Covered implantable medical device and preparation method thereof
A technology for implanting medical devices and coatings, applied in the field of implanted medical devices and their preparation, can solve problems such as increasing platelet attachment, activating the thrombin system, poor blood compatibility, and poor prognosis of patients, so as to promote chemical Effects of cross-linking, good anticoagulant properties, and good economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
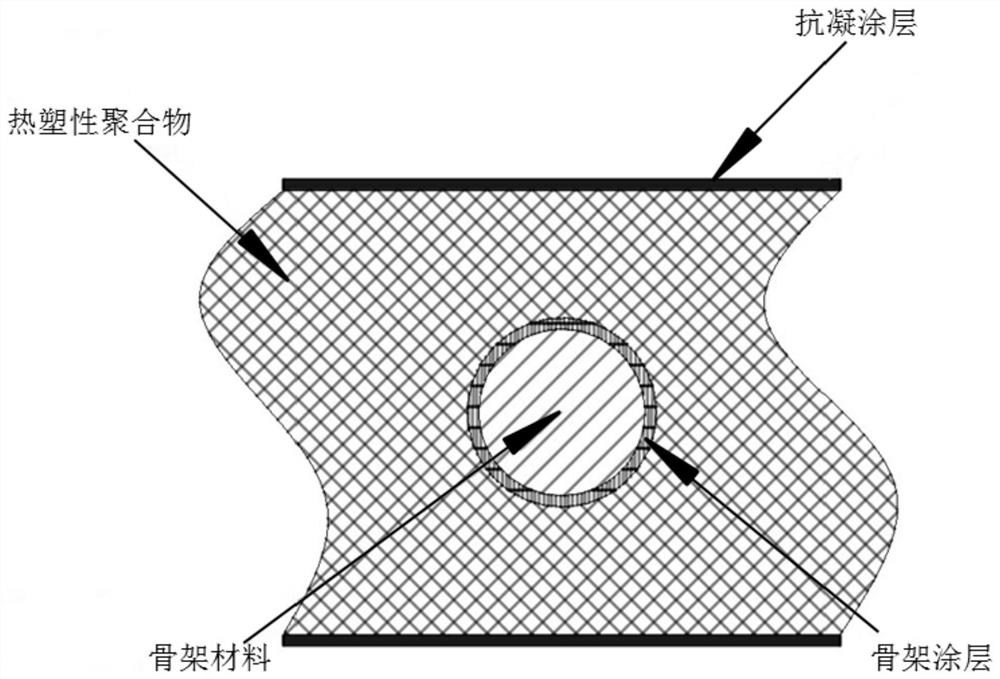
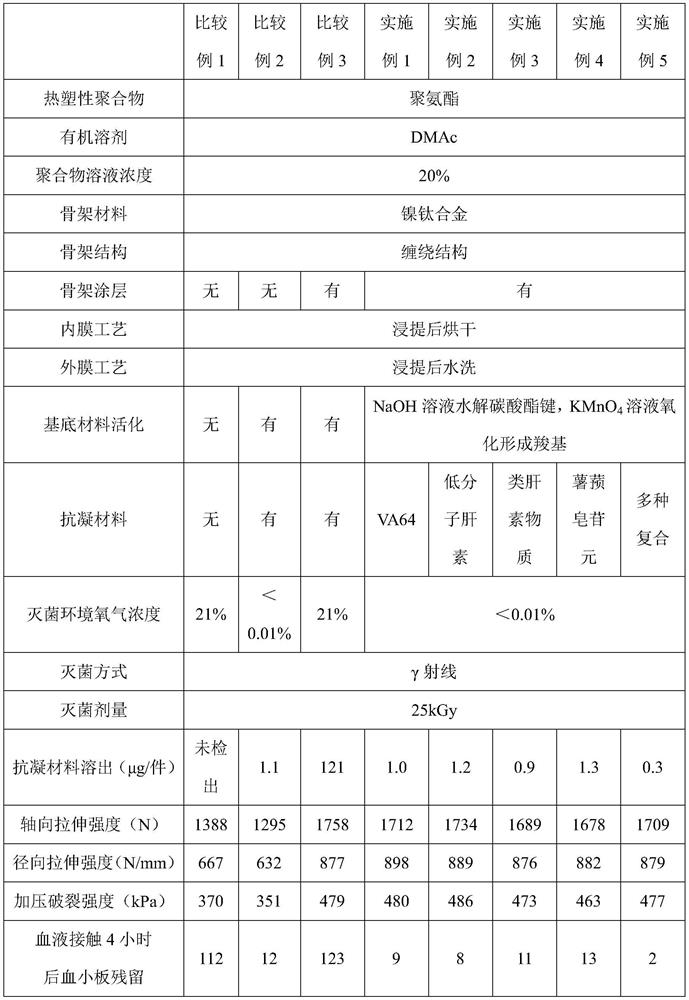
[0034] A covered artificial blood vessel, wherein the skeleton material is a polyester thread, the coating is a copolymer of hexamethylene diamine and pimelic acid, the thickness is 5 nm, the thermoplastic polymer is polyurethane, and the anticoagulant material is VA64.
[0035] The preparation method is specifically as follows:
[0036] Step 1: Dissolve the thermoplastic polymer polyurethane in the DMAc organic solvent to prepare a 20wt% thermoplastic polymer solution, defoam, attach the solution to the mold surface by electrostatic spinning, and dry to remove the organic solvent;
[0037] Step 2. After pretreatment with 5wt% sodium hydroxide solution for 8 hours, the surface molecular chains are partially hydrolyzed to form active carboxyl functional groups and then introduced into the coating. The coating is composed of hexamethylene diamine and pimelic acid in the skeleton through amidation reaction. The surface is polymerized in the form of ABAB head-to-tail bonding.
[0038] Ste...
Embodiment 2
[0043] A covered artificial blood vessel, wherein the coating is octamethylene diamine and tridecanedioic acid copolymer, the thickness is 5nm, the thermoplastic polymer is polyurethane, and the anticoagulant compound is low molecular heparin.
[0044] The preparation method is specifically as follows:
[0045] Steps together Example 1
[0046] Step 2: After pretreatment with 5wt% sodium hydroxide solution for 8 hours, the surface molecular chain is partially hydrolyzed to form active carboxyl functional groups and then introduced into the coating, the coating is octamethylene diamine and tridecanedioic acid through amidation reaction It is polymerized in the form of ABAB head-to-tail bonding on the surface of the framework.
[0047] Step three and step four are the same as in Example 1
[0048] Step 5: In 0.1wt% of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide aqueous solution, add 2wt% of polyethyleneimine (Mw=600Da) to make it and polyurethane For the reaction, the quaternization r...
Embodiment 3
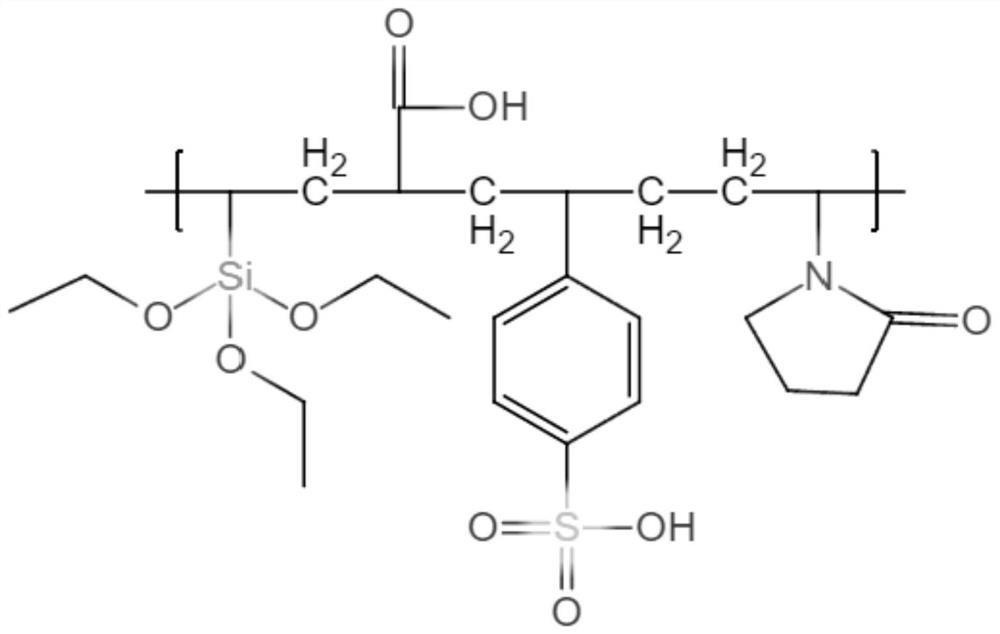
[0052] A covered artificial blood vessel, wherein the coating is: a copolymer of decamethylene diamine and eicosandioic acid, the thickness is: 5nm, the thermoplastic polymer is polyurethane, and the anticoagulant compound is a heparin-like copolymer, wherein Heparinoid copolymer is made by addition polymerization of vinyl triethoxy silane, N-vinyl pyrrolidone and sodium p-styrene sulfonate.
[0053] The preparation method is specifically as follows:
[0054] Steps together Example 1
[0055] Step 2. After pretreatment with 5wt% sodium hydroxide solution for 8 hours, the surface molecular chains are partially hydrolyzed to form active carboxyl functional groups and then introduced into the coating. The coating is hexamethylene diamine and adipic acid through an amidation reaction on the surface of the skeleton with ABAB head It is polymerized in the form of tail bonding.
[0056] Step three and step four are the same as in Example 1
[0057] Step five is specifically:
[0058] Take 5.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com