Method for inspecting quality of slender acanthopanax stilbenes powder
A kind of Acanthopanax powder, quality technology, applied in the direction of measuring device, color/spectral characteristic measurement, instrument, etc., can solve the problems of unfavorable quality and no quality standard, and achieve the effect of safe clinical use and good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0061] Example 1: Preparation of Acanthopanax powder
[0062] Acanthopanax powder: Prescription: Astragalus 800g, Acanthopanax 200g.
[0063] Preparation method: crush the above 2 traditional Chinese medicines into the coarsest powder, add 8 times the amount of water, soak for 1 hour, decoct and reflux for extraction for 2 hours, filter, add water to the filter residue and extract twice, add 8 times the amount of water each time and reflux for 2 hours , filtered several times, combined the filtrates, centrifuged while hot (50-60°C), concentrated the supernatant under reduced pressure to a relative density of about 1.2 (≤70°C), and spray-dried to obtain 300g of the finished product.
example 2
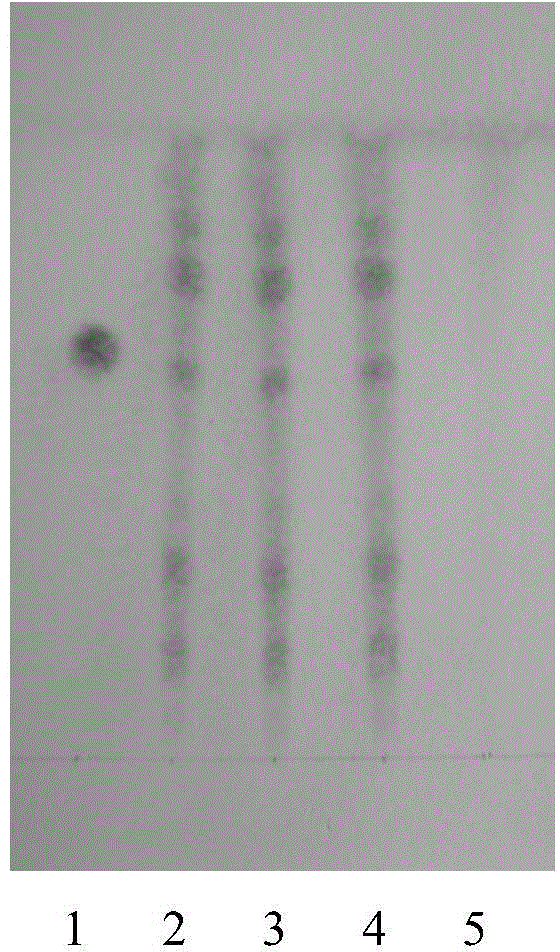
[0064] Example 2: Examination of the specificity of the identification method of Acanthopanax powder
[0065] (1) Reagents and samples
[0066] Control samples: Astragaloside IV, batch number: 110781-200613, purchased from China National Institutes for Food and Drug Control;
[0067] Acanthopanax powder: prepared in Example 1.
[0068] Negative control samples: Prepare negative control samples for each identification item according to the method in Example 1.
[0069] (2) Identification of Astragalus
[0070] Take 1 g of the sample prepared in Example 1, add 20 mL of methanol, heat and reflux for 1 hour, filter, add the filtrate to a neutral alumina column (100-120 mesh, 5 g, inner diameter 10-15 mm), wash with 100 mL of 40% methanol Remove, collect the eluent, evaporate to dryness, add 30 mL of water to the residue to dissolve, shake and extract with n-butanol saturated with water twice, 20 mL each time, combine the n-butanol solution, wash with water twice, 20 mL each tim...
example 3
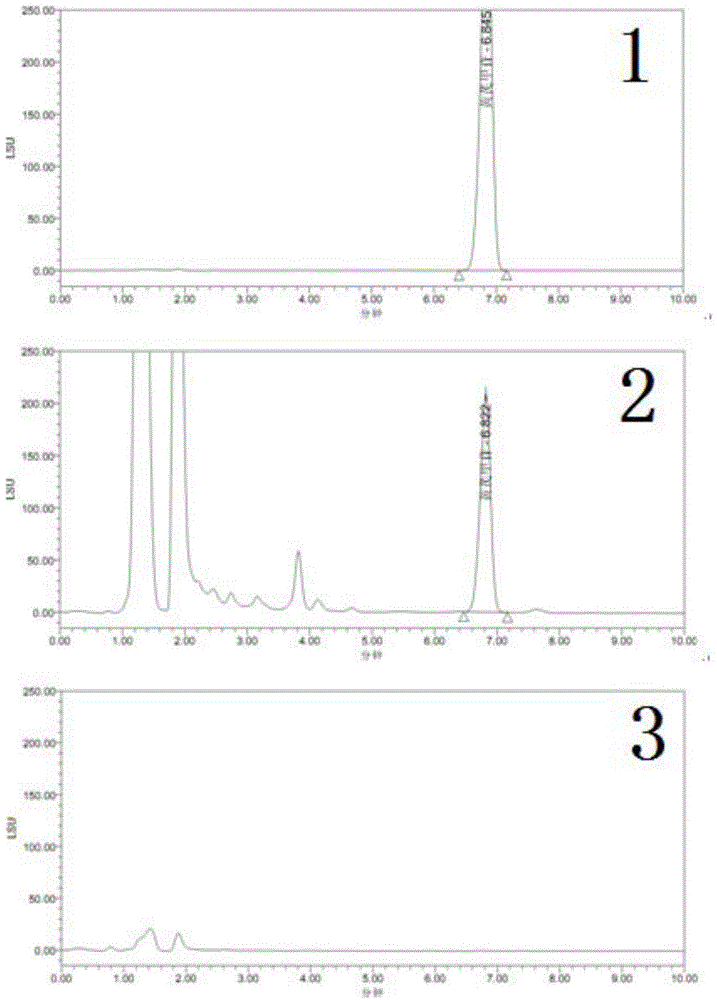
[0073] Example 3: Methodological investigation on the content determination of astragaloside IV
[0074] (1) Instruments and reagents, samples
[0075] Instrument: high performance liquid chromatography, e2695 type, Waters Corporation, USA
[0076] Evaporative light scattering detector, type 2424, Waters Corporation, USA
[0077] Electronic Analytical Balance, Model AB135-S, Mettler Toledo
[0078] Reagents: Acetonitrile is chromatographically pure; water is double distilled water; other reagents are analytically pure.
[0079] Control sample: Astragaloside IV, batch number: 110781-200613, purchased from China National Institutes for Food and Drug Control.
[0080] Acanthopanax powder: prepared in Example 1.
[0081] (2) Chromatographic conditions
[0082] The chromatographic column is a Kromasil100-5C18 column (150×4.6mm, 5μm); the mobile phase is acetonitrile-water (35:65); detection by an evaporative light scattering detector, the temperature of the drift tube is 60°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com