Cardiovascular implant for regulating and controlling immune response and promoting intimal regeneration and preparation method of cardiovascular implant
An immune response and cardiovascular technology, applied in the field of biomedicine, to achieve the effect of promoting mobilization and proliferation, promoting early endothelialization, and promoting inflammation regression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
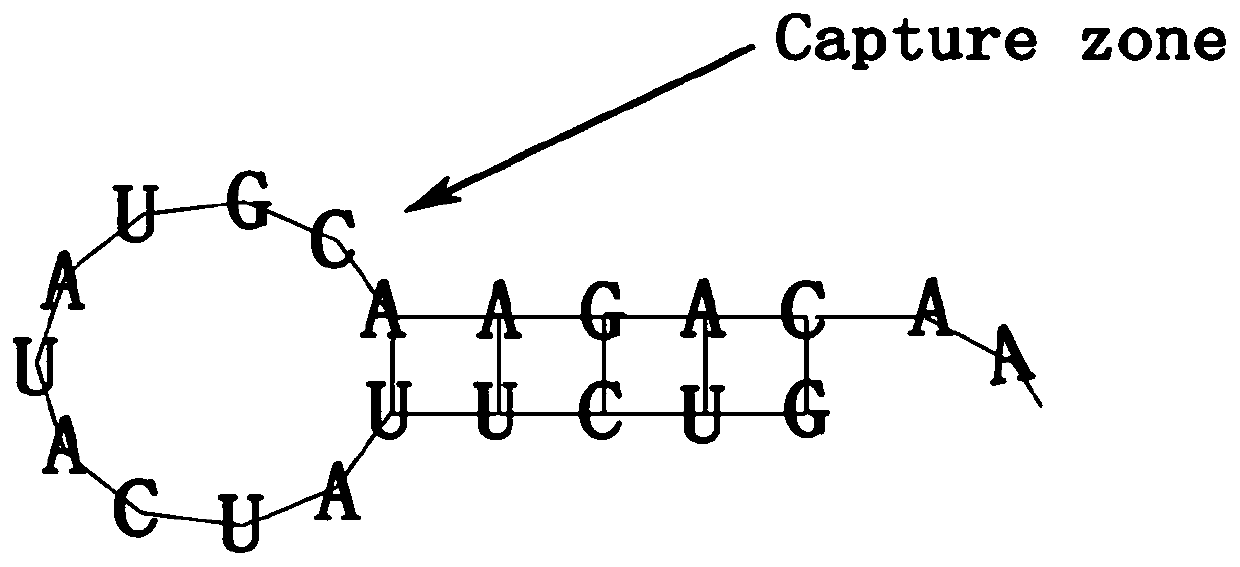
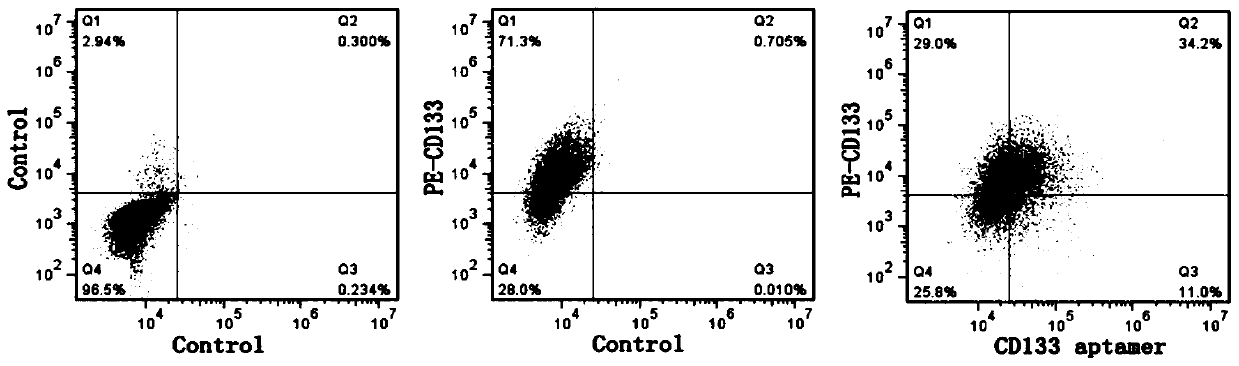
[0066] Example 1: Cardiovascular implants modified by simple CD133 aptamers
[0067] Preparation of natural decellularized vascular matrix material: Under sterile conditions, extract the common carotid artery from 250-300g SD rats, wash the blood with normal saline, separate and remove the outer connective tissue of the common carotid artery, and then cut it into a length of 0.5- Small blood vessels of 1 cm (generally, tissue engineered blood vessels with a length of 0.5-20 cm can meet the needs of clinical applications such as blood vessel transplantation, and the transplanted objects of the cardiovascular implants are rats, so small blood vessels with a length of 0.5-1 cm are used as Preferably), dilute trypsin with M199 medium to a concentration of 0.05% and digest blood vessels at 37°C for 30 minutes to remove cells, then use RNase, DNase and fat to remove nucleic acid and fat to obtain a vascular matrix material with only collagen and elastic fibers . Wherein, the calibe...
Embodiment 2
[0072] Example 2: Nano-sustained-release particles modified and surface-modified cardiovascular implants
[0073] In this example, nano-sustained-release particle modification and surface modification were performed on the cardiovascular implant body, but no CD133 aptamer modification was performed on the cardiovascular implant body, as follows:
[0074] Carry out the modification of slow-release nanoparticles on the body of the cardiovascular implant, mix 100ng / ml axon guidance molecule-1 (R&D, USA) and chitosan at a molar mass ratio of 1:2, and adjust the pH value of the solution 5.1-6.0, slowly add 0.15% TPP (sodium tripolyphosphate) during stirring, after continuous stirring to obtain a milky white liquid, centrifuge at 4°C for 10 minutes, wash the precipitate for 3 times with double distilled water, and then freeze-dry to obtain nano sustained-release particles. At 4°C, under the condition of 120rpm, use 0.1% (w / v) slow-release nanoparticles and 0.4% collagen solution to ...
Embodiment 3
[0080] Example 3: Triple Modified Cardiovascular Implants
[0081] In this example, the preparation method of the natural decellularized vascular matrix material and the cardiovascular implant body is the same as in Example 1, the difference is that the cardiovascular implant body is triple modified in this example, and the specific conditions are as follows:
[0082] CD133 aptamer anchoring modification: 5mM EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) was used to incubate the collagenous vascular matrix material (i.e. cardiovascular implant object body) for cross-linking for 24 h, and then washed 3 times with PBS. PEI / gold nanoparticles are synthesized by a one-step method. 1% PEI solution (polyethyleneimine) (1.44ml) is slowly added into 14mM or 28mM HAuCl4 aqueous solution (25ml), and stirred at room temperature for 24h to form low density and high density of PEI / gold nanoparticles. Then, centrifuge at 20000g / min at 4°C, wash with distilled water thr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| greyscale | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com