Method for precisely and quantitatively controlling chondroitin sulfate and dermatan sulfate contents of heparin/heparinoid
A technology of dermatan sulfate and chondroitin sulfate, applied in the field of precise and quantitative control of chondroitin sulfate and dermatan sulfate content in heparin/heparin, can solve the problems affecting the safety of heparin product use and small production scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1, refined danaparin sodium sample
[0092] 1. Content analysis method
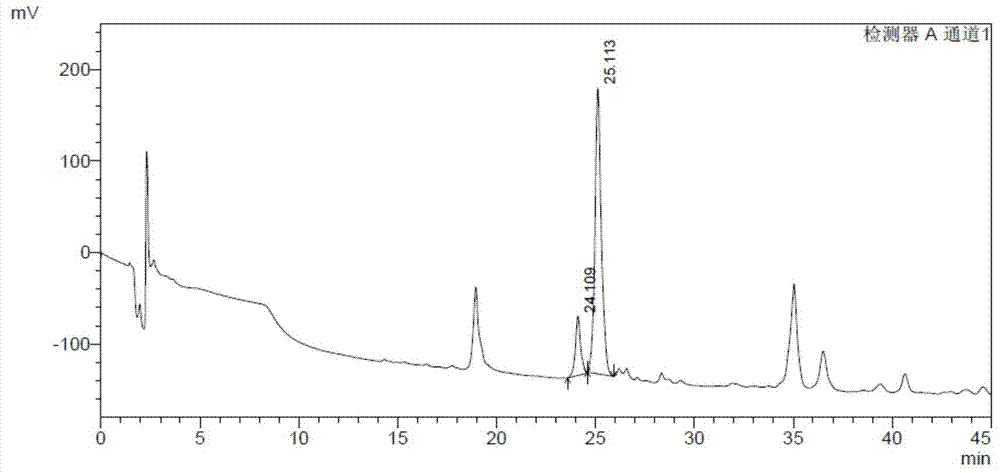
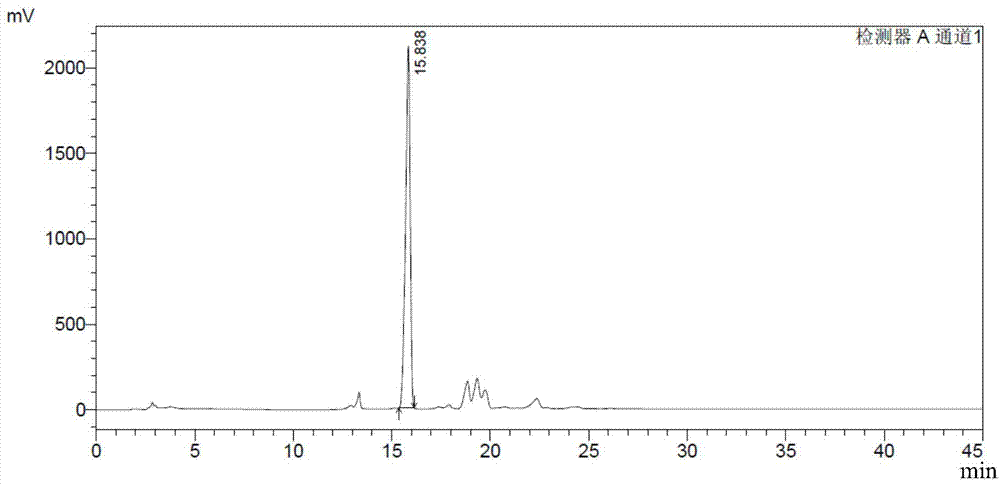
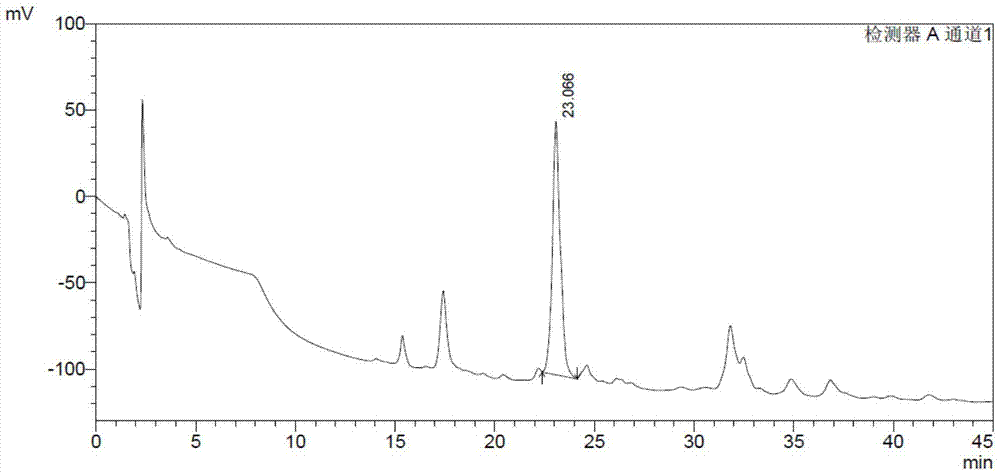
[0093] 1.1 DS content analysis method: prepare the test sample solution 10mg / ml with Tris buffer solution of pH 7.4, take 1ml of the sample solution, add 1IU of MBP-ChSase B at 25°C for 24h, and then use HPLC analysis (specific chromatogram The conditions are as follows), and the DS content was calculated by the external standard curve. The detection limit of DS content is 0.01%. Compared with the DS content analysis method recorded in European Pharmacopoeia 7.0 (EP7.0), the HPLC analysis method has high sensitivity and good repeatability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com