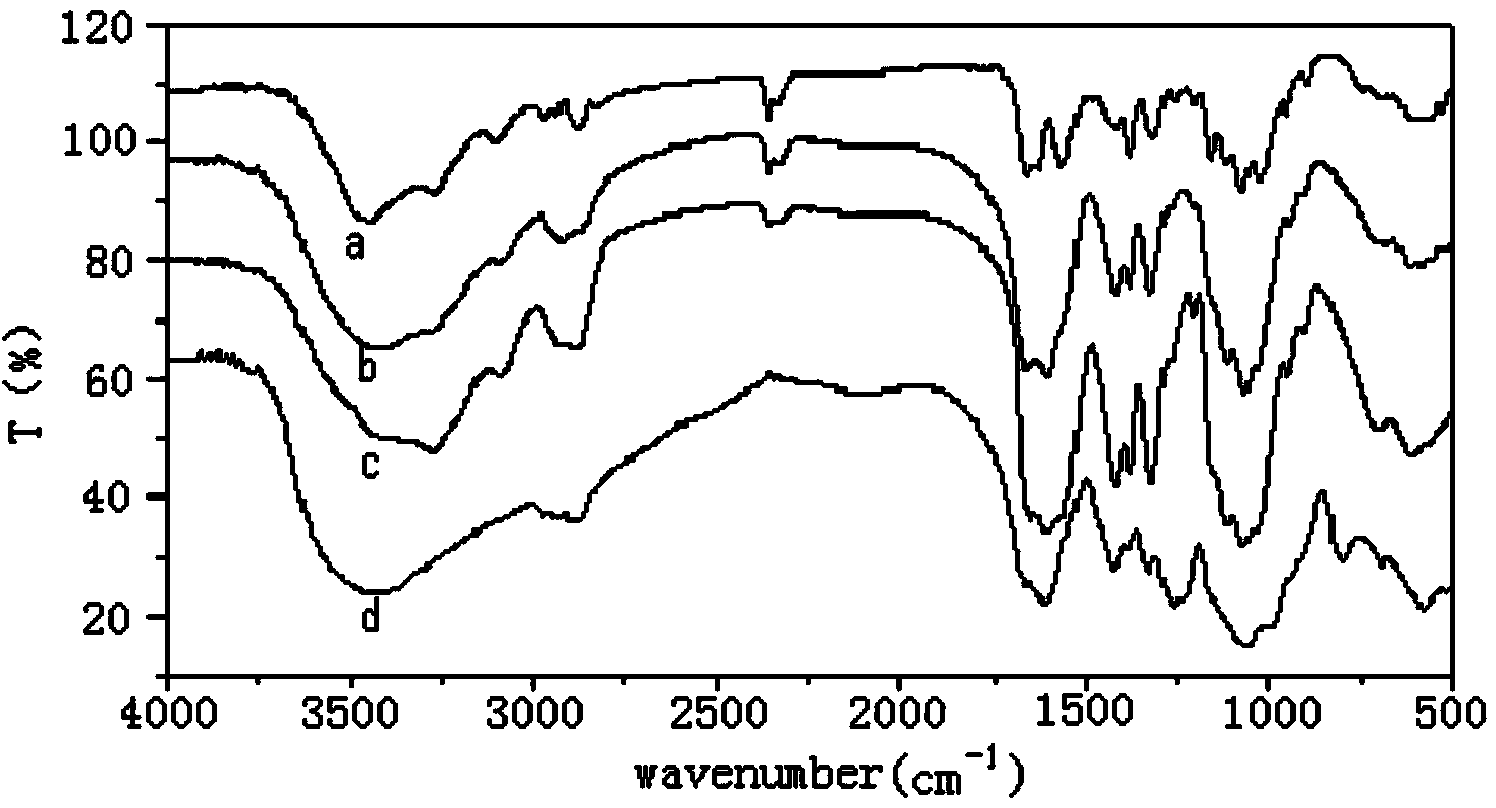
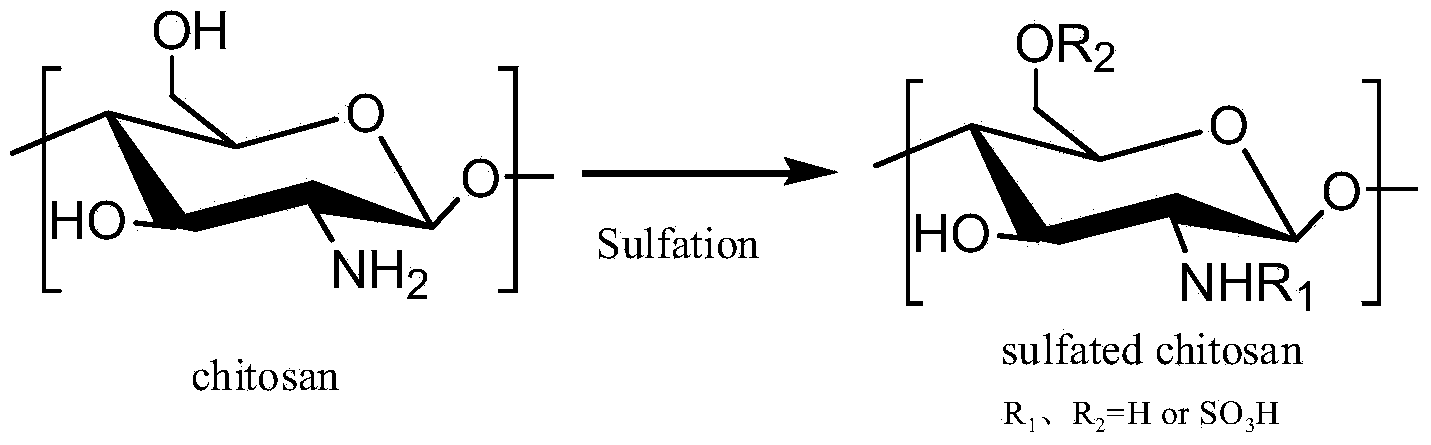
Synthesis method of 6-O-carboxymethyl chitosan sulfuric sulfation product
A technology of carboxymethyl chitosan sulfate and carboxymethyl chitosan, applied in the field of medical polymers, can solve the problems of selective substitution of carboxymethyl groups, difficulty in controlling the degree of substitution, unsatisfactory synthesis methods, etc. Virus contamination risk, excellent drug safety performance, mild effects of reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The invention provides a kind of synthetic method of 6-O-carboxymethyl chitosan sulfated product, in one embodiment, comprises the following steps:
[0036] (1) Chitin alkalization treatment
[0037] Mix chitin and 20wt%-60wt% NaOH aqueous solution according to the molar ratio of chitin and NaOH in the ratio of 1:5 to 1:10, stir and disperse at 20-25°C, and wait for NaOH aqueous solution under vacuum negative pressure for 1-5 hours Complete penetration, freeze at -20~0℃ for 12-48 hours;
[0038] (2) Chitin C 6 -O carboxymethylation
[0039] Add the chitin that has been alkalized in step (1) into the dispersant and stir until completely dispersed. The mass ratio of the volume of the dispersant to chitin is 10-20ml: 1g, slowly add chloroacetic acid, within 10min-1 hour After the addition, the mass ratio of chloroacetic acid to chitin is 0.47-2.33g: 1g, react at 25-65°C for 2-24 hours; after the reaction is completed, remove the dispersant by filtration, dissolve the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com