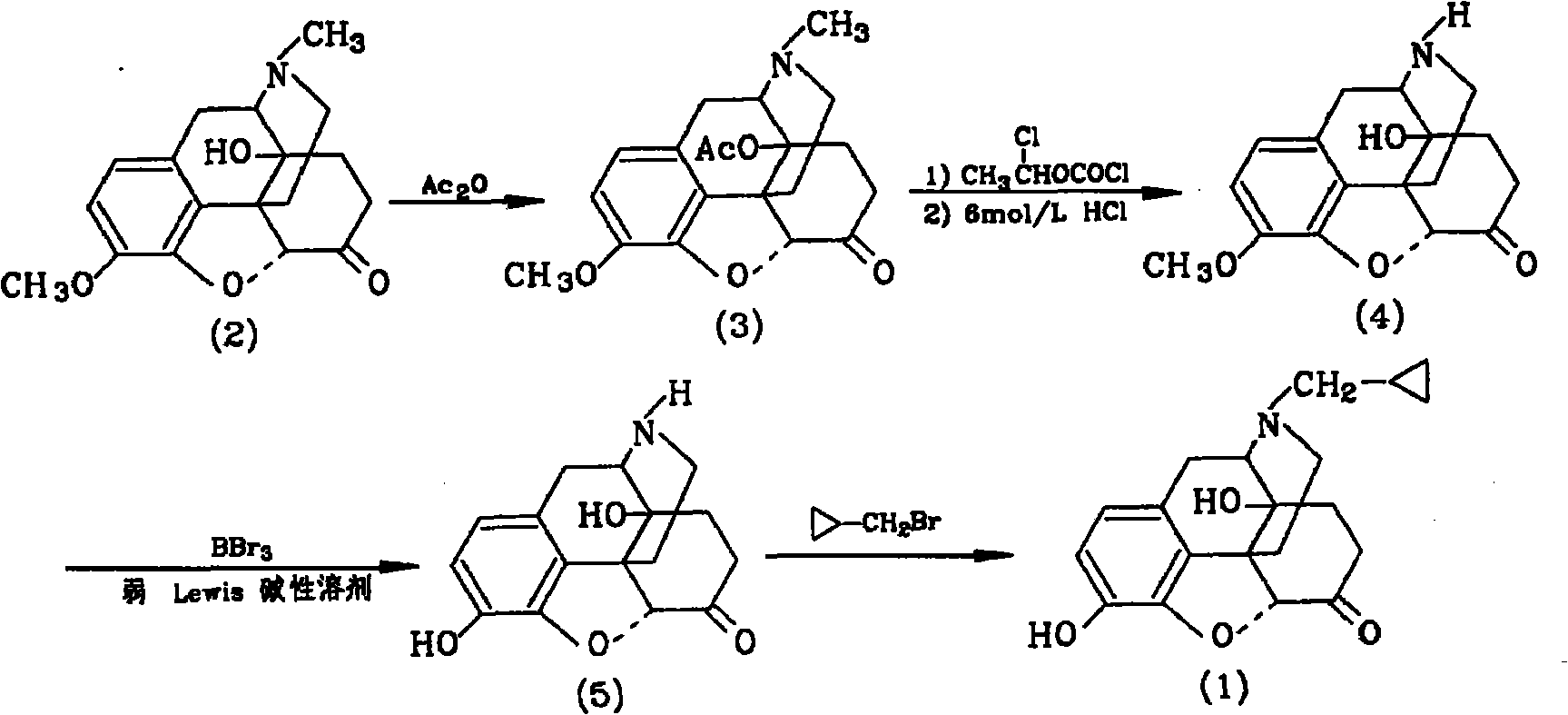
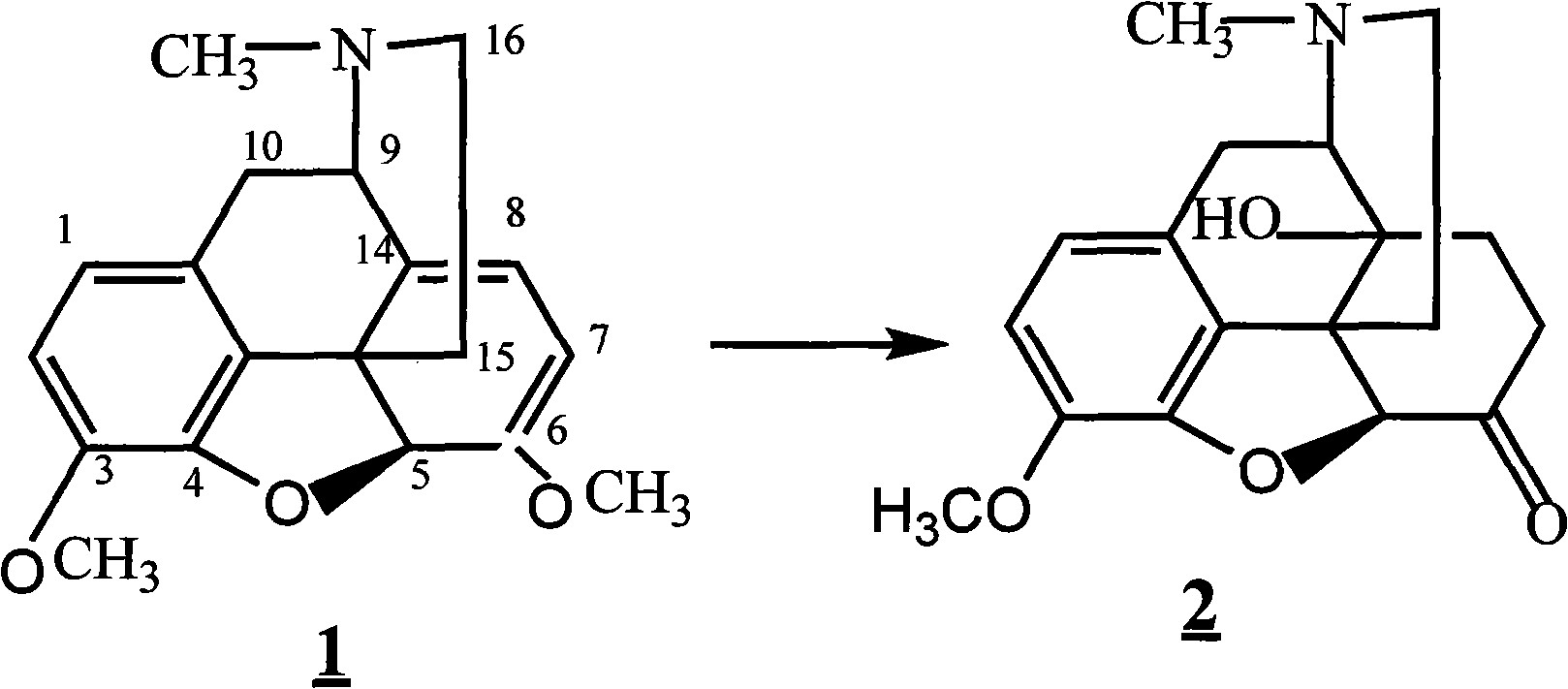
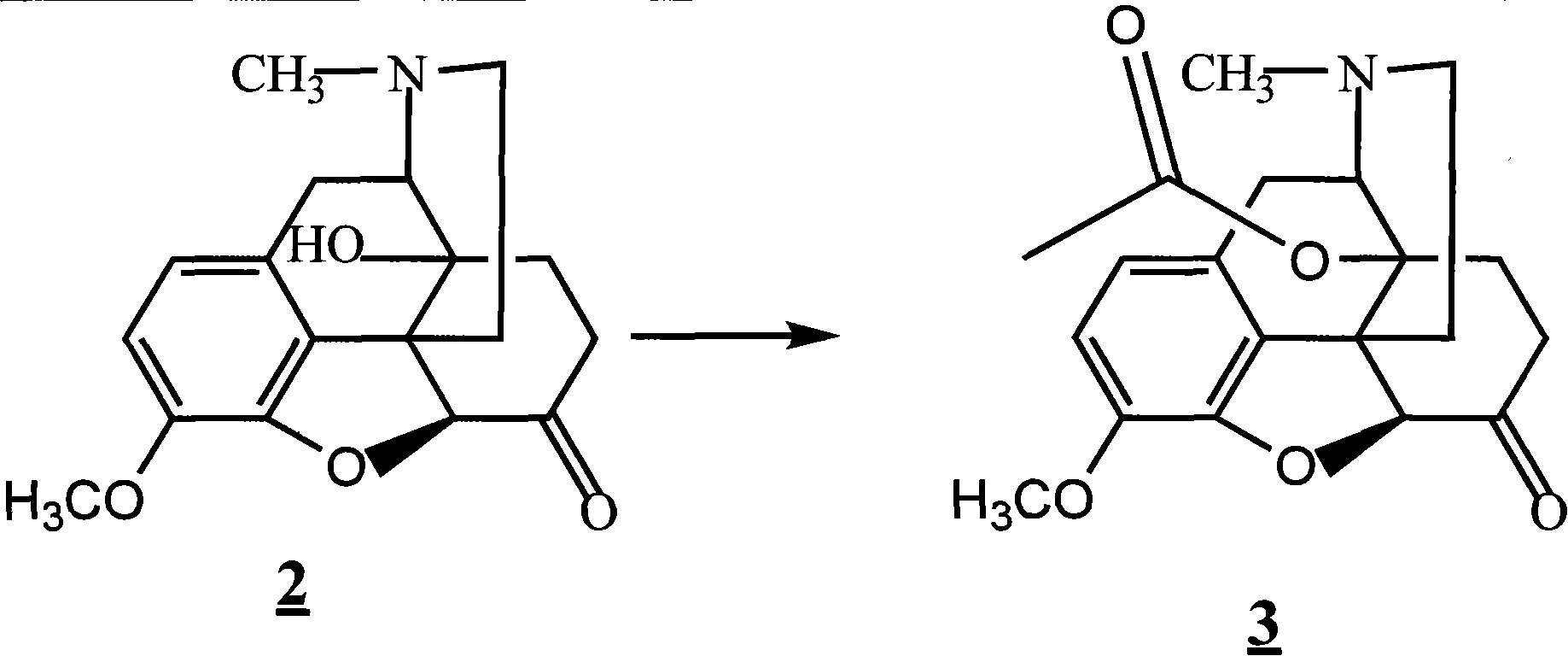
Method for synthesizing naloxone or naltrexone
A synthesis method and technology of naltrexone, which is applied in the field of pharmaceutical chemistry, can solve the problems of low reaction yield, environmental pollution, and serious injury to synthesis workers, and achieve increased reaction speed, no side reactions, and increased yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve 10g of thebaine in 100ml of formic acid, keep warm at 20°C, add 33g of m-chloroperoxybenzoic acid dropwise with stirring, keep warm for 5h, replace the system with nitrogen 4 times, add 0.5g of Raney nickel, replace with hydrogen 4 times, wait The system was stable, kept at 30°C, hydrogenated for 10 hours, filtered, the filter cake (the filter cake was the catalyst), washed twice with 10ml of water, the filtrate was adjusted to PH=8-9 with 30% sodium hydroxide solution, filtered, and the filter cake was vacuum-dried. Compound 28.2g was obtained, yield: 81.0%;
[0033] 28.2g of compound was added to the reaction flask, 82ml of acetic anhydride was added, the temperature was raised to 70°C, and after 1 hour of reaction, the system was concentrated to dryness in vacuo to obtain 38.8g of compound, yield: 94.7%;
[0034] Put 38.8g of the compound into the reaction flask, under nitrogen protection, add 90ml of toluene, add 14.7g of chloroformic acid-1-chloroethyl est...
Embodiment 2
[0039] Dissolve 10g of thebaine in 110ml of formic acid, keep warm at 25°C, add 35.2g of m-chloroperoxybenzoic acid dropwise with stirring, keep warm for 3 hours, replace the system with nitrogen for 5 times, add 0.6g of Raney nickel, and replace with hydrogen for 4 times, When the system is stable, keep warm at 25°C, hydrogenate for 12 hours, filter, filter the cake (the filter cake is the catalyst), wash twice with 10ml of water, adjust the filtrate to PH=8-9 with 30% sodium hydroxide solution, filter, and dry the filter cake in vacuum , to obtain compound 28.4g, yield: 82.9%;
[0040] Add 28.4g of compound into the reaction flask, add 84ml of acetic anhydride, raise the temperature to 60°C, and react for 1 hour, then concentrate the system to dryness in vacuo to obtain 38.9g of compound, yield: 93.5%;
[0041] Put 38.9g of the compound into the reaction flask, under nitrogen protection, add 90ml of toluene, add 14.7g of chloroformic acid-1-chloroethyl ester, add 0.9g of pot...
Embodiment 3
[0046] Dissolve 10g of thebaine in 130ml of formic acid, keep warm at 20°C, add 30g of m-chloroperoxybenzoic acid dropwise, keep warm for 7 hours, replace the system with nitrogen for 3 times, add 0.5g of Raney nickel, and replace with hydrogen for 5 times. Stable, keep warm at 30°C, hydrogenate for 10 hours, filter, filter cake (the filter cake is the catalyst), wash twice with 10ml of water, adjust the filtrate to PH=8-9 with 30% sodium hydroxide solution, filter, and vacuum dry the filter cake to obtain Compound 28.0g, yield: 79.0%;
[0047] Add 28.0 g of compound into the reaction flask, add 92 ml of acetic anhydride, raise the temperature to 80°C, and react for 1 hour, then concentrate the system to dryness in vacuo to obtain 38.7 g of compound, yield: 96%;
[0048] Put 38.7g of the compound into the reaction flask, under nitrogen protection, add 87ml of toluene, add 14.7g of chloroformic acid-1-chloroethyl ester, add 0.9g of potassium bicarbonate, heat up to 90°C for 40h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com