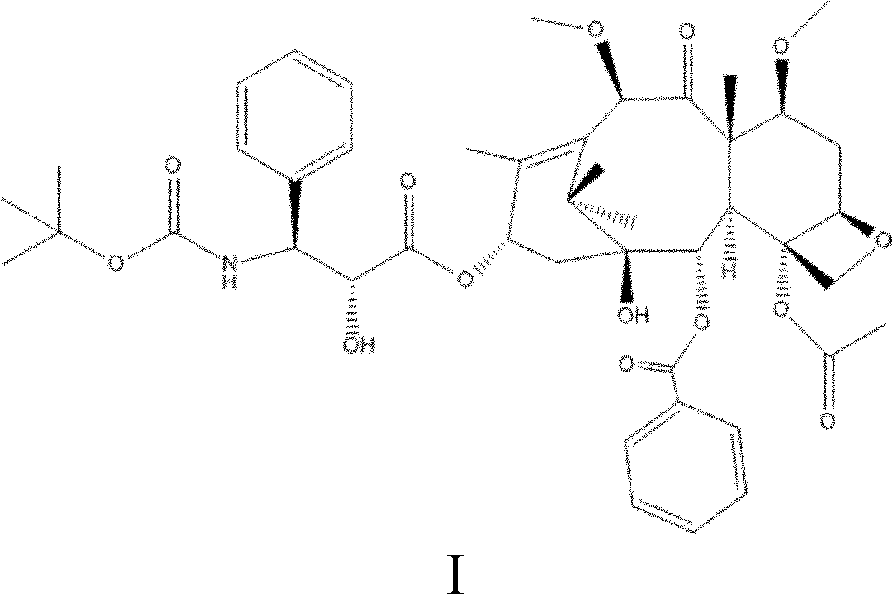
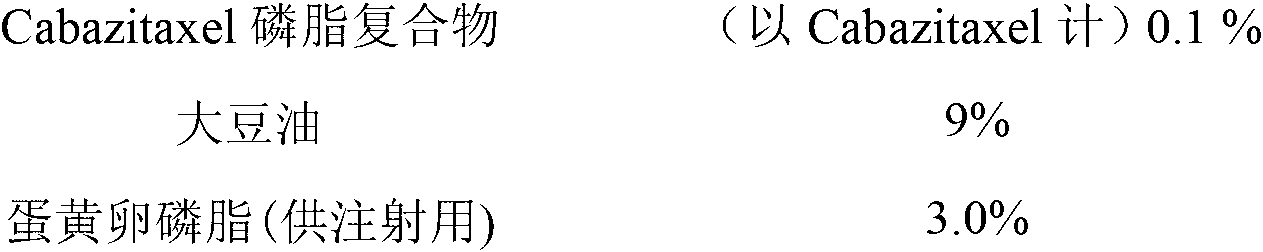Patents
Literature
182 results about "Cabazitaxel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
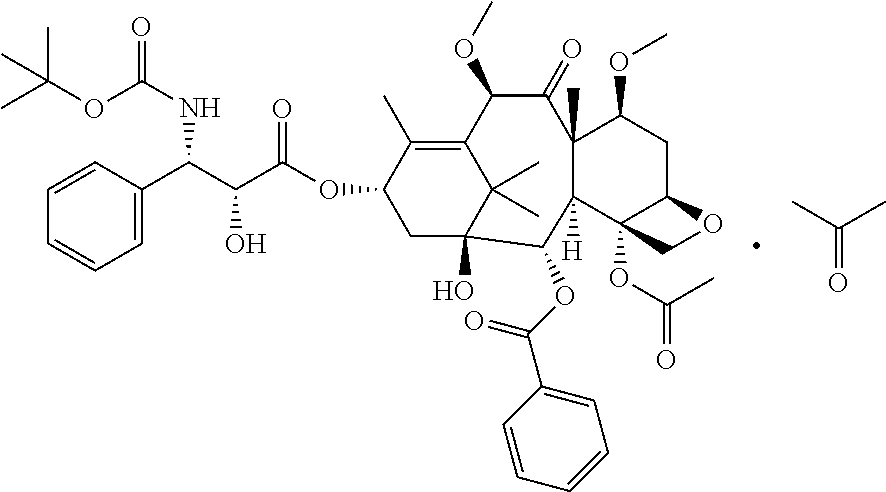
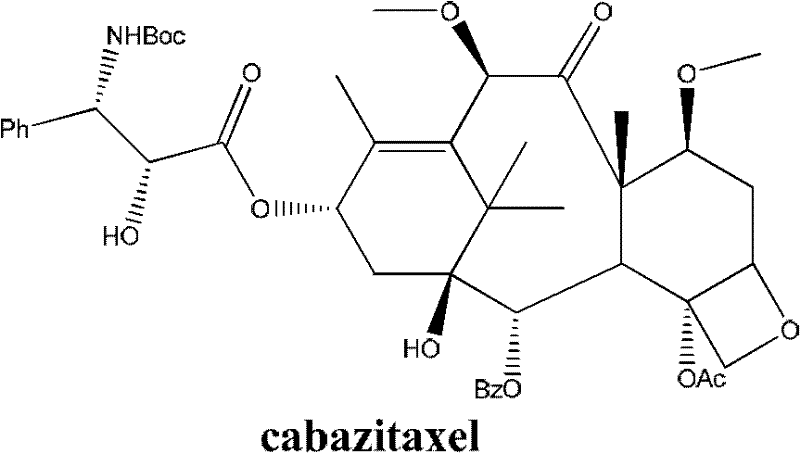
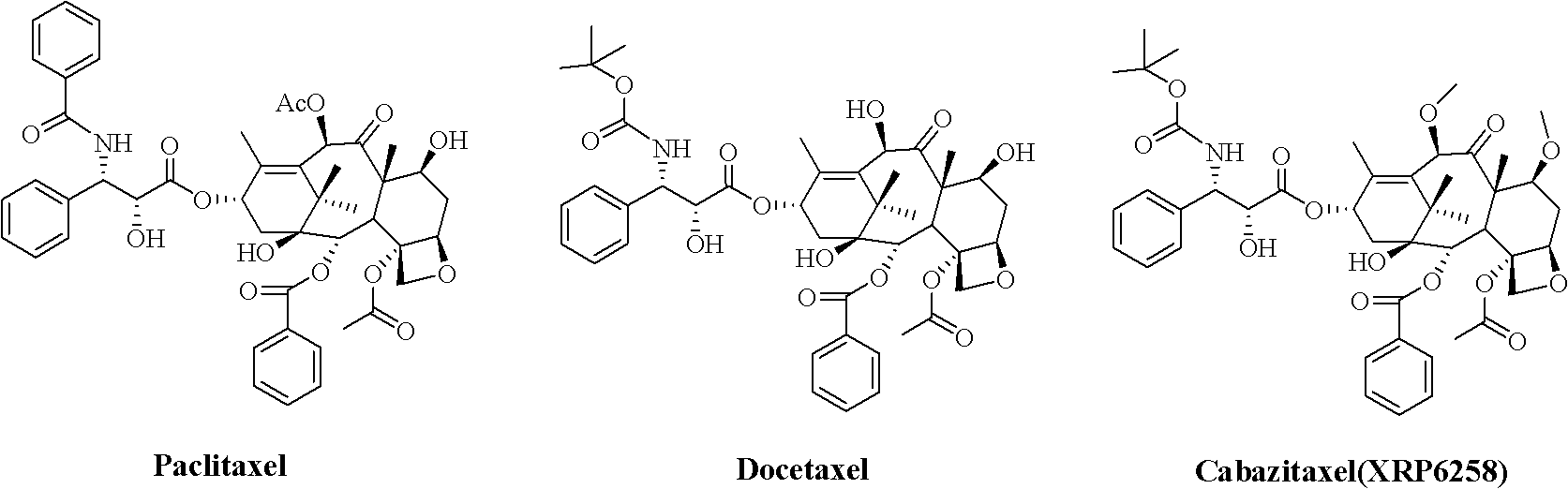
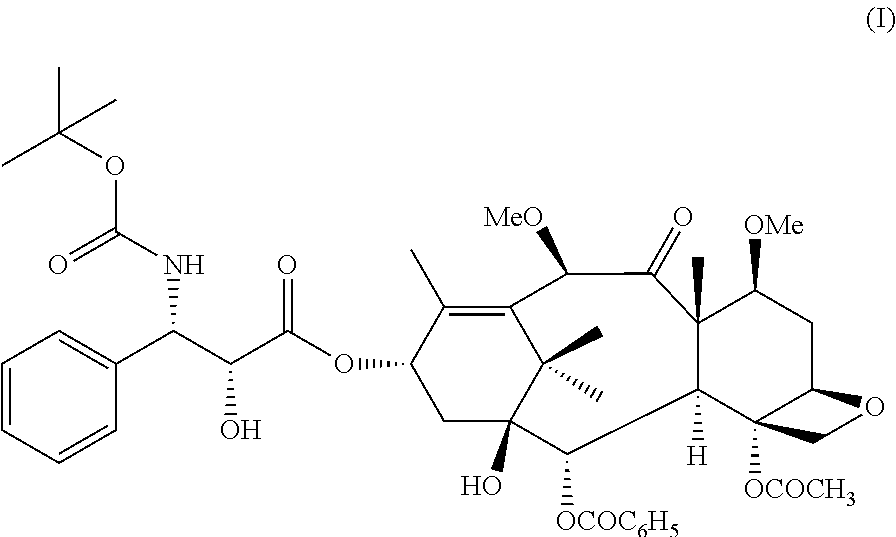
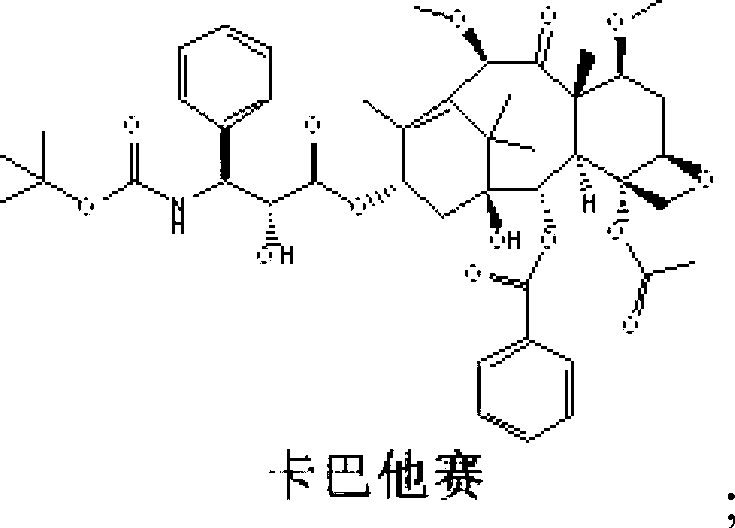
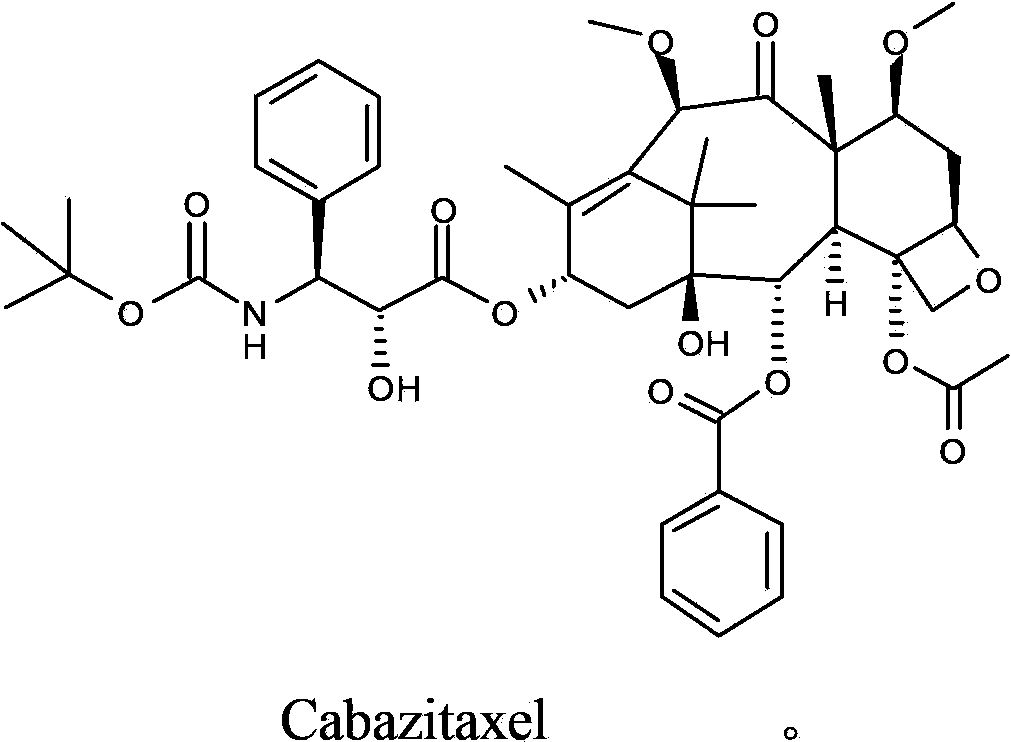
Cabazitaxel is used with another medication (prednisone) to treat prostate cancer.
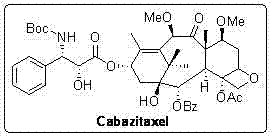
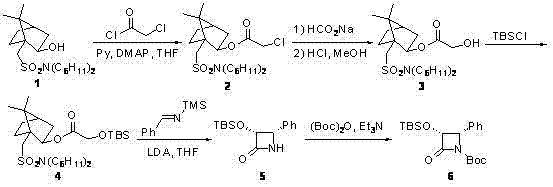
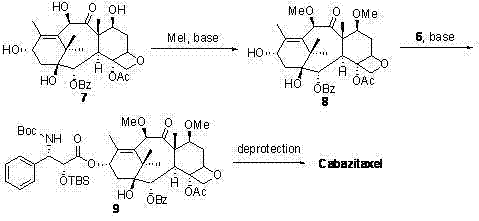
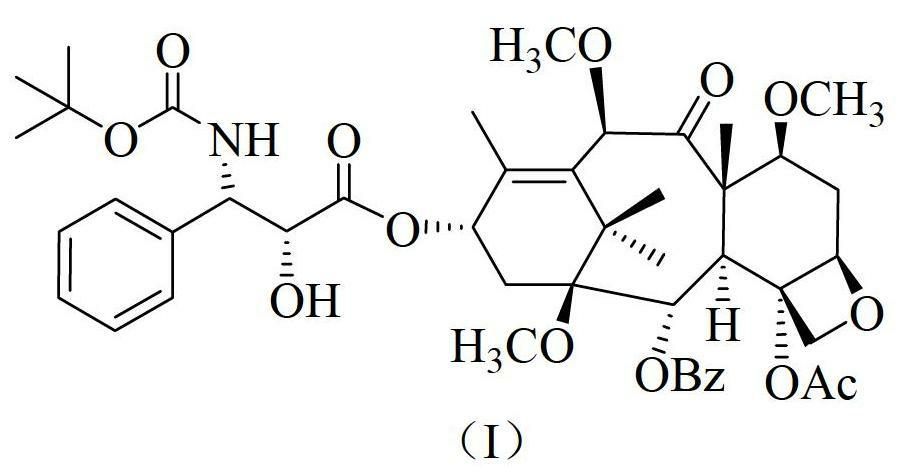
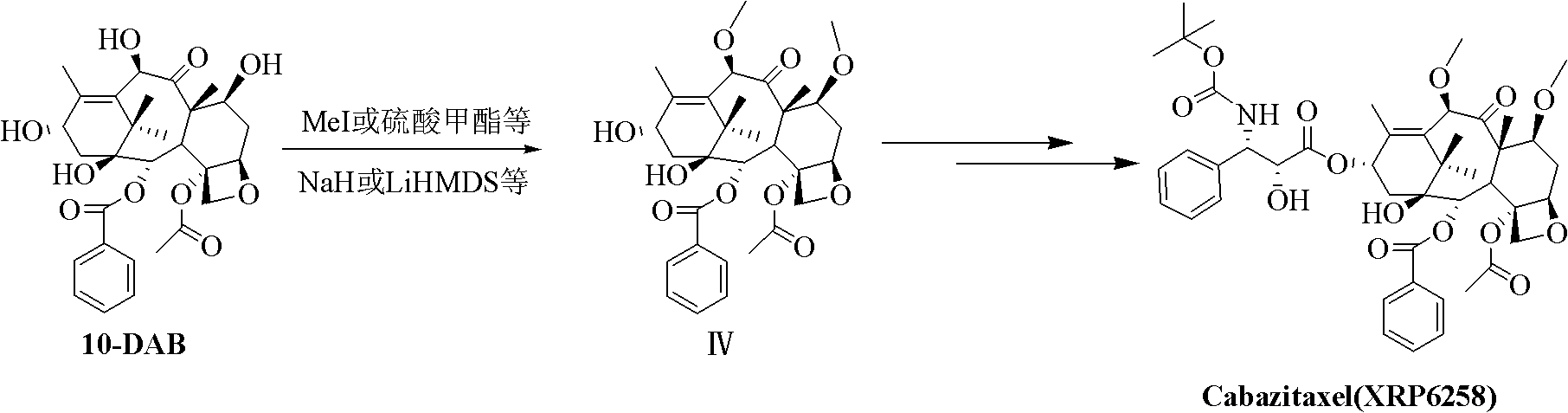
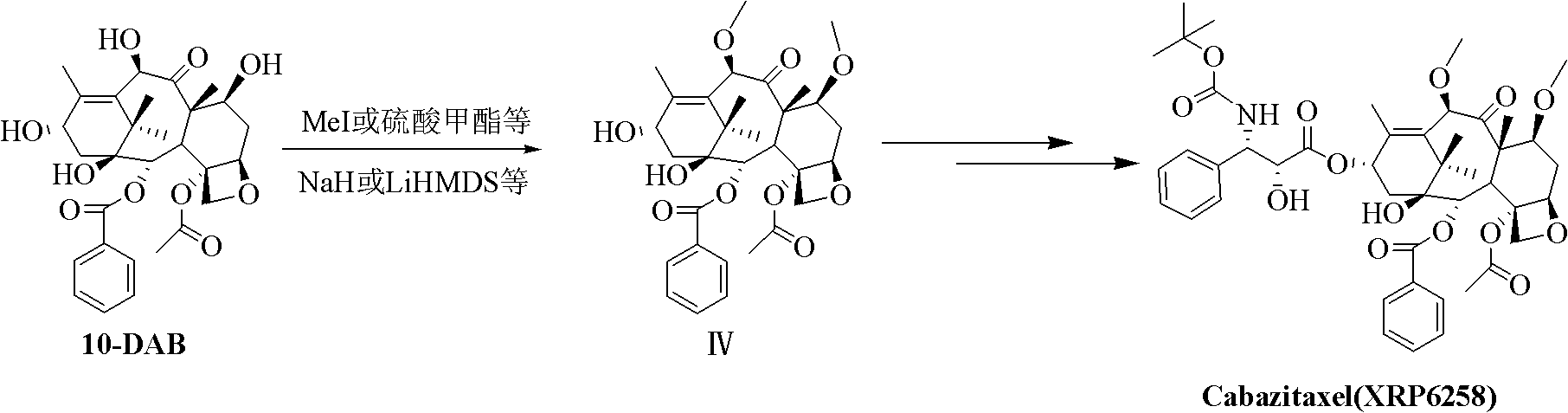
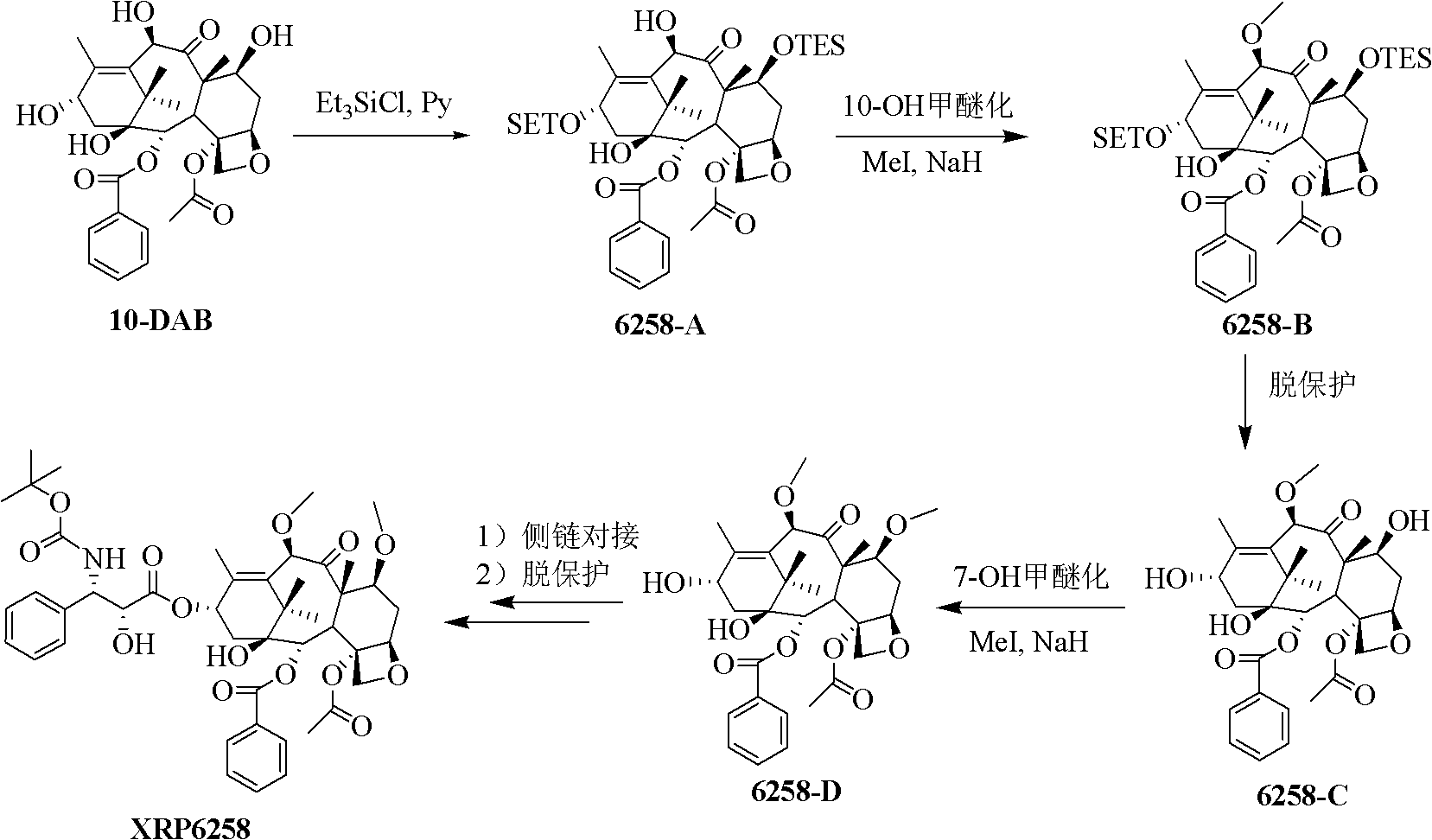
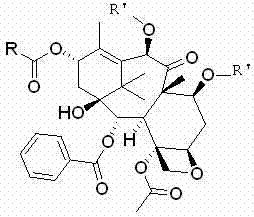
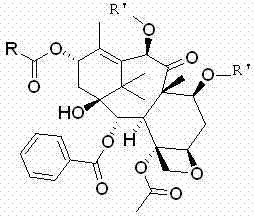
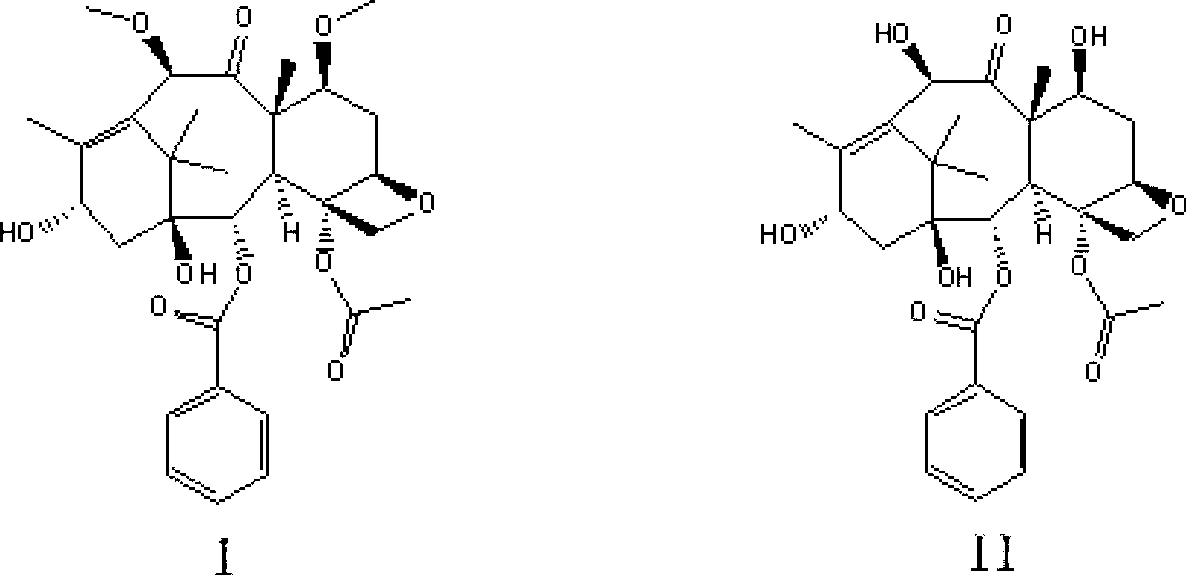
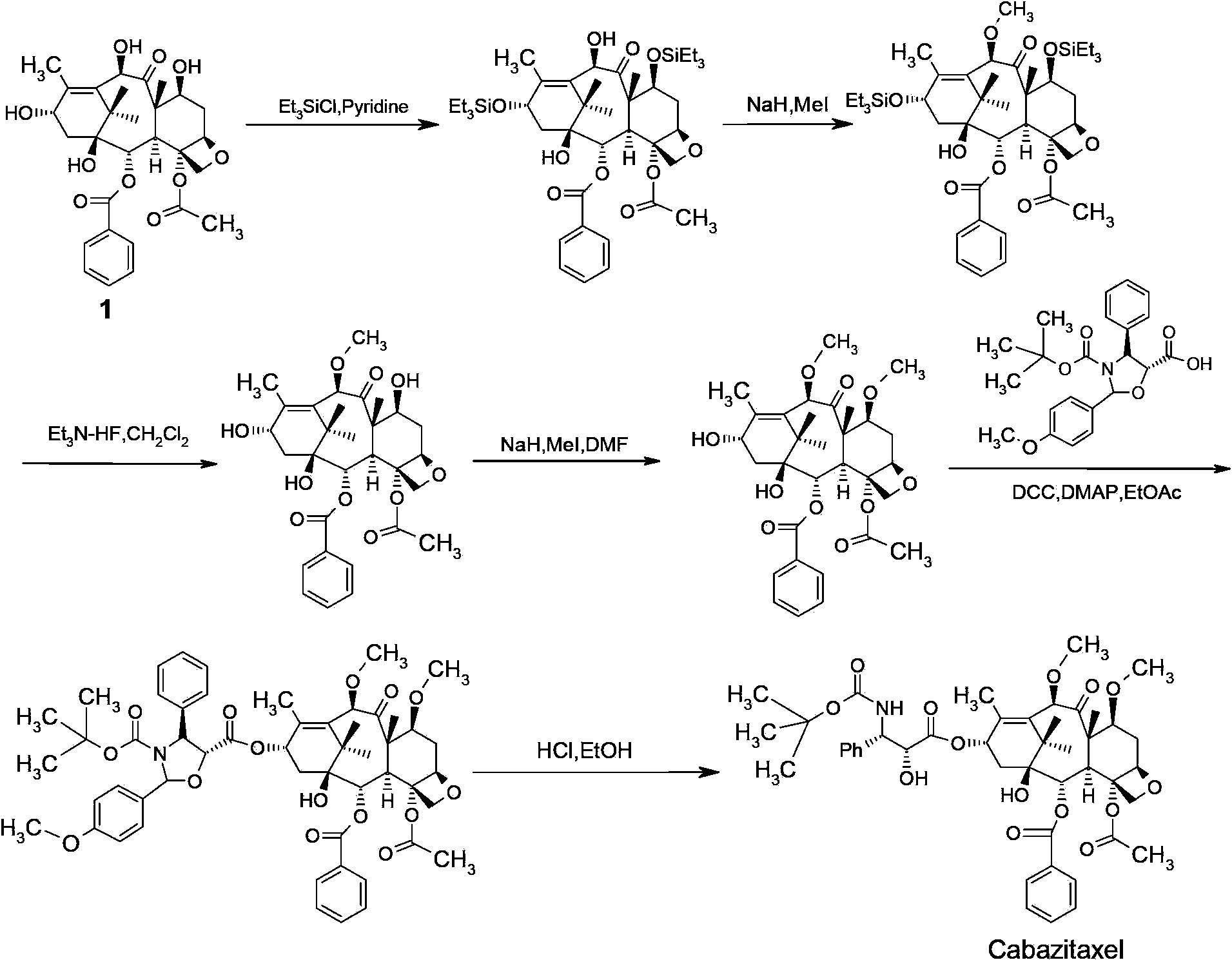
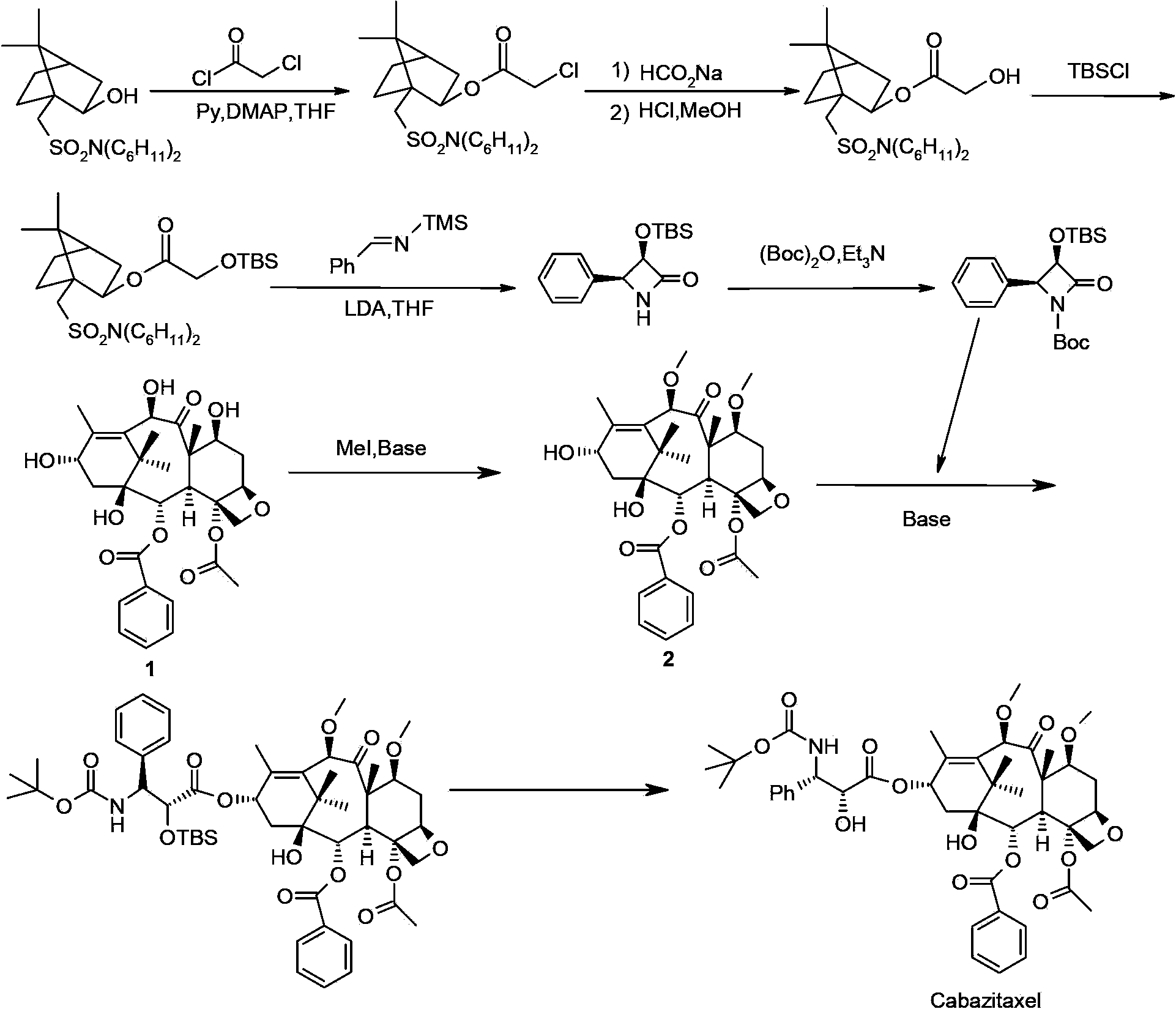
The synthetic method of cabazitaxel
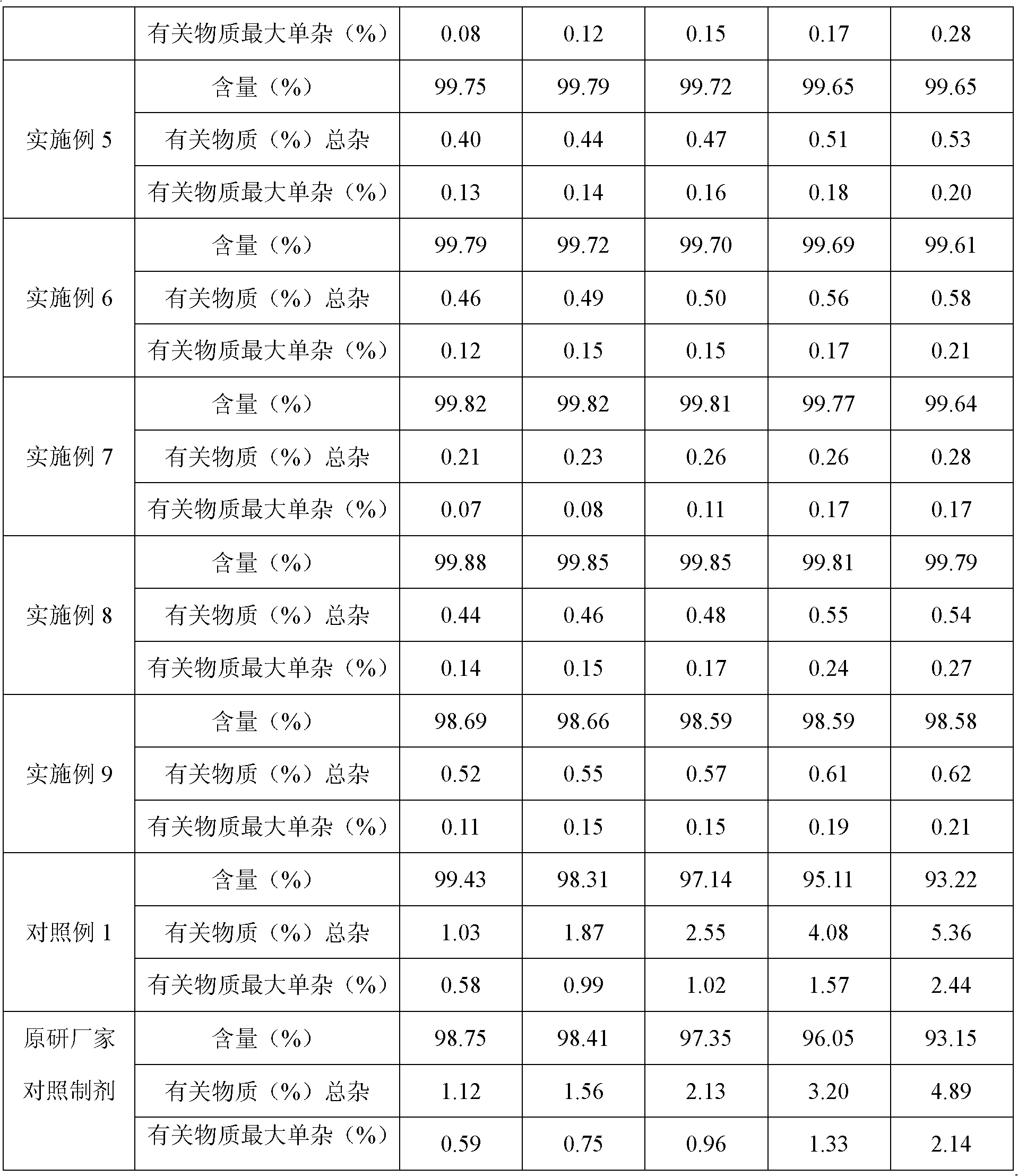
ActiveCN102285947AShort stepsMild conditionsOrganic chemistryChemical recyclingCabazitaxelDrugs synthesis
The invention discloses a method for synthesizing cabazitaxel, and belongs to the field of medicine synthesis. In the method, oppolzer reagent is used as a chiral source, and (3R,4S)-beta-lactam with optical purity ee of 98 percent is obtained by five steps. Under the action of a proper alkali, hydroxyls on C-7 and C-10 in 10-baccatine III are methylated selectively to form 7,10-dimethoxy-10-baccatine III in one step. Then beta-lactam is used to esterify the 7,10-dimethoxy-10-baccatine III under an alkaline condition, and then cabazitaxel with a purity of 99 percent can be obtained by a step of removing protective groups. In the synthesis method, the steps are short and the conditions are mild.
Owner:江苏宁录科技股份有限公司
Cabazitaxel formulations and methods of preparing thereof
InactiveUS20120065255A1Organic active ingredientsBiocideHydrotropeTocopherol polyethylene glycol succinate
Pharmaceutical formulations comprising cabazitaxel, solubilizer, tocopherol polyethylene glycol succinate (TPGS), one or more hydrotropes, optionally one or more agents having a pKa of about 3 to about 6, and optionally one or more antioxidizing agents, wherein the formulations are substantially free of polysorbates and polyethoxylated castor oil. The solubilizer may comprise glycofurol or ethanol. Pharmaceutical formulations may alternatively comprise cabazitaxel, solubilizer, optionally one or more agents having a pKa of about 3 to about 6, and optionally one or more antioxidizing agents, wherein the formulations are substantially free of polysorbates and polyethoxylated castor oil. These formulations may be combined with a diluent, which comprises TPGS and one or more hydrotropes. Methods of administering the cabazitaxel formulations include combining the formulations with an infusion solution.
Owner:SCIDOSE
Method for preparing cabazitaxel by taking 10-deacetylate-baccatin III as raw material
ActiveCN102417491AIncrease preparation meteringEasy to operateOrganic chemistryUrinary disorderAcetic acidCabazitaxel
The invention relates to a method for preparing cabazitaxel, particularly relates to a method for preparing the cabazitaxel by taking 10-deacetylate-baccatin III as a raw material and belongs to the technical field of drug synthesis. The method comprises the following steps: firstly, reacting 10-DAB with chlorocarbonate-2,2,2-trichloro ethyl ester, thereby obtaining a product; reacting the product with DMAP (dimethylamino pyridine), DCC (dicyclohexylcarbodiimide) and (4S, 5R)-2,2-dimethyl-4-phenyl-3-tert-butoxycarbonyl-3.5-oxazolidine formic acid, thereby obtaining a product; reacting the product with acetic acid and zinc powder, and then methylating the product; and lastly, adding a p-methyl benzenesulfonic acid, thereby reacting and obtaining a cabazitaxel product. The cabazitaxel prepared according to the method can be widely applied to the treatment of prostate cancer. Compared with a traditional technique for preparing the cabazitaxel, the method has the advantages that the preparation quantity is greatly increased, the operation steps are simplified, the manpower is saved and the method has industrial application value and prospect.
Owner:无锡紫杉药业股份有限公司
Method for preparing cabazitaxel
ActiveCN102336726AFew reaction stepsShort reaction cycleOrganic chemistryBulk chemical productionCabazitaxelChemical synthesis
The invention relates to the field of chemical synthesis, in particular to a method for preparing cabazitaxel. Reaction steps are reduced, a protective group is removed in a mild mode, the reaction period is shortened, and the high-purity cabazitaxel is obtained. The whole preparation method has the advantages of a few reaction steps, light pollution and suitability for industrial production.
Owner:重庆兴泰濠制药有限公司
Cabazitaxel drug composition and preparation method thereof
ActiveCN103393632AMeet treatment needsImprove stabilityOrganic active ingredientsGranular deliveryZeta potentialMass ratio
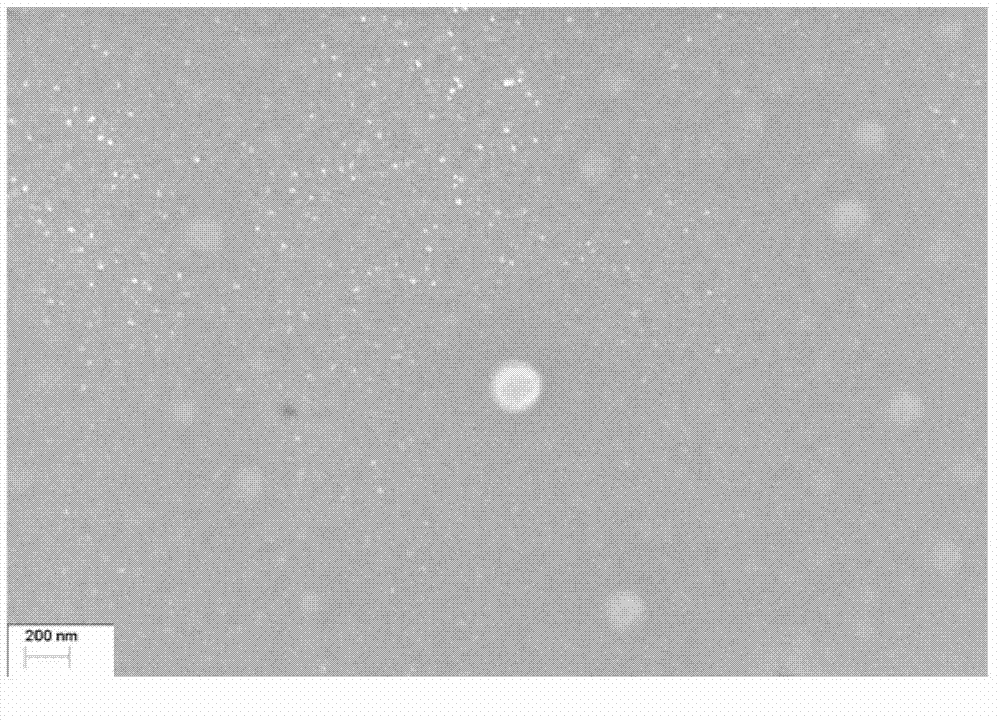
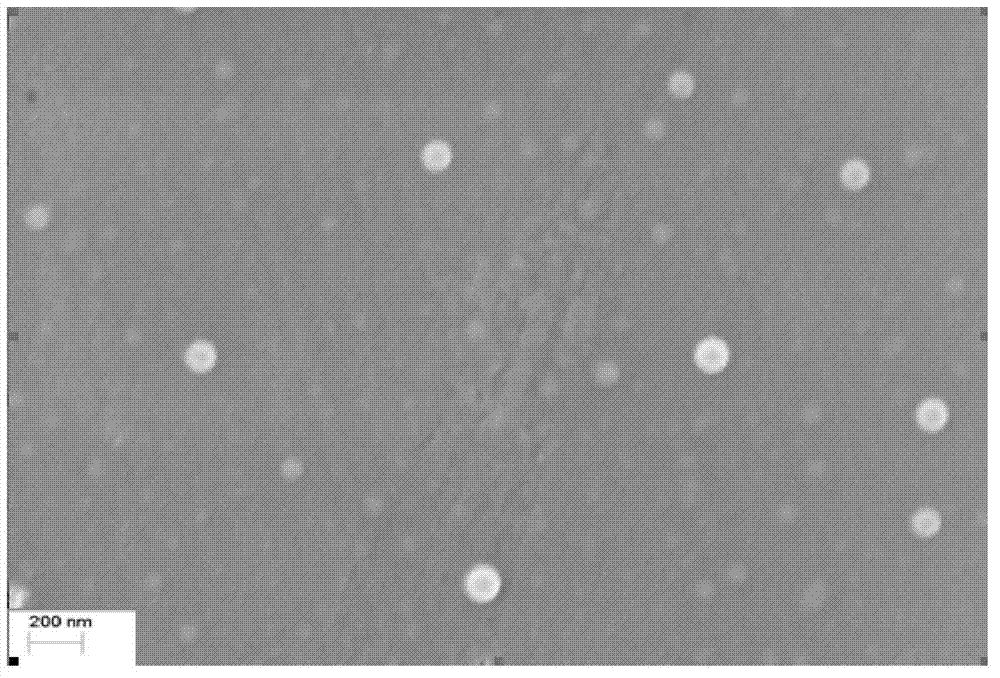
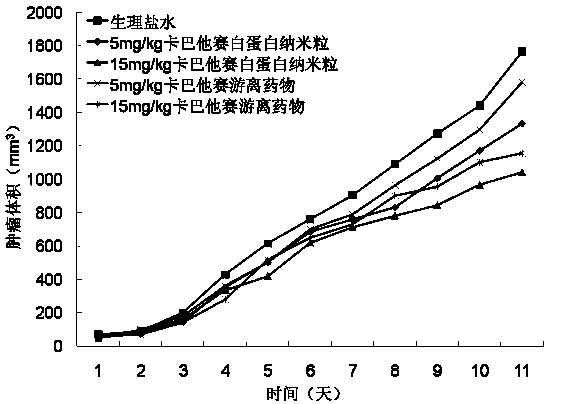
The invention provides a drug composition of cabazitaxel and a pharmaceutically acceptable biological carrier and a preparation method thereof. The cabazitaxel drug composition is actually a nanoparticle colloid dispersing system containing cabazitaxel. Cabazitaxel is encapsulated in a polymer shell made of proteins or is associated with the proteins by way of association to form nanoparticles, wherein the mass ratio of the cabazitaxel to the proteins is 1:(8-15); the pH value ranges from 5.0 to 7.0; the average diameter of the particles is not more than 200nm; the Zeta potential ranges from minus 10mv to minus 30mv; and the particles can be subjected to sterile filtration. The composition can be prepared by a high-pressure homogenating method or a protein denaturation and renaturation method. The composition prepared by the invention can be transformed to re-dispersable cakes or powder, can maintain stability for at least 48 hours at 37 DEG C after being re-dispersed in an aqueous medium, and can meet the requirements of intravenous drip therapy.
Owner:QILU PHARMA HAINAN
Preparation method of taxanes compound
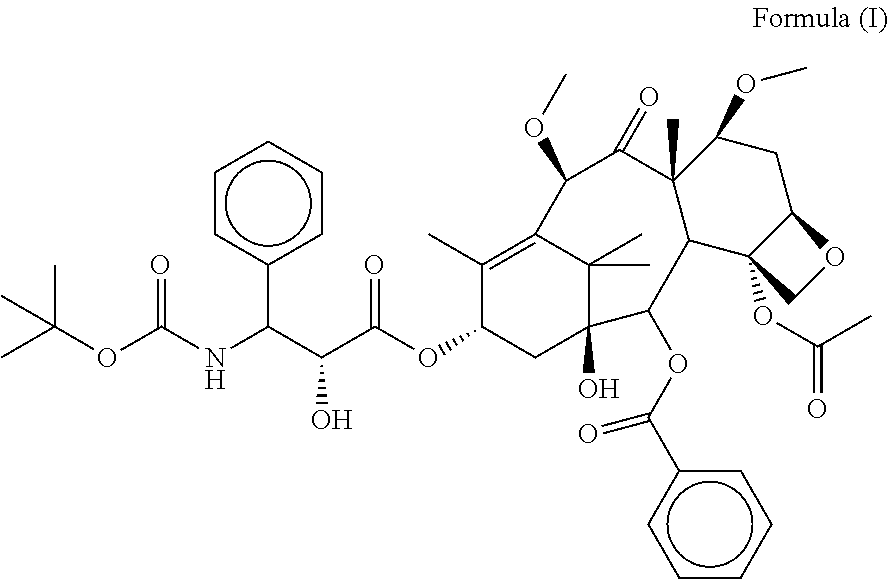
ActiveCN102060815AShort preparation timeImprove reaction efficiencyOrganic chemistryDocetaxelCabazitaxel
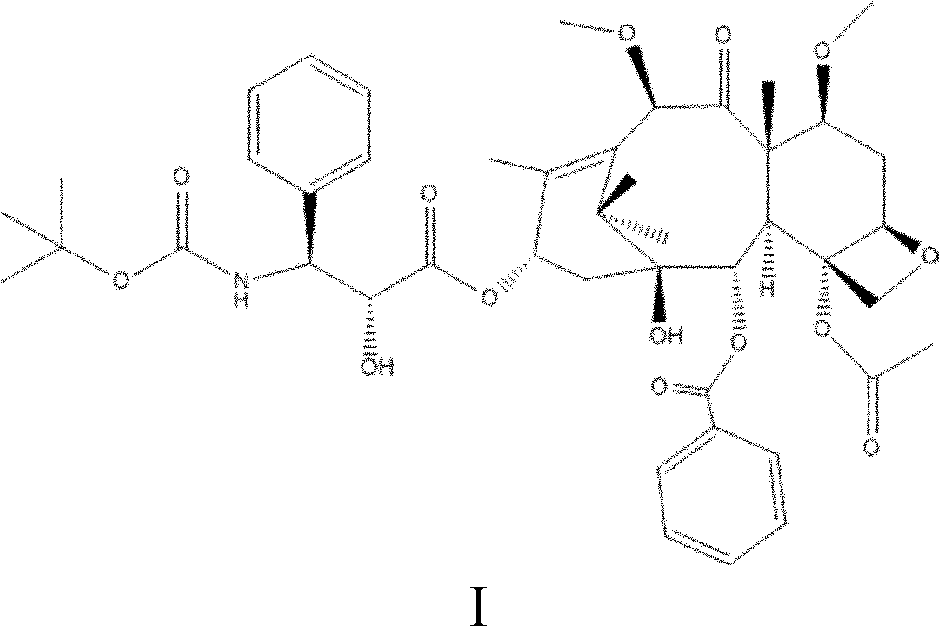
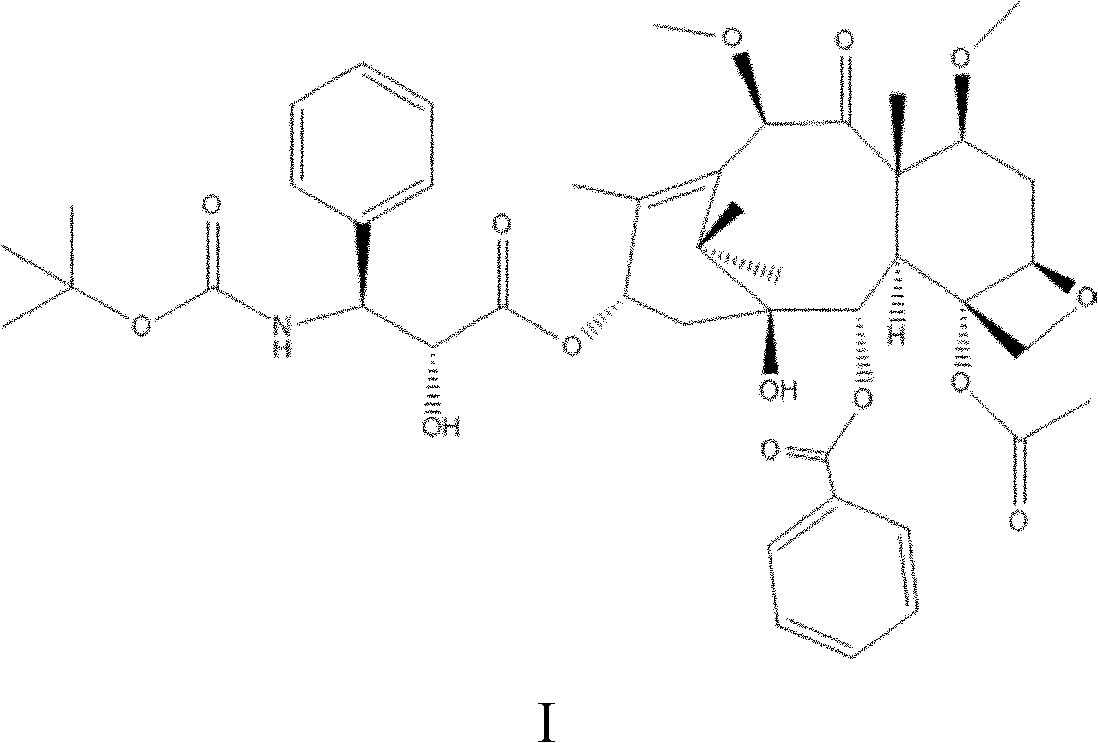
The invention discloses a preparation method of a taxanes compound shown as the formula I in the specification, relating to the field of pharmaceutical chemistry. The preparation method comprises the following steps of: under the water isolating condition, with docetaxel as a raw material and benzene alcohol, ketons or ethers compounds as a solvent, dropwise adding dimethyl sulfate, carrying out the alkylation reaction for 0.5 to 10 hours at the temperature of 10 to 60 DEG C, controlling the PH value of the reaction solution between 7 and 8 by an alkalescent organic solvent during the alkylation reaction, and adding water for separate out crystals after the alkylation reaction, wherein the crystals are the compound shown as the formula I. In the preparation method, a one-step method is adopted to prepare the taxanes compound cabazitaxel shown as the formula I. The preparation method has the advantages of few reaction steps, mild reaction condition, short reaction time and high reaction efficiency, and is favorable for industrial production. Meanwhile, through controlling the dropping speed of an alkylating agent and the PH value of the reaction solution, the docetaxel can be prevented from decomposing and the purity of the reaction product can be enhanced.
Owner:重庆兴泰濠制药有限公司
Crystal forms of cabazitaxel and preparation method thereof
ActiveCN102746258AImprove performanceWeak toxicityOrganic active ingredientsOrganic chemistry methodsCabazitaxelBiological activation
The invention relates to the field of medicinal chemistry, and discloses three crystal forms of cabazitaxel, i.e., an ester compound crystal form J of cabazitaxel, a hydrate crystal form G of cabazitaxel and a cabazitaxel crystal form I, and a preparation method of a novel crystal form of cabazitaxel. The novel cabazitaxel form has superior performance on the aspects of oral absorptivity, activation of metabolism and suppression of mitosis and interval cell function of cells, is weak in toxicity, has high storing and treating stability, and can be applied to preparation of a medicament for treating prostatitis.
Owner:重庆兴泰濠制药有限公司
Preparation method for cabazitaxel
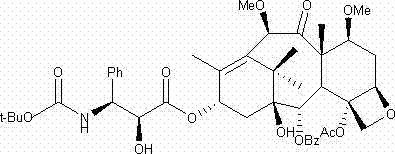
The invention relates to a preparation method for cabazitaxel. The method comprises that: (1) a compound 1 is subjected to deprotection to release hydroxyl groups positioned on the site 7 and the site 10; (2) a side chain is linked to the site 13 of taxane, then the dual methylation treatment is performed for the site 7 and the site 10 of the taxane; (3) the side chain positioned on the site 13 is subjected to ring opening to obtain a compound 4, wherein the compound 4 is the cabazitaxel. The reaction formula is as follow. The raw materials used in the method provided by the present invention are simple and easy to be obtained; the new process adopts the three-step reaction, and is simple; the yield is high; the cost is low; the prepared product has high purity, wherein the purity can reach more than 99.5%.
Owner:SHANGHAI HENGHE MEDICAL TECH
Cabazitaxel liposome injection and preparation method thereof
InactiveCN103768018AHigh encapsulation efficiencyStable drug loadingOrganic active ingredientsPharmaceutical non-active ingredientsPh gradientCabazitaxel
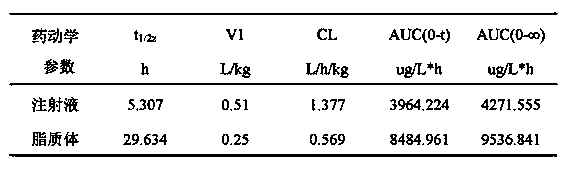
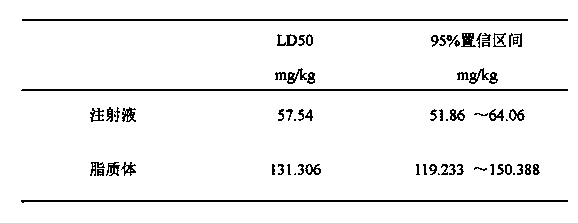
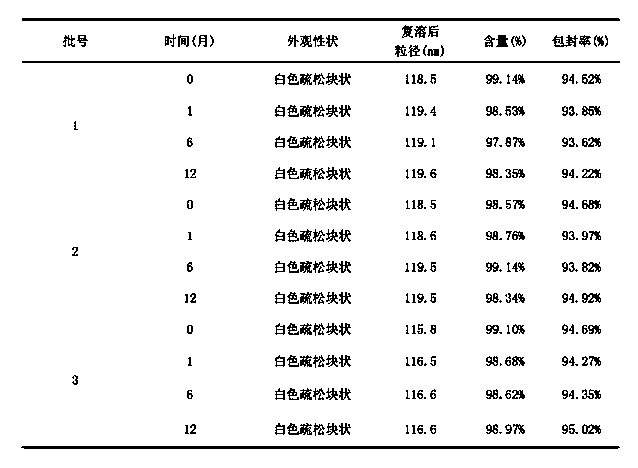
The invention provides a cabazitaxel liposome injection and a preparation method thereof. The cabazitaxel liposome injection comprises cabazitaxel, phosphatide, cholesterol, and mannitol or glucose, and can be prepared through the vacuum film condensation method, the rotary evaporation method, reverse evaporation method, high-pressure homogenization method, and pH gradient method. The cabazitaxel liposome injection has the advantages of reduced toxicity, convenience in clinic, and improved biological availability, and furthermore avoids the a plurality of changes of liposome during the storage process, such as oxidation and hydrolysis of phosphatide, agglomeration and fusion of liposome, and the like, wherein the changes can cause the leakage of coated substance. The invention provides a cabazitaxel liposome which has a good stability and is enough stable in the storage period.
Owner:NANJING LUYE PHARMA
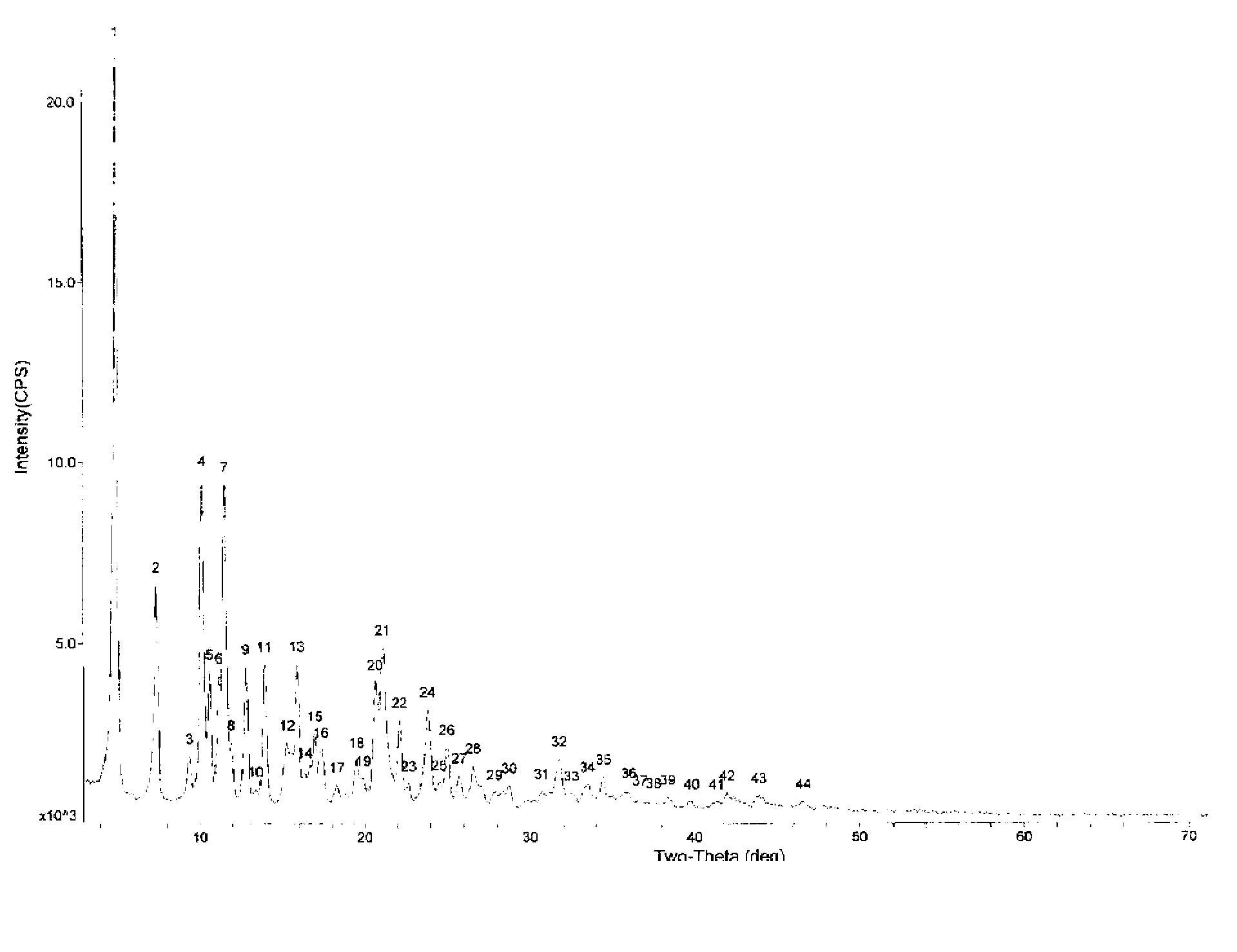
Cabazitaxel crystal and preparation method thereof
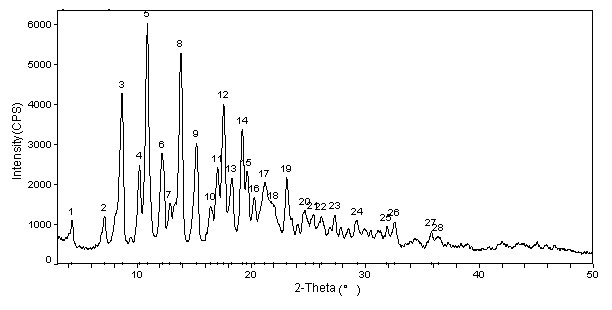
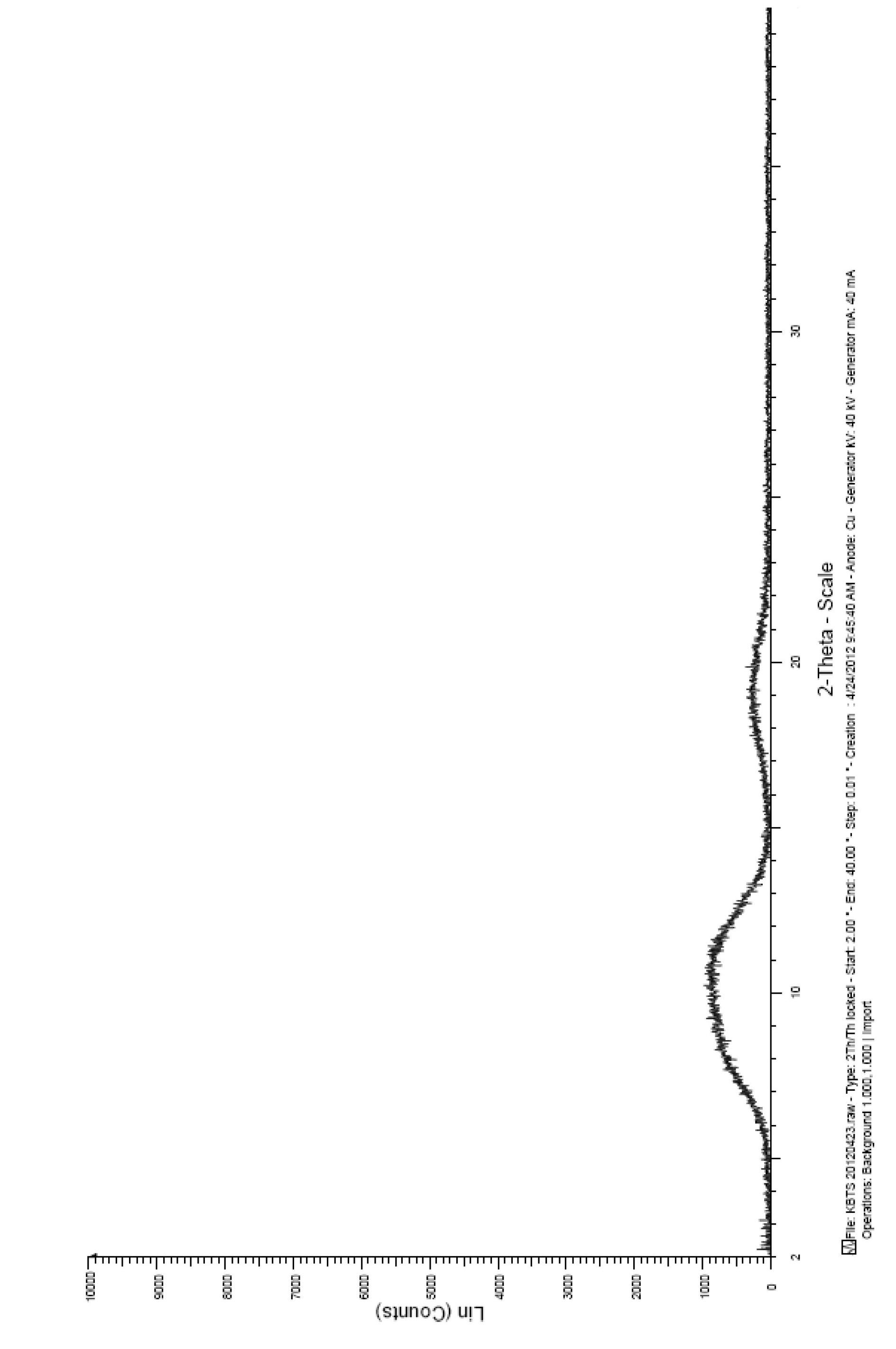
The invention relates to a cabazitaxel crystal which is solvent-free and non-crystallization water crystal form of 7, 10-dimethoxy docetaxel or (2R, 3S)-3-tert-butoxycarbonyl amino-2-hydroxyl-3- phenylpropionic acid 4-acetoxyl-2 alpha-benzoyloxy-5 beta, 20-epoxy-1-hydroxyl-7 beta, 10 beta- dimethoxy-9-oxo-11-taxadiene-13 alpha-ester; and the cabazitaxel crystal is shown to be positioned at 4.3, 7.1, 8.7, 10.2, 10.9, 12.2, 13.8, 15.2, 16.4, 17.0, 17.6, 18.3, 19.2, 19.6, 20.3, 21.2, 23.1, 24.7, 26.1, 27.3, 29.3, 31.9, 32.5 and 35.8 degrees 2 theta characteristic peak by powder X ray diffraction (PXRD). The invention also discloses a preparation method of the cabazitaxel crystal. The cabazitaxel crystal is prepared under the condition of reduced pressure at the room temperature, and is high in yield and good in purity.
Owner:SHANGHAI JINHE BIO TECH
Amorphous cabazitaxel and preparation method thereof
InactiveCN102659722AResidue reductionImprove stabilityOrganic active ingredientsOrganic chemistryCabazitaxelSolvent
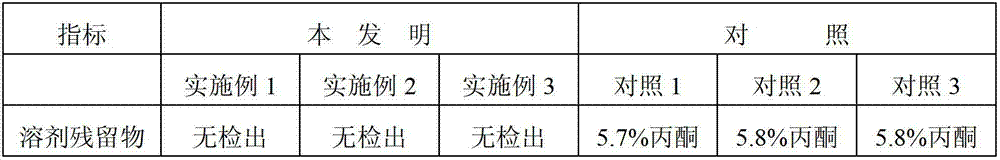
The invention relates to an amorphous cabazitaxel and a preparation method thereof and further relates to a medical application of a cabazitaxel amorphous substance. The amorphous cabazitaxel prepared according to the preparation method provided by the invention is low in solvent residual, is excellent in appearance form and stability and is preferably applied to clinic treatment.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Cabazitaxel injection and preparation method thereof
InactiveCN102068407AEasy to makeReduce adverse outcomesOrganic active ingredientsPharmaceutical delivery mechanismMedicineCabazitaxel
The invention provides cabazitaxel injection. The injection of each milliliter comprises the following components by weight: 10 to 100 milligrams of cabazitaxel, 250 to 800 milligrams of polysorbate 80, 200 to 600 milligrams of absolute ethanol, and 0.05 to 20 milligrams of citric acid. The cabazitaxel injection is a single preparation, does not need to be previously dissolved and prepared by using a special solvent, and can be directly diluted for administration; compared with the conventional preparation technology, the preparation has simple preparation process, low cost and good stability; meanwhile, the preparation shortens the time for preparing the medicinal liquor, improves the stability of clinical medicinal liquor, enhances the accuracy of administration dose, and reduces toxic or side reaction; and the preparation simplifies the preparation process of medicinal personnel, and reduces the risks of the preparation process on harm to the medicinal personnel and environmental pollution.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Multiphase-stable albumin conjunction type cabazitaxel
InactiveCN104490797AReduce aggregationEasy to separatePowder deliveryOrganic active ingredientsSolubilityNanoparticle
The invention belongs to the field of pharmaceutical preparations and discloses multiphase-stable albumin conjunction type cabazitaxel prepared by virtue of a novel albumin nanoparticle preparation technique. The prescription of a preparation comprises cabazitaxel, albumin, a stabilizer, a protective agent and a pH regulating agent. The preparation is prepared by virtue of the novel albumin nanoparticle preparation technique, and albumin conjunction type cabazitaxel is preferably prepared by virtue of high shearing force of a temperature-controllable planetary ball mill. According to the method, raw materials are crushed during the preparation, so that the particle size is reduced, and the solubility of drugs is increased.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Preparation and application of taxane prodrug
InactiveCN105884719AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryCabazitaxelDisulfide bond
The invention belongs to the technical field of drugs and relates to preparation and an application of a taxane prodrug. The prodrug is prepared from citronellol and an anticancer drug which are joined through a disulfide bond. The anticancer drug is one of paclitaxel and cabazitaxel. A dosage form which is clinically acceptable and capable of being injected or orally taken can be prepared from the prodrug of the anticancer drug and a pharmaceutically acceptable carrier. The dosage form comprises nanoparticles and lipidosome or emulsion. The prodrug and a preparation can obviously improve the activity of the anticancer drug, reduce the toxicity and increase the stability and the targeting property, so that development of the preparation is facilitated.
Owner:SHENYANG PHARMA UNIVERSITY
Cabazitaxel polymorphic form and preparation method thereof
The invention belongs to the technical field of medicines, relating to a cabazitaxel polymorphic form and a preparation method thereof, and particularly provides a novel crystalline form of anhydride, monohydrate and dehydrate of cabazitaxel and a preparation method thereof. The novel crystalline form provided by the invention is good in product stability, simple in preparation technology, good in quality reproducibility and easy to control for product purity, and is suitable for industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Cabazitaxel crystal and preparation method thereof
ActiveCN102898406AEfficient removalImprove solubilityOrganic chemistryTert-Butyloxycarbonyl protecting groupCabazitaxel
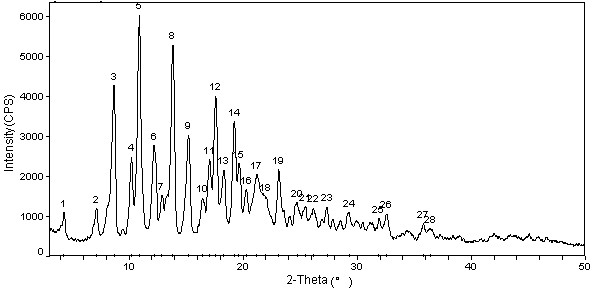
The invention relates to a solvate-free and crystal water-free crystal form of 4-accetoxy-2alpha-benzoyloxy-5beta,20-epoxy-1-hydroxy-7beta,10beta-dimethoxy-9-oxotax-11-en-13alpha-yl(2R,3S)-3tert-butoxycarbonylamino-2-hydroxy-3-phenylpropanoate 1-hydroxy-7beta,10beta-dimethoxy-9-oxo-5beta,20-epoxytax-11-ene-2alpha,4,13alpha-triyl4-acetate 2-benzoate13-[(2R,3S)-3-{[(tertbutoxycarbonyl]amino}-2-hydroxy-3-phenylpropanoate. A powder X-ray diffraction (PXRD) diagram shows that the crystal is positioned on the characteristic peaks of 4.3, 7.4, 8.6, 10.0, 11.0, 12.2, 12.6, 13.3, 13.6, 14.2, 15.0, 15.5, 16.4, 17.0, 18.1, 18.6, 20.2, 21.0, 21.6, 22.1, 22.8, 24.0, 24.6, 25.3, 25.8, 26.9, 28.0, 29.4, 31.5, 32.0, 34.4, 35.5, 36.8 and 41.7 DEG 2theta. The invention also discloses a preparation method for the crystal. The crystal prepared under reduced pressure at room temperature is high in yield and purity, and does not have any solvent residue.
Owner:SHANGHAI JINHE BIO TECH
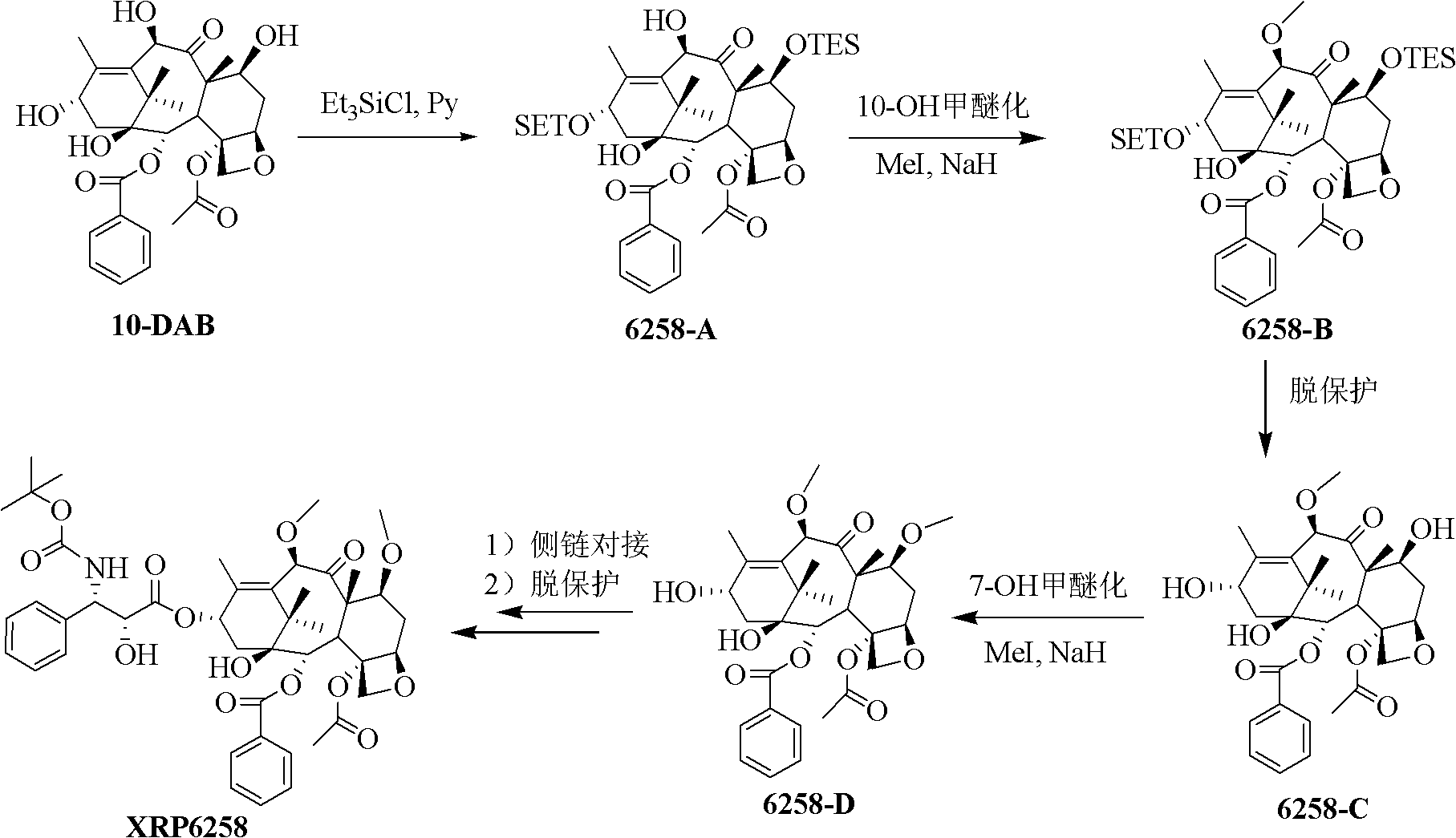
Synthetic method of cabazitaxel
ActiveCN102659721AHigh reaction yieldAddress rising costsOrganic chemistryBulk chemical productionCabazitaxelSide chain
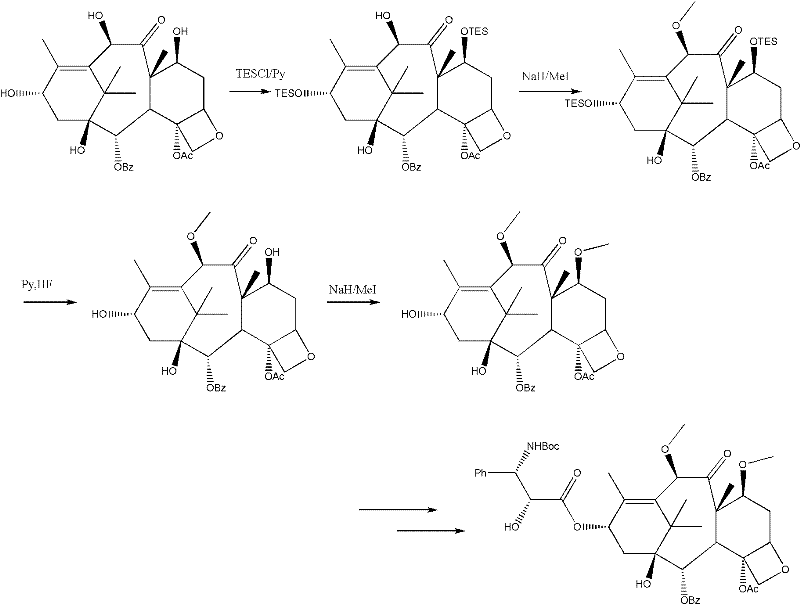
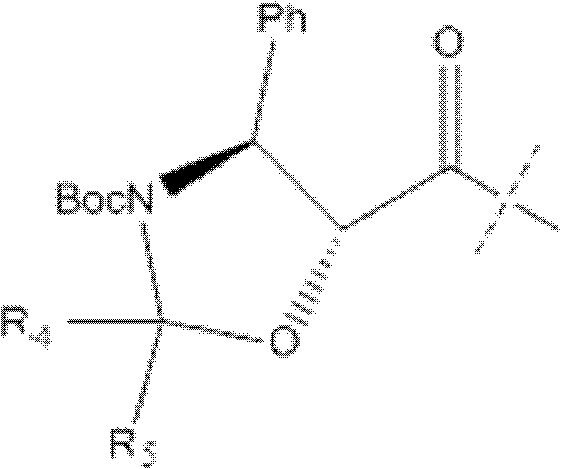
The invention relates to a synthetic method of cabazitaxel, which comprises the following steps: taking a compound 10-deacetylbaccatin III as a starting raw material and reacting with a protective agent to carry out selective protection on 7 and 10 hydroxyls; then carrying out selective protection on 13 hydroxyls of the compound, removing 7 and 10 protection radicals and carrying out methylation reaction; removing 13 protection radicals and carrying out condensation reaction with an oxazolidine carboxylic acid side chain; and removing and protecting a condensation product to obtain the cabazitaxel. The synthetic method has the beneficial effects that the proper protection radicals and a removal and protection method are selected, the oxazolidine carboxylic acid side chain is finally connected, and the synthetic method has the characteristics of low production cost, high yield, mild reaction conditions and simplicity in operation and is particularly suitable for industrial production.
Owner:BRIGHTGENE PHARMA
Preparation method of taxol anticancer drugs Cabazitaxel XRP6258
InactiveCN103012329AEasy to manufactureEasy to makeOrganic chemistryBulk chemical productionButt jointCabazitaxel
The invention belongs to the field of drug synthesis, and relates to a method for synthesizing a second-generation taxol anticancer drugs Cabazitaxel XRP6258. The method comprises the following steps of: obtaining C-7 and C-10 bit hydroxy methyl mercaptan methylene (MTM) and C-13 bit oxhydryl oxydic key intermediate (II) of 10-oxhydryl baccatin III(I) by high regioselectivity, reducing C-13 bit carbonyl to obtain a mother nucleus midbody (III), carrying out butt joint with various types of side chains to obtain butt joint product midbodies (IV-1) and (IV-2), and removing a side-chain protecting group after the di-methylthio is removed or removing the di-methylthio of the nuclear parent after the side-chain protecting group is removed to obtain a product Cabazitaxel XRP6258 (V). The method disclosed by the invention has the advantages of being simple in preparation process, high in yield, lower in cost, easy to operate and the like, so that the XRP6258 can be produced and prepared on a large scale.
Owner:FUDAN UNIV +1
Cabazitaxel albumin nanoparticle preparation for injection and preparation method thereof
InactiveCN104224750AImprove complianceImprove medication safetyOrganic active ingredientsMacromolecular non-active ingredientsOrganic solventCabazitaxel
The invention provides a cabazitaxel albumin nanoparticle preparation and a preparation method thereof. The cabazitaxel albumin nanoparticle preparation comprises cabazitaxel, albumin and pharmaceutically necessary auxiliary materials and can be prepared with a high-pressure homogenization method, a film shearing method, a solvent evaporation method and the like. According to the cabazitaxel albumin nanoparticle preparation and the preparation method thereof, problems of the drug loading capacity and the stability of existing albumin nanoparticles are solved; a low-toxicity organic solvent is adopted, so that highly allergic reactions due to the fact that Tween-80 is adopted in a cabazitaxel injection liquid can be avoided; by the aid of the high permeability and the retention effect of the nanoparticle on tumors, more drugs can be targeted passively to be concentrated in tumor tissue, so that the anti-tumor effect is improved; the preparation method is simple and suitable for industrial mass production.
Owner:SICHUAN UNIV
Synthetic method for cabazitaxel
InactiveCN102675256AHigh yieldHigh purityOrganic chemistryBulk chemical productionCompound aState of art
The invention discloses a synthetic method for cabazitaxel, and the synthetic method comprises the following steps: performing double methylation on the C7 site and C10 site of a compound A so as to obtain a compound B, and hydrolyzing the comound B under the acidity condition so as to obtain the cabazitaxel. The cabazitaxel has a reaction formula shown in the specification. Compared with the prior art, the synthetic method has the advantage that the compound A of which the C13-site hydroxyl is connected with a protecting group is used as a raw material, and a method of obtaining the cabazitaxel by virtue of double methylation of the C7-site and C10-site hydroxyl and hydrolysis, so that a step of generating by-products by the methylation of the C13-site hydroxyl of 10-Deacetylbaccatin III and a complex purification step are omitted. The synthetic method has the advantages of simple technology and moderate reaction condition, and can be used for improving the yield and purity of the cabazitaxel.
Owner:CHONGQING BEISHENG PHARMA TECH CO LTD
Method for purifying cabazitaxel
InactiveCN102887877ASolve technical bottlenecks that are difficult to removeIncrease productivityOrganic chemistryFluid phaseCabazitaxel
The invention discloses a method for purifying cabazitaxel. The method comprises the following steps of: obtaining a crystallized sample by taking cabazitaxel with purity over 98% as a start raw material and cyclohexane, ethyl acetate and ethanol as crystallization solvents; filtering, washing and drying the crystallized sample, and dissolving with methanol; adding a proper amount of adsorbent into the methanol solution of cabazitaxel; sufficiently adsorbing the sample by the adsorbent; filtering the sample again, and evaporating the solvent to dryness; and drying, wherein the impurity content of the dried sample in single technology is reduced to below 0.1% according to the high performance liquid chromatography detection. According to the invention, the impurity content of single cabazitaxel synthesis technology is effectively reduced by a separation and purification method combining crystallization and adsorption, and the requirement that the impurity content of single technology of cabazitaxel is lower than 0.1% is met.
Owner:JIANGSU HONGDOUSHAN BIOLOGICAL TECH
Albumin composition highly-carrying cabazitaxel medicine, preparation and preparation method thereof
ActiveCN105727303AReduce dosageExtend cycle timeOrganic active ingredientsPowder deliveryCabazitaxelLesion
The invention provides an albumin composition highly loaded with cabazitaxel and its preparation and preparation method. In the albumin composition highly loaded with cabazitaxel drug of the present invention, the carrier albumin in the form of albumin nanoparticles and non-nanoparticles encapsulates the drug loading of cabazitaxel up to 13% to 25% (weight), and The carrier albumin in the form of nanoparticles accounts for more than 50% by weight of the total albumin carriers. The composition of the present invention is convenient for clinical use, greatly reduces the amount of albumin as an auxiliary material, and more efficiently entraps drugs, delivers them to lesions, and plays a role.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
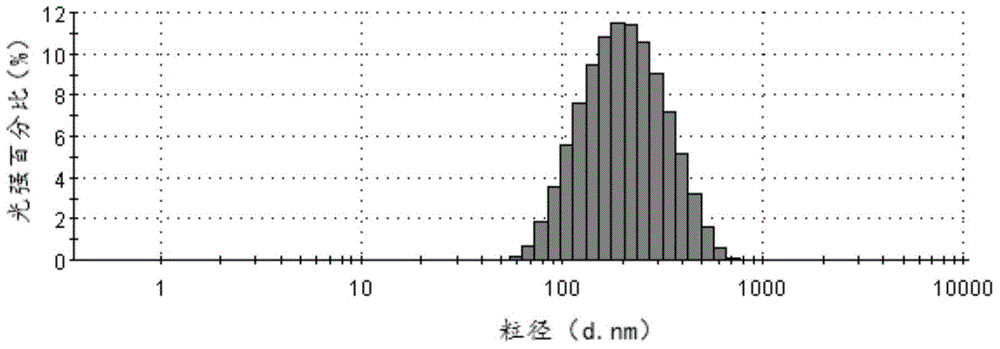
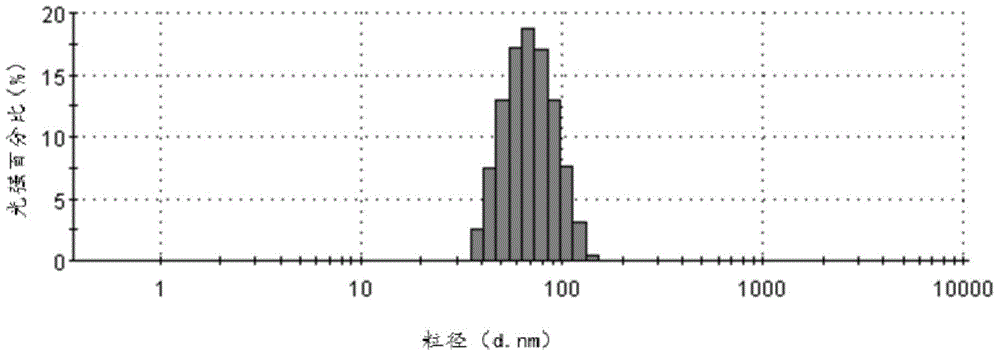
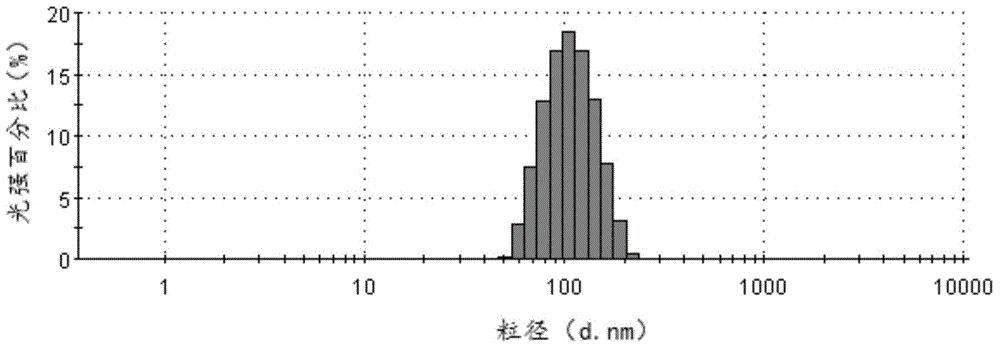
Cabazitaxel lipid microsphere injection and preparation method thereof
InactiveCN103006558AHigh drug loadingImprove solubilityOrganic active ingredientsEmulsion deliveryMicrospherePhospholipid complex
The invention discloses a Cabazitaxel phospholipid complex and a method for preparing a lipid microsphere injection which contains the phospholipid complex. The lipid microsphere injection consists of Cabazitaxel, injection phospholipid, injection oil, an emulsifying agent, a stabilizing agent, an antioxidant, an isoosmotic adjusting agent and water for injection. The lipid microsphere injection has the characteristics of high drug-loading rate, slow releasing, small adverse reaction, simplicity and practicability in preparation technology, good product stability and the like and is beneficial to clinic application and industrial production.
Owner:CHANGZHOU TARGET MEDICINE TECH CO LTD
Method for preparing second-generation taxol anticancer drug Cabazitaxel
InactiveCN103012328AEasy to manufactureEasy to makeOrganic chemistryBulk chemical productionCabazitaxelChemical compound
The invention belongs to the field of medicament synthesis, and relates to a method for synthesizing a second-generation taxol anticancer drug Cabazitaxel. The method comprises obtaining a key intermediate (II): a 10-deacetyl baccatin III (I) with C-7 and C-10 hydroxy-methylthio-methylene and C-13 hydroxy-oxidization, completing a de-methylthio operation for C-13 carbonyl and C-7 and C-10 methylthio methylene (MTM) of the compound II by a one-pot method to obtain a nucleus (IV) of an XRP6258, connecting the nucleus with various side chains, and then removing side chain protecting groups to obtain the product Cabazitaxel (V). The method has advantages of high efficiency in the preparation process, simple process, high yield, relatively low cost and easy operation, and is suitable for large-scale production and preparation of anti-cancer drugs XRP6258.
Owner:FUDAN UNIV +1
Cabazitaxel fat emulsion, and preparation method and use thereof
InactiveUS20180153848A1Excellent long-term storage stabilityExcellent resolubilityOrganic active ingredientsPharmaceutical non-active ingredientsCabazitaxelMedicine
Provided in the present invention is a cabazitaxel fat emulsion injection, wherein the cabazitaxel fat emulsion injection contains cabazitaxel, a medium chain triglyceride for injection, and lecithin. Also provided in the present invention are the method for preparing the cabazitaxel fat emulsion injection and the use thereof in preparing a drug for treating prostate cancer.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Compound, preparation method thereof and application of compound in preparation of cabazitaxel
InactiveCN102952102AReduce usageLow impurity contentOrganic chemistryEnvironmental effectCabazitaxel
The invention belongs to the field of pharmacy, particularly belongs to the field of the preparation of chemical drugs, and more particularly relates to a compound, a preparation method of the compound and the application of the compound in the preparation of cabazitaxel. In order to provide a cabazitaxel preparation method which is less in side reactions, high in product yield, less in byproducts, high in product quality, low in production cost, and suitable for the industrial production, the invention discloses a compound and a method for synthesizing cabazitaxel by the compound. The method disclosed by the invention is good in technical repeatability, less in solvent use level, lower in cost, and less in influence to operators and environment. According to the invention, the compound only needs to be refined by once or twice by normal reagent, so that the single impurity and the total impurity of midbodies and final products are less than 0.1% respectively, and the compound meets the quality requirement of bulk drug in ICH (international conference on harmonization) in the European union; and the compound can be taken as the raw material of cabazitaxel injection, and is very high in the value and the prospect of the industrial application.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
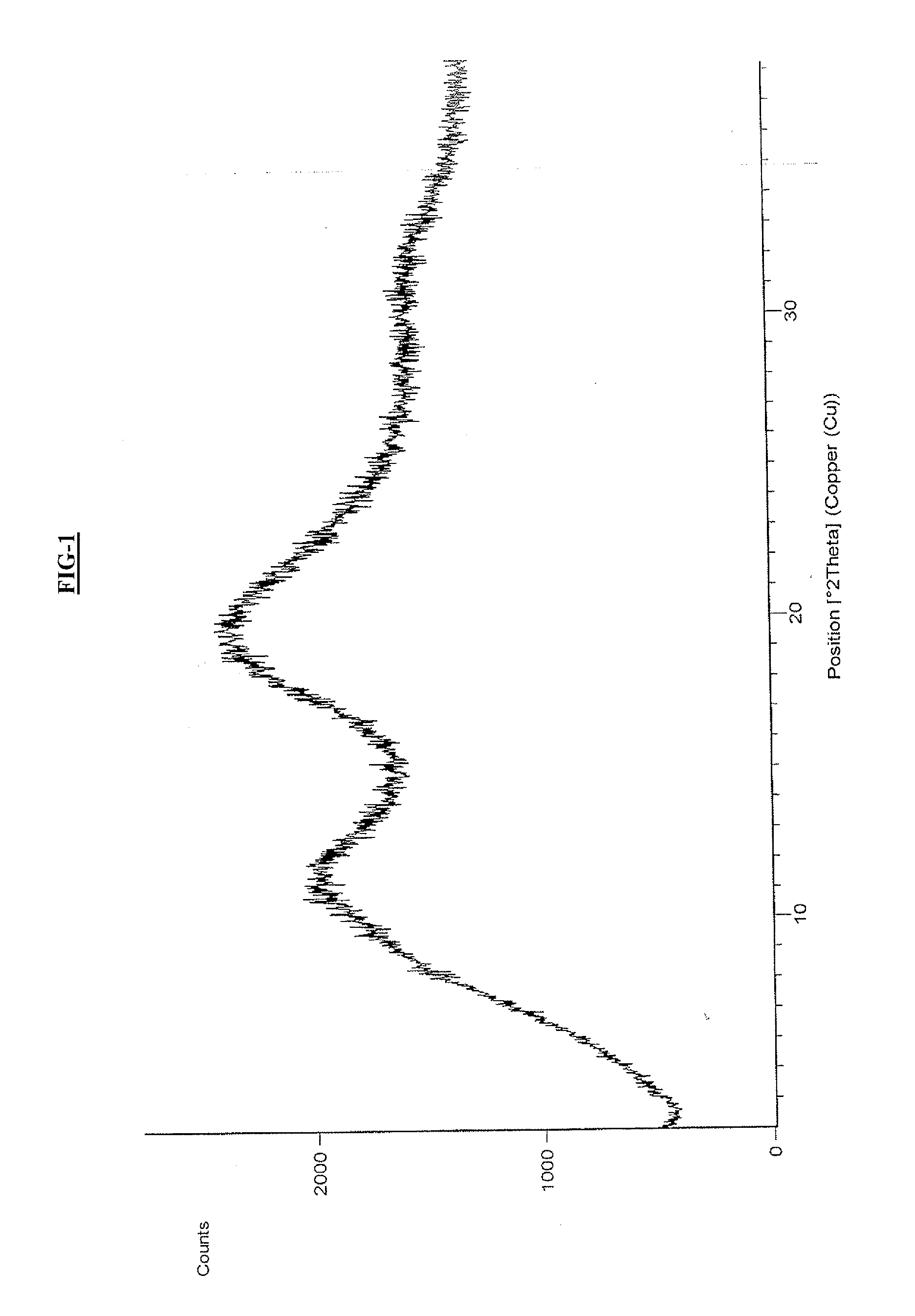
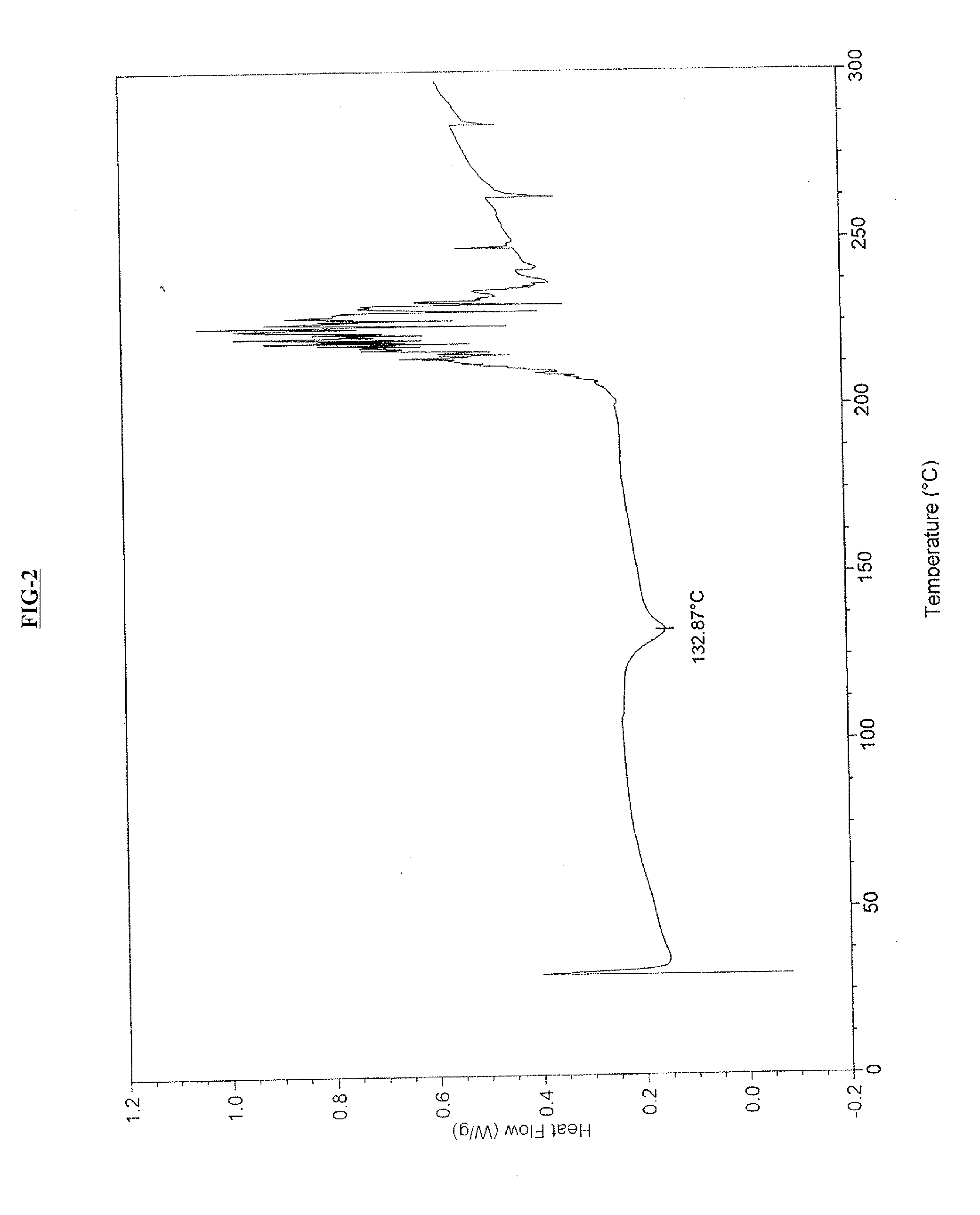
Amorphous form of cabazitaxel and process for its preparation
An Amorphous Form of Cabazitaxel is disclosed. It is preferably characterized by an X-ray powder diffraction (XRD) pattern as depicted in FIG.-1. It is prepared by (a) preparing a solution of Cabazitaxel in a suitable solvent and mixture thereof; and (b) recovering the Amorphous Forms of Cabazitaxel from the solution thereof by removal of the solvent.
Owner:FRESENIUS KABI ONCOLOGY LTD
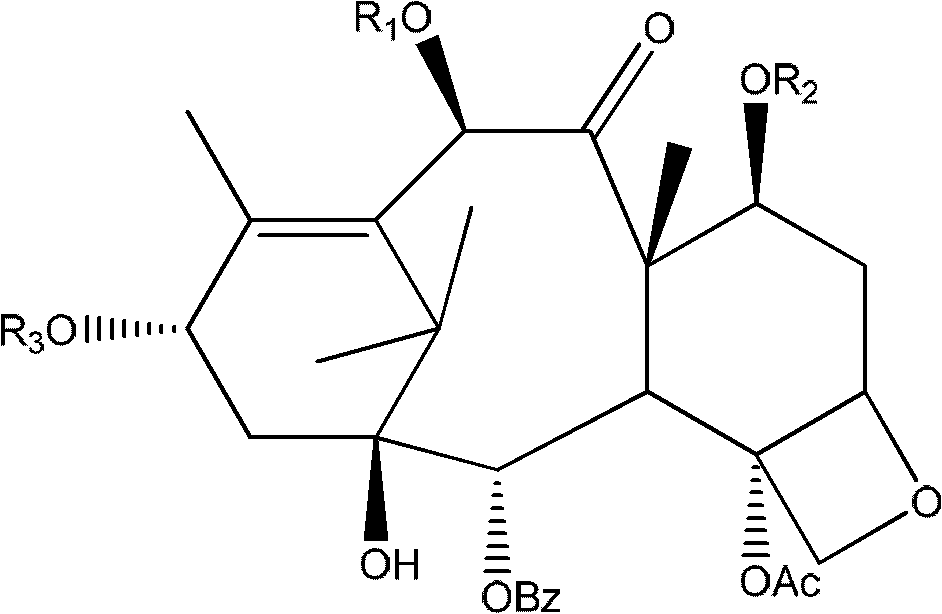
Preparation method of cabazitaxel and intermediate thereof
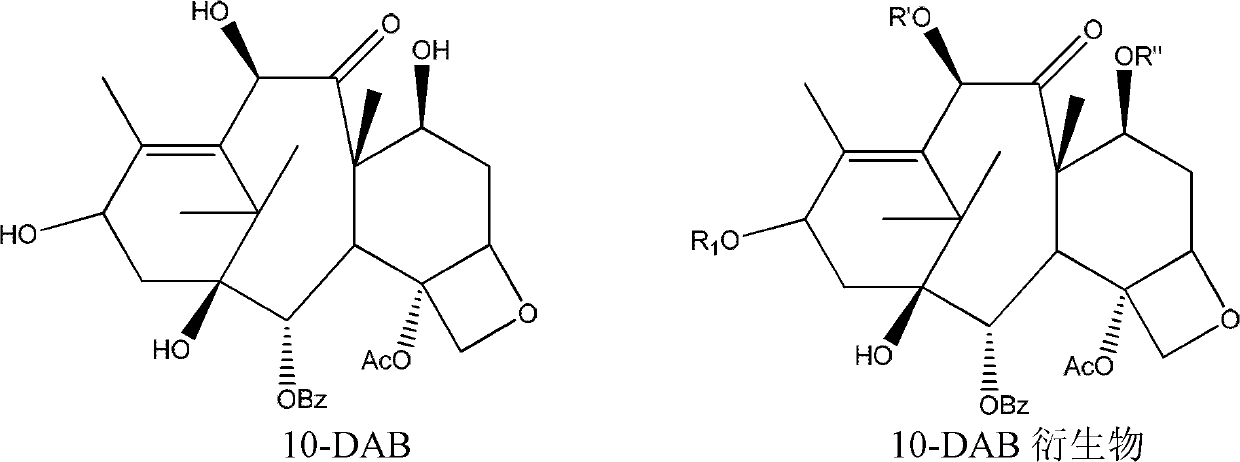
The invention discloses a preparation method of a cabazitaxel intermediate 7,10-dimethoxy-10-baccatin III. In the presence of alkali, 10-deacetylbaccatin III and a specific methylation reagent are subjected to selective methylation reaction at low temperature to obtain the cabazitaxel intermediate 7,10-dimethoxy-10-baccatin III. In the preparation method, the methylation reaction has high selectivity for C-7 and C-10 hydroxy sites on the 10-deacetylbaccatin III, so the yield is high. The invention also discloses a preparation method of cabazitaxel, which comprises the following steps: preparing the cabazitaxel intermediate according to the preparation method above; condensing the cabazitaxel intermediate with one side chain of docetaxel; and hydrolyzing the obtained condensation product under acidic conditions to obtain the cabazitaxel. The preparation method of cabazitaxel has the advantage of high total yield and is suitable for commercialized production.
Owner:BEIJING COLLAB PHARMA
10-deacetylbaccatin iii and method for methoxylation of its derivative
The invention relates to 10-deacetylbaccatin III and a method for methoxylation of its derivative, which belongs to the medicine synthesis technical field. The invention is characterized in that the under the condition that 10-DAB, the 7 position and the 10 position of its derivative are hydroxy or one of them is hydroxyl, a phase transfer catalyst quaternary ammonium salt N<+>R4X<-> is added under the existence of a methylating agent, and dissolved according to a weight ratio of 1:10-100 of solute to organic solvent, and reacted with low temperature, a lower layer water phase is separated after finishing the reaction, an organic phase is washed by a saturated salt solution, and the organic phase is concentrated by concentrated acid, a petroleum ether is added for completely deposing, filtered, deposed and dried to obtain the product. The invention provides a simple and easy method for preparing the 10-DAB and a methoxy compound of the 7 position and the 10 position hydroxy of its derivative. The method for large scale intermediate preparation enables possibility of preparation of docetaxel with large dosage, and is a key step for preparing cabazitaxel.
Owner:无锡紫杉药业股份有限公司
Cabazitaxel intermediate as well as preparation method and application thereof
ActiveCN103421036AMild reaction conditionsEasy to operateGroup 4/14 element organic compoundsBulk chemical productionChemical structureCabazitaxel
The invention discloses a cabazitaxel intermediate as well as a preparation method and an application thereof. The intermediate has a chemical structure formula shown as formula I shown in the description, and TES in the formula is the abbreviation of triethylsilane. The preparation method of the intermediate comprises a step B or a step A and the step B in the following synthetic route shown in the description, wherein the step A means that the compound of a formula 1 is subjected to a methylation reaction with a methylation agent for preparation of the compound of a formula 2; and the step B means that the compound of the formula 2 is subjected to a condensation reaction with the compound of a formula 3 for preparation of the intermediate I. The intermediate is subjected to hydrolysis under an acidic condition for removing the triethylsilane protective group, and thus cabazitaxel is prepared. According to the technical scheme, high-purity cabazitaxel can be synthesized by utilizing cheap and easily-available raw materials and with a low cost, large-scale industrialized production requirement on cabazitaxel is satisfied, and the preparation method is applicable to industrial application and has practical value.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com