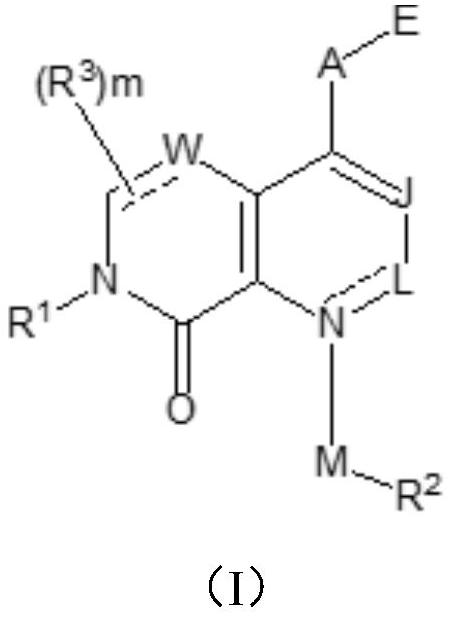
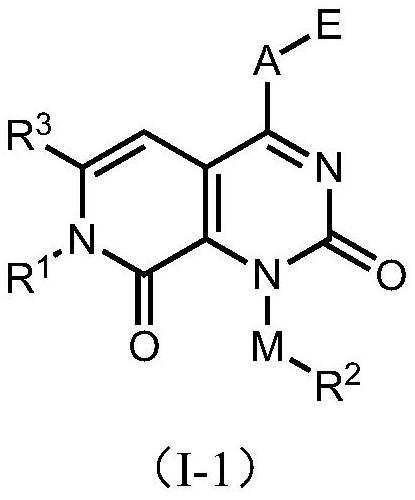
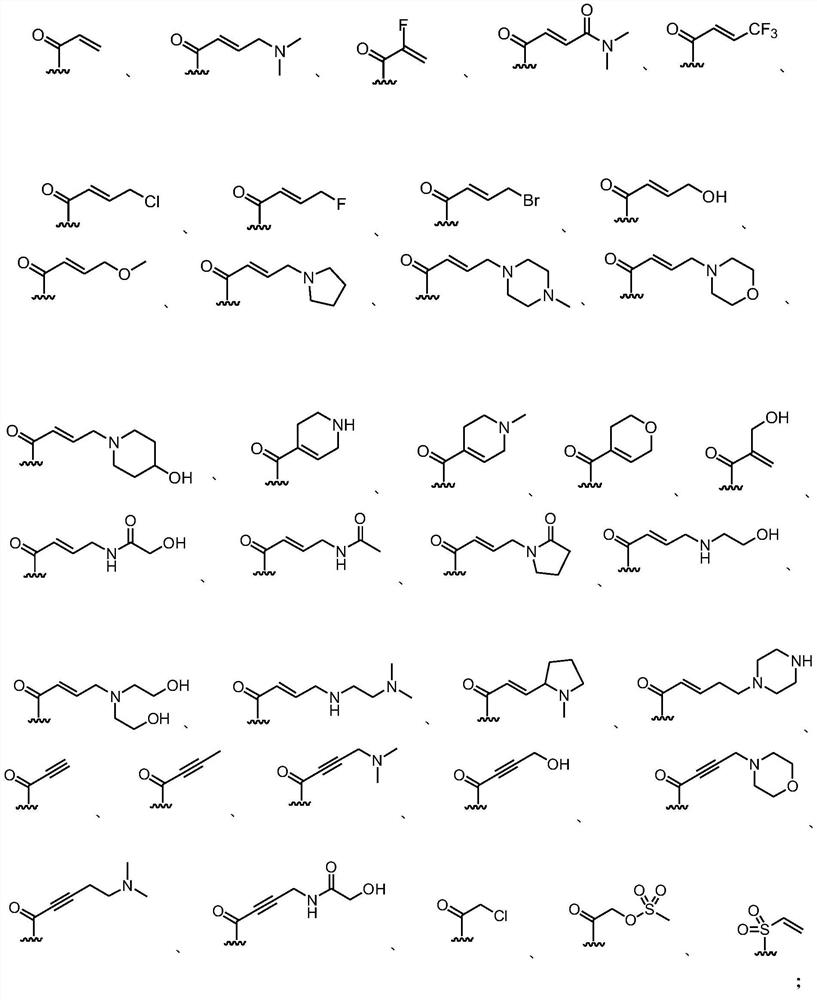
KRAS G12C mutant protein inhibitor, pharmaceutical composition comprising same, preparation method and application
A compound and pharmaceutical technology, applied in the field of preparation of KRASG12C mutant protein inhibitor and its pharmaceutical composition, can solve the problems of prolonging protein activation time and prolonging signal transduction cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1: example 1
[0191] Embodiment 1: the synthesis of example 1 compound and its intermediate
[0192] Referring to the synthesis method of WO2019141250A1, the synthetic routes of Intermediate J and Intermediate K are designed as follows:
[0193]
[0194] Synthesis of compound 1-2:
[0195] Compound 1-1 (1 g, 7.09 mmol, 1 eq) and compound 1-1A (1.26 g, 9.21 mmol, 982.46 uL, 1.3 eq) were dissolved in a solution in DCM (20 mL), TEA (1.43 g, 14.17 mmol , 1.97mL, 2eq), the mixture was stirred at 15-20°C for 2 hours. The mixture was diluted with DCM (30 mL), washed with 5% hydrochloric acid (50 mL) and saturated brine (30 mL), the organic phase was separated, washed with Na 2 SO 4 It was dried, filtered and concentrated under reduced pressure to obtain a crude product, which was purified by column chromatography (0-100% gradient elution, petroleum ether / ethyl acetate) to obtain compound 1-2 as a yellow solid (660 mg, yield 38.62%).
[0196] 1 H-NMR (400MHz, CDCl 3 )δ=8.58(s,1H),7.23-7.15...
example 1
[0238] Example 1b (1.5 mg, 13.20% yield, 98.9% purity). 1 H NMR (400MHz, CDCl 3 )δ=7.41-7.31(m,3H),7.24-7.17(m,1H),7.06(d,J=7.6Hz,1H),6.90(s,1H),6.78-6.69(m,2H),6.67 -6.57(m,1H),6.40(dd,J=1.6,16.8Hz,1H),5.85-5.78(m,1H),4.09-3.78(m,8H),3.71(s,3H),2.73-2.63 (m, 1H), 1.22 (d, J=6.8Hz, 3H), 1.11 (d, J=6.8Hz, 3H). MS: (M+H): 612.1.
[0239] The second preparation route of example 1a and example 1b is as follows:
[0240]
[0241] Synthesis of Compound 1-13:
[0242] Intermediate J (2g, 6.11mmol, 1eq), 2-isopropylphenylboronic acid (2g, 12.22mmol, 2eq), Cu 2 O (1.75g, 12.22mmol, 2eq) was dissolved in methanol (40mL), the mixture was stirred at 50°C for 2 hours under an oxygen atmosphere, concentrated under reduced pressure, diluted with water (80mL), extracted with ethyl acetate (80mL*2), and combined The organic phase was washed with saturated brine (50 mL), anhydrous Na 2 SO 4 It was dried, filtered, concentrated under reduced pressure, and purified by prep-TLC (petroleu...
Embodiment 2
[0257] Embodiment 2: the preparation of example 2 compound
[0258] Example 1a is demethylated by boron tribromide to obtain example 2 compound, MS: (M+H): 598.1
[0259]
[0260] Example 1a (120.0 mg, 182.47 μmol, 1 eq) was dissolved in DCM (3 mL), and BBr was added at 0 °C 3 (0.3mL), stirred for 1 hour, and the reaction solution was poured into iced NaHCO 3 (20mL), added water (10mL) and dichloromethane extraction (10mL*2), combined organic layer, dried over anhydrous sodium sulfate, concentrated crude product, preparative HPLC purified to obtain target product Example 2 (6.9mg, yield 6.1%) ).
[0261] 1 H NMR (400MHz, CDCl 3 )=7.39-7.31(m,2H),7.23-7.09(m,2H),7.00(d,J=6.4Hz,1H),6.91(s,1H),6.72-6.52(m,3H),6.45- 6.34(m,1H),5.85-5.78(m,1H),4.01-3.61(m,8H),2.65(q,J=6.7Hz,1H),1.21(d,J=6.8Hz,3H),1.10 (d,J=6.8Hz,3H).LCMS:(M+H) + :598.0
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com