Method for preparing methylnaltrexone bromide
A technology of bromomethylnaltrexone and naltrexone, applied in the field of pharmaceutical engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
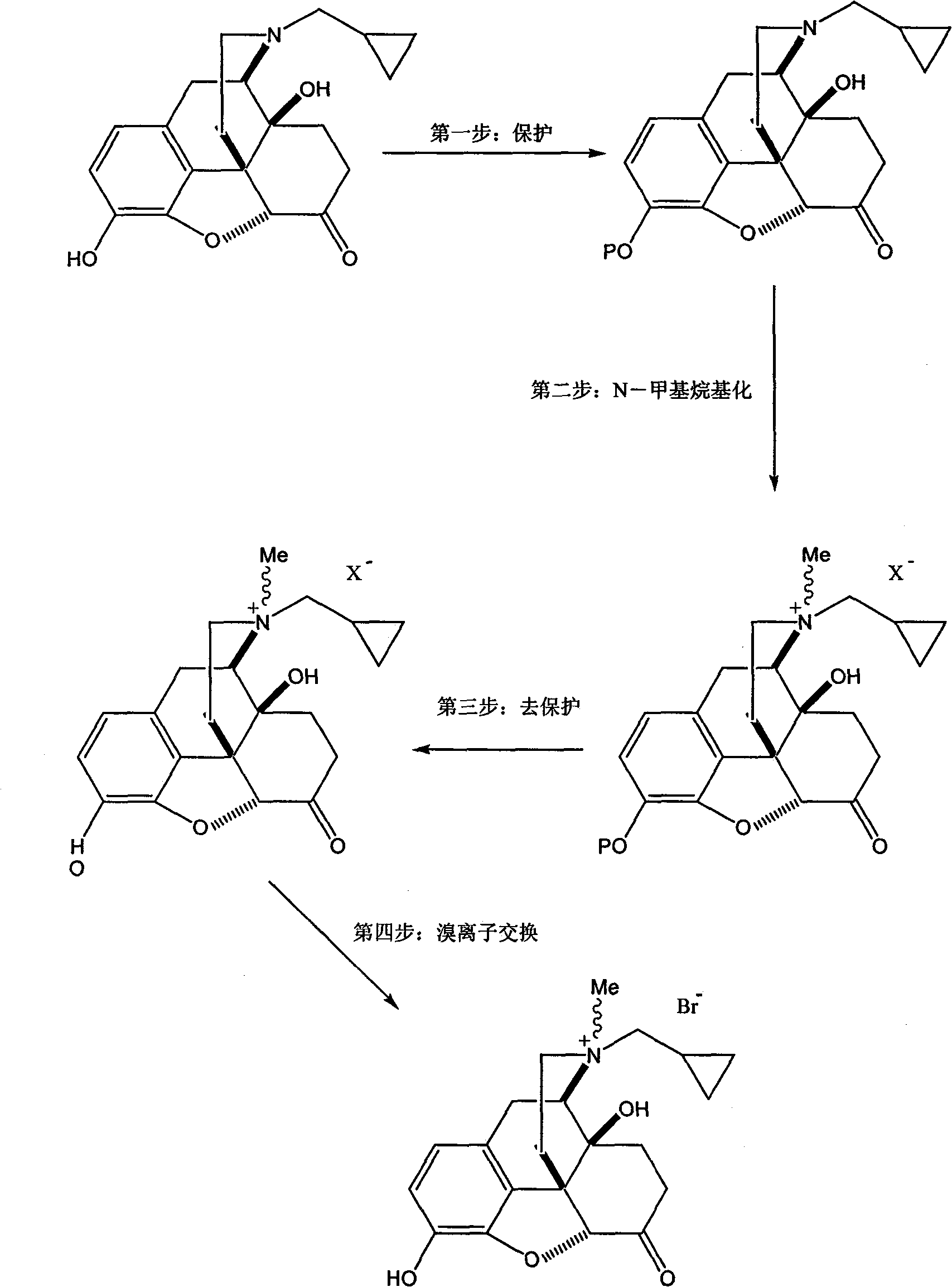
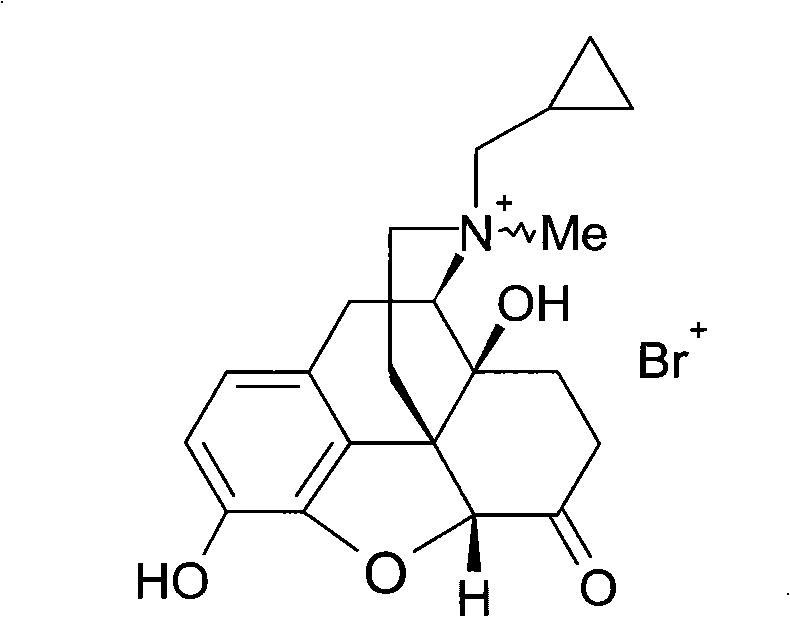
[0062] This example illustrates the preparation of bromomethylnaltrexone.
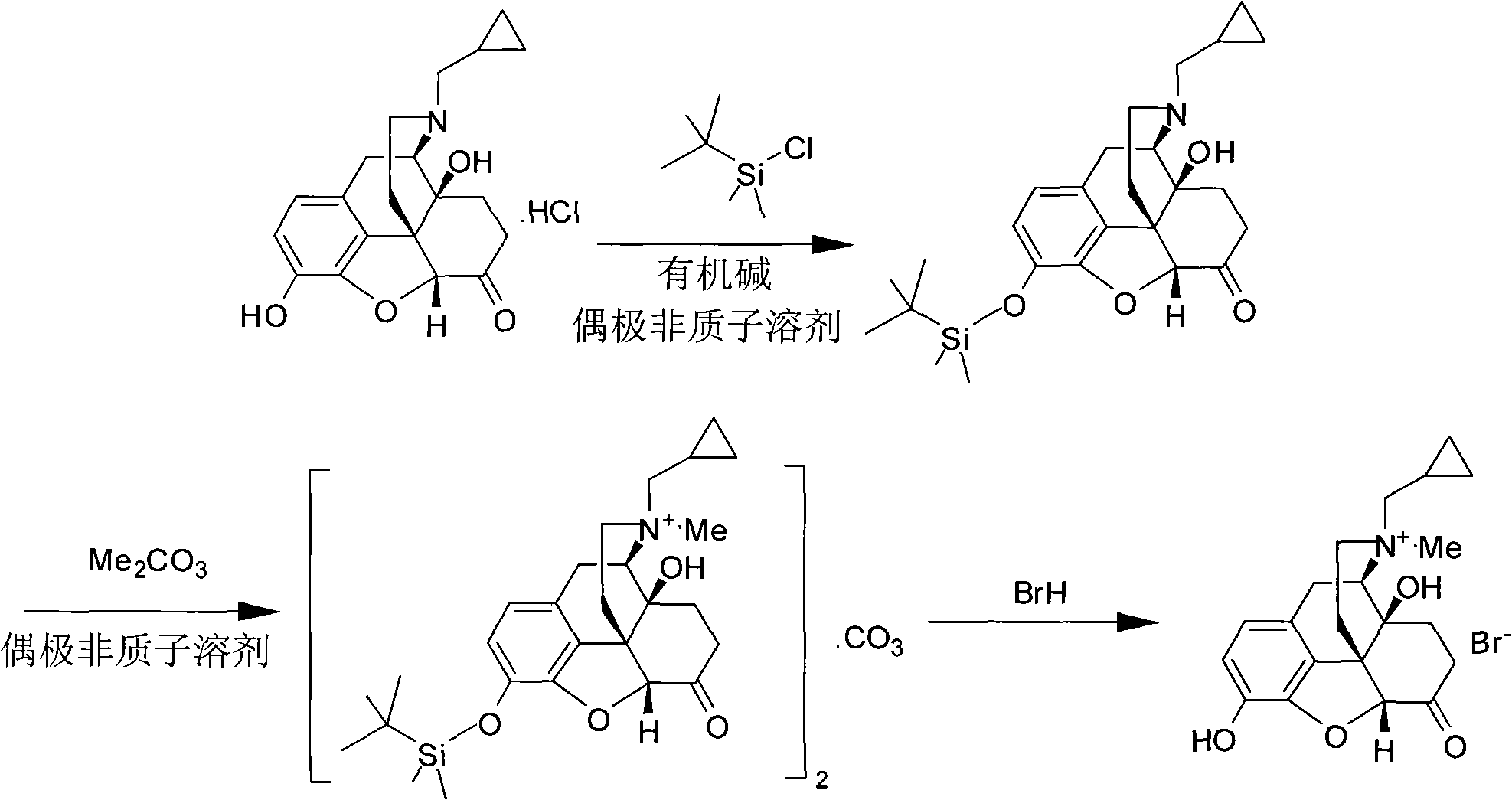
[0063] In the dry reaction flask, add naltrexone hydrochloride (94.5g, 0.25mol), N,N-dimethylformamide 1000mL, then add triethylamine (50.6g, 0.5mol), and stir the mixture at 20°C for 0.5 hours, Dimethyl tert-butylchlorosilane (37.7 g, 0.25 mol) was added slowly, and the mixture was stirred at 25° C. for 18 hours. The reaction solution was poured into 500 mL of water, and the resulting product was extracted with ethyl acetate. The ethyl acetate layer was dried and concentrated to dryness under reduced pressure to obtain 3-O-dimethyl tert-butylsilyl ether group-naltrexone (105 g, 0.23mol), yield 92%.
[0064] In the dry reaction flask, add 3-O-dimethyl tert-butylsilyl ether base-naltrexone (105g, 0.23mol), N,N-dimethylformamide 500mL, add dimethyl carbonate (62.2g , 0.69mol), stirred at 100°C for 8 hours, concentrated under reduced pressure to 100mL, filtered after cooling in an ice-water bath, and va...
Embodiment 2
[0067] This example illustrates the preparation of bromomethylnaltrexone.
[0068] In the dry reaction flask, add naltrexone hydrochloride (94.5g, 0.25mol), acetone 1000mL, then add pyridine (59.3g, 0.75mol), stir the mixture at 20°C for 0.5 hour, slowly add dimethyl tert-butyl chloride Silane (37.7 g, 0.25 mol), and the mixture was stirred at 30° C. for 12 hours. The reaction solution was poured into 500 mL of water, and the resulting product was extracted with ethyl acetate. The ethyl acetate layer was dried and then concentrated to dryness under reduced pressure to obtain 3-O-dimethyl tert-butylsilyl ether group-naltrexone (100 g, 0.22mol), the yield is 88%.
[0069] In the dry reaction flask, add 3-O-dimethyl tert-butylsilyl ether group-naltrexone (100g, 0.22mol), acetone 500mL, add dimethyl carbonate (99.2g, 1.1mol), stir at 105°C Concentrate under reduced pressure to 100 mL for 8 hours, filter after cooling in an ice-water bath, and dry the filter cake in vacuum at 35°...
Embodiment 3
[0072] This example illustrates the preparation of bromomethylnaltrexone.
[0073] In the dry reaction flask, add naltrexone hydrochloride (94.5g, 0.25mol), N-methylpyrrolidone 1000mL, then add N,N-dimethylaniline (75.7g, 0.625mol), and stir the mixture at 20°C for 0.5 hours , slowly added dimethyl tert-butylchlorosilane (37.7 g, 0.25 mol), and the mixture was stirred at 20° C. for 24 hours. The reaction solution was poured into 500 mL of water, and the resulting product was extracted with ethyl acetate. The ethyl acetate layer was dried and then concentrated to dryness under reduced pressure to obtain 3-O-dimethyl tert-butylsilyl-naltrexone (110 g, 0.23mol), yield 92%.
[0074] In the dry reaction bottle, add 3-O-dimethyl tert-butyl silyl ether base-naltrexone (110g, 0.23mol), N-methylpyrrolidone 500mL, add dimethyl carbonate (82.9g, 0.92mol) , stirred at 110°C for 6 hours, concentrated under reduced pressure to 100mL, filtered after cooling in an ice-water bath, and vacuum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com