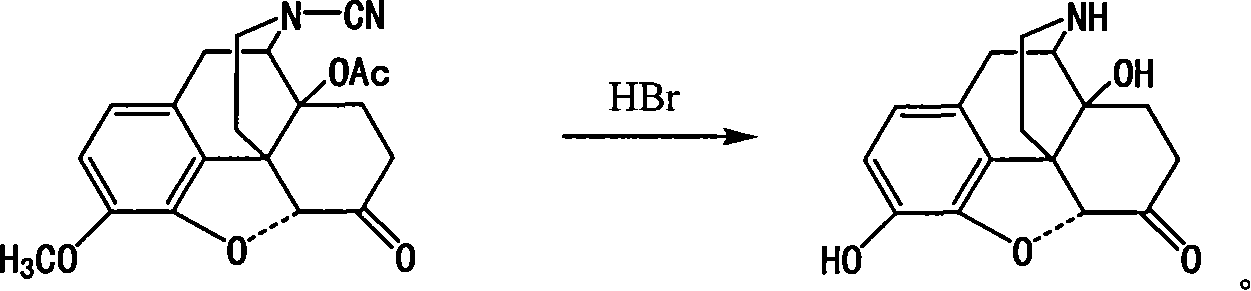
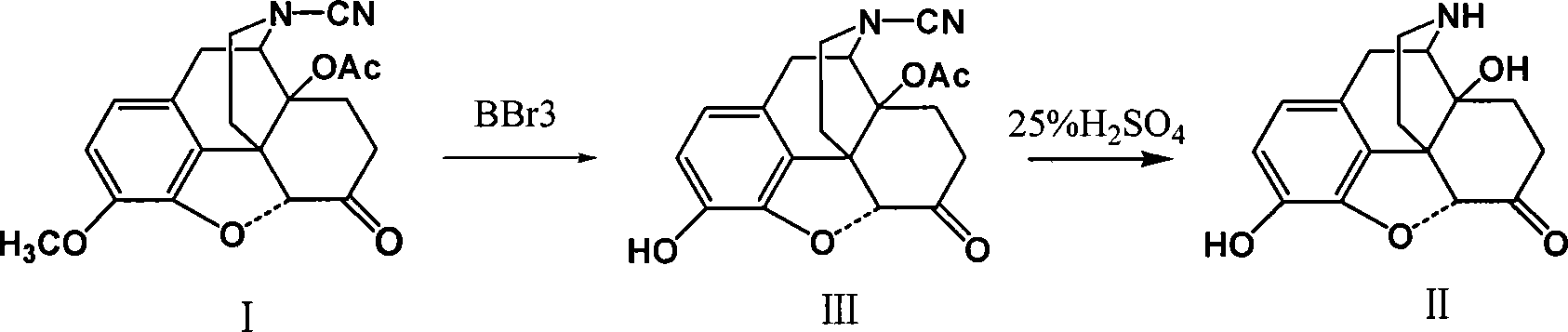
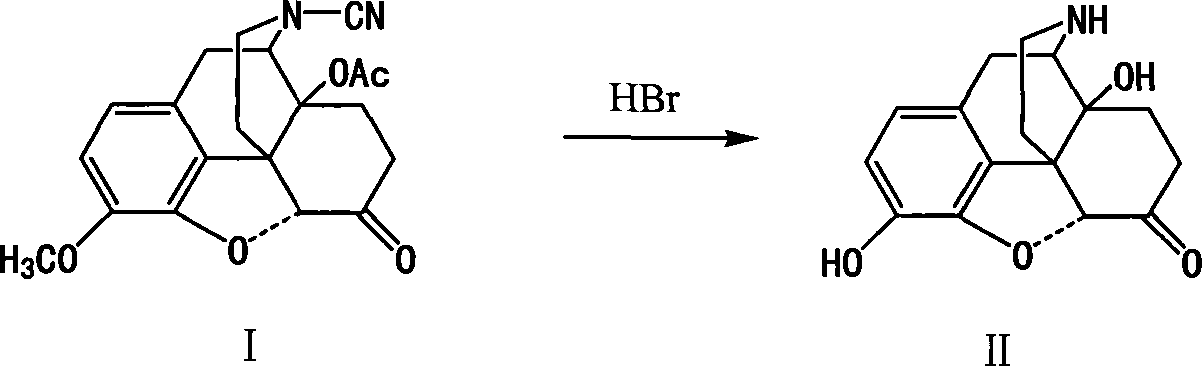
Method of preparing 14-hydroxy-7,8-dihydromorphone
A technology for dihydronormorphone and dihydronorcodeinone, which is applied in directions such as organic chemistry, can solve the problems of being unsuitable for large-scale production, high toxicity of boron tribromide, and high operating costs, and achieves low cost and simplification. Manipulation, effect of simplified reaction manipulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Compound I 10g (27.1mmol) was dissolved in 190ml of 20% hydrobromic acid, stirred at 118°C for 20h under nitrogen protection, cooled, and adjusted to alkaline with 25% ammonia water. Let stand, filter, wash with water, and dry to obtain 7.15g, yield 91%.
Embodiment 2
[0019] Compound I 10g (27.1mmol) was dissolved in 190ml of 25% hydrobromic acid, stirred at 118°C for 20h under nitrogen protection, cooled, and adjusted to alkaline with 25% ammonia water. Let stand, filter, wash with water, and dry to obtain 6.28g, yield 80%.
Embodiment 3
[0021] Compound I 10g (27.1mmol) was dissolved in 190ml of 30% hydrobromic acid, stirred at 118°C for 20h under nitrogen protection, cooled, and adjusted to alkaline with 25% ammonia water. Let stand, filter, wash with water, and dry to obtain 5.90 g, yield 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com