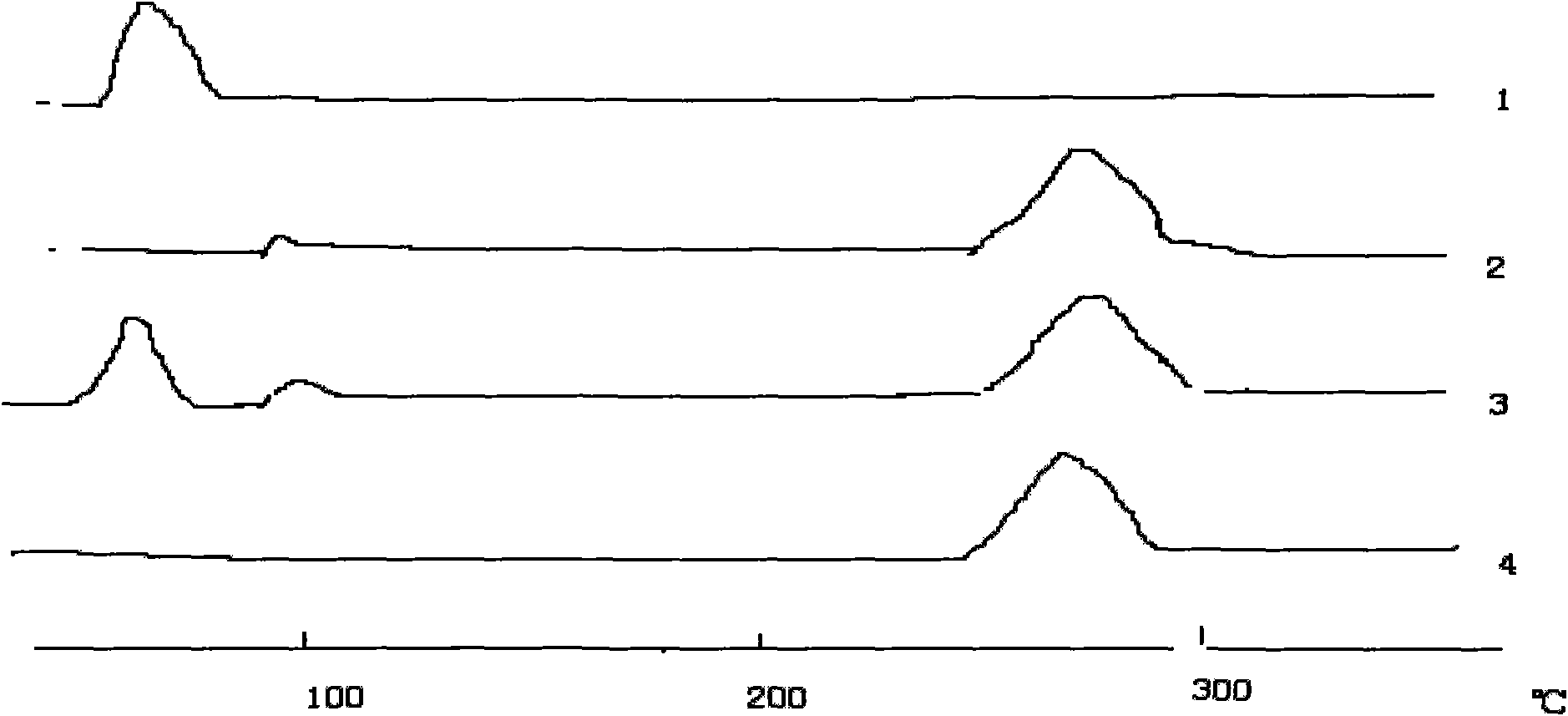
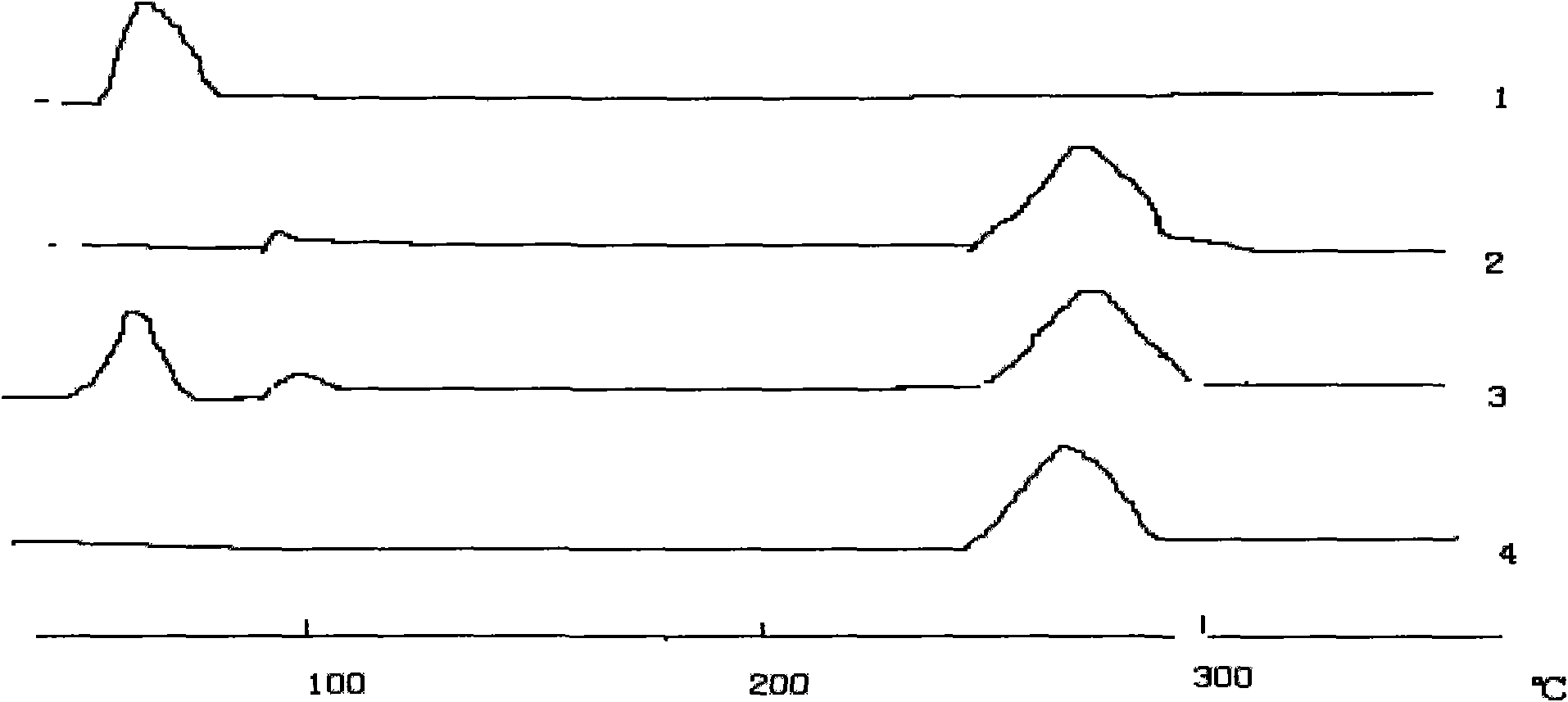
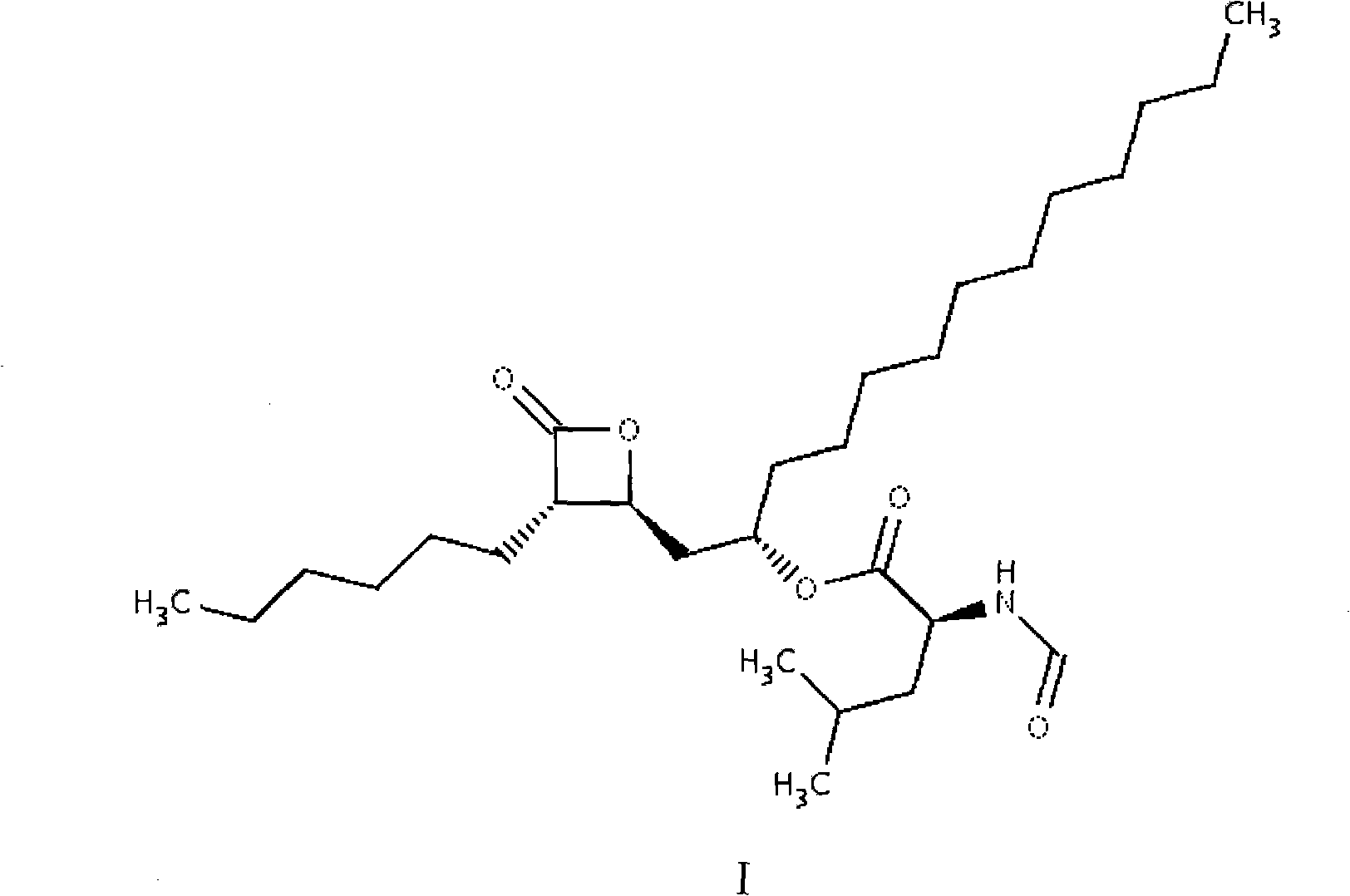
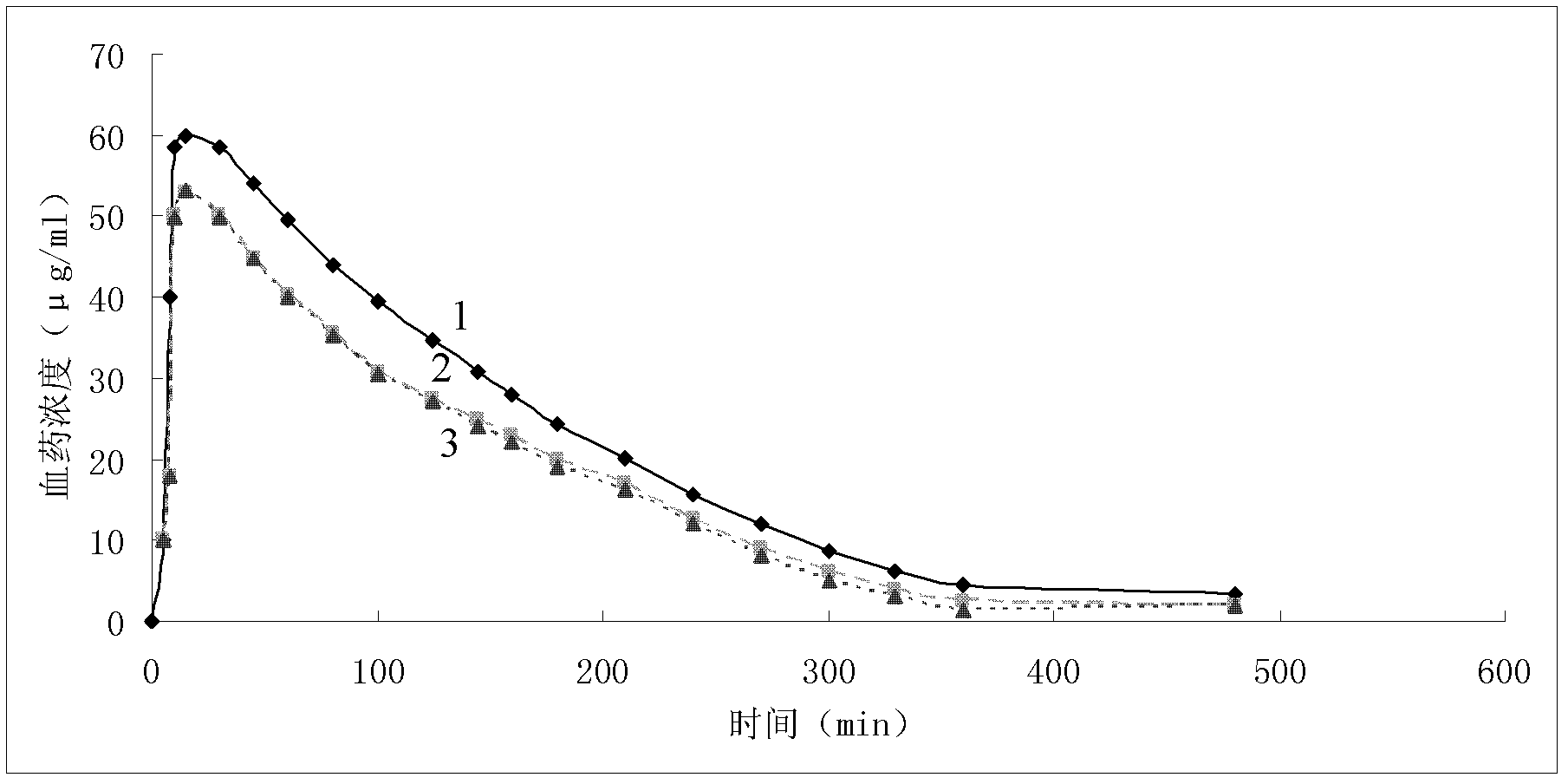
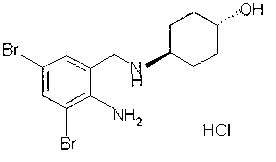
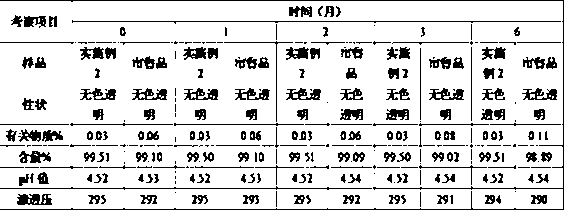
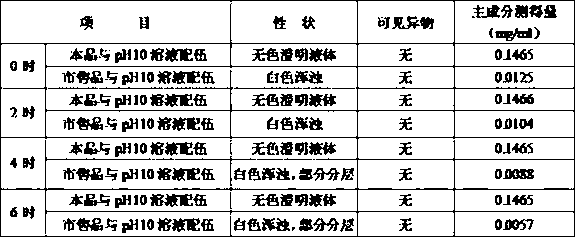
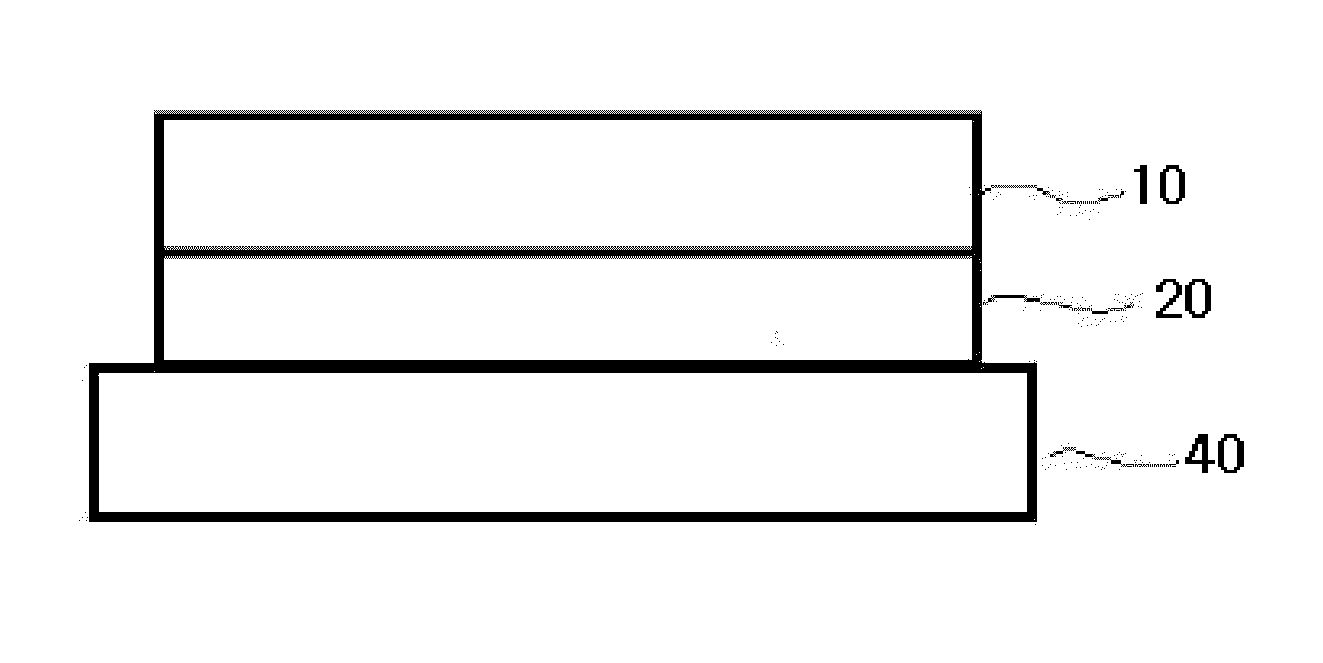
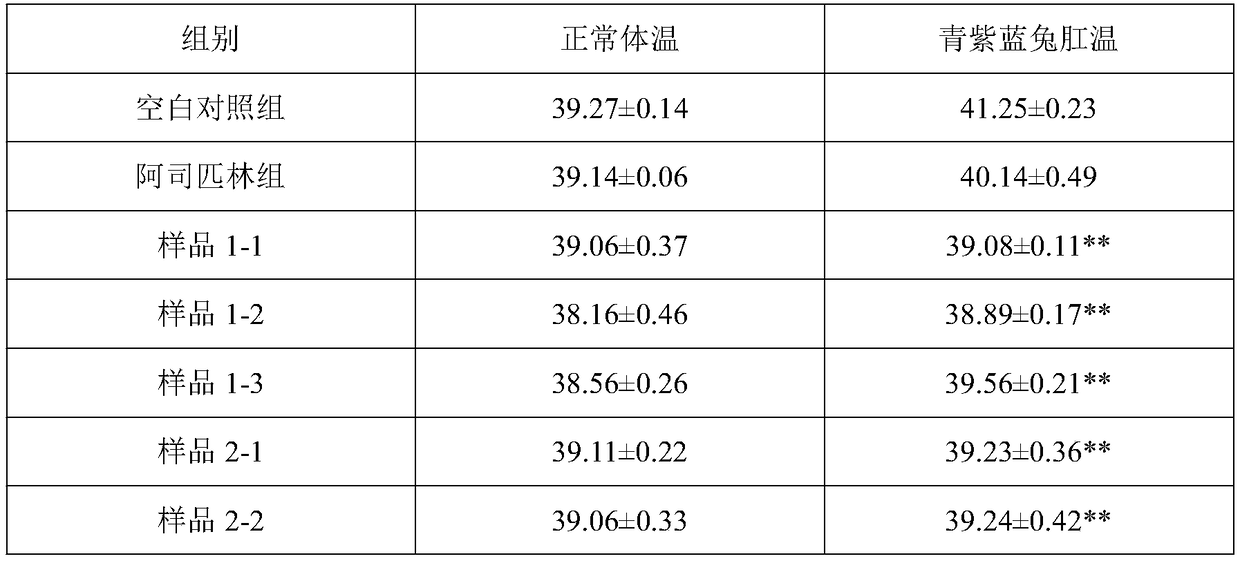
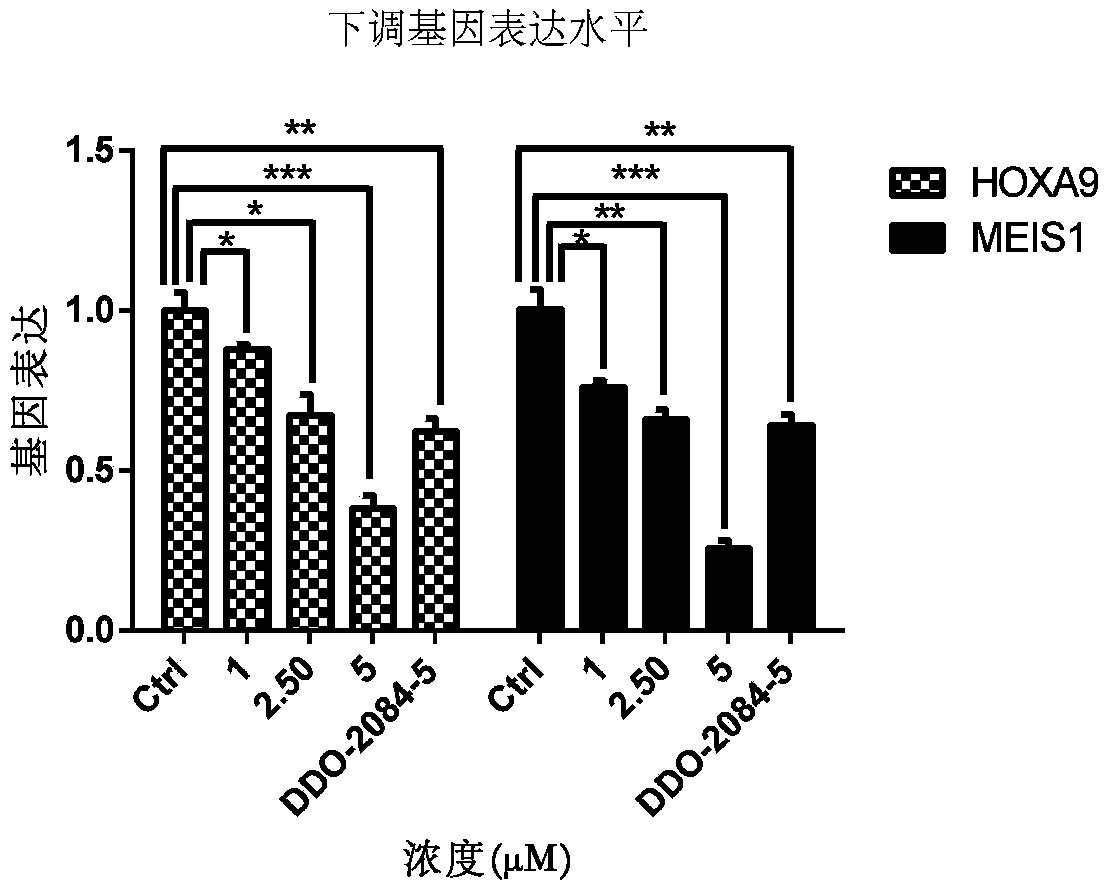
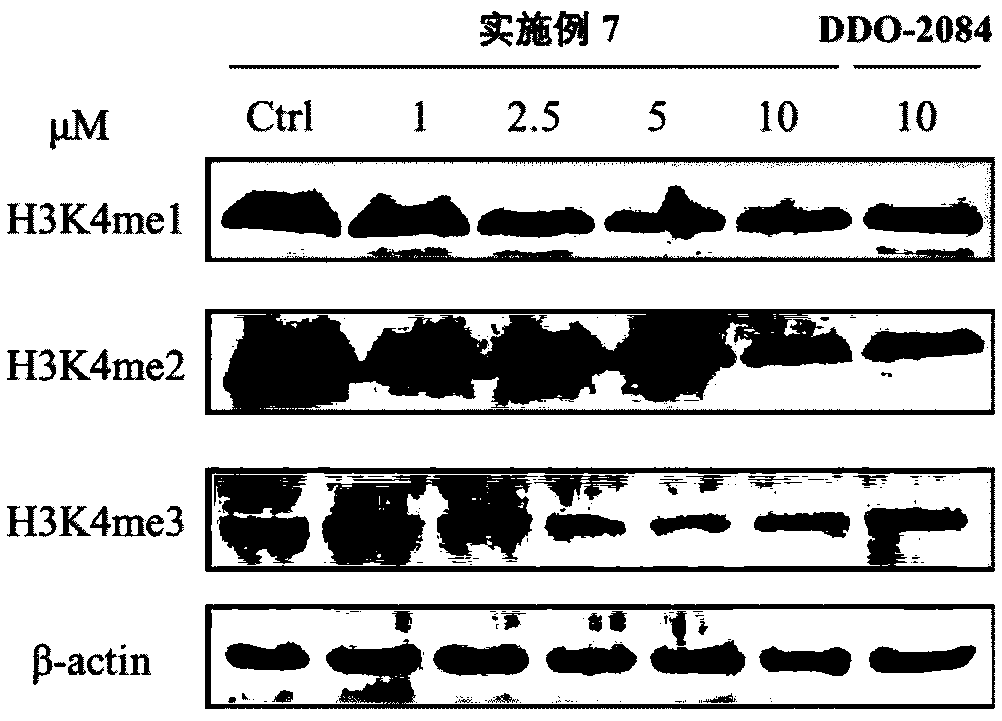
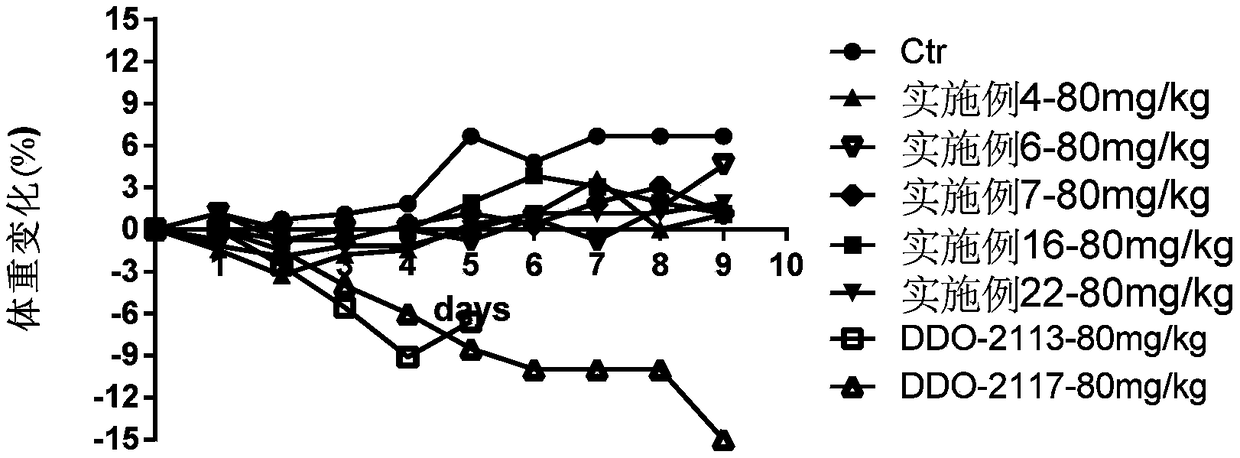
Patents
Literature
131results about How to "High drug safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
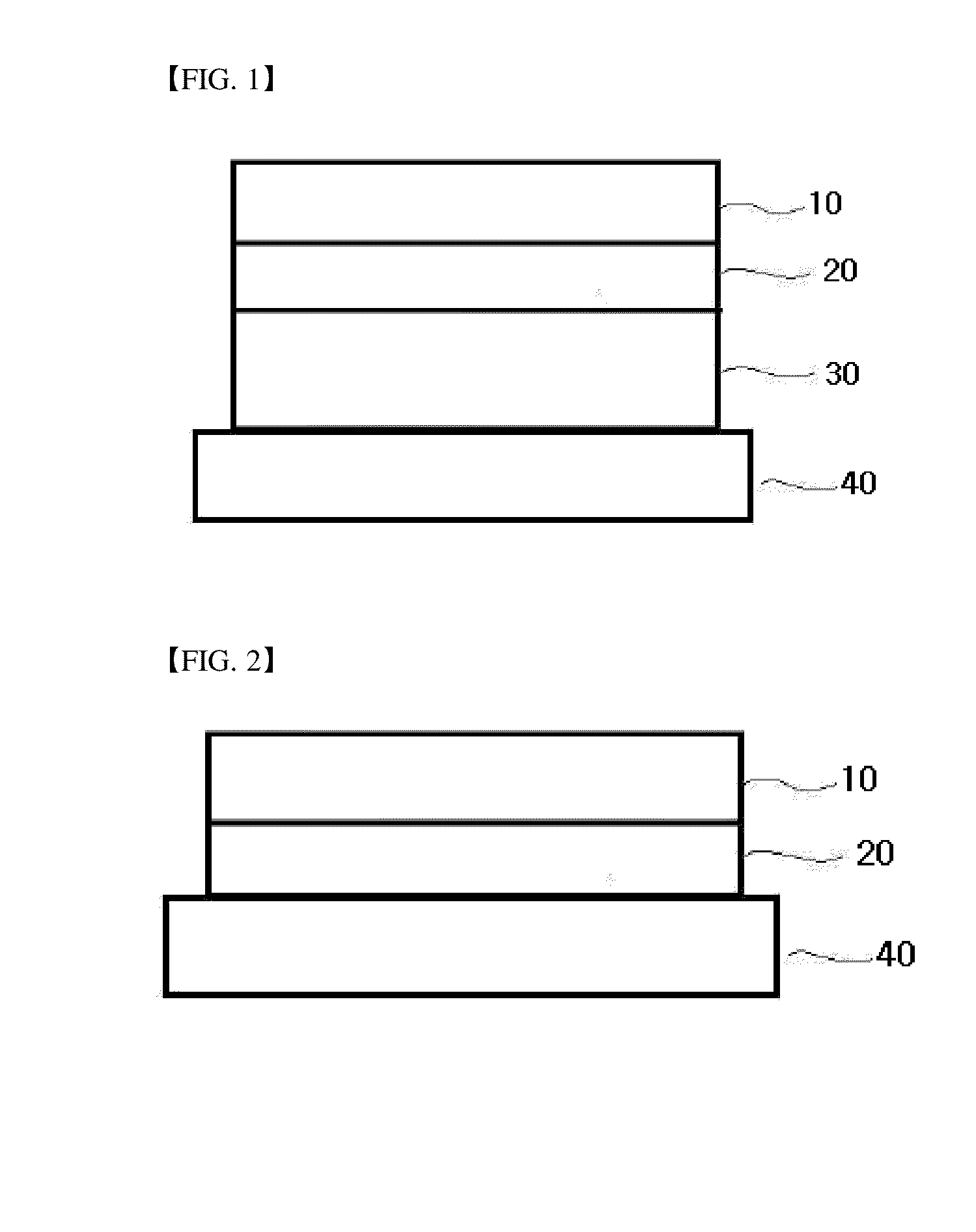
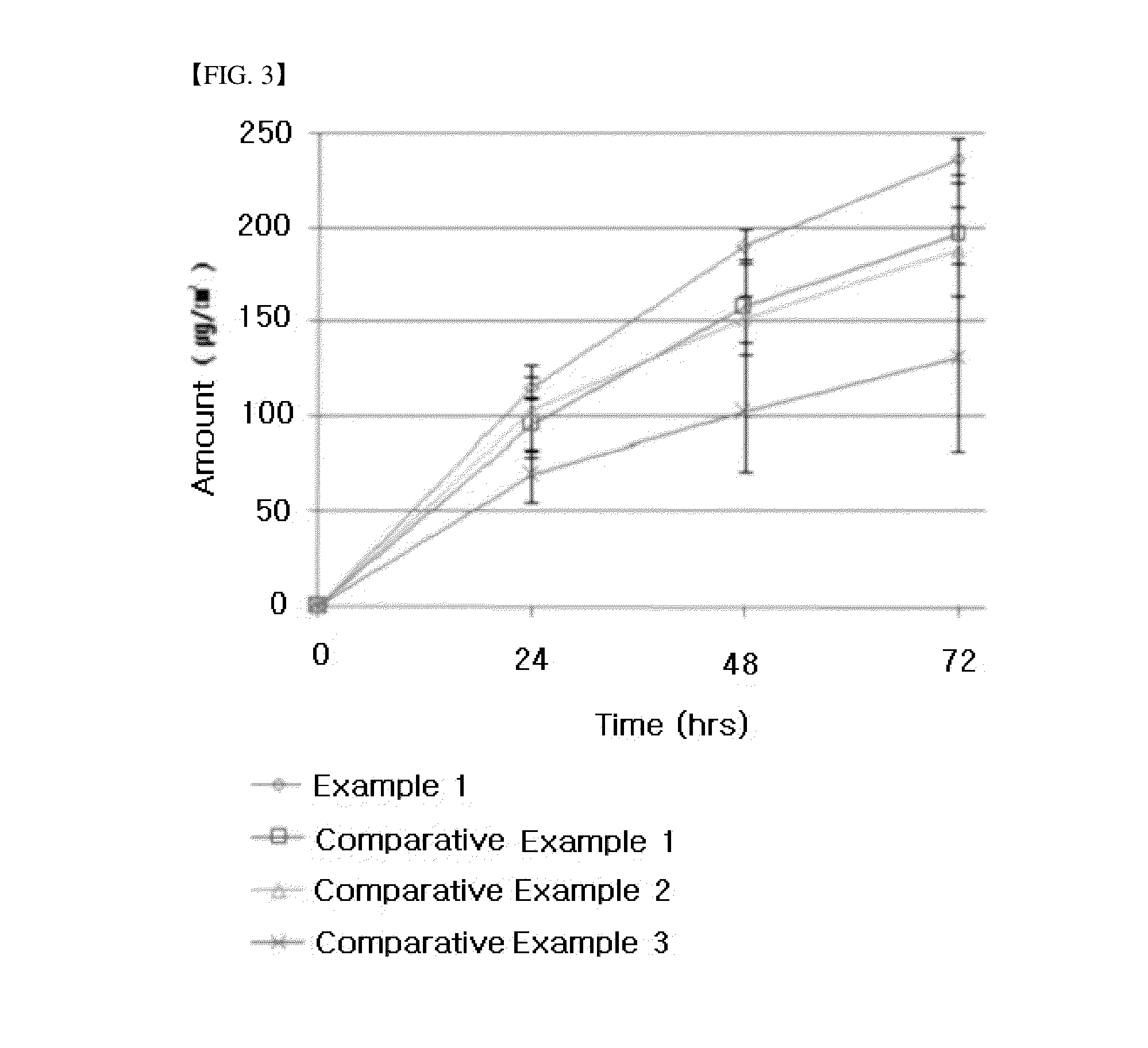
Ethanol-resistant sustained release formulations
InactiveUS20070212414A1High drug safetyLower potentialBiocideNervous disorderSustained Release FormulationsDelivery system
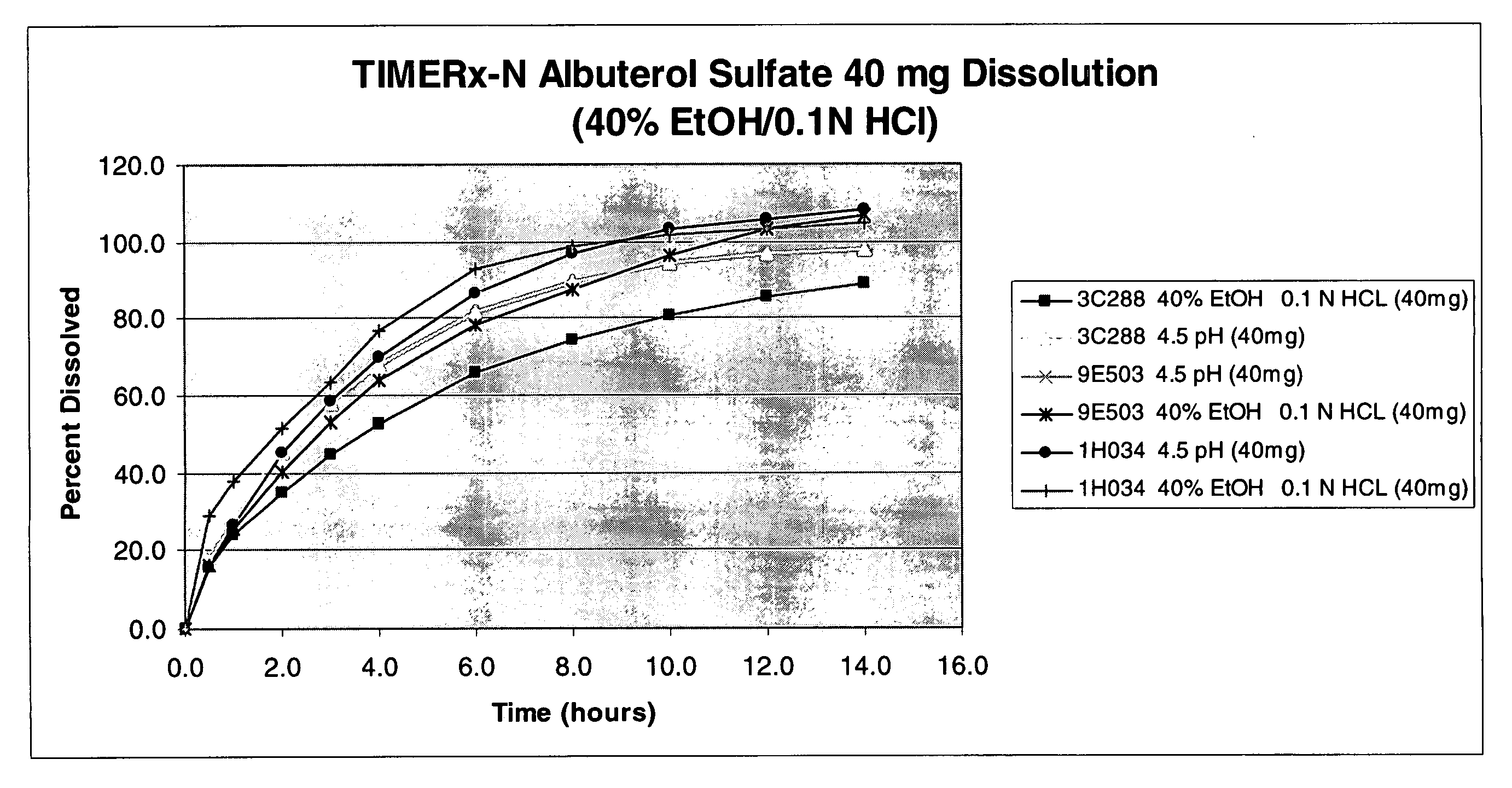
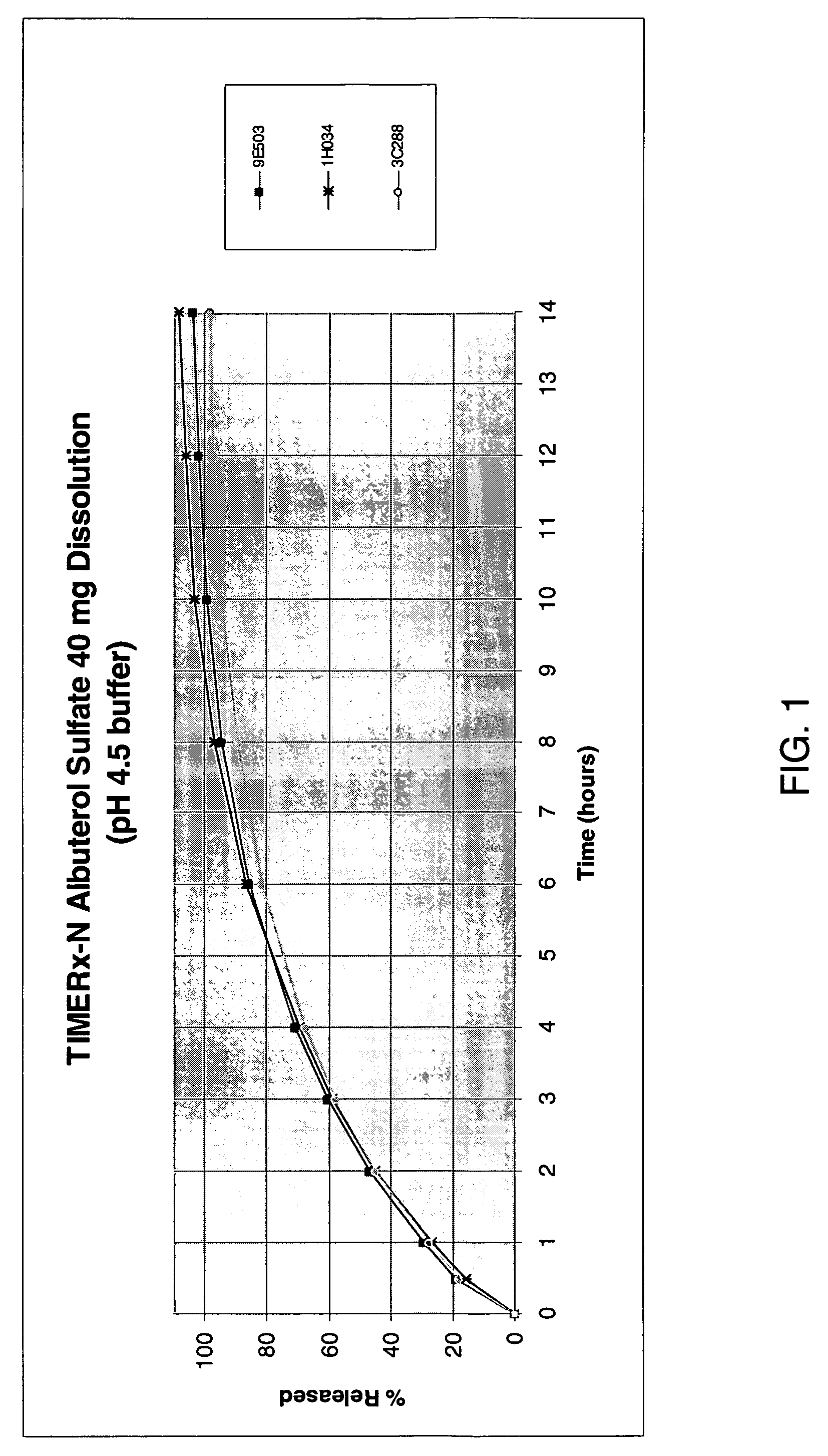
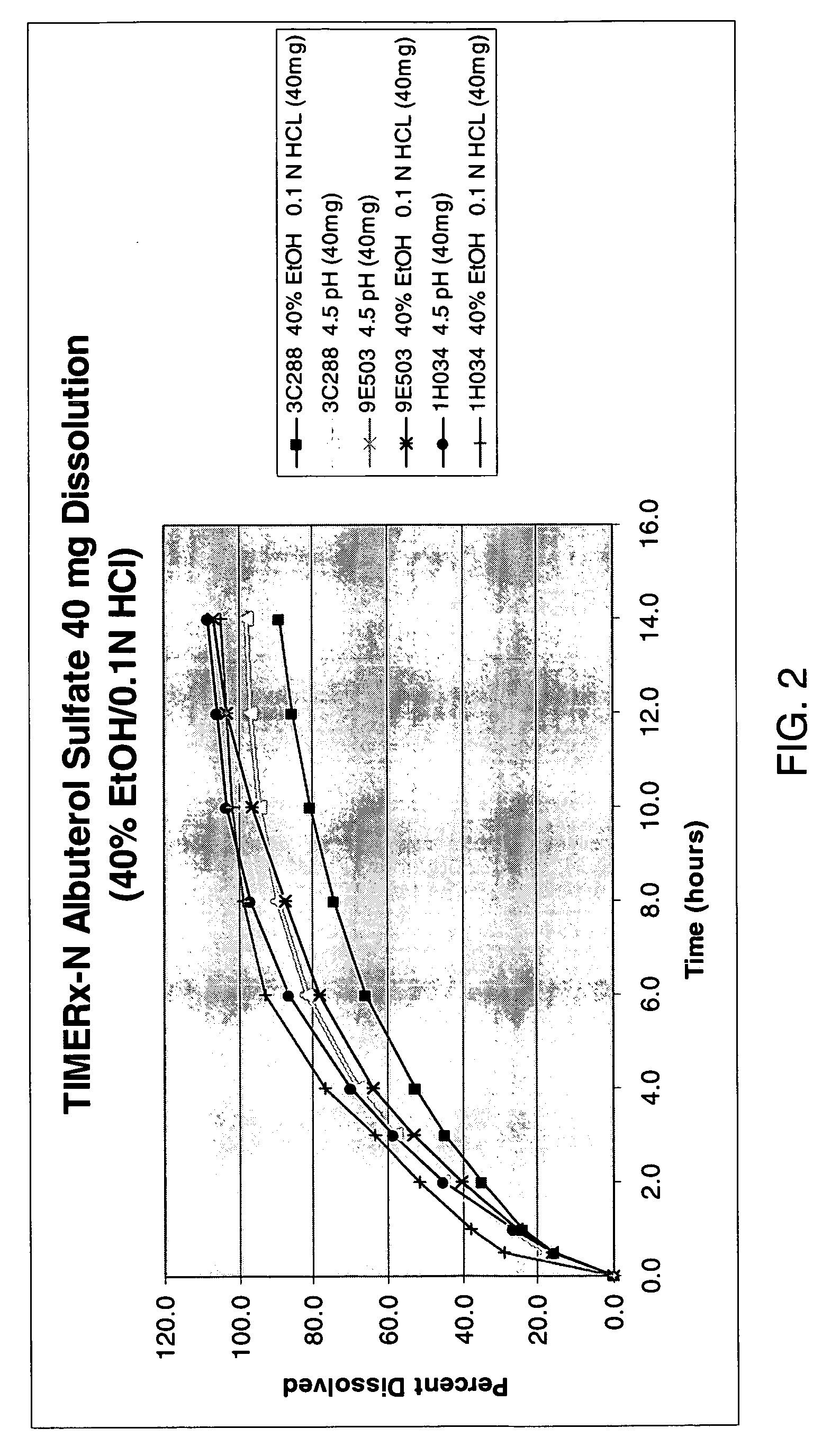
The invention provides formulations that resist dose dumping in the presence of ethanol and methods of use thereof. The formulations can be used to prevent dose dumping, to increase safety of drugs, and to reduce abuse of drugs prone to such abuse. The formulations comprise at least one drug and a sustained release delivery system. In one embodiment, the drug is an opioid.
Owner:ENDO PHARMA INC
Robust sustained release formulations of oxymorphone
InactiveUS20080085305A1Avoid dose dumpingHigh drug safetyPowder deliveryOrganic chemistryOxymorphoneSustained Release Formulations
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
American goldenrod herb total flavone extract and its preparing method and use
InactiveCN1899341AEfficient enrichmentHigh in flavonoidsSugar derivativesSugar derivatives preparationDiseaseChlorogenic acid
The present invention relates to American goldenrod herb total flavone extract and its preparation process and use. The American goldenrod herb total flavone extract contains quercetin, quercetin-3-O-beta-D-heteroside, kaempferol-3-O-alpha-L-rhamnoside, chlorogenic acid, etc. capable of preventing and treating senile dementia, various inflammatory respiratory diseases, esophagus cancer, etc. The preparation process is simple, high extracting rate, and the extract has high pharmacological effect, stable property, low toxicity and controllable quality.
Owner:LINSAIJIAO BIOLOGICAL SCI & TECH DEV SHANGHAI
E-configuration benzamide compounds, and pharmaceutical preparation application thereof
ActiveCN103880736AReduce solubilityReduced bioavailabilityOrganic active ingredientsPowder deliveryDiseaseSubtype selective
The invention discloses E-configuration benzamide compounds, and a pharmaceutical preparation application thereof. The structure of the E-configuration benzamide compounds is disclosed as Formula (I); the chemical name is N-(2-amino-4-fluorophenyl)-4-[N-[(E)-3-(3-pyridyl)acryloyl]aminomethyl]benzamide; and in the structural formula, the configuration of the 3-pyridylacryloyl group is E. The E-configuration benzamide compounds disclosed as Formula (I) have subtype selective histone deacetylase inhibition activity, and are mainly used for inhibiting HDAC1, HDAC2 and HDAC3 in Class I HDAC and HDAC10 in Class IIb HDAC. The E-configuration benzamide compounds disclosed as Formula (I) can be used for treating diseases related to histone deacetylase activity abnormity, such as cancers, including lymphomata, entity tumor, blood system tumors and the like.
Owner:SHENZHEN CHIPSCREEN BIOSCIENCES CO LTD
Cefoxitin sodium crystal compound and cefoxitin sodium composition powder injection
ActiveCN102358744AImprove solubilitySolubility stabilityAntibacterial agentsPowder deliveryCefoxitin SodiumSodium benzoate
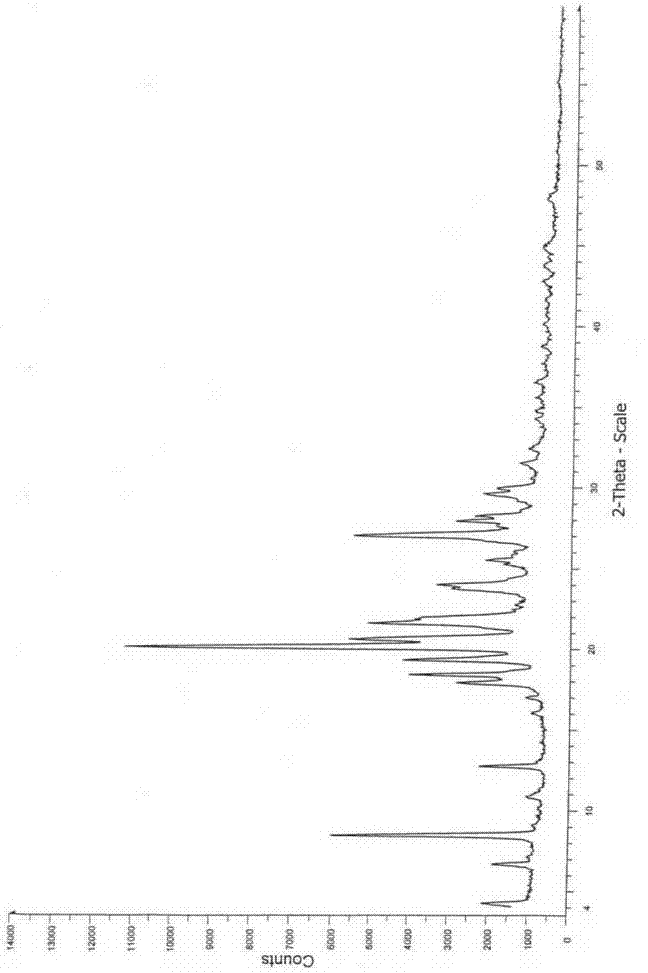
The invention relates to a cefoxitin sodium crystal compound. The method of powder X-ray diffractometry is utilized to determine the cefoxitin sodium crystal compound, and an X-ray powder diffraction pattern represented by the diffraction angle of 2theta+-0.2 degrees has characteristic diffraction peaks at 5.3 degrees, 8.6 degrees, 11.9 degrees, 13.2 degrees, 15.5 degrees, 16.1 degrees, 19.0 degrees, 22.3 degrees and 24.4 degrees. The invention also relates to a cefoxitin sodium composition powder injection containing the cefoxitin sodium crystal compound, and the cefoxitin sodium composition powder injection comprises 95 to 100 parts of the cefoxitin sodium crystal compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Orlistat tablets and preparation method thereof
ActiveCN101791296AGood chemical stabilityImprove physical stabilityOrganic active ingredientsMetabolism disorderOrlistatPharmacy
The invention belongs to the technical field of the medicament and in particular relates to tablets comprising orlistat and a preparation method thereof. Orlistat and cyclodextrin which are subjected to enclosing and auxiliary materials acceptable for pharmacy are pressed into the tablets. The tablets solve the sticking problem of carrying out pressing on the orlistat by the conventional process, obviously improve the chemical and physical stability of the orlistat, cover the unpleasant taste of the orlistat, improve the administration compliance of the dysphagia patients, have good dissolution and improve the curative effect of the orlistat.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of cefminox sodium crystalline compound and its composition powder injection
ActiveCN102276630AImprove solubilitySolubility stabilityAntibacterial agentsPowder deliveryCEFMINOX SODIUMX-ray
The invention relates to a cefminox sodium crystalline compound. The cefminox sodium crystalline compound is determined by a powder X-ray diffraction determination method; and characteristic diffraction peaks are displayed at 5.1, 6.9, 8.5, 10.3, 12.1, 15.1, 15.9, 17.4, 19.5, 21.7 and 24.6 degrees in an X-ray powder diffraction pattern represented by a diffraction angle of 2 theta+ / -0.2 degree. The invention also relates to cefminox sodium composition powder injection containing the cefminox sodium crystalline compound. The composition powder injection comprises the following components: 95 to 100 parts of cefminox sodium crystalline compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Cabazitaxel fat emulsion, and preparation method and use thereof
InactiveUS20180153848A1Excellent long-term storage stabilityExcellent resolubilityOrganic active ingredientsPharmaceutical non-active ingredientsCabazitaxelMedicine
Provided in the present invention is a cabazitaxel fat emulsion injection, wherein the cabazitaxel fat emulsion injection contains cabazitaxel, a medium chain triglyceride for injection, and lecithin. Also provided in the present invention are the method for preparing the cabazitaxel fat emulsion injection and the use thereof in preparing a drug for treating prostate cancer.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Managing research data for clinical drug trials
InactiveUS20140142961A1High transparencyExtension of timeData processing applicationsLaboratory analysis dataClinical researchLibrary science
A machine, computer program product, and computer-implemented method for performing a process of managing clinical research data by collating a plurality of clinical patient records into a database system and a process of allowing particular clinical users to access the clinical research records.
Owner:SOUTH TEXAS ACCELERATED RES THERAPEUTICS
Preparation method and application of antiviral extract of yin-nourishing Yinqiao (honeysuckle flower and fructus forsythiae) decoction prescription
InactiveCN106975021AThe method is simpleGood reproducibilityAntipyreticAnalgesicsChemistryHoneysuckle
The invention provides a preparation method and an application of an antiviral traditional Chinese medicine extract of a Yinqiao (honeysuckle flower and fructus forsythiae) decoction prescription. According to the invention, five preparation methods are provided, namely 1) a water extraction method, 2) an alcohol extraction method, 3) a water extraction and alcohol precipitation method, 4) a method combining water extraction and macroporous resin purification, and 5) a method combining alcohol extraction and macroporous resin purification. The invention is derived from a classic ancient prescription, namely 'Yinqiao Decoction' prescription prescribed within Chapter Middle-jiao of No.2 Volume in Differentiation on Febrile Diseases of WU Jutong who is a great master of febrile diseases in Qing dynasty; on the basis of the prescription, process optimization is conducted on the extract, various traditional Chinese medicinal preparations are prepared and major ingredients of active parts are defined. The traditional Chinese medicine extract of the Yinqiao decoction provided by the invention is in the form of an oral preparation. The prepared product (the traditional Chinese medicine extract) is detected, and a conclusion that the traditional Chinese medicine extract of the Yinqiao decoction is high in content of active ingredients is obtained.
Owner:江苏海王健康生物科技有限公司
Injection of ambroxol hydrochloride and preparation method thereof
InactiveCN102988281AReduce dosageLow costOrganic active ingredientsPharmaceutical delivery mechanismDrugs solutionPolyethylene glycol
The invention provides an injection of ambroxol hydrochloride and a preparation method thereof; the injection comprises 15 parts by weight of ambroxol hydrochloride, 0.01-0.03 part by weight of citric acid, 2-20 parts by weight of polyethylene glycol 400, 16-18 parts by weight of sodium chloride, and 2000 parts by weight of injection water, and preferably comprises 15 parts by weight of ambroxol hydrochloride, 0.02 part by weight of citric acid, 5 parts by weight of polyethylene glycol 400, 17 parts by weight of sodium chloride, and 2000 parts by weight of injection water. The injection of ambroxol hydrochloride provided by the invention can tolerate hot pressurized sterilization at 121 DEG C for 15 min, is compatible with drug solutions with a pH of more than 10, has good stability, and can better guarantee medication safety for human body.
Owner:辽宁科泰生物基因制药股份有限公司
Preparation method and application of Chinese medicine extract for 'prescription for treating diarrhoea with abdominal pain'
ActiveCN101474275AThe method is simpleGood reproducibilityDigestive systemPlant ingredientsSolventAlcohol
The invention discloses a preparation method for a Chinese herbal extract 'prescription for treating diarrhoea with abdominal pain', comprising the following steps: (1) fried rhizoma atractylodis macrocephalae, white paeony root, fried dried orange peel and divaricate saposhnikovia root are taken as raw materials, alcohol solution or water is taken as solvent to extract the raw materials to obtain the extracting solution; the weight ratio of the raw materials is as follows: fried rhizoma atractylodis macrocephalae: white paeony root: fried dried orange peel: divaricate saposhnikovia root is equal to 1:1:0.75:0.5; (2) the obtained extracting solution is concentrated until the volume of the extracting solution is 6-9 times of the weight of the raw materials, namely, 1kg of the raw materials corresponds to 6-9L of extracting solution; (3) the concentrated solution obtained in step (2) is diluted by water with 3-5 times of volume and then the supernatant is extracted; (4) the supernatant is absorbed by macroporous resin column, the eluted part of the alcohol is collected to obtain the alcohol eluent; (5) the obtained alcohol eluent is concentrated and dried to prepare the Chinese herbal extract. The invention also relates to the application of the Chinese herbal extract 'prescription for treating diarrhoea with abdominal pain' prepared by the method in preparing the medicines for curing irritable bowel syndrome of intestinal canal.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Water snowflake extract, preparation and use thereof
An extract of honeysuckle flower is prepared from the honeysuckle flower through distilling twice, collecting the distilled liquid, filtering to obtain filtrate, decocting the dregs, filtering to obtain filtrate, regulating pH value, purifying by macroreticular resin column, collecting the liquid, regulating pH value, purifying by macroreticular resin column, eluting with the water for injection, rejecting the eluting liquid, eluting with alcohol, collecting the eluting liquid removing alcohol to obtain extract, and storing the distiolled liquid and extract for furture use. Its preparations prepared from said extract and their usages are also disclosed.
Owner:JIANGSU KANION PHARMA CO LTD
Percutaneously absorbable preparation containing fentanyl and homologue thereof
InactiveUS20140243765A1Improve penetration efficiencyReduce drug doseBiocideAdhesive dressingsAdhesiveFentanyl
Disclosed is a transdermal preparation, comprising sequentially-stacked layers of a backing layer, a barrier layer, a drug adhesive layer and a release layer, wherein the drug adhesive layer contains a drug selected from the group consisting of fentanyl, an analogue thereof and a pharmaceutically-acceptable salt thereof, a skin permeation enhancer of the drug, and a polyacrylate adhesive, which shows a high skin permeation with a low drug dosage, equivalent to one with high drug dosage, by increasing the skin permeation rate of drug.
Owner:SAMYANG BIOPHARMLS CORP
Manchurian wildginger total polysaccharide extract with cough-relieving activity, and extraction method and application thereof
InactiveCN104027345ASignificant antitussive effectReduce cough sensitivityOrganic active ingredientsRespiratory disorderSolventIncubation period
The invention discloses a Manchurian wildginger total polysaccharide extract with cough-relieving activity and a preparation method and application thereof, belonging to the technical field of medicines. The Manchurian wildginger total polysaccharide extract is prepared by subjecting the root and / or tuber of Manchurian wildginger to extraction with a polar solvent, carrying out ethanol precipitation and then carrying out drying under reduced pressure, wherein the yield and the content of the total polysaccharide are 15 wt% and 60 to 80 wt%, respectively. Through a great number of pharmacodynamic tests, it is proved for the first time that the Manchurian wildginger total polysaccharide extract has a substantial cough inhibiting effect which is close to a treatment effect of codeine and shows a better effect on prolonging the incubation period of cough compared with codeine, and it is found out for the first time that the Manchurian wildginger total polysaccharide extract is capable of reducing sensitivity of cough and inhibiting airway inflammations, has good prevention and treatment effects on a variety of cough and can be used for preparation of drugs used for treating diseases related to cough. The Manchurian wildginger total polysaccharide extract has the advantages of definite activity, usage of original plants as experimental materials and low cost.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +1
Drug-loaded mixed micelle
InactiveCN102961322AImprove solubilityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical delivery mechanismPolyoxyethylene castor oilPolyester
The invention discloses a drug-loaded mixed micelle comprising taxane medicaments, amphiphilic chitosan derivatives and polyethylene glycol polyester block copolymer. The invention further discloses a preparation method of the drug-loaded mixed micelle. The product does not contain polyoxyethylene castor oil and ethanol, reduces adverse drug reactions, increases security of drug clinic application; by adding the amphiphilic chitosan derivatives and the polyethylene glycol polyester block copolymer, the stability of the preparation is enhanced and drug loading and drug efficacy are increased; and the preparation process is simple and controllable, and the production can be expanded easily.
Owner:HANGZHOU PUSH KANG BIOTECH CO LTD
Preparation method of traditional Chinese medicine extract of decoction capable of invigorating spleen yang and tonifying stomach and application thereof
PendingCN108743892AGuaranteed superiorityEfficient enrichmentDispersion deliveryDigestive systemActive componentTraditional medicine
The invention provides a preparation method of a decoction capable of invigorating spleen yang and tonifying stomach and application thereof. The decoction is derived from the decoctions capable of invigorating spleen yang and tonifying stomach in Treatise on Spleen and Stomach which is written by Li Dongyuan in Jin Dynasty and is abbreviated as Decoctions capable of Invigorating Spleen Yang and Tonifying Stomach in full texts of patents. The invention provides the following six preparation methods: (1) a water extraction method; (2) an alcohol extraction method; (3) a water extraction and alcohol precipitation method; (4) a method with combination of water extraction and macroporous resin purification; (5) a method with combination of alcohol extraction and macroporous resin purification;and (6) a classic decoction preparation method based on substances of classical famous prescriptions. The process for preparing the extract in the decoction can be optimized, so that the decoction can be prepared into various traditional Chinese medicine preparations; main components of the active component are defined. The invention provides powder of the decoction capable of invigorating spleenyang and tonifying stomach and various traditional Chinese medicine extract oral preparations; the prepared product is detected; the basis of the active substance of the traditional Chinese medicineextract of the decoction capable of invigorating spleen yang and tonifying stomach is clear and definite; the active substance is composed of a plurality of effective components according to a certainratio; and the content of the active substance is higher.
Owner:湖北颐仁中医药研究总院有限公司
Hydroxypropyl-beta-cyclodextrin inclusion of strychnine and preparation method thereof
InactiveCN102008476AImprove securityGood water solubilityOrganic active ingredientsAntipyreticWater solubleBioavailability
The invention discloses a hydroxypropyl-beta-cyclodextrin inclusion of strychnine and a preparation method thereof. The inclusion is prepared from the strychnine and hydroxypropyl-beta-cyclodextrin in weight ratio of 1: (1-100) by using the preferential inclusion process. Experimental results show that the hydroxypropyl-beta-cyclodextrin inclusion of the strychnine has the advantages of high inclusion rate, great drug loading and good stability, and can significantly improve the water solubility of the strychnine, lead the solubility in water to be more than 20 times that of the strychnine, significantly improve the bioavailability after administration of the strychnine, play a role in reducing the toxicity of the strychnine and lead the clinical application to be safer.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Sustained-release drug delivery system
ActiveCN113018248AGood biocompatibilityImprove formulation propertiesAerosol deliveryOintment deliveryDrug deliveryPharmaceutical medicine
The invention relates to a pharmaceutical composition, which comprises liquid oil, at least one compound as shown in the following formula I and at least one pharmaceutical active component. The pharmaceutical composition can be used for a sustained release preparation system, so that the medicine is in a semi-solid or solution form at normal temperature, the semi-solid preparation can be directly used as a medicine storage bank at the administration part, the liquid preparation is subjected to phase change to form the medicine storage bank after the administration part is in contact with body fluid, and meanwhile, the medication safety and tolerance are improved.
Owner:NANJING DELOVA BIOTECH CO LTD
Chinese medicinal composition for treating mental diseases, preparation method, application and quality control thereof
InactiveCN101590212AFacilitate removal of export barriersLow costHeavy metal active ingredientsNervous disorderDiseaseCoptis
The invention provides a Chinese medicinal composition for treating mental diseases, which is prepared from the following raw material drugs in proportion by weight: bezoar, amber, dandelion, pearl, pig bile, radix isatidis, forsythia fruit, borneol, honeysuckle, liquorice root, coptis root, abalone shell, radix trichosanthis, curcuma, rehmanniae, ochre, scutellaria, gypsum, uncaria stem, rhubarb, magnet, figwort, gardenia, radix puerariae and dwarf lilyturf root. Moreover, the invention also discloses a preparation method, application and a quality control method for the Chinese medicinal composition.
Owner:大连美罗中药厂有限公司
Traditional Chinese medicine composition for treating mental diseases, and preparation method, application and quality control thereof
InactiveCN102813873AFacilitate removal of export barriersLow costHeavy metal active ingredientsNervous disorderCoptisForsythia
The invention provides a traditional Chinese medicine composition for treating mental diseases. The composition is prepared from the following bulk drugs by weight: bezoar, amber, dandelion, pearl, porcine bile paste, isatis root, weeping forsythia capsule, borneol, honeysuckle flower, licorice, coptis, abalone shell, trichosanthes root, turmeric root tuber, rehmannia root, ochre, root of large-flowered skullcap, gypsum, gambir plant nod, rhubarb root and rhizome, magnetite, figwort root, cape jasmine fruit, root of kudzu vine and dwarf lilyturf root. Furthermore, the invention also discloses a preparation method, application and a quality control method for the traditional Chinese medicine composition.
Owner:大连美罗中药厂有限公司
Venenum bufonis extract with anti-infection action and preparation method thereof
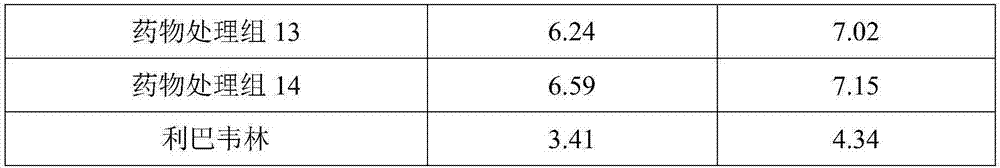
ActiveCN107397748AQuality is easy to controlHigh drug safetyAmphibian material medical ingredientsAntibacterial agentsArenobufaginMedicine
The invention relates to the technical field of traditional Chinese medicines and in particular discloses venenum bufonis extract with anti-infection action and a preparation method thereof. The venenum bufonis extract contains a phrynin effective component which contains the following components in parts by weight: 30-60 parts of arenobufagin, 35-60 parts of cinobufagin, 10-25 parts of telocinobufagin, 20-45 parts of bufotaline, 20-50 parts of cinobufotalin, 25-45 parts of bufalin, 15-40 parts of resibufogenin and 5-15 parts of hellebrigenin. The venenum bufonis extract provided by the invention is remarkable in anti-infection effect. In addition, the types and content of effective components of the extract are clear, the cytotoxicity is small, and quality control and medicinal safety of the extract are facilitated.
Owner:广州京泽健康科技有限公司
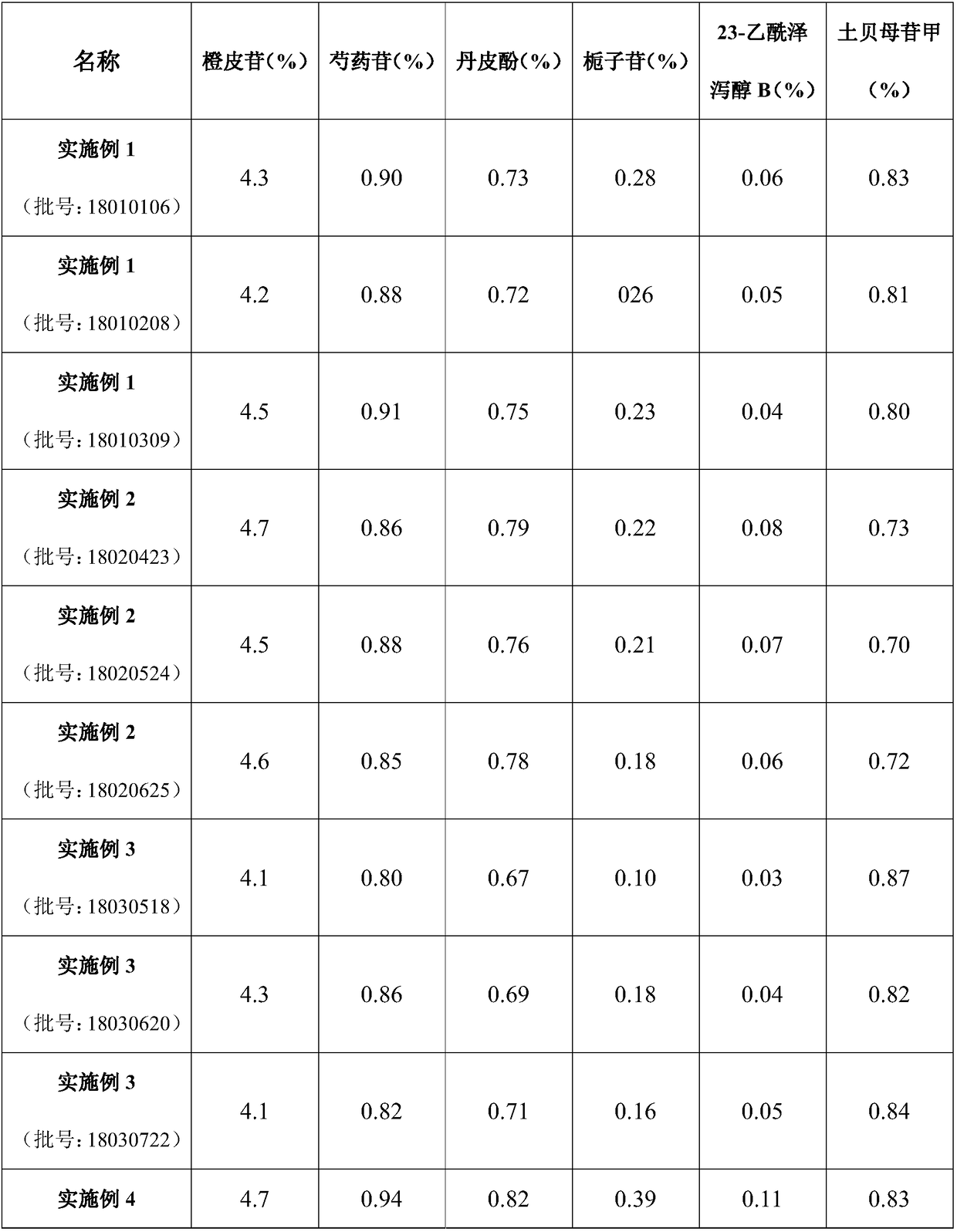
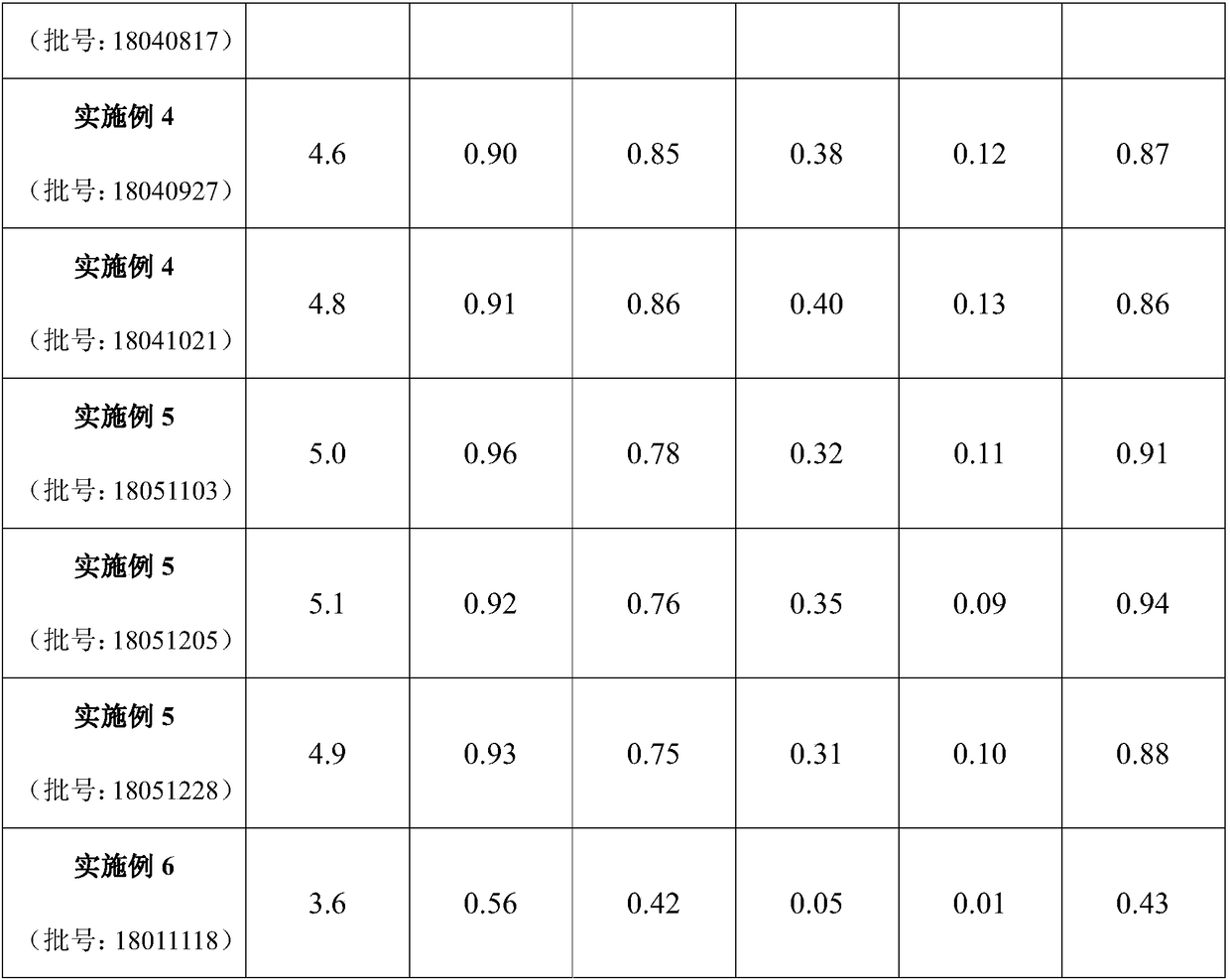
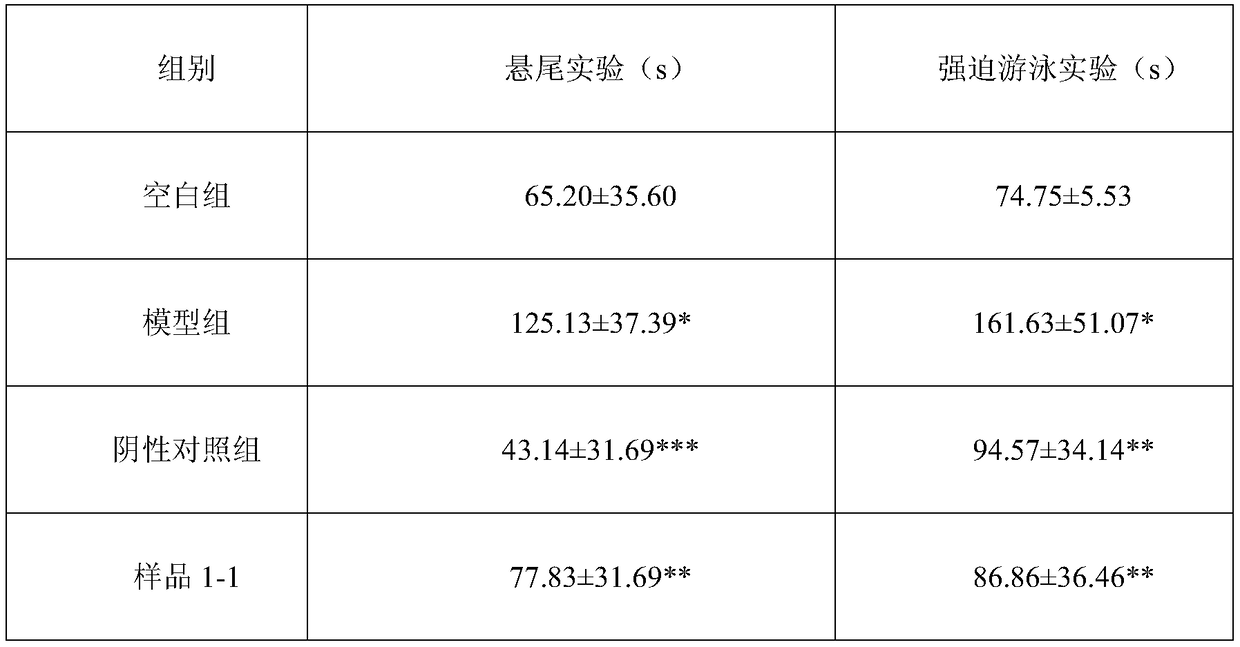
Traditional Chinese medicinal extract for treating stomachache, hypochondriac pain, gastroenteric ulcer and depression, as well as preparation methodS and application thereof
InactiveCN108888686ASimple Addition of Drug EffectsPrescription proportion is clear and clearNervous disorderDigestive systemPurification methodsAlcohol
The invention provides a traditional Chinese medicinal extract for treating stomachache, hypochondriac pain, gastroenteric ulcer and depression, as well as six preparation methods and application thereof. The traditional Chinese medicinal extract is prepared from the following raw materials: pericarpium citri reticulatae viride, pericarpium citri reticulatae, peony, moutan bark, cape jasmine (roasted), rhizoma alismatis and rhizoma bolbostemmae in a weight ratio of 4:4:4:3:3:3:6. The six preparation methods include (1) water extraction method; (2) alcohol extraction method; (3) water extraction and alcohol precipitation method; (4) water extraction combined macroporous resin purification method; (5) alcohol extraction combined macroporous resin purification method; and (6) classic decoction preparation method (classic prescribed substance standard). According to the preparation methods, the extract process is optimized to prepare traditional Chinese medicinal preparations, and the mainingredient of active parts can be defined. The traditional Chinese medicinal extract oral preparation, namely the formula of liver-depressed and gastropyretic epigastralgia, is provided, the preparedproduct is detected to reflect clear and definite active substance foundation and relatively high content of the traditional Chinese medicinal extract, namely the formula of liver-depressed and gastropyretic epigastralgia.
Owner:湖北颐仁中医药研究总院有限公司
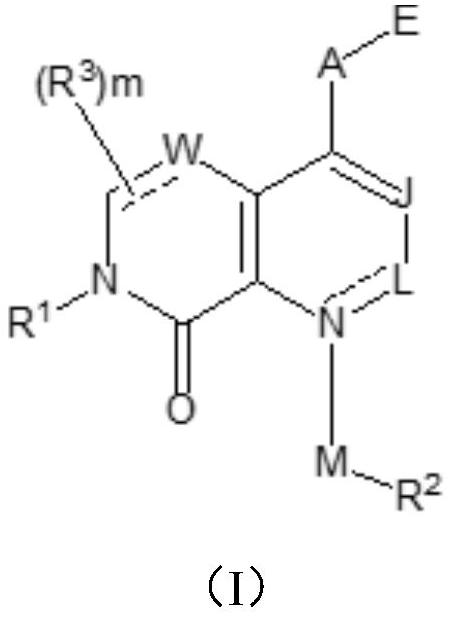
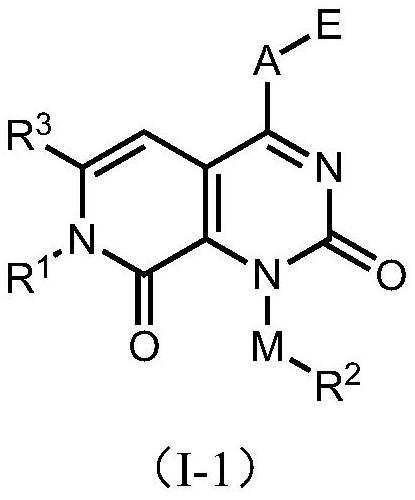
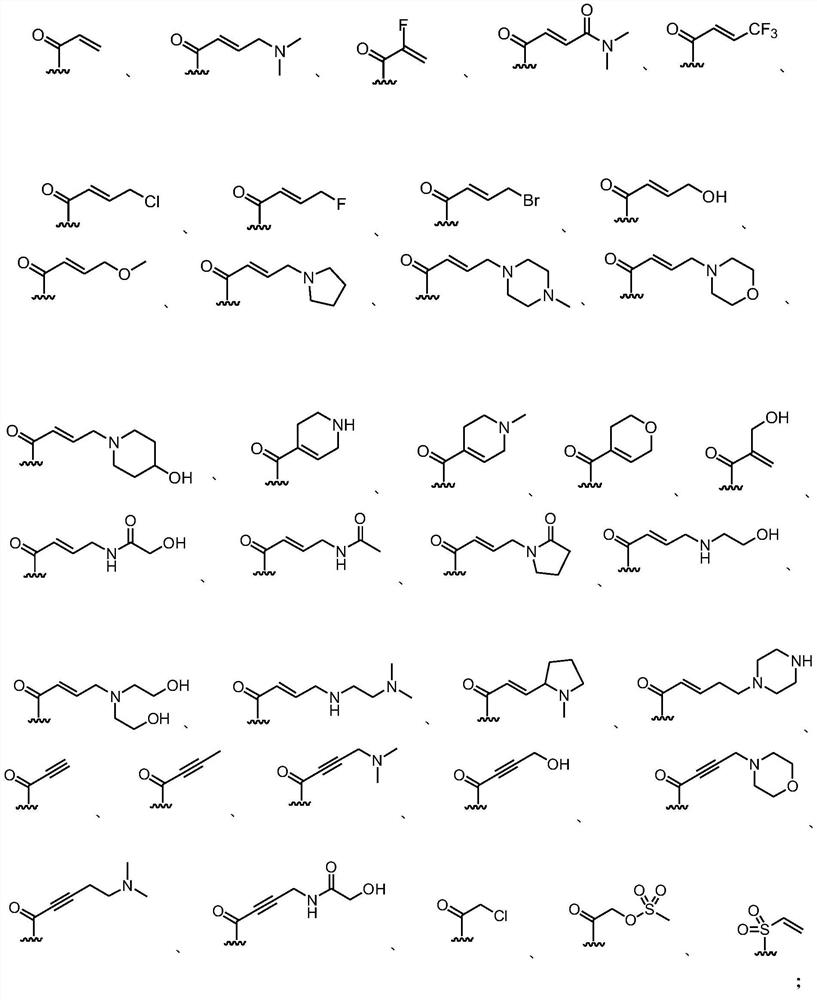
KRAS G12C mutant protein inhibitor, pharmaceutical composition comprising same, preparation method and application
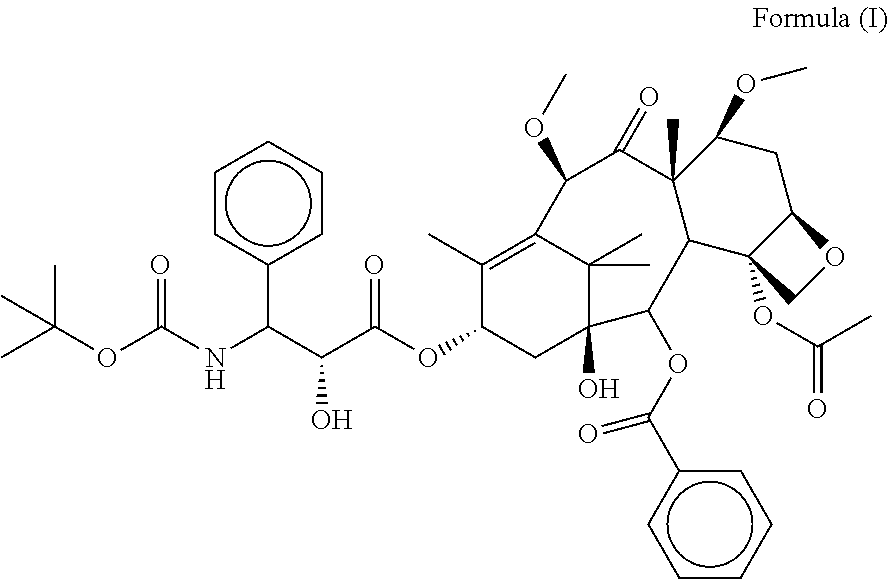
ActiveCN113527293AImprove mechanical propertiesImprove stabilityOrganic active ingredientsOrganic chemistryMutated proteinPharmaceutical drug
The invention provides a compound with irreversible inhibitor activity of G12C mutant KRAS protein, and a racemate, a stereoisomer, a pharmaceutically acceptable salt, a polymorphic substance or a solvate of the compound. The structure of the compound is shown as a formula (I) in the specification. Also provided are methods related to the preparation and use of the compounds, a pharmaceutical composition comprising the compounds, and a method of modulating G12C mutant KRAS protein activity for the treatment of conditions such as cancer.
Owner:SUZHOU INTRAGRAND PHARMA CO LTD
A method and a system for storing drugs in distribution packages, and a storage for drug distribution packages
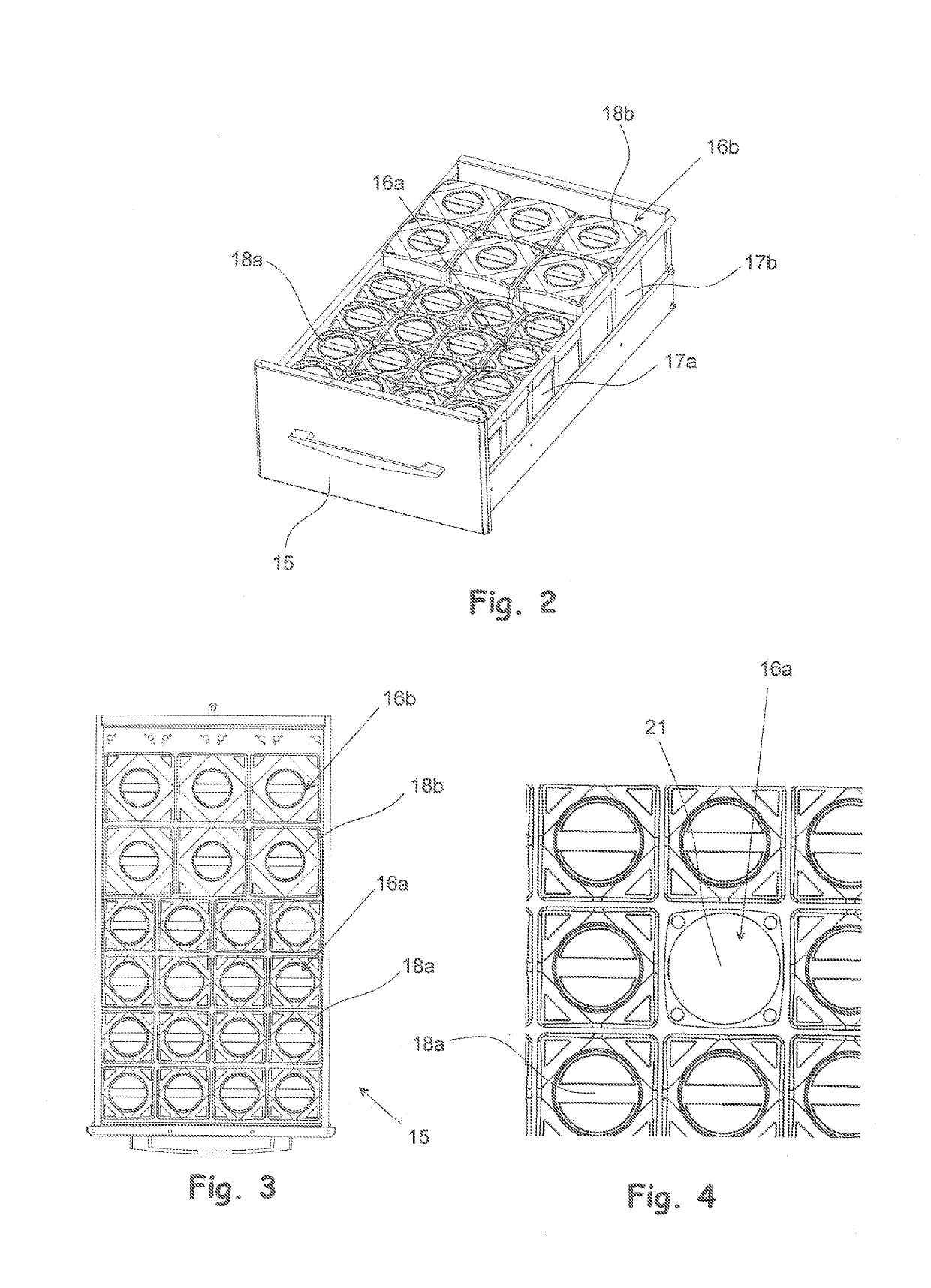
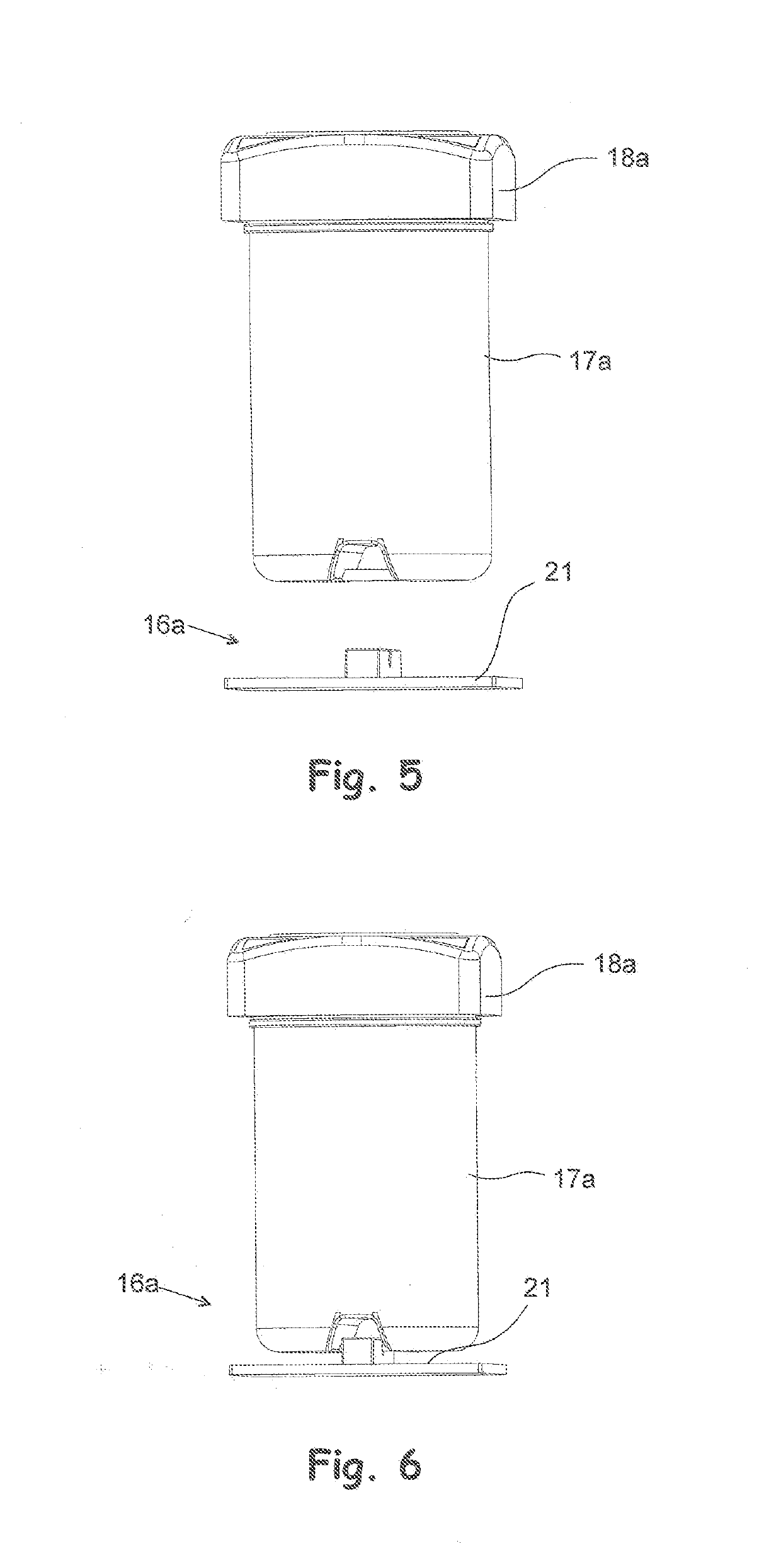
InactiveUS20190214123A1Avoid placingHigh drug safetyShow cabinetsDrug and medicationsData systemComputer science
A system for storing drugs in distribution packages of a storage for drug distribution packages includes a storage for drug distribution packages, including storage locations, where the distribution packages are placed; a data processing device with a memory for storing the number of distribution packages and / or drugs in the storage for drug distribution packages, the expiry date of the drugs, and at least one item of drug information and the personal data of a user of the storage for drug distribution packages; a stock data system stored in the memory of the data processing device configured to control the functions of the storage for drug distribution packages and to record stock data entries in the storage for drug distribution packages and to administer user rights of the stock data system; at least one detecting sensor placed in storage locations coupled to the data processing device.
Owner:NEWICON
Preparation method of traditional Chinese medicine extract of decoction capable of clearing lung heat and application thereof
InactiveCN108743893AGuaranteed superiorityEfficient enrichmentRespiratory disorderPlant ingredientsAlcoholActive component
The invention provides a preparation method of a decoction capable of clearing lung heat and application thereof. The decoction is derived from classical ancient prescriptions in Re-revised Yan's Health Preservation Prescriptions which is abbreviated as Decoctions capable of Clearing Lung Heat in full texts of patents. The invention provides the following six preparation methods: (1) a water extraction method; (2) an alcohol extraction method; (3) a water extraction and alcohol precipitation method; (4) a method with combination of water extraction and macroporous resin purification; (5) a method with combination of alcohol extraction and macroporous resin purification; and (6) a classical decoction preparation method based on substances of classical ancient prescriptions. The process forpreparing the extract in the decoction can be optimized, so that the decoction can be prepared into various traditional Chinese medicine preparations; main components of the active component are defined. The invention provides the extract of the decoction capable of clearing lung heat and various traditional Chinese medicine extract oral preparations; the prepared product is detected; the basis ofthe active substance of the the traditional Chinese medicine extract of the decoction capable of clearing lung heat is clear and definite; the active substance is composed of a plurality of effectivecomponents according to a certain ratio; and the content of the active substance is higher.
Owner:湖北颐仁中医药研究总院有限公司
External preparation containing phentolamine and preparation method thereof
InactiveCN101361725AGuaranteed validityImprove transdermal drug absorptionOrganic active ingredientsSexual disorderPharmacyWestern medicine
The invention belongs to the preparation field of western medicines and particularly relates to a high-efficiency and quick-acting phentolamine external preparation which takes natural plant naphtha and the extraction elaboration product thereof as a transdermal sorbefacient and a preparation method thereof. The invention selects the transdermal sorbefacient of natural volatile oils, takes phentolamine as the main active substance which plays a role in treatment in the preparation and is added with the accessories necessary in pharmacy, so as to prepare the phentolamine external transdermal preparation. Proved by experimentation on animals, the preparation can largely reduce the medication dosage and lower the systemic side effect and use cost when simultaneously maintaining the effectiveness of drugs. Compared with the prior art, the preparation can significantly improve the transdermal drug absorption amount of phentolamine, and shorten latent period of onset, has good medication safety and can offer patients more satisfactory curative effect.
Owner:FUDAN UNIV
Phenyl triazole MLL1-WDR5 protein-protein interaction inhibitor
PendingCN108715585AStrong interaction inhibitory activityReduce catalytic activityOrganic active ingredientsOrganic chemistryMedicinal chemistryIsrapafant
The invention relates to the field of medicinal chemistry, in particularly to a phenyl triazole MLL1-WDR5 protein-protein interaction inhibitor (I) and a preparation method thereof. Pharmacodynamic tests prove that a compound provided by the invention has strong MLL1-WDR5 protein-protein interaction inhibiting activity.
Owner:CHINA PHARM UNIV
Dexlansoprazole crystal form and preparation method
InactiveCN106866631AThe solvent is less toxicHigh drug safetyOrganic chemistry methodsIsopropyl etherDexlansoprazole
The invention belongs to the technical field of medicines and in particular relates to a dexlansoprazole crystal form and a preparation method. A dexlansoprazole crystal with good heat stability is obtained through a method comprising the steps of culturing a crystal nucleus and slowly crystallizing. The preparation method comprises the following steps: dissolving an anhydrous dexlansoprazole crude product into acetone and de-coloring with active carbon; dropwise adding one part of isopropyl ether at 20 DEG C to 25 DEG C; stirring and culturing a crystal; culturing the crystal nucleus; then slowly dropwise adding the other part of isopropyl ether; filtering and drying to obtain a product. The product prepared by the method provided by the invention is good in heat stability and slow in degradation speed; the problems of degradation, color change and the like during a storage process of the dexlansoprazole are effectively solved; the solubility and dissolvability of the product prepared by the method are also extremely improved and the bioavailability is improved.
Owner:SHANDONG YUXIN PHARMA CO LTD
Thermosensitive in-situ gel preparation for norcantharidin injection and preparation method and application of thermosensitive in-situ gel preparation
ActiveCN105902485APromote formationEasy to administerAerosol deliveryOintment deliveryGel preparationPolyethylene glycol
The invention discloses a thermosensitive in-situ gel preparation for norcantharidin injection and a preparation method and an application of the thermosensitive in-situ gel preparation. The thermosensitive in-situ gel preparation is prepared from a solvent and solute, wherein the solute is prepared from the following components by weight-to-volume ratio: 0.0025g / mL of norcantharidin, 0.19g / mL of poloxamer 407, 0.004g / mL of poloxamer 188, 0.0057g / mL of polypropylene, 0.0019g / mL of polyethylene glycol, 0.0008g / mL of hydroxypropyl methyl cellulose and 0.0002g / mL of chitosan. The gel temperature of the in-situ gel preparation is 34 DEG C, is close to the body temperature of a human body, and accords with the requirements of in vivo gel for injection; gel corrosion and drug release are carried out at a similar velocity; the drug is controlled to be slowly released by gel corrosion; the thermosensitive in-situ gel preparation has good drug slow-release and sustained-release effects; and the medication safety is good.
Owner:FIRST PEOPLES HOSPITAL OF YUHANG DISTRICT HANGZHOU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com