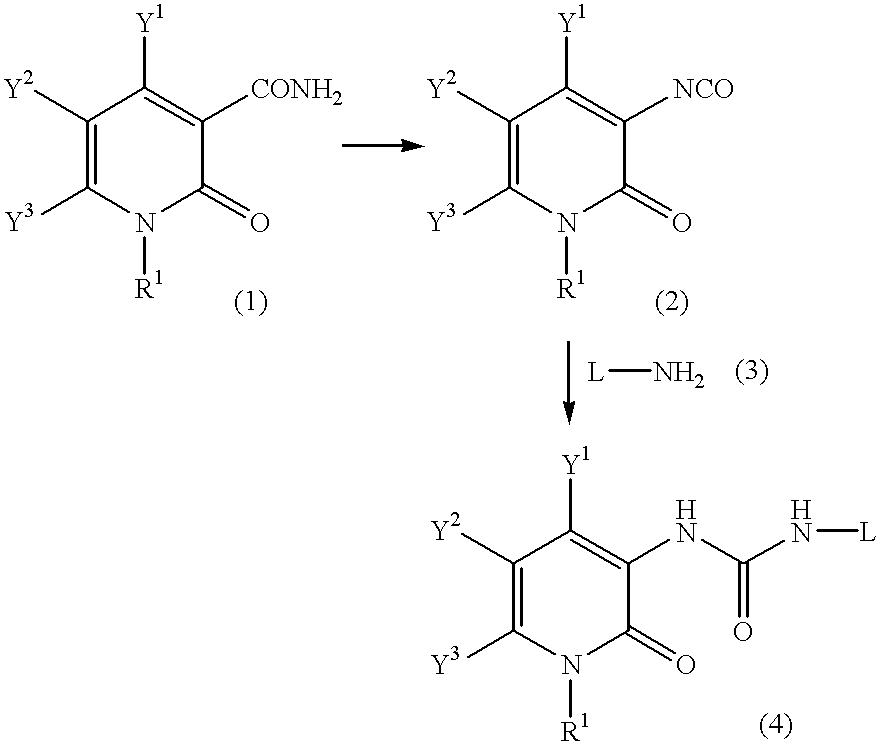
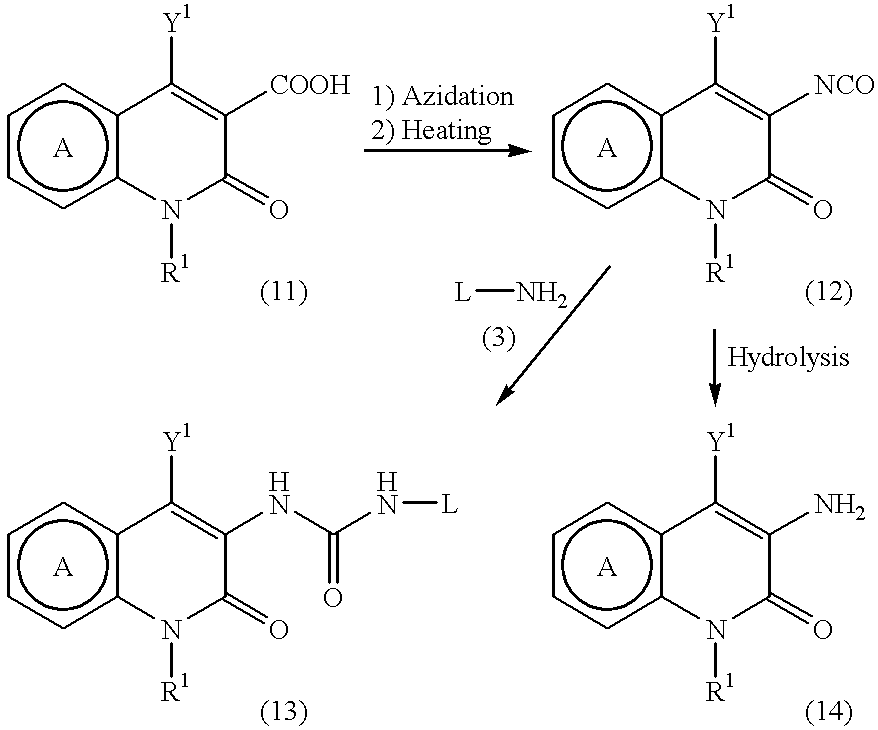
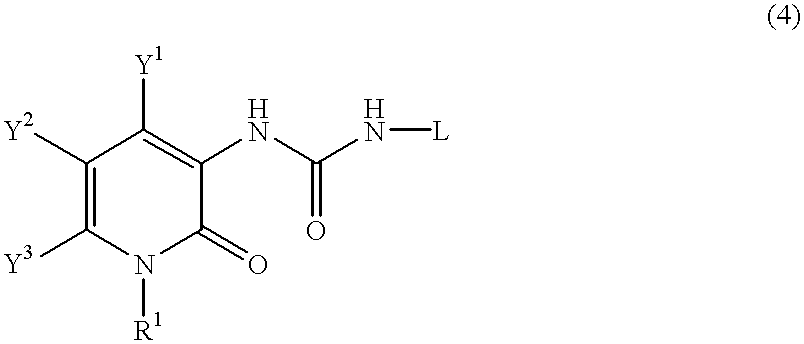
Pyridone derivatives and process for preparing the same
a technology of pyridone and derivatives, applied in the field of pyridone derivatives, can solve the problems of unsuitable large-scale production with respect to safety and explosion risk, and achieve the effects of improving the yield of reaction, facilitating reaction, and proceeding quickly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0218] Preparation of N-[1-butyl-4-(3-methoxyphenyl)-1,2-dihydro-2-oxo-1,8--naphthyridin-3-yl]-N'-(2,6-diisopropylphenyl) urea: 58
[0219] To a suspension of 1-butyl-3-carbamoyl-4-(3-methoxyphenyl)-1,2-dihy-dro-2-oxo-1,8-naphthyridine (10.0 g, 27.2 mmol) in N,N-dimethylformamide (100 ml) was added lead tetracetate (14.5 g, 32.6 mmol), and the mixture was stirred at room temperature for 0.5 hour. Subsequently, to the mixture was added 2,6-diisopropylaniline (5.3 g, 30 mmol) at the same temperature, and the mixture was stirred at 40.degree. C. for 50.degree. C. for 1.5 hour. After allowed to cool, ethyl acetate (500 ml) was added to the mixture, and the mixture was filtered through a celite pad. The filtrate was washed successively with water, 4 N aqueous hydrochloric acid solution, water and a saturated aqueous sodium chloride solution, dried over magnesium sulfate, and concentrated under reduced pressure to the volume of about 100 ml. The resultant was stirred for 2 hours under coolin...
example 2
[0221] Preparation of N-[1-butyl-4-(3-methoxyphenyl)-1,2-dihydro-2-oxo-1,8--naphthyridin-3-yl]-N'-(2,6-diisopropylphenyl)urea: 59
[0222] To a 1 N aqueuos sodium hydroxide solution (48 ml, 48 mmol) was added dropwise bromine (1.2 ml, 24 mmol) under ice-cooling, and the mixture was stirred for 30 minutes. The pale yellow solution thus obtained was added dropwise to a suspension of 1-butyl-3-carbamoyl-4-(3-m-ethoxyphenyl)-1,2-dihydro-2-oxo-1,8-naphthyridine (2.1 g, 6 mmol) and tetrabutylammonium hydrogen sulfate (102 mg, 0.3 mmol) in toluene (210 ml) at room temperature, and the mixture was stirred at the same temperature for 4 hours. To the mixture was added a solution of acetic acid (35 ml) and 2,6-diisopropylaniline (1.6 g, 9.0 mmol) in toluene (35 ml) at room temperature, and the mixture was stirred at the same temperature for 1.5 hour. Water was added to the reaction solution, and the mixture was extracted with ethyl acetate, washed with water, washed with a saturated aqueous sodiu...
example 3
[0224] Preparation of 1-butyl-3-amino-4-(3-methoxyphenyl)-1,2-dihydro-2-ox-o-1,8-naphthyridine; 60
[0225] To a 1 N aqueous sodium hydroxide-solution (88 ml, 88 mmol) was added dropwise bromine (1.0 ml, 19.4 mmol) under ice-cooling, and the mixture was stirred for 30 minutes. The pale yellow solution thus obtained was added dropwise to a suspension of 1-butyl-3-carbamoyl-4-(3-m-ethoxyphenyl)-1,2-dihydro-2-oxo- 1,8-naphthyridine (5.0 g, 14.2 ml) and tetrabutylammonium hydrogen sulfate (250 mg. 0.71 mmol) in tetrahydrofuran (500 ml) at room temperature, and the mixture was stirred at the same temperature for 6.5 hours. Water was added to the reaction solution, and the mixture was extracted with ethyl acetate, washed with water, washed with a saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. To the concentrated residue was added 2-propanol (40 ml), and the mixture was stirred for 3 hours under ice-cooling. The pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weights | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com