Separation method of heparan sulfate and dermatan sulfate in heparinoid
A technology of heparan sulfate and dermatan sulfate, which is applied in the field of medicine, can solve problems such as the inability to separate heparan sulfate and dermatan sulfate in heparan or precise quantitative control, and achieve the effect of easy amplification, good effect and avoiding loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] This embodiment provides a method for separating heparan sulfate and dermatan sulfate in heparan, which comprises the following steps:
[0022] (a) 40 g of heparinoid (specific optical rotation +5°, anticoagulant factor Xa activity 15 IU / mg) was dissolved in 400 ml of deionized water to prepare a 10% heparinoid solution;
[0023] (b) Inject the heparinoid solution into a polyacrylate anion exchange column with a column volume of 4 L for adsorption;
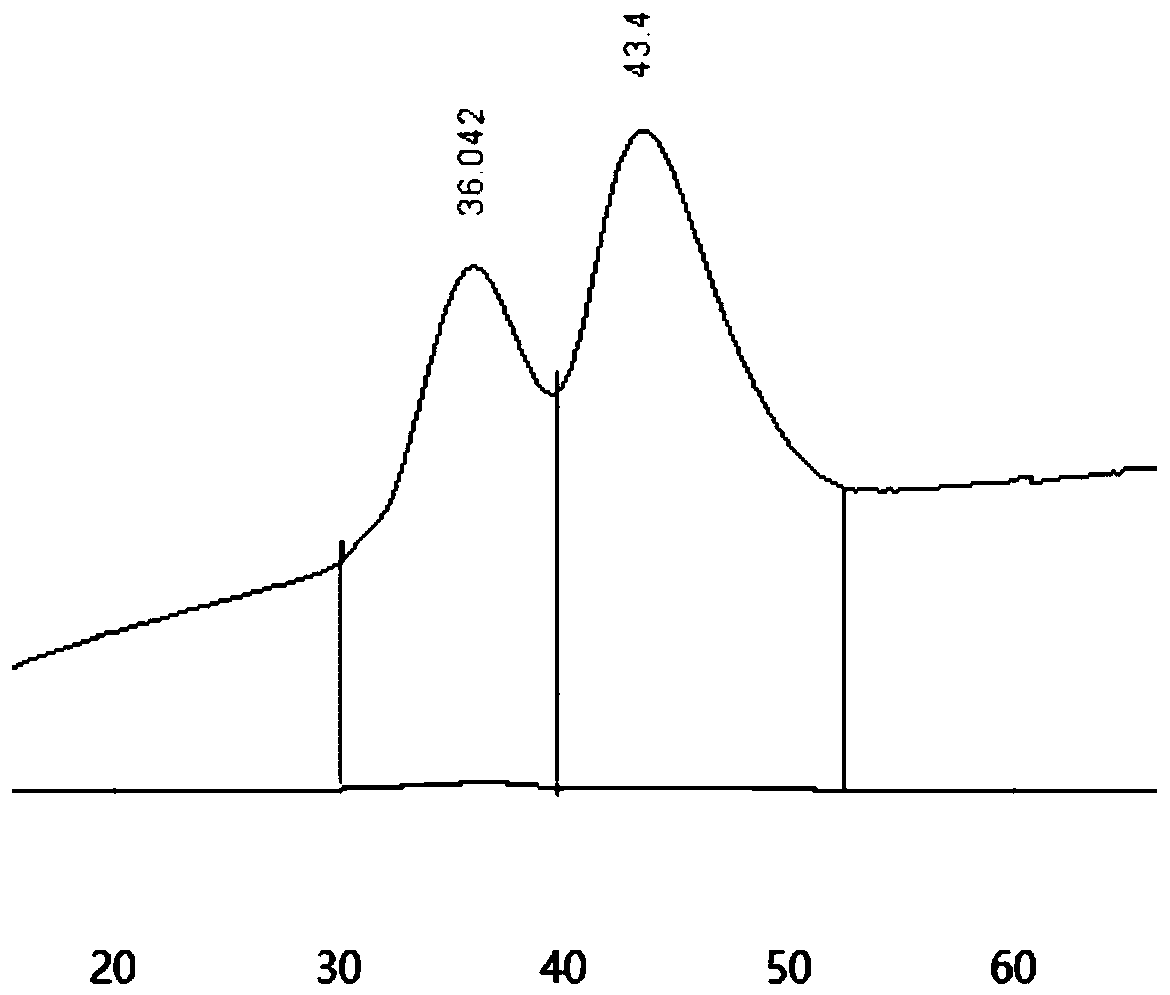
[0024] (c) Use 0~1mol / L sodium chloride solution for gradient elution (that is, linear gradient elution), the elution time is 100 minutes, and collect the eluent of each component; track with an ultraviolet detector (210nm ) to get as figure 1 The spectrogram shown;
[0025] (d) Concentrate the eluent of each component respectively (concentrate the eluent of each component to a sodium chloride concentration of 3~4wt%), add ethanol precipitation (so that each component elutes the ethanol in the mixed solution The concentr...
Embodiment 2
[0028] This example provides a method for separating heparan sulfate and dermatan sulfate in heparan, which is basically the same as the method in Example 1, except that: in step (a), a 5% heparan solution is prepared; finally 17 g of heparan sulfate and 17.5 g of dermatan sulfate were obtained.
Embodiment 3
[0030] This example provides a method for separating heparan sulfate and dermatan sulfate in heparan, which is basically the same as the method in Example 1, except that: in step (a), an 8% heparan solution is prepared; finally 17.2 g of heparan sulfate and 18.1 g of dermatan sulfate were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com