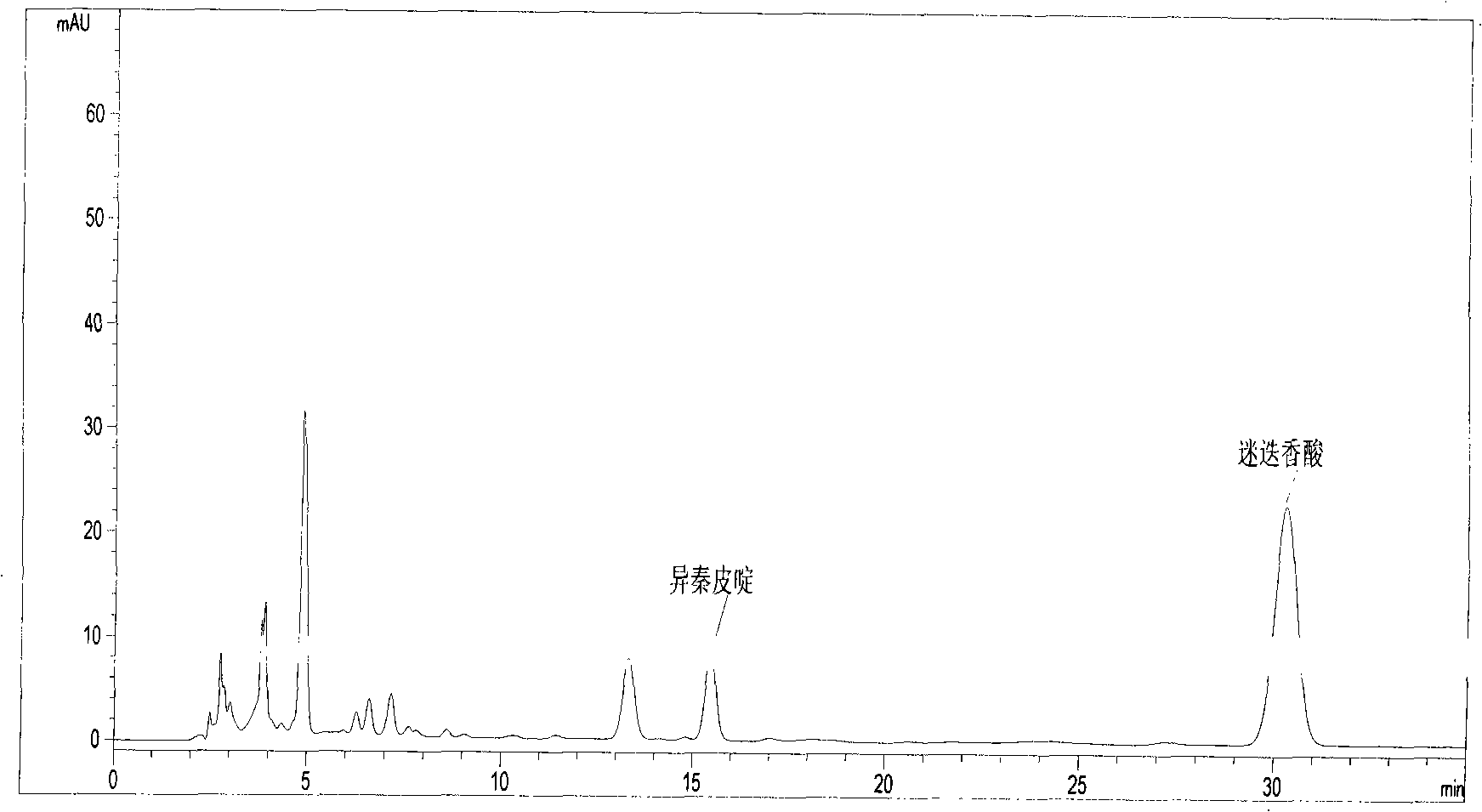
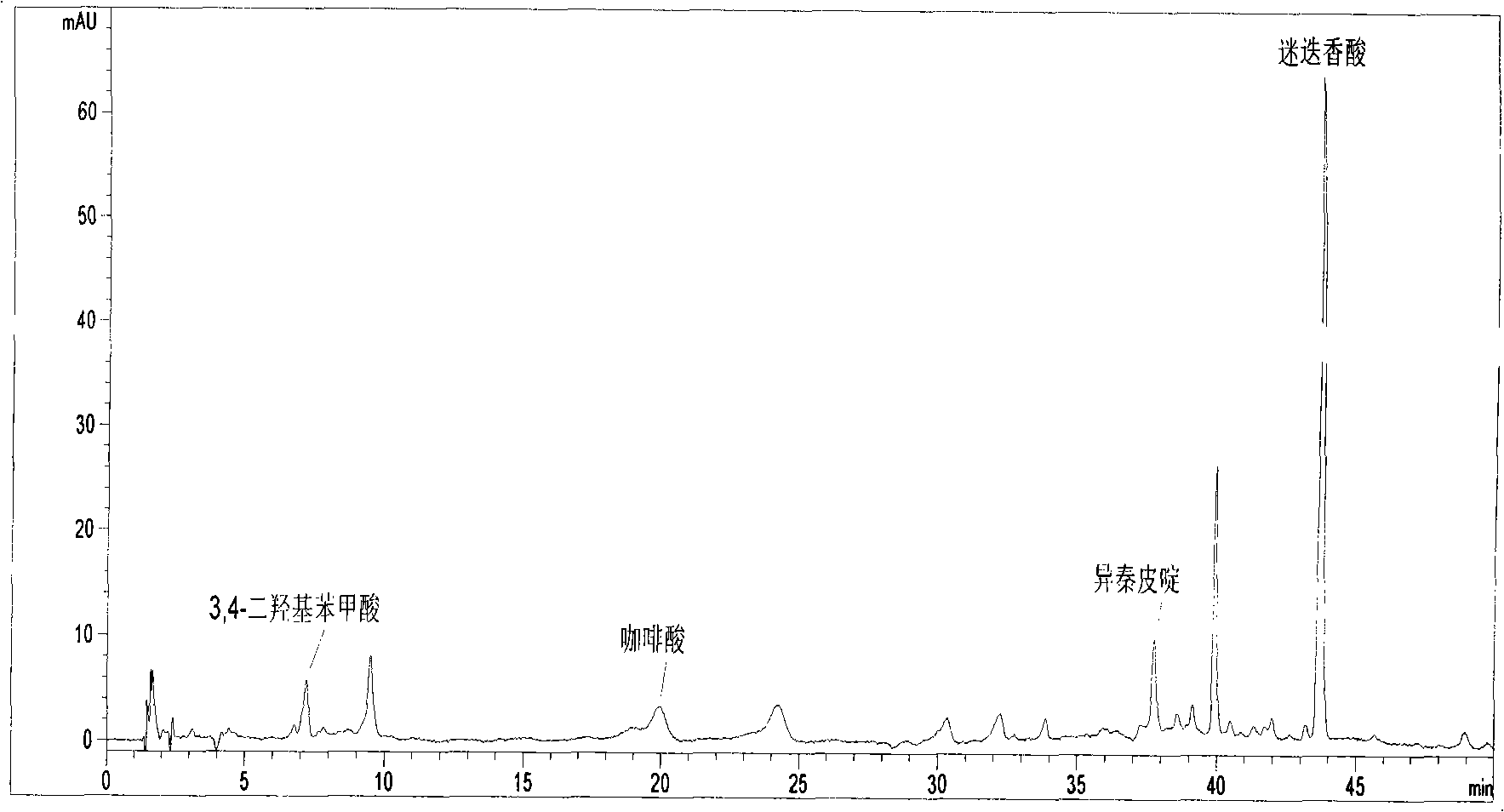
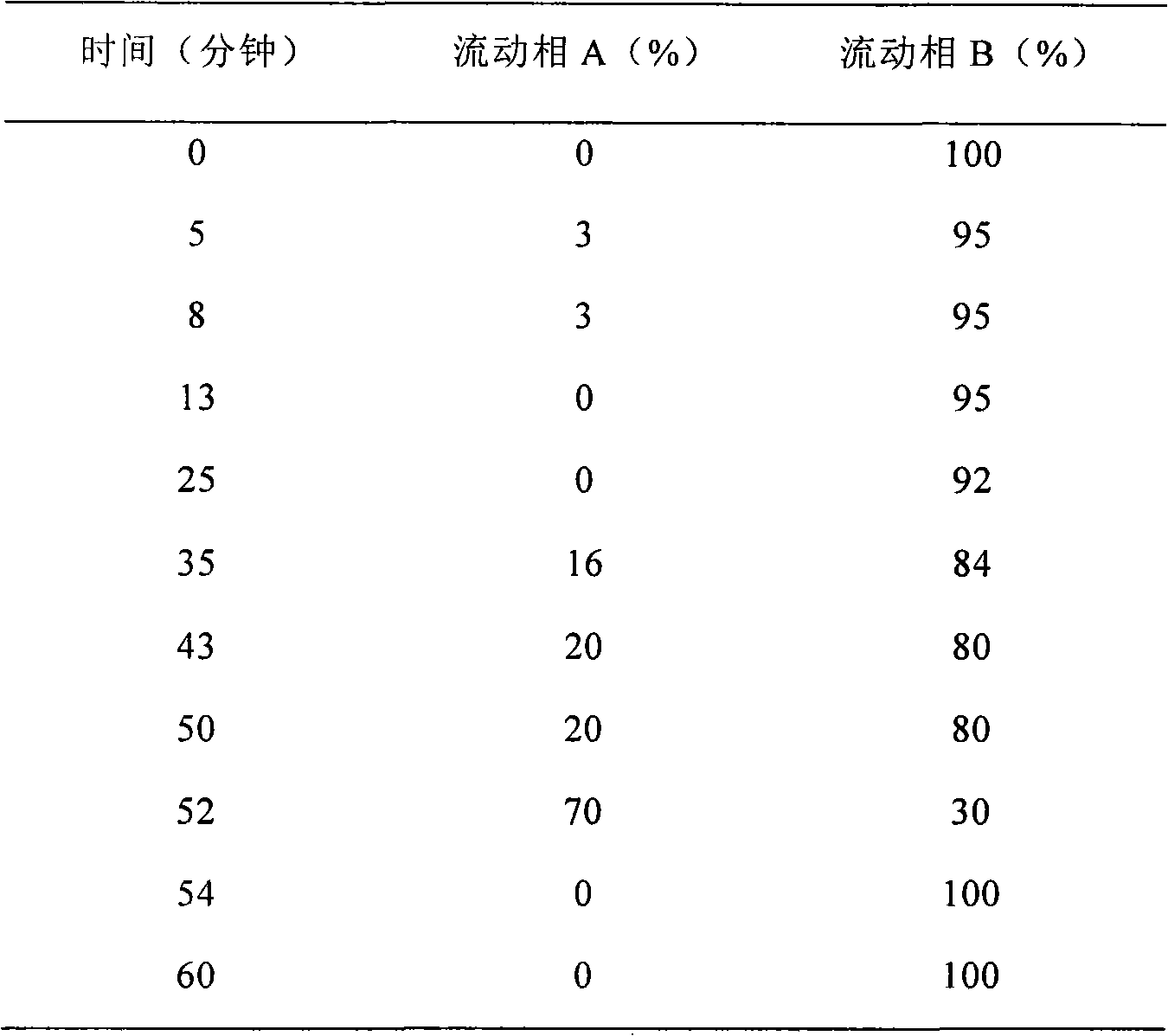
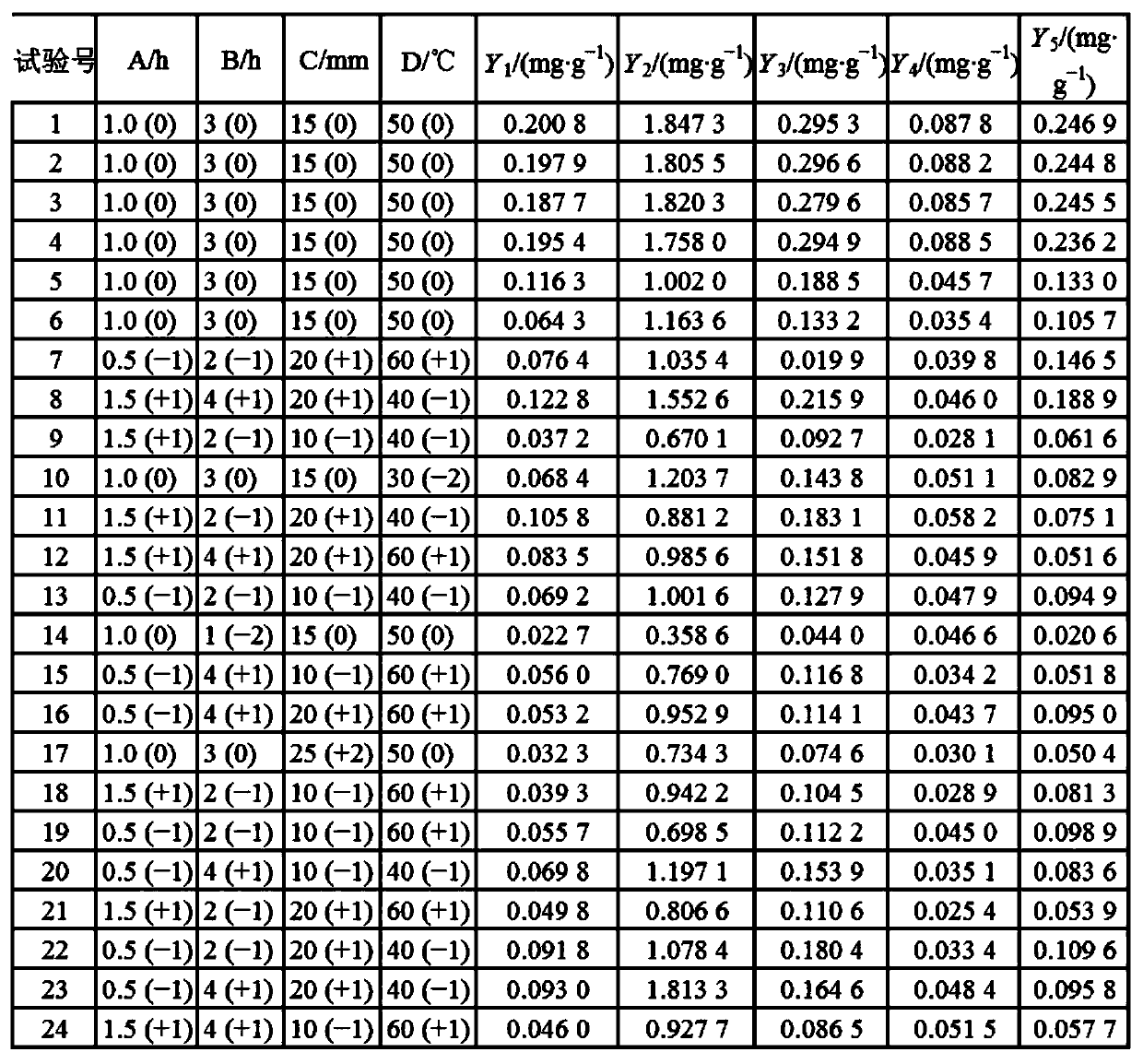
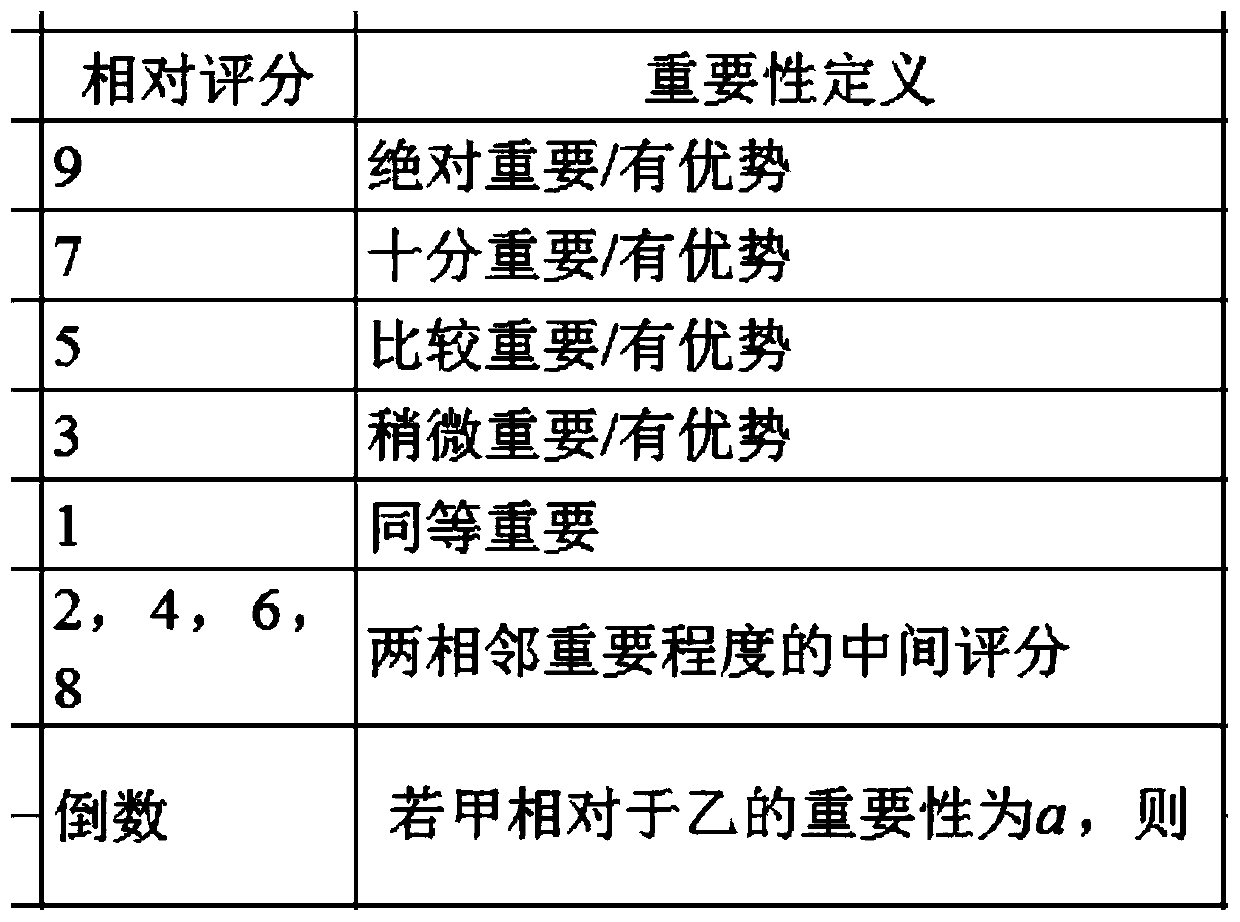
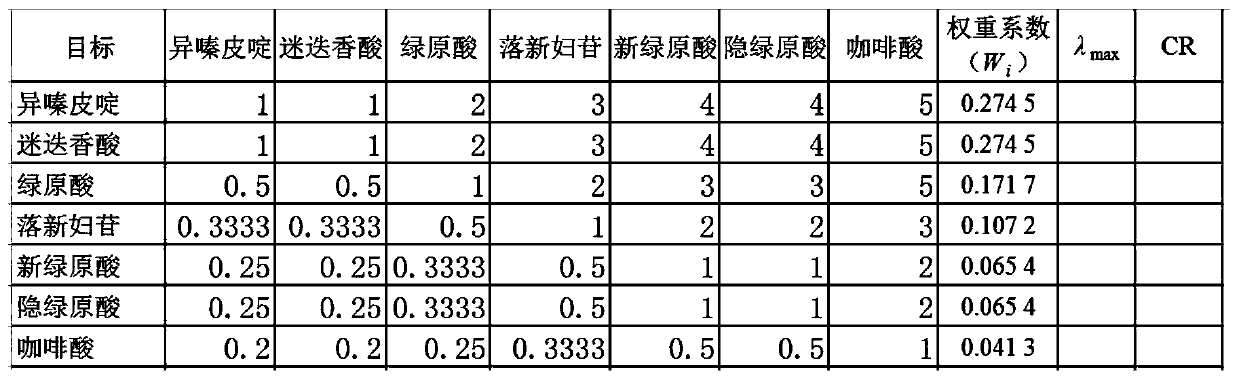
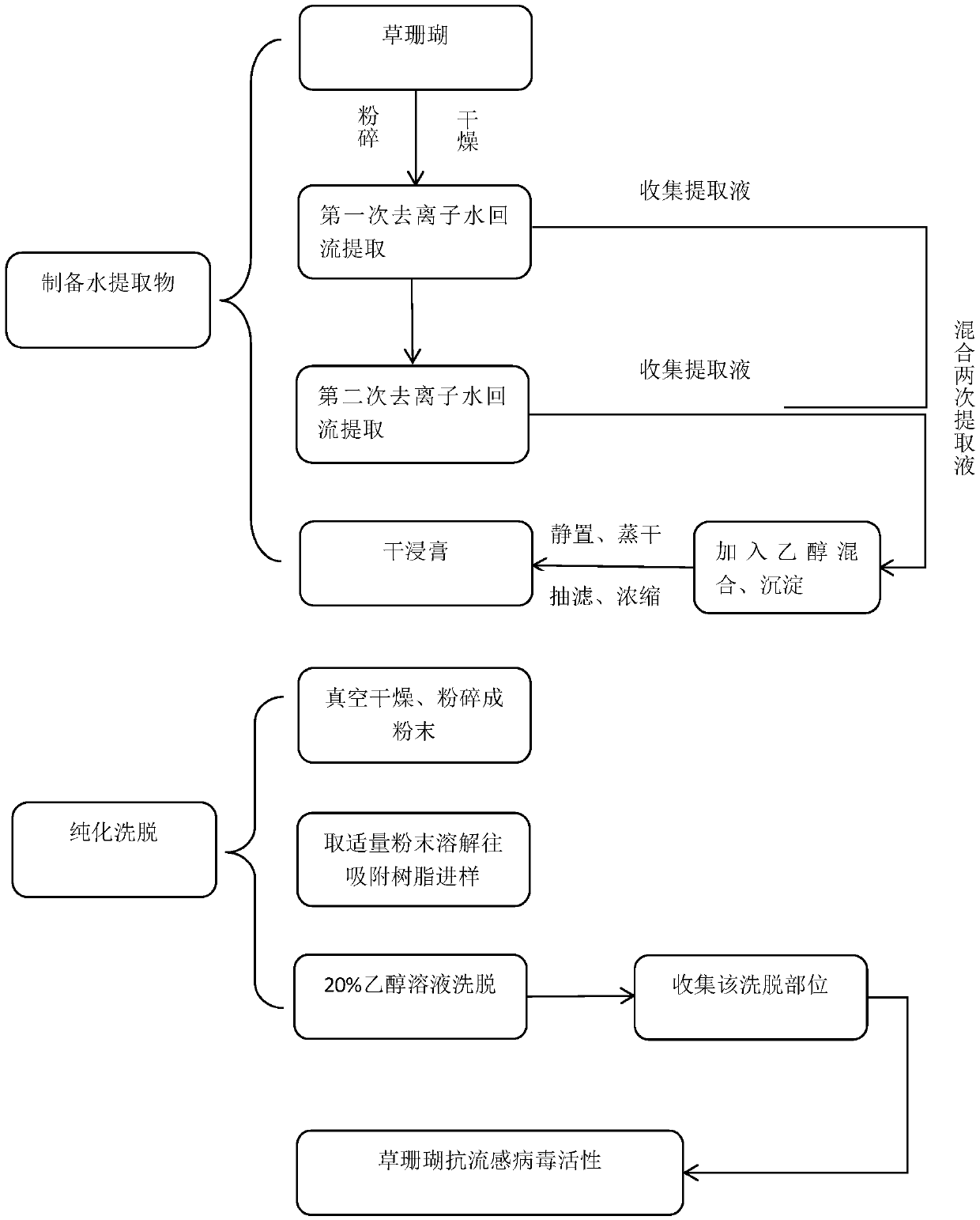
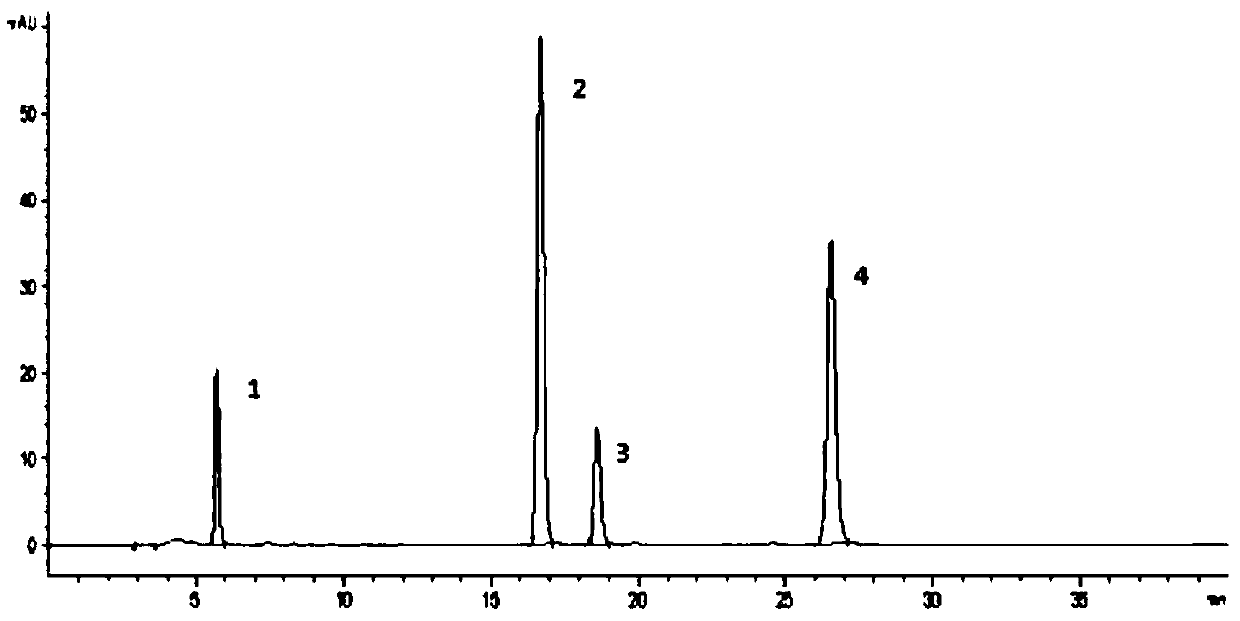
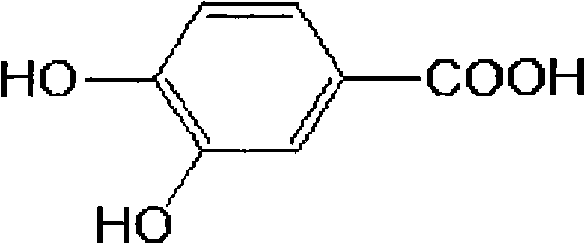
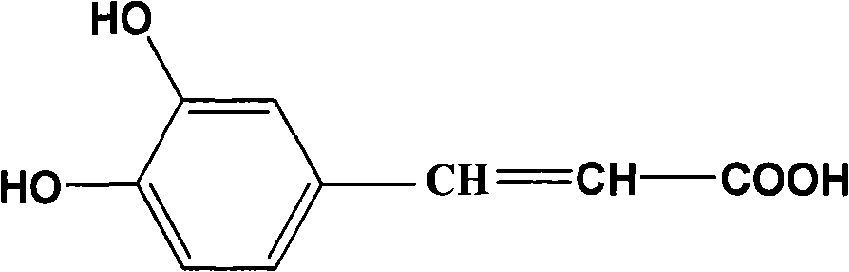
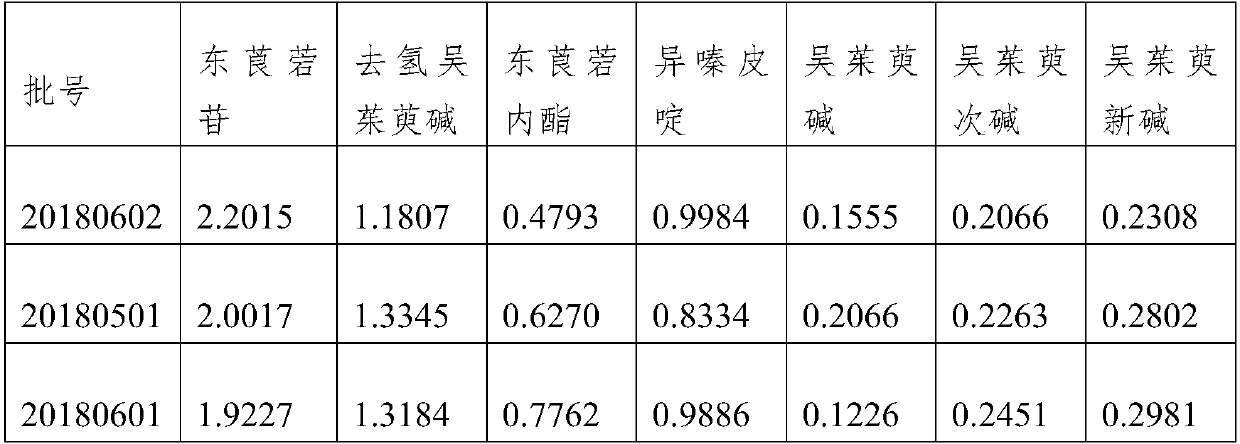
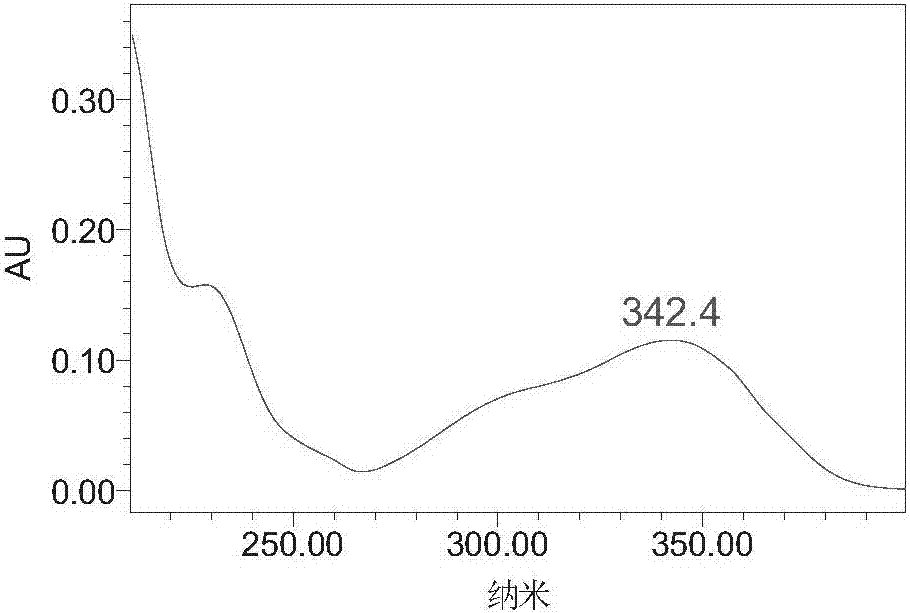
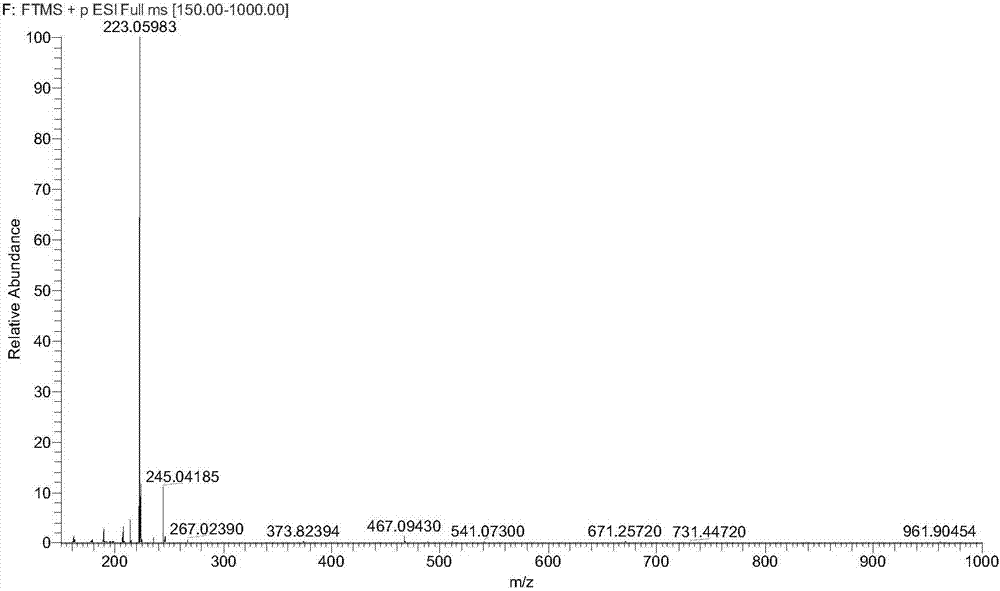
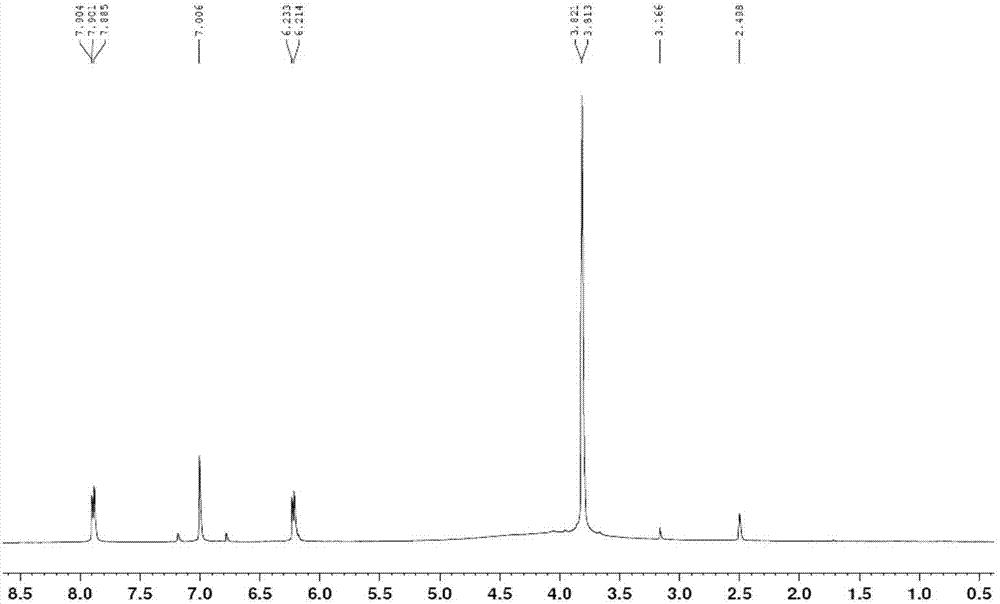
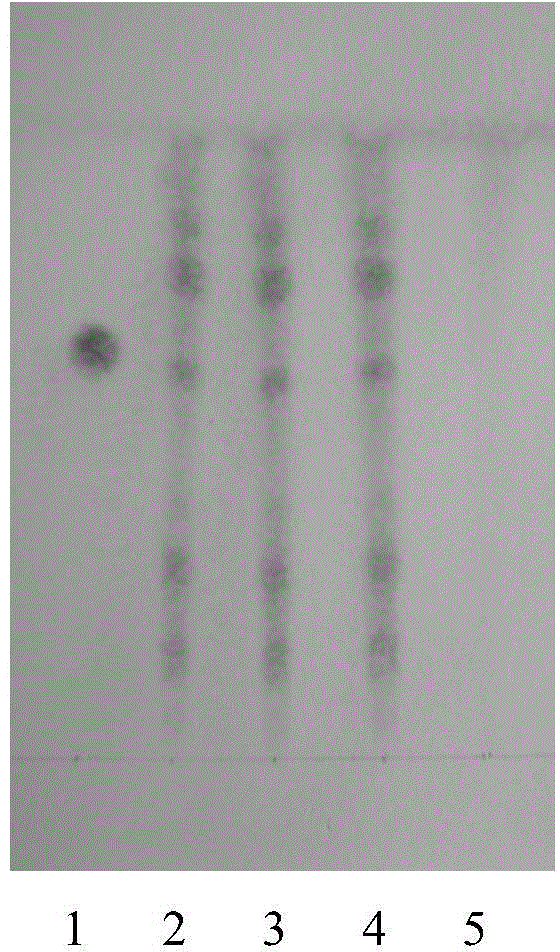
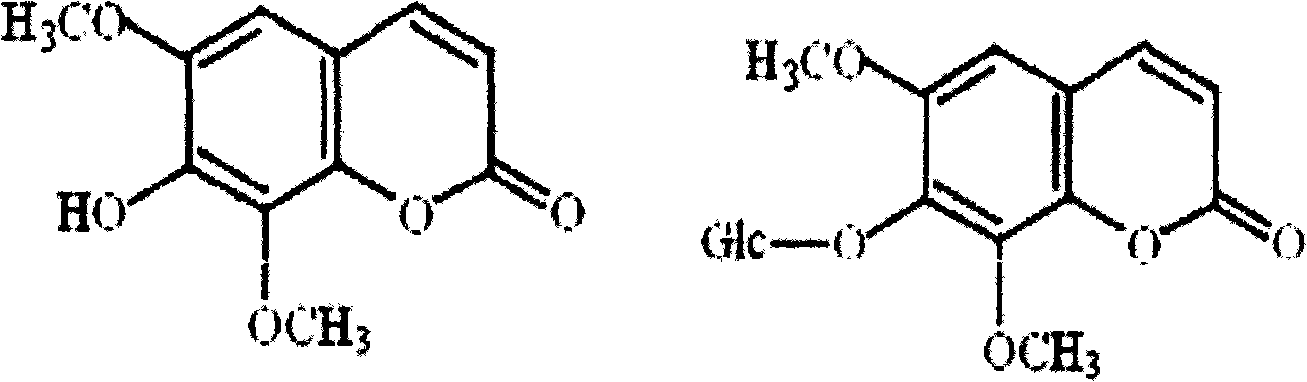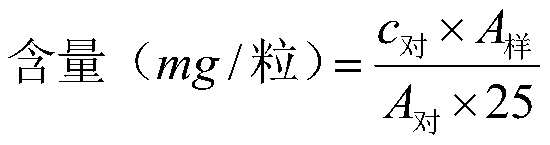Patents
Literature
42 results about "Isofraxidin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
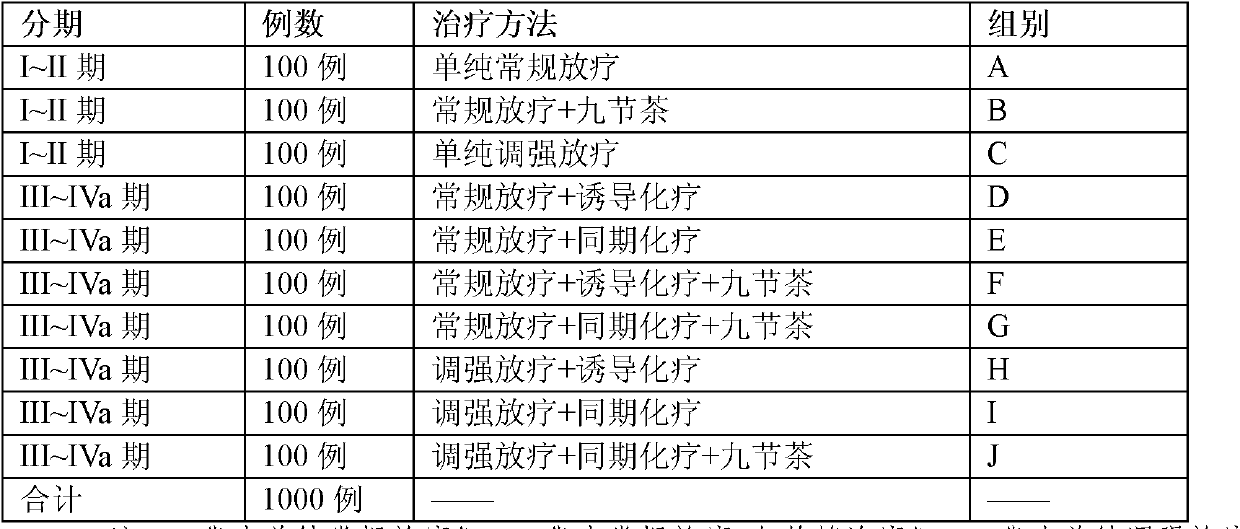
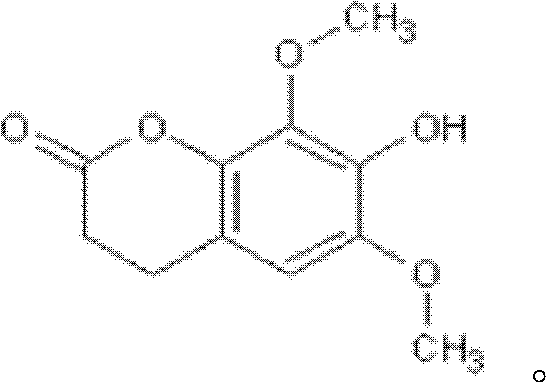
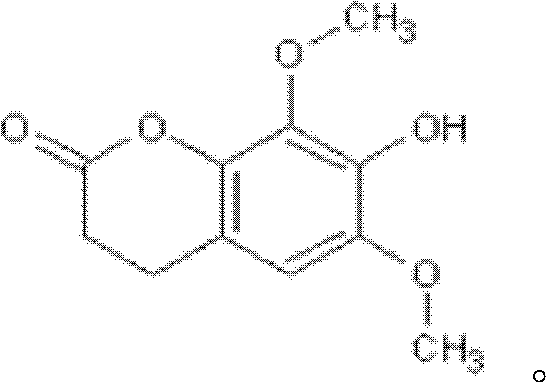
Isofraxidin is a chemical compound found in a variety of plants including Eleutherococcus senticosus.
Quality control method for traditional Chinese medicine oral liquid for treating psoriasis
InactiveCN105891404AAvoid overlappingAccurately reflectComponent separationChlorogenic acidTherapeutic effect
The invention discloses a quality control method for traditional Chinese medicine oral liquid for treating psoriasis. The medicine is prepared from seven types of medicinal materials such as radix paeoniae rubra, rhizoma smilacis glabrae and glabrous sarcandra herbs. According to the specific quality control method, the thin layer chromatography is applied for conducting thin-layer identification on radix paeoniae rubra and rhizoma smilacis glabrae in PSORI-CMO1, meanwhile, the high performance liquid chromatography is combined for measuring the contents of chlorogenic acid, paeoniflorin, isofraxidin, astilbin and engeletin in the oral liquid, it is guaranteed that detected constituents are accurate and reliable, and the purpose of effectively controlling quality of the traditional Chinese medicine oral liquid can be achieved. By means of the quality control method, the defect that a method for detecting multiple constituents of compound traditional Chinese medicine is complex is overcome; the method has the advantages that by identifying the two types of medicine materials and measuring the contents of important active constituents in the medicine, inherent quality, the treatment effect and stability of a preparation are effectively guaranteed, and controllability of the quality standards for the PSORI-CMO1 oral liquid is improved.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Content detection method for determining effective components in longshengzhi capsule by HPLC-QQQ/MS method
ActiveCN109307721AEasy to separateQuick analysisComponent separationBenzoylpaeoniflorinGradient elution
The invention provides a content detection method for determining effective components in a longshengzhi capsule by an HPLC-QQQ / MS method. The chromatograph condition is that the chromatographic column takes a octadecyl-bonded silica gel column as a filling agent, 0.1% formic acid-water as a mobile phase A, 0.1% formic acid-acetonitrile as a mobile phase B, and the volume ratio of the mobile phaseA and the mobile phase B is 80% to 0%:20 to 100%, so that gradient elution is performed. According to the content detection method for determining the effective components in the longshengzhi capsuleby the HPLC-QQQ / MS method, the effective substance components of traditional Chinese medicines such as astragaloside A, hydroxysafflor yellow A, calycosin 7-o-glucoside, calycosin, ferulic Acid, syringin, n-butylphthalide, ligustilide, senkyunolide A, senkyunolide I, senkyunolide H, ligustrazine, isofraxidin, dehydrocostus lactone, amygdalin, syringin E, paeoniflorin, oxypaeoniflora, benzoylpaeoniflorin, etc., in the longshengzhi capsule can be determined. The method has the advantages of being simple in operation method, high in sensitivity and accurate in content.
Owner:SHAANXI BUCHANG PHARMA
Glabrous sarcandra herb formula particle and preparation method thereof
InactiveCN101897740AEfficient removalEasy to deploy flexiblyDigestive systemAntiviralsDiseaseActive component
The invention relates to a glabrous sarcandra herb formula particle and a preparation method thereof, belonging to the traditional Chinese medicine formula particle preparation technology. Glabrous sarcandra herb is subject to water extraction and concentration, appropriate auxiliary material is added, and formula particle is prepared, content of active components isofraxidin and total flavones in the glabrous sarcandra herb is high, quality is stable and controllable, the specification of the prepared formula particle is that every 1-5g glabrous sarcandra herb formula particle is equivalent to 3-15g fresh glabrous sarcandra medicinal material. The invention can meet the requirements on clinic syndrome differentiation and treatment and add-minus according to the disease and is beneficial for doctors to make a prescription flexibly. The invention changes the traditional glabrous sarcandra medicinal material into particles, the stability of the invention is improved, the invention is easy to store and carry, formula is flexible, dosage is less, and use is convenient.
Owner:江西省药物研究所
Eleutherosides as adjuncts for vaccines and immune modulation
InactiveUS20030175777A1Reduce the amount requiredBiocideBacterial antigen ingredientsChlorogenic acidAdjuvant
Vaccines containing adjuvant comprising eleutherosides and related compounds are shown to be useful for the prevention of viral infections, bacterial infections and parasitic infections. The adjuvant compounds have been shown to modulate the expression of a wide variety or proteins involved in the immune response and inflammatory response. Exemplary eleutherosides and related compounds include eleutheroside A, eleutheroside B, eleutheroside C, eleutheroside D, eleutheroside E, eleutheroside F, and eleutheroside G, coniferylaldehyde, caffeic acid ethyl ester, chlorogenic acid, sinapinalcohol, isofraxidin, syringaresinol and 6,8-dimethoxy-7-hydroxycoumarin.
Owner:BONAGURA VINCENT R +3
Technology for synchronously extracting isofraxidin and flavonoids compounds from sarcandra glabra and applications thereof
InactiveCN101665479AHigh extraction rateImprove recycling ratesAntibacterial agentsOrganic active ingredientsSilica gelVacuum drying
The invention provides a technology for synchronously extracting isofraxidin and flavonoids compounds from sarcandra glabra and applications thereof, solving the problems that the prior art can not synchronously extract and separate isofraxidin and flavonoids compounds from sarcandra glabra, has low product purity and low productivity and the like. The technology adopts frequency rotating countercurrent extraction, obtains liquid extract by a climbing-film concentration evaporator and vacuum concentration, extracts the liquid extract and collects the liquid supernatant, crystallizes the liquidsupernatant after concentration, adds silica gel for adsorption after the crystal is dissolved, crystallizes the mixture at low temperature, and obtains the high-purity isofraxidin after vacuum drying; the liquid extract sedimentation is dissolved by adopting alkaline ethanol solution and then centrifugalized to collect the sedimentation, and the sedimentation is dissolved by water, crystallized,separated and vacuum-dried to obtain the flavonoids compounds. The technology realizes the synchronous high-efficiency extract and separation of high-purity isofraxidin and flavonoids compounds, andhas certain meaning and remarkable economic benefit on the wide application in the fields such as medicines, health care, make ups and the like.
Owner:三明华健生物工程有限公司
Method for determining content of compound Caoshanhu lozenge
ActiveCN101576545AImprove rationalityImprove reliabilityComponent separationMentholIsocratic elution
The invention relates to a method for determining the content of compound Caoshanhu lozenge prepared from glabrous sarcandra herb extractum, menthol and dementholised peppermint oil. The thin-layer chromatography is used for distinguishing the menthol and the glabrous sarcandra herb extractum in the lozenge. The method is characterized in that the high performance liquid chromatography is used forsimultaneously determining the content of two marker components, including isofraxidin and rosmarinic acid in the lozenge under the chromatographic condition of isocratic elution; or the high perform ance liquid chromatography is used for simultaneously testing the content of four marker components, including protocatechuic acid, caffeic acid, the isofraxidin and the rosmarinic acid in the lozengeunder the chromatographic condition of gradient elution.
Owner:JIANGZHONG PHARMA
Multi-index-based evaluating-optimizing glabrous sarcandra herb preparation technology
The invention discloses a multi-index-based evaluating-optimizing glabrous sarcandra herb preparation technology. The multi-index-based evaluating-optimizing glabrous sarcandra herb preparation technology comprises the following steps of HPLC determination of specified components, process optimization of glabrous sarcandra herb leaves, single factor experimental investigation of glabrous sarcandraherb stems, cutting-preparing design scheme of the glabrous sarcandra herb stems, establishment of an AHP model, a comprehensive scoring method and CCD-RSM optimized glabrous sarcandra herb stem cutting-preparing process and verification test. Through a single-factor test, the comprehensive scores of the content of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, isofraxidin, astilbin and rosmarinic acid are taken as the evaluation indicators, a central composite design-response surface methodology is used to screen glabrous sarcandra herb softening and cutting-preparing processes, the glabrous sarcandra herb stems and leaves subjected to process optimization by choosing different treatment methods, a multi-index analytic hierarchy process (AHP) is combined with the central composite design-response surface methodology to optimize the glabrous sarcandra herb preparation technology, the study is beneficial to the further development of glabrous sarcandra herb, and an experimental basis is expected to be provided for the establishment of related preparation specifications and quality standards of glabrous sarcandra herb.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for preparing isofraxidin from acanthopanax senticosus rhizomes
InactiveCN101851219AAbundant and easy to get resourcesHigh yieldOrganic chemistryLiquid ratioAqueous solution
The invention relates to a method for preparing isofraxidin from acanthopanax senticosus rhizomes, which is characterized by comprising the following steps: extracting crushed acanthopanax senticosus rhizomes and an ethanol solution in a certain feed liquid ratio; filtering an obtained mixture; carrying out decompression concentration on a filtrate to 1 / 2 volume to obtain a concentrated solution; then adding an acid aqueous solution and a given volume of 1,1-dichloroethane; separating out a 1,1-dichloroethane phase after hydrolysis extraction is carried out under the heating condition; carrying out the decompression concentration until dryness to obtain the product of the isofraxidin. The operation process hs the main characteristics of rich and available raw materials, simple operation and process, strong practicability and easy industrial operation, and high-yield products can be obtained by single extraction.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Sarcandra glabra extract with anti-influenza virus activity and preparation method thereof
ActiveCN108670973AStrong targetingAvoid interferenceAntiviralsHeterocyclic compound active ingredientsReflux extractionChlorogenic acid
The invention provides a sarcandra glabra extract with anti-influenza virus activity. Counted by the total weight of the sarcandra glabra extract, the sarcandra glabra extract comprises 0.020-0.040wt%of chlorogenic acid, 0.000-0.008wt% of isofraxidin, 0.025-0.060wt% of quercetin-3-O-beta-D-glucuronide and 0.083-0.158wt% of rosmarinic acid. The invention further provides a preparation method of the sarcandra glabra extract with the anti-influenza virus activity. The preparation method comprises the following steps: performing reflux extraction on sarcandra glabra for two times with deionized water, performing alcohol precipitation to obtain a dry sarcandra glabra water extract, then preparing the dry extract into a sample with a certain concentration, injecting the sample into macroporousadsorption resin, then eluting with an ethanol solution with the concentration of 20%, and collecting an eluate to obtain the sarcandra glabra extract with the anti-influenza virus activity. The sarcandra glabra extract and the preparation method have the advantages as follows: a help is provided for application of the sarcandra glabra extract to fighting against influenza; by the preparation method, the steps are simple and convenient, the operation is easy, quantification can be achieved, the repeatability is high; the preparation method is suitable for application of the extract to the large-scale industrial production process of a medicine in the later stage.
Owner:FUJIAN UNIV OF TRADITIONAL CHINESE MEDICINE
Extractive of effective part of chloranthus glaber and preparation method thereof
InactiveCN101574381ARegulatory immunityThrombocytopeniaAntibacterial agentsAntineoplastic agentsCentrifugationUltrafiltration
The invention relates to a phenolic acid effective part extracted from the traditional Chinese medicine-chloranthus glaber and preparation method thereof. Phenolic acid compound contained in the effective part includes rosmarinic acid, protocatechuic acid, isofraxidin and caffeic acid, and the total content of the phenolic acid compound in the extractive is no less than 50 percent. The chloranthus glaber is extracted by water or ethanol, extracting solution is processed by high-speed centrifugation, centrifugation solution is processed by ultrafiltration by an organic composite film, the pH value of ultrafiltrate is regulated to range from 1 to 7, and the ultrafiltrate is applied to a resin column to be absorbed; according to column volume, 2 to 3 times water is used for elution, and then 10 to 70 percent of ethanol with the pH value ranging from 8 to 14 is used for elution; and ethanol elution solution is collected, concentrated and dried, thereby obtaining the extractive of the effective part.
Owner:JIANGZHONG PHARMA
Method for preparing coumarin derivative
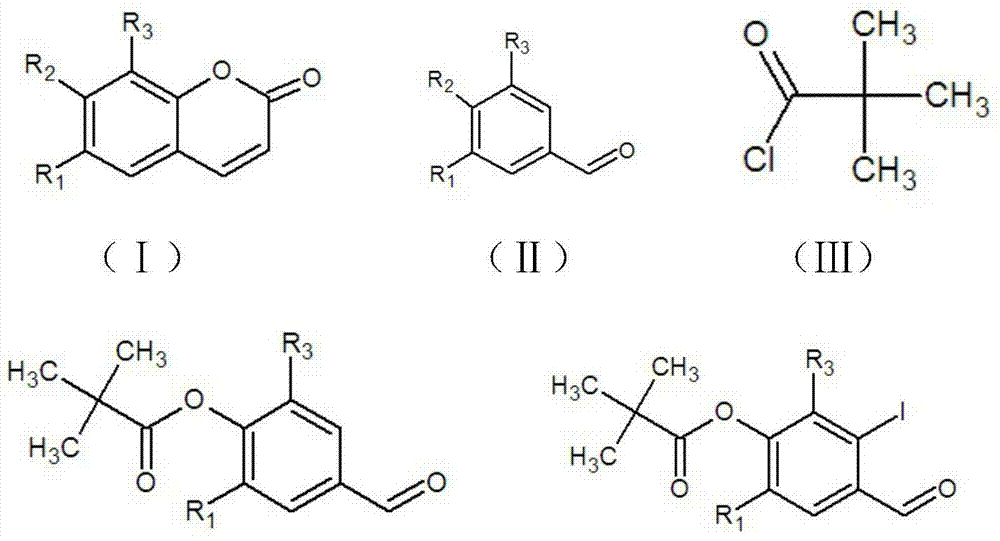
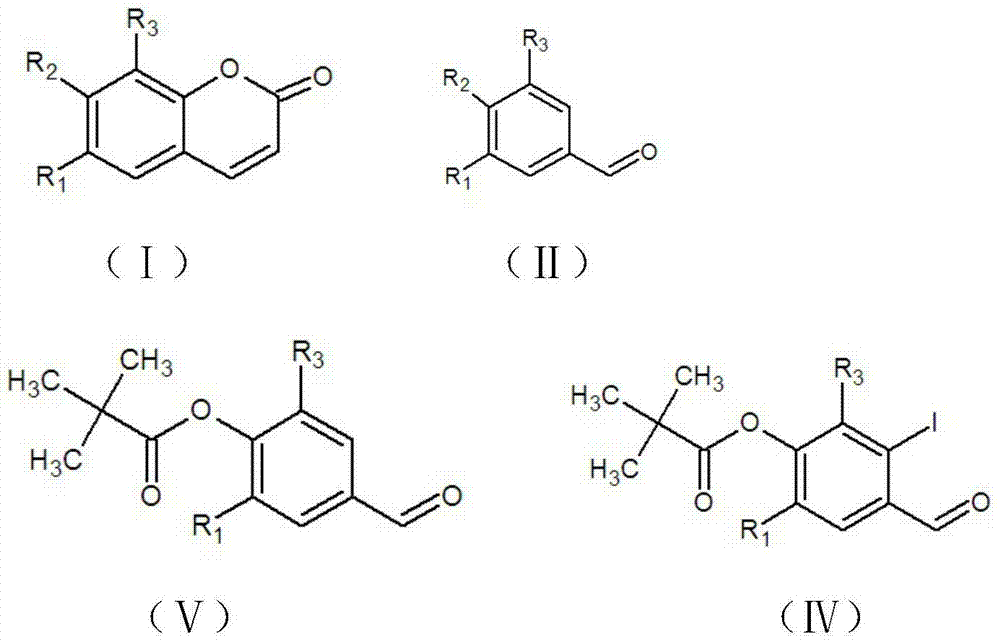
The invention discloses a method for preparing a coumarin derivative. According to the method, the coumarin derivative shown by formula (I) is prepared by using a compound shown by formula (II) as a raw material; the synthetic process route of the coumarin derivative is simple, feasible and safe, the method is suitable for industrial production, the raw materials are low-toxicity and economic, and the yield is universally high. The total yield of isofraxidin is 57.6% and is greater than 47.5% of the existing other synthetic route, the synthetic process step is shortened, and the process route is low-toxicity, environment-friendly and safe; 6,7,8-triethoxycoumarin is synthesized for the first time, the yield is up to 67%, and the synthetic method is simple, convenient, mild in conditions and low in pollution; the yield of scoparone is 77.4%, few byproducts are produced, the conditions are mild, and the experiment is easy in operation; by using the compound with relatively low toxicity, the pollution is low, and the cost is relatively low, so the method is suitable for industrialized production.
Owner:ZHEJIANG UNIV OF TECH
Quantitative analysis method of six chemical components in Chinese herbal medicine compound preparation using rhizoma dioscoreae nipponicae and acanthopanax roots as Chinese herbal medicines
ActiveCN104502485AImprove quality controlImprove accuracyComponent separationChlorogenic acidEleutherococcus senticosus
The invention discloses a quantitative analysis method of six chemical components in a Chinese herbal medicine compound preparation using rhizoma dioscoreae nipponicae and acanthopanax roots as Chinese herbal medicines. The quantitative analysis method comprises the following steps: (1) preparing a mixed control solution; (2) preparing a first test sample solution and a second test sample solution; (3) determining contents, namely, respectively taking the mixed control solution, the first test sample solution and the second test sample solution, performing gradient elution under high performance liquid chromatography detection conditions of using C18 bonded silica gel as a chromatographic column of a filling agent and acetonitrile-formic acid solution as a mobile phase, performing high performance liquid chromatography detection by combining a diode array detector and an evaporative light-scattering detector of detectors to obtain high performance liquid chromatograms of the mixed control solution, the first test sample solution and the second test sample solution, and determining the contents of dioscin, pseudoprotodioscin, protodioscin, chlorogenic acid, syringin and isofraxidin in a test sample by using an external standard method. The quantitative analysis method is simple, convenient and rapid to operate, high in accuracy and excellent in repeatability, and can be used for improving the quality control level of a product.
Owner:TIANJIN UNIV +1
Microbial agent for improving soil acidification and preparation method thereof
InactiveCN106748113AImprovement of dysplasia symptomsImprovement of rot symptomsDi-calcium phosphate fertilisersOrganic fertilisersSodium acetatePlant roots
The invention provides a microbial agent for improving soil acidification and a preparation method thereof. The microbial agent is prepared from the following components: sodium acetate, yeast extract, phycocyanin, folium artemisiae argyi, glucose, ammonium sulfate, astragalus smicus, isofraxidin, calcium hydrophosphate, dolomite dust, paprika powder, protamine lactobacillus powder, agrobacterium bacterial powder and torulaspora delbrueckii bacterial powder, wherein the protamine lactobacillus powder has a living cell concentration of protamine lactobacillus of 8*10<8>CFU / g; the agrobacterium bacterial powder has a living cell concentration of agrobacterium of 4*10<8>CFU / g; and the torulaspora delbrueckii bacterial powder has a living cell concentration of torulaspora delbrueckii of 2*10<9>CFU / g. By adopting the microbial agent, plant root rot and main root nondevelopment caused by soil acidification can be remarkably improved.
Owner:王民
Method for extracting isofraxidin
The invention relates to a method for extracting isofraxidin, which comprises the following steps of: pulverizing acanthopanax root serving as a raw material, and adding 10 to 20 percent water for wetting; adding biological enzyme for enzymolysis in the normal state for 3 to 10 days, and after the enzymolysis, extracting through the reflux in 80 to 90 percent methyl alcohol; and concentrating extracting solution under reduced pressure, adding ethyl acetate for dissolving to remove aqueous layer, decolorizing through an activated carbon alumina mixed column to remove impurities, concentrating and crystallizing, recrystallizing through the reflux of chloroform and acetone, and drying crystals at the low temperature to obtain the isofraxidin. In the method for extracting the isofraxidin, the product yield is high, the operation is simple, the repeatability is good, and the enlargement production is easy to perform.
Owner:NANJING ZELANG AGRI DEV
Sarcandra extract and application thereof
InactiveCN103623017AImprove the quality of lifeGood reliefDigestive systemAntineoplastic agentsThirstMedicine
The invention discloses a Sarcandra extract and application thereof, belonging to the field of medicine. The Sarcandra extract is prepared by subjecting the whole herb of Sarcandra to water extraction, concentration and drying. 1 g of the Sarcandra extract contains no less than 0.53 mg of isofraxidin (C11H10O5) and no less than 0.38 mg of rosmarinic acid (C18H16O8). The Sarcandra extract provided by the invention has good effects on treatment and prevention of thirst and radioactive dental caries caused by radiotherapy.
Owner:GUANGXI MEDICAL UNIVERSITY
Quality control method of capsule preparation for treating gastric ulcer
ActiveCN110187027AQuality improvementScientific and reasonable quality control methodsComponent separationEvodiamineClinical efficacy
The invention belongs to the technical field of capsule preparation quality control, and particularly relates to a quality control method of a capsule preparation for treating gastric ulcer. The quality control method is characterized in that the contents of scopolin, dehydroevodiamine, scopoletin, isofraxidin, rutaecarpin, evocarpine and evodiamine in galangal gastric ulcer capsules are determined by a HPLC (High Performance Liquid Chromatography) method, thereby building a quality control method of a galangal gastric ulcer capsule preparation for treating gastric ulcer. The adopted quality control method is scientific and reasonable, has high accuracy and high reproducibility, can comprehensively and effectively control the quality of the galangal gastric ulcer capsule preparation for treating duodenal ulcer, and can ensure the clinical effect of the preparation.
Owner:GUIZHOU MEDICAL UNIV
Application of glabrous sarcandra glabrous herb extract in reducing susceptibility of influenza virus
ActiveCN102793731BReduce morbidityReduce mortalityAntiviralsRespiratory disorderInflammatory factorsInfluenza A (H1N1) virus
The invention provides novel application of a glabrous sarcandra glabrous herb extract, belongs to the field of medicines, and provides an active extract of a medicinal material glabrous sarcandra glabrous herb through water extraction, alcohol precipitate and drying. 100g of the extract comprise 5 to 20g of tannin, and 0.02 to 1g of isofraxidin. The extract is confirmed and proved due to the effect that the incidence rate and death rate of a low influenza A H1N1 virus FM1 strain infected restraint stress loaded mouse can be reduced based on the glabrous sarcandra glabrous herb extract, and the lung tissue viral load of an influenza virus loaded mouse and inflammatory factor effects can be remarkably reduced by the extract. The glabrous sarcandra glabrous herb extract can be used for preparing medicaments applied to susceptibility of a human body to the influenza virus, relieving pneumonia and other respiratory inflammations due to secondary of body susceptible virus, pulmonary dysfunction and other clinical symptoms, and can be used for particularly effectively preventing, reducing or treating various clinical symptoms caused by virus injection occurring in an influenza virus susceptible body.
Owner:GUANGZHOU BAIYUNSHAN JINGXIUTANG PHARM CO LTD
Pharmaceutical composition for treating neurasthenia and preparation method thereof
ActiveCN104490904ASignificantly calms the mind and calms the mindComprehensive application effect is idealNervous disorderDigestive systemSyringinCurative effect
The invention relates to a pharmaceutical composition for treating neurasthenia and a preparation method thereof. The pharmaceutical composition comprises the following active ingredients: 0.03-0.5mg / ml eleutheroside E, 0.05-0.5mg / ml syringin, 0.005-0.05mg / ml isofraxidin and 0.03-0.5mg / ml schizandrin; preferably, 0.05-0.2mg / ml eleutheroside E, 0.1-0.3mg / ml syringin, 0.01-0.03mg / ml isofraxidin and 0.05-0.2mg / ml schizandrin. The pharmaceutical composition provided by the invention has the advantages that the preparation process is simple and feasible, the properties of the traditional Chinese medicine components are fully considered in the preparation process, excessive impurities are removed by macroporous adsorption resin to improve the concentration of active ingredients in the preparation and play the effects of the traditional Chinese medicine components; meanwhile, the used resin can be repeatedly used, so that resource consumption can be reduced. The traditional Chinese medicine composition prepared in the invention has a remarkable curative effect.
Owner:安徽九洲方圆制药有限公司
Method for preparing isofraxidin from herb sarcandra glabra
InactiveCN106967029AWide variety of sourcesLow priceOrganic chemistrySilica gelColumn chromatography
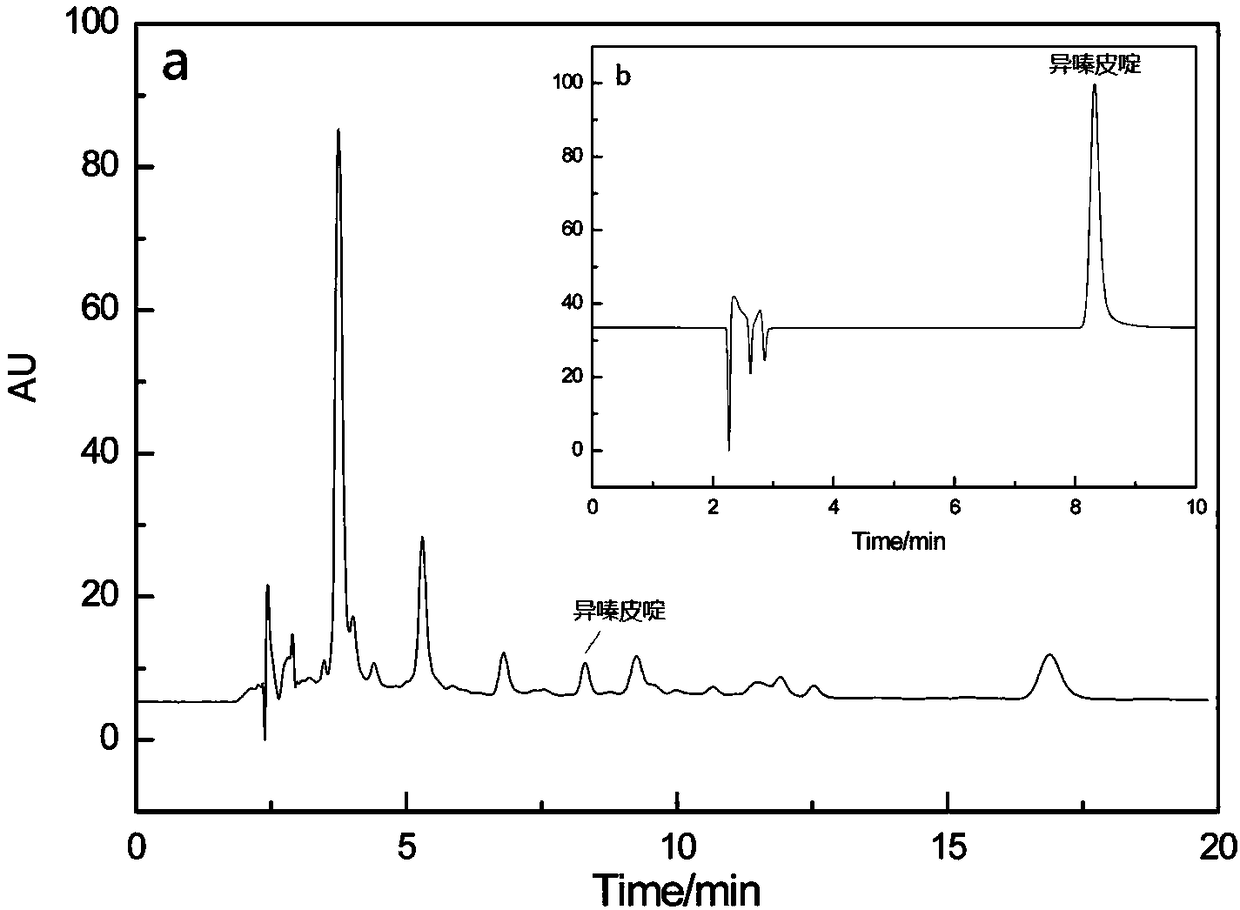
The present invention relates to a method for preparing isofraxidin from an herb sarcandra glabra. According to the method, complete plant sarcandra glabra decoction pieces are adopted as a raw material, a lot of non-related components are removed through water heating extraction and liquid-liquid extraction to obtain an isofraxidin crude product enrichment part, and silica gel column chromatography, gel column chromatography decolorization, reverse phase ODS column chromatography fine separation and recrystallization refining are performed so as to obtain the isofraxidin pure product with a purity of more than 99%. According to the present invention, the method has advantages of simple process, simple operation, low production cost, and high product purity.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Method for inspecting quality of slender acanthopanax stilbenes powder
InactiveCN104422664AIncrease the sugar contentStrong specificityComponent separationColor/spectral properties measurementsAstragalosideQuantitative determination
The invention relates to a method for inspecting the quality of slender acanthopanax stilbenes powder. The method comprises qualitative analysis and quantitative determination for the slender acanthopanax stilbenes powder. Compared with a method for inspecting the quality of a lingqijia oral solution, the method adopts a high performance liquid chromatography for measuring the content of astragaloside in the slender acanthopanax stilbenes powder, and a measuring method for the total sugar content is also adopted, a thin layer chromatography is adopted for respectively inspecting the astragaloside and isofraxidin in the slender acanthopanax stilbenes powder. The method is good in specificity, repeatability and accuracy and is used for performing comprehensive evaluation on the quality of the slender acanthopanax stilbenes powder so that the quality of a product is effectively controlled and safe, the effective and stable clinical use can be guaranteed.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Method for extracting isofraxidin from acanthopanax roots
ActiveCN108484557AReduce manufacturing costEase of industrial scale productionOrganic chemistryEleutherococcus senticosusBinding state
The invention provides a method for extracting isofraxidin from acanthopanax roots. According to the method disclosed by the invention, by virtue of extraction in combination hydrolysis in situ, glycosides prepared by binding isofraxidin and glucose, namely binding state isofraxidin, can be totally transformed into free state isofraxidin, so that the content of the isofraxidin in the extracting solution is increased, wherein an H<+> type ion exchange resin serves as a catalyst for promoting hydrolysis of the binding state isofraxidin, and a chlorinated solvent serves as an extraction agent forextracting the free state isofraxidin. Therefore, in the process of extraction in combination hydrolysis in situ, hydrolysis of the binding state isofraxidin and extraction of the free state isofraxidin are simultaneously completed. Moreover, the in-situ hydrolysis extraction method provided by the invention effectively avoids onerous two-step operations of hydrolysis and extraction, the operating procedures and the usage amount of the solvent are reduced, the production cost is low, and the yield of the isofraxidin is high.
Owner:NORTHEAST FORESTRY UNIVERSITY
Quality inspection method of Wujiaqi oral liquid (Chinese patent medicine prepared from acanthopanax root and radix astragali)
InactiveCN104422663AIncrease the sugar contentStrong specificityColor/spectral properties measurementsAstragalosideChinese patent medicine
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Living cell vitrification freezing fluid and freezing thawing method
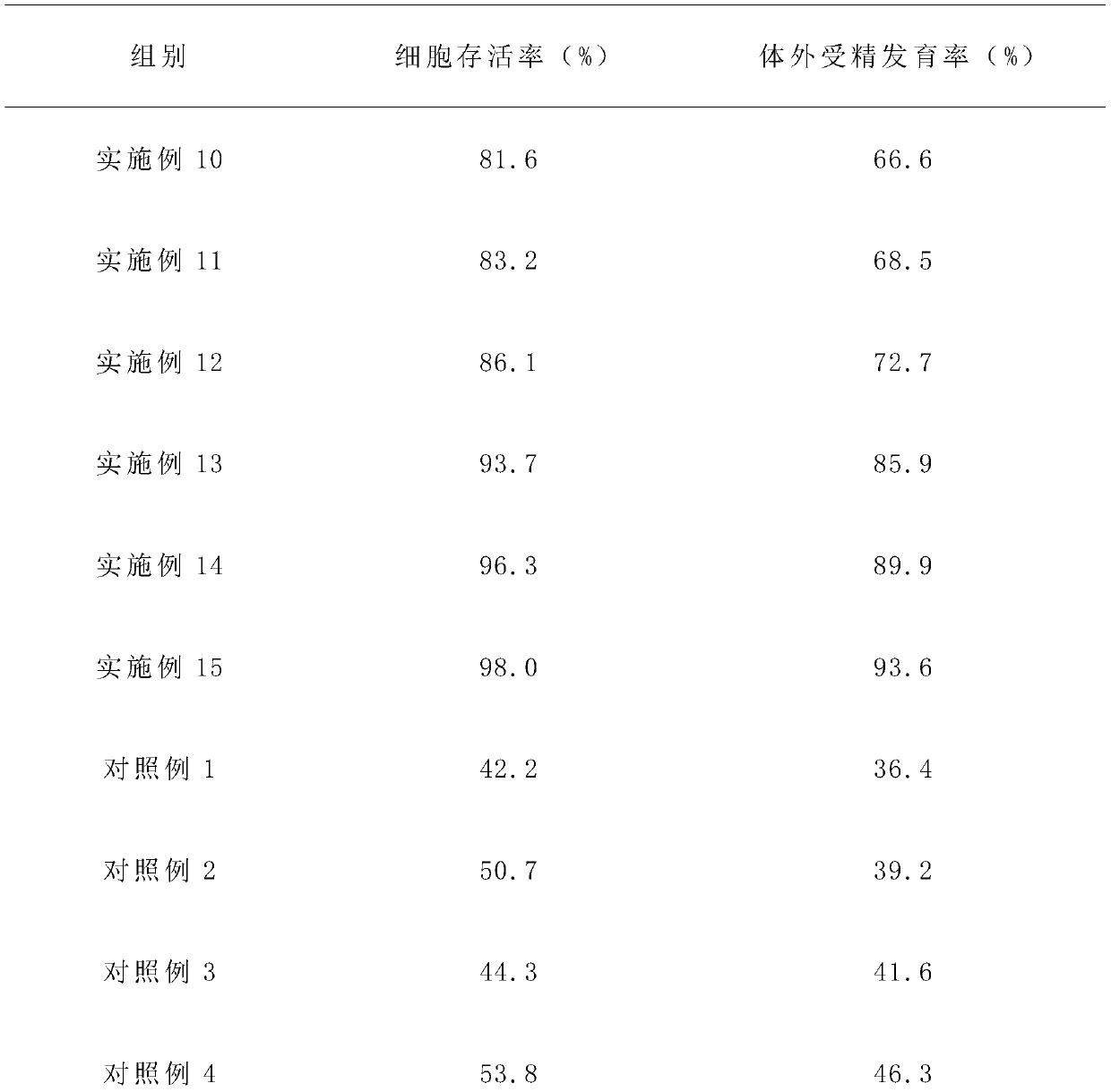
InactiveCN109819975AImprove the development rate of in vitro fertilizationImprove survival rateDead animal preservationHydroxyethyl starchChlorogenic acid
The invention provides a living cell vitrification freezing fluid and a freezing thawing method. The freezing fluid is prepared by that on the basis of an MEM culture medium and an F12 culture mediumin a volume ratio of 1:1, 50-60nmol / L of isofraxidin, 20-30nmol / L of chlorogenic acid and 40-48Mumol / L of a protective agent are added, wherein the protective agent comprises one or more of hydroxyethyl starch, tea polyphenol, 4-hydroxyethyl piperazine ethanesulfonic acid, lentinan and adenosine triphosphate. The living cell vitrification freezing fluid has the advantage that the living cell survival rate, the in-vitro fertilization and development rate, the cleavage rate and the blastocyst rate can be increased.
Owner:CENTURY BIOSTRENGTH BEIJING PTY LTD
Isofraxidin crystalline compound and glabrous sarcandra herb dispersible tablets and dropping pills containing isofraxidin crystalline compound
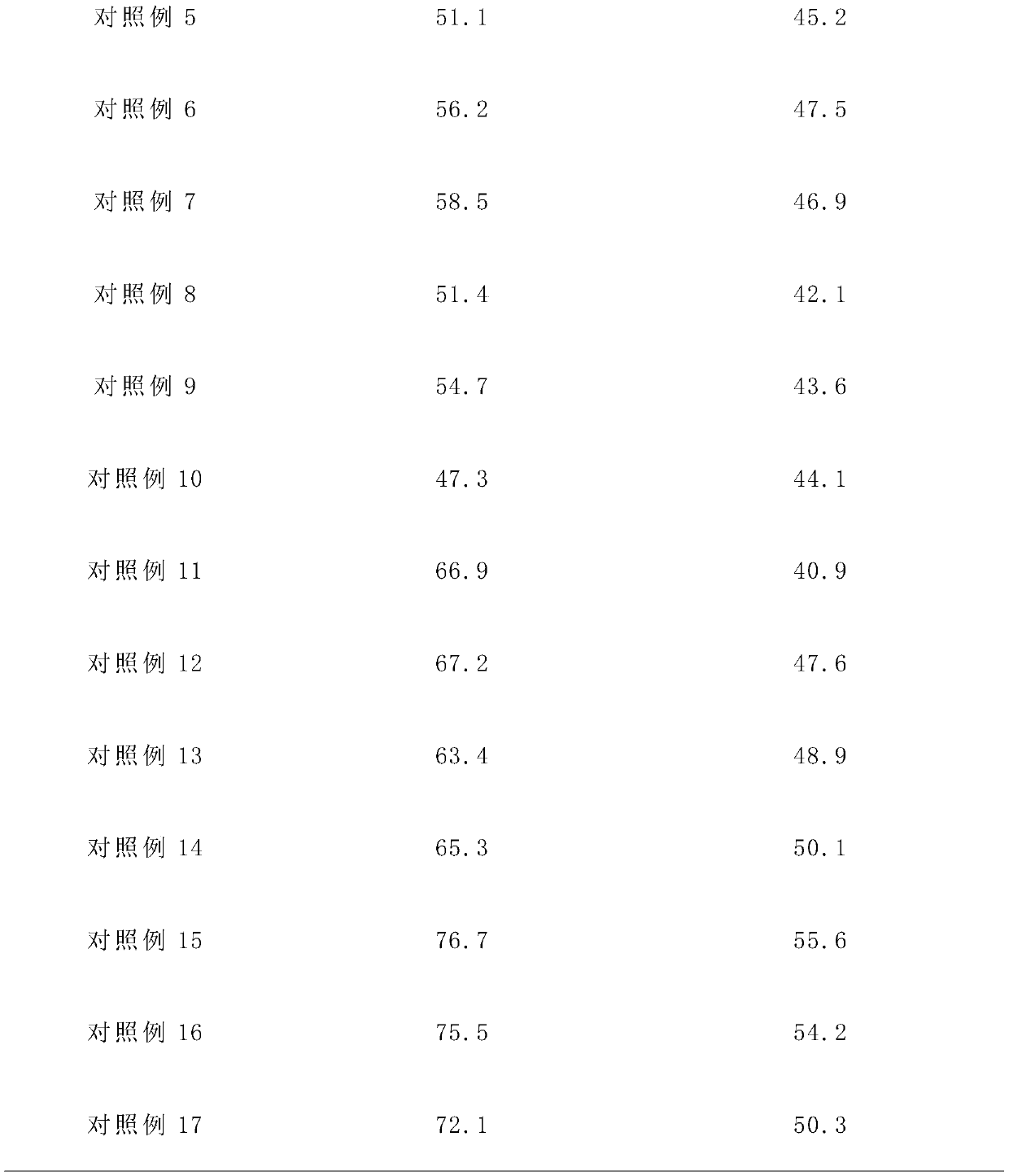
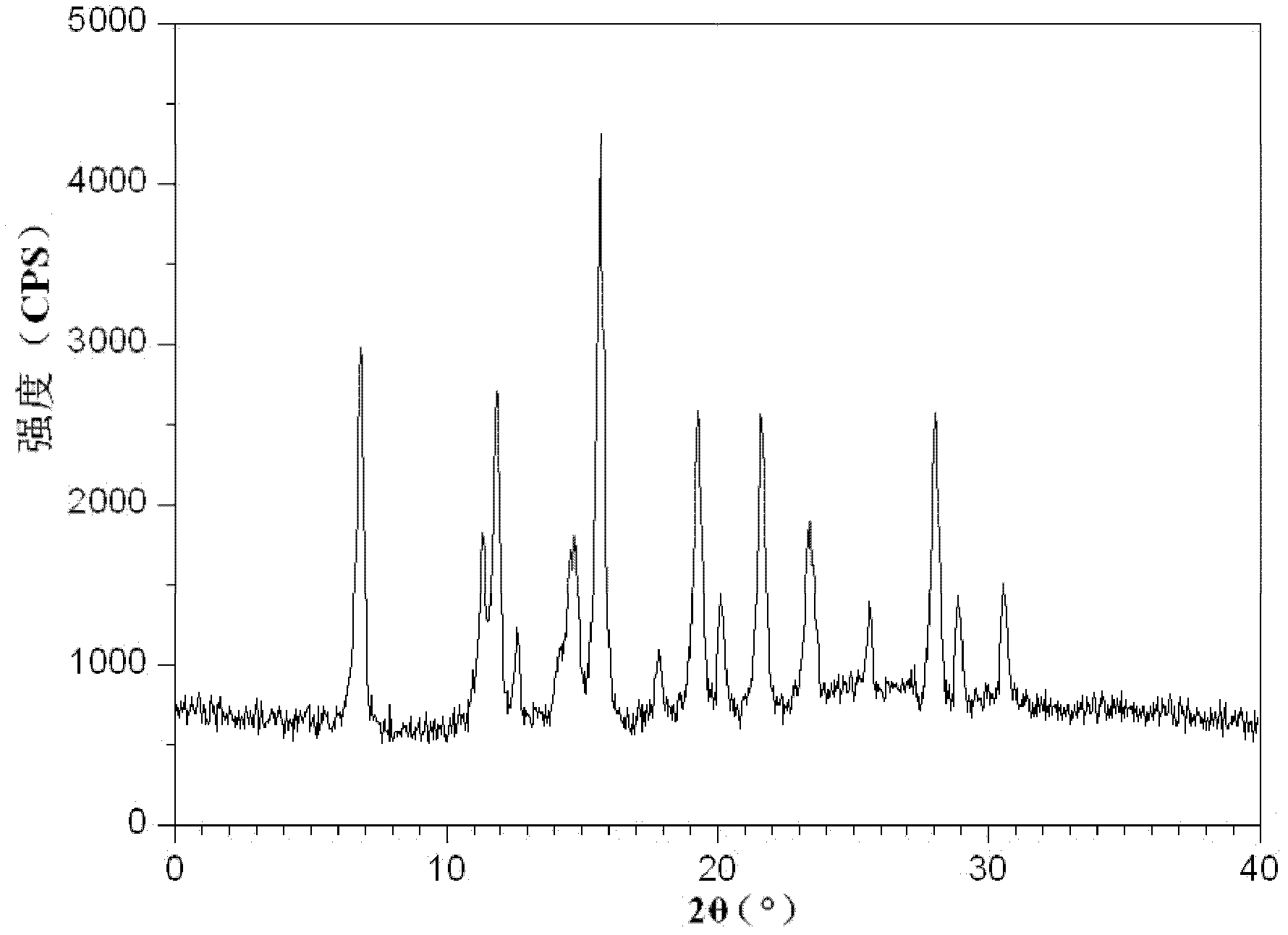
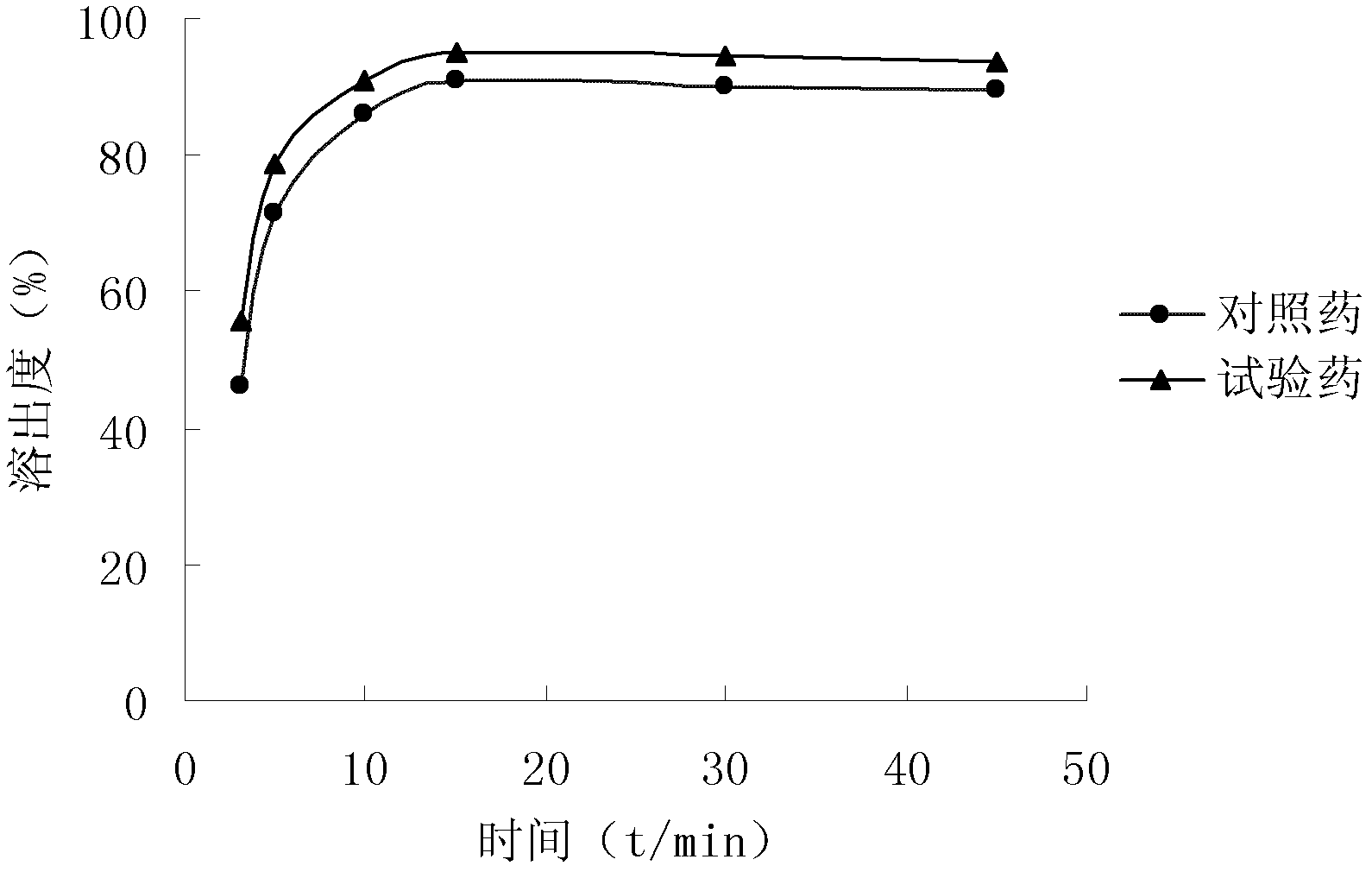
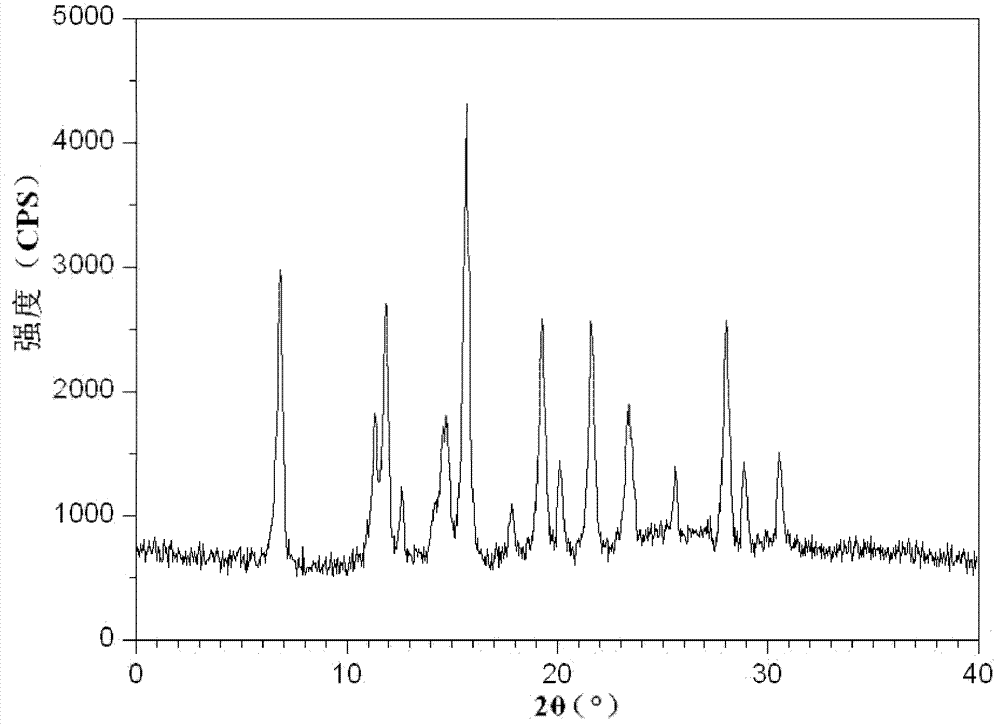
ActiveCN102633760AIncrease contentGuarantee drug safetyOrganic chemistryDigestive systemPowder diffractionDispersible tablet
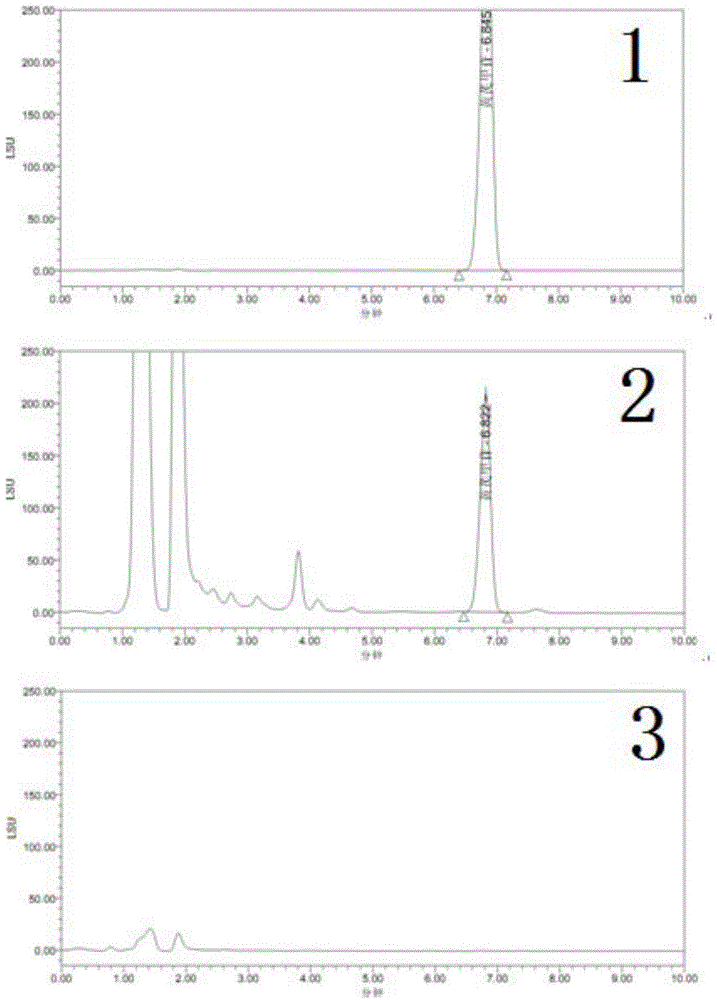
The invention relates to an isofraxidin crystalline compound and glabrous sarcandra herb dispersible tablets and dropping pills containing the isofraxidin crystalline compound. X-ray powder diffraction spectrogram characteristic peaks of the isofraxidin crystalline compound are measured by Cu-Ka rays and displayed at positions with 2theta being 6.8 degrees, 11.4 degrees, 12.0 degrees, 12.6 degrees, 14.8 degrees, 15.8 degrees, 18.0 degrees, 19.4 degrees, 20.1 degrees, 21.7 degrees, 23.4 degrees, 25.6 degrees, 28.0 degrees, 29.0 degrees and 30.6 degrees. Stability, bioavailability and quality of the glabrous sarcandra herb dispersible tablets and dropping pills are evidently improved, so that medication safety is guaranteed for patients. In addition, the glabrous sarcandra herb dispersible tablets and dropping pills have the advantages of stability in quality, accuracy in dosage, small difference in purity, easiness in implementation of mass mechanical production, high yield and low cost.
Owner:ZHEJIANG WECOME MEDICINE IND
Compositions of eleutherosides capable of modulating protein expresion
InactiveUS20070134711A1Reduce the amount requiredMicrobiological testing/measurementAntibody medical ingredientsChlorogenic acidAdjuvant
Vaccines containing adjuvant comprising eleutherosides and related compounds are shown to be useful for the prevention of viral infections, bacterial infections and parasitic infections. The adjuvant compounds have been shown to modulate the expression of a wide variety or proteins involved in the immune response and inflammatory response. Exemplary eleutherosides and related compounds include eleutheroside A, eleutheroside B, eleutheroside C, eleutheroside D, eleutheroside E, eleutheroside F, and eleutheroside G, coniferylaldehyde, caffeic acid ethyl ester, chlorogenic acid, sinapinalcohol, isofraxidin, syringaresinol and 6,8-dimethoxy-7-hydroxycoumarin.
Owner:BONAGURA VINCENT R +3
Fingerprint detection method of traditional Chinese medicine preparation for treating stroke
ActiveCN114646716AGuarantee internal quality stabilityEasy to operateComponent separationAgainst vector-borne diseasesChlorogenic acidGallic acid ester
The invention relates to a detection method of a fingerprint spectrum of a traditional Chinese medicine preparation for treating stroke, which comprises the following steps: establishing the fingerprint spectrum of the traditional Chinese medicine preparation by adopting an HPLC (High Performance Liquid Chromatography) method, and determining nine index components such as quercetin, gallic acid, protocatechuic acid, syringin, chlorogenic acid, paeoniflorin, eleutheroside E, isofraxidin and calycosin-7-glucoside; the internal quality of the traditional Chinese medicine preparation is effectively controlled, and safety and effectiveness of clinical medication are further guaranteed.
Owner:SHAANXI BUCHANG PHARMA
Plant fermentation product with dark circle resisting effect and preparation method thereof
ActiveCN113797127AEasily damagedExcellent anti-dark circle effectCosmetic preparationsBio-organic fraction processingBiotechnologyUrsolic acid
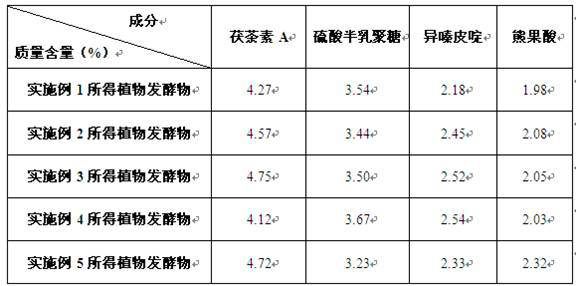
The invention relates to a plant fermentation product with a dark circle resisting effect and a preparation method thereof. The plant fermentation product is characterized by comprising the following components in percentage by mass: 4.12%-4.75% of Fuzhuan tea element A, 3.23%-3.67% of sulfuric acid galactan, 2.18%-2.54% of isofraxidin and 1.98%-2.32% of ursolic acid. The plant fermentation product has the advantage of excellent dark circle resisting effect.
Owner:PROYA COSMETICS
Chinese and western medicine composition for treating bronchial asthma and preparation method thereof
InactiveCN106727600AAchieve full recoveryQuick effectRespiratory disorderHeterocyclic compound active ingredientsSide effectAdditive ingredient
The invention discloses a Chinese and western medicine composition for treating bronchial asthma. The Chinese and western medicine composition for treating the bronchial asthma is prepared from, by weight, 9-13 parts of white mulberry root-bark, 25-30 parts of radix salviae miltiorrhizae, 7-10 parts of rhizoma atractylodis macrocephalae, 2-3 parts of 3-acetyl-11-keto-boswellic acid, 15-20 parts of tiliroside, 8-16 parts of jatrorrhizine hydrochloride, 5-14 parts of corynoline, 2-8 parts of etoposide, 9-12 parts of lupeol and 5-7 parts of isofraxidin. According to the medicine, the raw material ingredients are carefully selected according to the understanding mechanism to the bronchial asthma to achieve the comprehensive rehabilitation purpose, and the advantages of being capable of rapidly achieving the effects, stable in effect, convenient to carry and take and free of toxic and side effects after being taken for a long term are achieved.
Owner:HENAN BALING ELECTRONICS TECH CO LTD
Method for determining content of compound Caoshanhu lozenge
ActiveCN101576545BImprove rationalityImprove reliabilityComponent separationMentholIsocratic elution
The invention relates to a method for determining the content of compound Caoshanhu lozenge prepared from glabrous sarcandra herb extractum, menthol and dementholised peppermint oil. The thin-layer chromatography is used for distinguishing the menthol and the glabrous sarcandra herb extractum in the lozenge. The method is characterized in that the high performance liquid chromatography is used forsimultaneously determining the content of two marker components, including isofraxidin and rosmarinic acid in the lozenge under the chromatographic condition of isocratic elution; or the high perform ance liquid chromatography is used for simultaneously testing the content of four marker components, including protocatechuic acid, caffeic acid, the isofraxidin and the rosmarinic acid in the lozengeunder the chromatographic condition of gradient elution.
Owner:JIANGZHONG PHARMA
Isofraxidin crystalline compound and glabrous sarcandra herb dispersible tablets and dropping pills containing isofraxidin crystalline compound
ActiveCN102633760BIncrease contentGuarantee drug safetyOrganic chemistryDigestive systemPowder diffractionDispersible tablet
The invention relates to an isofraxidin crystalline compound and glabrous sarcandra herb dispersible tablets and dropping pills containing the isofraxidin crystalline compound. X-ray powder diffraction spectrogram characteristic peaks of the isofraxidin crystalline compound are measured by Cu-Ka rays and displayed at positions with 2theta being 6.8 degrees, 11.4 degrees, 12.0 degrees, 12.6 degrees, 14.8 degrees, 15.8 degrees, 18.0 degrees, 19.4 degrees, 20.1 degrees, 21.7 degrees, 23.4 degrees, 25.6 degrees, 28.0 degrees, 29.0 degrees and 30.6 degrees. Stability, bioavailability and quality of the glabrous sarcandra herb dispersible tablets and dropping pills are evidently improved, so that medication safety is guaranteed for patients. In addition, the glabrous sarcandra herb dispersible tablets and dropping pills have the advantages of stability in quality, accuracy in dosage, small difference in purity, easiness in implementation of mass mechanical production, high yield and low cost.
Owner:ZHEJIANG WECOME MEDICINE IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com