Method for preparing coumarin derivative
A technology of coumarin and substances, which is applied in the field of preparation of lactone compounds, can solve the problems of low content, complicated extraction, separation and purification process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of 7-hydroxy-6,8-dimethoxycoumarin (isozapyridine)
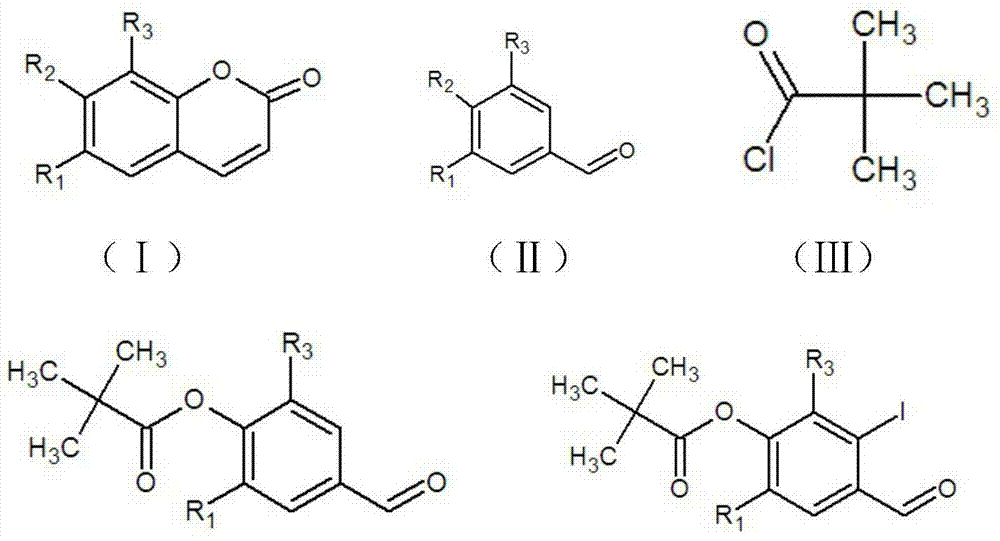
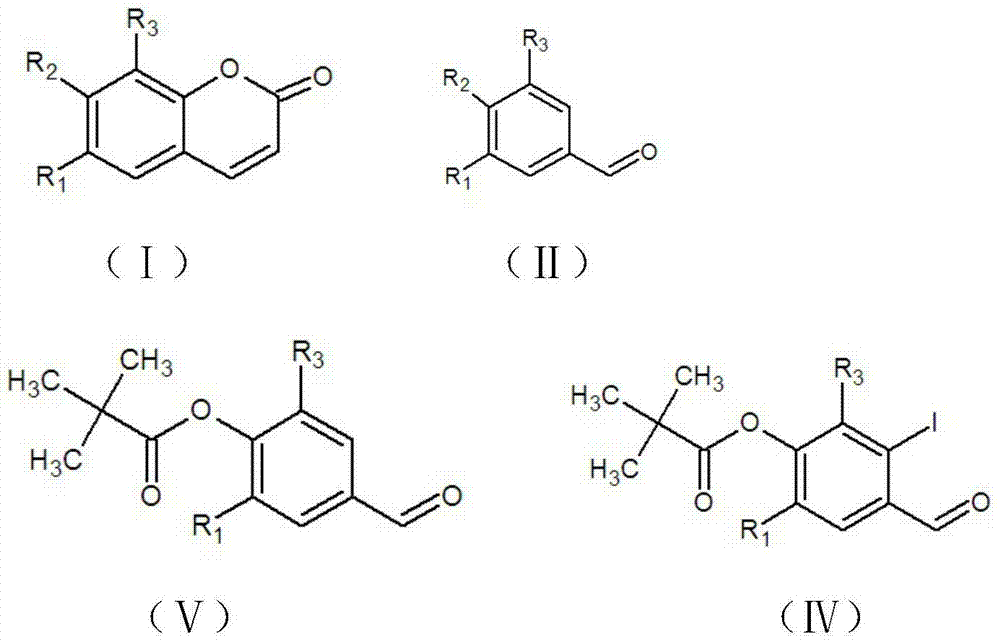
[0039] (1) Preparation of 4-formyl-2,6-dimethoxyphenyl-2,2-dimethylpropionate:
[0040] Add 3,5-dimethoxy-4-hydroxybenzaldehyde (Ⅱ) (546mg, 3mmol) and anhydrous dichloromethane (3ml) into a 25ml round bottom flask, stir to dissolve at 20°C, then add 4-dimethylamino Pyridine (15mg, 0.12mmol), stirred at 20°C for 0.5h, added dropwise pivaloyl chloride (732µl, 6mmol), triethylamine (940µl, 6mmol), stirred at 20°C for 2h, added 100 times the volume of the reactant (i.e. 100ml ) dichloromethane, washed successively with saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution, dried with anhydrous magnesium sulfate after removing the washing liquid, filtered with suction, concentrated the filtrate under reduced pressure to dryness, and obtained 790 mg of a light yellow solid crude product, namely 4-formyl-2,6-dimethoxyphenyl-2,2-dimethylpropionate (IV), yield 99%....
Embodiment 2
[0051] Example 2: Preparation of 7-hydroxy-6,8-dimethoxycoumarin (isozapyridine)
[0052] (1) Preparation of 4-formyl-2,6-dimethoxyphenyl-2,2-dimethylpropionate:
[0053] Add 3,5-dimethoxy-4-hydroxybenzaldehyde (Ⅱ) (546mg, 3mmol) and anhydrous dichloromethane (3ml) into a 25ml round bottom flask, stir to dissolve at 20°C, then add 4-dimethylamino Pyridine (15mg, 0.12mmol), stirred at 20°C for 0.5h, added dropwise pivaloyl chloride (549µl, 4.5mmol), triethylamine (705µl, 4.5mmol), stirred at 20°C for 2h, added 100 times the volume of the reactant ( (i.e. 100ml) dichloromethane, washed successively with saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution, dried with anhydrous magnesium sulfate after removing the washing liquid, filtered with suction, concentrated the filtrate to dryness under reduced pressure, and obtained 790 mg of crude product of light yellow solid , namely 4-formyl-2,6-dimethoxyphenyl-2,2-dimethylpropionate (Ⅳ), with...
Embodiment 3
[0065] Example 3: Preparation of 7-hydroxy-6-methoxycoumarin (scopolamine)
[0066] (1) Preparation of 4-formyl-22-methoxyphenyl-1,2-dimethylpropionate:
[0067] Add 3-methoxy-4-hydroxybenzaldehyde (456mg, 3mmol) and anhydrous dichloromethane (5ml) into a 25ml round bottom flask, stir and dissolve at 20°C, then add 4-dimethylaminopyridine (15mg, 0.12mmol ), stirred at 20°C for 0.5h, added dropwise pivaloyl chloride (732μl, 6mmol), triethylamine (940μl, 6mmol) successively, stirred at 20°C for 2h, added 100ml of dichloromethane to the reactant, followed by saturated sodium bicarbonate aqueous solution, washed with saturated sodium chloride aqueous solution, dried with anhydrous magnesium sulfate after removing the washing liquid, filtered with suction, concentrated the filtrate to dryness by rotary evaporation, and dried at 50°C for 5 minutes to obtain 700 mg of a light yellow solid crude product with a purity of 99% and a melting point of 83.8 -95.2°C.
[0068] 1 H NMR (500...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com