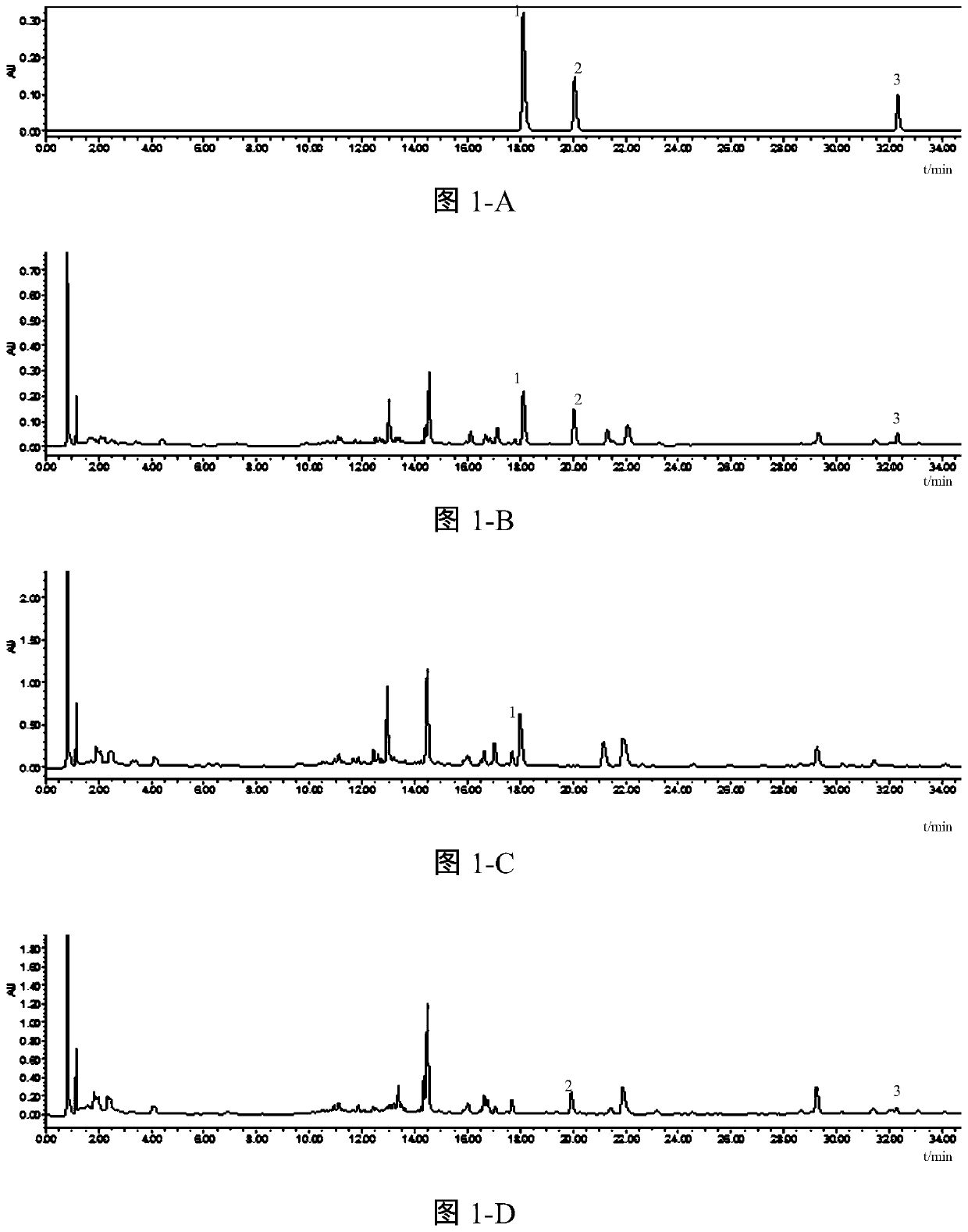
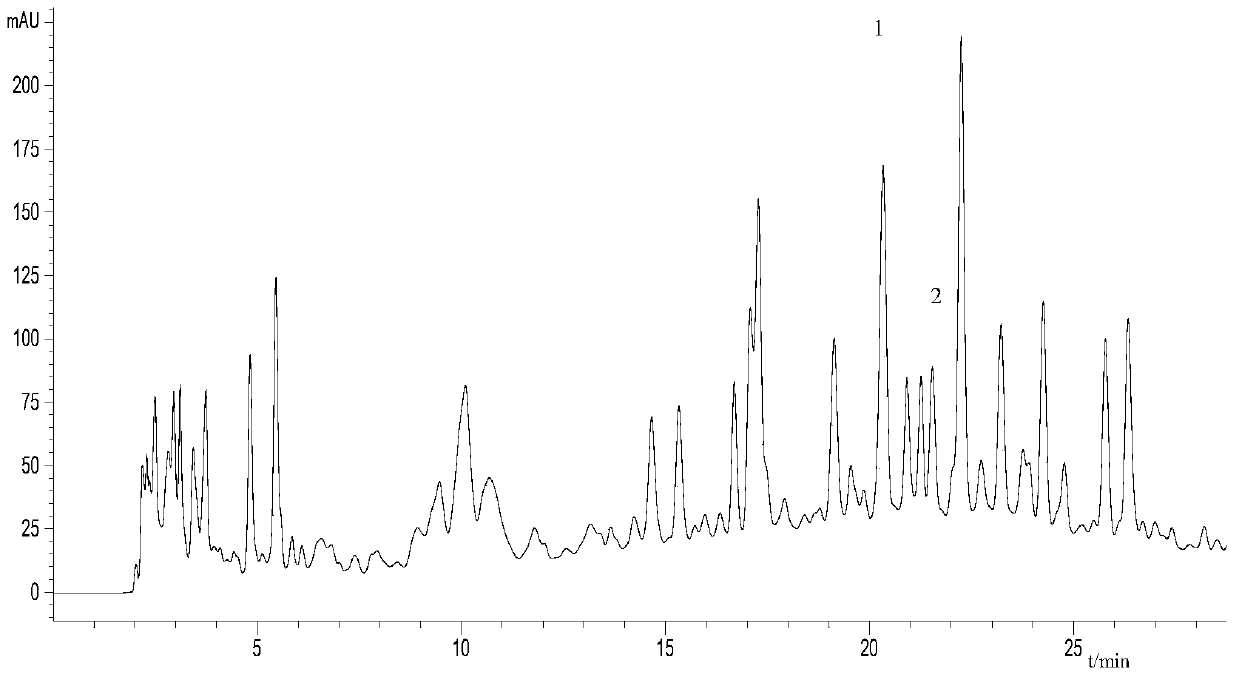
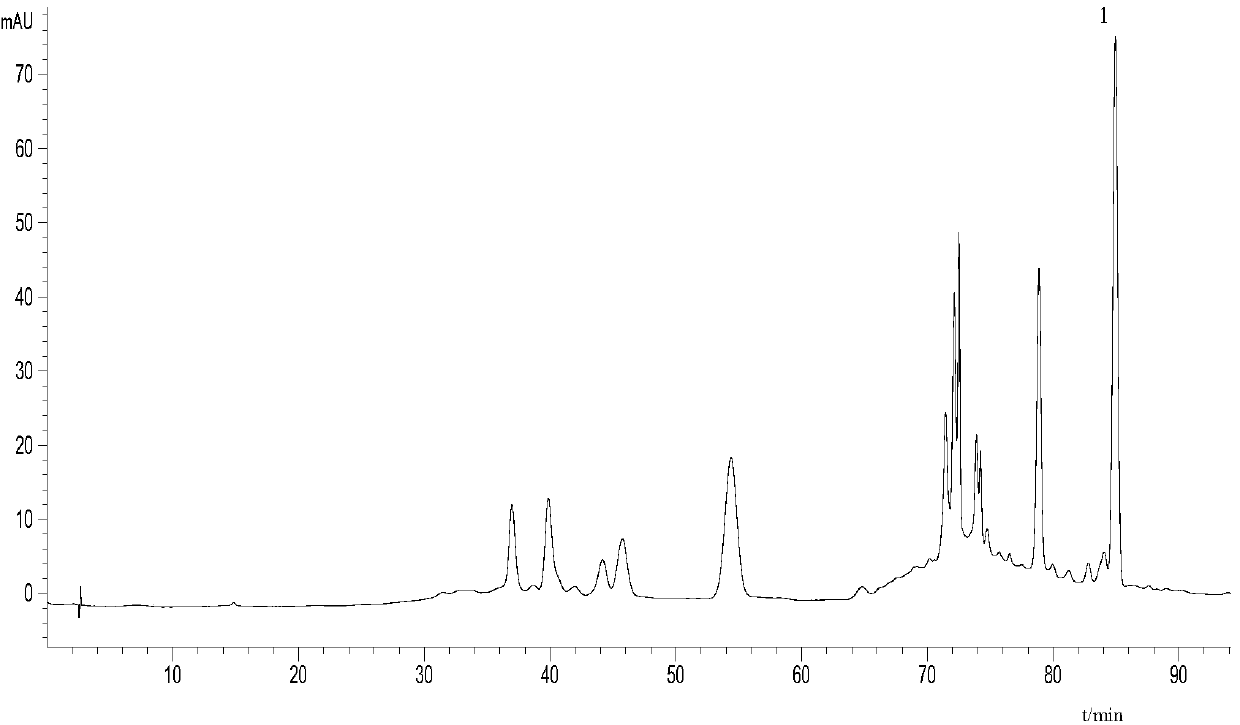
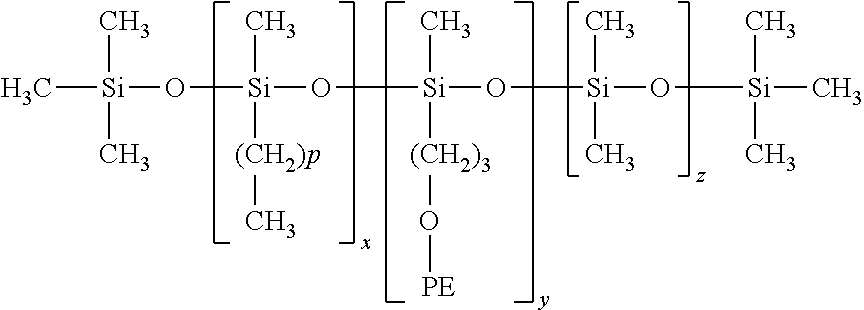Patents
Literature
78 results about "Astilbin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
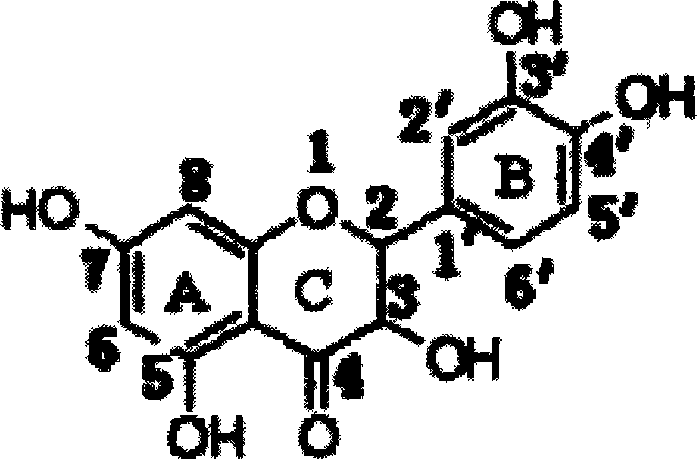
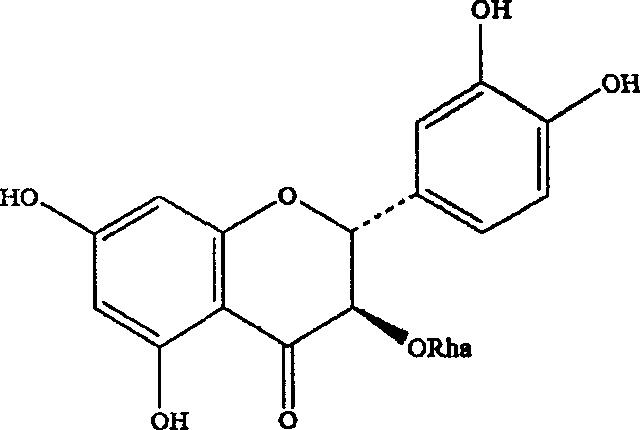
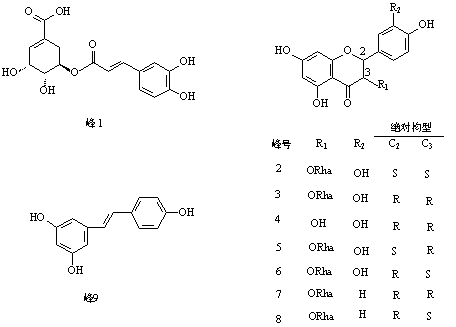
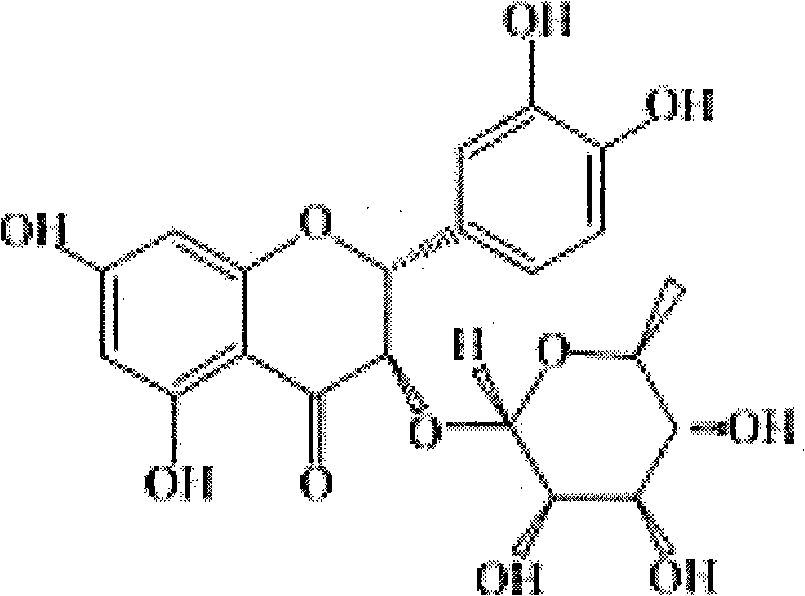
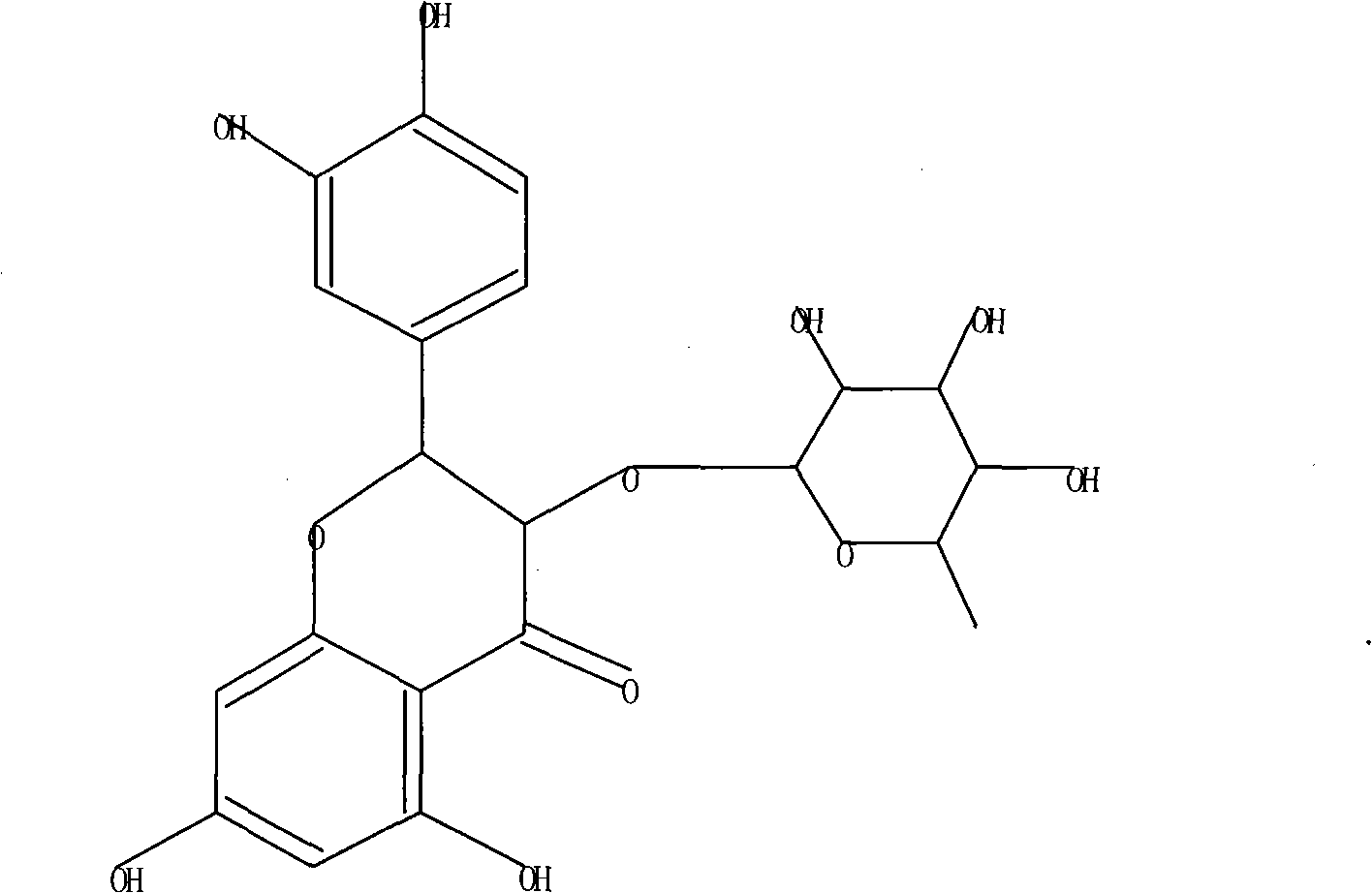
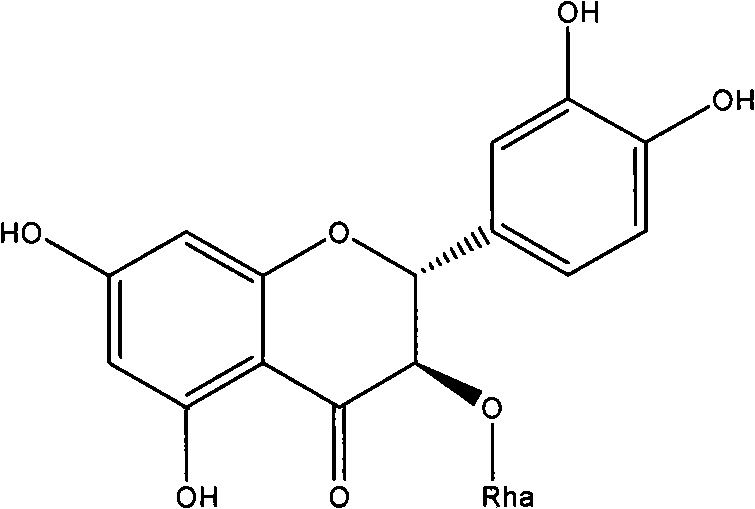
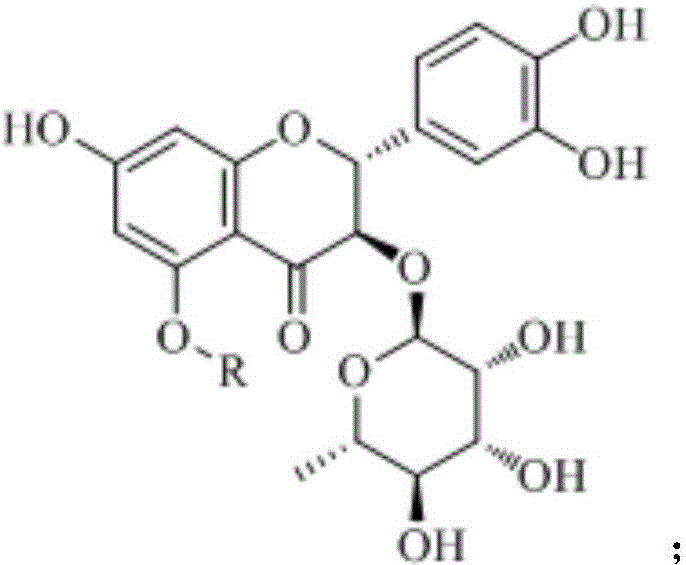
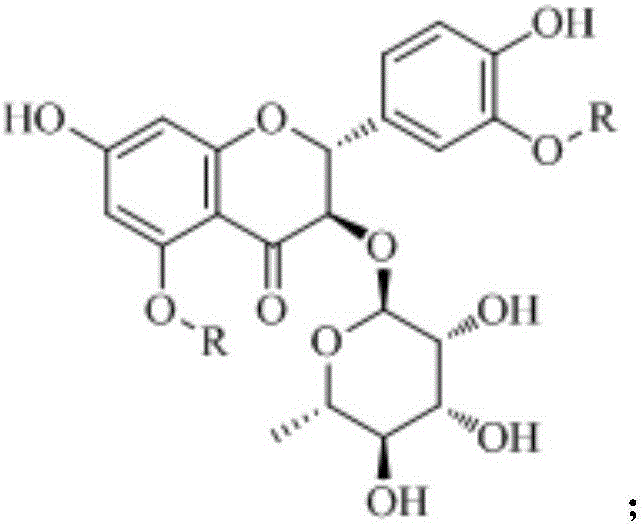
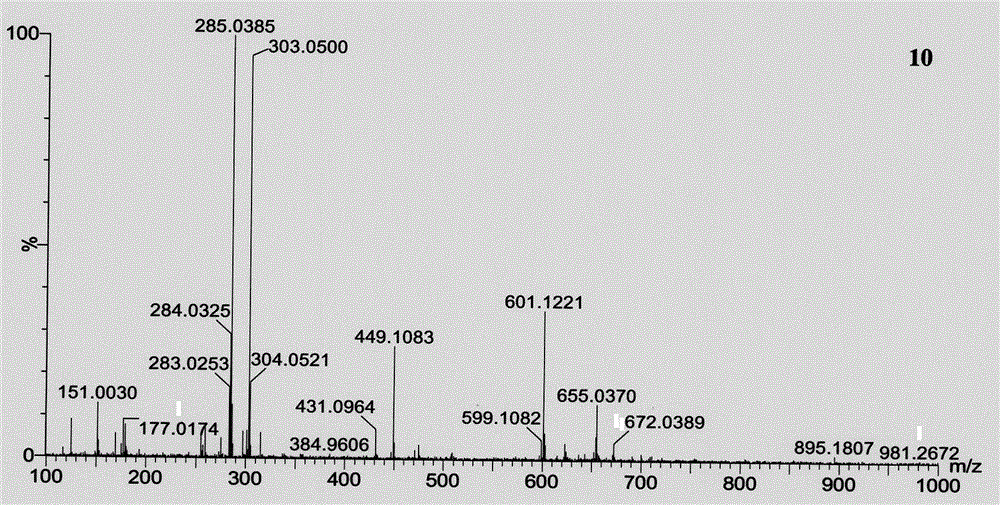
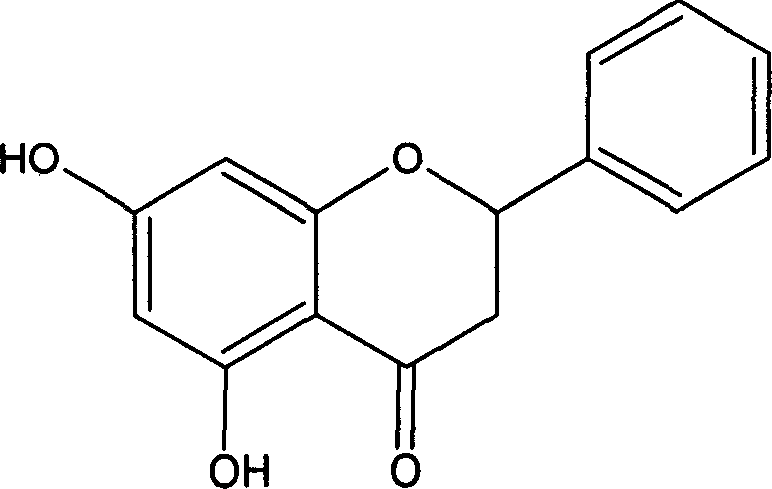
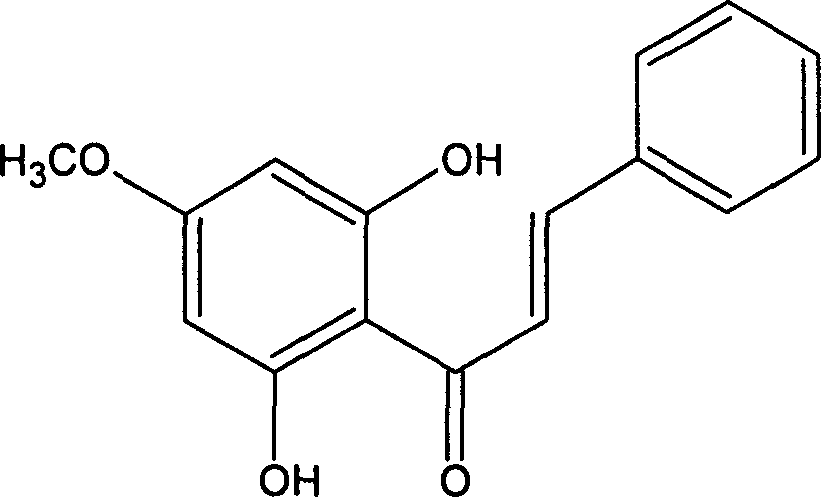
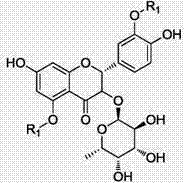
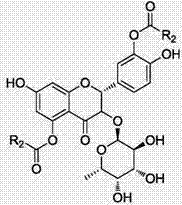
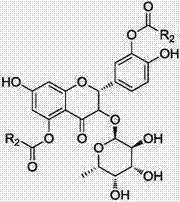
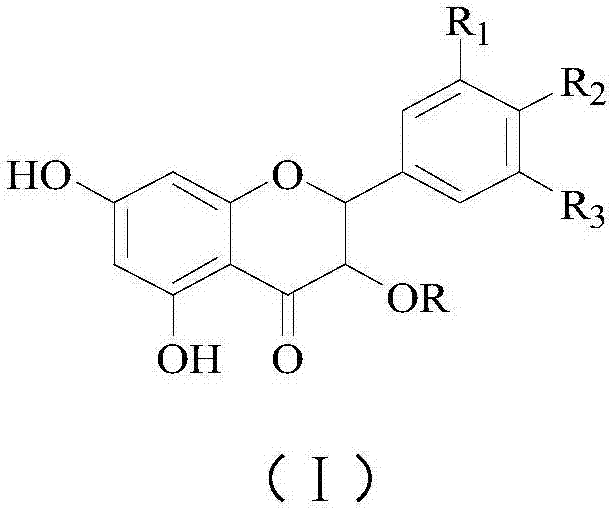
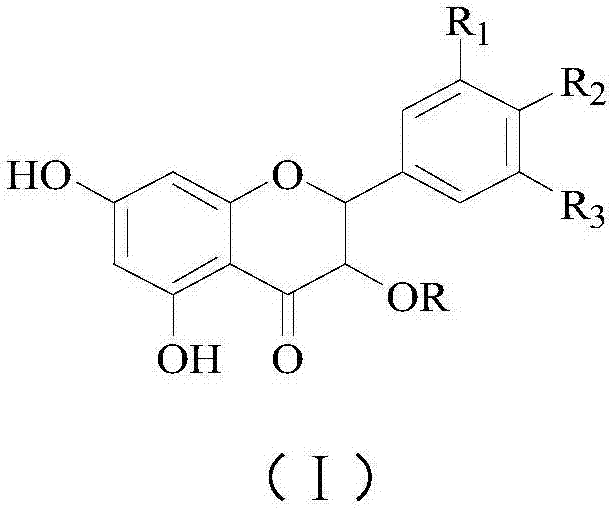
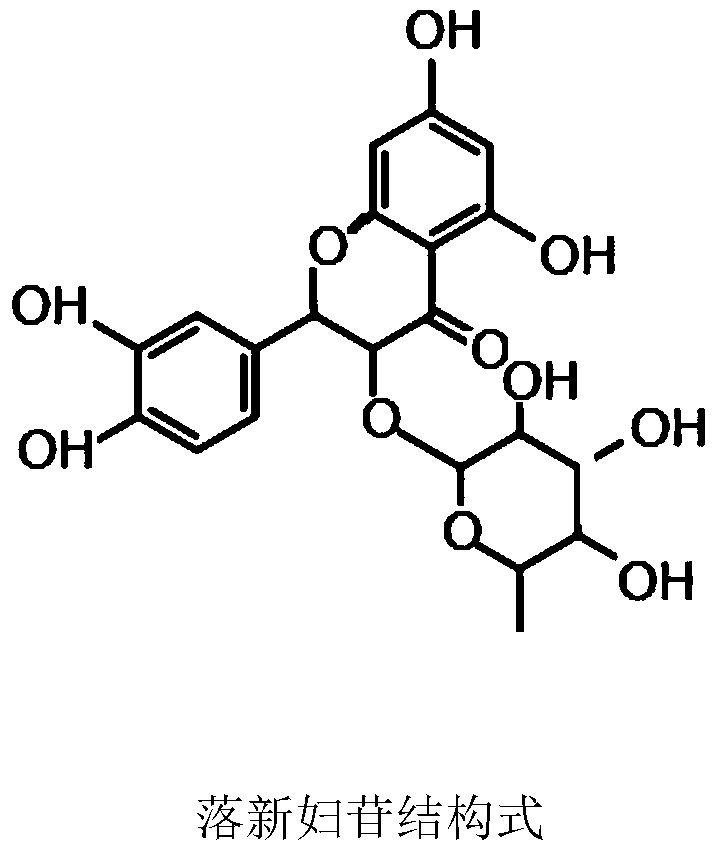
Astilbin is a flavanonol, a type of flavonoid. Astilbin is the (2R-trans)-isomer; neoisoastilbin is the (2S-cis)-isomer and isoastilbin is the (2R-cis)-isomer.
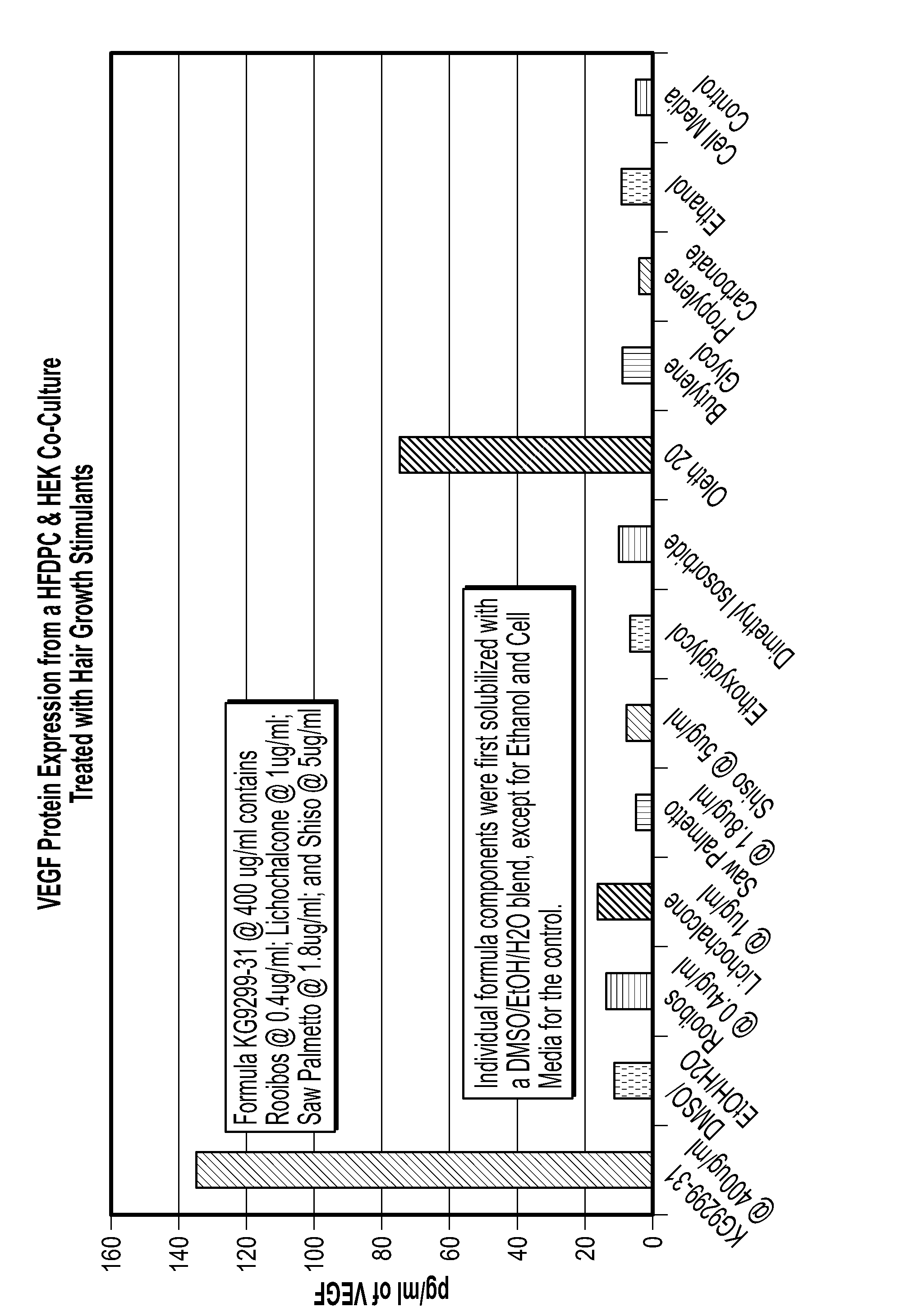
Methods and compositions for modulating hair growth or regrowth
InactiveUS20070036742A1Inhibit synthesisIncreased vascularizationBiocideCosmetic preparationsAstaxanthinRed Clover
The present invention relates to compositions and methods for modulating hair growth or regrowth. The compositions of the present invention comprise extracts of one or more of the following: Boswellia serrata, Undaria pinnatifida, green tea (e.g., Camellia sinensis), shiso, Pureraria mirifica, luteolin (e.g. Perilla ocymoides leaf extract), astilbin, vitamin E, amentoflavone, tetrahydropiperine, licochalcone, astaxanthin, red clover, Brassica juncea, unfermented green rooibos, enzyme CoQ10, salvia, ximenynic acid, hops oleoresin, apple, soy, saw palmetto, or ellagic extract, or any derivative thereof. In particular, the compositions and methods of the present invention can be used to stimulate or increase hair growth and / or prevent or slow the loss of hair by having one or more of the following functions: (a) inhibiting synthesis of DHT; (b) inhibiting proteasomal activity; (c) inhibiting IL-1 activity; (d) increasing vascularization; (e) increasing expression of vascular endothelial growth factor; (f) increasing expression of keratinocyte growth factor; (g) inhibiting inflammation; or (h) acting as an antibacterial.
Owner:ACCESS BUSINESS GRP INT LLC
Method of extracting and separating dihydroquercetin from roxburgh engelhardtia leaf
InactiveCN101054369AHigh yieldMature technologyOrganic chemistryPlant ingredientsLycium barbarum fruitAstilbin
The present invention discloses a method for extracting and separating dihydroquercetin from wolfberry leaf, in which dihydroquercetin is prepared from wolfberry leaf through extracting, concentrating, column chromatography, crystallizing and hydrolyzing to generate an acicular crystal white dihydroquercetin, wherein the content of dihydroquercetin more than 95%(HPLC test). The present invention can extract astilbin, quercetin and dihydroquercetin from wolfberry leaf simultaneously, which has advantages of high yield and ripe technology, and it is suitable for industrialization and continuous production.
Owner:宋云飞
Preparation method of astilbin
InactiveCN1724552AHigh purityIncrease contentSugar derivativesSugar derivatives preparationAlcoholPolyamide
The invention provides a process for preparing astilbin which comprises the steps of disintegrating medicinal materials, extracting with aqueous solutions of ethanol with two concentrations, degreasing the 90-95 wt% water raffinate of ethanol with petroleum ether, condensing into concrete, alcohol-depositing the concrete, merging the mother liquid with 20-80 wt% water raffinate of ethanol, concentrating and extracting with ethanol-containing acetic ester liquid, alcohol-depositing the extract and mixing with the precipitation, dissolving then passing through macro-porous absorption resin or polyamide columns, eluting with 20-80 wt% aqueous solution of ethanol, finally recrystallizing the eluent. The extraction rate of astilbin is over 50%, and the content of astilbin is over 90%.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
Use of rhizoma smilacis glabrae extract in preparation of lipid-lowering and fat-reducing medicines and health-care foods
ActiveCN103463531AIncrease useGood for weight lossMetabolism disorderFood preparationNeoisoastilbinAbdominal cavity
The invention belongs to the field of traditional Chinese medicines and relates to a use of rhizoma smilacis glabrae extract in preparation of lipid-lowering and fat-reducing medicines and health-care foods. The rhizoma smilacis glabrae extract comprises 5-O-caffeoylshikimic acid, astilbin, isoastilbin, neoastilbin, neoisoastilbin, taxifolin, engeletin, isoengelitin and resveratrol. An animal experiment shows that the rhizoma smilacis glabrae extract can obviously reduce mice abdominal cavity fat weight, reduce mice body weight, and reduce triglyceride and total cholesterol concentrations of mice serum. Effects of the rhizoma smilacis glabrae extract are stronger than effects of a single astilbin component. The rhizoma smilacis glabrae extract is extracted from traditional Chinese medicines, has synergism of components, has good fat-reducing effects and broadens a rhizoma smilacis glabrae application range.
Owner:JIANGI WENIR NUTRITION HIGH TECH
Quality control method for traditional Chinese medicine oral liquid for treating psoriasis
InactiveCN105891404AAvoid overlappingAccurately reflectComponent separationChlorogenic acidTherapeutic effect
The invention discloses a quality control method for traditional Chinese medicine oral liquid for treating psoriasis. The medicine is prepared from seven types of medicinal materials such as radix paeoniae rubra, rhizoma smilacis glabrae and glabrous sarcandra herbs. According to the specific quality control method, the thin layer chromatography is applied for conducting thin-layer identification on radix paeoniae rubra and rhizoma smilacis glabrae in PSORI-CMO1, meanwhile, the high performance liquid chromatography is combined for measuring the contents of chlorogenic acid, paeoniflorin, isofraxidin, astilbin and engeletin in the oral liquid, it is guaranteed that detected constituents are accurate and reliable, and the purpose of effectively controlling quality of the traditional Chinese medicine oral liquid can be achieved. By means of the quality control method, the defect that a method for detecting multiple constituents of compound traditional Chinese medicine is complex is overcome; the method has the advantages that by identifying the two types of medicine materials and measuring the contents of important active constituents in the medicine, inherent quality, the treatment effect and stability of a preparation are effectively guaranteed, and controllability of the quality standards for the PSORI-CMO1 oral liquid is improved.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Chinese herbal medicine compound preparation for treating skin and mucous membrane disease and preparation method thereof
InactiveCN108853404AImprove efficacyEasy to operateAntimycoticsDigestive systemAdditive ingredientUrsolic acid
The invention relates to a Chinese herbal medicine compound preparation for treating skin and mucous membrane disease. The Chinese herbal medicine compound preparation is prepared from the following raw materials: cortex moutan, polygonum chinense, radix salviae miltiorrhizae, herba lycopi, common dayflower herb, rhizoma smilacis glabrae, ramulus cinnamomi, caulis dendrobii officinalis, gorgon fruit and cortex cinnamomi; the ingredient of the Chinese herbal medicine compound preparation comprises a cortex moutan extract, a polygonum chinense extract, a radix salviae miltiorrhizae extract, a herba lycopi extract, a common dayflower herb extract, a rhizoma smilacis glabrae extract, a ramulus cinnamomi extract, a caulis dendrobii officinalis extract, a gorgon fruit extract and a cortex cinnamomi extract; and the effective ingredient of the cortex moutan extract is paeonol, the effective ingredient of the polygonum chinense extract is quercitrin, the effective ingredient of the radix salviae miltiorrhizae extract is tanshinone IIA, the effective ingredient of herba lycopi is ursolic acid, the effective ingredient of common dayflower herb is commelinin, the effective ingredient of rhizoma smilacis glabrae is astilbin, the effective ingredient of ramulus cinnamomi is cinnamaldehyde, the effective ingredient of caulis dendrobii officinalis is erianin, the effective ingredient of gorgon fruit is cyclo(profilum), and the effective ingredient of cortex cinnamomi is cinnamaldehyde.
Owner:近晟(上海)医药科技有限公司
Preparations containing an extract of eperua falcata and/or constituents of the latter
A cosmetic, pharmaceutical or dermatological preparation containing extract of the plant Eperua falcata, active principles of the plant Eperua falcata, astilbin or engeletin. The preparation is useful to inhibit release of pro-imflammatory mediators and neuropeptides, including CGRP and SP, for skin and hair treatment, including, sensitive skin, acne, scalp itch and neurogenous inflammation.
Owner:COGNIS FRANCE SA
Methods for extracting astilbin and dihydroquercetin from engelhardia roxburghiana wall leaves and application of extractives thereof
InactiveCN102234300AEasy to operateThe process is simple and feasibleSugar derivativesSugar derivatives preparationPolyamideAstilbin
The invention relates to a method for extracting astilbin and dihydroquercetin from engelhardia roxburghiana wall leaves and application of extractives thereof. The method for extracting the astilbin from the engelhardia roxburghiana wall leaves comprises the steps of: extracting by refluxing ethanol, degreasing by using ligarine, extracting by using ethyl acetate, blending two organic solvents with different polarities in proportion, and performing recrystallization so as to obtain the astilbin; and the method for extracting the dihydroquercetin from the engelhardia roxburghiana wall leaves mainly comprises the steps of: firstly, obtaining the astilbin by using the provided method, and then hydrolyzing the astilbin to obtain the dihydroquercetin. The methods provided by the invention are beneficial to simplification of the traditional operation processes such as resin absorption, polyamide column or silicagel column chromatography and the like, and have the characteristics of simple, convenient and feasible process, simplicity for operation, lower cost and higher extraction efficiency and content; and after being measured with the HPLC (High Performance Liquid Chromatography) method, the contents of the astilbin and the dihydroquercetin are both more than 90%, therefore the method is suitable for industrialized production.
Owner:广州医学院
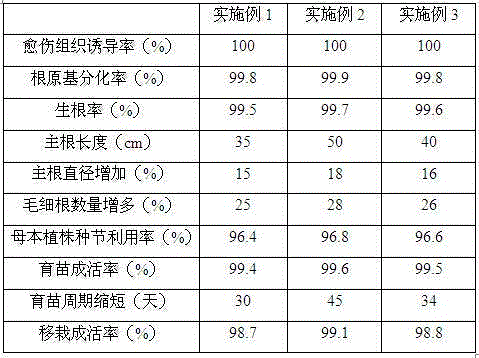
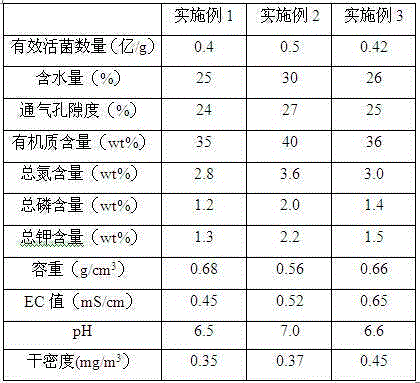
Seedling culturing substrate containing Chinese medicine residues and application of seedling culturing substrate in salt golden cypress bonsai seedling culturing
InactiveCN105123330AA lot of rootingNot easy to fall offGrowth substratesCulture mediaPeanut mealTotal nitrogen
The invention provides a seedling culturing substrate containing Chinese medicine residues. The seedling culturing substrate is prepared from the Chinese medicine residues, bean dregs, glucose powder, peanut meal, folia perillae acutae, pine needle meal, bagasse, micronutrient fertilizer, astilbin, organosilicone, humic acid ammonia, zeolite, kaolin and fermentation preparation. The invention further provides the application of the seedling culturing substrate in salt golden cypress bonsai seedling culturing. According to the prepared seedling culturing substrate, the number of effective living bacteria reaches 40-50 millions / g, the water content is 25%-30%, the aeration porosity is 24%-27%, the organic matter content reaches 35%-40%, the total nitrogen content reaches 2.8-3.6 wt%, the total phosphorus content is 1.2-2.0wt%, the total potassium content is 1.3-2.2wt%, the unit weight is 0.56-0.68 g / cm<3>, the EC value is 0.45-0.65 mS / cm, the pH is 6.5-7.0, and the dry density is 0.35-0.45 mg / m<3>.
Owner:WEIFANG YOURONG IND
Methods and compositions for modulating hair growth or regrowth
ActiveUS8197865B2Inhibit synthesisIncreased vascularizationBiocideCosmetic preparationsRed CloverOleoresin
The present invention relates to compositions and methods for modulating hair growth or regrowth. The compositions of the present invention include extracts of one or more of the following: Boswellia serrata, Undaria pinnatifida, green tea (e.g., Camellia sinensis), shiso, Pureraria mirifica, luteolin (e.g. Perilla ocymoides leaf extract), astilbin, vitamin E, amentoflavone, tetrahydropiperine, lichochalcone, astaxanthin, red clover, Brassica juncea, unfermented green rooibos, enzyme CoQ10, salvia, ximenynic acid, hops oleoresin, apple, soy, saw palmetto, or ellagic extract, or any derivative thereof.
Owner:ACCESS BUSINESS GRP INT LLC
Method for extracting astilbin from engelhardtia leaves
The invention provides a process for extracting astilbin from leaves of Engelhardtia roxburghiana wall, which consists of extracting with dissolvent, concentrating, disintegrating and purifying, crystallizing and drying, wherein the dissolvent can employ water or low carbon alcohols such as methanol, ethanol, propanol, isopropyl alcohol, butanol and amylic alcohol.
Owner:GUILIN NATURAL INGREDIENTS CORP
Method for extracting astilbin from china root
InactiveCN102432652APlay a separation effectGood recrystallization effect with waterSugar derivativesSugar derivatives preparationAlcoholAstilbin
The invention relates to a method for extracting an astilbin from a china root. The method comprises the following steps: taking the china root as a raw material; extracting in a manner of water backflow; adding salt and acid, and depositing flavonoid glycoside; after degreasing deposition, dissolving in alcohol and adding a macro-porous resin column; utilizing water to remove impurities; utilizing the alcohol to elute in a gradient form; collecting the astilbin stream; and concentrating and re-crystallizing, thereby obtaining a product. The method for producing the astilbin has the advantages of convenience in operation, low cost, environmental friendliness, strong technical specificity, high yield and high purity.
Owner:苏州北商智业管理咨询有限公司
Preparation method of astilbin
InactiveCN102020690AConducive to mass production operationsReduce energy consumptionSugar derivativesSugar derivatives preparationAcetic acidEthyl ester
The invention relates to a preparation method of astilbin with simple and convenient operation, little pollution and less energy consumption. The process comprises the following steps of: taking trester, adding ethanol to a microwave extraction device and carrying out microwave extraction, collecting extraction liquid, filtering, decompressing, recycling and concentrating ethanol to obtain an extract; carrying out counter-current extraction on a mixed solution of ethyl acetate and water in a ratio of 8 to 1, mixing the extraction liquid, decompressing and recycling the solvent; adding macroporous adsorptive resin for adsorption, eluting with the ethanol, collecting 3-8 times by measuring pin volume of eluent, decompressing, recycling and concentrating the solvent; and adding ethanol for crystallization, cleanly separating, washing and drying. The method of the invention is adopted for preparing astilbin with high product purity and is easy to realize industrial amplification.
Owner:SUZHOU PAITENG BIOLOGICAL MEDICAL TECH
Quality control method of xiaojiean preparation
ActiveCN102198210AAccurate identificationReliable identificationComponent separationAntineoplastic agentsMotherwortMedicine
The invention relates to a quality control method of a medicine preparation, and especially relates to thin layer chromatogram authentication and content determination of smilax glabra in a xiaojiean preparation. The method is a thin layer chromatogram authentication and / or content determination method formulated by using a special component in the smilax glabra of the xiaojiean preparation as an index component, wherein the special component is astilbin, and the xiaojiean preparation is a traditional Chinese medicine compound preparation prepared from 1100 parts of leatherleaf mahonia, 750 parts of thin evodia, 750 parts of motherwort, 750 parts of spatholobus stem, 900 parts of smilax glabra and 750 parts of fructus forsythiae. The thin layer chromatogram authentication method with a strong specialization can authenticate smilax glabra accurately and reliably, and a generally employed HPLC can determine the astilbin content in the xiaojiean preparation, so as to produce valuable significance for monitoring and controlling medicinal material purchase, preparation production process and preparation quality in market, and ensuring product safety, effectiveness and quality stabilization. According to the method, problem of confused smilax glabra basic material provided in the market can be solved effectively to ensure that xiaojiean preparation meets the national medicine standards strictly.
Owner:云南神威施普瑞药业有限公司
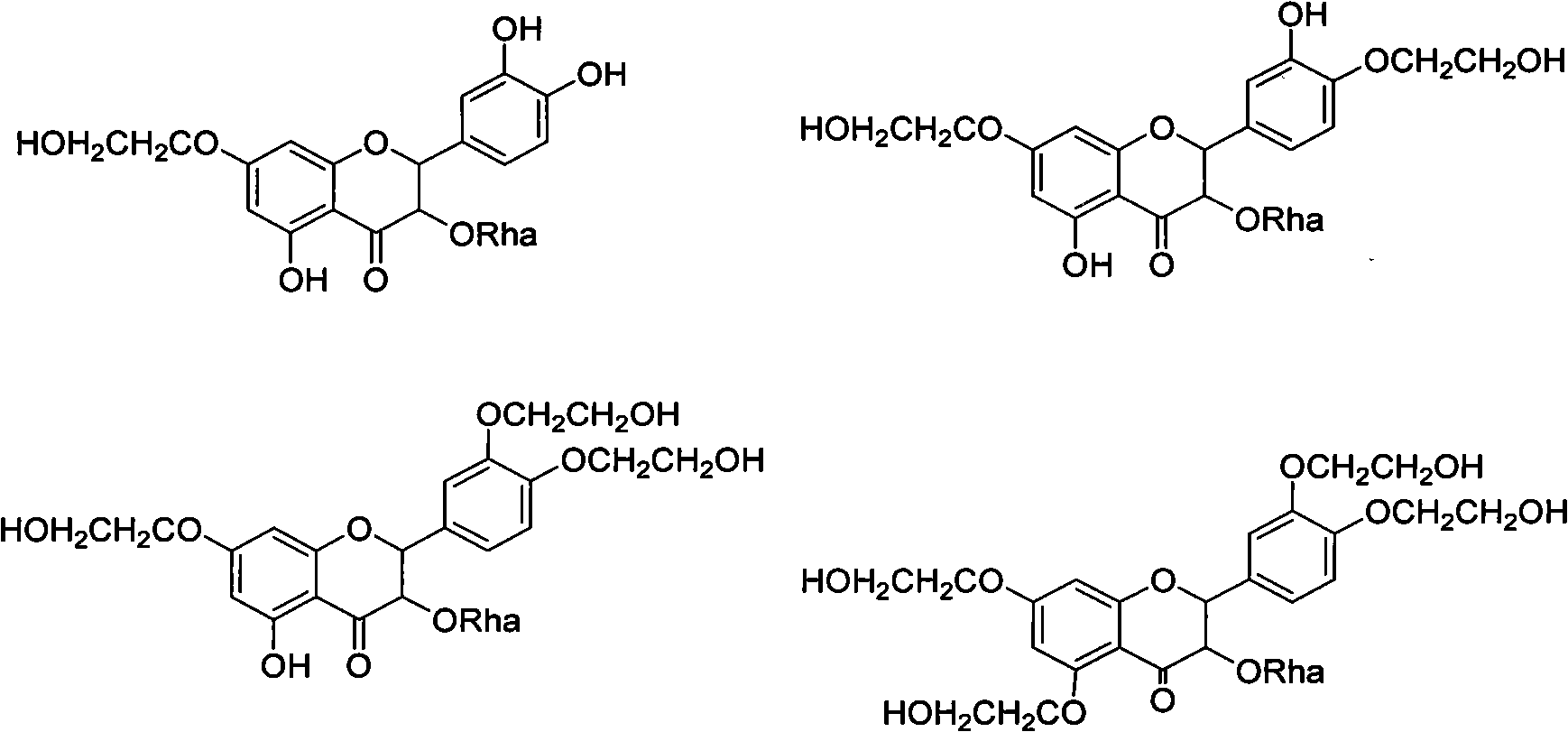
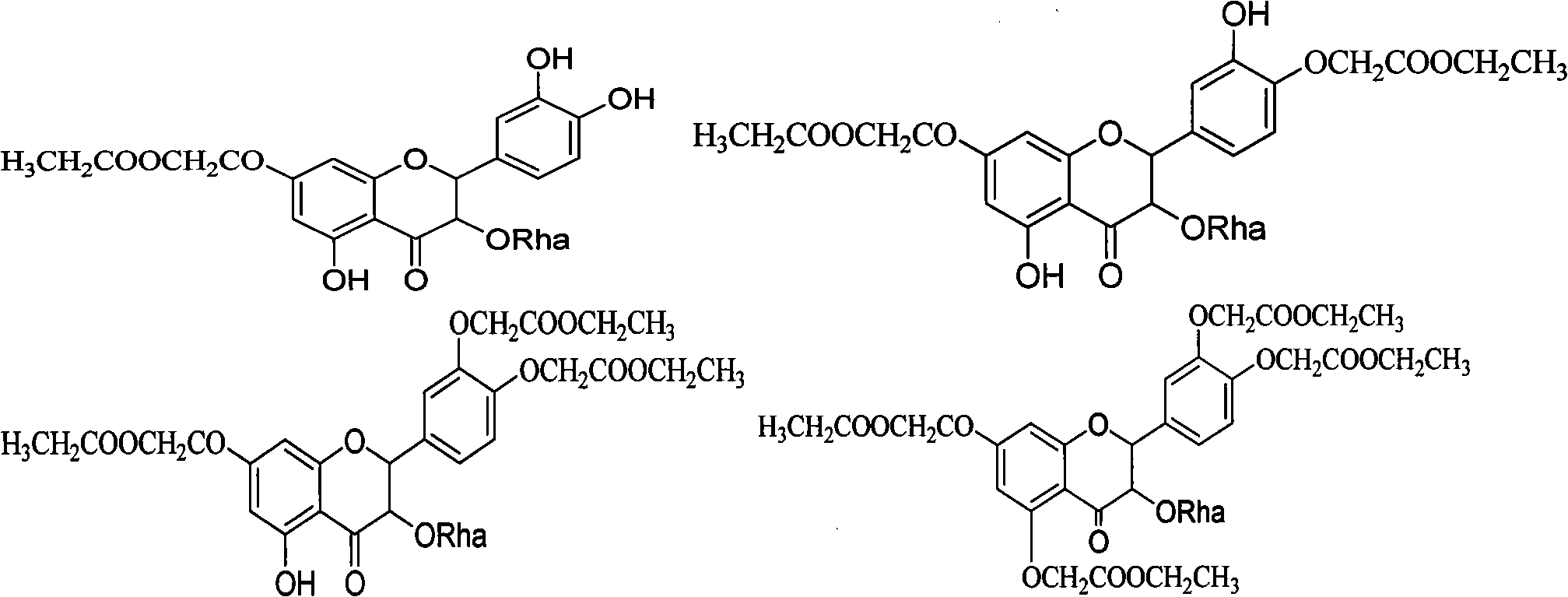
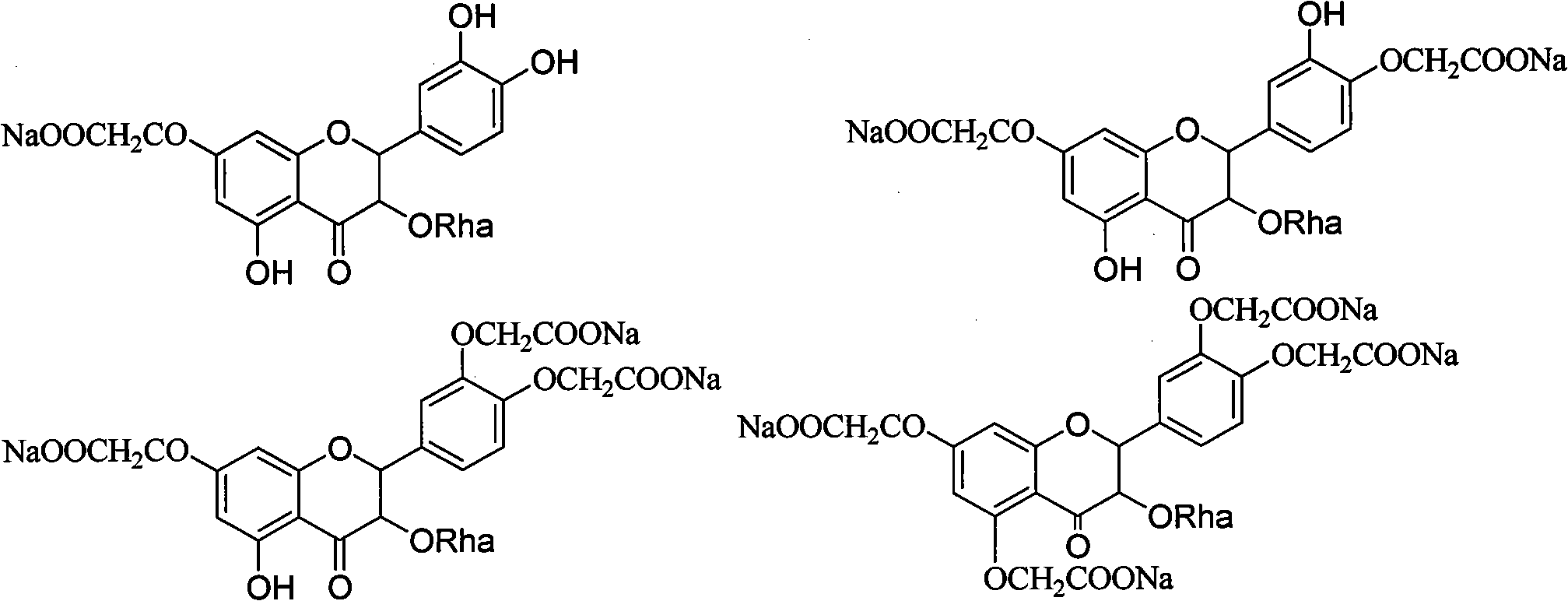
Derivatives of astilbin and preparation method thereof
Owner:SHANDONG GREENERY NATURAL MEDICINE RES & DEV
Method of quality control for smilax china
ActiveCN101549081AStable contentIncrease contentComponent separationPlant ingredientsSmilax chinaPhosphoric acid
The invention discloses a method of quality control for smilax china. In the method, the content of the smilax china is measured by using C18 or C8 as a filler; methanol- 0.1 percent of phosphoric acid solution with the proportion of 25-45 : 55-75 or acetonitrile- of 0.1 percent of phosphoric acid solution with the proportion of 10-20 : 80-90 or acetonitrile-the methanol-0.1 percent of phosphoric acid solution with the proportion of 0-10: 10-30 : 60-90 as a moving phase;, and using a detection wavelength ranging from 280 nm to 300 nm. Astilbin contained in the smilax china cannot be less than 0.030 percent, and engelitin contained in the smilax china cannot be less than 0.030 percent. The method of quality control for the smilax china is efficient, stable and accurate and has convenient operation and strong specialization, and can well control the quality of the smilax china or a preparation containing smilax china raw materials.
Owner:GUILIN SANJIN PHARMACEUTICALS CO LTD
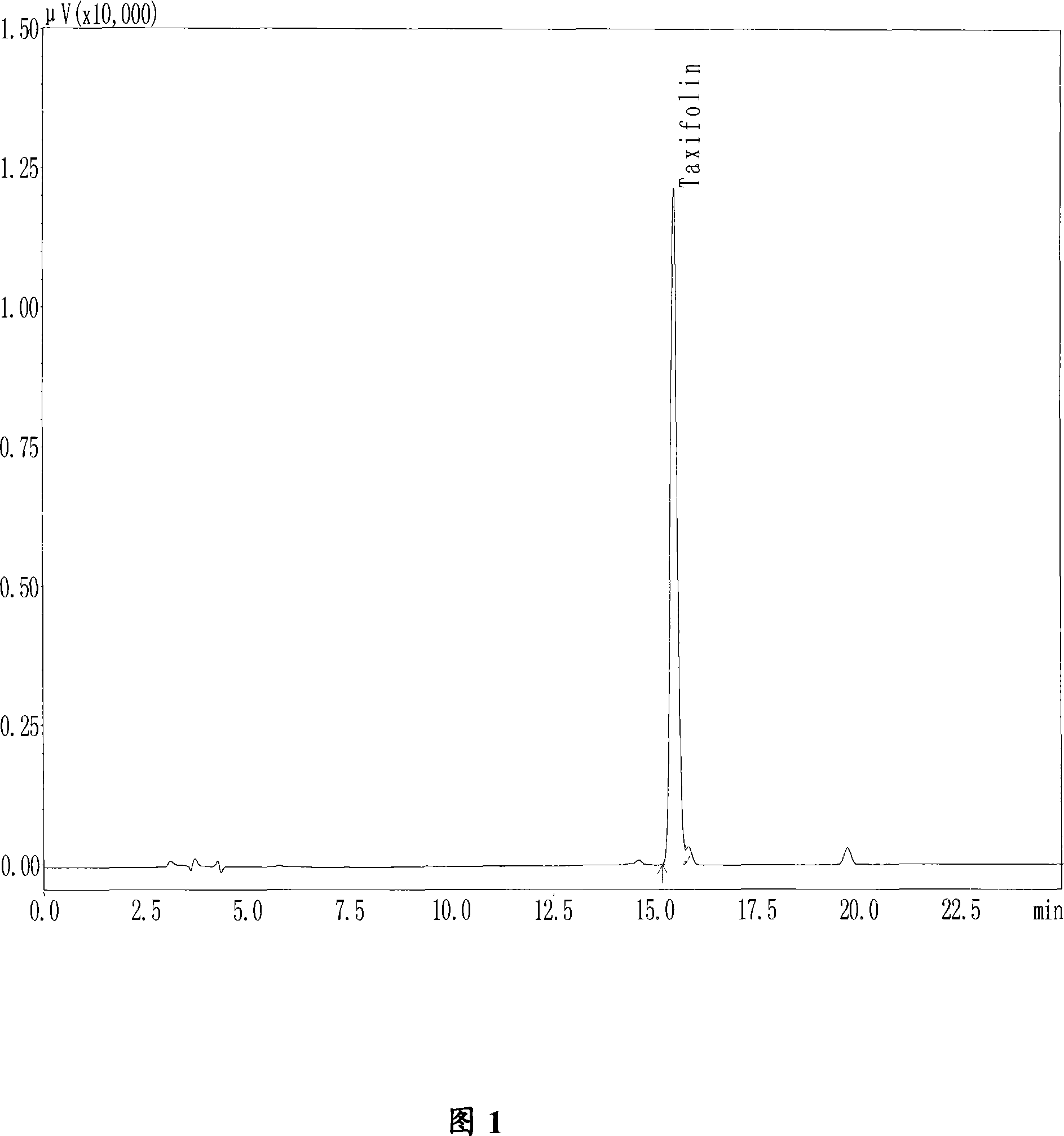
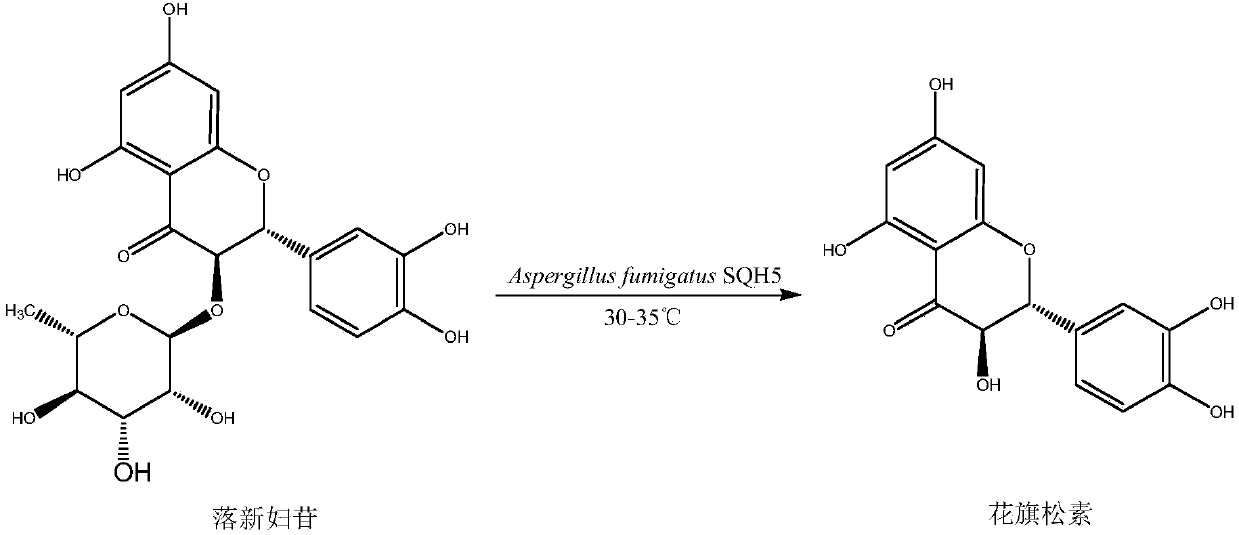
Aspergillus fumigates SQH4, and applications thereof in preparation of taxifolin through biotransformation
ActiveCN107893033ASolve the shortageReduce pollutionFungiMicroorganism based processesAspergillusAcid hydrolysis
The invention discloses aspergillus fumigates SQH4, and applications thereof in preparation of taxifolin through biotransformation. According to the applications, astilbin is taken as a substrate, a fermentation broth prepared via aspergillus fumigates SQH4 fermentation is taken as a catalyst, and the taxifolin conversion rate is 92.3% when the substrate feed concentration is controlled to be 3g / L. The technical advantages are that: the shortage problem of taxifolin extracted from plants is solved; the conversion specificity is better and product yield is higher than that of astilbin acid hydrolysis method. A production of taxifolin disclosed in the invention is low in production cost and environment pollution, and high in conversion rate.
Owner:ZHEJIANG UNIV OF TECH +1
Multi-index-based evaluating-optimizing glabrous sarcandra herb preparation technology
The invention discloses a multi-index-based evaluating-optimizing glabrous sarcandra herb preparation technology. The multi-index-based evaluating-optimizing glabrous sarcandra herb preparation technology comprises the following steps of HPLC determination of specified components, process optimization of glabrous sarcandra herb leaves, single factor experimental investigation of glabrous sarcandraherb stems, cutting-preparing design scheme of the glabrous sarcandra herb stems, establishment of an AHP model, a comprehensive scoring method and CCD-RSM optimized glabrous sarcandra herb stem cutting-preparing process and verification test. Through a single-factor test, the comprehensive scores of the content of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, isofraxidin, astilbin and rosmarinic acid are taken as the evaluation indicators, a central composite design-response surface methodology is used to screen glabrous sarcandra herb softening and cutting-preparing processes, the glabrous sarcandra herb stems and leaves subjected to process optimization by choosing different treatment methods, a multi-index analytic hierarchy process (AHP) is combined with the central composite design-response surface methodology to optimize the glabrous sarcandra herb preparation technology, the study is beneficial to the further development of glabrous sarcandra herb, and an experimental basis is expected to be provided for the establishment of related preparation specifications and quality standards of glabrous sarcandra herb.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
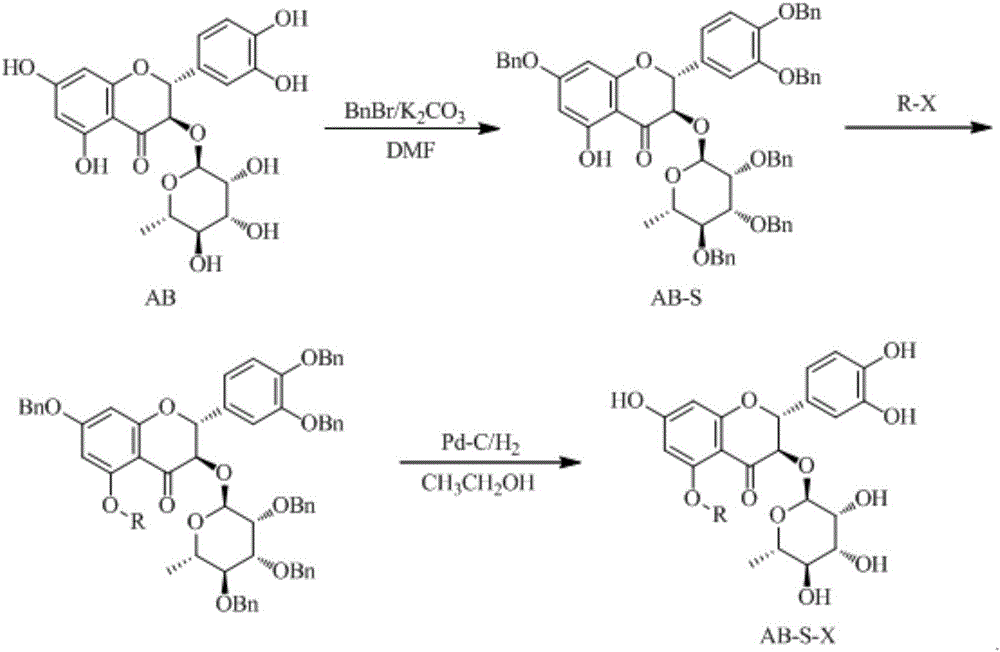
Astilbin derivatives and preparation method thereof
ActiveCN106083956AImprove bioavailabilitySugar derivativesSugar derivatives preparationBenzoyl bromideFlavanone
The invention relates to astilbin derivatives and a preparation method thereof and belongs to the technical field of fine chemical engineering. Astilbin is used as a raw material, a C-5 hydroxyl exposed single-hydroxyl intermediate AB-S and a C-5 and C-3' hydroxyl exposed double-hydroxyl intermediate AB-D are respectively prepared by controlling the molar ratio of benzyl bromide to the astilbin. The intermediates AB-S and AB-D respectively react with halogenated sugar, and the astilbin derivatives AB-S-X and AB-D-X are respectively prepared through deprotection. According to the astilbin derivatives and the preparation method of the astilbin derivatives, the bioavailability of the astilbin is improved by conducting selective protection and deprotection on phenolic hydroxyl groups and rhamnose hydroxyls, introducing monosaccharides or disaccharides to C-5 of flavanone parent nucleuses and introducing monosaccharides or disaccharides to C-5 and C-3'.
Owner:CHENGDU UNIV
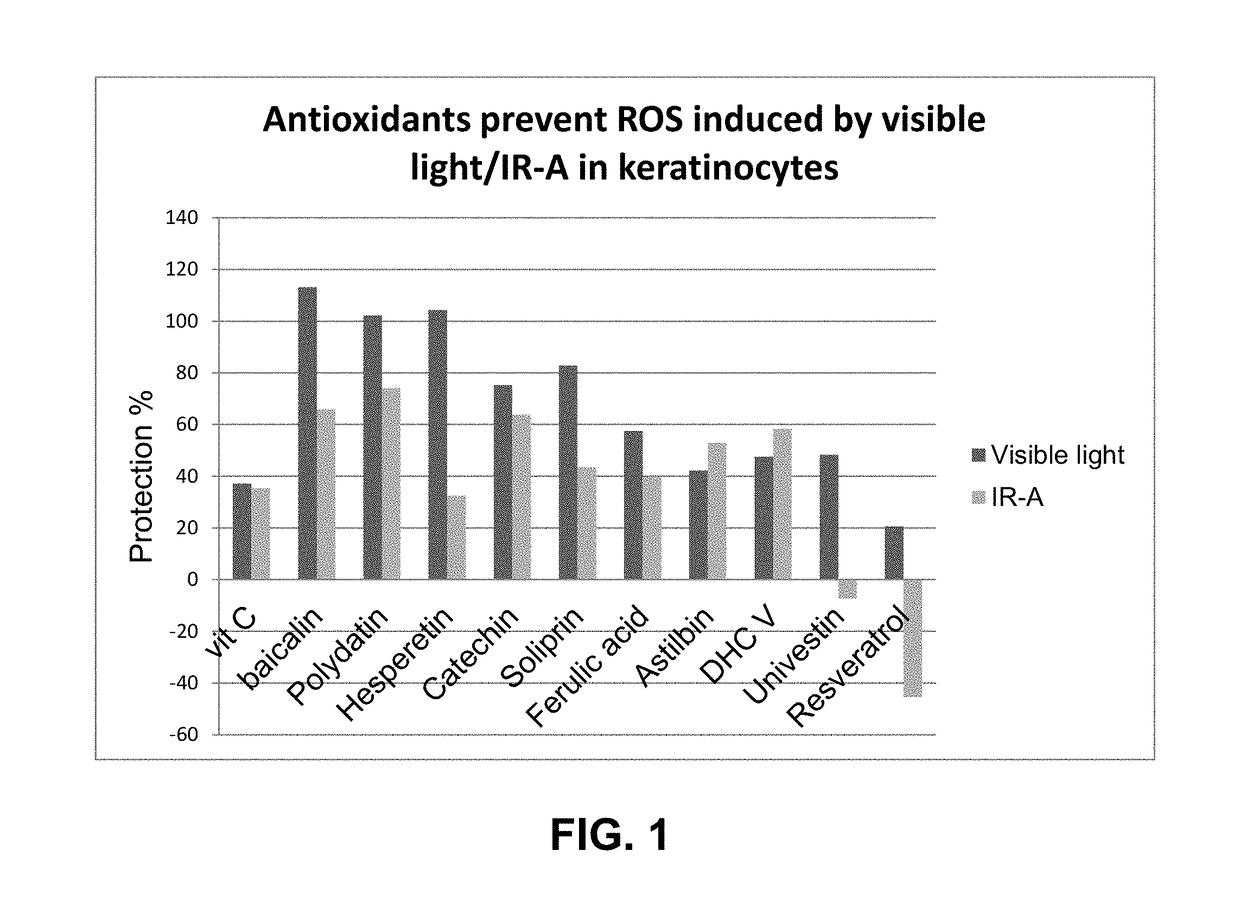
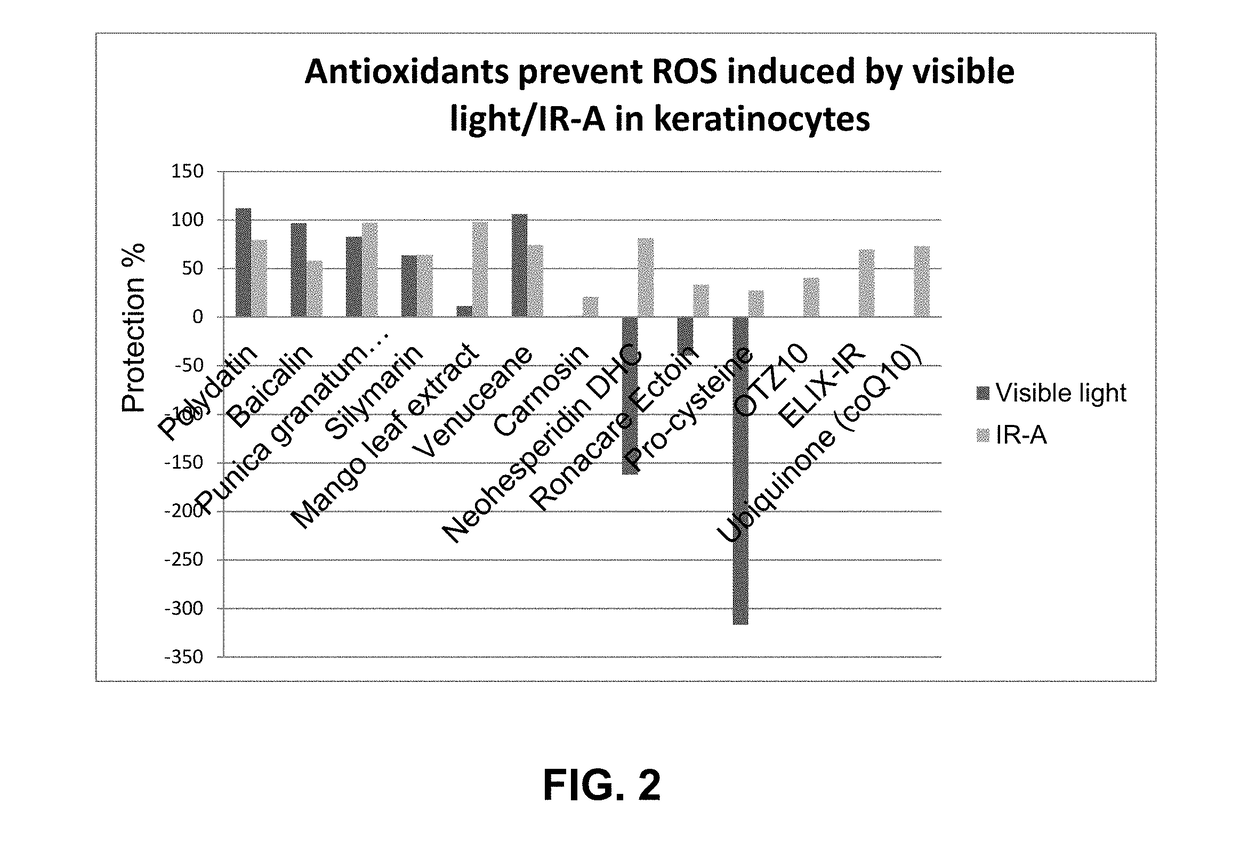
Methods and compositions for providing broad spectrum photo protection using antioxidants
ActiveUS10137072B2Good lookingGood for healthCosmetic preparationsToilet preparationsPunicaAntioxidant
The present disclosure relates to methods and compositions for providing at least broad spectrum photo protection to skin, which includes protection from at least infrared (IR) radiation and / or visible light. The methods typically entail applying to skin a cosmetic composition comprising: (a) one or more antioxidants selected from the group consisting of baicalin, Venuceane™, ferulic acid, polydatin, silymarin, punica granatum extract, mango leaf extract, soliprin, catechin, hesperetin, astilbin, and DHC V; (b) optionally, one or more solubilizers; and (c) a cosmetically acceptable carrier; wherein the combination of (a), optional (b), and (c) alone, provide at least broad spectrum protection from both infrared (IR) radiation and visible light. UV filters can also optionally be included to provide additional protection from UV light.
Owner:LOREAL SA
Traditional Chinese medicine active part for preventing chronic pelvic inflammation, and preparation method and applications thereof
ActiveCN106492069AImprove complianceDefinite curative effectOrganic active ingredientsSexual disorderPatient complianceToxic material
The invention discloses a traditional Chinese medicine active part for preventing chronic pelvic inflammation, and a preparation method and applications thereof. The content of the traditional Chinese medicine active part is 50-90%, and the traditional Chinese medicine active part comprises the following components in percentage by weight: 8.37-16.11% of astilbin, 4.52-12.55% of neoastilbin, 6.97-15.90% of isoastilbin, 15.47-22.65% of neoisoastilbin, 15.66-24.97% of engeletin and 3.75-7.15% of gracillin. The traditional Chinese medicine active part preparation for preventing chronic pelvic inflammation is prepared by combining smilax china flavonoid compound and saponin according to certain proportions, the curative effect is definite, the active ingredients are clear, and the traditional Chinese medicine active part has the efficacies of clearing away heat and toxic materials and invigorating blood circulation to remove blood stasis, is effectively used for preventing chronic pelvic inflammation, and is safe to use, convenient to take, small in dose and good in compliance of patients.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Flavone glycoside composition
The invention provides a flavone glycoside composition. The flavone glycoside composition consists of neoastilbin, astilbin, isoastilbin, neoisoastilbin, neoengelitin, engelitin, isoengelitin, neoisoengelitin, kaempferol-3-O-rhamnoside, taxifolin-3-O-(3''-O-p-(E)-galloyl)-[alpha]-L-rhamnoside and stereisomers thereof. The invention belongs to the fields of medicines, food, health care food and pharmaceutical preparations, and specifically includes a flavonoid glycoside composition, a preparation method, an application and a finished product.
Owner:苏州优诺康医药科技有限公司
Pharmaceutical composition containing glabrous sarcandra herb extract and its uses
The invention discloses a medicinal composition containing active ingredients extracted from glabrous sarcandra herb, including flavones, and / or saponin, and / or polysaccharides, or one or more of the following flavone active ingredients: isofraxidin-7-O-glucopyranoside, 5,7-dihydroxyl flavanone, 2',6'-dihydroxy-4'-methoxyl chalcone, pinostrobin, dihydromyricetin, Astilbin, naringenin 4',7-dimethyl ether.
Owner:JIANGXI BOSHILIAN SCI & TECH RES&DEV
Astilbin derivative and preparation method thereof
InactiveCN107141325AImprove bioavailabilityHigh yieldSugar derivativesSugar derivatives preparationAstilbinTert-leucine
The invention discloses an astilbin derivative and a preparation method thereof. The derivative is AB-N-X or AB-H-X; the general formula of the AB-N-X is shown in the description, wherein R1 represents glycine, leucine, proline, serine or alanine; the general formula of the AB-H-X is shown in the description, wherein the representation of R2 is shown in the description. The preparation method comprises the steps: 1, dissolving astilbin organic solvent, and carrying out the replacement reaction with Bian chlorine or Bian bromine under catalyst, obtaining AB-N-1 after extraction and purification; 2, dissolving the AB-N-1 by adding organic solvent, adding natural amino acid or acid anhydride for reaction, and obtaining AB-N-2 or AB-H-1 after purification; 3, injecting hydrogen into the AB-N-2 or AB-H-1 under the catalysis of the catalyst, taking off protection to obtain the final product AB-N-X or AB-H-X. The astilbin derivative has high bioavailability; the preparation method is reliable in process, simple to operate, and high in yield.
Owner:CHENGDU UNIV
Application of flavonone compounds in preparation of farnesol X receptor stimulant
InactiveCN107397743AStrong FXR activationEasily damagedOrganic active ingredientsDigestive systemTreatment effectBiliary excretion
The invention relates to application of flavonone compounds in the preparation of a farnesol X receptor stimulant. The flavonone compounds including dihydromyricetin, astilbin, isoastilbin, engeletin, isoengeletin, dihydroquercetin and dihydrokaempferol have a relatively strong FXR stimulation effect and can be used as a potential farnesol X receptor stimulant. The flavonone compounds including dihydromyricetin, astilbin, isoastilbin, engeletin, isoengeletin, dihydroquercetin and dihydrokaempferol have a remarkable inhibition effect to the active increase of Tbil, ALT, APL and gamma-GT caused due to ANIT-induced cholestasis, are capable of obviously improving the inhibition to ANIT-induced hepatic cell injury, inflammatory cell infiltration and bile excretion, have a relatively good treatment effect to cholestasis type hepatitis and can be used as potential drugs for treating the cholestasis type hepatitis.
Owner:CATCH BIO SCI & TECH
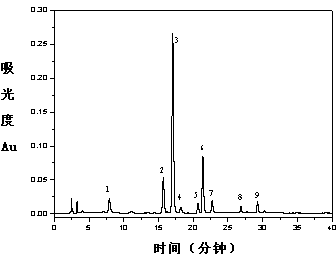
Method for determining active ingredients of rhizoma smilacis glabrae medicinal material
ActiveCN101716277AAvoid one-sidednessSimple methodAntibacterial agentsAntipyreticColumn temperatureGradient elution
The invention discloses a method for determining active ingredients of a rhizoma smilacis glabrae medicinal material, relating to the filed of medicament analysis. In the invention, a fingerprint taking an astilbin chromatographic peak as an internal reference peak is established. The method comprises the following steps of: (a) preparing a test article solution; (b) taking astilbin as a reference substance and taking 0.10mg / mL astilbin solution prepared from methanol as a reference substance solution; (c) chromatographic conditions: taking a chromatographic column made of octadecylsilane chemically bonded silica as filler; adopting gradient elution, detecting the wavelength of 325+ / -2 nm and the column temperature of 30+ / -5DEG C; calculating the theoretical column plate number not less than 3,000 according to astilbin peak; and (d) determining. The fingerprint established by rhizoma smilacis glabrae ethanol-soluble extractives contains active ingredients with main activity of rhizoma smilacis glabrae. The fingerprint is used for judging the quality of rhizoma smilacis glabrae and the one sidedness for judging the quality of rhizoma smilacis glabrae by determining one or two chemical components can be avoided. The method has the advantages of simpleness, convenience, stability, high precision, favorable reproduction, easy grasp and capability of rapidly and accurately identifying the authenticity and merits of products.
Owner:GUANGZHOU BAIYUNSHAN JINGXIUTANG PHARM CO LTD
Application of astilbin organic acid composition in treatment of psoriasis
ActiveCN111450107AGood preventionGood therapeuticOrganic active ingredientsDermatological disorderOrganic acidSide effect
The invention discloses an application of an astilbin organic acid composition in treatment of psoriasis. The astilbin organic acid composition comprises astilbin or a structural analogue thereof andan organic acid in any ratio. The composition has the beneficial effects that astilbin and organic acid are combined, so that the effect of preventing or treating psoriasis is good, and the pharmaceutical composition is safe and non-toxic and does not have side effects caused by hormones.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Process for extracting astilbin from Engelhardia roxburghiana Wall leaves
InactiveCN107056858AHigh extraction rateThe extraction yield is high above 65.48%Sugar derivativesSugar derivatives preparationEngelhardia roxburghianaBULK ACTIVE INGREDIENT
The invention discloses a process for extracting astilbin from Engelhardia roxburghiana Wall leaves, and belongs to the technical field of extraction of active ingredients from Engelhardia roxburghiana Wall leaves. The astilbin is prepared by the following steps: pulverizing, first decolorizing, coarse crystallization, second decolorizing, separation and purification by macroreticular resin, concentration and crystallization, drying and the like. The extraction yield of the prepared astilbin is 65.48% or above, the purity is 98.16% or above, and the extraction yield and the purity are higher than the extraction yield and the purity of the astilbin prepared in the prior art.
Owner:南宁馨艺荣生物科技有限公司
Quality detection method for traditional Chinese medicine formula
ActiveCN105510451AImprove resolutionEasy to separateComponent separationChromatographic separationActive component
A quality detection method for a traditional Chinese medicine formula which is named compound detoxication decoction. In the quality detection method, ultra performance liquid chromatography (UPLC) is employed for simultaneously detecting the contents of astilbin, forsythiaside A and forsythin in the compound detoxication decoction at 275 nm. According to the method, the various active components in the compound detoxication decoction are detected through the UPLC. The method is ultrahigh in separation rate, speed and sensitivity on the astilbin, the forsythiaside A and the forsythin. The method can achieve excellent chromatographic peak separation rate just through one injection without complex pre-treatment processes to a sample, so that the method is greatly increased in chromatographic separation rate and analysis speed, effectively saves analysis time, achieves quick and high-resolution quality detection for the compound detoxication decoction, and has excellent effects on clinical quality control.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Emulsion containing astilbin and preparation method thereof
InactiveCN104706583AIncrease contentChange distributionOrganic active ingredientsAntipyreticSide effectEmulsion
The invention provides an emulsion containing astilbin and a preparation method thereof. The emulsion comprises the components of an isotonic agent and a surfactant such as astilbin, water, oil as well as a stabilizing agent. The emulsion does not contain an organic solvent and a solubiliser with toxic and side effects, and has the advantages of no allergy and little side-effect.
Owner:张涛
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com