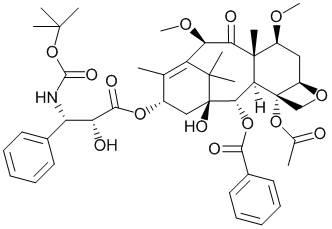
Synthetic method of cabazitaxel
A technique for the synthesis of cabazitaxel, which is applied in the production of bulk chemicals and organic chemistry, can solve the problems of general Raney nickel reduction yield, high cost of cabazitaxel, and many by-products, and achieve low price, Yield improvement and usage reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Route 1
[0058] (1) Synthesis of the type II-1 compound:
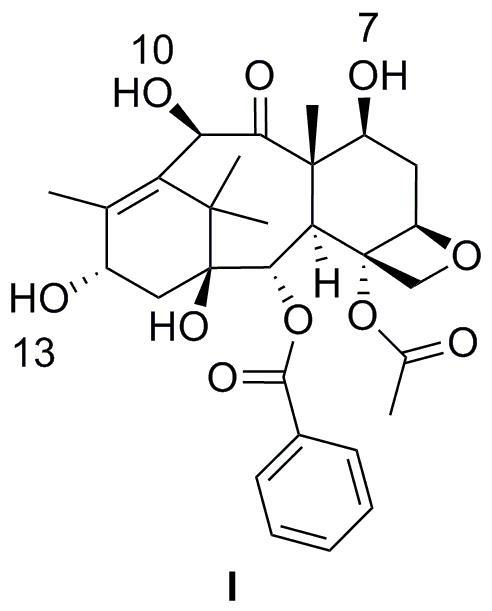
[0059] In dichloromethane, add the reaction solution slowly, keep it at 0 ° C for 30 minutes, rise to room temperature for another 30 minutes, slowly dripping a small amount of water to terminate the reaction, the concentrated reaction solution is oily, add 100ml dichloromethane to dissolve, and dissolve it.Wash 2m hydrochloride until the water layer is acidic, and then wash with saturated sodium bicarbonate solution (50ml × 2), washed (50ml × 2), washed with saturated sodium chloride solution (50ml × 2).Dry magnesium sulfate for 2 hours, filter, and dry.Add a mixed solution of 20ml ether / petroleum ether (volume ratio 1 / 1), stir at room temperature for 2 hours, filtrate, 40 ° C vacuum dry 7,10-dual (trichloroaceteoxyl) -10-de-acetylkbaka Pavilion PavilionIII 16.12g, revenue: 90.6%.
[0060] 1 H-NMR (400 MHz, CDCL 3 ) δ = 1.12 (s, 3H), 1.15 (s, 3H), 1.84 (s, 3H), 2.05 (m, 1H), 2.14 (m, 1H), 2.17 (S, 3H), 2.31 (m, ...
Embodiment 2
[0079] Route 2
[0080] (1) Format II-2 compound 7,10-dual (trichlorolyxyxyl) -10-synthesis of de-acetyl Kiba Kataki Pavilion III:
[0081] With reference to the method of Example 1, the type II-2 compound is obtained, and the yield is 91.2%.
[0082] (2) Synthesis of the type III-2 compound:
[0083] Solk the 3.44g 7,10-dual (trichlorolyxyxyl) -10-de-acetylkaba Kataki III in 50ml pyridine, and then slowly drip into TESCL (triathyl chloride) 0.62g, heated to 120℃, stir 4 hours, add 100ml water to the reaction solution, and then use acetate (100ml × 3) to extract the water layer, combine the organic layer, wash (50ml × 2), wash the saturated salt water (50ml × 2), add no additionDry water and sulfate, and use petroleum ether / dichloromethane (volume ratio 2 / 1) silicone column layer, that is, to obtain 2.92g of 7,10-dual (trichlorine steroxyl) -13-trTeechlor chothrose-10-de-acetyl Kuba Kaitting III, the earnings of 90.0%.
[0084] 1 H-NMR (400 MHz, CDCL 3 ) δ = 0.55 (m, 6H), 0.93 (...
Embodiment 3
[0099] Route 3
[0100] (1) Synthesis of the type II-3 compound:
[0101] Among the 250ml triangular bottle, nitrogen protection, 10.81g of 10-de-acetyl Kabata Pavilion III III at 100ml water pyridine, stir to 0 ° C for 10 minutes, dissolve 8ml methane methaneIn chloride, add the reaction solution slowly, keep it at 0 ° C for 30 minutes, rose to room temperature for another 40 minutes, slowly dripping a small amount of water to terminate the reaction, the concentrated reaction solution is oily, add 100ml dichloromethane dissolved, use2M hydrochloride was washed to the water layer as acidic, and then washed with saturated sodium bicarbonate solution (50ml × 2), washed (50ml × 2), washed with saturated sodium chloride solution (50ml × 2), stirred, and water -free sulfuric acidMagnesium dried for 2 hours, filtered, and dried.Add a mixed solution of 20ml ether / petroleum ether (volume ratio 1 / 1), stir at room temperature for 2.5 hours, filter, 40 ° C vacuum drying 7,10-double (metham...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com