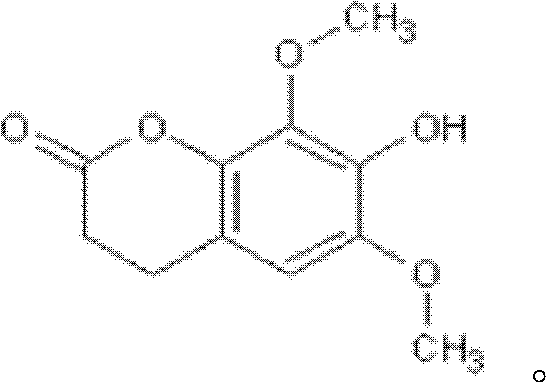
Isofraxidin crystalline compound and glabrous sarcandra herb dispersible tablets and dropping pills containing isofraxidin crystalline compound
A technique for isopicrin and compound, which is applied in the field of isopicrimidine crystalline compound and the dispersible tablets and dripping pills containing the compound, and can solve the problem of high cost of membrane separation method, low isopicrimidine content, unsatisfactory effect, etc. problems, to achieve the effect of ensuring drug safety, accurate dosage and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] [Example 1] Preparation of Isoferidin crystalline compound
[0053] 1) Take 2344g of sarcophagus, chop it up, decoct three times, add 12 times the amount of water to decoct for 1 hour each time, combine the decoction, filter, and concentrate the filtrate to extract A with a relative density of 1.05-1.10 at 60°C ;
[0054] 2) Dissolve the extract in hot water, filter through a microporous membrane, apply a macroporous adsorption resin on the obtained solution, the ratio of diameter to height of the macroporous adsorption resin column is 1:10, first elute with water at a flow rate of 3V / h, and then Elute with 40% ethanol solution of 5 times the volume of the column bed at a flow rate of 3 V / h, collect the 40% ethanol eluting solution, recover ethanol, and obtain extract B;
[0055] 3) Dissolve extract B in hot water, put the obtained solution on a 200-mesh silica gel column, and elute with n-hexane and ethyl acetate with a volume ratio of 10:1 for 100 min in sequence, an...
Embodiment 2-9
[0062]
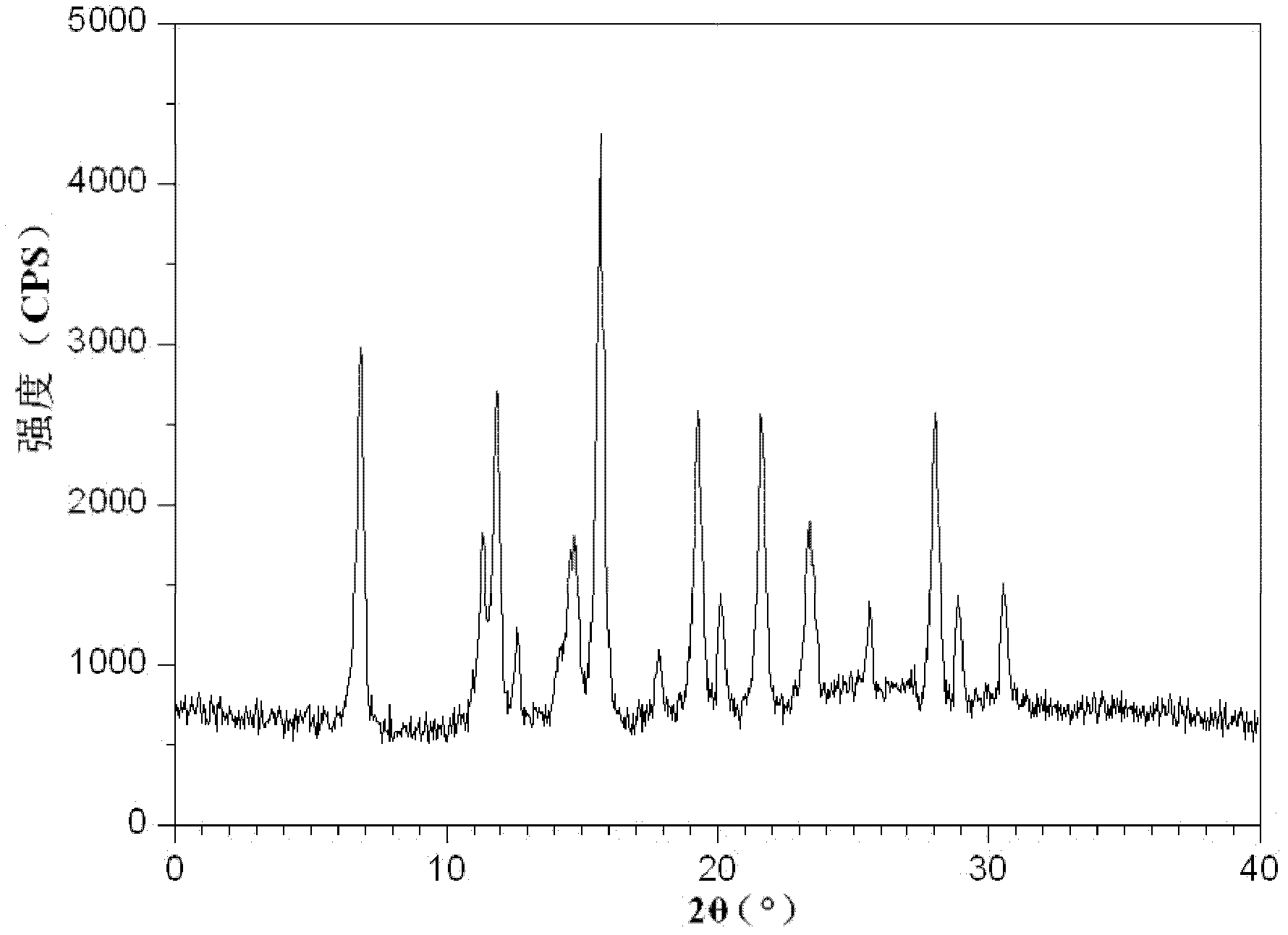
[0063] The X-ray powder diffraction spectrum obtained by measuring the isoferidin crystalline compound obtained in Examples 2-9 using Cu-Kα rays is similar to that in Example 1.
preparation Embodiment 1
[0065] [Preparation Example 1] Preparation of Zhongjiefeng Dispersible Tablets
[0066] Composition of raw materials: 2344g of sargassum, 70g of croscarmellose sodium, 70g of low-substituted hydroxypropyl cellulose, 8g of silicon dioxide, 75g of microcrystalline cellulose and 2.8g of magnesium stearate.
[0067] Preparation method: mix the yellow crystalline powder prepared in step 4) of Example 1 with the solid obtained in step 3) of Example 1 to obtain the Sargassum extract; Mix with 50g croscarmellose sodium, 70g low-substituted hydroxypropyl cellulose, 7.2g silicon dioxide and 75g microcrystalline cellulose, granulate, dry, then add 20g croscarmellose sodium , 0.8g silicon dioxide and 2.8g magnesium stearate are mixed evenly, are pressed into 1000, obtain described sargassum dispersible tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com