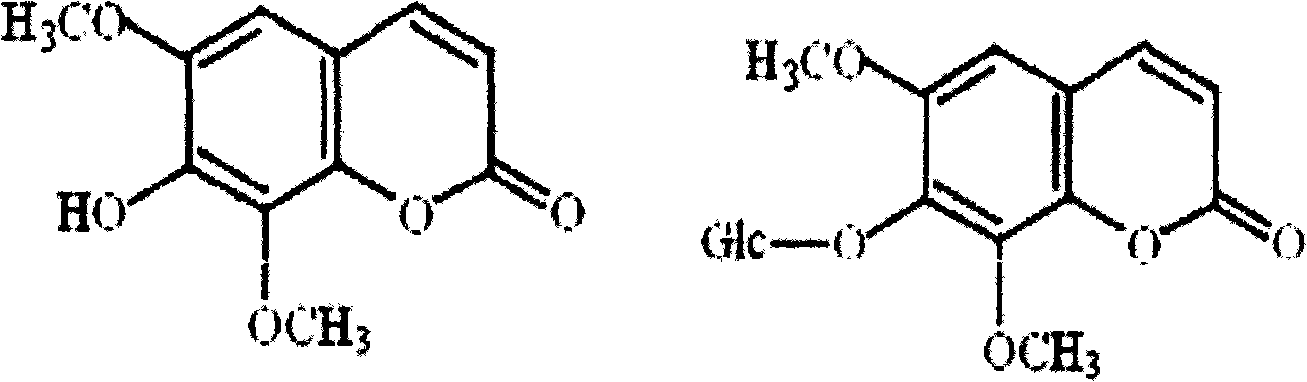Method for extracting isofraxidin
A technology of isoquinpin and an extraction method, which is applied in the field of biological enzymolysis and recrystallization to extract isoquinpin, can solve the problems of severe reaction, low extraction yield and poor reproducibility in the operation process, and achieves easy industrialization and high yield. , process mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Acanthopanax senticosus is removed and crushed to 40 meshes, take 1kg, add 200ml of water, mix well and add 5g of cellulase, natural enzymatic hydrolysis for 5 days, take it out and add 6L80% methanol to reflux extraction for 1 hour to extract 3 times, the extract is rotated The evaporator recovers methanol, adds 4BV ethyl acetate to the concentrated solution, heats to dissolve and remove the water layer, adds activated carbon alumina (1:4) column for decolorization, collects the injection liquid and recovers ethyl acetate under reduced pressure to 1 / 8 of the original volume, and adds a small amount of Stir with petroleum ether until precipitation occurs, and place to crystallize; filter out the crude crystals, reflux with chloroform and acetone in turn, dissolve and refrigerate, refrigerate to crystallize at 0-4°C, and dry under vacuum and low pressure to obtain 3.4 g of isoferidin, with a content of 95%.
Embodiment 2
[0033] Acanthopanax senticosus is removed and crushed to 80 mesh, take 5kg, add 800ml of water, mix well and add 5g of β-glucanase, natural enzymolysis for 10 days, take it out and put it into a multi-functional extraction tank, add 50L of 90% methanol for reflux extraction for 2 hours to extract 2 Once, combine the extracts to recover methanol, add 9BV ethyl acetate to the concentrated solution, heat to dissolve and remove the water layer, add activated carbon alumina (1:6) column for decolorization, collect the injection solution and recover ethyl acetate under reduced pressure to 1 / 3 of the original volume , add a small amount of petroleum ether and stir until precipitation occurs, leave to crystallize; filter out the crude crystals, reflux with chloroform and acetone in turn, dissolve and refrigerate, and refrigerate to crystallize at 0-4°C, dry under vacuum and low pressure to obtain 20 g of isoferidin, with a content of 98%.
Embodiment 3
[0035] Acanthopanax senticosus is removed and crushed to 60 mesh, take 5kg, add 1000ml of water, mix well and then add 15g of naringinase, natural enzymolysis for 7 days, take it out and put it into a multi-functional extraction tank, add 75L85% methanol for reflux extraction for 3 hours for extraction once, Combine the extracts to recover methanol, add 6BV ethyl acetate to the concentrated solution, heat to dissolve and remove the water layer, add activated carbon alumina (1:3) column for decolorization, collect the injection solution and recover ethyl acetate under reduced pressure to 1 / 5 of the original volume, add Stir with a small amount of petroleum ether until precipitation occurs, and place to crystallize; filter out the crude crystals, reflux with chloroform and acetone in turn to dissolve and refrigerate, refrigerate to crystallize at 0-4°C, and dry under vacuum and low pressure to obtain 14 g of isoferidin, with a content of 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com