Method for preparing heterocyclic compounds of methyl mercapto under ionic liquid catalysis
A technology of heterocyclic compounds and ionic liquids, applied in chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, chemical/physical processes, etc., can solve problems that no one has raised, and achieve simple and easy operation The effect of actual production and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
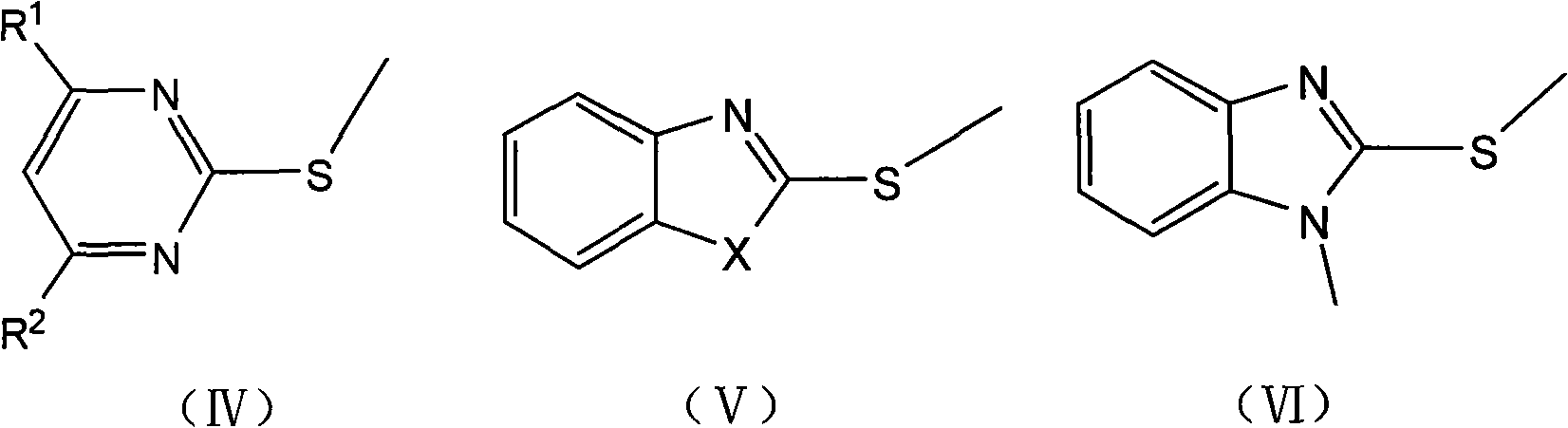
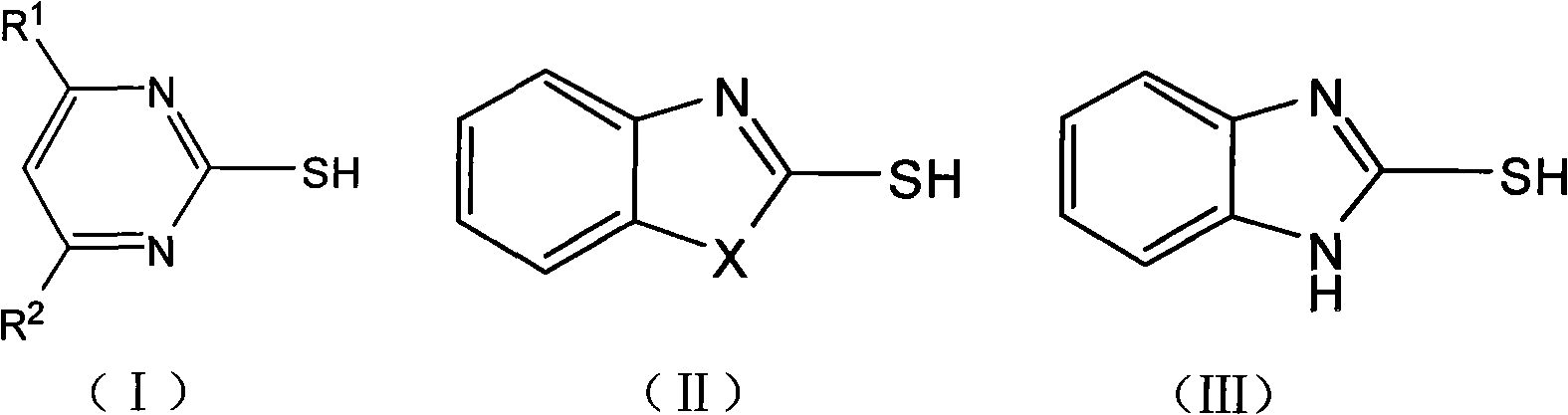
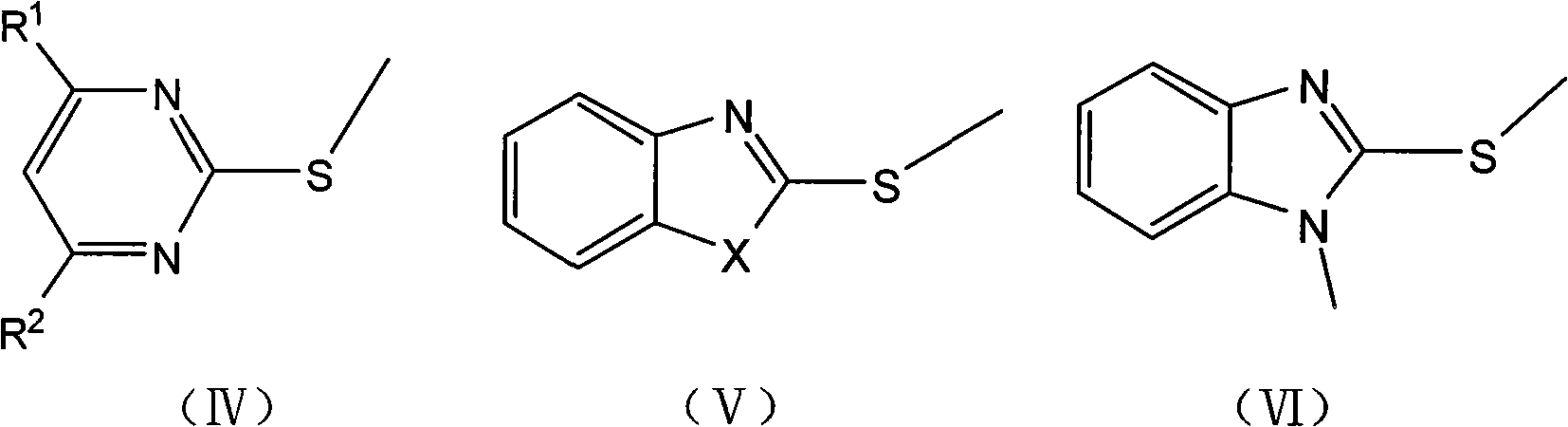
[0023] Preparation of 2-methylthiopyrimidine
[0024] Dissolve 2-mercaptopyrimidine (5.6g, 0.05mol) in 1-butyl-3-methylimidazole chloride (3.5g, 0.02mol), add dimethyl carbonate (5.4g, 0.06mol), and heat up under stirring to 90-130°C, react for 3-5 hours, and monitor by TLC or HPLC. After the reaction was completed, the product was cooled down to room temperature, extracted with ethyl acetate (10-30 ml), concentrated under reduced pressure, and the solvent was recovered to obtain the product as a colorless oil with a content of 97.6%. Add 2-mercaptopyrimidine and dimethyl carbonate to the ionic liquid, react under the same conditions, and cycle 5 times, the reaction results are shown in the following table:
[0025]
Embodiment 2
[0027] Preparation of 2-Methylthiobenzoxazole
[0028] Dissolve 2-mercaptobenzoxazole (12.1g, 0.08mol) in 1-butyl-3-methylimidazole chloride (7.0g, 0.04mol), add dimethyl carbonate (8.1g, 0.09mol), Heat up to 90-130°C with stirring, react for 2-4 hours, and monitor by TLC or HPLC. After the reaction was completed, it was lowered to room temperature, the product was extracted with ethyl acetate (10-30 ml), concentrated under reduced pressure, and the solvent was recovered. The product was vacuum-dried to obtain 10.0 g with a content of 95.2%. Yield 75.6%.
Embodiment 3
[0030] Preparation of 2-Methylthiobenzothiazole
[0031] Dissolve 2-mercaptobenzothiazole (16.7g, 0.1mol) in 1-butyl-3-methylimidazole chloride (10.5g, 0.06mol), add dimethyl carbonate (10.8g, 0.12mol), and stir Lower the temperature to 90-130°C, react for 2-4 hours, and monitor by TLC or HPLC. After the reaction was completed, it was lowered to room temperature, the product was extracted with ethyl acetate (10-30 ml), concentrated under reduced pressure, and the solvent was recovered. The product was vacuum-dried to obtain 14.9 g with a content of 95.0%. Yield 82.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com