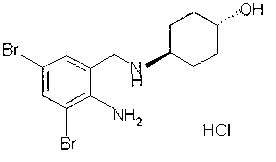
Injection of ambroxol hydrochloride and preparation method thereof
A technology of ambroxol hydrochloride and injection, which is applied in the field of high-stability ambroxol hydrochloride injection and its preparation, can solve the problems of no related reports and public use at home and abroad, affecting blood oxygen content, existing medication Potential safety hazards and other issues, to achieve good drug adaptability and safety, conducive to environmental protection, and conducive to the effect of drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
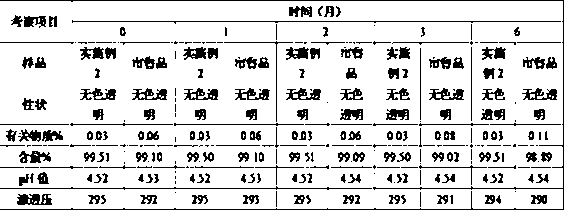
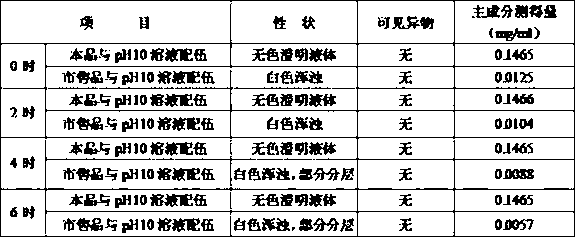
Image
Examples
Embodiment 1
[0067] Ambroxol Hydrochloride 15g
[0068] Citric acid 0.01g
[0069] Macrogol 400 2g
[0070] Sodium chloride 16g
[0071] Water for injection 2000g
[0072] -------------------------------------------------- ----
[0073] Preparation method:
[0074] 1) Take by weighing 90% of the prescription amount of water for injection, add the prescription amount of citrate, stir and dissolve;
[0075] 2) Weigh the prescribed amount of polyethylene glycol 400 and add it to the liquid medicine, and stir evenly;
[0076] 3) Weigh the prescribed amount of ambroxol hydrochloride and add it to the liquid medicine, stir to dissolve;
[0077] 4) Weigh the prescribed amount of sodium chloride, add it to the liquid medicine, and stir to dissolve;
[0078] 5) Weigh medicinal activated carbon according to the volume of 0.1% (w / v) of the liquid medicine, add it to the liquid medicine, stir for 20 minutes, filter to remove carbon, filter through a 0.45μm filter, rinse the pipeline and filter...
Embodiment 2
[0084] Ambroxol Hydrochloride 15g
[0085] Citric acid 0.02g
[0086] Macrogol 400 5g
[0087] Sodium chloride 17g
[0088] Water for injection 2000g
[0089] -------------------------------------------------- ----
[0090] Preparation method:
[0091] 1) Take by weighing 90% of the prescription amount of water for injection, add the prescription amount of citrate, stir and dissolve;
[0092] 2) Weigh the prescribed amount of polyethylene glycol 400 and add it to the liquid medicine, and stir evenly;
[0093] 3) Weigh the prescribed amount of ambroxol hydrochloride and add it to the liquid medicine, stir to dissolve;
[0094] 4) Weigh the prescribed amount of sodium chloride, add it to the liquid medicine, and stir to dissolve;
[0095] 5) Weigh medicinal activated carbon according to the volume of 0.1% (w / v) of the liquid medicine, add it to the liquid medicine, stir for 20 minutes, filter to remove carbon, filter through a 0.45μm filter, rinse the pipeline and filter...
Embodiment 3
[0101] Ambroxol Hydrochloride 15g
[0102] Citric acid 0.03g
[0103] Macrogol 400 10g
[0104] Sodium chloride 18g
[0105] Water for injection 2000g
[0106] -------------------------------------------------- ----
[0107] Preparation method:
[0108] 1) Take by weighing 90% of the prescription amount of water for injection, add the prescription amount of citrate, stir and dissolve;
[0109] 2) Weigh the prescribed amount of polyethylene glycol 400 and add it to the liquid medicine, and stir evenly;
[0110] 3) Weigh the prescribed amount of ambroxol hydrochloride and add it to the liquid medicine, stir to dissolve;
[0111] 4) Weigh the prescribed amount of sodium chloride, add it to the liquid medicine, and stir to dissolve;
[0112] 5) Weigh medicinal activated carbon according to the volume of 0.1% (w / v) of the liquid medicine, add it to the liquid medicine, stir for 20 minutes, filter to remove carbon, filter through a 0.45μm filter, rinse the pipeline and filter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com