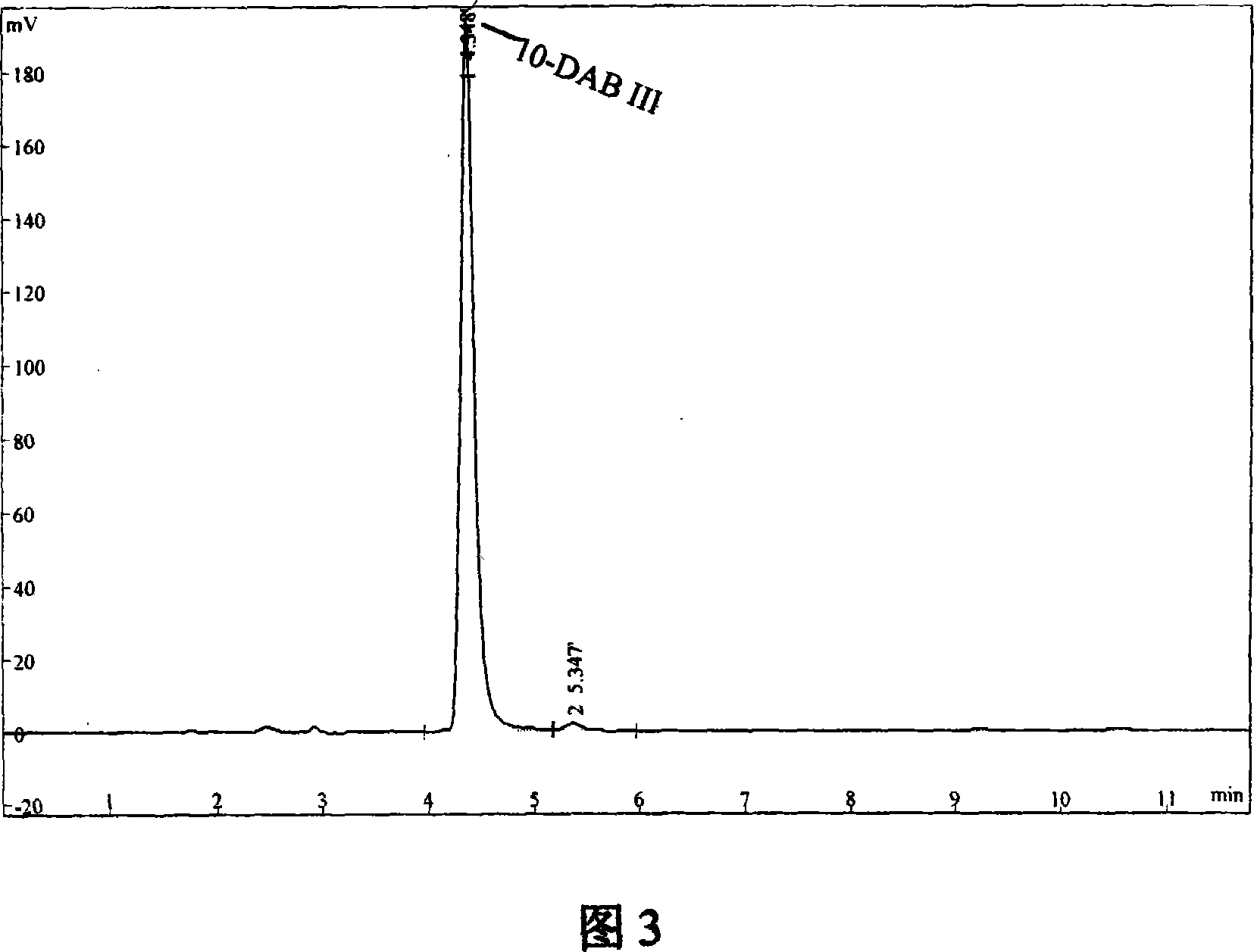
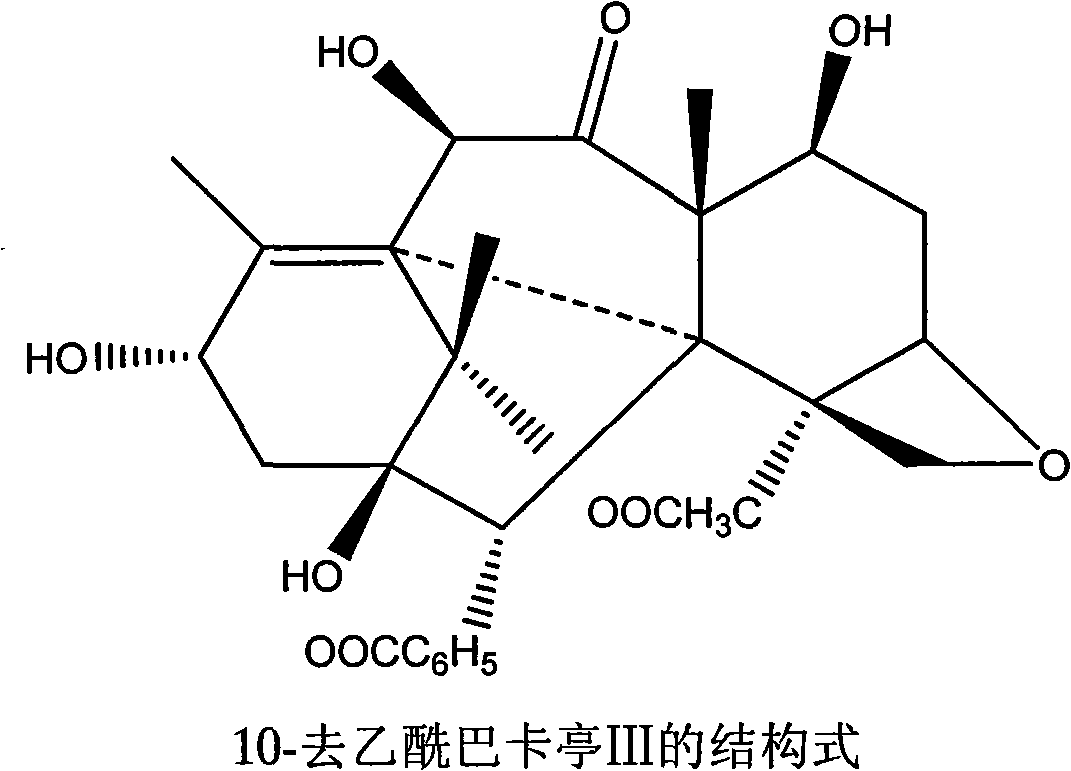
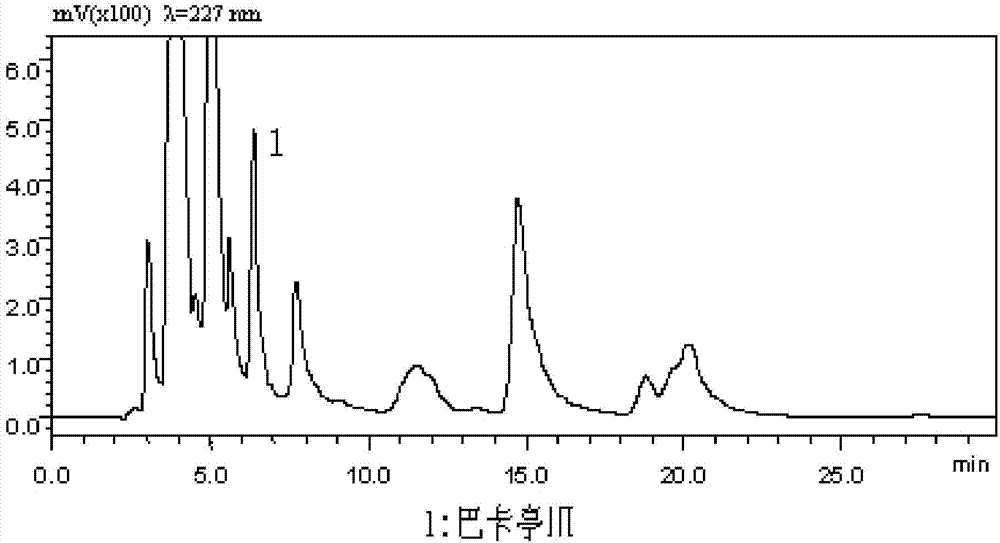
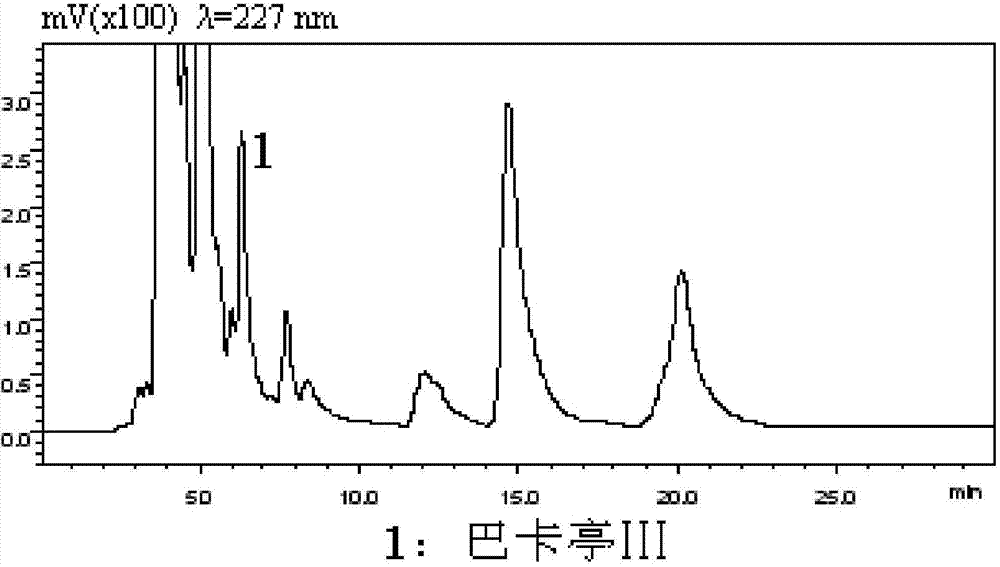
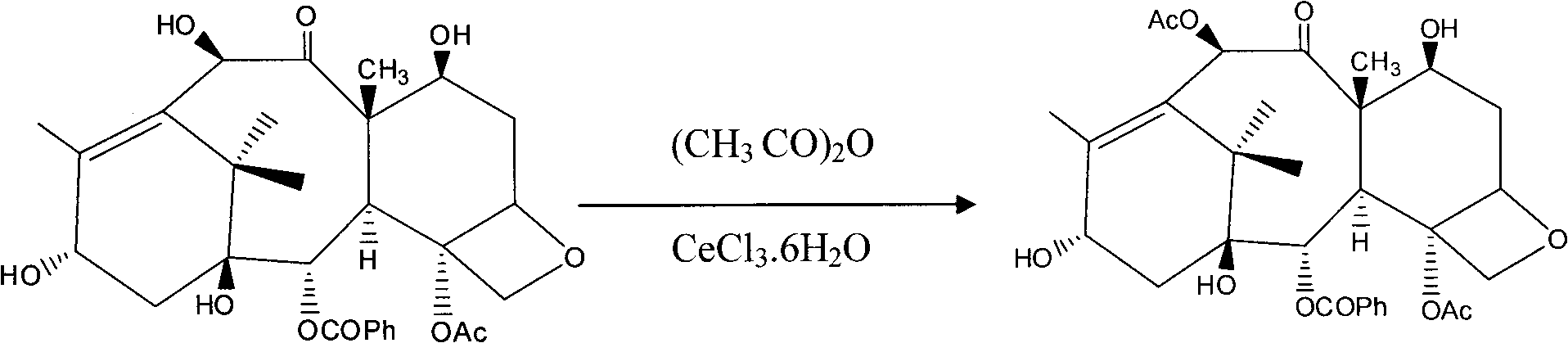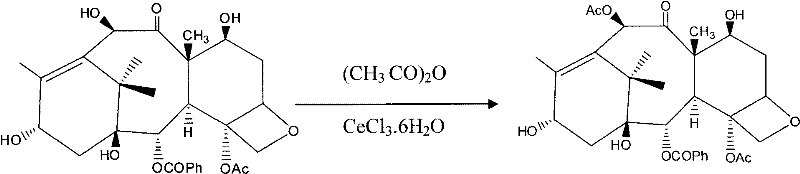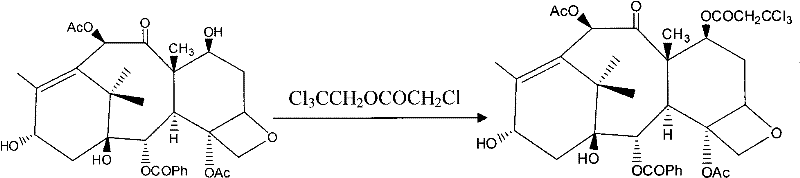Patents
Literature
106 results about "Baccatin III" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Baccatin III is an isolate from the yew tree (Genera Taxus). Baccatin III is a precursor to the anti-cancer drug paclitaxel (Taxol). In 2014, researchers reported introduction and expression of the endophytic fungal gene responsible for synthesizing baccatin III (10-deacetylbaccatin III 10-O-acetyltransferase), to the mushroom Flammulina velutipes. Researchers achieved the same accomplishment with Escherichia coli in 2000.
Facile method for synthesizing baccatin III compounds
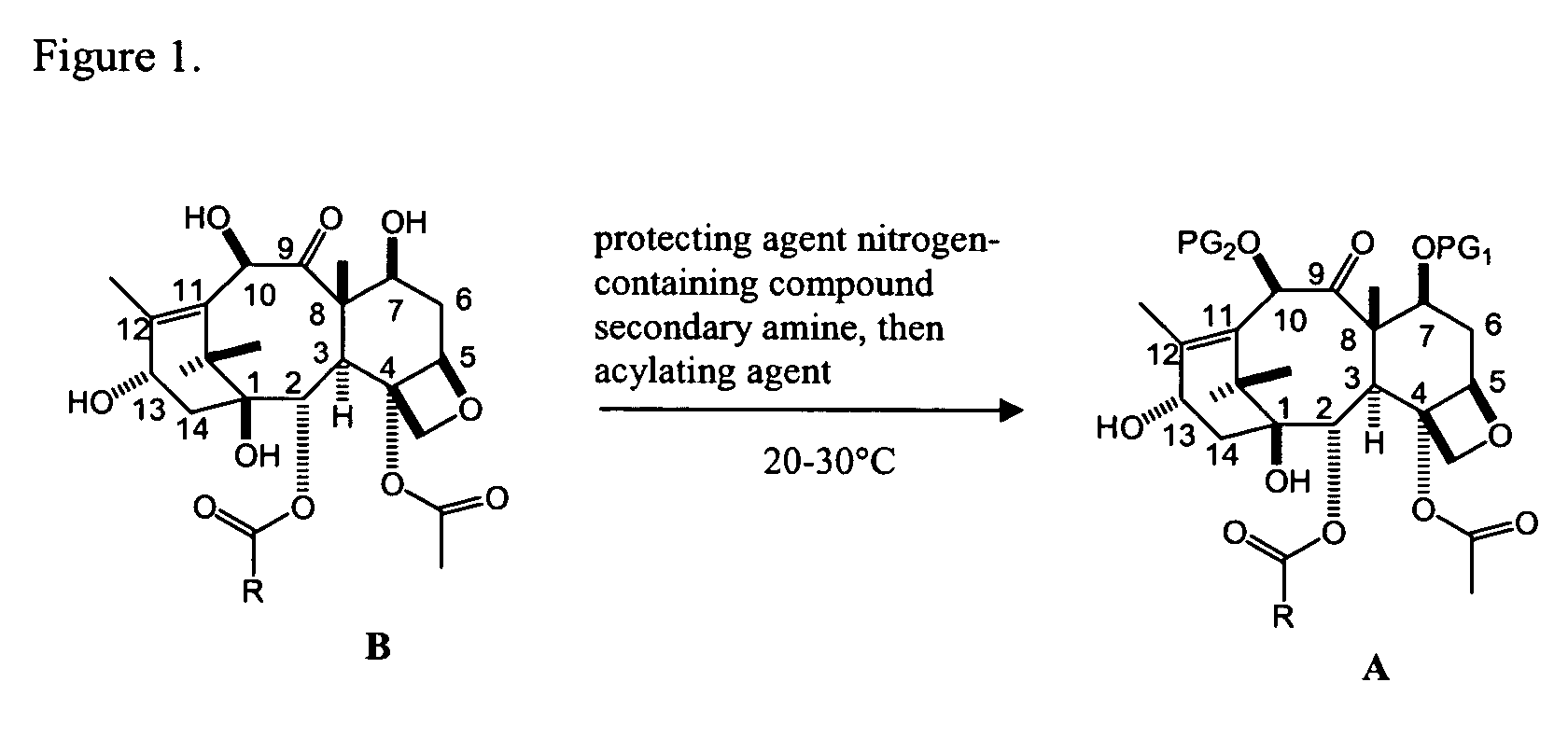
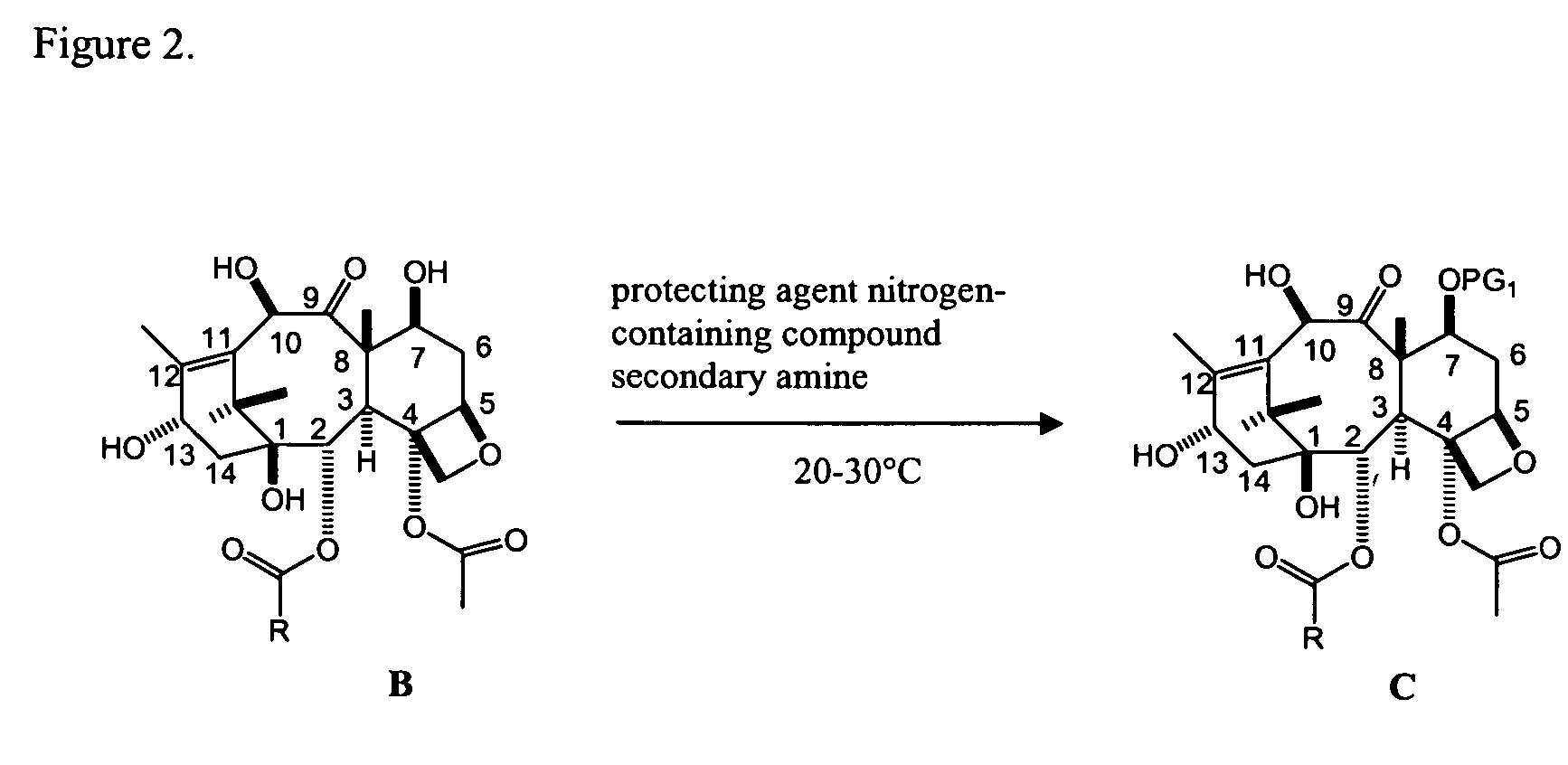
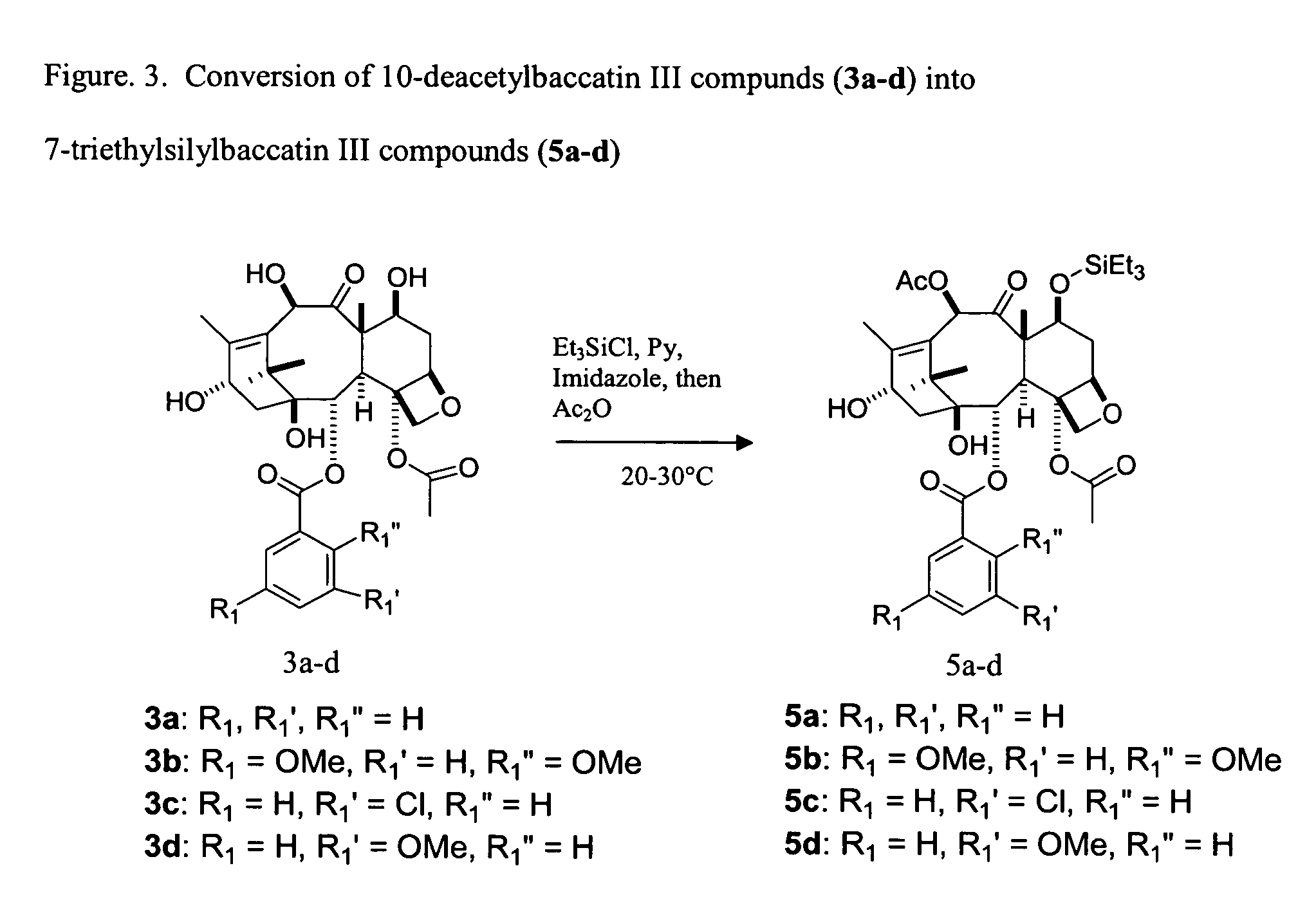
A process for synthesizing a C-7 protected baccatin III compound represented by formula (A), which comprises reacting a 10-deacetylbaccatin III compound represented by formula (B) with a protecting agent and an acylating agent in the presence of a secondary amine and a nitrogen-containing compound. Also, a process for synthesizing a C-7 protected 10-deacetylbaccatin III compound represented by formula (C), which comprises reacting a 10-deacetylbaccatin III compound represented by formula (B) with a protecting agent in the presence of a secondary amine and a nitrogen-containing compound. In both processes the nitrogen-containing compound is selected from a nitrogen-containing heterocycle or a trialkylamine. When the nitrogen-containing heterocycle is selected, it may be an unsubstituted or a substituted pyridine or an unsubstituted or a substituted pyrazine. When a trialkylamine is selected, it may be, for example, triethylamine or diisopropylethylamine.wherein PG1 represents the organic residue of the protecting agent, PG2 represents the organic residue of the acylating agent, and R represents a simple or substituted aryl group or a heterocyclic group.
Owner:IMMUNOGEN INC
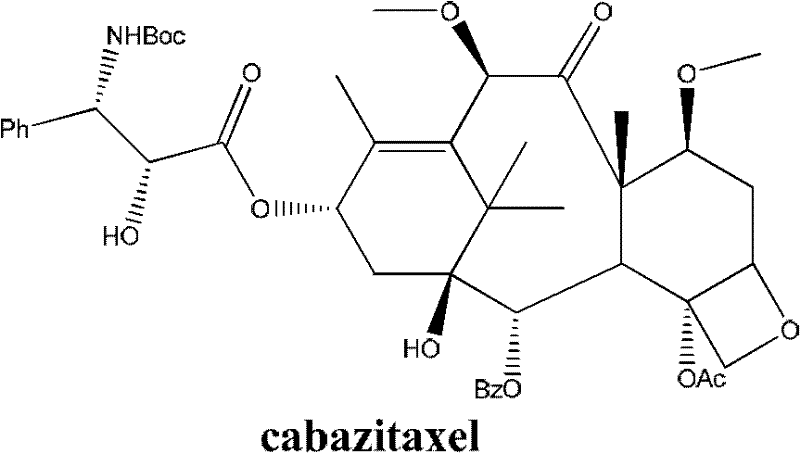
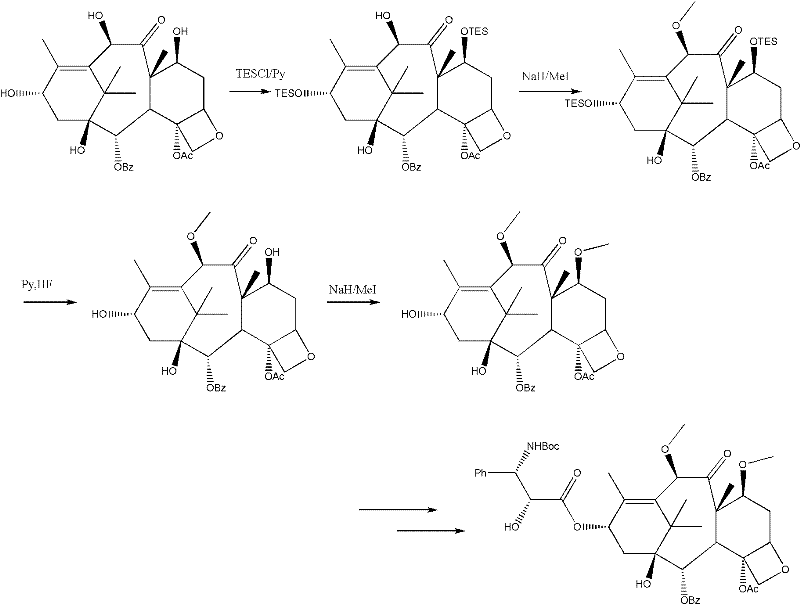
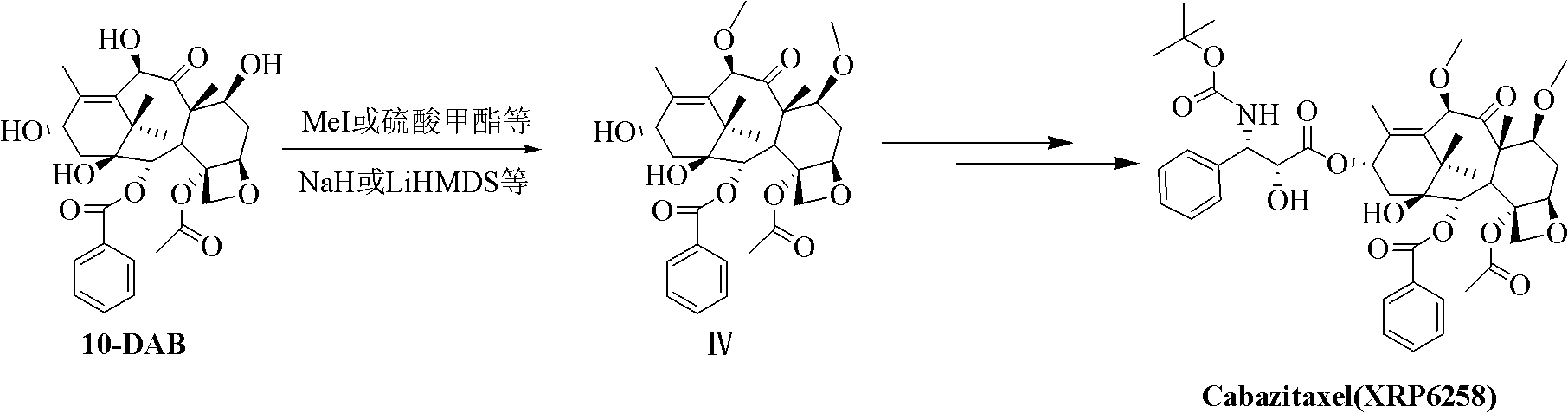
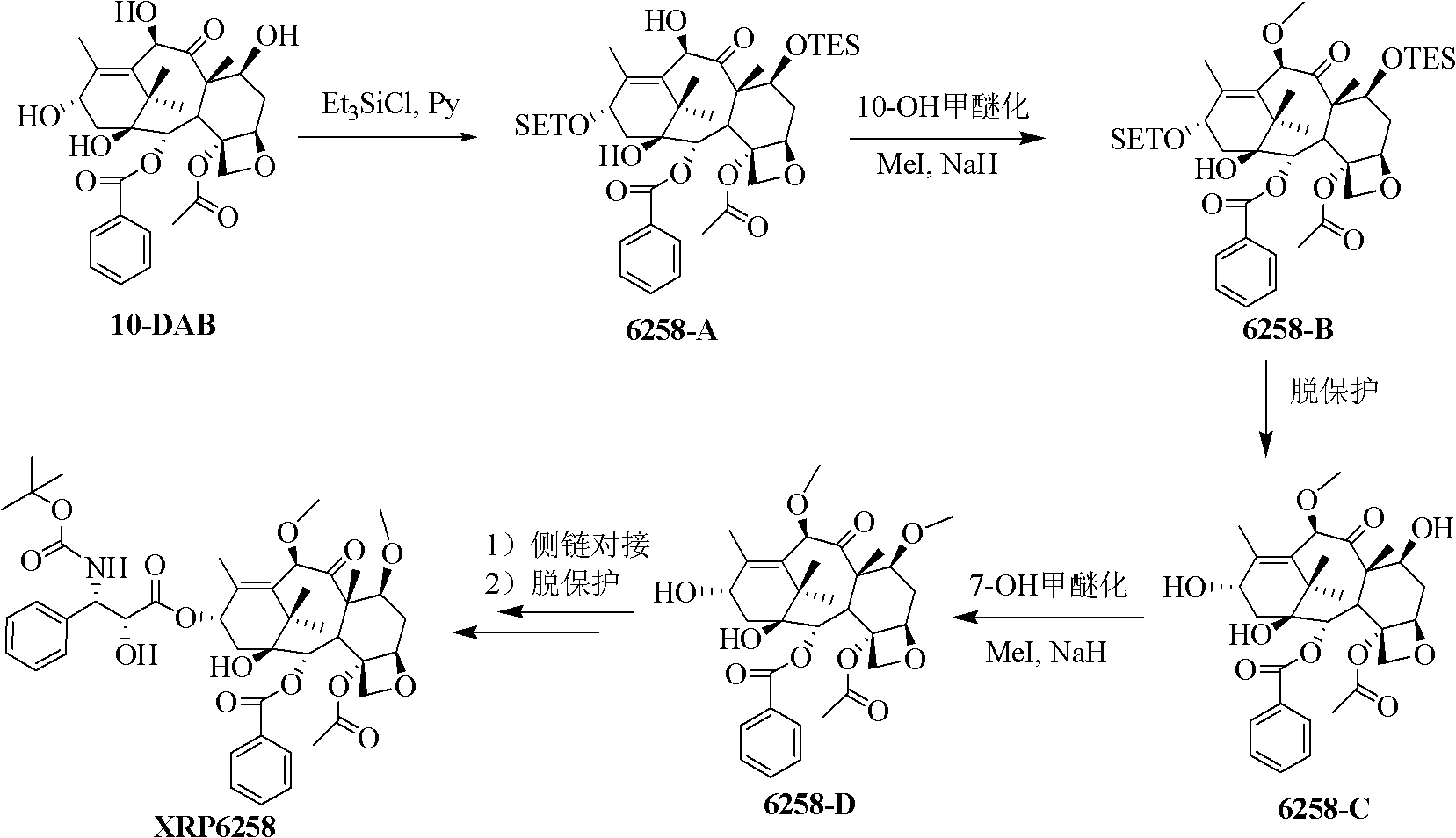
Method for preparing cabazitaxel by taking 10-deacetylate-baccatin III as raw material
ActiveCN102417491AIncrease preparation meteringEasy to operateOrganic chemistryUrinary disorderAcetic acidCabazitaxel
The invention relates to a method for preparing cabazitaxel, particularly relates to a method for preparing the cabazitaxel by taking 10-deacetylate-baccatin III as a raw material and belongs to the technical field of drug synthesis. The method comprises the following steps: firstly, reacting 10-DAB with chlorocarbonate-2,2,2-trichloro ethyl ester, thereby obtaining a product; reacting the product with DMAP (dimethylamino pyridine), DCC (dicyclohexylcarbodiimide) and (4S, 5R)-2,2-dimethyl-4-phenyl-3-tert-butoxycarbonyl-3.5-oxazolidine formic acid, thereby obtaining a product; reacting the product with acetic acid and zinc powder, and then methylating the product; and lastly, adding a p-methyl benzenesulfonic acid, thereby reacting and obtaining a cabazitaxel product. The cabazitaxel prepared according to the method can be widely applied to the treatment of prostate cancer. Compared with a traditional technique for preparing the cabazitaxel, the method has the advantages that the preparation quantity is greatly increased, the operation steps are simplified, the manpower is saved and the method has industrial application value and prospect.
Owner:无锡紫杉药业股份有限公司
Taxoid derivatives and process for producing the same
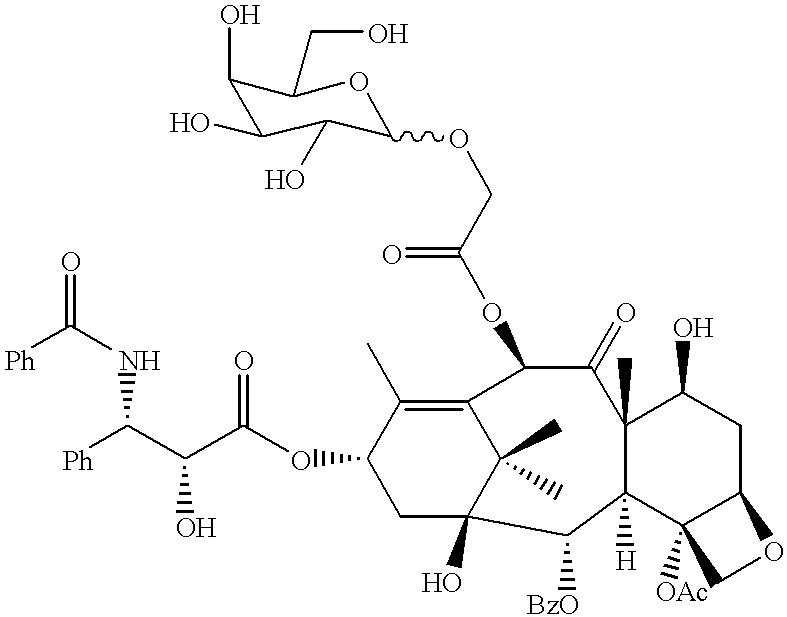
An object of the invention is to develop galactose or mannose derivatives of docetaxel, etc. having improved solubility and physiological activity, to alleviate burden imposed on patients and to provide effective therapeutic drug for tumors.The present invention provides taxoid derivatives comprising any of paclitaxel, docetaxel and 10-deacetyl-baccatin III to which galactose or mannose is linked through a spacer, and methods for producing taxoid derivatives comprising reacting paclitaxel, docetaxel or 10-deacetyl-baccatin III with tetrabenzyl acetyloxygalactoside or tetrabenzyl acetyloxymannoside, subjecting the product to debenzylation reaction, and optionally to detriethylsilylation reaction.
Owner:BIO RES CORP OF YOKOHAMA +2
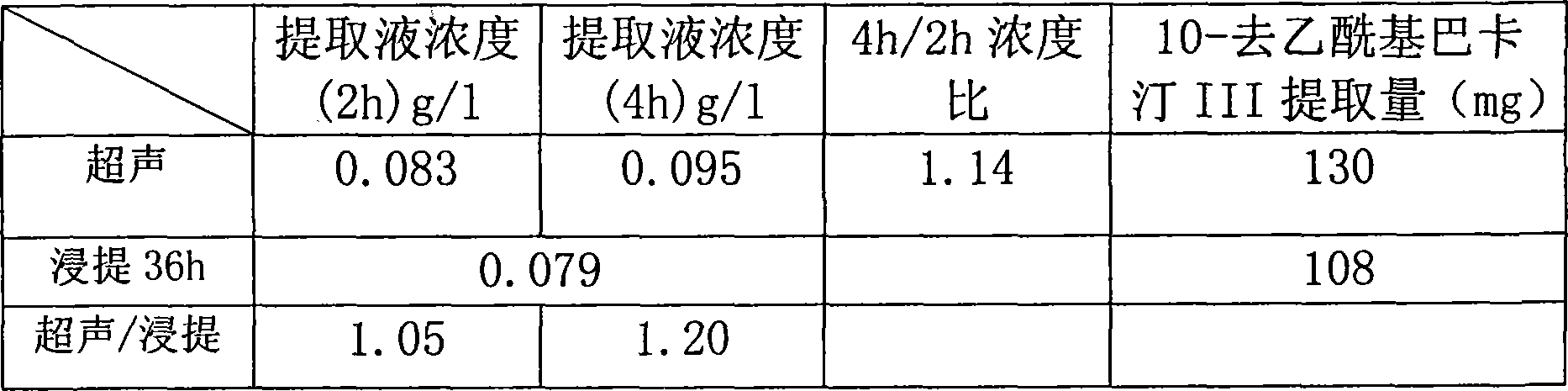
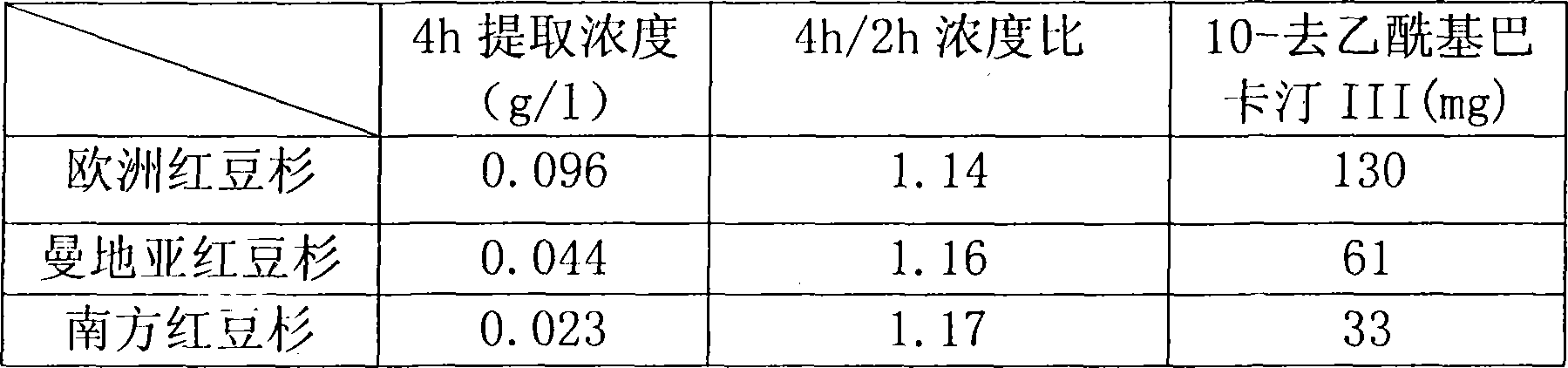
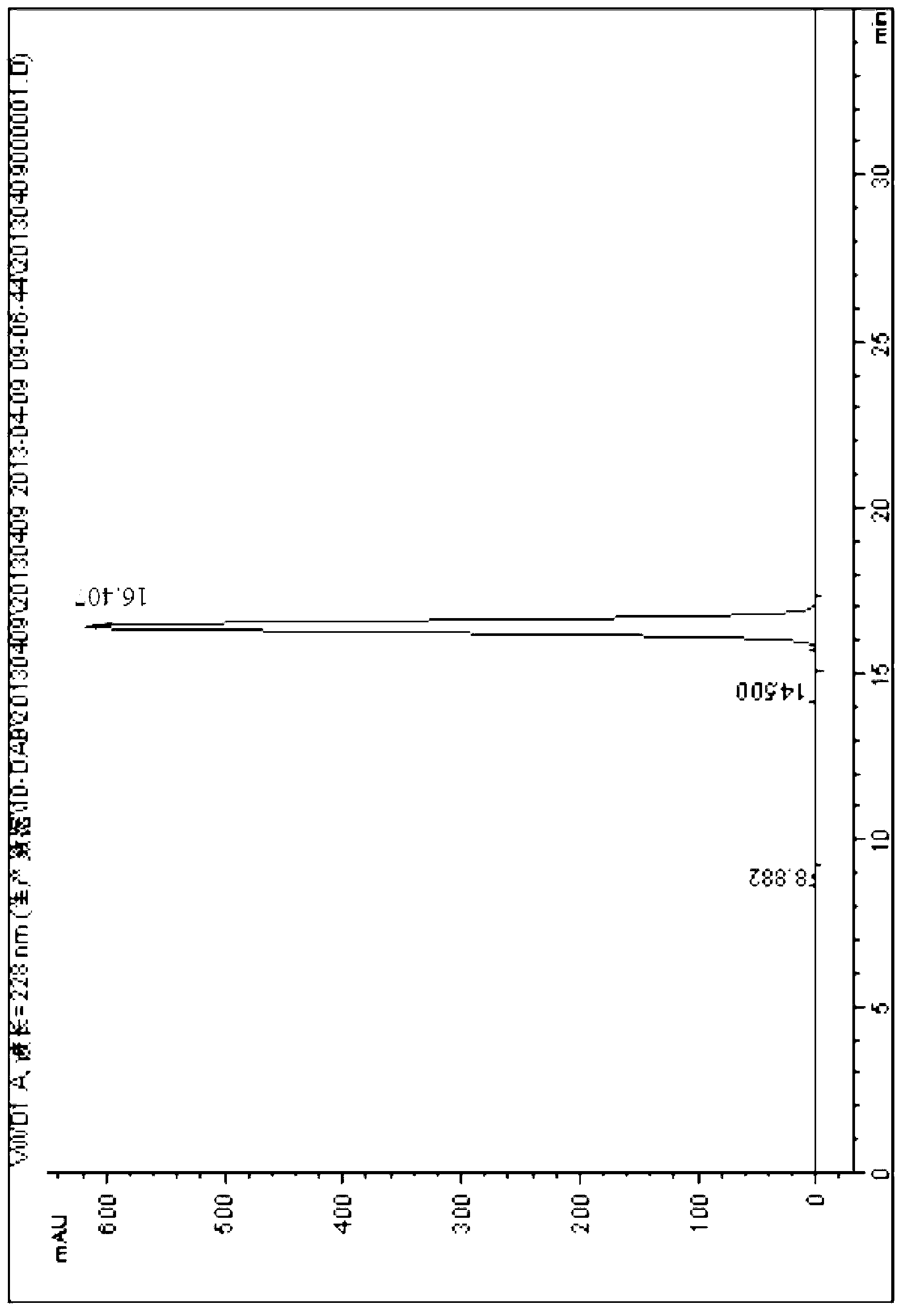
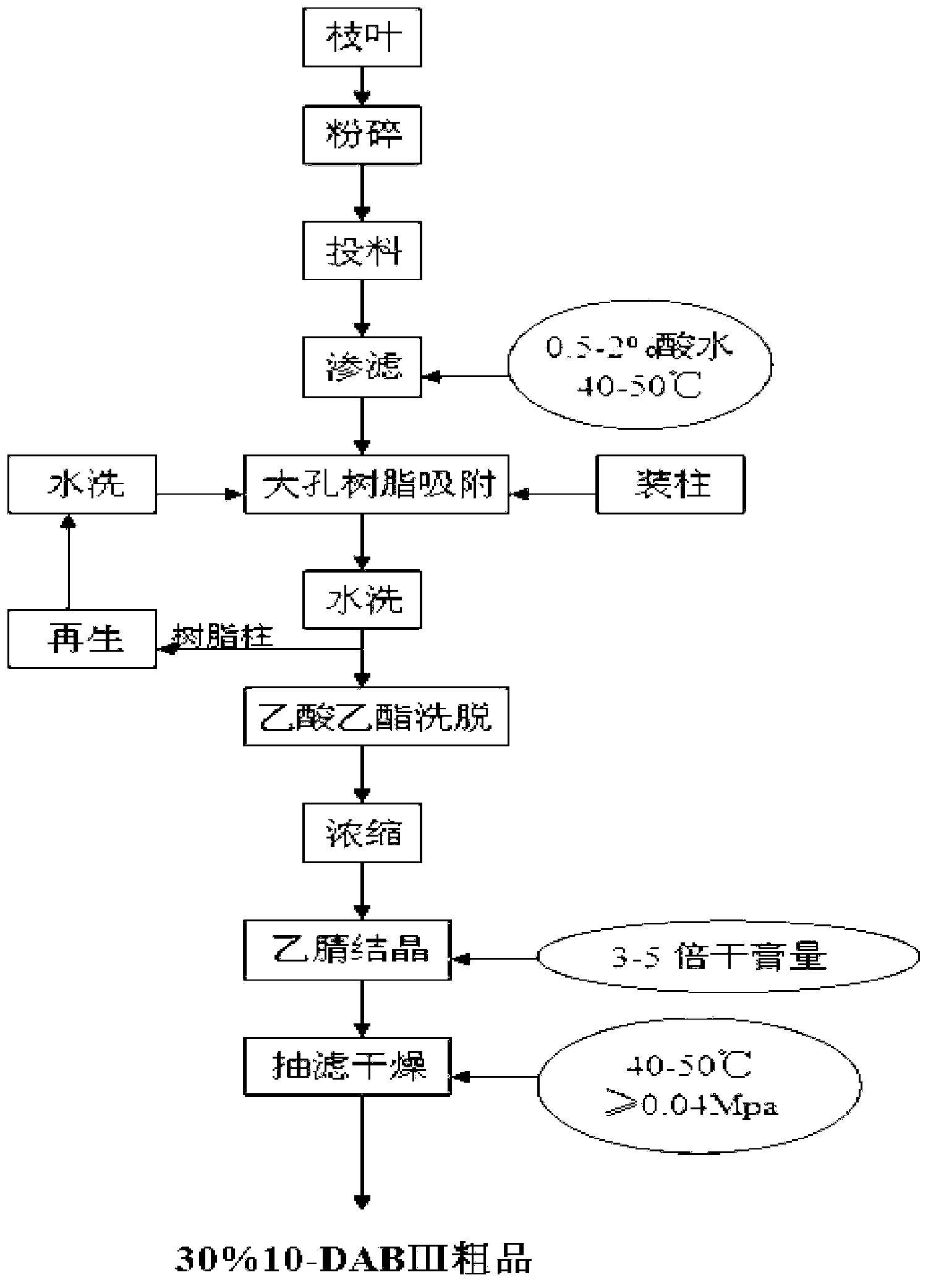
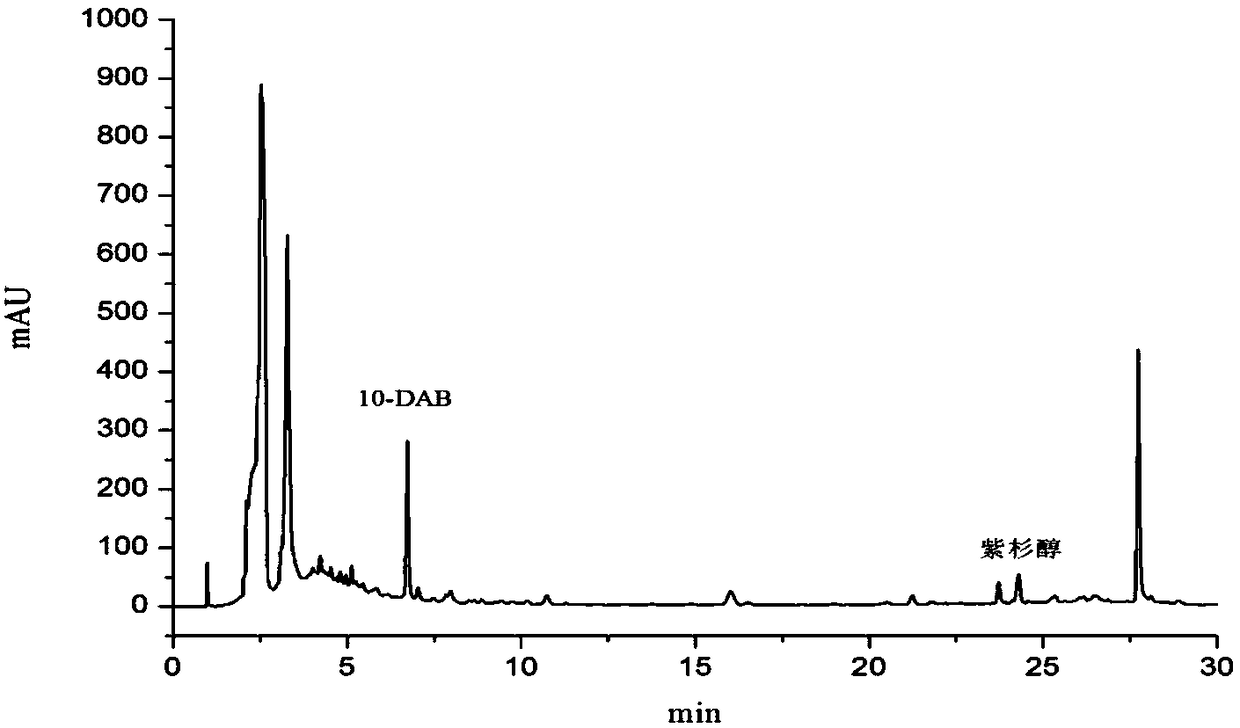
Method for ultrasonic extraction and separation of 10-deacetyl baccatin III from yew branch leaves
ActiveCN101230053AAvoid disadvantages such as large recovery lossOrganic chemistryOrganic solventAlcohol
The invention discloses an improved method for extracting 10-deacetylbaccatin III (10-DAB) from a yew. A citric acid aqueous solution with the density of 0.1 to 0.5 percent according to the weight percentage is used for extracting crushed yew branches and leaves through continuous ultrasound. The obtained extracting solution is adsorbed through macroporous adsorptive resin, then eluted through ethyl alcohol, and the solvent is distilled to dryness to obtain a fulvous condensed extract. An organic solvent is used for dissolving condensed extract, insolubles is removed, the solvent is distilled to dryness, yellow solid which contains 10-DAB and has the weight ratio of approximately 15-17 percent is then obtained. The purity of recrystal can reach above 98 percent. By utilizing the continuous ultrasound, the extraction time can be greatly reduced, and the solvent can be saved by using the macroporous adsorptive resin, thereby the invention is favorable for large-scale industrial production.
Owner:江苏拜瑞生物医药科技有限公司
Method for elementary separating 10-noracetyl Baccatins III from branches and leaves of yew
A process for primarily extracting 10-deacetyl palcatine C from the twig and leaf of enqlish yew includes extracting in alcohol solution, concentrating laying aside, deposing taxol and low-polarity impurities, filtering, regulating pH=5-6, macroreticular resin adsorbing, eluting with absolute alcohol, and concentrating.
Owner:GUANGDONG KELUN PHARMACEUTICAL CO LTD
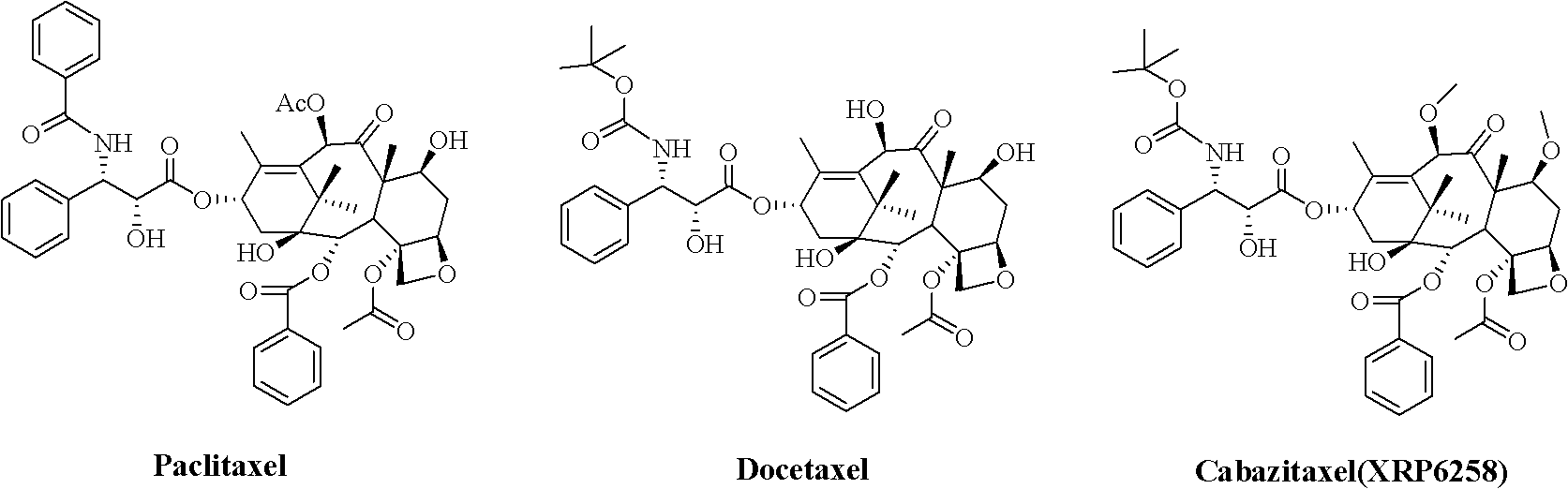
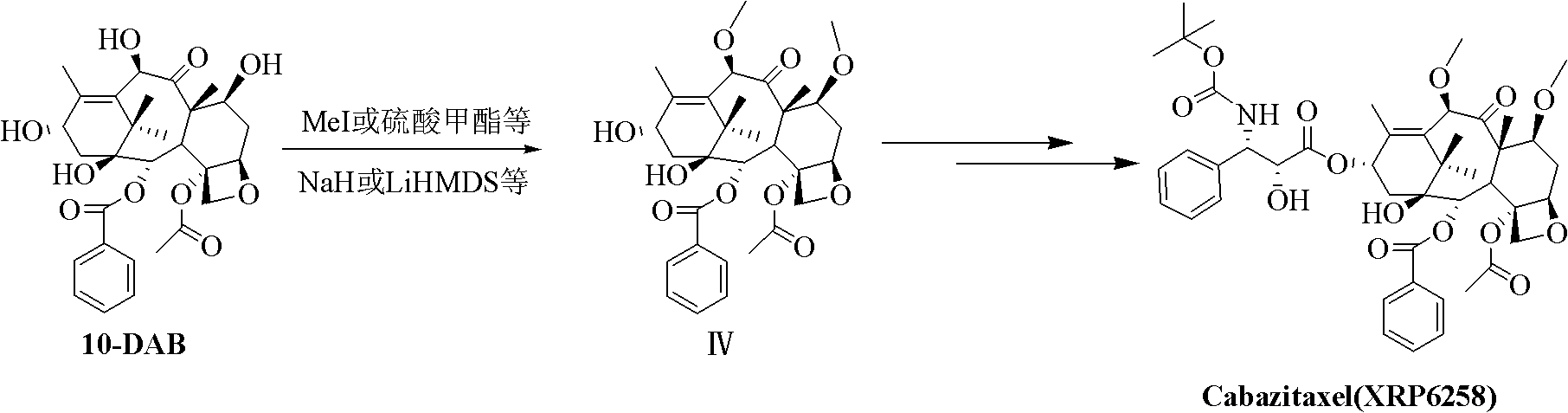
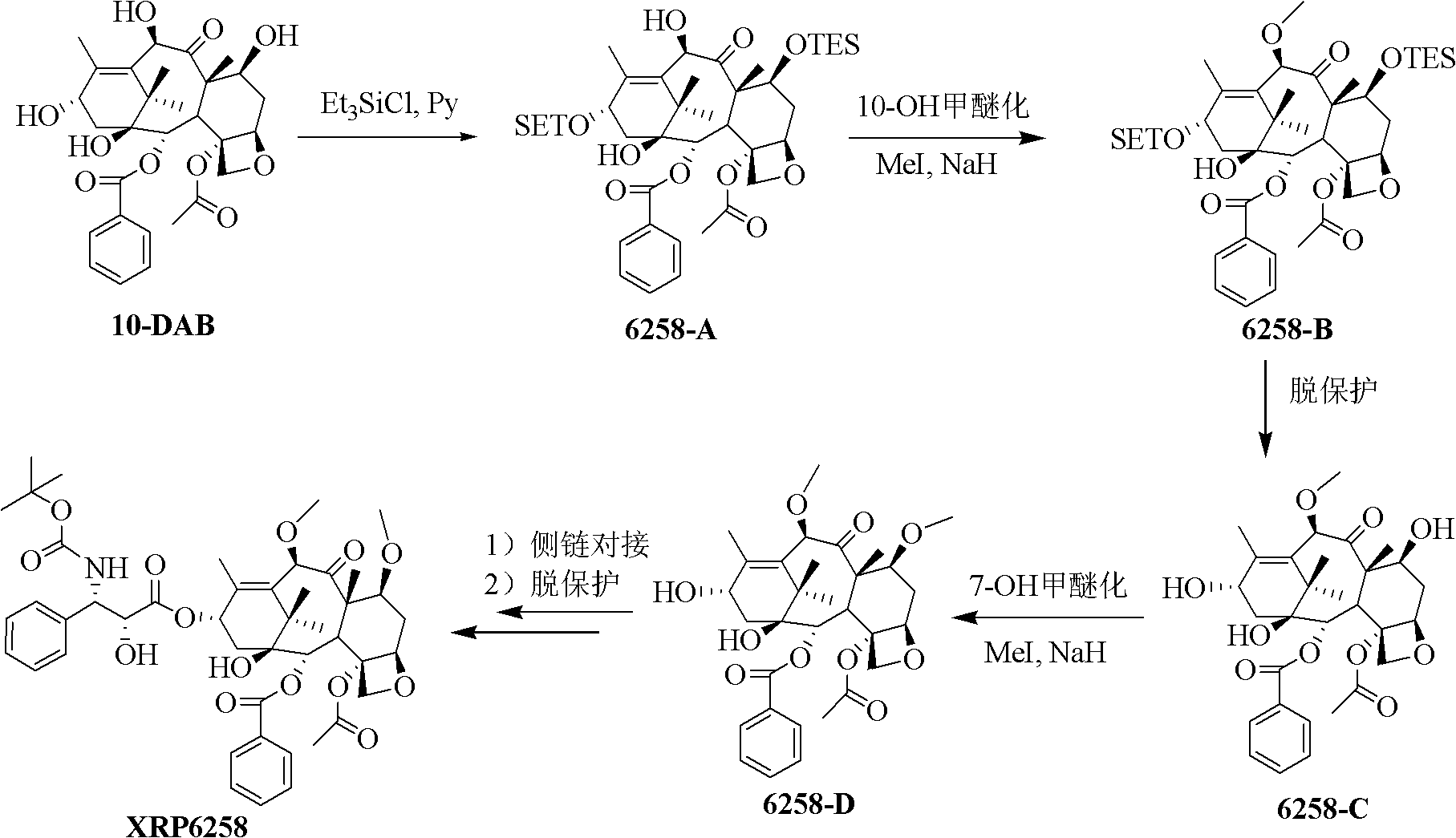
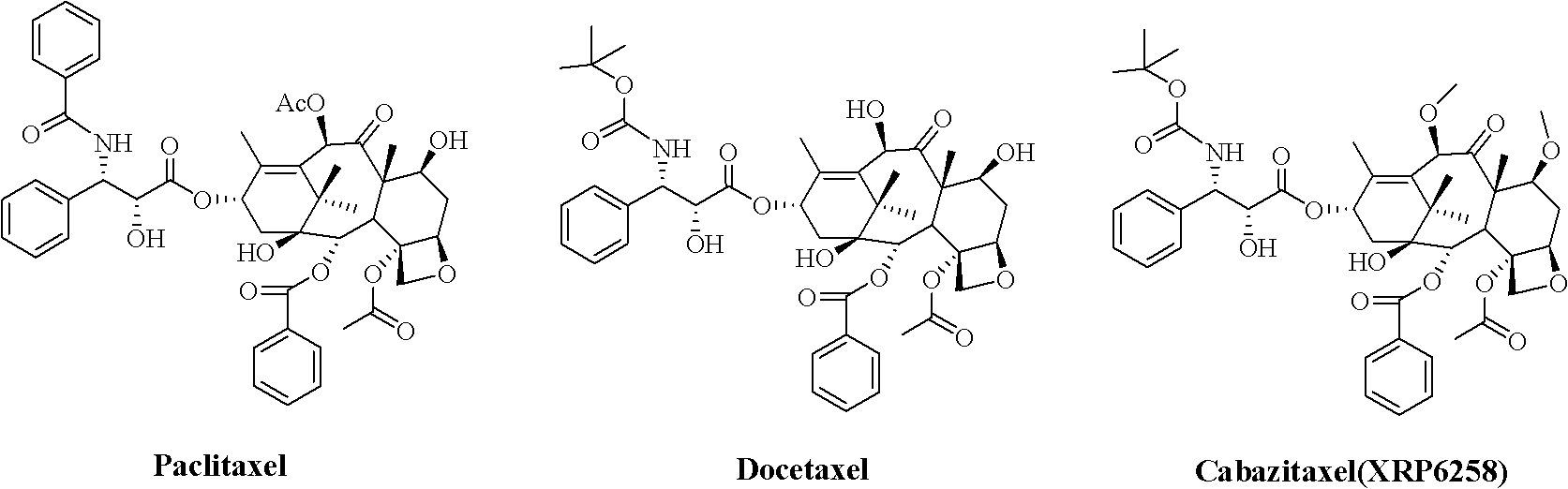
Preparation method of taxol anticancer drugs Cabazitaxel XRP6258
InactiveCN103012329AEasy to manufactureEasy to makeOrganic chemistryBulk chemical productionButt jointCabazitaxel
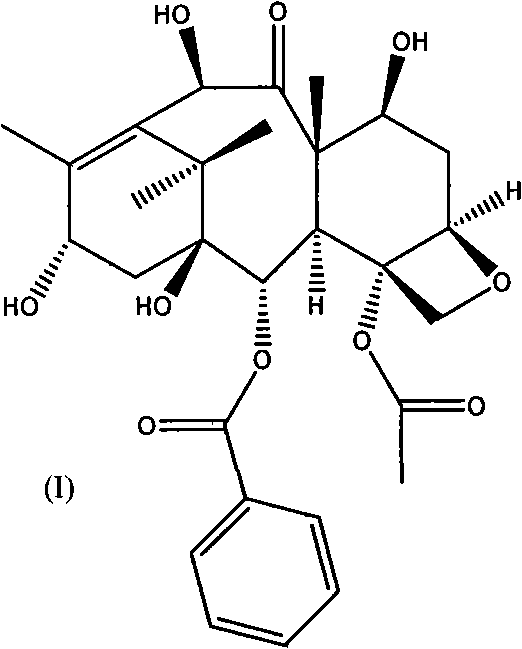
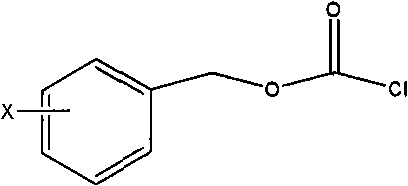
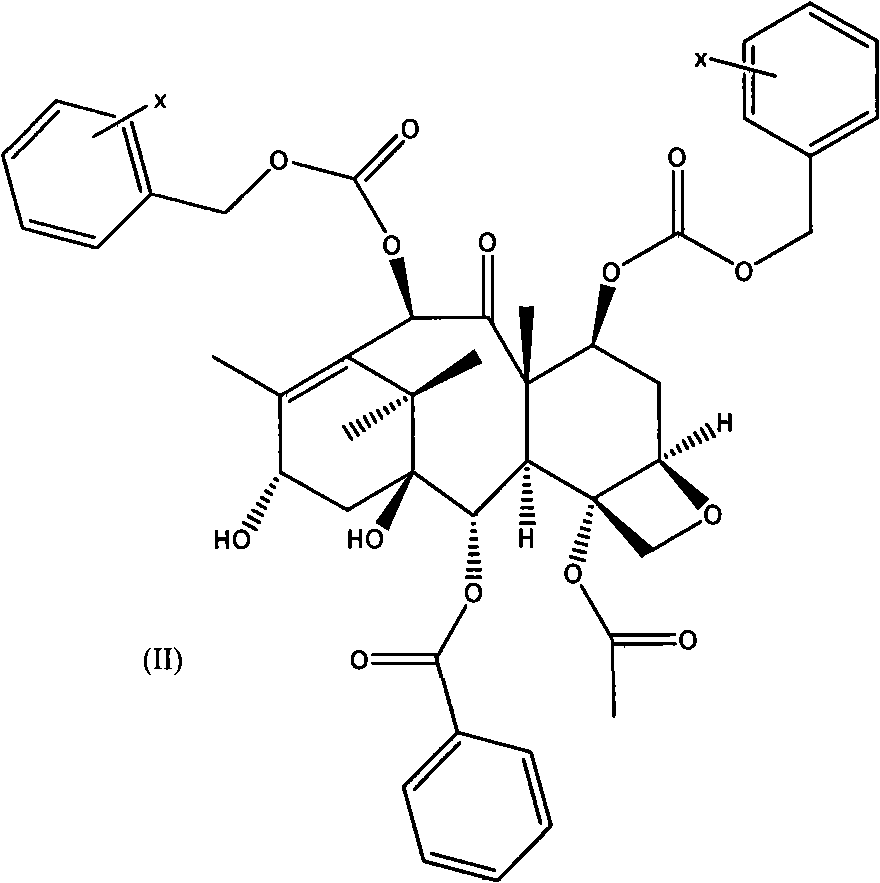
The invention belongs to the field of drug synthesis, and relates to a method for synthesizing a second-generation taxol anticancer drugs Cabazitaxel XRP6258. The method comprises the following steps of: obtaining C-7 and C-10 bit hydroxy methyl mercaptan methylene (MTM) and C-13 bit oxhydryl oxydic key intermediate (II) of 10-oxhydryl baccatin III(I) by high regioselectivity, reducing C-13 bit carbonyl to obtain a mother nucleus midbody (III), carrying out butt joint with various types of side chains to obtain butt joint product midbodies (IV-1) and (IV-2), and removing a side-chain protecting group after the di-methylthio is removed or removing the di-methylthio of the nuclear parent after the side-chain protecting group is removed to obtain a product Cabazitaxel XRP6258 (V). The method disclosed by the invention has the advantages of being simple in preparation process, high in yield, lower in cost, easy to operate and the like, so that the XRP6258 can be produced and prepared on a large scale.
Owner:FUDAN UNIV +1
Extraction method of taxus active extract
ActiveCN103211844AHigh yieldEasy to degradeOrganic active ingredientsConiferophyta medical ingredientsIsomerizationSlag
The invention relates to an extraction method of a taxus active extract. According to the invention, taxus branches and leaves are crushed to 20-50 meshes; petroleum ether with a 3-5-time amount is added; the mixture is placed under 0-5 DEG C for 10-12h; filtering is carried out, such that a filter slag is obtained; and leaching is carried out. According to the process adopted by the invention, low-temperature leaching is adopted, and methanol leaching with different concentration gradient is adopted, such that taxol is greatly protected and is prevented from conversion during the separation process. Taxol is a biological macromolecular substance, and is susceptible to degradation or isomerization under the influences of environmental conditions such as temperature, organic solvent, acid, alkali, and the like, such that other taxane substances might be produced. For example, under strongly acidic or weakly alkaline environmental conditions, taxol is degraded into baccatin III or is subjected to epitope isomerization to form 7-epipaclitaxel. Under high temperature, taxol is subjected to a degradation reaction to form corresponding small-molecualr substances. With the process provided by the invention, taxol yield is greatly improved.
Owner:安徽和华生物医药科技有限公司
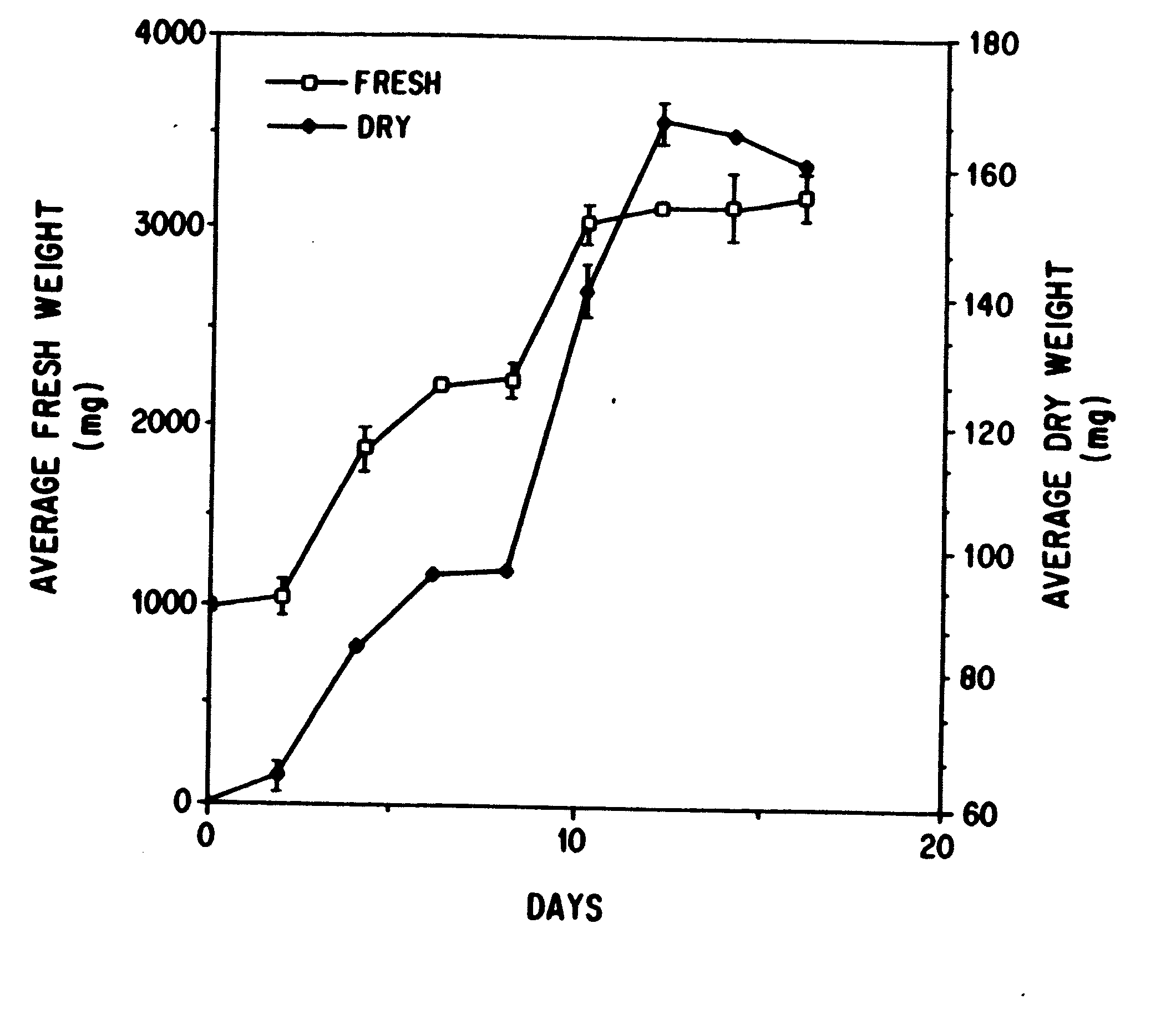
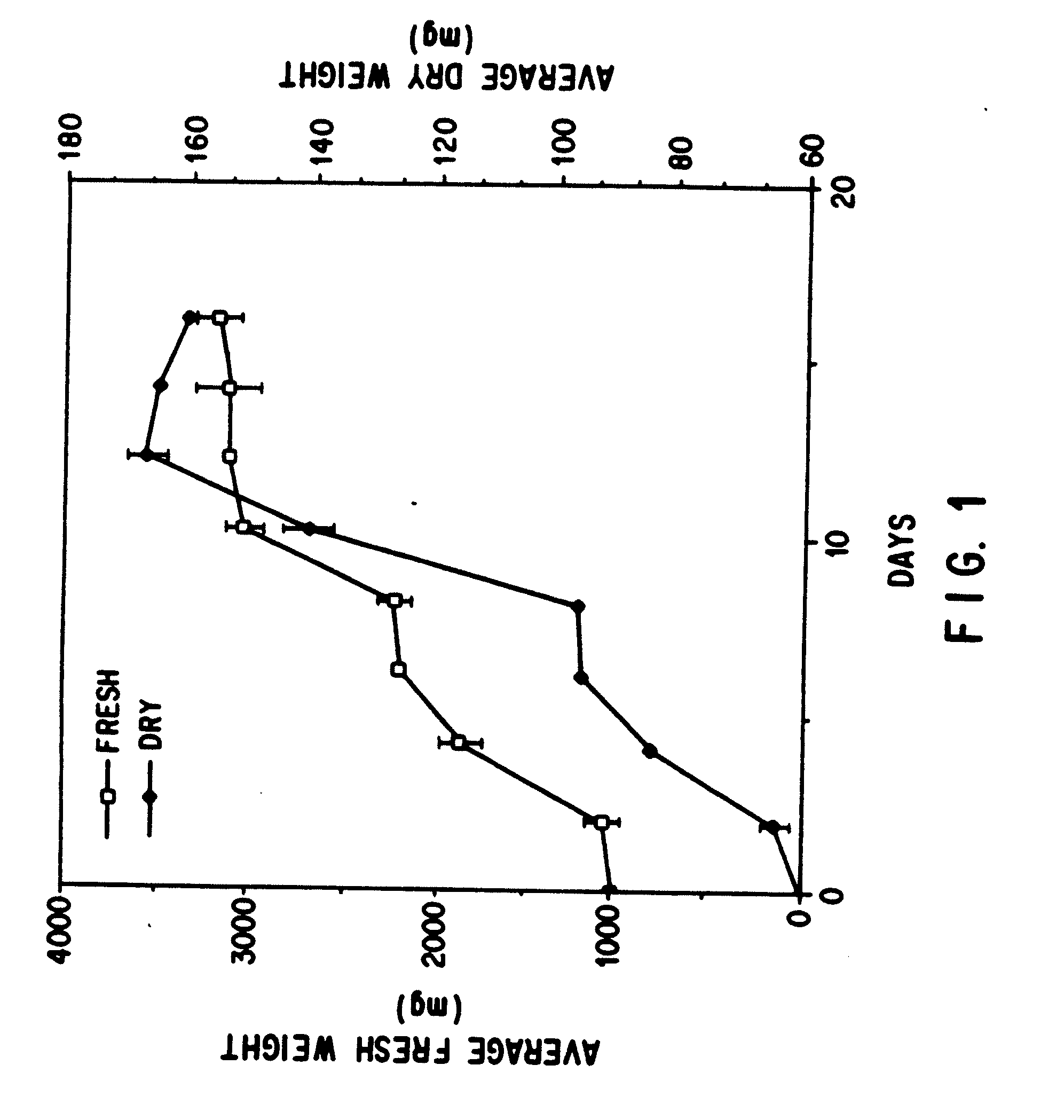
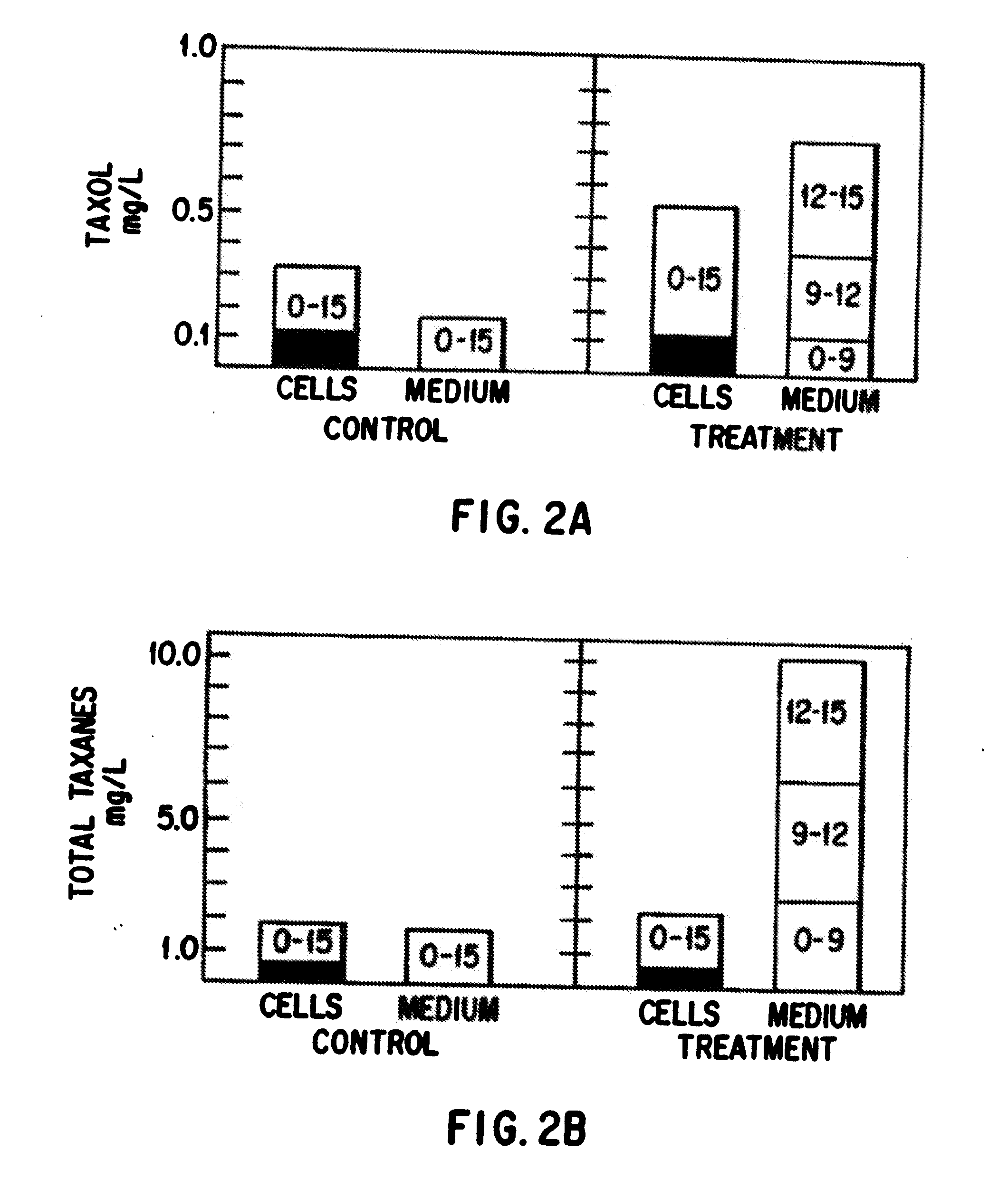
Enhanced production of taxol and taxanes by cell cultures of Taxus species
InactiveUS7264951B1Promote rapid growthIncrease cell densityOrganic chemistryTissue cultureBiotechnologyTaxus species
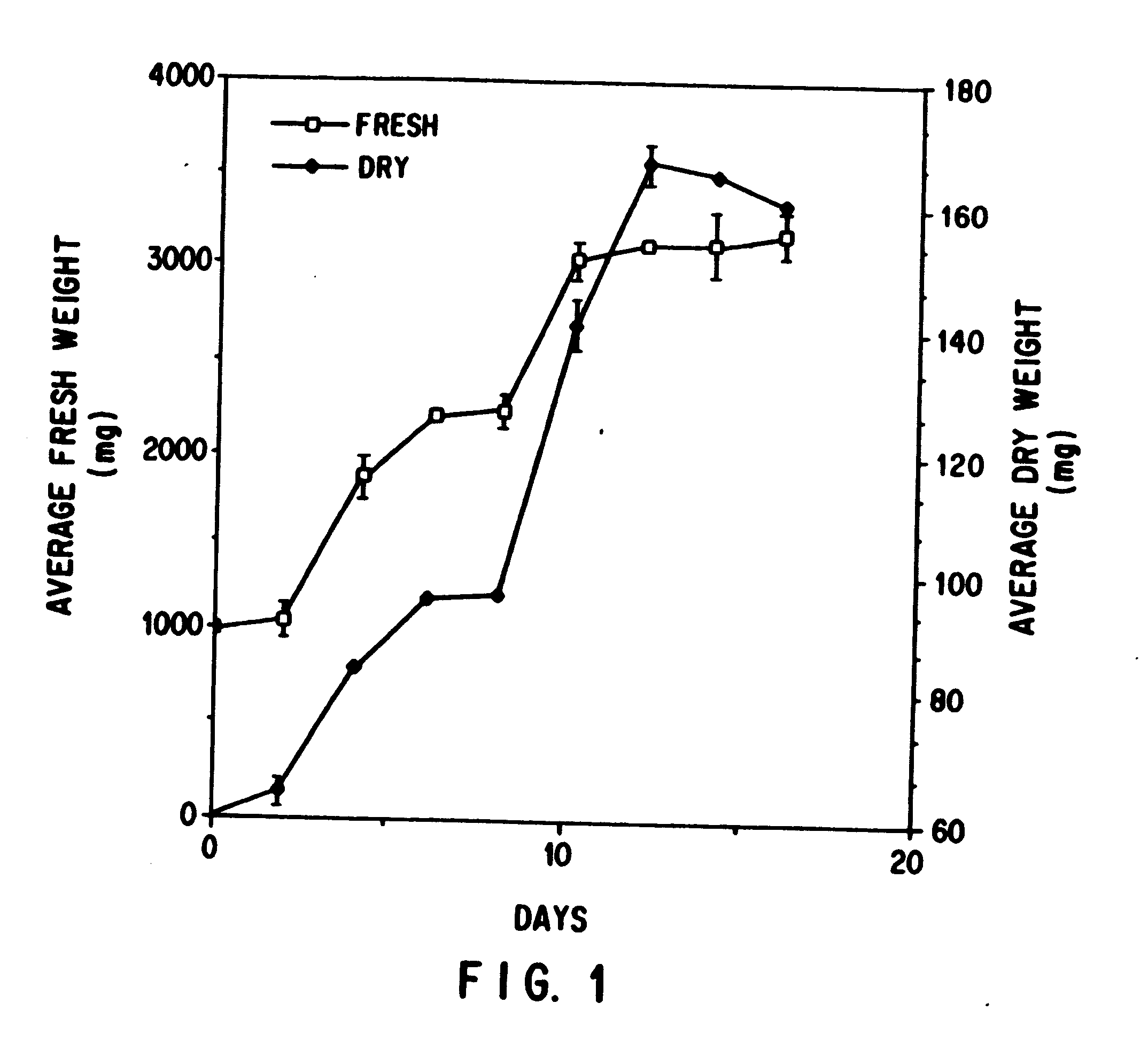
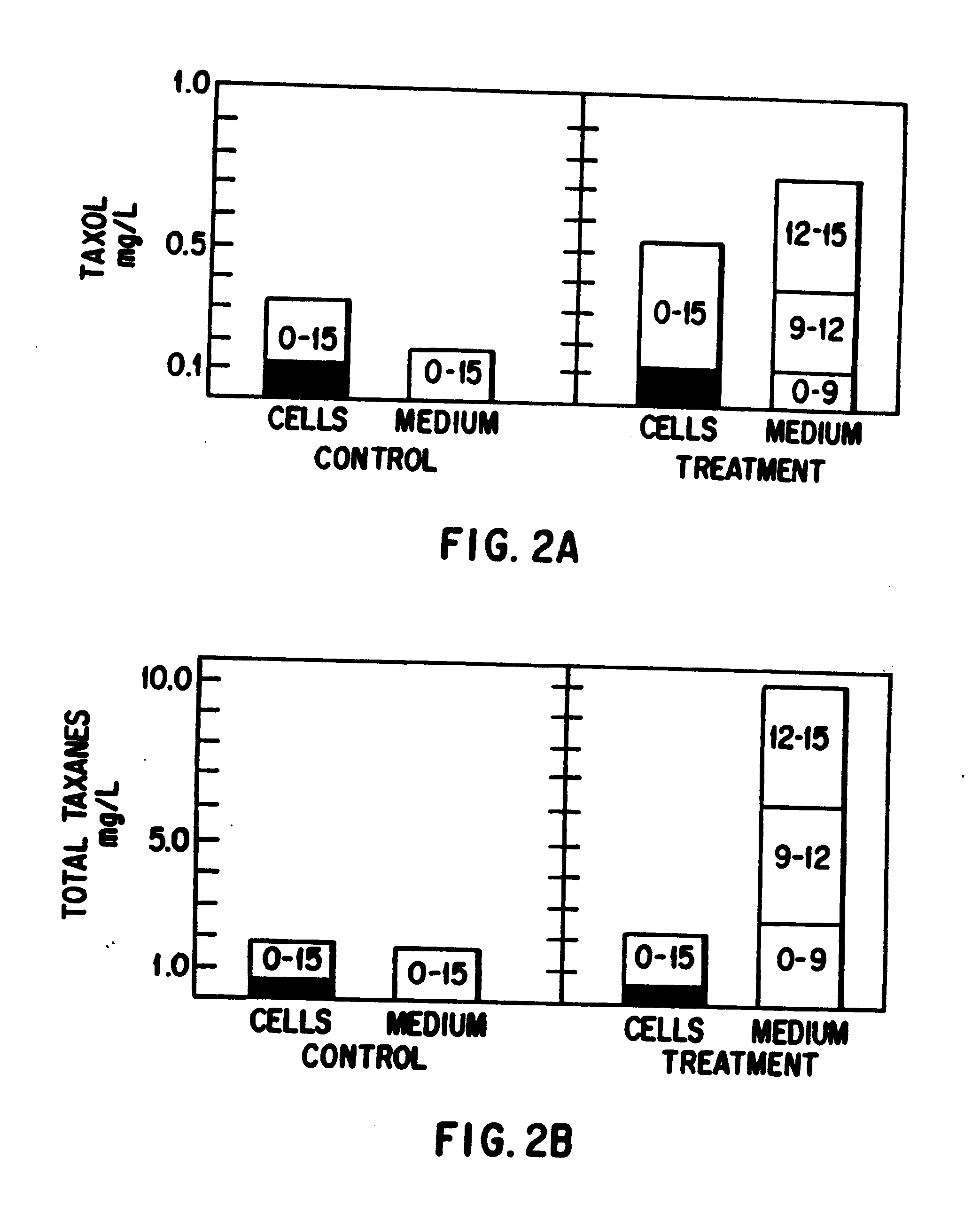
This invention provides methods whereby taxol, baccatin III, and other taxol-like compounds, or taxanes, can be produced in very high yield from all known Taxus species, e.g., brevifolia, canadensis, cuspidata, baccata, globosa, floridana, wallichiana, media and chinensis. Particular modifications of culture conditions (i.e., media composition and operating modes) have been discovered to enhance the yield of various taxanes from cell culture of all species of Taxus. Particularly preferred enhancement agents include silver ion or complex, jasmonic acid (especially the methyl ester), auxin-related growth regulators, and inhibitors of the phenylpropanoid pathway, such as 3,4-methylenedioxy-6-nitrocinnamic acid. These enhancement agents may be used alone or in combination with one another or other yield-enhancing conditions. While the yield of taxanes from plant cell culture of T. chinensis is particularly enhanced by use of one or more of these conditions, yield of taxanes for all Taxus species has been found to benefit from use of these conditions.
Owner:PHYTON HLDG
Method for preparing second-generation taxol anticancer drug Cabazitaxel
InactiveCN103012328AEasy to manufactureEasy to makeOrganic chemistryBulk chemical productionCabazitaxelChemical compound
The invention belongs to the field of medicament synthesis, and relates to a method for synthesizing a second-generation taxol anticancer drug Cabazitaxel. The method comprises obtaining a key intermediate (II): a 10-deacetyl baccatin III (I) with C-7 and C-10 hydroxy-methylthio-methylene and C-13 hydroxy-oxidization, completing a de-methylthio operation for C-13 carbonyl and C-7 and C-10 methylthio methylene (MTM) of the compound II by a one-pot method to obtain a nucleus (IV) of an XRP6258, connecting the nucleus with various side chains, and then removing side chain protecting groups to obtain the product Cabazitaxel (V). The method has advantages of high efficiency in the preparation process, simple process, high yield, relatively low cost and easy operation, and is suitable for large-scale production and preparation of anti-cancer drugs XRP6258.
Owner:FUDAN UNIV +1
Enhanced production of taxol and taxanes by cell cultures of taxus species
This invention provides methods whereby taxol, baccatin III, and other taxol-like compounds, or taxanes, can be produced in very high yield from all known Taxus species, e.g., brevifolia, canadensis, cuspidata, baccata, globosa, floridana, wallichiana, media and chinensis. Particular modifications of culture conditions (i.e., media composition and operating modes) have been discovered to enhance the yield of various taxanes from cell culture of all species of Taxus. Particularly preferred enhancement agents include silver ion or complex, jasmonic acid (especially the methyl ester), auxin-related growth regulators, and inhibitors of the phenylpropanoid pathway, such as 3,4-methylenedioxy-6-nitrocinnamic acid. These enhancement agents may be used alone or in combination with one another or other yield-enhancing conditions. While the yield of taxanes from plant cell culture of T. chinensis is particularly enhanced by use of one or more of these conditions, yield of taxanes for all Taxus species has been found to benefit from use of these conditions.
Owner:DFB BIOTECH
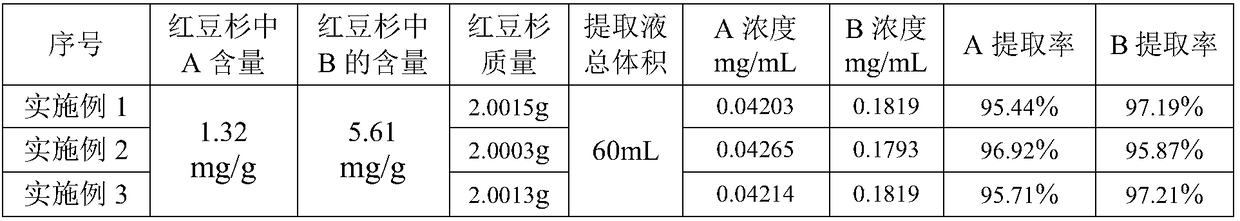
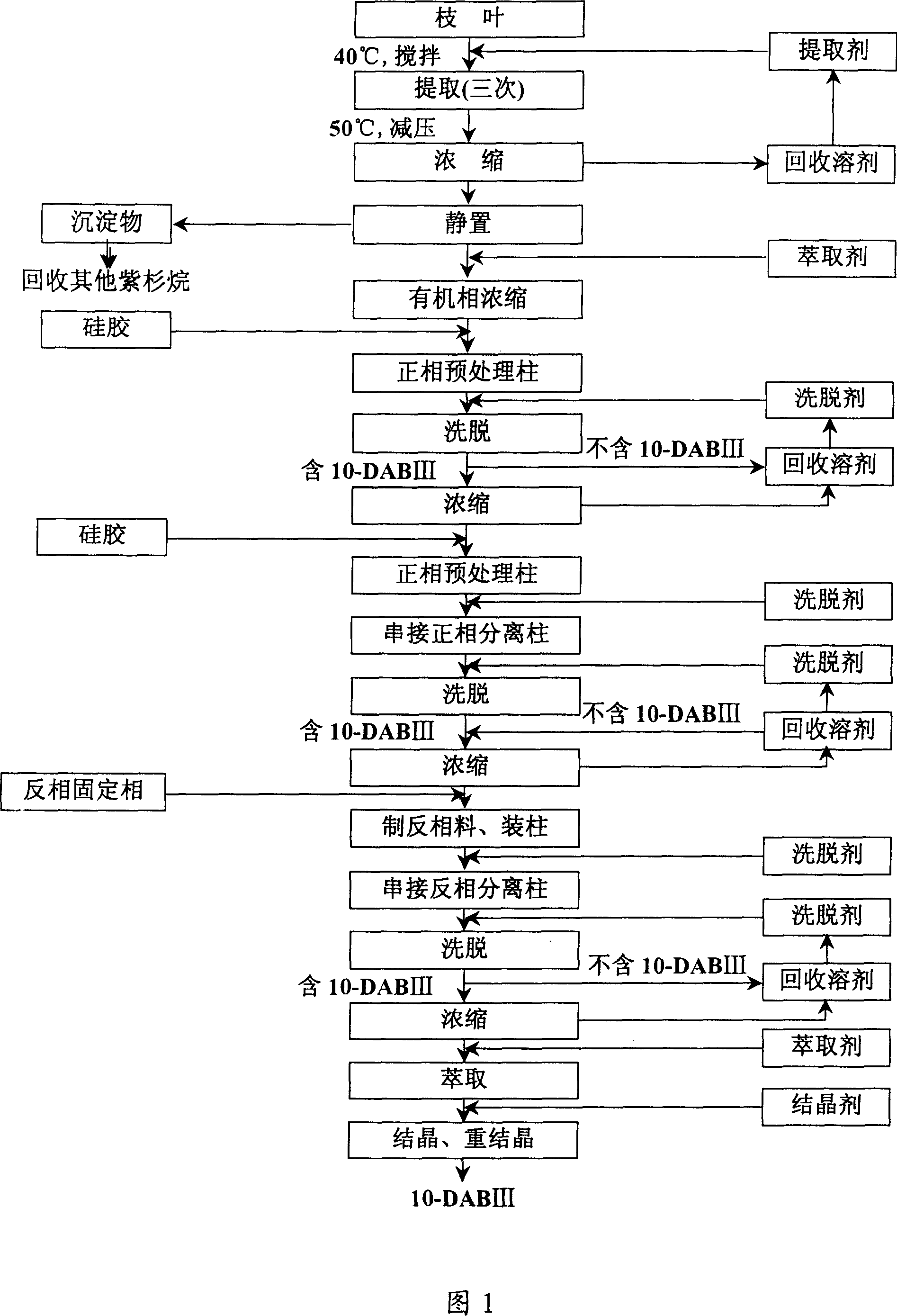
Method for separating and purifying 10-deacetyl baccatin III from Chinese yew branches and leaves
The invention relates to a method for preliminarily separating 10-deacetyl baccatin III from Chinese yew branches and leaves. The method comprises the following steps of: performing percolation to extract crushed Chinese yew branches and leaves; then performing adsorption through a macroporous adsorption resin column, and performing elution by using an organic solvent; removing impurities to obtain a crude product of 10-deacetyl baccatin III; dissolving the crude product of 10-deacetyl baccatin III by using the organic solvent, and then adding a reverse phase filler to perform adsorption; putting the mixture into a preparation column, and then performing elution; and concentrating and removing impurities from a main section to obtain a pure product of 10-deacetyl baccatin III. According to the method, the use of a large amount of the organic solvent is not needed during extraction of the branches and leaves, the macroporous adsorption resin and a reverse phase filler can be repeatedly used, the type of the solvent is simplified, the operation is convenient to perform, the cost is lowered, and the recovery rate of 10-DAB III is higher. Meanwhile, low-content percolate during continuous production can be entirely recycled, so that the discharge and treatment of wastewater are reduced, and the method can be well applied in production.
Owner:无锡紫杉药业股份有限公司
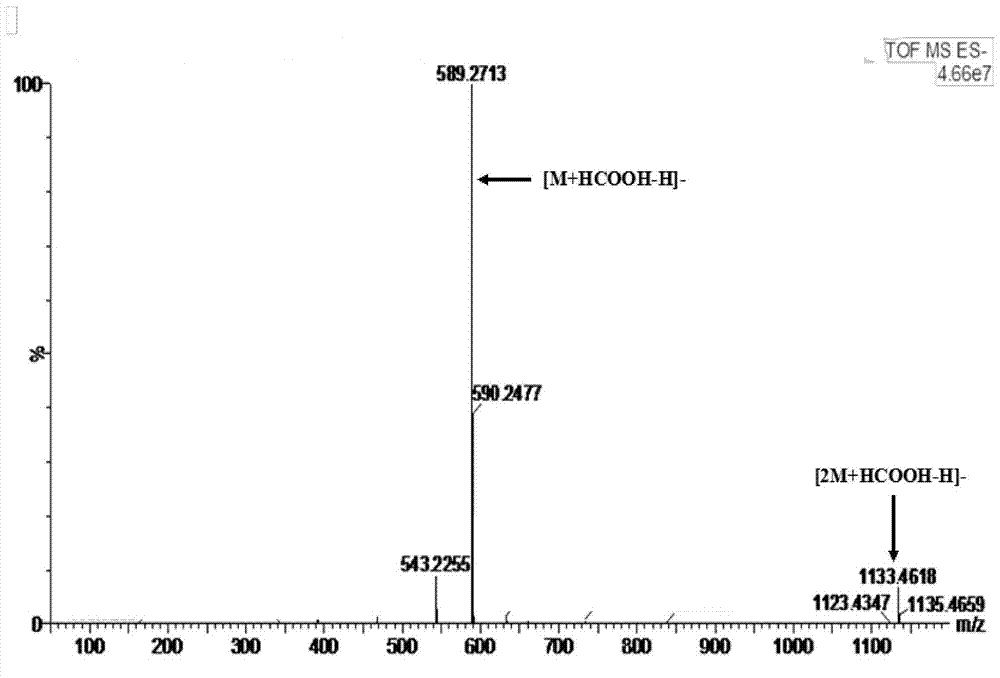
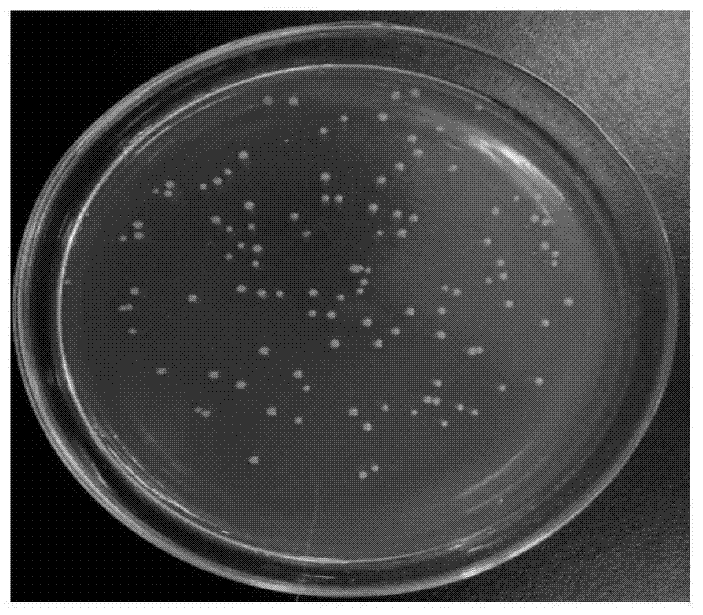
Raoultella ornithinolytica and applications thereof
ActiveCN104726369AImprove electrochemical activityBacteriaMicrobiological testing/measurementBiotechnologyEngineering
The invention discloses a raoultella ornithinolytica P1-A-19, which is prepared by carrying out enrichment, separation, purification and screening on sludge produced by sewage treatment plants. The raoultella ornithinolytica P1-A-19 is characterized in that the strain shows an electricity-production capacity when applied to a microbial fuel cell, and also has a capacity of converting baccatin III so as to generate 10-deacetyl baccatin III (10-DAB), and the Preservation Number of the strain is CGMCC No. 10267. The raoultella ornithinolytica P1-A-19 disclosed by the invention has a great significance for the enrichment of diversity of electricity-production microorganisms and the excavation of more microbial strains with high electrochemical activity; and microbial strains for 10-DAB biosynthesis are further widened, thereby providing new materials and new methods for the development of new taxane compound synthesis approaches.
Owner:TIANJIN UNIV OF SCI & TECH
Preparation method of taxol anticancer drugs XRP6258
InactiveCN103012330AEasy to manufactureEasy to makeOrganic chemistryAntineoplastic agentsButt jointDrugs synthesis
The invention belongs to the field of drug synthesis, and relates to a method for preparing taxol anticancer drugs XRP6258. The method comprises the following steps of: obtaining C-7 and C-10 bit hydroxy methyl mercaptan methylene (MTM) and C-13 bit oxhydryl oxydic key intermediate (II) of 10-oxhydryl baccatin III(I) by high regioselectivity, sequentially removing methylthio, reducing C-13 bit carbonyl or sequentially reducing C-13 bit carbonyl and removing methylthio to obtain a mother nucleus (IV) of the product XRP6258, carrying out butt joint with side chains with various types and removing a side-chain protecting group to obtain a product Cabazitaxel (V). The method disclosed by the invention has the advantages of being high in efficiency during a preparation process, simple in preparation process, high in yield, lower in cost, easy to operate and the like, so that the XRP6258 can be produced and prepared on a large scale.
Owner:FUDAN UNIV +1
Method for planting Taxus baccata in Zhejiang, China
Owner:曹县花仙子摄影服饰有限公司
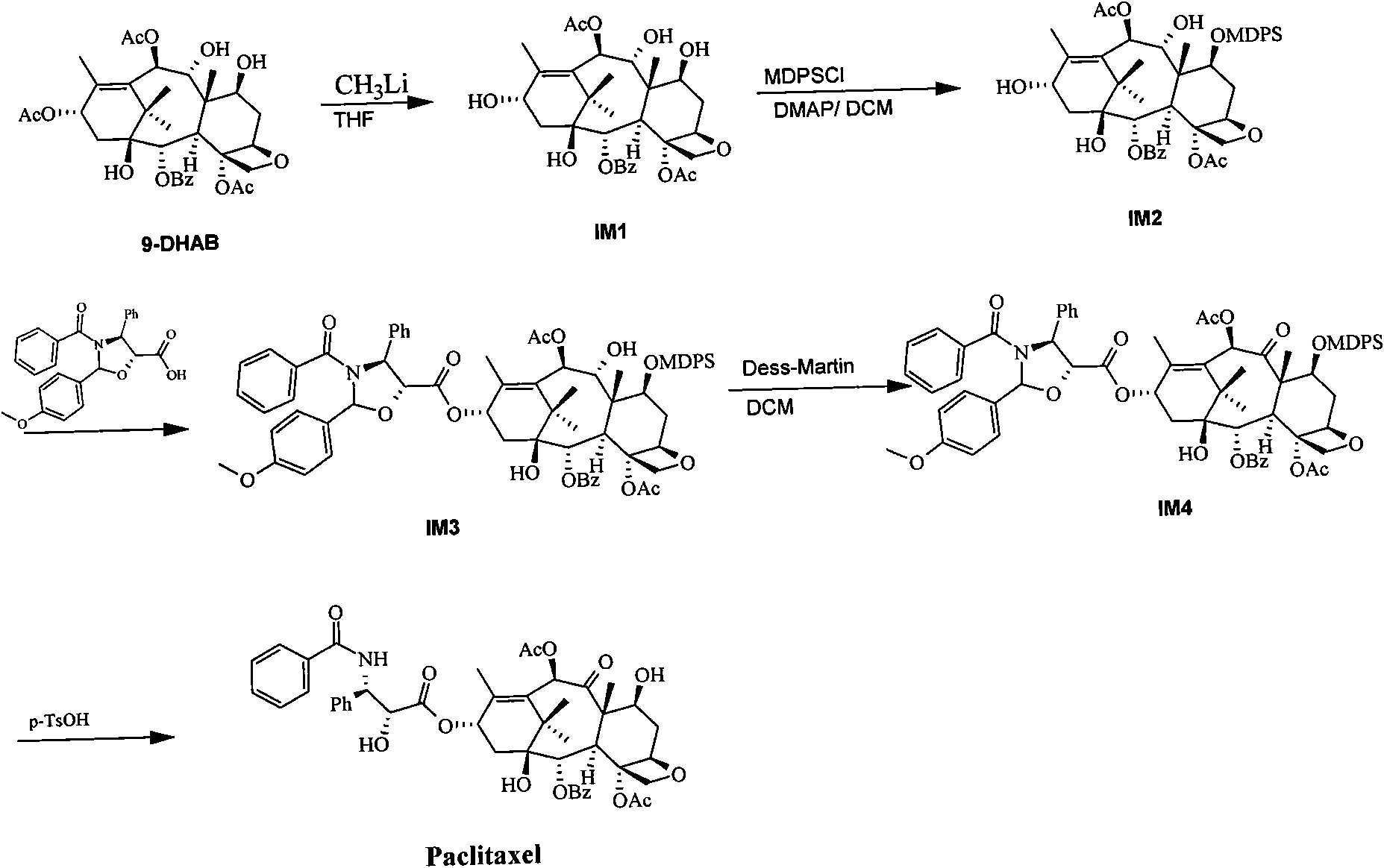
Method for semi-synthesis of paclitaxel on industrialized basis
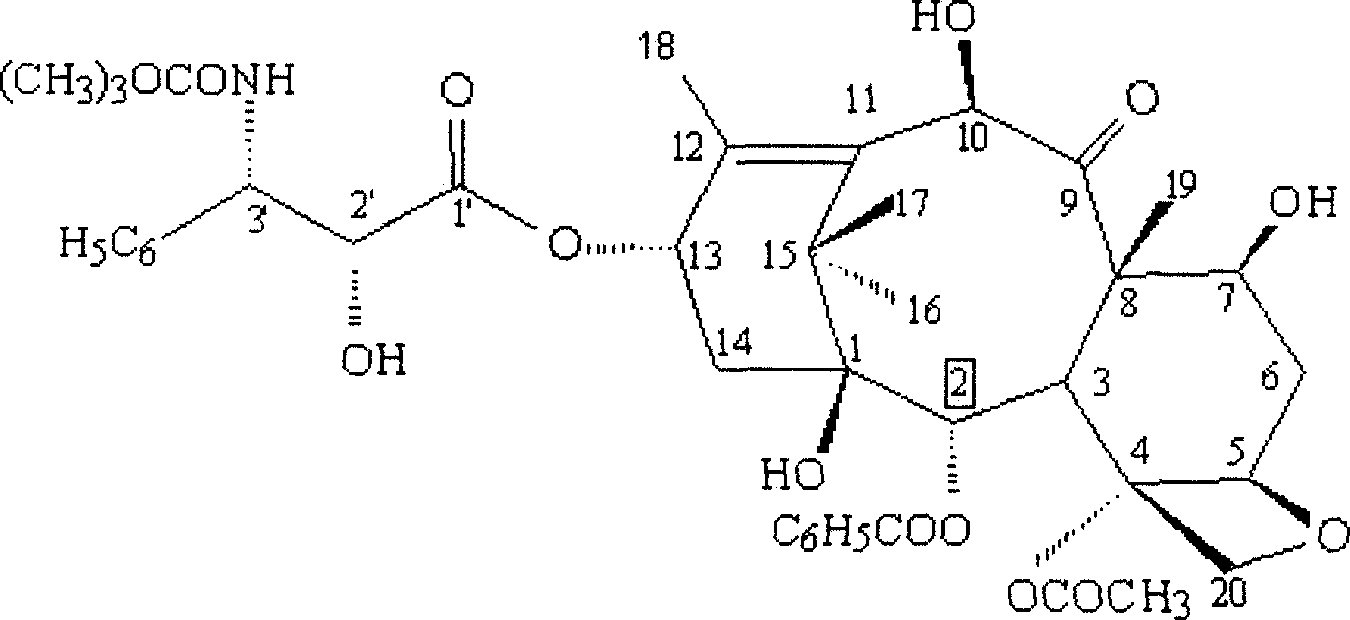
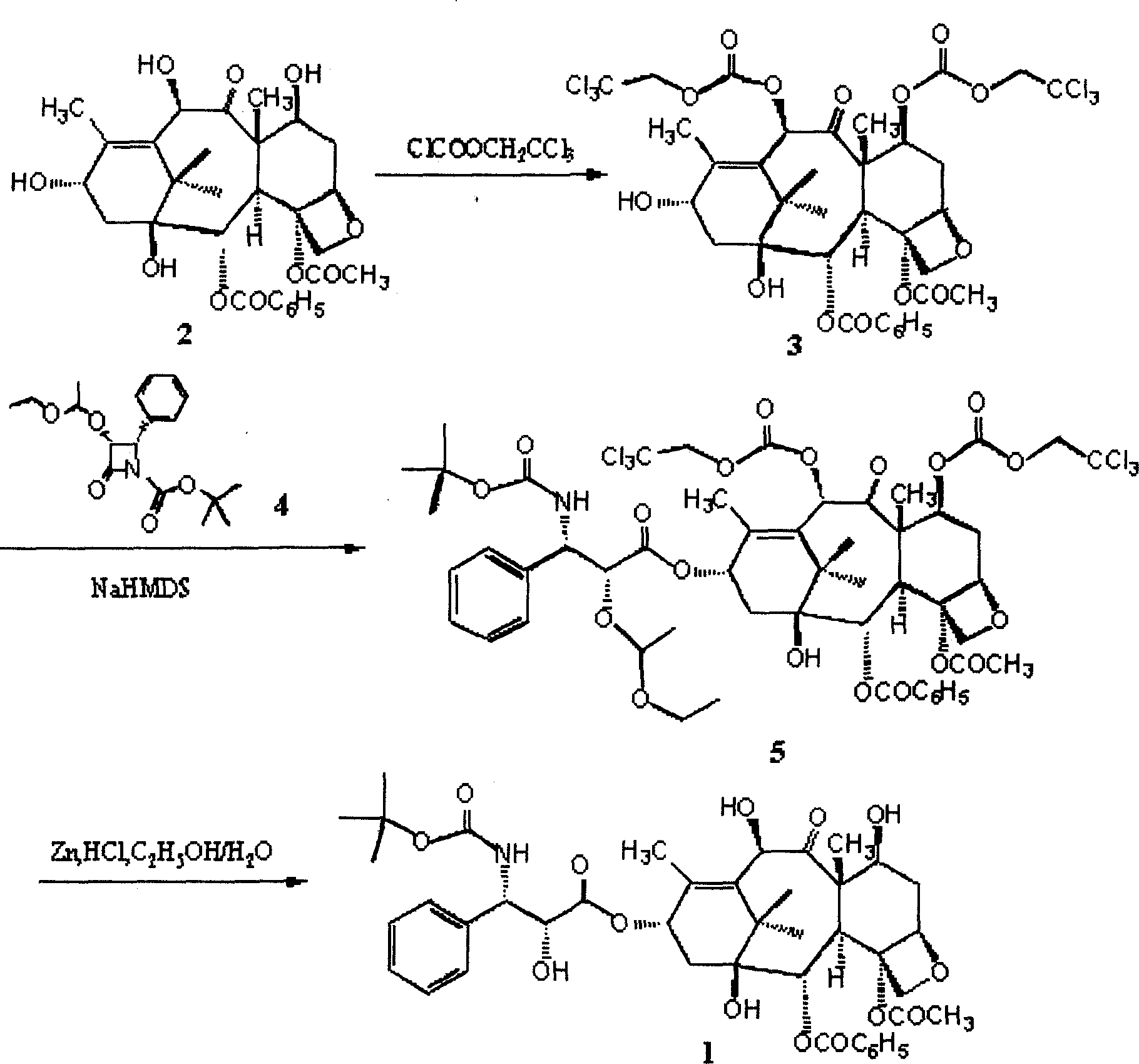
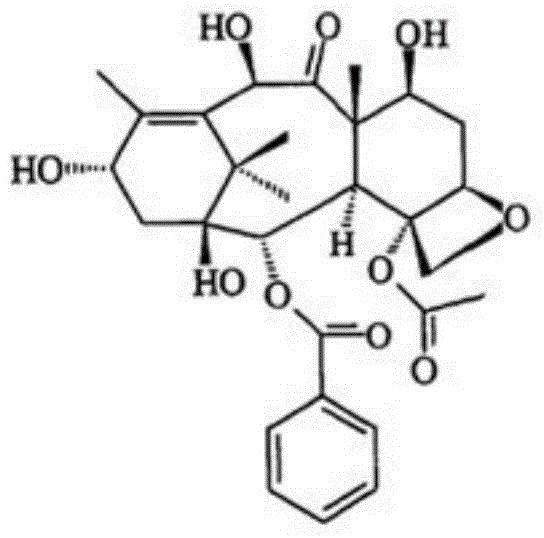
ActiveCN101863862AShort reaction timeSuitable for industrial productionOrganic chemistryBulk chemical productionSide chainBaccatin III
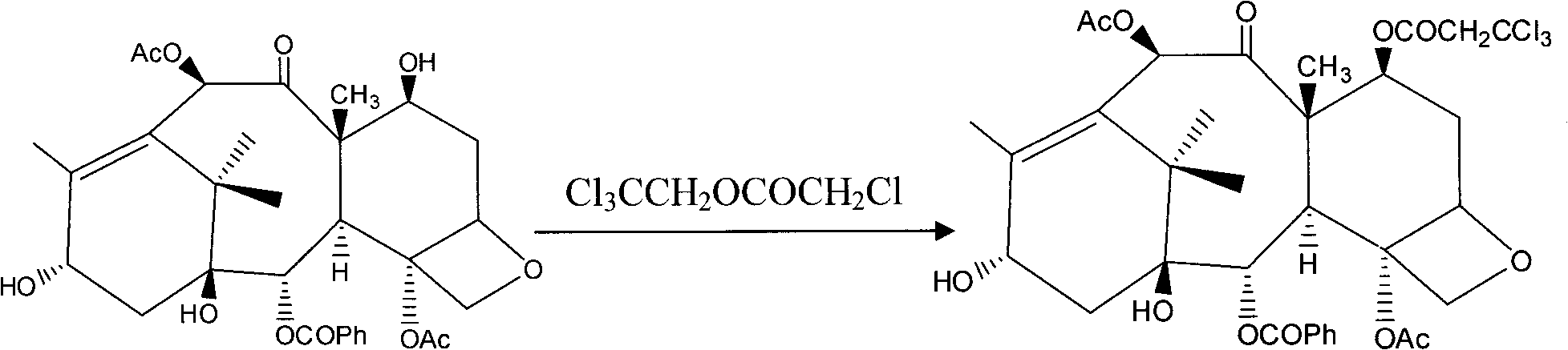
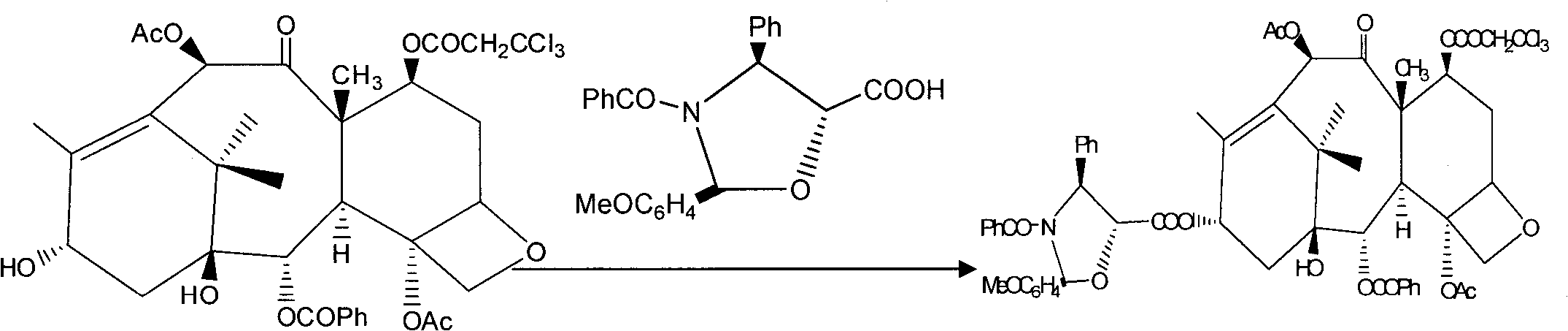
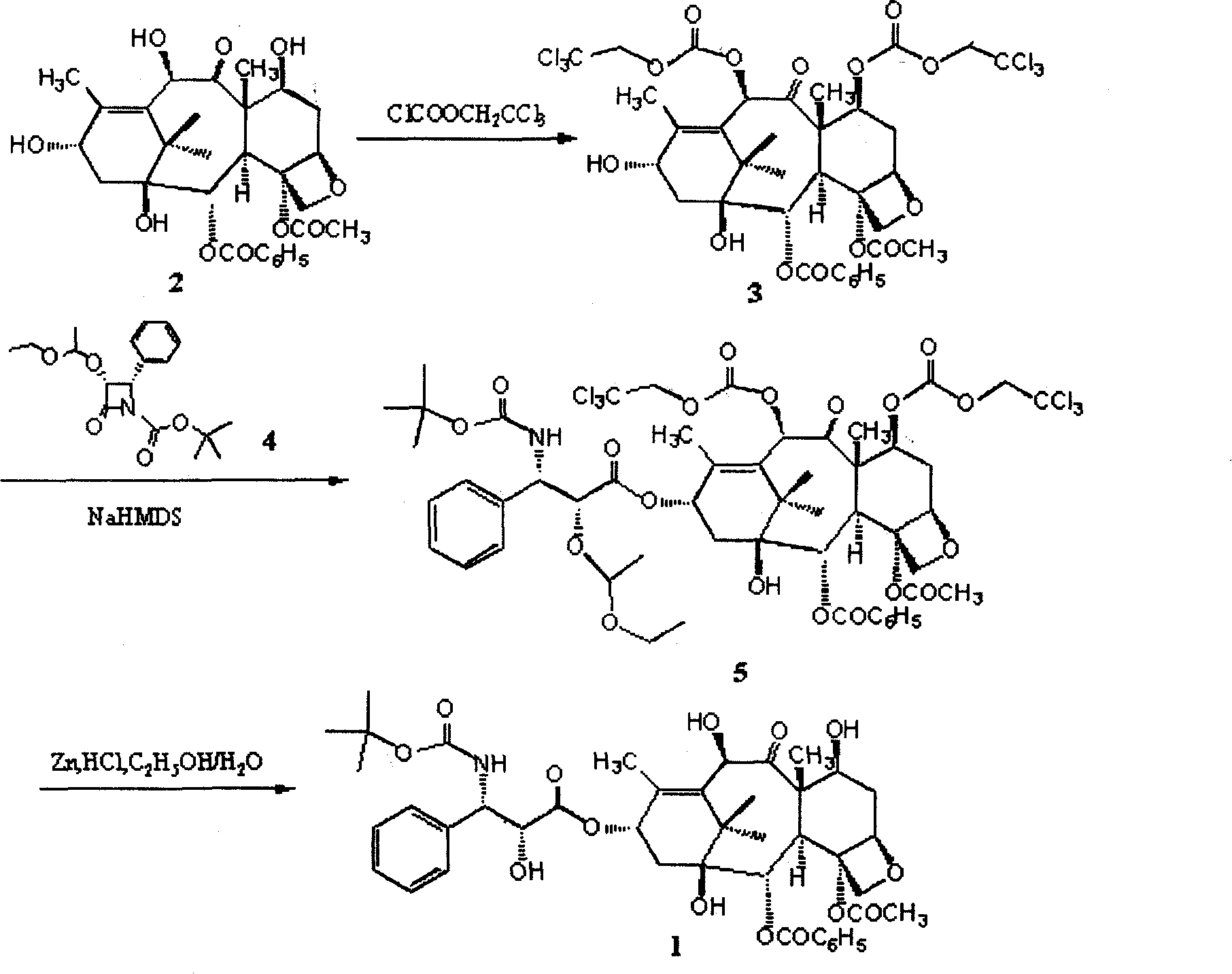
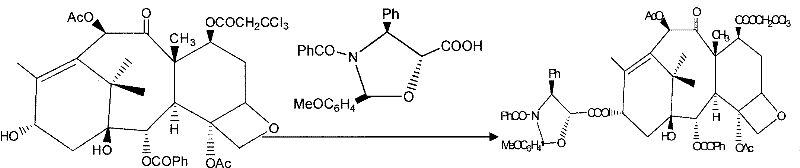
The invention provides a method for the semi-synthesis of paclitaxel on an industrialized basis. The method comprises the following steps: a) acetylating the 10-hydroxyl in the presence of CeCl3.6H2O as a high-efficiency specific catalyst to obtain baccatin III through the reaction; b) protecting the C-7-hydroxyl in the presence of trichloroethyl chloroformate to obtain baccatin III, wherein the C-7 position of baccatin III is protected; c) conducting the esterification reaction between the baccatin III obtained in the reaction of step b) and (4S, 5R)-3-benzoyl-2-(4-methoxyphenyl)-4-phenyl-5-oxazolinyl carboxylate to obtain the following derivatives; and d), opening the oxazole ring on the ring-shaped lateral chain of (4S, 5R)-3-benzoyl-2-(4-methoxyphenyl)-4-phenyl-5-oxazolinyl baccatin III, and synchronously removing the 7-protective group to obtain paclitaxel. The method of the invention breaks with the conventional method which sequentially comprising the steps of protecting the 7-position hydroxyl and acetylating the 10-position hydroxyl, so that the method has the advantages of shorter reaction time and less by-products; the yield of single-step reaction reaches 90%; and each step of reaction is conducted at normal pressure in the absence of protective inert gases. Therefore, the method is suitable for industrialized production.
Owner:上海卓鼎生物技术有限公司
Synthesis process of polyene taxol
The present invention provides synthesis of polyene taxol, and the polyene taxol synthesizing path consists of three steps, including protection, condensation and hydrolysis, mainly. The material 10-deacetyl Baccatin III first has its 7th and 10th positions hydroxyl groups protected, then optical side chain introduced to its 13th position to obtain intermediate, and finally hydrolyzed to obtain the target product polyene taxol. The preparation process of the present invention has less steps, no isomer impurity, less side reactions, high utilization of material 10-deacetyl Baccatin III, high yield, low cost and other advantages. The product is for treating tumor clinically.
Owner:SHANGHAI JINHE BIO TECH +1
Method for high efficiency separating and purifying 1-deacetyl Baccatins III (10-DABIII)
This invention relates to a high efficiency method of separation and purification 10 - DAB III. It utilizes tetrahydrofuran to dissolve semi-manufactured 10-DBA III, then add it to dynamic axial compression liquid phase preparative chromatographic column, adopts blend solvent of tetrahydrofuran, normal hexane and methanol as elution mobile phase, through one to twice separation, then be able to obtain 10 - DAB III of purity reaching 99 percent.
Owner:CHENGDU PUSH BIOLOGICAL TECH
Method for extracting 10-deacetyl baccatin III from branches and leaves of taxus chinensis
The invention discloses a method for extracting 10-eacetyl baccatin III from branches and leaves of taxus chinensis, comprising the following steps: 1, extraction and concentration: proportionally mixing smashed branches and leaves of taxus chinensis with water, carrying out ultrasonic extraction, adding solvent into filtrate obtained from ultrasonic extraction to extract, and concentrating extract liquor to obtain extract; and 2, recrystallization: firstly dissolving the extract into acetonitrile by heating, cooling and crystallizing, and filtering to obtain the crystalline powder of 10-eacetyl baccatin III. In the invention, the method for extracting and preparing 10-eacetyl baccatin III with high content, high yield and low cost is provided.
Owner:无锡紫杉药业股份有限公司
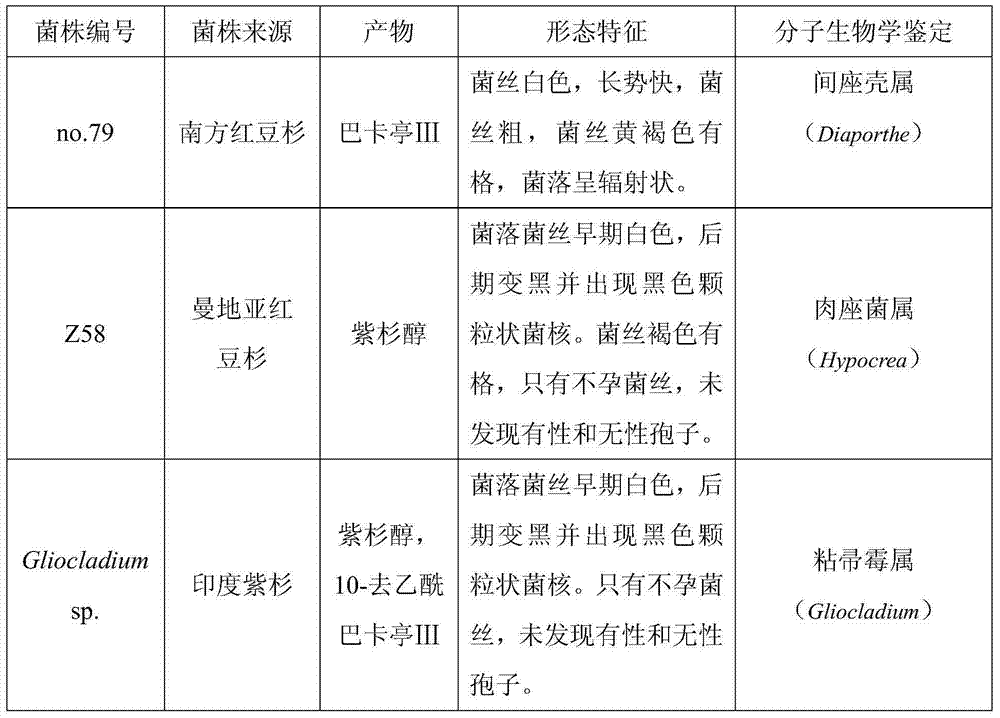
Combined strains for highly producing baccatin III and method for producing baccatin III
InactiveCN104726345AIncrease the content of baccatin ⅢReduce dependenceFungiFermentationBiotechnologySynthesis methods
The invention discloses a group of combined strains for highly producing baccatin III and a method for producing baccatin III, belonging to the technical field of biology. The combined strains for highly producing baccatin III are combined strains of baccatin III production strains and paclitaxel production strains, wherein the baccatin III production strains are Taxus chinensis var mairei endophytic fungi no.79, and paclitaxel production strains are Taxus media endophytic fungi Z58 or Taxus india endophytic Gliocladium SP. By sequentially carrying out independent culture and mixed culture on the baccatin III production strains and the paclitaxel production strains, the yield of baccatin III in an fermented object is substantially increased; by virtue of metabolic pathway complementation and a feedback inhibition theory, the yield of baccatin III is greatly increased and is far higher than that of an independent culture manner. According to the method, the industrial fermentation culture and production of baccatin III by virtue of endophytic fungi are realized, relatively cheap raw materials are provided for the production of paclitaxel by virtue of a semi-synthesis method, and the production cost is lowered; the dependence on the Taxus chinensis resource is reduced, and the damage to the environment is alleviated.
Owner:HENAN INST OF SCI & TECH
Process for preparing paclitaxel
InactiveUS6130336ASpeed up the processEasy to disassembleOrganic chemistryAntineoplastic agentsOxazolidine EBenzoyl chloride
PCT No. PCT / KR97 / 00157 Sec. 371 Date Feb. 23, 1999 Sec. 102(e) Date Feb. 23, 1999 PCT Filed Aug. 25, 1997 PCT Pub. No. WO98 / 08832 PCT Pub. Date Mar. 5, 1998The present invention elates to a process for preparing paclitaxel represented by formula (1) characterized in that: (a) an oxazolidine derivative represented by formula (2) or its salt in which X represents halogen, is coupled with a 7-trihaloacetyl-baccatin III represented by formula (3) in which R1 represents trihaloacetyl, in a solvent in the presence of a condensing agent to produce an oxazolidine substituent-containing taxane represented by formula (4) in which X and R1 are each as previously defined; (b) the oxazolidine ring is opened in a solvent in the presence of an acid, and the product thus obtained is reacted with benzoyl chloride in the presence of a base to produce a protected paclitaxel wherein the hydroxy group at 7-position is protected with trihaloacetyl group represented by formula (5) in which R1 is as previously defined; (c) then the protecting group at 7-position is removed by ammonia or a salt of ammonia with a weak acid in a solvent.
Owner:HANMI PHARMA
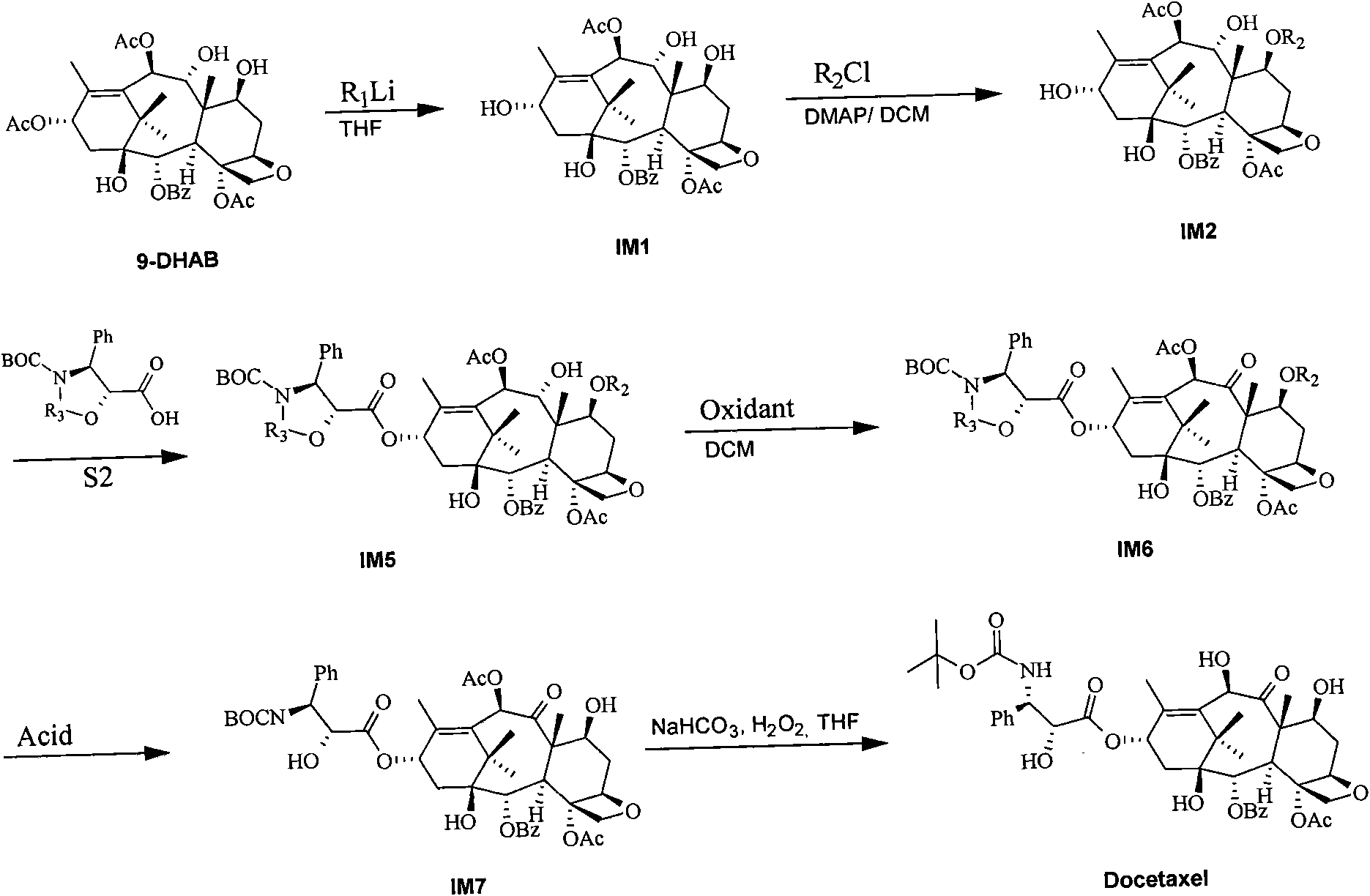
Method for preparing docetaxel
InactiveCN101353334AHigh purityHigh reaction yieldOrganic chemistryAntineoplastic agentsTert-Butyloxycarbonyl protecting groupBenzyl chloroformate
The invention relates to a new method for preparing Docetaxel which includes a new synthesizing method for forming 10-deacetyl baccatin III(II) for protecting C-7, C-10-carbobenzoxy by using the 10-deacetyl baccatin III(I) for reacting with a benzyl chloroformate compound, and a new method for forming a new compound of 2'-(1-ethoxyethyl)-N-debenzoyl-N-(boc)-C-7, C-10-II-X-CBZ-10-deacetyl paclitaxel(IV) by the reaction of (II) with 1-boc-(3R,4S)-4-phenyl azetidine-2-ketone(III) as well as the new compound. Simultaneously, the invention also provides a new method for preparing a new compound of 2'- (1-ethoxyethyl)-N-debenzoyl-N-(boc)-10-deacetyl paclitaxel(V) and a new method for removing the 1-ethoxyethyl from the (IV) to form the Docetaxel.
Owner:重庆医科大学医药研究所
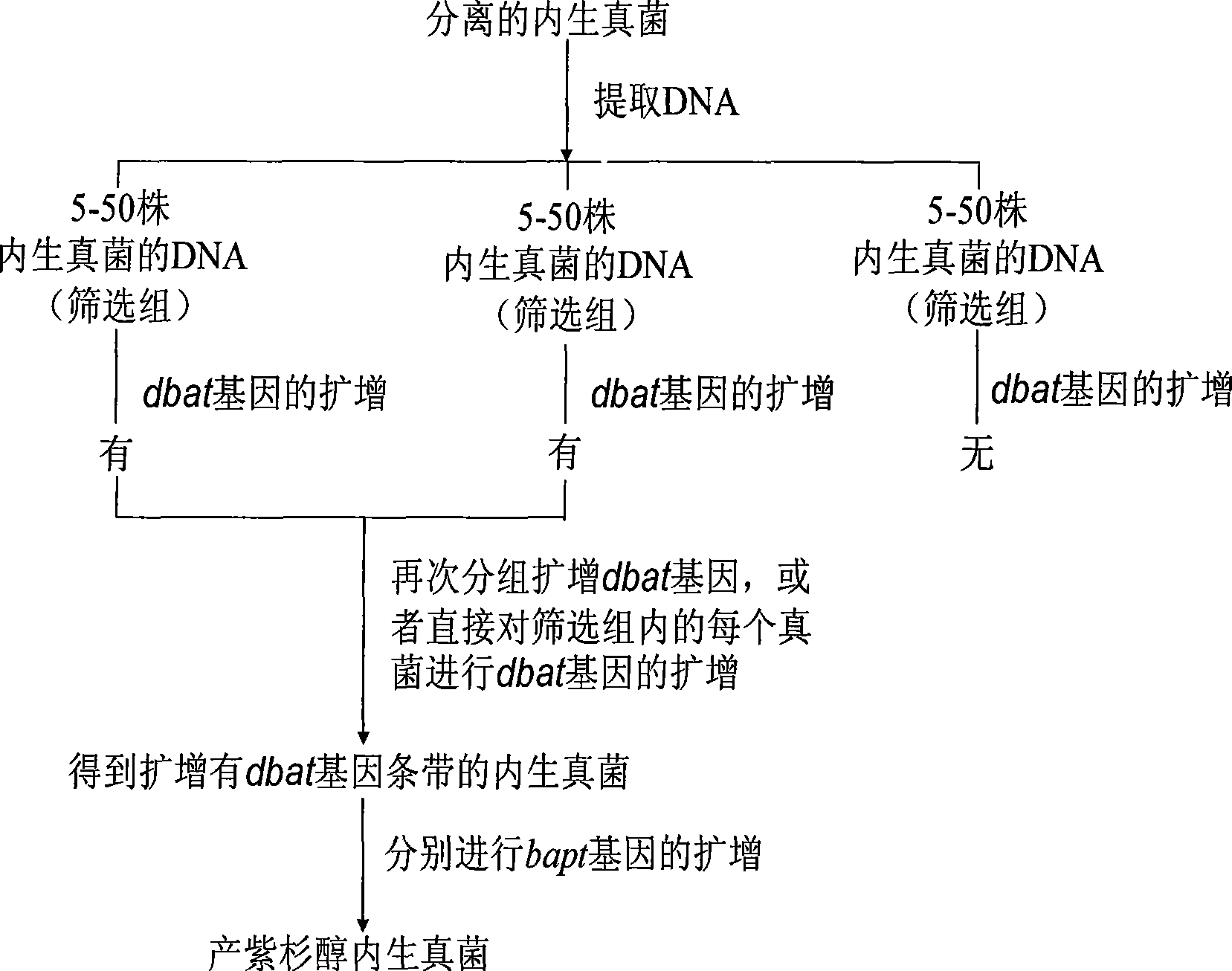
Method for quickly screening taxol-producing endophytic fungi
InactiveCN101418341ASimplify the screening processImprove screening efficiencyMicrobiological testing/measurementBiotechnologyEnzyme Gene
The invention discloses a method for quickly screening taxol-producing endogenetic fungi. The method quickly screens the taxol-producing endogenetic fungi by adoption of key enzyme genes synthesized by taxol as molecular markers and on the basis of the PCR amplification technology. The method designs primers according to conserved regions of 10-Deacetyl Baccatin III-10-O- acetylase genes and C-13phenylalanine side chain CoA acetylase genes of reported yew, performs amplification screening on fungi which is obtained by separation of yew barks in turn, performs fermentation culture on the fungi which can generate the two genes by means of amplification, extracts the taxol, analyzes the taxol of the fungi by HPLC and MS, and finally determines the taxol-producing endogenetic fungi. The invention provides an effective strain separating and screening method and a batch of effective original strains in order to realize replacement of extraction and conversion of the taxol from yew plant tissue by the microorganism fermentation method.
Owner:HUAZHONG UNIV OF SCI & TECH
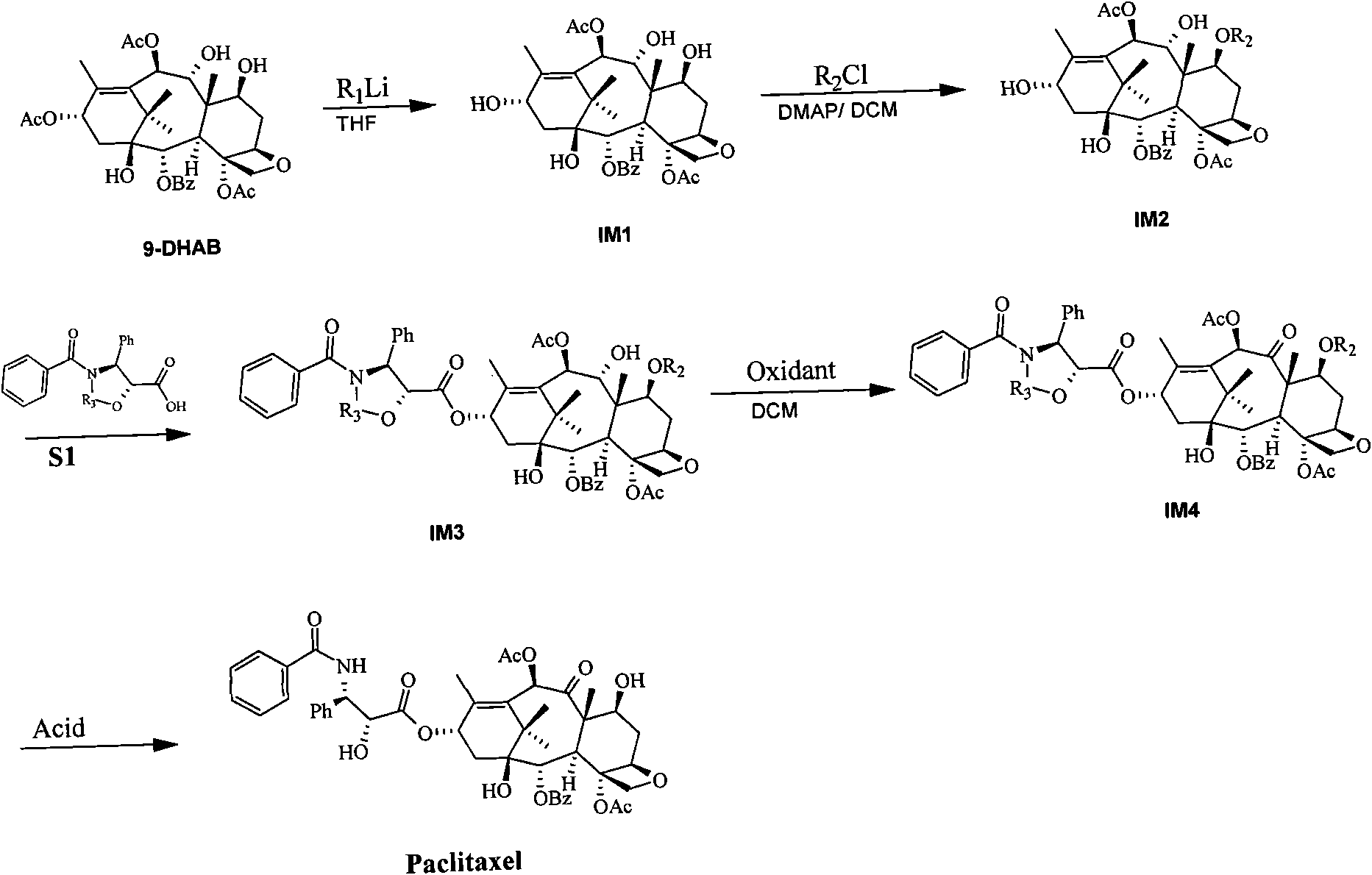
Method for semi-synthesizing paclitaxel and docetaxel
InactiveCN101838251ASimple processHigh yieldOrganic chemistryBulk chemical productionDocetaxel-PNPDocetaxel
The invention discloses a method for semi-synthesizing paclitaxel and docetaxel. The method for semi-synthesizing the paclitaxel sequentially comprises the following steps of: selectively stripping off C-13 acetyl of 13-acetyl-9-dihydro baccatin III to obtain a compound IM1; protecting C-7 hydroxy of the compound IM1 with a protective group to obtain a compound IM2; carrying out condensation reaction on the compound IM2 and a compound S1 and a compound S2 respectively to obtain a compound IM3 and a compound IM5; oxidizing C-9 hydroxy of the compound IM3 and the compound IM5 with oxidant to obtain a compound IM4 and a compound IM6 respectively; stripping off the C-7 protective group and the R3 protective group of the compound IM4 to obtain the paclitaxel; and stripping off the C-7 protective group, R3 protective group and acetyl of the compound IM6 to obtain the docetaxel. The method has the advantages of high yield of final products, low cost, and easy implementation.
Owner:翟雄 +1
Method for simultaneously extracting paclitaxel and 10-DABIII (10-Deacetyl Baccatin III) from taxus chinensis
InactiveCN108467373AHigh extraction rateAvoid oxidative degradationOrganic chemistryBaccatin IIISolvent
The invention discloses a method for simultaneously extracting paclitaxel and 10-DABIII (10-Deacetyl Baccatin III) from taxus chinensis. The method comprises the following steps: crushing taxus chinensis leaves into powder; soaking with an extraction solvent; putting a mixed liquid into a crushing extraction device; extracting for 1-3 times; performing crushing extraction at a low temperature andhigh pressure; after paclitaxel and 10-DABIII are rapidly dissolved into the extraction solvent, performing centrifugal separation on supernate and precipitate, thereby obtaining the supernate, that is, extracts of paclitaxel and 10-DABIII. Due to the low temperature and the high pressure, paclitaxel and 10-DABIII can be simultaneously extracted in one process, and in addition, the extraction rateof paclitaxel and 10-DABIII is as high as 96%. The method disclosed by the invention has the advantages of being good in stability, simple to operate, short in preparation time, high in extraction efficiency, safe and non-toxic, and the like, and meanwhile paclitaxel and 10-DABIII can be effectively prevented from oxydative degradation.
Owner:梅州市中大南药发展有限公司 +1
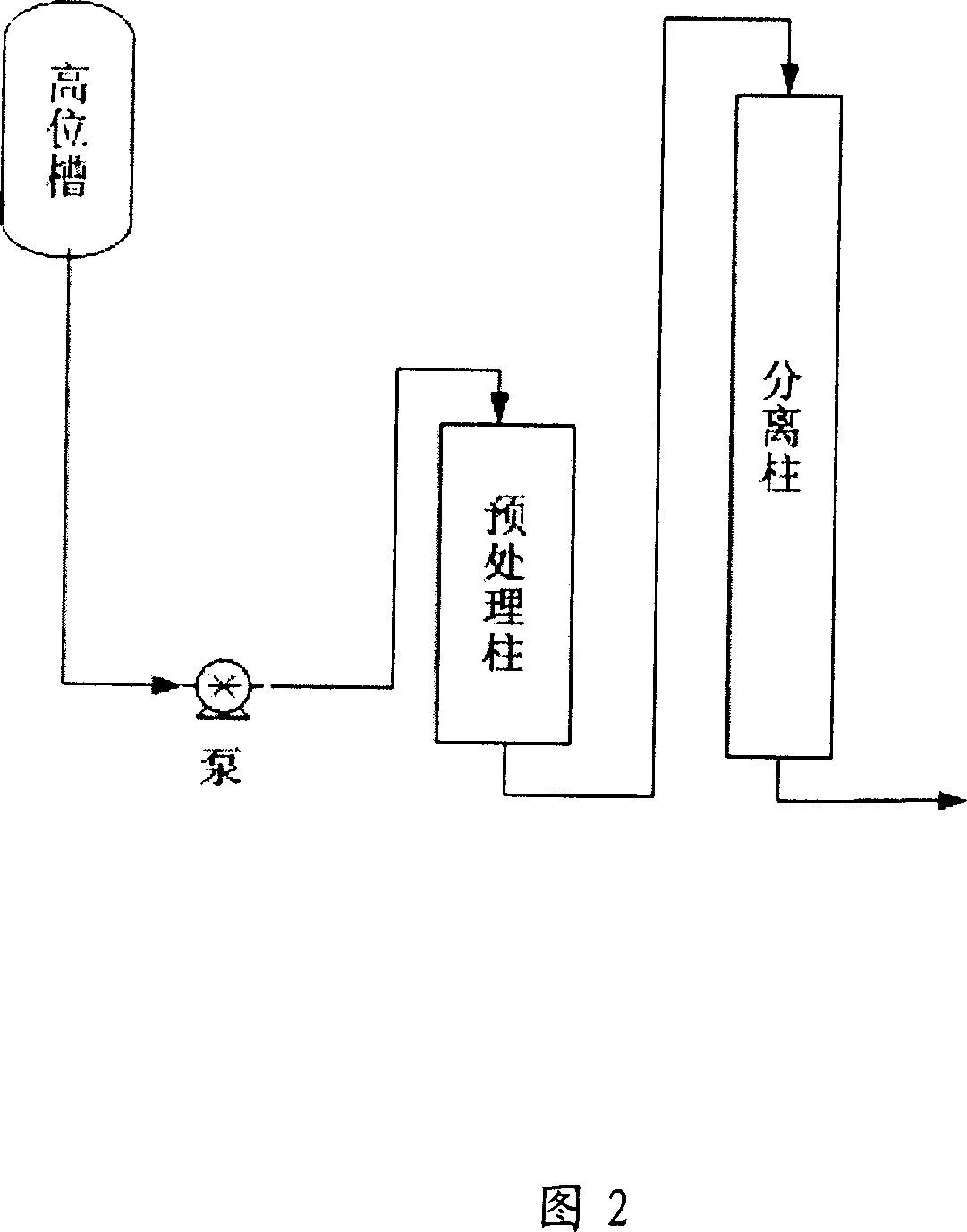
Method for separating and purifying 10-deacetyl Baccatins III
ActiveCN101045718AGood removal effectExtended service lifeOrganic chemistryPurification methodsNegative phase
This invention relates to a kind of separation and purification method of 10- DABIII. This invention adopts positive and negative phase chromatographic technique combinding, takes normal phase color spectrum as first order hand ling, remove most impurity, in favor of protecting high cost reversion phase chromatography support, prolong its service life; whereas reversion phase chromatography could hand larger sample quantity, and has better removal effect to the impurity that normal phase colour spectrum unease separate off, solvent is easy reclaim and cost is cheaper. So the combinding of positive and negative phase chromatographic technique not only could obtain 10 -DABIII, but also be able to reduce cost. Besides, this invention also adopts two-stage color spectrum tand em isolation techniques, through pretreatment post to separate a part of impurity, then cascaded separating column to carry out subdivision, advance separating effect, increase load sample quantity of chromatographic column.
Owner:北京信汇科技有限公司
Semi-synthesis of a protected baccatin III compound
InactiveUS20020128498A1High yieldSimple and inexpensiveGroup 4/14 element organic compoundsDocetaxelBaccatin III
The present invention relates to a semi-synthetic process to convert a naturally occurring taxane into a suitable starting material for the synthesis of paclitaxel and related compounds. Specifically, the present invention relates to a process for the conversion of 9-dihydro-13-acetylbaccatin III into a 7-protected baccatin III which can then be used as starting material for the synthesis of taxane derivatives such as paclitaxel, docetaxel, cephalomannine and other taxanes structurally related to baccatin III. The method as described uses a preparative scale technique which is amenable to commercial scale-up.
Owner:CHATHAM BIOTEC LTD
Method for using Taxus media branches and leaves as raw material to prepare high-purity 10-deacetyl baccatin III
InactiveCN105085443AImprove solubilityImprove leaching efficiencyOrganic chemistryBaccatin IIIDissolution
A method for using Taxus media branches and leaves as a raw material to prepare high-purity 10-deacetyl baccatin III comprises the technological steps of (1) coarse extraction, (2) sedimentation, (3) adsorption, (4) dissolution, (5) crystallization and (6) recrystallization. According to the method, a crude product is dissolved by adopting different single solvents through macro-porous resin adsorption to remove a large amount of undissolved impurities, and then the high-purity 10-deacetyl baccatin III is obtained through multiple-time crystallization. The method is simple in process and low in cost and is beneficial to environmental protection, and operation is easy to master.
Owner:SICHUAN AGRI UNIV
Method for extracting 10-deacetyl baccatin III from Taxus chinensis var.mairei
The invention discloses a method for extracting 10-deacetyl baccatin III from Taxus chinensis var.mairei, and relates to the technical field of natural product extraction. The method comprises the following steps: (1) crushing is conducted, liquid nitrogen treatment is conducted, and ammonia gas blasting treatment is conducted; (2) petroleum ether is subjected to ultrasonic impurity removal; (3) ethanol is subjected to high-pressure and constant-temperature extraction and ultrasonic extraction; (4) decompression concentration is conducted, dissolution is conducted though acetone, filtering andconcentration are conducted, and thus a crude extract I is obtained; (5) macroporous adsorption resin is washed though an alkali solution and deionized water in sequence and then eluted with the ethanol, eluent is collected and concentrated, and thus a crude extract II is obtained; (6) primary recrystallization is conducted, specifically, recrystallization is conducted through acetonitrile; and (7) secondary recrystallization is conducted, specifically, recrystallization is conducted through an acetone / petroleum ether system. The method is high in operability, the steps are reasonably set, operation parameters are strictly controlled, the 10-deacetyl baccatin III with the excellent purity and yield can be obtained, the purity reaches 99.3% or above, and the yield is 0.0242% or above.
Owner:无锡紫杉药业股份有限公司
Method for semi-synthesis of paclitaxel on industrialized basis
ActiveCN101863862BShort reaction timeSuitable for industrial productionOrganic chemistryBulk chemical productionSide chainCarboxylic salt
The invention provides a method for the semi-synthesis of paclitaxel on an industrialized basis. The method comprises the following steps: a) acetylating the 10-hydroxyl in the presence of CeCl3.6H2O as a high-efficiency specific catalyst to obtain baccatin III through the reaction; b) protecting the C-7-hydroxyl in the presence of trichloroethyl chloroformate to obtain baccatin III, wherein the C-7 position of baccatin III is protected; c) conducting the esterification reaction between the baccatin III obtained in the reaction of step b) and (4S, 5R)-3-benzoyl-2-(4-methoxyphenyl)-4-phenyl-5-oxazolinyl carboxylate to obtain the following derivatives; and d), opening the oxazole ring on the ring-shaped lateral chain of (4S, 5R)-3-benzoyl-2-(4-methoxyphenyl)-4-phenyl-5-oxazolinyl baccatin III, and synchronously removing the 7-protective group to obtain paclitaxel. The method of the invention breaks with the conventional method which sequentially comprising the steps of protecting the 7-position hydroxyl and acetylating the 10-position hydroxyl, so that the method has the advantages of shorter reaction time and less by-products; the yield of single-step reaction reaches 90%; and eachstep of reaction is conducted at normal pressure in the absence of protective inert gases. Therefore, the method is suitable for industrialized production.
Owner:上海卓鼎生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com