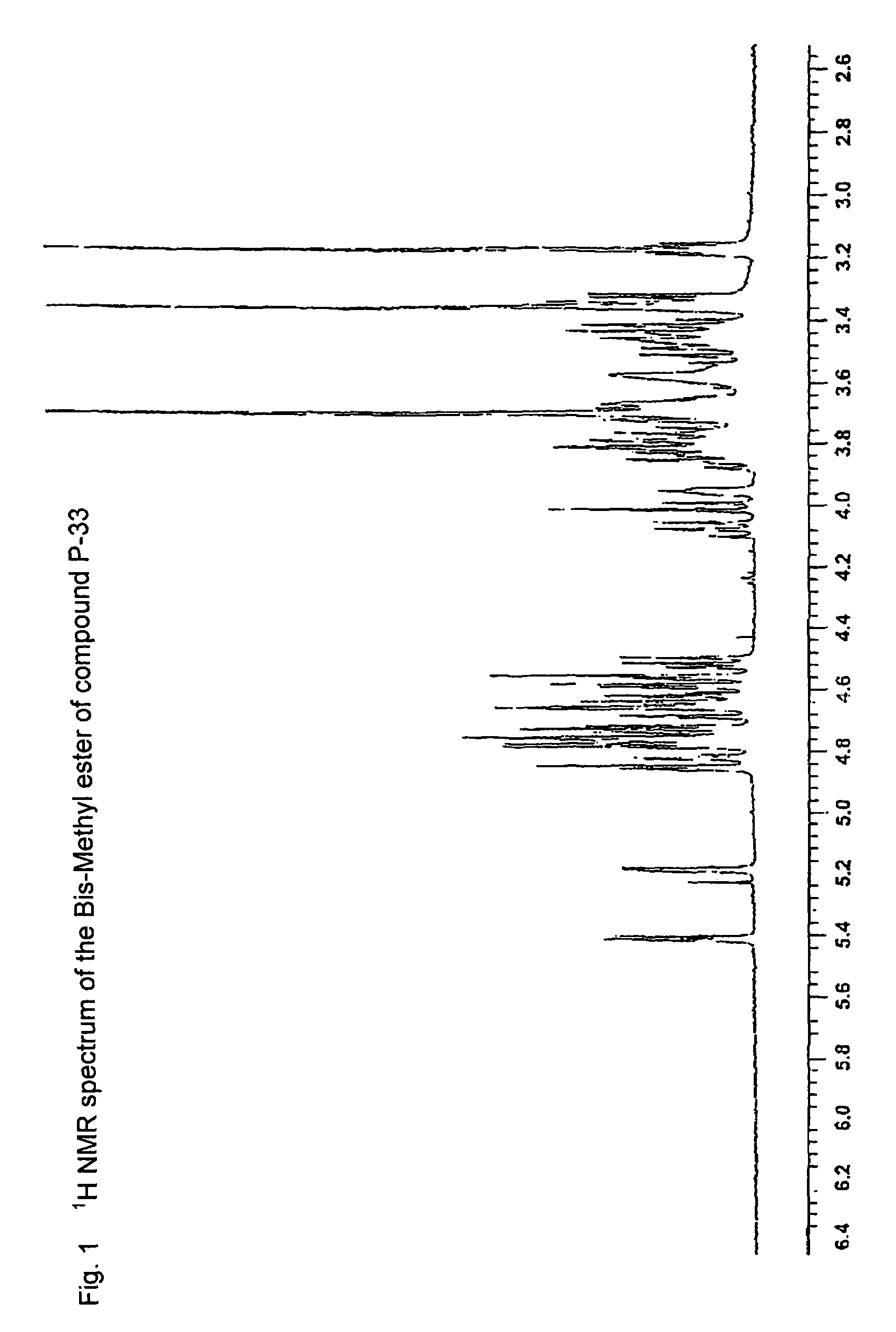
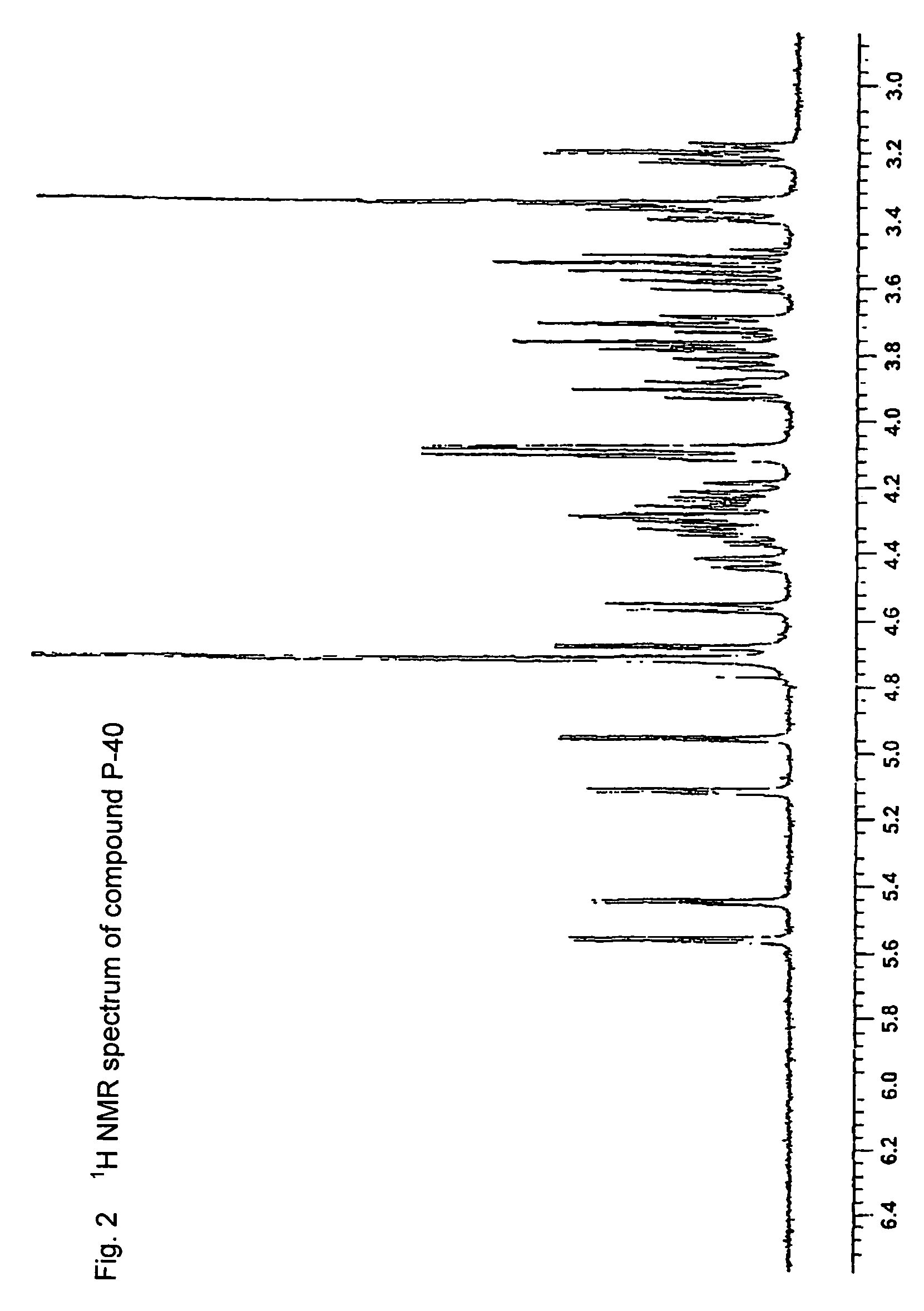
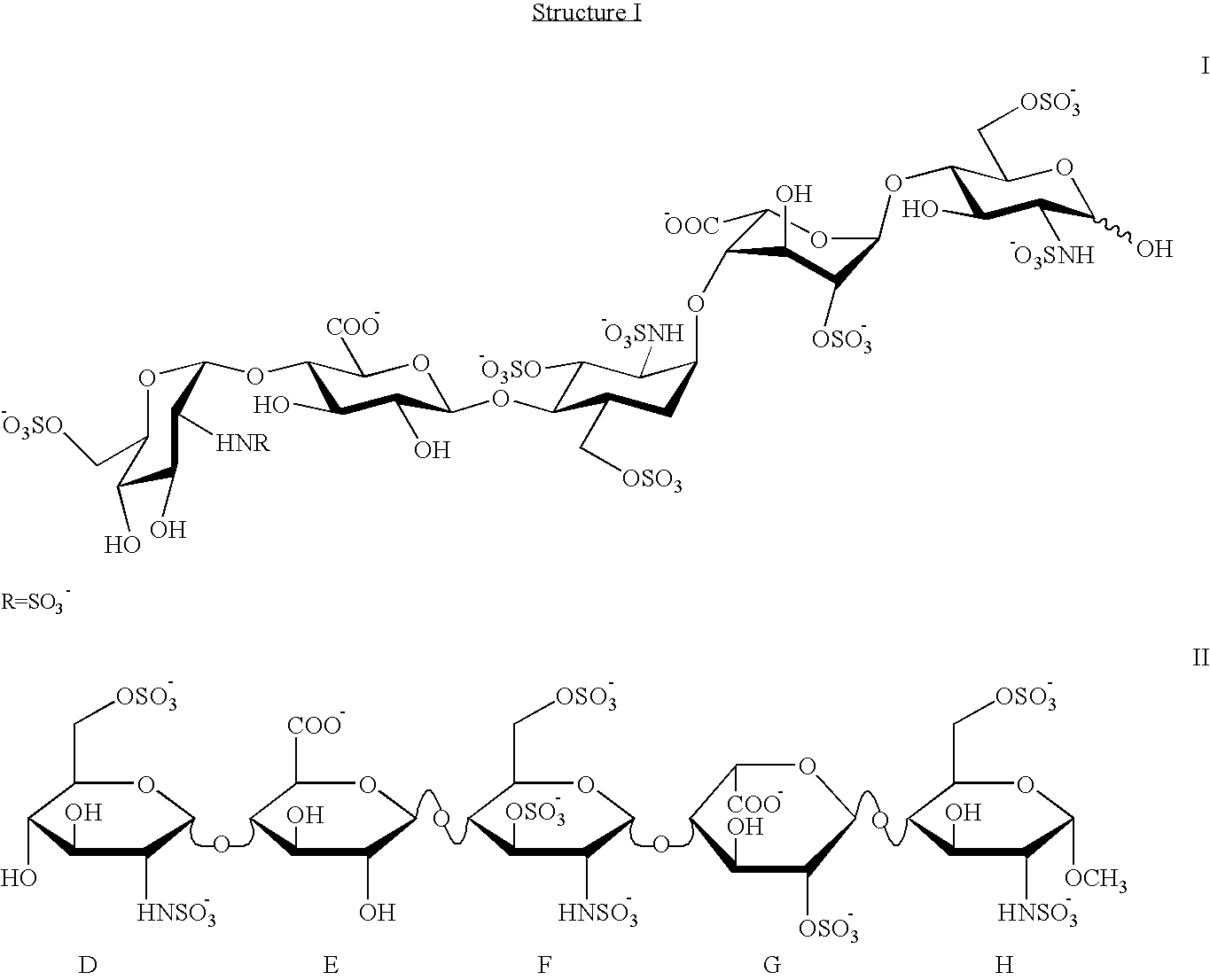
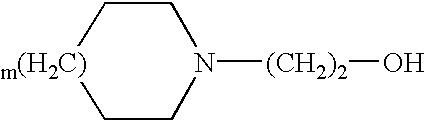
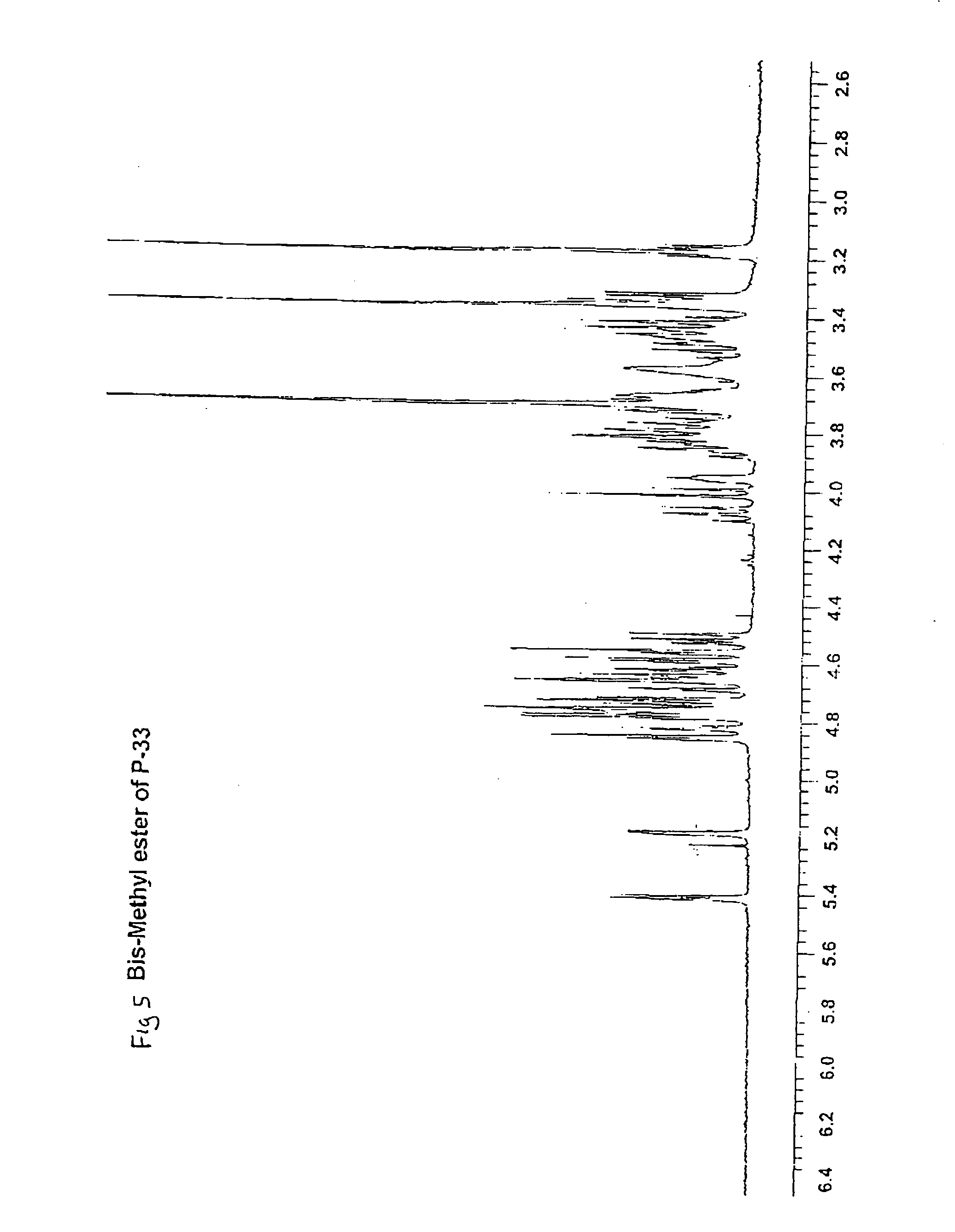
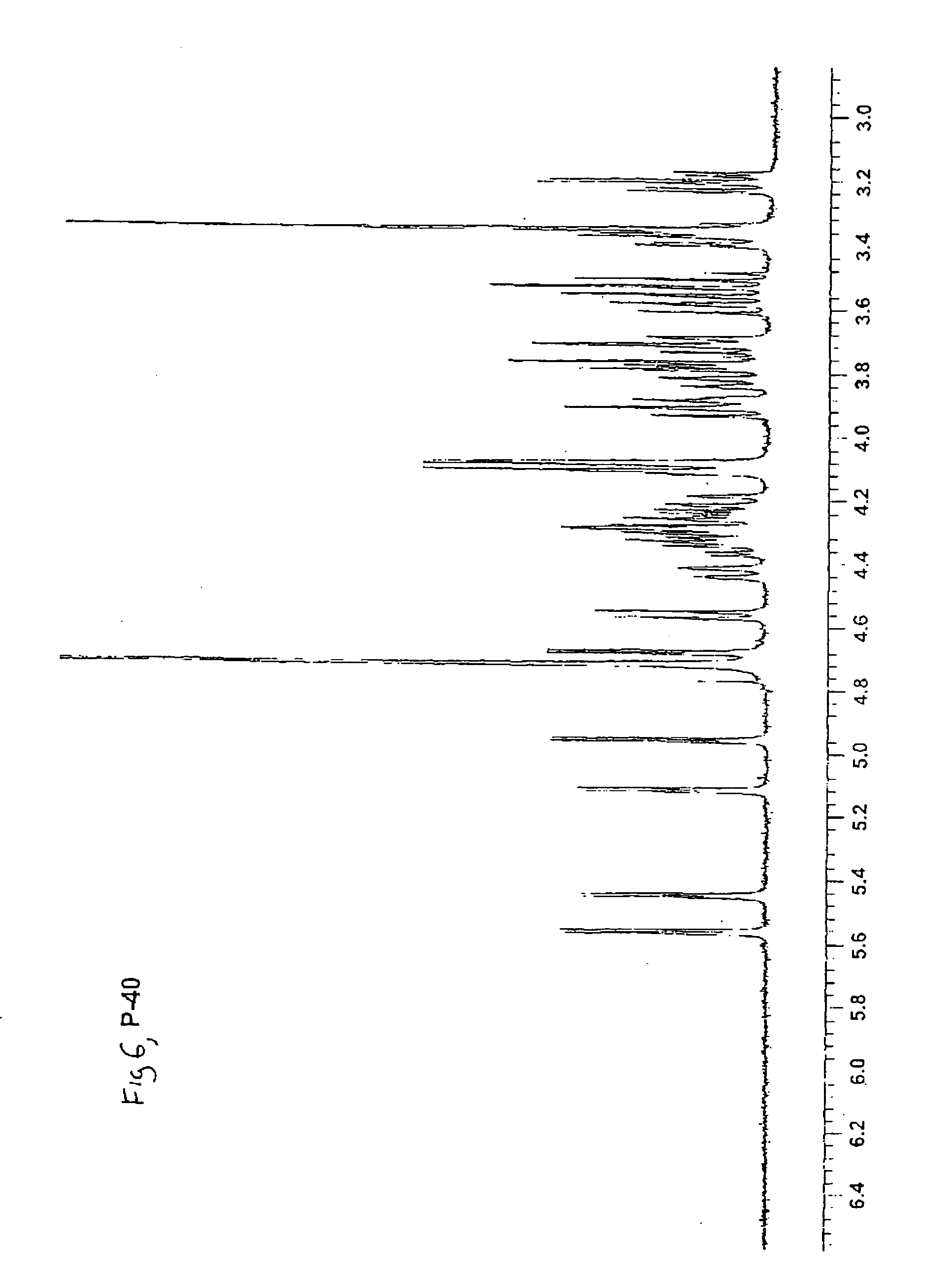
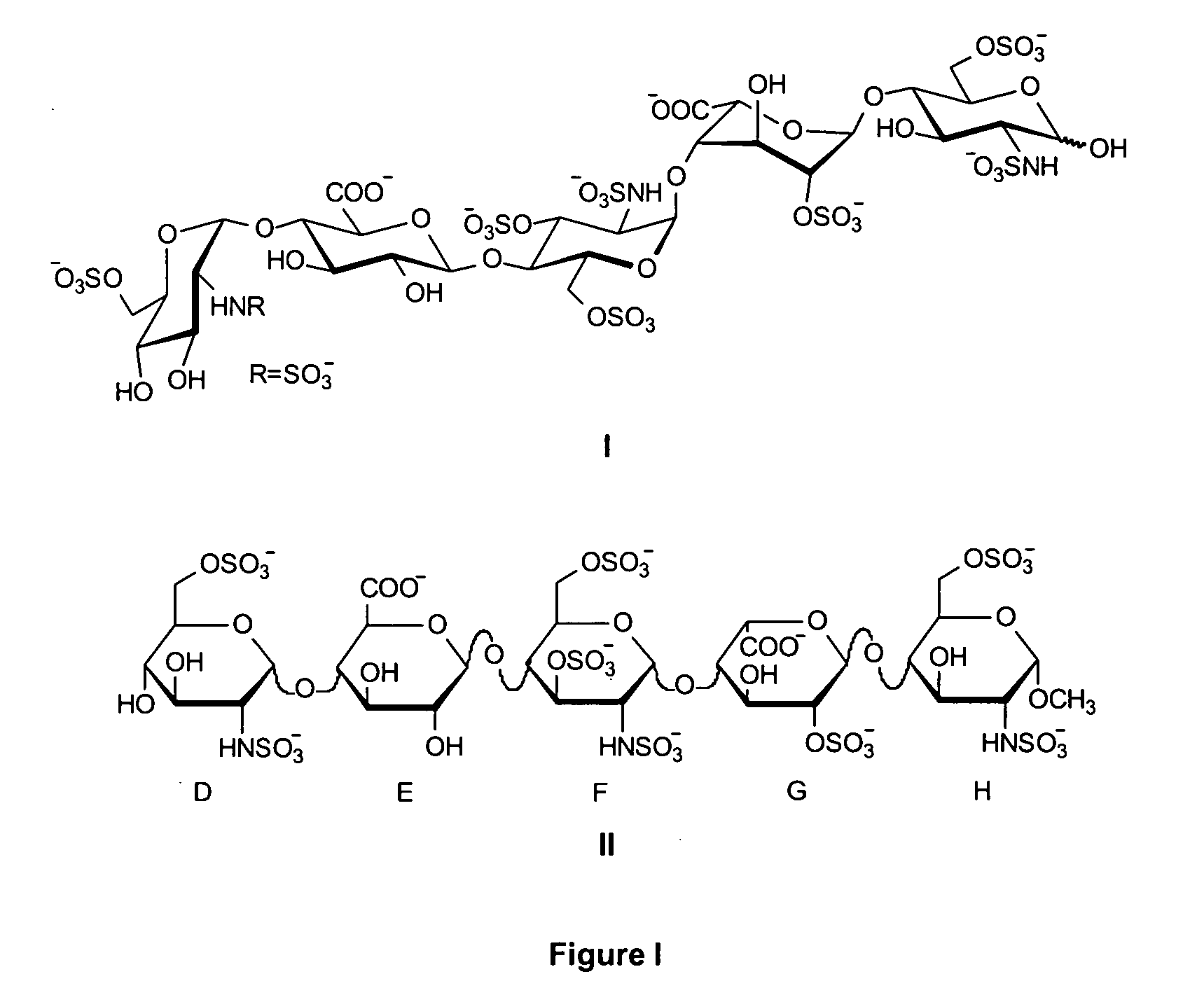
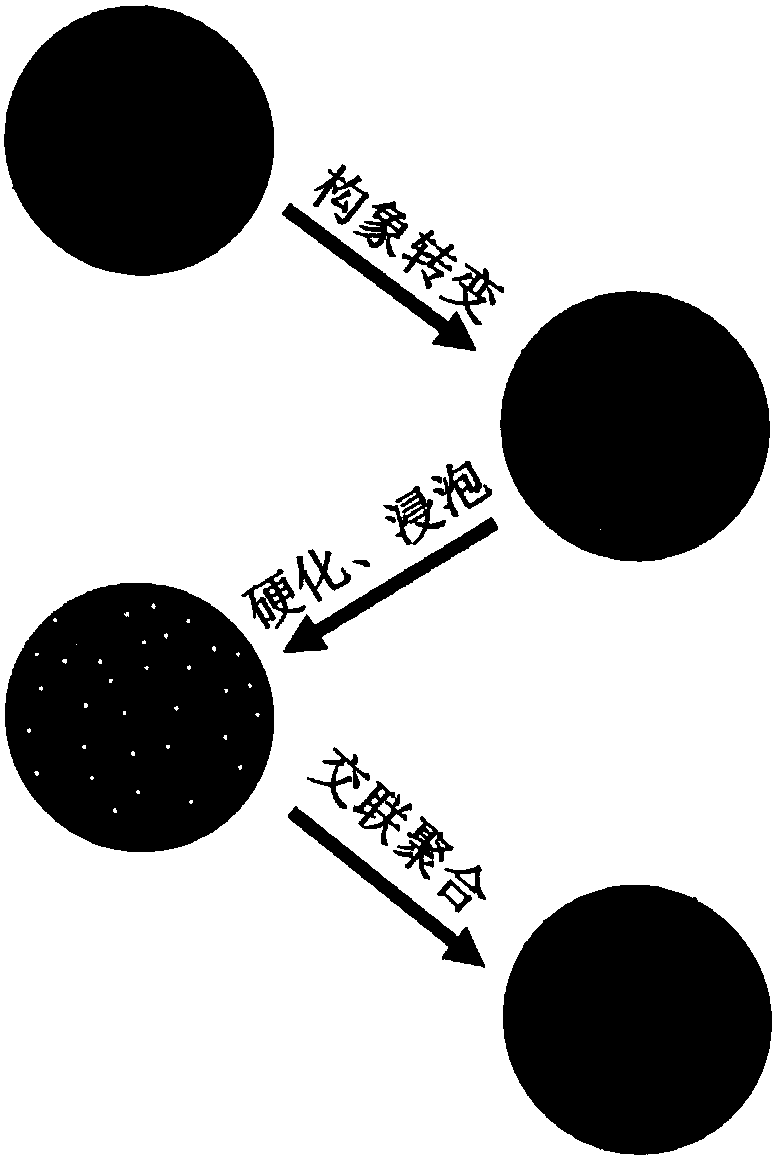
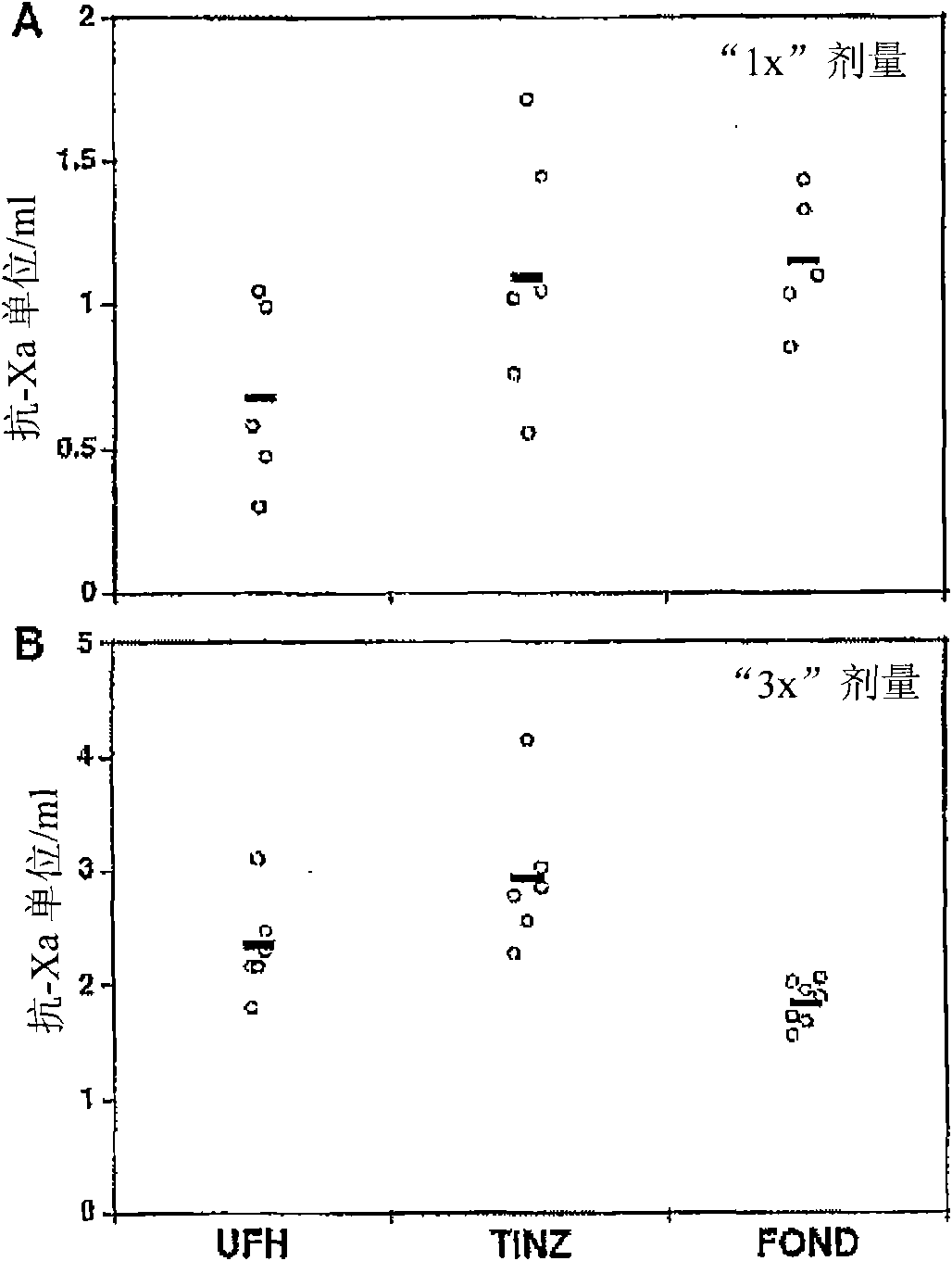
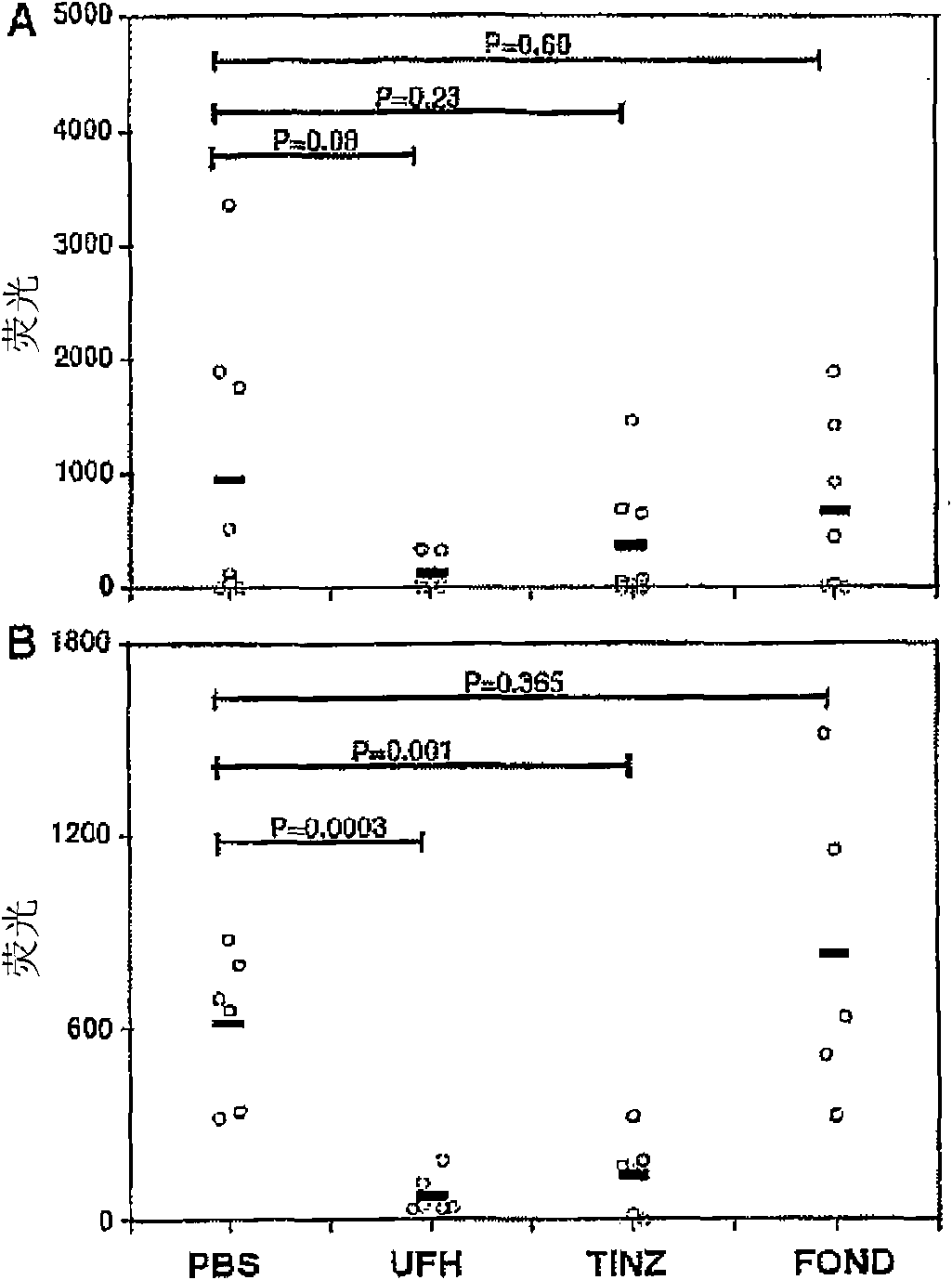
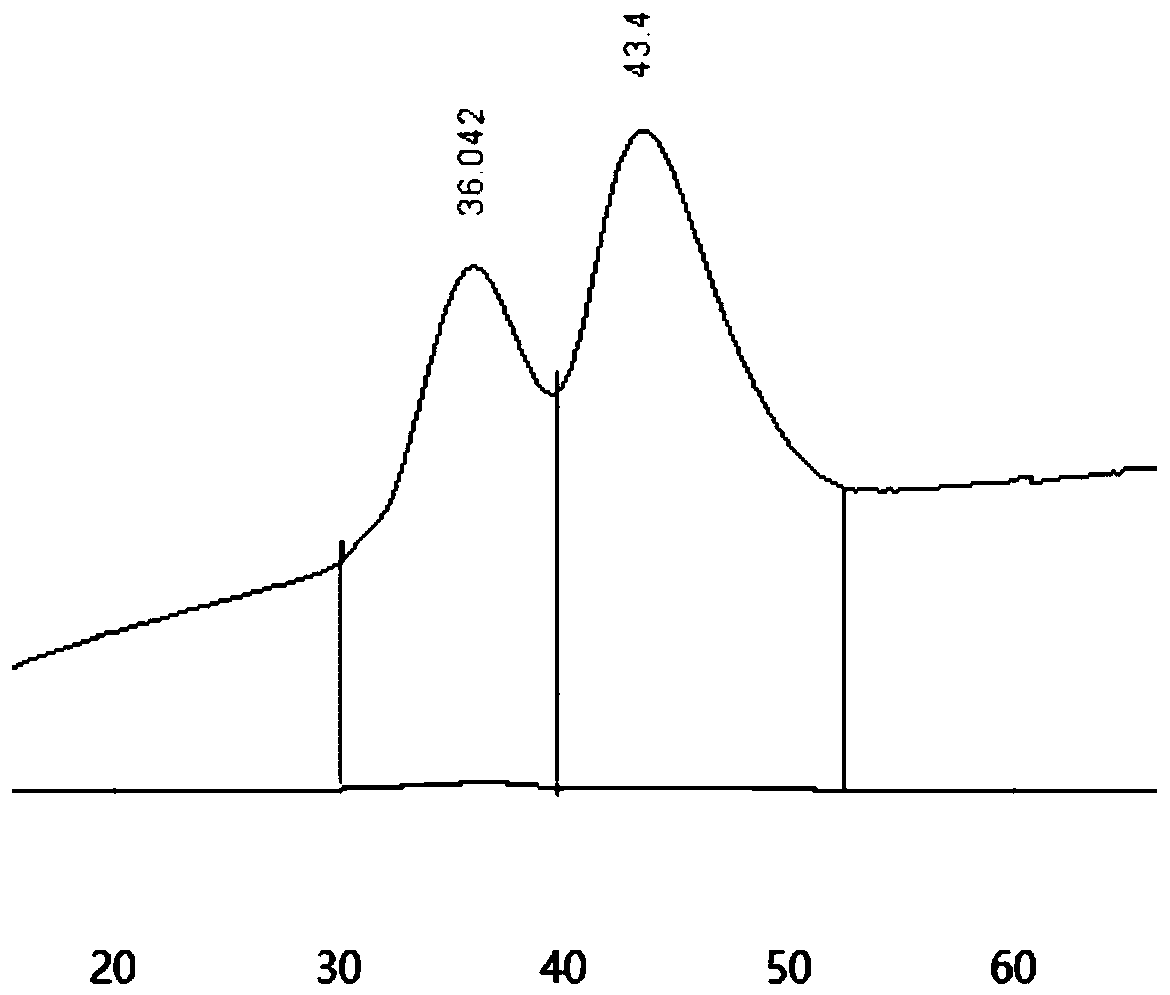
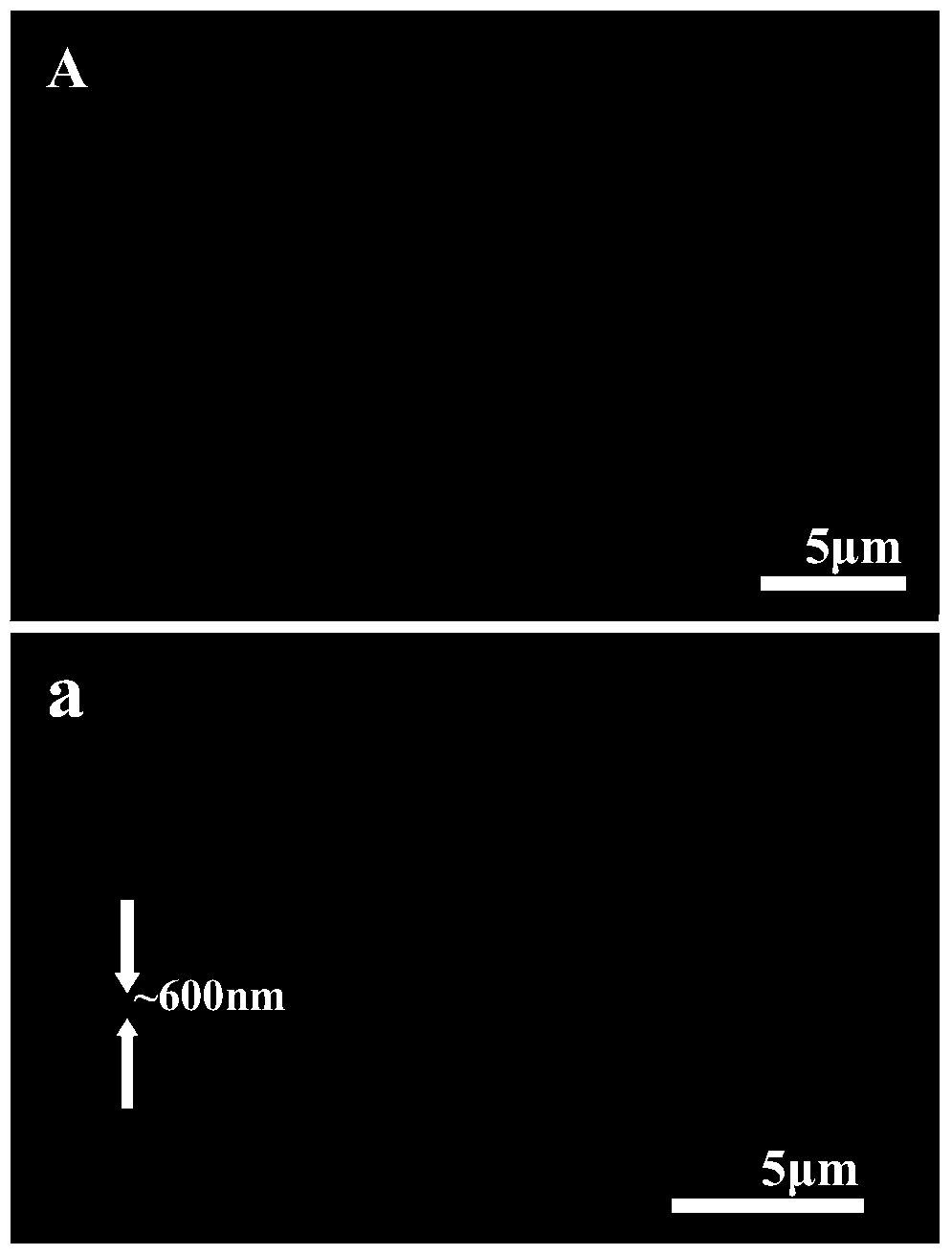
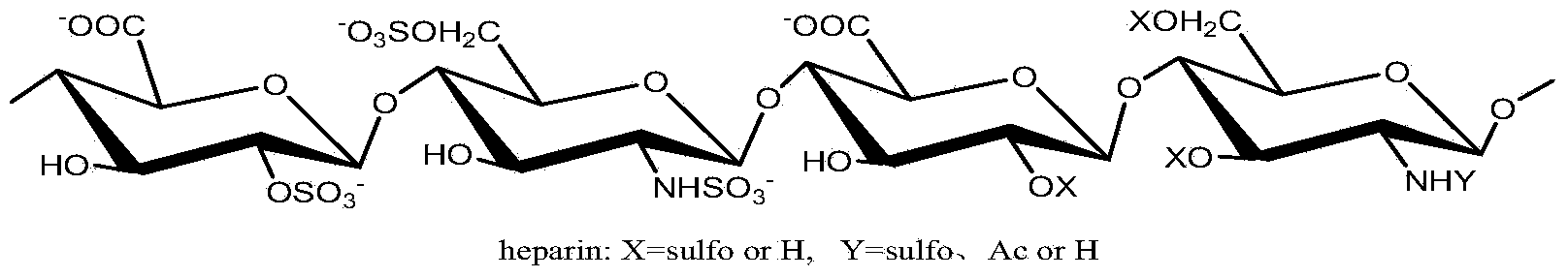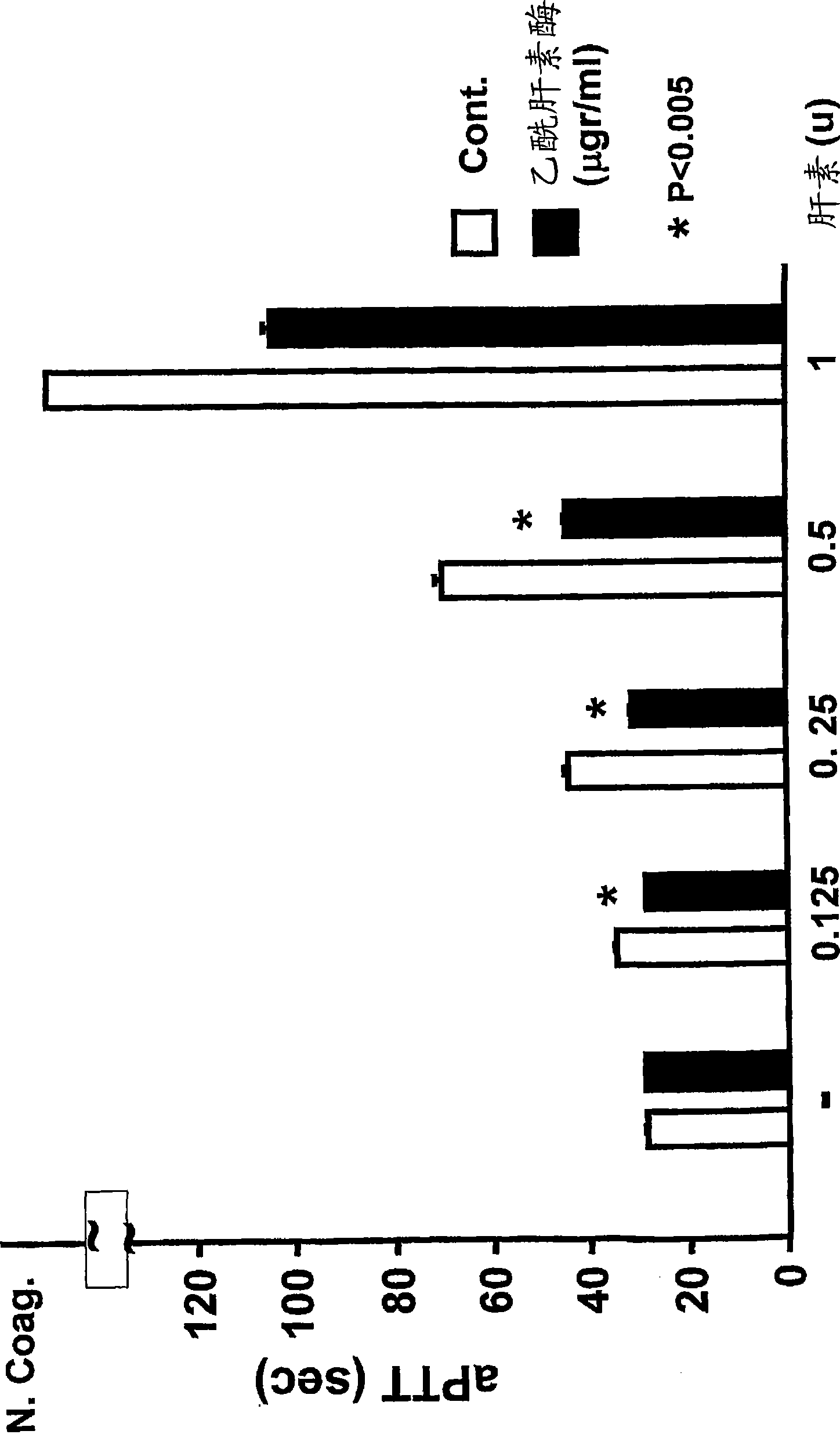Patents
Literature
46 results about "Heparinoids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
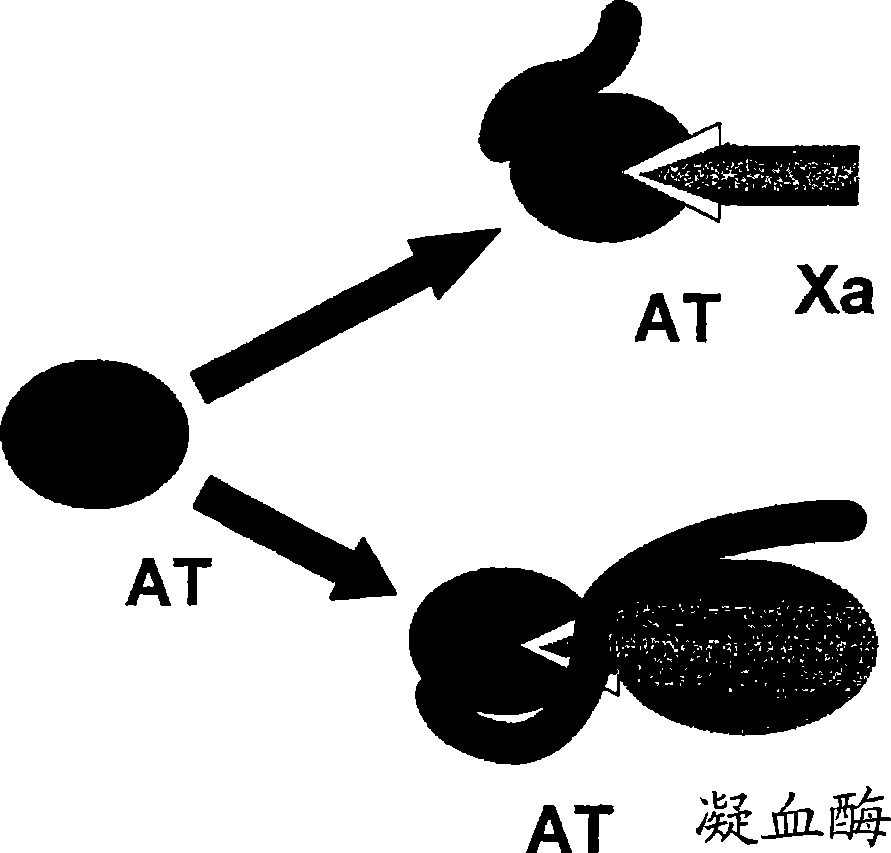
Heparin derivatives. The term has also been used more loosely to include naturally occurring and synthetic highly-sulphated polysaccharides of similar structure. Heparinoid preparations have been used for a wide range of applications including as anticoagulants and anti-inflammatories and they have been claimed to have hypolipidemic properties. (From Martindale, The Extra Pharmacopoeia, 30th, p232)
Synthetic heparin pentasaccharides
InactiveUS7541445B2Simple ideaImpact successEsterified saccharide compoundsOrganic active ingredientsHeparinoidsTetrasaccharide
Preparation of synthetic monosaccharides, disaccharides, trisaccharides, tetrasaccharides and pentasaccharides for use in the preparation of synthetic heparinoids.
Owner:DR REDDYS LAB SA
Plaster for topical use containing heparin and diclofenac
Plaster for topical use having an analgesic activity and at the same time being able to re-absorb haematomas, comprising:a substrate layer;an adhesive layer in the form of a hydrogel matrix containing a pharmaceutically acceptable diclofenac salt, heparin or a heparinoid;a protective film which can be removed at the moment of use.
Owner:ALTERGON
Heparin oligosaccharides
ActiveUS20060079483A1Reduces or eliminates at least one symptom of the diseaseOrganic active ingredientsSugar derivativesHeparinoidsCombinatorial chemistry
The present invention provides composition and methods for the synthesis of low molecular weight heparins and heparinoids. The present invention also provides compositions having substantially homogenous populations of desired heparin molecules, or molecules useful in the synthesis of heparin oligosaccharides.
Owner:ACAD SINIC
Synthetic heparin pentasaccharides
InactiveUS20050080042A1Simple ideaImpact successEsterified saccharide compoundsOrganic active ingredientsHeparinoidsDisaccharide
Preparation of synthetic monosaccharides, disaccharides, trisaccharides, tetrasaccharides and pentasaccharides for use in the preparation of synthetic heparinoids.
Owner:DR REDDYS LAB SA
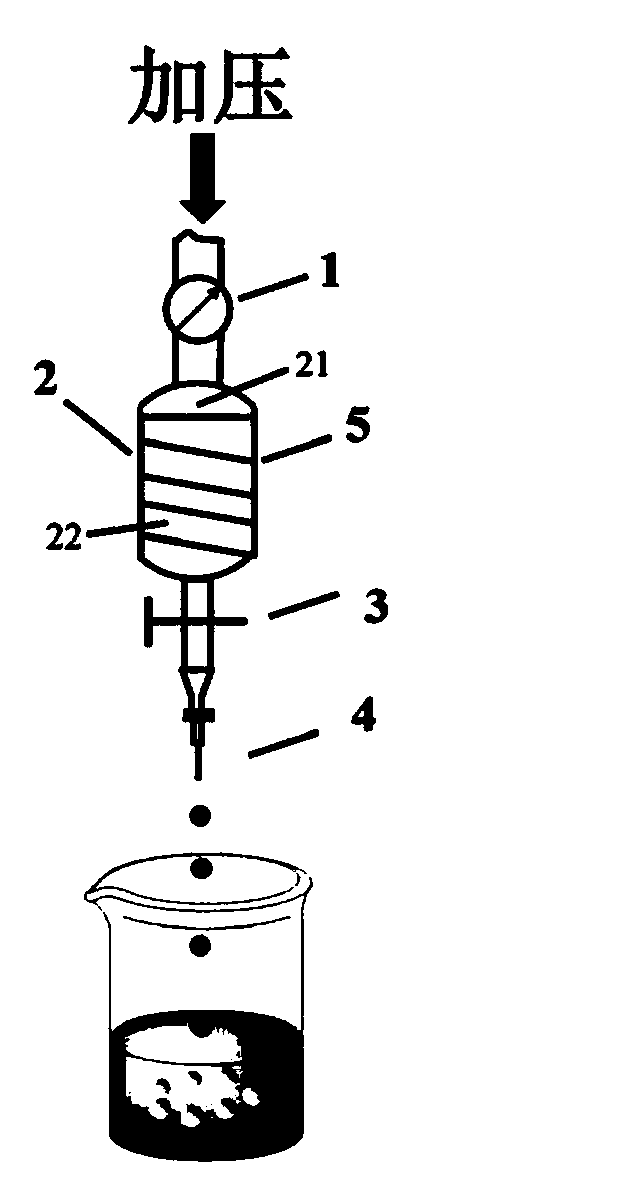
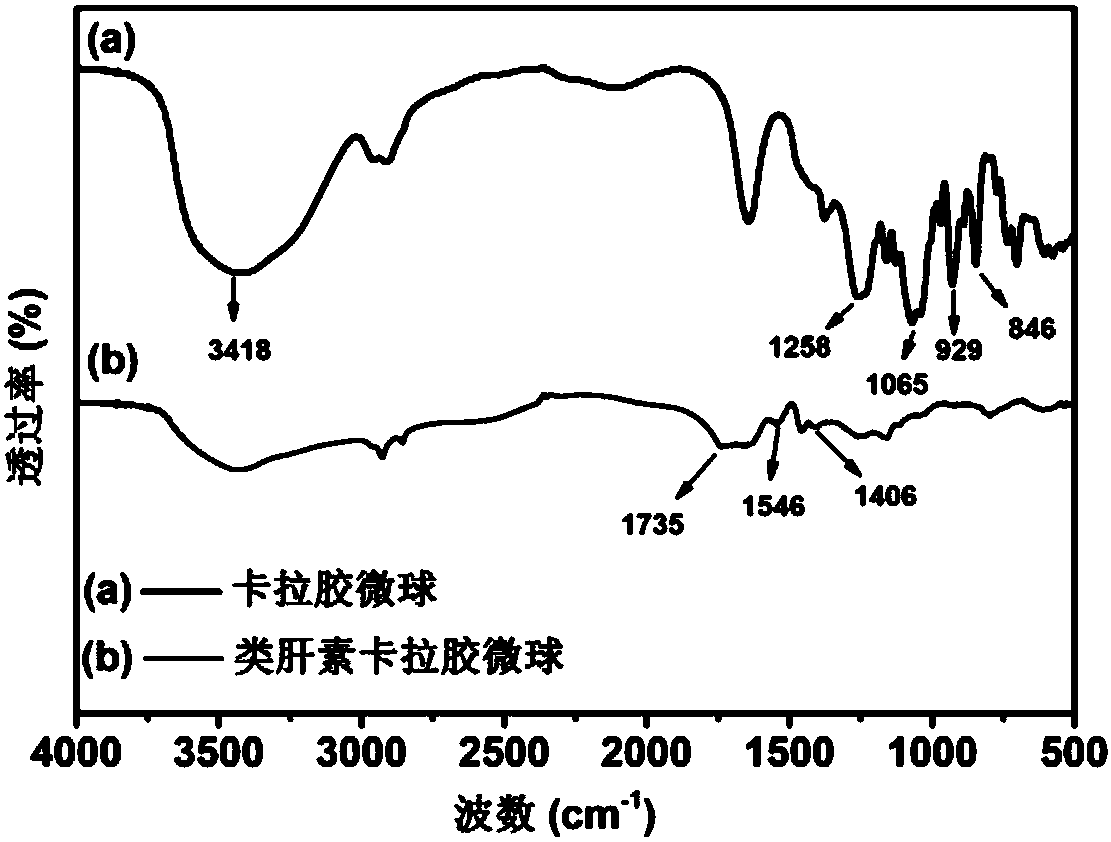
Carrageenan-based self-anticoagulant heparitin microsphere, as well as preparation method and application thereof
ActiveCN108530670AGood biocompatibilityLow cytotoxicityOther chemical processesAlkali metal oxides/hydroxidesCarrageenanDouble network
The invention discloses a carrageenan-based self-anticoagulant heparitin microsphere, as well as a preparation method and application thereof. The carrageenan-based self-anticoagulant heparitin microsphere comprises a carrageenan microsphere of a network structure and a polyacrylic acid crosslinked network constructed by a crosslinking agent and interspersed with a carrageenan microsphere network.The microsphere is low in cytotoxicity and high in biocompatibility and anticoagulant property and has a structure and functional group similar to anticoagulant heparitin. The interspersed carrageenan network and polyacrylate crosslinked network form double networks and the double networks are restricted by each other, so that the mechanical strength of the microsphere is strengthened, the swelling rate is limited, breakage of the microsphere in a using process can be effectively avoided, the dimensional stability of the microsphere can be maintained in use and the microsphere is a novel adsorption material with application prospect for a hemoditoxifier.
Owner:SICHUAN UNIV
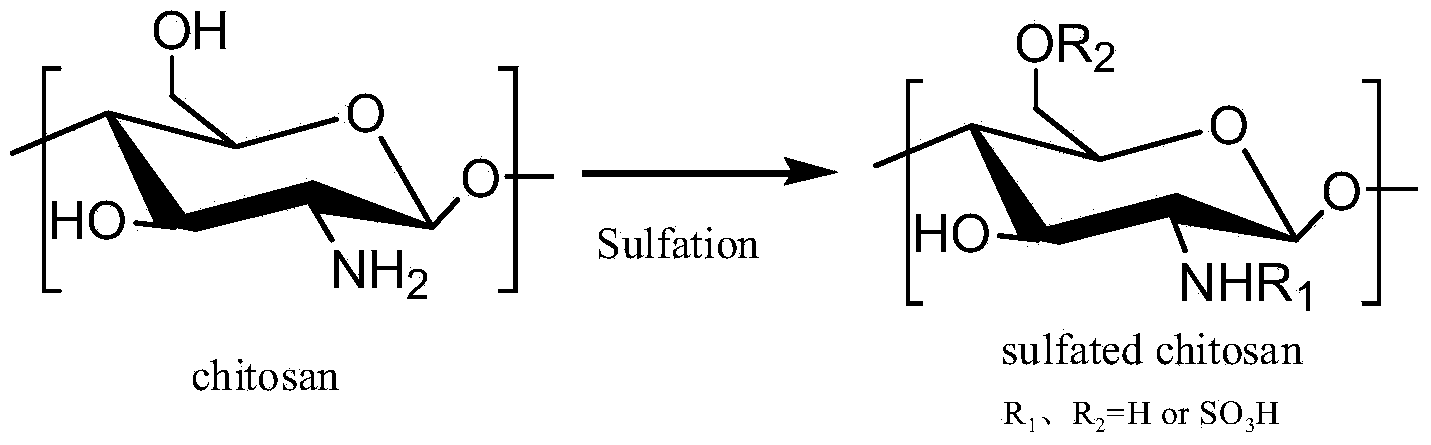
Synthesis method of 6-O-carboxymethyl chitosan sulfuric sulfation product
The invention discloses a synthesis method of a 6-O-carboxymethyl chitosan sulfation product. The method comprises the following steps: (1) chitin alkaline treatment of chitin; (2) carboxymethylation of C6-O site of chitin; (3) deacetylation reaction of 6-O-carboxymethyl chitin; and (4) sulfation of 6-O-carboxymethyl chitosan. The synthesis method disclosed by the invention is a bran-new method for selectively replacing and controlling the replacement rate by using chitin. The synthesis product of the method provides N-site -SO3H with main anticoagulant activity and introduces -COOH, so that a lot of -COOH and SO3H which have negative electricity in the molecular structure are regularly distributed, and an anticoagulant effect of heparinoid is generated by the synergistic effect. High molecular polysaccharide is a heparinoid drug which is selectively modified by chitin via a safe reagent, so that reagent pollution of bulk drugs is reduced, the virus contamination risk of heparin biological extraction is avoided, the safety performance of the drug in the clinical experiment is more excellent in theory as compared with heparin sodium, so the 6-O-carboxymethyl chitosan sulfation product provided by the invention is expected to serve as a cheap direct thrombin inhibitor to replace heparin sodium anti coagulation drugs.
Owner:SHENZHEN BRIGHT WAY NOVEL BIO MATERIALS TECH CO LTD
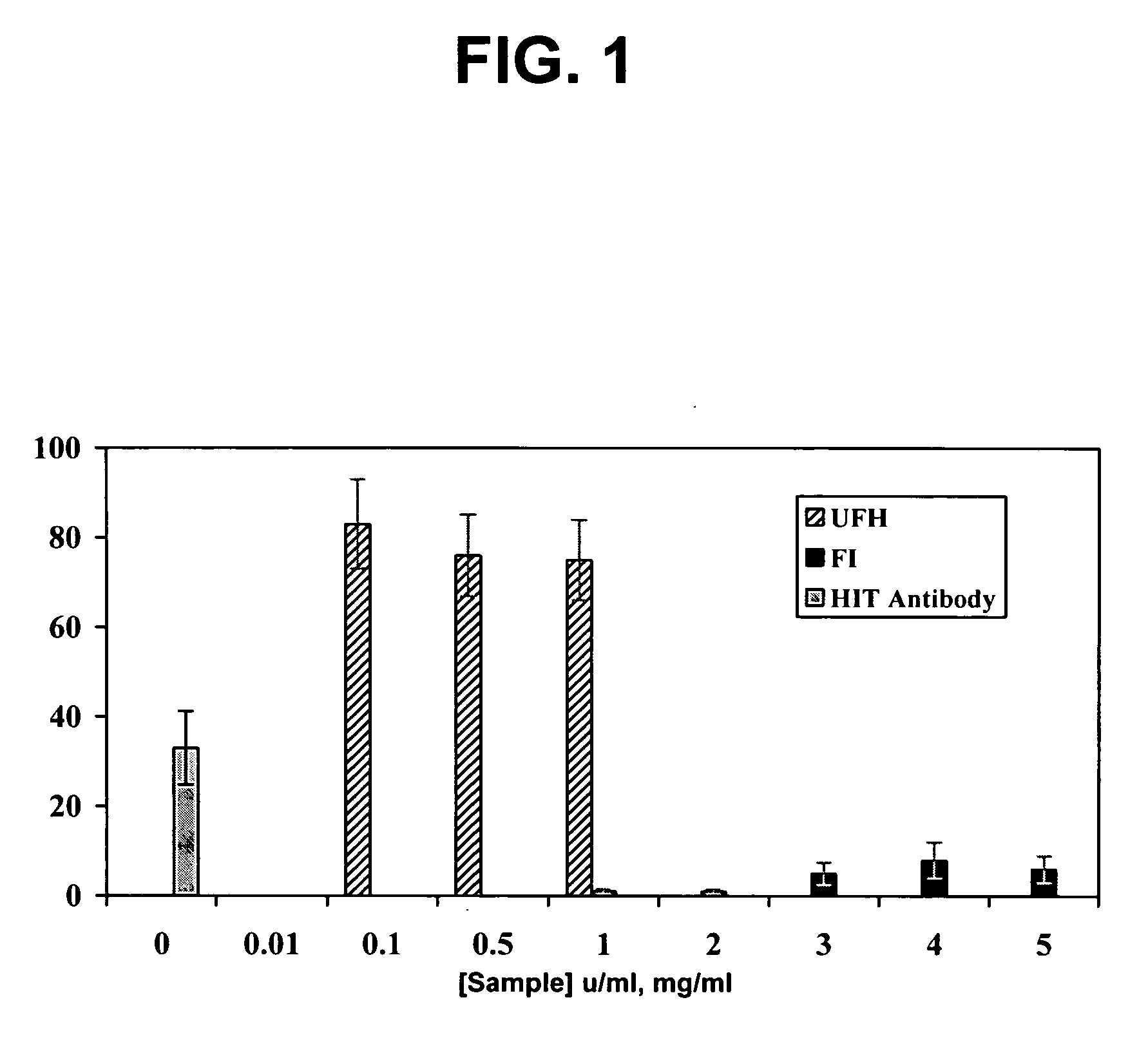
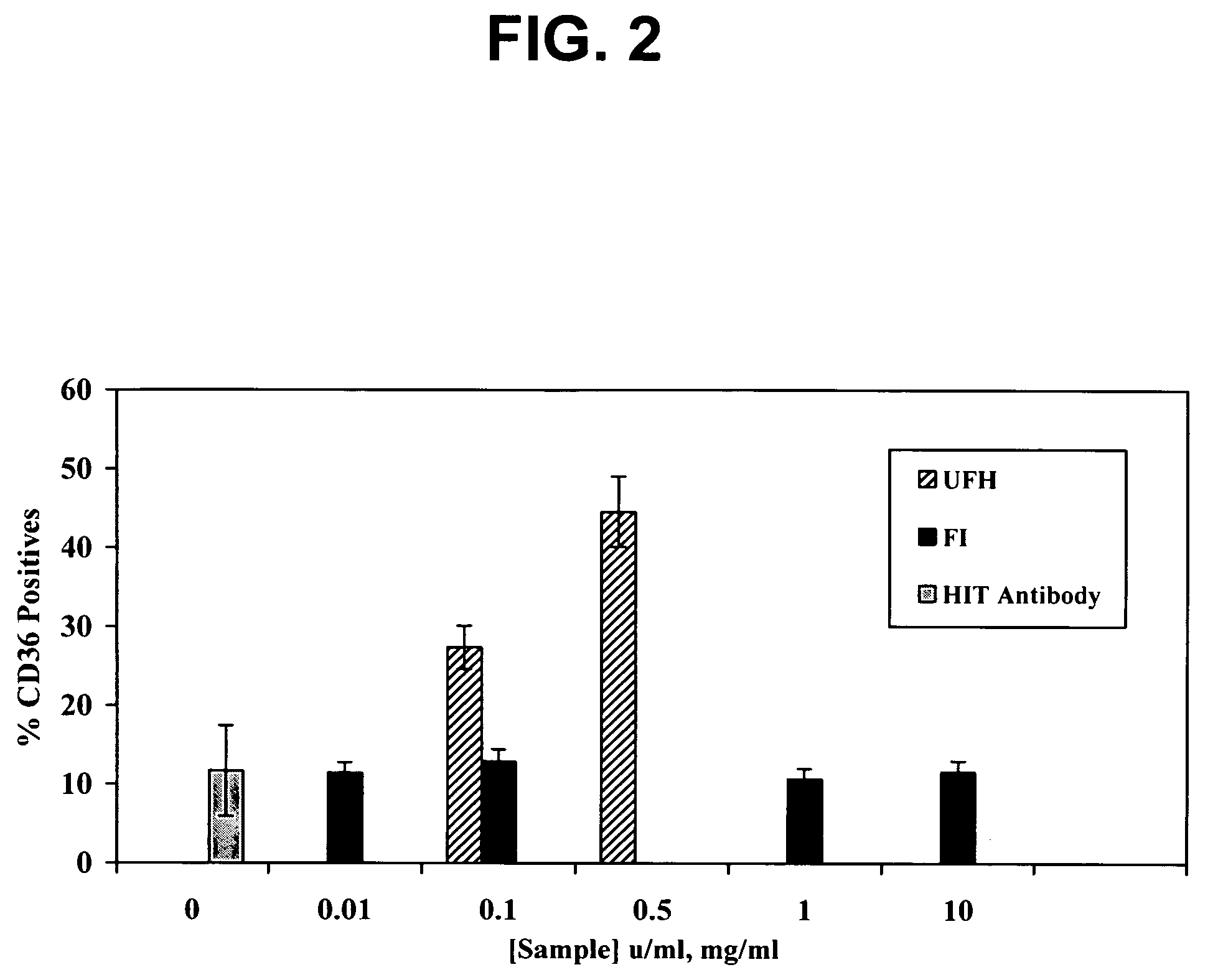
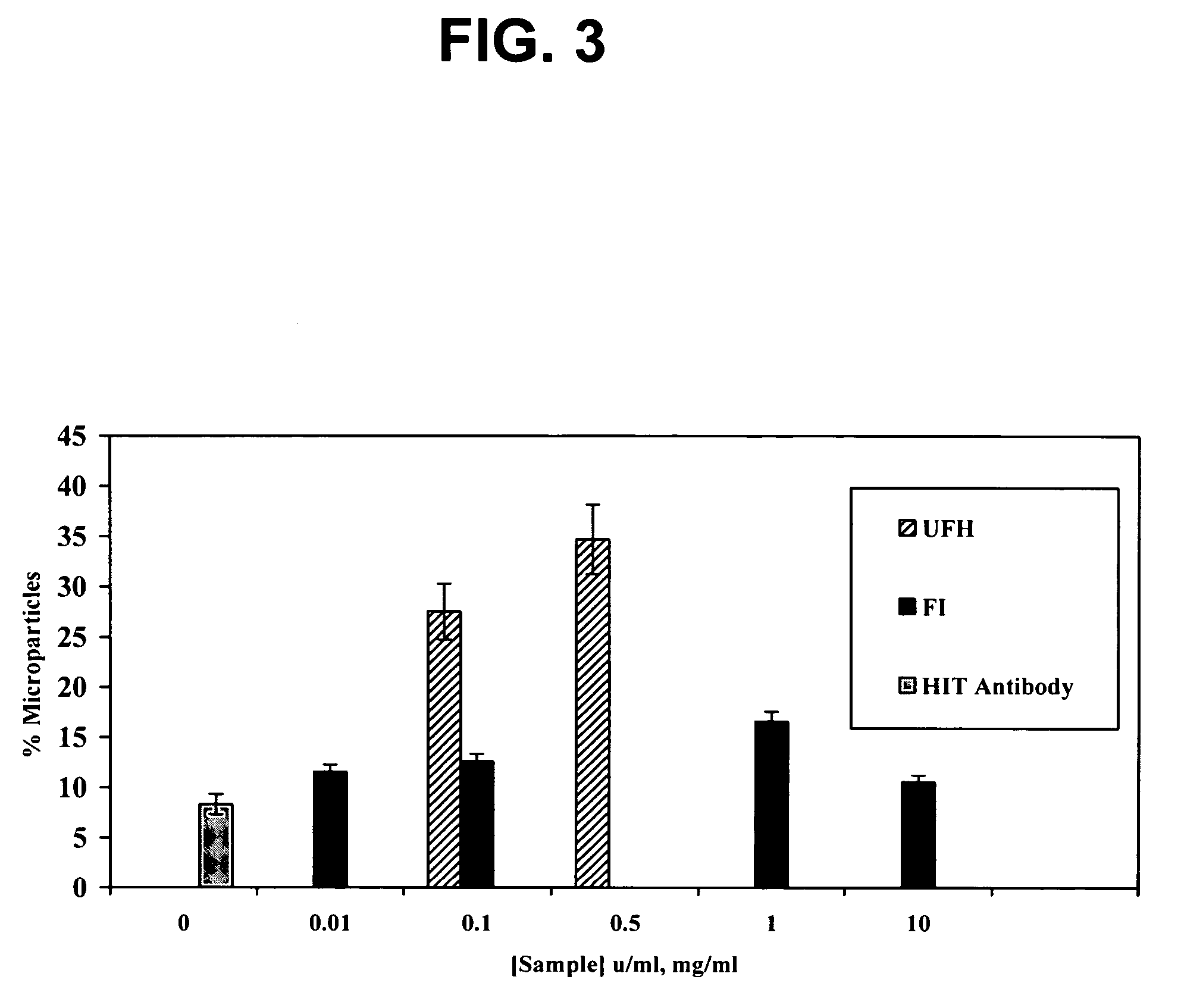
Use of dermatan sulfates and/or desulfated heparins to treat or prevent heparinoid-induced autoimmune responses
InactiveUS20050261241A1Inhibit platelet activationSuppress autoimmune responseOrganic active ingredientsBiocideInduced autoimmune reactionHeparinoids
Owner:CELSUS BIOPHARMACEUTICALS INC
Heparinoid gel microsphere and preparation method thereof and purpose thereof
ActiveCN109331750AExcellent anticoagulantGood dimensional stabilityGel preparationColloidal chemistry detailsBlood treatmentsMicrosphere
The invention discloses a heparinoid gel microsphere. The heparinoid gel microsphere is prepared through coagulating bath reaction between a polymer solution and a heparinoid reaction fluid; and the heparinoid gel microsphere has a functional group similar to anticoagulant heparin. The heparinoid gel microsphere disclosed by the invention has the advantages that the anticoagulation property is excellent, the dimension is stable, and breakage cannot be caused by a reason of swelling or a pressure in the blood treatment process. The invention further provides a preparation method of the heparinoid gel microsphere; and the preparation method of the heparinoid gel microsphere is simple and suitable for industrialization. The invention further discloses a purpose of the heparinoid gel microsphere and can be used for the field of blood purification.
Owner:SICHUAN UNIV
Heparinoid-modified polyvinyl alcohol hydrogel thin nano-compound hematodialysis film and preparation method thereof
InactiveCN105727771AImprove responsePermanent hydrophilicitySemi-permeable membranesMembranesFiberBiocompatibility Testing
The invention relates to a heparinoid-modified polyvinyl alcohol hydrogel thin nano-compound hematodialysis film and a preparation method thereof. A heparinoid hydrogel skin layer is arranged as an outer layer of a thin nano-compound hematodialysis film and a porous supporting layer with nano-pores communicated with each other is arranged as an inner layer of the thin nano-compound hematodialysis film. The preparation method comprises the following steps: performing electrostatic spinning on a PAN solution, thereby acquiring a nanometer fiber as the supporting layer of a compound film; adding sodium-hydrogen into the PVA solution, and then adding 1,3-propane sultone, reacting for 8-24h at 40-80 DEG C, filtering and drying, thereby acquiring s-PVA; mixing s-PVA with PVA, adding a solvent, adjusting pH, adding a cross-linking agent, coating on the supporting layer after cross-linking, and sealing at room temperature, thereby acquiring the heparinoid-modified polyvinyl alcohol hydrogel thin nano-compound hematodialysis film. The preparation method provided by the invention is simple in reaction process and is easily performed. The prepared thin nano-compound hematodialysis film has the characteristics of permanent hydrophily, low protein adsorbability and excellent biocompatibility.
Owner:DONGHUA UNIV
Plaster for topical use containing heparin and diclofenac
Plaster for topical use having an analgesic activity and at the same time being able to re-absorb haematomas, comprising: a substrate layer; an adhesive layer in the form of a hydrogel matrix containing a pharmaceutically acceptable diclofenac salt, heparin or a heparinoid; a protective film which can be removed at the moment of use.
Owner:DONATI ELISABETTA +1
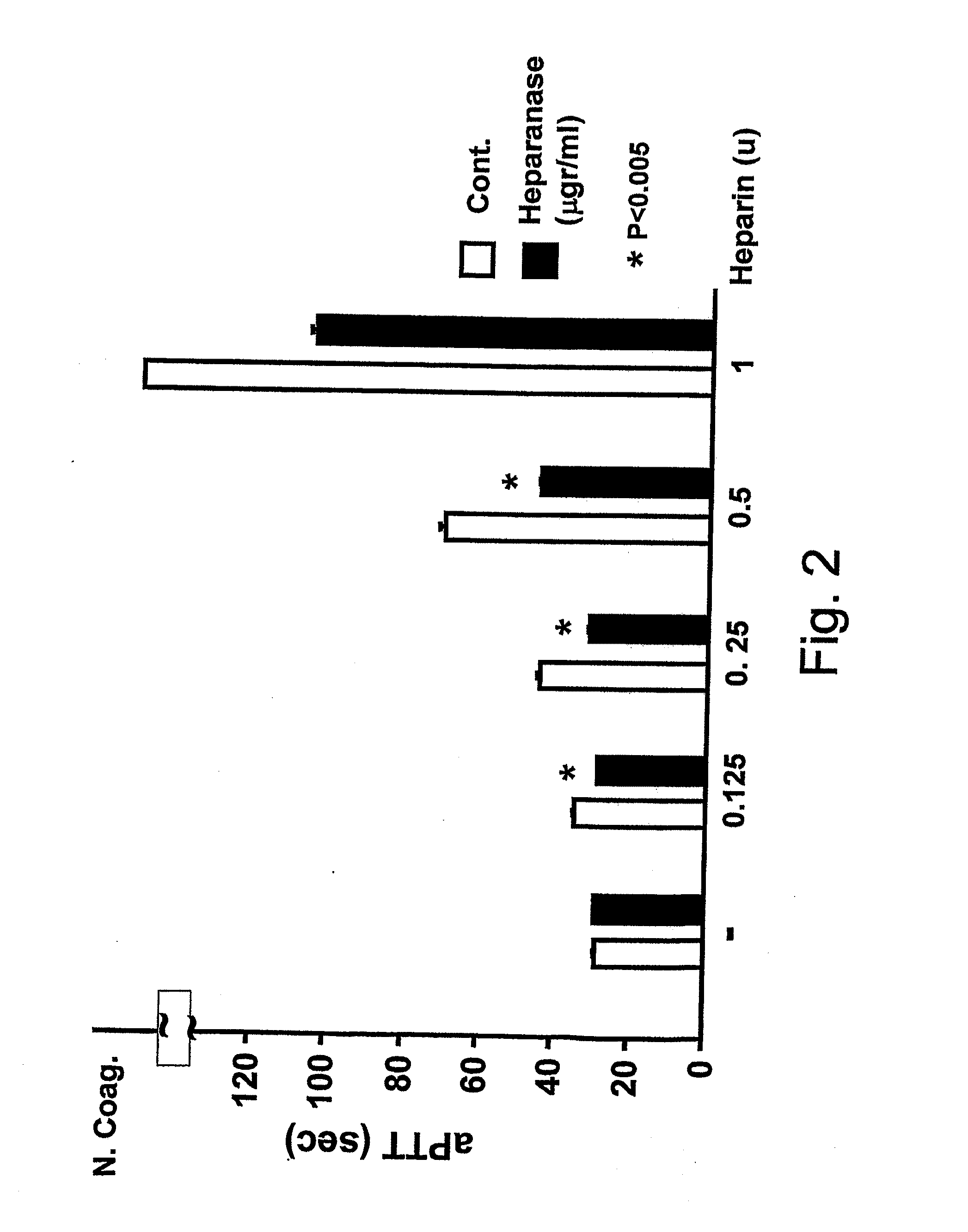
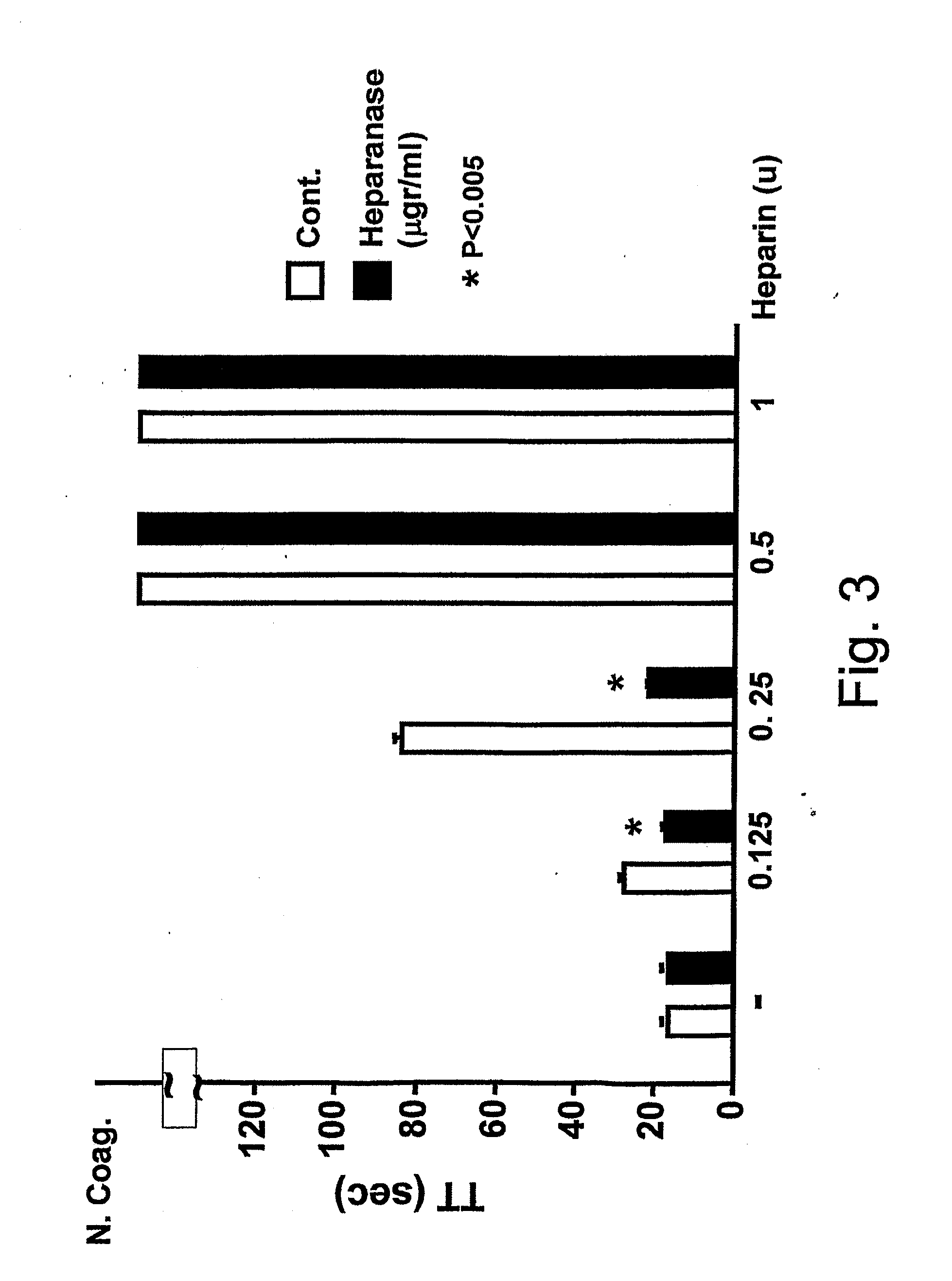
Use of non-catalytic form of heparanase and peptides thereof for reversing the Anti-coagulant effects of heparinoids
InactiveUS20110104140A1Peptide/protein ingredientsMicrobiological testing/measurementMedicineHeparinoids
The present invention relates to inhibition of heparinoids anti-coagulation activity by a non-active form of a eukaryotic endoglycosidase or any fragment or peptide thereof comprising at least one heparin-binding domain. More particularly, the invention provides compositions and methods for the inhibition of heparinoids anti-coagulation activity and for the treatment of coagulation related pathologic clinical conditions, using a non-active form of mammalian heparanase or peptides thereof comprising at least one heparin-binding domain.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
Heparin Oligosaccharides
ActiveUS20080171722A1Reduces or eliminates at least one symptom of the diseaseOrganic active ingredientsSugar derivativesHeparinoidsCombinatorial chemistry
The present invention provides composition and methods for the synthesis of low molecular weight heparins and heparinoids. The present invention also provides compositions having substantially homogenous populations of desired heparin molecules, or molecules useful in the synthesis of heparin oligosaccharides.
Owner:ACAD SINIC
Preparation method of heparinoid
ActiveCN104877042AThe synthesis process is simpleProduction operation is easy to controlSulfo/sulfonyldioxy group formation/introductionHeparinoidsSulfur trioxide
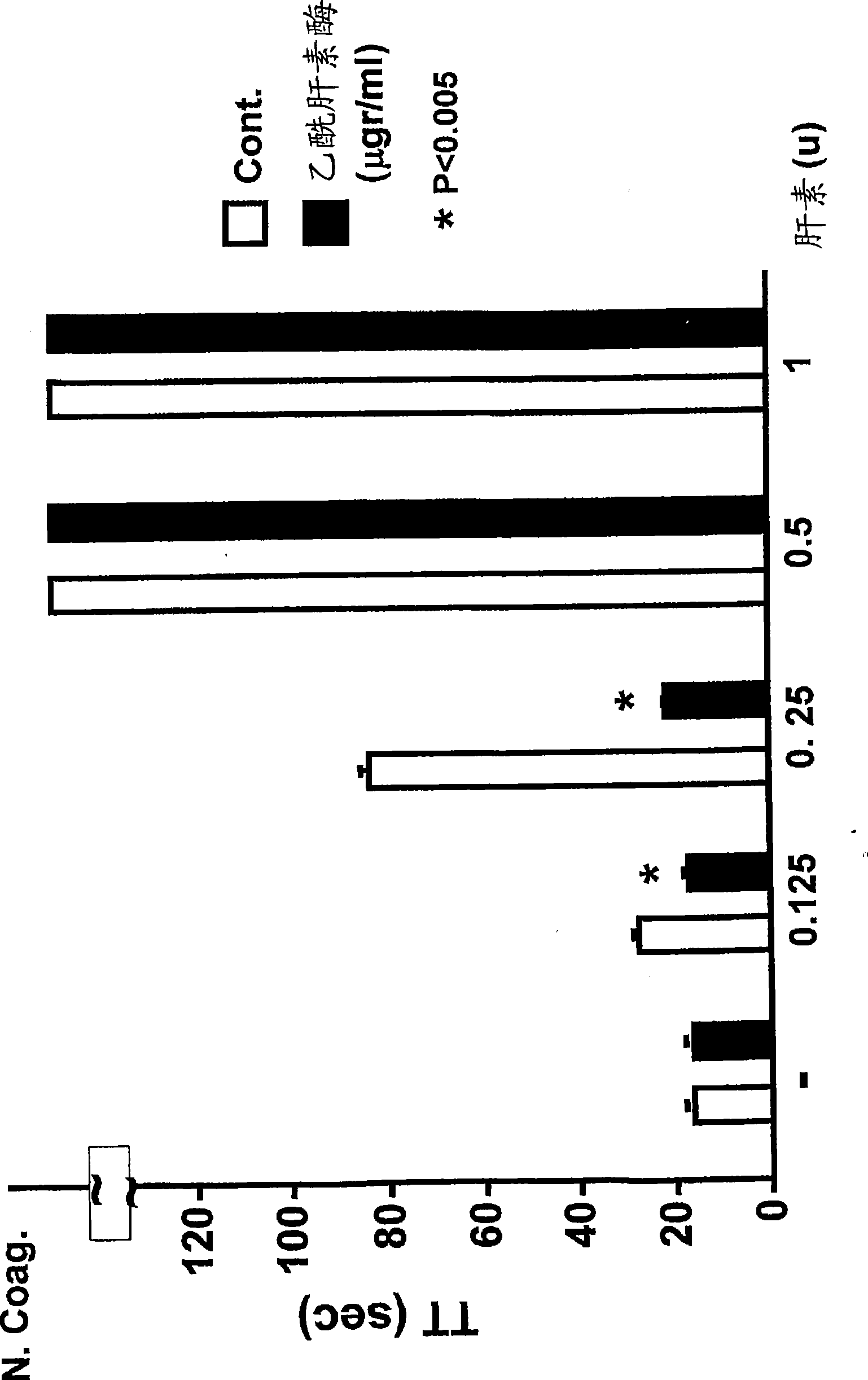
The invention discloses a preparation method of heparinoid. The preparation method comprises the following steps : adopting chondroitin sulfate as a raw material, and treating to obtain heparinoid after sulfonation reaction. In the sulfonation reaction, the adopted solvent is formamide and the adopted sulfonating agent is free sulfur trioxide, fuming sulfuric acid or pyridine sulfur trioxide. The preparation method of heparinoid, disclosed by the invention, has the advantages that the synthesis process is simple, the production and the operation are easily controlled, no special requirement exists for production equipment, the molecular weight range of a product is stable, the physicochemical indexes of the product are controlled in the quality standard range, and simultaneously the product is high in yield and the method is suitable for large-scale industrial production.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
Plaster for topical use containing heparin and diclofenac
Plaster for topical use having an analgesic activity and at the same time being able to re-absorb haematomas, comprising: a substrate layer; an adhesive layer in the form of a hydrogel matrix containing a pharmaceutically acceptable diclofenac salt, heparin or a heparinoid; a protective film which can be removed at the moment of use.
Owner:ALTERGON
Method for precisely and quantitatively controlling chondroitin sulfate and dermatan sulfate contents of heparin/heparinoid
The invention discloses a method for precisely and quantitatively controlling the chondroitin sulfate and dermatan sulfate contents of heparin / heparinoid. The method comprises the following steps: 1) adding proteins with chondrosulphatase B activity into a heparinoid / heparin containing solution, and after the DS content reaches an expected value, terminating the enzymatic hydrolysis; and 2) further adding proteins with chondrosulphatase AC activity into the solution, and after the CS content reaches an expected value, terminating the enzymatic hydrolysis, thus obtaining a heparin sample or heparinoid sample with expected CS and DS contents. The method disclosed by the invention realizes the efficient and precise control over the CS and DS contents of heparin / heparinoid, thereby establishing a novel low-cost and high-yield production process of heparin products. The method is simple and easy to perform, has an application value of large-scale production, and can be used for producing danaparoid sodium meeting the standards of European Pharmacopoeia.
Owner:TSINGHUA UNIV
Process for preparing high-purity dermatan sulfate from heparin sodium leftovers
ActiveCN106188341AGood treatment effectIncrease economic benefitsHazardous substanceSpecific rotation
The invention relates to a process for preparing high-purity dermatan sulfate from heparin sodium leftovers. The process comprises the following steps: precipitating leftovers produced during production of high-quality heparin sodium serving as a raw material in a grading way by ethanol to obtain a heparinoid crude product and a dermatan sulfate crude product, and precipitating the heparinoid crude product in a grading way by the ethanol and oxidizing by hydrogen peroxide to obtain positive specific rotation heparinoid and negative specific rotation heparinoid; degrading the dermatan sulfate crude product by nitrous acid in order to degrade heparin substances contained in the crude product into low-molecular heparin substances, precipitating by ethanol in a grading way, and oxidizing by hydrogen peroxide to obtain over 98 percent dermatan sulfate. By adopting the process, the problem of low purity of the dermatan sulfate in the market for a long time is solved, the leftovers are changed into treasure, and large extra benefit can be created for an enterprise; the process has the advantages of simple steps, environmental friendliness, no production of toxic and harmful substances, low cost and easiness for scale production.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Method of manufacturing composition comprising local anesthetic, heparinoid, and buffer
PendingUS20140194380A1Maintains bioavailabilityMaintenanceBiocideOrganic active ingredientsAnesthetic AgentMedicine
An improved method for preparing a composition including a heparinoid, a local anesthetic, and a buffer for treatment of a lower urinary tract disease or condition can comprise either: (A) (i) providing a heparinoid in solid form or liquid form; (ii) providing a local anesthetic in solid form or liquid form; (iii) adding a liquid buffer to the heparinoid in solid form or liquid form; (iv) adding the local anesthetic to the mixture of the liquid buffer and the heparinoid; and (v) if necessary, adjusting the pH of the mixture of the liquid buffer, the local anesthetic, and the heparinoid so that a pH is achieved of from about 6.8 to about 8.3 is achieved without precipitation of the local anesthetic; or (B) (i)providing a heparinoid in solid form or liquid form; (ii) providing a local anesthetic in solid form or liquid form; (iii) mixing the heparinoid in solid form or liquid form and the local anesthetic in solid form or liquid form; (iv) adding a liquid buffer to the mixture of the heparinoid and the local anesthetic to form a mixture of liquid buffer, the heparinoid, and the local anesthetic; and (v) if necessary, adjusting the pH of the mixture of the liquid buffer, the local anesthetic, and the heparinoid so that a pH is achieved of from about 7.0 to about 7.8 is achieved without precipitation of the local anesthetic. The invention also encompasses a stable premixed liquid composition that avoids precipitation of the local anesthetic.
Owner:PARSONS LOWELL C
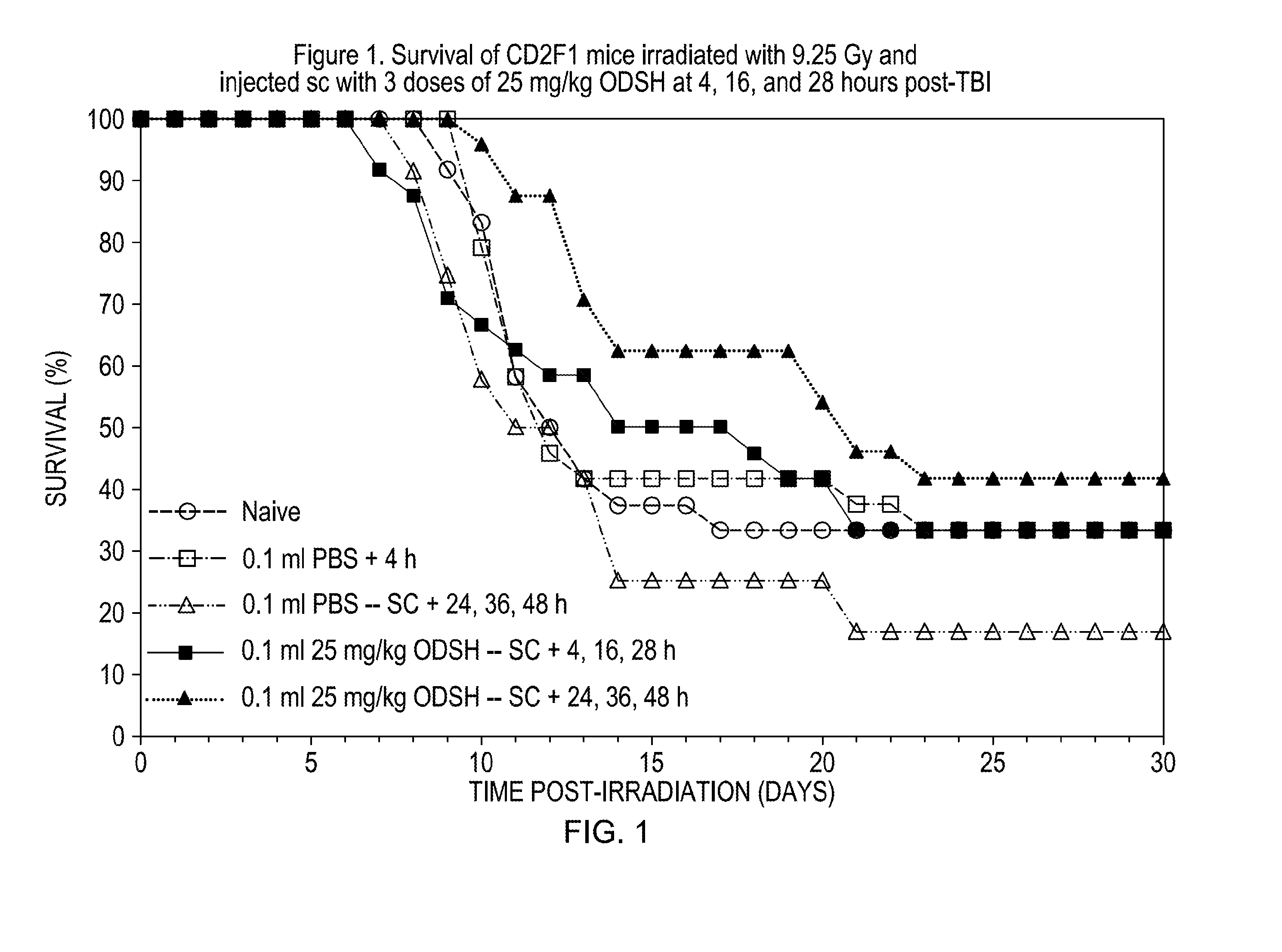
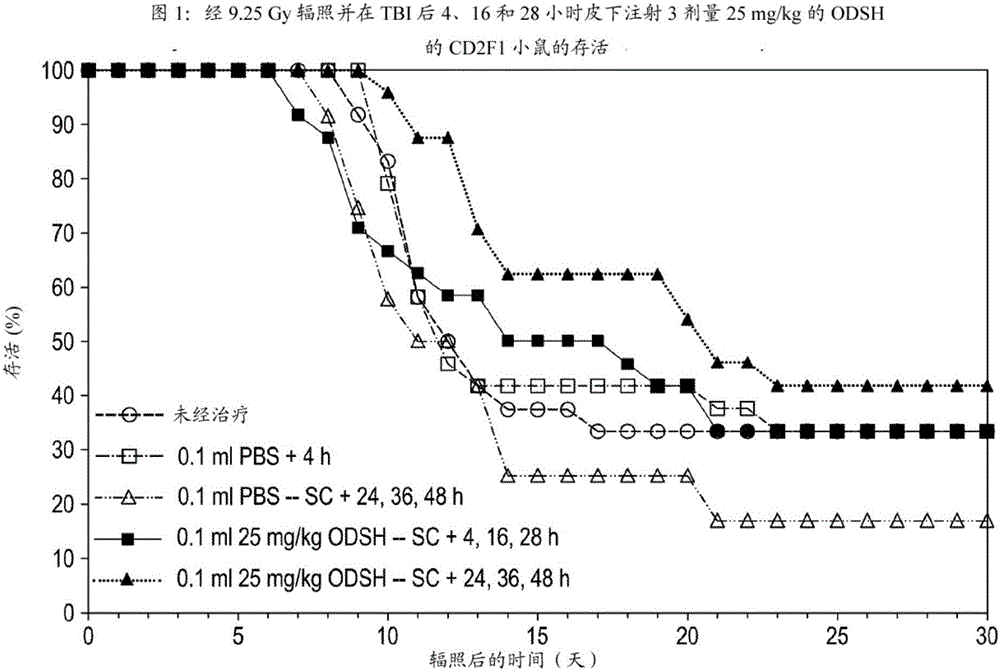
Methods of treating and preventing radiation damage
The invention relates to methods of treating and preventing radiation damage from whole-body exposure. According to the methods of the invention, subjects are treated therapeutically and / or prophylactically with low-anticoagulant heparinoids. The invention also relates to methods of extending the life of subjects exposed to whole-body radiation.
Owner:CANTEX PHARMA
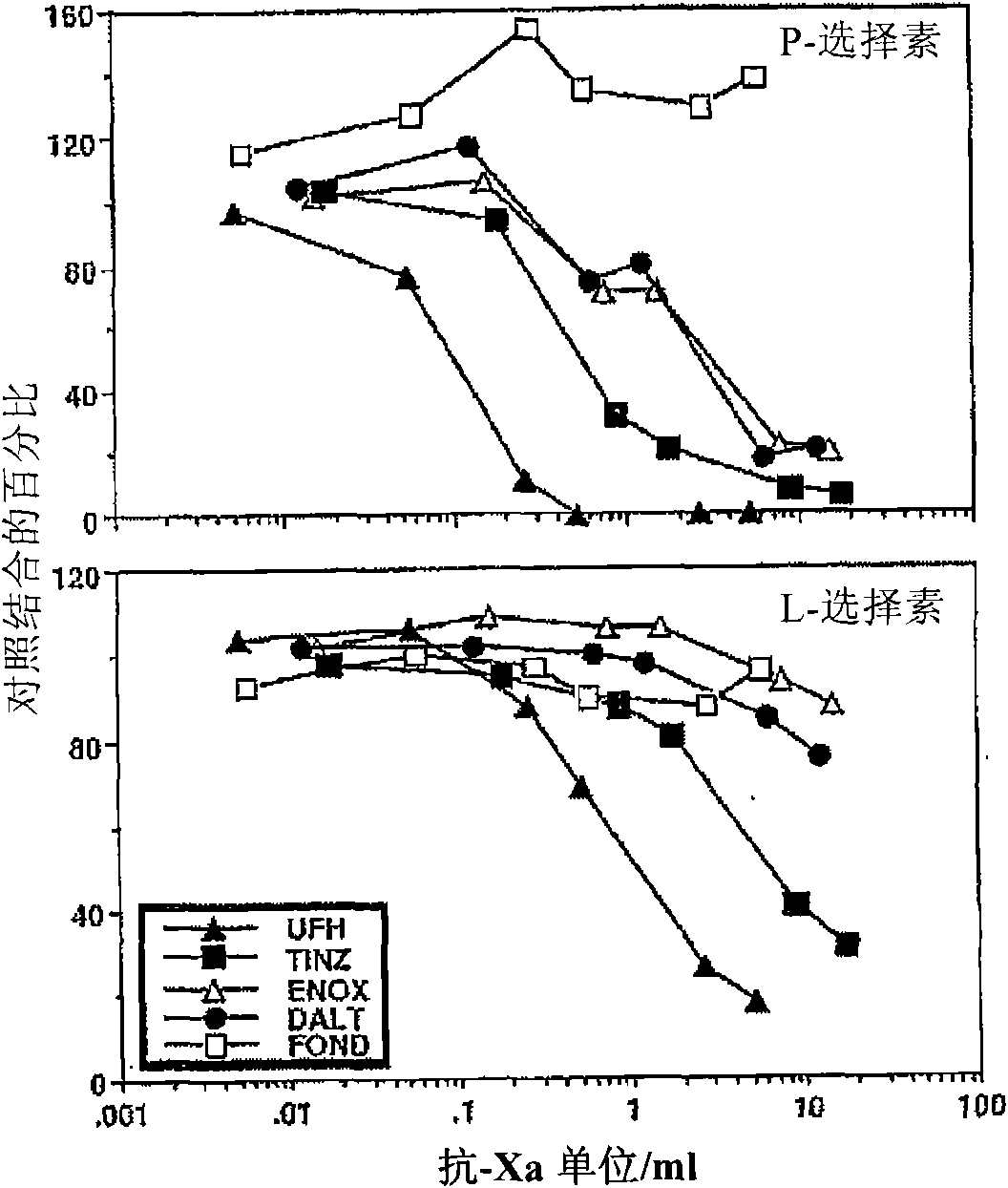
Heparin compostions and selectin inhibition
The disclosure provides in vitro and in vivo methods for identifying Heparins and Heparinoids that modulate the activity of selectins . The disclosure also provides Heparins and Heparinoids that modulate the activity of selectins . The identification and isolation of these heparin formulations has the potential to mediate a wide variety of pathologies mediated by P- and / or L-selectin, including hematogenous metastasis, diseases associated with inflammation (e.g., asthma, arthritis, allergic dermatitis), ischemia-reperfusion injury, or other pathologies such as sickle cell anemia. Selectin inhibition can be achieved at plasma concentrations lower than those that cause excessive anticoagulation or unwanted bleeding in a human subject.
Owner:RGT UNIV OF CALIFORNIA
Process for extracting heparinoid or protein from heparin by-products
ActiveCN109402205AHigh yieldEfficient separationPeptide preparation methodsFermentationUltrafiltrationHeparinoids
The invention relates to a process for extracting heparinoid or protein from heparin by-products. The method comprises the following steps: (1) adding hot water into the heparin by-products for dissolving, and then adding sodium chloride until the heparin by-products are completely dissolved; (2) after the heparin by-products are completely dissolved, adding an enzyme reagent A, adjusting the pH to 8-10, heating and then cooling to the room temperature, and centrifuging to obtain first centrifugate; (3) adding a precipitant B into the first centrifugate, stirring, and centrifuging to obtain second centrifuged wet solid; (4) adding hot water into the second centrifuged wet solid for dissolving, and then adding the sodium chloride until the solid is completely dissolved; (5) after the secondcentrifuged wet solid is completely dissolved, adding ethanol for precipitating, and collecting precipitate I; (6) adding water for dissolving the precipitate I, and carrying out ultrafiltration to obtain ultrafiltrate; (7) adding the sodium chloride into the ultrafiltrate for dissolving, then adding ethanol, and collecting precipitate II; (8) drying the precipitate II to obtain the heparinoid orthe protein. The process has the advantages that the process can improve the recovery rate and titer of the heparinoid or the protein.
Owner:如皋市桦儒肠衣有限公司
Separation method of heparan sulfate and dermatan sulfate in heparinoid
The invention relates to a separation method of heparan sulfate and dermatan sulfate in heparinoid. The separation method comprises the following steps of a, dissolving the heparinoid in water to prepare a heparinoid solution; b, adsorbing the heparinoid solution through anion resin; c, adopting a 0-1 mol / L sodium chloride solution for gradient elution to collect all component eluents; d, concentrating the component eluents separately and adding ethanol for precipitation to obtain the heparan sulfate and dermatan sulfate. In this way, the byproducts of heparinoid generated during production offine products of heparin from crude products of heparin can serve as raw materials, the loss of effective ingredients in the prior art is avoided, no impurities are introduced, the process is simple,the separation cost is low, the effect is excellent, and amplification is easy.
Owner:SUZHOU WISMED PHARMA CO LTD
A heparin-like modified polyvinyl alcohol hydrogel thin-layer nanocomposite hemodialysis membrane and its preparation method
InactiveCN105727771BGood antifoulingImprove responseSemi-permeable membranesMembranesFiberPolymer science
The invention relates to a heparinoid-modified polyvinyl alcohol hydrogel thin nano-compound hematodialysis film and a preparation method thereof. A heparinoid hydrogel skin layer is arranged as an outer layer of a thin nano-compound hematodialysis film and a porous supporting layer with nano-pores communicated with each other is arranged as an inner layer of the thin nano-compound hematodialysis film. The preparation method comprises the following steps: performing electrostatic spinning on a PAN solution, thereby acquiring a nanometer fiber as the supporting layer of a compound film; adding sodium-hydrogen into the PVA solution, and then adding 1,3-propane sultone, reacting for 8-24h at 40-80 DEG C, filtering and drying, thereby acquiring s-PVA; mixing s-PVA with PVA, adding a solvent, adjusting pH, adding a cross-linking agent, coating on the supporting layer after cross-linking, and sealing at room temperature, thereby acquiring the heparinoid-modified polyvinyl alcohol hydrogel thin nano-compound hematodialysis film. The preparation method provided by the invention is simple in reaction process and is easily performed. The prepared thin nano-compound hematodialysis film has the characteristics of permanent hydrophily, low protein adsorbability and excellent biocompatibility.
Owner:DONGHUA UNIV
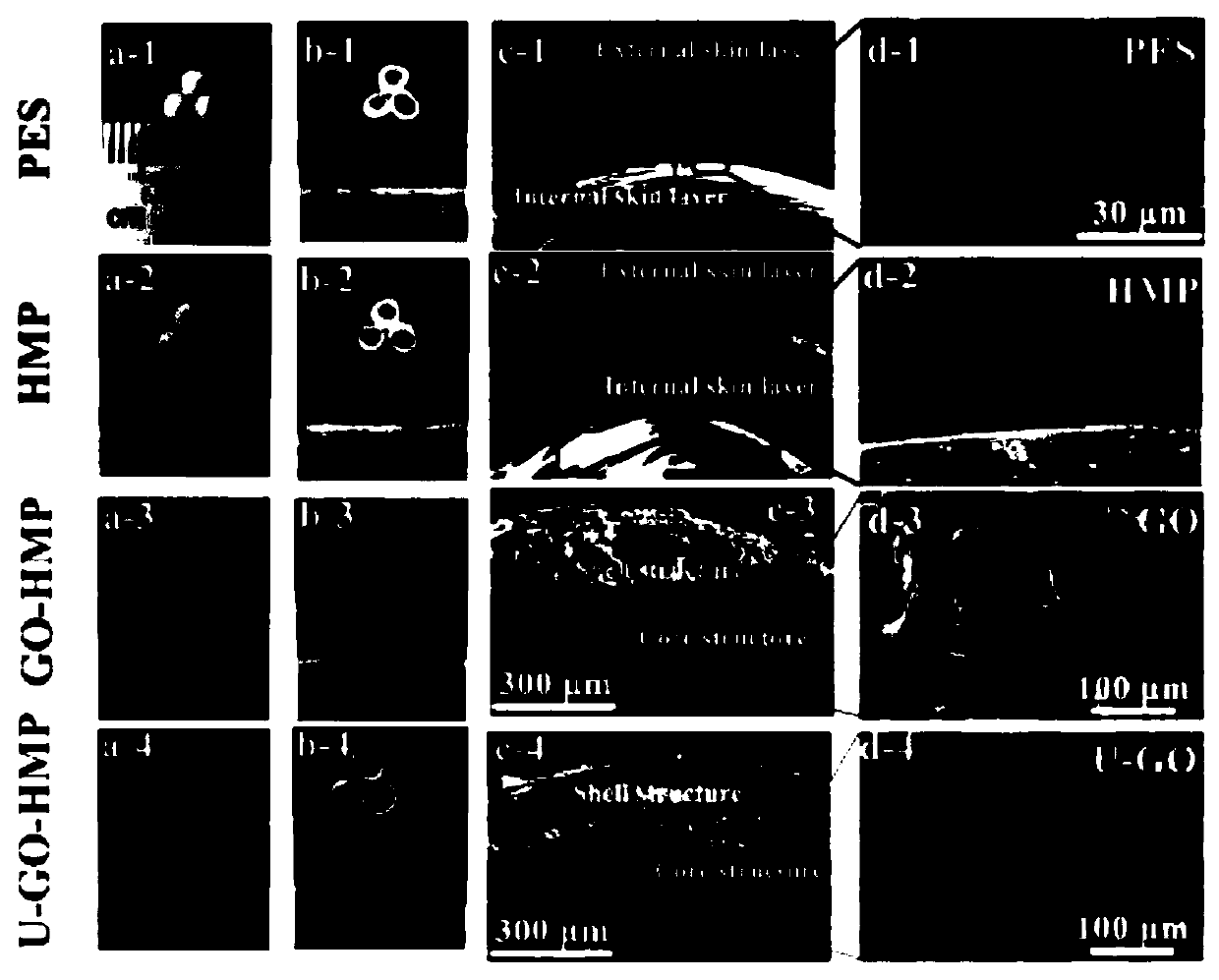
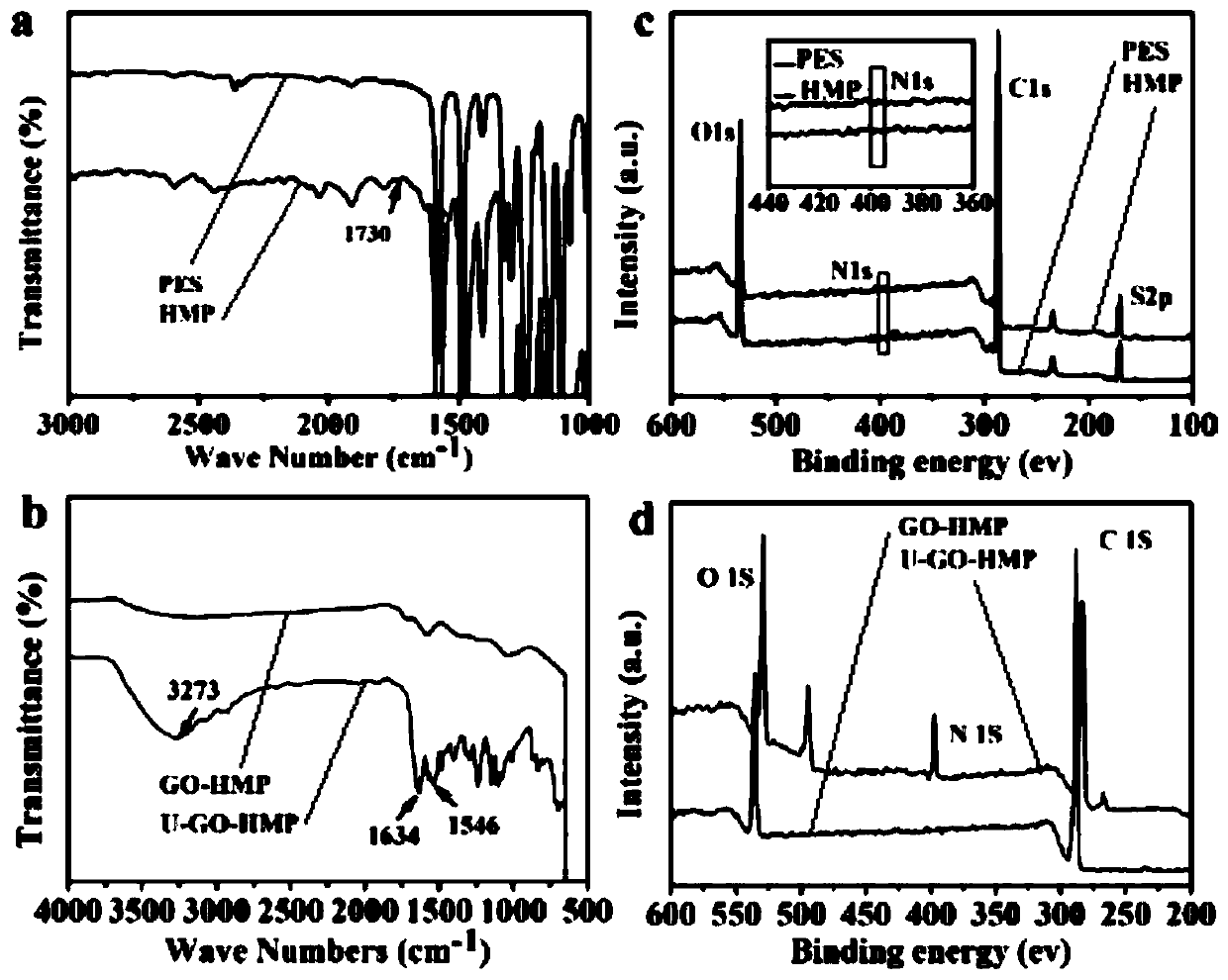
Core-shell structured heparinoid microsphere based on graphene oxide grafted urease, and preparation method and applications of core-shell structured heparinoid microsphere
ActiveCN110624512AAllow diffusionGood blood compatibilityIon-exchange process apparatusOther chemical processesMicrospherePolymer solution
The invention discloses a core-shell structured heparinoid microsphere based on graphene oxide grafted urease, and a preparation method and applications of the core-shell structured heparinoid microsphere. According to the preparation method, a graphene oxide two-dimensional nanosheet layer is taken as a base material, immobilizing urease on the surface of the base material by carbodiimide methodso as to obtain a core solution; in-situ cross-linking polymerization is adopted to prepare a heparinoid polymer solution to obtain a shell solution; and reverse-forward phase inversion method is adopted to obtain the core-shell structured heparinoid microsphere. The core-shell structured heparinoid microsphere is capable of removing urea with high efficiency in hemoperfusion, relieving patient pain, and can also be used for dialysate regeneration in hemodialysis, and provide technical support for a wearable artificial kidney.
Owner:SICHUAN UNIV
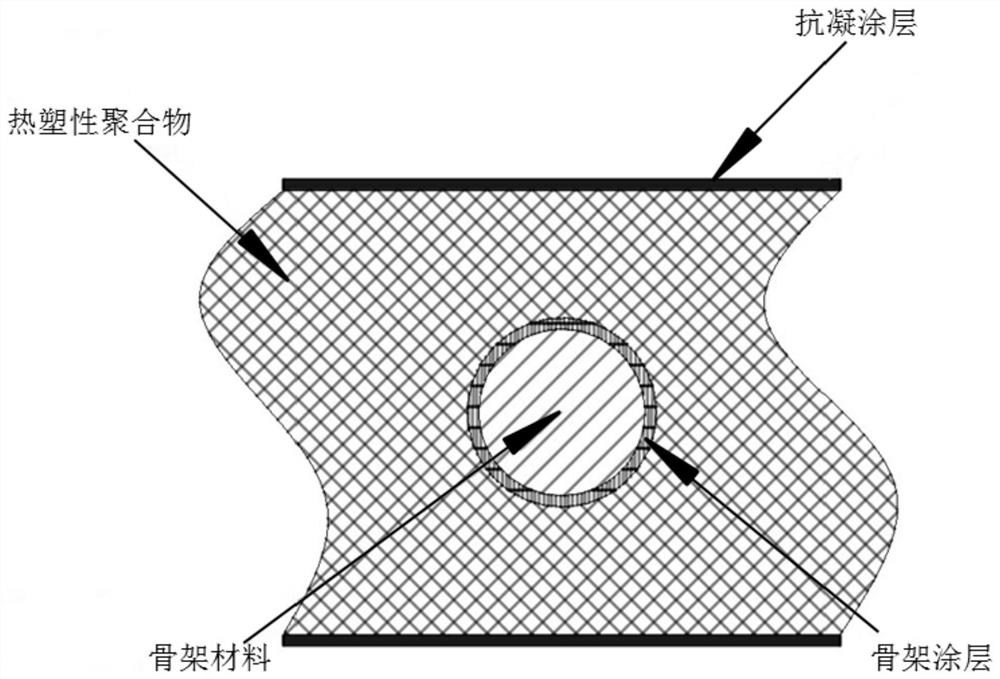
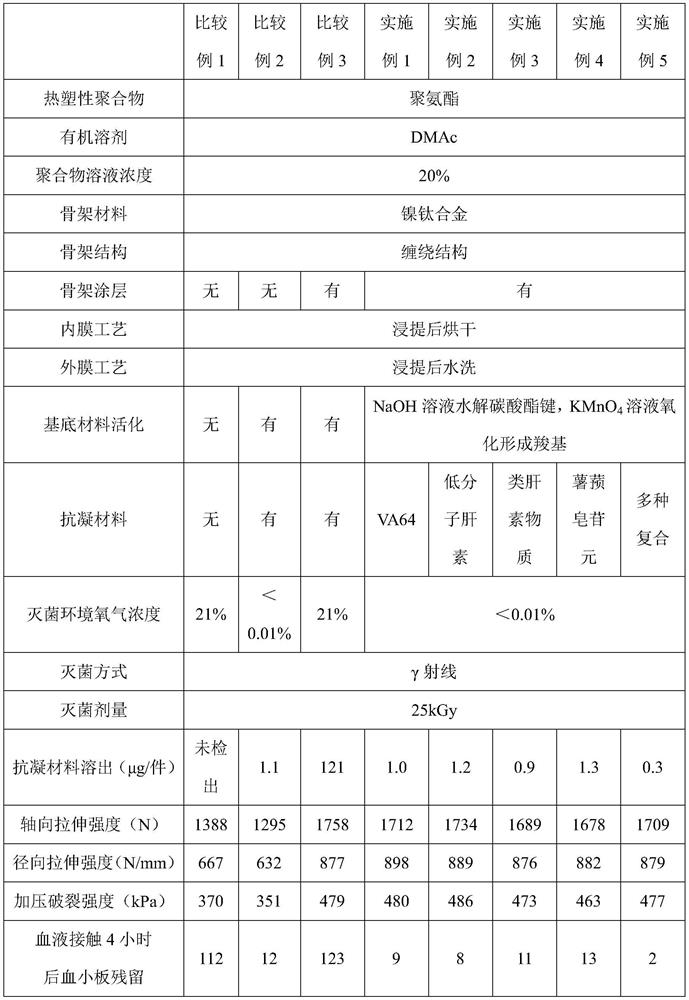
Covered implantable medical device and preparation method thereof
ActiveCN111760073AExcellent anticoagulant propertiesGood economic valuePharmaceutical containersMedical packagingCopolymerCompatibilization
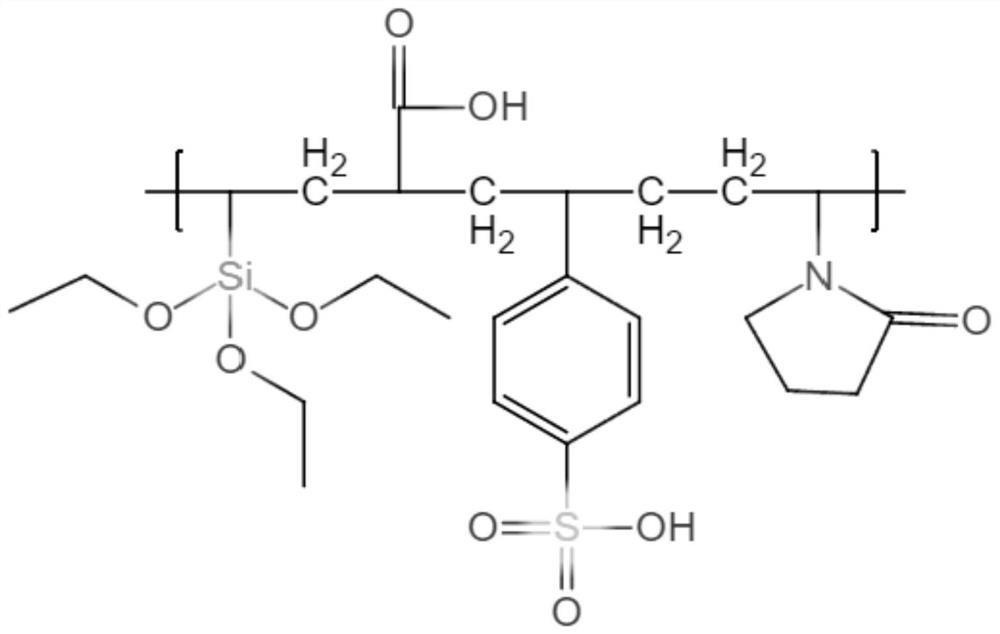
The invention belongs to the technical field of medical devices, and discloses a covered implantable medical device and a preparation method thereof. The implantable medical device comprises a skeleton material, the surface of the skeleton material is sequentially covered with a coating and a thermoplastic polymer film, the surface of the thermoplastic polymer material film is grafted with an anticoagulant compound; the coating is formed by condensation polymerization of diamine A and dibasic acid B in the form of ABAB head-to-tail bonding on the skeleton surface through amidation reaction; the anticoagulant compound has carboxyl or sulfonic acid groups, specifically one or more of VA64, low molecular weight heparin, heparinoid copolymer and diosgenin. Bonding strength between the polymermaterial and the skeleton material is improved by arranging the coating on the surface of the skeleton material, anticoagulation modification is performed on the covered skeleton material, and the problem about blood compatibility of the implantable device is solved.
Owner:WUHAN YONGSEN BIOTECH
A method for separating heparinoids from heparin by-product waste protein
The invention relates to a method for separating heparinoids from heparin by-product waste protein. The purpose is to provide a method for separating heparinoids from by-products of heparin to utilize waste and save cost, and it should have the characteristics of simple process and low cost. The technical solution is: a method for separating heparinoids from heparin by-product waste protein, which is characterized in that it includes the following steps: 1) making a solution of heparin by-product waste protein; 2) adding an adsorbent to the solution for adsorption; 3 ) washing and eluting the adsorbent, collecting the eluent and precipitating to obtain a precipitated solid 1; 4) making the precipitated solid 1 into a solution, followed by adjusting pH, oxidizing, adjusting pH, reducing, adjusting pH, adjusting salt concentration and precipitation to obtain the precipitated solid II; 5) drying the precipitated solid II to obtain the heparinoid.
Owner:ZHEJIANG CASING ANIMAL BY PROD CO LTD
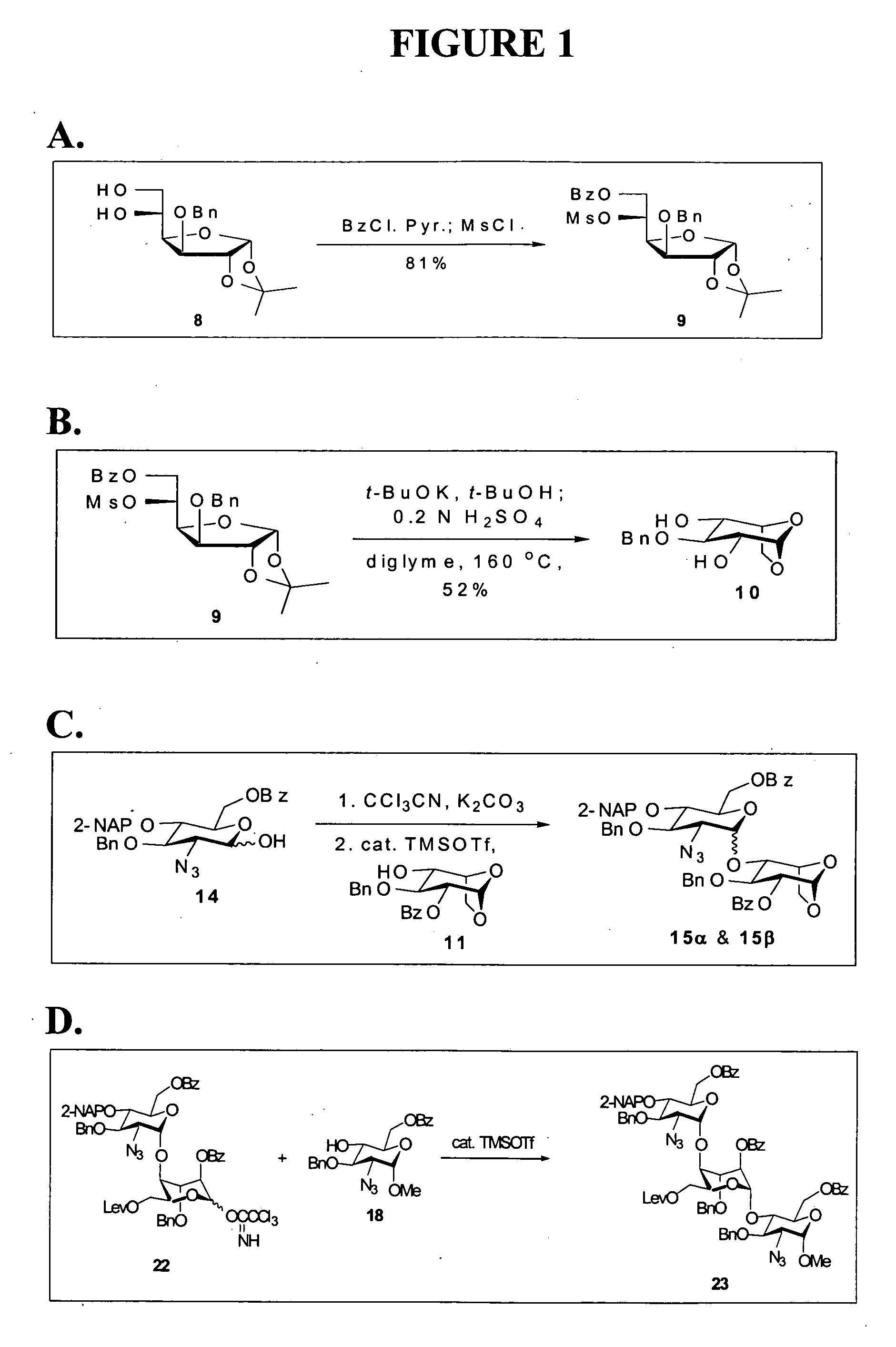
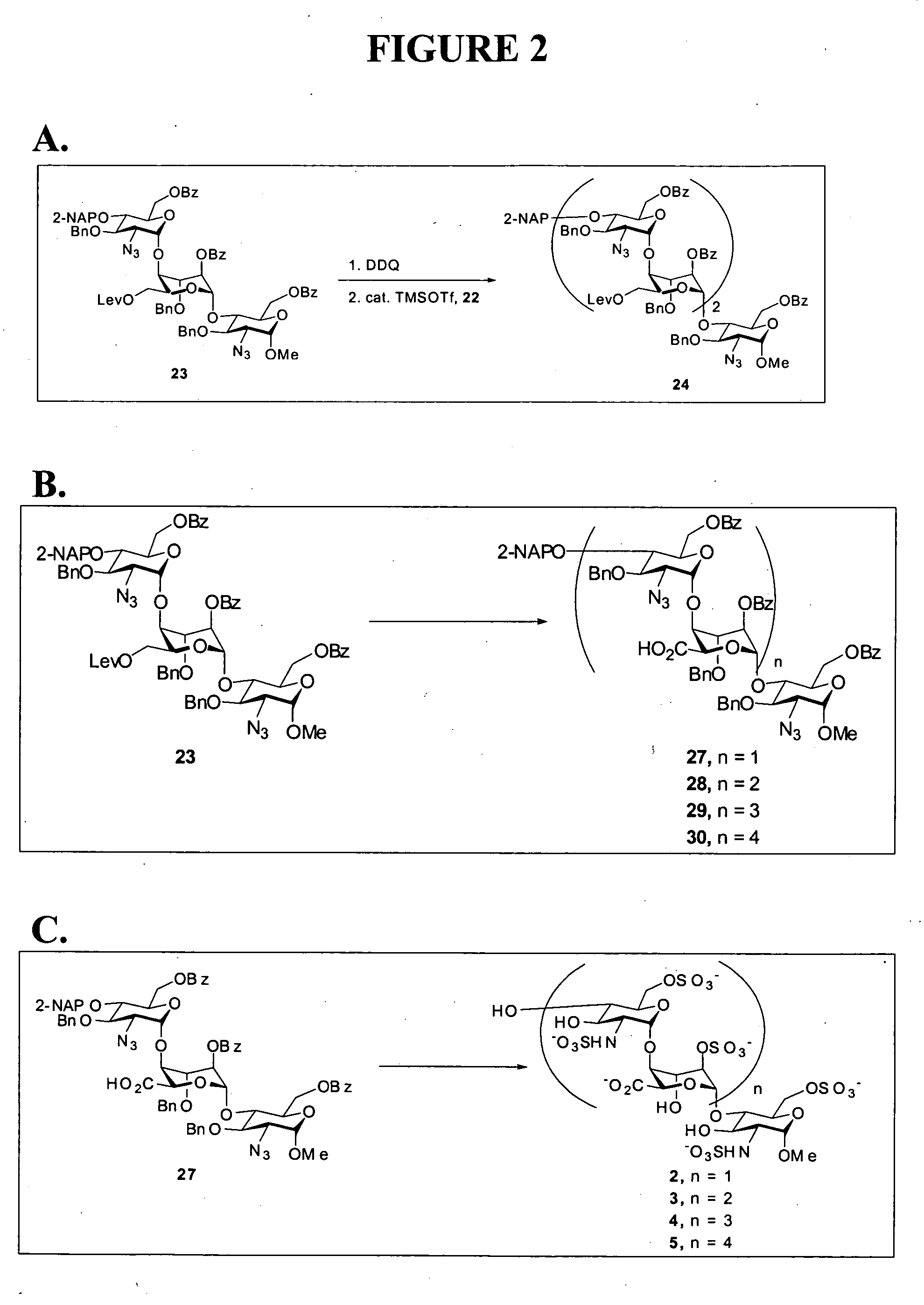
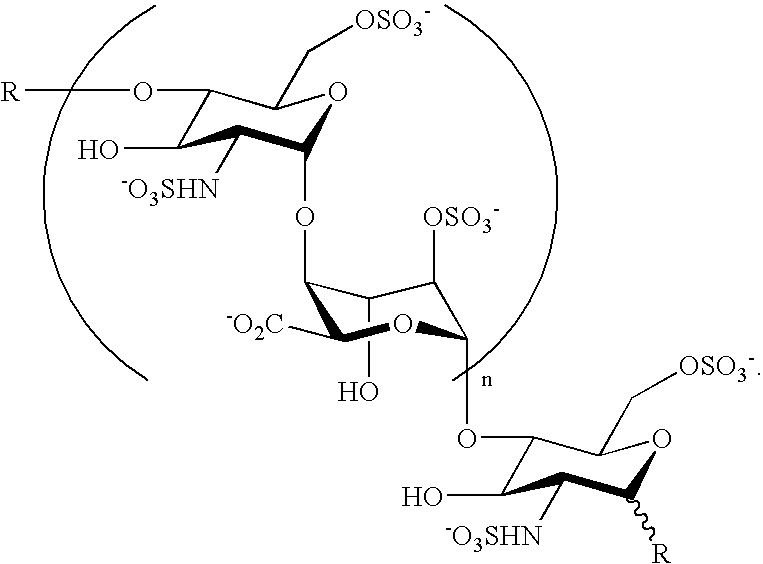
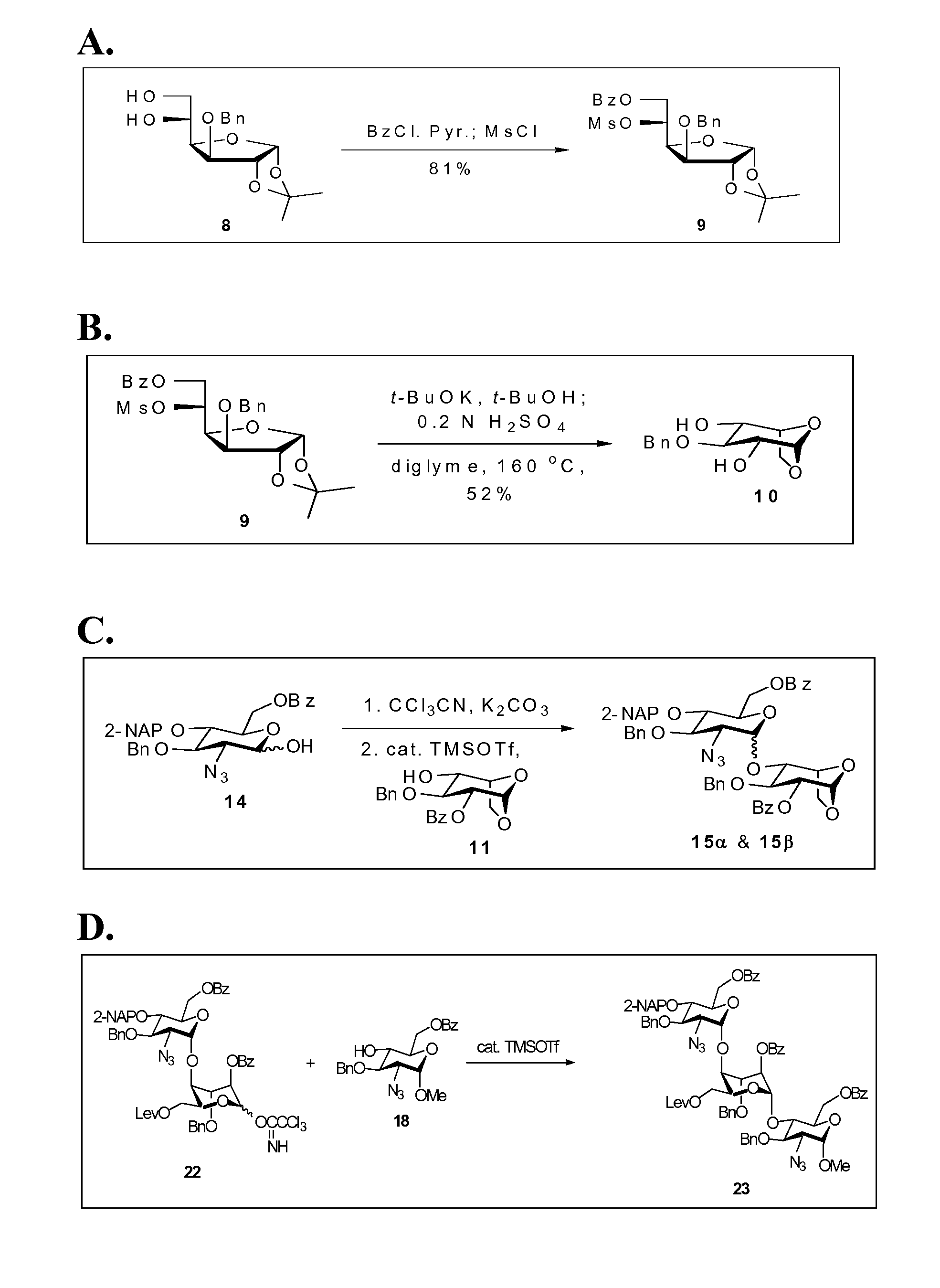
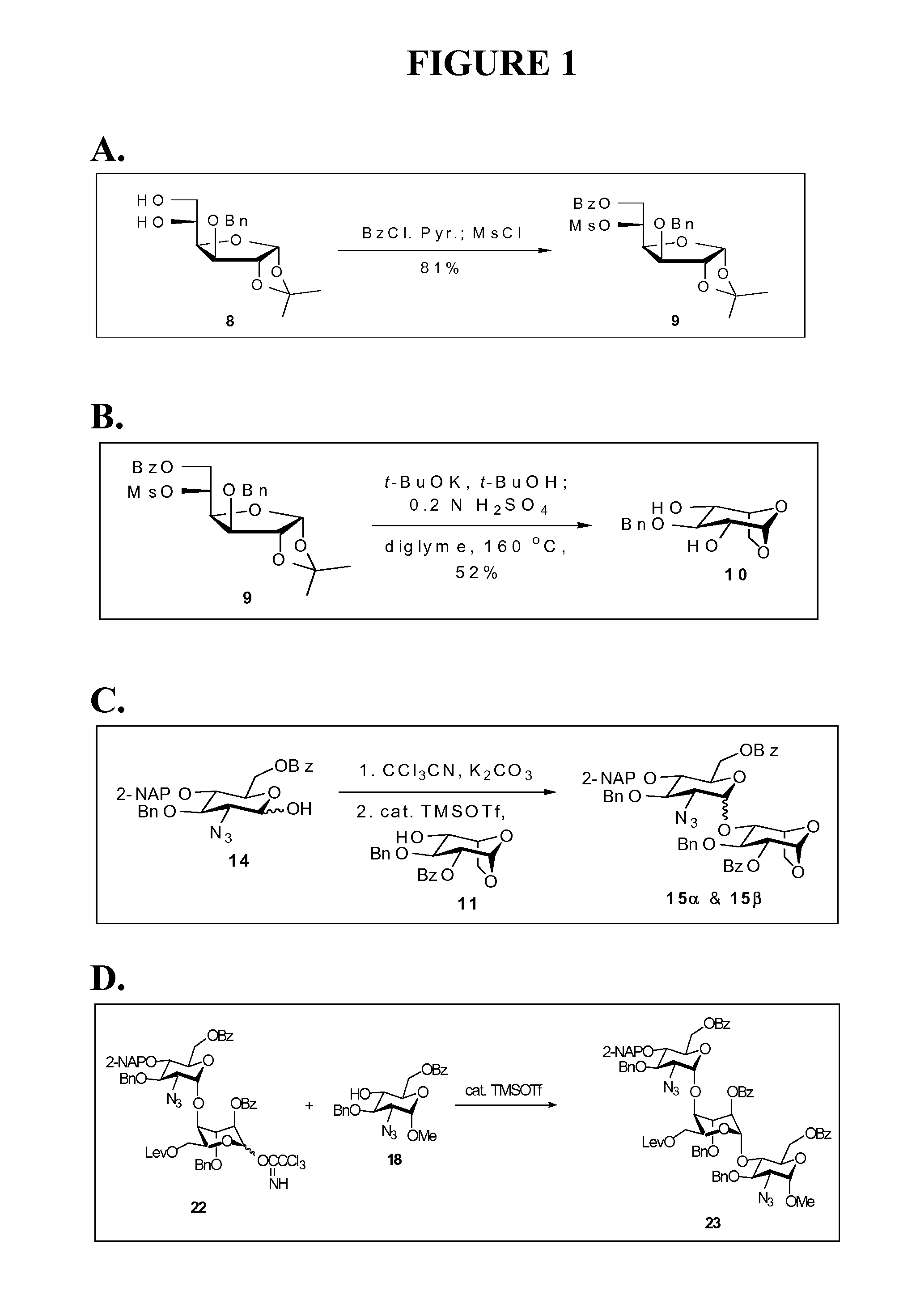
Disaccharide intermediate and synthesis method thereof
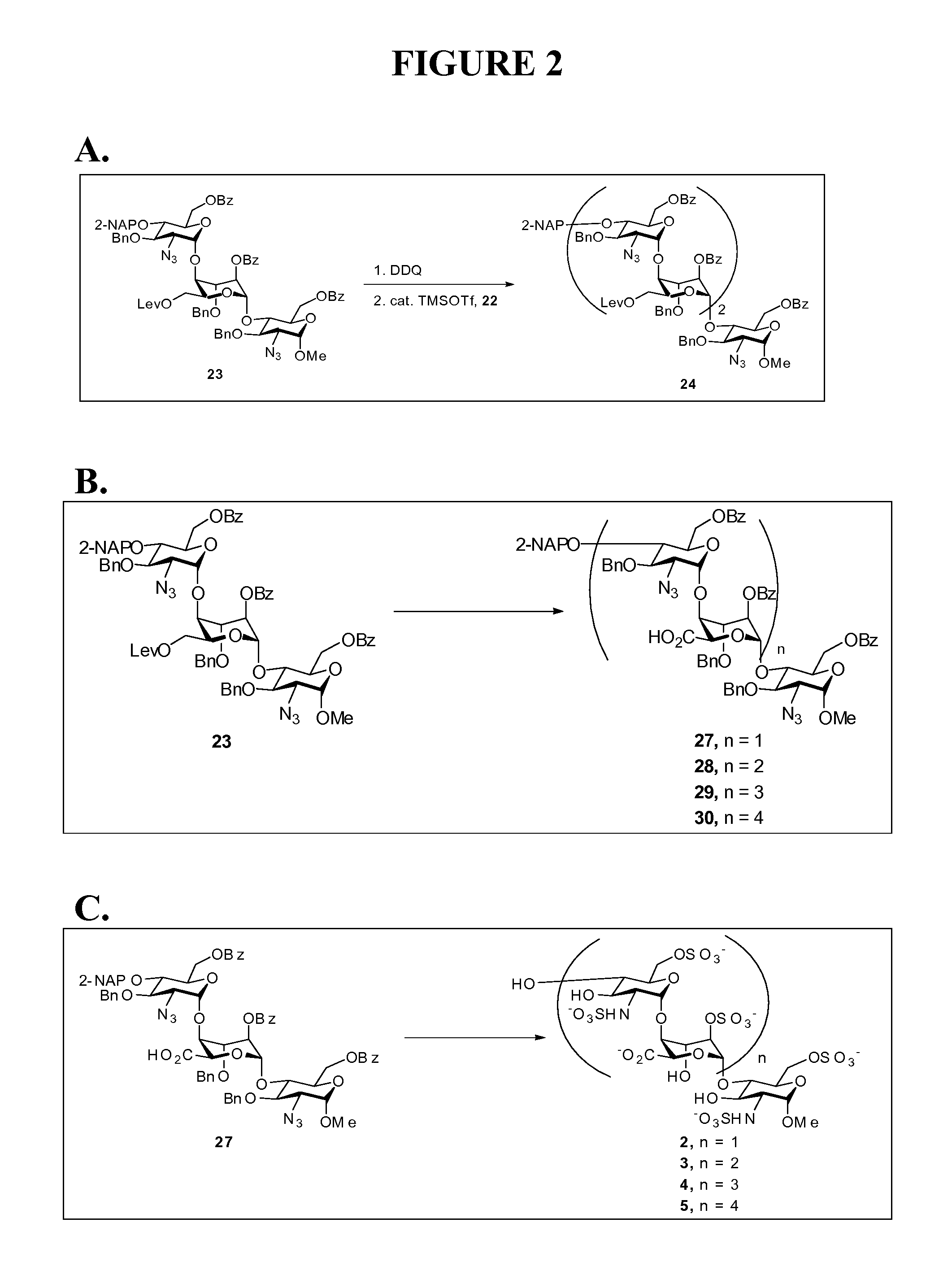
InactiveUS10287309B2High yieldHigh stereoselectivityEsterified saccharide compoundsSugar derivativesChemical synthesisSynthesis methods
The present invention relates to a disaccharide intermediate and a synthesis method thereof, relates to the chemical pharmaceutical field, and more specifically relates to a method for preparing a disaccharide segment of a key intermediate for chemically synthesizing heparin and heparinoid compounds. Disclosed are a new disaccharide intermediate and three methods for synthesizing the disaccharide intermediate, that is, compounds of a formula (4) and glucopyranose protected by different anomeric carbon are made to react in the presence of an active agent, to obtain the disaccharide intermediate. According to the technical solutions of the present invention, synthetic raw materials are easy to obtain, have a mild reaction condition, and are suitable for industrialized production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Use of non-catalytic form of heparanase and peptides thereof for reversing the anti-coagulant effects of heparinoids
The present invention relates to inhibition of heparinoids anti-coagulation activity by a non-active form of a eukaryotic endoglycosidase or any fragment or peptide thereof comprising at least one heparin-binding domain. More particularly, the invention provides compositions and methods for the inhibition of heparinoids anti-coagulation activity and for the treatment of coagulation related pathologic clinical conditions, using a non-active form of mammalian heparanase or peptides thereof comprising at least one heparin-binding domain.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
Method for manufacturing composition comprising local anesthetic, heparinoid, and buffer
InactiveCN103747790ARetain bioavailabilityRetain efficacyOrganic active ingredientsNervous disorderAnesthetic AgentUrinary Tract Diseases
An improved method for preparing a composition including a heparinoid, a local anesthetic, and a buffer for treatment and used for treating a lower urinary tract disease or condition, can comprise: (i) providing a heparinoid in solid form or liquid form; (ii) providing a local anesthetic in solid form or liquid form; (iii) adding a liquid buffer to the heparinoid in solid form or liquid form; (Iv) adding the local anesthetic to the mixture of the liquid buffer and the heparinoid; and (v) if necessary, adjusting the pH of the mixture of the liquid buffer, the local anesthetic, and the heparinoid so that a pH from about 6.8 to about 8.3 is achieved without precipitation of the local anesthetic.
Owner:C·洛维尔·帕森斯
Low density lipoprotein adsorption microsphere and preparation method and adsorption material
ActiveCN109092262AHigh anticoagulant activityIncrease costIon-exchange process apparatusOther chemical processesFiberDelta-Tocopherol
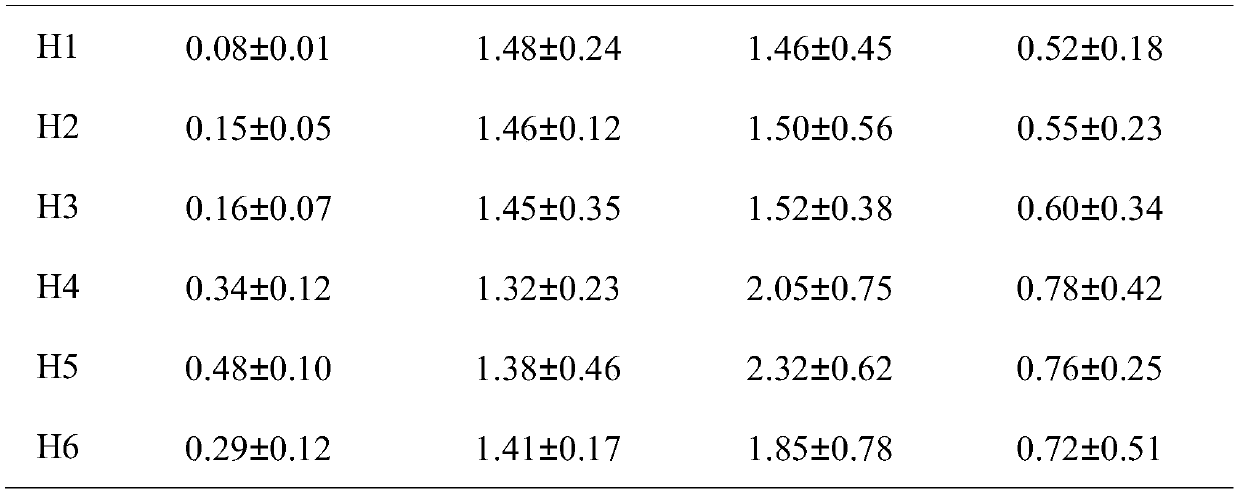
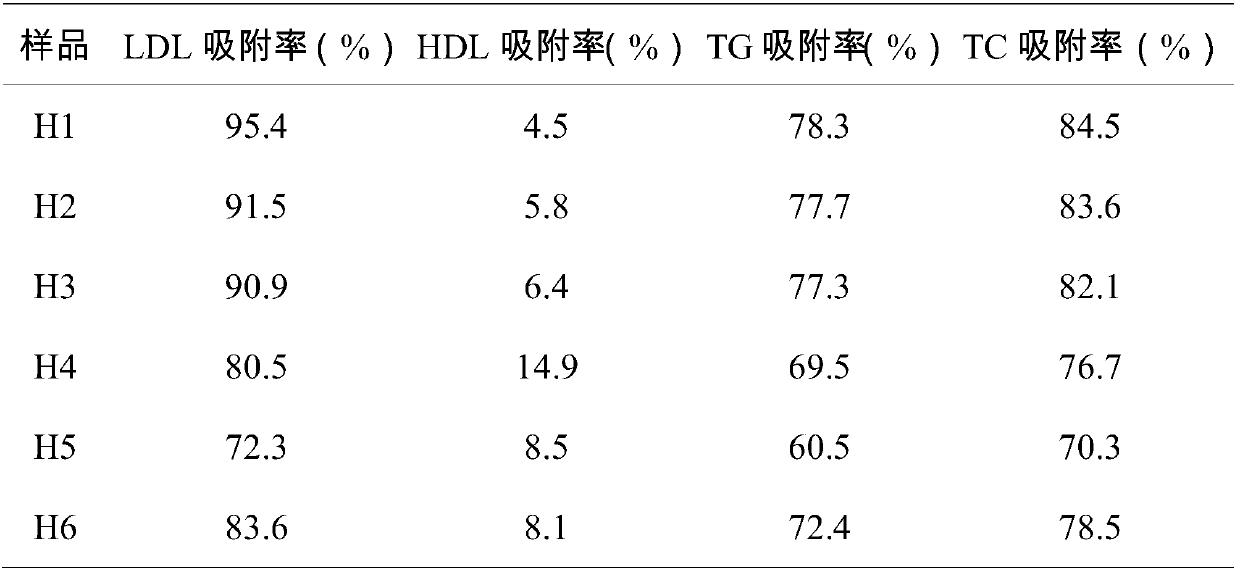
The invention belongs to the technical field of biological fibers and functional materials and specifically relates to a low density lipoprotein adsorption microsphere and a preparation method and anadsorption material. The low density lipoprotein adsorption microsphere comprises a carrier; a hydrophobic functional group and heparinoid are coupled to the carrier; the mass ratio of the carrier, the heparinoid and the hydrophobic functional group is 1:(0.1-0.5):(0.1-0.5); the hydrophobic functional group consists of Delta-tocopherol and Alpha-tocopherol, wherein the content of the Delta-tocopherol does not exceed 5-8wt%. The low density lipoprotein adsorption microsphere provided by the invention has the advantages that the adsorption selectivity for LDL in whole blood is high, the adsorption rate of the LDL can reach up to 95% and above; more importantly, the damage to erythrocyte is low; the erythrocyte decrement rate is only 3.21%; and meanwhile, the filtering speed is high, the costis low, and the low density lipoprotein adsorption microsphere is suitable for large-scale promotion and application.
Owner:广州达济医学科技有限公司
Methods of treating and preventing radiation damage
The invention relates to methods of treating and preventing radiation damage from whole-body exposure. According to the methods of the invention, subjects are treated therapeutically and / or prophylactically with low-anticoagulant heparinoids. The invention also relates to methods of extending the life of subjects exposed to whole-body radiation.
Owner:CANTEX PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com