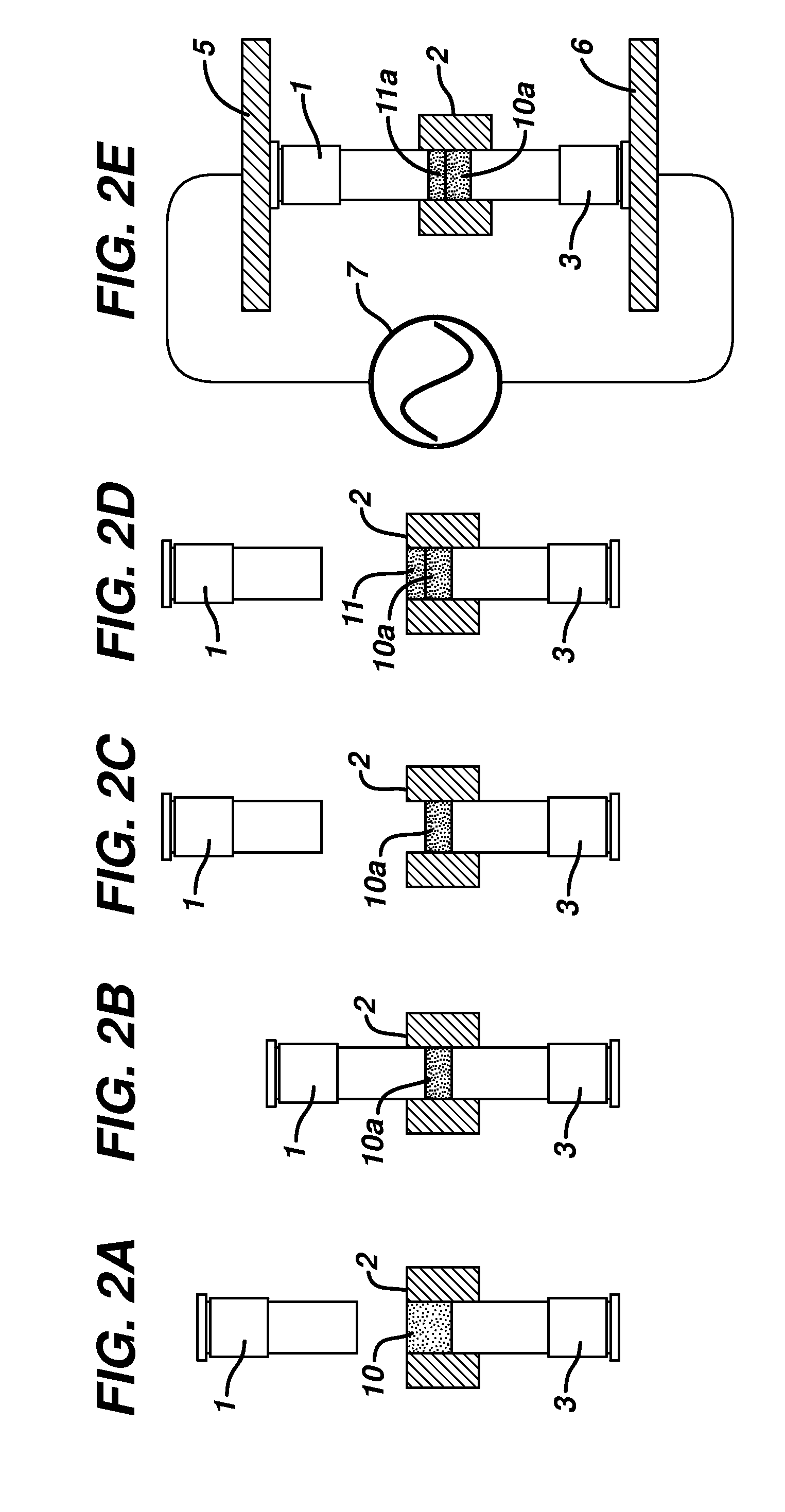
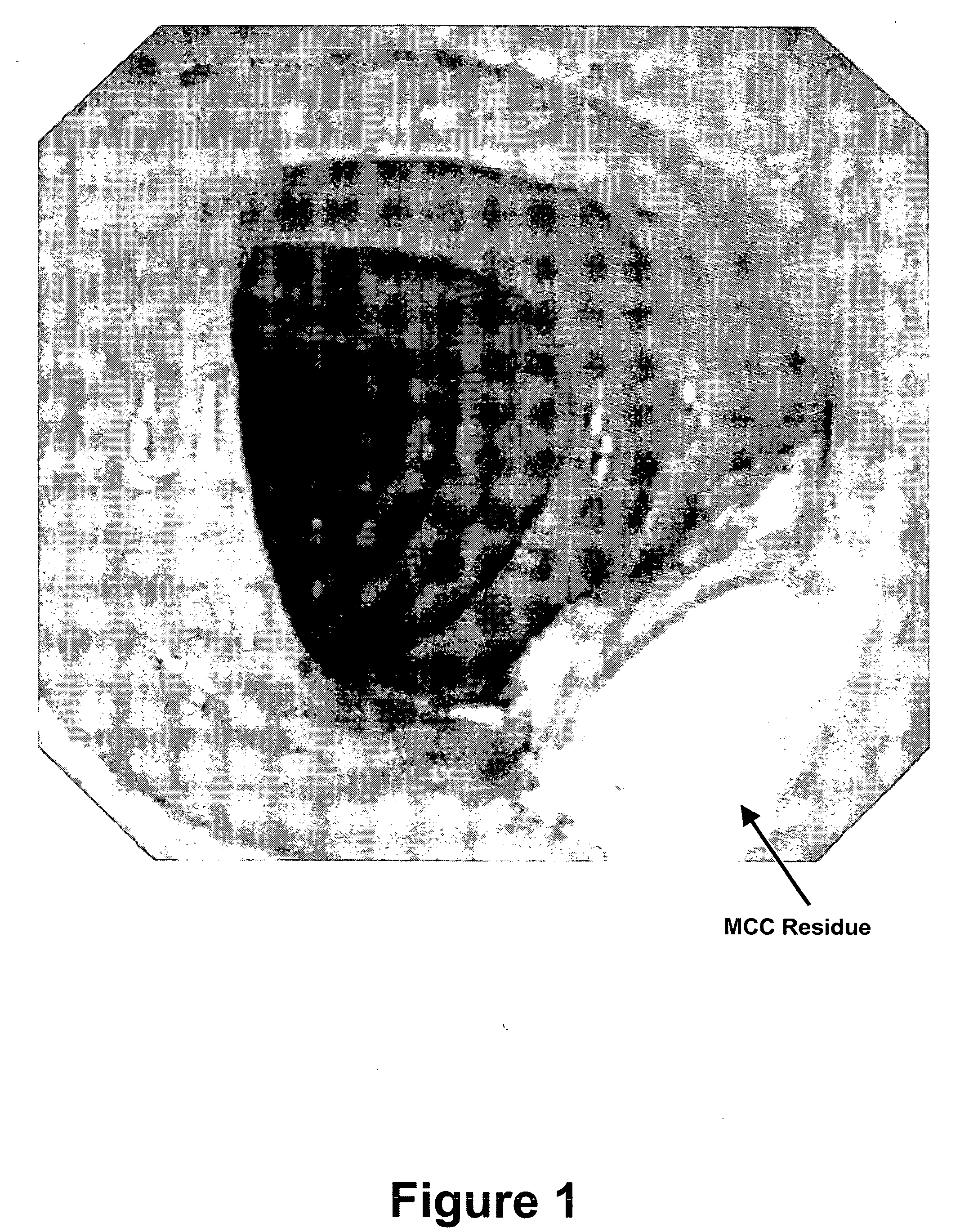
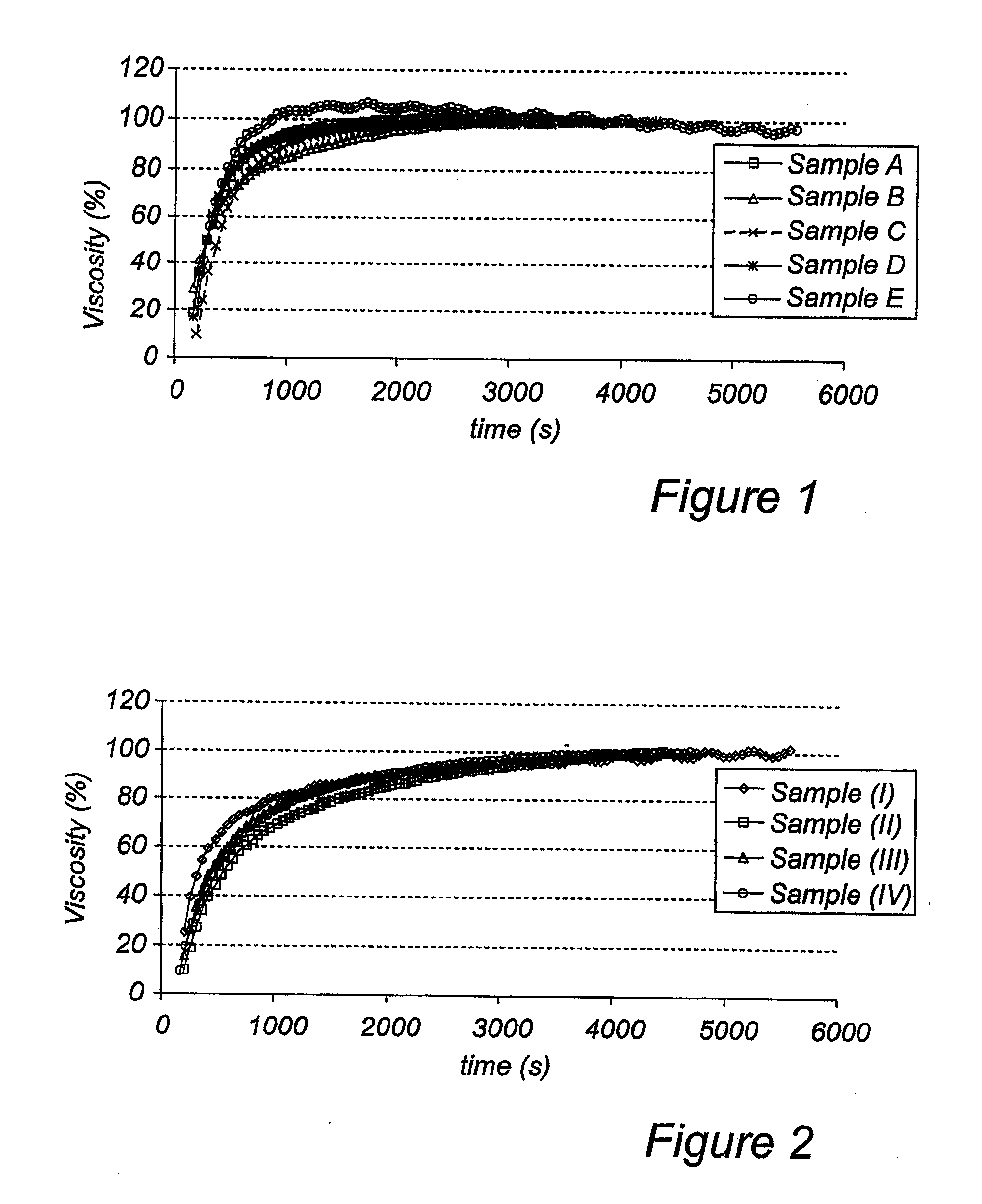
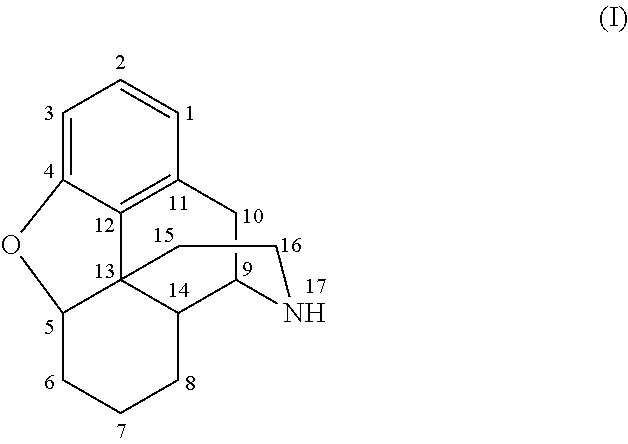
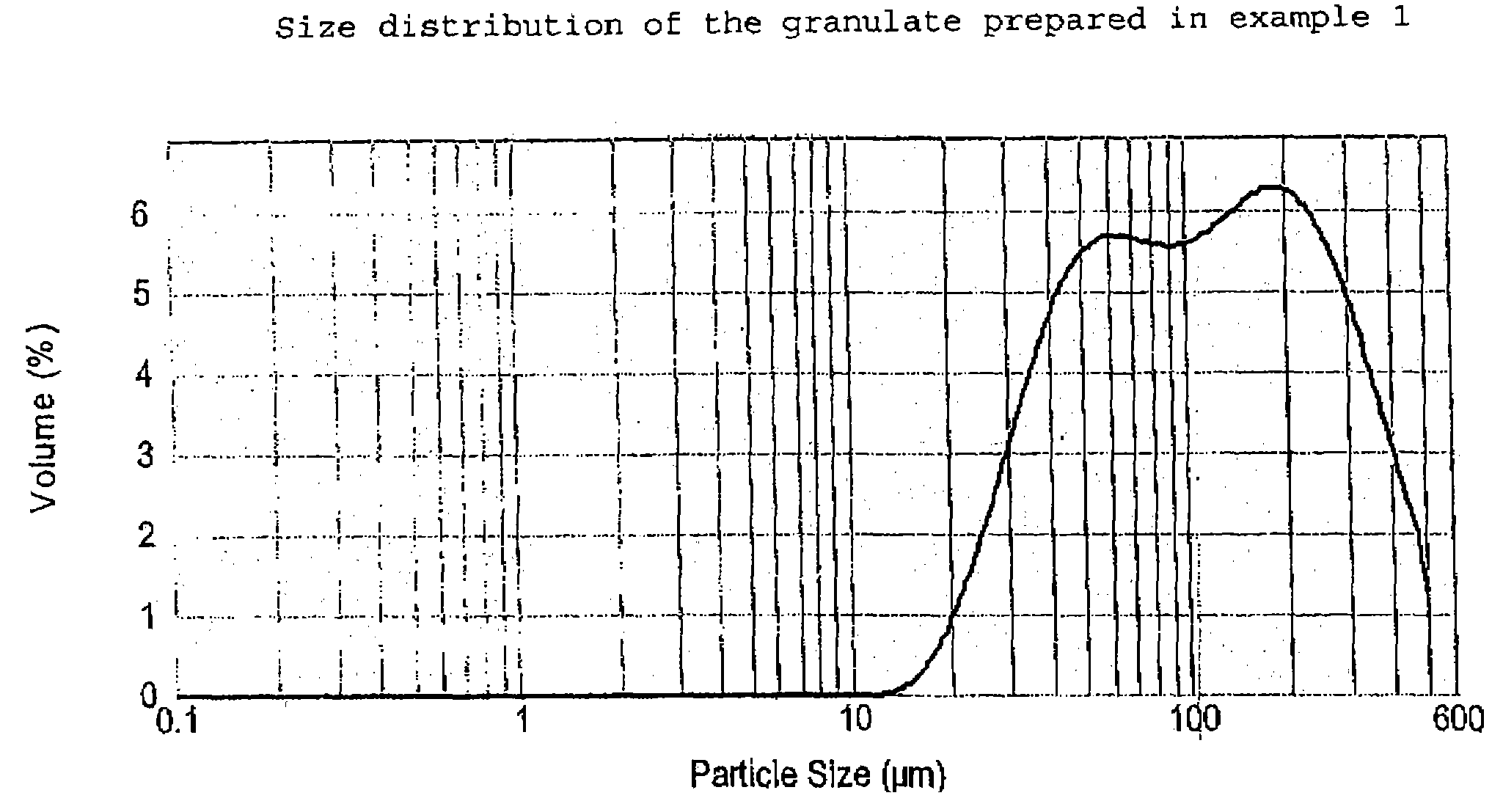
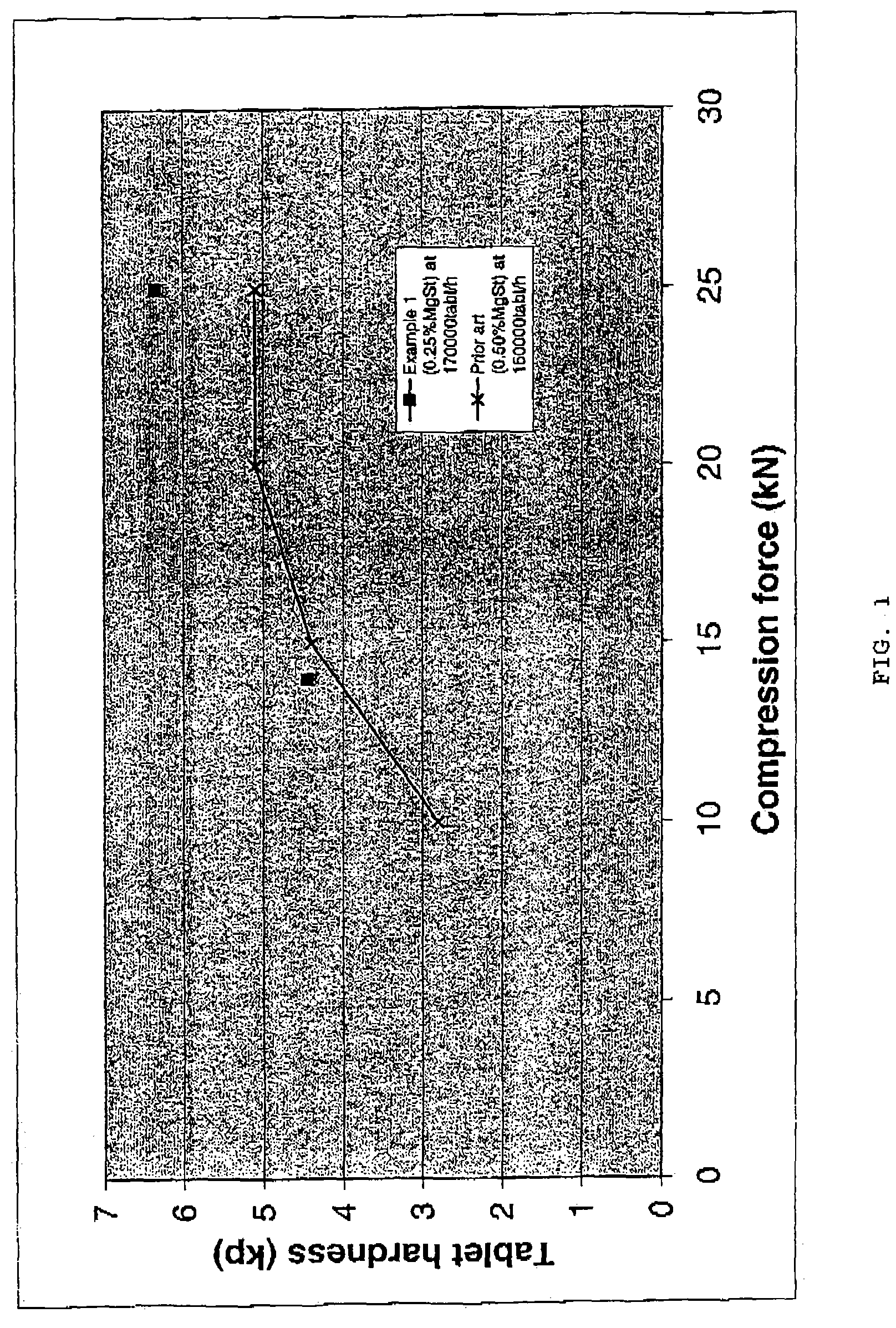
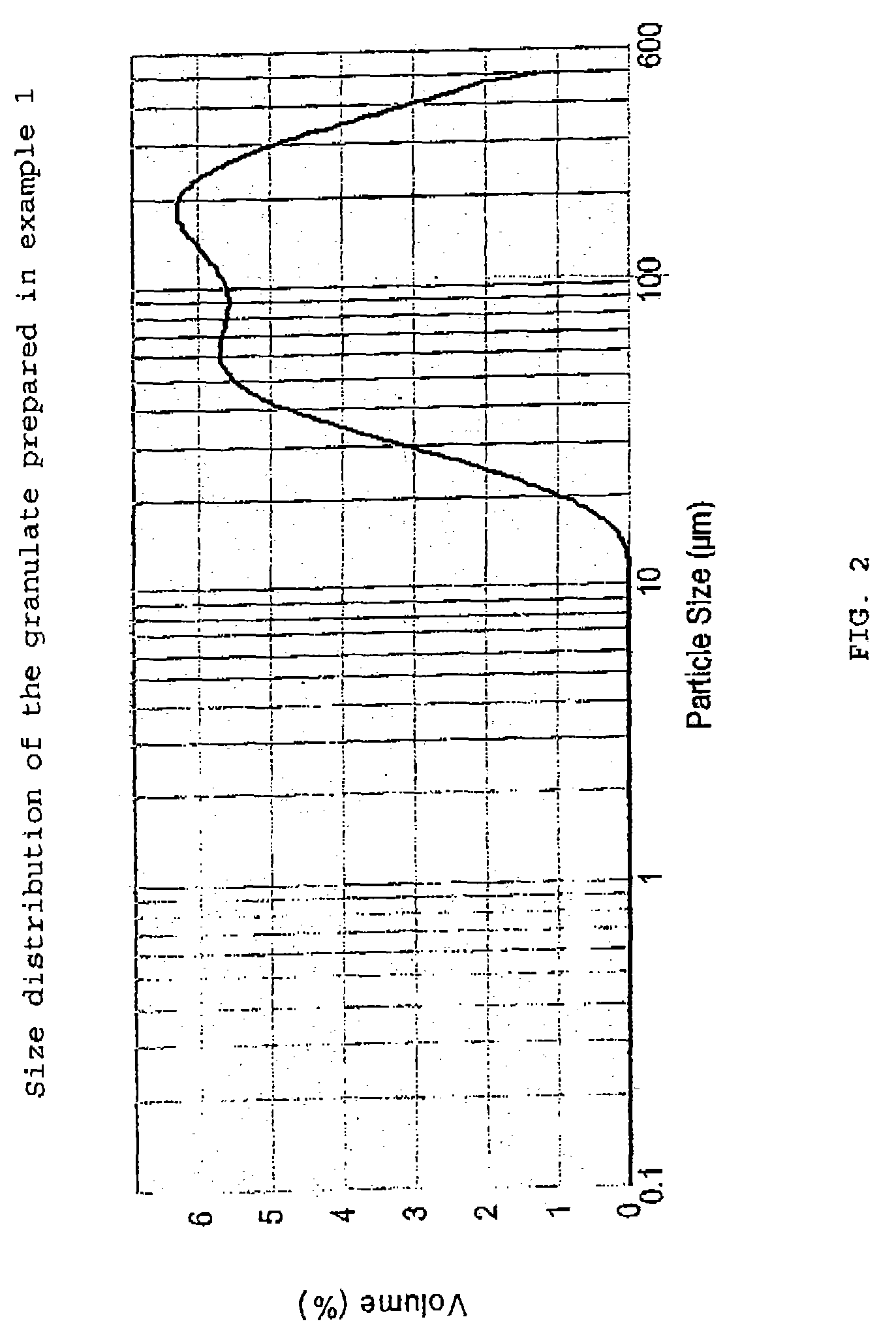
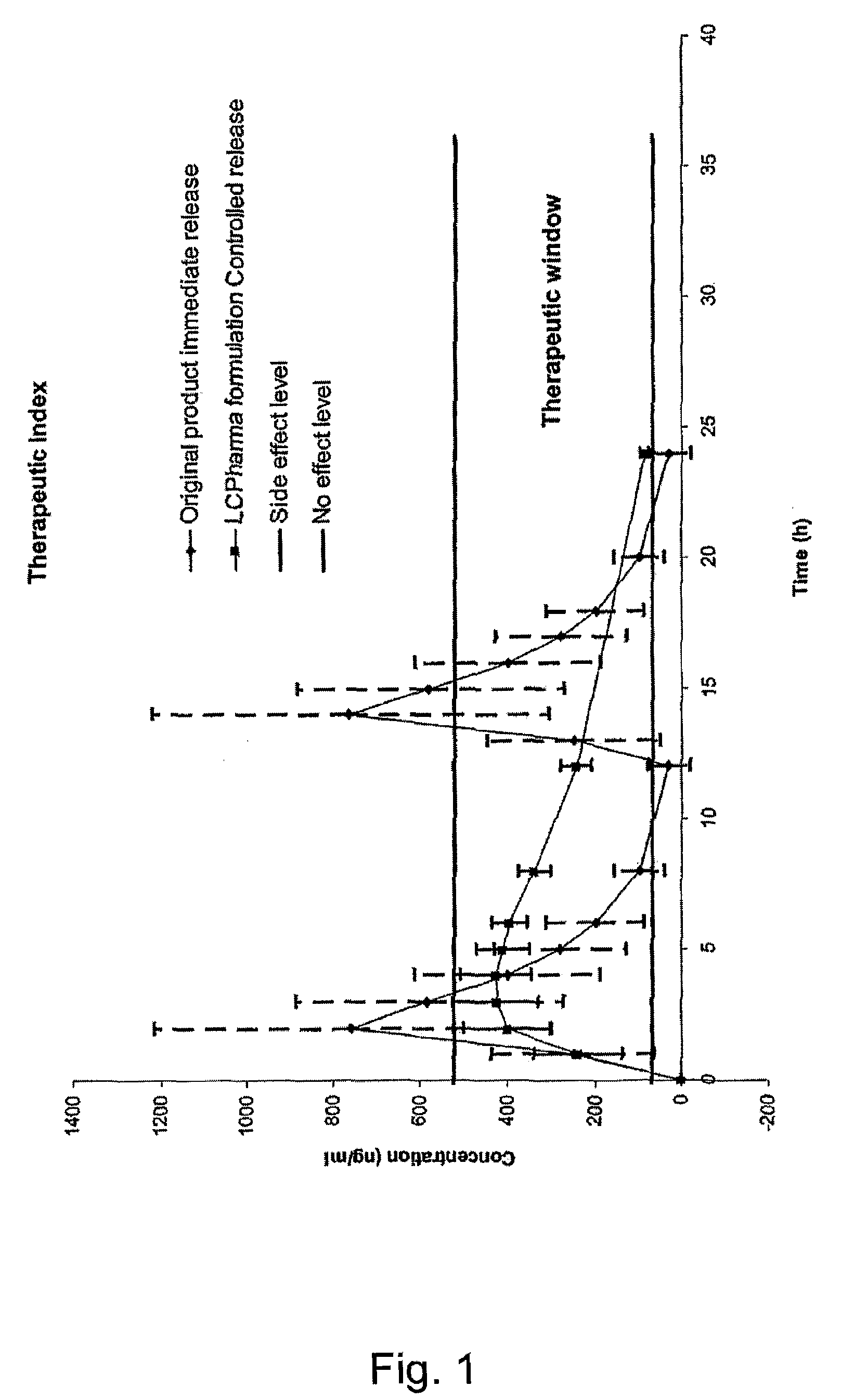
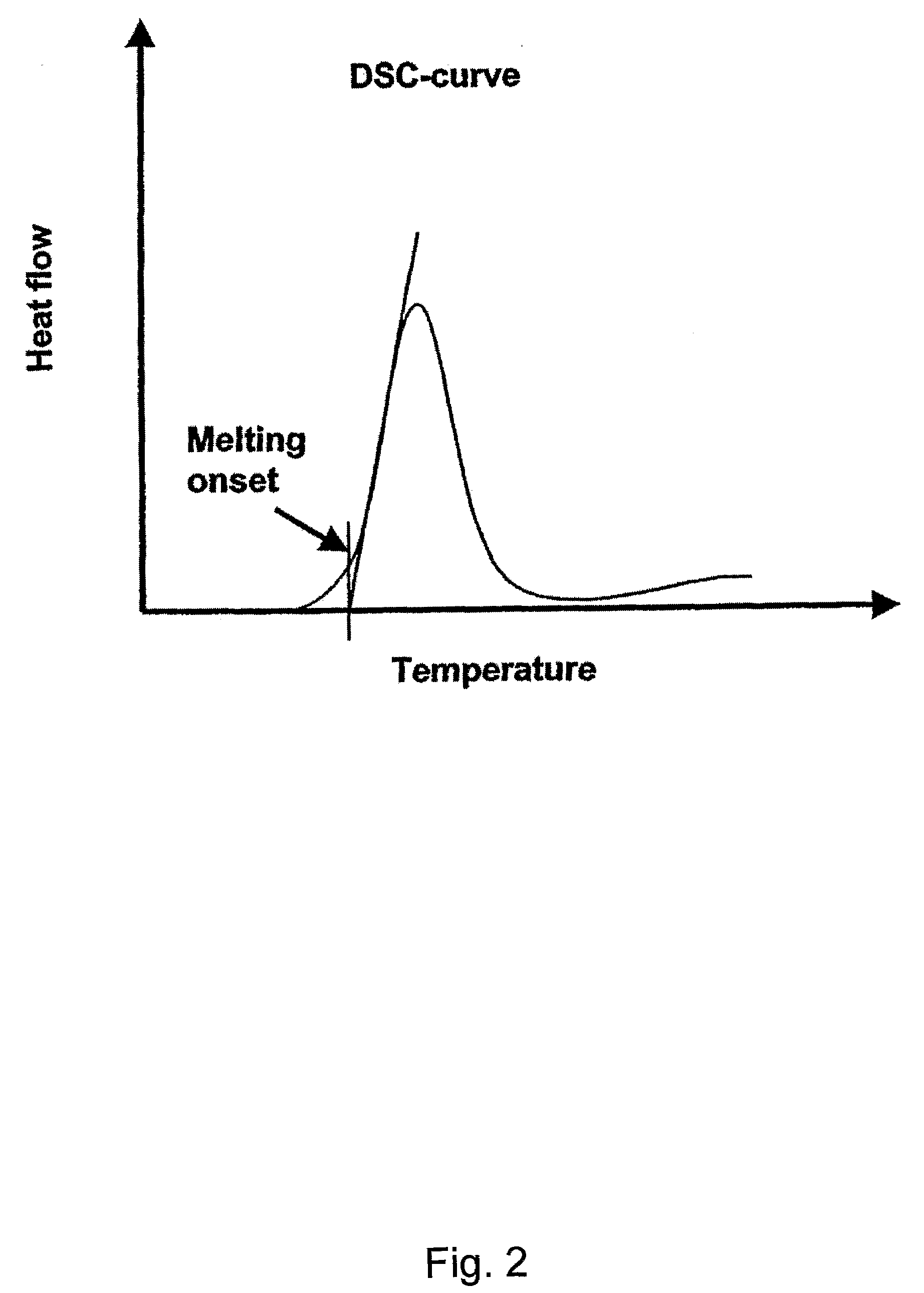
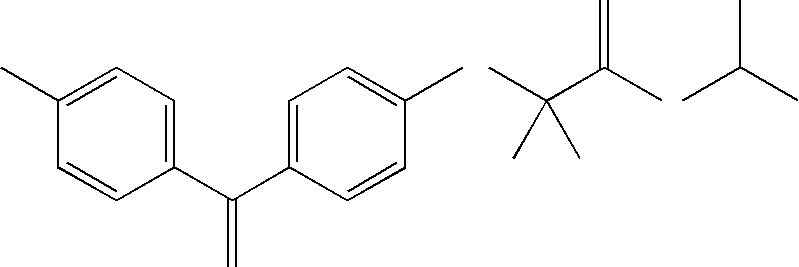
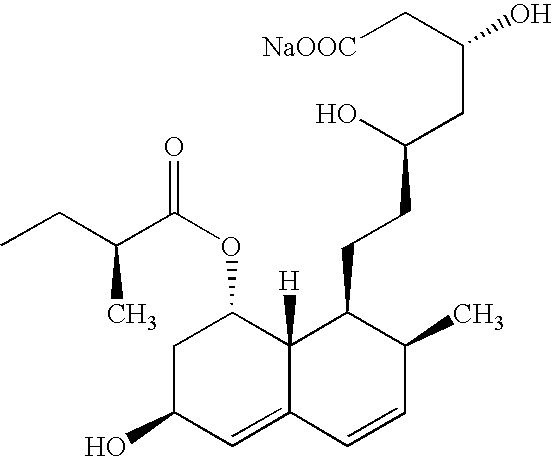
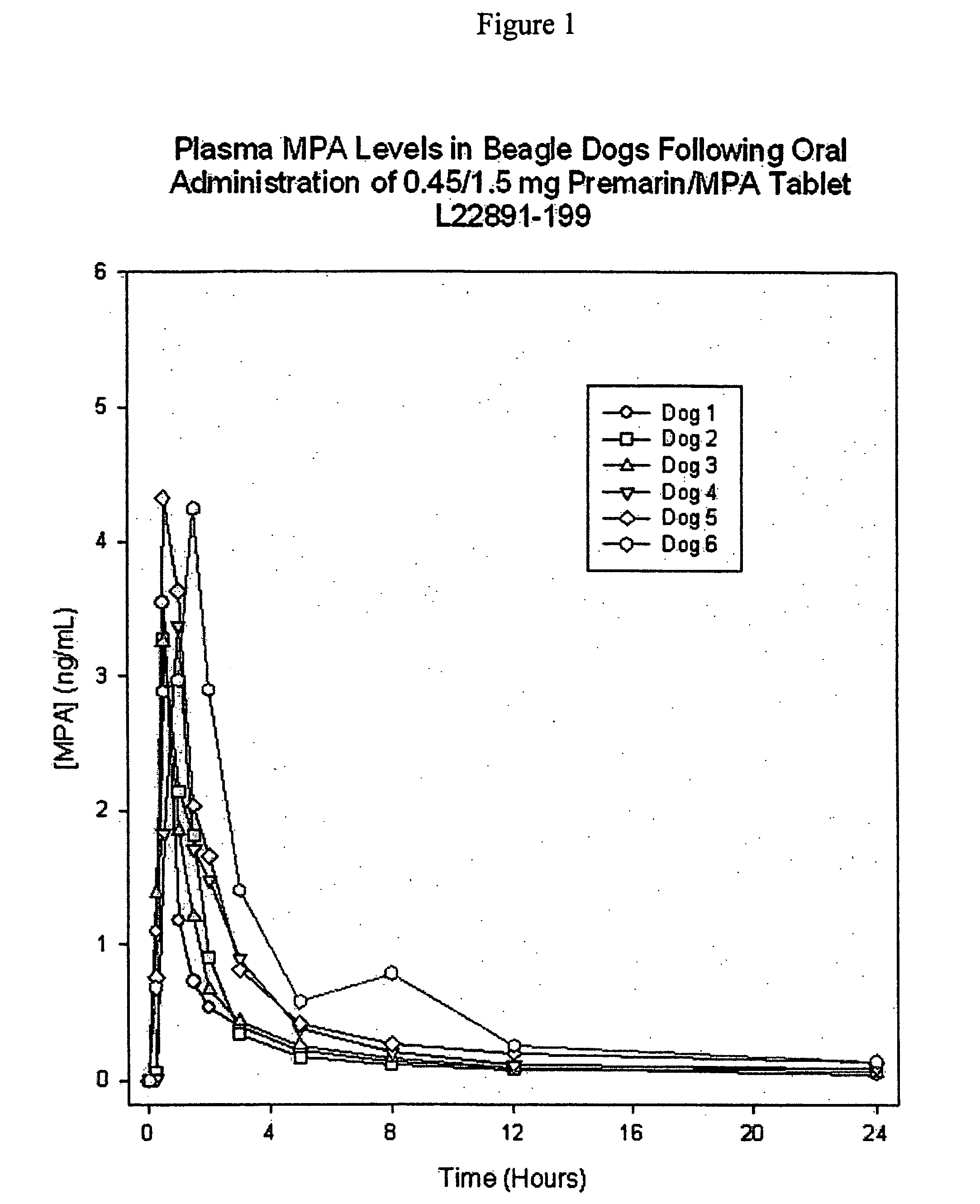
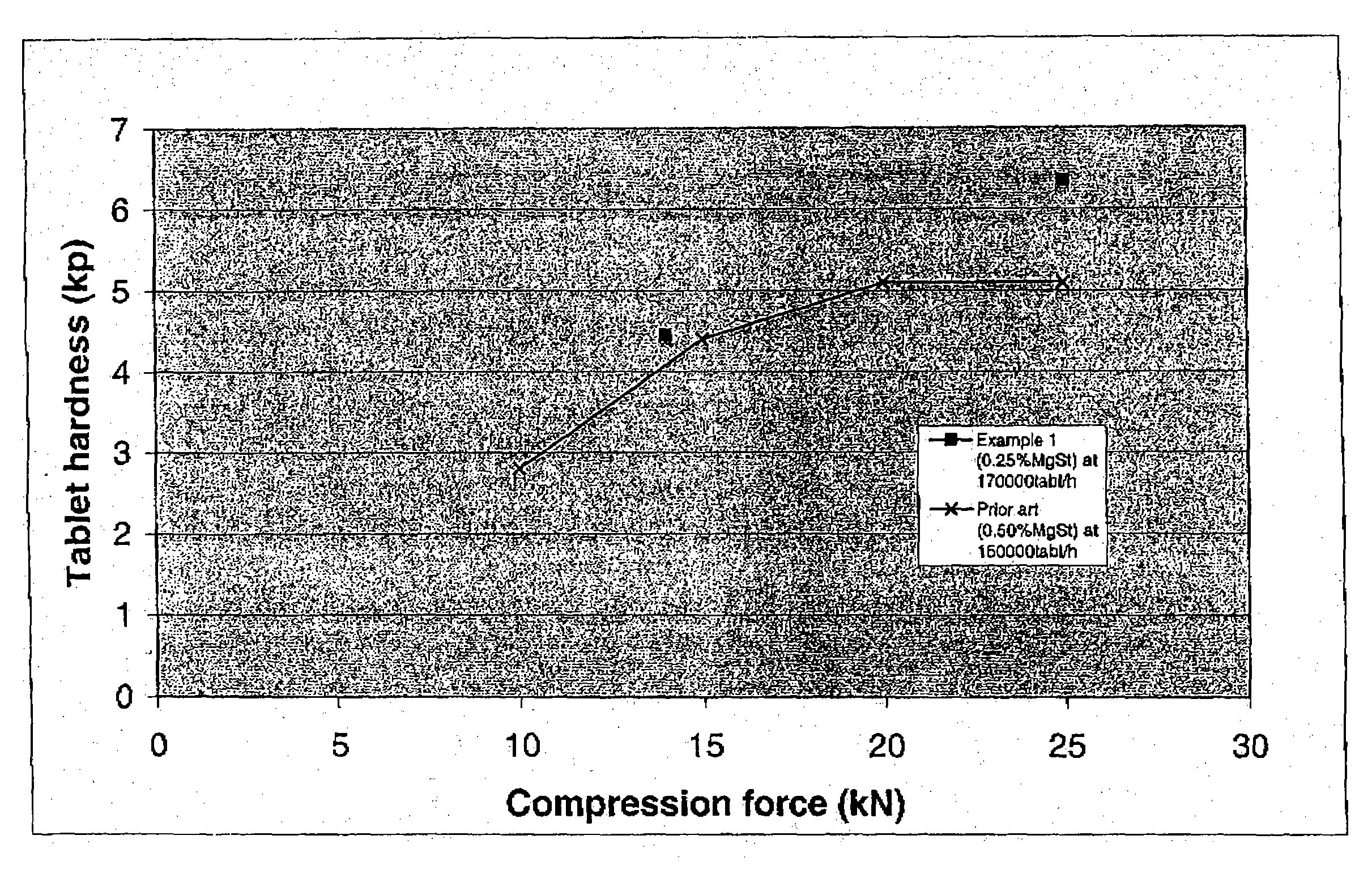
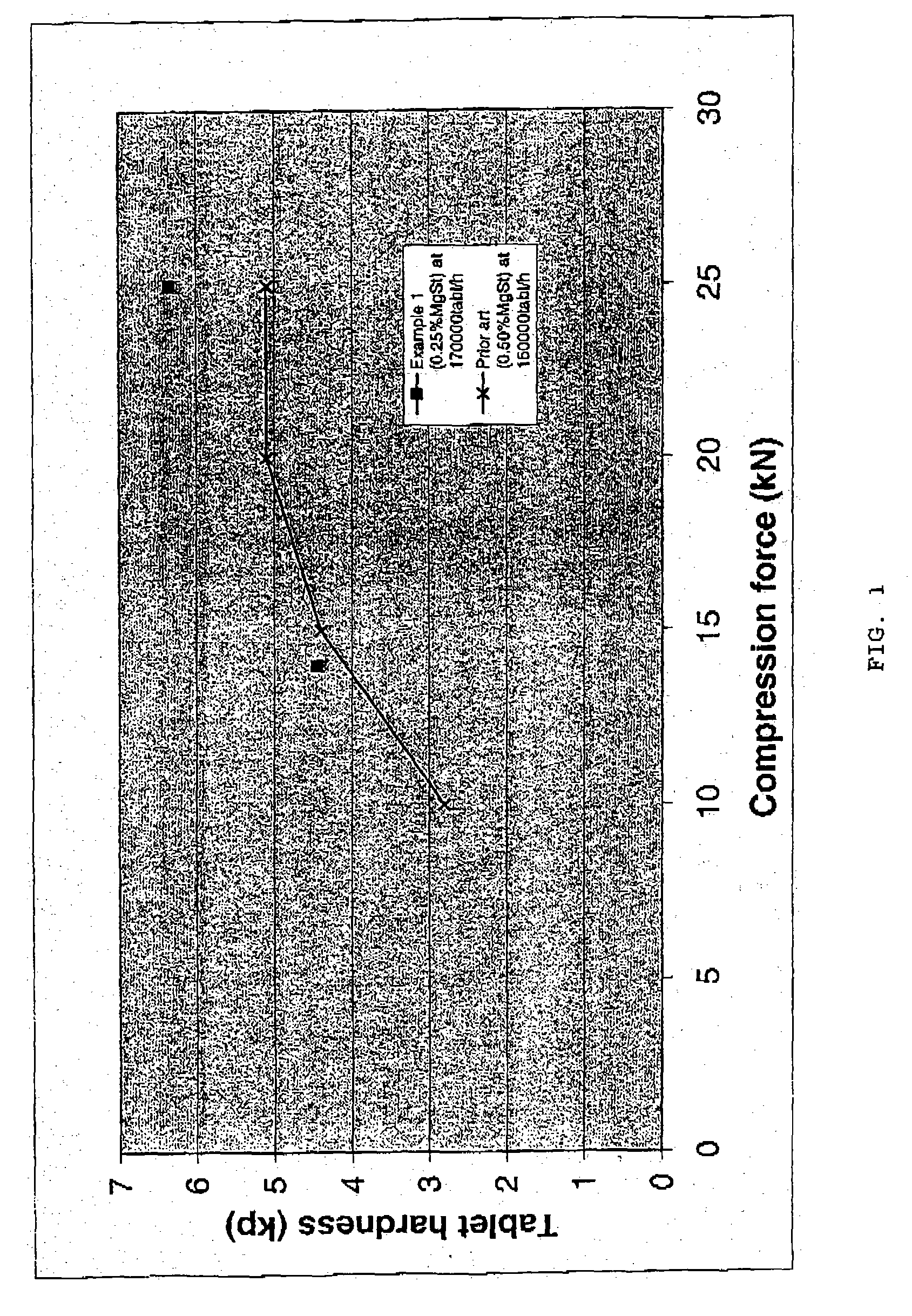
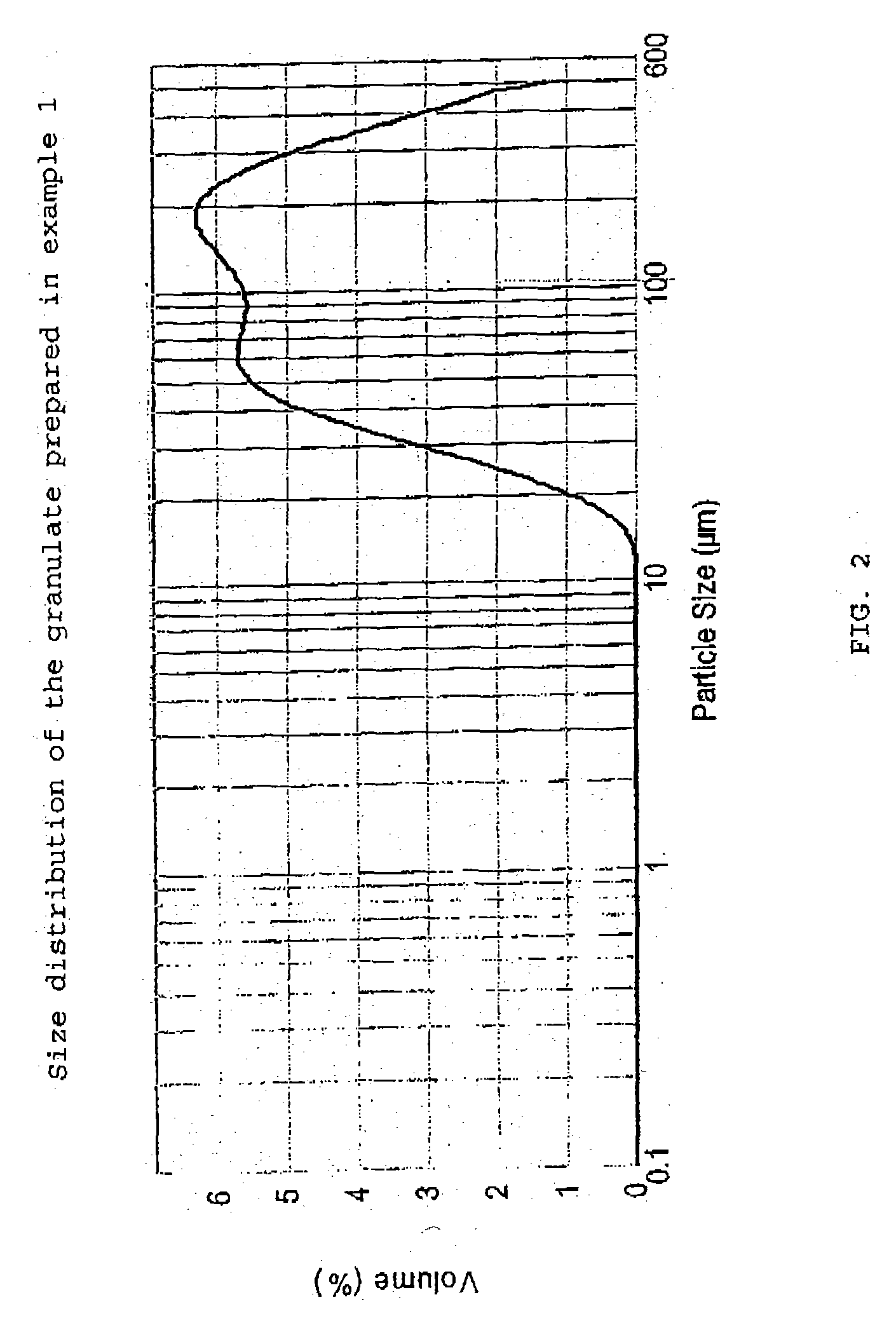
Patents
Literature
283 results about "Solid Dose Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
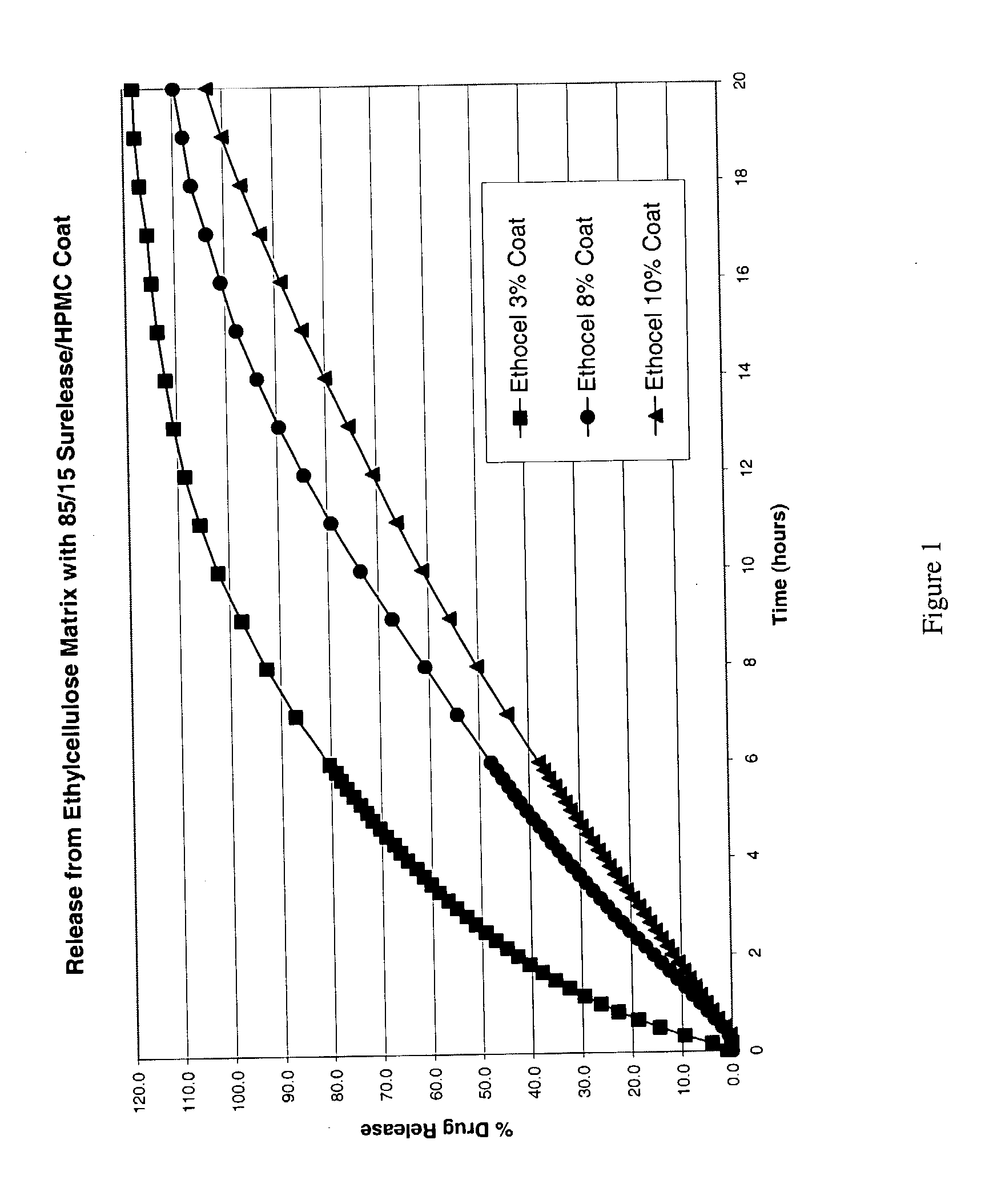
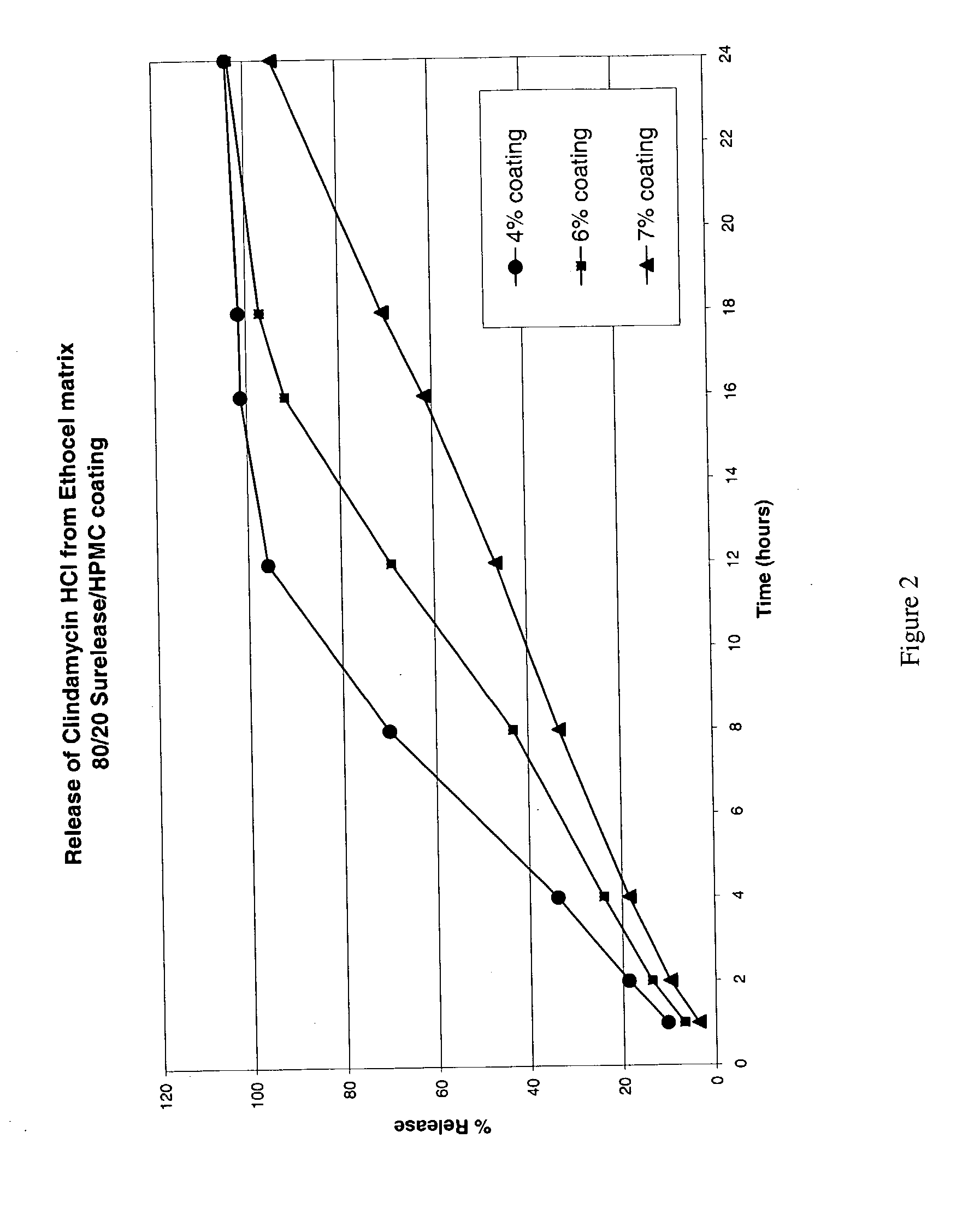
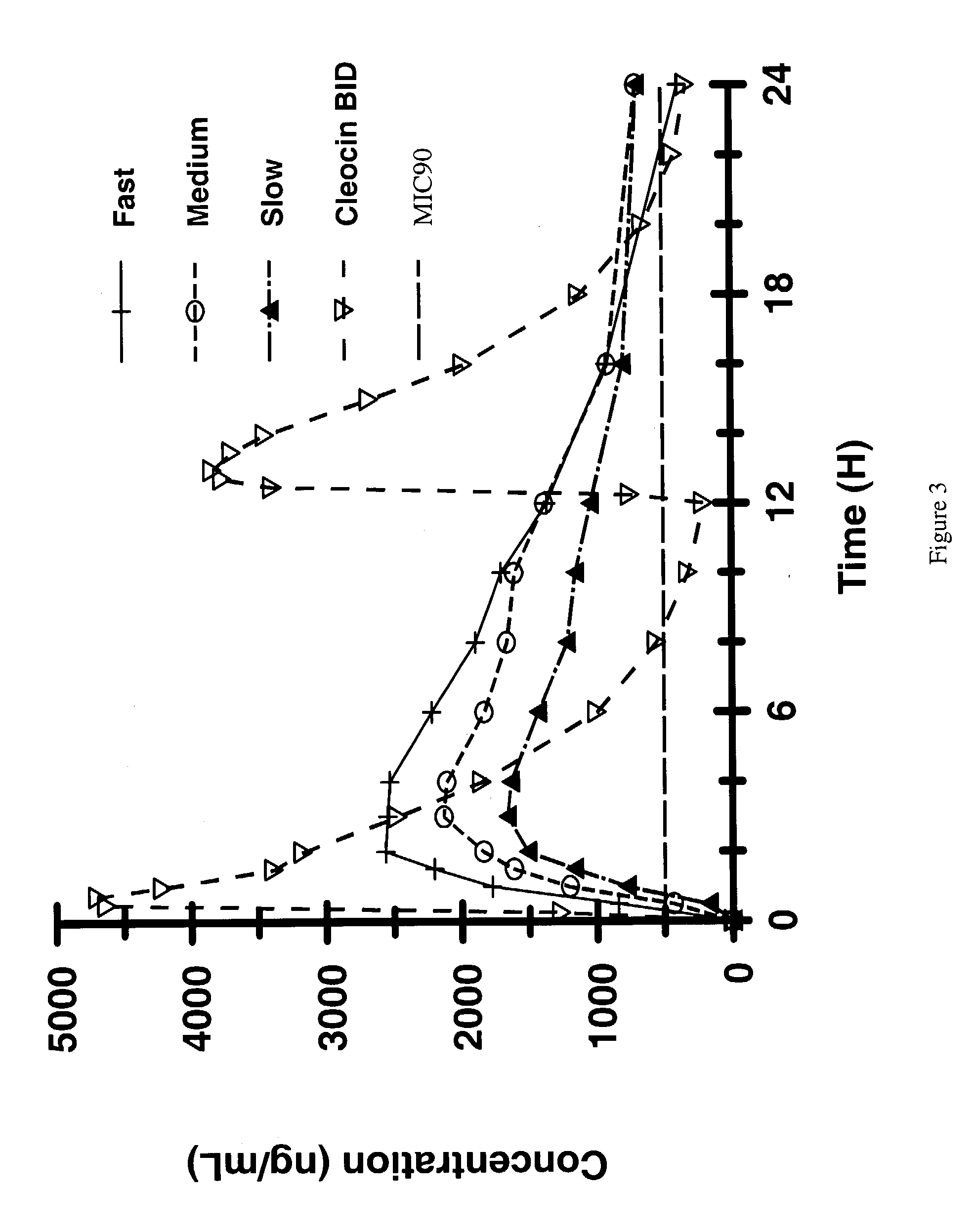
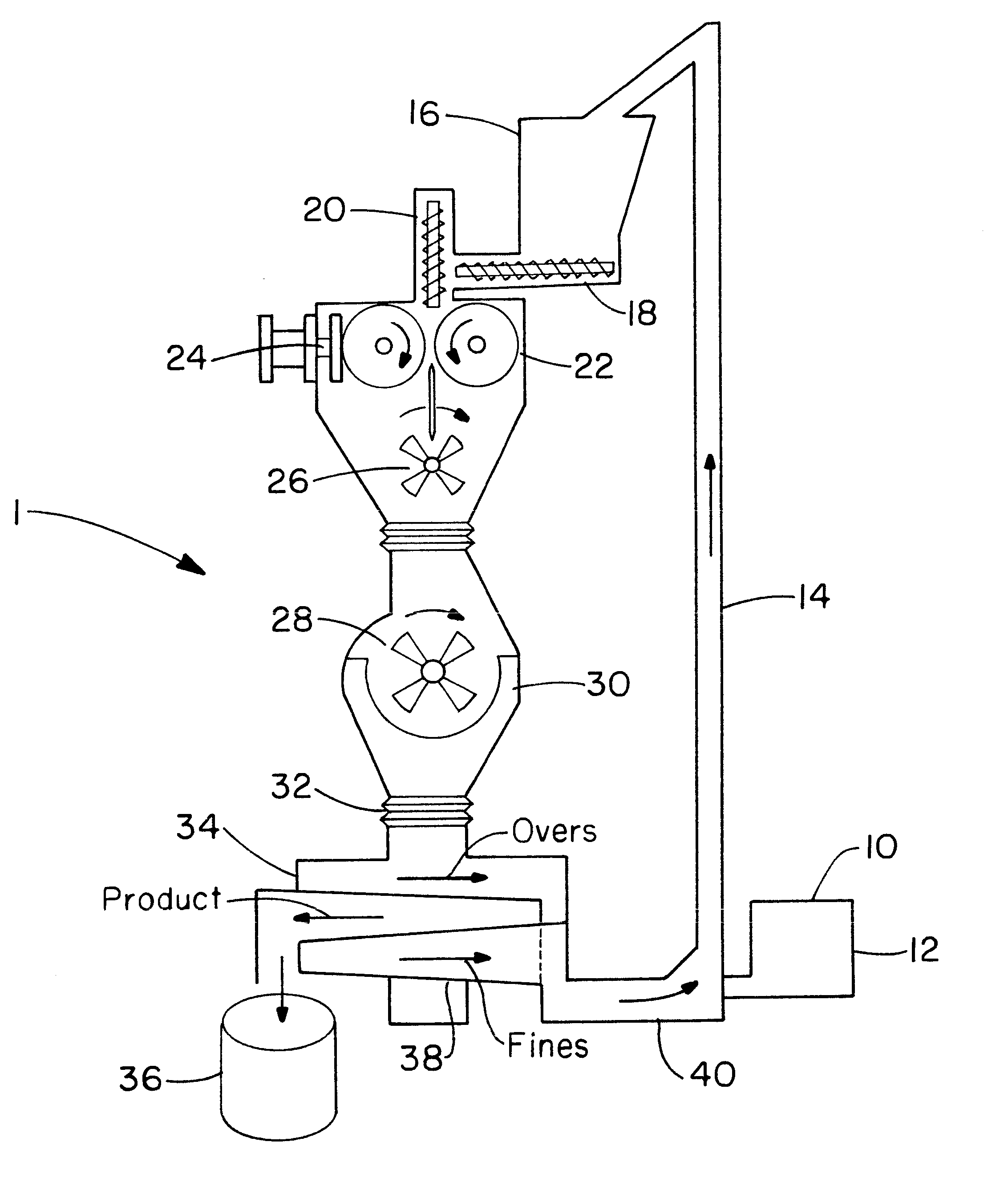
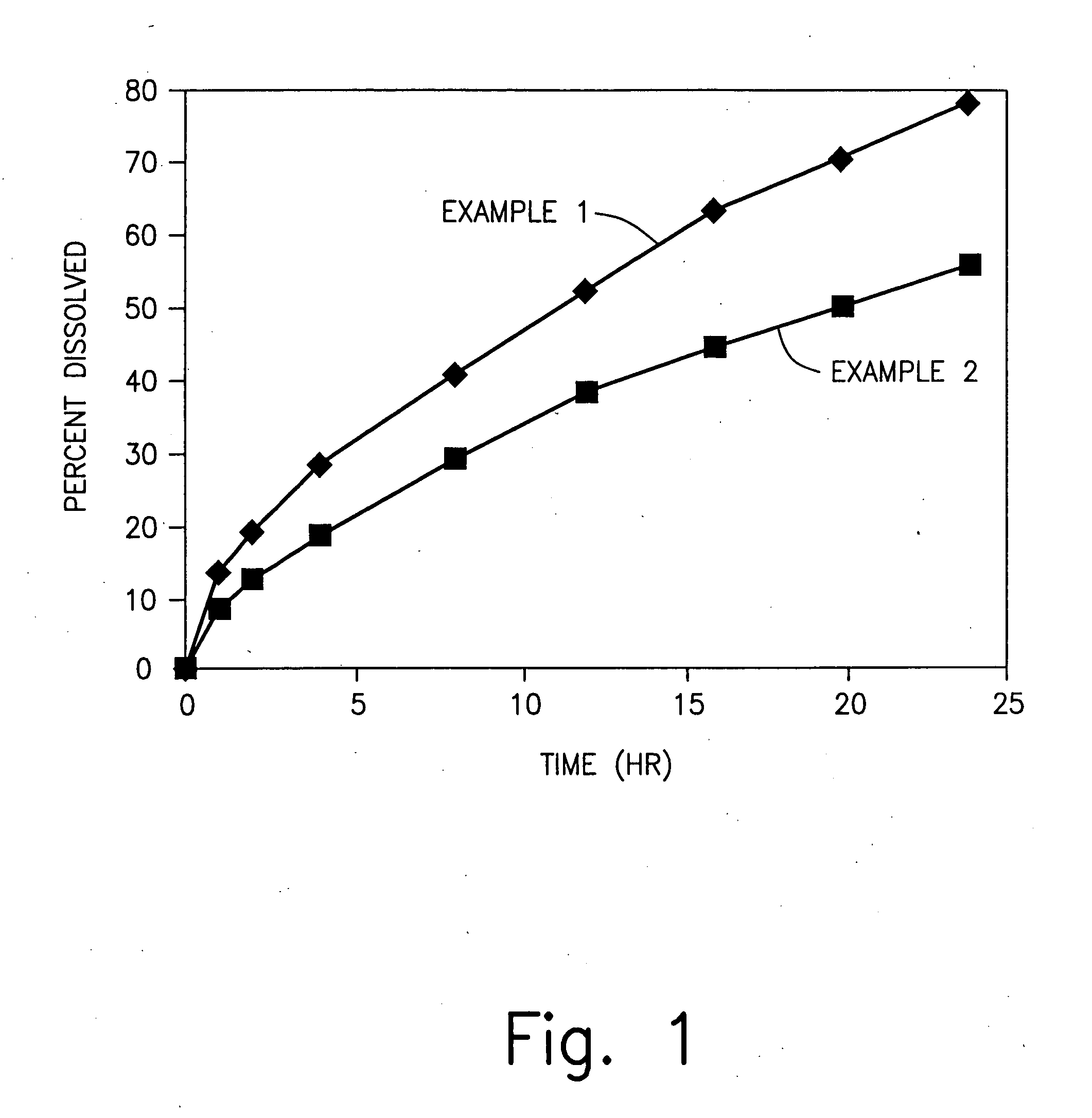
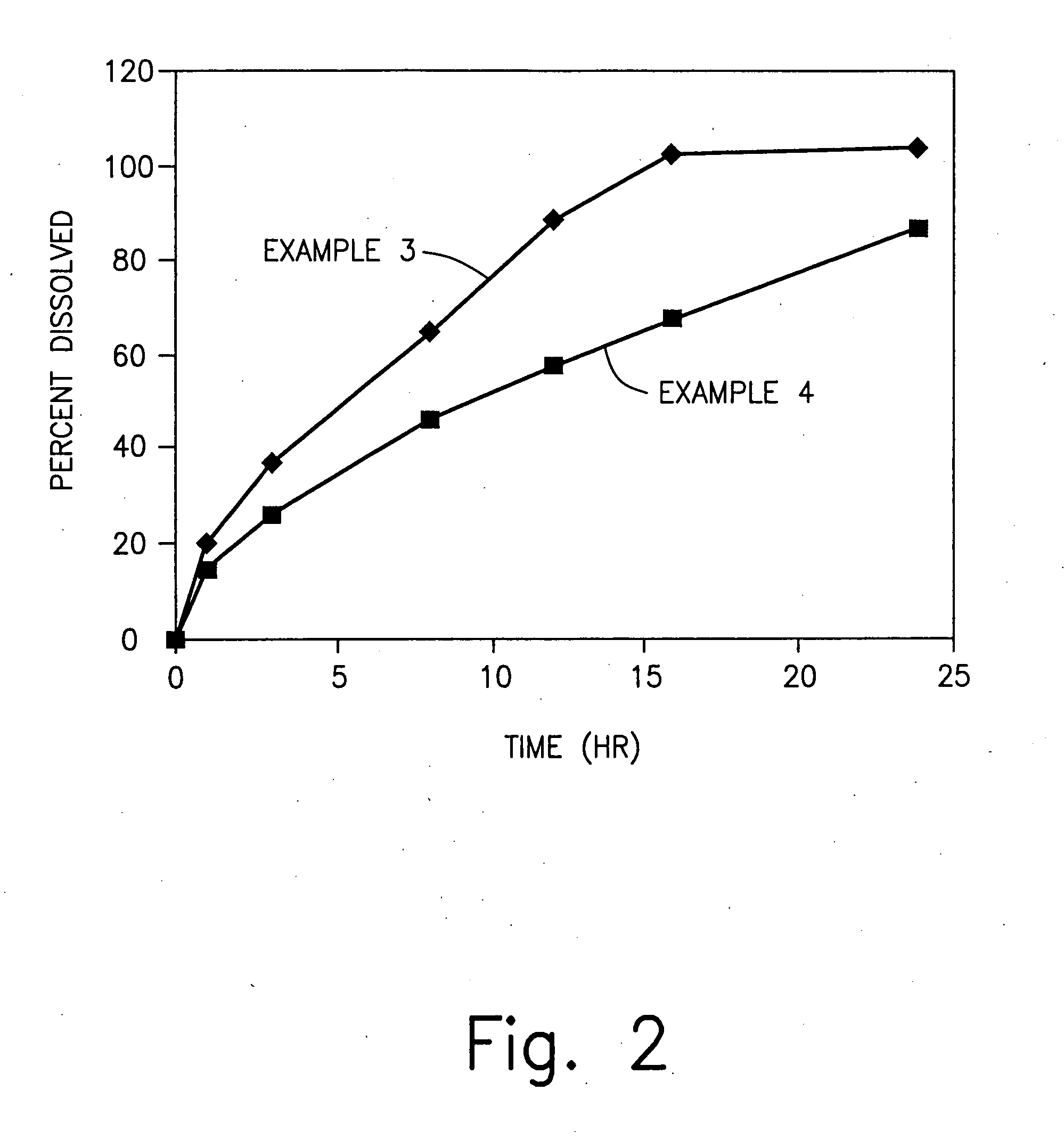
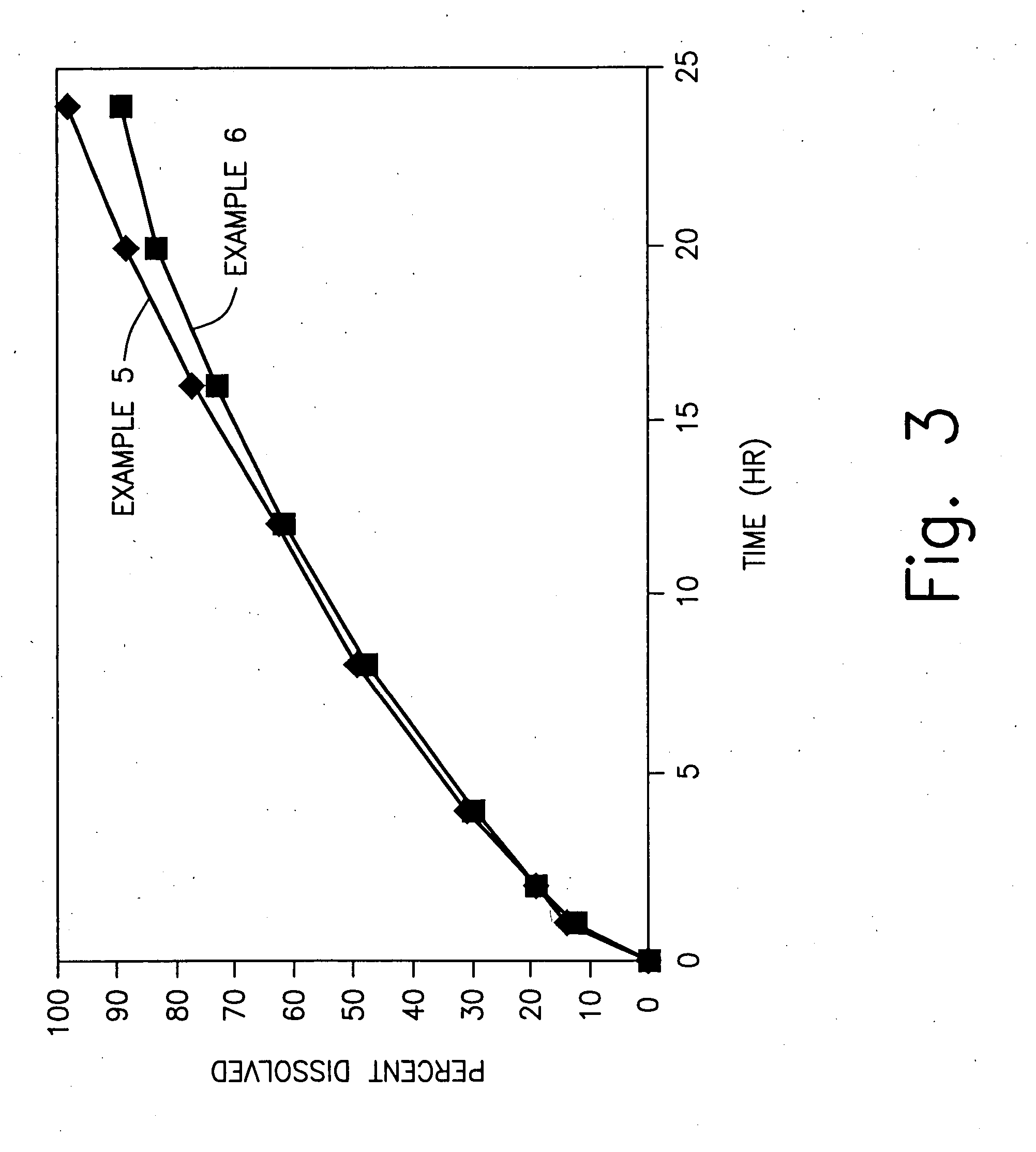
Zero-order sustained release dosage forms and method of making same
InactiveUS20030133982A1High drug loadingReduce releasePowder deliveryBiocideSustained release drugHydrophobic polymer
The present invention relates to zero-order sustained release solid dosage forms suitable for administration of a wide range of therapeutically active medicaments, especially those that are water-soluble, and to a process of making same. The solid dosage form comprises (a) a matrix core comprising ethylcellulose and the active agent and (b) a hydrophobic polymer coating encasing the entire matrix core.
Owner:PHARMACIA CORP
Sustained release heterodisperse hydrogel systems-amorphous drugs
Sustained release oral solid dosage forms comprising agglomerated particles of a therapeutically active medicament in amorphous form, a gelling agent, an ionizable gel strength enhancing agent and an inert diluent, as well as processes for preparing and using the same are disclosed. The sustained release oral solid dosage forms are useful in the treatment of hypertension in human patients.
Owner:PENWEST PHARMA CO
Directly compressed solid dosage articles
InactiveUS6623756B1Low amountImprove flow characteristicsPowder deliveryPharmaceutical non-active ingredientsCross-linkControl release
Solid dosage articles such as pharmaceutical tablets for the controlled release of a desired compound such as an active ingredient are directly compressed from a flowable, compressible mixture of the active ingredient, a slightly cross-linked rheology modifying polymer or copolymer, and one or more excipients. The rheology modifying polymer or copolymer is a granulated powder of suitable particle size and is generally made from one or more unsaturated (di)carboxylic acids, half ester thereof, and other optional monomers.
Owner:LUBRIZOL ADVANCED MATERIALS INC
Simethicone solid oral dosage form
The present invention provides a composition for forming a compressed solid dosage form that is a free-flowing compressible admixture of simethicone, an adsorbant, and an optional active agent, wherein the weight ratio of simethicone to adsorbent is at least 1:2.22. Also included are solid dosage forms made from a free-flowing compressible admixture of simethicone, an adsorbant, and an optional active agent, wherein the weight ratio of simethicone to adsorbent is at least 1:2.22.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
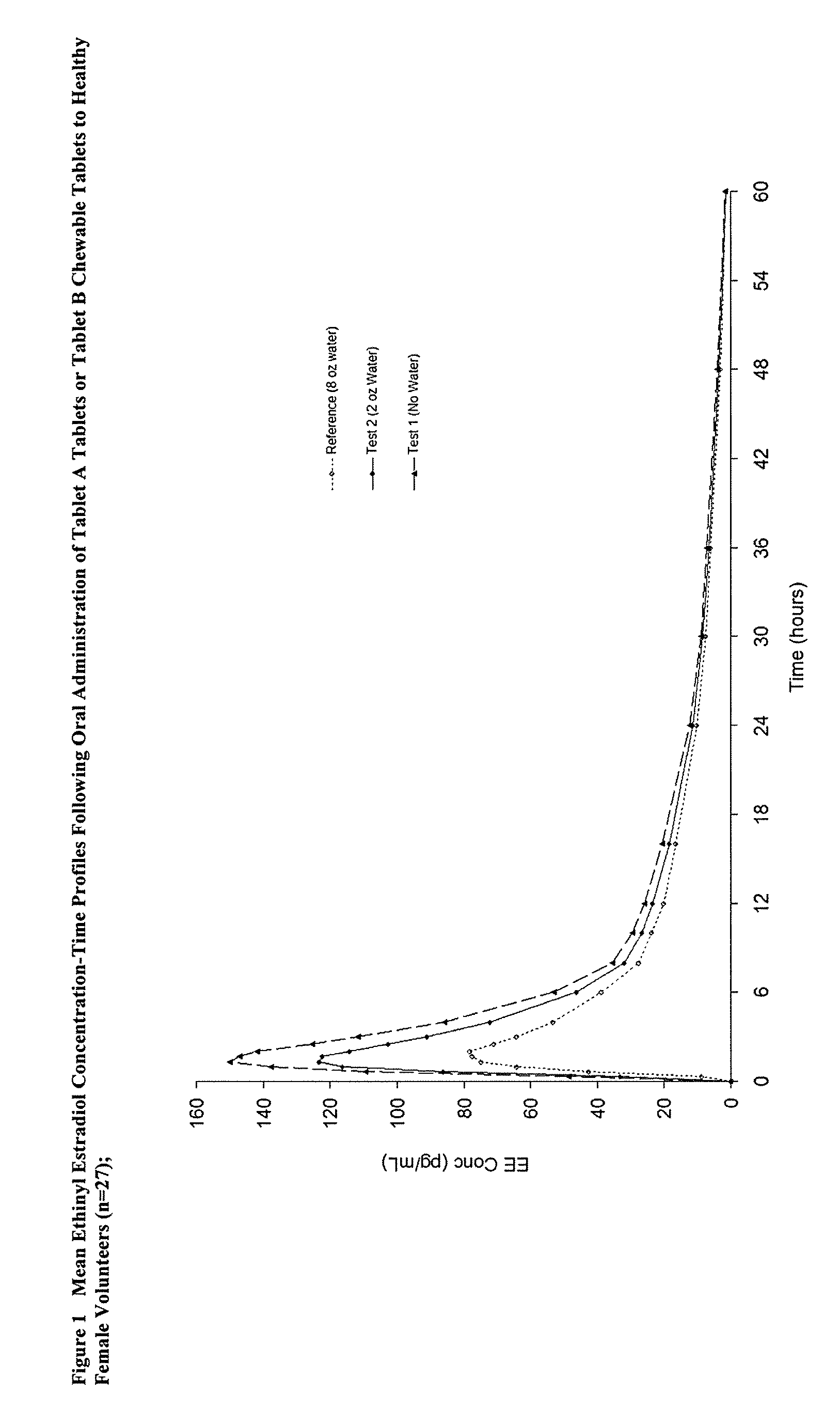
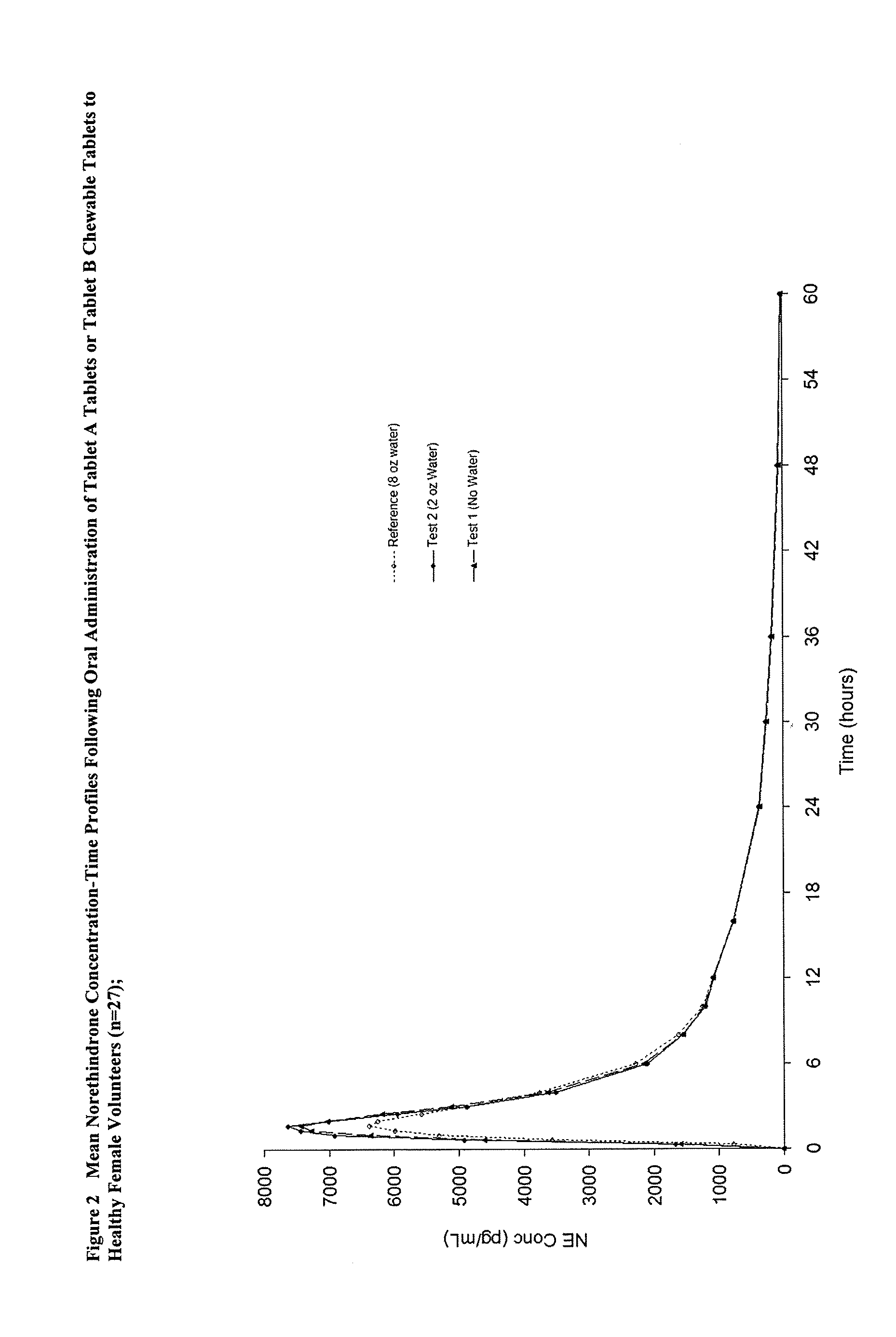
Methods to administer ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20070286819A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPill deliveryHormone replacementBioavailability
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:WARNER CHILCOTT CO LLC
Robust sustained release formulations
InactiveUS20080085304A1Avoid dose dumpingHigh drug safetyPowder deliveryPill deliverySolid Dose FormOxymorphone
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
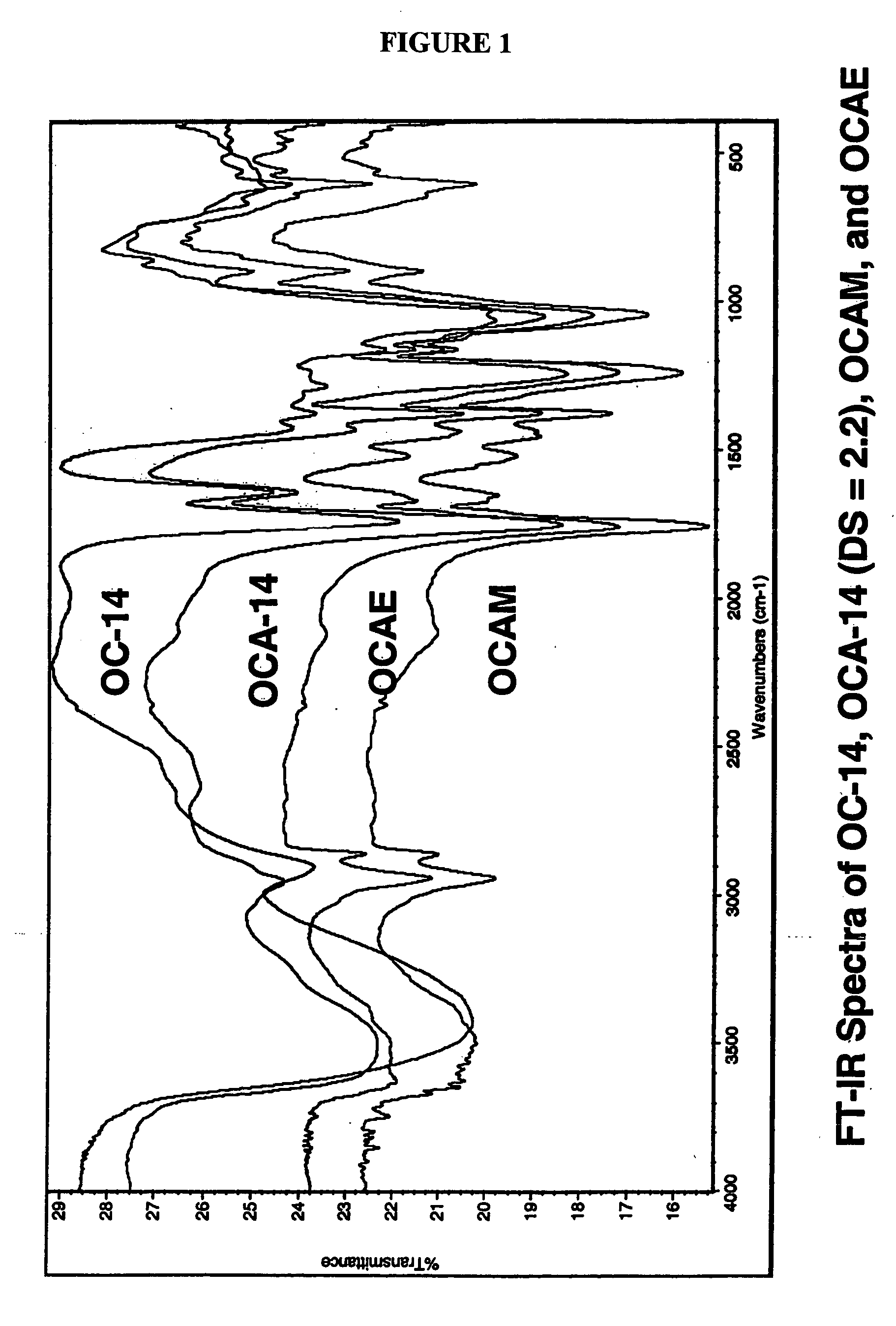
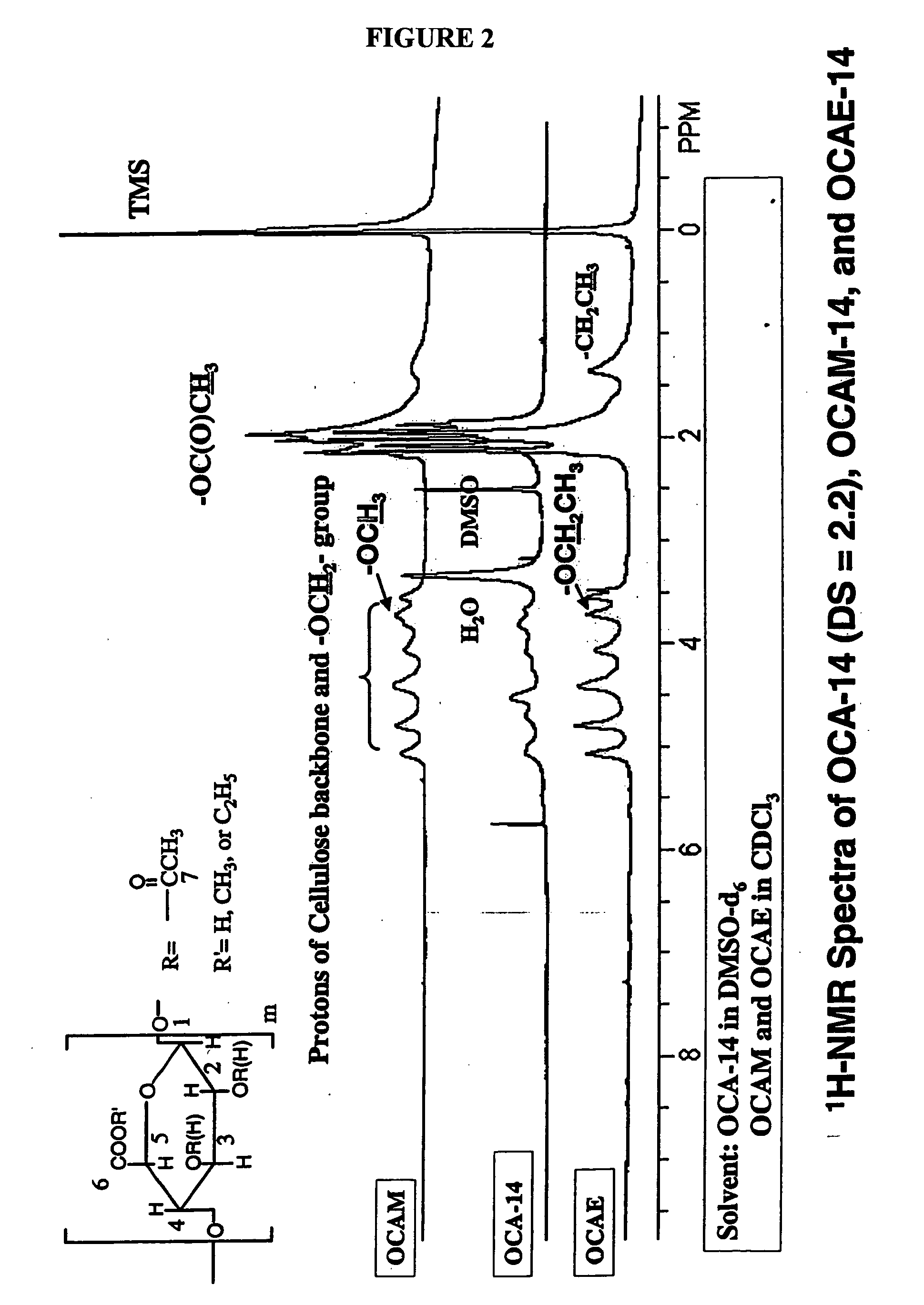
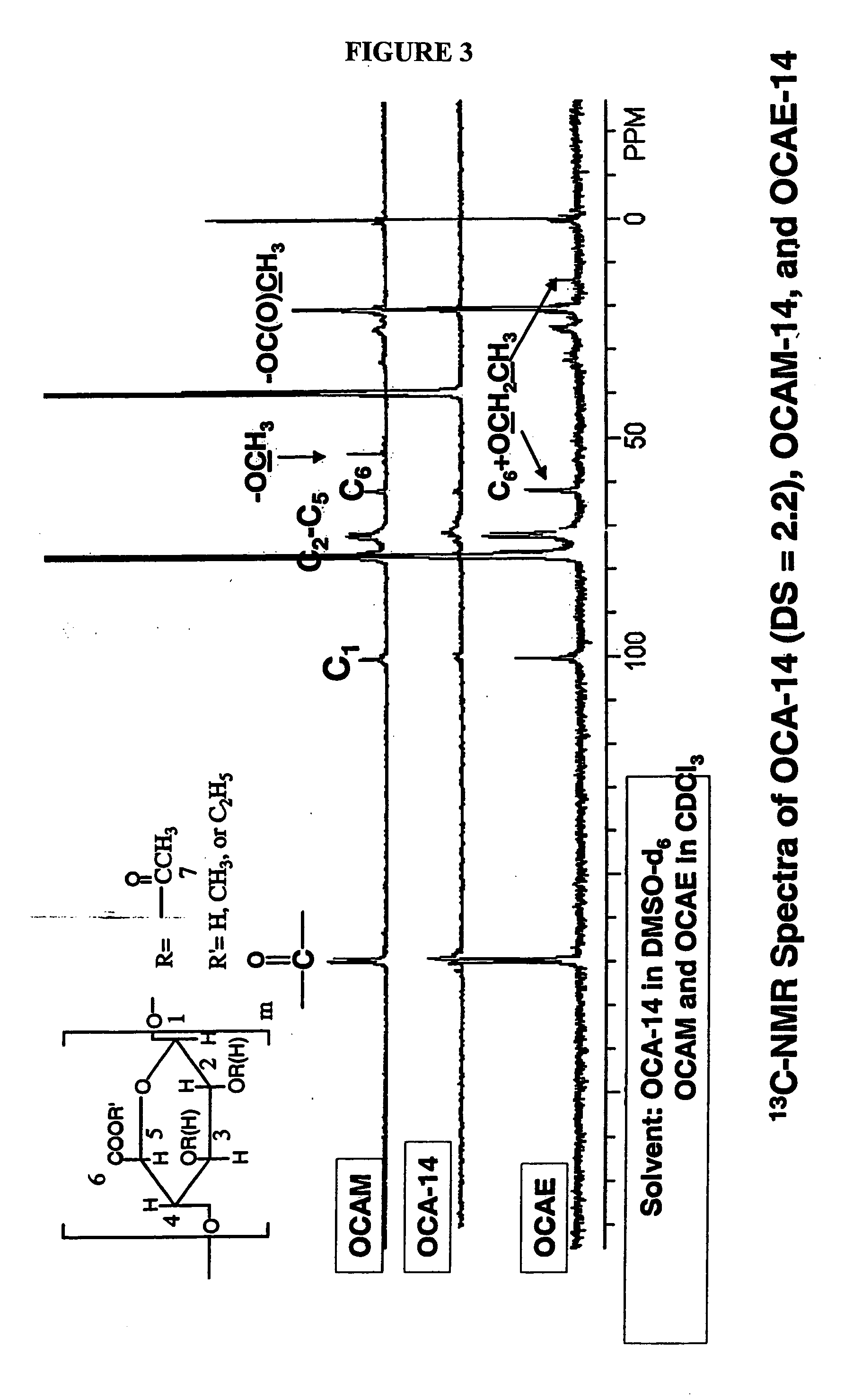
Biodegradable oxidized cellulose esters and their uses as microspheres
A new cellulose excipient, OCCAE, suitable for use as a binder, filler, and / or disintegrant in the development of solid dosage forms and as a bodying agent or a drug carrier in the preparation of topical formulations is described. The cellulose excipient is formed by reacting an oxidized cellulose ester with an alcohol in the presence of a catalyst. The invention also describes the formation of controlled release microspheres using OCCAE and / or oxidized cellulose esters that may be used to control the release of drug in a patient over a time period of several hours to several days.
Owner:UNIV OF IOWA RES FOUND
Fast dissolving composition with prolonged sweet taste
A novel fast dissolving pharmaceutical composition in solid dosage form with prolonged sweet taste which comprises (a) At least one pharmaceutically active agent, (b) At least one water soluble sugar, (c) At least one non-sugar sweetner in normal fast release form and (d) At least one non-sugar sweetner in a mucoadhesive slow release form.
Owner:PANACEA BIOTEC
Machine for the manufacture of dosage forms utilizing radiofrequency energy
ActiveUS20110068511A1Organic active ingredientsNervous disorderSolid Dose FormBiomedical engineering
The present invention features the present invention features a machine for the production of a solid dosage form including: (a) a die platen having one or more forming cavities each having an inner wall, a first opening at the surface of one side of the die platen, and a second opening at the surface on the opposite side of the die platen; (b) one or more first forming tools each adapted to move into one of the forming cavities through the first opening of the forming cavity; (c) one or more second forming tools each adapted to move adjacent to one of the second openings or into one of the forming cavities through the second opening of the forming cavity; (d) at least one first RF electrode operably associated with the one or more first forming tools, the one or more second forming tools, or the inner wall of the one or more forming cavities; and (e) at least one second RF electrode operably associated with the one or more first forming tools.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Solid dosage form for acid-labile active ingredient
InactiveUS20050281876A1Prevent and inhibit acid degradationPreserve bioavailabilityPowder deliveryMedical devicesBULK ACTIVE INGREDIENTSolid Dose Form
The present invention relates to solid, orally administrable dosage forms for acid-labile actives having at least one molded insert or core containing an acid-labile active ingredient, such as a proton pump inhibitor that is surrounded by barrier layer that is subsequently coated with an enteric layer. The present invention also relates to a dosage form that combines the barrier coated active ingredient containing insert with a second active ingredient.
Owner:MCNEIL PPC INC
Directly compressible sustained release formulation containing microcrystalline cellulose
The present invention provides an improved process for the preparation of a agglomerated solid dosage form, comprising: (1) preparing an aqueous slurry of (a) microcrystalline cellulose; (b) a microcrystalline cellulose compressibility augmenting agent which (i) physically restricts the proximity of the interface between adjacent cellulose surfaces; (ii) inhibits interactions between adjacent cellulose surfaces, for example, via the creation of a hydrophobic boundary at cellulose surfaces; or (iii) accomplishes both (i) and (ii) above; and (c) an active agent; (2) thereafter drying the resultant aqueous slurry in a manner which inhibits quasi-hornification, thereby obtaining an agglomerated material which is directly compressible into a solid dosage form.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Colonic purgative composition with soluble binding agent
ActiveUS20050129781A1Improve dosage form characteristicSimple preparation processBiocideInorganic phosphorous active ingredientsBowel cleansingTolerability
This invention relates to novel colonic purgative compositions in a solid dosage form, comprising at least one purgative and at least one soluble, or soluble, nonfermentable binder, such as polyethylene glycol. Further, this invention relates to methods of using the colonic purgative compositions. The present compositions and methods are designed to improve patient tolerance and compliance, while at the same time improving the quality of bowel cleansing. The formulations and methods of this invention are particularly useful to cleanse the bowel prior to diagnostic and surgical procedures and can also be employed in lower dosages as a laxative to promote elimination and / or to relieve constipation.
Owner:SALIX PHARMA INC
Process for preparing a directly compressible solid dosage form containing microcrystalline cellulose
The present invention provides an improved process for the preparation of a agglomerated solid dosage form, comprising: (1) preparing an aqueous slurry of (a) microcrystalline cellulose; (b) a microcrystalline cellulose compressibility augmenting agent which (i) physically restricts the proximity of the interface between adjacent cellulose surfaces; (ii) inhibits interactions between adjacent cellulose surfaces, for example, via the creation of a hydrophobic boundary at cellulose surfaces; or (iii) accomplishes both (i) and (ii) above; and (c) an active agent; (2) thereafter drying the resultant aqueous slurry in a manner which inhibits quasi-hornification, thereby obtaining an agglomerated material which is directly compressible into a solid dosage form.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Solid Dosage Form Comprising Proton Pump Inhibitor and Suspension Made Thereof
InactiveUS20080020053A1Stable levelConvenient coatingAntibacterial agentsPowder deliveryBULK ACTIVE INGREDIENTSolid Dose Form
A solid rapidly gelling oral pharmaceutical dosage form, as well as aqueous suspensions prepared thereof, comprising an acid sensitive proton pump inhibitor as active ingredient distributed in a multitude of enteric coated pellets and a suspension modifying granulate comprising a rapidly dissolving diluent granulated together with a gelling agent chosen among xanthan gums, and an acidic pH-regulating agent and a binder. The suspension modifying granulate is rapidly disintegrating and gelling when suspended in an aqueous medium and thus forming a homogenous stable and robust suspension having a reproducible and stable viscosity. Furthermore the invention relates to an improved process for its manufacture and the use of such formulation in medical treatment including prevention of gastrointestinal disorders in humans.
Owner:ASTRAZENECA AB
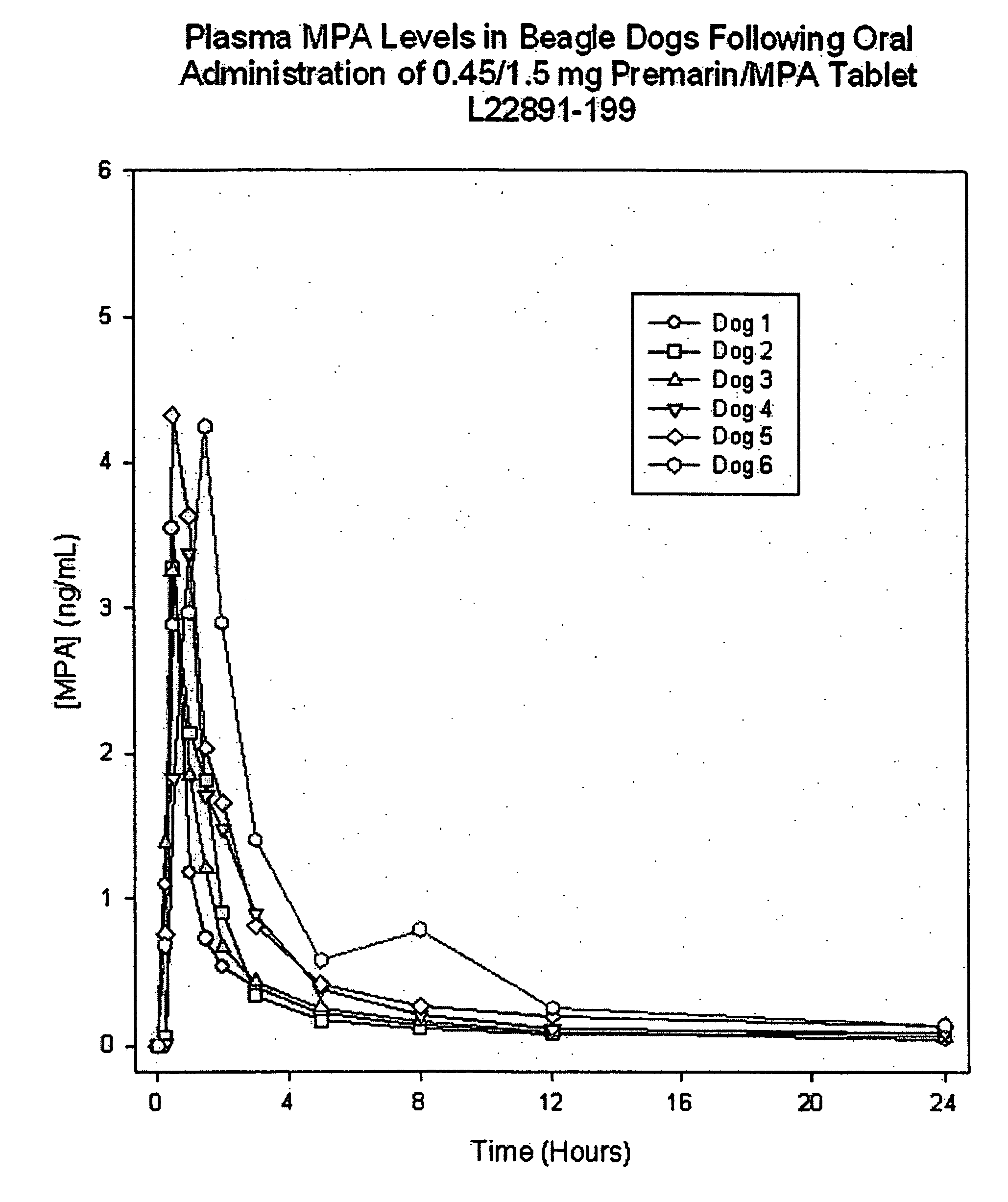
Compositions comprising fenofibrate and rosuvastatin
InactiveUS20050096391A1Improve bioavailability in vivoSubstance may accumulateBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor rosuvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCrosuvastatin) of between about 150 and about 12,000. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and rosuvastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
Zero-Order Modified Release Solid Dosage Forms
The invention comprises a solid dosage form for delivery of water soluble pharmaceutical agents. The solid dosage form comprises a matrix core containing the pharmaceutical agent and a hydrophobic material, and a coating containing a hydrophilic pore-forming agent and a hydrophobic polymer. The dosage form exhibits a zero-order release profile upon dissolution.
Owner:SPECGX LLC
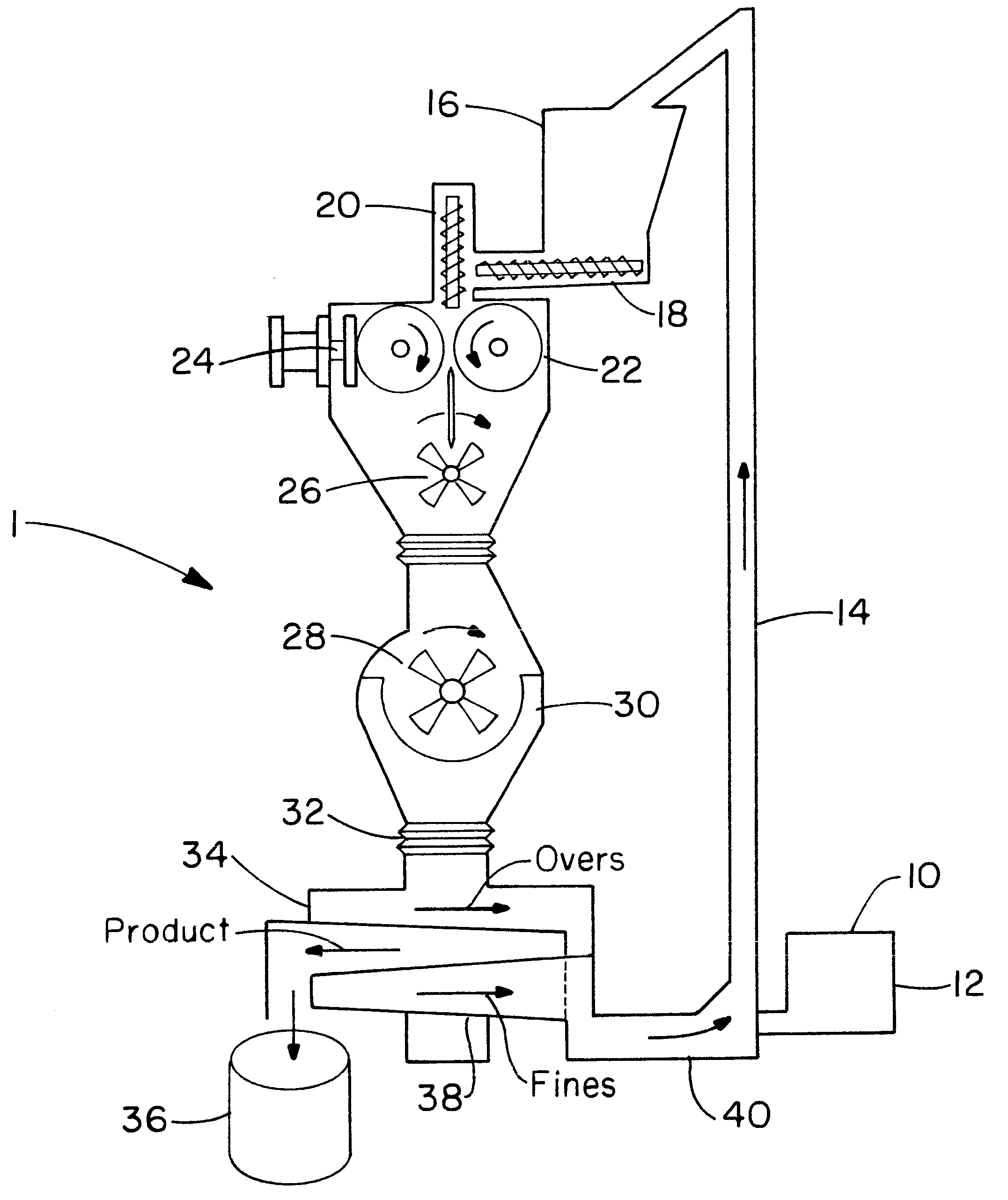
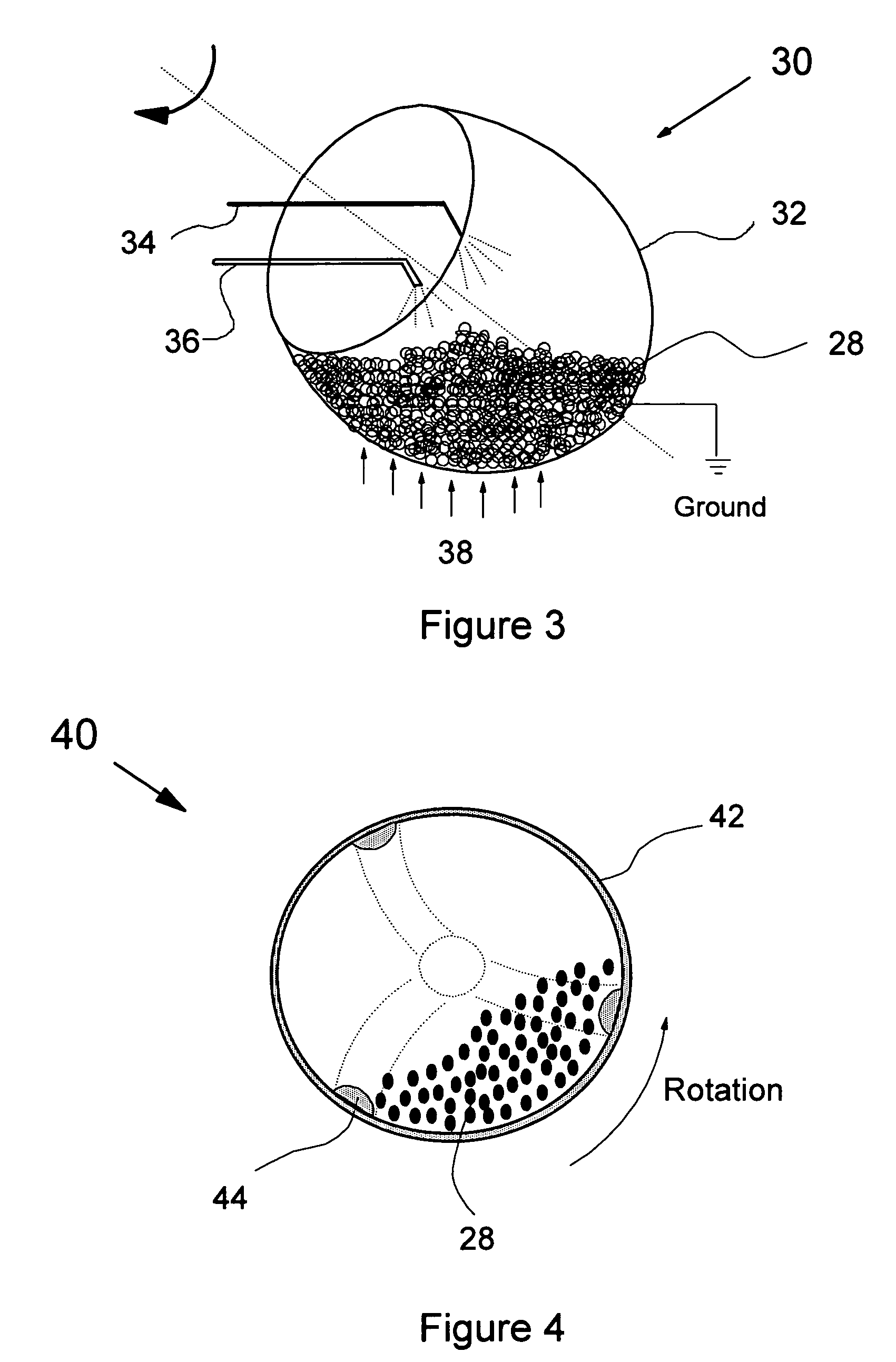
Direct coating solid dosage forms using powdered materials
The present invention provides a method and apparatus for dry coating solid dosage forms. The method includes the steps of placing solid dosage forms in a rotatable, electrically grounded housing, and spraying a film forming polymer powder composition into the housing during rotation thereof to form a polymer coating on the solid dosage forms, the polymer powder composition being sprayed using an electrostatic spray gun, and curing the coated solid dosage forms.
Owner:WESTERN ONTARIO THE UNIV OF +1
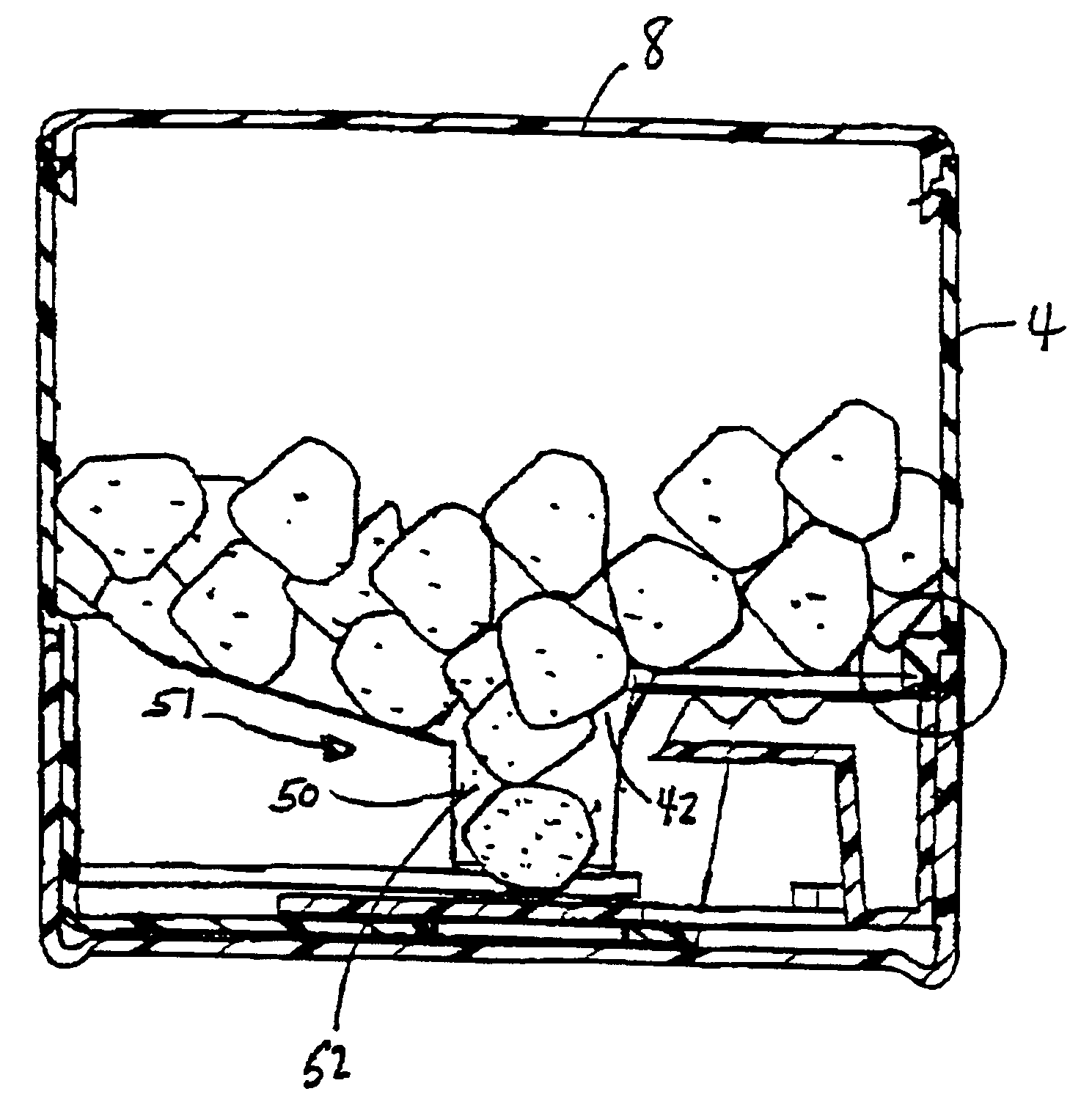
Solid dosage form dispenser
A dispenser for dispensing solid dosing forms which include a storage compartment and a releasing portion at the bottom of the storage compartment. The releasing portion is adapted to receive a releasing device which dispenses a single solid dosage form while providing security against the release of multiple solid dosage forms as well as clogging which may prevent the release of a single solid dosage form.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Methods of Producing Stabilized Solid Dosage Pharmaceutical Compositions Containing Morphinans
Methods for producing stabilized solid dosage form pharmaceutical compositions are provided. In particular, methods for preparing protected granules containing morphinans, and solid dosage form pharmaceutical compositions produced using the morphinan-protected granules are provided.
Owner:MALLINCKRODT INC
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
Owner:FERRING B B
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
ActiveUS7094545B2Improve manufacturing speedIncrease speed and capacityOrganic active ingredientsPeptide/protein ingredientsSolid Dose FormBULK ACTIVE INGREDIENT
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient, and to a method for manufacturing thereof. The invention relates to a pharmaceutical composition as a solid dosage form comprising desmopressin, or a pharmaceutically acceptable salt thereof, as a therapeutically active ingredient together with a pharmaceutically acceptable excipient, diluent or carrier, or mixture thereof, wherein at least one of said excipient, diluent and carrier is a substance selected from a monosaccharide, disaccharide, oligosaccharide and a polysaccharide, wherein the said substance has an average particle size in the range of from 60 to 1,000 μm. A method according to the present invention provides an improved production of solid dosage forms of desmopressin.
Owner:FERRING BV
Sustained release heterodisperse hydrogel systems-amorphous drugs
InactiveUS6048548AHigh total specific surfaceMacromolecular non-active ingredientsCoatingsDiluentHuman patient
Sustained release oral solid dosage forms comprising agglomerated particles of a therapeutically active medicament in amorphous form, a gelling agent, an ionizable gel strength enhancing agent and an inert diluent, as well as processes for preparing and using the same are disclosed. The sustained release oral solid dosage forms are useful in the treatment of hypertension in human patients.
Owner:PENWEST PHARMA CO
Sustained release matrix systems for highly soluble drugs
Disclosed are sustained release oral solid dosage forms comprising a therapeutically effective amount of a medicament having a solubility of more than about 10 g / l; a pH modifying agent; and a sustained release matrix comprising a gelling agent, said gelling agent comprising a heteropolysaccharide gum and a homopolysaccharide gum capable of cross-linking said heteropolysaccharide gum when exposed to an environmental fluid, said dosage form providing a sustained release of said medicament after oral administration to human patients.
Owner:PENWEST PHARMA CO
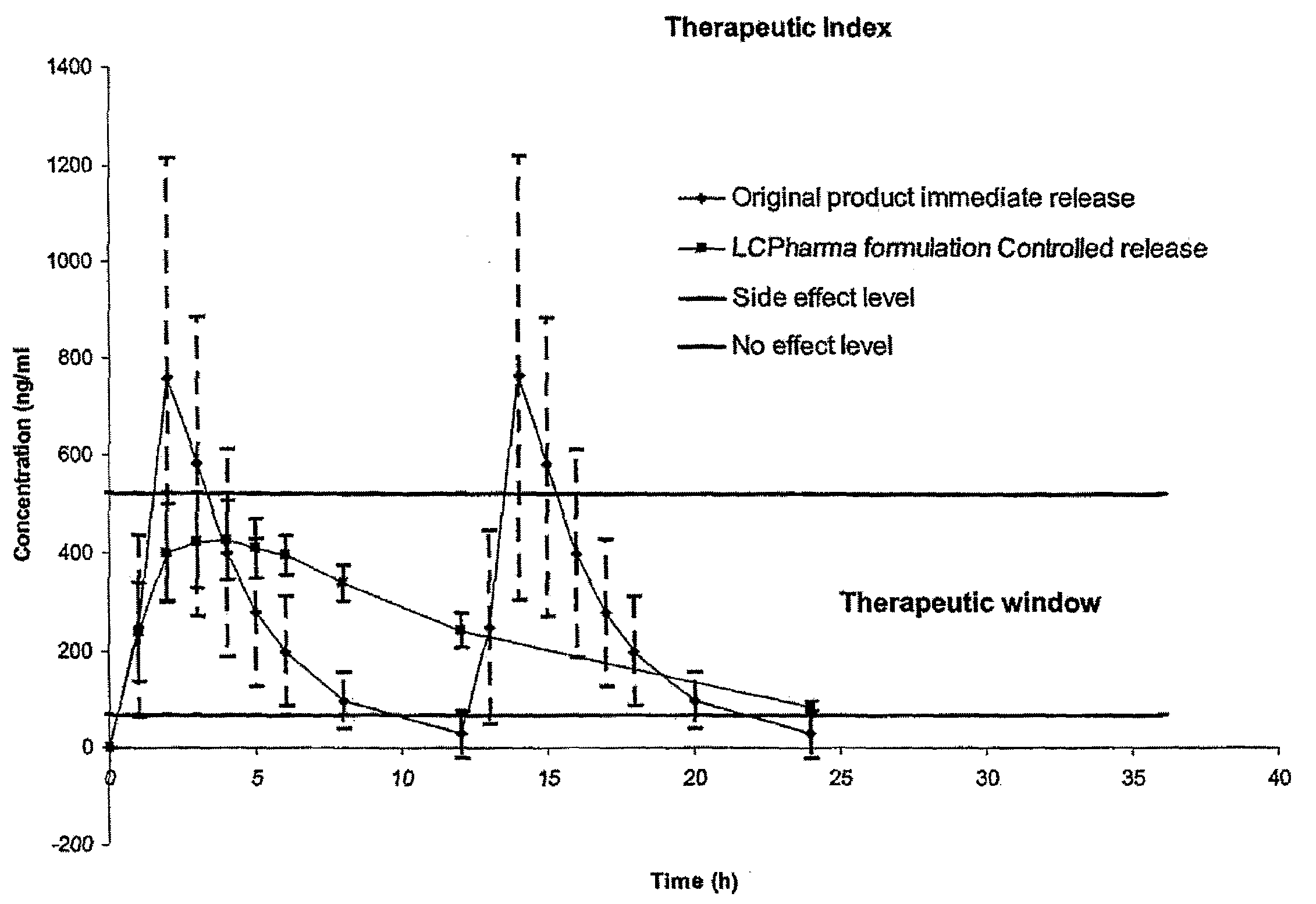
Pharmaceutical Compositions Comprising Sirolimus and/or an Analogue Thereof
InactiveUS20080275076A1Improve safety/efficacy ratioReduce impactAntibacterial agentsBiocideParticulatesSide effect
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising sirolimus (rapamycin) and / or derivatives and / or analogues thereof. Compositions of the invention exhibit an acceptable bioavailability of sirolimus and / or a derivative and / or an analogue thereof. The pharmaceutical compositions of the invention are designed to release sirolimus in a controlled manner so that the plasma levels stays within the narrow therapeutic window that exist for this class of substances. An extended release profile, where the peak concentration has been reduced without loosing significant bioavailability, together with less variable absorption, is expected to improve the safety / efficacy ratio of the drug. Furthermore, compositions according to the invention provide for a significant reduced food effect and a delayed release of sirolimus is expected to reduce the number of gastro-intestinal related side effects.
Owner:LIFECYCLE PHARMA AS
Compositions comprising fenofibrate and pravastatin
InactiveUS20050096390A1Substance may accumulateImprove bioavailability in vivoBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor pravastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCpravastatin) of between about 90 and about 6300. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and pravastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
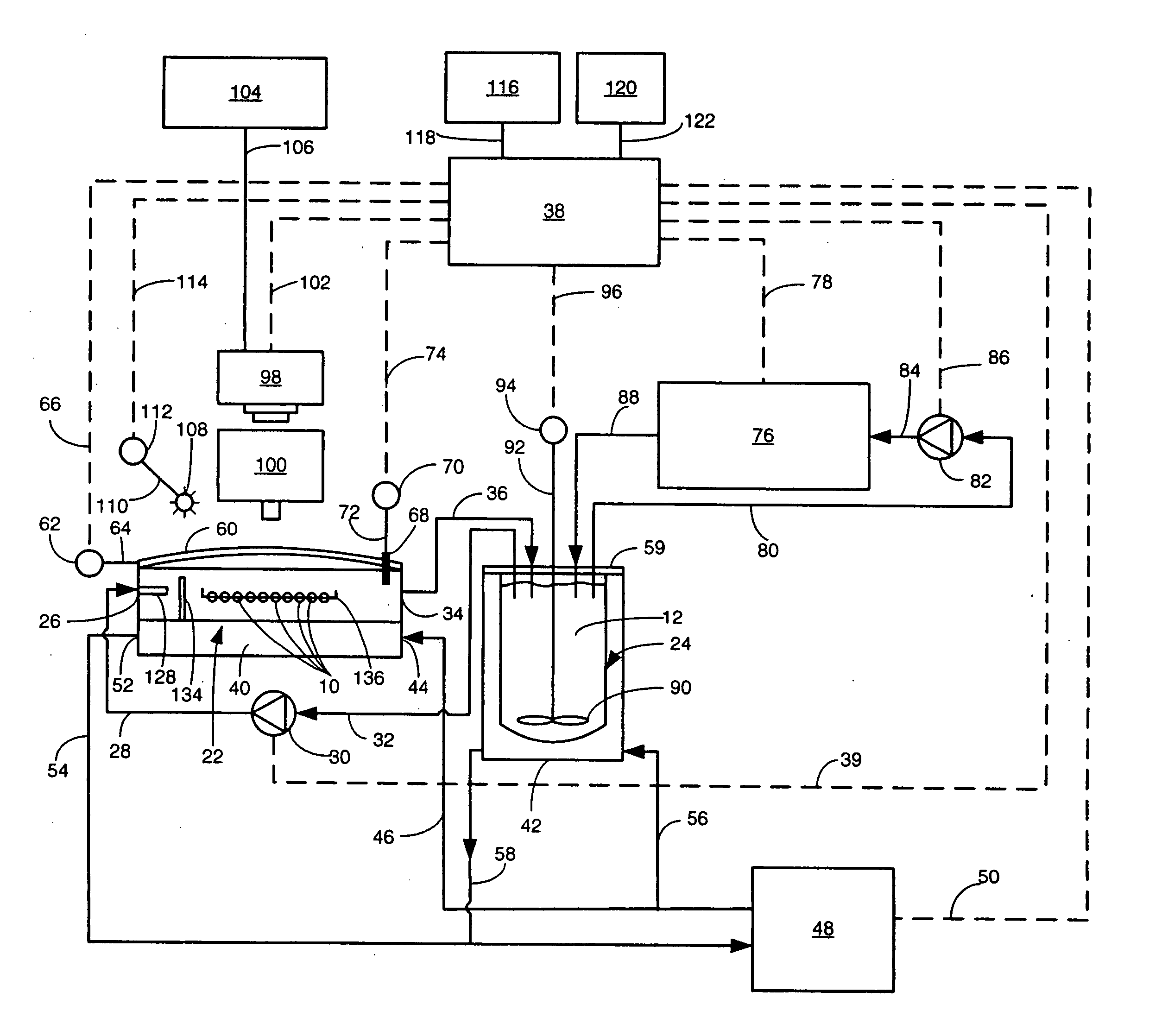
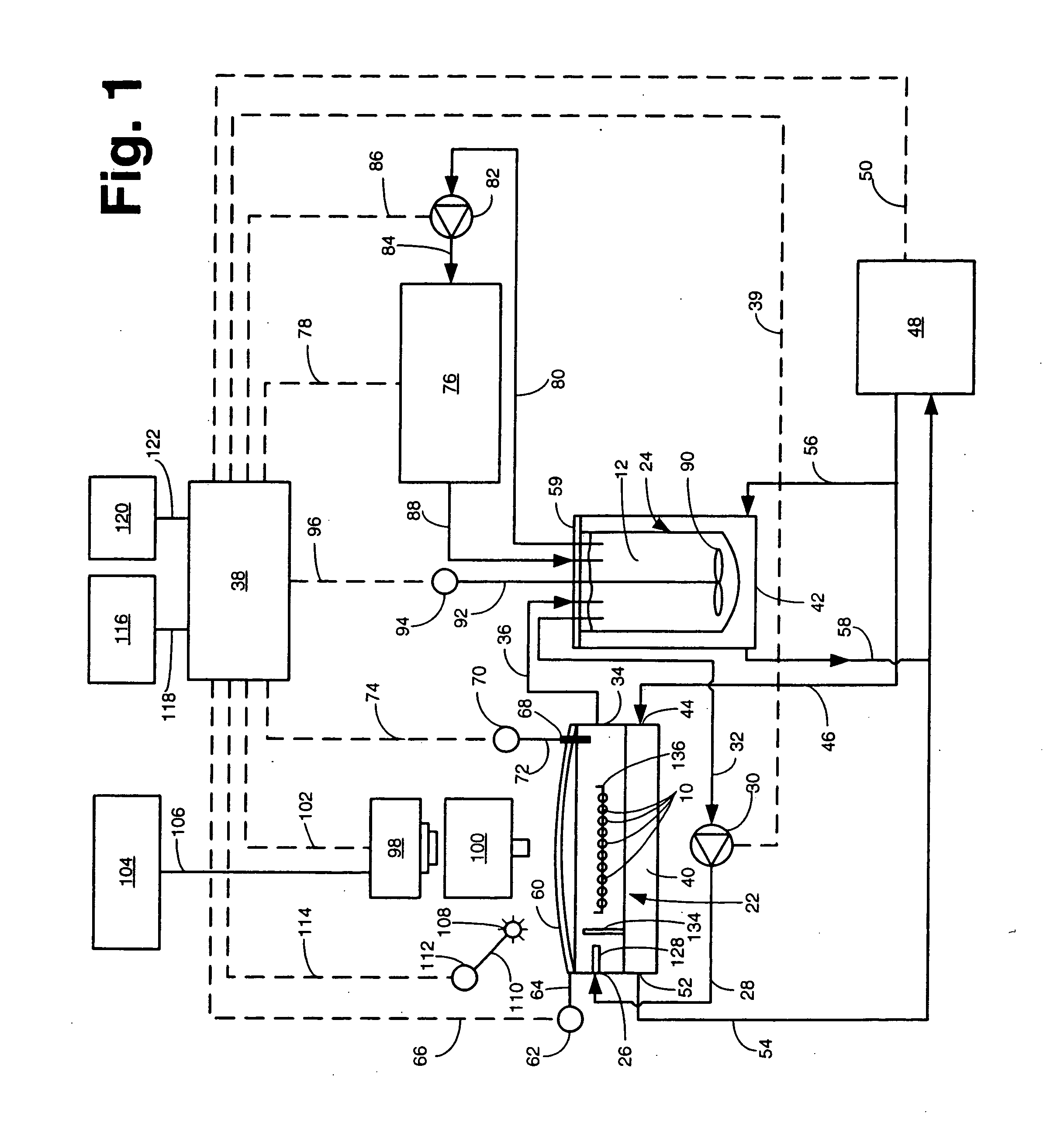
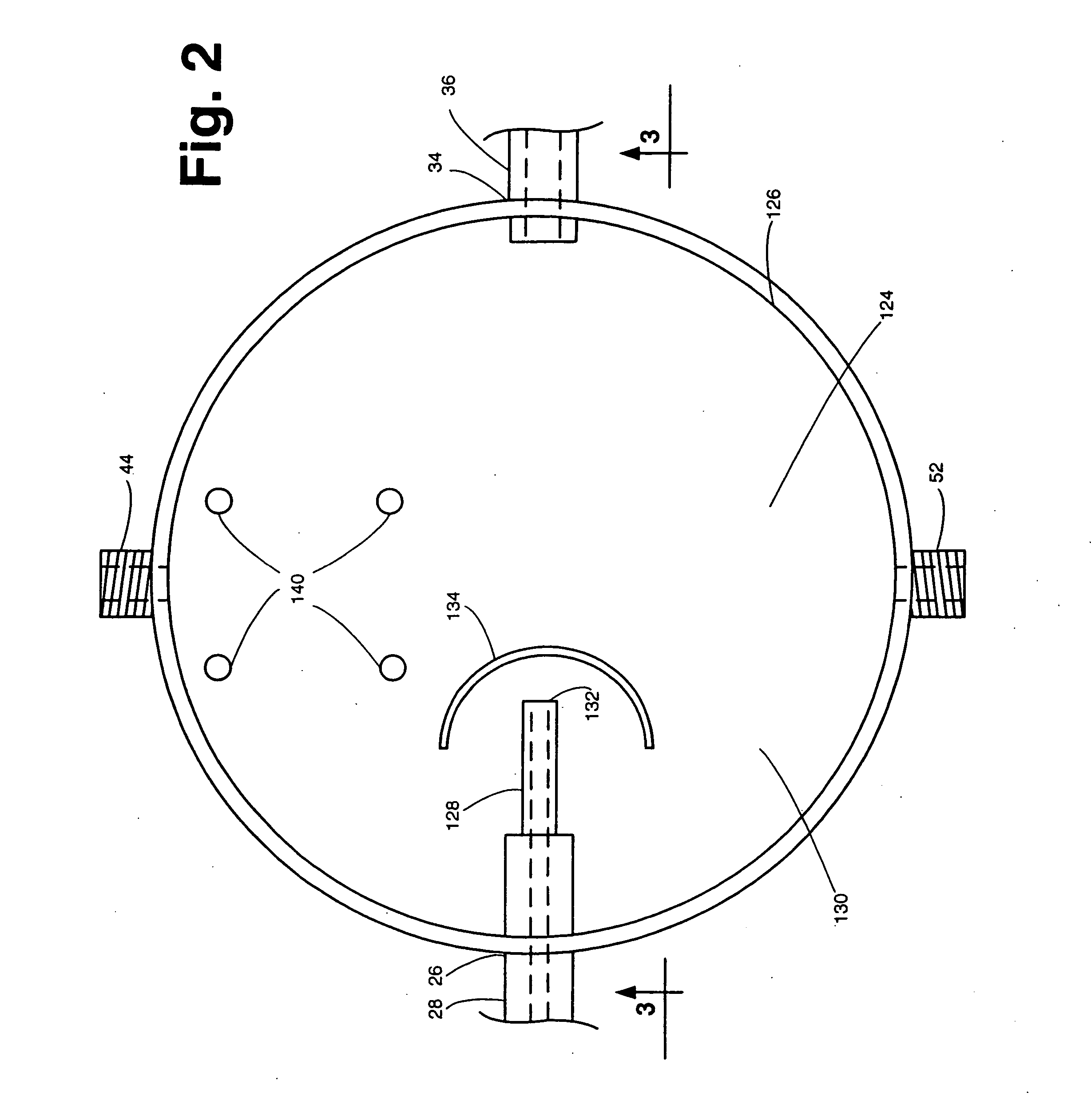
Apparatus and method for concurrently monitoring active release and physical appearance of solid dosage form pharmaceuticals
InactiveUS20050003550A1Quality improvementAvoid displacementComponent separationMaterial analysis by optical meansVideo monitoringCompound (substance)
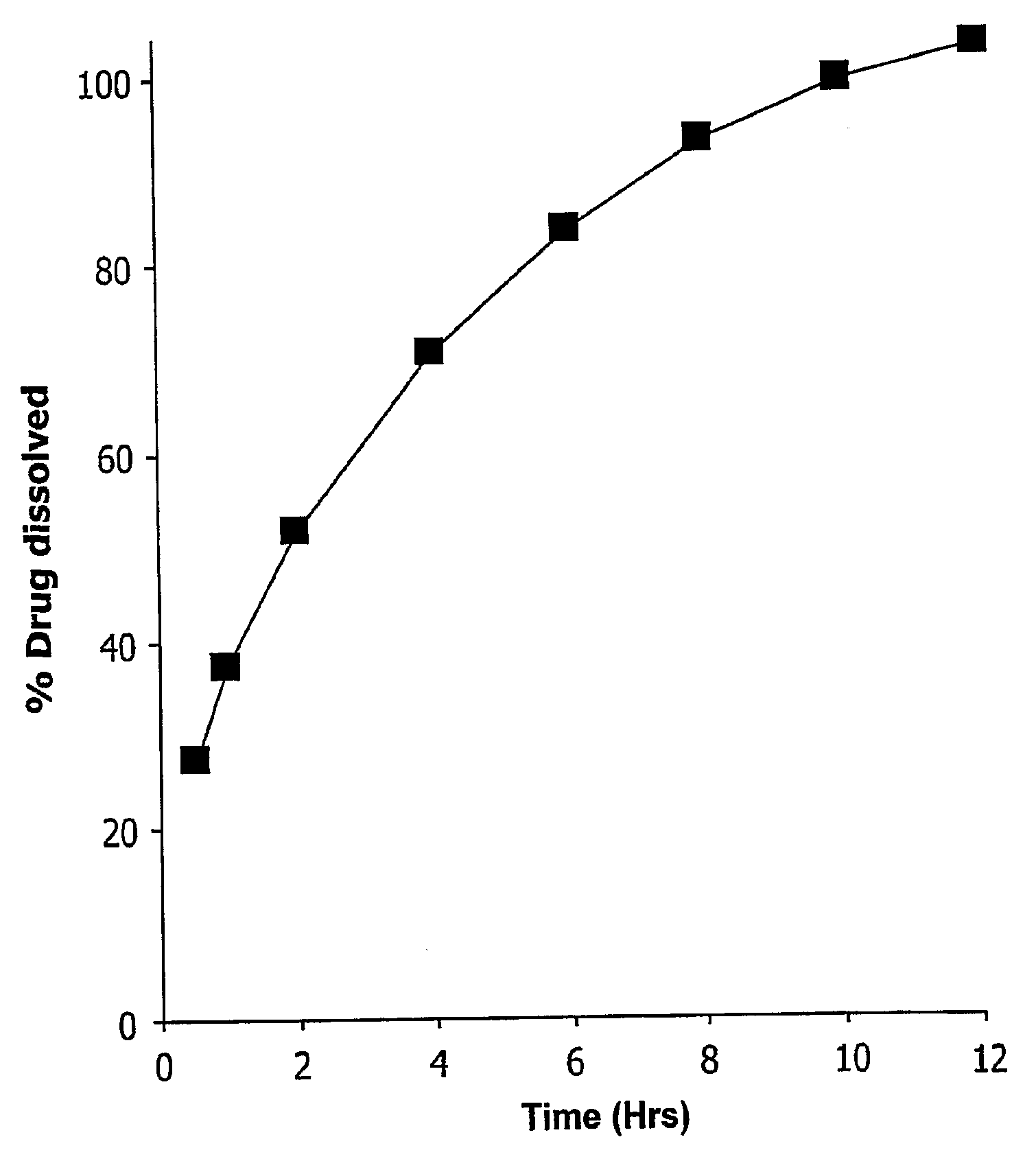
An apparatus and method for monitoring the dissolution properties of a solid dosage form pharmaceutical or other material. The apparatus includes a hollow dissolution chamber for supporting the dosage form and subjecting it to a dissolution liquid so that the dosage form dissolves in the liquid. A dissolution liquid analyzing device (e.g., a spectrophotometer) analyzes the properties of the dissolution liquid as the dosage form dissolves. A video monitoring means (e.g., a stereo-microscope and video camera) provides a series of images of the dosage form as it dissolves. The series of images and data resulting from the analysis are recorded and correlated. The temperature, flow rate and chemical parameters of the dissolution liquid can be controlled (e.g., held constant or altered), if desired.
Owner:ALKERMES PHARMA IRELAND LTD
Sugar coatings and methods therefor
InactiveUS20050271724A1Convenient coatingOrganic active ingredientsCoatingsSugarUltimate tensile strength
Compositions particularly useful as coatings for solid dosage forms of therapeutic agents are provided, as are solid dosage forms comprising such coatings, processes for preparing such solid dosage forms, and the products of those processes. The coating compositions generally provide excellent strength and resistance to cracking, even when applied to flexible / swellable tablet cores such as hydrogel-type cores. The compositions also exhibit excellent odor-blocking characteristics.
Owner:WYETH LLC
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
InactiveUS20050019392A1Reduce wearPeptide/protein ingredientsPharmaceutical non-active ingredientsDiluentBULK ACTIVE INGREDIENT
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient, and to a method for manufacturing thereof. The invention relates to a pharmaceutical composition as a solid dosage form comprising desmopressin, or a pharmaceutically acceptable salt thereof, as a therapeutically active ingredient together with a pharmaceutically acceptable excipient, diluent or carrier, or mixture thereof; wherein the pharmaceutical composition is composed of a compressed granulate and contains lubricant in an amount of from 0.05 to less than 0.50 percent by weight of said pharmaceutical composition.
Owner:FERRING B B
Method for preparing solid dosage form of desmopressin
InactiveUS20050158378A1Simple preparation processShort processing timePeptide/protein ingredientsWood working apparatusSolid Dose FormUrology
The present invention relates to a novel method for the preparation of a solid dosage form of desmopressin, or a pharmaceutically acceptable salt thereof, comprising providing a desmopressin containing granulate suitable for compression to a pharmaceutically acceptable tablet, as well as to solid dosage forms, preferably tablets, obtainable by said method.
Owner:FERRING BV
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
ActiveUS20040220080A1Organic active ingredientsPeptide/protein ingredientsBULK ACTIVE INGREDIENTSolid Dose Form
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient, and to a method for manufacturing thereof. The invention relates to a pharmaceutical composition as a solid dosage form comprising desmopressin, or a pharmaceutically acceptable salt thereof, as a therapeutically active ingredient together with a pharmaceutically acceptable excipient, diluent or carrier, or mixture thereof, wherein at least one of said excipient, diluent and carrier is a substance selected from a monosaccharide, disaccharide, oligosaccharide and a polysaccharide, wherein the said substance has an average particle size in the range of from 60 to 1,000 mum. A method according to the present invention provides an improved production of solid dosage forms of desmopressin.
Owner:FERRING BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com