10-deacetylbaccatin iii and method for methoxylation of its derivative
A deacetylation bacca, methoxylation technology, applied in organic chemistry, silicon organic compounds, etc., can solve the problem that the reaction cannot be carried out smoothly and quickly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
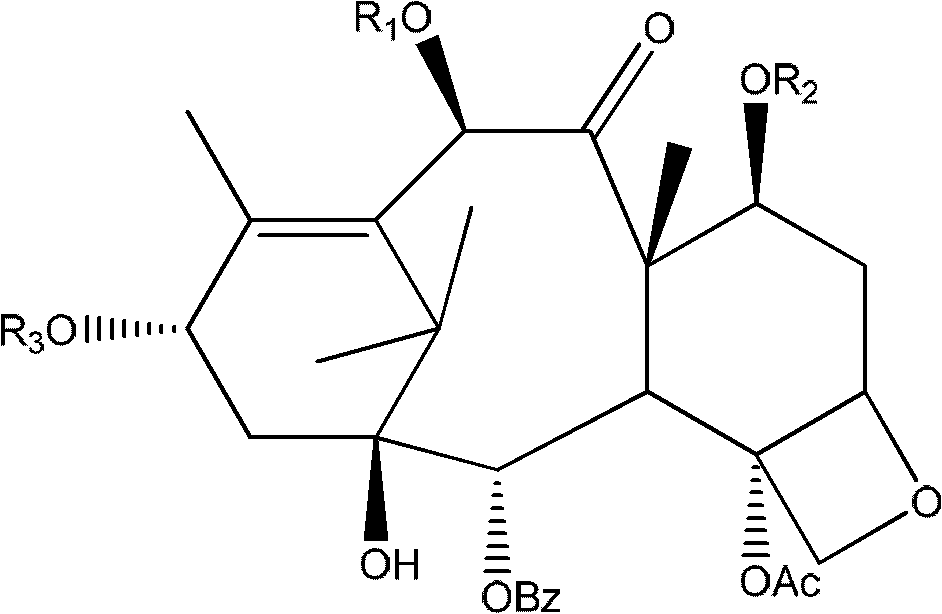
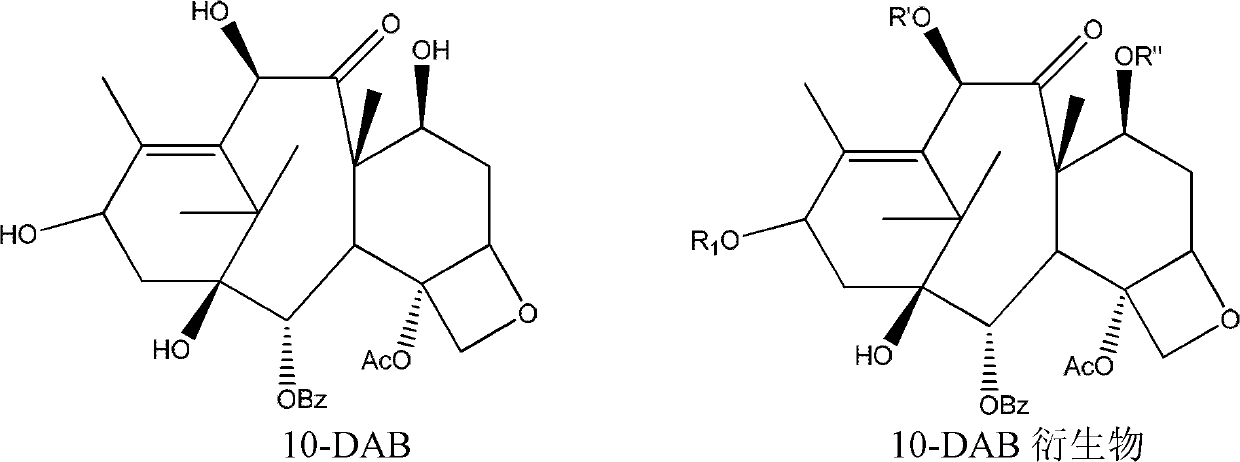
[0031] In a 5L reaction flask, 100.0g (0.15mol) of 13-triethylsilyl-10-DAB was dissolved in 2.5L of anhydrous ether, and 1g (0.003mol) of tetrabutyl bromide was added under mechanical stirring Ammonium chloride, cryogenically cooled to -40 degrees Celsius. Then, a solution of dimethyl sulfate (1 mol, 126 g) in 200 mL of anhydrous ether was added dropwise into the reaction flask. Then add 60% NaH 134.7g (3.3mol) powder in batches from the feeding tube. The addition is completed in about 1 hour, and the addition rate is controlled so that the temperature of the reaction solution in the reaction bottle is below -15 degrees Celsius. After stirring for another hour, the ice-salt bath was removed; according to the detection of TLC, after the reaction of the starting materials was completed, 1000 mL of 0.5 mol / L hydrochloric acid was added dropwise with the ice bath turned on, and then the lower layer of water was separated, and then 1000 mL was added. .5mol / L hydrochloric acid sol...
Embodiment 2
[0033] Suspend 100.0 g (0.18 mol) of 10-DAB in 2.5 L of anhydrous ether in a 5 L reaction flask, add 1 g of tetrabutylammonium bromide with mechanical stirring, and bathe in ice-salt water to 0 °C. Then, a solution of dimethyl sulfate (1 mol, 126 g) in 200 mL of anhydrous ether was added dropwise into the reaction flask. Then add 60% NaH 134.7g (3.3mol) powder in batches from the feeding tube. The addition was completed in about 1 hour, and the addition rate was controlled so that the temperature of the reaction solution in the reaction bottle was below 5 degrees Celsius. After stirring for another hour, the ice-salt bath was removed; after the reaction of the starting materials was completed according to TLC detection, 1000 mL of 0.5 mol / L hydrochloric acid was added dropwise with the ice bath turned on, and the lower aqueous phase was separated, and then 1000 mL of After the 0.5mol / L hydrochloric acid solution was stirred, the lower aqueous phase was separated. Then, after...
Embodiment 3
[0035]Suspend 100.0 g (0.18 mol) of 10-DAB in 2.5 L of anhydrous toluene in a 10 L reaction flask, add 1 g of tetrabutylammonium bromide with mechanical stirring, and bathe in ice-salt water to 0 degrees Celsius. Then a solution of dimethyl sulfate (0.5 mol, 63 g) in 1000 mL of anhydrous toluene was added dropwise into the reaction flask. Then add 6.58 g (0.24 mol) of 90% NaH solid powder in batches from the feeding tube. The addition was completed in about 1 hour, and the addition rate was controlled so that the temperature of the reaction solution in the reaction bottle was below 5 degrees Celsius. After stirring for another 1 hour, the ice-salt bath was removed; after the reaction of the starting materials was completed according to TLC detection, 1000 mL of 0.5 mol / L hydrochloric acid was added dropwise with the ice bath turned on, and the lower aqueous phase was separated, and then 1000 mL of After the 0.5mol / L hydrochloric acid solution was stirred, the lower aqueous ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com