C18 chromatographic column (3.0*100mm, 1.8[mu]m), a flow phase is acetonitrile (A)-0.1% phosphoric acid (B), gradient elution is adopted, a flow speed is 0.35-0.45mL . min<-1>, a detection wave length is 324-326nm and 236-238nm, and a column temperature is 25-35 DEG C. The method disclosed by the invention is accurate, quick and acute, and the quality control level of the Reduning injection is further improved.
Owner:JIANGSU KANION PHARMA CO LTD
Traditional Chinese medicine composition content determination method
ActiveCN107149623AHydroxy compound active ingredientsComponent separationEphedra sinicaRHODIOLA ROSEA ROOT
The present invention relates to a traditional Chinese medicine composition content determination method, wherein the traditional Chinese medicine composition comprises the following herbs such as forsythia suspensa, honeysuckle, radix isatidis, bitter almond, menthol, herba houttuyniae, rheum palmatum l, pogostemon cablin, dryopteris crassirhizoma, rhodiola rosea l, ephedra sinica stapf, licorice root and gypsum. According to the present invention, with the content determination method, the contents of seven components such as neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, isoforsythiaside A, 4,5-di-O-caffeoylquinic acid, quercitrin and glycyrrhizic acid are simultaneously determined, such that the energy source can be effectively saved, and the analysis cost can be reduced, wherein the traditional Chinese medicine composition content determination method of the present invention is not disclosed in the prior art.
Owner:SHIJIAZHUANG YILING PHARMA
Quality control method for simultaneous realization of content analysis and similarity evaluation of 18 components in Ilex kudingcha
ActiveCN106198782ARealize simultaneous content determinationComprehensive evaluationComponent separationIlex kudingchaHydroxytyrosol
The invention discloses a quality control method for simultaneous realization of content analysis and similarity evaluation of 18 components in Ilex kudingcha. Rutin, isochlorogenic acid A and kudinoside A are used as internal references; correction factors of rutin for 6-hydroxy-7,7a-dihydro-2(6H)-benzofuran and hydroxytyrosol glucoside, correction factors of isochlorogenic acid A for protocatechuic acid, kudinoside E, kudinoside D, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, isochlorogenic acid B, and isochlorogenic acid C, and correction factors of kudinoside A for latifoloside G, kudinoside G, ilex kudingcha ilexoside T and latifoloside H are calculated, and the factors are used as constants for determining content. Only three common reference substances are needed for simultaneous determination of contents of 18 kinds of components in ilex kudingcha, quality of the ilex kudingcha can be rapidly, economically and scientifically controlled, and further cluster analysis, main component analysis and similarity calculation of medicinal materials can be carried out by using contents of the 18 kinds of components, in order to comprehensively control quality of ilex kudingcha.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
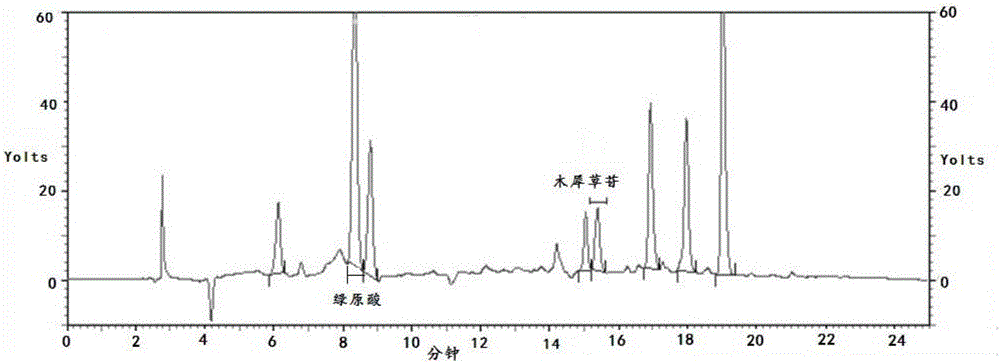
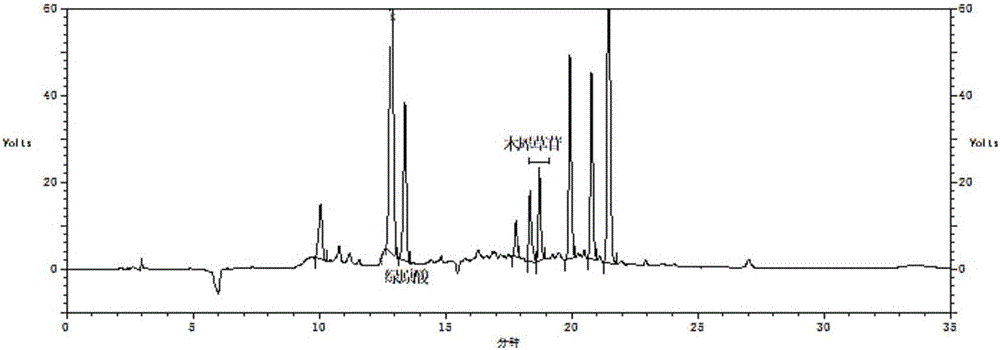
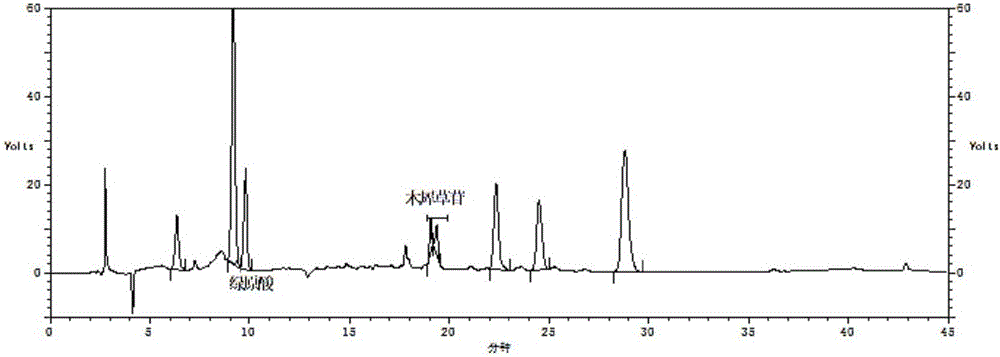
Method for establishing fingerprint of flos lonicerae medicinal preparation
The invention relates to a method for establishing a fingerprint of a flos lonicerae medicinal preparation. The method comprises the following steps of by taking flos lonicerae formula granules as detection objects, establishing a method for a fingerprint of a medicinal preparation, and obtaining more comprehensive fingerprint information; determining common characteristic peaks: first peak neochlorogenic acid, second peak chlorogenic acid, third peak cryptochlorogenic acid, fourth peak rutin, fifth peak galuteolin, sixth peak, seventh peak isochlorogenic acid A and eighth peak isochlorogenic acid C; selecting the second peak chlorogenic acid as an inner reference peak, and determining relative retention time of the common characteristic peaks of the flos lonicerae formula granules. In addition, by combining information of multiple chromatographic peaks in the fingerprint, the quality of the flos lonicerae formula granules can be detected comprehensively and quickly, and comprehensive quality detection and integral quality control of the flos lonicerae formula granules are facilitated, thereby benefiting the improvement on the safety and the stability of medicine use. Meanwhile, the method has the advantages of high stability, high precision, good repeatability and the like.
Owner:华润三九现代中药制药有限公司
Methods for separation and content determination of chlorogenic acid type components in gynura procumbens
The invention discloses two methods for quantitative determination of chlorogenic acid, neochlorogenic acid and three isochlorogenic acids in gynura procumbens extract. The two methods comprise the steps that 1, the best chromatogram conditions are determined; 2, reference substances of the neochlorogenic acid, the chlorogenic acid and the three isochlorogenic acids are obtained, methyl alcohol is added to the reference substances, and then a reference solution is prepared; 3, the gynura procumbens extract is obtained, methyl alcohol is added to the gynura procumbens extract, and after filtering, a sample is obtained; 4, the reference solution and the sample solution are precisely absorbed, chromatography sample introduction is conducted, and the contents of the chlorogenic acid, the neochlorogenic acid and the three isochlorogenic acids are measured; 5, or, only a chlorogenic acid reference solution is prepared in the step 2, the chlorogenic acid is used as a reference substance, the relative correction factors of the isochlorogenic acid C, the isochlorogenic acid A, the isochlorogenic acid B and the neochlorogenic acid are 1.21, 1.12, 1.07 and 0.92, and the contents of the five substances are measured. According to the two methods, the chlorogenic acid is used as reference, fk / s between the chlorogenic acid and other components is established, the content of each component is calculated, the external standard method and the method for quantitative analysis of multi-components by a single marker are similar in accuracy and reliability, and therefore a brand-new mode is provided for evaluating the quality of gynura procumbens more authentically.
Owner:谭玉莲
Analysis method for simultaneously determining six polyphenol contents in flue-cured tobacco
ActiveCN104777256AMeet the testing requirementsThe pre-processing process is simpleComponent separationChlorogenic acidRelative standard deviation
The invention discloses an analysis method for simultaneously determining six polyphenol content in flue-cured tobacco. The method comprises the following steps: grinding leaves of flue-cured tobacco, extracting through a methanol solution, analyzing filtrate through a high performance liquid chromatography after filtering, adopting a HSST3 chromatographic column and a diode array detector, wherein the detection wavelength of the detector is 342mm, the reference wavelength is 480nm, the bandwidth of the detection wavelength is 4nm, the bandwidth of the reference wavelength is 100nm, and the flow velocity of the flowing phase is 0.4mL / min; the flowing phase A is aqueous solution of glacial acetic acid, and the flowing phase B is methanol solution of glacial acetic acid, simultaneously determining contents of six polyphenols in the flue-cured tobacco: neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, scopoletin, rutin and kaempferol alcohol-3-rutinoside in a gradient elution manner. The adding standard recovery of the method is between 95.6-109.3%, the detection limit is 3.7-9.9 migrogram / g, the relative standard deviation is 0.5-3.9% (n equals to 4), the relative coefficients of the standard solution are more than 0.9995, and the pre-treatment of the method is simple and the analysis time is short.
Owner:CHINA TOBACCO SICHUAN IND CO LTD +1
Phlegm-heat clearing injection fingerprint spectrum establishment method and fingerprint spectrum thereof
ActiveCN110187041AAvoid one-sidednessHigh precisionComponent separationDicaffeoylquinic acidInjection solution
The invention relates to the technical field of medicine detection, and in particular relates to a phlegm-heat clearing injection fingerprint spectrum establishment method and a fingerprint spectrum thereof; the phlegm-heat clearing injection is prepared from scutellaria baicalensis, bear gall powder, cornu gorais, honeysuckle and fructus forsythiae, and a phlegm-heat clearing injection fingerprint spectrum is established by detecting the phlegm-heat clearing injection components by adopting an ultra-high performance liquid chromatography method; the method specifically comprises the followingsteps of S1, preparing a reference substance solution: taking a proper amount of caffeic acid, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3, 4-dicaffeoylquinic acid and 3, 5-dicaffeoylquinic acid, 4, 5-dicaffeoylquinic acid, forsythiaside D, baicalin, scutellarin, chrysin-7-O-glucuronide, oroxylin-7-O-glucuronide and a wogonin reference substance, and adding methanol to prepare a solution which is 20-50 [mu]g in 1ml. according to the phlegm-heat clearing injection fingerprint spectrum establishment method provided by the invention, the method is simple and convenient to operate, stable in result, high in reproducibility and high in precision.
Owner:SHANGHAI KAIBAO PHARMA
Method for preparing lonicerae flos monomer with dynamic axial compression column
ActiveCN106317141AImprove resource utilizationSimple preparation processSugar derivativesOrganic compound preparationPurification methodsChlorogenic acid
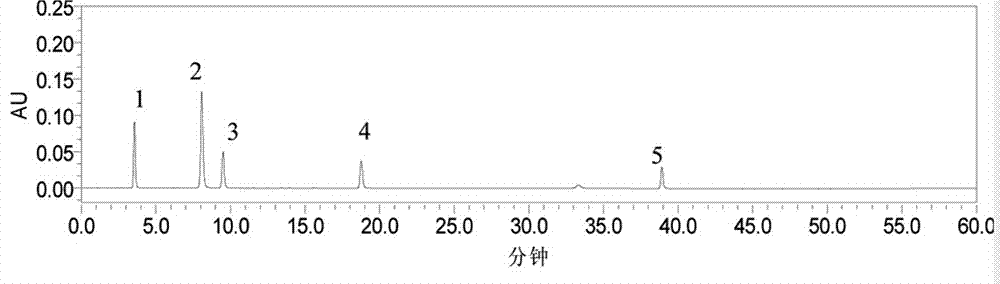
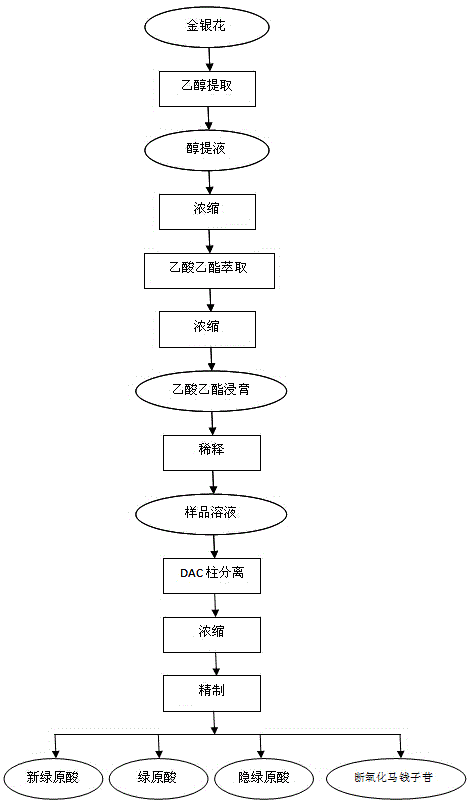
The invention relates to the technical field of medicine, and discloses a method for separating and purifying the four monomers neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid and secoxyloganin from lonicerae flos with a dynamic axial compression column. The method comprises the steps of mobile phase preparation, dissolution and filtration, dynamic axial compression column packing, system balancing, elution and separation, concentration and the like. With the method, the four monomers neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid and secoxyloganin can be prepared at a same time in one separation purification process. As a result of high performance liquid chromatography detection, the purities of the four monomers are all above 90%. The method provided by the invention has the advantages of simple operation, short production period, high preparation efficiency, and controllable product quality. All adopted organic reagents can be recycled. The method is a highly efficient, environment-friendly and highly applicable separation purification method for industrial preparation of lonicerae flos monomers.
Owner:SHENYANG PHARMA UNIVERSITY
Pharmaceutical composition useful for treating chronic myeloid leukemia
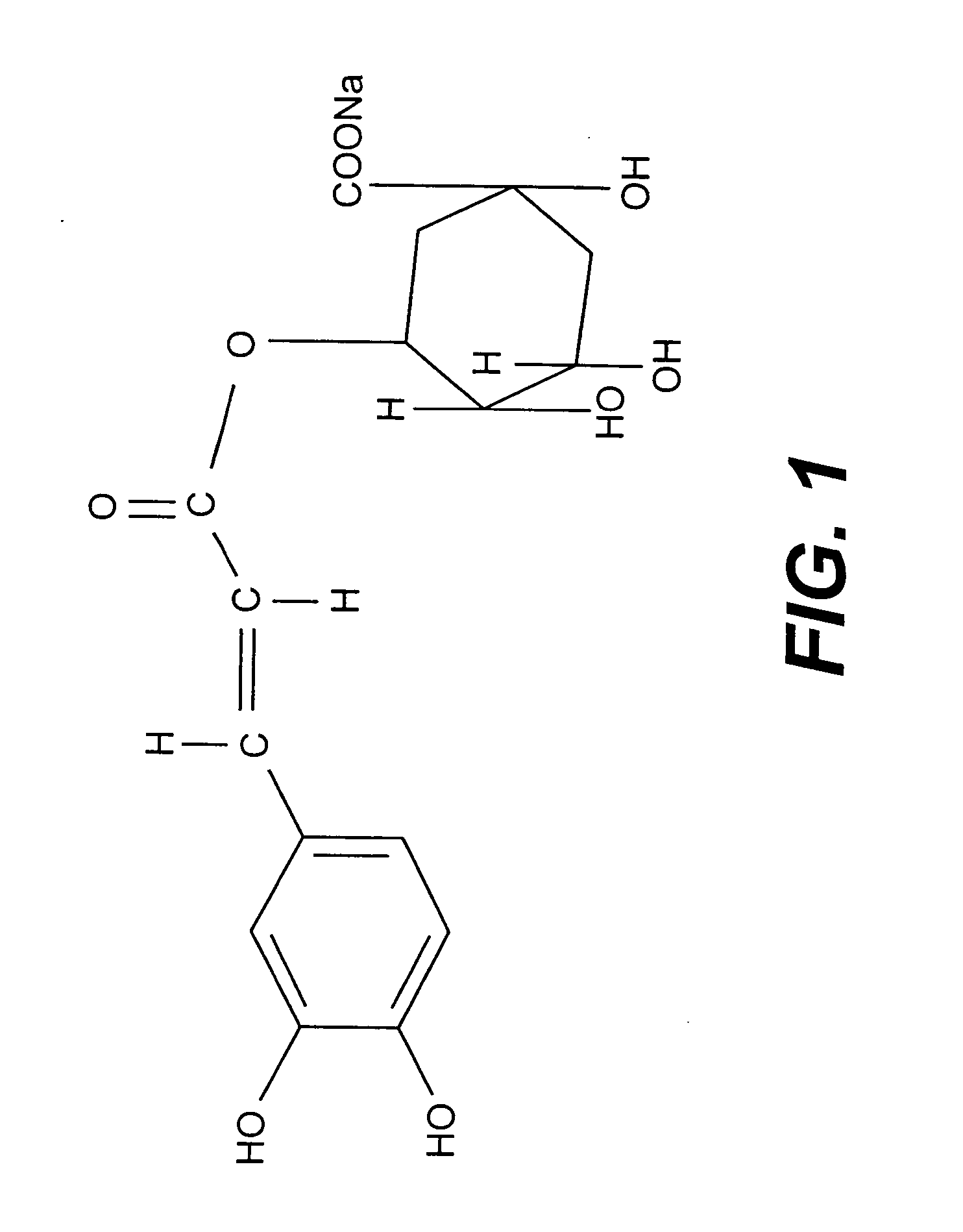
The present invention relates to a pharmaceutical composition useful for treating chronic myeloid leukemia where Bcr-Abl kinase is constitutively expressed in animals and humans, said composition comprising an effective amount of analogs of chlorogenic acid such as neochlorogenic acid (5-O-caffeoyl quinic acid), cryptochlorogenic acid (4-O-Caffeoyl quinic acid), 3-O-(3'-methylcaffeoyl) quinic acid and 5-O-(Caffeoyl-4'-methyl) quinic acid and / or its salts such as sodium, potassium and ammonium together with pharmaceutically acceptable additives.
Owner:COUNCIL OF SCI & IND RES
Multi-index-based evaluating-optimizing glabrous sarcandra herb preparation technology
The invention discloses a multi-index-based evaluating-optimizing glabrous sarcandra herb preparation technology. The multi-index-based evaluating-optimizing glabrous sarcandra herb preparation technology comprises the following steps of HPLC determination of specified components, process optimization of glabrous sarcandra herb leaves, single factor experimental investigation of glabrous sarcandraherb stems, cutting-preparing design scheme of the glabrous sarcandra herb stems, establishment of an AHP model, a comprehensive scoring method and CCD-RSM optimized glabrous sarcandra herb stem cutting-preparing process and verification test. Through a single-factor test, the comprehensive scores of the content of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, isofraxidin, astilbin and rosmarinic acid are taken as the evaluation indicators, a central composite design-response surface methodology is used to screen glabrous sarcandra herb softening and cutting-preparing processes, the glabrous sarcandra herb stems and leaves subjected to process optimization by choosing different treatment methods, a multi-index analytic hierarchy process (AHP) is combined with the central composite design-response surface methodology to optimize the glabrous sarcandra herb preparation technology, the study is beneficial to the further development of glabrous sarcandra herb, and an experimental basis is expected to be provided for the establishment of related preparation specifications and quality standards of glabrous sarcandra herb.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Production method of Lonicera japonica flower cells rich in chlorogenic acid substances
InactiveCN109757372AControllableEasy to operate and managePlant tissue cultureHorticulture methodsFlosChlorogenic acid
The invention relates to a production method of Lonicera japonica flower cells rich in chlorogenic acid substances. The method comprises the following steps: pretreatment; disinfection; culture mediumpreparation; callus treatment; subculture; establishment of a Lonicera japonica flower cell suspension culture system and posttreatment. Content of the chlorogenic acid substances in the product produced with the method is as high as 9.68% or higher which is increased by 100%-100.83% as compared with the content 4.82%-4.84% of chlorogenic acid of natural Lonicera japonica flower buds on the basisof the conventional production method (Determination of eight components in Lonicerae japonicae flos by HPLC [J]. Chinese Traditional and Herbal Drugs, 2014, 45(7):1006-1009 by Limiao, Wangyongxiangand Mengjin), production period is shortened by 300-330 days, and cost is reduced by 70%-80% through cost accounting. Indusial and automatic production is easy to realize, and the method can be widelypromoted and used.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Method for detecting effective part group of periploca forrestii schltr
ActiveCN109655558AHarvest GuideThe detection method is accurateComponent separationChlorogenic acidRepeatability
The invention discloses a method for detecting an effective part group of periploca forrestii schltr. The method is used for detecting the content of chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid, isochlorogenic acid C and procyanidine A2, the detection method is accurate, the sensitivity is high, the repeatability is good, results are reliable, a basis is provided for quality control and evaluation of the periploca forrestii schltr medicinal material, and a foundation is laid for determining the optimal production place of the medicinal material. The method is beneficial for determining the appropriate growth area of the medicinal material on production, and is used for guiding medicinal material harvesting.
Owner:贵州中医药大学
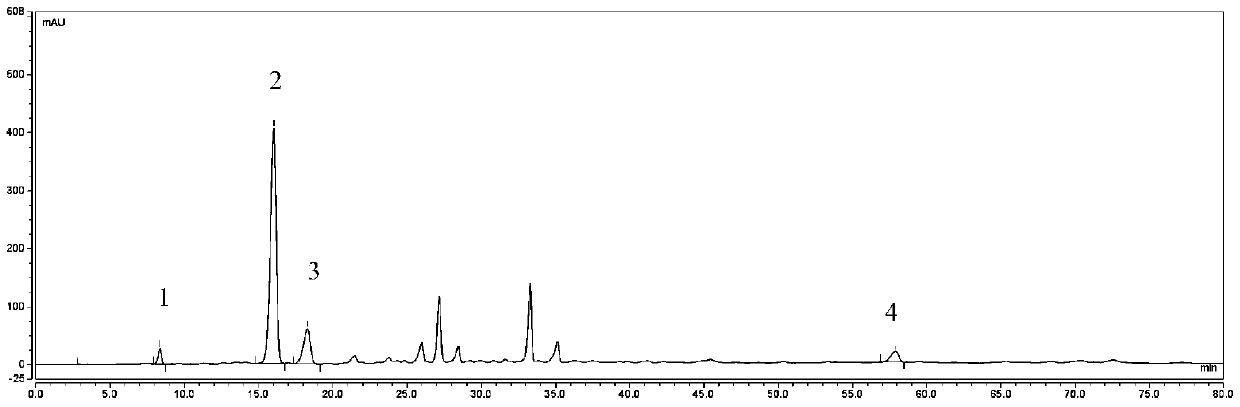
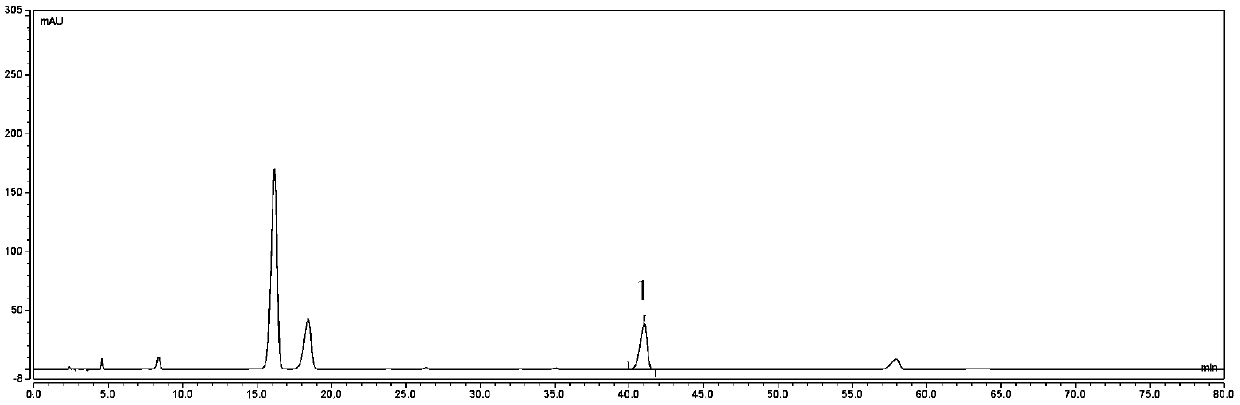
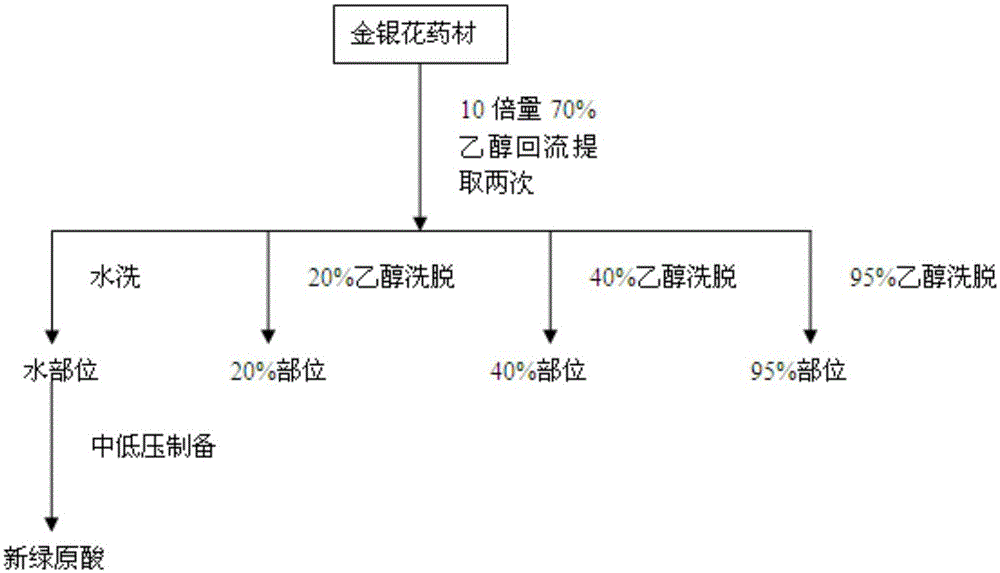
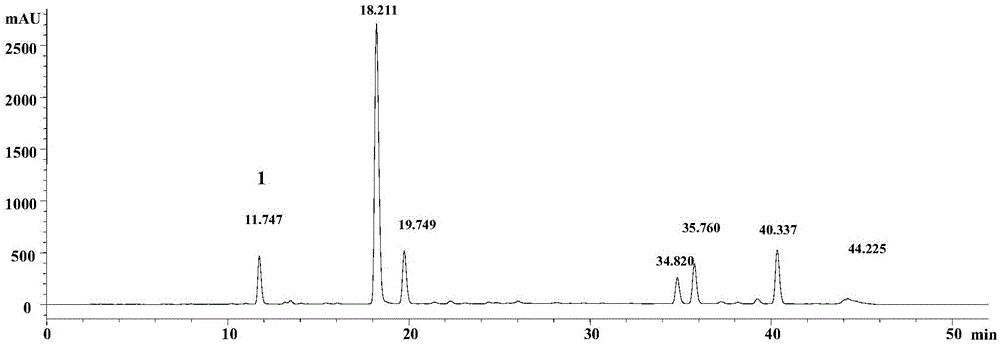
Preparation method of neochlorogenic acid
ActiveCN105061212AMeet the requirements of reference substance for content determinationOrganic compound preparationCarboxylic acid esters separation/purificationReflux extractionChlorogenic acid
The invention relates to the chemical field, in particular to a preparation method of neochlorogenic acid. According to the preparation method, Flos Lonicerae japonicae is subjected to reflux extraction through ethanol, filtered, concentrated, separated with macroporous adsorption resin and subjected to gradient elution through ethanol and water; elution parts of neochlorogenic acid are collected after HPLC (high performance liquid chromatography) analysis and detection; neochlorogenic acid is prepared quickly with a medium-low pressure preparative chromatograph and is above a gram level; meanwhile, neochlorogenic acid is subjected to structural identification and purity detection, and the purity can be as high as 98.86%. Neochlorogenic acid prepared with the method meets requirements of reference substances for content detection, and a new method for preparing high-purity neochlorogenic acid on a large scale is provided.
Owner:JIANGSU KANION PHARMA CO LTD
Quality monitoring method for quantitative fingerprint spectrum of Shuanghuanglian oral liquid
InactiveCN112858549ARealize fingerprint monitoringRealize quantitative detectionComponent separationChlorogenic acidPharmacology
The invention relates to a quantitative fingerprint quality monitoring technology for Shuanghuanglian oral liquid, and belongs to the technical field of compound quality analysis. A dual-wavelength quantitative fingerprint spectrum quality control method is established on the basis of a high performance liquid chromatography-ultraviolet detection technology, fingerprint spectrum monitoring of the Shuanghuanglian oral liquid is achieved through one-time analysis, content determination of three index components of chlorogenic acid, baicalin and forsythin is achieved at the same time, and one-standard multi-determination amount of neochlorogenic acid and cryptochlorogenic acid is achieved. According to the method, fingerprint spectrum and quantitative detection are simultaneously realized in one-time analysis, the quality of the Shuanghuanglian oral liquid can be more comprehensively and accurately evaluated, the Shuanghuanglian oral liquid of different manufacturers can be distinguished, meanwhile, the detection time is shortened to the greatest extent, and the resource consumption is reduced.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for rapidly separating and purifying polyphenol compounds in sorbus pohuashanensis berries
ActiveCN109503373AOvercome operabilityOvercome the cycleSugar derivativesOrganic compound preparationDicaffeoylquinic acidPolyamide
The invention discloses a method for rapidly separating and purifying polyphenol compounds in sorbus pohuashanensis berries. The method comprises the steps as follows: mashing sorbus pohuashanensis berries, adding an ethanol solution for ultrasonic extraction, and performing concentration under reduced pressure to obtain crude extract of sorbus pohuashanensis berries; performing gradient elution by a polyamide chromatographic column with ethanol as an eluant, and performing concentration under reduced pressure to obtain eluates with different gradient fractions; separating and purifying the eluates with different gradient fractions with HSCCC (high-speed counter-current chromatography) to obtain neochlorogenic acid, chlorogenic acid, quercetin-3-O-(6''-alpha-L)-rhamnosyl-4'''-alpha-L-rhamnosyl)-beta-D-glucoside, 3,5-O-dicaffeoylquinic acid and rutin with purity higher than 95% respectively. The method adopts a simple process, is high in separation speed and low in comprehensive cost and has very good promotional and use values, and the product purity is high.
Owner:LIAONING UNIVERSITY
Construction method and detection method of UPLC characteristic chromatogram of stemona tuberosa medicinal material
The invention relates to a construction method and a detection method of a UPLC characteristic chromatogram of a stemona tuberosa medicinal material. The construction method of the characteristic chromatogram comprises the following steps: preparing a reference substance solution of a control sample from chlorogenic acid, neochlorogenic acid and cryptochlorogenic acid as a control sample respectively; preparing a reference substance solution of a control medicinal material from a stemona tuberosa control medicinal material; taking a stemona tuberosa medicinal material and a standard decoctionsample respectively to prepare a test sample solution and a standard decoction test sample solution; sucking the reference substance solution, the test sample solution and the standard decoction testsample solution respectively, and injecting the solutions into an ultra-high performance liquid chromatograph for measurement; and comparing a test sample chromatogram of the stemona tuberosa medicinal material with a test sample chromatogram of a standard decoction sample and calibrating characteristic peaks of 8 water-soluble components to obtain the UPLC characteristic chromatogram of the stemona tuberosa medicinal material. The characteristic chromatogram can be used for qualitative and quantitative analysis of the quality of the stemona tuberosa medicinal material, can ensure the qualityof a stemona tuberosa traditional decoction prepared from the medicinal material, and is also suitable for detecting other preparations containing stemona tuberosa.
Owner:GUANGDONG YIFANG PHARMA +1
Multi-component content determination method of Miao medicine Laportea bulbifera
InactiveCN109270177ASimple methodFast wayComponent separationChlorogenic acidCyclohexanecarboxylic acid
The invention discloses a multi-component content determination method of Miao medicine Laportea bulbifera. The method comprises the following steps: S100, preparation of mixed reference solution, respectively and accurately weighing right amount of reference substances of Gallocatechin, neochlorogenic acid, Epigallocatechin, catechinic acid, chlorogenic acid, Cyclohexanecarboxylic acid, epicatechin, rutin, isoquercitrin, kaempferol-3-0-rutinoside and quercitrin; and simultaneously determining contents of eleven components including the Gallocatechin, the neochlorogenic acid, the Epigallocatechin, the catechinic acid, the chlorogenic acid, the Cyclohexanecarboxylic acid, the epicatechin, the rutin, the isoquercitrin, the kaempferol-3-0-rutinoside and the quercitrin. The method is simple, efficient, highly sensitive and excellent in specificity, and can provide baisis for quality control of Laportea bulbifera medicinal materials. Compared with the ordinary liquid phase, the method has the advantages of being accurate, reliable and highly sensitive, and having lower specificity detection limit and quantification limit, and is more effective in medicinal material component content analysis through a liquid chromatography-mass spectrometry analysis technology.
Owner:GUIZHOU MEDICAL UNIV
Method for extracting chlorogenic acid and neochlorogenic acid from bunge pricklyash leaves
InactiveCN104016864ASimple processEasy to operateOrganic compound preparationCarboxylic acid esters separation/purificationChlorogenic acidGradient elution
The invention discloses a method for extracting chlorogenic acid and neochlorogenic acid from bunge pricklyash leaves. The method is characterized by comprising the following steps of: crushing dried bunge pricklyash leaves till the average particle size is 400-600 micrometers; adding to methanol with the concentration of 50wt%-80wt% according to the material-to-liquid ratio of 1g:(8-30)mL, oscillating at room temperature for 24-36 hours, separating supernatant, concentrating, and drying to obtain a methanol extract; dispersing the methanol extract into distilled water at the material-to-ratio of 1g:(10-30)mL, adding ethyl acetate according to the volume ratio of 1:2, carrying out oscillating extraction for 2-3 times, collecting a water-phase extractive, concentrating and drying, wherein the yield is 19.8%-25.8%; carrying out gradient elution on a reveres-phase resin SciBioChem MCI-GEL chromatographic column positioned on a dried sample by using methanol-water, wherein the filler height is 20cm, the column diameter is 3.2 cm, the concentrations of methanol-water are 0wt%, 10wt%, 20wt%, 30wt%, 40wt% and 50wt%, and the elution flow velocity is1-2mL / min; respectively collecting 10wt% methanol eluent and 20wt% methanol eluent, concentrating, drying, and then separating and purifying by using liquid chromatography to obtain the neochlorogenic acid and the chlorogenic acid, wherein the yield is 0.03%-0.05% and 0.03%-0.06% respectively.
Owner:SICHUAN UNIV
Method for evaluating quality of cold-treating and cough-relieving granules by combining multi-index components with fingerprint spectrum
ActiveCN112051350AGood reference valueQuality improvementComponent separationChlorogenic acidO-Phosphoric Acid
The invention belongs to the field of traditional Chinese medicine quality evaluation, and relates to a method for evaluating the quality of granules for treating cold and relieving cough by combiningmulti-index components with fingerprints, which adopts reversed-phase high-performance liquid chromatography and gradient elution by using acetonitrile and 0.1% phosphoric acid aqueous solution to simultaneously analyze and determine 10 components of 13 batches of granules for treating cold and relieving cough, and researches the fingerprints of the 10 components. Results show that 10 components(neochlorogenic acid, chlorogenic acid, puerarin, 3 '-methoxypuerarin, daidzein, baicalin, forsythin, wogonoside, baicalein and wogonin) are well separated, the sampling recovery rate is 98.98%-100.6%, and the similarity of 13 batches of samples at 250 nm is lower than 0.90 or above. Principal component analysis and thermogram analysis results show that the established content determination and characteristic spectrum analysis combined method proves that the cold-relieving and cough-relieving granules of different manufacturers are obviously different.
Owner:淄博市食品药品检验研究院
Method for measuring content of chemical components in traditional Chinese medicine compound preparation
The invention provides a method for determining the content of chemical components in a traditional Chinese medicine compound preparation, belongs to the technical field of Chinese patent medicine content determination, and aims to detect the components with greatly different contents in traditional Chinese medicines, accurately determine the contents of high-content components and low-content components in the same traditional Chinese medicine sample and judge the mass change of the traditional Chinese medicines. The traditional Chinese medicine is Shuanghuanglian injection. In order to solve the problem of simultaneous determination of components with great content differences in a traditional Chinese medicine complex system, a multiple dilution method is constructed, a test solution with a low dilution ratio is prepared to detect low-content components (21 components such as neochlorogenic acid), and a test solution with a high dilution ratio is prepared to detect high-content components (baicalin). Rapid detection of components with great content differences in the sample is realized through different dilution methods. The dilution ratio and dilution times of the test solution can be determined according to the content difference degree of the detection components in the to-be-detected sample. The method effectively solves the problem of detection of the components with the content difference.
Owner:河南福森药业有限公司 +1
Composition for substituting fructus xanthii to prepare glioma treatment medicine and measuring method
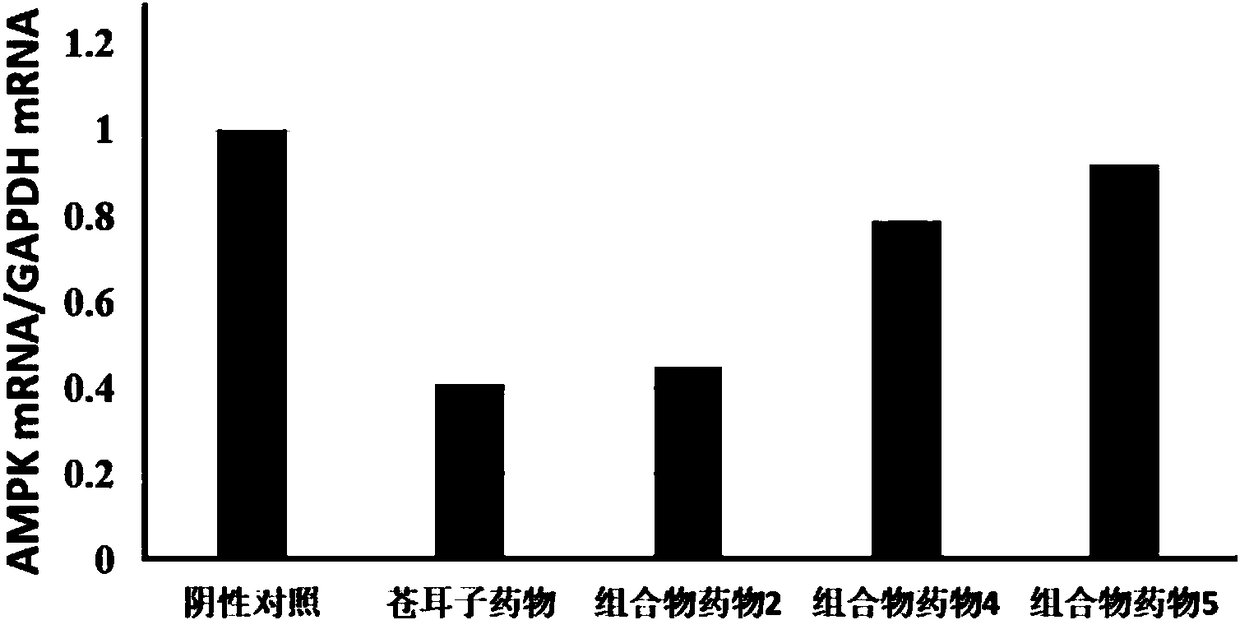
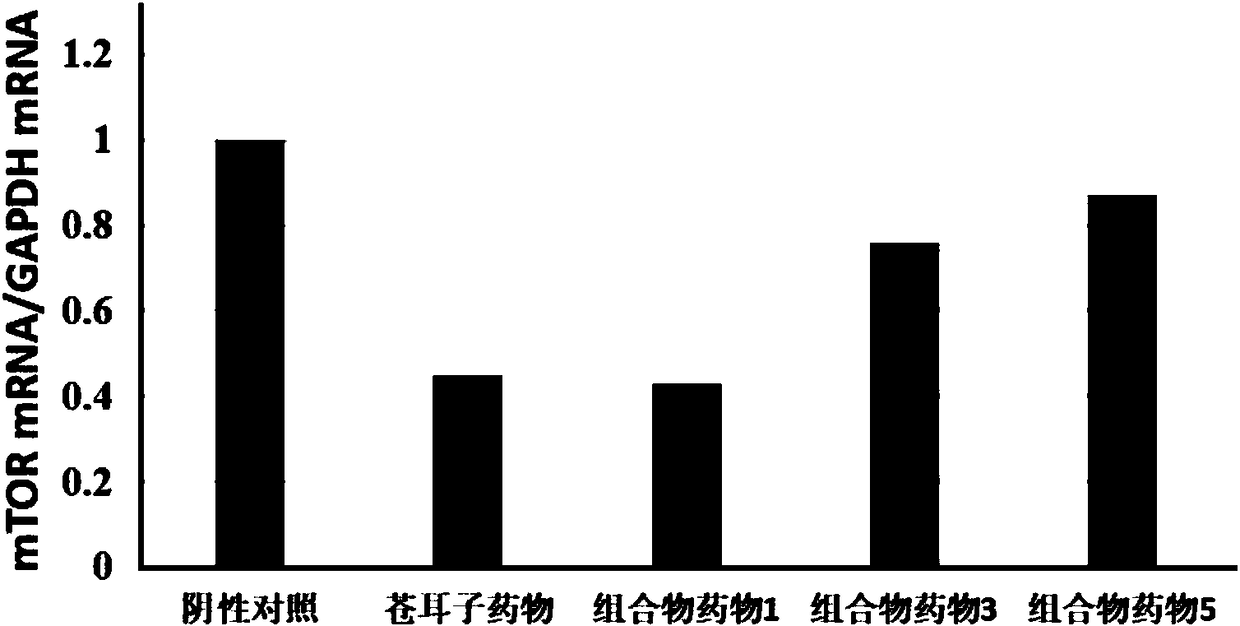
InactiveCN108714147APrevent proliferationInhibition of Biological Behavioral EffectsOrganic active ingredientsComponent separationDicaffeoylquinic acidMedicine
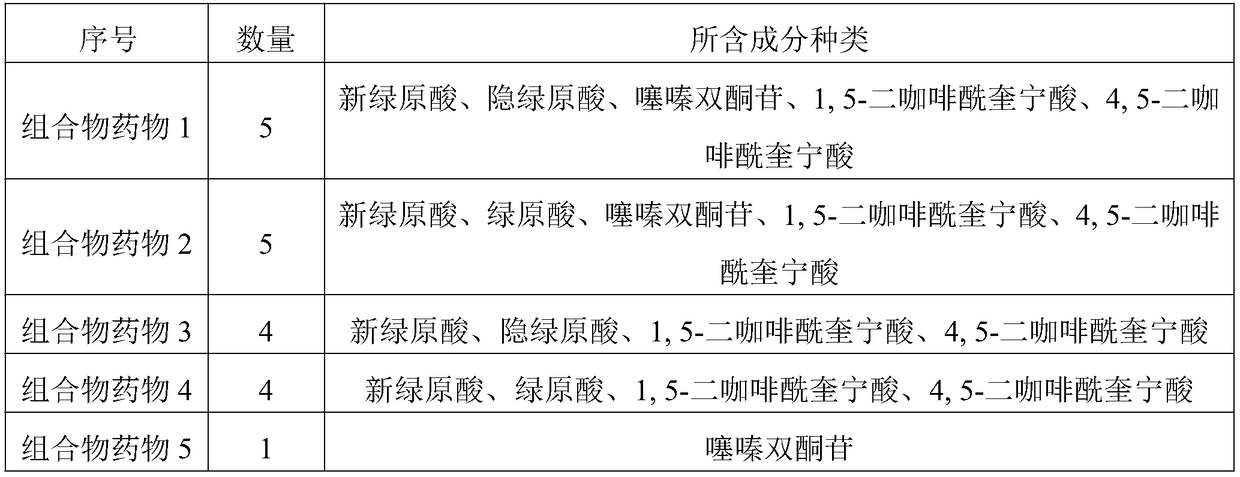
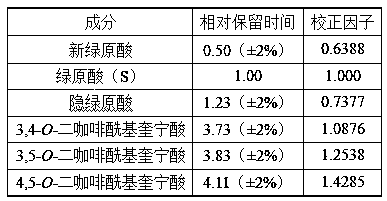
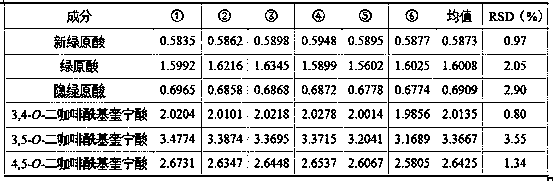
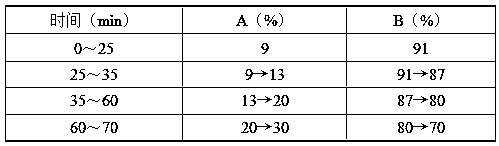
The invention discloses a composition for substituting fructus xanthii to prepare a glioma treatment medicine and a measuring method. The composition composed of neochlorogenic acid, chlorogenic acid,xanthiside, 1,5-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid basically can substitute an inhibitory effect of the fructus xanthii on mTOR gene expression equivalently, wherein the compositionand the fructus xanthii have equivalent strength; the composition can be used for equivalently substituting the fructus xanthii to prepare an mTOR gene expression inhibitor. Those skilled in the art know that inhibition of the mTOR gene expression can effectively inhibit proliferation of glioma cells (miRNA-99b negatively regulates ability of mTOR for inhibiting invasion of glioma cells. Chinese Pharmacological Bulletin, April 2018, Vol. 34, No. 4); therefore, the composition can be used for equivalent substitution of the fructus xanthii to prepare the glioma treatment medicine.
Owner:杨真慧
Multi-component content determination method of mussaenda hirsutula
InactiveCN107677750AEffective quality controlImprove quality inspection standardsComponent separationOrganic acidDicaffeoylquinic acid
The invention discloses a multi-component content determination method of mussaenda hirsutula. According to the invention, a QMSA method is employed for simultaneously determining 6 organic acid components in mussaenda hirsutula: neochlorogenic acid, cryptochlorogenic acid, chlorogenic acid, 3,5-O-dicaffeoylquinic acid, 3,4-O-dicaffeoylquinic acid, and 4,5-O-dicaffeoylquinic acid. The selected organic acid components of the mussaenda hirsutula are taken as an index for content determination, and the method has the advantages of high precision, good reappearance, good stability, high recovery rate, and accurate determination result, so that the quality of mussaenda hirsutula can be effectively controlled.
Owner:广西壮族自治区食品药品检验所
Method for measuring concentration of 7 blood inflow ingredients in Eucommia ulmoides extract
InactiveCN110057938AHigh sensitivityWide linear rangeComponent separationHplc esi msChlorogenic acid
The invention discloses a method for measuring the concentration of 7 blood inflow ingredients in an eucommia extract. The method comprises the following steps: firstly preparing a standard control solution by using geniposidic acid, protocatechuic acid, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, pinoresinol diglucoside and terpineol monoglucoside; preparing an internal standard solution by using puerarin; then establishing an analysis method for simultaneous measuring the concentration of 7 ingredients in the eucommia extract in rat plasma by using an HPLC-ESI-MS / MS analysis; and the result shows that the measurement method disclosed by the invention has the advantages of wide linear range, high sensitivity and low limit of quantification, and can be used for accurately and reliably measuring the concentration of the 7 ingredients in the eucommia extract in the rat plasma extracted at 15 different time points after a spontaneously hypertensive rat is gavaged with the eucommia extract at the same time, and can be used for providing a technical support for the researches on the pharmacokinetic characteristics of the 7 ingredients in the eucommia extract.
Owner:GUIZHOU MEDICAL UNIV
A quality control method capable of simultaneous content analysis and similarity evaluation of 18 components in Kudingcha
ActiveCN106198782BRealize simultaneous content determinationComprehensive evaluationComponent separationIlex kudingchaHydroxytyrosol
The invention discloses a quality control method for simultaneous realization of content analysis and similarity evaluation of 18 components in Ilex kudingcha. Rutin, isochlorogenic acid A and kudinoside A are used as internal references; correction factors of rutin for 6-hydroxy-7,7a-dihydro-2(6H)-benzofuran and hydroxytyrosol glucoside, correction factors of isochlorogenic acid A for protocatechuic acid, kudinoside E, kudinoside D, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, isochlorogenic acid B, and isochlorogenic acid C, and correction factors of kudinoside A for latifoloside G, kudinoside G, ilex kudingcha ilexoside T and latifoloside H are calculated, and the factors are used as constants for determining content. Only three common reference substances are needed for simultaneous determination of contents of 18 kinds of components in ilex kudingcha, quality of the ilex kudingcha can be rapidly, economically and scientifically controlled, and further cluster analysis, main component analysis and similarity calculation of medicinal materials can be carried out by using contents of the 18 kinds of components, in order to comprehensively control quality of ilex kudingcha.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Method for measuring content of active ingredients in Chinese honeylocust fruit
ActiveCN114674958AEasy to operateHigh sensitivityComponent separationAgainst vector-borne diseasesBiotechnologyApigenin
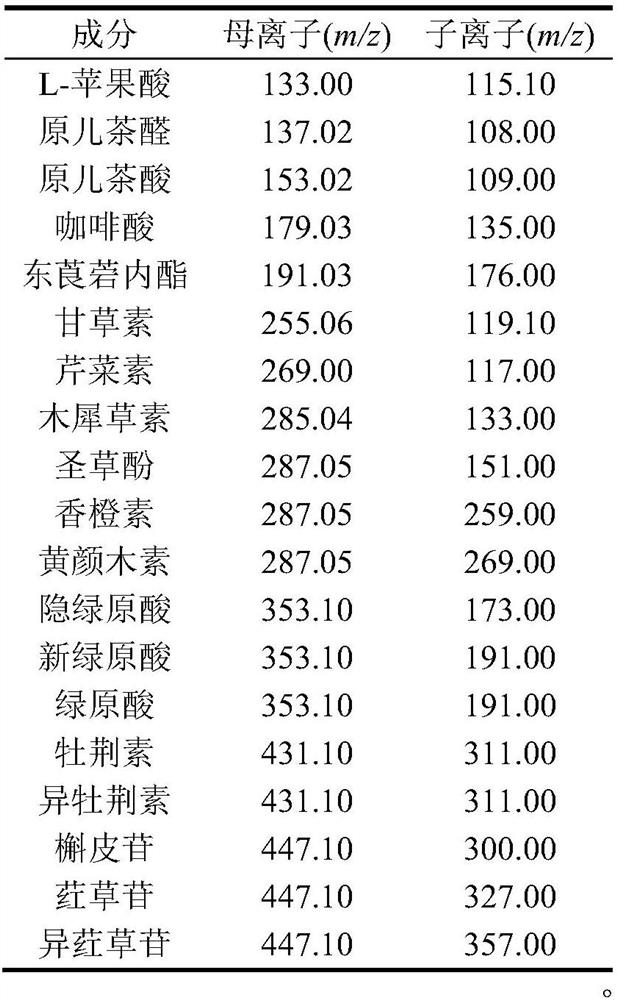
The invention provides a method for determining the content of active ingredients in Chinese honeylocust fruit, which adopts ultra-high performance liquid chromatography-mass spectrometry. The content of L-malic acid, protocatechuic aldehyde, protocatechuic acid, caffeic acid, scopoletin, liquiritigenin, apigenin, luteolin, eriodictyol, citrus aurantium, fustin, cryptochlorogenic acid, neochlorogenic acid, chlorogenic acid, vitexin, isovitexin, quercitrin, orientin and isoorientin in the Chinese honeylocust fruit can be rapidly detected. The method provided by the invention has the advantages of simple operation, high sensitivity, fast analysis speed and strong specificity, and can be used for quality control of Chinese honeylocust fruit.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com