Patents
Literature
1003 results about "Disaccharide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A disaccharide (also called a double sugar or bivose) is the sugar formed when two monosaccharides (simple sugars) are joined by glycosidic linkage. Like monosaccharides, disaccharides are soluble in water. Three common examples are sucrose, lactose, and maltose.
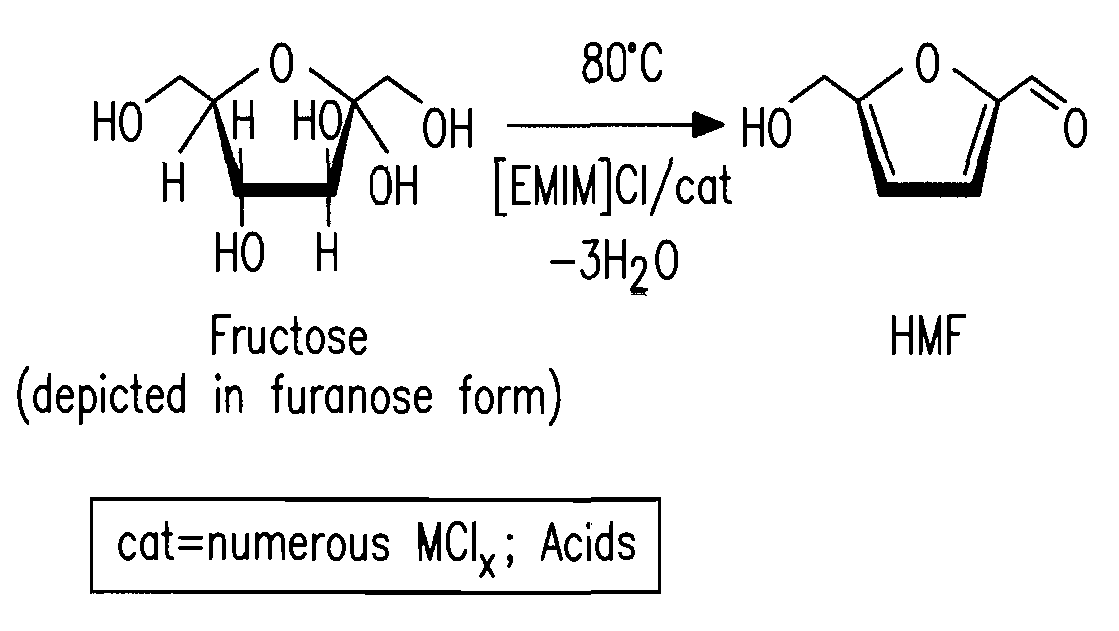
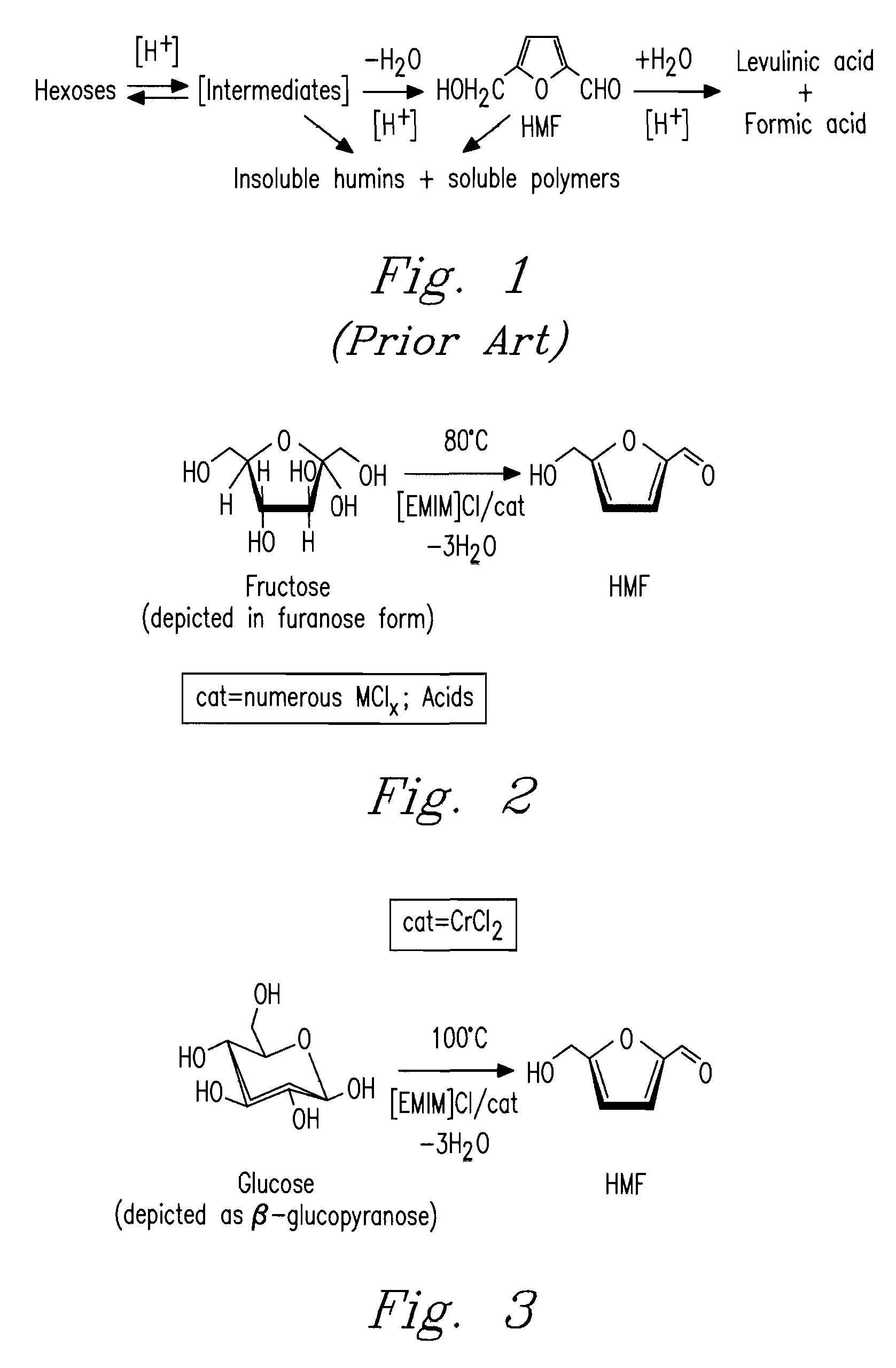
Methods for conversion of carbohydrates in ionic liquids to value-added chemicals
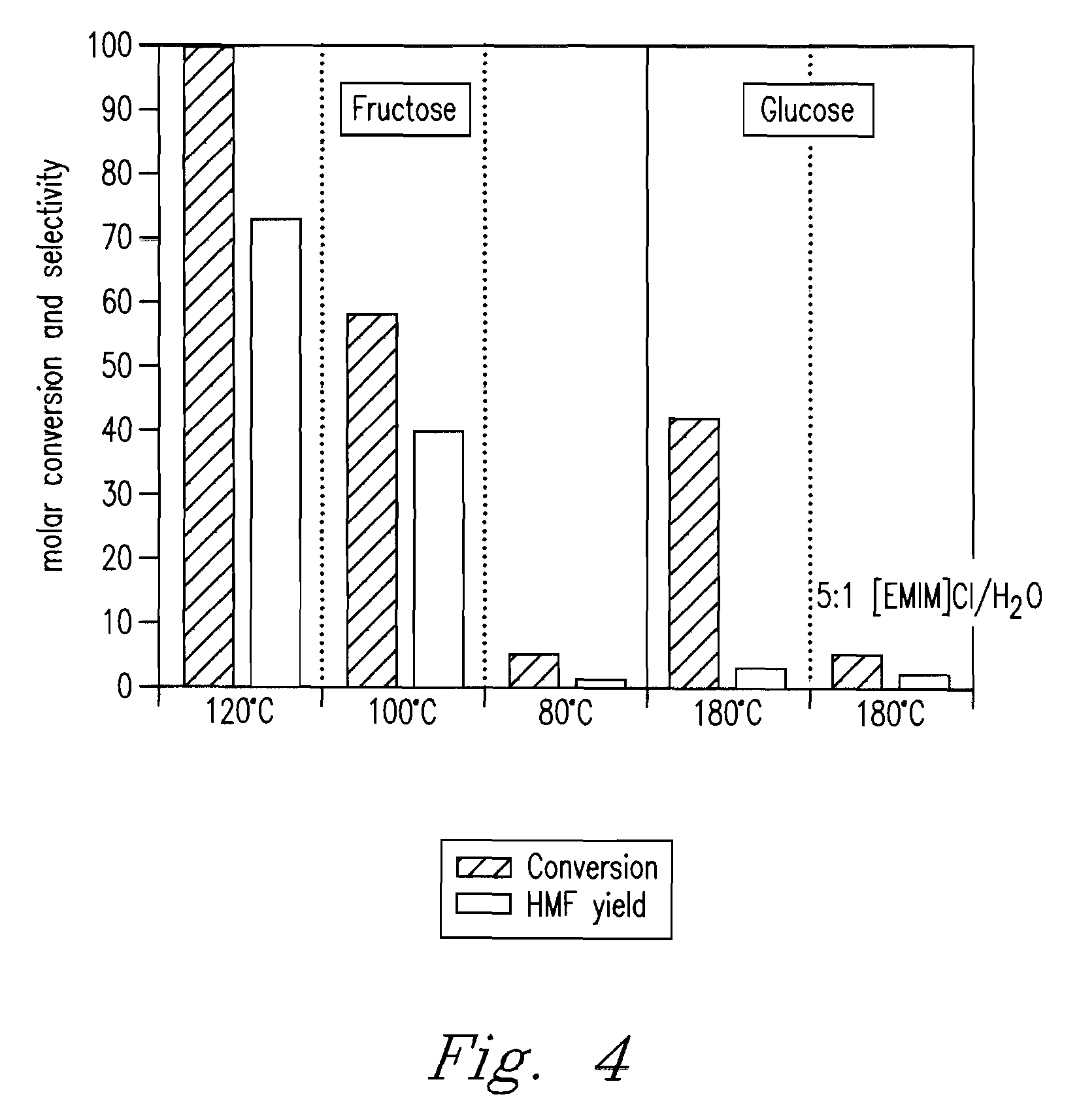
Methods are described for converting carbohydrates including, e.g., monosaccharides, disaccharides, and polysaccharides in ionic liquids to value-added chemicals including furans, useful as chemical intermediates and / or feedstocks. Fructose is converted to 5-hydroxylmethylfurfural (HMF) in the presence of metal halide and acid catalysts. Glucose is effectively converted to HMF in the presence of chromium chloride catalysts. Yields of up to about 70% are achieved with low levels of impurities such as levulinic acid.
Owner:BATTELLE MEMORIAL INST
Formulation of preservation mixtures containing sensitive biologicals to be stabilized for ambient temperature storage by drying
This invention relates to formulations and methods for preserving sensitive biologicals, viruses, bacteria and eukaryotic cells by drying. More particularly, the invention relates to preservation mixtures containing viruses or cells and protectants, including a combination of a methylated monosaccharide and a disaccharide, or oligosaccharide, wherein the mixtures are adapted to stabilize these during dehydration and subsequent storage at ambient and higher temperature.
Owner:AVANT IMMUNOTHERAPEUTICS
Liposome compositions of porphyrin photosensitizers
Liposomal pharmaceutical formulations incorporating porphyrin photosensitizers useful for photodynamic therapy or diagnosis of malignant cells. The liposomal formulations comprise a porphyrin photosensitizer, particularly the hydro-mono benzoporphyrins (BPD) having light absorption maxima in the range of 670-780 nanometers, a disaccharide or polysaccharide and one or more phospholipids.
Owner:QLT INC
Pharmaceutical Formulation and Process
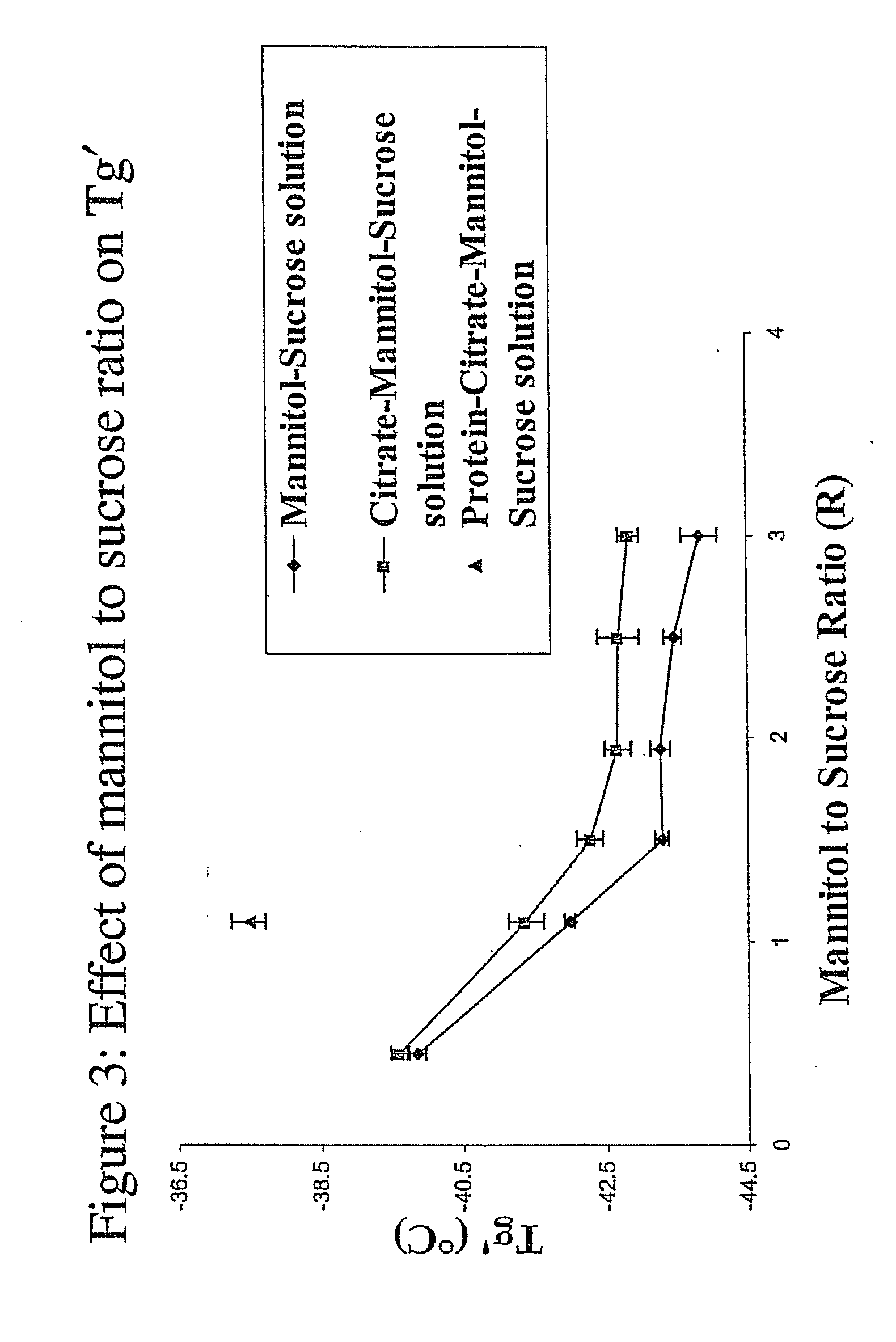
InactiveUS20070196364A1Increased Tg′Less-dramatic effectOrganic active ingredientsBiocideSucroseActive agent
A process for lyophilization or freeze-drying of a pharmaceutical product is provided and a liquid formulation suitable for lyophilization. In particular, a process for lyophilization or freeze-drying a liquid formulation that includes a protein active agent, a bulking agent and a saccharide stabilizing agent is provided. The saccharide to bulking agent ratio and the protein concentration of the formulation are important factors that affect crystallization of the bulking agent during lyophilization and storage as are some processing conditions. In one embodiment, the saccharide is a disaccharide, such as sucrose and the crystalline bulking agent is mannitol. The protein can be an antibody or a non-antibody protein.
Owner:HUMAN GENOME SCI INC
Novel Candy and Process for Producing the Same
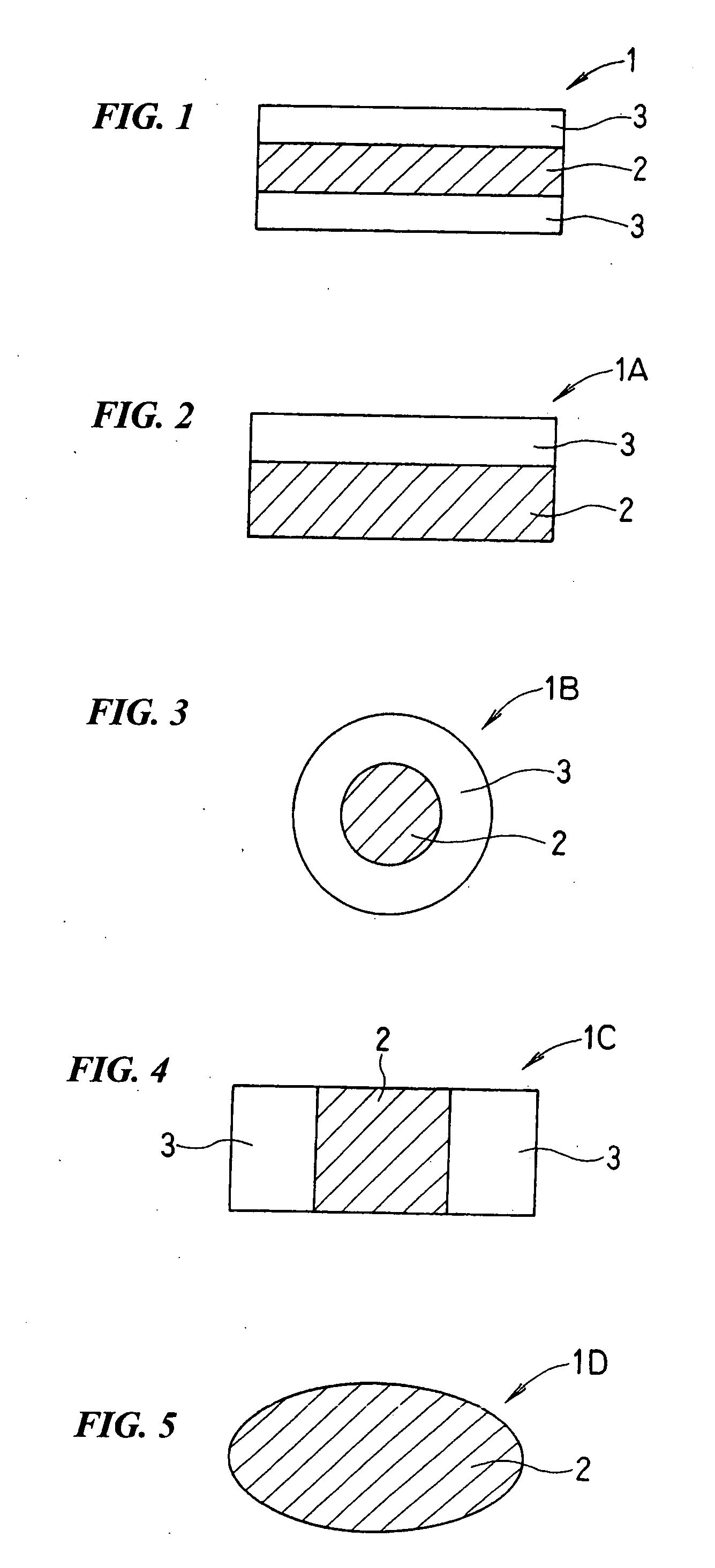
InactiveUS20090053390A1Low viscosityImprove liquidityConfectionerySweetmeatsAdditive ingredientAlcohol sugars
A delicious candy in which crystallized xylitol has been molded in a desired shape and which has a beautiful shape and gives a cooling feeling attributable to xylitol; and a process by which the candy can be easily and industrially continuously produced. The novel candy (1) has a layered constitution which comprises two opposed candy part layers (3) and, sandwiched between these, a xylitol crystal part layer (2) comprising xylitol as the main component, at least one ingredient selected among monosaccharides, disaccharides, and sugar alcohols obtained by reducing these, and a viscosity-lowering agent as essential components, the at least one ingredient and the viscosity-lowering agent being contained in given amounts based on the xylitol as the main component.
Owner:MONDELEZ JAPAN
Liposome compositions of porphyrin photosensitizers
InactiveUS6890555B1Good reproducibilityHydrate fastBiocideEnergy modified materialsPhotodynamic therapyPhotosensitizer
Liposomal pharmaceutical formulations incorporating porphyrin photosensitizers useful for photodynamic therapy or diagnosis of malignant cells. The liposomal formulations comprise a porphyrin photosensitizer, particularly the hydro-mono benzoporphyrine (BPD) having light absorption maxima in the range of 670-780 nanometers, a disaccharide or polysaccharide and one or more phospholipids.
Owner:QLT INC
Concentrated, water-soluble, granular plant growth regulator formulation and methods for use of same
Owner:VALENT BIOSCIENCES CORP
Stabilizing Excipient for Inactivated Whole Virus Vaccine
The invention relates to a vaccine composition comprising:a) inactivated whole virus, andb) a stabilizing excipient which comprises:i. a buffer solution,ii. a mixture of essential and nonessential amino acids,iii. a disaccharide,iv. a polyol,v. a chelating agent,vi. urea or a urea derivative, andvii. a nonionic surfactant.
Owner:SANOFI PASTEUR SA
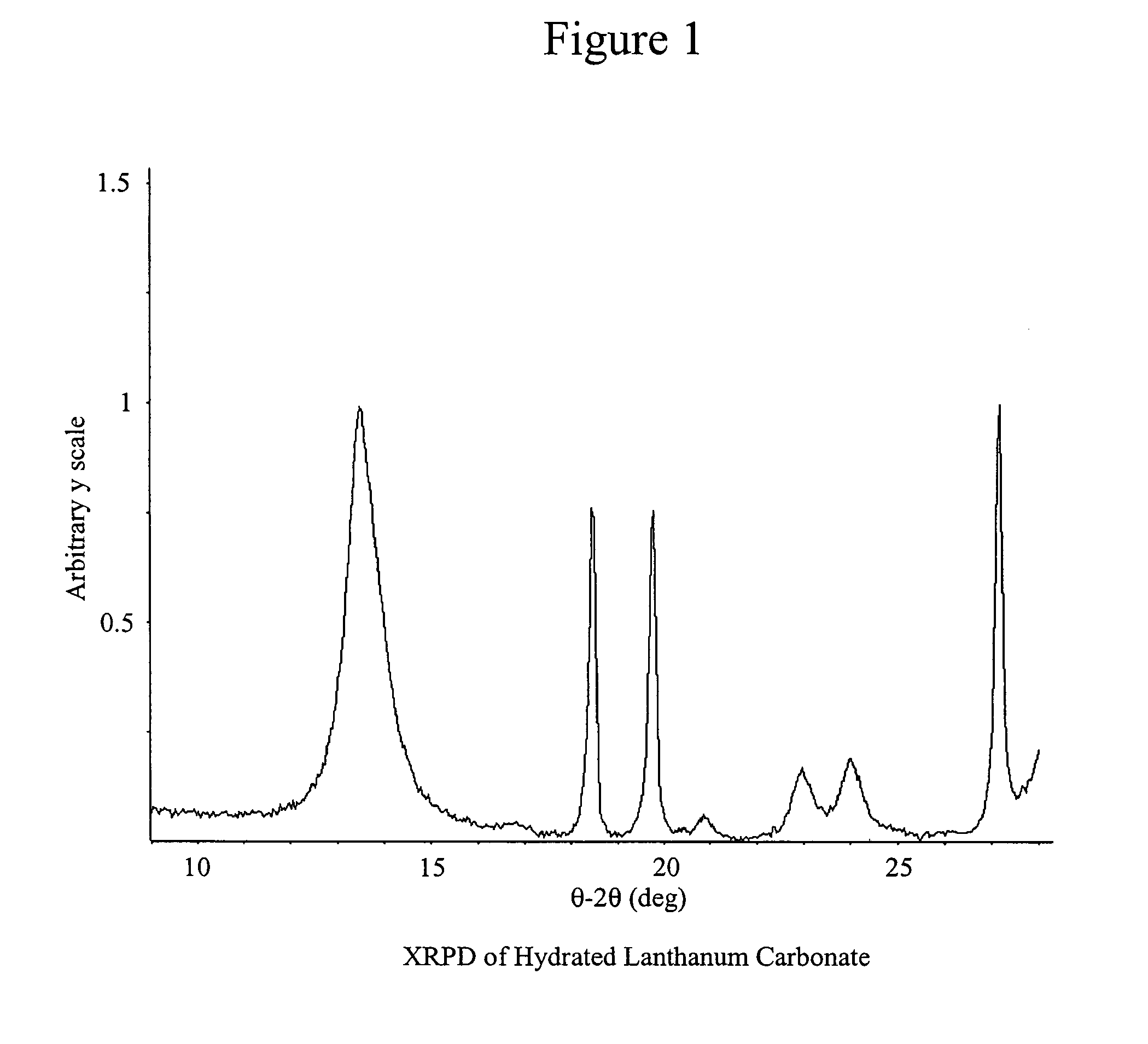
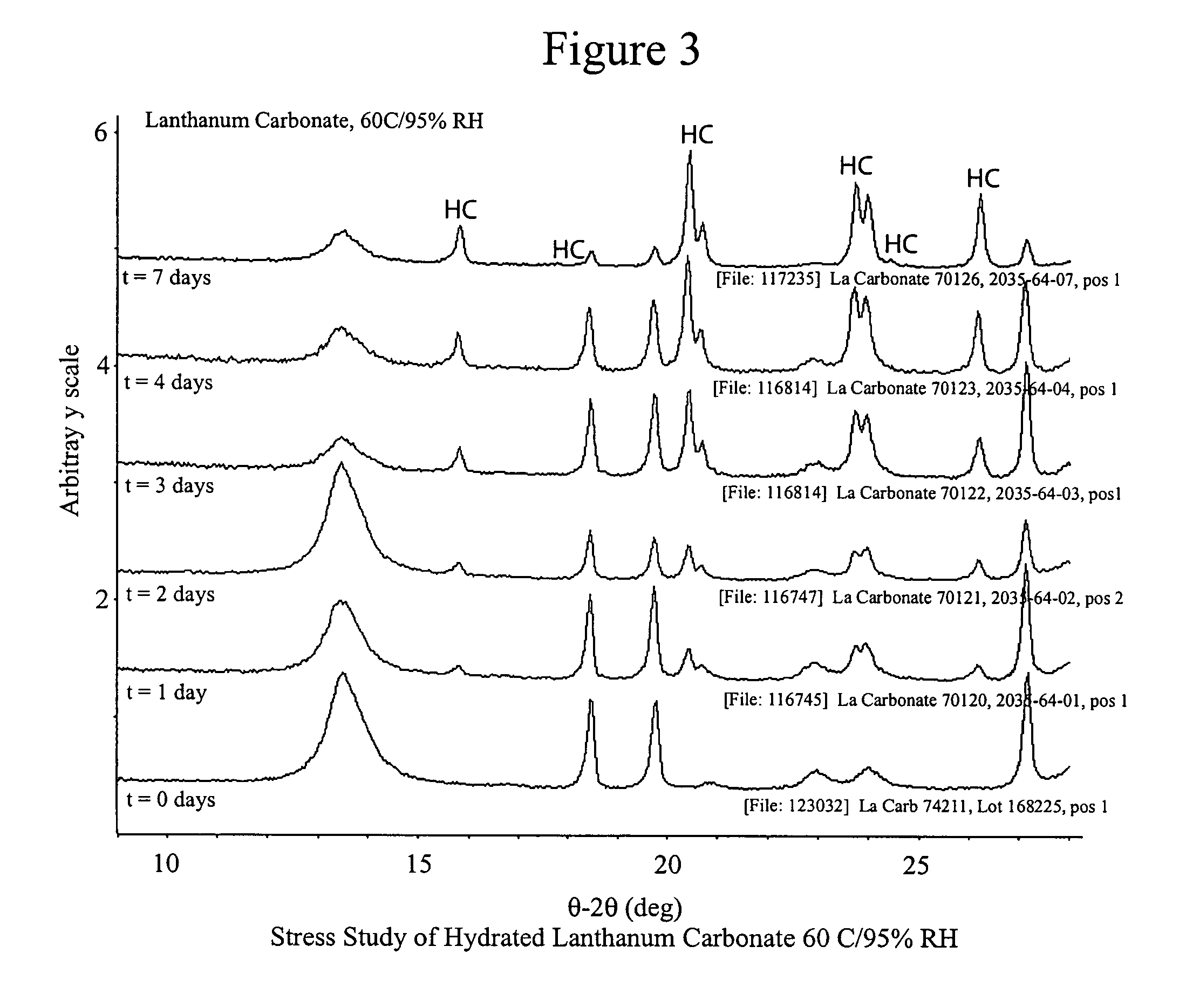
Stabilized lanthanum carbonate compositions
Owner:TAKEDA PHARMA CO LTD
Use of lipid conjugates in the treatment of diseases
InactiveUS7101859B2Reduce molecular weightIncrease rangeAntibacterial agentsBiocideLymphatic SpreadContact dermatitis
The invention provides novel methods for treating disease based upon the medicinal use of lipids and phospholipids covalently bound to physiologically acceptable monomers or polymers. Phosphatidylethanolamine moieties conjugated to physiologically acceptable monomers and polymers (PE conjugates) manifest an unexpectedly wide range of pharmacological effects, including stabilizing cell membranes; limiting oxidative damage to cell and blood components; limiting cell proliferation, cell extravasation and (tumor) cell migratory behavior; suppressing immune responses; and attenuating physiological reactions to stress, as expressed in elevated chemokine levels. The surprisingly manifold pharmacological properties of the PL-conjugates allow for the invention, disclosed herein, of novel methods for the treatment of a diverse range of disease states, including obstructive respiratory disease, including asthma; colitis and Crohn's disease; central nervous system insult, including blood brain barrier compromise, ischemic stroke, and multiple sclerosis; contact dermatitis; psoriasis; cardiovascular disease, including ischemic conditions and prophylaxis for invasive vascular procedures; cellular proliferative disorders, including anti-tumor vasculogenesis, invasiveness, and metastases; anti-oxidant therapy; hemolytic syndromes; sepsis; acute respiratory distress syndrome; tissue transplant rejection syndromes; autoimmune disease; viral infection; and hypersensitivity conjunctivitis. The therapeutic methods of the invention include administration of phosphatidylethanolamine bound to carboxymethylcellulose, heparin, hyaluronic acid, polyethylene glycol, and Polygeline (haemaccel). Disclosed herein are also new compounds comprised of phospholipid moieties bound to low molecular weight monomers and dimers, including mono- and disaccharides, carboxylated disaccharides, mono- and dicarboxylic acids, salicylates, bile acids, and fatty acids.
Owner:YEDGAR SAUL
Use of monosaccharides and disaccharides as complete replacements for synthetic fixative and styling polymers in hair styling products
InactiveUS20090074697A1Remarkable hard-holdReduced flaking characteristicCosmetic preparationsHair cosmeticsCopolymerDisaccharide
Owner:HENKEL KGAA
Qualitative and quantitative analysis method for polyoses
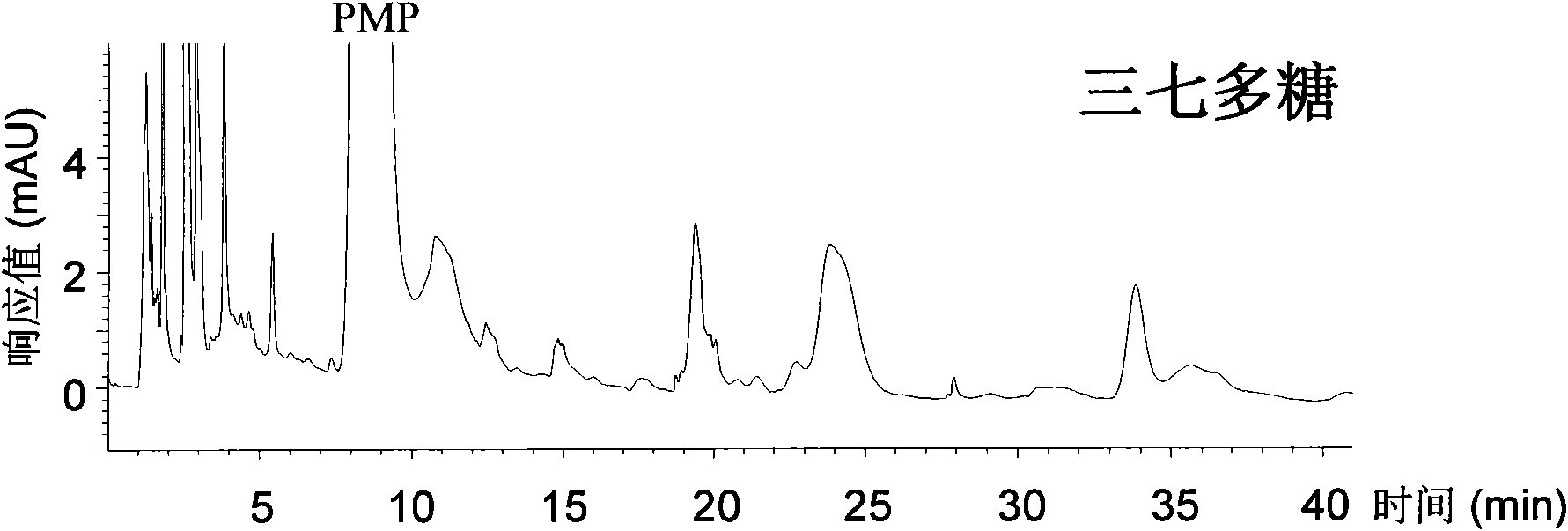
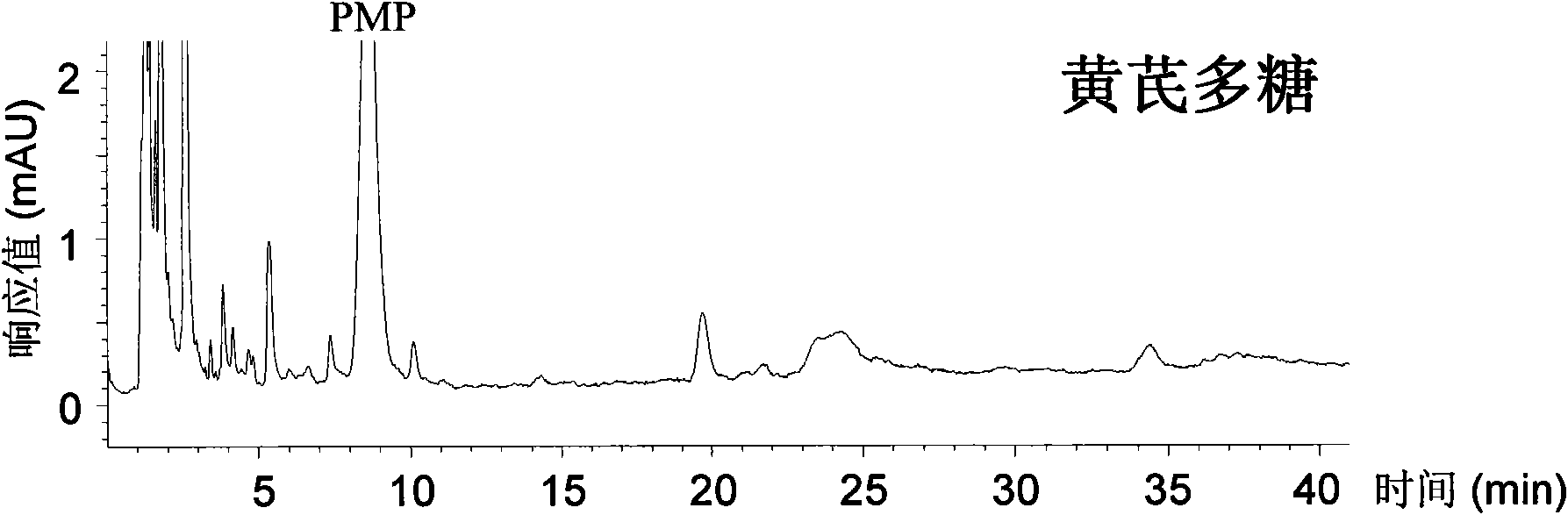
ActiveCN101539550AEasy to separateImprove featuresComponent separationMaterial analysis by observing effect on chemical indicatorAdditive ingredientUronic acid
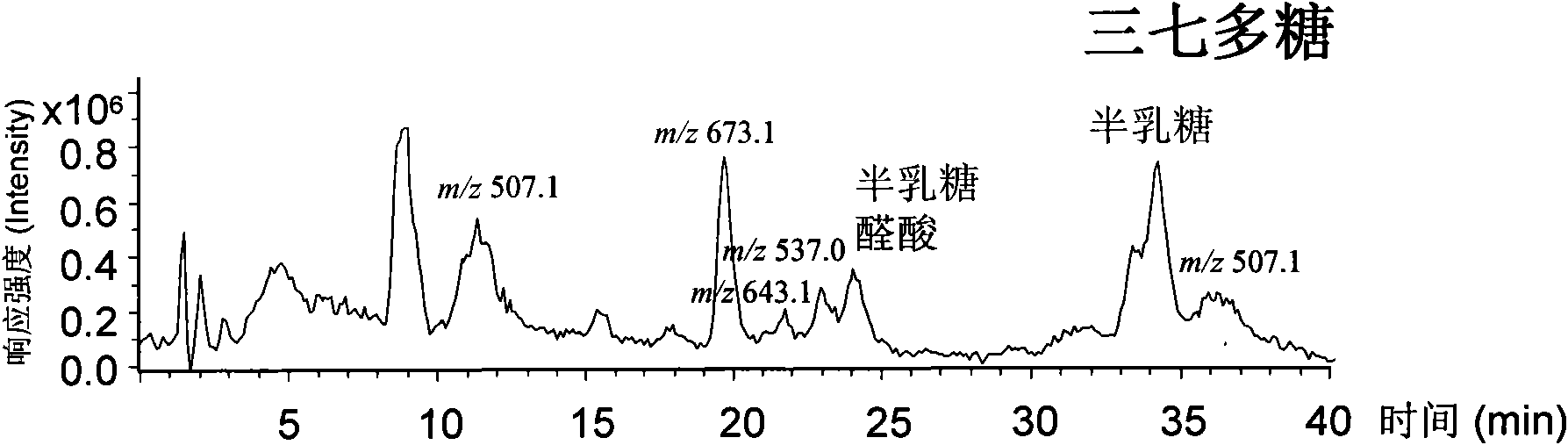
The invention relates to a qualitative and quantitative analysis method for polyoses, which comprises the following steps: (1) the zymohydrolysis and the verification of the polyoses: measuring the content of a polyoses water solution by a colorimetric method; (2) comparing the change of a polyoses mapping before and after zymohydrolysis by HPSEC-ELSD analysis; (3) saccharide ingredient direct HPLC analysis of a polyoses zymohydrolysis solution or HPLC analysis after pre-column derivatization: identifying polyoses mapping and chromatogram peaks by taking a standard monosaccharose, a uronic acid, a disaccharide, and the like as contrasts and taking a chromatogram peak mass-to-charge ratio as reference, and using stable characteristic sections as a quantitative analysis index; and (4) realizing the qualitative and quantitative analysis of the polyoses by establishing polyoses zymohydrolysis responding characteristics and polyoses zymohydrolysis mapping which are based on structural information through the independent or combining application of the steps. The qualitative and quantitative analysis method can be used for the identification and quantitative measurement of the polyoses of traditional Chinese medicine, such as panax, panax pseudoginseng, gen-seng, aweto, cordyceps, ganoderma lucidum, fomes japonica, milk veteh, angelica, and the like and provides an effective method for controlling the quality of the polyoses and the products thereof.
Owner:李绍平
Synthetic heparin pentasaccharides
InactiveUS7541445B2Simple ideaImpact successEsterified saccharide compoundsOrganic active ingredientsHeparinoidsTetrasaccharide
Preparation of synthetic monosaccharides, disaccharides, trisaccharides, tetrasaccharides and pentasaccharides for use in the preparation of synthetic heparinoids.
Owner:DR REDDYS LAB SA
Special gestation compound feed for sows at later stage of pregnancy and preparation method thereof
InactiveCN102919570AReduce the incidence of constipationReduce laborFood processingAnimal feeding stuffConstipationFetus
The invention discloses a special gestation compound feed for sows at later stage of pregnancy. The special gestation compound feed is prepared from the following raw materials: corn, bran, rice bran meal, DDGS (dried distiller grains with soluble), swelling soya dregs, swelling soybean flour, fermented soybean meal, compound sugar, mountain flour, calcium hydrogen phosphate, choline chloride, common salt, compound premix, L-lysine hydrochloride, L-threonine, acidifier, clostridium butyricum and yeast cell wall polysaccharides. The invention, starts from nutrition of the sows at the later stage of the pregnancy, gives consideration to the growth of fetuses and the physiological characteristics of the sows per se, designs daily ration with high protein, high amino acid and low energy, adds the compound sugar constituted by different monosaccharides and disaccharides, and can provide energy which can be utilized quickly and improve the birth weight and the uniformity of piglets; and by adding the clostridium butyricum and the yeast cell wall polysaccharides, the special gestation compound feed can improve the intestinal health of the sows, improve immunity, regulate intestinal peristalsis, reduce the constipation incidence of the sows, shorten the labor stage of the sows, reduce the incidence rate of mastitis and endometritis of the sows, and assist the sows in rapid postpartum recovery.
Owner:HUAIAN ZHENGCHANG FEED
Compositions and methods for regulation of active TNF- alpha
InactiveUS6020323AReduced activityReduced availabilityEsterified saccharide compoundsBiocideSulfationMedicine
Substances comprising disaccharides and substances comprising carboxylated and / or sulfated oligosaccharides in substantially purified form, and methods of using same, are disclosed for the regulation of cytokine activity in a host. For instance, the secretion of active Tumor Necrosis Factor Alpha (TNF- alpha ) can be either inhibited or augmented selectively by administration to the host of an effective amount of a substance of the invention. Thus, the present invention also relates to pharmaceutical compositions and their use for the prevention and / or treatment of pathological processes involving the induction of active cytokine secretion, such as TNF- alpha . The invention also relates to the initiation of a desirable immune system-related response by the host to the presence of activators, including pathogens. The substances and pharmaceutical compositions of the present invention may be administered daily, at very low effective doses, typically below 0.1 mg / kg human, or at intervals of up to about 5-8 days, preferably once a week.
Owner:YEDA RES & DEV CO LTD
Sugar reduction of food products
ActiveUS20180146699A1Hydrolytic activity was lowHigh activityConfectionerySweetmeatsFood materialSugar
A process for reducing the monosaccharide and / or disaccharide content in a food material, the process comprising contacting the food material with a glucosyltransferase that comprises an amino acid sequence having at least 95% identity to SEQ ID NO: 1.
Owner:SOC DES PROD NESTLE SA
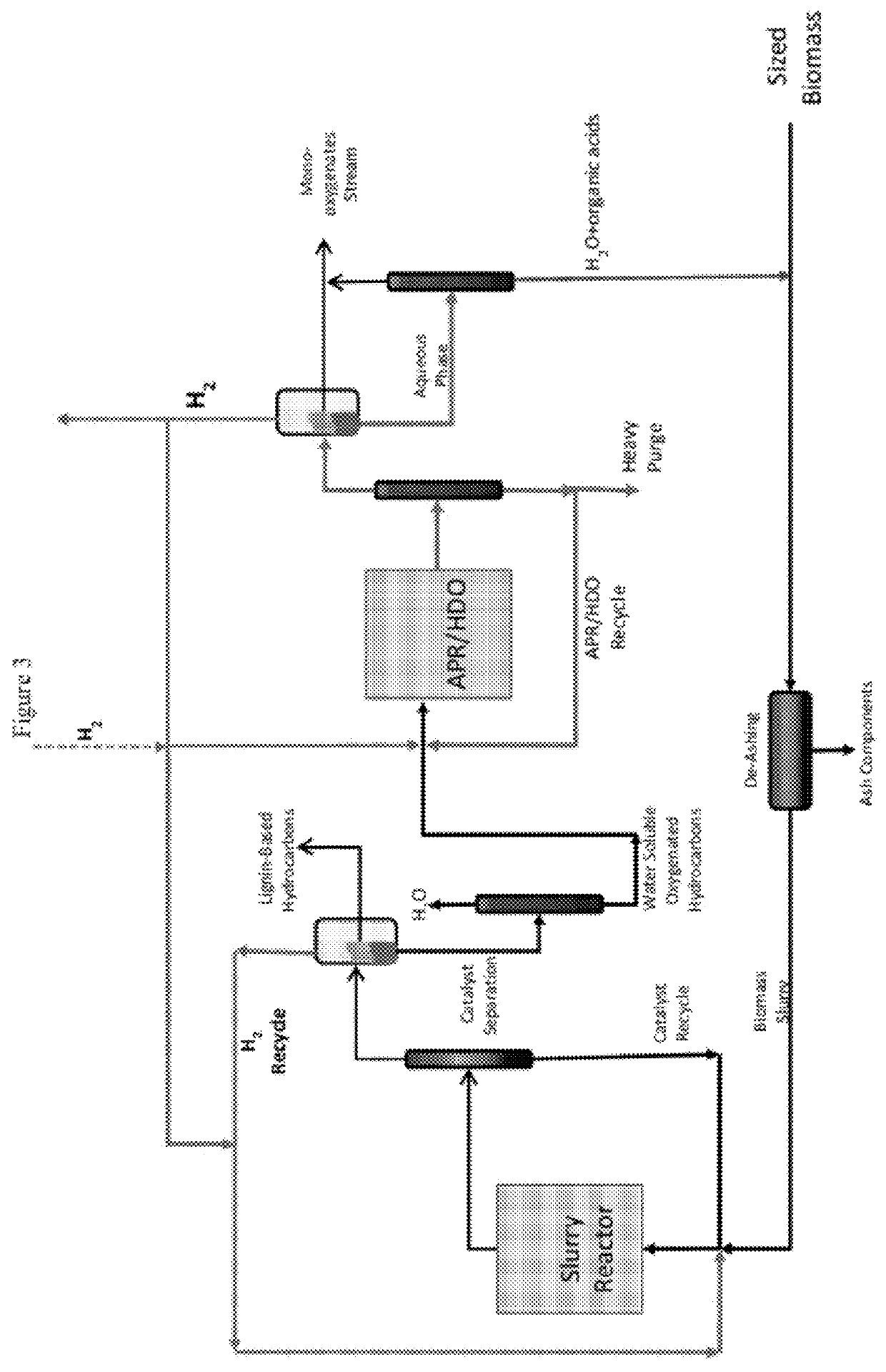
Reductive biomass liquefaction
ActiveUS20120172579A1Oxygen-containing compound preparationLiquid hydrocarbon mixture productionCyclic etherBiofuel
The present invention provides methods, reactor systems, and catalysts for converting in a continuous process biomass to less complex oxygenated compounds for use in downstream processes to produce biofuels and chemicals. The invention includes methods of converting the components of biomass, such as hemicellulose, cellulose and lignin, to water-soluble materials, including lignocellulosic derivatives, cellulosic derivatives, hemicellulosic derivatives, carbohydrates, starches, polysaccharides, disaccharides, monosaccharides, sugars, sugar alcohols, alditols, polyols, diols, alcohols, ketones, cyclic ethers, esters, carboxylic acids, aldehydes, and mixtures thereof, using hydrogen and a heterogeneous liquefaction catalyst.
Owner:VIRENT
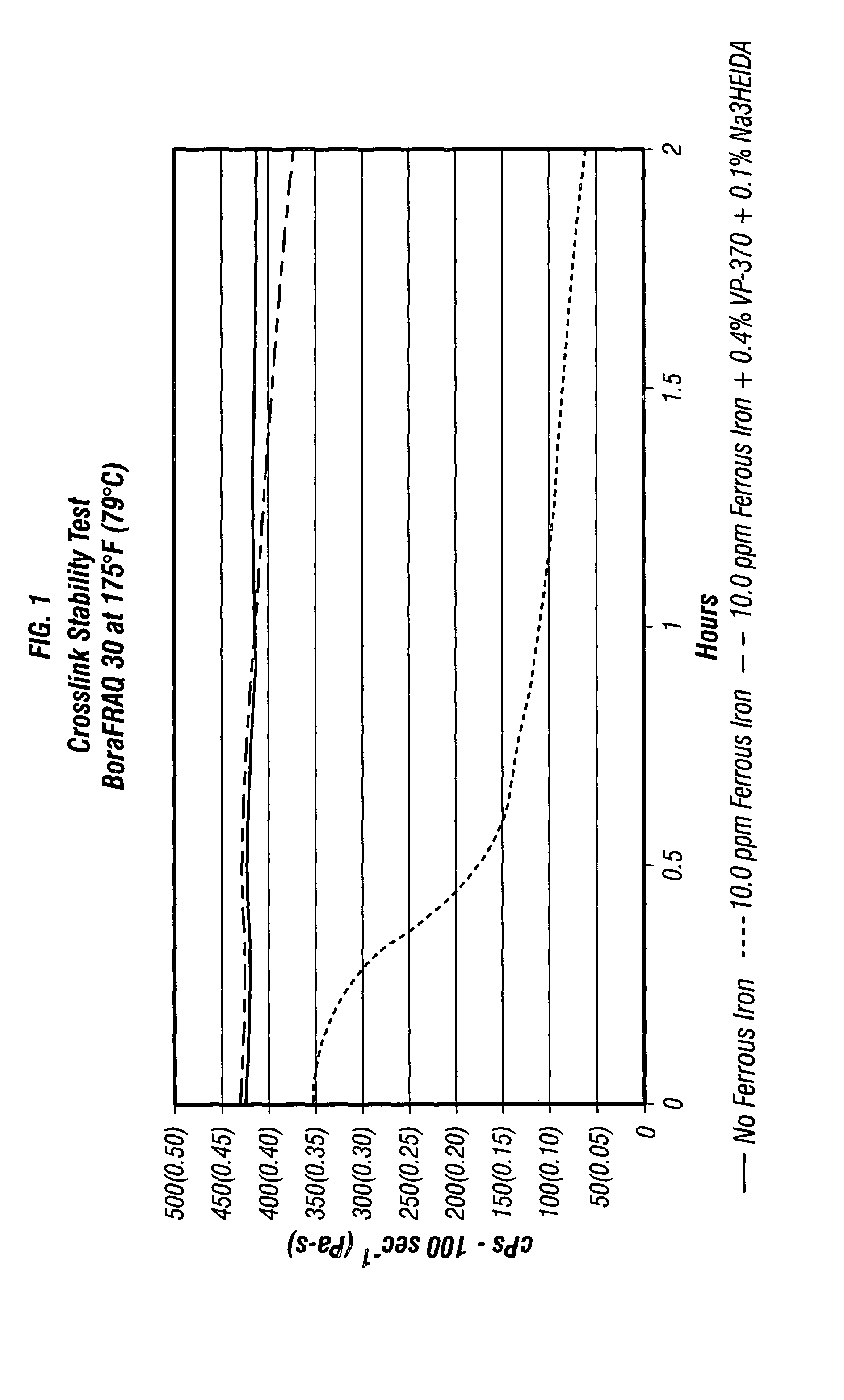
Biodegradable chelant compositions for fracturing fluid
InactiveUS7078370B2Improved fracture propertyImprove propertiesFluid removalFlushingFracturing fluidAlcohol sugars
It has been discovered that biodegradable and non-toxic chelant compositions can perform multiple beneficial functions in an aqueous fracturing fluid through the chelation of ions. Some of the multiple functions include various combinations of the following: demulsifier, demulsifier enhancer, scale inhibitor, crosslink delay agent, crosslinked gel stabilizer, enzyme breaker stabilizer, and the like. Some of the chelants used in the compositions include, but are not necessarily limited to, sodium polyaspartate; sodium iminodisuccinate; disodium hydroxyethyleneiminodiacetate (Na2HEIDA); sodium gluconate; sodium glucoheptonate; sugar alcohols; monosaccharides; disaccharides; and mixtures thereof.
Owner:SUPERIOR ENERGY SERVICES LLC
Composition for coating frozen confectionery and a process for manufacturing same
ActiveUS20180092378A1Reduce the amount requiredHigh viscosityFrozen sweetsCocoaGlucose-Fructose SyrupWater activity
The invention relates to a composition for coating a frozen confection, the composition comprising 10 to 50 wt. % dry glucose syrup with a DE (Dextrose Equivalent) below 40, and a total amount of mono and di-saccharides below 10 wt. %, and a water activity below 1.0 and 35 to 70 wt. % fat. The invention also relates to a process for making the composition.
Owner:SOC DES PROD NESTLE SA
Fat emulsion providing improved health and taste characteristics in food
InactiveUS20120053251A1Low in fatHigh emulsionBiocideEdible oils/fats with reduced calorie/fat contentFiberAntioxidant
The embodiments relate to fat emulsion structures based on both an aqueous and non-aqueous glycerin component as the primary aqueous component in which the fat emulsion can create a wide range of viscosities that mimic fat structures similar to cream, or all the way to hardened fat structures like Trans Fat. The fat emulsion can be added to a wide group of foods that use a monosaccharide or disaccharide as the basis for its sweetener component, can lower the sugar content of foods, can improve mouth feel while lowering the fat content in high fat foods, can add a balance of dietary fats and fiber to foods, and can add antioxidant content to food products.
Owner:ANTIOXIDANT SUPERFOODS
Cross-linked hyaluronic acid cell-scaffold material and preparation method and application
ActiveCN104225677APromote regenerationProvide temporary protectionOrganic active ingredientsSurgical drugsCross-linkFreeze-drying
The invention relates to a cross-linked hyaluronic acid cell-scaffold material and its preparation method and application. The cross-linked hyaluronic acid cell-scaffold material is obtained by crosslinking of high-molecular hyaluronate and low-molecular hyaluronate. The proportion of hyaluronate disaccharide molecules which take part in the crosslinking is 0.5%-20%, and expansion rate of the hyaluronate disaccharide molecules in an isotonic solution is 80%-110%. The preparation method of the cell-scaffold material comprises two freeze drying steps. Hyaluronate mixed with a cross-linking agent is formed firstly; after a heating reaction, water is added for swelling to form gel; and freeze drying is then carried out to obtain the porous scaffold material. The scaffold has abundant pores, has a certain mechanical strength and pore size and has good hydroscopicity and biocompatibility. The scaffold can be used as a tissue engineering cell-scaffold in promoting cartilage injury repairing and also can be used for preparing a haemostasis anti-adhesion material.
Owner:INST OF BIOPHARM OF SHANDONG PROVINCE
Feeding combination for brooding layers before six-week old
ActiveCN102578426AReduce stressImprove liquidityAnimal feeding stuffAccessory food factorsAnti stressPhytase
The invention provides a feeding combination for brooding layers before six-week old, which includes a feeding I for layers before two-week old and a feeding II for layers between three to six-week old, wherein the feeding I comprises corn, puffed grains, puffed soybean meal, puffed soybeans, fermented soybean meal, insect protein, one level steam fish meal, plant oil, monosaccharide or disaccharides, calcium hydrogen phosphate, limestone powder, baking soda, compound trace elements, amino acid, choline chloride, salt, anti-stress agent, multi-vitamin, compound enzyme preparation and phytase raw materials; and the feeding II comprises corn, soybean meal, fermented soybean meal, puffed soybeans, insect protein, one level steam fish meal, plant oil, monosaccharide or disaccharides, calcium hydrogen phosphate, limestone powder, baking soda, compound trace elements, amino acid, choline chloride, salt, anti-stress agent, multi-vitamin, compound enzyme preparation and phytase. Compared with the existing layer feeding, the phase combination feeding is more reasonable and scientific, and the immunity and the survive rate of layers are obviously improved and the morbidity is obviously decreased.
Owner:山东众成饲料科技有限公司
Delivery vehicle for probiotic bacteria comprising a dry matrix of polysaccharides, saccharides and polyols in a glass form and methods of making same
Owner:ADVANCED BIONUTRITION CORP
Ophthalmic and contact lens solutions containing simple saccharides as preservative enhancers
InactiveUS20070098818A1Effective preservationDegree of reductionBiocideHydroxy compound active ingredientsTagatoseSucrose
The present invention relates to an ophthalmic solution comprising 0.00001 to 10.0 weight percent of a simple saccharide, at least 0.00001 weight percent of a preservative, and not more than about 0.2 percent by weight chloride. The simple saccharide is chosen from the group consisting of: inositol; mannitol; sorbitol; sucrose; dextrose; glycerin; propylene glycol; ribose; triose; tetrose; erythrose; threose; pentose; arabinose; ribulose; xylose; xylulose; lyxose; hexose; allose; altrose; fructose; galactose; glucose; gulose; idose; mannose; sorbose; talose; tagatose; adlose; ketose; heptose; sedoheptulose; monosaccharides; disaccharides; sugar alcohols; xylitol; and polyol.
Owner:FXS VENTURES LLC
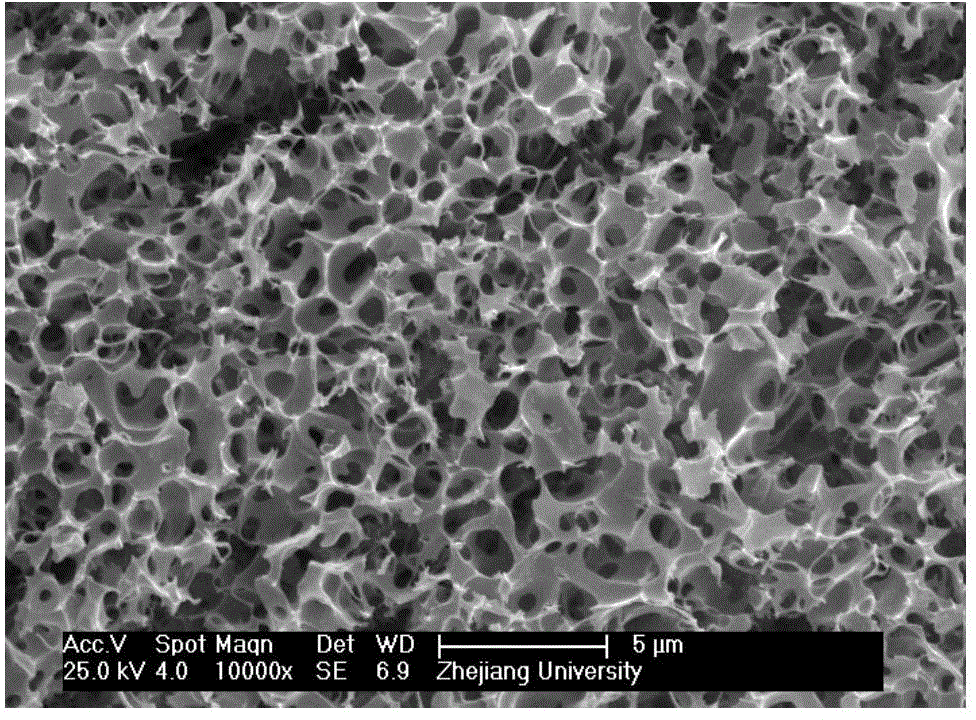
Preparation method and product of multistage porous carbon material
The invention discloses a preparation method of a multistage porous carbon material. The preparation method comprises the following steps: blending a carbon source with an activator, and performing two-step carbonization and after treatment to obtain the multistage porous carbon material, wherein the two-step carbonization comprises low-temperature carbonization and high-temperature carbonization, the temperature of the low-temperature carbonization is 200-400 DEG C, and the temperature for the high-temperature carbonization is 800-1200 DEG C; the carbon source is a biomass, monosaccharide, disaccharide or polysaccharide of which the cellulose content is more than 20%; and the activator is selected from ammonium oxalate, potassium oxalate, potassium hydrogen oxalate, potassium tetroxalate, sodium oxalate, sodium hydrogen oxalate, sodium tetroxalate, sodium hydrogen carbonate or potassium hydrogen carbonate. The invention provides the preparation method of the multistage porous carbon material rich in macropore, and according to the preparation method, physical expansion and chemical activation are utilized, and a carbon source is used as a raw material to match with a specific activator in order to prepare the multistage porous carbon material rich in macropore; and the raw material is low in price and easy to obtain, the method is simple and strong in sustainability and has a potential for large-scale production.
Owner:ZHEJIANG UNIV
Superparamagnetic Nanoparticles Based on Iron Oxides with Modified Surface, Method of Their Preparation and Application
InactiveUS20090309597A1Less loadImprove abilitiesPigmenting treatmentMaterial nanotechnologyArginineDextran
The subject of the invention is superparamagnetic nanoparticle probes based on iron oxides, to advantage magnetite or maghemite, with modified surface, coated with mono-, di- or polysaccharides from the group including D-arabinose, D-glucose, D-galactose, D-mannose, lactose, maltose, dextrans and dextrins, or with amino acids or poly(amino acid)s from the group including alanine, glycine, glutamine, asparagine, histidine, arginine, L-lysine, aspartic and glutamic acid or with synthetic polymers based on (meth)acrylic acid and their derivatives selected from the group containing poly(N,N-dimethylacrylamide), poly(N,N-dimethylmethacrylamide), poly(N,N-diethylacrylamide), poly(N,N-diethylmethacrylamide), poly(N-isopropylacrylamide), poly(N-isopropylmethacrylamide), which form a colloid consisting of particles with narrow distribution with polydispersity index smaller than 1.3, the average size of which amounts to 0.5-30 nm, to advantage 1-10 nm, the iron content is 70-99.9 wt. %, to advantage 90 wt. %, the modification agent content 0.1-30 wt. %, to advantage 10 wt. %.The particles of size smaller than 2 nm with polydispersity index smaller than 1.1 can be obtained by a modified method of preparation.Superparamagnetic nanoparticle probes according to the invention are prepared by pre-precipitation of colloidal Fe(OH)3 by the treatment of aqueous 0.1-0.2M solution of Fe(III) salt, to advantage FeCl3, with less than an equimolar amount of NH4OH, at 21° C., under sonication, to which a solution of a Fe(II) salt, to advantage FeCl2, is added in the mole ratio Fe(III) / Fe(II)=2 under sonication and the mixture is poured into five- to tenfold, to advantage eightfold, molar excess of 0.5M NH4OH. The mixture is left aging for 0-30 min, to advantage 15 min, and then the precipitate is repeatedly, to advantage 7-10 times, magnetically separated and washed with deionized water. Then 1-3 fold amount, to advantage 1.5 fold amount, relative to the amount of magnetite, of 0.1 M aqueous solution of sodium citrate is added and then, dropwise, 1-3 fold amount, to advantage 1.5 fold amount, relative to the amount of magnetite, of 0.7 M aqueous solution of sodium hypochlorite. The precipitate is repeatedly, to advantage 7-10 times, washed with deionized water under the formation of colloidal maghemite to which, after dilution, is added dropwise, to advantage under 5-min sonication, an aqueous solution of a modification agent, in the weight ratio modification agent / iron oxide=0.1-10, to advantage 0.2 for amino acids and poly(amino acid)s and 5 for saccharides.The particles smaller than 2 nm with polydispersity index smaller than 1.1 are prepared by mixing at 21° C. 1 volume part of 10-60 wt. %, to advantage 50 wt. %, of an aqueous solution of a saccharide, disaccharide or polysaccharide, such as D-arabinose, D-glucose, D-galactose, D-mannose, lactose, maltose, dextran and dextrins, and 1 volume part of aqueous solution of a Fe(II) and Fe(III) salt, to advantage FeCl2 and FeCl3, where the molar ratio Fe(III) / Fe(II)=2. A 5-15%, to advantage 7.5%, solution of NH4OH is added until pH 12 is attained and the mixture is heated at 60° C. for 15 min. The mixture is then sonicated at 350 W for 5 min and then washed for 24 h by dialysis in water using a membrane with molecular weight cut-off 14,000 until pH 7 is reached. The volume of solution is reduced by evaporation so that the final dry matter content is 50-100 mg / ml, to advantage 80 mg per 1 ml.Superparamagnetic nanoparticle probes according to the invention can be used for labelling cells used in magnetic resonance imaging for monitoring their movement, localization, survival and differentiation especially in detection of pathologies with cell dysfunction and of tissue regeneration and also for labelling and monitoring cells administered for cell therapy purposes, in particular embryonal stem cells, fetal stem cells, stem cells of an adult human including bone marrow stem cells, olfactory glial cells, fat tissue cells, in the recipient organism by magnetic resonance.The preparation of labelled cells proceeds by adding to the complete culture medium 5-20 μl, to advantage 10 μl, of a colloid containing 0.05-45 mg iron oxide per ml, to advantage 1-5 mg iron oxide per ml of the medium, and culturing the cells for a period of 1-7 days, to advantage for 1-3 days, at 37° C. and 5% of CO2.
Owner:INST OF MACROMOLECULAR CHEM ASCR V V I +1
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
ActiveUS7094545B2Improve manufacturing speedIncrease speed and capacityOrganic active ingredientsPeptide/protein ingredientsSolid Dose FormBULK ACTIVE INGREDIENT
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient, and to a method for manufacturing thereof. The invention relates to a pharmaceutical composition as a solid dosage form comprising desmopressin, or a pharmaceutically acceptable salt thereof, as a therapeutically active ingredient together with a pharmaceutically acceptable excipient, diluent or carrier, or mixture thereof, wherein at least one of said excipient, diluent and carrier is a substance selected from a monosaccharide, disaccharide, oligosaccharide and a polysaccharide, wherein the said substance has an average particle size in the range of from 60 to 1,000 μm. A method according to the present invention provides an improved production of solid dosage forms of desmopressin.
Owner:FERRING BV
Base-facilitated production of hydrogen from biomass
InactiveUS20050163704A1Improve thermodynamic spontaneityReduce the temperatureProductsElectrolysis componentsCelluloseBy-product
A base-facilitated reaction for the production of hydrogen from biomass or component(s) thereof. Hydrogen is produced from a reaction of naturally occurring organic matter with a base to form a bicarbonate or carbonate compound as a by-product. The base-facilitated hydrogen-producing reactions are thermodynamically more spontaneous than conventional-type reformation reactions and are able to produce hydrogen gas at less extreme reaction conditions than conventional-type reformation reactions. The preferred reactants are biomass, components of biomass, and mixtures of components of biomass. Especially preferred are base-facilitated reactions in which hydrogen is produced from carbohydrates or mixtures of carbohydrates, include monosaccharides, disaccharides, polysaccharides, and cellulose. The instant reactions can occur in the liquid phase or solid.
Owner:OVONIC BATTERY CO INC
Polysaccharide containing botulinum toxin pharmaceutical compositions and uses thereof
InactiveUS7758873B2High potencyImprove stabilityCosmetic preparationsOrganic active ingredientsToxinPolysaccharide
This invention relates to the use of a composition comprising a polysaccharide and a botulinum toxin for reducing a skin wrinkle. In some embodiments, the polysaccharide comprises disaccharides. In some embodiments, the average molecular weight of a disaccharide unit of the polysaccharide is between about 345 D and about 1,000 D.
Owner:ALLERGAN INC
Alpha-selective sialyl phosphate donors for preparation of sialosides and sialoside arrays for influenza virus detection
ActiveUS8507660B2Esterified saccharide compoundsSaccharide with carbocyclic radicalsHemagglutininCancer cell
A novel N-acetyl-5-N,4-O-carbonyl-protected dibutyl sialyl phosphate donor for sialylation of both primary and sterically hindered secondary acceptors to prepare sialosides with high yield and α-selectivity is disclosed. Methods for making disaccharide building blocks comprising α(2→3), α(2→6), α(2→8), α(2→8) / α(2→9) alternate, and α(2→9) sialosides are provided. methods for one-pot synthesis of complex sialosides are disclosed. Libraries of sialosides and methods for using the libraries for detection and receptor binding analysis of surface glycoproteins or pathogens and cancer cells are disclosed. Methods for distinguishing between hemagglutinin (HA) from various strains of influenza are provided.
Owner:ACAD SINIC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com