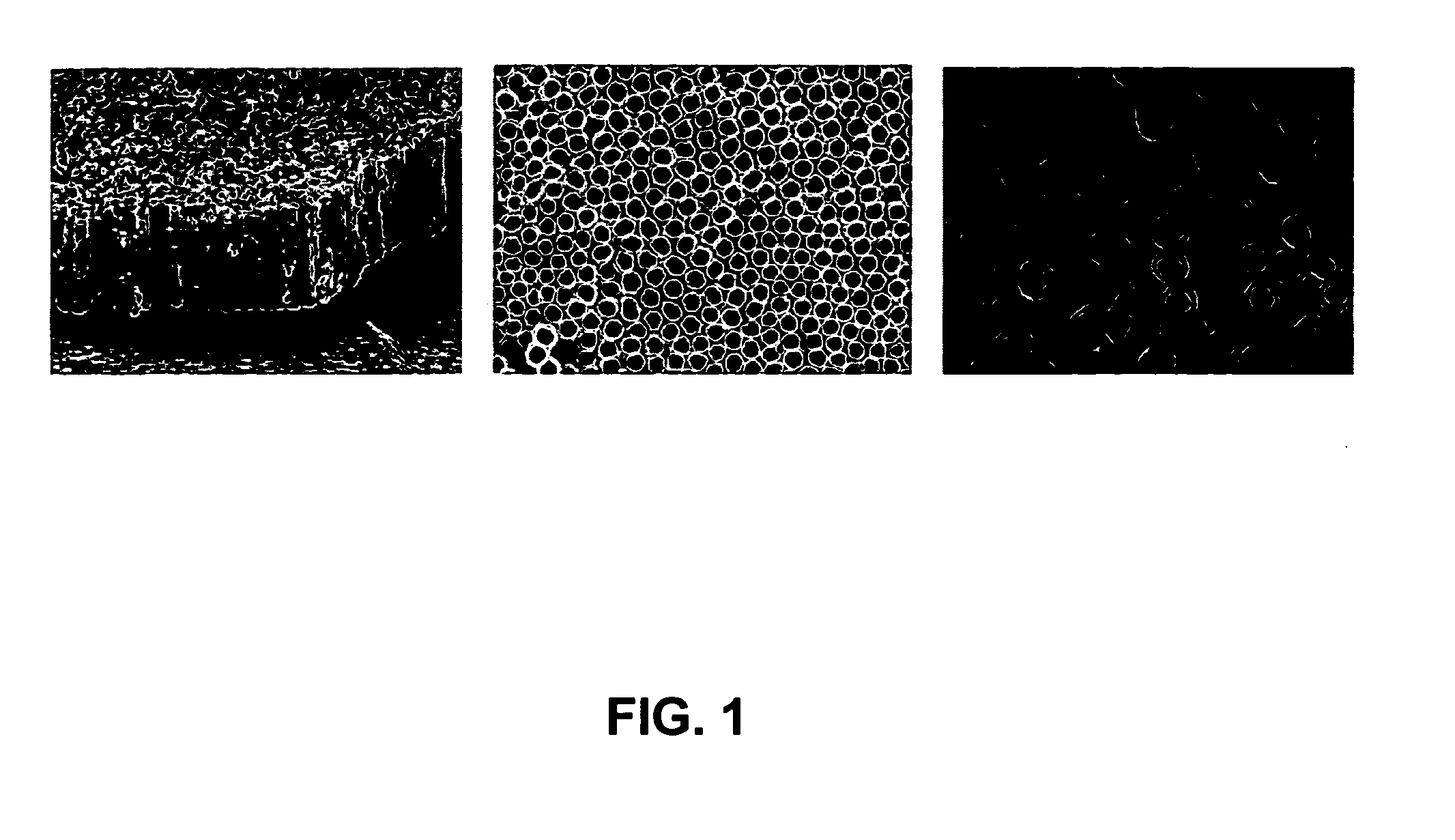
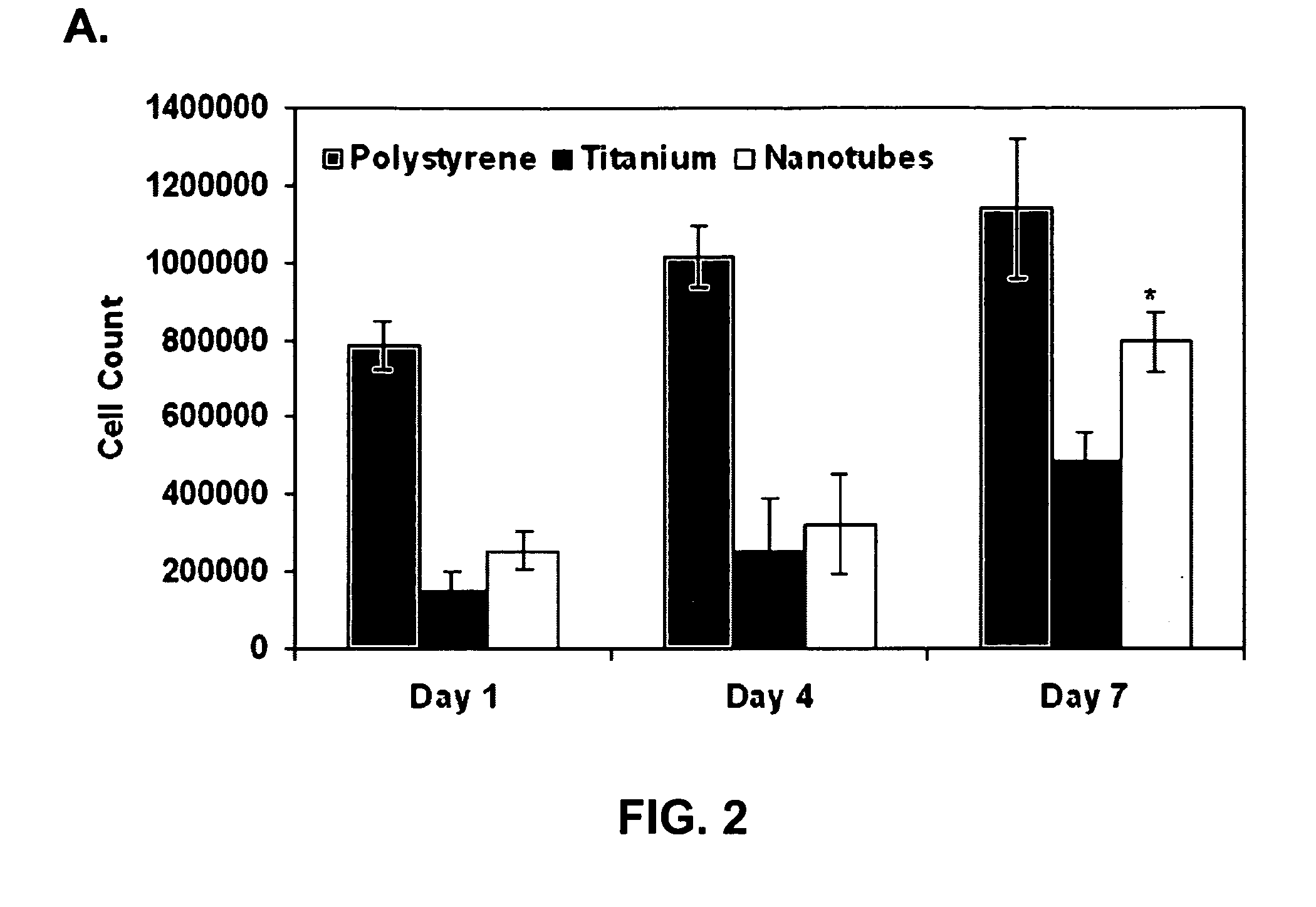
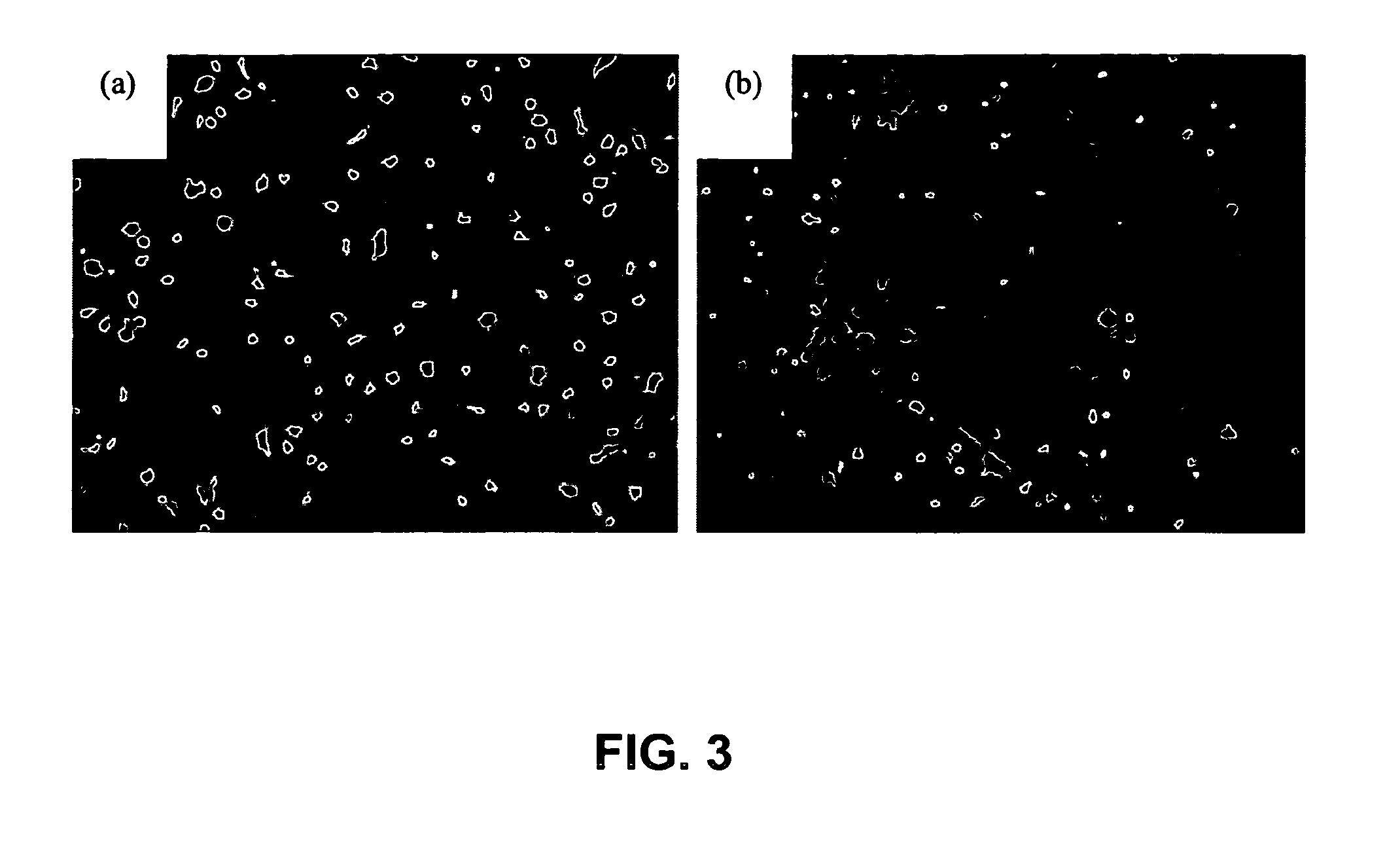
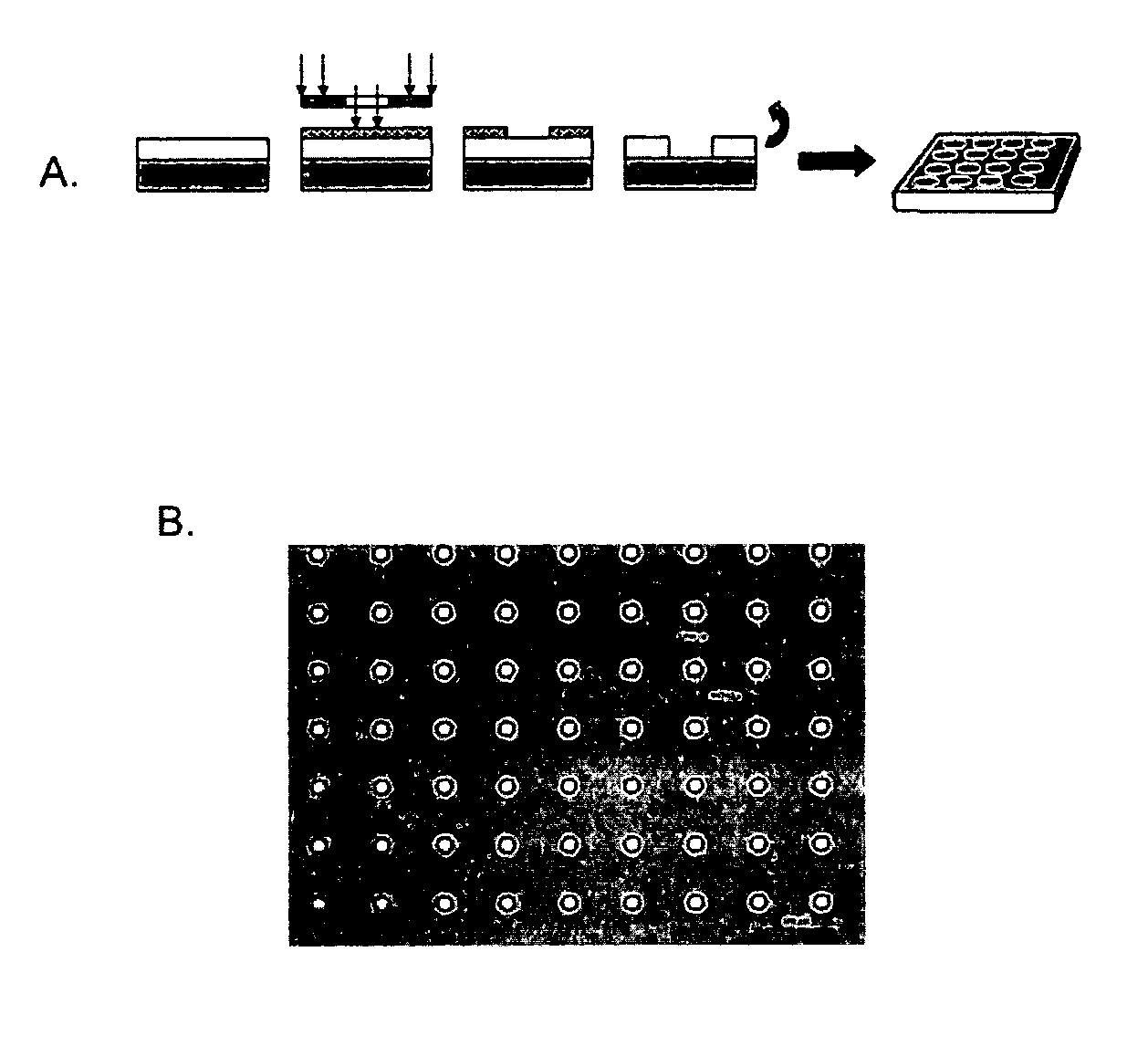
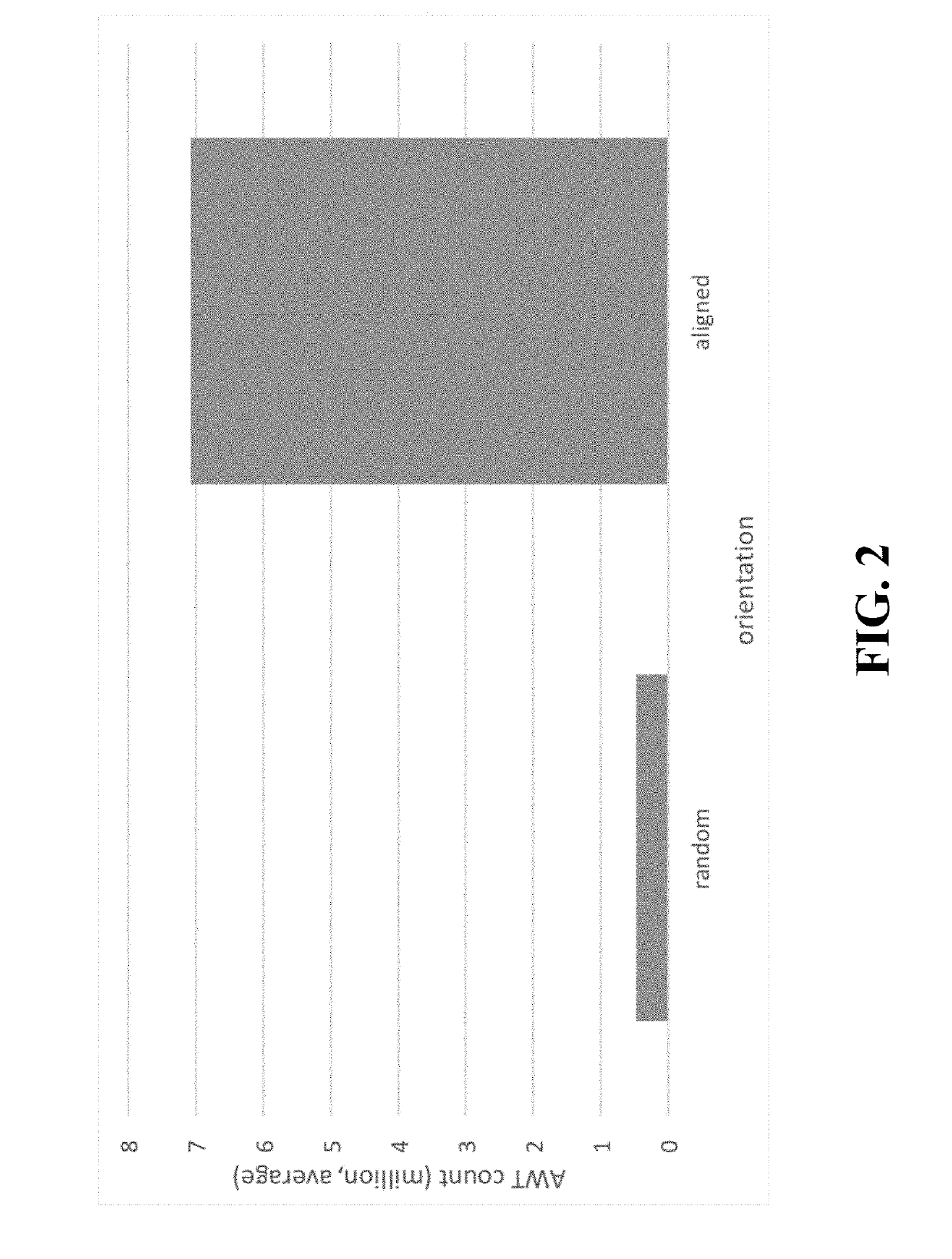
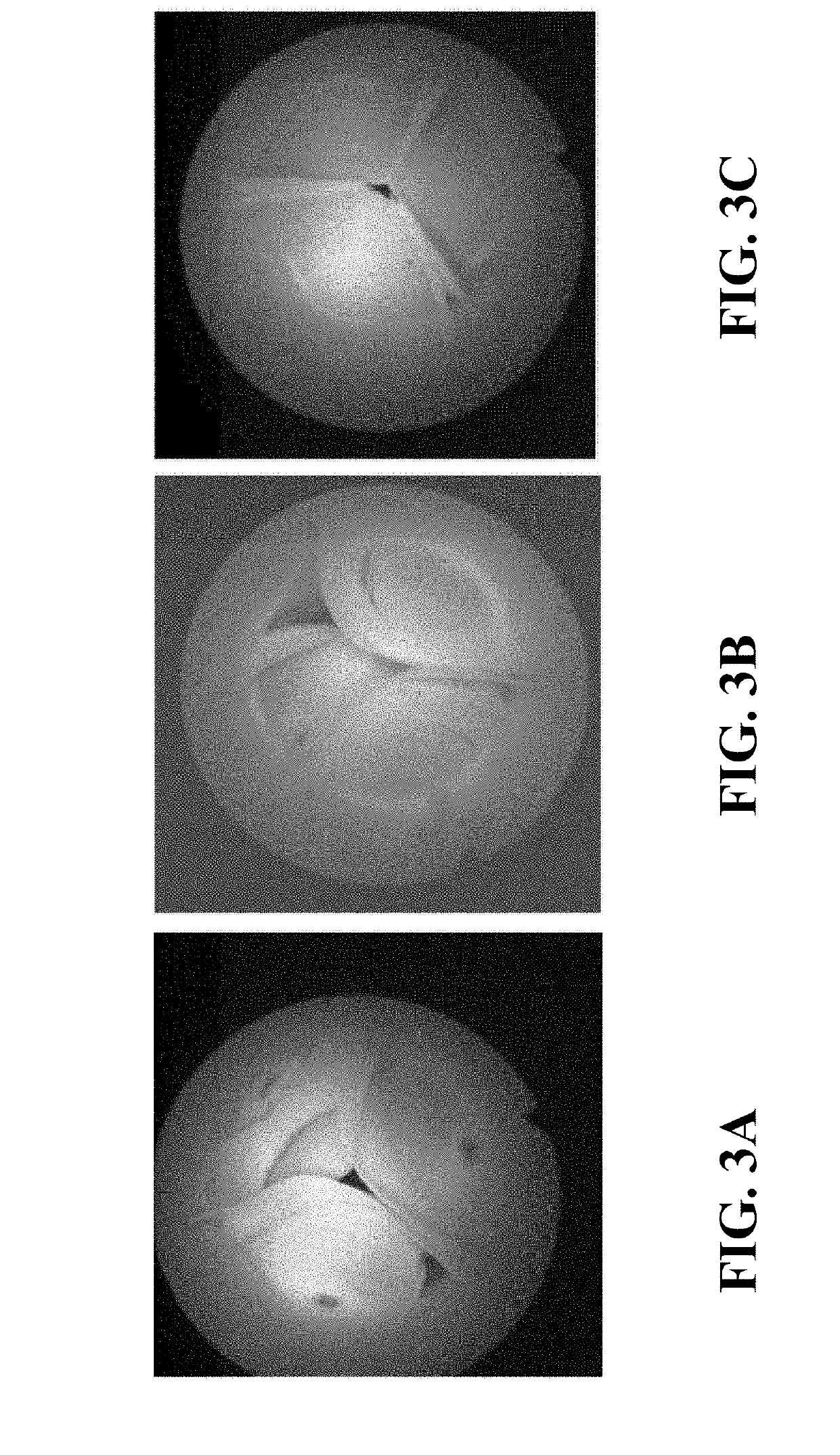
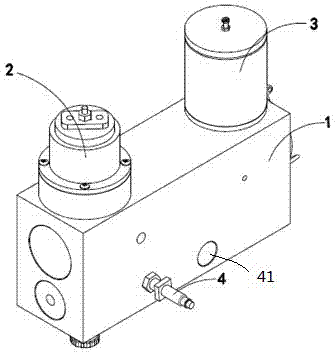
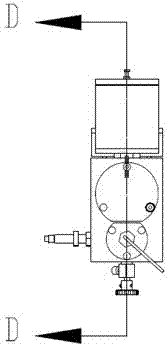
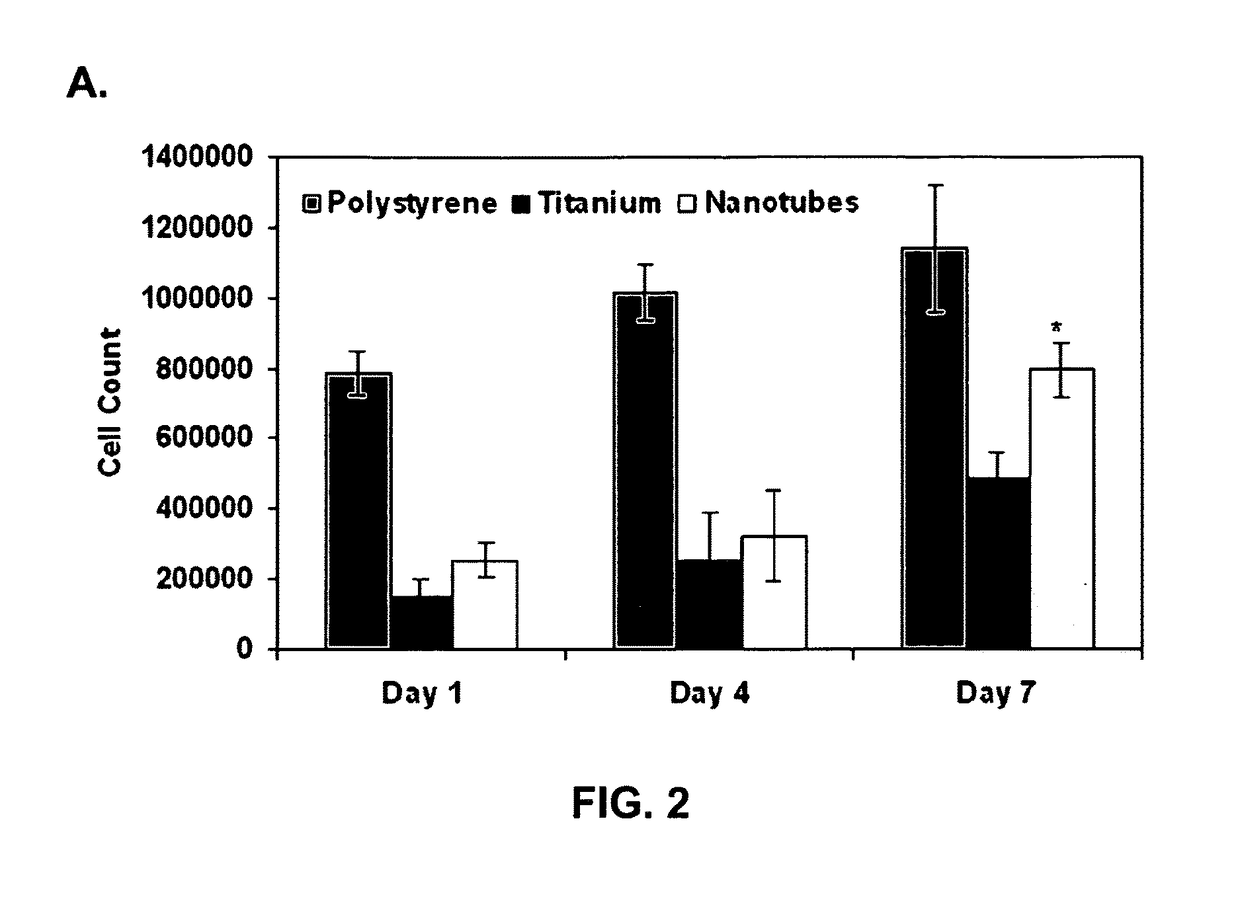
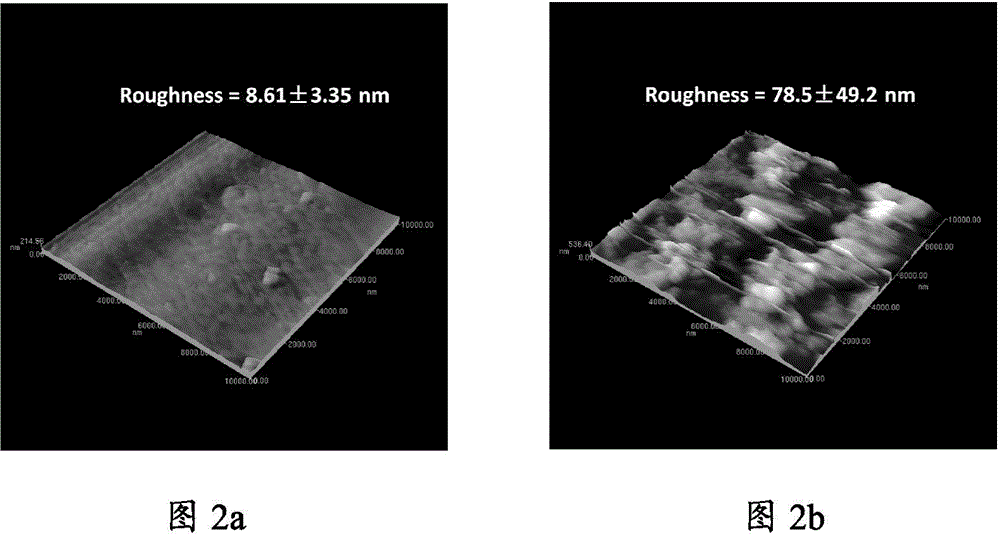
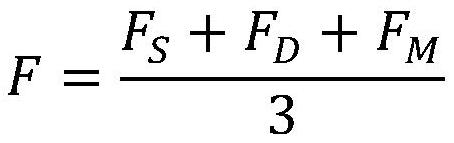Patents
Literature
36 results about "Cardiovascular implant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
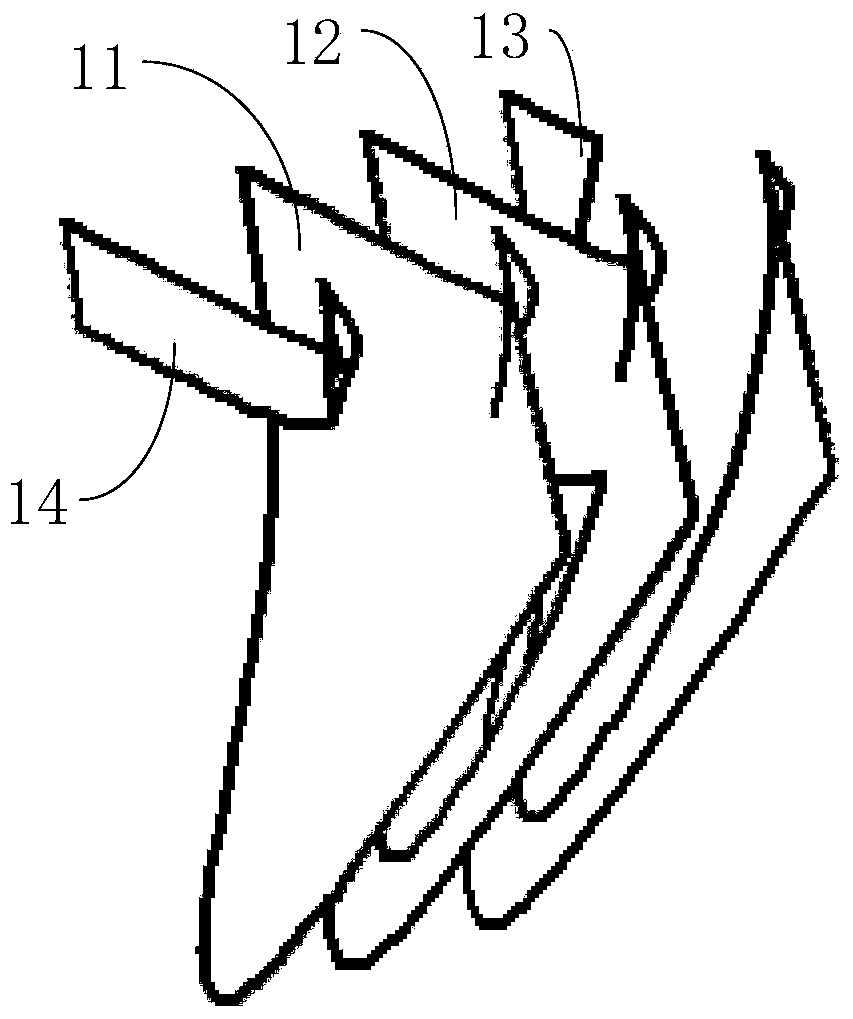
Patent Type
Patent Status
Application Year
Inventor
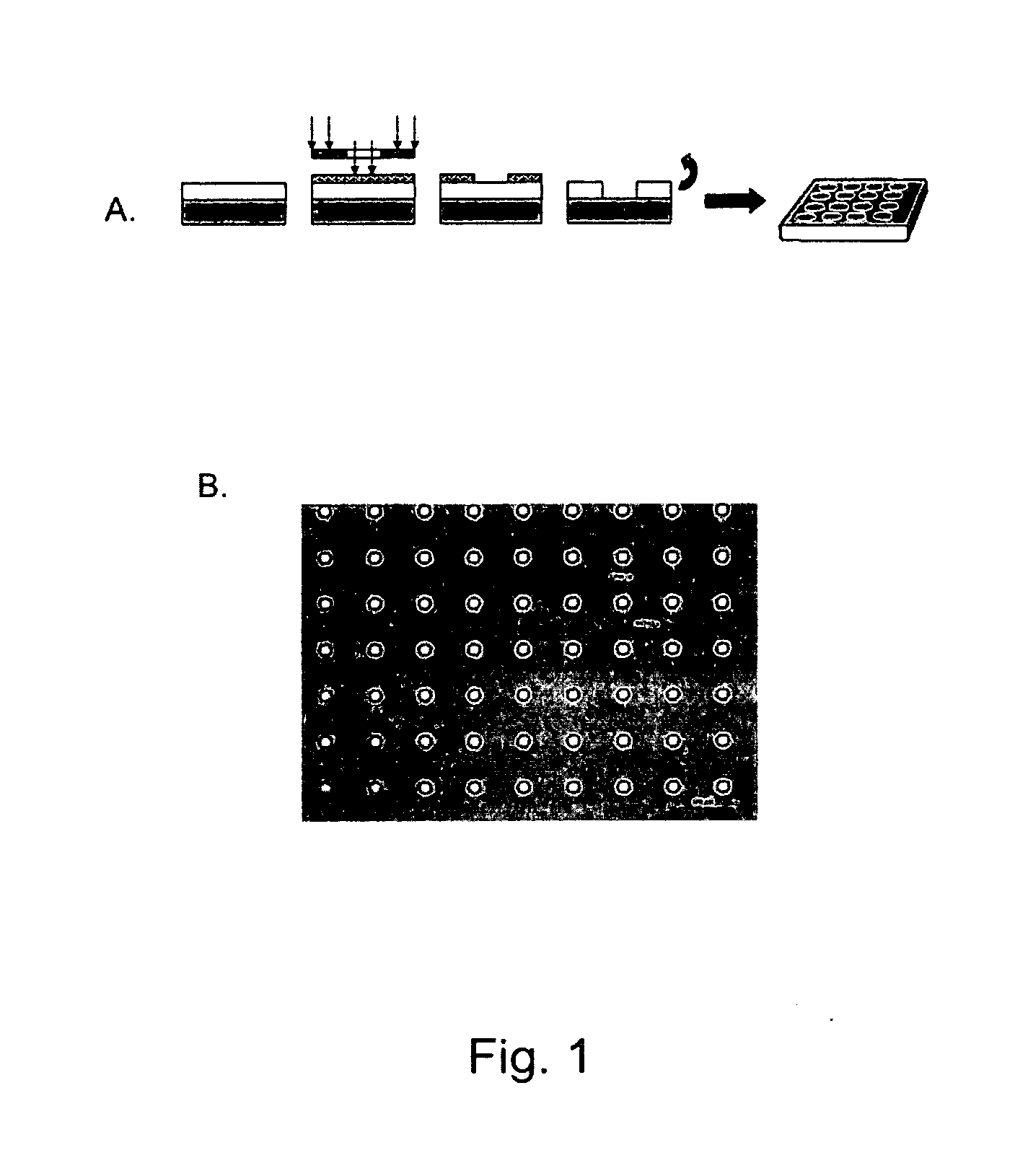
Nanostructure surface coated medical implants and methods of using the same
Compositions including a surface or film comprising nanofibers, nanotubes or microwells comprising a bioactive agent for elution to the surrounding tissue upon placement of the composition in a subject are disclosed The compositions are useful in medical implants and methods of treating a patient in need of an implant, including orthopedic implants, dental implants, cardiovascular implants, neurological implants, neurovascular implants, gastrointestinal implants, muscular implants, and ocular implants.
Owner:PENN STATE RES FOUND +1
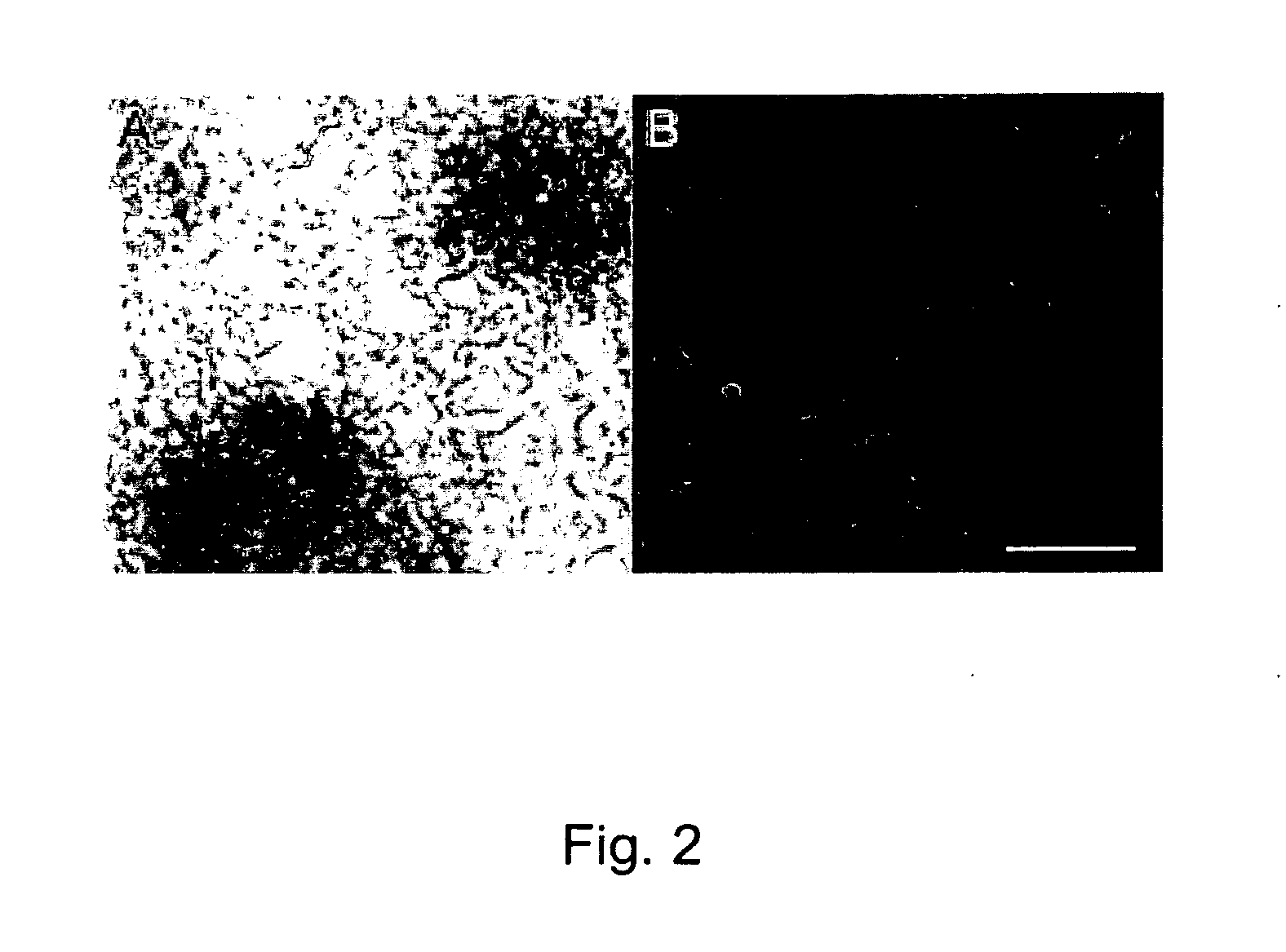
Topographically engineered structures and methods for using the same in regenerative medicine applications
InactiveUS20100318193A1Enhancing and promoting cell differentiation and cellEnhancing and promoting cell and cell viabilityPharmaceutical delivery mechanismProsthesisNanotopographyGastrointestinal implant
The present invention provides compositions including a cell contacting surface or film comprising nanotopography of nanofibers, nanotubes, nanochannels, microchannels or microwells, which are capable of enhancing or promoting cell differentiation or cell viability. The compositions are useful as medical implants, including orthopedic implants, dental implants, cardiovascular implants, neurological implants, neurovascular implants, gastrointestinal implants, muscular implants, and ocular implants. The present invention also provides methods of treating a patient in need of such an implant.
Owner:RGT UNIV OF CALIFORNIA +1
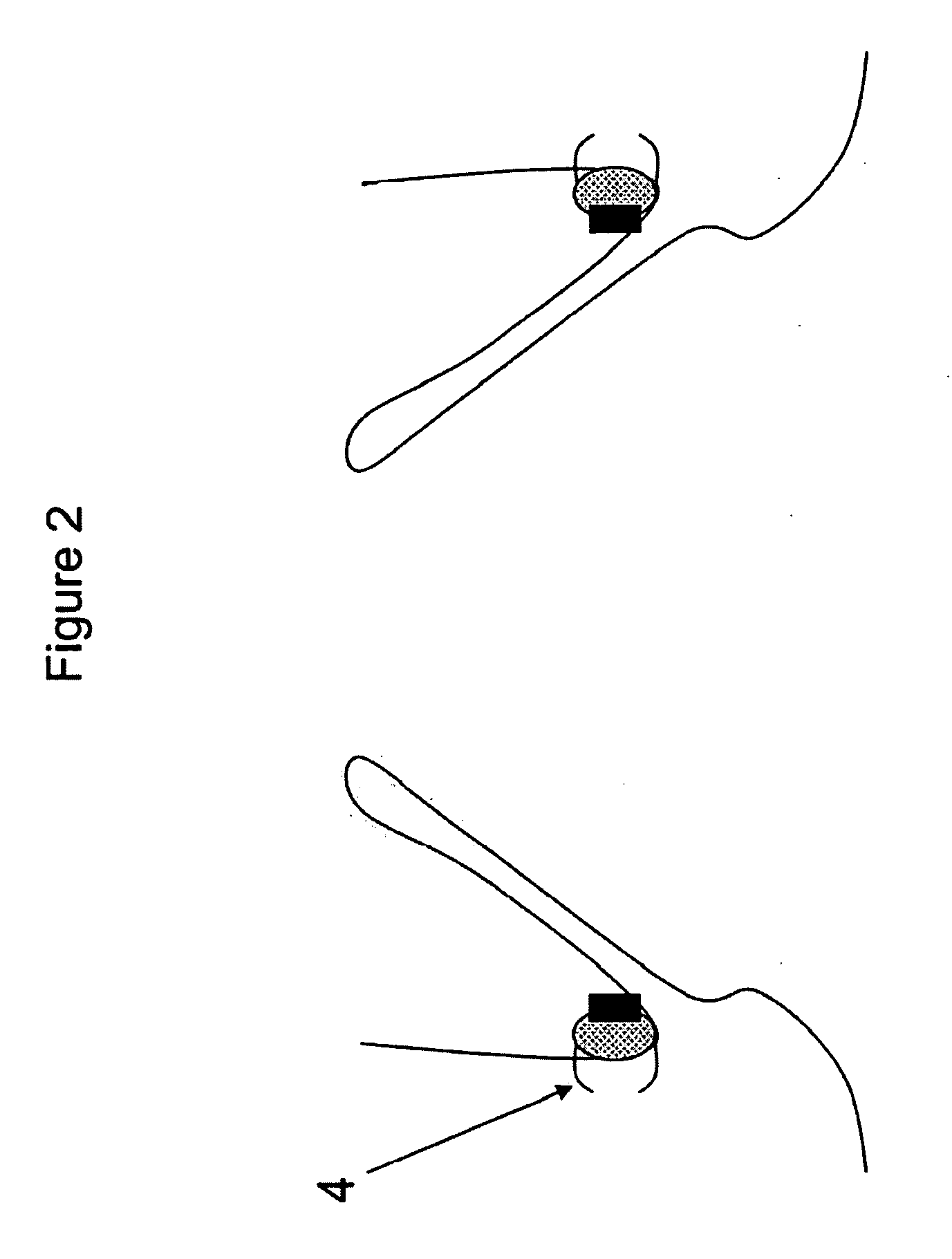
Method and apparatus for anchoring cardiovascular implants
InactiveUS20090030435A1Reduce risk of migrationReduce intrusionSuture equipmentsStentsVascular implantTherapeutic Devices
Methods, devices and systems facilitate retention of a variety of therapeutic devices. Devices generally include an anchoring element, which has been designed to promote fibrotic ingrowth, and an anchored device, which has been designed to firmly engage the complementary region of the anchoring element. The anchoring element may be placed in a minimally invasive procedure temporally separated from the deployment of the anchored device. Once enough time has passed to ensure appropriate fixation of the anchoring element by tissue and cellular ingrowth at the site of placement, the anchored device may then be deployed during which it firmly engages the complementary region of the anchoring element. In this manner, a firm attachment to the implantation site may be made with a minimum of required hardware. Some embodiments are delivered through a delivery tube or catheter and while some embodiments may require laparoscopy or open surgery for one or more of the placement procedures. Some embodiments anchor devices within the cardiovascular tree while others may anchor devices within the gastrointestinal, peritoneal, pleural, pulmonary, urogynecologic, nasopharyngeal or dermatologic regions of the body. An alternative embodiment provides for the placement of the anchoring element and anchored device simultaneously, but allows for their removal separately. This embodiment allows the device, which may be placed only temporarily and be designed to be removed, to experience significant fibrotic ingrowth, but then to be easily detached from the ingrowth-anchored region to allow for simple and quick device removal.
Owner:THERANOVA LLC
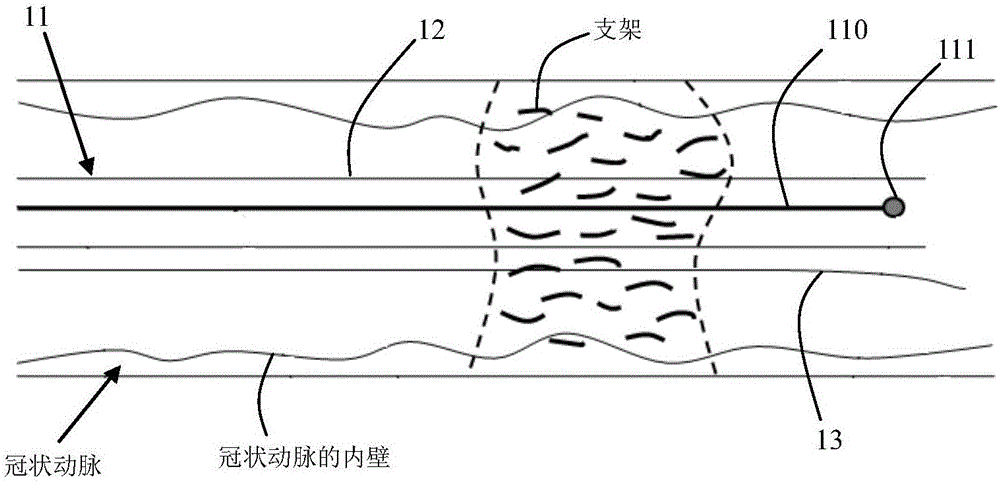
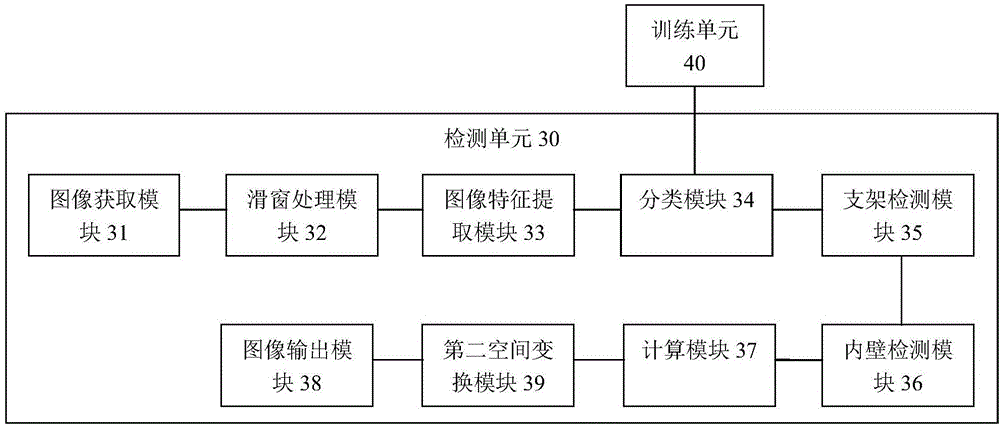
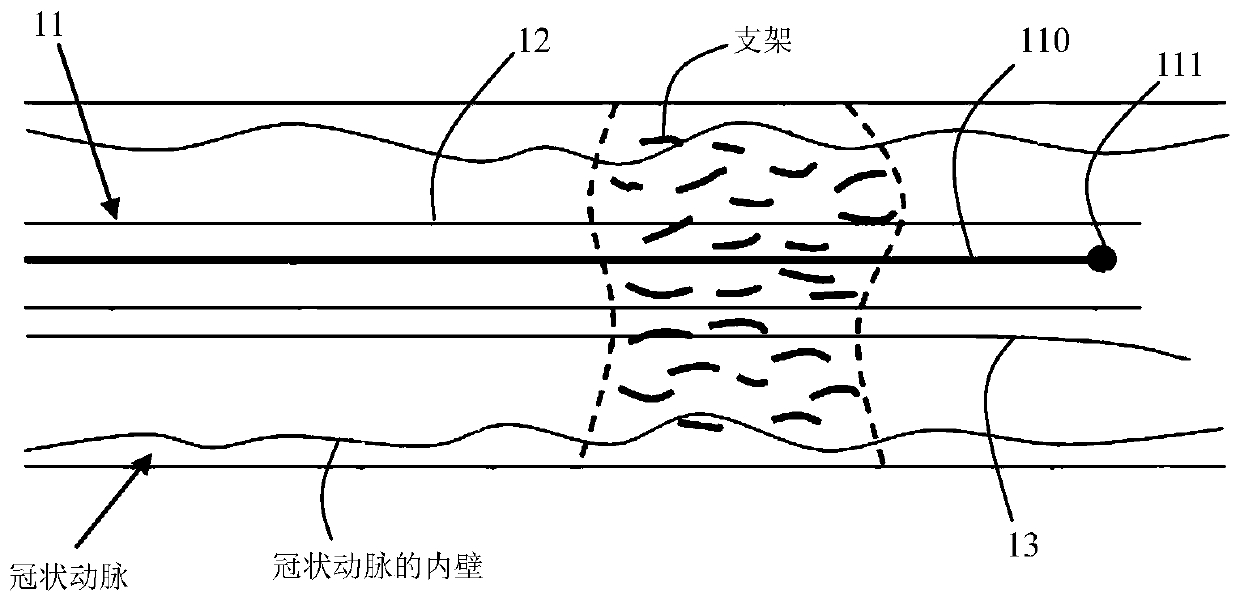
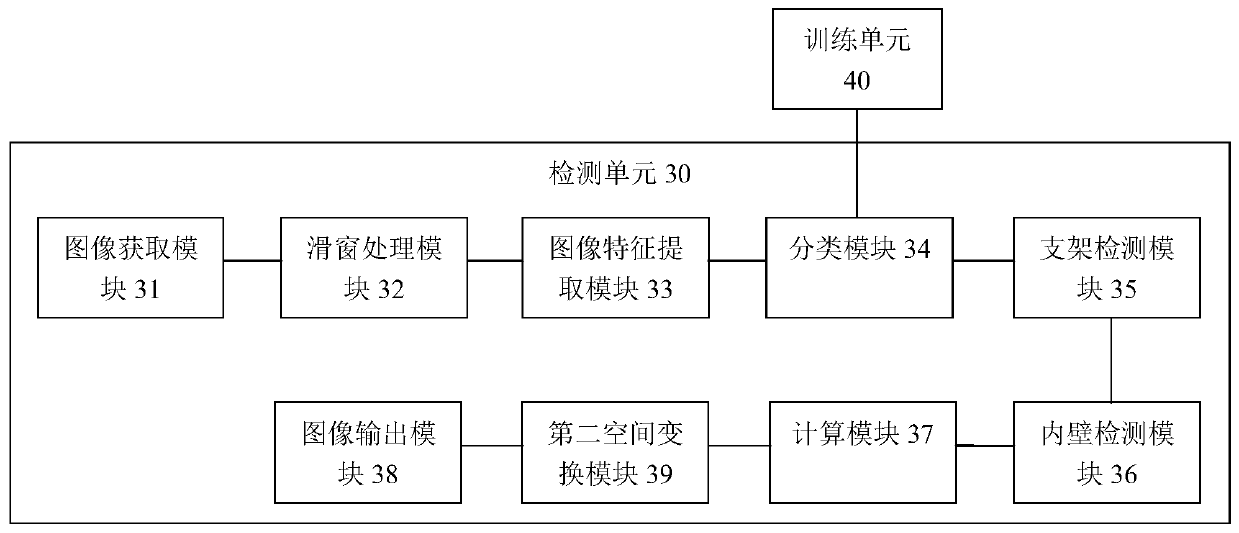
Method and system for automatically detecting and evaluating cardiovascular implanted stents on basis of OCT (optical coherence tomography)
ActiveCN106780495AImprove detection accuracyImprove robustnessImage enhancementImage analysisCoronary arteriesFeature extraction
Owner:SHENZHEN VIVOLIGHT MEDICAL DEVICE & TECH CO LTD
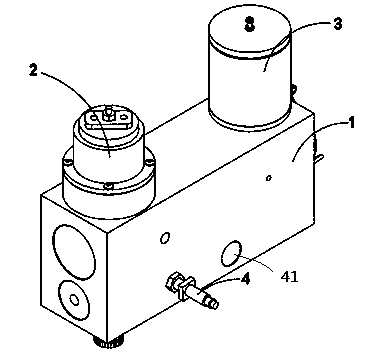
Valve fatigue-life testing device
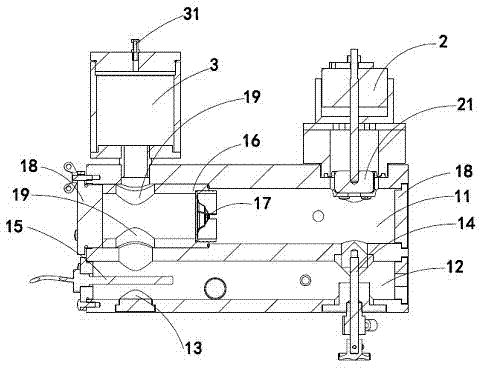
The invention provides a valve fatigue-life testing device. The valve fatigue-life testing device comprises a body at least, wherein the body is composed of an upper channel and a lower channel which is communicated with the upper channel, one side of the bottom of the body is provided with an opening, a linear motor is arranged on one side of the top of the body, a liquid container which is communicated with the body is arranged on the other side of the top of the body, and a cover is arranged at the top of the liquid container. A damper is arranged at one end of the lower channel and under the linear motor, and a temperature sensor is arranged at the other end of the lower channel. The upper channel of the body further comprises a valve fixer provided with a through hole, wherein the valve fixer is horizontally placed inside the upper channel of the body and arranged below the liquid container. A shaft of the linear motor is connected with a piston provided with a rolling membrane, and the piston is arranged inside the body in a stretching mode. The linear motor of the valve fatigue-life testing device enables loads to be directly applied to test liquid in a waveform mode under current control and to be transmitted to a test valve or other test pieces according to the Pascal law, and high-frequency oscillation signals in the test liquid are filtered through the liquid container. All test indexes can meet relevant national standards for artificial cardiac valves which are cardiovascular implants, and the final testing result is accurate and reliable.
Owner:上海心瓣测试设备有限公司
Artificial polymer aorta valve
ActiveCN103961192AImprove performanceExtend your lifeHeart valvesAnatomical structuresProsthetic valve
The invention discloses an artificial polymer aorta valve, belongs to the technical field of medical instruments, and particularly belongs to angiocarpy implantation materials. The artificial polymer aorta valve comprises three valve leaflets and a valve ring. The valve leaflets are fixed to the valve ring, and the valve ring is sutured to the human aortic root in a valve replacement operation. The valve leaflets have the function of controlling blood to move in one direction under the drive of differential pressure. According to the diameter of the aorta valve ring of a patient, the artificial polymer aorta valve of the proper size is selected. The artificial polymer aorta valve has the advantages that the artificial polymer aorta valve is suitable for replacing the human aorta valve; stress borne by the free edges of the valve leaflets, the coaptation area, the connecting positions of the valve leaflets and the aortic root and the middles of the valve leaflets is increased in sequence; the middles of the valve leaflets are thinnest and bear the largest deformation, and therefore the performance of the materials can be achieved fully; the service life of the valve is prolonged in the high circulating bending force bearing environment; the artificial valve is designed according to the anatomical structure of the human aorta valve, and the blood flows through the valve in the excellent hemodynamic environment.
Owner:BEIJING UNIV OF TECH
Medical implant component comprising composite biotextile and method of making
ActiveCN113518633AEnhanced interactionGood biocompatibilityFibre treatmentSurgeryPolyolefinMaterials science
Disclosed herein is a medical implant component comprising a composite biotextile, which biotextile comprises i) a polyolefin fibrous construct comprising at least one strand with titer of 2-250 dtex, tensile strength of at least 10 cN / dtex and comprising high molar mass polyolefin fibers; and ii) a coating comprising a biocompatible and biostable polyurethane elastomer comprising polysiloxane in soft segments and / or having one or more hydrophobic endgroups, wherein the polyurethane coating has been applied to at least part of the surface of the biotextile and in an amount of 2.5-90 mass% based on composite biotextile. At least one strand of such composite biotextile, for example a partly coated woven fabric, shows an advantageous combination of good biocompatibility, especially hemocompatibility, high strength and pliability, and laser cuttability; allowing to make pieces of fabric having well-defined regular edges that have high suture retention strength. The invention also provides a method of making the composite biotextile. Further embodiments concern the use of such biotextile in a medical implant component for an implantable medical device and the use of such medical implant component in making an implantable medical device; such as in orthopedic applications and cardiovascular implants. Other embodiments include such medical devices or implants comprising the medical implant component.
Owner:DSM IP ASSETS BV
Method for forming albumen layer by mediating albumin on surface of material, biological material and application thereof
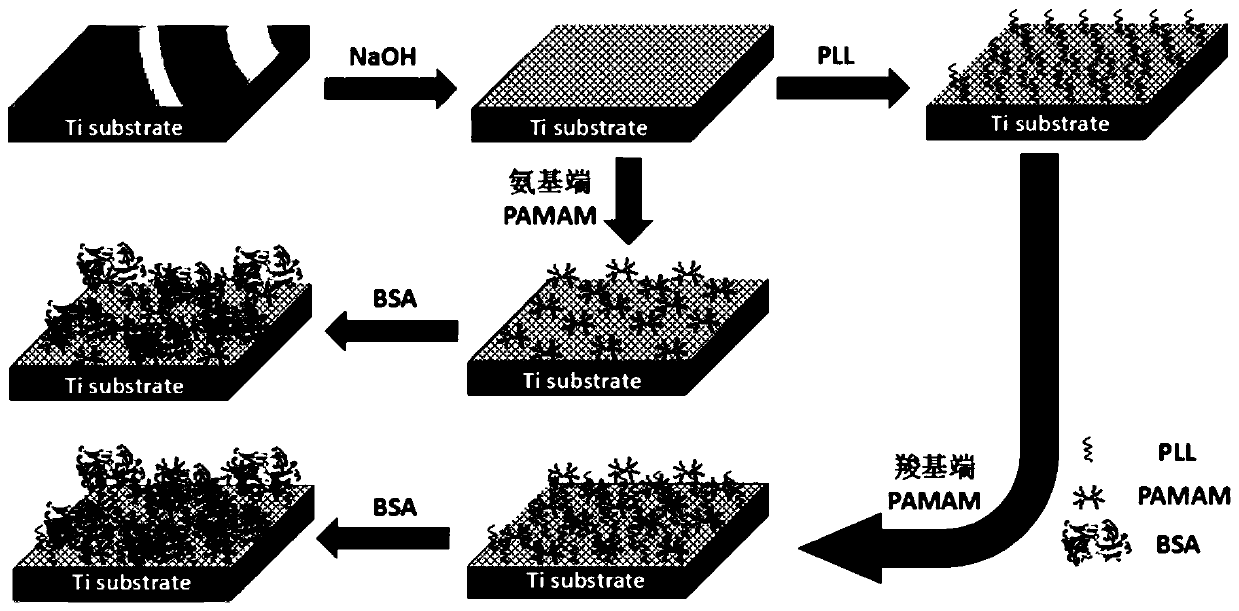
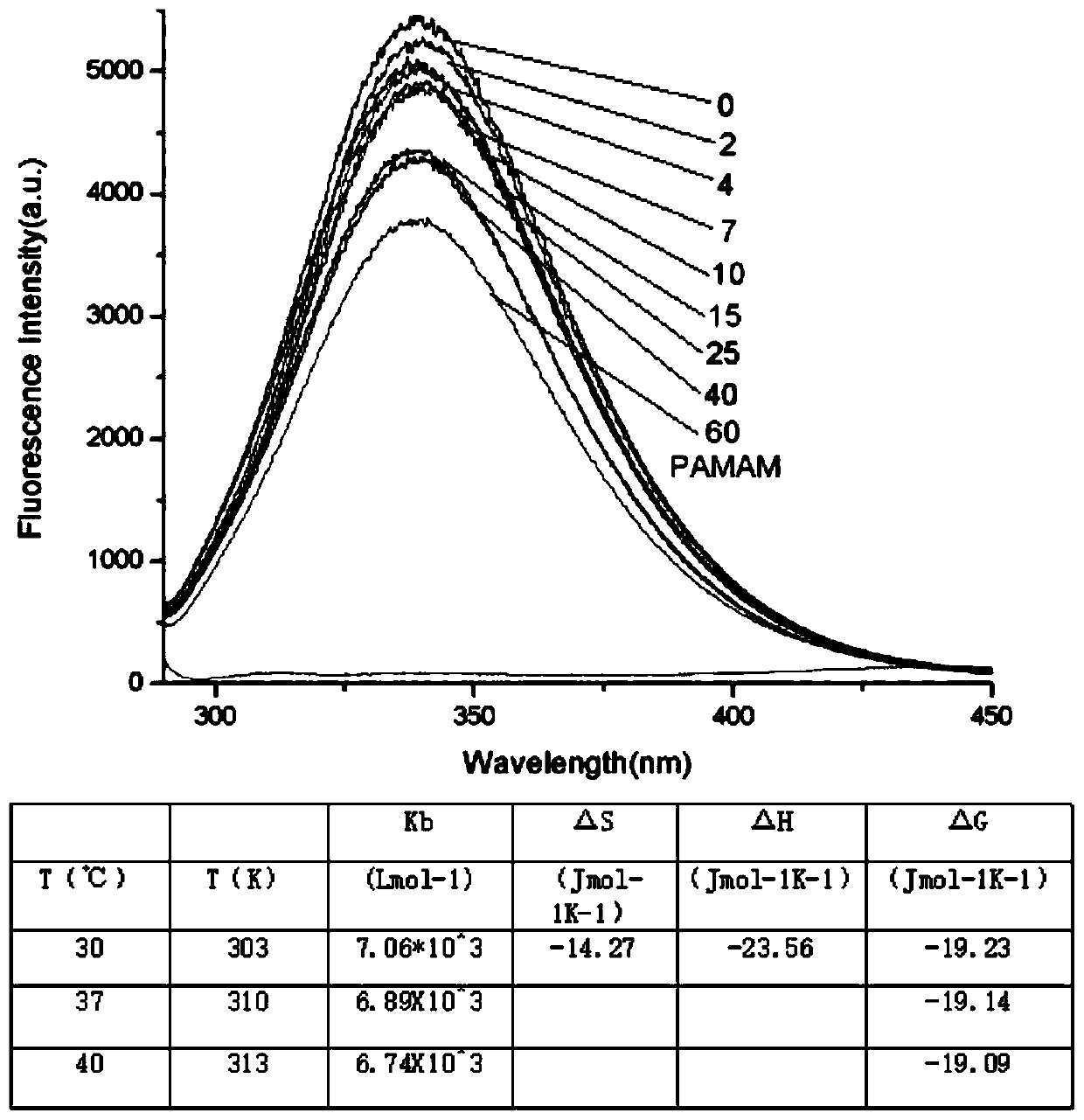
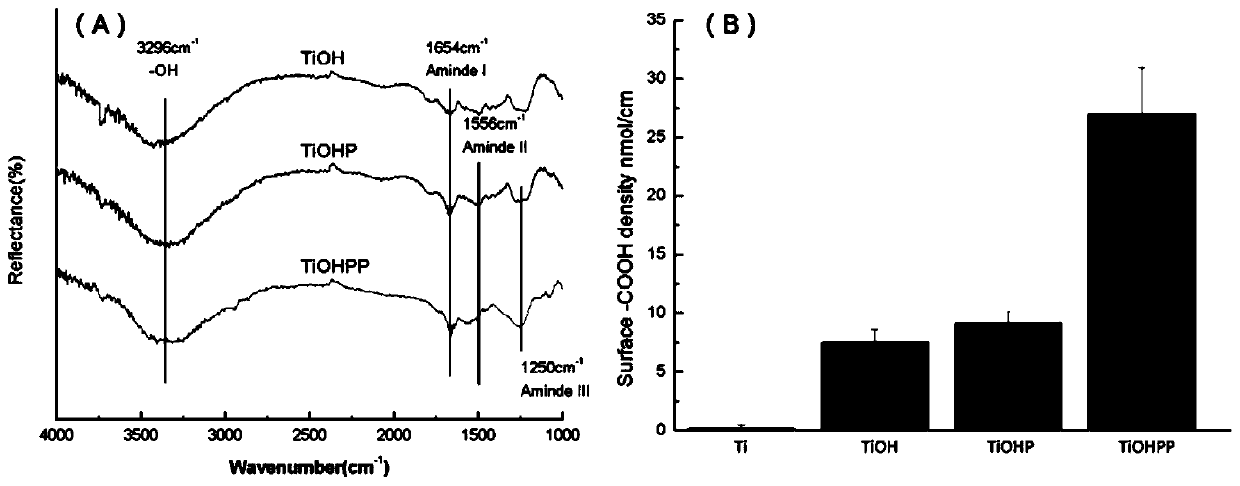
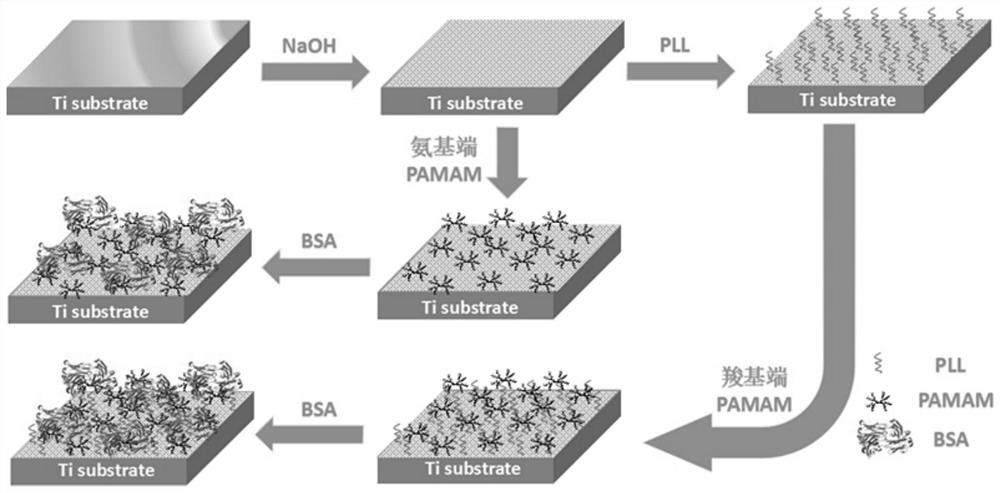
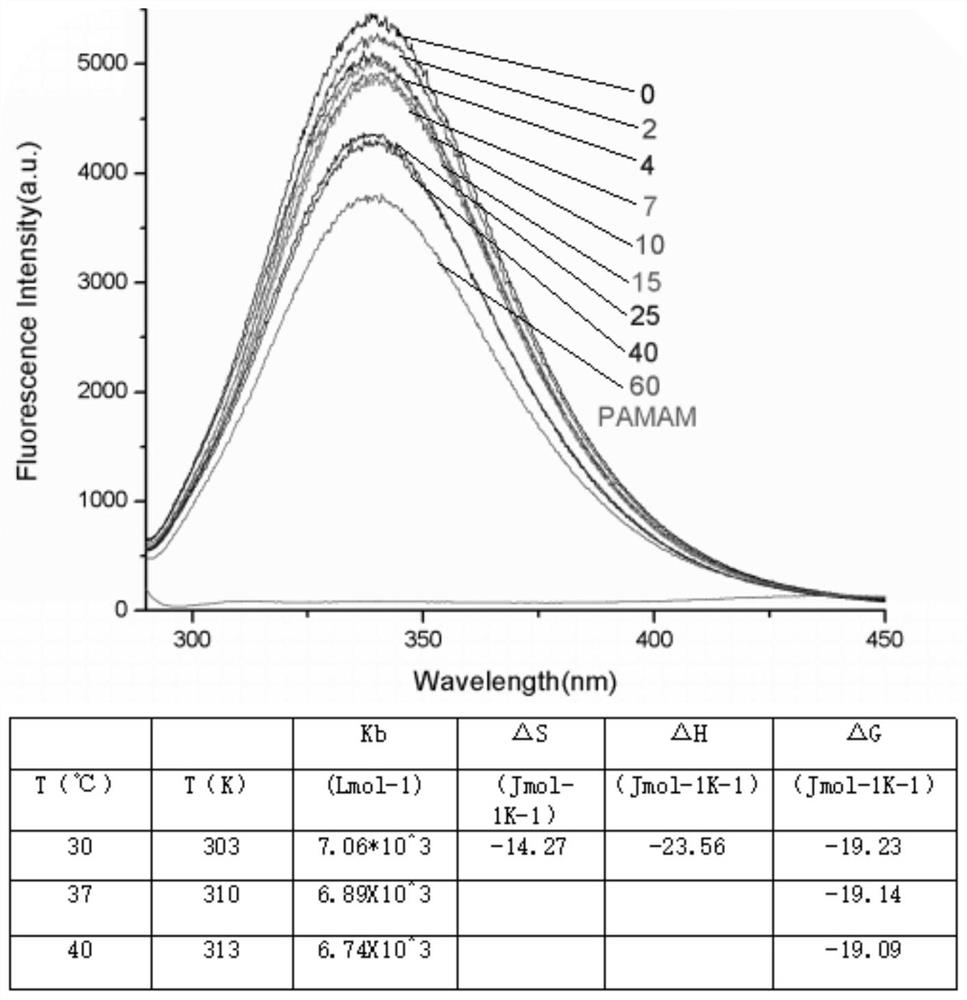
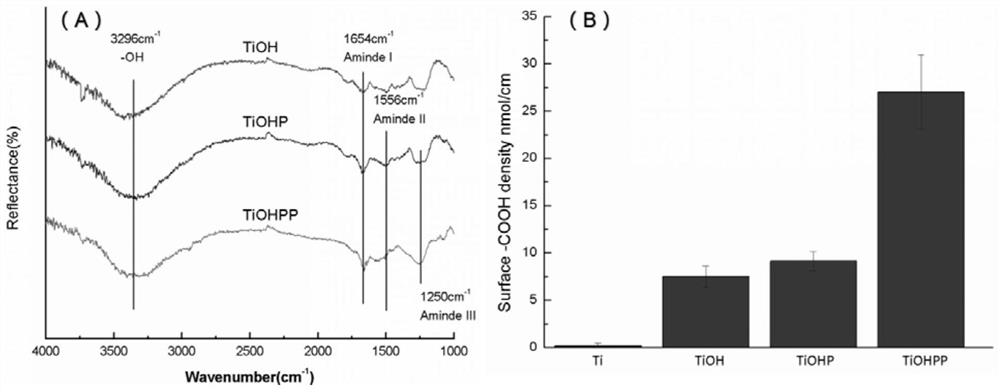
The invention discloses a method for forming an albumen layer by mediating albumin on the surface of a material, a biological material and application thereof and relates to the technical field of biomaterials. The method for forming an albumen layer by mediating albumin on the surface of a material includes standing a to-be-supported material in an alkaline solution and then obtaining an alkali-activated material; fixing dendrimers on the surface of the alkali-activated material, and then immersing the modified material in an albumin solution for standing reaction. Preferably, the dendrimer is a polyamide-amine dendrimer PAMAM; and more preferably, the molecular weight of PAMAM is 6000 to 10000. The biological material forms a PAMAM layer and the albumin layer on the surface of the to-be-supported material, and the albumin is mediated to form the albumen layer. The biological material is used for preparing a cardiovascular implant material and can reduce the adhesion of platelets to achieve anticoagulation, and the biocompatibility of the material is improved.
Owner:SOUTHWEST JIAOTONG UNIV
Electro-Spun Cardiovascular Implant
ActiveUS20190201588A1Increased durabilityImprove fatigue resistanceHeart valvesElectro-spinningVascular implantBiomedical engineering
A biodegradable cardiovascular implant is provided for growing cardiovascular tissue in a patient. The implant distinguishes an electro-spun network with supramolecular compounds having hard-blocks covalently bonded with soft-blocks resulting in much enhanced durability and fatigue resistance, while maintaining the effectiveness as a cardiovascular implant.
Owner:XELTIS AG
Valve fatigue-life testing device
The invention provides a valve fatigue-life testing device. The valve fatigue-life testing device comprises a body at least, wherein the body is composed of an upper channel and a lower channel which is communicated with the upper channel, one side of the bottom of the body is provided with an opening, a linear motor is arranged on one side of the top of the body, a liquid container which is communicated with the body is arranged on the other side of the top of the body, and a cover is arranged at the top of the liquid container. A damper is arranged at one end of the lower channel and under the linear motor, and a temperature sensor is arranged at the other end of the lower channel. The upper channel of the body further comprises a valve fixer provided with a through hole, wherein the valve fixer is horizontally placed inside the upper channel of the body and arranged below the liquid container. A shaft of the linear motor is connected with a piston provided with a rolling membrane, and the piston is arranged inside the body in a stretching mode. The linear motor of the valve fatigue-life testing device enables loads to be directly applied to test liquid in a waveform mode under current control and to be transmitted to a test valve or other test pieces according to the Pascal law, and high-frequency oscillation signals in the test liquid are filtered through the liquid container. All test indexes can meet relevant national standards for artificial cardiac valves which are cardiovascular implants, and the final testing result is accurate and reliable.
Owner:上海心瓣测试设备有限公司
Nickel-titanium alloy containing biologically-active coating, and preparation method and application thereof
InactiveCN104129113AMeet the need for rapid endothelializationExpand the scope of clinical applicationLayered productsMetallic material coating processesPhosphateSolvent
The invention discloses a nickel-titanium alloy containing a biologically-active coating. A preparation method for the alloy comprises the following steps: polishing the surface of the nickel-titanium alloy so as to allow the surface of the nickel-titanium alloy to be shining; then soaking the alloy in a Tris-dopamine hydrochloride solution with a concentration of 1 to 3 mg / mL for 12 to 36 h so as to allow a polydopamine film to be formed on the surface; preparing a chitosan solution with a concentration of 1 to 10 mg / mL with 0.1 to 0.5% (v / v) acetic acid as a solvent, preparing a gelatin solution with a concentration of 1 to 10 mg / mL and an endothelial cell growth factor solution with a concentration of 100 to 500 ng / mL by using a phosphate buffer solution, soaking the treated nickel-titanium alloy in the gelatin solution for 12 to 36 h and then carrying out self-assembling of chitosan-gelatin-endothelial cell growth factor-gelatin layer by layer, wherein soaking in each solution lasts for 5 to 20 min and there are 4 to 10 cycles of layer-by-layer self-assembling; and finally, carrying out blow-drying with nitrogen. The coating can effectively promote adhesion and growth of endothelial cells on the surface of the nickel-titanium alloy, so demands of rapid endothelialization of the nickel-titanium alloy as a cardiovascular implant material are met, the problems of induction of thrombi and generation of coagulation on the surface of the nickel-titanium alloy are overcome, and the clinical application scope of the nickel-titanium alloy is broadened.
Owner:CHONGQING UNIV
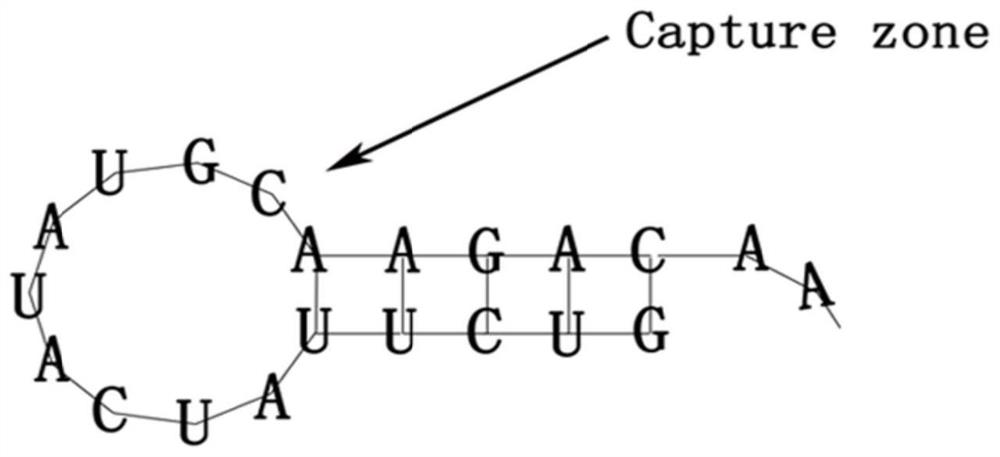
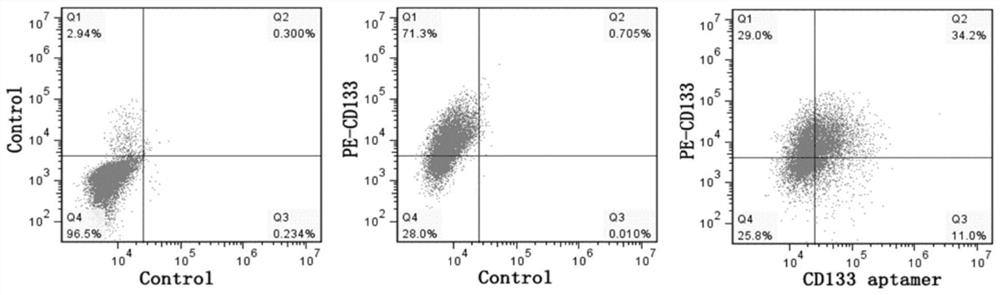
Cardiovascular implant for regulating and controlling immune response and promoting intimal regeneration and preparation method of cardiovascular implant
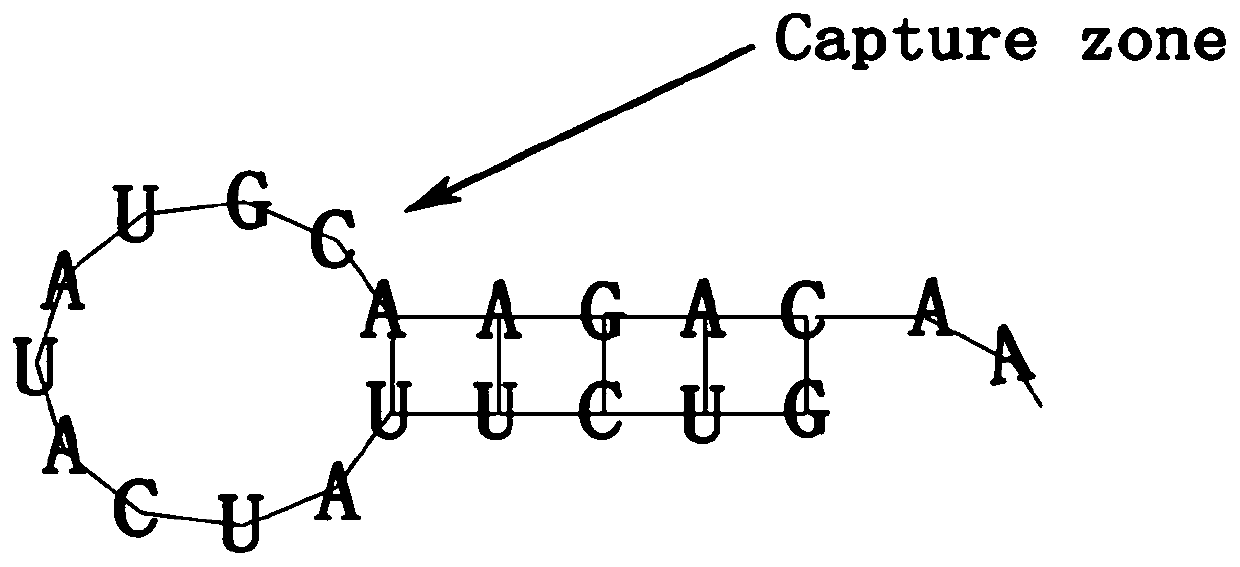
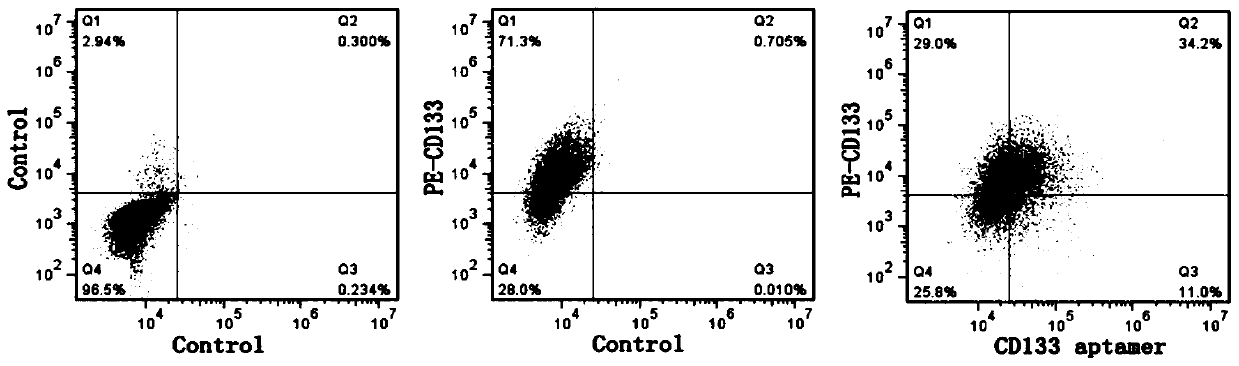
The invention belongs to the technical field of biological medicines, and relates to a cardiovascular implant and a preparation method thereof, in particular to a cardiovascular implant for regulatingimmune response in situ and promoting intimal regeneration and a preparation method thereof. The cardiovascular implant comprises a cardiovascular implant body, and the cardiovascular implant body ismodified with a CD133 aptamer and sustained-release nanoparticles. The cardiovascular implant body is modified with the CD133 aptamer and the sustained-release nanoparticles containing the nerve axonguidance molecule-1, the immune response is regulated and controlled in situ and intimal regeneration is promoted, thrombosis is effectively overcome, and long-term unobstructed blood vessels are promoted. Especially for patients with clinically inflammatory basal lesions, the invention provides the cardiovascular implant which is simple, convenient and rapid to prepare, wide in applicability andcapable of regulating excessive inflammatory reaction in situ and promoting intimal regeneration to maintain long-term functions and has a good application prospect.
Owner:ARMY MEDICAL UNIV
Nanostructure surface coated medical implants and methods of using the same
Owner:PENN STATE RES FOUND +1
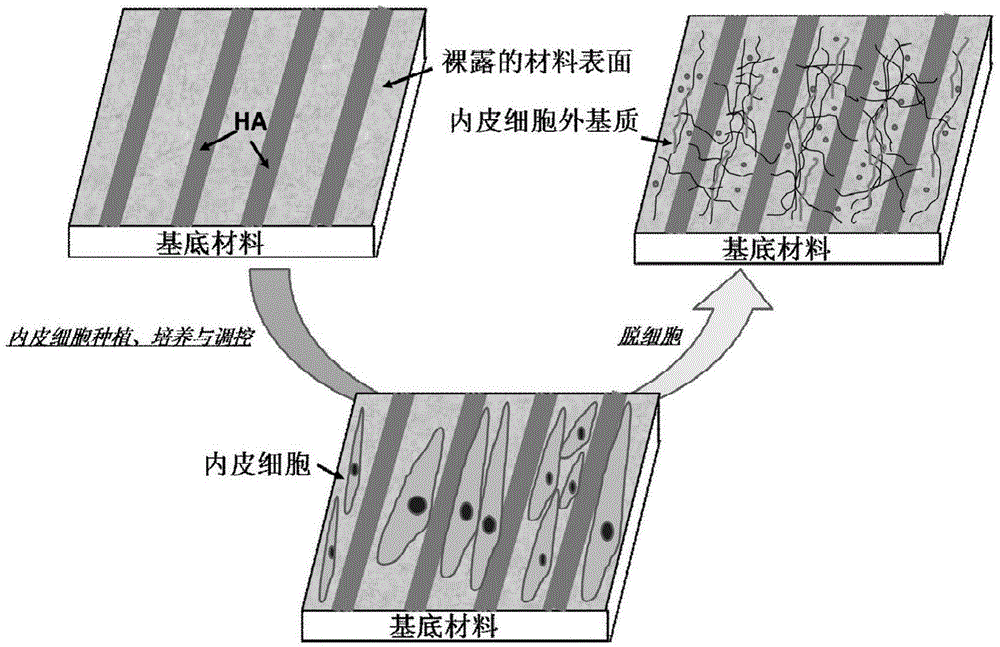
Method for improving various bionic functions on surface of cardiovascular implant material
InactiveCN103656750BHigh degree of bionicsSimple preparation processSurgeryArtificial cell constructsCell-Extracellular MatrixVascular endothelium
The invention discloses a method for improving various bionic functions on the surface of a cardiovascular implant material. The method comprises the following steps: first, preparing an HA (Hyaluronic Acid) stripe-like micrograph with high molecular weight, cultivating vascular endothelial cells on the surface, and regulating in vitro construction of extracellular matrixes of vascular endothelial cells through the HA stripe-like micrograph; and then, peeling the endothelial cells on the surface of the material by a decellularizing technology so as to prepare a multifunctional biological modified surface with anticoagulation / endothelialization promotion / smooth muscle cell adhesion inhibition effects and macrophage adhesion proliferation / inhibition and spreading on the surface of the cardiovascular implant material. The method disclosed by the invention constructs the multifunctional bionic endothelial cell extracellular matrix modified layer which is of anticoagulation and reendothelialization, inhibits adhesion and proliferation of smooth muscle cells, promotes phenotype shrink and inhibits adhesion and spreading of macrophage. The blood compatibility of the material, the structural and functional repair capacity of endothelial injury are remarkably improved.
Owner:SOUTHWEST JIAOTONG UNIV
Cardiovascular implant intervention material/instrument biological film coating and preparation method thereof
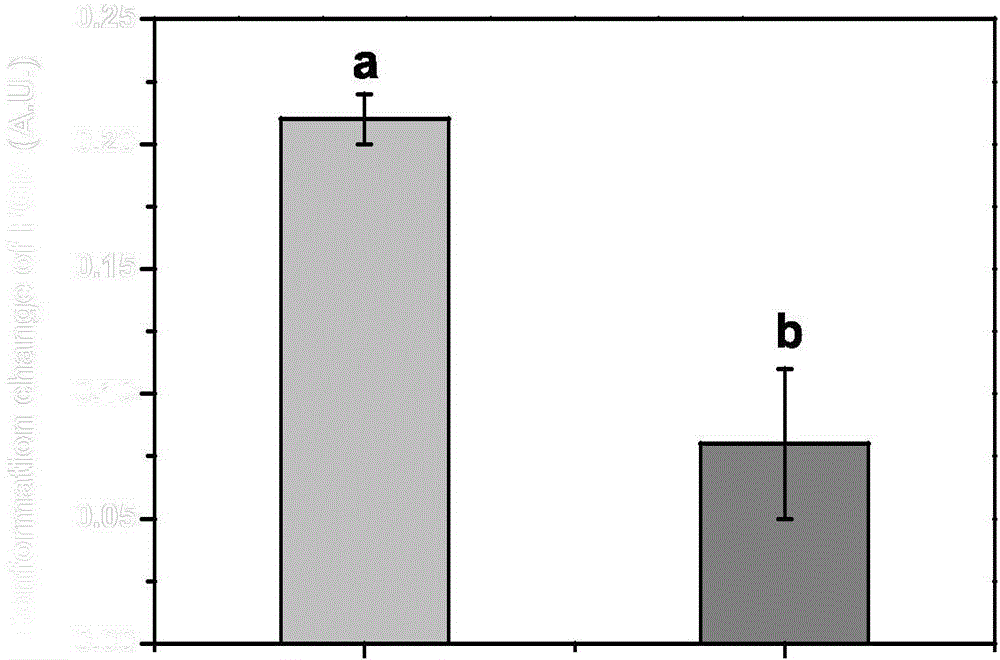
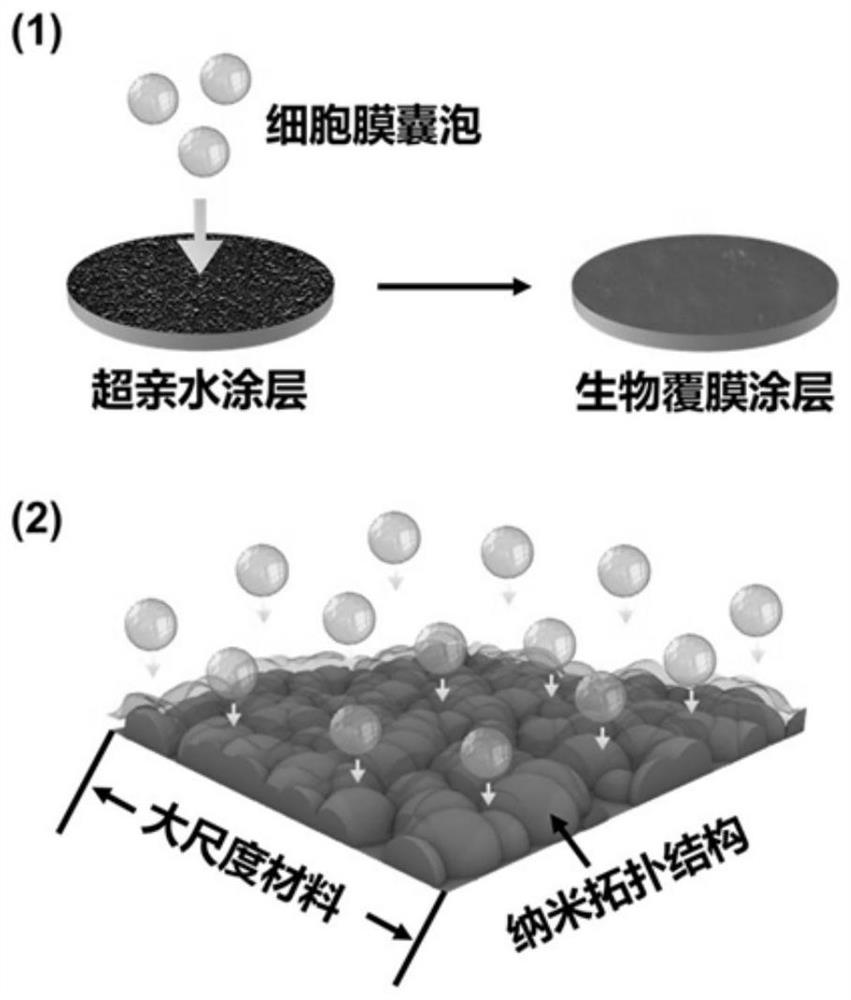
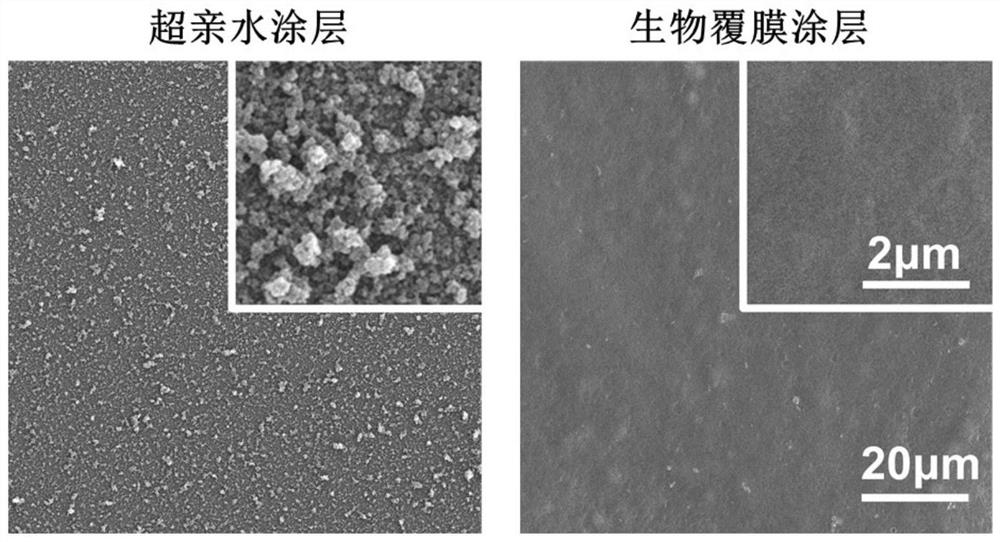
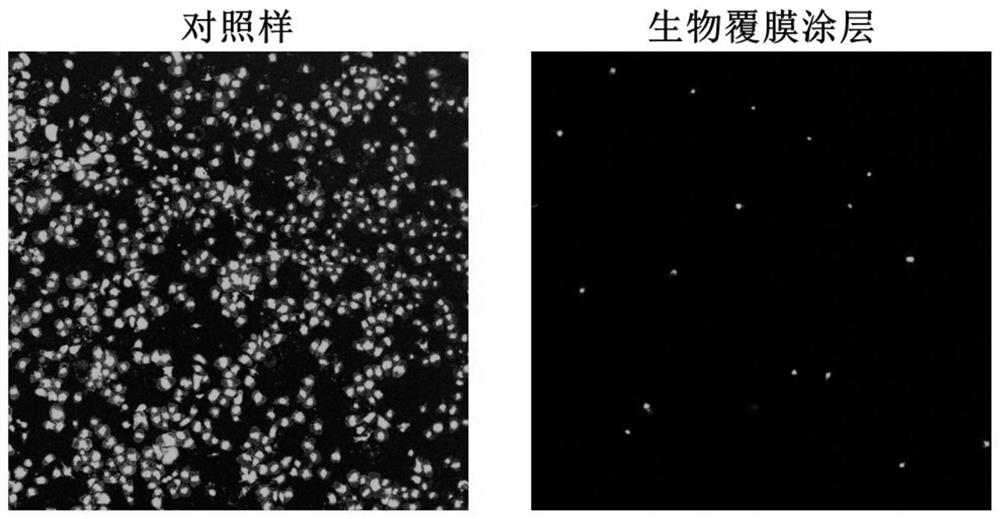
ActiveCN114099785AImprove anticoagulant performanceGood anti-inflammatory effectSurgeryPharmaceutical delivery mechanismThrombusCell membrane
The invention discloses a cardiovascular implant intervention material / instrument biological film coating and a preparation method thereof. The preparation method comprises the following steps: (1) preparing a cell membrane vesicle suspension; (2) constructing a super-hydrophilic coating on the surface of the substrate material; and (3) co-incubating the cell membrane vesicle suspension obtained in the step (1) and the super-hydrophilic coating obtained in the step (2), and cleaning with deionized water to obtain the cardiovascular implant intervention material / instrument biological membrane coating. The invention also comprises the cardiovascular implant intervention material / instrument biological film coating prepared by the method. According to the coating, the surface of a large-scale medical device is coated with a cell membrane for the first time, the anticoagulation performance and the anti-inflammatory performance of a blood contact material can be effectively enhanced, and the coating has the capacity of promoting endothelial repair and can be suitable for modification of medical interventional devices such as heart occluders, heart valves, intravascular stents and artificial blood vessels; the inflammatory reaction degree and the thrombosis risk after the instrument is implanted can be obviously reduced.
Owner:SICHUAN UNIV
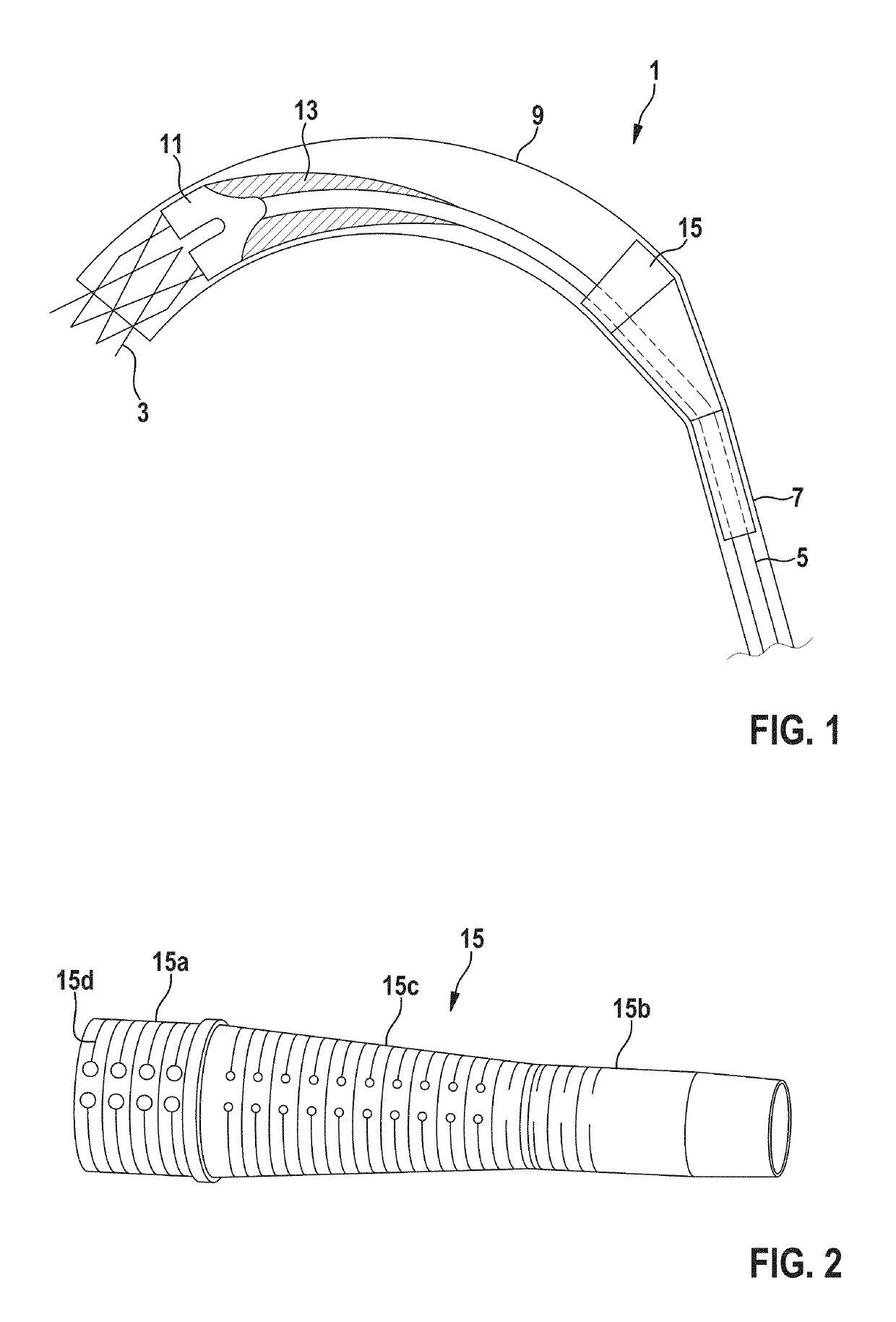
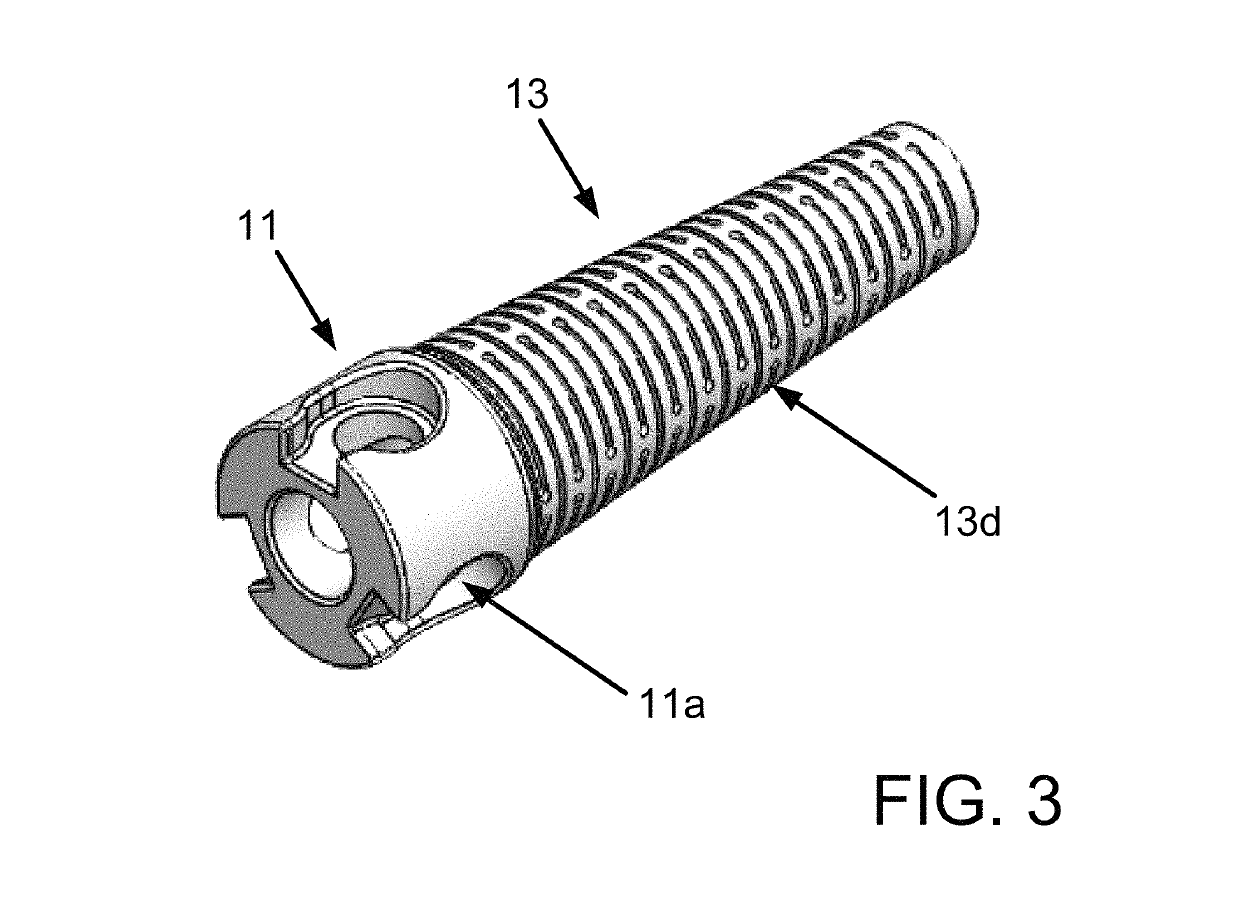
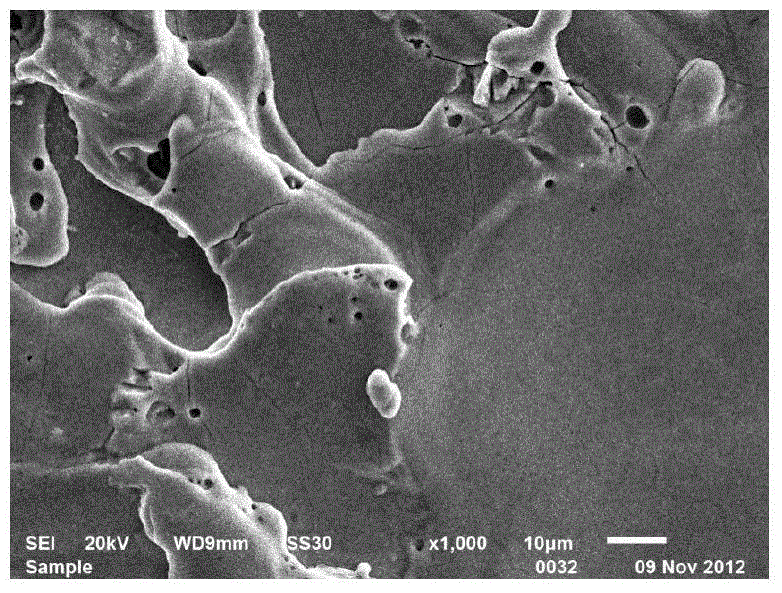
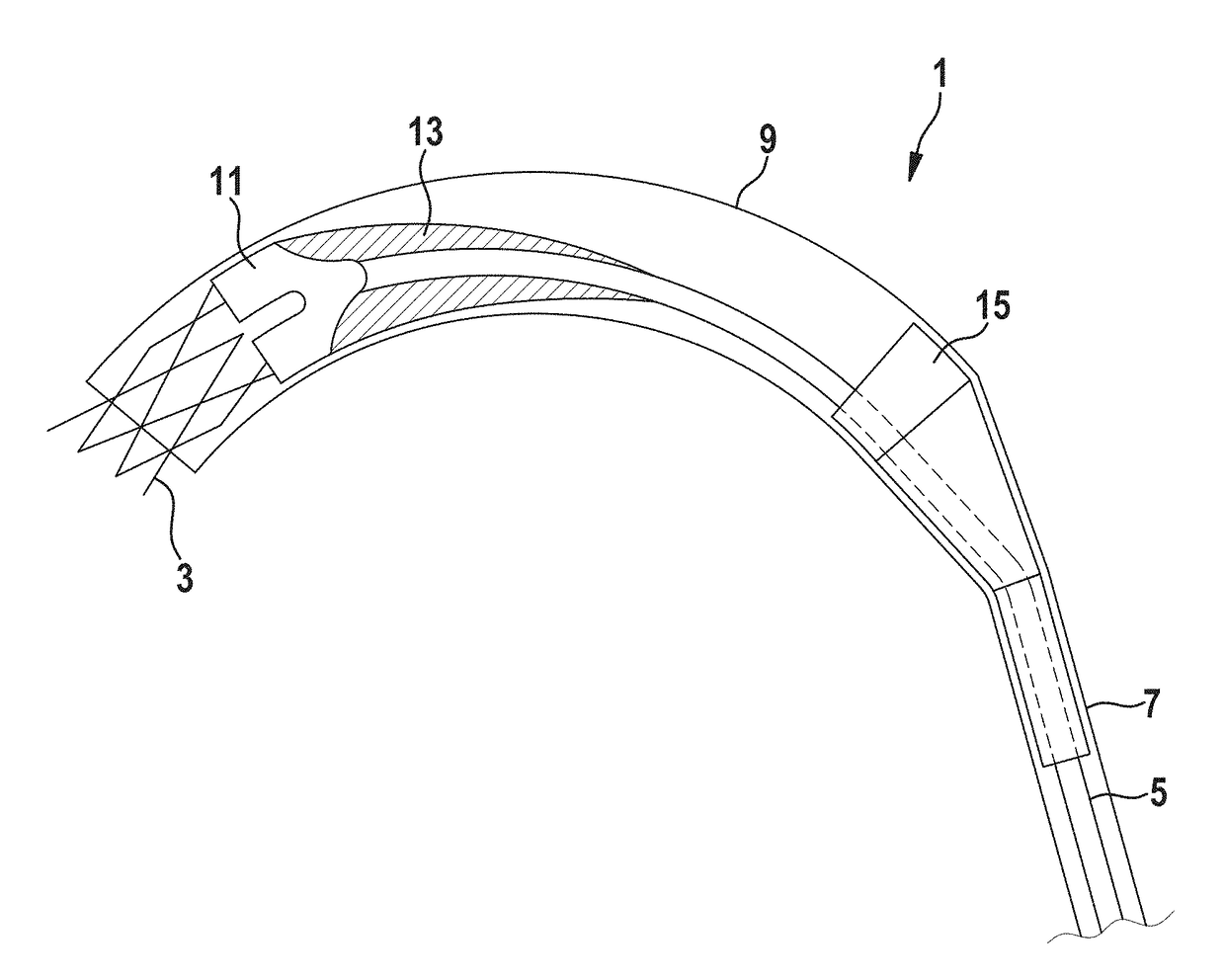
Delivery catheter and catheter arrangement
A delivery catheter for implanting a self-expanding implant such as a cardiovascular implant. An outer catheter shaft is formed as an implant capsule for encasing the implant during delivery. A flexible distal support element having a substantially truncated cone-shaped portion, which tapers in the proximal direction, is attached, directly proximally of the implant, in a fixed position to a first, inner catheter shaft, and / or a flexible proximal support element having a substantially truncated cone-shaped portion, which tapers in the proximal direction, is inserted at the proximal end of the implant capsule into the second, outer catheter shaft in a fixed position.
Owner:BIOTRONIK AG
Preparation method of medical implant material surface function atom doped molecular sieve layer
ActiveCN104532213AHigh bonding strengthImprove corrosion resistanceLiquid/solution decomposition chemical coatingSynthesis methodsBiocompatibility Testing
The invention provides a preparation method of a medical implant material surface function atom doped molecular sieve layer and belongs to the technical field of preparation of the molecular sieve layer having high biological activity. New-generation medical implant alloy materials (a bone repair material, a dental implant material and a cardiovascular implant material) having low elasticity modulus and high biocompatibility are used as a substrate, a Silicalite-1 type molecular sieve layer doped with different function atoms grows on the surface of the substrate by means of a co-synthesis method, and accordingly the biological activity, corrosion resistance and bone growing induction capacity of a composite material is improved. The preparation method is suitable for various low-elasticity-modulus medical implant materials, has the advantages of being moderate in preparation conditions, simple in process, low in cost and the like, can be applied to implant materials of any shapes and surface roughness and shows the potential of further development of novel hard-tissue implant materials.
Owner:JILIN UNIV
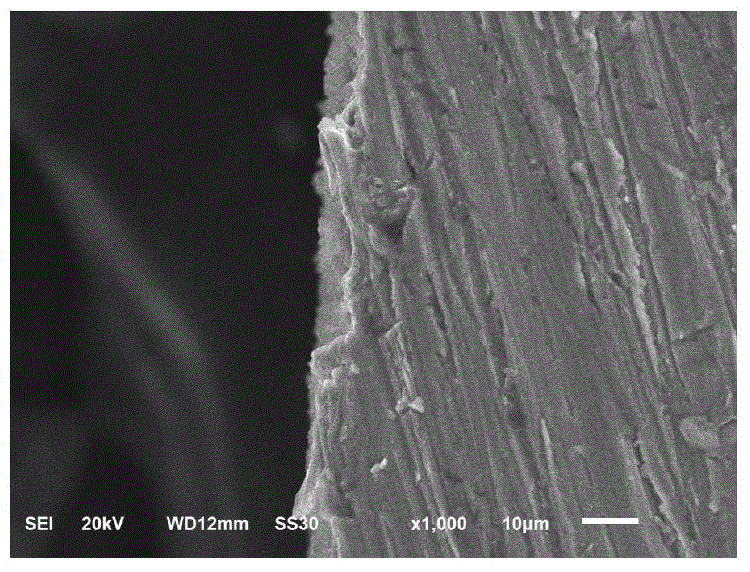
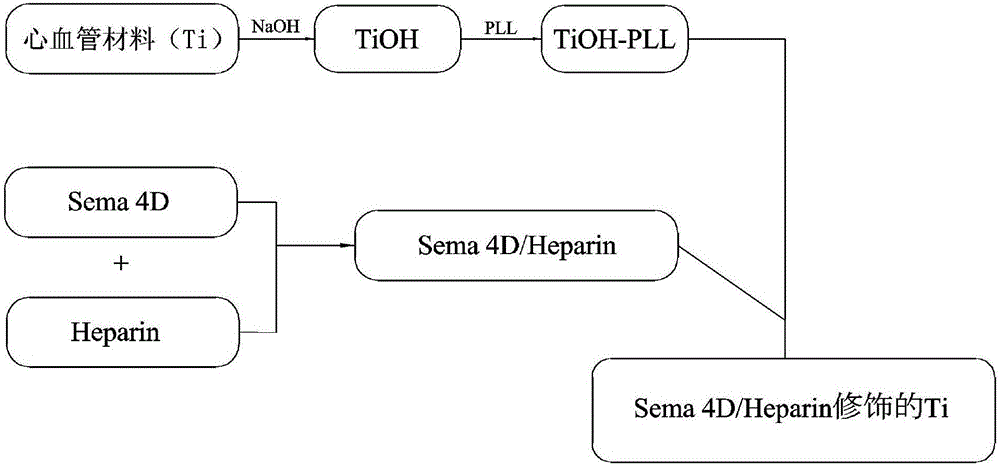
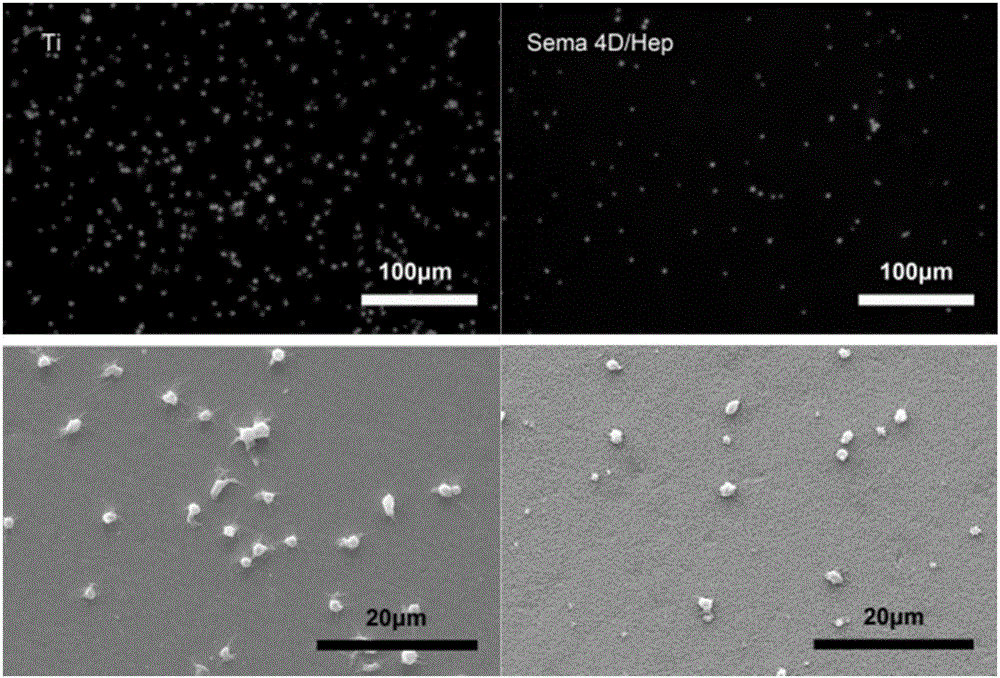
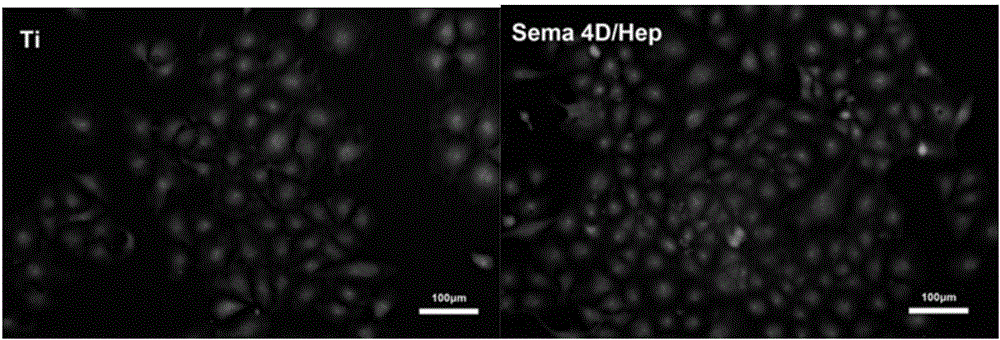
Method for constructing Sema 4D/Heparin microenvironment on surface of cardiovascular implanted material
ActiveCN106474559ADelayed half-lifeEasy to identifySurgeryPharmaceutical delivery mechanismBiological activationCell migration
The invention discloses a method for constructing a Sema 4D / Heparin microenvironment on the surface of a cardiovascular implanted material. The method comprises the following steps: A, performing alkali activation on a material by adopting NaOH; B, performing surface amino functionalization on the sample prepared in the step A; C, preparing a Heparin / Sema 4D compound; and D, fixing the Heparin / Sema 4D compound on the surface of the sample prepared in the step B. According to the method, Sema 4D and heparin are combined and together fixed on a material surface, so that Sema 4D can be protected and is prevented from being degraded by protease, identification and combination of Sema 4D and corresponding receptors can be promoted, and functions of the Sema 4D can be achieved. The method can be used for well simulating a body blood injury repairing process, has the advantages of simple process, is easy to operate, does not need expensive complicated equipment, and is applicable to surfaces of various complicated cardiovascular implanted appliances and other devices with requirement for resisting coagulation / promoting endothelial cell migration.
Owner:SOUTHWEST JIAOTONG UNIV
polymer prosthetic aortic valve
ActiveCN103961192BImprove performanceExtend your lifeHeart valvesAnatomical structuresProsthetic valve
Owner:BEIJING UNIV OF TECH
Method for fixing polypeptide aptamers on surface of cardiovascular implanting device
InactiveCN102085125BImprove efficiencyAchieving directional fixationStentsSurgeryProgenitorHuman body
The invention discloses a method for fixing polypeptide aptamers on the surface of a cardiovascular implanting device, which ensures that endothelial progenitor cells in the blood can be captured after the ardiovascular implanting device is implanted into a human body. The method comprises the steps of: surface activation, silanization treatment, biotin fixation, avidin fixation, albumin fixation, biotinylated aptamers fixation and the like. The method for fixing specific polypeptide aptamers for capturing the endothelial progenitor cells on the surface of the cardiovascular implanting device ensure that the cardiovascular implanting device can be used for capturing the endothelial progenitor cells in the blood after being implanted into a human body, and is favorable for inducing the rapid endothelium transition of the surface of the implanting device and improving the blood compatibility of a material, and thus, the implanting device has favorable functions of blood anticoagulation and restenosis inhibition.
Owner:CO WITH LTD LIABILITY OF MEDICAL SCI +1
Preparation method of molecular sieve coating doped with functional atoms on the surface of medical implant material
ActiveCN104532213BHigh bonding strengthImprove corrosion resistanceLiquid/solution decomposition chemical coatingSynthesis methodsBiocompatibility Testing
The invention provides a preparation method of a medical implant material surface function atom doped molecular sieve layer and belongs to the technical field of preparation of the molecular sieve layer having high biological activity. New-generation medical implant alloy materials (a bone repair material, a dental implant material and a cardiovascular implant material) having low elasticity modulus and high biocompatibility are used as a substrate, a Silicalite-1 type molecular sieve layer doped with different function atoms grows on the surface of the substrate by means of a co-synthesis method, and accordingly the biological activity, corrosion resistance and bone growing induction capacity of a composite material is improved. The preparation method is suitable for various low-elasticity-modulus medical implant materials, has the advantages of being moderate in preparation conditions, simple in process, low in cost and the like, can be applied to implant materials of any shapes and surface roughness and shows the potential of further development of novel hard-tissue implant materials.
Owner:JILIN UNIV
A kind of hydrogel coating with long-term antibacterial function and preparation method thereof
ActiveCN108815587BAdjustable thicknessCompact structureSurgeryCoatingsAntimicrobial drugOrthopedic department
The invention discloses a hydrogel coating with a long-term antibacterial function and a preparation method thereof. The method comprises the following steps that 1, pre-treatment is conducted on a base material; 2, at room temperature, the base material subjected to pre-treatment is placed in a polycation electrolyte solution and a mixed solution of a polyphenol compound and an antibacterial medicine in sequence, and after reaction is carried out in a polyanion electrolyte solution for 5-20 min, washing is conducted for 3-5 times; 3, operation of step 2 is repeated for 5-20 times to obtain the hydrogel coating. The method is simple and convenient to operate and cheap in raw material, and the prepared hydrogel coating has good biocompatibility and excellent antibacterial performance; the hydrogel coating has good medicine loading capacity and stable medicine releasing performance, and can be used for preparing and modifying cardiovascular implant endovascular devices, orthopedics filling materials and antibacterial wound dressings.
Owner:JILIN VENUS HAOYUE MEDICAL LTD
Method for mediating albumin to form protein layer on material surface, biomaterial and application thereof
The invention discloses a method for mediating albumin to form a protein layer on the surface of a material, a biological material and an application thereof, and relates to the technical field of biological materials. The method for mediating albumin to form a protein layer on the surface of the material comprises: standing the material to be loaded in an alkaline solution to obtain an alkali-activated material; immobilizing dendrimers on the surface of the alkali-activated material, and then immersing the modified material in Standing reaction in the albumin solution; preferably, the dendritic macromolecule is polyamidoamine type dendritic macromolecule PAMAM; more preferably, the molecular weight of PAMAM is 6000-10000. The biomaterial forms a PAMAM layer and an albumin layer on the surface of the material to be loaded, and then mediates the albumin to form a protein layer. The biomaterial is used to prepare a cardiovascular implant material, which can reduce the adhesion of platelets to play an anticoagulant effect and improve the biocompatibility of the material.
Owner:SOUTHWEST JIAOTONG UNIV
Nickel-titanium alloy containing bioactive coating and its preparation method and application
InactiveCN104129113BMeet the need for rapid endothelializationExpand the scope of clinical applicationLayered productsMetallic material coating processesPhosphateLayer by layer self assembly
The invention discloses a nickel-titanium alloy containing a biologically-active coating. A preparation method for the alloy comprises the following steps: polishing the surface of the nickel-titanium alloy so as to allow the surface of the nickel-titanium alloy to be shining; then soaking the alloy in a Tris-dopamine hydrochloride solution with a concentration of 1 to 3 mg / mL for 12 to 36 h so as to allow a polydopamine film to be formed on the surface; preparing a chitosan solution with a concentration of 1 to 10 mg / mL with 0.1 to 0.5% (v / v) acetic acid as a solvent, preparing a gelatin solution with a concentration of 1 to 10 mg / mL and an endothelial cell growth factor solution with a concentration of 100 to 500 ng / mL by using a phosphate buffer solution, soaking the treated nickel-titanium alloy in the gelatin solution for 12 to 36 h and then carrying out self-assembling of chitosan-gelatin-endothelial cell growth factor-gelatin layer by layer, wherein soaking in each solution lasts for 5 to 20 min and there are 4 to 10 cycles of layer-by-layer self-assembling; and finally, carrying out blow-drying with nitrogen. The coating can effectively promote adhesion and growth of endothelial cells on the surface of the nickel-titanium alloy, so demands of rapid endothelialization of the nickel-titanium alloy as a cardiovascular implant material are met, the problems of induction of thrombi and generation of coagulation on the surface of the nickel-titanium alloy are overcome, and the clinical application scope of the nickel-titanium alloy is broadened.
Owner:CHONGQING UNIV
Cardiovascular implant based on in-situ regulation immune response and preparation method thereof
ActiveCN114246986APromote regenerationPromote long-term patencyPowder deliveryHydrolasesAntiendomysial antibodiesEngineering
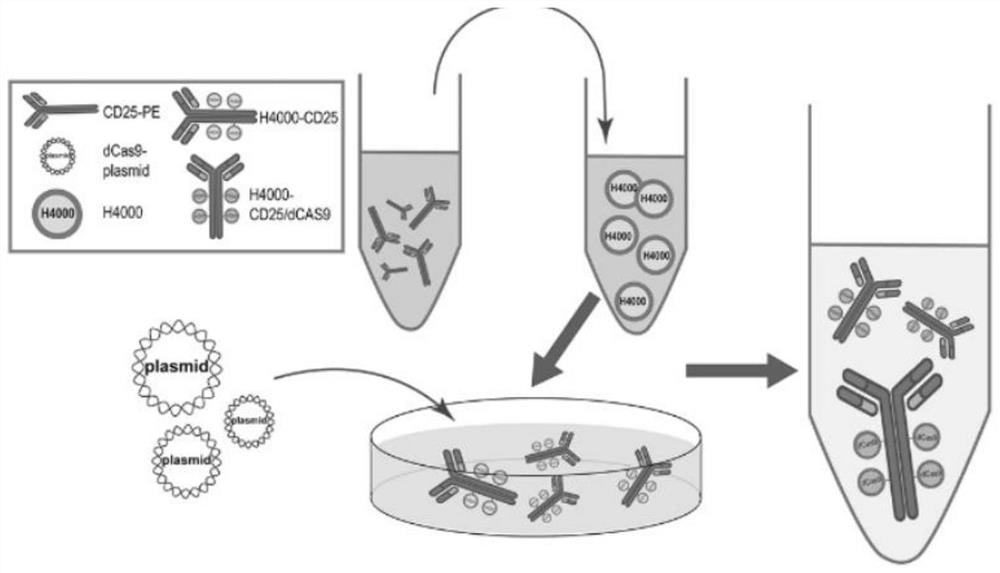
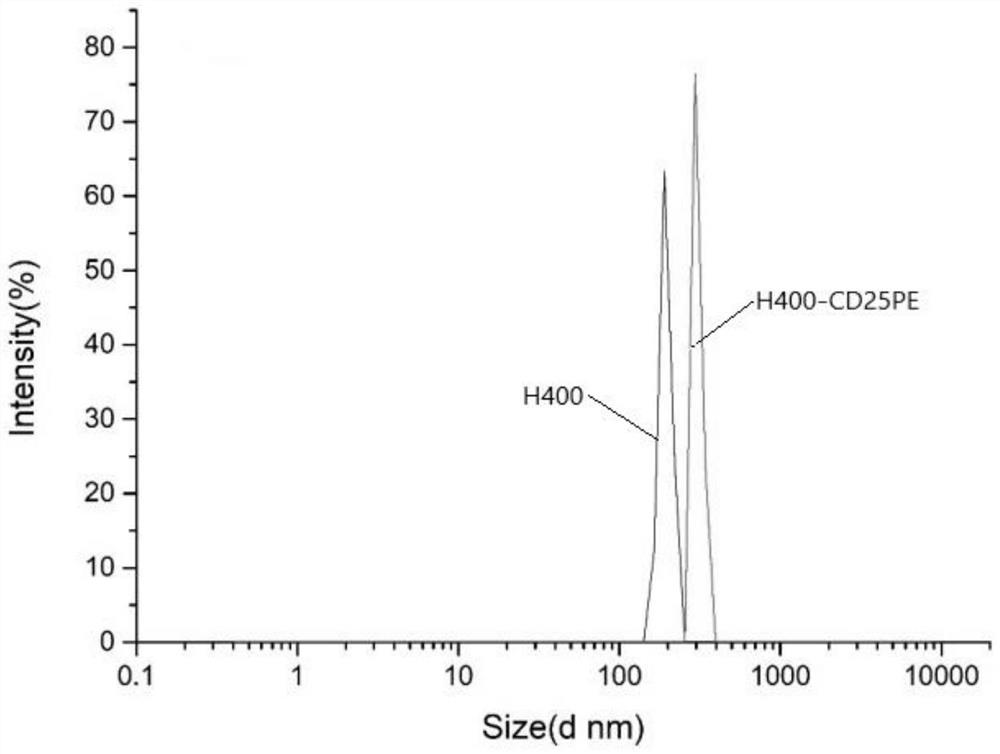
The invention discloses a cardiovascular implant based on in-situ regulation and control of immune response and a preparation method thereof, and belongs to the technical field of biological medicine, and the technical scheme is characterized in that the cardiovascular implant comprises a cardiovascular implant body and H4000-CD25 / dcas9 slow-release nanoparticles modified on the cardiovascular implant body; the H4000-CD25 / dcas9 sustained-release nano particle comprises an H4000 plasmid nano carrier (Engreen), a CD25 antibody and a dcas9 plasmid sequence, and is characterized in that the H4000-CD25 / dcas9 sustained-release nano particle comprises a H4000 plasmid nano carrier (Engreen), the preparation method of the cardiovascular implant comprises the following steps: constructing the cardiovascular implant body, preparing the H4000-CD25 nano transfection carrier, preparing the H4000-CD25 / dcas9 sustained-release nano-particles, and coupling the H4000-CD25 / dcas9 sustained-release nano-particles to the cardiovascular implant body. The method is mainly used for constructing the cardiovascular implant modified with the H4000-CD25 / dcas9 sustained-release nanoparticles, nerve fibers can be induced to grow into engineering blood vessels, and the anti-thrombus function of the cardiovascular implant is improved and in-situ regeneration of the cardiovascular implant is promoted by utilizing the regulation and control capability of Treg cells on immune response.
Owner:ARMY MEDICAL UNIV
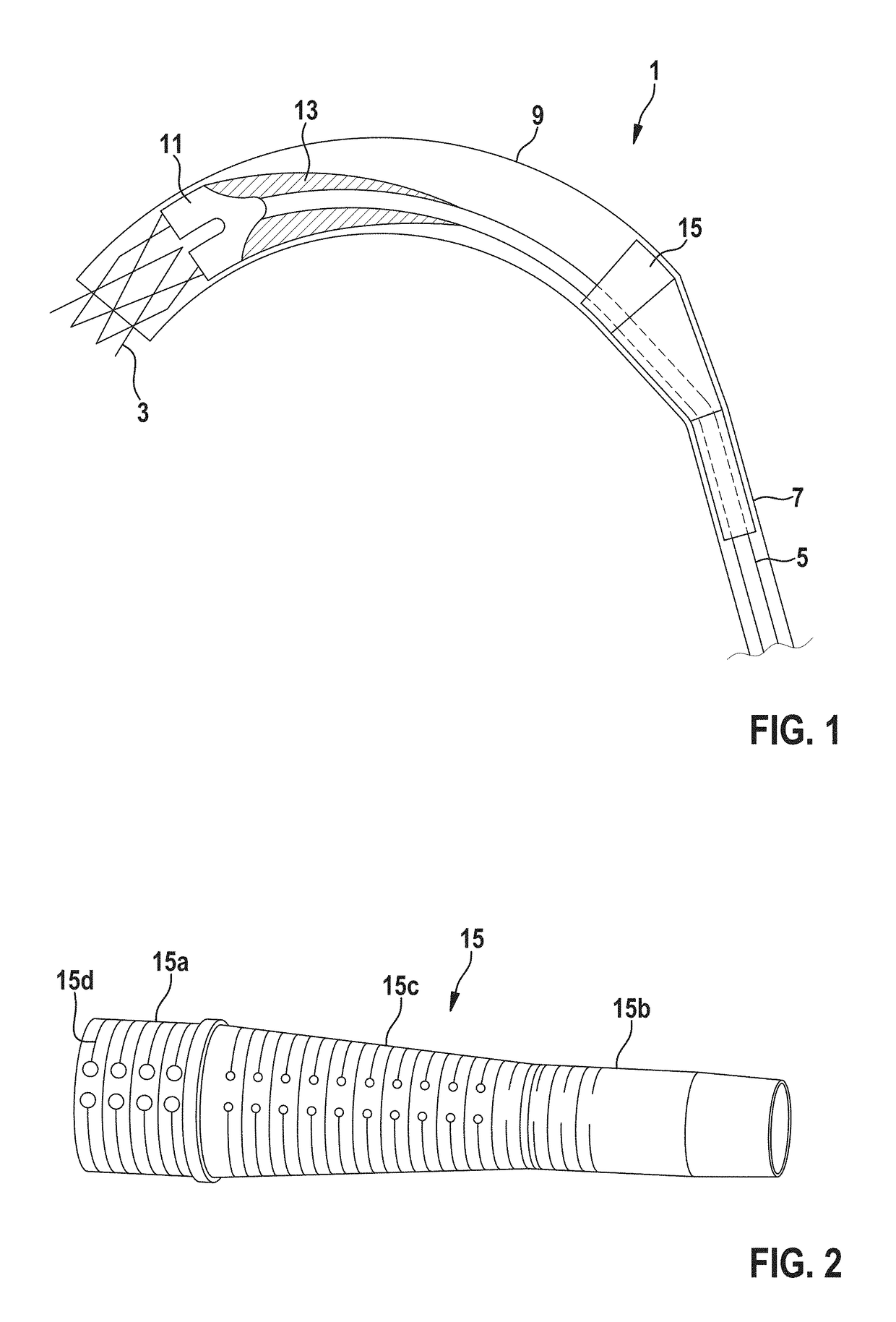
Delivery catheter and catheter arrangement
A delivery catheter for implanting a self-expanding implant such as a cardiovascular implant. An outer catheter shaft is formed as an implant capsule for encasing the implant during delivery. A flexible distal support element having a substantially truncated cone-shaped portion, which tapers in the proximal direction, is attached, directly proximally of the implant, in a fixed position to a first, inner catheter shaft, and / or a flexible proximal support element having a substantially truncated cone-shaped portion, which tapers in the proximal direction, is inserted at the proximal end of the implant capsule into the second, outer catheter shaft in a fixed position.
Owner:BIOTRONIK AG
Cardiovascular implant for regulating immune response and promoting intima regeneration and preparation method thereof
The invention belongs to the technical field of biomedicine and relates to a cardiovascular implant and a preparation method thereof, in particular to a cardiovascular implant capable of regulating immune response in situ to promote intima regeneration and a preparation method thereof. The cardiovascular implant includes a cardiovascular implant body, and the cardiovascular implant body is modified with CD133 aptamers and slow-release nanoparticles. Modified the CD133 aptamer and slow-release nanoparticles containing axon-guiding molecule-1 onto the body of the cardiovascular implant to regulate the immune response in situ and promote intima regeneration, effectively overcome thrombus formation, and promote long-term patency of blood vessels . Especially for patients with clinical inflammatory basic lesions, the present invention provides a cardiovascular implant that is easy and quick to prepare, has wide applicability, can regulate excessive inflammatory reactions in situ, promote intimal regeneration and maintain long-term function, and has the advantages of Good application prospects.
Owner:ARMY MEDICAL UNIV
OCT-based automatic detection and evaluation method and system for cardiovascular implanted stents
ActiveCN106780495BImprove detection accuracyImprove robustnessImage enhancementImage analysisCoronary arteriesFeature extraction
The present invention provides a method and system for automatic detection and evaluation of an OCT-based cardiovascular implant stent, the method comprising the following steps: acquiring a coronary artery OCT image to be detected; Perform preprocessing; perform sliding window traversal on the preprocessed image; perform feature extraction on the sliding window image; judge whether the feature value of the extracted sliding window image is greater than the feature threshold corresponding to the classifier model; detect where the bracket is located in the sliding window image The area and the center of the stent; detect the inner wall contour of the coronary artery in the preprocessed image; calculate the shortest distance between the center of the stent and the inner wall contour of the coronary artery to obtain the adhering situation or coverage of the stent; the adhering situation of the stent or coverage is displayed. The method and system proposed by the invention are not affected by noise such as blood artifacts, can work in a complex imaging environment, have high detection accuracy of the stent, and have good robustness.
Owner:SHENZHEN VIVOLIGHT MEDICAL DEVICE & TECH CO LTD
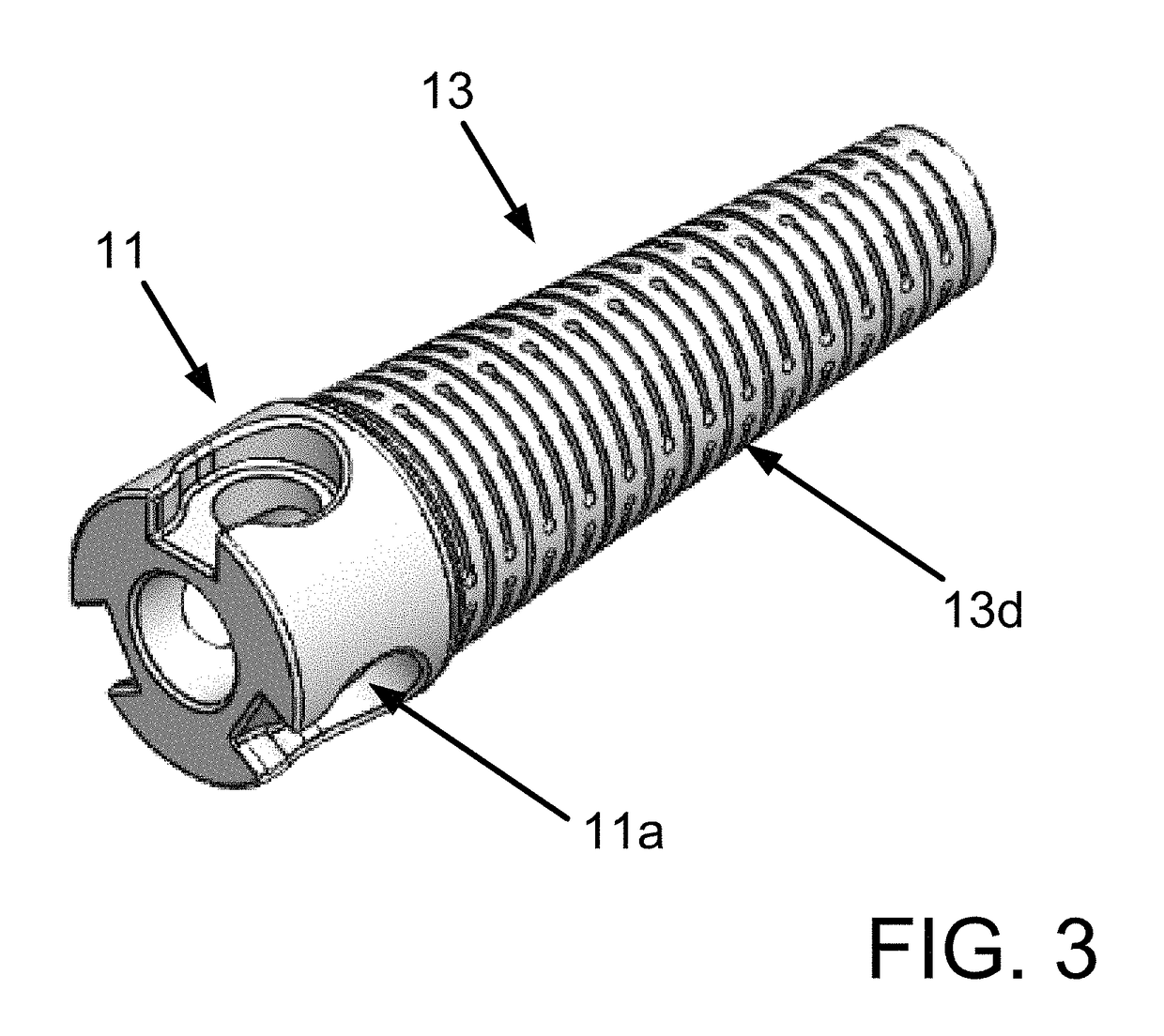
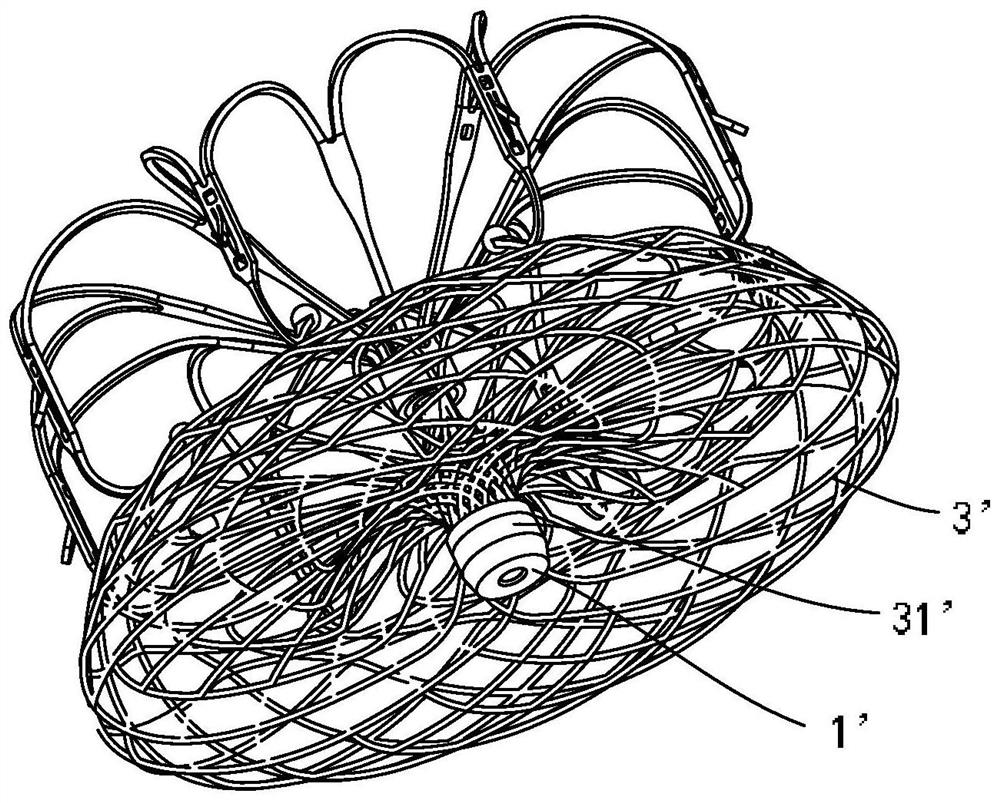
Connecting piece and assembling process of cardiovascular implant and conveying equipment
PendingCN113440322ALower protrusion heightReduces the risk of microclotsStentsProsthesisForeign matterVascular implant
The invention relates to the technical field of cardiovascular implant implantation, in particular to a connecting piece and an assembling process of a cardiovascular implant and conveying equipment, the connecting piece is used for connecting the cardiovascular implant and the conveying equipment, the connecting piece comprises a main body, a connecting hole and a threaded hole are formed in the main body, the connecting hole is used for inserting a mounting part of the cardiovascular implant, and the threaded hole is used for being in threaded connection with the conveying equipment. When the connecting piece is used for connecting the cardiovascular implant and the conveying equipment, a connecting end or a closing-up structure is reduced, the risk of generating micro thrombus can be effectively reduced, stress on each part of the implant is uniform, the structure is simple, welding spots are reduced, in addition, the foreign matter content and the surface area are also reduced, and the risk of corrosion is further reduced.
Owner:GUANGDONG PULSE MEDICAL SCI & TECH CO LTD
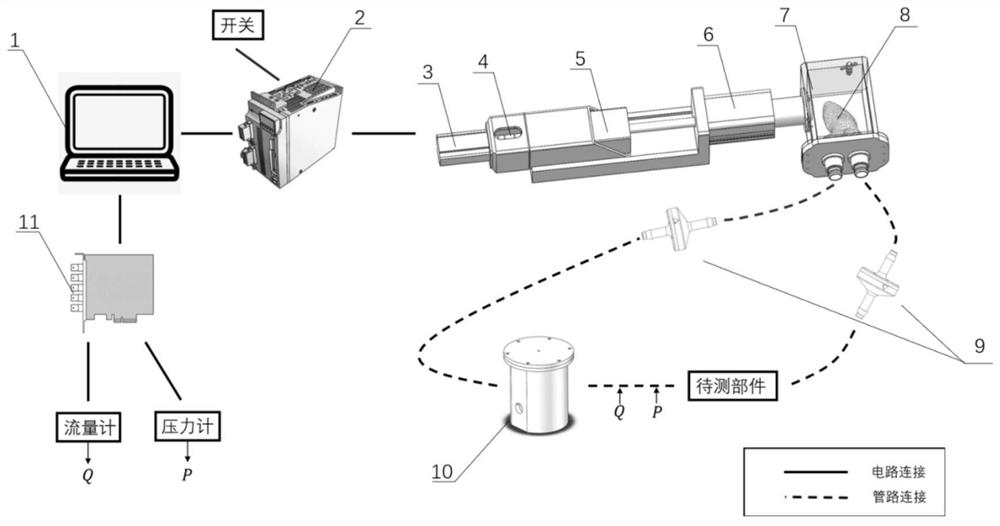
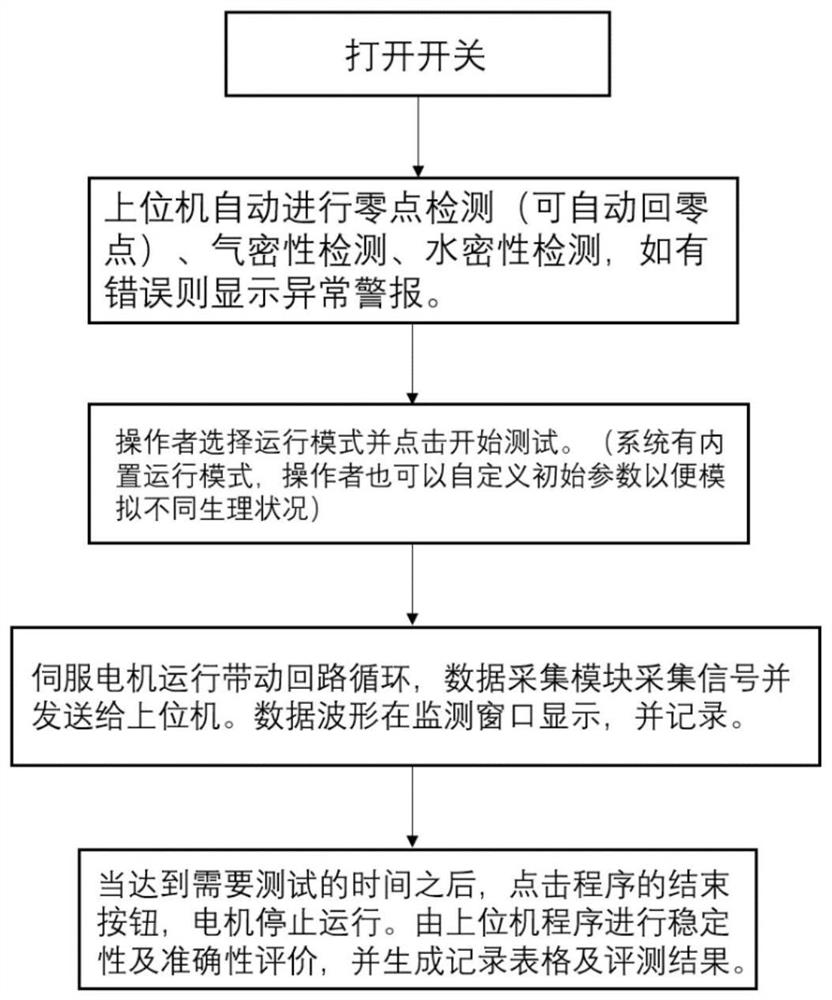
In-vitro evaluation test system and test method for cardiovascular implant interventional material
The invention discloses an in-vitro evaluation test system and test method for a cardiovascular implant interventional material, and belongs to the technical field of test. The evaluation system is mainly composed of an upper computer, a servo driving system, a water circulation loop and a data acquisition module. According to the system, the servo motor system is controlled by the upper computer to serve as power input to drive the water circulation loop to operate, and then the data of the water circulation loop is collected by the data collection module, so that the performance of the cardiovascular implant interventional material placed in the circulation loop is evaluated. By means of the mode, the system can be used for evaluating the performance of various cardiovascular implant interventions, data recording and sorting are facilitated, the data can be compared with standard data, a detection result can be generated, and the system has the advantages of being accurate, stable and the like.
Owner:BEIJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com