Plaster for topical use containing heparin and diclofenac
a technology of diclofenac and heparin, which is applied in the field of topical use of heparin and diclofenac, and can solve the problems of greasy residue on the skin, inability to achieve uniform dosing of active ingredients, and considerable inconvenien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
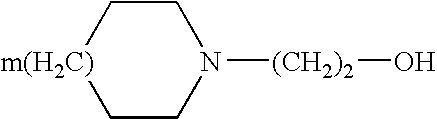
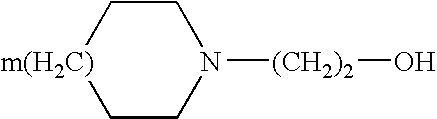
[0013] In the plaster according to the present invention, diclofenac is generally present in the form of a pharmaceutically acceptable salt, and preferably it is a salt with a heterocyclic amine of general formula: 1
[0014] where m is 0 or1.
[0015] According to a particularly preferred embodiment, the heterocyclic amine is N-hydroxyethyl pyrrolidine (epolamine).
[0016] The diclofenac salt is contained in the plaster according to the present invention in concentrations generally ranging from 0.1 to 5 wt %, preferably in concentrations of between 0.3 and 3 wt % with respect to the total weight of the composition used for the preparation of the hydrogel matrix.
[0017] According to a particularly preferred embodiment, the concentration of the diclofenac salt is 1.3 wt % with respect to the total weight of the composition used for the preparation of the hydrogel matrix.
[0018] When the plaster according to the present invention contains a heparinoid, the latter preferably presents a molecular...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adhesive | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| total weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com