Traditional Chinese medicine active part for preventing chronic pelvic inflammation, and preparation method and applications thereof
A technology of chronic pelvic inflammatory disease and effective parts, applied in the field of effective parts of traditional Chinese medicine for the prevention and treatment of chronic pelvic inflammatory disease and its preparation, can solve problems such as difficulty in effective control of drug stability, imperfect control of quality standards, and unclear material basis of drug efficacy. To achieve the effect of good patient compliance, safe use, and clear active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 prevents and treats the Chinese medicine effective part preparation (tablet) of chronic pelvic inflammatory disease
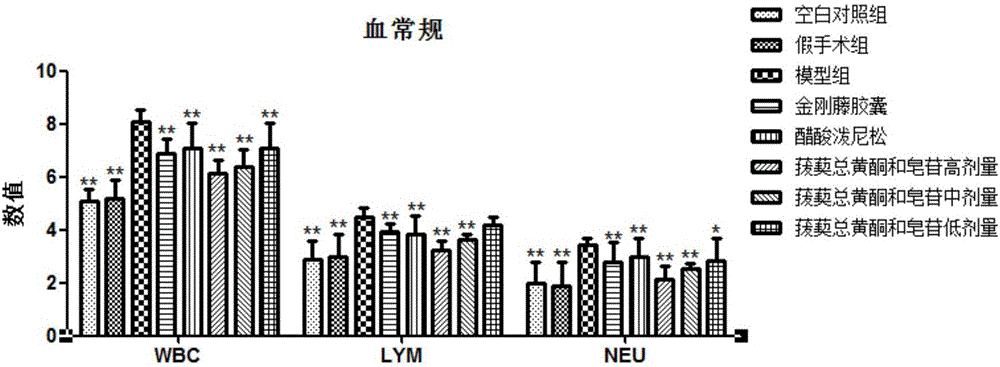
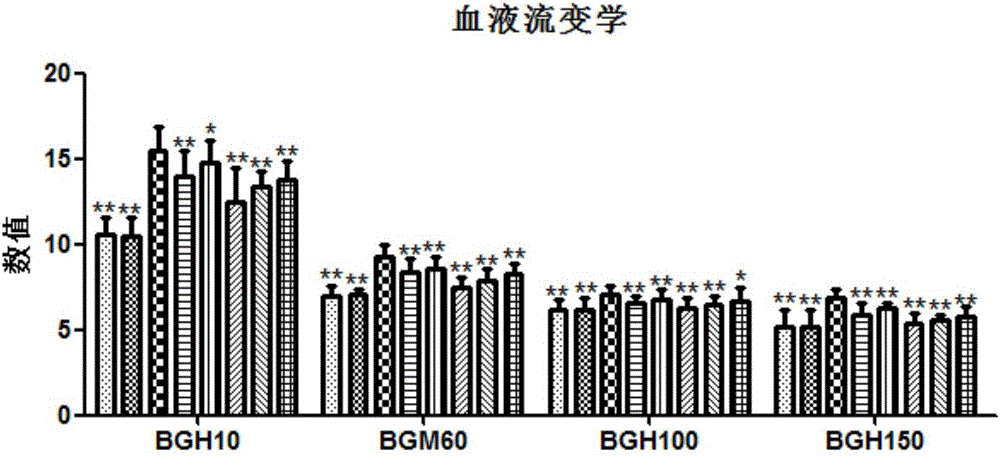
[0030] The effective part of the traditional Chinese medicine for preventing and treating chronic pelvic inflammatory disease in this embodiment includes the following components in terms of weight percentage: 12.23% astilbin, 6.82% astilbin, 10.39% isoastilbin, 17.39% astilbin, 17.32% astilagin, 5% diosgenin.
[0031] The preparation method of the effective part preparation of traditional Chinese medicine for preventing and treating chronic pelvic inflammatory disease of the present embodiment, comprises the following steps:
[0032] (1), 5 kg of the rhizome of Smilax smilax was crushed into coarse powder, and the crushed medicinal material was reflux extracted with 95% ethanol for 3 times, boiled for 1 hour each time, the extracts were filtered and combined, concentrated under reduced pressure until no alcohol smell, to obtain a concentrat...
Embodiment 2
[0038] The Chinese medicine effective part preparation (capsule) of embodiment 2 prevention and treatment of chronic pelvic inflammatory disease
[0039] The effective parts of the traditional Chinese medicine for preventing and treating chronic pelvic inflammatory disease in this embodiment include the following components in terms of weight percentage: 8.37% astilbin, 4.52% astilbin, 15.90% isoastilbin, 22.65% astilbin, 15.66% astilagin, 3.75% diosgenin.
[0040] The preparation method of the effective part preparation of preventing and treating chronic pelvic inflammatory disease of the present embodiment, comprises the following steps:
[0041](1) 5 kg of the rhizome of Smilax smilax was crushed into a coarse powder, and the crushed medicinal material was reflux extracted with 75% ethanol for 3 times, boiled for 1 hour each time, the extracts were filtered and combined, concentrated under reduced pressure until no alcohol smell, to obtain a concentrate;
[0042] (2), take...
Embodiment 3
[0047] The Chinese medicine effective part preparation (injection) of embodiment 3 prevention and treatment of chronic pelvic inflammatory disease
[0048] The effective part of the traditional Chinese medicine for preventing and treating chronic pelvic inflammatory disease in this embodiment includes the following components in terms of weight percentage: astilbin 16.11%, new astilbin 12.55%, isoastilbin 6.97%, new different 15.47% astilbin, 24.97% astilagin, 7.15% diosgenin.
[0049] The preparation method of the effective part preparation of traditional Chinese medicine for preventing and treating chronic pelvic inflammatory disease of the present embodiment, comprises the following steps:
[0050] (1), 5 kg of the rhizome of Smilax smilax was crushed into coarse powder, and the crushed medicinal material was reflux extracted with 95% ethanol for 3 times, boiled for 1 hour each time, the extracts were filtered and combined, concentrated under reduced pressure until no alcoh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com