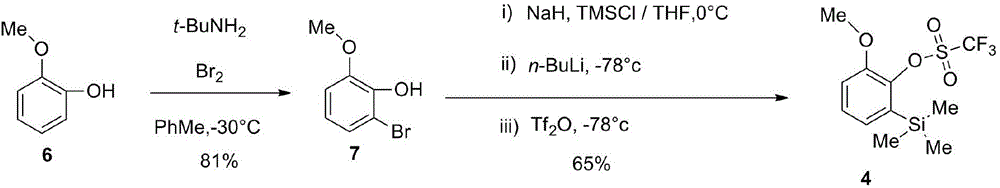Patents
Literature
137 results about "Boron tribromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
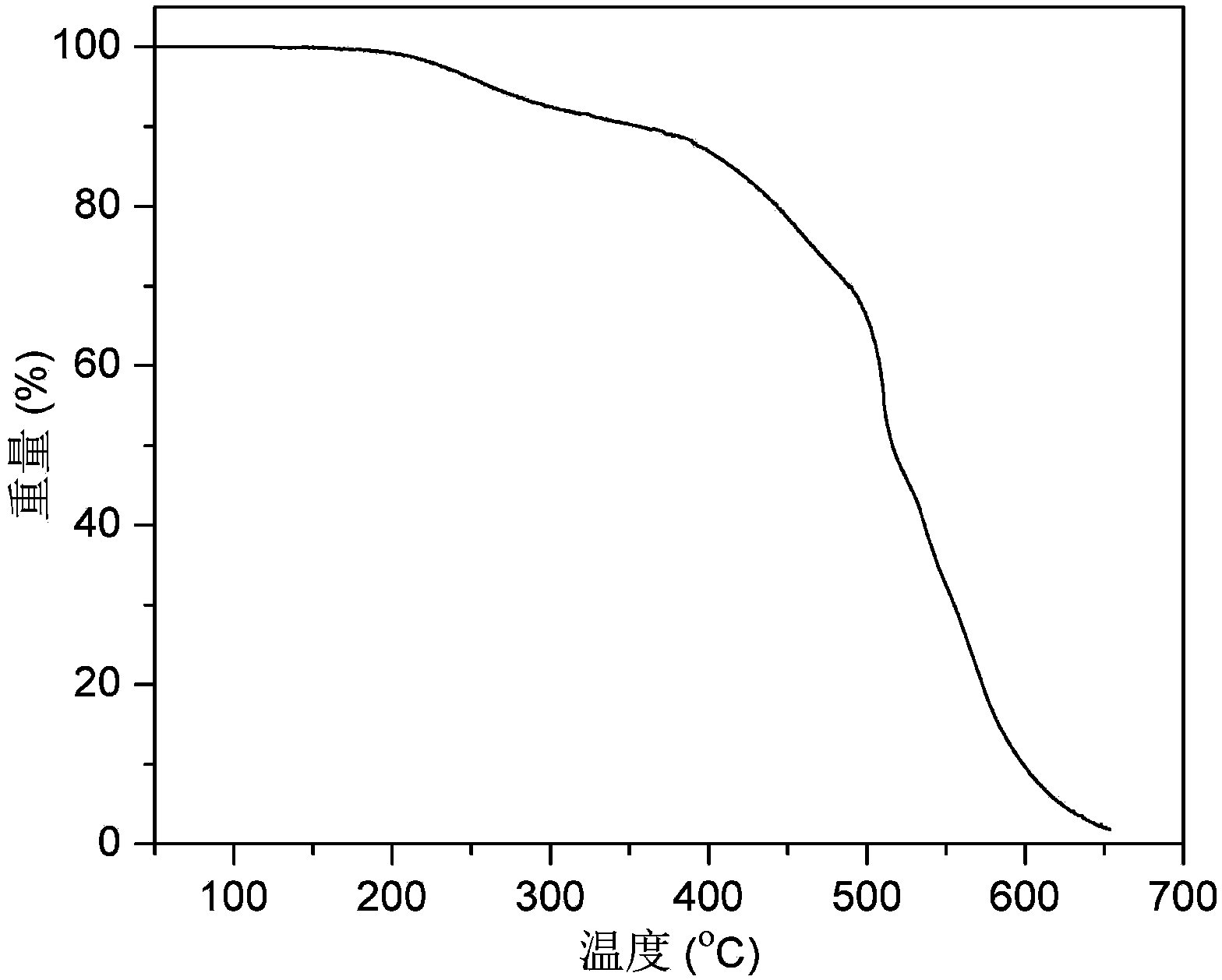
Boron tribromide, BBr₃, is a colorless, fuming liquid compound containing boron and bromine. Commercial samples usually are amber to red/brown, due to weak bromine contamination. It is decomposed by water and alcohols.
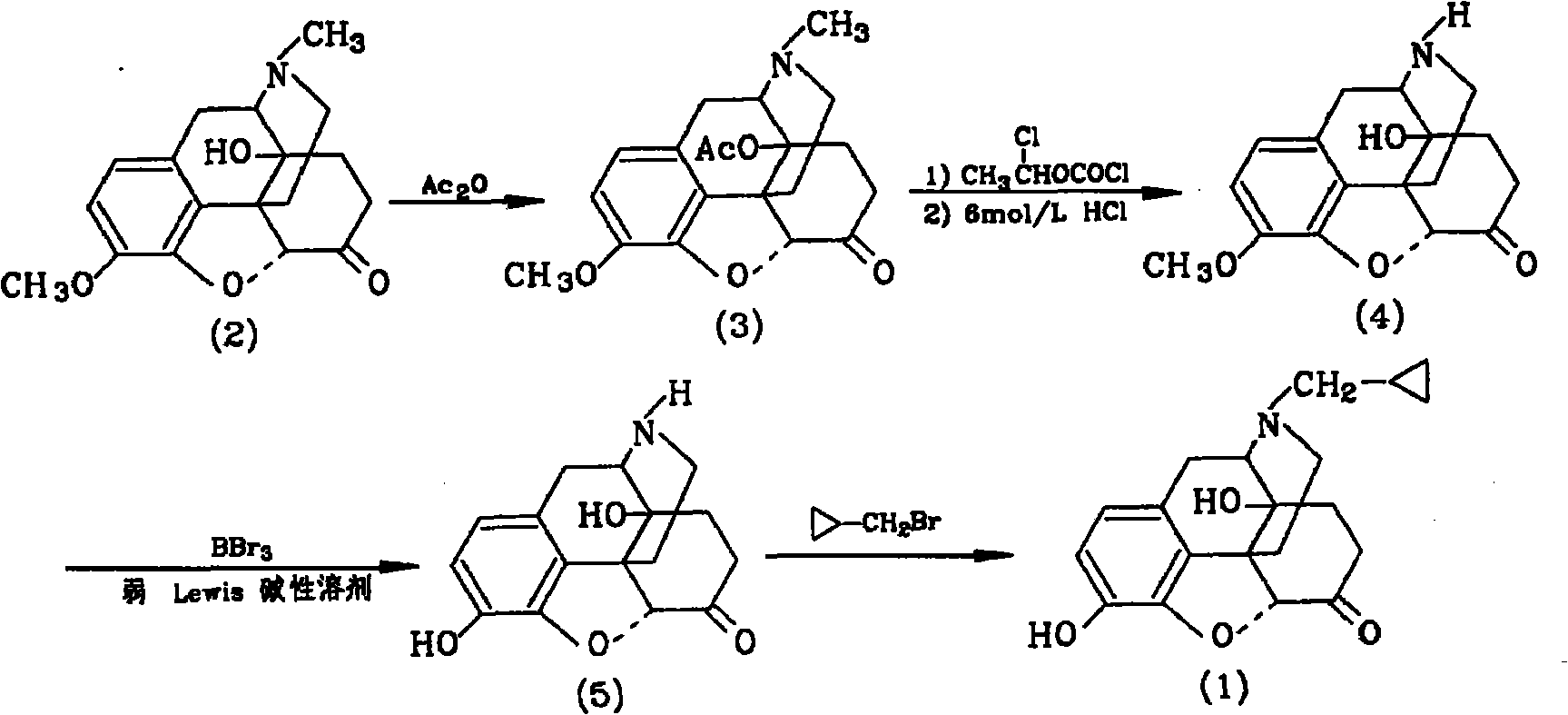
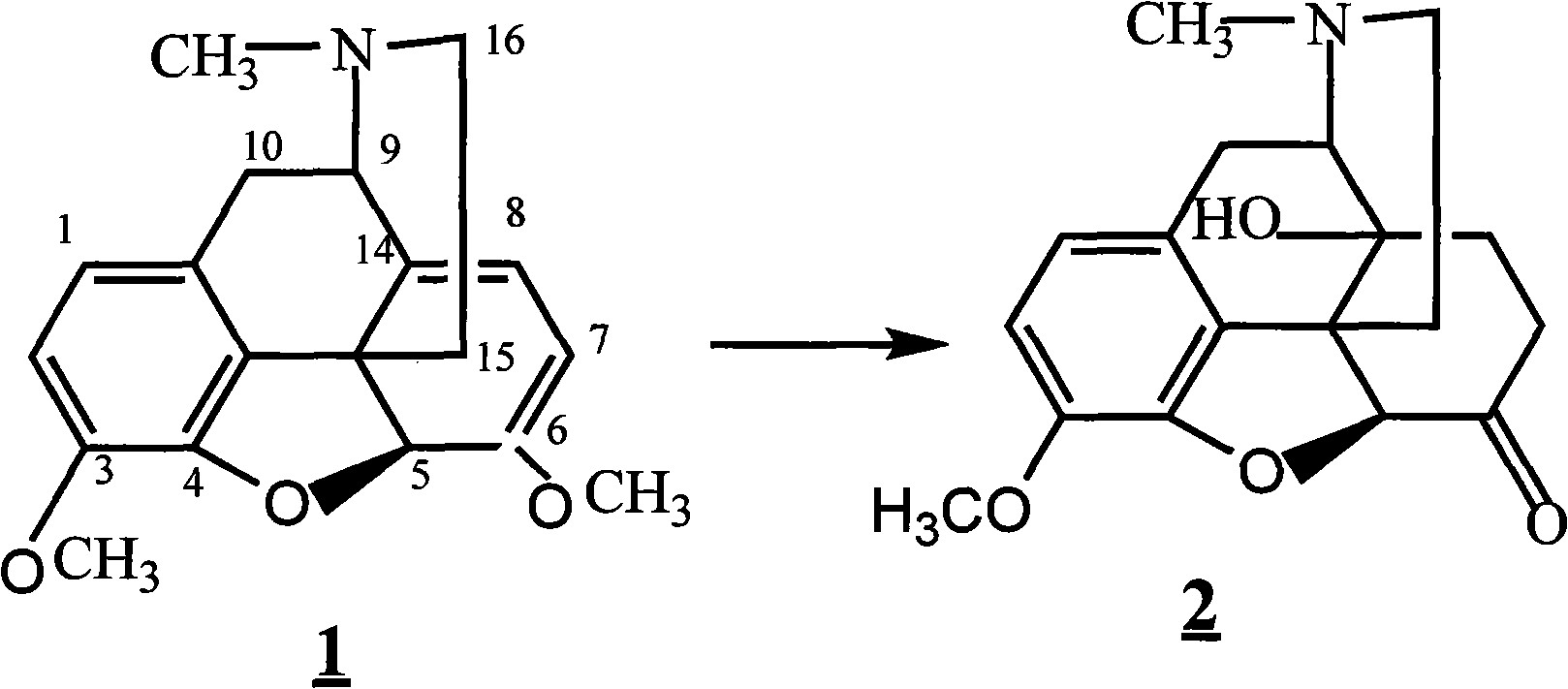
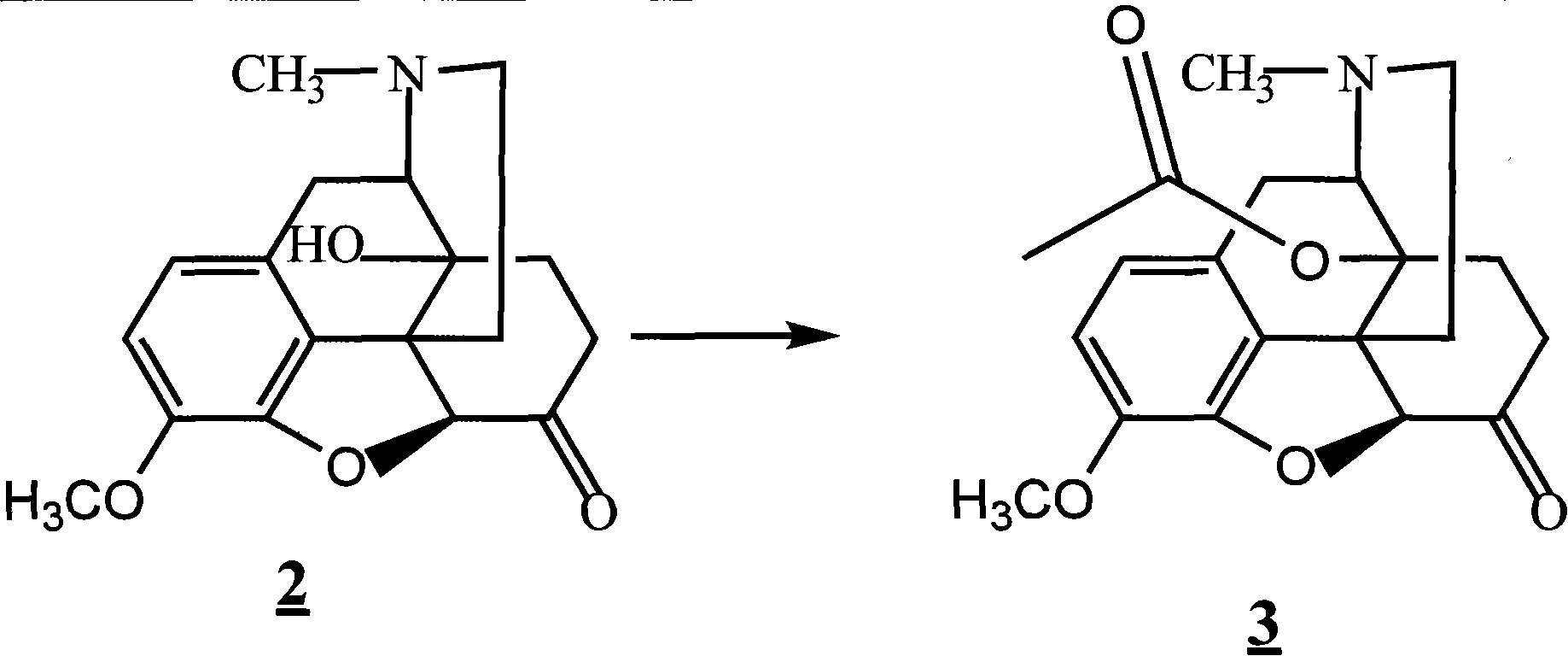
Method for synthesizing naloxone or naltrexone
The invention provides a method for synthesizing naloxone or naltrexone, which comprises the following steps of: dissolving thebaine in formic acid, uniformly stirring, dripping an oxidant, keeping the temperature of between 20 and 40 DEG C for 3 to 7 hours, displacing gas in a reaction vessel by inert gas serving as protective gas for 3 to 5 times, adding a metallic framework catalyst, displacing the gas by hydrogen for 3 to 5 times, keeping the temperature of between 25 and 45 DEG C and stabilizing a system for 7 to 13 hours to obtain a compound 2; reacting the compound 2 with acetic anhydride at the temperature of between 60 and 100 DEG C for 1 to 2 hours to obtain a compound 3; taking the inert gas as the protective gas, adding toluene, chloroformic acid-1-chloroethyl ester and potassium bicarbonate into the compound 3, heating to the temperature of between 75 and 100 DEG C and reacting for 20 to 40 hours, concentrating under reduced pressure until the system is fully dry, adding 10 percent hydrochloric acid, and heating and refluxing for 2 to 6 hours to obtain a compound 4; dissolving the compound 4 and at least one alkylation reagent in an organic solvent 1 and reacting with alkali at the temperature of between 50 and 100 DEG C to obtain a compound 5; and reacting the compound 5 with boron tribromide in an organic solvent 2 at the temperature of between -10 and 40 DEG C for 2 to 4 hours to obtain a compound 6, namely the naloxone or naltrexone.
Owner:甘肃普安制药股份有限公司
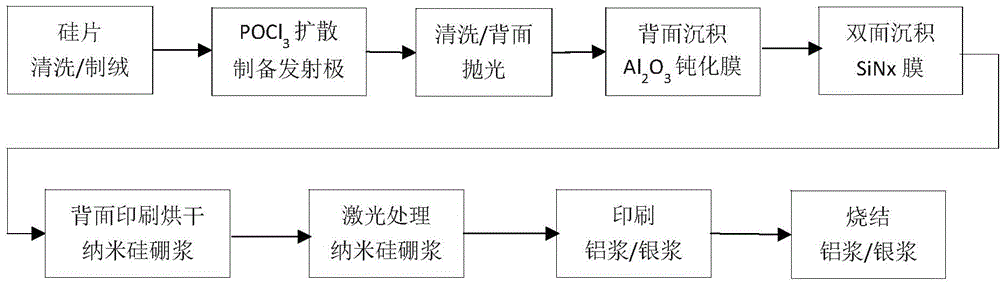
Nano silicon boron slurry and method for preparing PERL solar battery by utilizing nano silicon boron slurry
InactiveCN104638033ALimit industrial mass productionSimple processFinal product manufacturePhotovoltaic energy generationNano siliconScreen printing
The invention discloses nano silicon boron slurry and a method for preparing a PERL solar battery by utilizing the nano silicon boron slurry and a PERL battery structure. A silk screen printing technology which is suitable for the industrialized mass production is adopted, the nano silicon boron slurry is printed on the surface of a silicon chip, and the local boron diffusion is completed by virtue of high temperature diffusion or laser doping process route. A boron tribromide gas diffusion source with high toxicity is avoided, and the corresponding complicated technological procedures such as masking, etching, washing and the like for realizing the local boron diffusion is avoided. Compared with a produced PERC battery, the upgrading of the PERC battery can be completed, and the battery efficiency can be improved by only adding a printer and a drying furnace to the production process of the PERL battery.
Owner:苏州金瑞晨科技有限公司
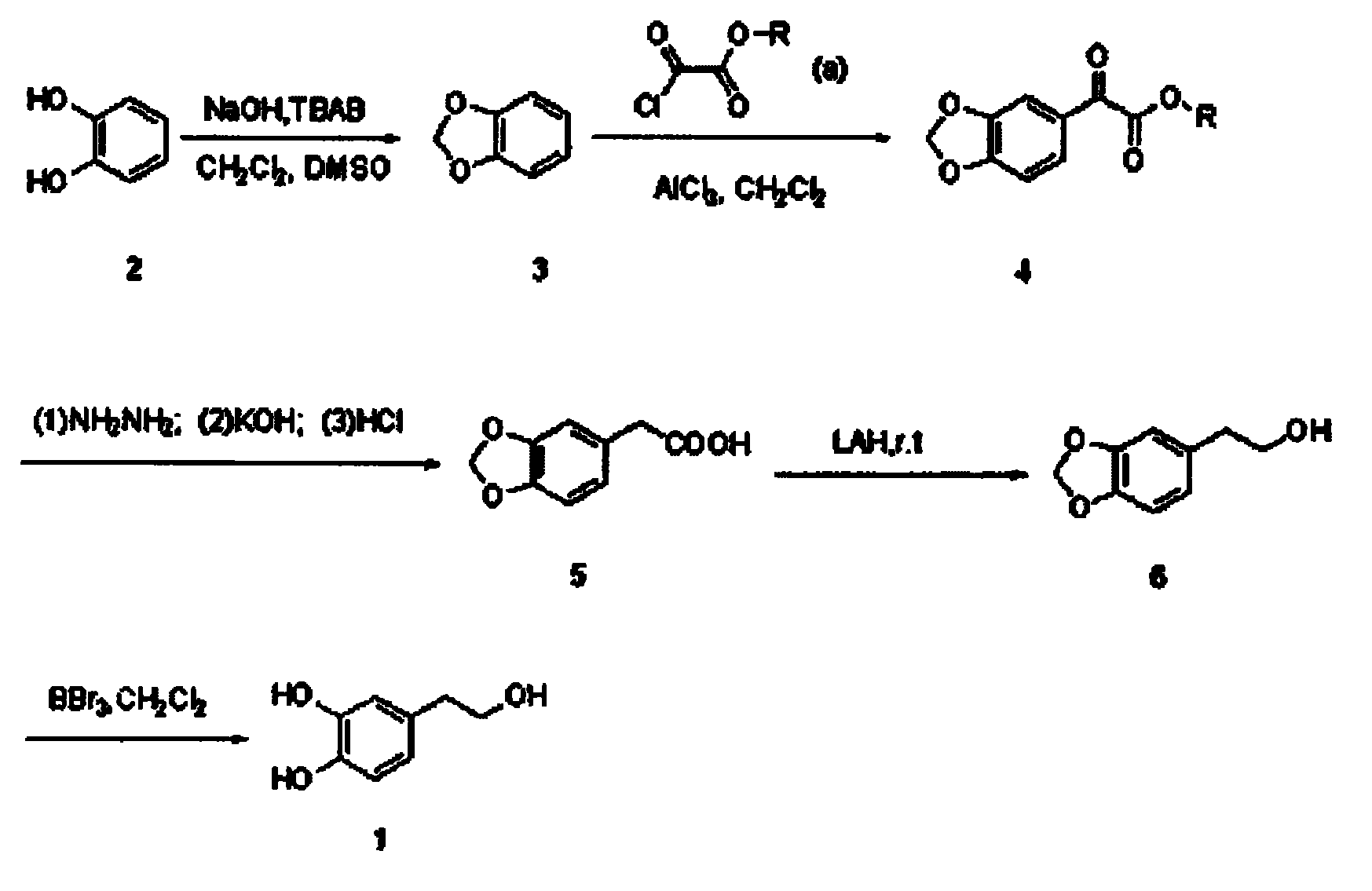
Synthetic method of hydroxytyrosol
InactiveCN103664536AMild reaction conditionsLower reaction costOrganic chemistryOrganic compound preparationChemical synthesisHydroxytyrosol
The invention belongs to the technical field of medicament synthesis, and in particular relates to a chemical synthetic method of hydroxytyrosol. The chemical synthetic method comprises the steps of (1) protecting two phenolic hydroxyl groups of catechol by using dichloromethane, and enabling catechol to react with dichloromethane to prepare 1,2-methylenedioxybenzene; (2) enabling 1,2-methylenedioxybenzene to react with various monoesters of oxalyl chloride to prepare 3,4-methylenedioxy phenylglyoxylic acid ester; (3) preparing 3,4-methylenedioxy phenylacetic acid by using 3,4-methylenedioxy phenylglyoxylic acid ester through a Wollff-kishner-Huang Minglong reduction reaction; and (4) reducing the 3,4-methylenedioxy phenylacetic acid by using lithium aluminum hydride, lithium borohydride or sodium borohydride to prepare 3,4-methylenedioxy phenethyl alcohol, and then removing methylene protection of the 3,4-methylenedioxy phenethyl alcohol by using boron tribromide or palladium on activated carbon to prepare hydroxytyrosol. A reactive reagent used in the synthetic method disclosed by the invention is easy to obtain and low in price, the reaction condition is mild, and the final yield of the whole reaction is 23%.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
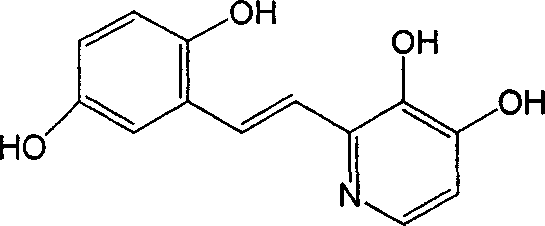
Polyhydroxy stilbenes compound preparation and uses as drugs for suppressing SARS
InactiveCN1736986AMeet the requirements of therapeutically effective doseOrganic chemistryHydroxy compound active ingredientsMethyl aldehydeTriethylphosphite
The invention provides a group of polyhydroxy stilbene compounds, their preparing process and use for suppressing and eradicating SARS coronavirus. The preparing process comprises, preparing phosphonic ester by reacting multi-alkyl substituted chloro (bromo) methoxyl or pyridine compounds with triethyl phosphate, then reacting phosphonate ester compound with multi-alkyl (oxy) phenylpyridine methyl aldehyde to obtain multi-alkyl (oxy) stilbene compounds. Finally acting with boron tribromide to obtain polyhydroxy stilbene compounds with substituent groups.
Owner:DALIAN UNIV OF TECH +1
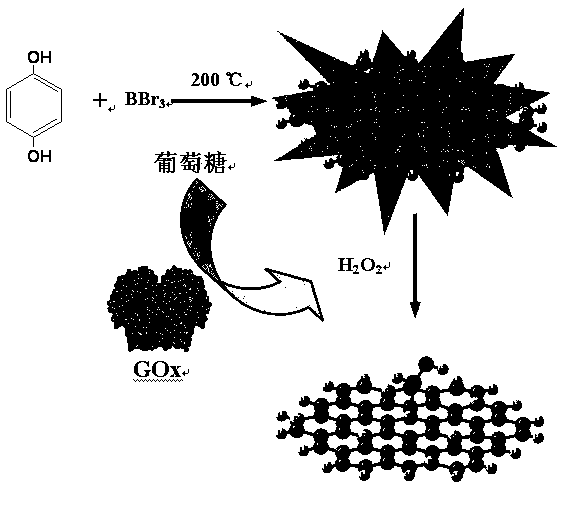
Method for preparing boron-doped carbon quantum dots by one-step solvothermal method and application of boron-doped carbon quantum dots
InactiveCN103881708AHigh fluorescence quantum yieldLow toxicityFluorescence/phosphorescenceLuminescent compositionsEvaporationBoron doped carbon
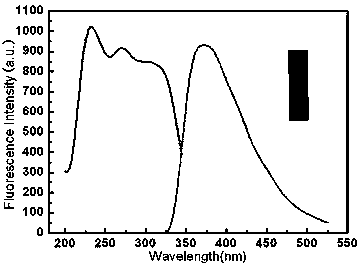
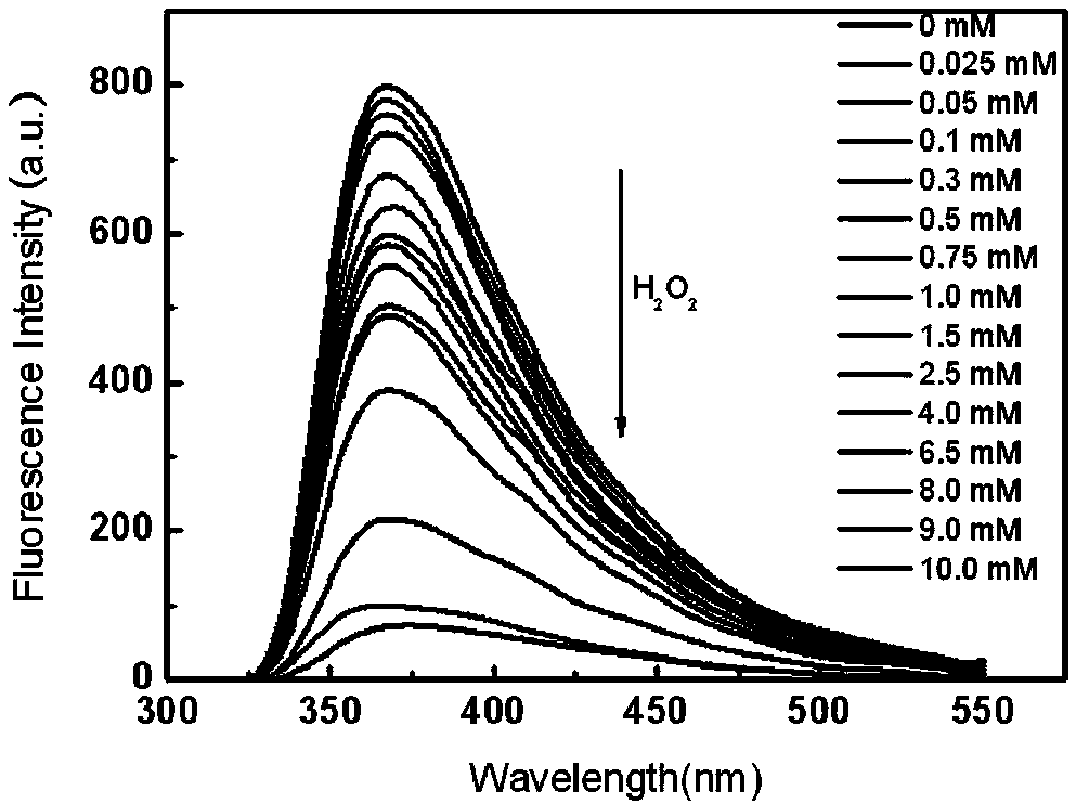
The invention relates to the field of carbon quantum dot preparation and in particular relates to a method for preparing boron-doped carbon quantum dots by a one-step solvothermal method and an application of the boron-doped carbon quantum dots. The preparation method comprises the steps of putting hydroquinone into a stainless steel high pressure reactor with a polytetrafluoroethylene lining, adding acetone according to the proportion of hydroquinone to acetone being 1.0g:5mL, and then dropping boron tribromide into the reactor slowly; putting the sealed reactor into an air dry oven, heating the reactor at 150-220 DEG C for 1-5 hours, and then cooling the reactor to the room temperature; concentrating and evaporating the mixed liquid in the reactor to dryness by using the mode of rotary evaporation, thus obtaining the boron-doped carbon quantum dots. The boron-doped carbon quantum dots prepared by the method can be used for detecting the contents of hydrogen peroxide and glucose.
Owner:ZHEJIANG NORMAL UNIVERSITY
Synthetic method of 6(4-hydroxyl ethyoxyl) cyclotriphophazene
InactiveCN102766168ASimple post-processingHigh reaction yieldGroup 5/15 element organic compoundsDistillationSodium hydride
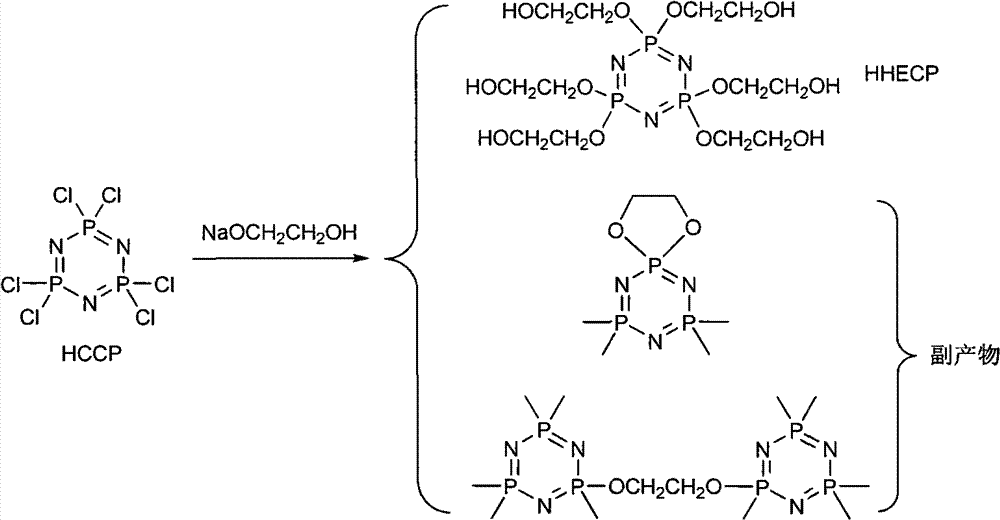
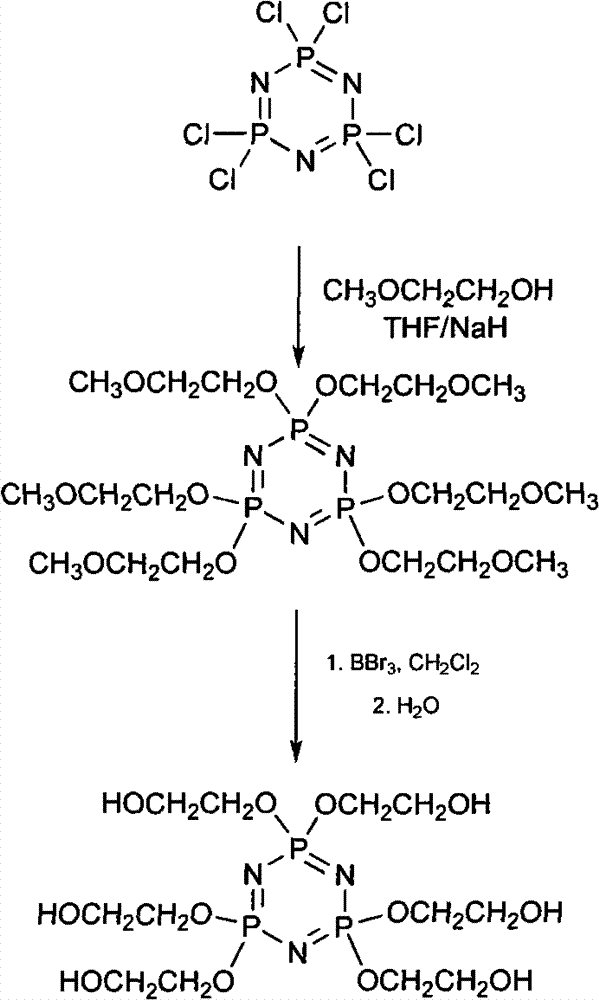
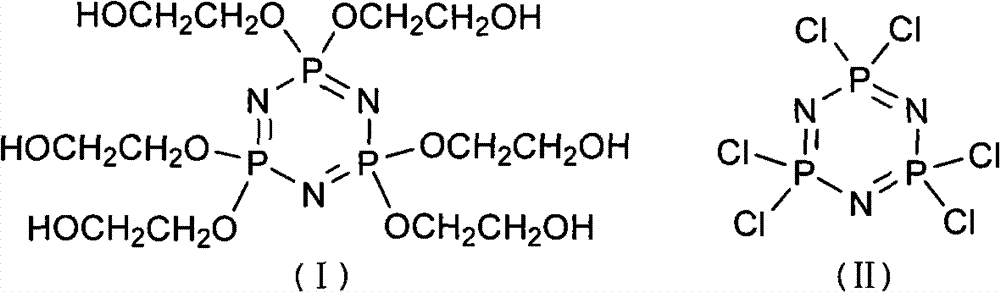
The invention discloses a synthetic method of 6(4-hydroxyl ethyoxyl) cyclotriphophazene. The method includes: (1) enabling methyl cellosolve and sodium hydride to react to obtain methyl sodium ethoxide, adding a tetrahydrofuran solution of hexachlorocyclotriphosphazene, reacting for 30min-45min at the temperature of 20-35 DEG C, filtering, removing solvent by steaming, extracting, washing and conducting reduced pressure distillation to obtain 6(4-methoxyl ethyoxyl) cyclotriphophazene; (2) enabling the 6(4-methoxyl ethyoxyl) cyclotriphophazene and boron tribromide to react for 2h-5h at the temperature of 0 DEG C-20 DEG C under the condition of stirring, after the reaction, adding water to conduct cancellation reaction, standing to separate a water phase, and conducting reduced pressure distillation to remove water to obtain the 6(4-hydroxyl ethyoxyl) cyclotriphophazene. The synthetic method of the 6(4-hydroxyl ethyoxyl) cyclotriphophazene is high in reacting yield and is mainly used for synthesis of the 6(4-hydroxyl ethyoxyl) cyclotriphophazene.
Owner:XIAN MODERN CHEM RES INST
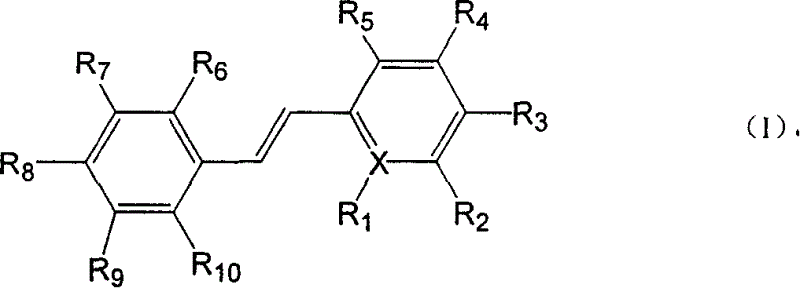
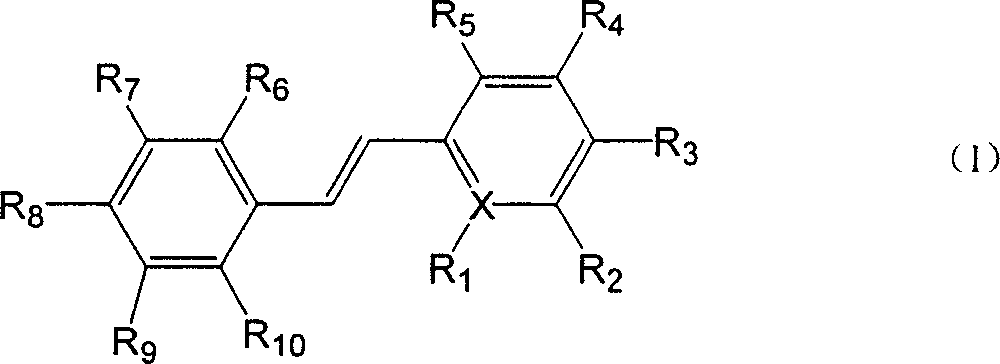
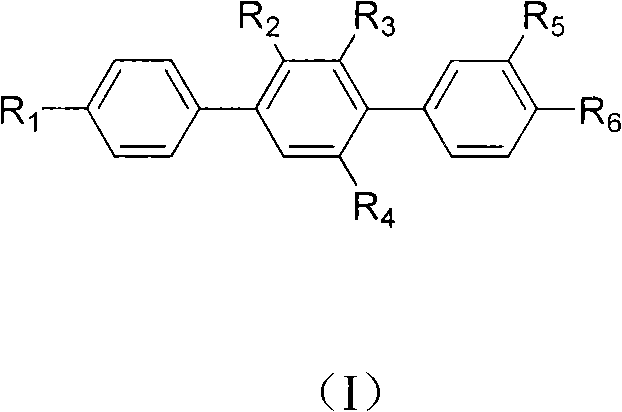
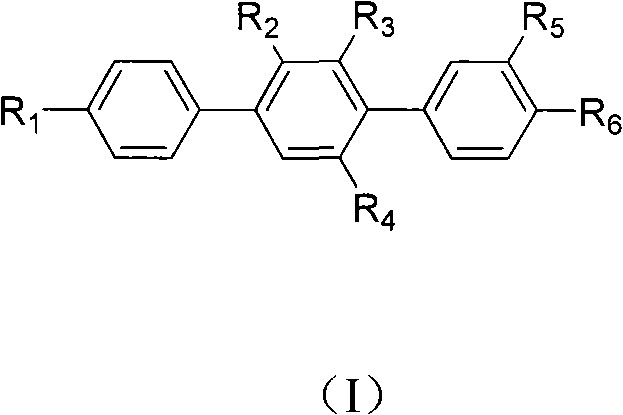
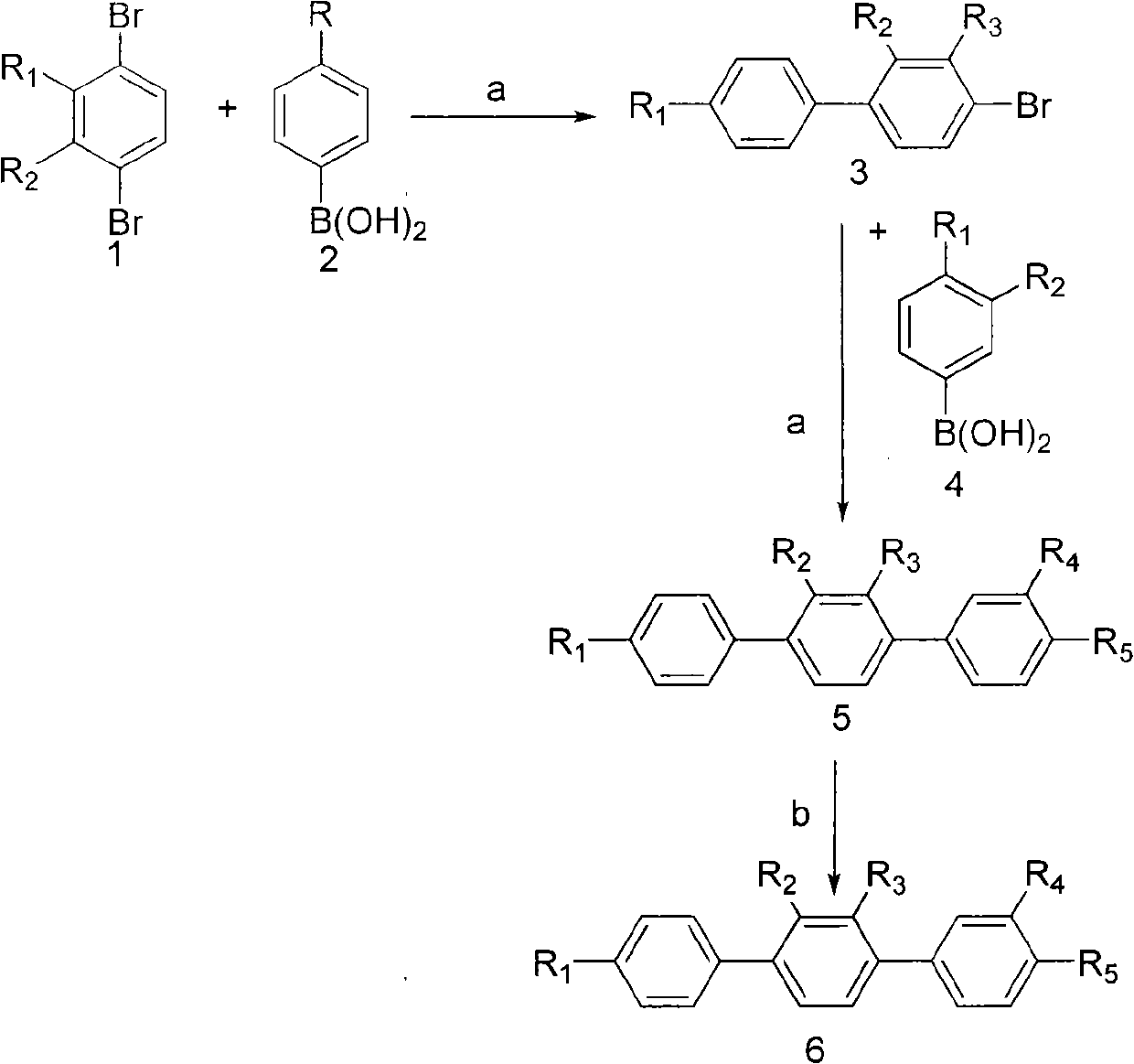
Paraterphenyl derivative and application thereof to preparation of antitumor medicaments
The invention discloses a paraterphenyl derivative. The paraterphenyl derivative has a compound structural formula shown as a formula (I) in the specification, wherein R1 is hydroxyl or methoxyl; R2 is hydrogen, hydroxyl or methoxyl; R3 is hydrogen, hydroxyl or methoxyl; R4 is hydrogen, hydroxyl or methoxyl; R5 is hydroxyl or methoxyl; and R6 is hydroxyl or methoxyl. The paraterphenyl derivative is obtained by coupling Suzuki with demthylating boron tribromide. A test shows that the paraterphenyl derivative has higher cytotoxin activity and potential of being developed into antitumor medicaments.
Owner:SHANDONG UNIV
Organic field effect transistor material based on oxa-condensed ring, and synthetic method and application thereof
ActiveCN106749318AImprove solubilityImprove mobilityOrganic chemistrySolid-state devicesSolubilityTrimethyltin chloride
Owner:SUZHOU JOYSUN ADVANCED MATERIALS CO LTD
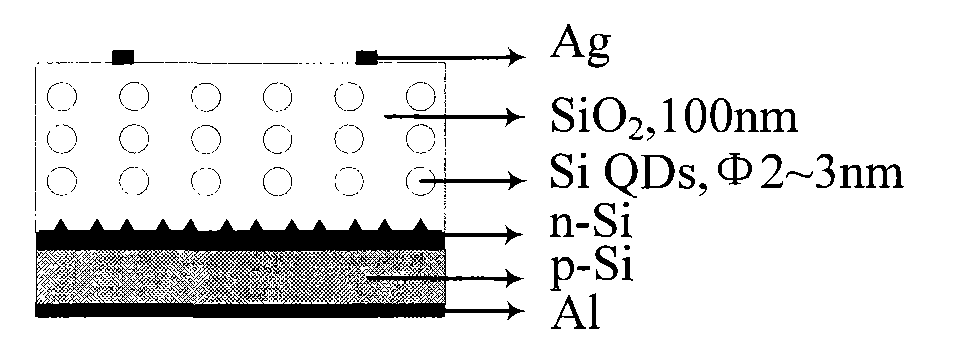
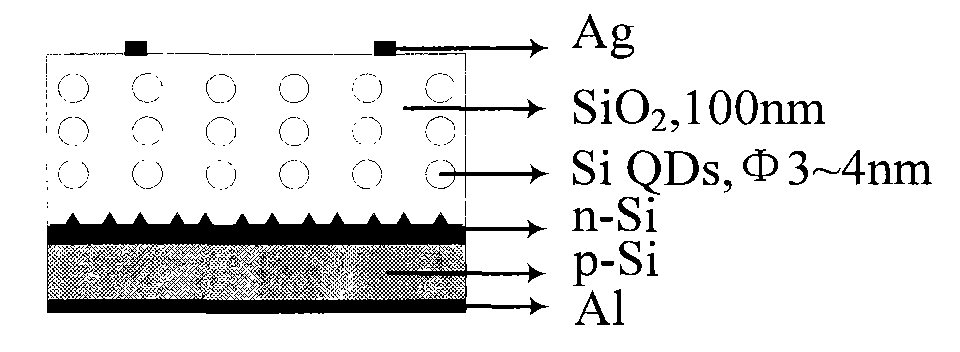
Silicon quantum dot solar cell and preparation method thereof
InactiveCN101834215AReduce usageEasy to prepareFinal product manufacturePhotovoltaic energy generationBack surface fieldSilicon dioxide
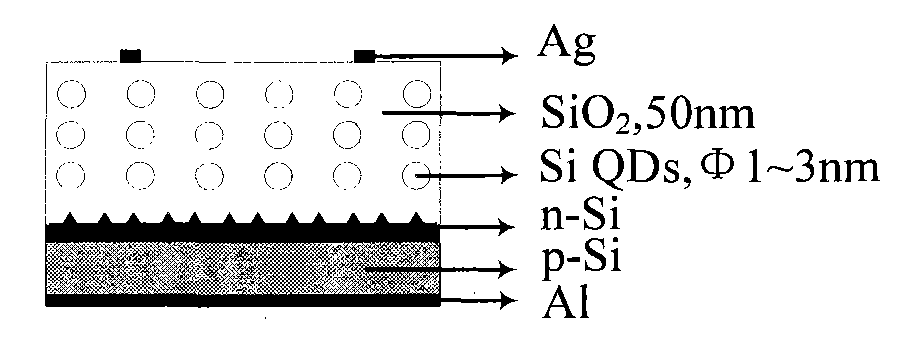
The invention relates to a novel silicon quantum dot solar cell which belongs to the field of solar cells and is characterized in that a silicon quantum dot layer is added on a pn junction of a traditional crystalline silicon cell. The structure of the solar cell is as follows: phosphorus oxychloride (or boron tribromide) is diffused on a napped p type (or n type) crystalline silicon substrate, then a silicon dioxide layer containing n type (or p type) silicon quantum dots is prepared, silver positive electrodes are finally respectively added on the front surface and the back surface, and an aluminum back surface field of a silver aluminum back electrode is embedded. The cell has simple structure, strong light absorption capacity and large photo-generated current, and the preparation steps are compatible with the preparation process of the existing crystalline silicon solar cell, thereby providing a good solution way for improving the conversion efficiency of the existing crystalline silicon cell.
Owner:TSINGHUA UNIV
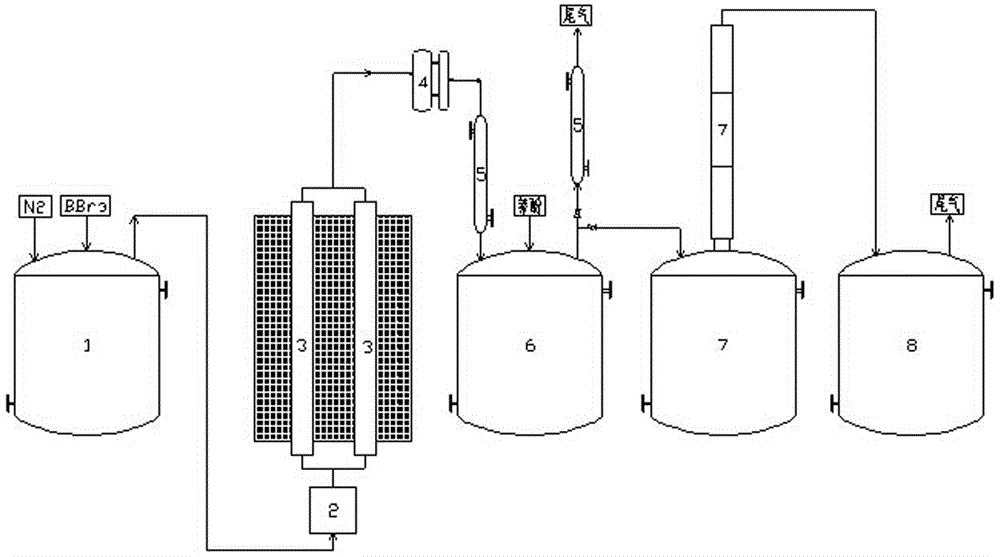
Method and device for preparing high-purity boron tribromide
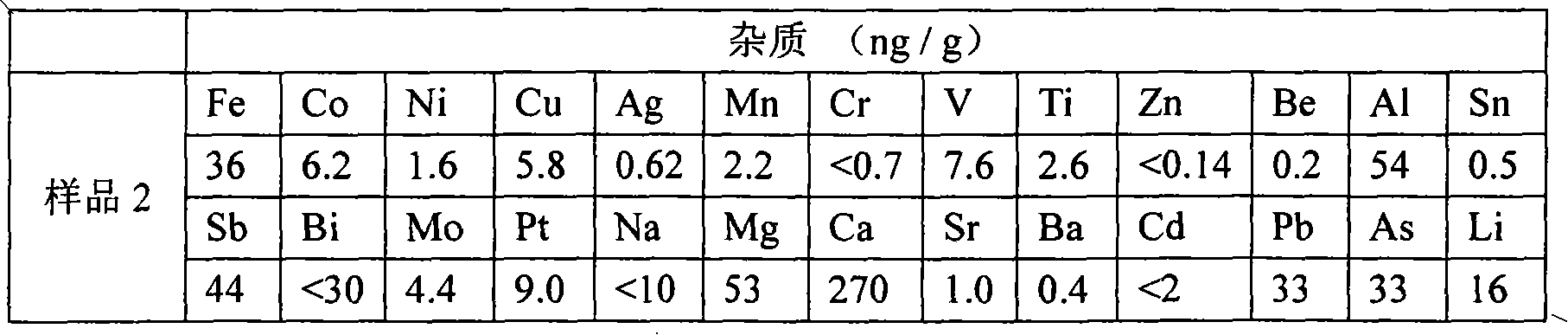
InactiveCN101955189ALess impuritiesHigh purityBoron halogen compoundsAcid washingReaction temperature
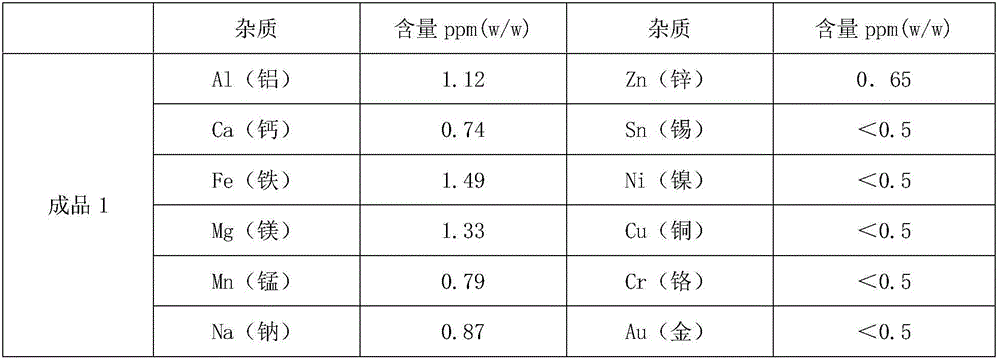
The invention provides a method and a device for preparing high-purity boron tribromide. The method comprises the following steps of: performing acid-washing on industrial boron powder to prepare boron blocks; performing separatory purification on pure chemical liquid bromine, and performing a reaction on the purified liquid bromine and the industrial boron blocks at the high temperature of between 600 and 850 DEG C in a bromination furnace to obtain boron tribromide liquid containing impurities; removing redundant Br2 in boron tribromide by using high-purity aluminum serving as a debromination agent to obtain white boron tribromide gas, and hydrocooling the white boron tribromide gas to form white boron tribromide liquid; and removing high-boiling point and low-boiling point impurities through fractional distillation of a fractional column to obtain a BBr3 product of which the purity is more than 6N, wherein the fractionation temperature of the boron tribromide is between 85 and 120 DEG C. The device for preparing the high-purity boron tribromide is formed by connecting a vaporizer, the bromination furnace, a bromine removing furnace, a collector, the fractional column and a finished product storage tank sequentially through a pipeline made of high-purity quartz glass. The produced boron tribromide has a few impurities and high purity which can reach over 6N, and the method has the advantages of readily available materials, low cost, simple device, small equipment investment and industrialized production.
Owner:GRIMAT ENG INST CO LTD
Preparation method of TTZ (thiotriazinone)
The invention discloses a preparation method of TTZ (thiotriazinone), which comprises the following steps: 1) adding 5-10 kg of acetic acid and 50-150 kg of ammonium acetate into 1,800-2,200 kg of ethyl alcohol to prepare a buffer system with the pH value of 6-7; 2) adding 480-500 kg of 2-methyl-3-thiosemicarbazide, boron tribromide and diethyl oxalate, heating to 80-82 DEG C, and performing circulation reflux reaction for 4-6 hours, wherein the molar ratio of 2-methyl-3-thiosemicarbazide to diethyl oxalate is 1:(1.15-1.25), and the dosage of boron tribromide is 4-6% of the weight of 2-methyl-3-thiosemicarbazide; 3) cooling for crystallization, and separating to obtain a TTZ crude product and a mother liquor; 4) adding 1,800-2,200 kg of water in the TTZ crude product, heating to 70-80 DEG C, then adding 1,250-1,400 kg of hydrochloric acid with the concentration of 30%, cooling for crystallization, separating and drying to obtain the TTZ finished product. The preparation method has the advantages that the common problems of high cost, low yield, large yield variability, instability of product quality, difficulty in solvent recovery, large amount of waste water and the like in the prior art can be solved.
Owner:SHANDONG HUIHAI PHARMA & CHEM
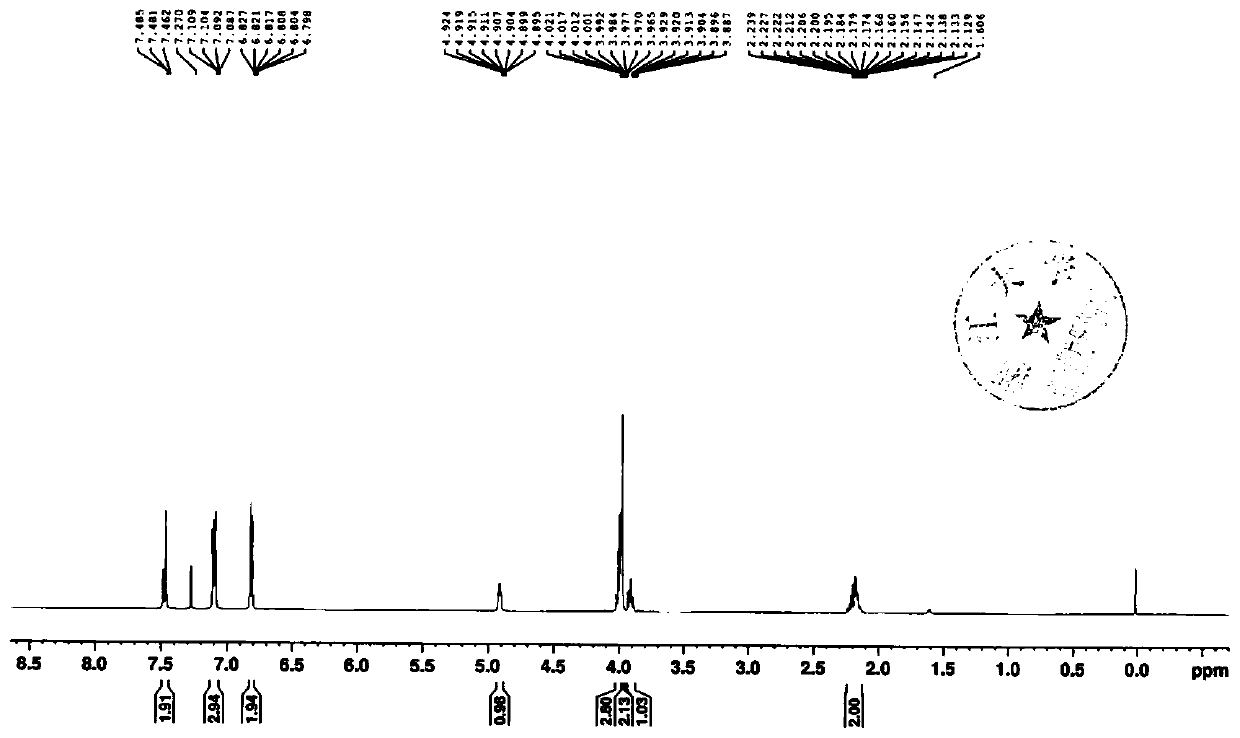
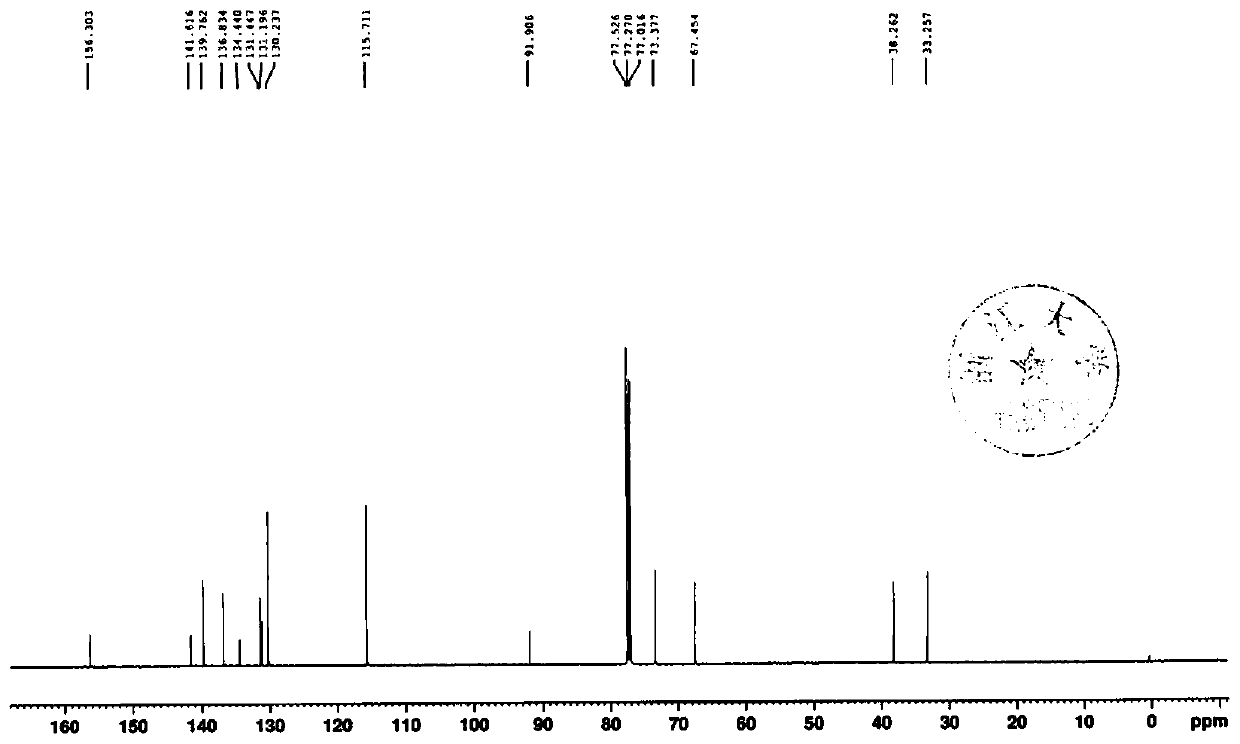
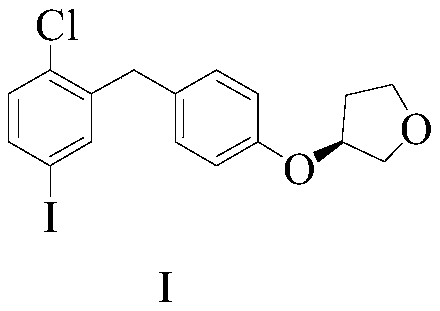
Empagliflozin intermediate preparation method
The invention discloses an empagliflozin intermediate preparation method, which comprises: carrying out substitution by using p-methoxybenzyl chloride and p-iodoaniline as starting raw materials to obtain a compound IV, performing diazotization and a Sandmeyer reaction to obtain a compound III, further performing demethylation under the action of boron tribromide to obtain a compound II, and finally performing condensation with (S)-3-p-toluenesulfonyloxy tetrahydrofuran to obtain the target compound I, wherein the product purity is greater than or equal to 99.0%. According to the invention, the preparation method has advantages of simple and easily available raw materials, low cost, simple operation steps, simple post-treatment and high product yield, and is suitable for industrial production.
Owner:ZHEJIANG HUAYI PHARMA CO LTD OF HANGZHOU HUADONG PHARMA GRP +1
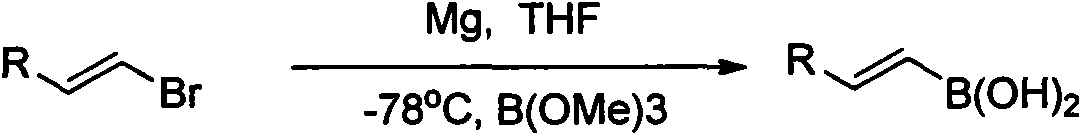
Synthesis and application of novel vinyl boronizing reagent
ActiveCN104211723AImprove processing stabilitySimple and fast operationGroup 3/13 element organic compoundsHydrogen fluorideSynthesis methods
The invention relates to synthesis and application of a novel vinyl boronizing reagent, the reagent can be coupled with other organic boric acid to obtain trans potassium alkenyltrifluoroborate in various forms. The synthesis method is as below: slowly introducing propyne into a dichloromethane solution of boron tribromide at -10 to 0 DEG C; directly adding potassium fluoride aqueous solution or quenching under acidic conditions to obtain boric acid; then directly addingg potassium hydrogen fluoride to obtain a target boronizing reagent trans-bromoethenyl trifluoro borane potassium (1), which is a white flake solid, wherein the yield in the step is 71-82%. The boronizing reagents can be subjected to Suzuki coupling to be effectively applied in synthesis of trans potassium alkenyltrifluoroborate (3) in different forms. The invention has the advantages of high stereoselectivity and strong stability of the product, suitability for long time storage, and simple, fast and high yield synthesis method.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
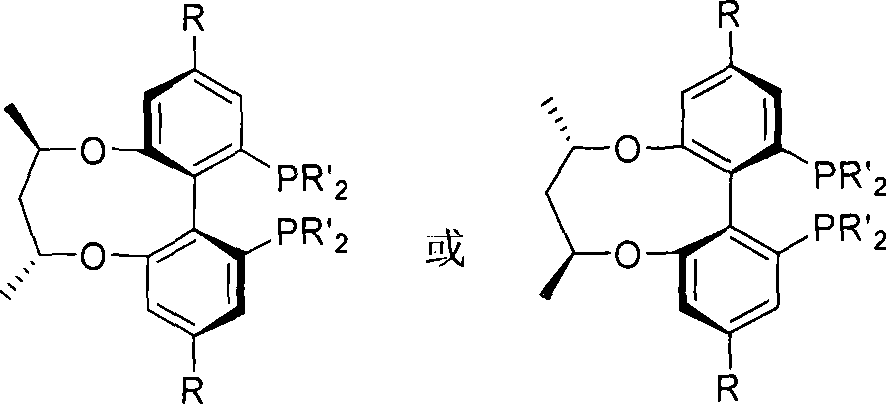
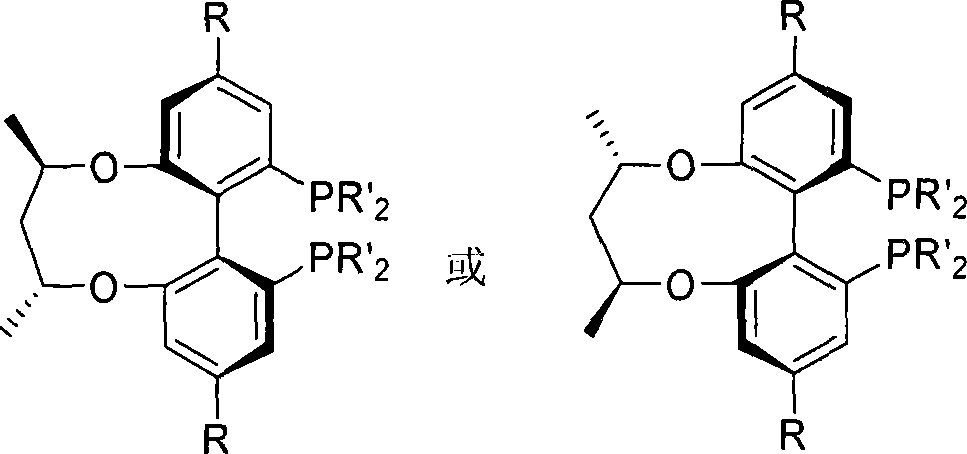
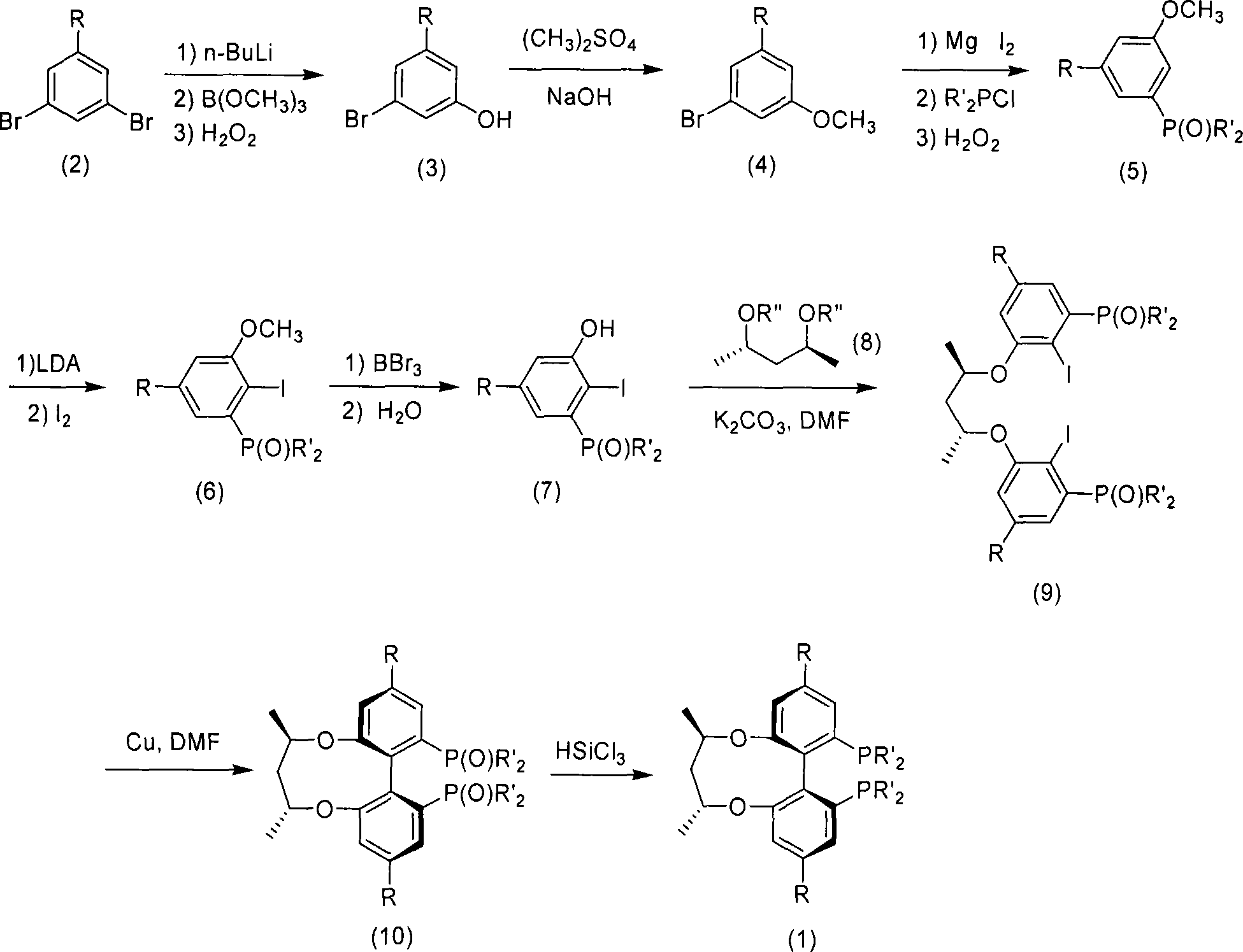
Central chirality induced axial chirality diphosphine ligand and method for synthesizing same
InactiveCN101230075AAvoid splittingEasy to synthesizeGroup 5/15 element organic compoundsDiphosphinesStructural formula
The invention relates to central chiral induced axial chiral diphosphine ligand, which adopts the structural formula that in the formula, R1 is benzyl or substituted benzyl; R is tertiary butyl or trifluoromethyl. The invention adopts the preparation method that 1-R-3, 5-dibromo benzene is used as the raw material, to produce 3-R-5- bromophenol; and then methylation is performed to obtain 1-R-3- bromine -5- anisole; after lithium realization of 3-dihydro pjosphinyl-5-R methyl phenoxide which reacts with dihydro phosphonic chloride and is obtained through oxidation, the product of the reaction with iodine reacts with boron tribromide, 2-iodine-3-dihydro pjosphinyl-5-R phenol obtained through demethylation and then reacts with central chiral 2, 4- pentanediol derivative, to obtain 2, 4-di (2-iodine-3- dihydro pjosphinyl-5- substituent phenoxy oxygen) pentane; <6, 61 -(2, 4- pentanediol oxygen)>-(4, 41)- disubstituent -(2, 21)-di (dialkyl pjosphinyl)-(1, 11)- biphenyl is produced through coupling, and finally reaction is performed with tri-butylamine and trichlorosilane, to obtain the diphosphine ligand.
Owner:WUHAN UNIV
Method for synthesizing bisdiboron
ActiveCN104151342AHarm reductionEasy to operateGroup 3/13 element organic compoundsChemical recyclingTriethylamine hydrobromideHydrogen
The invention relates to a method for synthesizing bisdiboron. The method comprises the following steps: preparing a tri-substituted boron midbody from raw materials, namely, nafoxidine and boron tribromide which are easy to obtain from the market, in the presence of an acid-binding agent triethylamine; subsequently reacting with boron tribromide to obtain a bromo-boron midbody; coupling the product in the presence of metal sodium to obtain nafoxidine-substituted coupled boron; finally adding pinacol to react, thereby obtaining a target product, namely, the bisdiboron. The method has the advantages that nafoxidine can be directly recycled, and the triethylamine hydrobromide can be also directly recycled after being simply neutralized and dried. The midbodies obtained in the method disclosed by the invention can be directly used after the solvent is simply distilled, except the compound, the midbodies are all solids when being purified, the operation is easy, the solvent and the reagents can be recycled, the damage to the environment is reduced, and the process can be successfully expanded to be the scale greater than 10kg.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
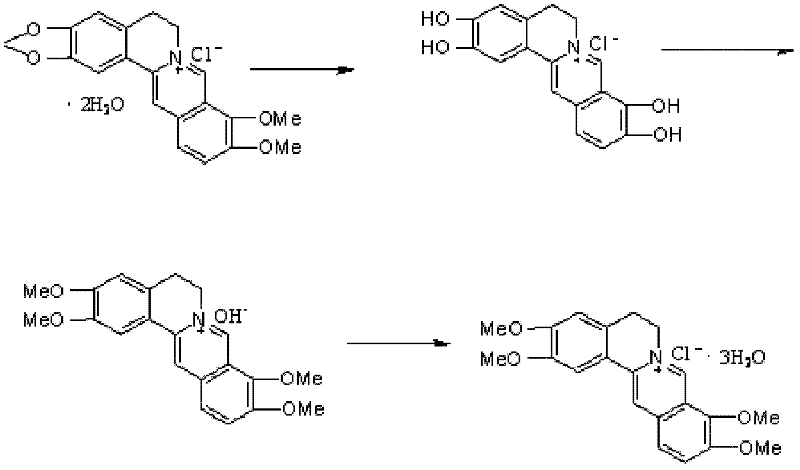
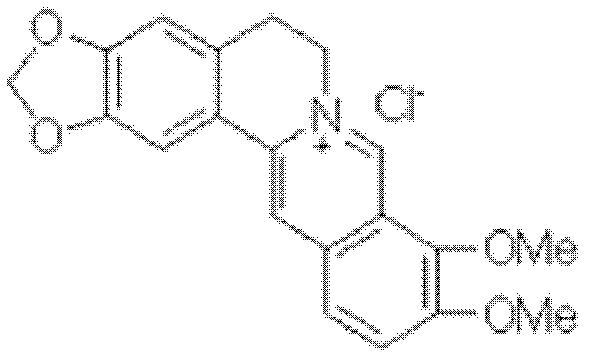
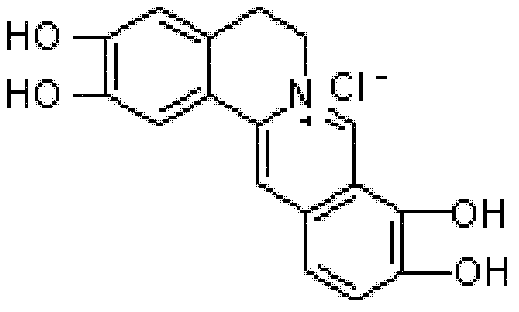
The preparation method of palmatine
ActiveCN102285981ASave resourcesShort reaction stepsOrganic chemistryChemical recyclingChemical synthesisAluminium chloride
The invention discloses a preparation method of fibrauretin which is applied in the chemical synthesis field of broad-spectrum antibiotics. The preparation method comprises the following steps: adding berberine hydrochloride in an organic solvent, and reacting at 100-150 DEG C under the catalysis of a Lewis acid to remove methylene and obtain an intermediate 2,3,9,10-tetrahydroxyberberine; placing the intermediate 2,3,9,10-tetrahydroxyberberine in a basic solvent to react under the action of a methylating agent at 30-40 DEG C and obtain an intermediate fibrauretin base; and heating the intermediate fibrauretin base in distilled water to dissolve, and adding hydrochloric acid to form the salt and obtain fibrauretin, wherein the organic solvent is selected from one of toluene and xylene, the Lewis acid is selected from one of aluminium chloride, boron tribromide and zinc chloride, and the methylating agent is dimethyl sulfate. The chemical synthesis method is adopted to prepare fibrauretin, thus the plant resource can be saved and the ecological environment can be protected; and berberine hydrochloride is used as the raw material, the raw material is cheap and easily available, the reaction steps of the synthetic route are short and the operations are simple, thus the cost can be saved and the method is suitable for industrial production.
Owner:NORTHEAST PHARMA GRP
Synthon and method for preparing dibenzocoronene compounds by same
ActiveCN103570714AImprove solubilityEasy to purifyLiquid crystal compositionsOrganic chemistryCoroneneSynthon
The invention relates to a synthon and a method for preparing dibenzocoronene compounds by the same. The synthon is a tetrahydroxy-substituted perylene bisimide compound, and has a structure as shown in general formula (I), wherein R is a C1-C18 linear chain primary alkyl or a primary alkyl with a branched chain. According to the invention, dibromo-perylene anhydride or mixtures are used for preparing dibromo-imide; dibromo-imide and phenylboronic acid substituted by alkoxys at 3 and 4 positions are subjected to a Suzuki coupling reaction under the catalysis of Pd(PPh3)4 to prepare aryl-substituted perylene bisimide compounds; further dealkylation reaction is carried out under the action of boron tribromide so as to form tetrahydroxy-substituted perylene bisimide compound synthon; then the synthon is used as a raw material to synthesize charge transfer compounds of coronene compounds with imide groups at two sides of the molecule. The synthetic method of the invention is simple, high in yield, and widely applicable to organic light-emitting diodes, field effect transistors, and solar cells. The general formula (I) is shown in the description.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
Bis(pinacolato)diboron production process
ActiveCN102558209AHigh reaction conversion rateImprove securityGroup 3/13 element organic compoundsBoron trichlorideReaction temperature
The invention discloses a bis(pinacolato)diboron production process which is characterized in that boron trichloride is used as a raw material and is aminated with dimethylamine gas in an n-hexane system, the desalinated reaction solution and boron tribromide react at the room temperature, the prepared intermediate and magnesium are coupled in toluene, 1, 2-dichloroethane solution of pinacol is dropped into the desalinated reaction solution, and the target product is prepared via transesterification. The invention has the advantages that: n-hexane is used as a reaction solvent for the first two steps, the conversion rate of reaction is increased, and the ultra low temperature can be avoided; the safety of the process is improved due to use of magnesium; and the yield of the product is increased in transesterification through controlling the reaction temperature.
Owner:HAIMEN RUIYI MEDICAL TECH
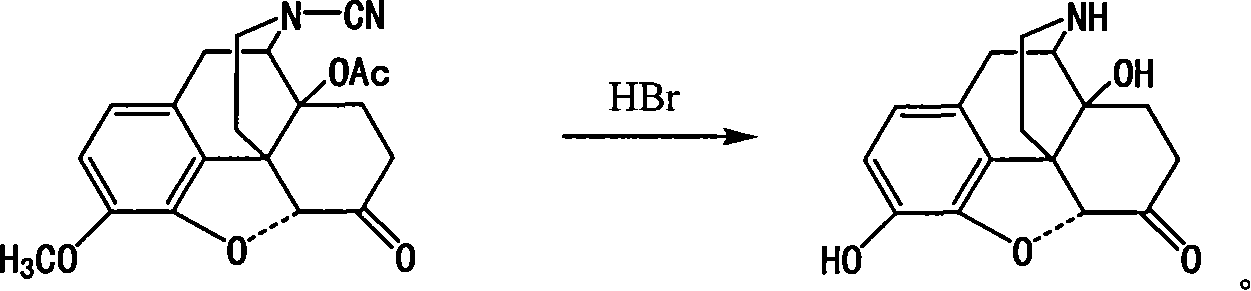
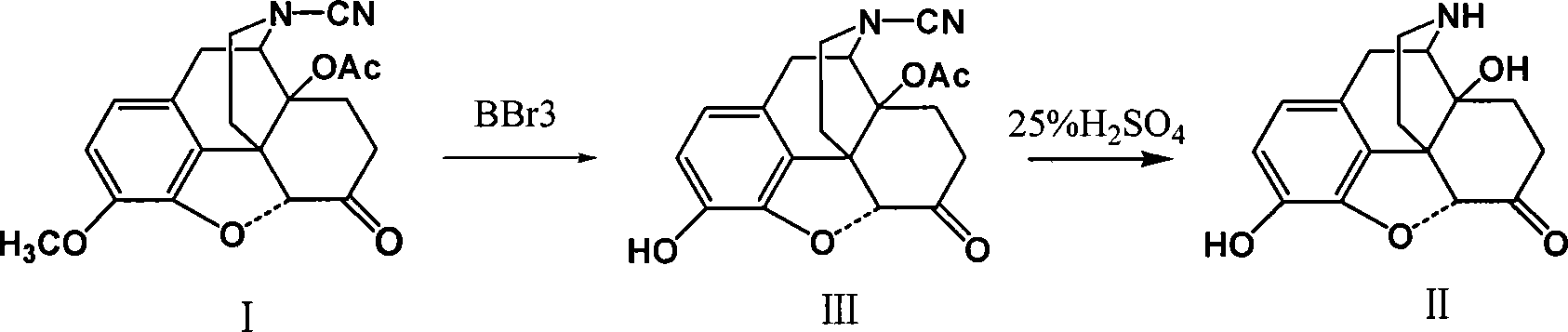
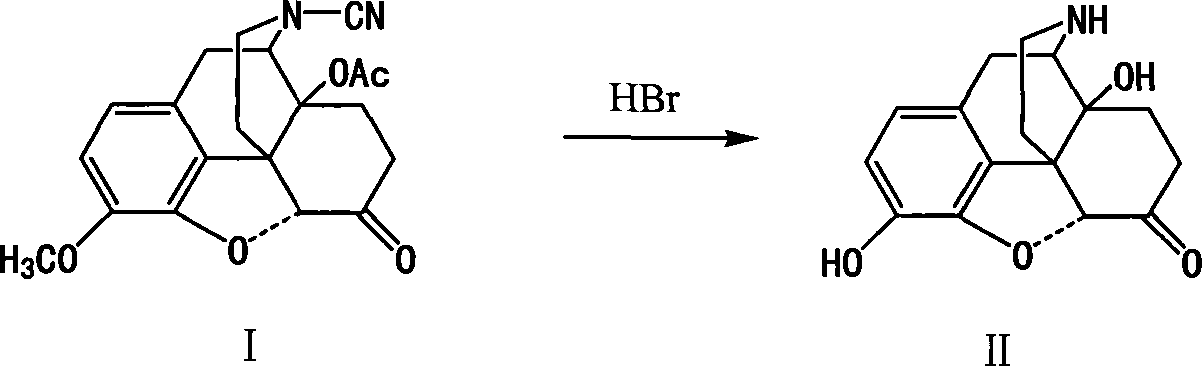
Method of preparing 14-hydroxy-7,8-dihydromorphone
This invention discloses a method for preparing 14-hydroxide-7, 8-One drop of morphine dihydro, which takes 14-acyloxy-17- Cyano-7, 8-dihydro - codeine drop one as the raw material, hydro-bromic acid as the deoxidation methyl and hydrolyzation reaction reagent to process14-acyloxy-7, 8-dihydro-morphia ketone.
Owner:FUDAN UNIV
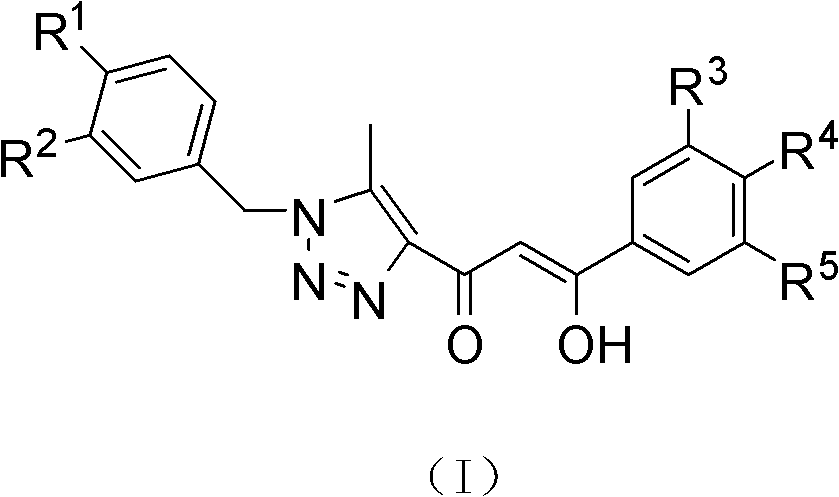
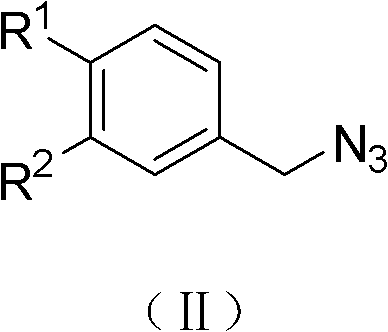
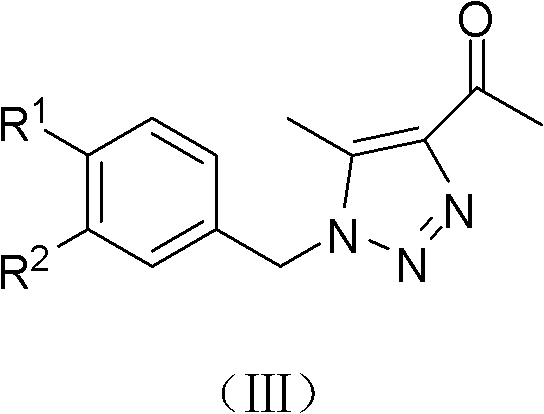
(Z)-1-(1-substituted benzyl-5-methyl-1H-1,2,3-triazole-4-yl)-3-substituted benzyl-3-hydroxy-2-propylene-1-ketone compound and preparation method and application thereof
InactiveCN102503900AMild reaction conditionsOrganic active ingredientsOrganic chemistryBenzoyl bromideCarboxylic acid
Owner:BEIJING UNIV OF TECH
Sulfonated perfluorocyclobutyl polyarylether polymers as well as preparation method and application thereof
ActiveCN104277216AEasy to prepareRaw materials are easy to getSolid electrolyte fuel cellsFuel cell detailsPolymer sciencePhenol
The invention discloses sulfonated perfluorocyclobutyl polyarylether polymers as well as a preparation method and application thereof, belonging to the technical field of fuel cells. The preparation method of sulfonated perfluorocyclobutyl polyarylether polymers comprises the following steps: preparing a trifluorovinyl aryl ether monomer containing methoxy by taking different methoxy aromatic dihydric phenols and 1,2-dibromotetrafluoroethane as raw materials; obtaining perfluorocyclobutyl polyarylether polymer containing methoxy by utilizing (2phi+2phi) thermal cyclization polymerization; reacting the perfluorocyclobutyl polyarylether polymer with boron tribromide so as to remove a protecting group methyl to prepare perfluorocyclobutyl polyarylether polymers with different hydroxyl contents; introducing an sulfonation lateral group by adopting a grafting method to obtain the sulfonated perfluorocyclobutyl polyarylether polymers which are used for preparing a proton exchange membrane. A 5% weight loss temperature of the obtained sulfonated perfluorocyclobutyl polyarylether polymers can be over 217 DEG C. The sulfonated perfluorocyclobutyl polyarylether polymers have the advantages of low water absorption, low swelling rate, high proton conductivity, and can be used as proton exchange membrane material of a fuel cell.
Owner:GUANGXI UNIV
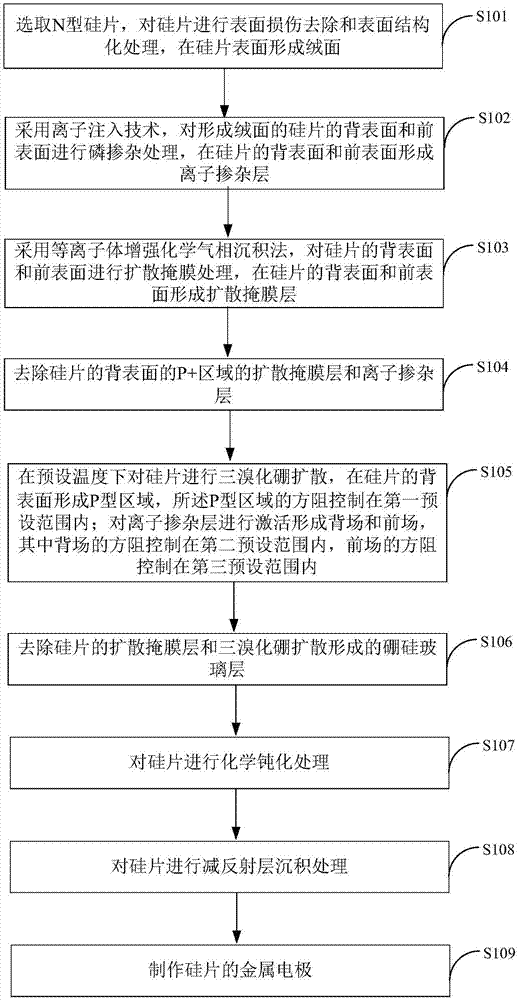
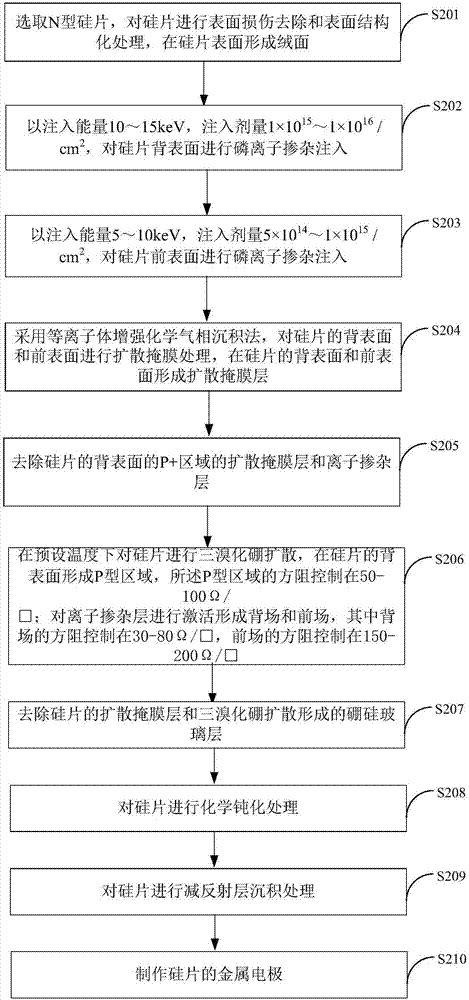
Preparation method of IBC battery
InactiveCN107275443AThe production process is simpleReduce manufacturing costFinal product manufacturePhotovoltaic energy generationBiological activationSolar cell
The invention is applicable to the technical field of the preparation of solar cells. The invention provides a preparation method of an IBC battery. Through using the ion implantation technology, a back surface and a front surface of a silicon wafer which forms a suede are subjected to phosphorus doping treatment. The plasma enhanced chemical vapor deposition method is used to carry out diffusion mask processing on the back surface and the front surface of the silicon wafer. A diffusion mask and an ion-doped layer in a P+ area on the back surface of silicon wafer are removed. The boron tribromide diffusion of the silicon wafer is carried out in a preset temperature, a P type area is formed at the back surface of the silicon wafer, and the square resistance of the P type area is controlled in a first preset range. An ion doping layer is activated to form a back field and a front field, the square resistance of the back field is controlled in a second preset range, and the square resistance of the front field is controlled in a third preset range. According to the embodiment of the invention, the ion implantation can be realized to carry out phosphorus doping treatment, the high temperature of the boron tribromide diffusion is used to realize the activation of the ion implantation impurities, a flow of IBC battery manufacturing can be simplified, and the production cost is reduced.
Owner:YINGLI ENERGY CHINA
2-phenylnaphthalene derivative and application thereof in preparation of anti-tumor medicaments
InactiveCN103193601ASimple structureEasy to synthesizeHydroxy compound active ingredientsOrganic compound preparationProstate cancerStructural formula
The invention discloses a 2-phenylnaphthalene derivative. The structural formula is as shown in formula (I), wherein R1 is hydrogen, R2 is hydroxy or hydrogen, R3 is hydroxy or hydrogen, R4 is hydrogen, R5 is hydrogen, R6 is hydrogen, R7 is hydroxy or hydrogen, R8 is hydroxy or hydrogen and R9 is hydrogen. The compound is obtained by utilizing suzuki coupling and boron tribromide demethylation. Tests prove that the 2-phenylnaphthalene derivative disclosed by the invention can significantly inhibit proliferation and migration of tumor cells, has development potential in preparation of medicaments for resisting breast cancer, breast ductal cancer, lung cancer, cervical cancer, prostatic cancer, colon cancer and gastric cancer, and has good application prospects.
Owner:SHANDONG UNIV
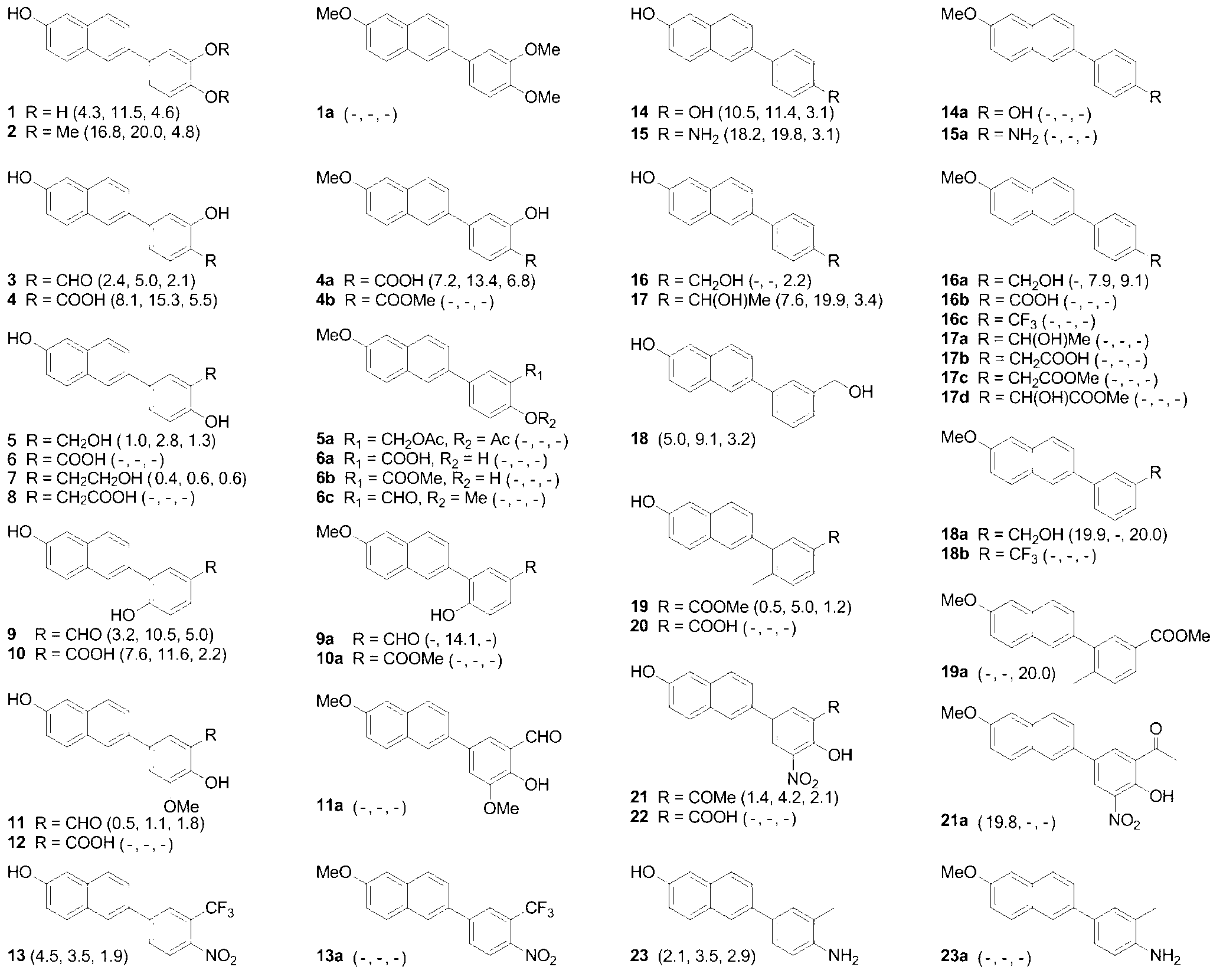
Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate
The invention belongs to the field of chemical synthesis of drugs, particularly relates to a synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and an intermediate and aims to provide a synthesis method of a compound (1). The method comprises steps as follows: a, a compound (4) and boron tribromide react, and a compound (3) is synthesized; b, the compound (3) and difluorodibromomethane have a ring closing reaction in the presence of a phase-transfer catalyst, and a compound (2) is synthesized; c, the compound (2) is hydrolyzed, and the compound (1) is synthesized. According to the synthetic method and the intermediate, one novel compound (4) is provided, one novel preparation method of the compound (1) is obtained, with the adoption of the method, not only can the overall yield of the reaction be increased, but also expensive reaction raw materials can be avoided, and the reaction cost is reduced to the great extent; besides, according to the method, utilization of a palladium catalyst and a dangerous sodium cyanide reagent can further be avoided, the safety of pharmaceutical production is guaranteed, and the method is applicable to industrial production.
Owner:ZHEJIANG YONGNING PHARMA
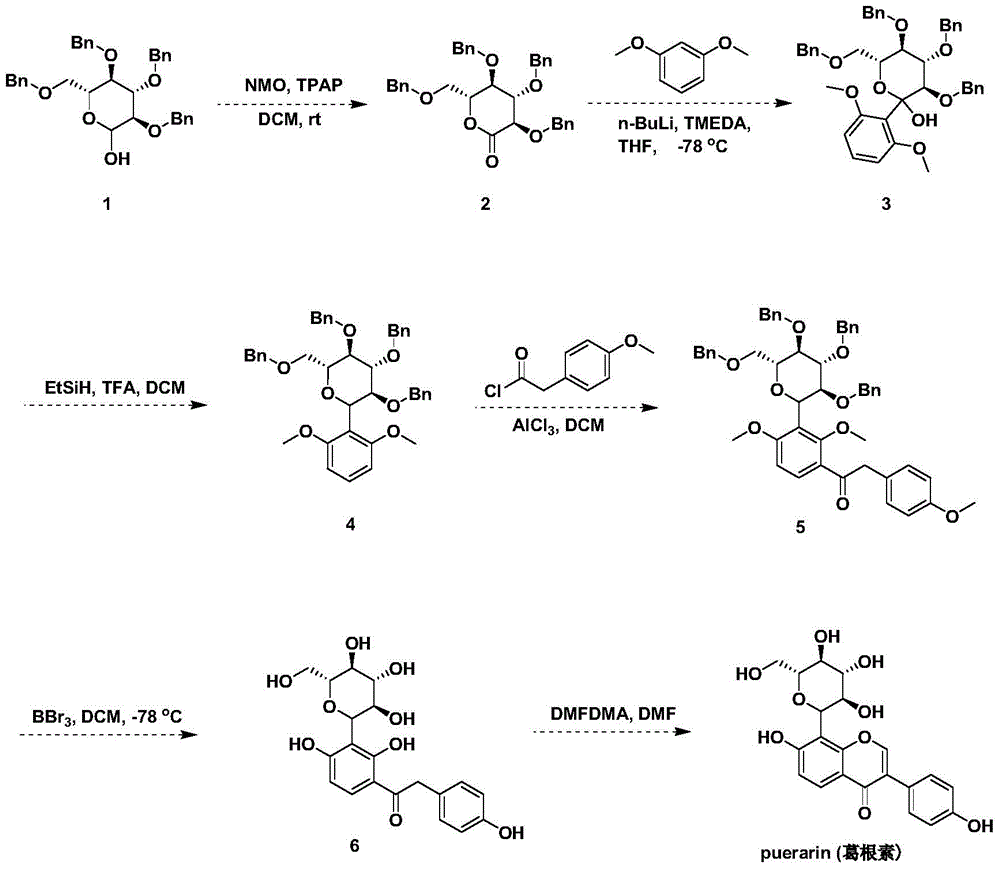
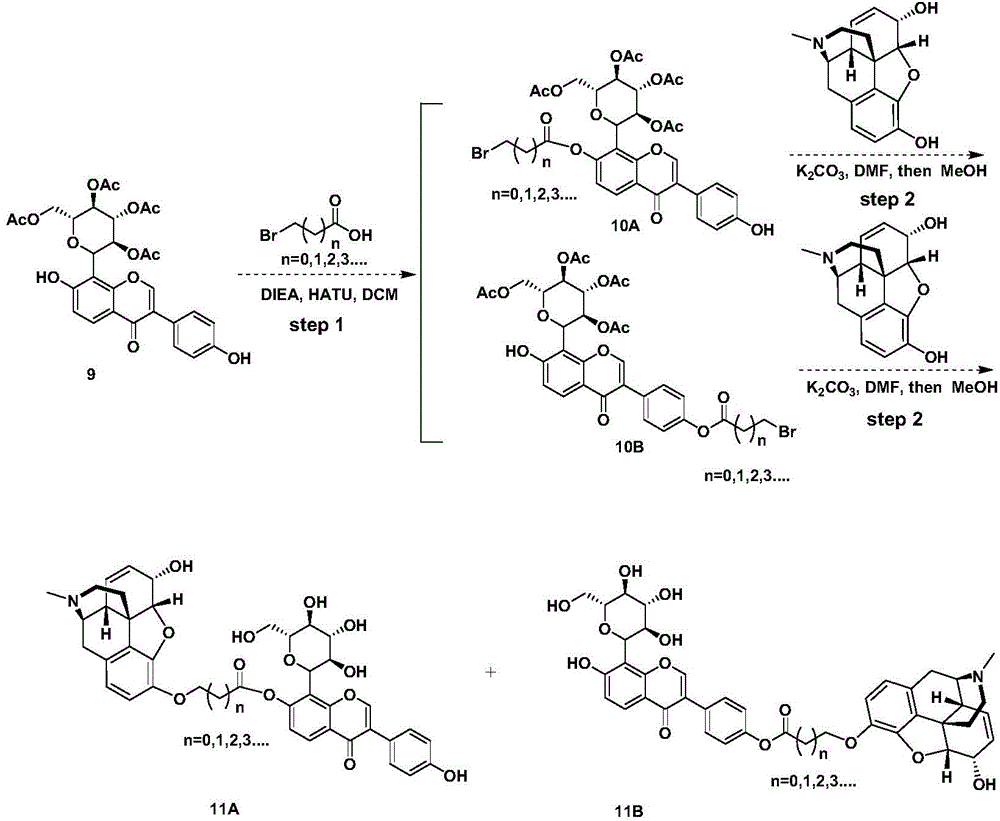
Preparation method for puerarin morphine
InactiveCN104876951AReduce dosageReduce addictionNervous disorderOrganic chemistrySide effectMorphine
The invention discloses a preparation method for puerarin morphine. The preparation method comprises the following steps: (1) the synthesis of puerarin, to be specific, 1) preparing a compound 2 by taking a compound 1 as a raw material; 2) modifying the compound 2 to prepare a compound 3; 3) removing hydroxide radicals of the compound 3 to prepare a compound 4; 4) enabling the compound 4 to react with p-methoxyphenylacetyl chloride to prepare a compound 5; 5) reducing the compound 5 into a compound 6 through boron tribromide; 6) synthesizing the puerarin by taking the compound 6 as a raw material; (2) the synthesis of puerarin morphine. Through the use of the puerarin morphine, the dosage of morphine is reduced, the addiction and tolerance of the morphine are reduced, side effects of the morphine in the aspects of the respiratory system, cardiovascular system and central nervous system are effectively slowed down, and the puerarin morphine can be used for pain treatment as a substitute for morphine.
Owner:XI AN JIAOTONG UNIV
Boron diffusion method and N-type solar cell preparation method
ActiveCN111146311AImprove uniformitySimple processFinal product manufactureSemiconductor/solid-state device manufacturingPhysical chemistryThin membrane
The invention provides a boron diffusion method. The boron diffusion method comprises the following steps: 1) selecting an N-type silicon substrate as a substrate to carry out double-sided texturing treatment; 2) carrying out wet oxidation on the N-type silicon substrate to form a silicon oxide layer on the silicon surface; 3) preparing a double-sided p+ doped region on the surface of the N-type silicon substrate by using boron tribromide as a boron source; 4) putting one surface of the N-type silicon substrate into a mixed solution of HF, HNO3 and H2SO4 for etching treatment to remove the silicon oxide layer on the back surface and the p+ doped region on the back surface and obtain an etched smooth pyramid surface, and putting an HF solution into the other surface of the N-type silicon substrate to remove the silicon oxide layer on the front surface. According to the invention, a silicon oxide film is prepared on the silicon surface before boron diffusion to prevent the formation of BRL; the silicon oxide layer prepared by a wet method is good in uniformity; the process is simple, and procedures do not need to be increased; the oxidation and HF cleaning processes are separately carried out, so the process window is greatly widened.
Owner:江苏杰太光电技术有限公司
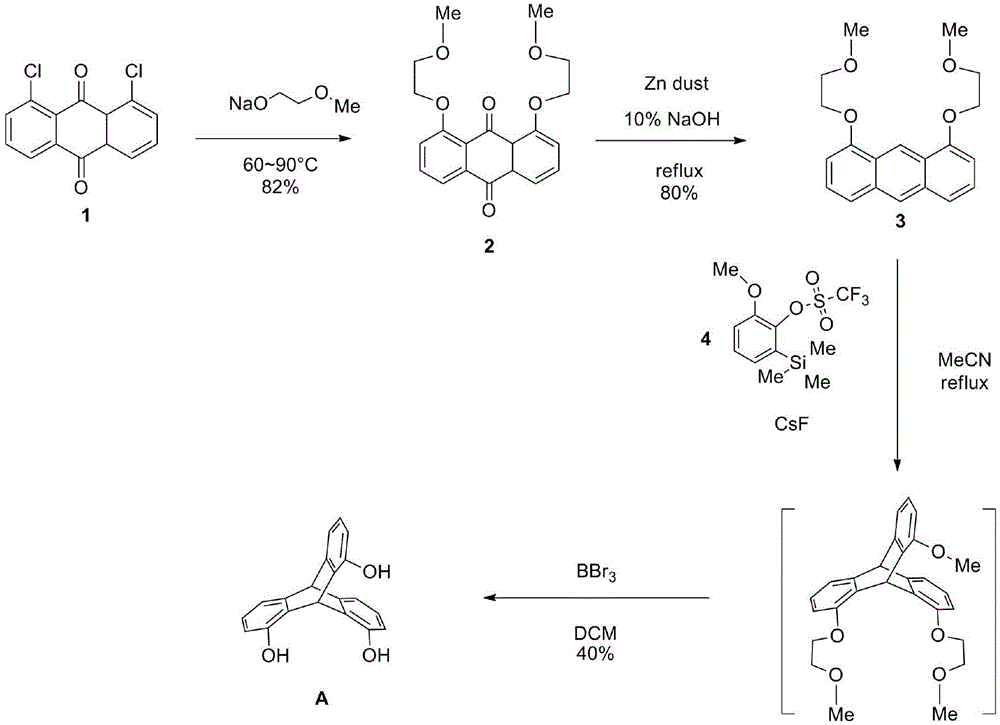
1,1',1''-trishydroxy triptycene and synthesis method thereof
InactiveCN104529716AGroup 4/14 element organic compoundsOrganic compound preparationTrifluoromethanesulfonic anhydrideSynthesis methods
The invention provides a novel compound 1,1',1''-trishydroxy triptycene A. The product is synthesized by the following steps: (1) producing 1,8-disubstituted anthraquinone from ethylene glycol monomethyl ether, metallic sodium and 1,8-dichloroanthraquinone; (2) reducing 1,8-disubstituted anthraquinone with zinc powder to obtain 1,8-disubstituted anthracene; (3) simultaneously, reacting bromine, tert-butylamine and guaiacol to obtain 2-bromo-6-methoxyphenol and reacting 2-bromo-6-methoxyphenol, sodium hydride, trimethylchlorosilane, n-butyllithium and trifluoromethanesulfonic anhydride to obtain a dehydrobenzene precursor; and (4) finally reacting 1,8-disubstituted anthracene and the dehydrobenzene precursor to produce triptycene and carrying out deprotection reaction on the crude product triptycene and boron tribromide to obtain the product 1,1',1''-trishydroxy triptycene. The product 1,1',1''-trishydroxy triptycene synthesized by the method has unique spatial structure and has potential application values in such fields as chemical bionics, molecular recognition, organic materials, and polymers with special functions.
Owner:CHONGQING UNIV
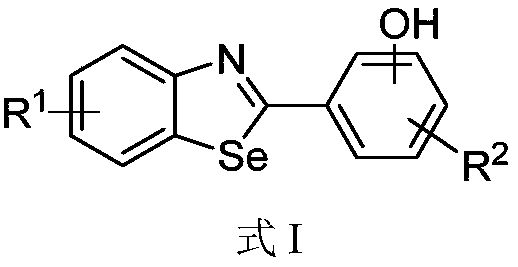
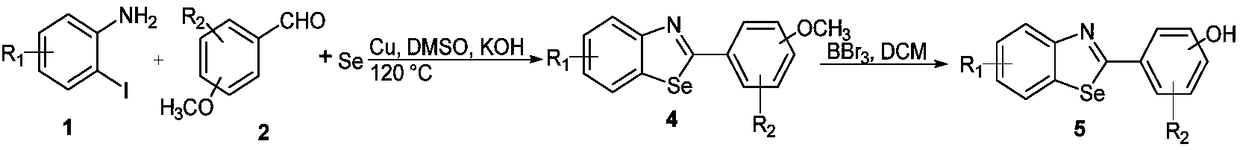
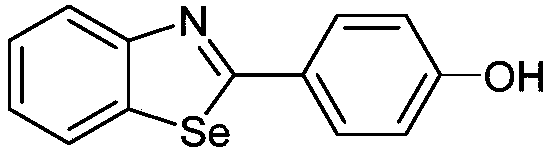
Benzoselenazole compound as well as preparation method and application thereof
InactiveCN108863985AERβ agonistic activity is goodMild conditionsOrganic chemistryAntineoplastic agentsDiseaseOestrogen receptor
The invention discloses a benzoselenazole compound as well as a preparation method and application thereof and belongs to the technical field of medicines. The preparation method of the benzoselenazole compound is characterized by preparing a series of methoxy-containing benzoselenazole compounds from o-iodoaniline derivatives, benzaldehyde derivatives and selenium under catalysis of copper through a one-pot process, and finally carrying out boron tribromide demethylation to obtain a hydroxy-containing benzoselenazole compound. In-vitro experiments show that the benzoselenazole compound as a ligand of an oestrogen receptor ER beta shows excellent agonist effect; the correlation research shows that the compound as an ERb ligand has treatment effect in animal model of rheumatic arthritis andintestinal diseases caused by inflammation, so that the benzoselenazole compound has potential treatment effect in treatment of rheumatic arthritis and intestinal diseases.
Owner:WUHAN UNIV
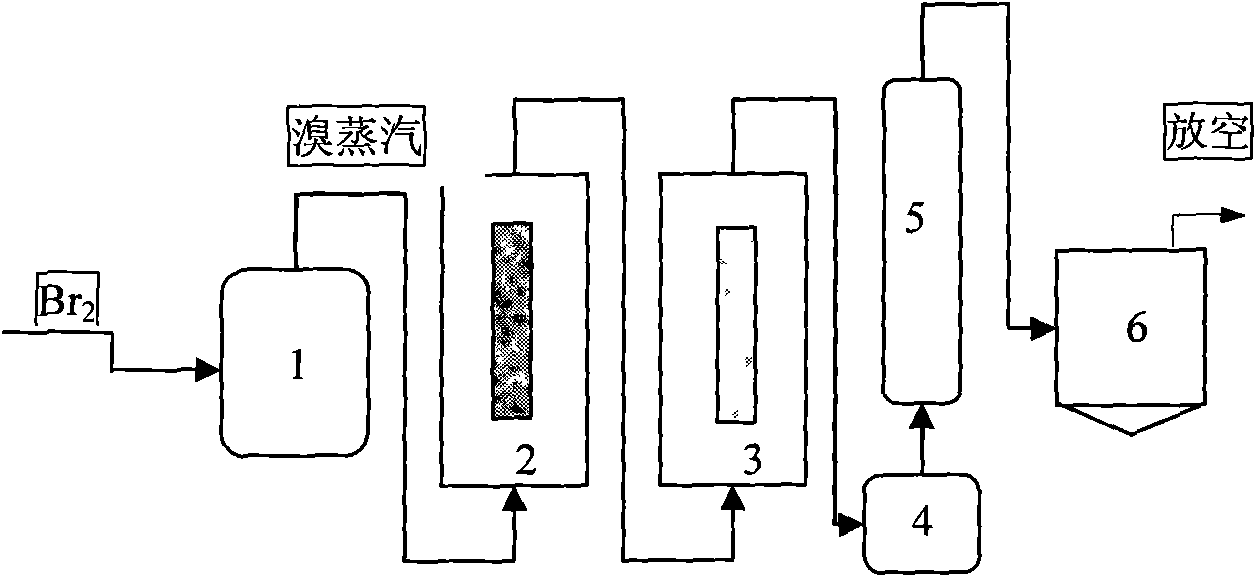
Method and device for producing high-purity boron tribromide
ActiveCN106829989AReduce manufacturing costSuitable for large-scale productionBoron halidesDistillationReaction temperature
The invention provides a method and device for preparing high-purity boron tribromide (BBr3) from boron carbide used as a raw material. The method comprises the following steps: vaporizing industrial grade liquid bromine through a vaporization kettle, introducing the bromine vapor into a drying chamber under the guidance action of a trace of nitrogen gas, removing moisture by means of anhydrous calcium chloride, feeding into a reaction furnace, and reacting with boron carbide particles at a reaction temperature of 650-850 DEG C to obtain boron tribromide containing impurities, dust and colors; performing ash removal on the boron tribromide through an ash blocker, removing residual bromine and iron ions by means of naphthol in a distillation kettle, and distilling to obtain white boron tribromide; and rectifying through a rectification tower, and removing high boiling point and low boiling point impurities to obtain a boron tribromide product of which the purity is greater than 5N, wherein the boron tribromide rectifying temperature is 120 DEG C, and the reflux ratio is 2-3.The high-purity boron tribromide preparation device provided by the invention is composed of the vaporization kettle, a dryer, the reaction furnace, the ash blocker, a condenser, the distillation kettle (for iron and bromine removal), the rectification tower and a finished product kettle. The purity of the boron tribromide produced by the invention can be up to 5N or above; and the method and device are low in cost and high in efficiency, and can realize industrial continuous production.
Owner:江西瑞合特种材料有限公司
Resveratrol synthesis preparation method
InactiveCN104326880AMild reaction conditionsHigh yieldOrganic compound preparationGroup 5/15 element organic compoundsXylyleneFood additive
The invention relates to a resveratrol preparation method which belongs to the field of food additives and preparing methods thereof. The resveratrol synthesis preparation method comprises the following steps: (1) using 4-methoxybenzyl bromide, triphenyl phosphine and xylene as raw materials for synthesis of triphenyl 4-methoxybenzyl phosphorus bromide (compound I); (2) using the compound I, tetrahydrofuran and 3, 5-dimethoxy benzaldehyde as raw materials for synthesis of a cis / trans-3, 4, 5-trimethoxy-1, 2-diphenylethene mixture (compounds II); (3) using the compound II, tetrahydrofuran and diphenyl disulfide as raw materials for synthesis of trans-3, 4, 5-trimethoxy-1, 2-diphenylethene (compound III); and (4) using the compound III, boron tribromide and methylene chloride as raw materials for synthesis of trans-3, 4, 5-trihydroxy-1, 2-diphenylethene (compound IV), namely resveratrol. The resveratrol synthesis preparation method has the advantages of mild reaction conditions, high yield (on the basis of the 4-methoxybenzyl bromide, the total yield is 66%), high purity (more than 98%) and the like.
Owner:西安莹朴生物科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
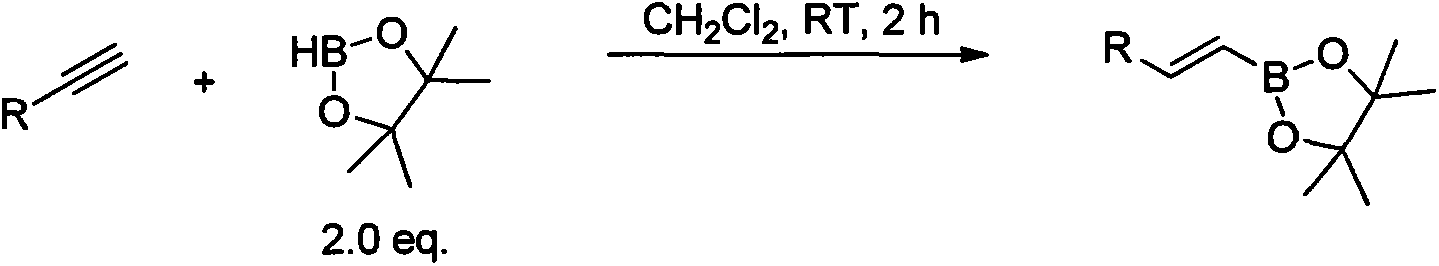

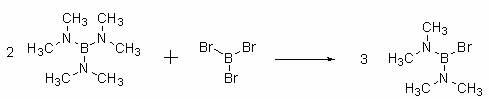
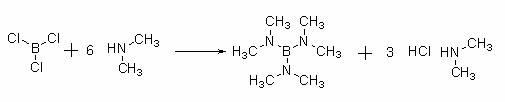
![Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate](https://images-eureka.patsnap.com/patent_img/a697c075-5f54-4317-bd82-eb08d7e49b7e/BDA0000782155620000011.PNG)
![Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate](https://images-eureka.patsnap.com/patent_img/a697c075-5f54-4317-bd82-eb08d7e49b7e/BDA0000782155620000021.PNG)
![Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate](https://images-eureka.patsnap.com/patent_img/a697c075-5f54-4317-bd82-eb08d7e49b7e/BDA0000782155620000022.PNG)