Sustained release matrix systems for highly soluble drugs
a matrix system and drug technology, applied in the direction of drug compositions, non-active ingredients of oil/fat/waxes, cardiovascular disorders, etc., can solve the problems of variable drug release, soluble to highly soluble drugs present formulation difficulties, sustained release formulations with soluble drugs are susceptible to "dose dumping"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
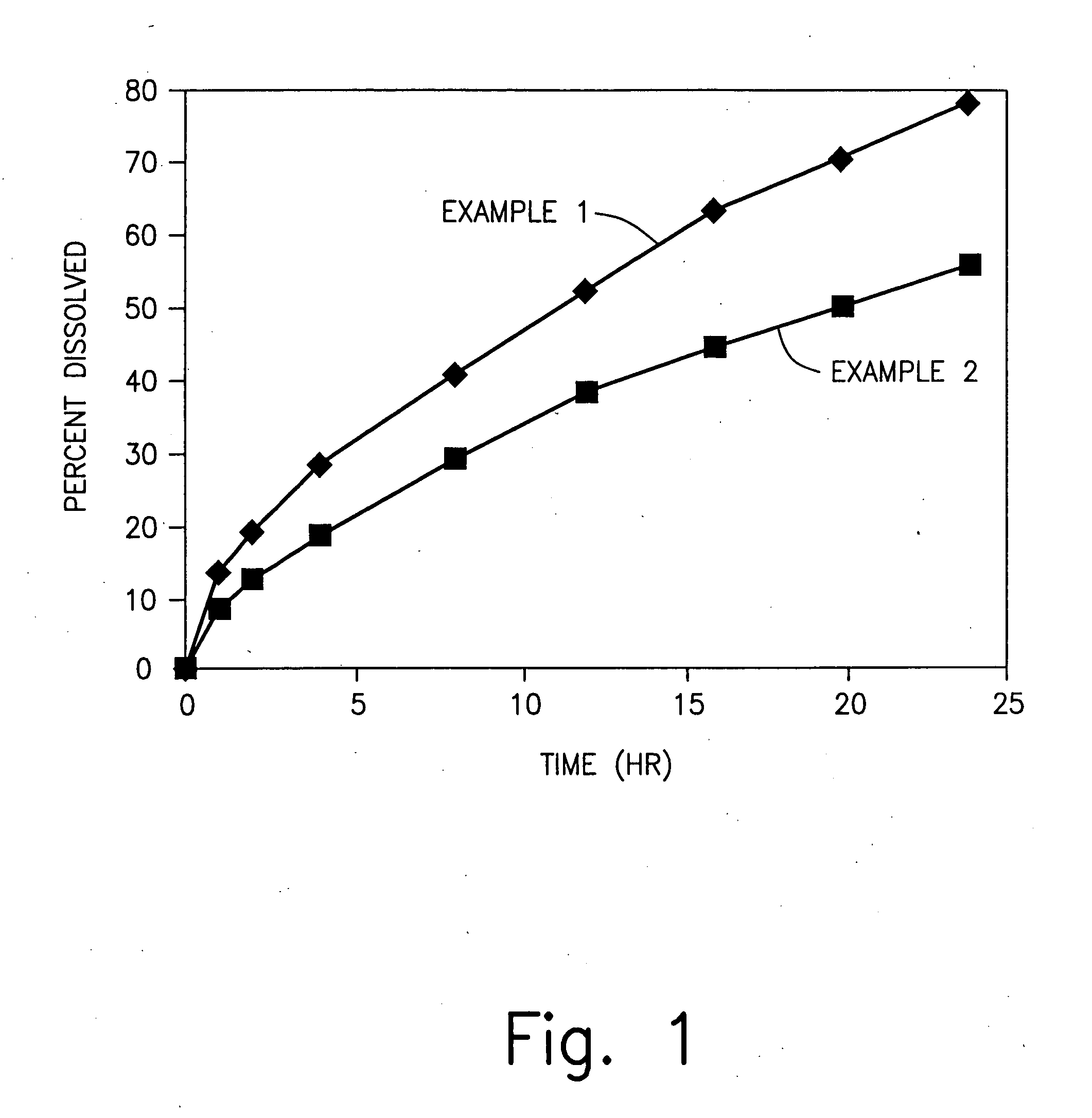
Examples
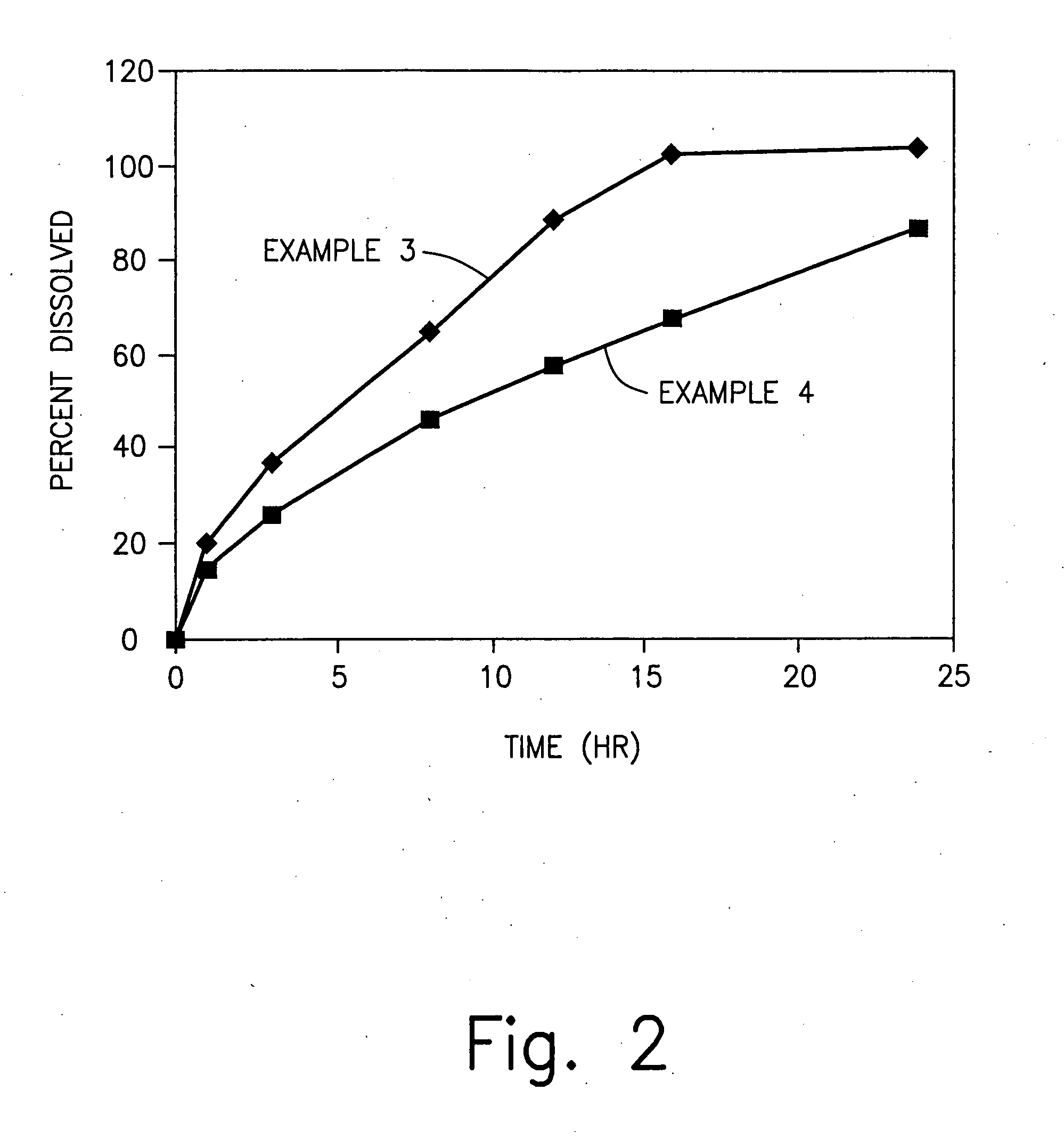
examples 3-4
Effect of Gum:Dextrose Ratio
[0127] In Examples 3-4, a sustained release excipient is prepared in accordance with the procedure set forth in Examples 1 and 2. The ingredients of the sustained release excipient of Examples 3 and 4 are set forth in table 4 below:
4TABLE 4 Component Amount (%) - Ex. Amount (%) -Ex. 2 1 Xanthan Gum 12 20 2 Locust Bean Gum 18 30 3 Dextrose 70 50 4 Water* 25 35 *removed during processing
[0128] Thereafter, diltiazem tablets are prepared as follows:
[0129] The desired amount of diltiazem, fumaric acid and the sustained release excipient are placed in a granulator and mixed for 3 minutes at low speed. Water is added over a 2 minute interval while the impeller is running at low speed (additional water and granulation time may be used to form proper granules). The resultant granules are then dried in a fluid bed dryer until LOD is less than 5% and milled with hammer forward at 2000-3000 rpm using screen #0050. The milled granulation is then placed in a V-Blender ...
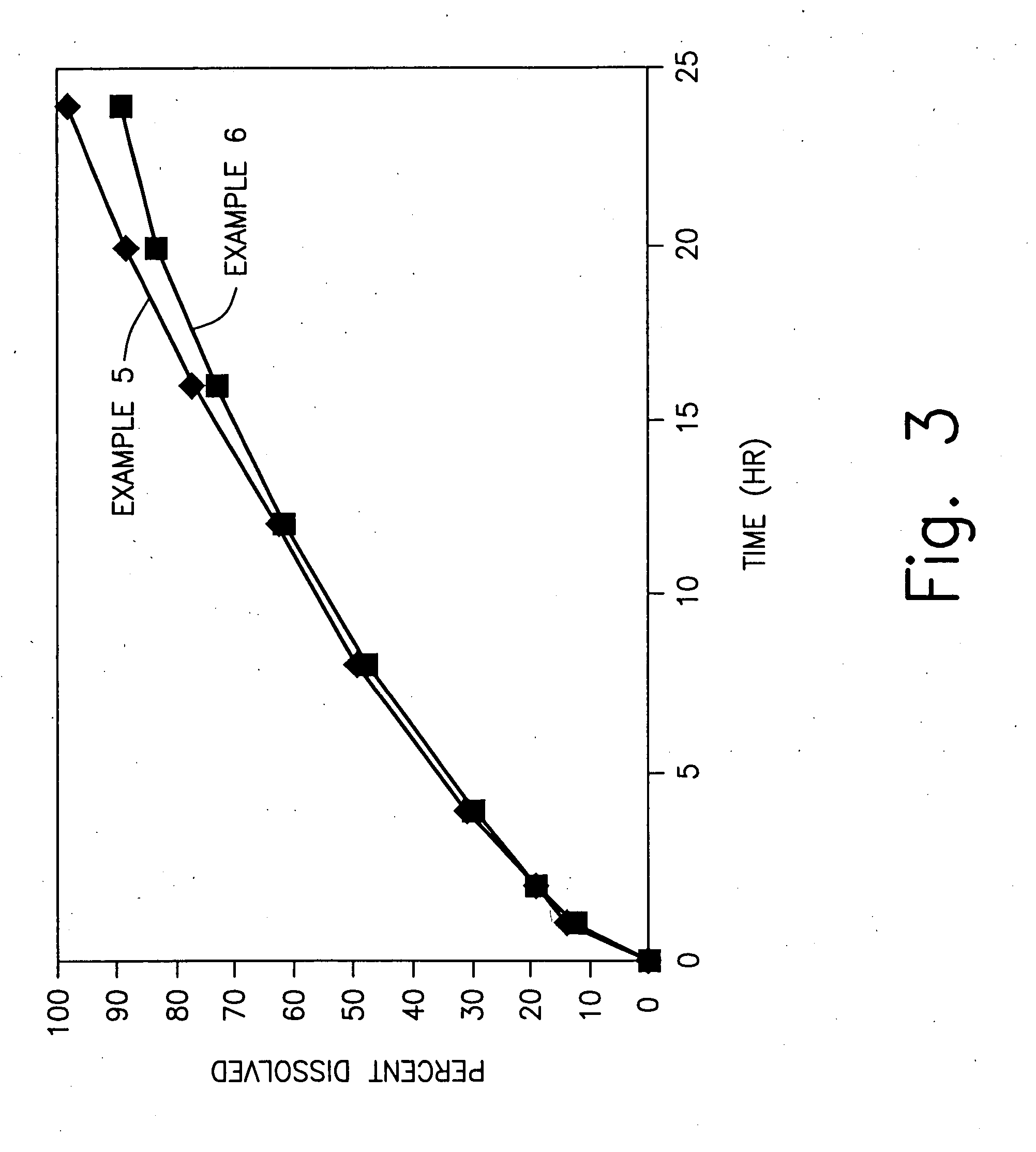
examples 5-6
Effect of Surfactant Type
[0133] In Examples 5-6, a sustained release excipient is prepared accordance with the procedure set forth in Examples 1 and 2. The ingredients of the sustained release excipient of Examples 5 and 6 are set forth in table 7 below:
7 TABLE 7 Component Amount (%) - Ex. 5-6 1 Xanthan Gum 12 2 Locust Bean Gum 18 3 Dextrose 70 4 Water* 25 *removed during processing
[0134] Thereafter, diltiazem tablets are prepared as follow:
[0135] The desired amount of diltiazem, fumaric acid and a suitable amount of water are mixed for 5 minutes with an impeller type mixer to form a slurry. The slurry is then added to the sustained release excipient over a 1 minute interval in the granulator, with the impeller running on low speed. Next, the mixture is granulated for 2 minutes with the chopper and impeller on high speed (additional water and granulation time may be used to form proper granules). The resultant granules are then dried in a fluid bed dryer until LOD is less than 5% an...
examples 7-8
Effect of Surfactant Level
[0140] In Examples 7-8, a sustained release excipient is prepared accordance with the procedure set forth in Examples 1 and 2. The ingredients of the sustained release excipient of Examples 7 and 8 are set forth in table 10 below:
10 TABLE 10 Component Amount (%) - Ex. 7-8 1. Xanthan Gum 12 2. Locust Bean Gum 18 3. Dextrose 70 4. Water* 25 *Removed during processing
[0141] Thereafter, diltiazem tablets are prepared as follows:
[0142] The desired amount of diltiazem, fumaric acid and a suitable amount of water are mixed for 5 minutes with an impeller type mixer to form a slurry. The slurry is then added to sustained release excipient over a 1 minute interval in the granulator, with the impeller running on low speed. Next, the mixture is granulated for 2 minutes with the chopper and impeller on high speed (additional water and granulation time may be used to form proper granules). The resultant granules are then dried in a fluid bed dryer until LOD is less than ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com