Cabazitaxel polymorphic form and preparation method thereof
A technology of cabazitaxel, which is applied in the field of medicine, can solve the problems that acetonide is not very stable, is not suitable for industrialized large-scale production, and the quality of raw materials has declined, so as to achieve good purity and content stability, little harm to the environment and personnel, and Good repeatability and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
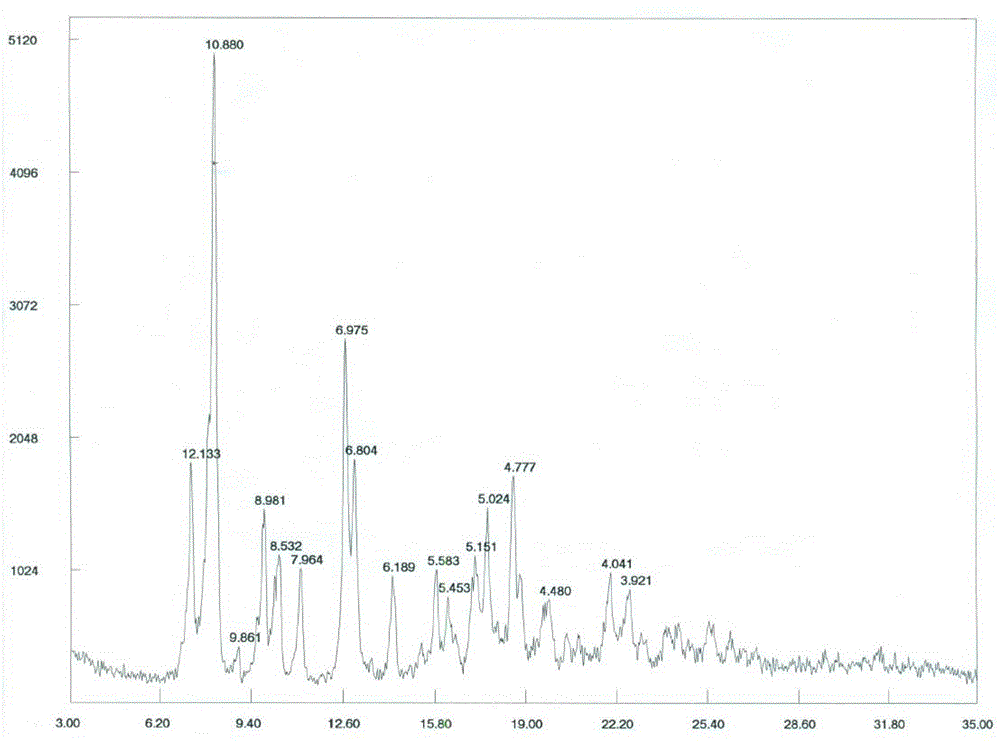
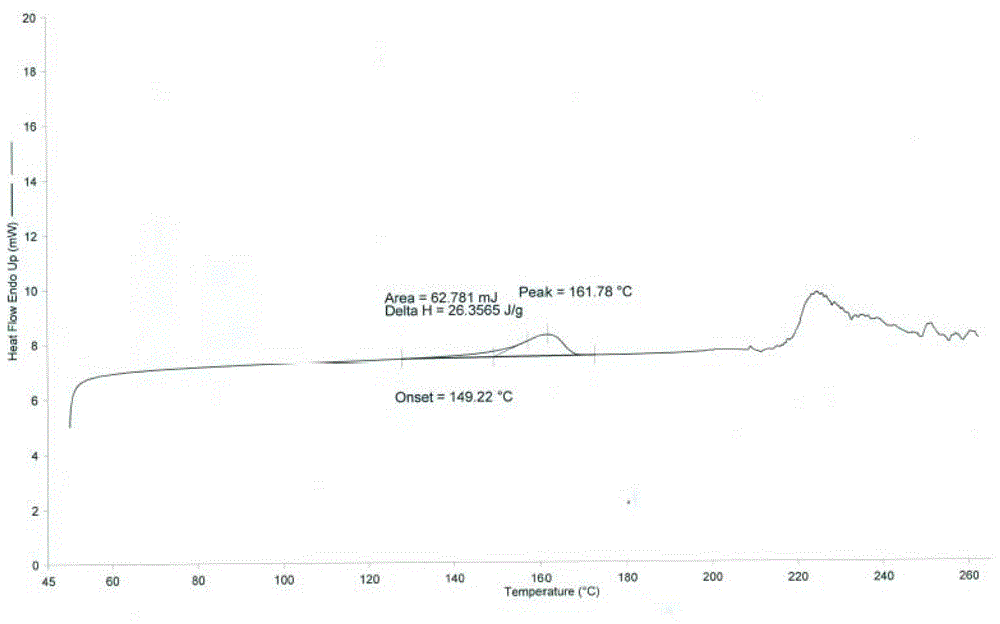
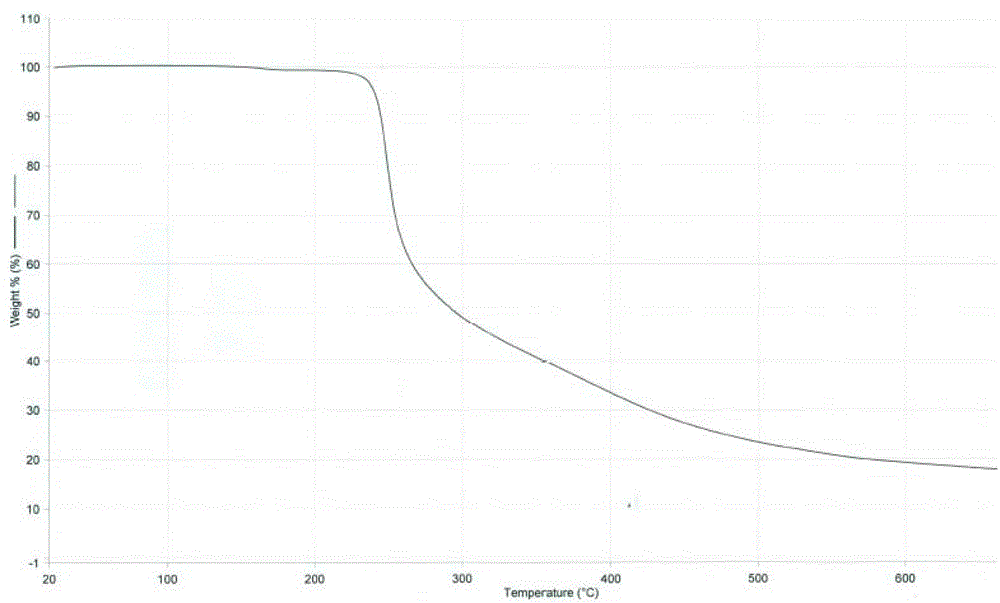
[0040]500 mg of cabazitaxel acetonide was weighed and dissolved in 5 ml of acetone with stirring, and 50 ml of purified water was added dropwise to the above solution at room temperature, resulting in a large amount of white solids. After stirring at room temperature for 30min, filter, the obtained white filter cake was washed with purified water for 3 times, and after vacuum drying, 458 mg of white solid powder was obtained with a yield of 98.0%; the XPRD spectrum of the product is shown in figure 1 , see DSC spectrum figure 2 , see TGA spectrum image 3 , according to the spectrum and data, the compound is in the form of anhydrous cabazitaxel.
Embodiment 2
[0042] 500 mg of cabazitaxel acetonide was weighed and dissolved in 10 ml of acetone with stirring, and 100 ml of purified water was added dropwise to the above solution at room temperature, resulting in a large amount of white solids. After stirring at room temperature for 45min, filter, the obtained white filter cake was washed 3 times with purified water, and 452 mg of white solid powder was obtained after vacuum drying, and the yield was 96.6%; Basically the same, the product is in the form of cabazitaxel anhydrous.
Embodiment 3
[0044] 500 mg of cabazitaxel acetonide was weighed and dissolved in 5 ml of ethanol with stirring, and 50 ml of purified water was added dropwise to the above solution at room temperature, resulting in a large amount of white solids. After stirring at room temperature for 30min, filter, the obtained white filter cake was washed 3 times with purified water, and after vacuum drying, 459 mg of white solid powder was obtained with a yield of 96.2%; the XPRD spectrum of this product is shown in Figure 4 , see DSC spectrum Figure 5 , see TGA spectrum Image 6 , and there is no organic solvent residue in the crystal form by gas phase detection. According to the spectrum and data, the product is in the form of cabazitaxel monohydrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com