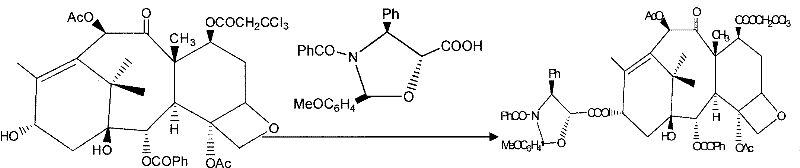
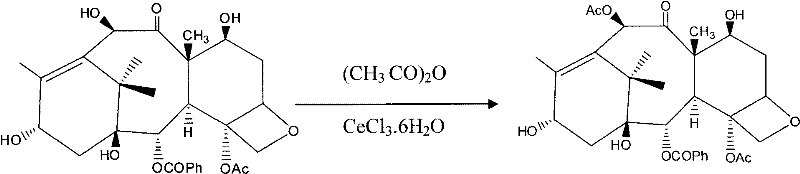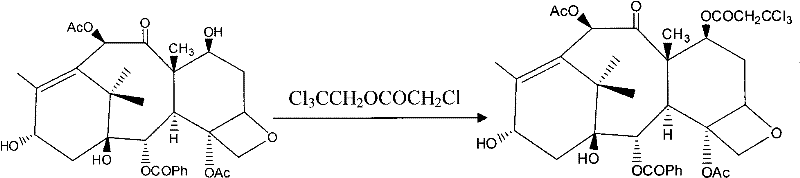Method for semi-synthesis of paclitaxel on industrialized basis
A paclitaxel and semi-synthetic technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of many by-products and long reaction time, and achieve the effects of less by-products, short reaction time, and reduced operation links.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] a, the synthesis of baccatin III:
[0034] Add 1 g of 10-DAB to a dry round-bottomed flask, dissolve in 20 ml of THF, stir magnetically to dissolve, then add 80 mg of CeCl 3 .6H 2 O, add 3ml of acetic anhydride again, stir and react at room temperature for 1.5 hours, TLC detects that the reaction is complete, the reaction solution is poured into ice water and left to stand, white particles crystallize out, and filtered with a glass sand funnel.
[0035] After drying, 1.04 g of bacatin III solid was obtained with a yield of 95%.
[0036] b. Preparation of baccatin III in which trichloroethyl chloroformate protects the 7-hydroxyl group:
[0037] Dissolve 1 g of baccatin III in 20 ml of anhydrous dichloromethane, stir to dissolve, add 0.62 ml of anhydrous pyridine, 0.5 ml of trichloroethyl chloroformate, and react at 10°C for 1.5 hours. TLC detects that the reaction is complete. Add ice water to the bottle to stop the reaction. The organic phase was washed three times wi...
Embodiment 2
[0043] a, the synthesis of baccatin III:
[0044] Add 1 g of 10-DAB to a dry round-bottomed flask, dissolve it in 15 ml of tetrahydrofuran, and stir to dissolve it, then add 50 mg of anhydrous CeCl 3 , then add 5ml of acetic anhydride, stir and react at room temperature for 2 hours, TLC detects that the reaction is complete. After drying, 0.95 g of bacatin III solid was obtained with a yield of 88%.
[0045] b. Preparation of baccatin III in which trichloroethyl chloroformate protects the 7-hydroxyl group:
[0046] Dissolve 1 g of baccatin III in 15 ml of anhydrous dichloromethane, stir to dissolve, add 0.7 ml of anhydrous pyridine, 0.7 ml of trichloroethyl chloroformate, and react at 20 ° C for 1 hour. TLC detects that the reaction is complete. Add ice water to the bottle to stop the reaction. The organic phase was washed three times with 5% hydrochloric acid, then washed with saturated sodium carbonate, and finally washed with saturated brine, the organic phase was dried ...
Embodiment 3
[0051] a, the synthesis of baccatin III:
[0052] Add 1 g of 10-DAB to a dry round-bottomed flask, dissolve in 25 ml of THF, stir magnetically to dissolve and add 150 mg of CeCl 3 .7H 2 O, add 4ml of acetic anhydride again, stir and react at room temperature for 3 hours, TLC detects that the reaction is complete, the reaction solution is poured into ice water and left to stand, white particles are crystallized, and filtered with a glass sand funnel.
[0053] After drying, 0.93 g of bacatin III solid was obtained with a yield of 86%.
[0054] b. Preparation of baccatin III in which trichloroethyl chloroformate protects the 7-hydroxyl group:
[0055] Dissolve 1g of baccatin III in 25ml of anhydrous dichloromethane, stir to dissolve, add 0.55ml of anhydrous pyridine, 0.8ml of trichloroethyl chloroformate, and react at 5°C for 2 hours. TLC detects that the reaction is complete. Add ice water to the bottle to stop the reaction. The organic phase was washed three times with 5% h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com