E-configuration benzamide compounds, and pharmaceutical preparation application thereof
A technology of benzamide and compounds, which is applied in the field of chemical pharmaceuticals, can solve the problems of unobtained, undisclosed, unobtainable solid dispersion of benzamide and polyvinylpyrrolidone, etc., and achieve the effect of improving dissolution rate and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
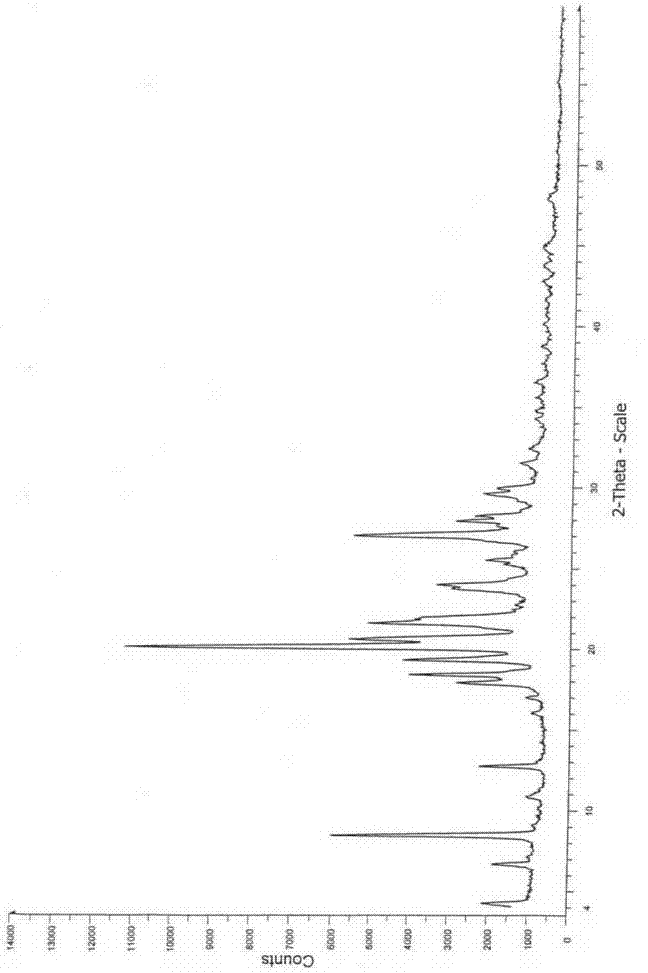
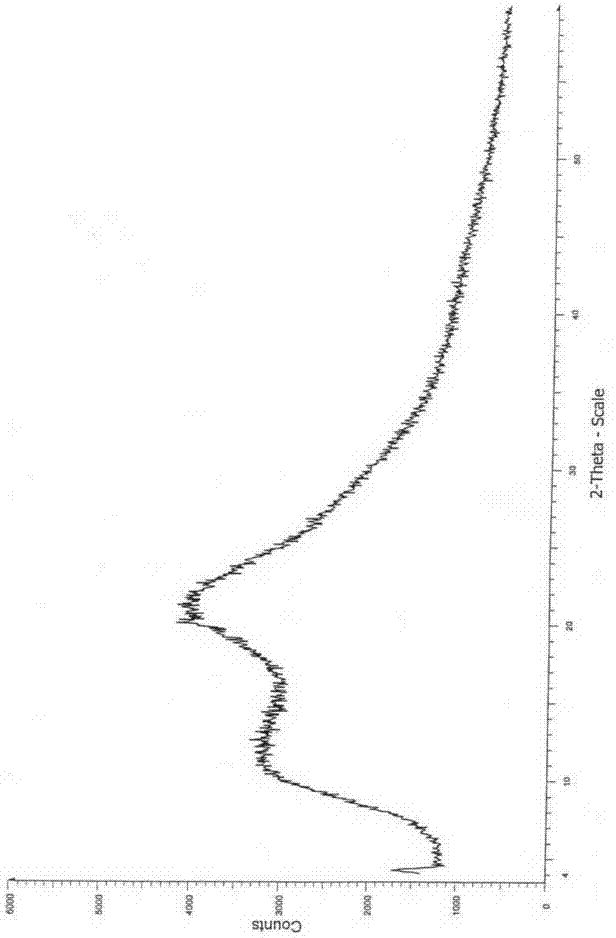
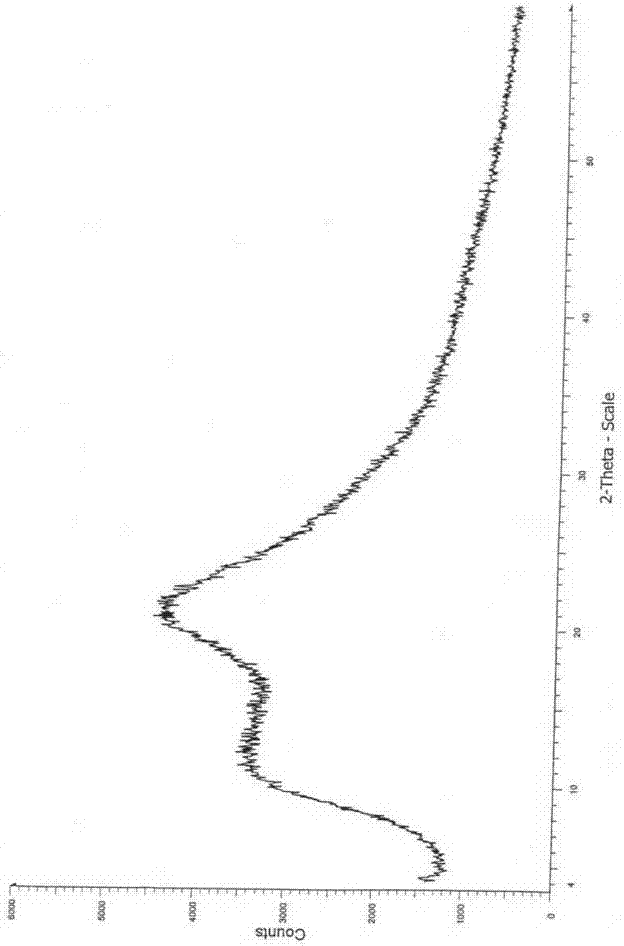
Image
Examples
Embodiment 1
[0053] Example 1: Preparation of 4-[N-[(E)-3-(3-pyridine)acryloyl]aminomethyl]benzoic acid
[0054]
[0055] Add 298g (2.00mol) trans-3-(3-pyridine)acrylic acid, 324g (2.00mol) N,N'-carbonyldiimidazole and 3000mL tetrahydrofuran into a 5-liter three-neck glass flask equipped with mechanical stirring and reflux condenser , react at about 45°C for about 3 hours to prepare (E)-3-(3-pyridine)acryloyl imidazole active intermediate solution.
[0056] Add 302g (2.00mol) p-aminomethylbenzoic acid, 80g (2.00mol) sodium hydroxide and 2000mL water into another 10-liter three-neck glass flask equipped with mechanical stirring, stir at room temperature for about 30 minutes, and then dropwise add the above ( E) -3-(3-pyridine) acryloyl imidazole active intermediate solution, react at room temperature for about 8 hours. Concentrate the reaction mixture in vacuo to remove tetrahydrofuran, add 2000 mL of saturated sodium chloride solution, neutralize with concentrated hydrochloric acid unt...
Embodiment 2
[0061] Example 2: Preparation of N-(2-amino-4-fluorophenyl)-4-[N-[(E)-3-(3-pyridine)acryloyl]aminomethyl]benzamide
[0062]
[0063] Add 282g (1.00mol) 4-[N-[(E)-3-(3-pyridine)acryloyl]aminomethyl]benzoic acid, 162g (1.00mol) N,N'-carbonyldiimidazole and 2820mL tetrahydrofuran were reacted at 45°C for about 3 hours to obtain 4-[N-[(E)-3-(3-pyridine)acryloyl]aminomethyl] Benzoyl active intermediate solution.
[0064]Add 168g (1.33mol) of 4-fluoro-o-phenylenediamine and 800mL of tetrahydrofuran into another 5-liter three-neck glass flask equipped with mechanical stirring, protect it with nitrogen, stir at room temperature for about 10 minutes, add the above 4-[N-[ (E)-3-(3-pyridine)acryloyl]aminomethyl]benzoyl active intermediate solution, react at room temperature for about 24 hours. Filter, rinse the filter cake with 400 mL of tetrahydrofuran, and dry in vacuo to obtain a crude product. Dissolve the crude product in 1200mL2mol / L hydrochloric acid, add dropwise 960mL1mol / ...
Embodiment 3
[0070] Example 3: Determination of the inhibitory activity of test compounds on different subtypes of HDAC
[0071] 1. Experimental plan
[0072] Utilizing 11 subtypes of purified HDAC (HDAC1-11) in class I, II, and IV, the sirtuin detection kit produced by BSP Bioscoence was used to measure the inhibitory activity of the test compound on different subtypes of HDAC, and calculated Its half inhibitory concentration of enzyme activity (IC 50 ).
[0073] 2. Experimental materials and reagents
[0074] (1) N-(2-amino-4-fluorophenyl)-4-[N-[(E)-3-(3-pyridine)acryloyl]aminomethyl]benzamide, according to the embodiment of the present invention 2 preparation, which was dissolved in DMSO (100mM).
[0075] (2) N-(2-amino-4-fluorophenyl)-4-[N-(3-pyridylacryloyl)aminomethyl]benzamide, prepared according to Example 2 of US7,244,751B2, dissolved in in DMSO (100 mM).
[0076] (3) Bovine serum albumin (1mg / mL)
[0077] (4) Purified HDAC1-11 subtype protein (0.4ng / μL, N-GST tagged, BSP B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com