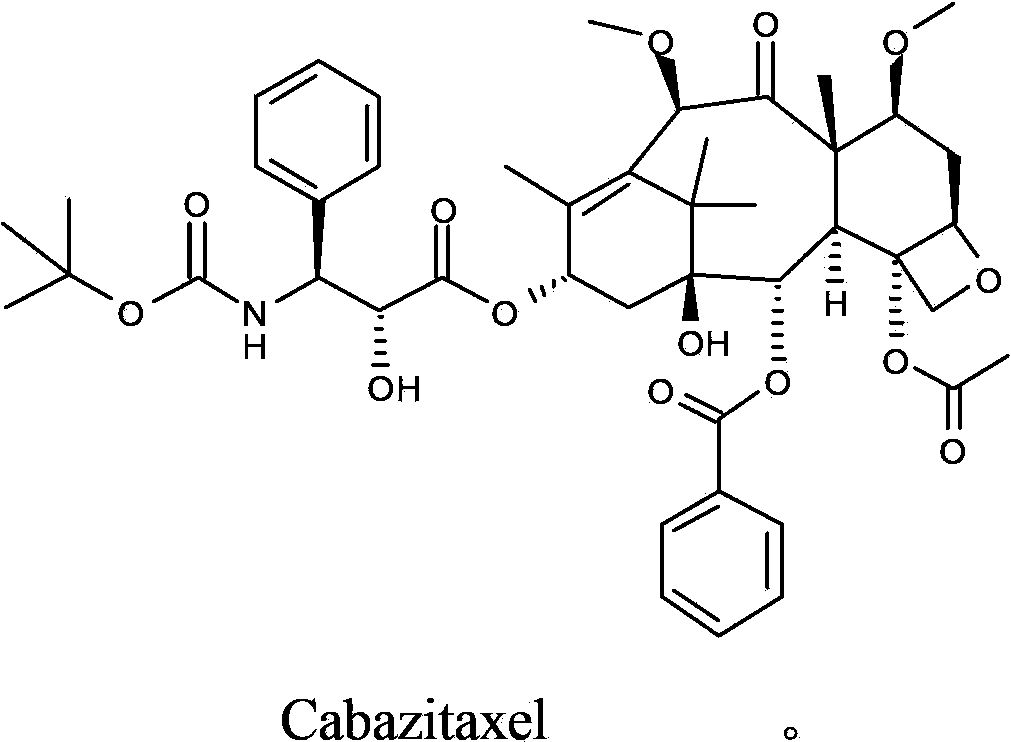
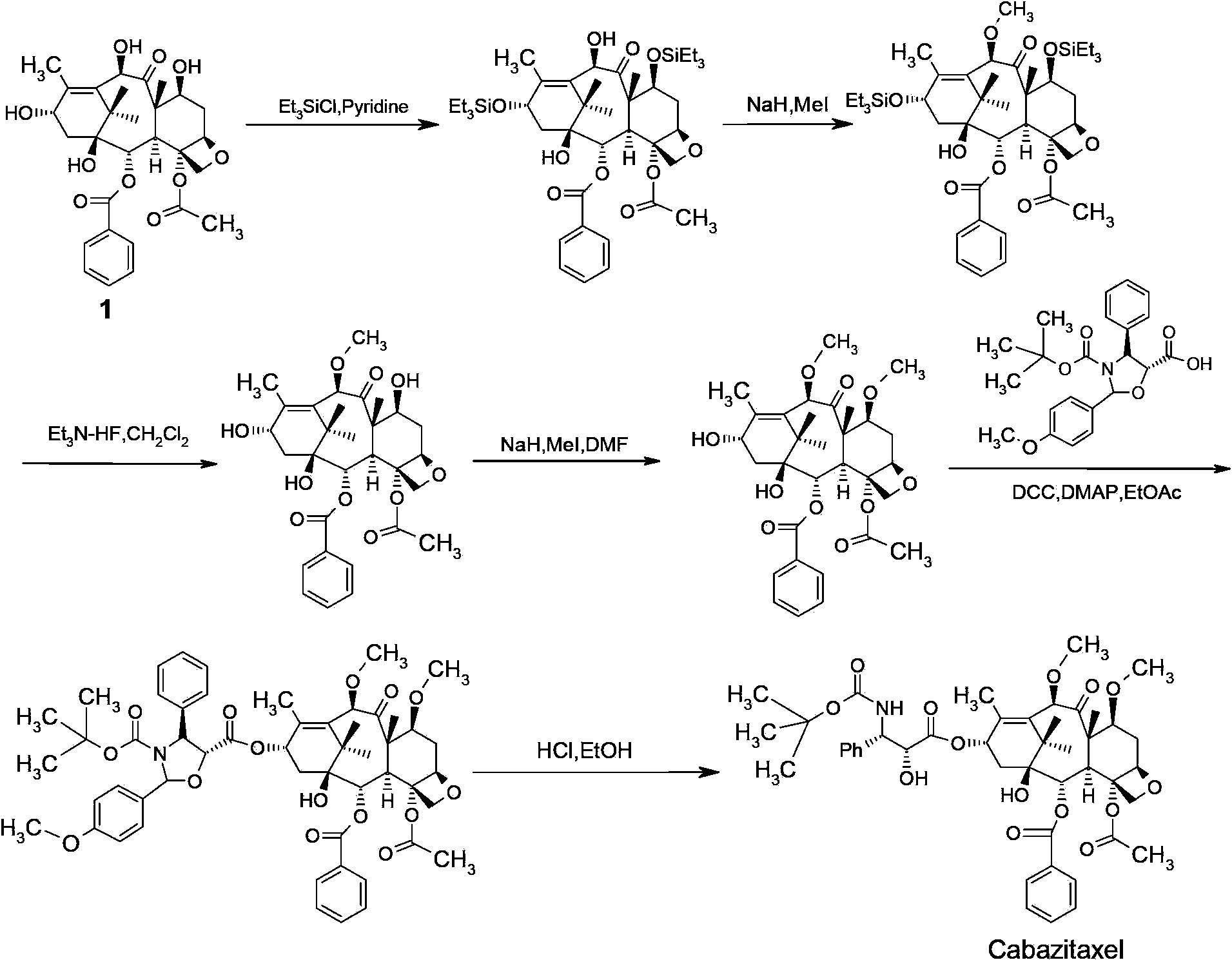
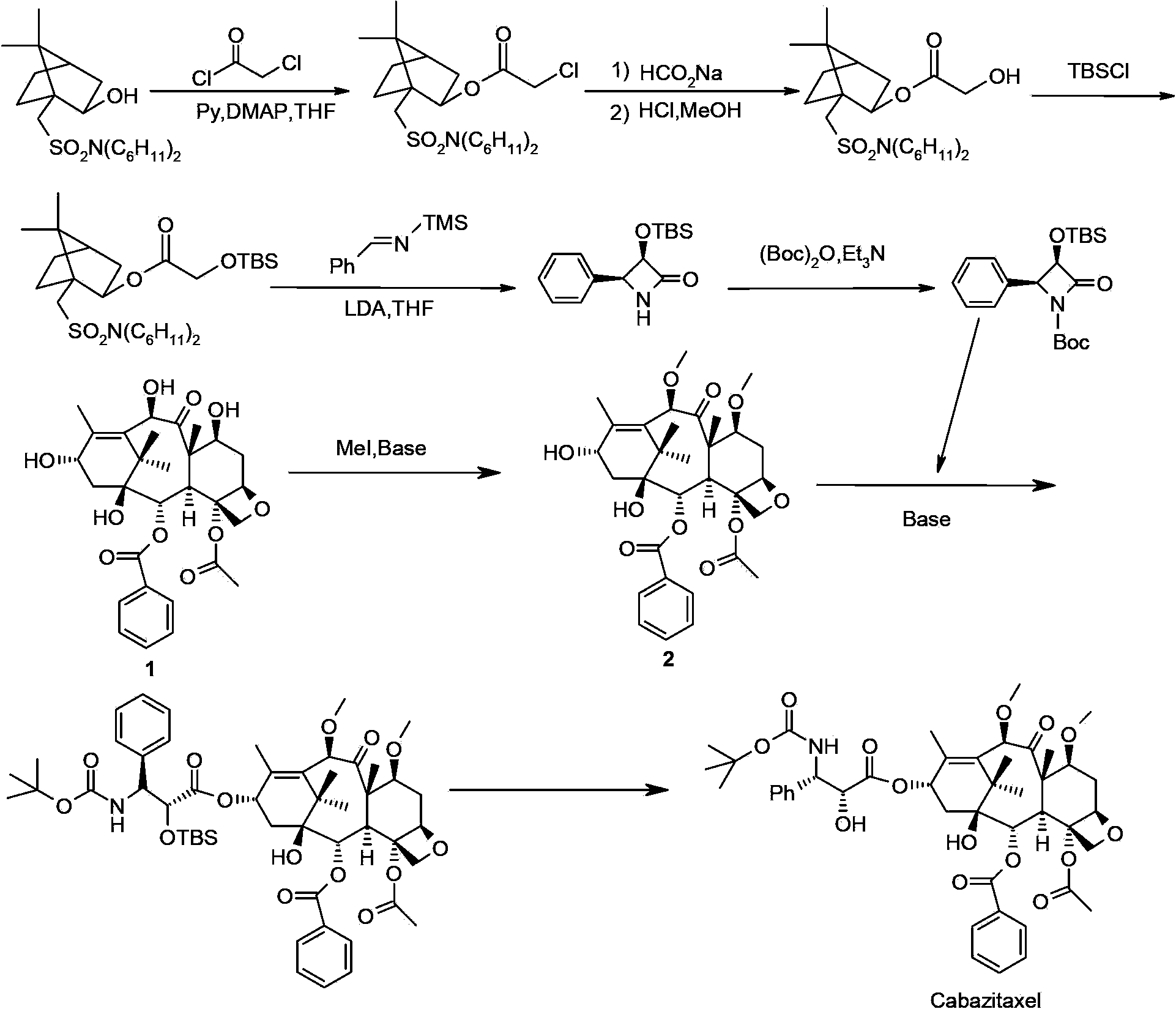
Cabazitaxel intermediate as well as preparation method and application thereof
A cabazitaxel and intermediate technology, which is applied in the field of pharmaceutical synthesis, can solve the problems of harsh three-step synthesis reaction conditions, unsuitable for industrial production requirements, and low purity of the final product, and achieves simple preparation method, mild reaction conditions, and yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: preparation intermediate of the present invention
[0050]
[0051] Step A:
[0052] Under the protection of argon, 10.9 g (20 mmol, commercially available) of 10-deacetylbaccatin III (compound of formula 1) was dissolved in 100 mL of N-methylpyrrolidone (NMP); cooled to -30 ° C, and 1.68 g of sodium hydride was added (70mmol), insulated and stirred for 10 to 20 minutes; 11.36g (80mmol) of methyl iodide was added dropwise, and the insulated and stirred for 3 hours after dropping; naturally warming up to room temperature, stirring and reacting for 6 to 8 hours, TLC detection (developing agent is dichloromethane / Methanol=25 / 1, V / V) After the reaction of the raw materials is completed, the reaction solution is cooled to 0°C, and then 50mL of saturated ammonium chloride aqueous solution is added dropwise; Continue to stir for 1 hour, filter to obtain a light yellow solid; beat with 30% methanol aqueous solution; filter; vacuum-dry at 50°C for 8 hours; the...
Embodiment 2
[0059] Embodiment 2: preparation intermediate of the present invention
[0060] Step A:
[0061] Under argon protection, 10.9 g (20 mmol, commercially available) of 10-deacetylbaccatin III (compound of formula 1) was dissolved in 60 mL of N-ethylpyrrolidone (NEP) and 30 mL of tetrahydrofuran; cooled to -30 ° C, added tert Potassium butoxide 4.5g (40mmol), keep stirring for 10-20 minutes; add 4.24g (40mmol) of trimethyl orthoformate dropwise, keep stirring for 3 hours after dropping; naturally warm to room temperature, stir for 8-10 hours, TLC detection (The developer is dichloromethane / methanol=25 / 1, V / V) After the reaction of the raw materials is completed, the reaction solution is cooled to 0°C, and then 50mL of saturated ammonium chloride aqueous solution is added dropwise; Butyl ether, the solid slowly precipitated, and continued to stir for 1 hour after adding, and filtered to obtain a light yellow solid; slurred with 30% methanol aqueous solution; filtered; vacuum dried...
Embodiment 3
[0064] Embodiment 3: preparation intermediate of the present invention
[0065] Step A:
[0066] Dissolve 5.5g (10mmol) of 10-deacetylbaccatin III (compound of formula 1) in 60mL of pyridine and 40mL of toluene under the protection of argon; cool to -30°C, add 1.0g (41.7mmol) of sodium hydride, and keep stirring 10 to 20 minutes; add 3.8 g (40 mmol) of methyl bromide dropwise, and keep stirring for 3 hours after dropping; naturally warm up to room temperature, stir and react for 6 to 8 hours, and detect by TLC (developing agent is dichloromethane / methanol=25 / 1, V / V) After the reaction of the raw materials is completed, the reaction solution is cooled to 0° C., and then 50 mL of saturated ammonium chloride aqueous solution is added dropwise; after the drop is completed, 300 mL of isopropyl ether is added under stirring, and the solid is slowly precipitated out. After the addition, continue to stir for 1 hour, and filter. A small amount of toluene was rinsed to obtain a light ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com