Use of dermatan sulfates and/or desulfated heparins to treat or prevent heparinoid-induced autoimmune responses
Inactive Publication Date: 2005-11-24
CELSUS BIOPHARMACEUTICALS INC
View PDF26 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
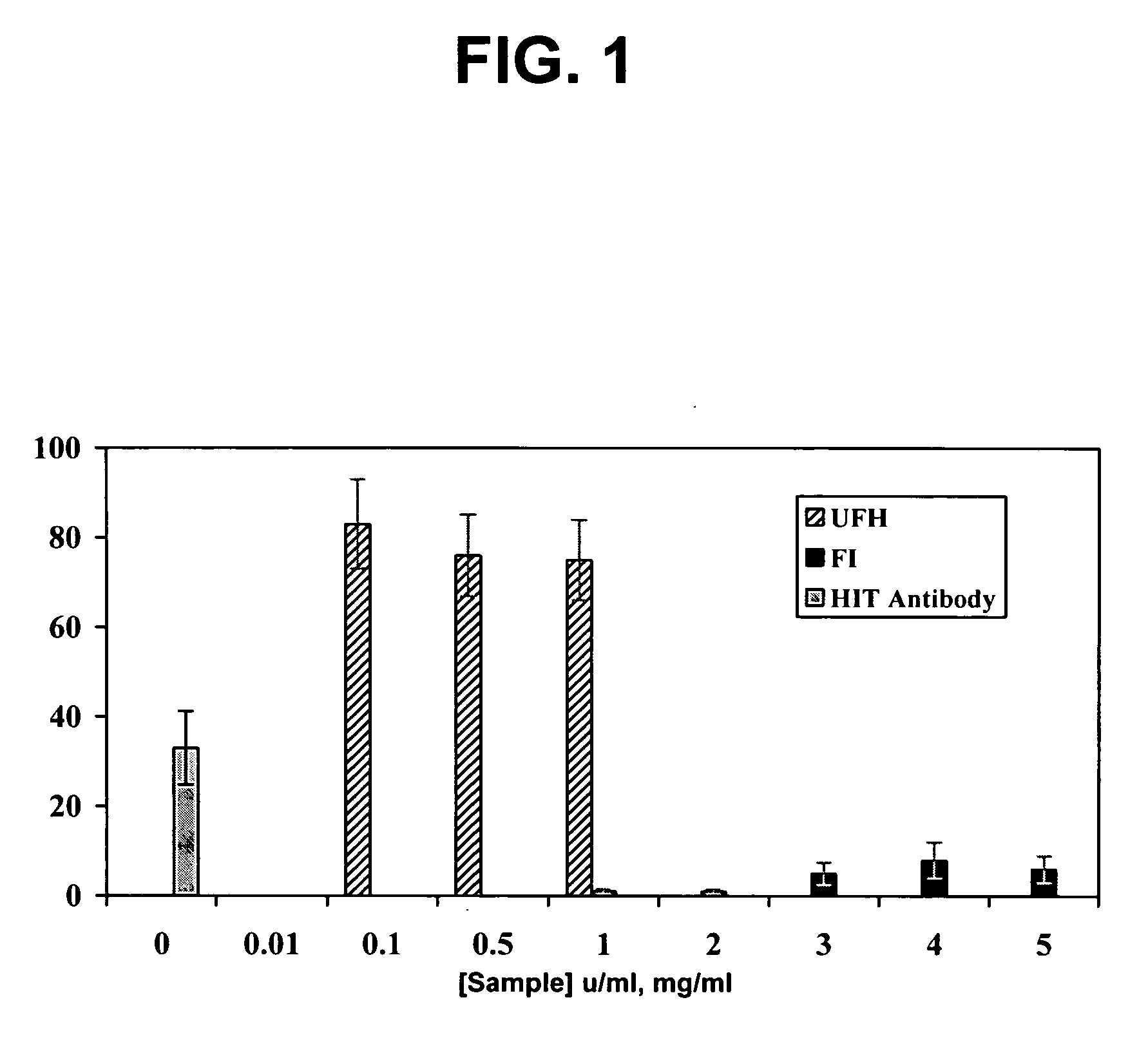
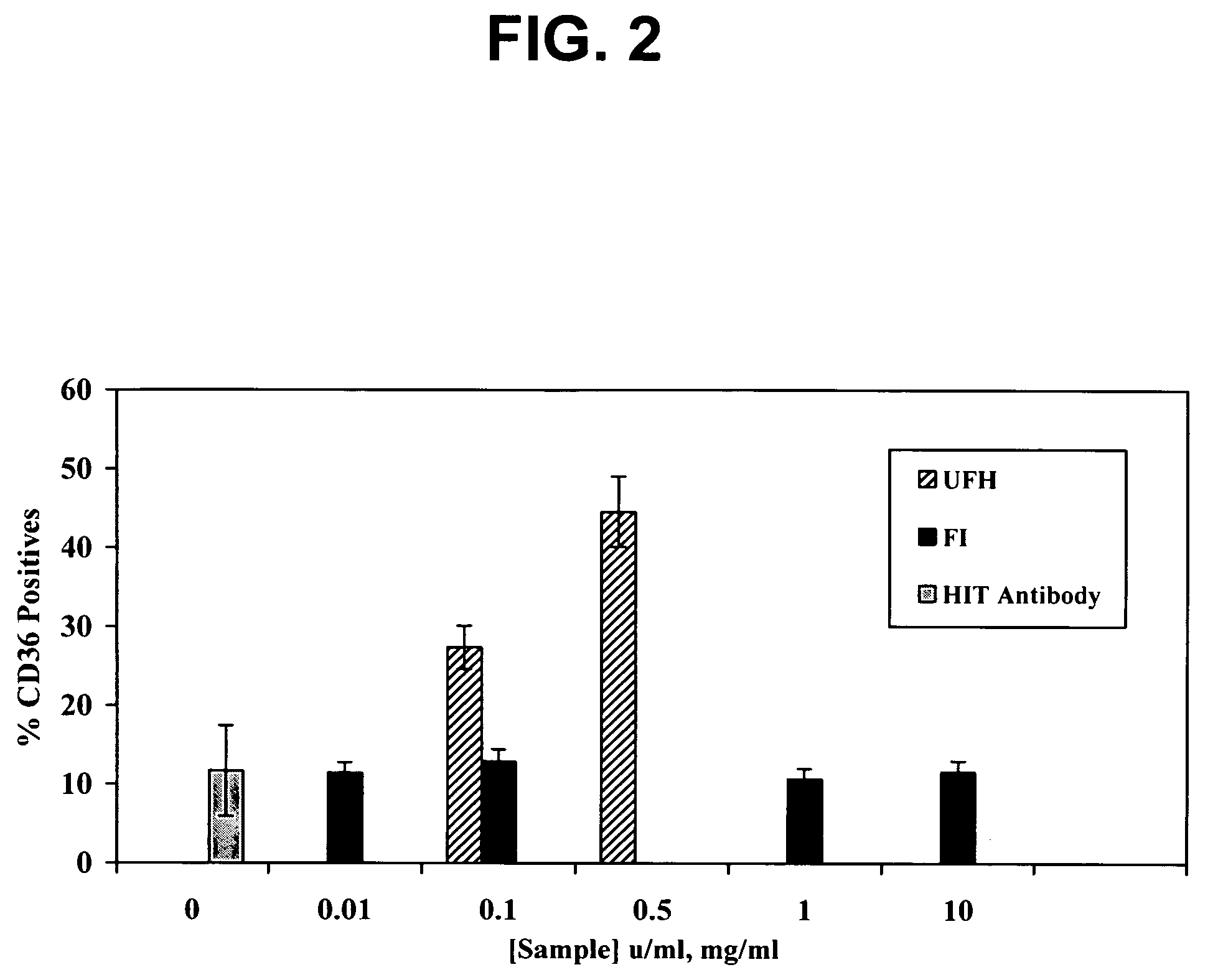
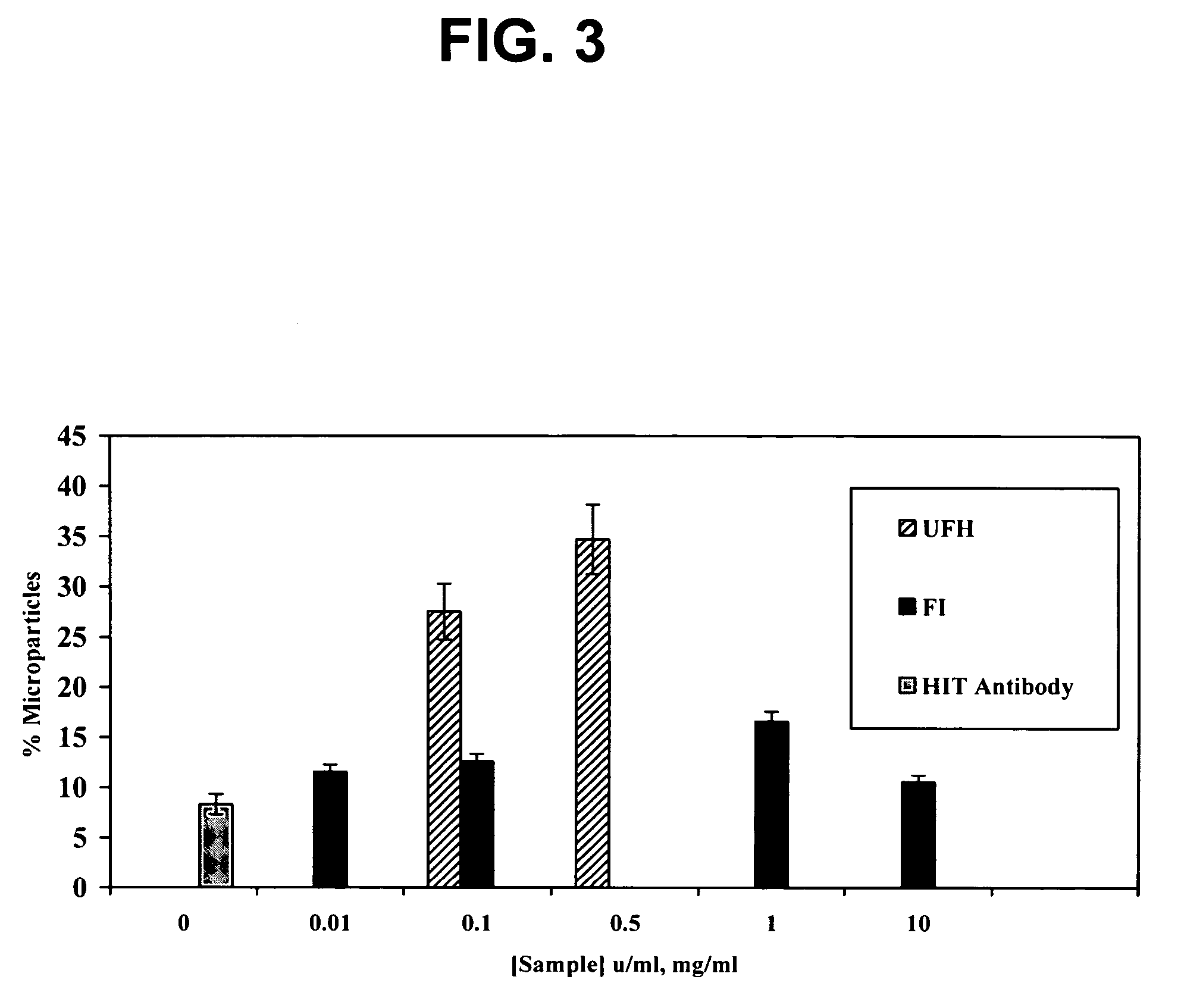
[0020] The dermatan sulfates and/or O-desulfated heparins of this invention unexpectedly prevent the activation of platelets and endothelial cell damage caused by the immune complexes of HIT, even in the presence of administered heparin, a property or activity referred to as “HIT antagonism.” The HIT antagonist properties of the dermatan sulfates and/or O-desulfated heparins of this invent
Problems solved by technology
In contrast to the hemorrhagic thrombocytopenia disease states, HIT is associated with an increased risk of thrombosis.
Historically, about 40% of these HIT patients develop a life-threatening thrombosis or HITT syndrome that produces devastating complications including necrosis of the extremities, stroke, myocardial infarction and pulmonary embolism.
Thus, it has been previously concluded that hyper- or oversulfation of GAGs is an inappropriate approach to anticoagulation based on the HIT inducing potential of sulfated GAGs, and that to reduce the ability of a GAG to induce a HIT response: (i) the nature of the glycosidic bond is unimportant; (ii) the molecule should be unbranched; (iii) the molecule should have a degree of sulfation (i.e., the number of sulfate groups/monosaccharide) of less than 0.60 (i.e., a sulfate to carboxylate (S/C) ratio of no more than 1.2) for chain lengths greater than 10 monosaccharides (i.e., greater than 3000 Daltons); (iv) the molecule should be smaller than 2400 Daltons if its degree of sulfation is 1.0-1.3; and (v)
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Login to View More
Abstract
Dermatan sulfates and/or O-desulfated heparins useful in treating and preventing heparinoid-induced autoimmune responses, in particular heparin-induced thrombocytopenia (HIT) and its associated disease states. The dermatan sulfates comprise repeating disulfated and/or trisulfated disaccharide units of L-iduronic acid and N-acetyl-D-galactosamine. The O-desulfated heparins comprise heparin molecules selectively O-desulfated at the 2-O and/or 3-O positions of the uronic acid and glucosamine saccharide residues. Particularly effective dermatan sulfate HIT antagonists have a mean molecular weight of from about 2000 to about 10,000 Daltons.
Description
CROSS-REFERENCE TO RELATED APPLICATIONS [0001] This application makes reference to the following co-pending U.S. Provisional Patent Application No. 60 / 572,364, filed May 19, 2004. The entire disclosure and contents of the above application is hereby incorporated by reference.GOVERNMENT INTEREST STATEMENT [0002] This invention was made in part with Government support under Grant No. HL-66-646-01 and HL-70-453-01 awarded by the National Institute of Health, National Heart, Lung & Blood Institute. The Government may have certain rights to the invention.BACKGROUND [0003] 1. Field of the Invention [0004] The present invention relates to treating and / or preventing heparinoid-induced autoimmune responses with certain dermatan sulfates and / or certain O-desulfated heparins. The present application particularly relates to the treatment and / or prevention of heparin-induced thrombocytopenia (HIT) and its associated disease states with certain dermatan sulfate and / or certain O-desulfated heparin...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/737
CPCA61K31/737A61L27/54A61L29/16A61L31/16A61L2300/236A61K2300/00A61P37/00
Inventor CARDIN, ALAN D.
Owner CELSUS BIOPHARMACEUTICALS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com