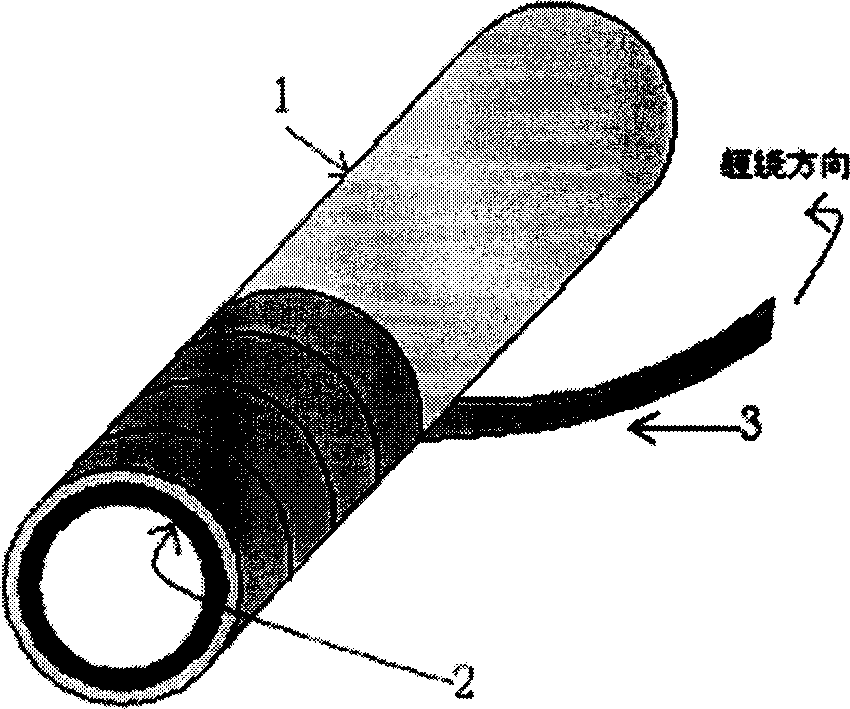
Angiosupport made of polyamide and polyhydroxy fatty acid ester comixture and its preparation method
A technology of polyhydroxyalkanoate and vascular stent, which is applied in the field of biomedical engineering and can solve the problems such as no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of a PHB-HV / PU vascular stent with a diameter of 8 mm and a length of 50 mm
[0034] 1. Preparation of PHB-HV / PU solid film
[0035] ①, Mix PHB-HV and chloroform in a flask, mixing ratio: 1gPHB-HV plus 20ml chloroform, heat in a water bath, reflux for 15 minutes, and prepare a uniform solution. Mix PU and tetrahydrofuran in another flask, mixing ratio: 1gPU plus 20ml tetrahydrofuran, heat in a water bath, reflux for 15 minutes, and prepare a homogeneous solution.
[0036] ② Use a pipette to pipette the PU solution according to the proportion (the mass percentage of PU is 5%), slowly add it dropwise to the PHB-HV solution, stir while adding, condense and reflux for 15 minutes.
[0037] ③, using the method of cast film formation, cool the solution prepared in ② and pour it into a pair of evaporating dishes with a diameter of 10cm, fasten the upper and lower evaporating dishes, and slowly volatilize chloroform and tetrahydrofuran within 2 days , an...
Embodiment 2
[0053] Example 2: Preparation of a PHB / PU vascular stent with a diameter of 3 mm and a length of 10 mm
[0054] 1. Preparation of PHB / PU solid film
[0055] The preparation method is the same as the method for preparing the PHB-HV / PU solid film in Example 1, except that the PHB-HV / PU is replaced by PHB / PU, the percentage of PU is adjusted to 10%, and the diameter of the evaporating dish is adjusted to 6cm.
[0056] 2. Preparation of PHB / PU porous membrane
[0057] The preparation method is the same as the method for preparing the PHB-HV / PU solid film in Example 1, except that the PHB-HV / PU is replaced by PHB / PU, the percentage of PU is adjusted to 10%, and the diameter of the evaporating dish is adjusted to 6cm, the particle size of NaCl is adjusted to 200-300μm.
[0058] 3. Preparation of PHB / PU reinforcement
[0059] The preparation method is the same as the method for preparing the PHB-HV / PU reinforcing rib in Example 1, except that PHB-HV / PU is replaced by PHB / PU.
[...
Embodiment 3
[0062] Example 3: Preparation of a PHB-HH / PU vascular stent with a diameter of 12 mm and a length of 100 mm
[0063] 1. Preparation of PHB-HH / PU solid film
[0064] The preparation method is the same as the method for preparing the PHB-HV / PU solid film in Example 1, except that PHB-HV / PU is replaced by PHB-HH / PU, the percentage of PU is adjusted to 20%, and the diameter of the evaporating dish Adjusted to 19cm.
[0065] 2. Preparation of PHB-HH / PU porous membrane
[0066] The preparation method is the same as the method for preparing the PHB-HV / PU porous membrane in Example 1, except that PHB-HV / PU is replaced by PHB-HH / PU, the percentage of PU is adjusted to 20%, and the diameter of the evaporating dish is adjusted to 20%. Adjust to 19 cm, and adjust the particle diameter of NaCl to 200 to 300 μm.
[0067] 3. Preparation of PHB-HH / PU reinforcement
[0068] The preparation method is the same as the method for preparing the PHB-HV / PU reinforcing rib in Example 1, except tha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com