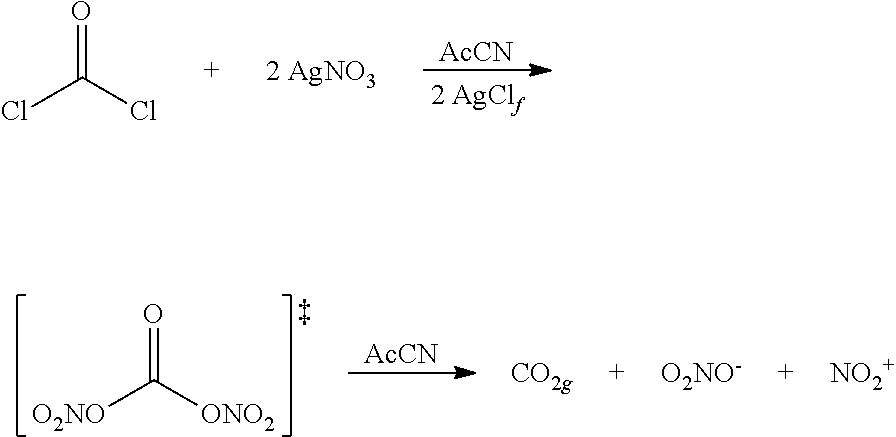Patents
Literature
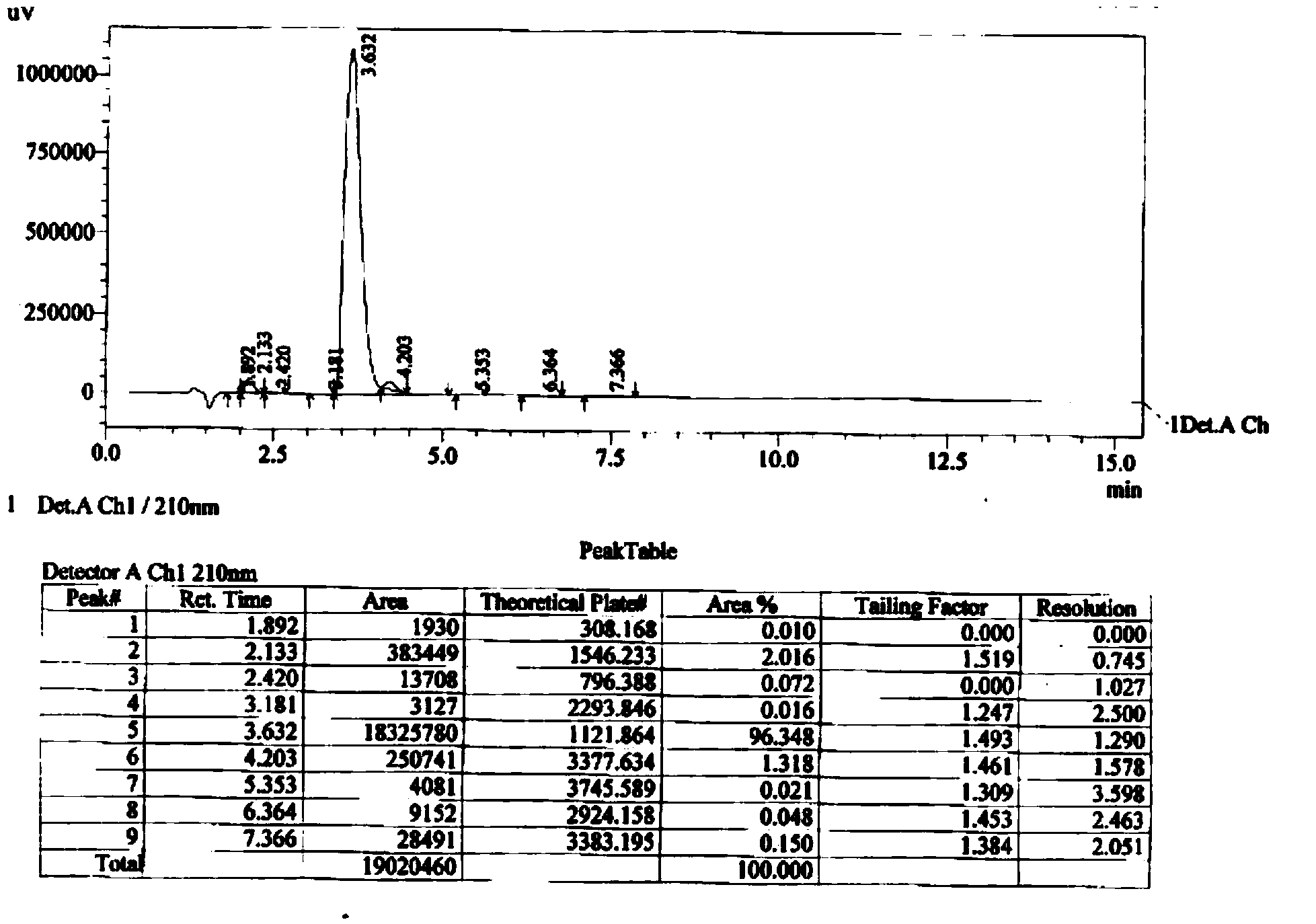
169 results about "Chloroformic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chloroformic acid is an unstable chemical compound with the formula ClCO₂H. It is the single acyl-halide derivative of carbonic acid (phosgene is the double acyl-halide derivative). Chloroformic acid is also structurally related to formic acid, which has a hydrogen instead of the chlorine. Despite the similar name, it is very different from chloroform.
Method for synthesizing naloxone or naltrexone
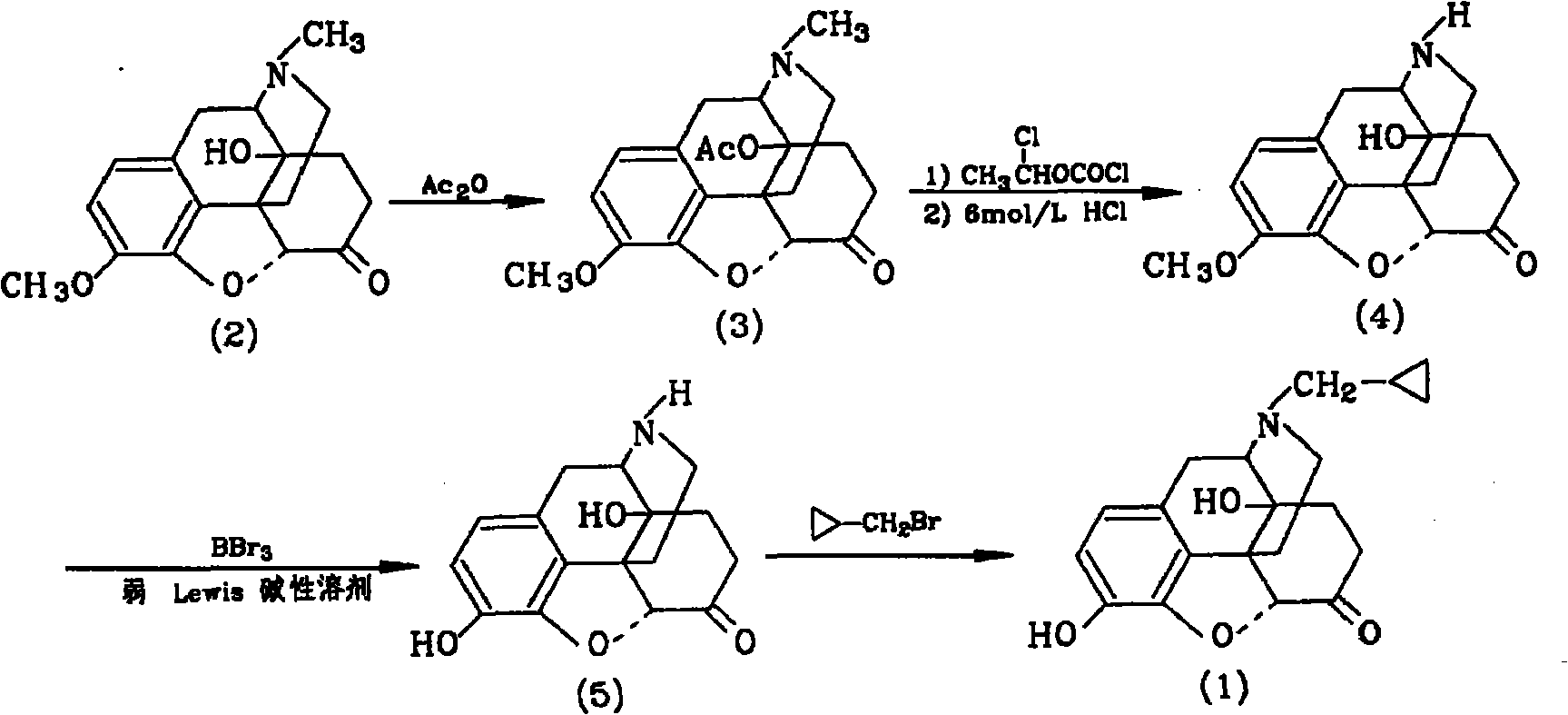
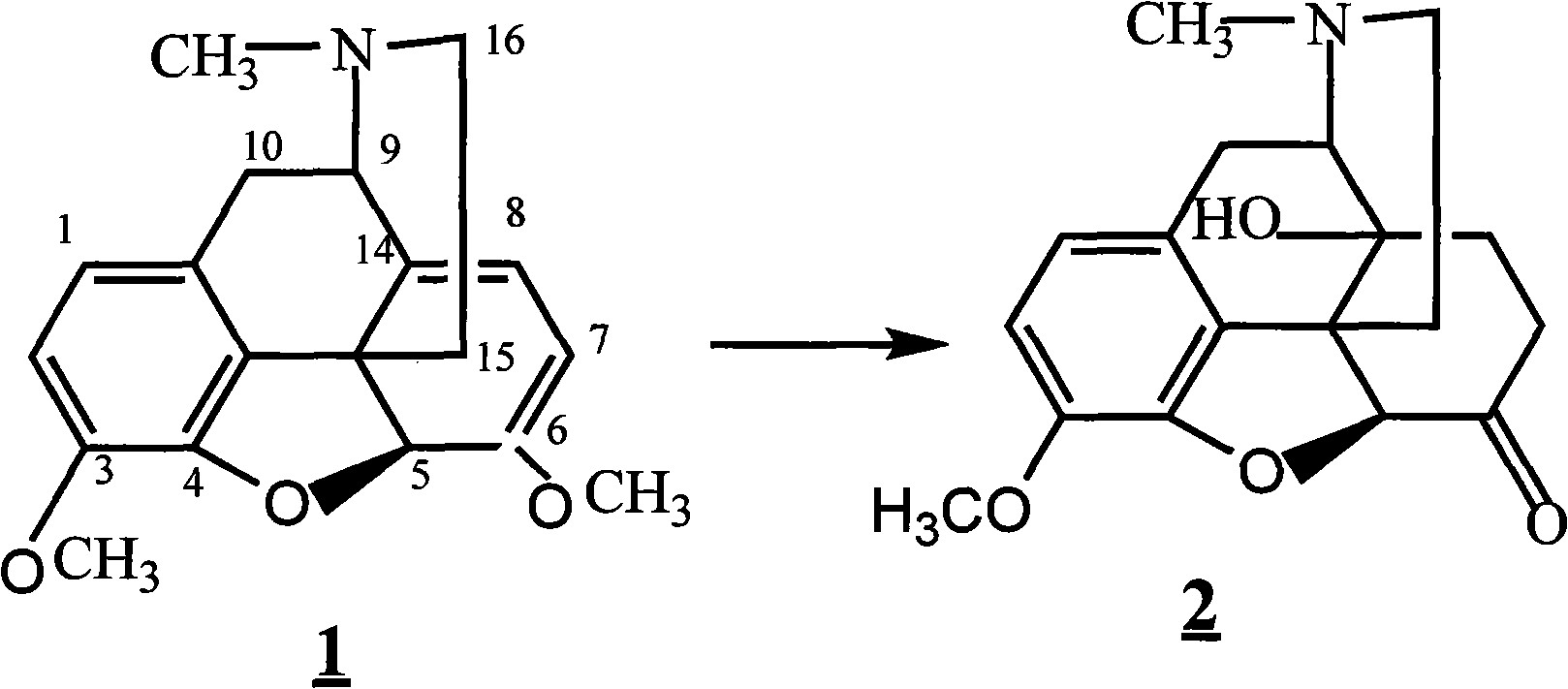
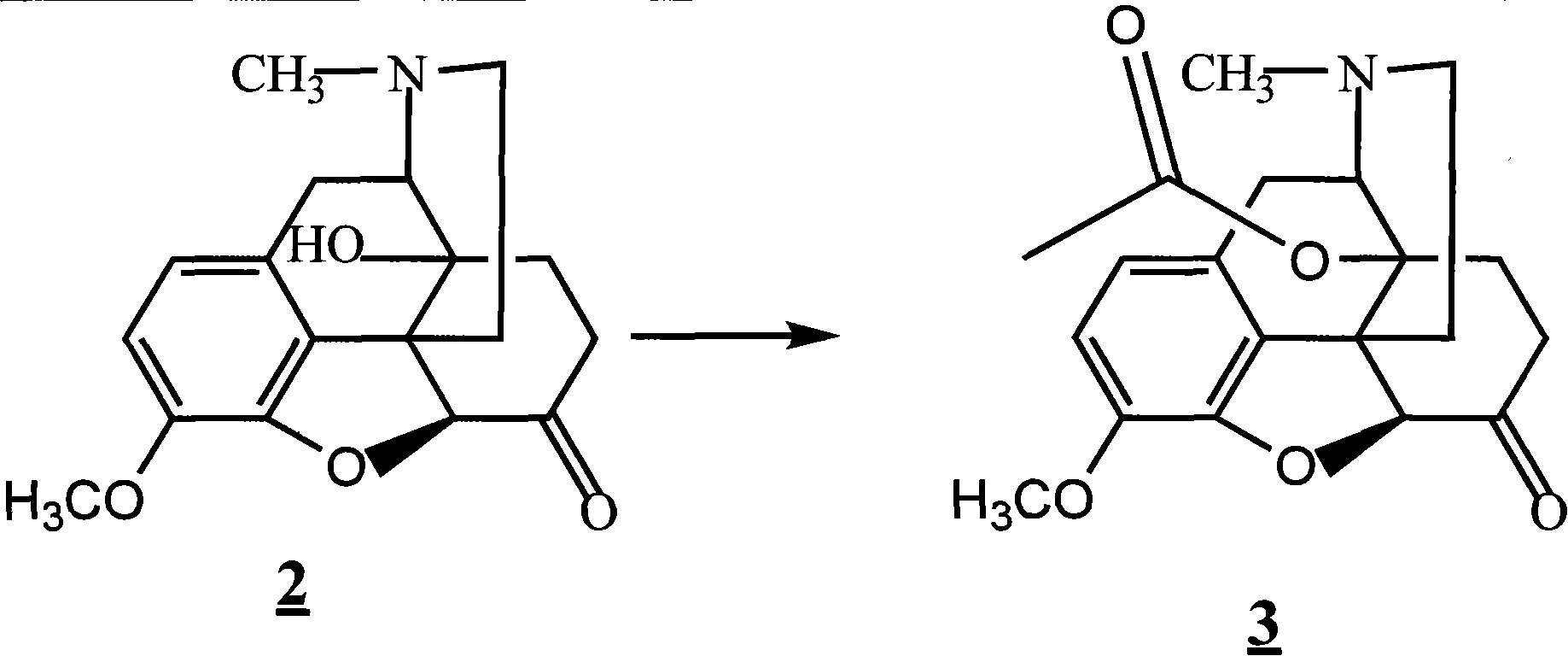
The invention provides a method for synthesizing naloxone or naltrexone, which comprises the following steps of: dissolving thebaine in formic acid, uniformly stirring, dripping an oxidant, keeping the temperature of between 20 and 40 DEG C for 3 to 7 hours, displacing gas in a reaction vessel by inert gas serving as protective gas for 3 to 5 times, adding a metallic framework catalyst, displacing the gas by hydrogen for 3 to 5 times, keeping the temperature of between 25 and 45 DEG C and stabilizing a system for 7 to 13 hours to obtain a compound 2; reacting the compound 2 with acetic anhydride at the temperature of between 60 and 100 DEG C for 1 to 2 hours to obtain a compound 3; taking the inert gas as the protective gas, adding toluene, chloroformic acid-1-chloroethyl ester and potassium bicarbonate into the compound 3, heating to the temperature of between 75 and 100 DEG C and reacting for 20 to 40 hours, concentrating under reduced pressure until the system is fully dry, adding 10 percent hydrochloric acid, and heating and refluxing for 2 to 6 hours to obtain a compound 4; dissolving the compound 4 and at least one alkylation reagent in an organic solvent 1 and reacting with alkali at the temperature of between 50 and 100 DEG C to obtain a compound 5; and reacting the compound 5 with boron tribromide in an organic solvent 2 at the temperature of between -10 and 40 DEG C for 2 to 4 hours to obtain a compound 6, namely the naloxone or naltrexone.
Owner:甘肃普安制药股份有限公司
R-amisulpride medicine salt, preparation method, crystal form and application thereof
ActiveCN106995397AGood curative effectLower blood sugarMetabolism disorderOrganic chemistry methodsPyrrolidineTriethylamine
The invention concretely relates to R-amisulpride medicine salt, a preparation method, a crystal form and application thereof in preparing a medicine for treating diabetes. The preparation method comprises the following steps: by adopting 2-aminomethyl-N-ethyl pyrrolidine as a raw material, and adopting L-tartaric acid, for splitting to obtain (R)-2-aminomethyl-N-ethyl pyrrolidine; directly condensing amisulpride acid and the (R)-2-aminomethyl-N-ethyl pyrrolidine under the catalysis of isopropyl chlorocarbonate and triethylamine, thus obtaining R-amisulpride; reacting the R-amisulpride obtained in the step (2) and acid to obtain the R-amisulpride medicine salt. The preparation method has the advantages of simplicity in operation, high safety, good product quality, low cost and the like, and is convenient for scale production.
Owner:SHENZHEN FONCOO PHARMACEUTICAL CO LTD
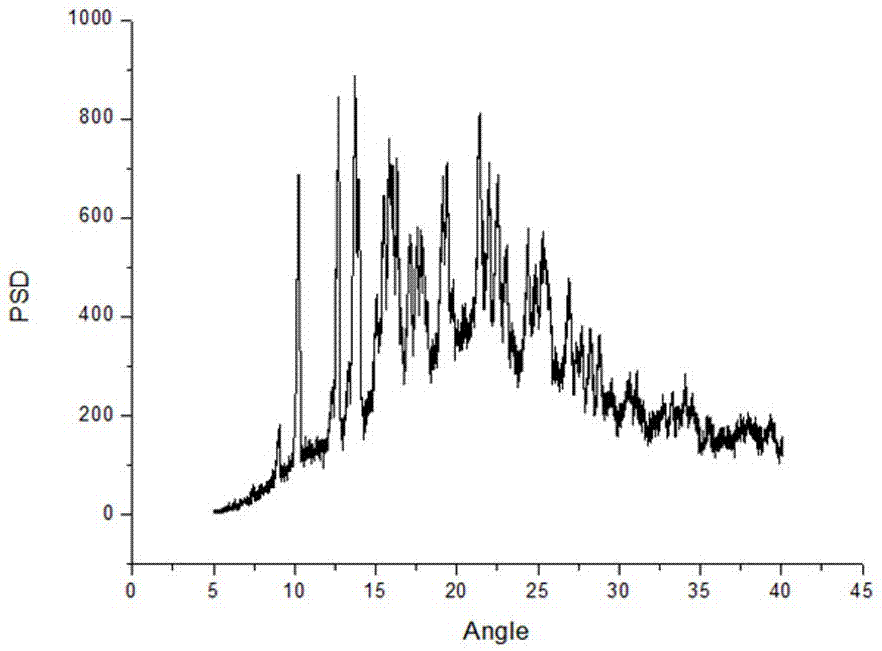
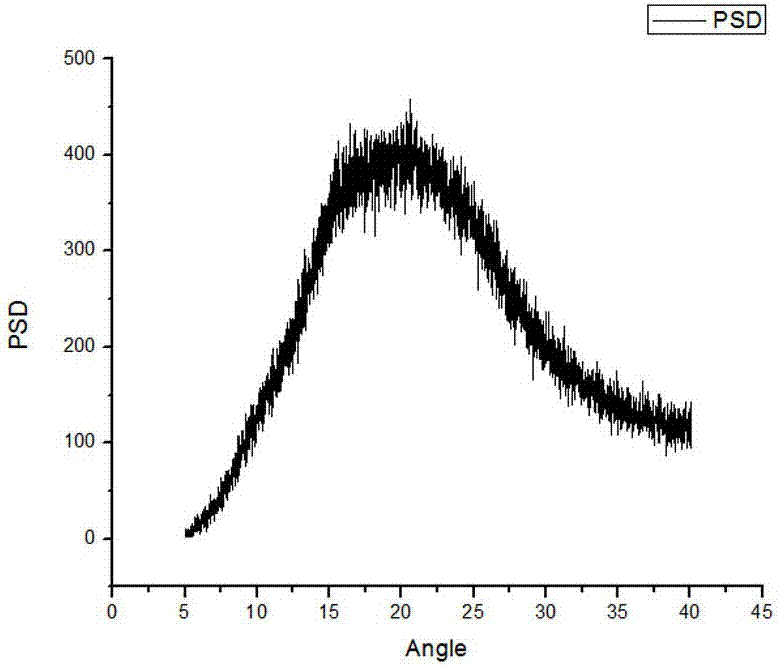
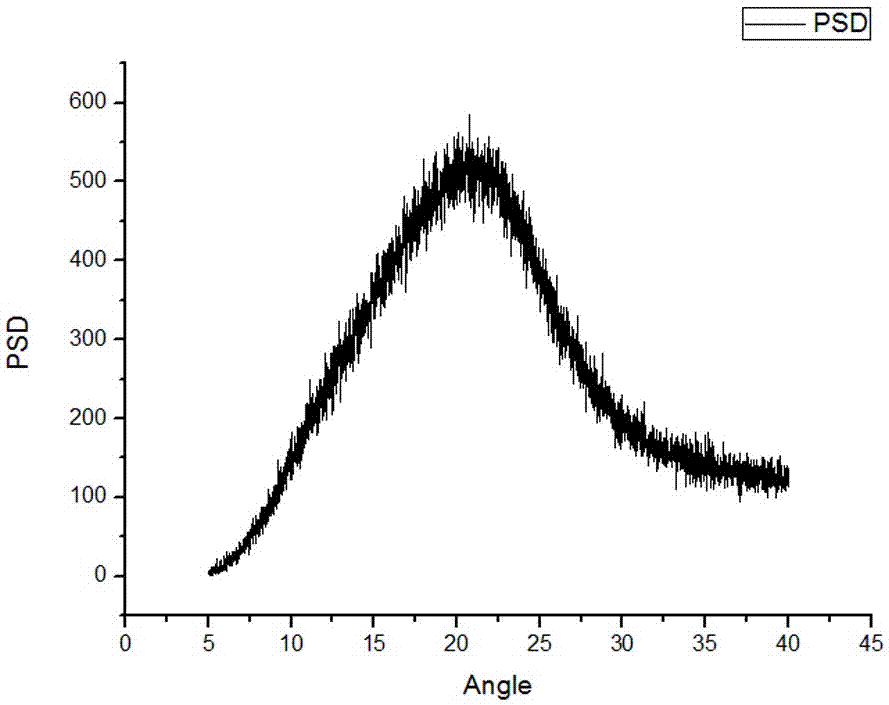
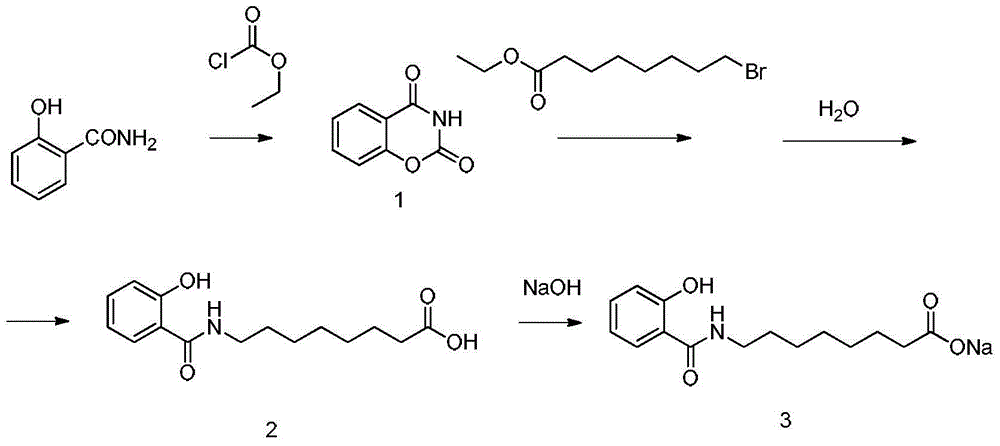
Method for preparing sodium, 8-(2-hydroxybenzamido)octanoate
InactiveCN104974060AGood choiceHigh yieldOrganic compound preparationCarboxylic acid amides preparationEthyl chloroformateOctanoic Acids
The invention discloses a method for preparing sodium, 8-(2-hydroxybenzamido)octanoate which is a drug intermediate. The method comprises the following steps of reacting salicylamide, which serves as a raw material, with ethyl chloroformate to produce an intermediate 2H-benzo[e][1,3]oxazine-2,4(3H)-dione, reacting the intermediate with 8-bromooctanoic acid ethyl ester, carrying out hydrolysis so as to obtain 8-(2-hydroxybenzamido)octanoic acid, and then, reacting 8-(2-hydroxybenzamido)octanoic acid with sodium hydroxide so as to obtain the final product, namely sodium, 8-(2-hydroxybenzamido)octanoate. According to the method, the raw material reacts with ethyl chloroformate to produce the intermediate 2H-benzo[e][1,3]oxazin-2,4(3H)-dione, so that the yield of the reaction is greatly increased, the selectivity of reaction is greatly improved, the production cost is reduced, the production of byproducts is lowered, industrial production is facilitated; and the method is environment-friendly.
Owner:CHEMSOON CO LTD
Preparation method of capecitabine and intermediate thereof
InactiveCN101993463AAdequate responseHigh yieldSugar derivativesSugar derivatives preparationInosineIodine
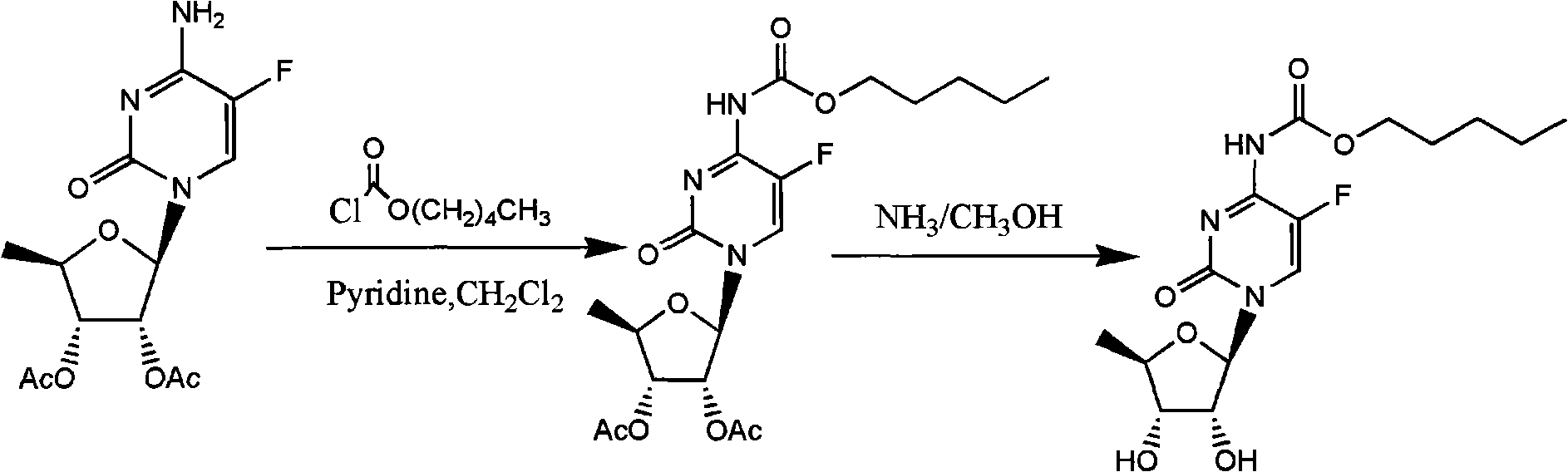
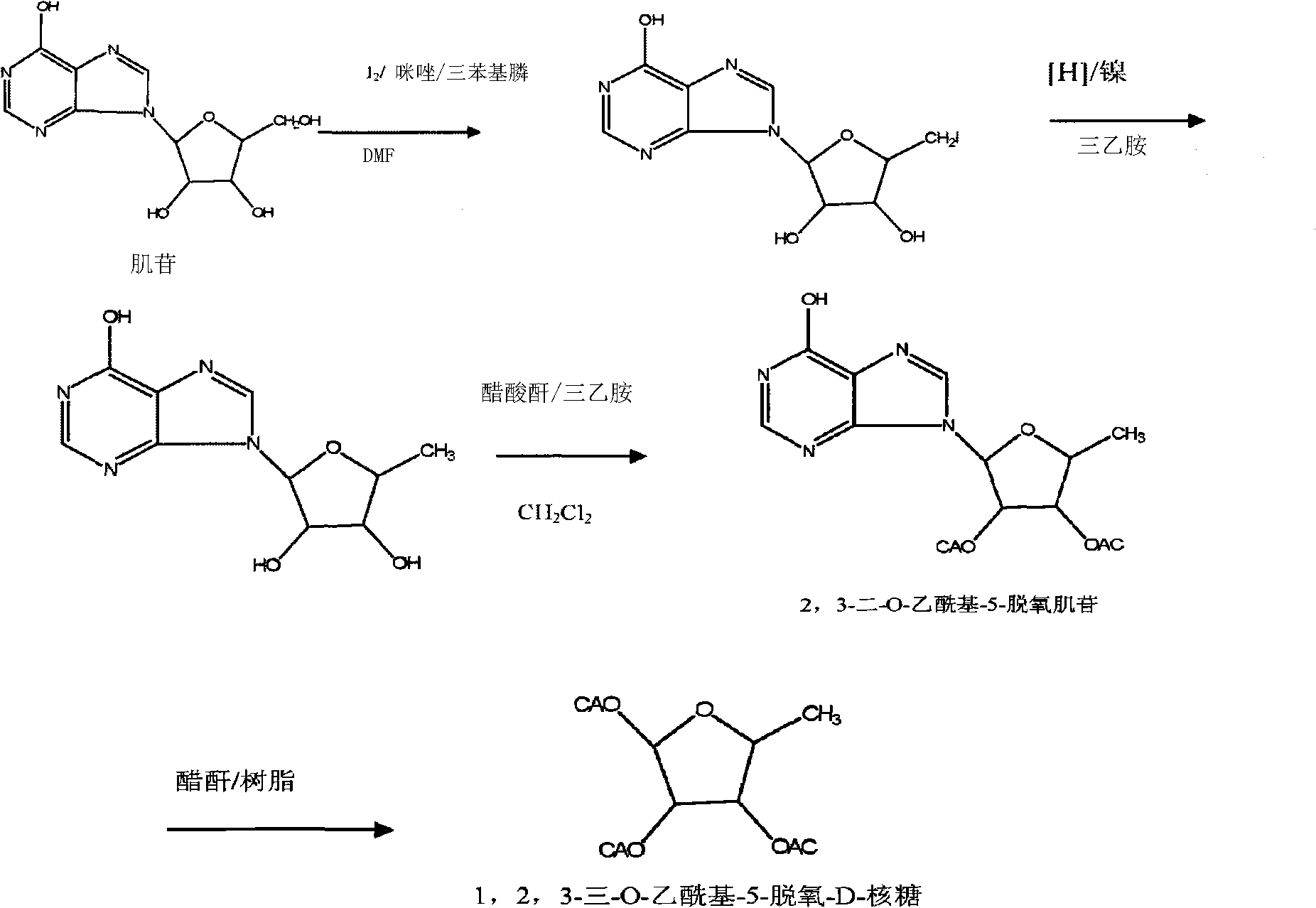
The invention relates to a preparation method of capecitabine and an intermediate thereof. The material conversion rate is high with less side reaction under the condition that bicarbonate is used as alkali by changing the reaction condition of synthesizing a compound of a formula I by a 5'-deoxidized-5-gemcitabine derivative and chloroformic acid n-amyl ester. Meanwhile, the applicant researches and finds that: Since 5-deoxidized-tri-O-acetyl-D-ribose prepared by using inosine reactrion substituted by iodine and protected by hydroxyl is adopted, the post-treatment process is simplified, and the yield is improved. The 5-deoxidized-tri-O-acetyl-D-ribose is a material for synthesizing the compound of the formula I while the compound of the formula I is a significant intermediate of capecitabine. The whole capecitabine synthesis process has high yield and low cost.
Owner:CHENGDU KANGHONG PHARMA GRP
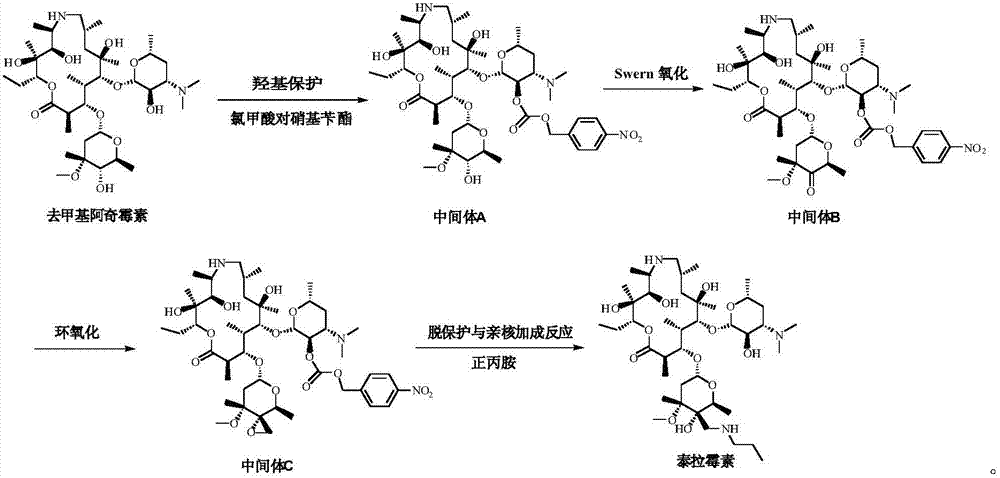
Preparation method of tulathromycin
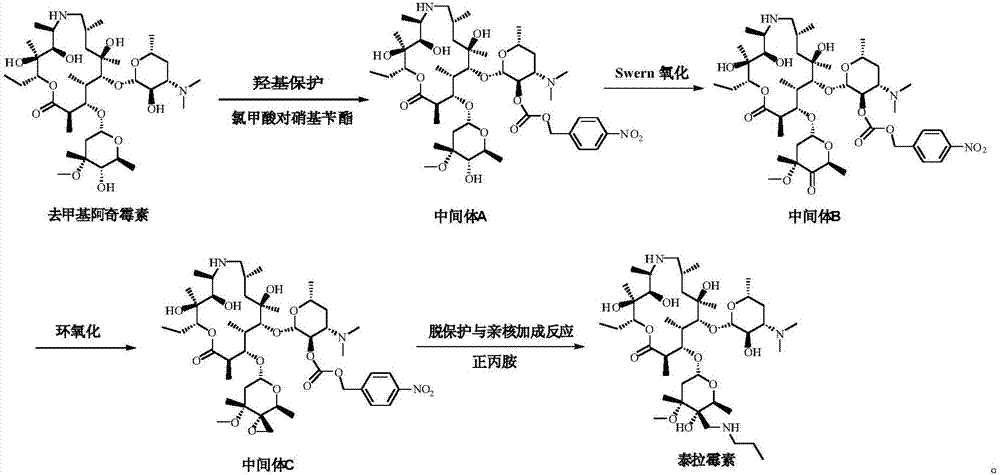
ActiveCN106939029AImprove protectionAvoid catalytic hydrogenation methodsSugar derivativesSugar derivatives preparationAzithromycinChemical synthesis
The invention relates to the field of chemical synthesis and particularly relates to a preparation method of tulathromycin. The method comprises protecting a 2-hydroxyl group of demethyl azithromycin through 4-nitrobenzyl chloroformate, carrying out oxidation and epoxidation on a 4"-hydroxyl group, and carrying out deprotection and 4"-epoxy nucleophilic addition through n-propylamine to obtain tulathromycin. Comprised with the prior art, the preparation method has simple processes, mild conditions and a high yield, is free of palladium-carbon hydrodeprotection and is conducive to industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
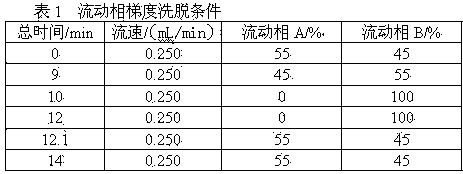
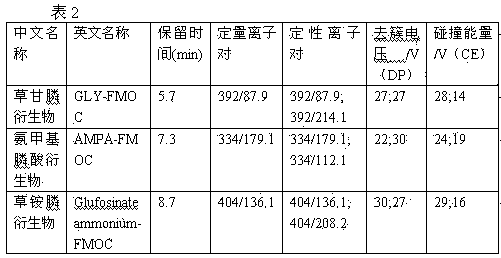
Pretreatment method for measuring pesticides of glyphosate and phosphinothricin in tea leaves
ActiveCN103869028AExtraction does not affectImprove derivation efficiencyComponent separationPretreatment methodPhenolic content in tea
The invention provides a pretreatment method for measuring pesticides of glyphosate and phosphinothricin in tea leaves. In general, acidic precipitation, solid phase extraction, precolumn derivatization and a liquid chromatograph / mass spectrometer are used for measuring, and a method for measuring the residual amounts of glyphosate and phosphinothricin in the tea leaves by using the liquid chromatograph / mass spectrometer is established. In the method, a large quantity of tea polyphenols in the tea leaves are precipitated, so that the deviation efficiency of solid phase extraction liquid and 9-fluorenylmethyl chloroformate is increased, the interference of impurities is reduced, and the high sensitivity is realized. The method is accurate, efficiency and stable, can meet the requirement of residue detection, and can be applied to the qualitative and quantitative analysis of the glyphosate and the phosphinothricin in the various tea leaves.
Owner:TEA RES INST CHINESE ACAD OF AGRI SCI
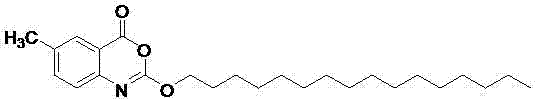
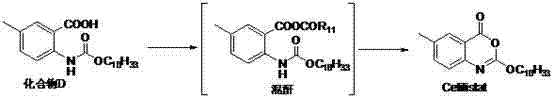
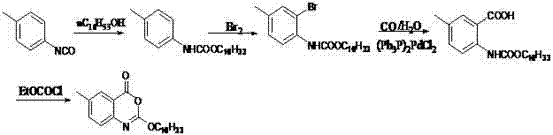
Method for preparing cetilistat
The invention discloses a method for preparing cetilistat, which belongs to the technical field of the medicine preparation method, and is used for solving the problems of high three wastes, low yield and high cost in the current preparation method. The method comprises the following steps: 1)esterifying a compound A(2-amino-5-methyl benzoic acid) or its salt; 2) acylating an esterification object in the step 1) and chloroformic acid n-cetanol ester; 3)removing ester group of the compound obtained in the step 2) to obtain 2-(cetane carbonyl oxygen)amino-5-methyl benzoic acid; and 4)cyclizing the compound in the step 3) to obtain the target products. By improving the production steps, the invention provides the synthetic method with simple operation, high efficiency, and convenient industrial production for the cetilistat product.
Owner:CHONGQING TOPTECH PHARMA TECH
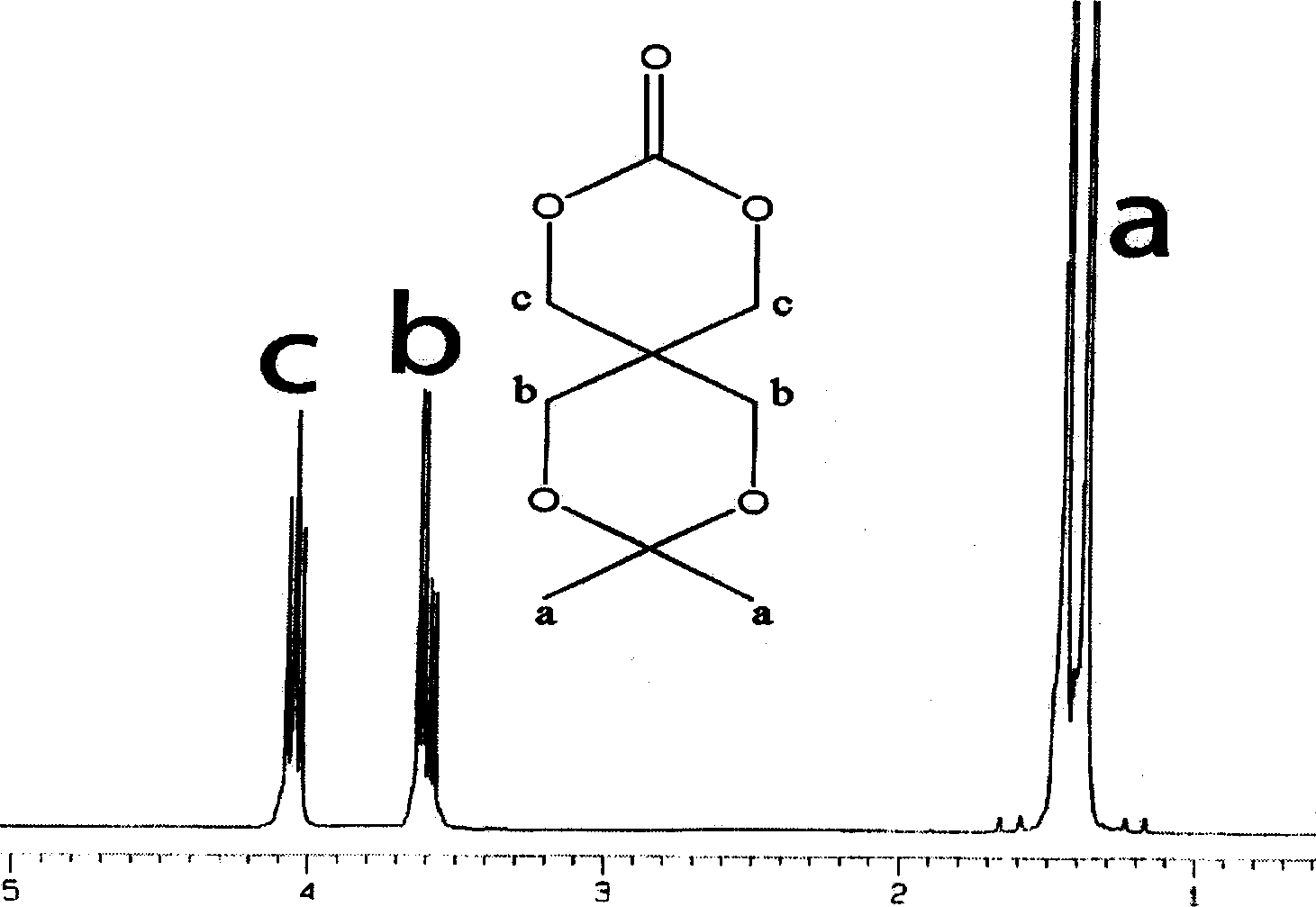
Cyclic aliphatic acid ester carbonate, its polymer, synthesis method and uses thereof
The provided preparation methdo for cyclic aliphatic carbonate comprises: protecting two hydroxyl groups of the pentaerythritol to let another couple of hydroxyl react with ethyl chloroformate; further, ring-opening polymerizing or copolymerizing with aliphatic cyclic ester, removing the protection, and obtaining the product. This cyclic esters is fit to biodegradation without toxin, can connect the drug and active short peptide to improve the polymer bio- compatibility and bio-compatibility, and has wide application.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
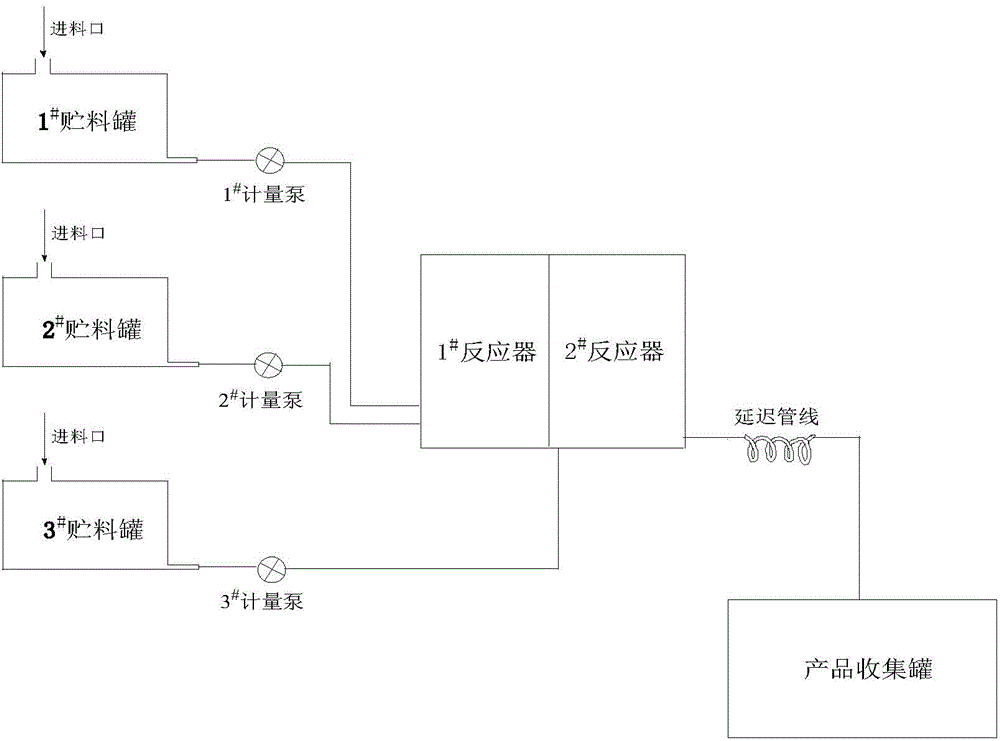
Method for preparing peroxidized dicarbonate (2-ethylhexyl) ester (EHP) by continuous flow
InactiveCN104370789AEmission reductionReduce labor intensityOrganic compound preparationPeroxy compound preparationDicarbonateEmulsion
The invention provides a method for preparing peroxidized dicarbonate (2-ethylhexyl) ester (EHP) by a continuous flow. The method comprises the following steps: preparing an alkaline aqueous liquid; carrying out reaction on the alkaline aqueous liquid and hydrogen peroxide in a reactor; adding a chloroformic acid-2-ethyl caproate liquid to react with a product of the alkaline aqueous liquid and hydrogen peroxide; and further carrying out full reaction on the product to obtain a product by virtue of a delayed pipeline, wherein the reaction process is carried out in a micro-reactor. The solvent or emulsion type peroxidized dicarbonate (2-ethylhexyl) ester (EHP) prepared by the method provided by the invention is stable in product quality and good in repeatability. As reaction is carried out in the micro-reactor, the process flow is simplified, so that the reaction is continuously carried out under mild, safe and environment-friendly conditions. Therefore, the product content and yield are greatly improved, and the yield can reach over 98%.
Owner:LINZIZHENGHUA ACCESSORY INGREDIENT ZIBO
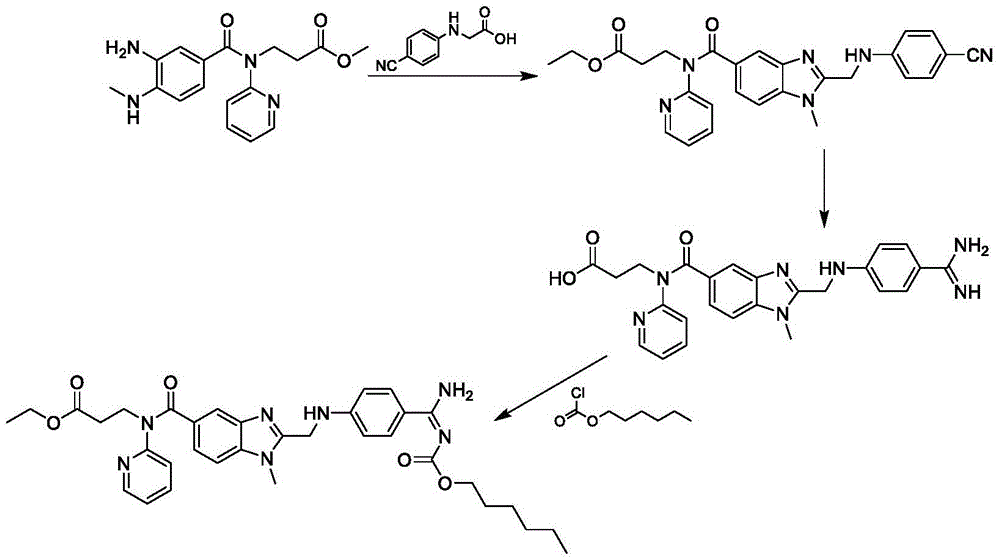
Preparation method of dabigatran etexilate mesylate
InactiveCN105566297ASimple and fast operationHigh selectivityOrganic chemistryPropanoic acidChloroformate
The invention discloses a preparation method of dabigatran etexilate mesylate, and belongs to the technical field of medicine. The preparation method comprises the following steps: taking 3-[(3-amino-4-methylaminobenzoyl)pyridine-2-ylamino]ethyl propanoate and N-(4-cyanphenyl)amino acetic acid as the raw materials to synthesize an intermediate (S3); making the intermediate (S3) carry out ring-closure reactions to generate an intermediate (S4); subjecting the intermediate (S4) to acid splitting in the presence of a hydrogen chloride-ethanol solution at first, then carrying out ammonification in the presence of ammonia water to generate an intermediate (S5); carrying out reactions between the intermediate (S5) and n-hexyl chloroformate under an alkaline condition to generate an intermediate (S6); dissolving the intermediate (S6), and finally carrying out reactions between the intermediate (S6) and methylsulfonic acid to obtain dabigatran etexilate mesylate. The preparation method has the advantages of simpleness, controllable and mild conditions, high yield, high product purity, stable product property, and suitability for industrial production.
Owner:HARBIN PHARMA GROUP TECH CENT
Preparation method of 2'3'-di-O-acetyl-5'-desoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine
InactiveCN102977169AHarm reductionHigh yieldSugar derivativesSugar derivatives preparation5-fluorocytidineChloroformate
The invention relates to a preparation method of 2'3'-di-O-acetyl-5'-desoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine, belonging to the technical field of medicine. The compound is an important intermediate of Capecitabine. The preparation method comprises the following step: by using anhydrous sodium carbonate or anhydrous potassium carbonate as alkali, quaternary ammonium salt as a phase-transfer catalyst and 4-substituted-pyridine as a catalyst, carrying out amidation reaction on 2'3'-di-O-acetyl-5'-desoxy-5-fluorocytidine and amyl chloroformate to obtain the 2'3'-di-O-acetyl-5'-desoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine. The invention adopts the anhydrous sodium carbonate or anhydrous potassium carbonate instead of the high-toxicity organic alkali pyridine, and obtains ideal yield and product purity. The method has the advantages of accessible raw materials, low cost, small environmental hazard, high safety and reliability, short reaction time and good product quality.
Owner:QILU TIANHE PHARMA
Synthesis method of dabigatran etexilate
InactiveCN104650037AEasy to routeRaw materials are easy to getOrganic chemistrySynthesis methodsChloroformate
The invention relates to the technical field of medicine synthesis and purification, in particular to a synthesis method for preparing dabigatran etexilate. The method comprises the following steps: with N-(4-cyanophenyl) glycine and 3-[(3-amino-4-methylamino benzoyl) (pyridine-2-yl) amino] ethyl propionate as raw materials, carrying out dehydration-condensation reaction, sequentially introducing hydrogen chloride and an ammonia gas to the reactants in an ethanol solution; adding hexyl chloroformate, and carrying out condensation reaction; and carrying out recrystallization to obtain the dabigatran etexilate of which the purity is 99%. The method is simple, safe, easy to operate, short in reaction cycle, and relatively high in yield and purity; impurities are easy to remove; column chromatography isolation is not needed in purification; and the method is suitable for industrialized production.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Synthesis process for ethoxy carbonyl isothiocyanate
ActiveCN104761479AReduce dosageReduce pollutionOrganic chemistryEthyl chloroformateChemical synthesis
Belonging to the technical field of chemical product synthesis and beneficiation reagent production, the invention in particular relates to a synthesis process for ethoxy carbonyl isothiocyanate. The process includes: dissolving 45 parts of 99% sodium thiocyanate in 80 parts of water, adding 0.55 part of polyethylene glycol with an average molecular weight of 200 into the solution, lowering the temperature of the solution to less than 10DEG C, adding 54.8 parts of 99% ethyl chloroformate slowly into the reaction system under stirring, controlling the reaction temperature not higher than 15DEG C, and carrying out stirring reaction for 3h, at the end of the reaction, adding 30 parts of water, performing stirring for 10min, then conducting standing for 1h, separating a lower layer water phase so as to obtain an upper layer oily liquid, i.e. N-ethoxy carbonyl isothiocyanate. The ethyl chloroformate based product yield is 83.42%, the environmental pollution is reduced, energy consumption is also lowered, and the catalyst dosage is reduced.
Owner:SHENYANG YOUYAN MINERAL CHEM CO LTD
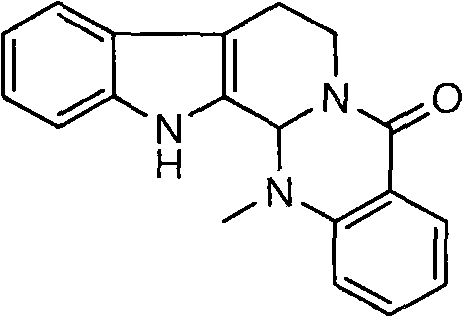
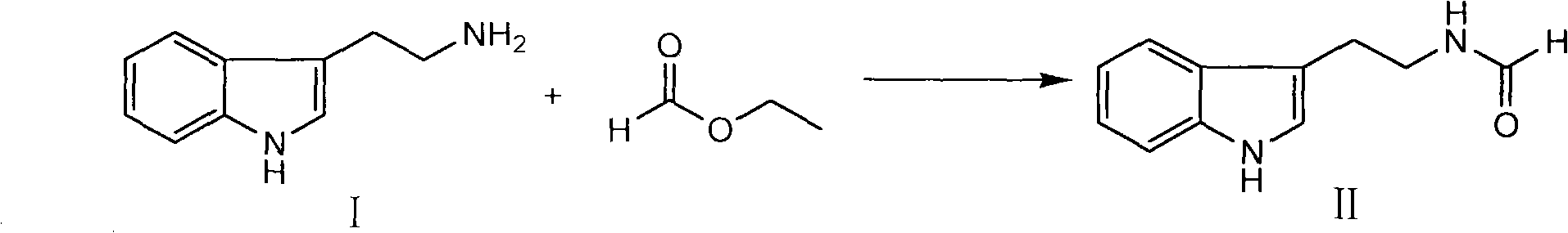
Method for synthesizing evodiamine
The invention discloses an organic chemical synthesis method, in particular a method for chemically synthesizing evodiamine. Indole acetonitrile is taken as a raw material, and the method comprises the following steps of: performing catalytic hydrogenation on palladium carbon to prepare tryptamine; mixing with ethyl formate to form solution for a formylation reaction, and dissolving the reactants in solution of dichloromethane; adding trifluoroacetic acid for cyclization, and preparing another intermediate in a reflux state from N-methylanthranilic acid and excessive ethyl chloroformate; and finally, subjecting two intermediates to condensation reaction in a non-polar solvent system. The method for chemically synthesizing the evodiamine has the advantages of rich raw material source, low price of the raw material, capacities of improving the yield and fulfilling the aim of reducing the production cost by optimizing reaction conditions, environmental friendliness and suitability for large-scale industrial production. The product can be widely applied to pharmaceutical industry.
Owner:HANGZHOU FST PHARMA
Process for the preparation of polycarbonates and diaryl carbonate
InactiveUS20090240021A1Pump componentsChemical/physical/physico-chemical stationary reactorsArylChloroformate
The present invention relates to a process for the continuous preparation of polycarbonates or diaryl carbonates by the method of the phase boundary process, in which both the mixing of the organic and aqueous phase and the upstream oligomerization step or aryl chloroformate and / or diaryl carbonate preparation step are effected in a special pump.
Owner:COVESTRO DEUTSCHLAND AG
Novel biological contamination-resistant ultrathin compound film and preparation method thereof
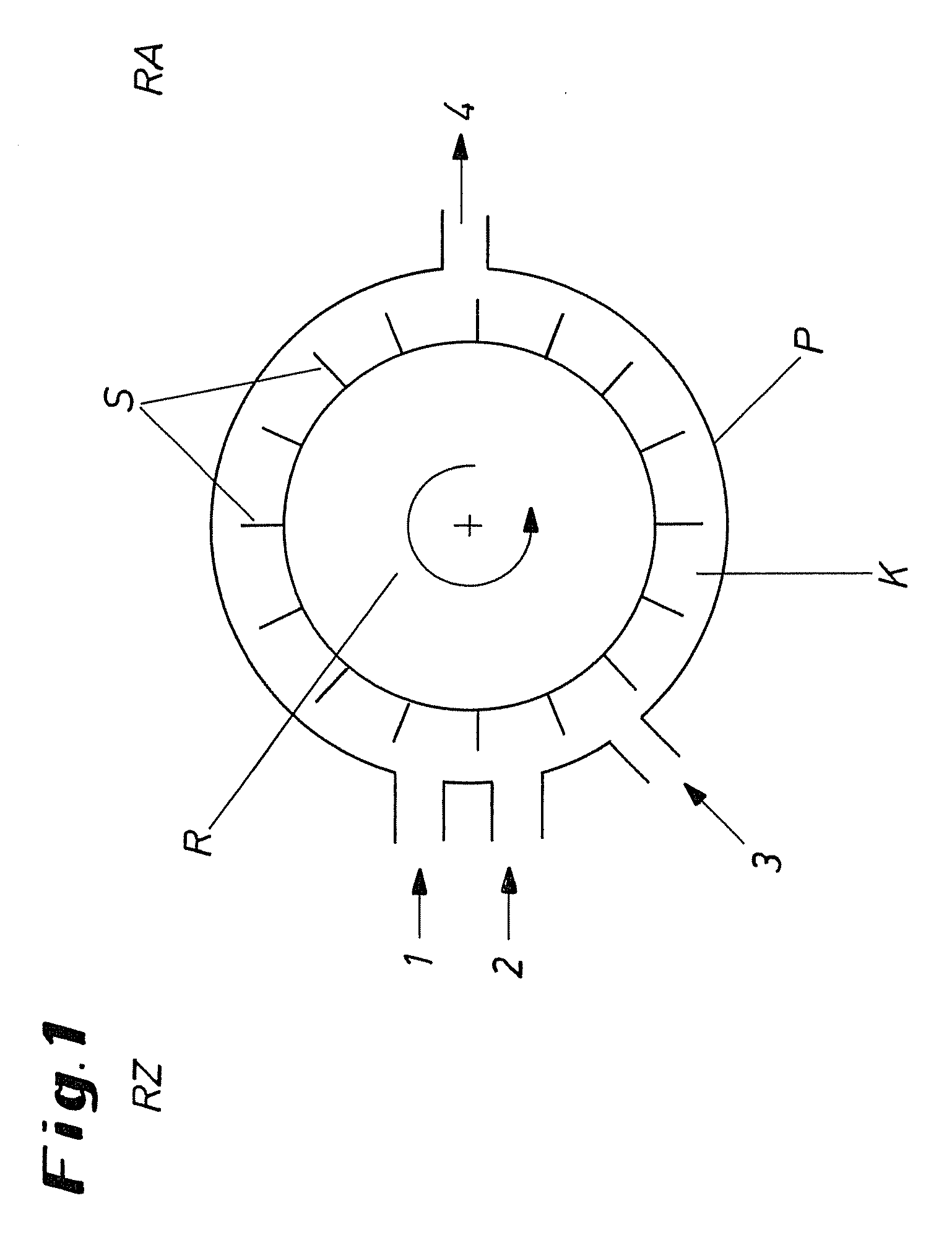
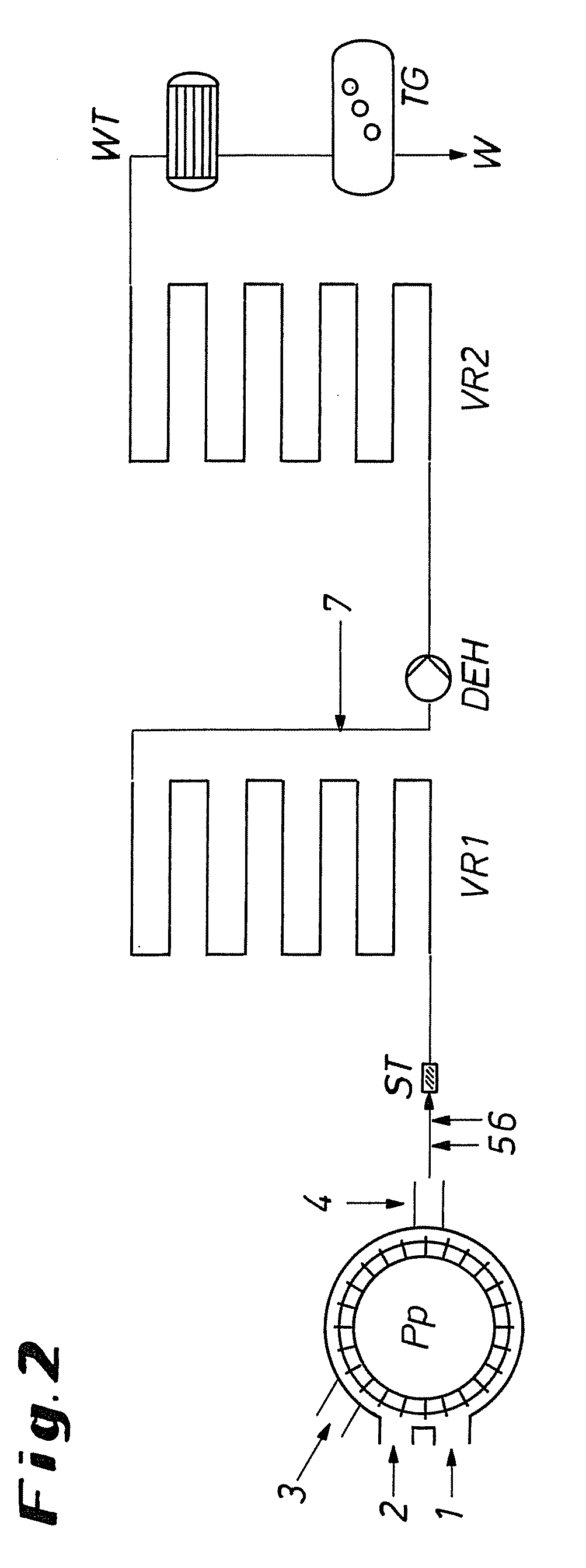
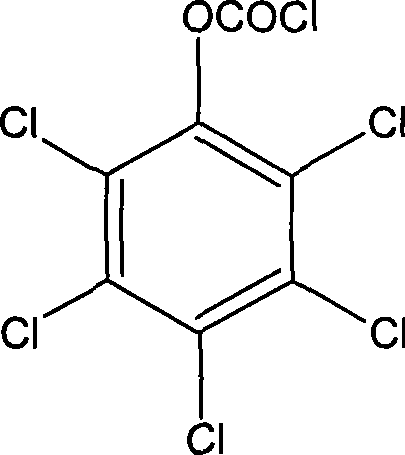
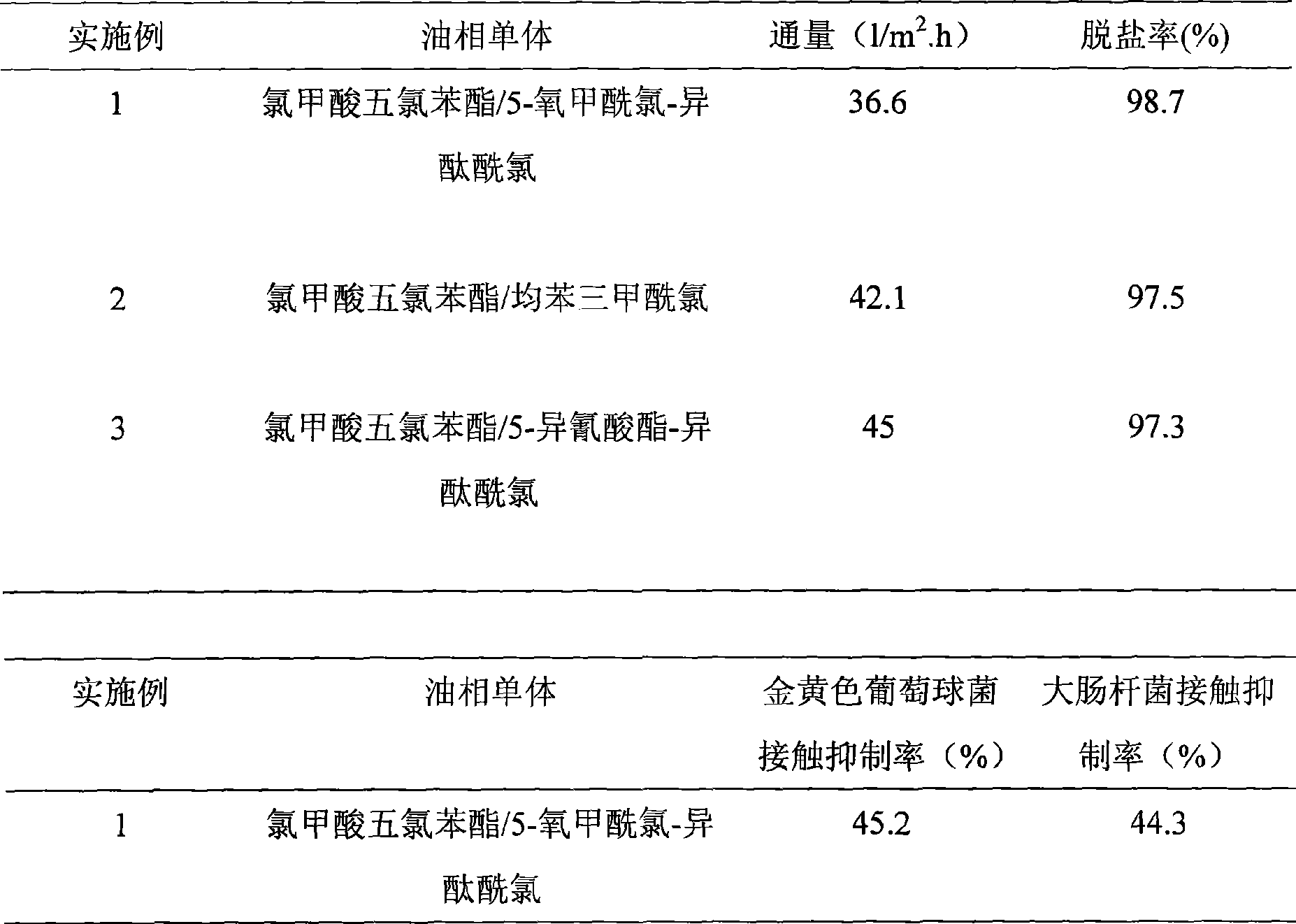
ActiveCN101502763AEasy to manufactureBacteria work wellSemi-permeable membranesChloroformic acidAramid
The invention discloses an organic composite membrane, in particular to a pollution-resistant composite membrane and a preparation method thereof. The invention compounds an aromatic polyamide lamina on a porous supporting membrane by polyamine and aromatic acyl chloride or isocyanate or oxygen formyl chloride interfacial polycondensation, the surface of the aromatic polyamide lamina has chloroformic acid pentachlorobenzene groups; polysulfone supporting membrane is directly soaked into polyamide solution, single interfacial polymerization reaction is carried out on the supporting membrane and aromatic acyl chloride solution after a rubber roll is used for rolling the surface of the supporting membrane, the supporting membrane is dried in the shade for 1-3 minutes in air, heat treatment is carried out for 3-10 minutes at 40-70 DEG C, the ultra-thin composite membrane is prepared by washing. The invention is characterized in that the prepared membrane has good bacteriostatic effect, efficient desalting property and simple preparation. The products and the method of the invention can be widely applied in the processing procedure of organic liquids.
Owner:HANGZHOU WATER TREATMENT TECH DEV CENT
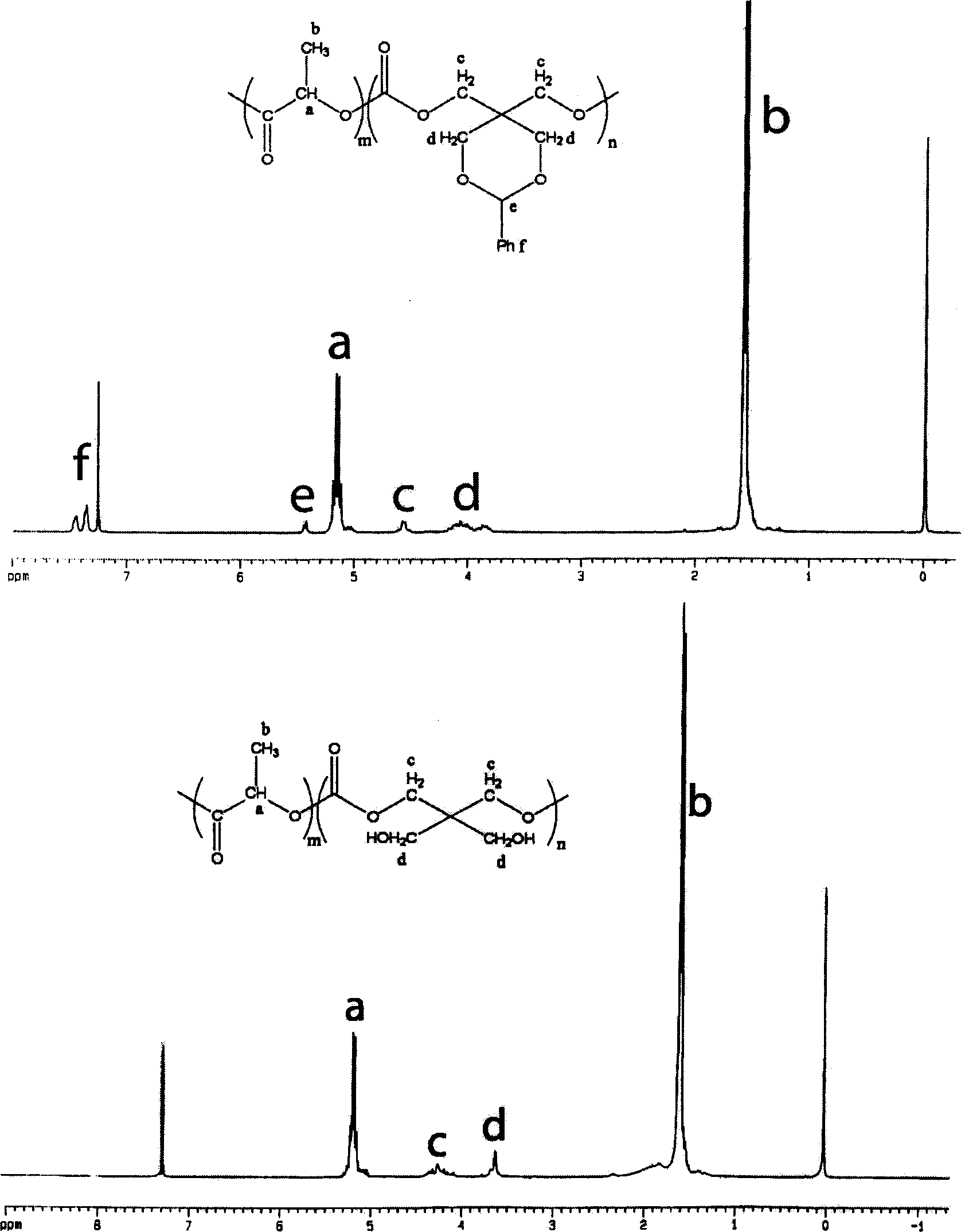
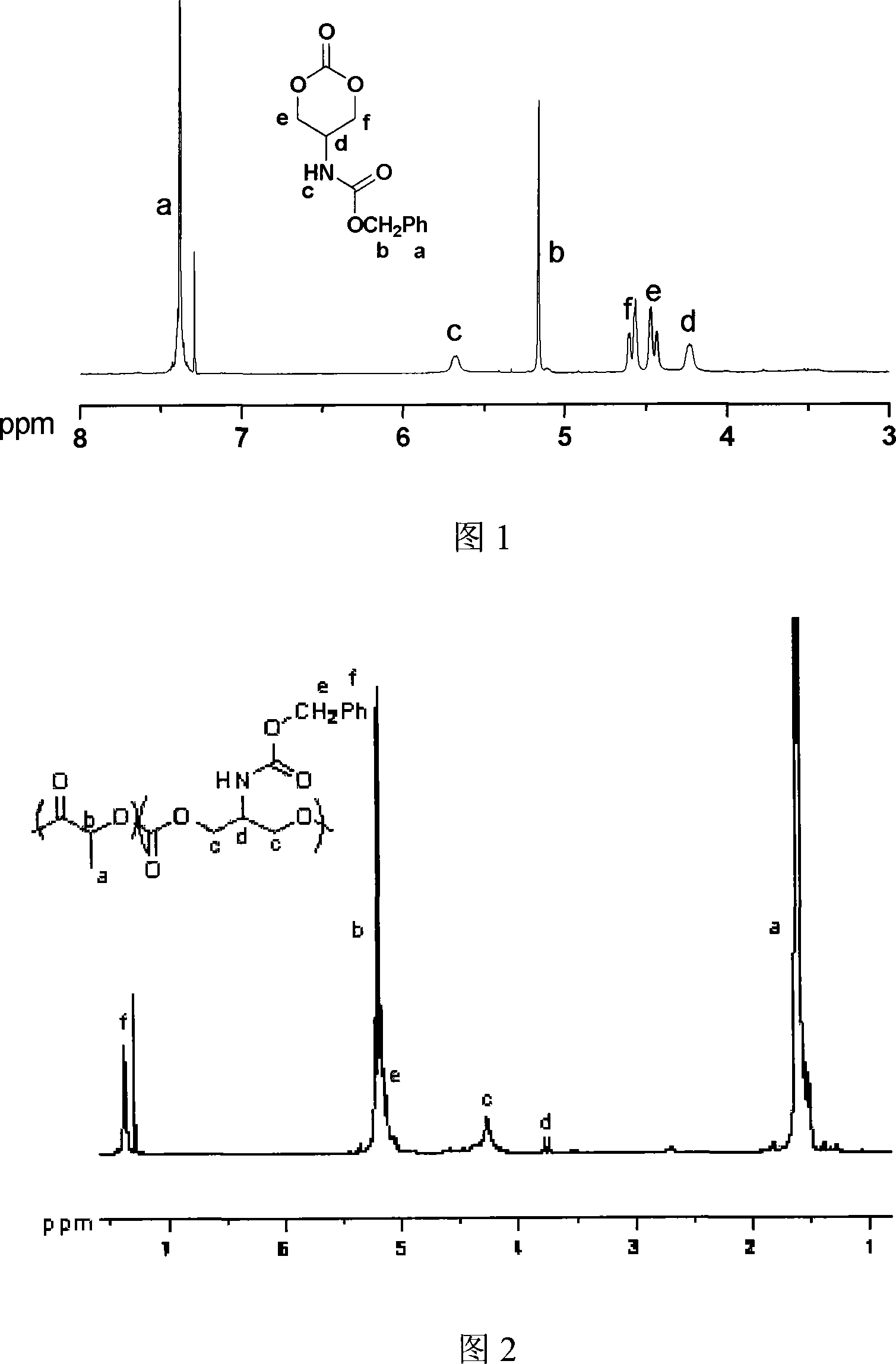
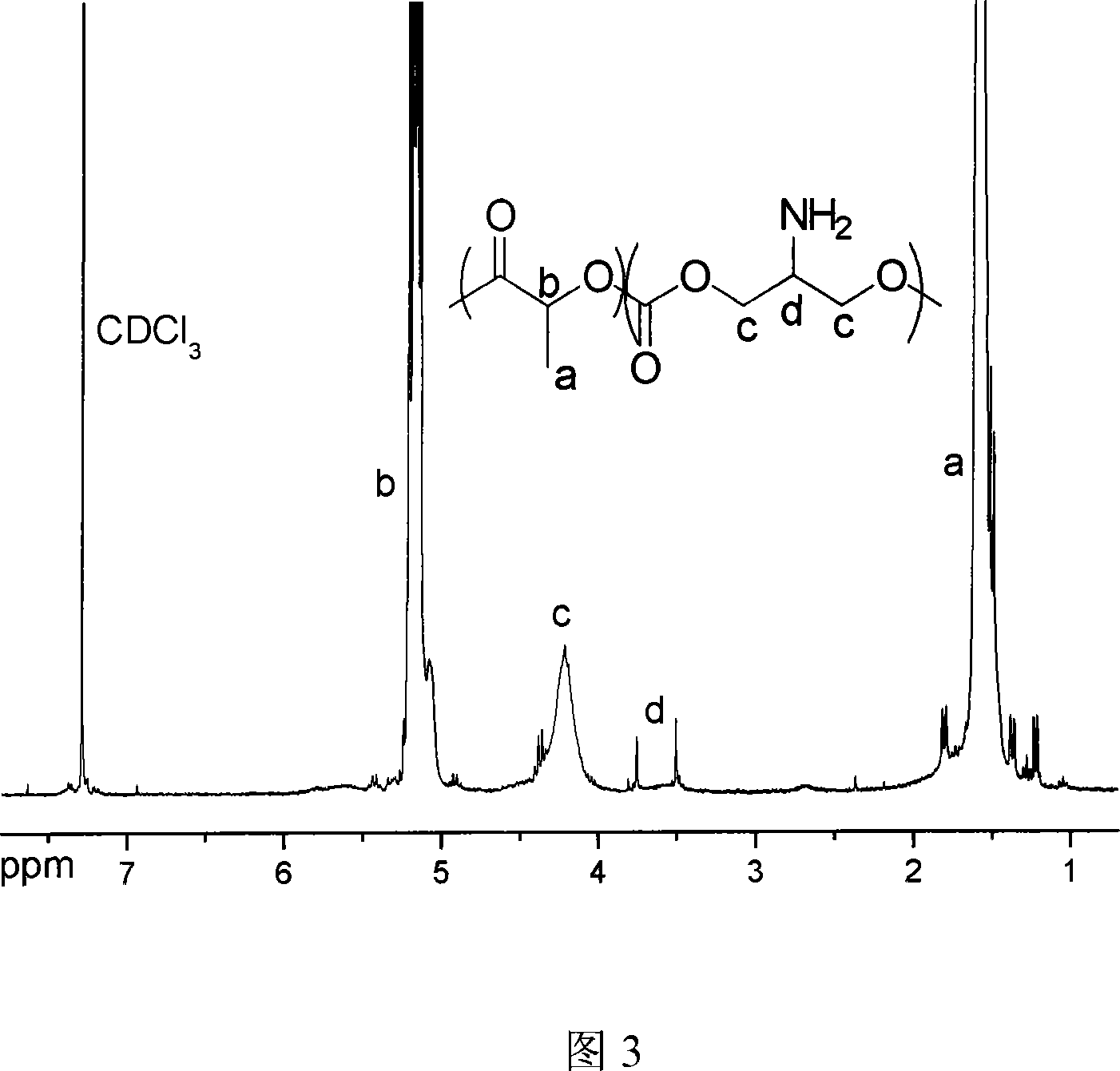
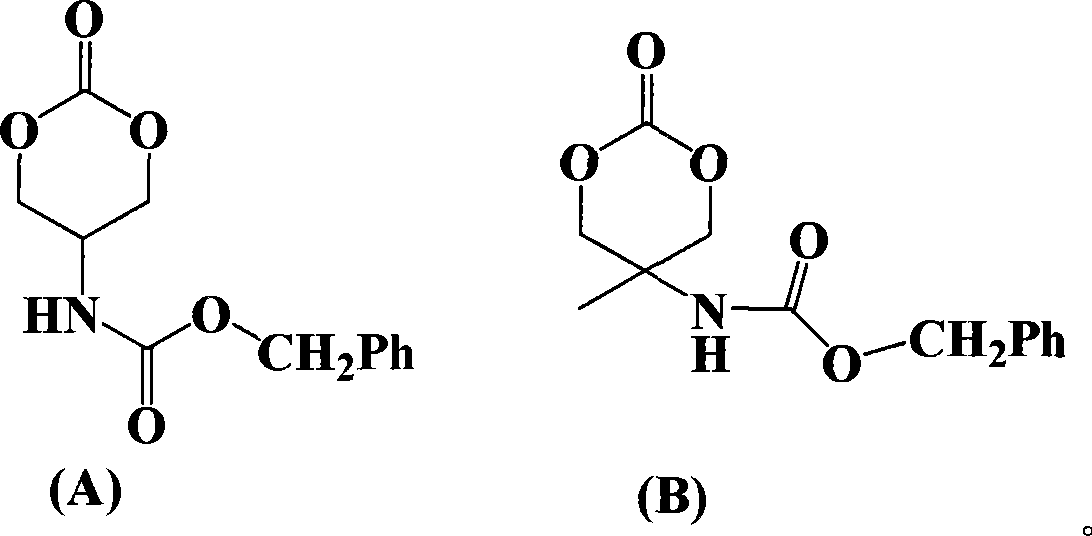
Ring aliphatic carbonate monomer, polymer and preparation method thereof
The invention provides a cyclic aliphatic carbonate monomer including 2'-benzyloxygenamide trimethylene carbonate and 2-methyl-2-benzyloxygenamide trimethylene carbonate and the polymer and the preparation method thereof. The raw material for the preparation of the two monomers is 2-amino-1,3-propanediol and 2-methyl-2-amino-1,3-propanediol, the amino group is protected, and the hydroxyl group at two ends and ethyl cacodylic acid chloroformate react to produce the monomer. The polymer with the amino at the protective side is obtained by the homopolymerization of the monomer or the copolymerization of the monomer and the aliphatic cyclic ester, and then the polymer with the side amino is obtained after escaping the protection of the polymer. The functional side amino of the polymer can be loaded with the drug molecules and the bioactive peptide by the covalent bond and the ionic bond. Thereby the invention can be applied in the fields of the drug controlled release, the gene therapy and the tissue engineering.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
New crystal form of mianserin hydrochloride, detection method and applications thereof
InactiveCN105012864ADefinite curative effectLittle side effectsNervous disorderOrganic chemistryBenzaldehydeTherapeutic effect
The present invention provides a new crystal form of mianserin hydrochloride, a detection method and applications, and relates to a traditional Chinese medicine and Western medicine preparation for depression, especially to applications of a compound drug prepared from a Western medicine mianserin hydrochloride adopted as the main component and Chinese herbs in treatment of depression. According to the mianserin hydrochloride, benzaldehyde and ethanolamine are adopted as starting raw materials and are subjected to chemical combination to obtain an intermediate I, the intermediate I reacts with styrene oxide to obtain an intermediate II, the intermediate II and thionyl chloride are subjected to chemical combination under an alkaline condition to obtain an intermediate III, the intermediate III, o-aminobenzyl alcohol and fumaric acid are subjected to chemical combination to obtain an intermediate IV, an intermediate V is obtained from the intermediate IV under the action of concentrated sulfuric acid and sodium carbonate, the intermediate V reacts with ethyl chloroformate under an alkaline condition to obtain an intermediate VI, the intermediate VI reacts with formaldehyde and formic acid to obtain an intermediate VII, and the intermediate VII is acidified with hydrochloric acid-ethyl acetate to obtain the mianserin hydrochloride. The new crystal form of the mianserin hydrochloride of the present invention has characteristics of significant treatment effect, symptom and root cause treatment, and wide application prospets.
Owner:仁和堂药业有限公司
Synthetic method for capecitabine key intermediate
ActiveCN104926901AHigh yieldControl ratioSugar derivativesSugar derivatives preparationChloroformateChloroform
The invention discloses a synthetic method for capecitabine key intermediate 2`,3`-O-diacetylpyridine-5`-deoxygenation-5-fluorine-N4-[(pentyloxy) carbonyl] cytidine. The synthetic method for the capecitabine key intermediate comprises the following steps that 1, 5- fluorocytosine, an acid-binding agent, chloroform, water and phase transfer catalyst are mixed, pentyl chloroformate is added dropwise under stirring, and the chloroform solution of (5-fluorine-2-oxo-1,2-dihydropyrimidine-4-base) amylcarbamate is obtained; 2, 1,2,3-three-O-acetyl-5-deoxygenation-6- ribofuranose is added into the chloroform solution obtained in the step 1, lewis acid is added dropwise, the reaction is performed for 2-10 hours after adding, and the capecitabine key intermediate is obtained after post-processing. The synthetic method is simple and convenient in operation, a silicane protective agent and intermediate product purification are not needed, the high yield of finished products is achieved, the proportion of alpha isomer in the products is effectively controlled, and compared with literature data, the purity of the obtained products is greatly improved.
Owner:广安凯特制药有限公司
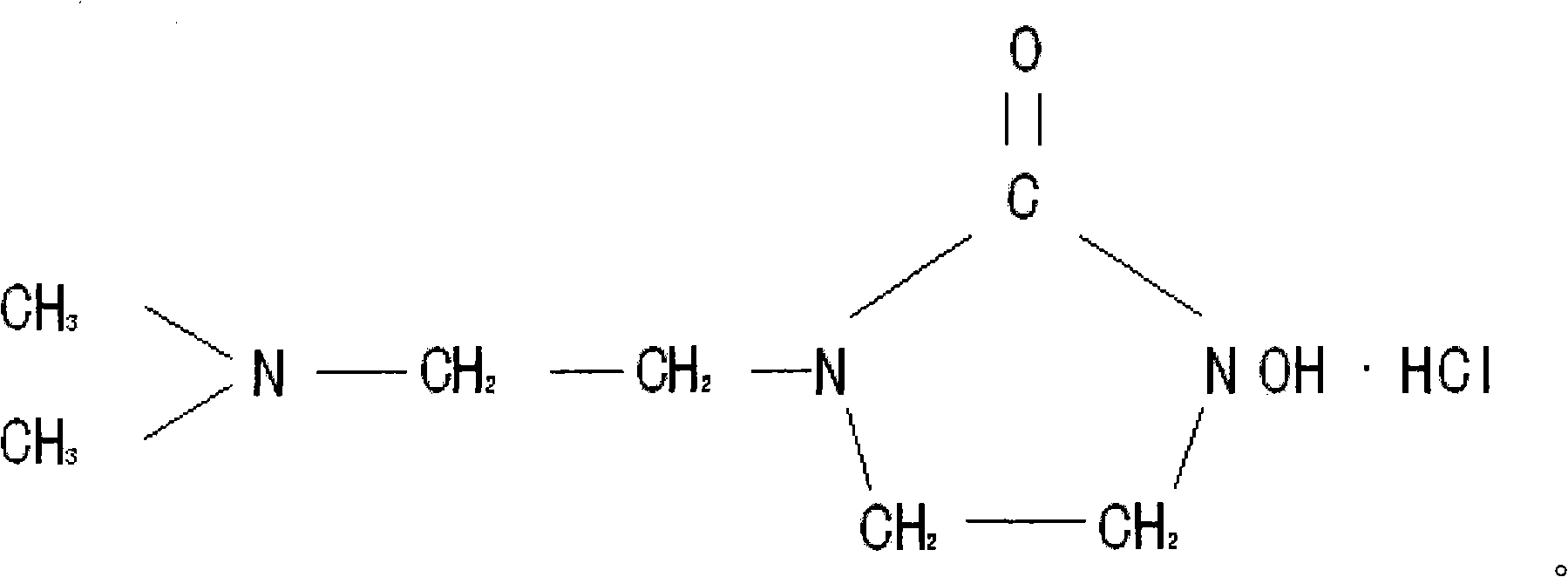
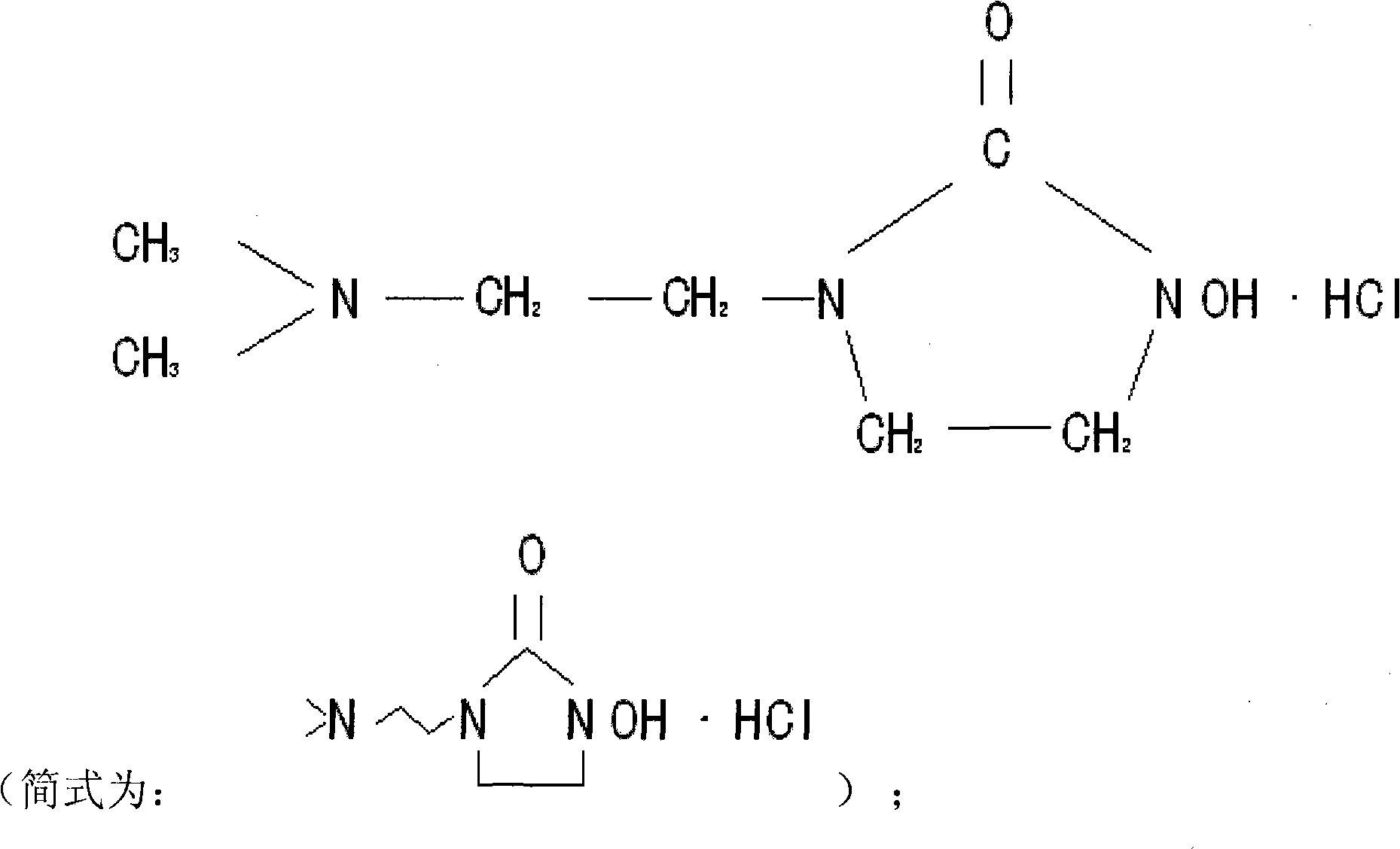
1 (dimethylamino) ethyl, 3-hydroxy-imidazoline hydrochloride and preparation method thereof
The invention relates to 1 (dimethylamino) ethyl, 3-hydroxy-imidazolone hydrochloride and a preparation method thereof for effectively solving the problem that electroplating wastewater treatment can not reach the state environmental protection standard. The preparation method of the 1 (dimethylamino) ethyl, 3-hydroxy-imidazolone hydrochloride comprises the steps of: mixing dimethylamino ethylamine and p-Nitrophenyl chloroformate, and then adding a sodium carbonate solution for reaction; mixing an oil layer at the top and benzyl hydroxylamine, and then adding the sodium carbonate solution for reaction; adjusting pH value, standing and distilling to obtain dimethylamino ethyl benzyl hydroxyl urea; putting in a steel kettle together with dichloroethane and the sodium carbonate solution for reaction; adding a sodium hydroxide solution to obtain 1-dimethylamino-ethyl, 3-acrinyl-imidazolone; then adding a hydrogenation kettle for removing benzyl; adding in petroleum ether for separating out 1 (dimethylamino) ethyl, 3-hydroxy-imidazolone; dissolving in water; adding hydrochloric acid for adjusting pH value; and distilling to obtain 1 (dimethylamino) ethyl, 3-hydroxy-imidazolone hydrochloride. The invention is mainly used for the electroplating wastewater treatment.
Owner:聂世保
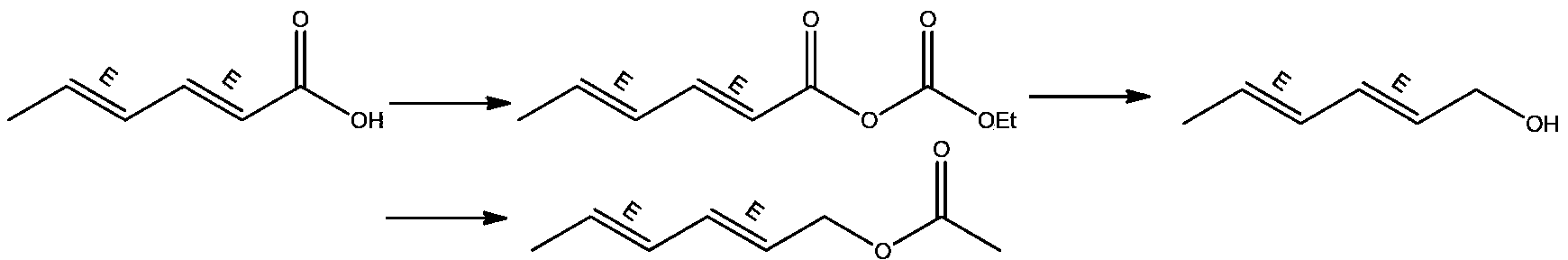
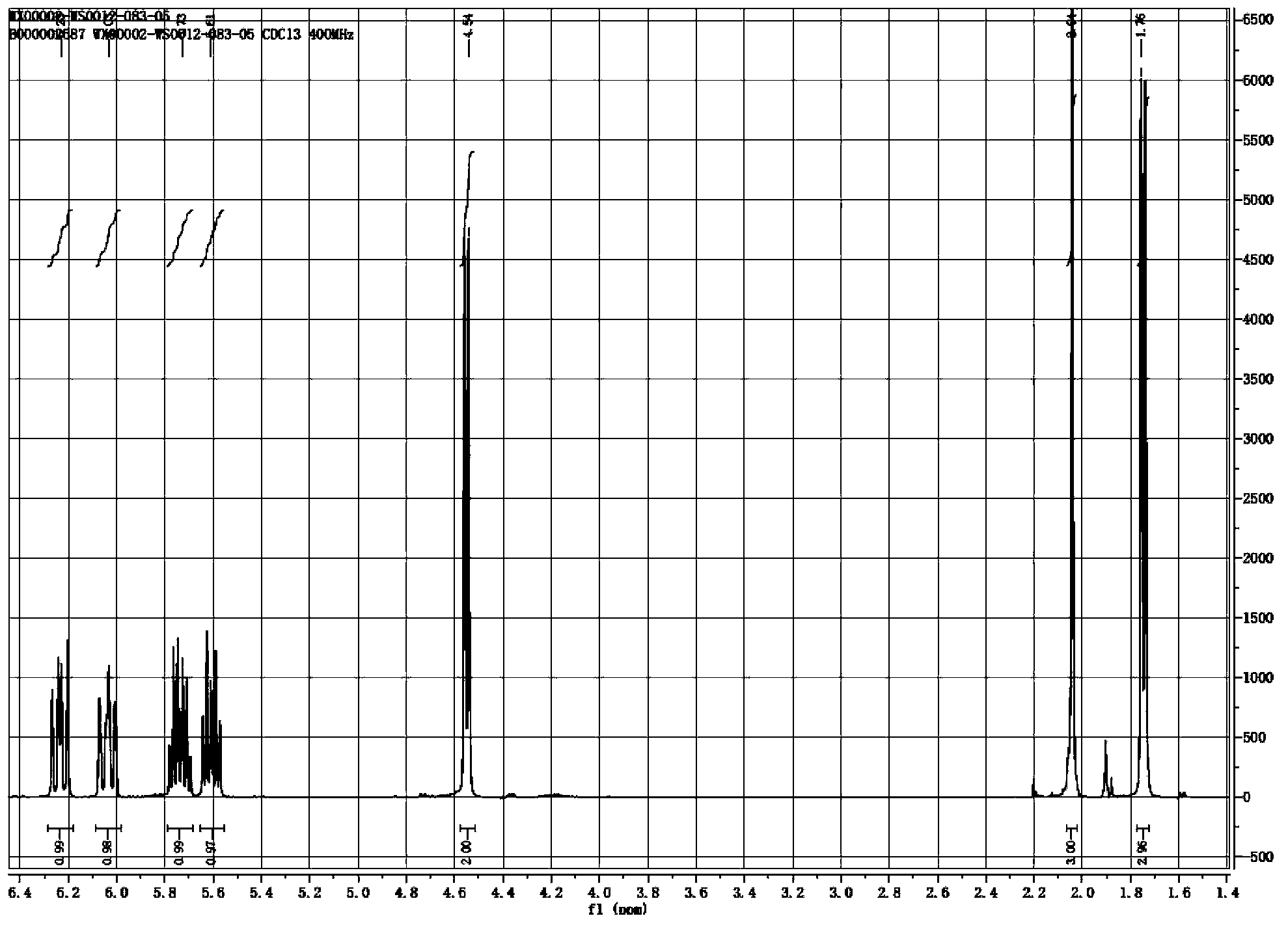
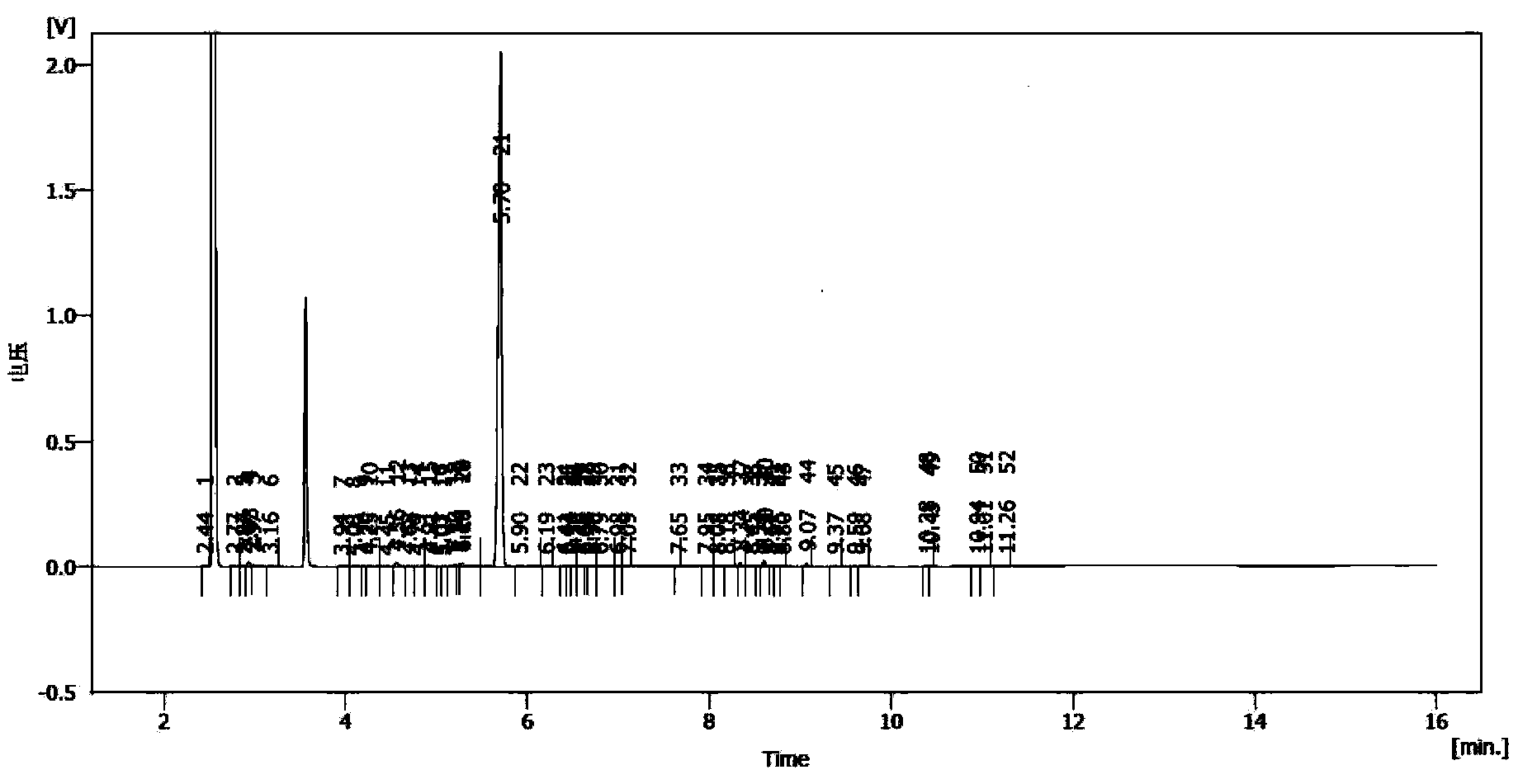
Synthesis method of laspeyresia pomonella sex pheromone intermediate (2E, 4E)-2,4-hexadienol acetate
InactiveCN103360248AImprove conversion rateAvoid the defects of incomplete conventional esterification reactionOrganic compound preparationCarboxylic acid esters preparationChemical synthesisEthyl chloroformate
The invention relates to the field of chemical synthesis of an insect sex pheromone intermediate, and particularly relates to a synthesis method of a laspeyresia pomonella sex pheromone intermediate (2E, 4E)-2,4-hexadienol acetate. According to the method provided by the invention, sorbic acid is used as a start material and generates an active acid anhydride with ethyl chloroformate; the active acid anhydride generates (2E, 4E)-hexadiene-1-ol in a reducing system of hydroboron anion reducing agent / inorganic aqueous alkali; and (2E, 4E)-2,4-hexadienol acetate is finally obtained through an esterification reaction. The synthesis method provided by the invention has the advantages of easily available raw materials, mild reaction conditions, convenience in operation, low requirements for equipment pipelines, high product yield and simple after-treatment, and is environment-friendly and suitable for large-scale industrial production.
Owner:WATERSTONE PHARMA WUHAN
Preparation method of 2'-3'-bis-O-acetyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine
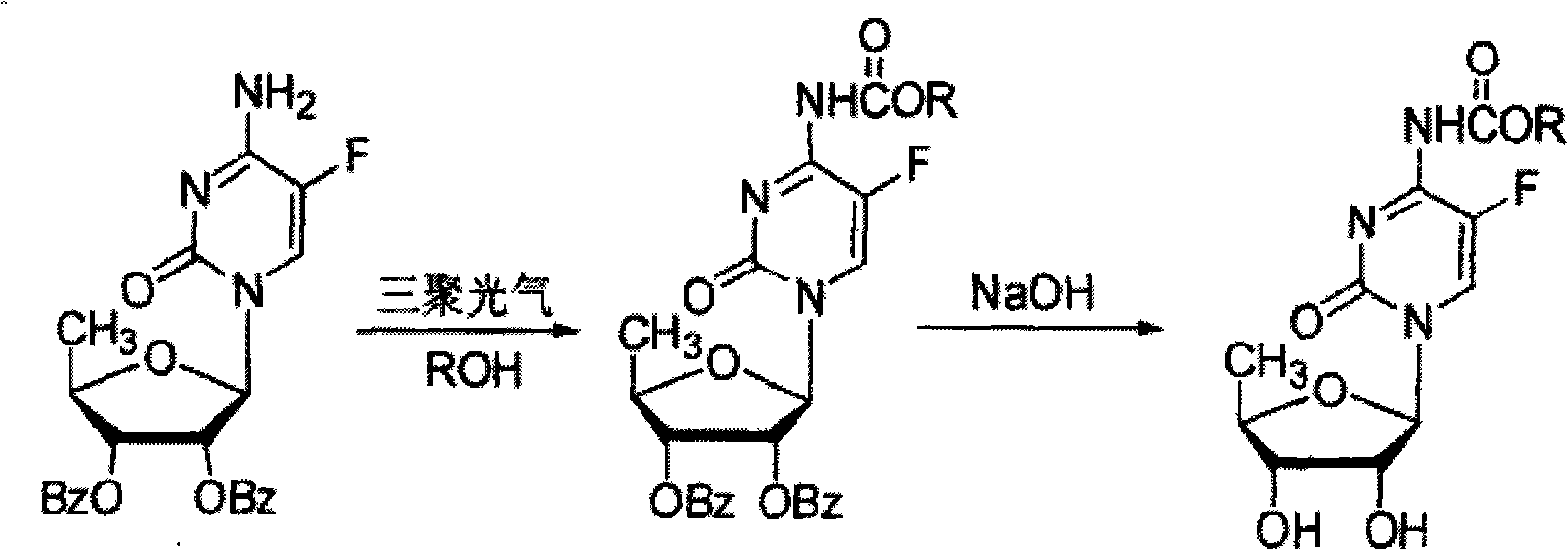
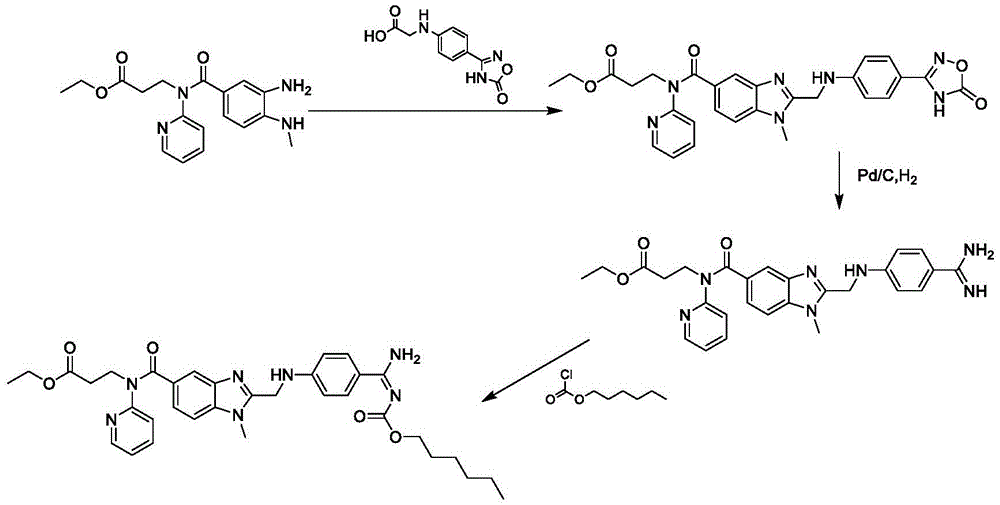
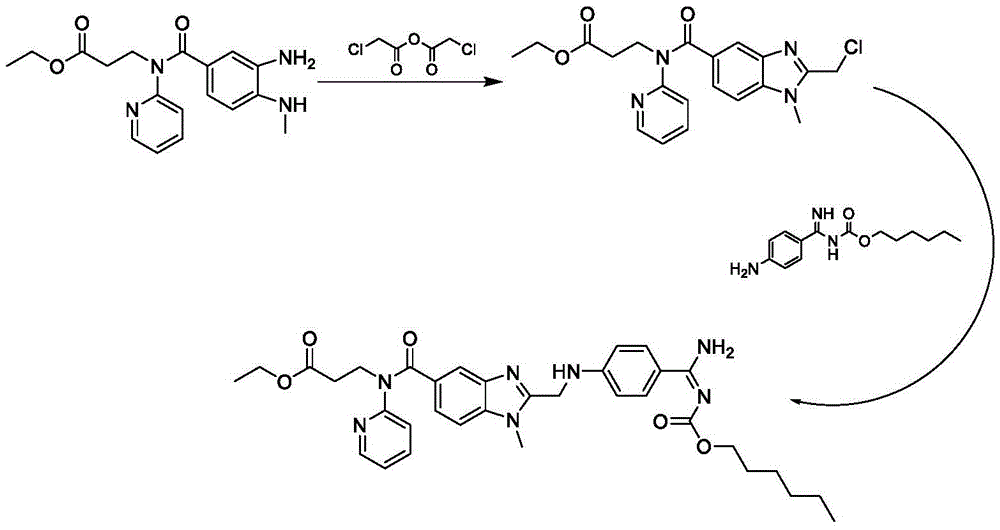
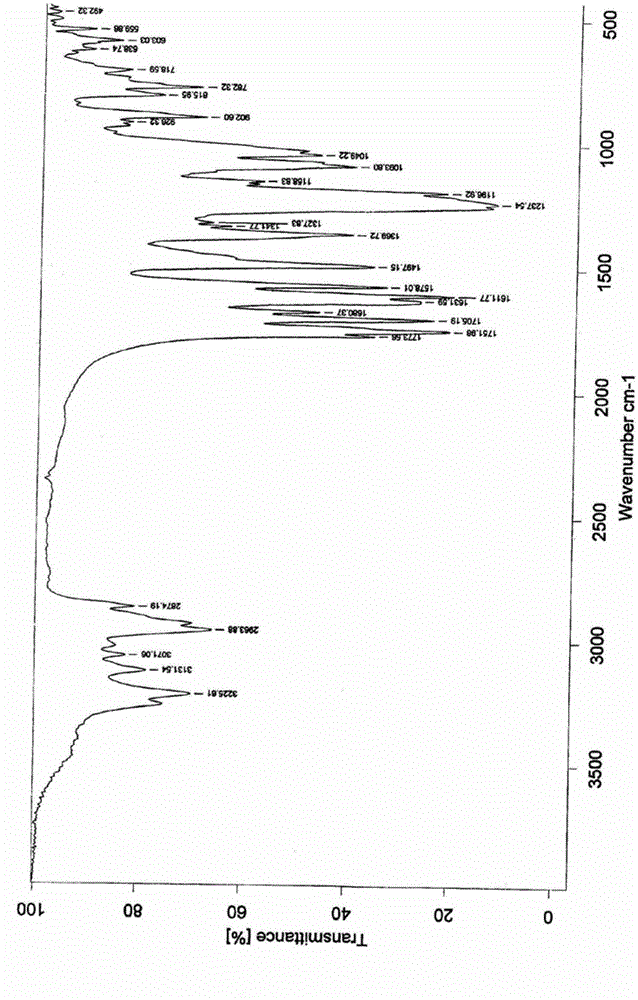
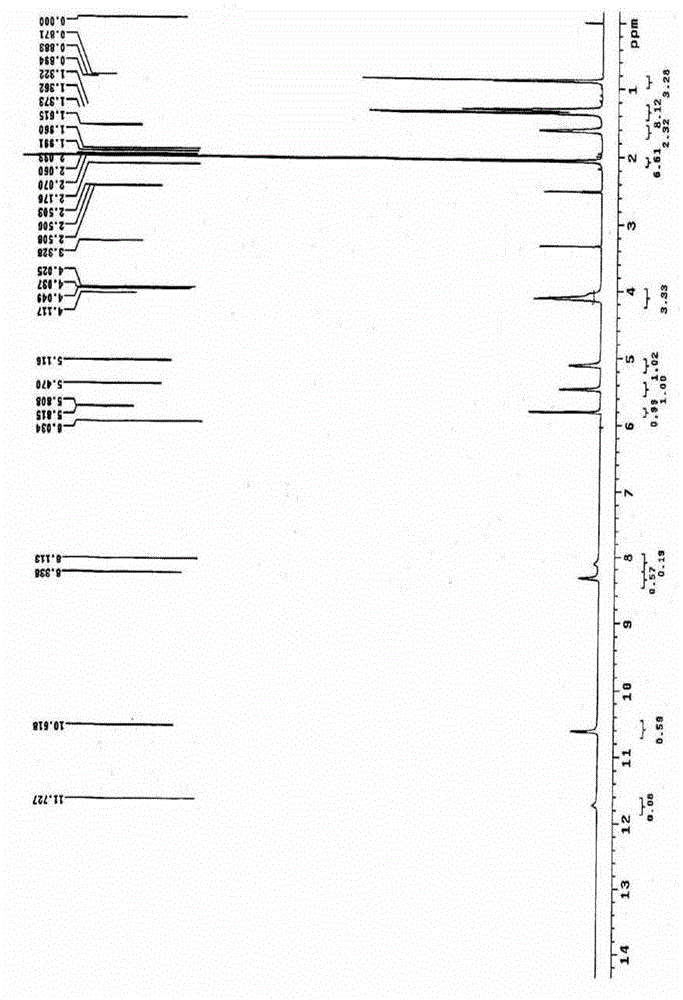
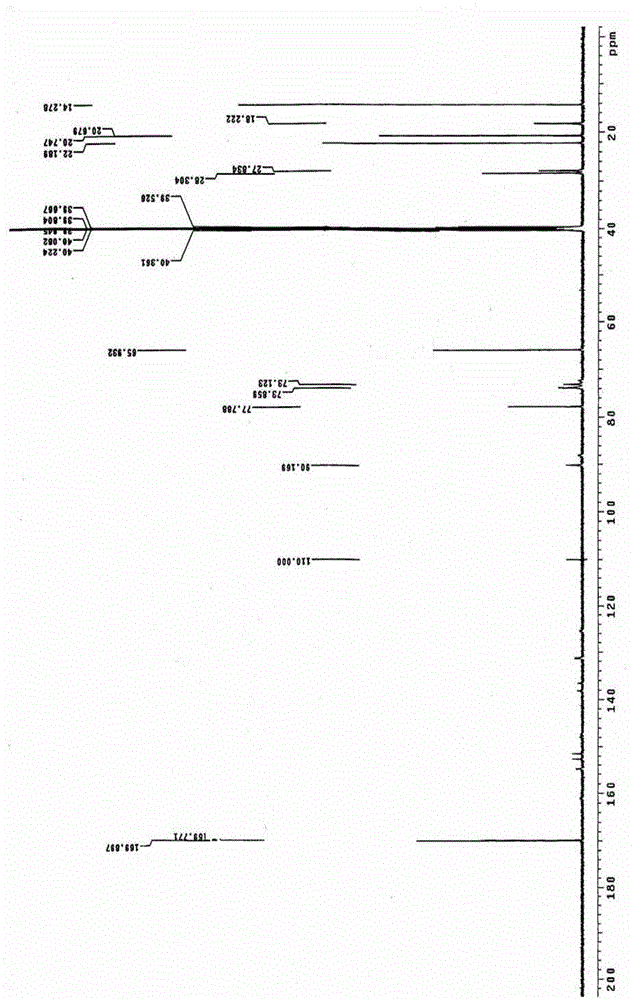
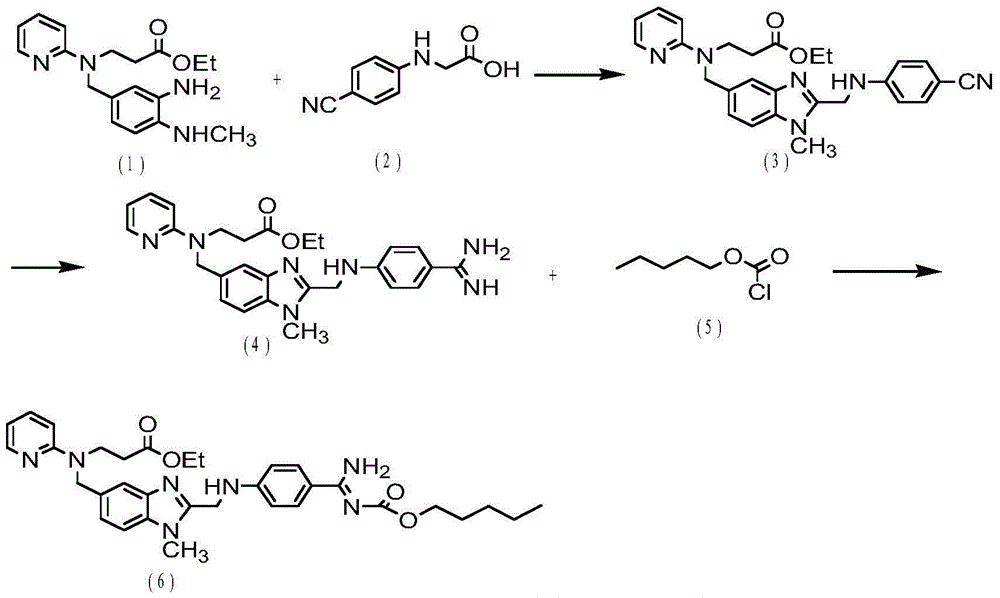
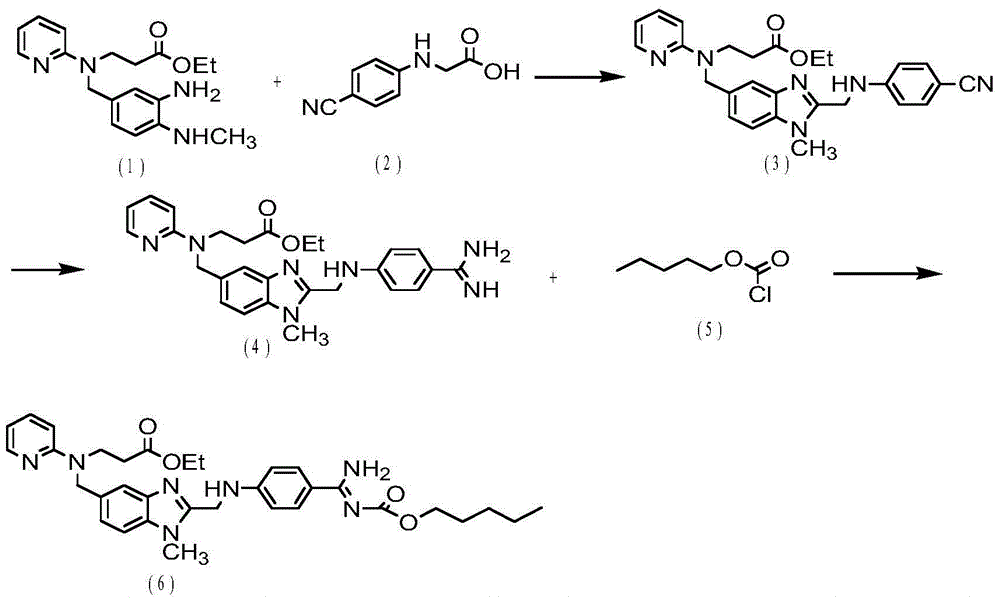
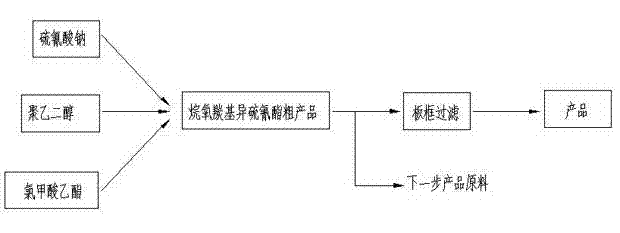
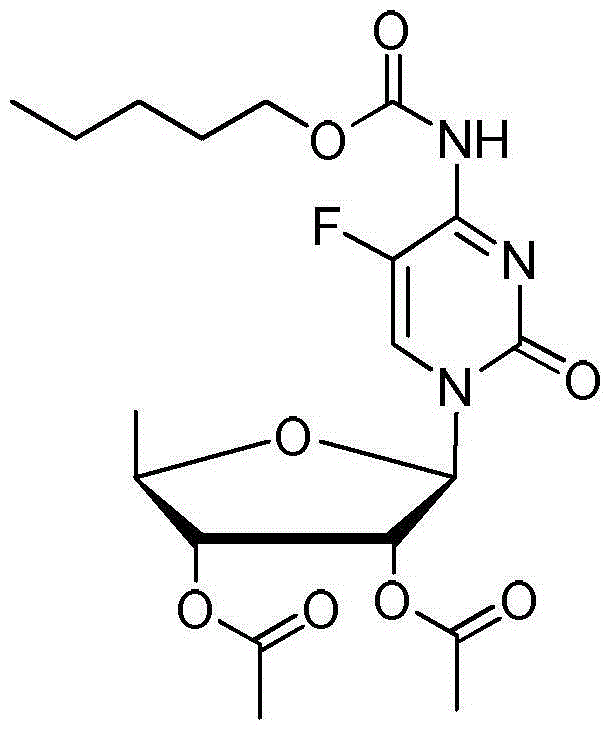
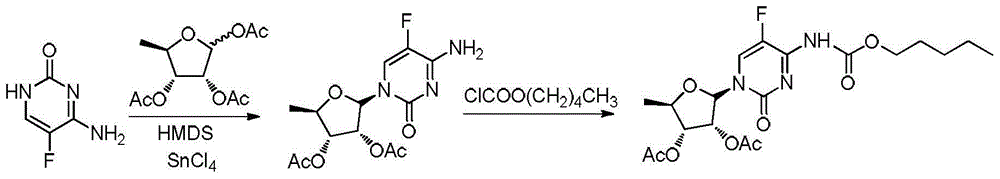
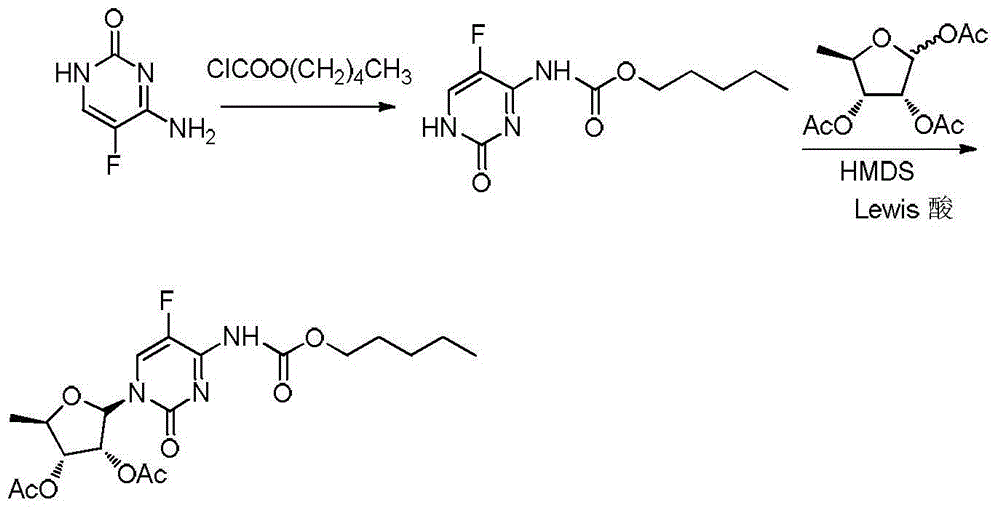
ActiveCN106478751AHigh yieldHigh puritySugar derivativesSugar derivatives preparationChloroformatePyridine
The invention relates to the field of pharmaceutical chemistry, in particular to a preparation method of 2'-3'-bis-O-acetyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine; the method comprises: reacting a compound of formula IV as a raw material with n-amyl chloroformate under the action of K3PO4 to obtain the compound of formula V, 2',3'-bis-O-acetyl-5'-deoxy-5-fluorine-N4-[(pentyloxy)carbonyl]cytidine. The invention also provides application of the method in the preparation of capecitabine. The preparation method has the advantages that reaction yield can be significantly increased, product purity is high, reaction conditions are mild, the use of pyridine is avoided, pyridine residue in the product is avoided, and the method is suitable for industrial production of medicine.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
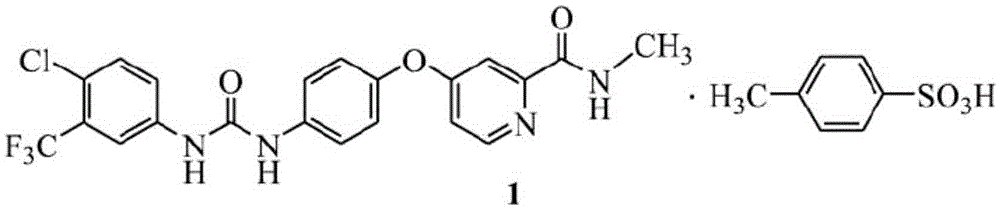
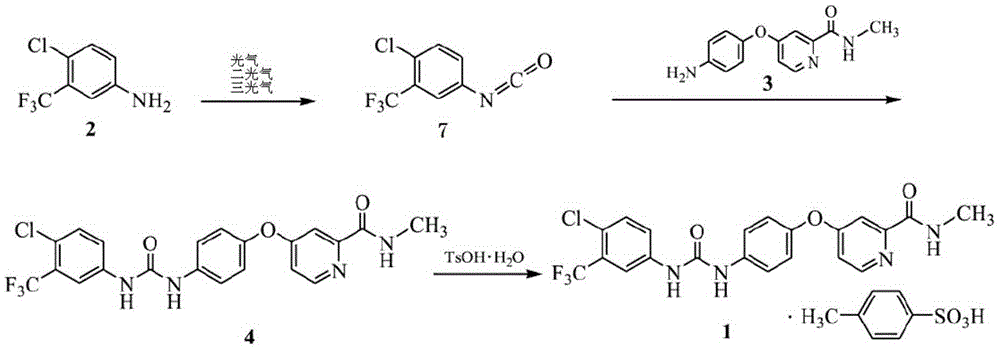
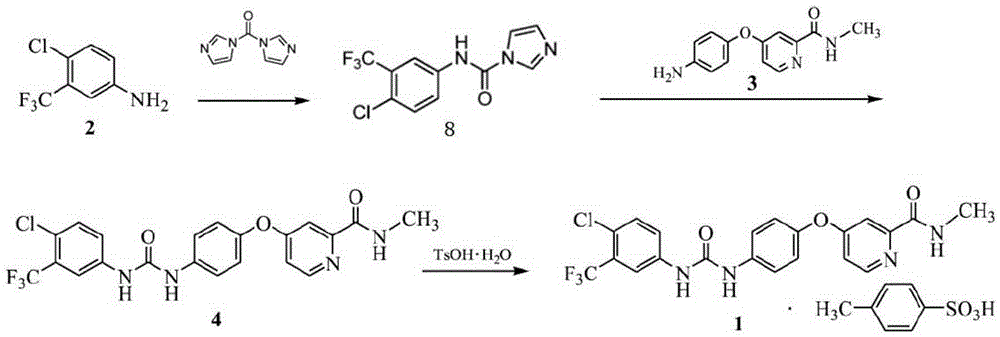
Preparation method of sorafenib tosylate
The invention relates to a preparation method of sorafenib tosylate. The preparation method comprises the steps of reacting propylene chloroformate (5) with low cost and 4-chloro-3-trifluoromethyl phenylamine (2) with low cost to generate activated ester (6); reacting activated ester and 4-(4-aminophenoxy)-N-methyl-2-pyridine carboxamide (3) under the catalysis of N-methylpyrrolidine to obtain sorafenib with high yield; carrying out simple aftertreatment on the reaction to obtain sorafenib with relatively high purity; and then, reacting sorafenib and p-toluene sulphonic acid to generate the target product. The method is low in cost, simple in operation, few in reaction step, short in period, low in energy consumption, safe in process, free of high-toxicity reagents and suitable for industrial production, and the obtained product is high in yield and purity and free of potential safety problems.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
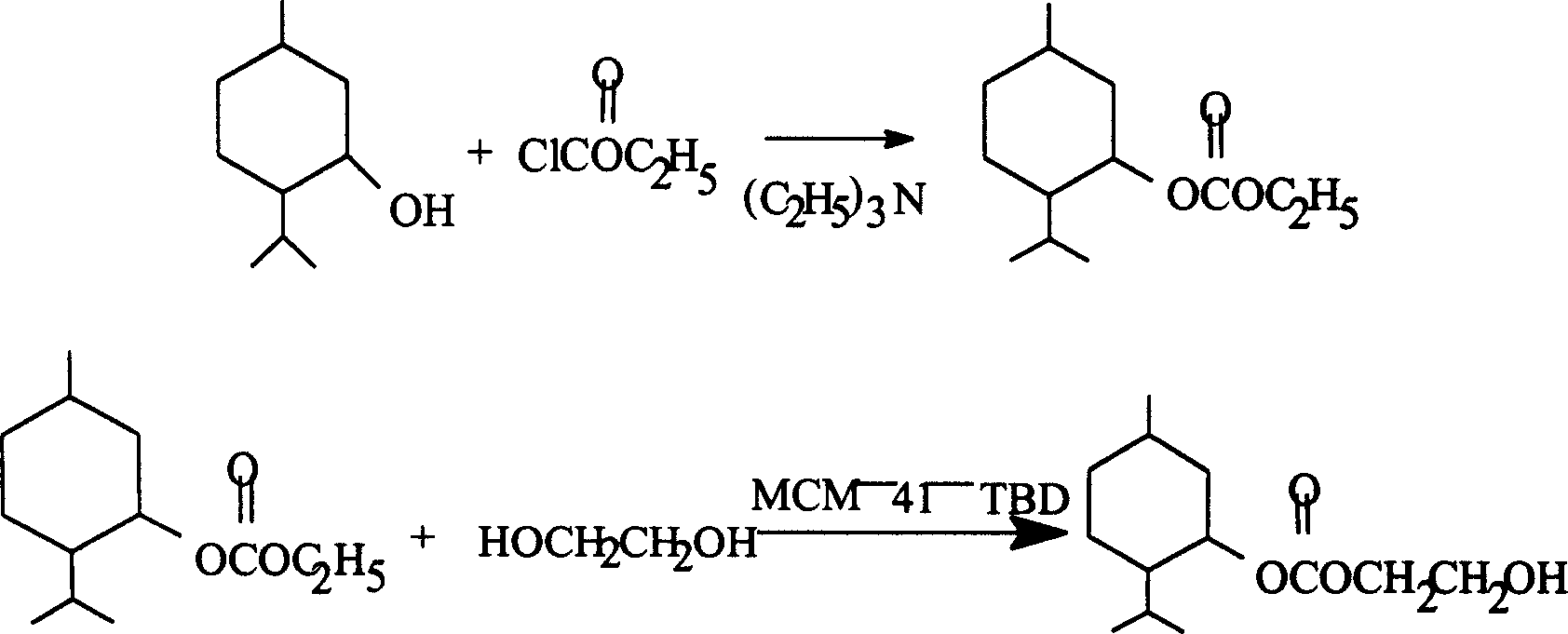
Method for synthesizing L-menthol glycol carbonic ester
InactiveCN1616406AHigh yieldOrganic compound preparationCarbonic/haloformic acid esters preparationEthyl chloroformateReaction temperature
The present invention discloses a kind of L-menthol glycol carbonic ester synthesizing process. In the first step, menthol and ethyl chloroformate are reacted at 60 deg.c for 9-11 hr in the presence of catalyst pyridine or triethylamine to prepare to prepare L-menthol ethanol carbonic ester. In the second step, L-menthol ethanol carbonic ester and glycol produce alcohol exchange reaction of 10-15 hr to obtain L-menthol glycol carbonic ester at 125 deg.c and in the presence of catalyst MCM-41-TBD or HIGH-TDB. The present invention has the advantages of no use and production of phosgene and the catalyst MCM-41-TBD or HIGH-TDB used in the second step with high catalytic activity and capable of being reused.
Owner:上海香料研究所
4-methyl-2-nitroaniline synthesis method
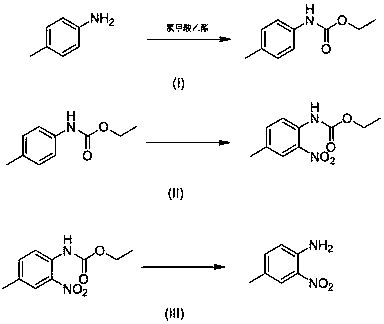
InactiveCN107759479AMild reaction conditionsNo need for high temperatureCarbamic acid derivatives preparationOrganic compound preparationNitrosoMethylaniline
The invention discloses a 4-methyl-2-nitroaniline synthesis method, which comprises: carrying out amino protection with ethyl chloroformate by using 4-methylaniline as a raw material to generate N-(p-toluene)ethyl carbamate; adding an oxidant and a copper salt catalyst, and carrying out a reaction for a certain time at a temperature of 50-120 DEG C in a reaction solvent by using a nitroso-containing compound as a nitrating agent to prepare a corresponding protected o-nitro-p-toluidine; and carrying out hydrolysis to obtain the target product. According to the present invention, the method hasadvantages of simple preparation process, mild reaction condition, high yield and environment protection.
Owner:NANJING UNIV OF SCI & TECH
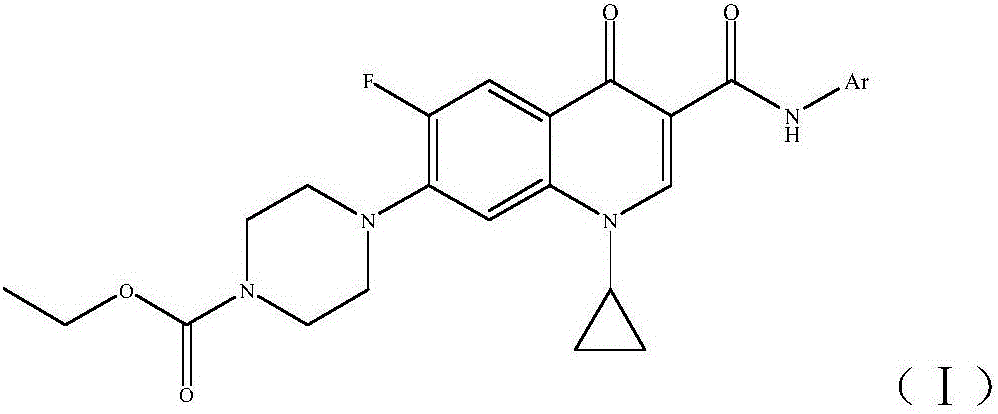
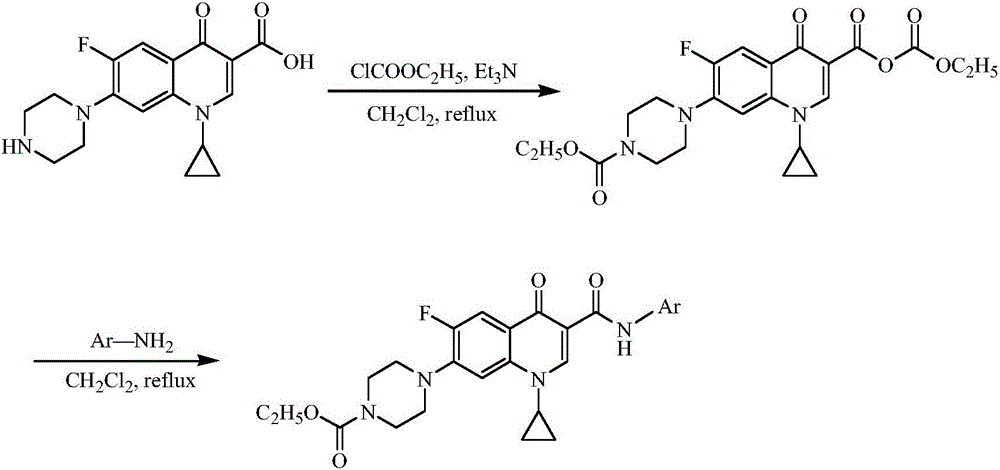
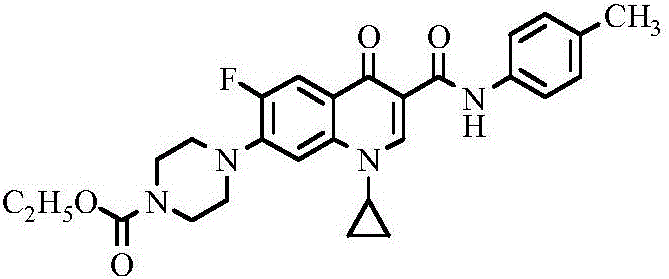
Ciprofloxacin C3 amide derivative, preparation method thereof and application
ActiveCN106432076AThe synthesis method is simpleThe synthesis method is safeAntibacterial agentsOrganic active ingredientsEthyl chloroformateHydrogen
The invention discloses a ciprofloxacin C3 amide derivative, a preparation method thereof and an application. The ciprofloxacin C3 amide derivative is provided with a structure as shown in a formula (I). Ciprofloxacin hydrochloride is added into excessive methylene dichloride, triethylamine is dripped to adjust the pH (potential of hydrogen) of reaction liquid to be 7.5-8.5, an appropriate amount of ethyl chloroformate is added, the reaction liquid is subjected to return flow agitation at the temperature of 20 DEG C and is clear, triethylamine is dripped into the reaction liquid so that the pH of the reaction liquid is 7.5-8.5, arylamine is added, return flow is continued for 1-2 hours, and the reaction liquid is cooled, reduced pressure distillation and concentration to obtain a target product. The ciprofloxacin C3 amide derivative has good sonodynamic activity and is obviously superior to ciprofloxacin in terms of microwave synergistic antibacterial effect.
Owner:LIAONING UNIVERSITY
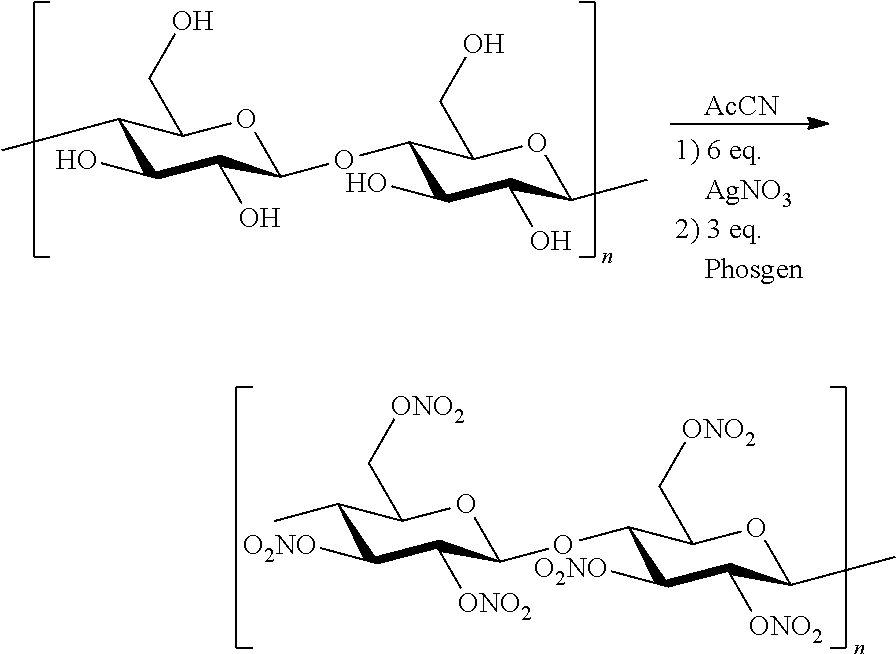
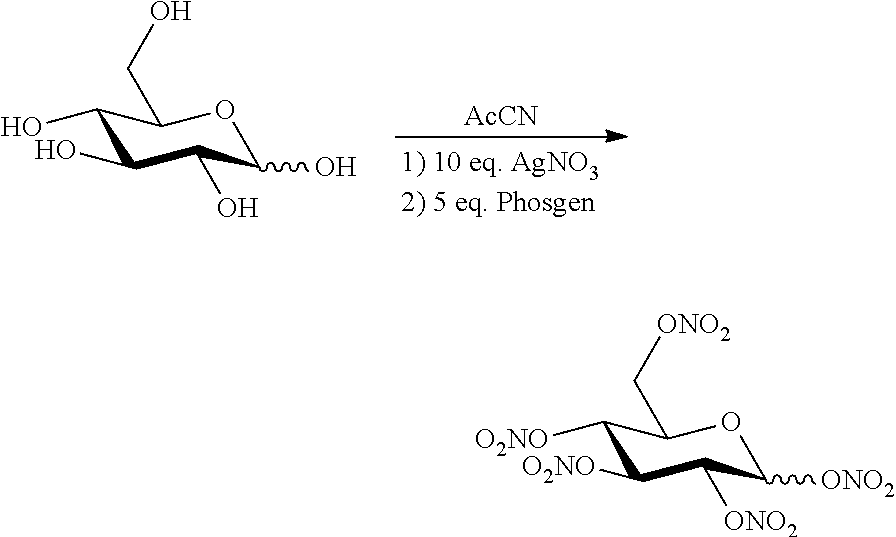
Novel Method for Directly Nitration of OH-, SH-and NHR-Functions in Organic Molecules by Means of in Situ Generated Carbonic Acid Dinitrate
The invention relates to a nitration method having the following principles: a phosgene species is converted with two equivalent silver nitrates into a double-mixed anhydride of carbonic acid and nitric acid, known here as carbonic acid dinitrate (I). Said operation is carried out in situ, and the formed dinitrate decomposes spontaneously. In addition to carbon dioxide, nitrate ions and nitronium ions are formed, said ions comprising electrophiles which are necessary for nitration. The solution which is used is acetonitrile, and is insignificant if the alcohol species is dissolved or suspended. The necessary equivalent silver nitrates are introduced into the system and optionally heated or cooled to the desired temperature. Subsequently, the acid chloride is introduced slowly, drop by drop or slowly little by little. Phosgene, diphosgene, triphosgene and chloroformic acid ester can be used as carbonic acid dichloride and monochloride, and their thiocarbonic acid analogues. A brown colouration and precipitated silver chloride display the formation of the carbonic acid reactants, said brown colouration rapidly discolouring due to an immediate reaction of the nitronium ions with the substrate with is to be nitrated. Towards the end of the addition of phosgene, the brown colouration remains for longer and longer until it no longer disappears. Then, it is stirred for another hour at room temperature. In the event of high acid-sensitive educts, non-nucleophilic nitrogen bases such as DBU can be added to the system in order to intercept the formation of nitric acid.
Owner:SYNOVO
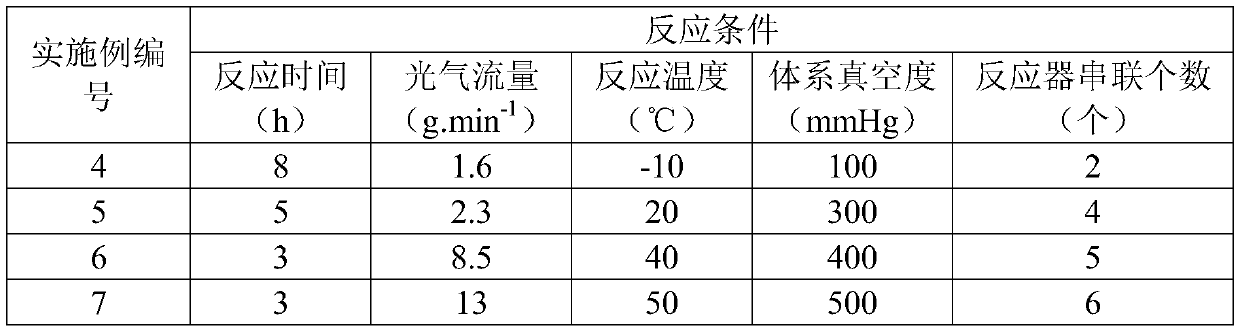
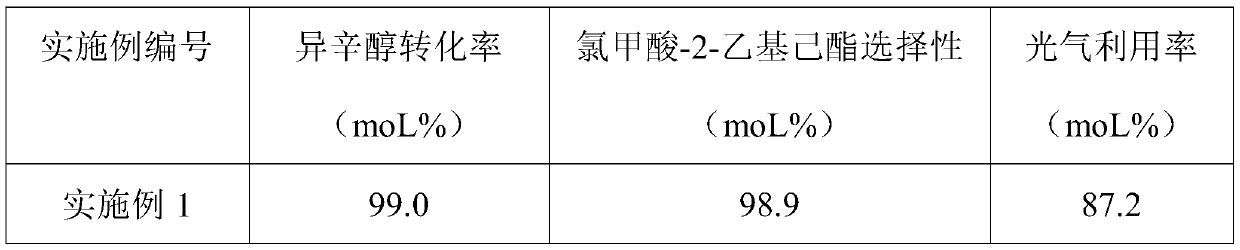
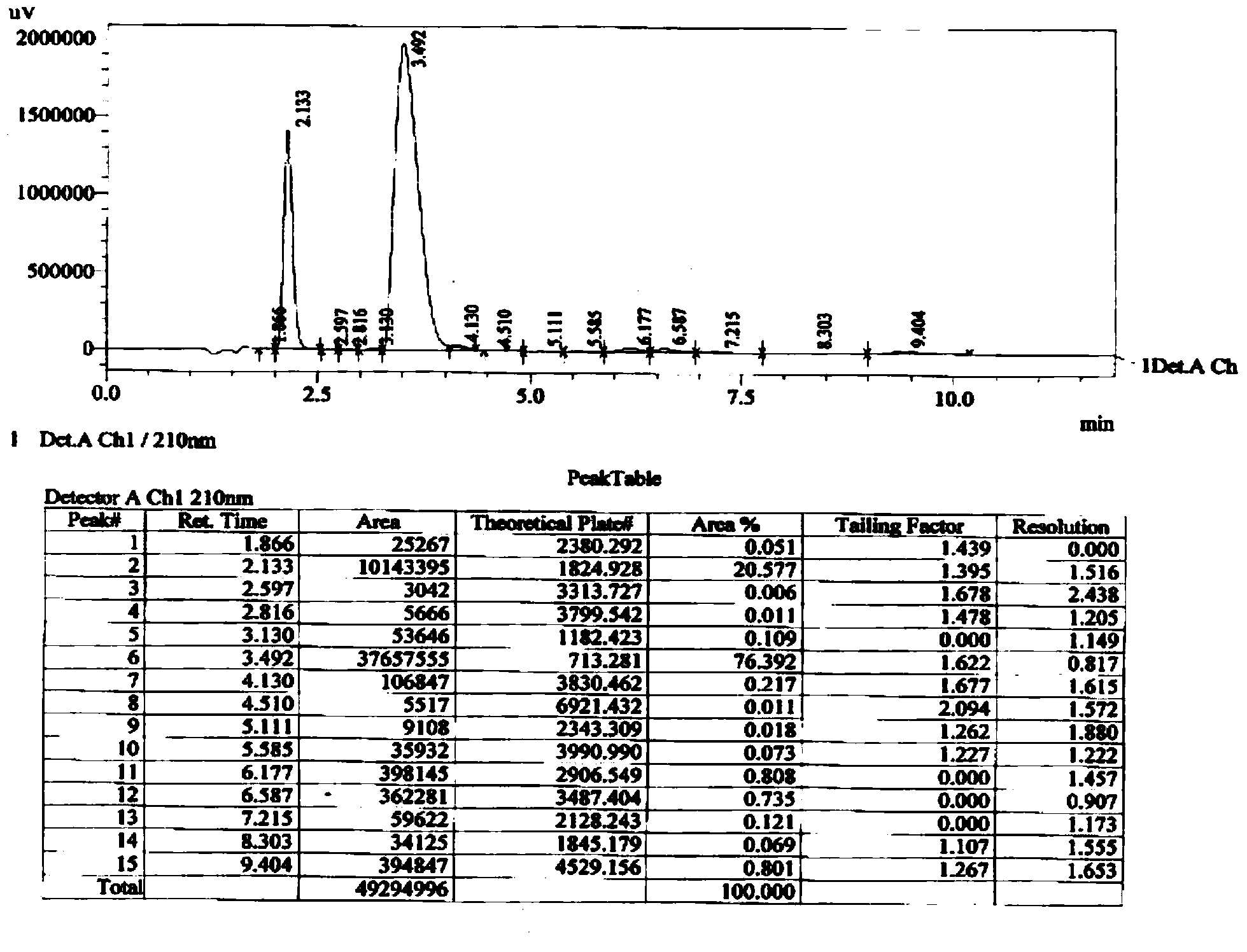
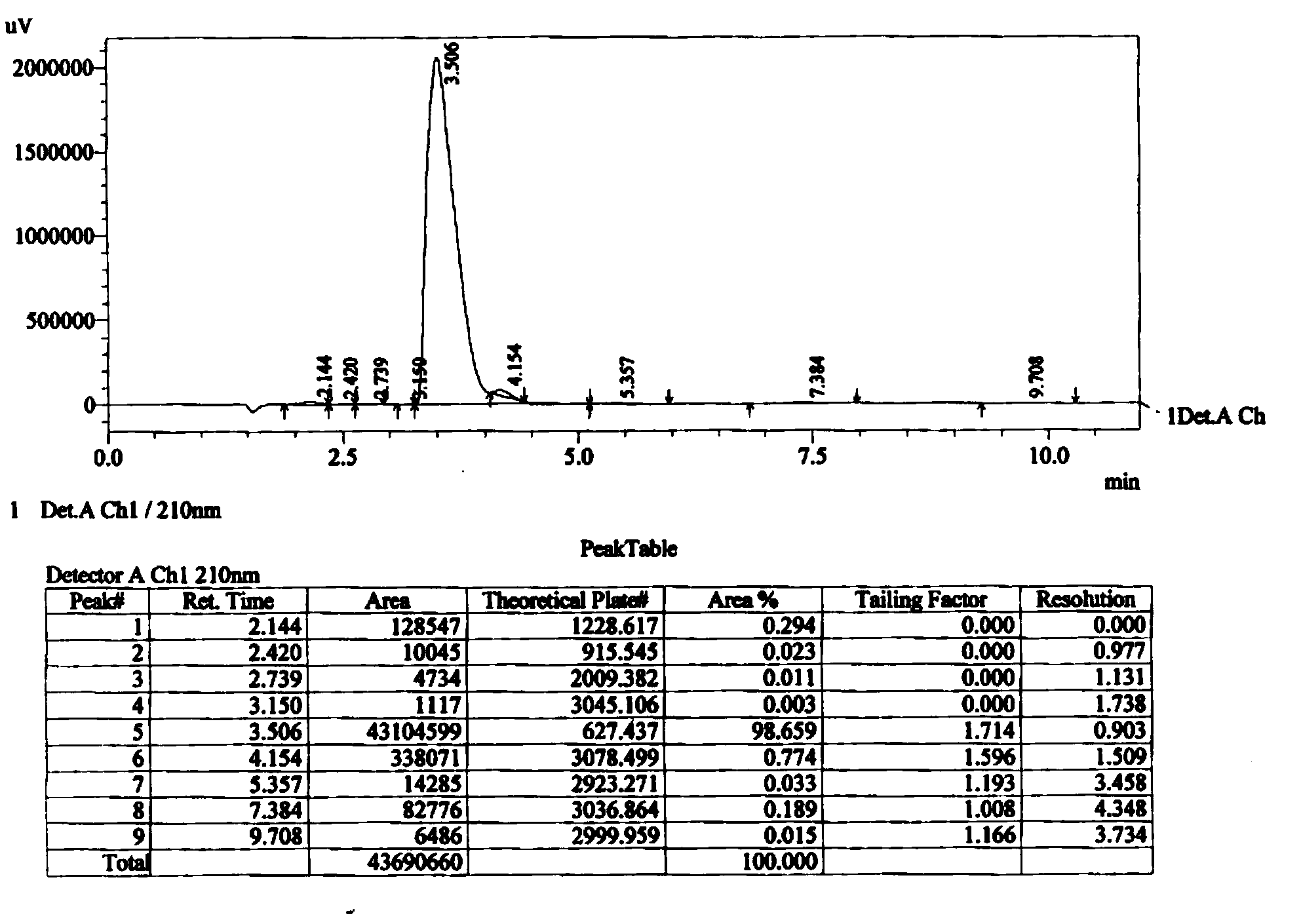
Method for preparing 2-ethylhexyl chloroformate
PendingCN111302941AIncrease profitTransfer in timeChemical/physical/physico-chemical stationary reactorsLiquid-gas reaction processesIsooctyl alcoholEthyl group
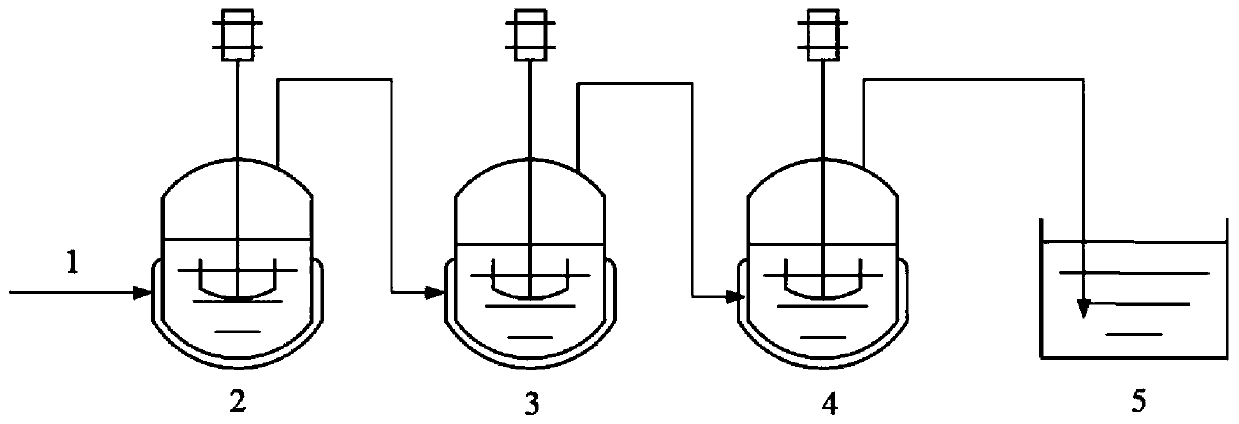
The invention provides a method for preparing 2-ethylhexyl chloroformate. The method comprises a step of reacting isooctyl alcohol with phosgene by using multiple stages of reaction kettles which areconnected in series. By adopting a micro-negative pressure system, hydrogen chloride generated by the reaction can be transferred in time, and unreacted phosgene in one reaction kettle can enter the next-stage reaction kettle for further reaction, so the utilization rate of phosgene and the conversion rate of isooctyl alcohol are improved, reaction time is greatly shortened, and industrial application value is relatively high.
Owner:JIANGSU YANGNONG CHEM GROUP +3
Preparation method of chiral intermediate for synthesizing statins
The invention discloses a preparation method of a chiral intermediate for synthesizing statins. The preparation method comprises the following steps: 1, in the presence of a solvent, enabling (3R)-3-[(t-butyldimethylsilane)oxy]-glutarate monoester and 4-nitrophenyl chloroformate to react with alkali so as to obtain 1,5-p-nitrocarbobenzoxyalkyl-(3S)-3-[(t-butyldimethylsilane)oxy]-glutarate diester; 2, in the presence of a solvent, enabling triphenyl methyl phosphonium bromide to react with alkali and then react with 1,5-p-nitrocarbobenzoxyalkyl-(3S)-3-[(t-butyldimethylsilane)oxy]-glutarate diester to obtain a target product, namely (3R)-3-[(t-butyldimethylsilane)oxy]-5-oxo-6-triphenylphosphine caproate. According to the preparation method disclosed by the invention, the reaction process is more stable, the raw material utilization ratio is relatively high, and the yield is obviously improved. More importantly, due to the reduction of impurities, the product can obtain purity according with industrial production through simple crystallization operation, so that the production efficiency is greatly improved, the cost is saved, and the environment friendliness is ensured at the same time.
Owner:JIANGSU LONG HEALTHCARE
Preparation method of thymopeptide-5
The invention discloses a preparation method of thymopeptide-5, comprising the following steps: using tyrosine benzyl ester hydrochloride as a starting material; using N-methyl morpholine as a catalyst; adopting alkyl chloroformate as an activating agent and activating protected amino acid to form high-activity mixed anhydride which reacts with exposed amino acid or peptide of an amino end. The high-purity thymopeptide-5 is prepared by the steps: dipeptide combination, kyrine combination, tetrapeptide combination, pentapeptide combination, crude generation and purification. The method simplifies a technology, has little three-waste pollution and is convenient to implement industrially.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
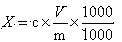
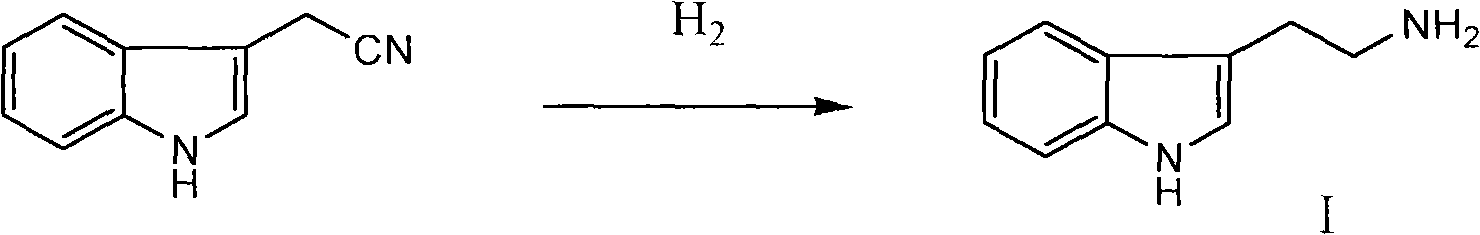

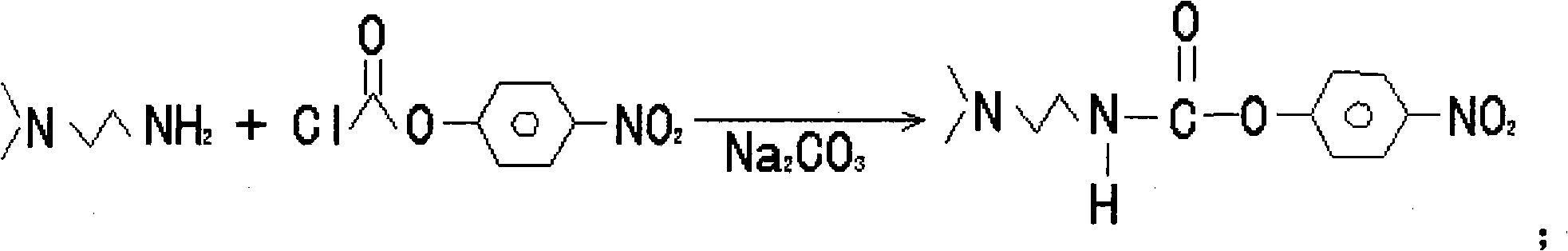
![Preparation method of 2'-3'-bis-O-acetyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine Preparation method of 2'-3'-bis-O-acetyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine](https://images-eureka.patsnap.com/patent_img/d9c115da-e3ad-45d9-bb9c-e3718ec9cf8b/BDA0000795204420000011.png)
![Preparation method of 2'-3'-bis-O-acetyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine Preparation method of 2'-3'-bis-O-acetyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine](https://images-eureka.patsnap.com/patent_img/d9c115da-e3ad-45d9-bb9c-e3718ec9cf8b/BDA0000795204420000012.png)
![Preparation method of 2'-3'-bis-O-acetyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine Preparation method of 2'-3'-bis-O-acetyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine](https://images-eureka.patsnap.com/patent_img/d9c115da-e3ad-45d9-bb9c-e3718ec9cf8b/BDA0000795204420000021.png)