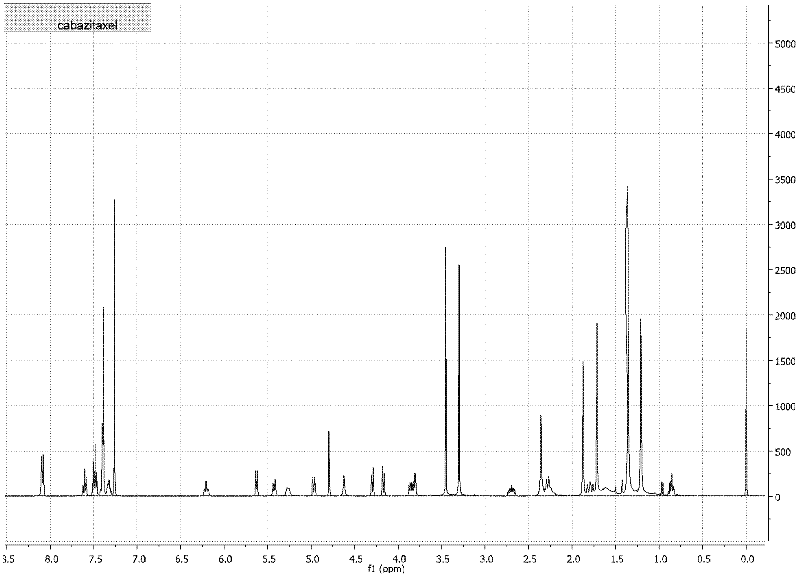
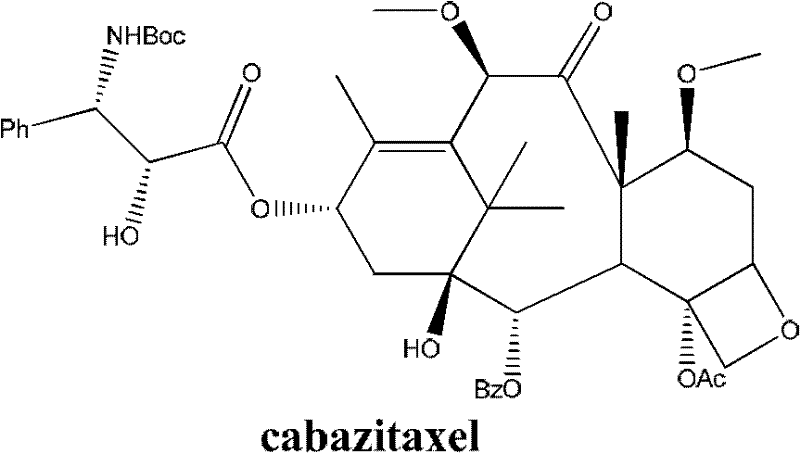
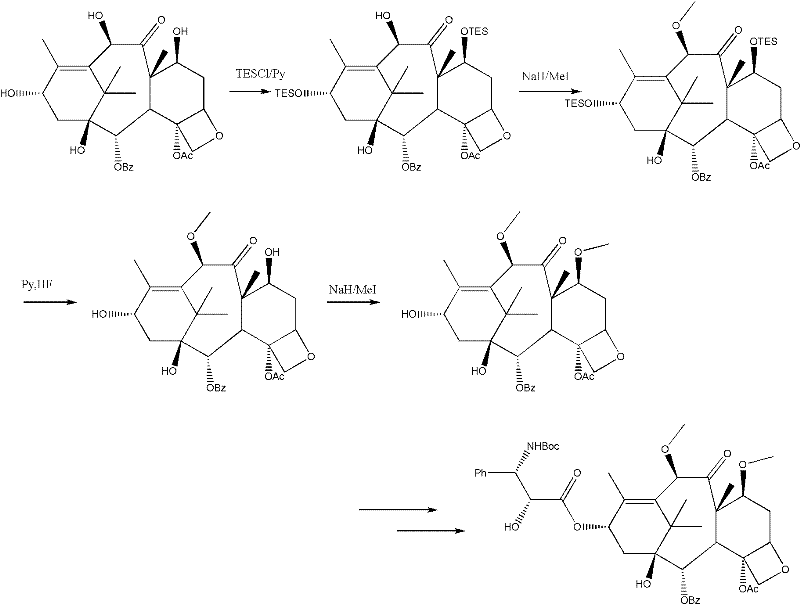
Method for preparing cabazitaxel by taking 10-deacetylate-baccatin III as raw material
A technology for deacetylation and cabazitaxel, which is applied in the field of drug synthesis, can solve problems such as long synthesis paths, and achieve the effects of saving labor, simplifying operation steps, and increasing preparation and metering.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Suspend 10.0g (0.018mol) of 10-DAB in 250mL of chloroform in a 500mL reaction flask, add 5mL of pyridine under mechanical stirring, and control the temperature to 0°C with an ice-salt bath. Then, a solution of 5.46 mL (0.040 mol) of trichloroethyl chloroformate in 20 mL of chloroform was added dropwise into the reaction flask. The dropwise addition is completed in about 1 hour, and the rate of addition is controlled so that the temperature of the reaction solution in the reaction bottle is below 5 degrees Celsius. After stirring for another hour, the ice-salt bath was removed; according to the detection of TLC, after the reaction of 10-DAB was completed, 100 mL of 0.5 mol / L hydrochloric acid was added dropwise with the ice-salt bath turned on, and the lower aqueous phase was separated by standing, and then added 100mL of 0.5mol / L hydrochloric acid solution was stirred and the lower aqueous phase was separated. Then, after washing with 50 mL of saturated brine, the ...
Embodiment 2
[0032] (1) Suspend 100.0 g (0.18 mol) of 10-DAB in 2.5 L of dichloromethane in a 500 mL reaction flask, and add 50 mL of pyridine in ice-salt water bath to 0 degrees Celsius under the condition of mechanical stirring. Then, a solution of 54.6 mL (0.40 mol) of trichloroethyl chloroformate in 200 mL of dichloromethane was added dropwise into the reaction flask. The dropwise addition is completed in about 1 hour, and the rate of addition is controlled so that the temperature of the reaction solution in the reaction bottle is below 5 degrees Celsius. After stirring for another 1 hour, the ice-salt bath was removed; after the 10-DAB reaction was completed according to the detection of TLC, 1000 mL of 0.5 mol / L hydrochloric acid was added dropwise under the condition of opening the ice bath, and the lower aqueous phase was separated, and then 1000 mL was added. .5mol / L hydrochloric acid solution was stirred and the lower aqueous phase was separated. Then, after washing with 500 mL ...
Embodiment 3
[0039] (1) Suspend 200.0 g (0.36 mol) of 10-DAB in 6 L of chloroform in a 10 L reaction flask, and add 100 mL of pyridine to ice-salt water bath to 0 degrees Celsius under the condition of mechanical stirring. Then, a solution of 109.2 mL (0.80 mol) of trichloroethyl chloroformate in 400 mL of dichloromethane was added dropwise into the reaction flask. The dropwise addition is completed in about 1 hour, and the rate of addition is controlled so that the temperature of the reaction solution in the reaction bottle is below 5 degrees Celsius. After stirring for another 1 hour, the ice-salt bath was removed; after the 10-DAB reaction was completed according to the detection of TLC, 2000 mL of 0.5 mol / L hydrochloric acid was added dropwise with the ice bath turned on, and the lower aqueous phase was separated, and then 2000 mL was added. .5mol / L hydrochloric acid solution was stirred and the lower aqueous phase was separated. Then, after washing with 1000 mL of saturated brine, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com