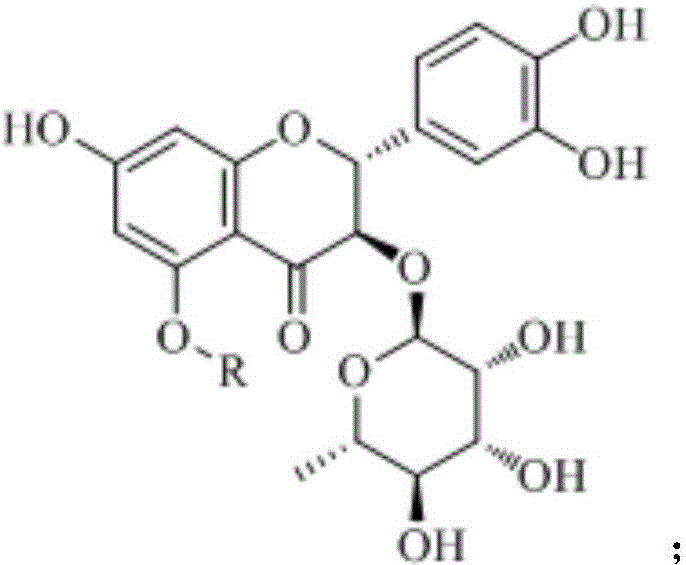
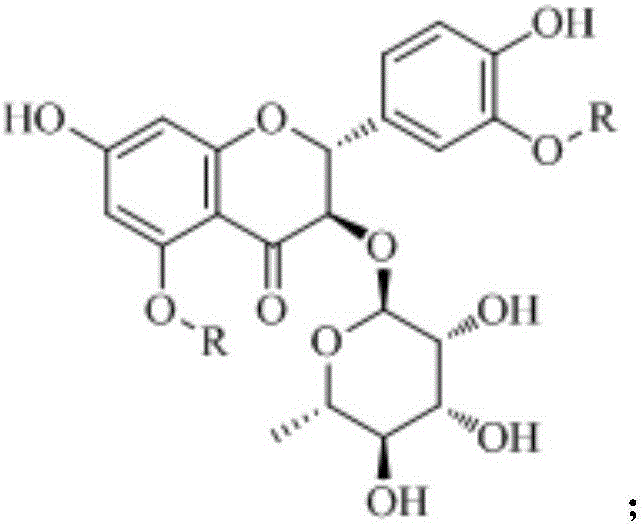
Astilbin derivatives and preparation method thereof
A technology of fucoside derivatives and astilbin, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve problems such as limited effect, and achieve the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
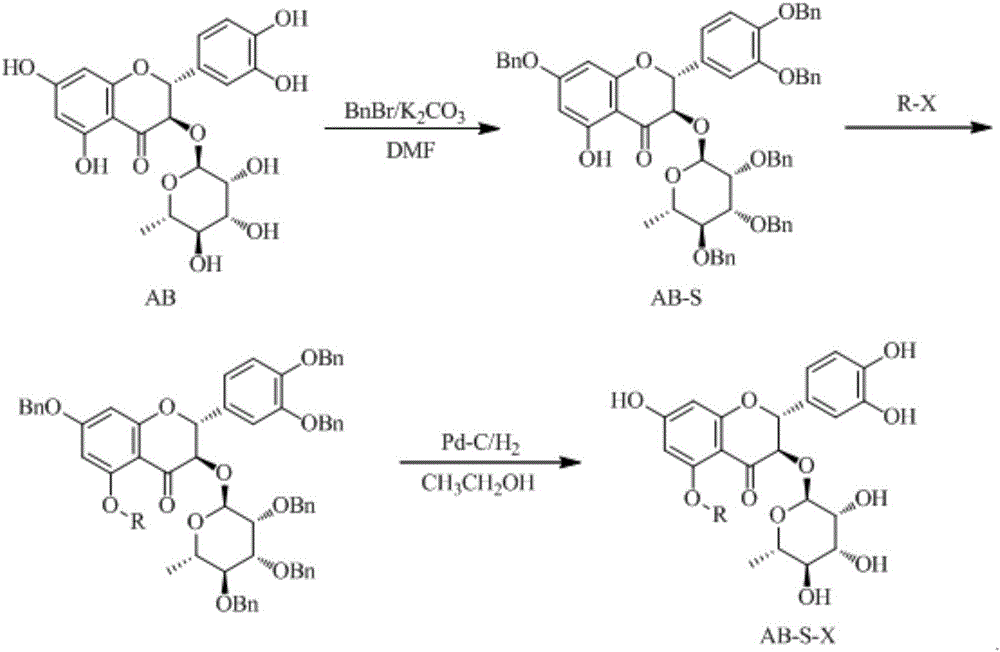
[0027] Embodiment 1: the preparation of compound AB-S
[0028] Add 1.0g of raw material AB, 3mL of N,N-dimethylformamide into the single-necked bottle to dissolve, add 2.37mL of Bian bromide, 2.7g of anhydrous K 2 CO 3 , Stir the reaction at 45°C for 3h. After the reaction, add 30mL water to the system, extract with ethyl acetate (20mL×3), that is, extract 3 times with 20mL each time; wash the organic phase with dilute hydrochloric acid (15mL×3), wash with water (20mL×3), and concentrate under reduced pressure To dryness, 0.65 g of light yellow solid was obtained.
Embodiment 2
[0029] Embodiment 2: the preparation of compound AB-D
[0030] Add 1.0g of raw material AB, 4mL of N,N-dimethylformamide into the single-necked bottle to dissolve, add 1.85mL of Bian bromide, 2.1g of anhydrous K 2 CO 3 , Stir the reaction at 25°C for 3h. After the reaction was completed, 30 mL of water was added to the system, extracted with ethyl acetate (20 mL×3), the organic phase was washed with dilute hydrochloric acid (15 mL×3), washed with water (20 mL×3), and concentrated to dryness under reduced pressure to obtain a light yellow solid 0.53 g.
Embodiment 3
[0031] Embodiment 3: the preparation of compound AB-S-1 (glucose derivative)
[0032] Add 1.0 g AB-S, 0.31 g bromoglucose, 10 mL CH 2 Cl 2 dissolved, adding anhydrous K 2 CO 3 1.0g, reflux at 50°C for 4h. After the reaction was complete, the reaction solution was spin-dried, then 30 mL of water was added, extracted with ethyl acetate (30 mL×3), washed with dilute hydrochloric acid (15 mL×3), and concentrated to dryness under reduced pressure to obtain 0.61 g of a light yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com