Synthesis process of polyene taxol
A technology of docetaxel and its synthetic method, which is applied in the direction of organic chemistry, can solve the problems of reduced yield, high product cost, difficult separation, etc., and achieve the effect of less impurities, low cost and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
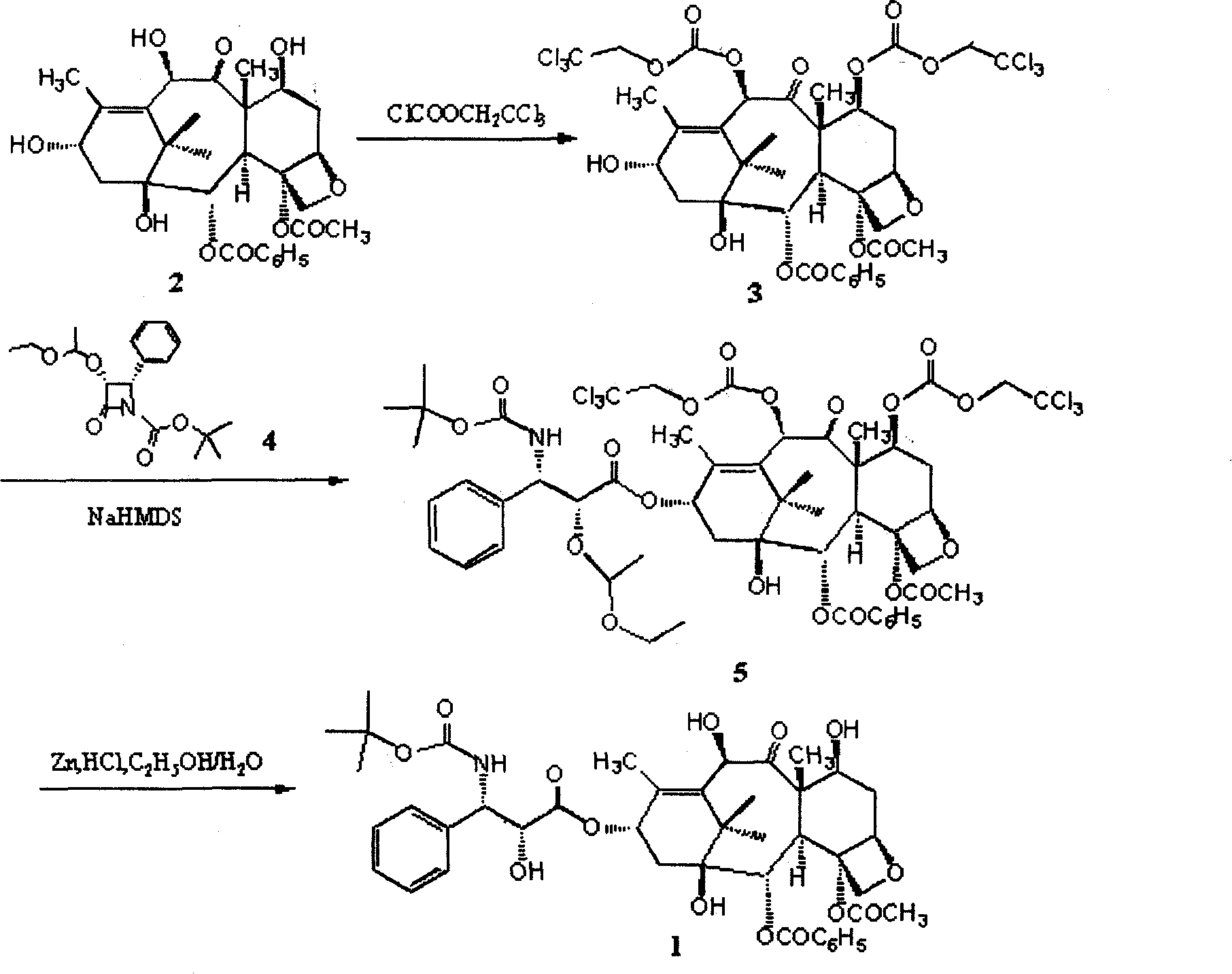
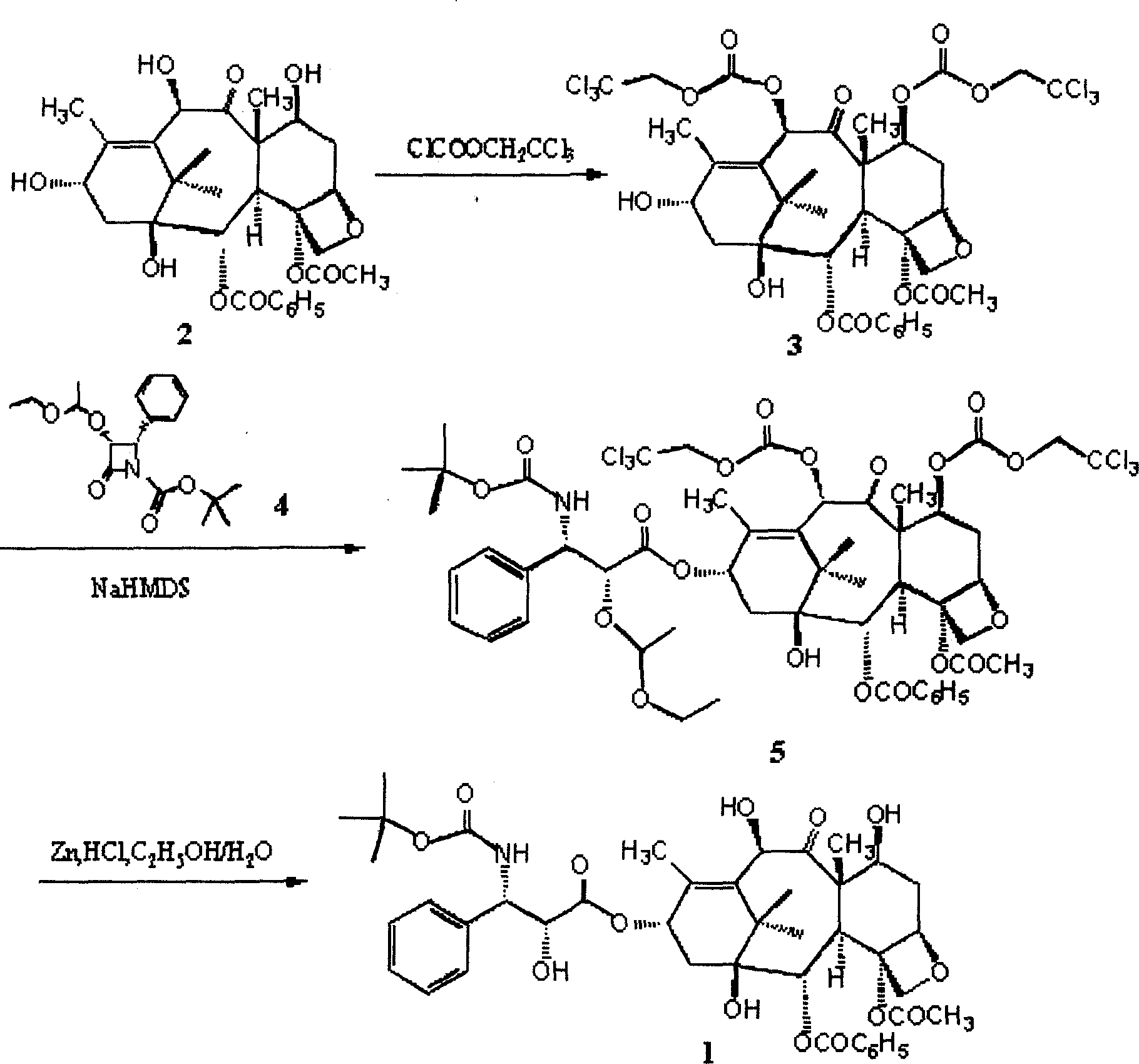
[0034] Example 1, the protection of the 7-position and 10-position hydroxyl of 10-deacetyl baccatin III
[0035] Take 20 grams of 10-deacetylbaccadin III that has been dried in vacuum and add it to a four-necked reaction flask, vacuumize nitrogen or argon for three times, pass nitrogen or argon protection, add 360ml of anhydrous pyridine, stir, and control the reaction The system is at 0°C, and 17.4ml of (2,2,2-trichloro)ethyl chloroformate is extracted with a syringe, and slowly added dropwise to the reaction flask, and the temperature of the reaction system is controlled at 0±1°C. After the dropwise addition, spot the plate to judge the reaction end point (developing agent: ethyl acetate / n-hexane=4 / 6), and stop the reaction with an appropriate amount of deionized water after the reaction. Concentrate the reaction solution below 60°C to dryness, add an appropriate amount of dichloromethane to completely dissolve the concentrate, add 2mol / l hydrochloric acid to wash to acidic ...
example 2
[0036] Example 2, the protection of the 7-position and 10-position hydroxyl of 10-deacetyl baccatin III
[0037] Take 300 grams of 10-deacetylbaccatin III and add it to a 5-liter four-necked reaction flask, add pyridine, stir, control the reaction system at 10-15°C, and slowly add chloroformic acid-(2, 2,2-Trichloro)ethyl ester / dichloromethane (254ml / 280ml) solution, the dropwise addition time is about 10-20 minutes, and the temperature of the reaction system is controlled at 15±5°C. After the dropwise addition, continue to react for 10 minutes, point the plate to judge the end point of the reaction (developing agent: ethyl acetate / n-hexane=4 / 6), transfer to a 15-liter extraction bottle after the reaction, add 2L deionized water to terminate the reaction , extracted three times with 6L dichloromethane. Combine the extracts, add 4mol / l hydrochloric acid to wash to acidic (PH=4-5), add saturated sodium bicarbonate to wash to basic (PH=7-8), and then wash with deionized water to...
example 3
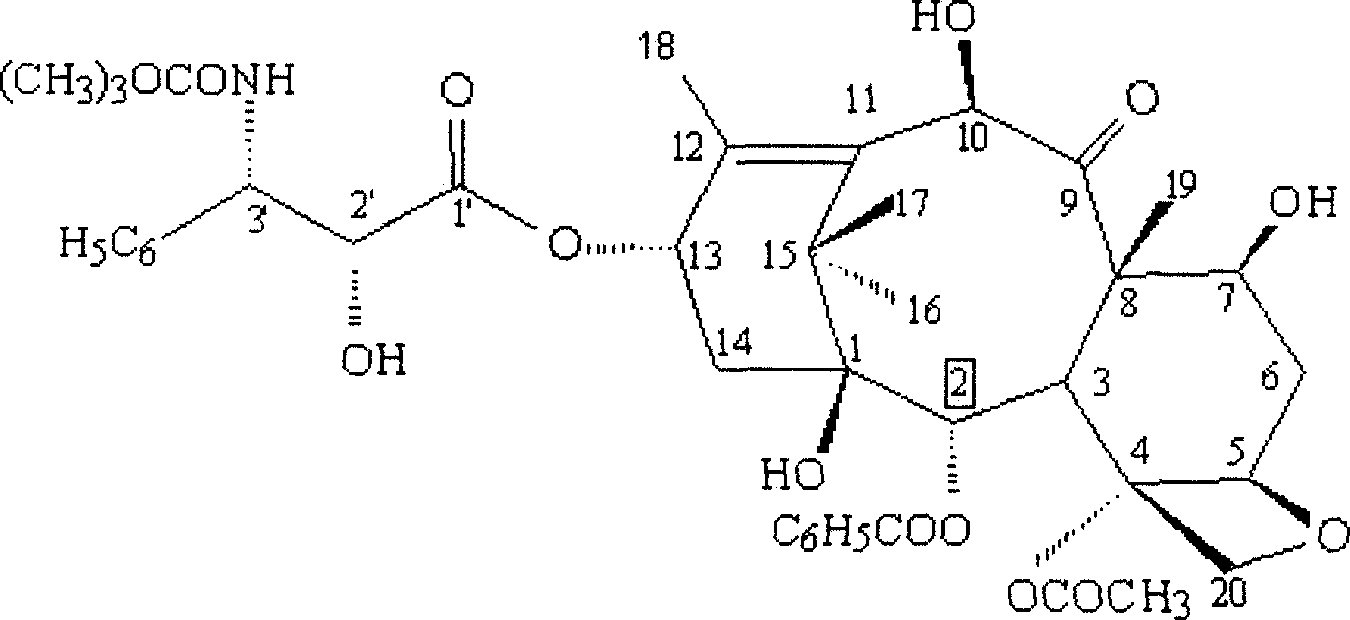
[0038] Example 3, Condensation Step of Intermediate 3 and Side Chain
[0039] Take 30.2 grams of 7,10-bis(trichloroethoxycarbonyl)-10-deacetylbaccatin III and add it to a 250ml four-necked reaction flask, vacuumize nitrogen or argon for three times, and pass nitrogen or argon Under gas protection, add 80ml of anhydrous tetrahydrofuran, stir, cool to -60°C, then slowly add 30% NaHMDS / THF 33ml dropwise, keep the reaction temperature not exceeding -60°C, after the addition is complete, continue the reaction at this temperature for about 30 minutes. Then 18.1 grams of side chains were added dropwise, keeping the reaction temperature not exceeding -60°C. After the addition was complete, the temperature was slowly raised to 0°C, and the reaction was completed in about 1 hour. Add 200 ml of saturated sodium bicarbonate to the reaction solution, extract three times with 400 ml of dichloromethane, combine the organic phases, wash with 300 ml of saturated brine, dry over anhydrous magne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com